Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Macrocyclic geminal diols: synthesis, structures, stability and photophysical properties†
Bo
Zou
a,
Xiaolin
Chen
a,
Haoran
Liu
a,
Sijie
Wen
a,
Jieqing
Huang
a,
Hengshan
Wei
a,
Jinqing
Huang
 b,
Yucheng
Gu
b,
Yucheng
Gu
 c,
Bingjia
Xu
c,
Bingjia
Xu
 *d,
Jun
Fan
*d,
Jun
Fan
 *a and
Hua-Wei
Jiang
*a and
Hua-Wei
Jiang
 *a
*a
aSchool of Chemistry, South China Normal University, Guangzhou 510006, China. E-mail: fanj@scnu.edu.cn; hwjiang@m.scnu.edu.cn
bDepartment of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
cSyngenta Jealott's Hill International Research Centre, Bracknell, Berkshire, UK
dSchool of Environmental and Chemical Engineering, Wuyi University, Jiangmen, 529020, China. E-mail: bingjiaxu@m.scnu.edu.cn
First published on 12th November 2025
Abstract
Geminal diols are generally unstable and prone to dehydration, yielding carbonyl compounds and making their isolation as discrete species highly challenging. Herein, we report the synthesis, structural characterization, and stability of a series of crystalline, stable, and rigid macrocyclic gem-diols obtained via acid hydrolysis of macrocyclic ketal precursors at −25 °C. Single-crystal X-ray diffraction analysis of these compounds reveals O–C–O bond angles of approximately 111°, along with extensive hydrogen-bonding networks that contribute to stabilizing the gem-diols. Thermogravimetric and hydrolytic analyses reveal a pronounced size-dependent stability trend. Theoretical calculations indicate that the enhanced stability of smaller gem-diol macrocycles stems from their ability to relieve substantial angle strain via sp3 hybridization at the methylene carbon, an effect that diminishes as ring size increases. Based on our experimental and computational results, the inner angle value of the macrocyclic ketone is proposed as a criterion for evaluating the relative stability of macrocyclic ketones versus their gem-diol forms. The photophysical properties of the macrocyclic geminal diols and macrocyclic diketones are also examined. This work broadens the scope of stable geminal diols and provides fundamental insights into their structure–stability relationships, thereby laying the groundwork for the strategic design and synthesis of structurally diverse macrocyclic geminal diols.
Introduction
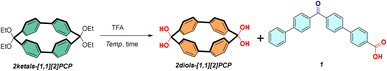
A geminal diol (gem-diol) is defined as a diol in which two hydroxyl groups are bonded to the same carbon atom. These species are recognized as key intermediates in a variety of organic and biochemical transformations,1–5 as well as in atmospheric processes,6–8 and they hold considerable potential for applications in organic synthesis,9 environmental chemistry,10 biochemistry,11,12 and coordination chemistry.13–20gem-Diols readily undergo dehydration to yield carbonyl compounds, making them generally difficult to isolate (Fig. 1a). For example, methanediol—the simplest geminal diol—although known to exist predominantly as a stable species in equilibrium with formaldehyde in aqueous solution,21 was not successfully isolated in the gas phase until 2022 by Kaiser et al.22 The stability of geminal diols is primarily influenced by steric hindrance, hydrogen bonding, strong electron-withdrawing effects, and ring strain.23–27 For instance, acetone forms its hydrate less readily than formaldehyde owing to steric hindrance,21 while chloral hydrate28 and ninhydrin hydrate29 are stabilized by strong electron-withdrawing groups. Although ring strain is recognized as an important contributor to the stability of geminal diols, only a limited number of strain-stabilized examples have been reported to date. 1,1-Cyclopropanediol, the smallest cyclic diol, can be synthesized via hydration of cyclopropenone,30,31 and its stability is attributed to the substantial angle strain of the three-membered ring, which enables its isolation as a stable compound.21,23 However, 1,1-cyclobutanediol, 1,1-cyclopentanediol, and 1,1-cyclohexanediol can only be formed in small amounts at equilibrium with their corresponding ketones and water (Fig. 1b).21,32 Additionally, coordination of a metal ion to certain polycyclic compounds can increase strain and promote gem-diol formation.33,34 Recent years have witnessed substantial progress in the synthesis of strained macrocyclic organic compounds.35–50 Macrocyclic organic compounds, in contrast to aliphatic small-ring analogues, exhibit greater structural diversity and offer enhanced potential for rational design and functional modification. Therefore, investigating the synthesis, structures, and stability of strained macrocyclic diols is of considerable interest.We previously accessed two series of rigid porphyrin wheel β–β connected and biphenylene-linked—via platinum-mediated cyclization, with the highest strain energies of each series reaching 77.4 kcal mol−1 and 49.3 kcal mol−1, respectively.51,52 One-dimensional porphyrin nanotubes were also constructed via hydrogen-bond-driven self-assembly of porphyrin wheels bearing carboxylic acid groups.53 In 2022, we first reported [n]CTPE and [n]CHPE—two rigid macrocycles composed of para-biphenyl units and ylidene linkers (Fig. 1c, left).54 [2]CTPE exhibited distinctive dual-state emission properties and a long solid-state fluorescence lifetime, whereas larger macrocycles displayed enhanced aggregation-induced emission relative to the TPE monomer, indicating size-dependent physicochemical behavior. We subsequently made considerable efforts to synthesize analogous biphenylene macrocycles with varied bridging units. During this period, in 2025, the Yamago group reported similar rigid macrocycles, [1.1][n]paracyclophanes ([1,1][n]PCPs) (Fig. 1c, right),55 composed of para-biphenyl units connected by methylene bridges, and confirmed the presence of through-space conjugation between biphenyl units within the macrocycles. Here, we report the synthesis of macrocyclic gem-diols (2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, diol-2Me-[1.1][2]PCP) via hydrolysis of the corresponding ketal precursors (2ketals-[1.1][2]PCP, 2ketals-[1.1][3]PCP, 2ketals-[1.1][4]PCP, ketal-2Me-[1.1][2]PCP). The structures of these macrocyclic gem-diols, as well as two macrocyclic diketones (2ketones-[1.1][3]PCP, 2ketones-[1.1][4]PCP), were elucidated. The thermal stability of these macrocyclic gem-diols was investigated using thermogravimetric analysis (TGA) and further evaluated through theoretical calculations.
Results and discussion
Synthesis of macrocyclic gem-diols and macrocyclic diketones
The precursors of the 2ketals-[1.1][n]PCPs were synthesized through platinum-mediated cyclization followed by reductive elimination (see SI). The macrocyclic gem-diol 2diols-[1.1][2]PCP was synthesized by subjecting 2ketals-[1.1][2]PCP to an excess of trifluoroacetic acid (TFA) (Table 1). After complete conversion of 2ketals-[1.1][2]PCP, the acid was neutralized with a saturated aqueous sodium bicarbonate solution. The resulting mixture was filtered, and the crude product was recrystallized to yield the pure macrocyclic gem-diol. The acid hydrolysis conditions were optimized using 2ketals-[1.1][2]PCP as a model compound. It was found that TFA proved to be a suitable acid for the efficient hydrolysis of 2ketals-[1.1][2]PCP. Parallel reactions were performed over 30 minutes at 70 °C, 25 °C, 0 °C, and −25 °C (Table 1), and yields were determined by 1H NMR spectroscopy. At 70 °C, the desired gem-diol product was not obtained; instead, the starting material was completely converted into the ring-opening product 1, identified as 4′-([1,1′-biphenyl]-4-carbonyl)-[1,1′-biphenyl]-4-carboxylic acid. At 25 °C, the desired 2diols-[1.1][2]PCP was obtained in 43% yield together with product 1 in 57% yield, whereas at 0 °C, the yield of 2diols-[1.1][2]PCP increased to 87%, with 13% of 1 formed. At −25 °C,the desired product was obtained in 96% yield, with only 4% of 1 observed. The reaction time also affected the product distribution. Thin-layer chromatography showed incomplete conversion at 20 min, while full conversion was achieved at 30 min with the highest yield of 2diols-[1.1][2]PCP. Prolonged reaction times led to increased formation of byproduct 1. Thus, TFA at −25 °C for 30 min was selected as the optimal condition for hydrolysis, affording 2diols-[1.1][2]PCP in 78% isolated yield after recrystallization. The hydrolysis of 2dbts-[1.1][2]PCP, in which methylene carbons are connected to 1,4-dioxabutan-1,4-diyl units, did not proceed under the optimized conditions, likely due to the enhanced stability of the five-membered rings (see SI).| Entry | Temp. | Time | Yield b of 2diols-[1.1][2]PCP | Yield b of 1 |
|---|---|---|---|---|
| a All the reactions were carried out using 2ketals-[1.1][2]PCP (20 mg, 0.04 mmol) and trifluoroacetic acid (3 ml). b Determined by 1H NMR spectroscopy unless otherwise noted. c Isolated yield. | ||||
| 1 | −25 °C | 30 min | 96% (78%)c | 4% |
| 2 | 0 °C | 30 min | 87% | 13% |
| 3 | 25 °C | 30 min | 43% | 57% |
| 4 | 70 °C | 30 min | 0% | 100% |
Acid hydrolysis of the macrocyclic ketal precursors 2ketals-[1.1][3]PCP and ketal-2Me-[1.1][2]PCP under the optimized conditions afforded 2diols-[1.1][3]PCP and diol-2Me-[1.1][2]PCP in isolated yields of 79% and 58%, respectively (Scheme 1a and 1c). However, hydrolysis of 2ketals-[1.1][4]PCP afforded the macrocyclic diketone 2ketones-[1.1][4]PCP in 80% isolated yield, and no gem-diol product was detected (Scheme 1b). The transformation of 2diols-[1.1][2]PCP and 2diols-[1.1][3]PCP into their respective ketone counterparts was then attempted by heating in toluene under reflux for 5 h. While 2diols-[1.1][2]PCP remained unchanged, 2diols-[1.1][3]PCP was successfully converted into 2ketones-[1.1][3]PCP (78% isolated yield) (Scheme 1a). The characterization data of 2ketones-[1.1][3]PCP were consistent with those reported by Yamago group.56 Interestingly, when the ethyl acetate solution of 2ketones-[1.1][3]PCP was exposed to ambient air for 48 h, it quantitatively reverted to 2diols-[1.1][3]PCP (Scheme 1a). In contrast, 2ketones-[1.1][4]PCP remained unchanged under identical conditions (Scheme 1b).
 | ||
| Scheme 1 Synthesis of (a) 2diols-[1,1][3]PCP and 2ketones-[1,1][3]PCP, (b) 2ketones-[1,1][4]PCP (c) diol-2Me-[1,1][2]PCP. | ||
Structure of macrocyclic gem-diols and macrocyclic diketones
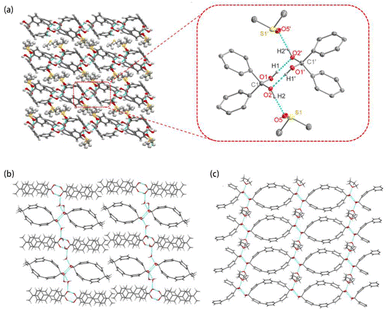
The 1H NMR spectra of 2diols-[1.1][2]PCP and diol-2Me-[1.1][2]PCP each exhibited a singlet at δ = 6.86 ppm, attributable to the geminal diol protons (see SI, Fig. S1a and S1c). The geminal diol carbon in both compounds appeared at δ = 97.48 ppm in the 13C NMR spectrum (see SI, Fig. S32 and S36). For 2diols-[1.1][3]PCP, a similar singlet appeared at δ = 6.96 ppm in the 1H NMR spectrum (see SI, Fig. S1b), and the corresponding carbon resonance was detected at δ = 96.64 ppm in the 13C NMR spectrum. In contrast, the 13C NMR spectra of 2ketones-[1.1][3]PCP and 2ketones-[1.1][4]PCP exhibited resonances at δ = 201.06 ppm and δ = 200.83 ppm, respectively, which were assigned to the carbonyl carbons. MALDI-TOF mass spectra of the three macrocyclic geminal diols (2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and diol-2Me-[1.1][2]PCP) and two macrocyclic diketones (2ketones-[1.1][3]PCP and 2ketones-[1.1][4]PCP) showed molecular ion peaks at m/z = 396.138, m/z = 548.158, m/z = 392.176, m/z = 513.138 and m/z = 664.247, in agreement with their calculated values of C26H20O4+ [M]+: 396.136, C38H28O4+ [M + H]+: 548.199, C28H24O2+ [M]+: 392.178, C38H25O2+ [M + H]+: 513.184 and C50H32O2+ [M]+: 664.240, respectively.To gain further insight into the structural characteristics of these macrocyclic geminal diols, single crystals were grown of 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and diol-2Me-[1.1][2]PCP, as well as the macrocyclic diketone 2ketones-[1.1][4]PCP and the ketal precursors ketal-2Me-[1.1][2]PCP, 2ketals-[1.1][2]PCP, 2ketals-[1.1][3]PCP, and 2ketals-[1.1][4]PCP. Their structures were unambiguously confirmed by single-crystal X-ray diffraction analysis (Fig. 2).
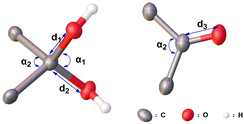
Each molecule exhibited an elliptical cavity, with the cavity size increasing as the ring size expanded. The exocyclic O–C–O bond angle (α1) in 2diols-[1.1][2]PCP (111.74°) is slightly larger than that in 2diols-[1.1][3]PCP (110.55°), whereas the endocyclic C–C–C angle (α2) in 2diols-[1.1][2]PCP (101.20°) is about 3° smaller than that in 2diols-[1.1][3]PCP (104.03°), indicating greater angular strain in the former due to its more constrained ring size. The α2 in 2diols-[1.1][2]PCP and 2diols-[1.1][3]PCP are close to those observed in 2ketals-[1.1][2]PCP (101.11°) and 2ketals-[1.1][3]PCP (103.44°), suggesting that the overall macrocyclic strain remains nearly unchanged before and after hydrolysis. For 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and diol-2Me-[1.1][2]PCP, the C–O bond lengths of the geminal hydroxyl groups (1.41–1.42 Å) are longer than those in hexafluoroacetone hydrate (1.38 Å), confirming that the stabilization of these macrocyclic geminal diols arises from ring strain rather than electron effects.57 In diol-2Me-[1.1][2]PCP, the intramolecular O⋯O distance (2.33 Å) and exocyclic O–C–O angle (111.05°) are nearly identical to those in 2diols-[1.1][2]PCP, indicating that despite its lower symmetry, the geminal diol geometry remains essentially unchanged.
Geminal diols can easily form hydrogen bonds with solvent molecules,58 which may contribute to their stabilization. Extensive hydrogen-bonding networks are observed in the macrocyclic geminal diol 2diols-[1.1][2]PCP (Fig. 3a). The hydroxyl groups of the geminal diol moieties form eight-membered ring structures through intermolecular hydrogen bonding with adjacent macrocycles. In addition, one hydroxyl hydrogen of the geminal diol moiety forms a hydrogen bond with an oxygen atom of the solvent molecule dimethyl sulfoxide. These intermolecular hydrogen bonds (O(1)–H(1)⋯O(2′) and O(1′)–H(1′)⋯O(2)) exhibit identical O⋯O distances of 2.74 Å. Hydrogen bonds are observed between 2diols-[1.1][2]PCP and dimethyl sulfoxide solvent molecules, with O⋯O distances of 2.64 Å (O(2)–H(2)⋯O(5) and O(2′) –H(2′)⋯O(5′)). Similar hydrogen-bonding networks are also observed in 2diols-[1.1][3]PCP and diol-2Me-[1.1][2]PCP (Fig. 3b and c). It is worth mentioning that while 2diols-[1.1][2]PCP contains two geminal diol moieties, diol-2Me-[1.1][2]PCP contains only one. As a result, the density of the hydrogen-bonding network in diol-2Me-[1.1][2]PCP is lower, which may contribute to its reduced structural stability compared to 2diols-[1.1][2]PCP.
For the macrocyclic diketone 2ketones-[1.1][4]PCP, the distance between the oxygen atom and its closest carbon atom is 1.22 Å, consistent with a typical carbonyl double bond. The bond angles C(3)–C(1)–C(50) and C(26)–C(2)–C(27) are 112.84° and 113.19°, respectively, which are significantly smaller than the corresponding angle in benzophenone (116.90°),59 indicating considerable ring strain. Upon conversion from 2ketals-[1.1][4]PCP to 2ketones-[1.1][4]PCP, the hybridization of the bridging carbon changes from sp3 to sp2 due to the formation of carbonyl groups. This transformation leads to a substantial increase in the biphenyl dihedral angle α from 106.01° to 112.92°, as well as an increase in the bending angle β of the internal phenyl units from 5.47° to 8.46°. These changes indicate that the elliptical cavity of 2ketones-[1.1][4]PCP has a smaller eccentricity than that of 2ketals-[1.1][4]PCP, suggesting that 2ketones-[1.1][4]PCP accommodates greater ring strain (Table 2).
| Compound | d 1 (Å) | d 2 (Å) | d 3 (Å) | α 1 (°) | α 2 (°) |
|---|---|---|---|---|---|
a Define as the C–O bond lengths d1.
b Define as the C–O bond lengths d2.
c The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths of the carbonyl group in the macrocyclic diketones.
d Define α1 as the exocyclic O–C–O bond angle.
e Define α2 as the intracyclic C–C–C bond angle.
f Not applicable. O bond lengths of the carbonyl group in the macrocyclic diketones.
d Define α1 as the exocyclic O–C–O bond angle.
e Define α2 as the intracyclic C–C–C bond angle.
f Not applicable.
|
|||||
| diol-2Me-[1,1][2]PCP | 1.41 | 1.41 | —f | 111.05 | 101.45 |
| 2diols-[1,1][2]PCP | 1.41 | 1.41 | —f | 111.74 | 101.20 |
| 2diols-[1,1][3]PCP | 1.42 | 1.41 | —f | 110.55 | 104.03 |
| 2ketones-[1,1][4]PCP | —f | —f | 1.22 | —f | 113.19 |
Thermal stability of the macrocyclic gem-diols
The thermal stability of 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and diol-2Me-[1.1][2]PCP was evaluated by thermogravimetric analysis (TGA). The powders of 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and diol-2Me-[1.1][2]PCP used for TGA were prepared by recrystallization from ethyl acetate/n-hexane, followed by vacuum drying for 24 hours. As shown in Fig. 4, 2diols-[1.1][2]PCP (black line) exhibited no weight loss until 150 °C. Then, between 150 °C and 210 °C, an approximate 8% weight loss was observed, corresponding to the release of two water molecules. Upon continued heating above 205 °C, the sample gradually underwent complete decomposition. The results implied that, although 2diols-[1.1][2]PCP could dehydrate and likely form 2ketones-[1.1][2]PCP, the latter is not sufficiently stable to be isolated. This observation is consistent with the results of the heating experiments on 2diols-[1.1][2]PCP mentioned above. For diol-2Me-[1.1][2]PCP (Fig. 4, cyan line), an initial approximate 2% weight loss from 30 °C to 110 °C was observed, which can be attributed to the loss of solvent molecules engaged in hydrogen bonding with the geminal diols. Between 140 °C and 190 °C, an approximate 6% weight loss may be attributed to the release of a water molecule. In the case of 2diols-[1.1][3]PCP (Fig. 4, green line), a weight loss of approximately 4% between 100 °C and 160 °C corresponds to the elimination of two water molecules. Above 160 °C, the TGA curve exhibited a distinct plateau, indicating the formation of 2ketones-[1.1][3]PCP. 2ketones-[1.1][3]PCP remained thermally stable over a broad temperature range, consistent with the dehydration experiments described in the synthesis section.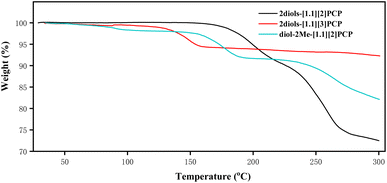 | ||
| Fig. 4 Thermogravimetric analysis of 2diols-[1,1][2]PCP, 2diols-[1,1][3]PCP and diol-2Me-[1,1][2]PCP, measured under nitrogen atmosphere. | ||
TGA measurements indicate that 2diols-[1.1][2]PCP is more thermally stable than diol-2Me-[1.1][2]PCP, which may be attributed to the more extensive intermolecular hydrogen-bonding network present in 2diols-[1.1][2]PCP. 2diols-[1.1][2]PCP and diol-2Me-[1.1][2]PCP exhibit greater thermal stability than 2diols-[1.1][3]PCP. These results, along with the observation that acid hydrolysis of 2ketals-[1.1][4]PCP did not yield the macrocyclic geminal diol product, suggest that the stability of macrocyclic geminal diols depends on ring size; specifically, increasing the ring size leads to reduced thermal stability. This trend is likely attributed to the reduction of angular strain in larger macrocycles, which renders the geminal diol functionality less thermodynamically favored.
DFT calculation
To further elucidate the structure–stability relationships, gas-phase geometry optimizations and strain energy calculations60–62 were performed at the WB97XD/6-311G(d) level for macrocyclic gem-diols—including diol-2Me-[1,1][2]PCP and 2diols-[1,1][n]PCPs—their corresponding macrocyclic diketones (ketone-2Me-[1,1][2]PCP and 2ketones-[1,1][n]PCPs), and the respective macrocyclic ketals (ketal-2Me-[1,1][2]PCP and 2ketals-[1,1][n]PCPs) (See SI, Fig. S74). The optimized core structures are in good agreement with the corresponding single-crystal X-ray structures (Table 3).| Entry | Compound | Strain energy (kcal mol−1) | The inner angle (°) |
|---|---|---|---|
| 1 | ketal-2Me-[1,1][2]PCP | 50.29 | 100.97 |
| diol-2Me-[1,1][2]PCP | 45.96 | 101.86 | |
| ketone-2Me-[1,1][2]PCP | 57.51 | 104.11 | |
| 2 | 2ketals-[1,1][2]PCP | 43.89 | 100.90 |
| 2diols-[1,1][2]PCP | 45.33 | 101.82 | |
| 2ketones-[1,1][2]PCP | 68.94 | 104.23 | |
| 3 | 2ketals-[1,1][3]PCP | 29.64 | 102.74 |
| 2diols-[1,1][3]PCP | 31.33 | 103.65 | |
| 2ketone-[1,1][3]PCP | 52.98 | 108.58 | |
| 4 | 2ketals-[1,1][4]PCP | 25.70 | 104.10 |
| 2diols-[1,1][4]PCP | 26.12 | 105.96 | |
| 2ketones-[1,1][4]PCP | 46.40 | 112.71 |
The strain energies of 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and 2diols-[1.1][4]PCP were calculated to be 45.33, 31.33, and 26.12 kcal mol−1, respectively, whereas those of 2ketones-[1.1][2]PCP, 2ketones-[1.1][3]PCP, and 2ketones-[1.1][4]PCP were 68.94, 52.98, and 46.40 kcal mol−1, respectively. In all three comparisons the strain energies of the gem-diol forms are more than 20 kcal mol−1 lower than those of the corresponding ketone forms. The strain energy of diol-2Me-[1.1][2]PCP is 11.55 kcal mol−1 lower than that of ketone-2Me-[1.1][2]PCP; this difference is approximately half of the aforementioned strain energy gaps, consistent with the fact that diol-2Me-[1.1][2]PCP contains only one gem-diol moiety. The strain energies of the macrocyclic ketals are similar to those of the corresponding macrocyclic gem-diols but are lower than those of the corresponding macrocyclic diketones.
The calculated internal angles for 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and 2diols-[1.1][4]PCP are 101.82°, 103.65°, and 105.96°, respectively, which are smaller than those of the corresponding diketones (2ketones-[1.1][2]PCP, 2ketones-[1.1][3]PCP, 2ketones-[1.1][4]PCP: 104.23°, 108.58°, 112.71°, respectively). The calculated internal angles of the ketals (2ketals-[1.1][2]PCP, 2ketals-[1.1][3]PCP, 2ketals-[1.1][4]PCP) are 101.86°, 102.74°, and 104.10°, respectively, showing an increasing trend with macrocycle size that aligns well with single-crystal X-ray data. On the basis of these computational and experimental results, we propose that the calculated internal angle of macrocyclic diketones can be used as a predictive indicator of relative stability: for internal angles ≈ 108° both ketone and gem-diol forms may be thermodynamically accessible; for internal angles ≤ 104° the gem-diol form is strongly favored; and for internal angles ≥ 112° the ketone form is strongly favored.
We compared the strain distribution of 2diols-[1,1][n]PCPs (n = 2, 3, 4) and 2ketones-[1,1][n]PCPs (n = 2, 3, 4) by strain visualization using StrainViz.63 For 2diols-[1,1][n]PCPs (n = 2, 3, 4) and 2ketones-[1,1][n]PCPs, the highest strain energy is located at the Cphenyl–Cgem-diol and Cphenyl–Ccarbonyl bonds, respectively (see SI, Fig. S84). The highest strain energies in 2ketones-[1,1][n]PCPs are approximately 1.5 to 2.5 times higher than those in the corresponding 2diols-[1,1][n]PCPs.
These results indicate that an increase in macrocycle size leads to a pronounced decrease in ring strain energy, while dehydration of gem-diols to the corresponding sp2-hybridized carbonyl compounds significantly increases the ring strain energy across different macrocycle sizes. Thus, from the perspective of ring strain energy, the formation of geminal diols within strained macrocyclic frameworks enhances stability.
Photophysical properties
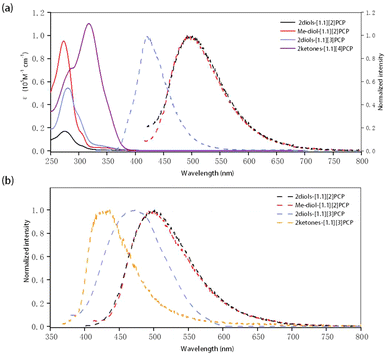
The UV-vis absorption properties of these macrocycles in dilute THF solution were investigated, and the results are presented in Fig. 5, Tables 4 and S24. The absorption bands of the macrocyclic gem-diols, attributed to π–π* electronic transitions, were observed in the range of 273 to 280 nm. A distinct redshift in the maximum absorption wavelengths was observed with increasing macrocycle size, which is likely due to the extended conjugation of biphenyl units as the ring size increases. For macrocyclic diketones, the maximum absorption wavelength (λmax) of 2ketones-[1.1][4]PCP was measured to be 317 nm. However, due to the rapid conversion of 2ketones-[1.1][3]PCP to 2diols-[1.1][3]PCP in THF, the photophysical properties of 2ketones-[1.1][3]PCP could not be obtained in this solvent.| Compound | λ abs (nm) | λ PL,s (nm) | λ PL,sl (nm) | Φ PL,s (%) | Φ PL,sl (%) |
|---|---|---|---|---|---|
| a UV-visible maximum absorption in THF solution. b PL maximum in solid state. c PL maximum in THF solution. d Absolute photoluminescence quantum yield in solid state. e Absolute photoluminescence quantum yield in THF solution. | |||||
| diol-Me-[1,1][2]PCP | 273 | 503.5 | 491.5 | 10.13 | 12.73 |
| 2diols-[1,1][2]PCP | 274 | 501 | 491.5 | 17.74 | 6.92 |
| 2diols-[1,1][3]PCP | 280 | 425.5 | 420.5 | 20.55 | 38.23 |
| 2ketones-[1,1][3]PCP | — | 474 | — | 6.72 | — |
| 2ketones-[1,1][4]PCP | 317 | — | — | — | — |
These macrocyclic gem-diols exhibit strong fluorescence in both THF solution and the solid state. In THF solution, the maximum emission wavelengths (λPL,sl) of 2diols-[1.1][2]PCP, diol-2Me-[1.1][2]PCP, and 2diols-[1.1][3]PCP were determined to be 491.5 nm, 491.5 nm, and 420.5 nm, respectively, comparable to those of their corresponding ketal precursors (see SI). A blueshift in emission was observed with increasing macrocycle size, likely due to the relaxation of ring strain. The photoluminescence quantum yields (ΦPL,sl) of 2diols-[1.1][2]PCP, diol-2Me-[1.1][2]PCP, and 2diols-[1.1][3]PCP in THF were 6.92%, 12.73%, and 38.23%, respectively. Similar to their behavior in THF, 2diols-[1.1][2]PCP, diol-2Me-[1.1][2]PCP, and 2diols-[1.1][3]PCP exhibit solid-state maximum emission wavelengths (λPL,s) of 501 nm, 503.5 nm, and 425.5 nm, respectively. Their corresponding solid-state ΦPL,s values were 17.74%, 10.13%, and 20.55%. For macrocyclic diketones, no visible emission was detected for 2ketones-[1.1][4]PCP. In contrast, 2ketones-[1.1][3]PCP exhibits solid-state emission with a maximum emission wavelength of 474 nm and a ΦPL,s of 6.72%. Such shifts and changes in quantum yields may be attributed to alterations in conjugation length and ring strain, which modulate the electronic structures and non-radiative decay pathways of these macrocycles.
Conclusion
In summary, a series of crystalline, stable, and rigid macrocyclic gem-diols and macrocyclic diketones were successfully synthesized via acid hydrolysis of macrocyclic ketals at −25 °C. Single-crystal X-ray diffraction analysis of 2diols-[1.1][2]PCP, 2diols-[1.1][3]PCP, and diol-2Me-[1.1][2]PCP reveals O–C–O bond angles of approximately 111°. Notably, these macrocycles form a robust eight-membered hydrogen-bonded structure in the solid state, which likely contributes to their ordered packing and enhanced stability. Thermogravimetric analysis reveals a clear, ring size-dependent thermal stability. 2diols-[1.1][2]PCP remains stable up to 150 °C, and diol-2Me-[1.1][2]PCP begins to dehydrate at around 140 °C. 2diols-[1.1][3]PCP starts to dehydrate at approximately 100 °C, whereas 2diols-[1.1][4]PCP could not be obtained under similar conditions. The strain energies of macrocyclic gem-diols are consistently lower than those of their dehydrated ketone counterparts. This difference can be attributed to the sp3-hybridized gem-diol carbon atoms, which better accommodate angular strain than the planar sp2-hybridized carbons in the ketones. We propose that the calculated inner angles of macrocyclic ketones can be used to assess the relative stability of macrocyclic ketones versus their gem-diol forms. The successful synthesis of this new class of macrocyclic geminal diols not only expands the library of stable gem-diol compounds but also provides deeper insights into the structural features and stability factors of this important functional group, thereby laying a solid foundation for the design and synthesis of more diverse macrocyclic gem-diol molecules.Author contributions
H.-W. Jiang initiated and designed the project. Supervision of the research was provided by H.-W. Jiang, B. Xu, and J. Fan. B. Zou performed the primary experiments and conducted corresponding data analysis. X. Chen contributed to part of the experimental work. H. Wei was responsible for the majority of the photophysical measurements. H. Liu, S. Wen, and J. Huang aided in data collection and analysis. The initial draft of the manuscript was prepared by B. Zou. All authors participated in the discussion and revision of the manuscript.Conflicts of interest
The authors declare no competing financial interests.Data availability
CCDC 2479706 (diol-2Me-[1.1][2]PCP), 2479718 (2diols-[1.1][2]PCP), 2479720 (2diols-[1.1][3]PCP), 2479725 (2ketones-[1.1][4]PCP), 2479728 (2dbts-[1.1][2]PCP), 2479740 (ketal-2Me-[1.1][2]PCP), 2479742 (2ketals-[1.1][4]PCP), 2479743 (2ketals-[1.1][2]PCP) and 2479744 (2ketals-[1.1][3]PCP) contain the supplementary crystallographic data for this paper.64a–iSupplementary information: experimental procedures, characterization data for compounds, NMR spectra, thermogravimetric analysis, and X-ray crystallographic data. See DOI: https://doi.org/10.1039/d5sc08216a.
Acknowledgements
This work was supported by the Natural Science Foundation of Guangdong Province, China (grant no. 2022A1515012199) and the 2022 Guangdong–Hong Kong–Macao Greater Bay Area Exchange Programs of South China Normal University. The authors gratefully acknowledge Prof. Ji-Chang Xiao and Prof. Xiaopeng Li at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Prof. Jianxin Song at the College of Chemistry and Chemical Engineering of Hunan Normal University for their assistance with MALDI-TOF mass spectrometry measurements.Notes and references
- J.-J. Li, C. Li, C. A. Blindauer and T. D. H. Bugg, Evidence for a gem-Diol Reaction Intermediate in Bacterial C–C Hydrolase Enzymes BphD and MhpC from 13C NMR Spectroscopy, Biochemistry, 2006, 45, 12461–12469 CrossRef CAS.
- F. K. Yoshimoto and F. P. Guengerich, Mechanism of the Third Oxidative Step in the Conversion of Androgens to Estrogens by Cytochrome P450 19A1 Steroid Aromatase, J. Am. Chem. Soc., 2014, 136, 15016–15025 CrossRef CAS.
- K. Xu, Y. Wang and H. Hirao, Estrogen Formation via H-Abstraction from the O–H Bond of gem-Diol by Compound I in the Reaction of CYP19A1: Mechanistic Scenario Derived from Multiscale QM/MM Calculations, ACS Catal., 2015, 5, 4175–4179 CrossRef CAS.
- E. G. Kovaleva and J. D. Lipscomb, Intermediate in the O–O Bond Cleavage Reaction of an Extradiol Dioxygenase, Biochemistry, 2008, 47, 11168–11170 CrossRef CAS PubMed.
- G. J. Christian, S. Ye and F. Neese, Oxygen activation in extradiol catecholate dioxygenases–a density functional study, Chem. Sci., 2012, 3, 1600–1611 RSC.
- B. Franco, T. Blumenstock, C. Cho, L. Clarisse, C. Clerbaux, P. F. Coheur, M. De Mazière, I. De Smedt, H. P. Dorn, T. Emmerichs, H. Fuchs, G. Gkatzelis, D. W. T. Griffith, S. Gromov, J. W. Hannigan, F. Hase, T. Hohaus, N. Jones, A. Kerkweg, A. Kiendler-Scharr, E. Lutsch, E. Mahieu, A. Novelli, I. Ortega, C. Paton-Walsh, M. Pommier, A. Pozzer, D. Reimer, S. Rosanka, R. Sander, M. Schneider, K. Strong, R. Tillmann, M. Van Roozendael, L. Vereecken, C. Vigouroux, A. Wahner and D. Taraborrelli, Ubiquitous atmospheric production of organic acids mediated by cloud droplets, Nature, 2021, 593, 233–237 CrossRef CAS.
- A. Parandaman, M. Kumar, J. S. Francisco and A. Sinha, Organic acid formation from the atmospheric oxidation of gem diols: Reaction mechanism, energetics, and rates, J. Phys. Chem. A, 2018, 122, 6266–6276 CrossRef CAS.
- J. L. Axson, K. Takahashi, D. O. De Haan and V. Vaida, Gas-phase water-mediated equilibrium between methylglyoxal and its geminal diol, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6687–6692 CrossRef CAS.
- Z.-Z. Xie, Y. Zheng, C.-P. Yuan, J.-P. Guan, Z.-P. Ye, J.-A. Xiao, H.-Y. Xiang, K. Chen, X.-Q. Chen and H. Yang, Photoredox-Catalyzed Deoxygenation of Hexafluoroacetone Hydrate Enables Hydroxypolyfluoroalkylation of Alkenes, Angew. Chem., Int. Ed., 2022, 61, e202211035 CrossRef CAS PubMed.
- H. A. Rypkema, A. Sinha and J. S. Francisco, Carboxylic Acid Catalyzed Hydration of Acetaldehyde, J. Phys. Chem. A, 2015, 119, 4581–4588 CrossRef CAS PubMed.
- A. Das, S. Mahale, V. Prashar, S. Bihani, J. L. Ferrer and M. V. Hosur, X-ray Snapshot of HIV-1 Protease in Action: Observation of Tetrahedral Intermediate and Short Ionic Hydrogen Bond SIHB with Catalytic Aspartate, J. Am. Chem. Soc., 2010, 132, 6366–6373 CrossRef CAS.
- A. Krzemińska, V. Moliner and K. Świderek, Dynamic and Electrostatic Effects on the Reaction Catalyzed by HIV-1 Protease, J. Am. Chem. Soc., 2016, 138, 16283–16298 CrossRef.
- L. Bravo-García, G. Barandika, B. Bazán, M. K. Urtiaga and M. I. Arriortua, Thermal stability of ionic nets with CuII ions coordinated to di-2-pyridyl ketone: Reversible crystal-to-crystal phase transformation, Polyhedron, 2015, 92, 117–123 CrossRef.
- C. G. Efthymiou, C. P. Raptopoulou, V. Psycharis, A. J. Tasiopoulos, A. Escuer, S. P. Perlepes and C. Papatriantafyllopoulou, Copper(II)/di-2-pyridyl ketone chemistry: A triangular cluster displaying antisymmetric exchange versus an 1D coordination polymer, Polyhedron, 2013, 64, 30–37 CrossRef CAS.
- G. Yang, M.-L. Tong, X.-M. Chen and S. W. Ng, Bis(di-2-pyridylmethanediol-N,O,N')copper(II) Diperchlorate, Acta Crystallogr., Sect. C:Cryst. Struct. Commun., 1998, 54, 732–734 CrossRef.
- D. G. Mantero, M. Altaf, A. Neels and H. Stoeckli-Evans, Pyridin-4-ylmethanediol: the hydrated form of isonicotinaldehyde, Acta Crystallogr., Sect. E:Struct. Rep. Online, 2006, 62, o5204–o5206 CrossRef CAS.
- D. P. Giannopoulos, L. Cunha-Silva, R. Ballesteros-Garrido, R. Ballesteros, B. Abarca, A. Escuer and T. C. Stamatatos, New structural motifs in Mn cluster chemistry from the ketone/gem-diol and bis(gem-diol) forms of 2,6-di-(2-pyridylcarbonyl)pyridine: {Mn II4Mn III2} and {Mn II4Mn III6} complexes, RSC Adv., 2016, 6, 105969–105979 RSC.
- H.-S. Wang, F.-J. Yang, Q.-Q. Long, Z.-Y. Huang, W. Chen and Z.-Q. Pan, Syntheses, crystal structures, and magnetic properties of a family of heterometallic octanuclear [Cu6Ln2] (Ln = Dy(iii), Tb(iii), Ho(iii), Er(iii), and Gd(iii)) complexes, New J. Chem., 2017, 41, 5884–5892 RSC.
- T. C. Stamatatos, V. Nastopoulos, A. J. Tasiopoulos, E. E. Moushi, W. Wernsdorfer, G. Christou and S. P. Perlepes, High Nuclearity Single-Molecule Magnets: a Mixed-Valence Mn26 Cluster Containing the Di-2-pyridylketone Diolate Dianion, Inorg. Chem., 2008, 47, 10081–10089 CrossRef CAS PubMed.
- A. J. Tasiopoulos and S. P. Perlepes, Diol-type ligands as central ‘players’ in the chemistry of high-spin molecules and single-molecule magnets, Dalton Trans., 2008, 5537–5555 RSC.
- K. B. Wiberg, K. M. Morgan and H. Maltz, Thermochemistry of Carbonyl Reactions. 6. A Study of Hydration Equilibria, J. Am. Chem. Soc., 1994, 116, 11067–11077 CrossRef CAS.
- C. Zhu, N. F. Kleimeier, A. M. Turner, S. K. Singh, R. C. Fortenberry and R. I. Kaiser, Synthesis of methanediol [CH2(OH)2]: The simplest geminal diol, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2111938119 CrossRef CAS PubMed.
- M. S. Yu, gem-Polyols — a unique class of compound, Russ. Chem. Rev., 1991, 60, 1035 CrossRef.
- J. M. Lázaro Martínez, P. N. Romasanta, A. K. Chattah and G. Y. Buldain, NMR Characterization of Hydrate and Aldehyde Forms of Imidazole-2-carboxaldehyde and Derivatives, J. Org. Chem., 2010, 75, 3208–3213 CrossRef.
- A. F. Crespi and J. M. Lázaro-Martínez, Can a gem-Diol Moiety Be Isolated? A Reaction Study by NMR and X-ray Spectroscopies, J. Chem. Educ., 2023, 100, 4536–4542 CrossRef CAS.
- A. F. Crespi, A. J. Byrne, D. Vega, A. K. Chattah, G. A. Monti and J. M. Lázaro-Martínez, Generation and Stability of the gem-Diol Forms in Imidazole Derivatives Containing Carbonyl Groups. Solid-State NMR and Single-Crystal X-ray Diffraction Studies, J. Phys. Chem. A, 2018, 122, 601–609 CrossRef CAS PubMed.
- A. F. Crespi, D. Vega, A. K. Chattah, G. A. Monti, G. Y. Buldain and J. M. Lázaro-Martínez, gem-Diol and Hemiacetal Forms in Formylpyridine and Vitamin-B6-Related Compounds: Solid-State NMR and Single-Crystal X-ray Diffraction Studies, J. Phys. Chem. A, 2016, 120, 7778–7785 CrossRef CAS PubMed.
- F. I. Luknitskii, Chemistry of chloral, Chem. Rev., 1975, 75, 259–289 CrossRef CAS.
- D. J. McCaldin, The Chemistry of Ninhydrin, Chem. Rev., 1960, 60, 39–51 CrossRef CAS.
- P. Lipp, J. Buchkremer and H. Seeles, Studien in der Cyclopropan-Reihe. Cyclo-propanon, Adv. Cycloaddit., 1932, 499, 1–25 CAS.
- S. E. Schaafsma, H. Steinberg and T. J. de Boer, The chemistry of cyclopropanones. Part VI: Addition of 1,1-dihydroxycyclopropane, Recl. Trav. Chim. Pays-Bas, 1967, 86, 651–654 CrossRef CAS.
- P. Zuman, Additions of water, hydroxyde ion, alcohols and alkoxide ions to carbonyl and azomethine bonds, Arkivoc, 2002, 1, 85–140 Search PubMed.
- H. Cui, R. Goddard, K.-R. Pörschke, A. Hamacher and M. U. Kassack, Bispidin-9,9-diol Analogues of Cisplatin, Carboplatin, and Oxaliplatin: Synthesis, Structures, and Cytotoxicity, Inorg. Chem., 2016, 55, 2986–2997 CrossRef CAS.
- K. Bleher, P. Comba, M. Gast, S. Kronenberger and T. Josephy, Copper-bispidine-catalyzed aziridination–A new twist in small molecule activation, Inorg. Chim. Acta, 2022, 532, 120752 CrossRef CAS.
- S. E. Lewis, Cycloparaphenylenes and related nanohoops, Chem. Soc. Rev., 2015, 44, 2221–2304 RSC.
- H. Kono, Y. Li, R. Zanasi, G. Monaco, F. F. Summa, L. T. Scott, A. Yagi and K. Itami, Methylene-Bridged [6]-, [8]-, and [10]Cycloparaphenylenes: Size-Dependent Properties and Paratropic Belt Currents, J. Am. Chem. Soc., 2023, 145, 8939–8946 CrossRef CAS.
- H. Shudo, M. Kuwayama, M. Shimasaki, T. Nishihara, Y. Takeda, N. Mitoma, T. Kuwabara, A. Yagi, Y. Segawa and K. Itami, Perfluorocycloparaphenylenes, Nat. Commun., 2022, 13, 3713 CrossRef CAS.
- Y. Han, S. Wu, K. Y. S. Khoo and C. Chi, Synthesis of fully π-conjugated non-alternant carbon nanobelts, Nat. Synth., 2025, 4, 947–955 CrossRef CAS.
- N. Narita, Y. Kurita, K. Osakada, T. Ide, H. Kawai and Y. Tsuchido, A dodecamethoxy [6] cycloparaphenylene consisting entirely of hydroquinone ethers: unveiling in-plane aromaticity through a rotaxane structure, Nat. Commun., 2023, 14, 8091 CrossRef CAS PubMed.
- K. Y. Cheung, K. Watanabe, Y. Segawa and K. Itami, Synthesis of a zigzag carbon nanobelt, Nat. Chem., 2021, 13, 255–259 CrossRef CAS PubMed.
- T. D. Clayton, J. M. Fehr, T. W. Price, L. N. Zakharov and R. Jasti, Pinwheel-like Curved Aromatics from the Cyclotrimerization of Strained Alkyne Cycloparaphenylenes, J. Am. Chem. Soc., 2024, 146, 30607–30614 CrossRef CAS PubMed.
- P. C. Hall, H. W. Reid, I. Liashenko, B. Tandon, K. L. O'Neill, N. C. Paxton, G. C. J. Lindberg, R. Jasti and P. D. Dalton, [n] Cycloparaphenylenes as compatible fluorophores for melt electrowriting, Small, 2024, 20, 2400882 CrossRef CAS.
- A. A. Kamin, T. D. Clayton, C. E. Otteson, P. M. Gannon, S. Krajewski, W. Kaminsky, R. Jasti and D. J. Xiao, Synthesis and metalation of polycatechol nanohoops derived from fluorocycloparaphenylenes, Chem. Sci., 2023, 14, 9724–9732 RSC.
- E. J. Leonhardt and R. Jasti, Emerging applications of carbon nanohoops, A Donor–Acceptor 10-Cycloparaphenylene and Its Use as an Emitter in an Organic Light-Emitting Diode, Nat. Rev. Chem., 2019, 3, 672–686 CrossRef CAS.
- D. Chen, Y. Wada, Y. Kusakabe, L. Sun, E. Kayahara, K. Suzuki, H. Tanaka, S. Yamago, H. Kaji and E. Zysman-Colman, Org. Lett., 2023, 25, 998–1002 CrossRef CAS PubMed.
- T. Terabayashi, E. Kayahara, Y. Zhang, Y. Mizuhata, N. Tokitoh, T. Nishinaga, T. Kato and S. Yamago, Synthesis of twisted [N] cycloparaphenylene by alkene insertion, Angew. Chem., Int. Ed., 2023, 135, e202214960 CrossRef.
- Y. Yoshigoe, Y. Tanji, Y. Hata, K. Osakada, S. Saito, E. Kayahara, S. Yamago, Y. Tsuchido and H. Kawai, Dynamic Au–C σ-Bonds Leading to an Efficient Synthesis of [n]Cycloparaphenylenes (n = 9–15) by Self-Assembly, JACS Au, 2022, 2, 1857–1868 CrossRef CAS.
- Y. Miyazawa, Z. Wang, M. Matsumoto, S. Hatano, I. Antol, E. Kayahara, S. Yamago and M. Abe, 1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane Aromaticity, J. Am. Chem. Soc., 2021, 143, 7426–7439 CrossRef CAS PubMed.
- A. Usami, H. Kono, V. Austen, Q. M. Phung, H. Shudo, T. Kato, H. Yamada, A. Yagi, K. Amaike, K. J. Fujimoto, T. Yanai and K. Itami, In-insect synthesis of oxygen-doped molecular nanocarbons, Science, 2025, 388, 1055–1061 CrossRef CAS PubMed.
- B. Zou, J.-A. Li, Z. Zhai, T. Yu, H. Liu, J. Huang, H. He, B. Xu and H.-W. Jiang, cis-Linked Cyclotetraphenylenes: Synthesis, Structures and Fluorescence Properties, Eur. J. Org Chem., 2024, 27, e202400135 CrossRef CAS.
- H.-W. Jiang, T. Tanaka, H. Mori, K. H. Park, D. Kim and A. Osuka, Cyclic 2,12-Porphyrinylene Nanorings as a Porphyrin Analogue of Cycloparaphenylenes, J. Am. Chem. Soc., 2015, 137, 2219–2222 CrossRef CAS.
- H.-W. Jiang, T. Tanaka, T. Kim, Y. M. Sung, H. Mori, D. Kim and A. Osuka, Synthesis of [n]Cyclo-5,15-porphyrinylene-4,4′-biphenylenes Displaying Size-Dependent Excitation-Energy Hopping, Angew. Chem., 2015, 127, 15412–15416 CrossRef.
- S. Idrees, Z. Li, F. Fang, H. He, I. Majeed, Y. Zhang, A. Osuka, Y. Cao, Z. Zeng, X. Li and H.-W. Jiang, Porphyrin nanotubes based on a hydrogen-bonded organic framework, Nanoscale, 2022, 14, 14630–14635 RSC.
- H. He, J.-A. Li, Y. Zhang, S. Idrees, J. Cai, Y. Li, A. Osuka, B. Xu and H.-W. Jiang, Synthesis, structures and fluorescence properties of gem-linked cyclic tetraphenylethylenes and cyclic hexaphenylethylenes, Org. Chem. Front., 2022, 9, 2932–2938 RSC.
- E. Kayahara, S. Hirata, Y. Mizuhata, Y. Yasuda, Y. Kusakabe, H. Kaji and S. Yamago, Synthesis of π-Extended [1.1]Paracyclophanes, [1.1][n]PCP (n=2, 3, and 4), and Their Through-Space Conjugation, Chem.–Eur. J., 2025, 31, e202402225 CrossRef CAS PubMed.
- E. Kayahara, T. Hayashi, K. Takeuchi, F. Ozawa, K. Ashida, S. Ogoshi and S. Yamago, Strain-Induced Double Carbon–Carbon Bond Activations of Cycloparaphenylenes by a Platinum Complex: Application to the Synthesis of Cyclic Diketones, Angew. Chem., Int. Ed., 2018, 57, 11418–11421 CrossRef CAS PubMed.
- G. Mlostoń, M. Jasiński, A. Linden and H. Heimgartner, Reactions of 2-Unsubstituted 1H-Imidazole 3-Oxides with 2,2-Bis(trifluoromethyl)ethene-1,1-dicarbonitrile: A Stepwise 1,3-Dipolar Cycloaddition, Helv. Chim. Acta, 2006, 89, 1304–1316 CrossRef.
- E. T. Urbansky, Carbinolamines and Geminal Diols in Aqueous Environmental Organic Chemistry, J. Chem. Educ., 2000, 77, 1644 CrossRef CAS.
- H. Kutzke, H. Klapper, R. B. Hammond and K. J. Roberts, Metastable [beta]-phase of benzophenone: independent structure determinations via X-ray powder diffraction and single crystal studies, Acta Crystallogr., Sect. C:Cryst. Struct. Commun., 2000, 56, 486–496 CrossRef.
- Y. Segawa, H. Omachi and K. Itami, Theoretical Studies on the Structures and Strain Energies of Cycloparaphenylenes, Org. Lett., 2010, 12, 2262–2265 CrossRef CAS PubMed.
- S. M. Bachrach and D. Stück, DFT Study of Cycloparaphenylenes and Heteroatom-Substituted Nanohoops, J. Org. Chem., 2010, 75, 6595–6604 CrossRef CAS PubMed.
- T. Iwamoto, Y. Watanabe, Y. Sakamoto, T. Suzuki and S. Yamago, Selective and Random Syntheses of [n]Cycloparaphenylenes (n = 8–13) and Size Dependence of Their Electronic Properties, J. Am. Chem. Soc., 2011, 133, 8354–8361 CrossRef CAS PubMed.
- C. E. Colwell, T. W. Price, T. Stauch and R. Jasti, Strain visualization for strained macrocycles, Chem. Sci., 2020, 11, 3923–3930 RSC.
- (a) CCDC 2479706: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7bjt; (b) CCDC 2479718: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7bx6; (c) CCDC 2479720: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7bz8; (d) CCDC 2479725: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7c4g; (e) CCDC 2479728: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7c7k; (f) CCDC 2479740: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7cmy; (g) CCDC 2479742: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7cp0; (h) CCDC 2479743: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7cq1; (i) CCDC 2479744: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7cr2.
Footnote |
| † On the auspicious occasion of her 95th birthday, this paper is dedicated to Professor Youyou Tu, the esteemed recipient of the 2015 Nobel Prize in Physiology or Medicine, in recognition of her groundbreaking discovery of Artemisinin, which has saved millions of lives worldwide. |
| This journal is © The Royal Society of Chemistry 2026 |