Open Access Article
Open Access ArticleCross-scale design of abiotic–biotic interfaces for semi-artificial photosynthesis
Hao
Wang†
ab,
Jialu
Li†
bc,
Yuhua
Feng†
a,
Donghao
He
b,
Xiaolei
Fan
 cd,
Bo
Wang
cd,
Bo
Wang
 b,
Zhonghua
Cai
*a,
Cuiping
Zeng
b,
Zhonghua
Cai
*a,
Cuiping
Zeng
 *b and
Kemeng
Xiao
*b and
Kemeng
Xiao
 *b
*b
aMarine Ecology and Human Factors Assessment Technical Innovation Center of Natural Resources Ministry, Tsinghua Shenzhen International Graduate School, Shenzhen, Guangdong Province 518055, PR China. E-mail: caizh@sz.tsinghua.edu.cn
bState Key Laboratory of Quantitative Synthetic Biology, Shenzhen Key Laboratory of Materials Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: cp.zeng@siat.ac.cn; km.xiao@siat.ac.cn
cNingbo China Beacons of Excellence Research and Innovation Institute, University of Nottingham Ningbo China, 211 Xingguang Road, Ningbo 315048, China
dDepartment of Chemical Engineering, School of Engineering, The University of Manchester, Oxford Road, Manchester M13 9PL, UK
First published on 4th January 2026
Abstract
By coupling semiconductor nanomaterials with living microbes, nanomaterial–microorganism hybrid systems (NMHSs) create powerful biohybrids that unlock new routes for efficient and sustainable solar-to-chemical conversion. Central to the performance of this system is the biotic–abiotic interface, where photoelectrons must efficiently traverse from inorganic materials into complex cellular redox networks. This review highlights recent progress in understanding and engineering these interfaces across three dimensions: material architecture, microbial electron-handling machinery, and interfacial construction strategies. By dissecting how composition, size, and morphology of photosensitizers align with extracellular matrices, transmembrane conduits, and intracellular compounds, we reveal principles for minimizing interfacial resistance and maximizing charge transfer. We further classify interface communication modes into extracellular wiring, transmembrane bridging, and intracellular embedding, and evaluate corresponding construction approaches. By drawing connections between interfacial features and electron-transfer performance, we propose a multidimensional framework that integrates material engineering, microbial adaptation, and interface optimization. This perspective emphasizes the synergistic co-design of both abiotic and biotic components to achieve efficient and stable solar-to-chemical conversion, offering new opportunities for rational design of high-performance NMHSs.
1 Introduction
In the face of a deepening energy crisis and mounting environmental pressures, efficiently capturing and converting clean resources—especially solar energy—has become paramount for sustainable development.1–3 Yet, in the life sciences, leveraging light to power microbial metabolism remains a formidable challenge: natural photosynthetic microbes convert under 3% of solar energy into biomass, while industrially optimized strains lack any inherent light-harvesting capability.4 Semiconductor nanomaterials, prized for their exceptional photon-capture prowess, can drive redox reactions under illumination to emulate natural photosynthesis.5–7 However, their limited catalytic selectivity constrains both product diversity and value.2 To surmount the inherent limitations of both natural and artificial photosynthesis, the NMHSs has emerged by incorporating microbial cells with semiconductor materials, with light-harvesting materials capture photons to generate charge carriers, while microbial metabolisms channel those electrons into value-added chemical production.8,9 For discussion on NMHSs involving in various solar energy conversion applications, we direct the reader to additional comprehensive reviews.10,11Despite these advances, most NMHSs remain proof-of-concept demonstrations under tightly controlled conditions and deliver overall low solar-to-chemical efficiencies.12 A major contributor to this performance gap is the biotic–abiotic interface, where the transfer of photoelectrons from the material to cellular metabolism undergoes substantial losses.13 Although state-of-the-art photosensitizers can convert ∼80% of absorbed photons into free carriers,14 typically fewer than 10% of those electrons ultimately reach intracellular enzymes.15 This indicates that the dominant losses occur at the interface rather than in light capture. An efficient interface directs photoelectrons into target biological pathways rather than dissipation as recombination or side reactions. Besides, the interface must reconcile the divergent physicochemical needs of the two components, maintaining catalyst stability and favorable charge energetics while preserving cell viability and membrane integrity.2,16 Taken together, these constraints underscore that the interface is not a passive boundary but the central design element that controls the fate of photoelectrons and ultimately dictates the performance ceiling of NMHSs. Consequently, a comprehensive review that systematically elucidates the biotic–abiotic interface is essential for guiding the rational design of semi-artificial photosynthesis systems (SAPSs).
Recently, several insightful reviews have begun to address aspects of the NMHS interface. Our previously published review analyzed the solar-energy flow for NMHSs, discussing light capture, energy transport and conversion process.10 Although it briefly touched on interfacial electron transfer within the broader context of energy flow, its emphasis remained on system-level energetics rather than the fundamental nature of the interface. Other excellent reviews have highlighted more specialized dimensions of NMHSs operation. For example, one review provided an elegant overview of strategies for coupling materials with the native electron-uptake machinery of electroactive microbes;17 however, its focus on a specific class of electroactive microorganisms naturally limits the broader applicability of NMHSs, and the crucial influence of material properties design on interfacial behavior was not extensively discussed. Likewise, a recent electron transfer related perspective offered a comprehensive survey of biological, physicochemical, and electrochemical characterization tools, especially advances in spatiotemporal imaging at the single-cell level, yet focused primarily on measurement techniques rather than mechanistic determinants of interface structure and function.18 Reviews centered on quantum-efficiency analysis have also contributed valuable insights by quantifying the role of interfacial processes in overall energy-conversion efficiency,19 though these discussions largely address performance metrics rather than the underlying architectural and mechanistic basis of the interface itself. Together, these works have significantly advanced the field, each illuminating a particular facet of NMHSs interfacial behavior. Nevertheless, a unified and cross-scale framework that connects material architecture, microbial electron-handling pathways, and the design principles that govern biotic–abiotic coupling remains missing.
The communication of materials and cells involves the physical contact form and photoelectrons transfer behavior, which originated from the material physico-chemical structure and the microbial electron-handling machinery. Realizing efficient electron flow demands co-design on both material and microbial fronts. On the material side, photosensitizer composition (metal-based semiconductors, MOFs, quantum dots), size (quantum vs. meso vs. micro),20–24 and morphology (nanoparticles, sheets, layers, porous scaffolds) govern light absorption, carrier mobility,25–27 and physical contact with cells.28,29 On the biological side, extracellular polymeric substances (EPS),30,31 conductive appendages (e.g., OmcS and OmcZ),32,33 transmembrane complexes (such as the Mtr cytochromes),34,35 and intracellular redox cofactors create multistep electron-relay pathways or coupling effect with nanomaterials.36–38 Beyond the bandgap effects covered in previous reviews, efficiently aligning a material's photo-physicochemical properties with microbial binding motifs and electron-shuttle pathways shortens charge-transfer distances and lowers interfacial resistance, thereby maximizing electron-transfer efficiency at the material–cell interface. To date, the relationships between specific semiconductor–microbe interfacial features and their corresponding electron transfer rates remain poorly understood. Unraveling these underlying mechanisms will enable the rational design of NHMSs systems for efficient solar-to-chemical conversion.
In this review, we develop a cross-scale framework for revealing interface design in NMHSs by examining three interwoven dimensions: material architecture, microbial structure, and interfacial construction strategies. First, the influence of photosensitizer composition, size, and morphology—from quantum-confined dots to macro structure—affect cell coupling and charge delivery (Sections 1.1–1.3) were systematically discussed. Next, we dissect microbial electron-handling mechanisms as well as on biohybrid coupling and electrons transfer—extracellular EPS networks, transmembrane conduits, periplasmic relays, and intracellular cofactors—and their structural determinants (Sections 2.1–2.6). Finally, we analyze the above two parts on material and cell communication modes, and classified interface construction and electron transfer modes into extracellular wiring, transmembrane bridging, and intracellular embedding of photosensitizers (Sections 3.1–3.6), comparing physical adsorption, electrostatic/hydrogen binding, and biomineralization approaches. Drawing these threads together, we propose a multidimensional synergistic optimization strategy—combining topology, tailored surface chemistry, and enhanced bio-affinity—to guide the rational design of high-performance NMHSs interfaces.
2 Synergizing material geometry with charge transfer at the interface
In NMHSs, photocatalytic materials generate electron–hole pairs upon exposing to light irradiation. Photoelectrons are subsequently conveyed across the biohybrid interface into microbial cells, enabling intracellular metabolic pathways for the selective production of target molecules.39,40 Optimizing materials is essential for enhancing charge carrier transport and impacting the system's stability and energy conversion efficiency. Photocatalytic materials function as light-harvesting elements in NMHSs, similar to chlorophyll and other pigments in natural photosynthesis, by absorbing solar energy and transferring it into photogenerated charge carriers. The photoelectrochemical properties of these materials substantially affect the efficacy of solar-to-chemical energy conversion. Effective photocatalysts must exhibit excellent photon absorption and excited-state charge carriers generation ability.41 Ideal light-harvesting materials should have broad spectrum absorption and high absorption coefficients, enhancing light usage and promoting the generation of excited-state electrons. Inefficient light absorption leads to an initial energy loss that cannot be mitigated by enhanced downstream electron transfer and catalytic mechanisms. If the material components of NMHSs demonstrate inadequate absorption at certain wavelengths, the associated portion of solar energy is not fully utilized, consequently diminishing the overall light-to-energy conversion efficiency at the interface.28 Besides, the photostability of these materials is also essential for preserving energy transfer at the interface. Photocatalysts that degrade or undergo structural alterations upon exposure to light may see a gradual decrease in energy capture efficiency, thereby impacting the long-term stability and operational efficacy of the system.42 Therefore, these characteristics of photocatalysts determine the theoretical maximum for solar-to-chemical energy conversion efficiency at the material–biological interface in NMHSs.The influence of photoelectrical properties of materials, including photocurrent density, bandgap, and charge carrier mobility on photoelectron generation and subsequent transfer efficiency, have been thoroughly reviewed.43–47 However, the underlying mechanisms that shape these properties have received far less attention. In particular, how material size and structural characteristics modulate intrinsic photoelectrical behavior and, in turn, dictate the modes by which materials interface with microorganisms remains insufficiently understood. These factors are key determinants of both the resulting photoelectrochemical performance and the efficiency of interfacial electron transfer. This study systematically examines the size (from quantum-scale to mesoscale and microscale) and morphology (including particulate, sheet-like, and layered structures) of photocatalytic materials. It clarifies how their spatial compatibility, contact modes, and mass transport architectures collectively influence interfacial electron transfer networks. Rational interface design that leverages the intrinsic dimensions and structural characteristics of materials is essential for attaining multiscale compatibility and synchronized functionality among modules, thus potentially resolving current efficiency constraints. Consequently, comprehensive analysis and targeted optimization of materials are crucial for advancing NMHSs from theoretical models to practical applications, as well as for attaining efficient and sustainable solar energy conversion. This section will focus on the dimensional and structural parameters of light-harvesting materials, examine their effects on overall system performance, and suggest strategies for enhancing interfacial electrons transfer efficiency.
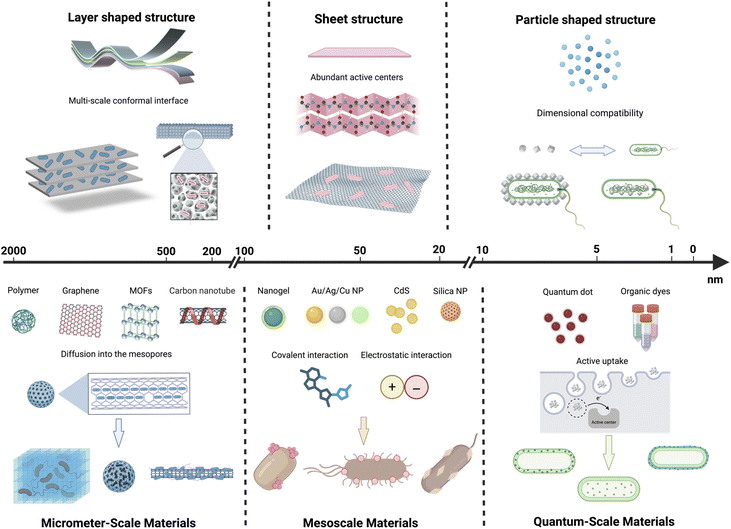
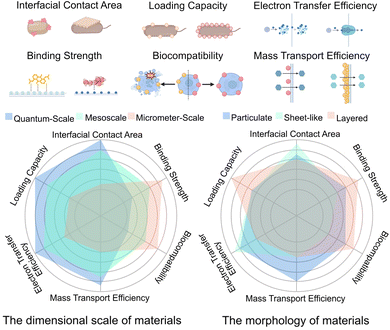
2.1 Classification of material dimensions and their interfacial characteristics
The solar-to-chemical energy conversion efficiency hinges on two physical parameters: (i) band gap determining spectral utilization and driving force, and (ii) electron transfer efficiency governing carrier utilization at bio–abiotic interfaces.10 Material size and structure play a crucial role in regulating both parameters. The size scale of materials has a significant impact on their physical properties, interface interaction modes, and electron transfer efficiency. As material dimensions shift from the quantum to the nanoscale, notable changes arise in both physical and chemical properties, observable in several critical domains: quantum-scale materials exhibit pronounced quantum confinement effects. When the dimensions of a semiconductor approach the exciton Bohr radius, the continuous band structure transitions to discrete energy levels. This feature enables precise modulation of the bandgap through size control, thus enhancing light absorption characteristics and electronic structure. Therefore, materials of different sizes exhibit distinct properties in the generation, separation, and transport of photogenerated charge carriers. Material size also has a significant impact on the interactions occurring at the material–biological interface. Quantum-scale materials possess a high specific surface area and numerous surface-active sites, enhancing interfacial interactions with biological components and thereby improving electron transfer efficiency. Minimally small sizes can jeopardize interfacial stability and lead to unwanted side reactions. An increase in particle size results in a decrease in specific surface area, potentially reducing spatial coordination with biological partners. The variations in photoelectrochemical properties and integration modes directly affect the efficiency of photoelectrons transfer at the interface, thus determining the overall performance of solar-to-chemical energy conversion within the system. This section examines how materials of different dimensions, ranging from quantum-scale (<10 nm) to mesoscale (10–100 nm) and macroscale (>100 nm), modulate interfacial electron-transfer behavior during the construction of nanomaterial–microorganism hybrid systems. It highlights how dimensionality governs charge-generation, transport, and coupling modes within the hybrid light-harvesting module.| Classification | Material type | Advantages | Binding mechanisms | Ref. |
|---|---|---|---|---|
| Quantum-scale materials (<10 nm) | QDs | Quantum confinement effect, ultrasmall dimensions, facile surface functionalization | Intracellular self-assembly, internalization/active uptake | 52 and 53 |
| Photosensitizer molecules | High membrane permeability, excellent lipophilicity | Free diffusion | 54–56 | |
| Mesoscale materials (10–100 nm) | Non-metallic compounds | Size-tunable visible-light response, biosynthetic compatibility | Biomineralization, surface display | 13 and 57 |
| Metallic compounds | Bandgap engineering for enhanced light harvesting & charge separation, carrier design balances catalytic activity and biosafety | Adsorption via carrier micropores, electrostatic adsorption, covalent bonding | 15 and 58–61 | |
| Metal nanoparticles | Surface plasmon resonance effect enabling photothermal-catalytic synergy | Internalization/active uptake | 62–64 | |
| Micrometer-scale materials (>100 nm) | MOFs | Tunable porous architecture enabling compartmentalized encapsulation of semiconductors/microbes | In situ self-assembly, electrostatic adsorption, covalent bonding | 65–68 |
| Carbon-based composites | Exceptional biocompatibility and environmental sustainability | Electrostatic adsorption, covalent bonding | 69–71 | |
| Conjugated organic polymers | Inherent multiple electron donor–acceptor units, strong membrane affinity via hydrophobic/π–π interactions | Surface self-assembly, electrostatic adsorption | 72–74 |
Previous studies have synthesized various core–shell QDs with tunable band gaps, leveraging the unique properties of quantum-scale materials, and spanning excitation energies from ultraviolet to near-infrared ranges. QDs were coupled with targeted enzyme sites in bacteria via chemical bonding and self-assembly. Upon exposure to light, photoelectrons from QDs are transferred to bacteria, which subsequently utilize substrates including carbon dioxide, nitrogen, and water to synthesize valuable products such as isopropanol, 2,3-butanediol, and methyl ketones.75 The benefits of quantum-scale materials in surface redox reactions have been extensively utilized in battery technologies. The modification of graphene using carbon QDs enhances the specific capacity of lithium and sodium-ion batteries, leading to improved performance compared to conventional graphene-based batteries.76 NMHSs leverage the electronic properties of quantum-scale materials, facilitating the rapid transfer of photoelectrons to low-potential biological catalytic centers, thereby improving the efficiency of light-harvesting modules.
Besides, the small size of quantum-scale materials shortens the distance for electron delivery to biological catalytic sites, enabling their internalization by bacteria and allowing direct interaction with intracellular electron-transfer chains (such as hydrogenase active centers). Such dimensions not only facilitate physical proximity but also enhance quantum confinement effects, which can increase charge-carrier density and improve the driving force for photoelectron injection into biological redox cofactors. For example, the design of gold nanocluster/organic semiconductor heterostructures (AuNC@OFTF) provides dimensions suitable for penetrating bacterial cells and interacting directly with the thylakoid membrane. The resulting efficient photoelectron injection into photosystem II leads to markedly enhanced photocurrent relative to conventional systems.62 Similar strategies have been demonstrated using CdS@ZnS quantum dots for specifically binding to the nitrogenase in the Azotobacter vinelandii and Cupriavidus necator bacteria.75 These particles establish close contact with intracellular redox centers of PSI/PSII components and metabolic enzymes, thereby enabling multistep intracellular photo-reducing cascades and improving solar-to-chemical conversion efficiencies.
Despite their potential, the inherent limitations of quantum-scale materials—including aggregation, structural instability, and cytotoxicity constrain their broader applicability in hybrid systems.77 The extremely small size of QDs also makes them prone to aggregation, which decreases the availability of active sites and compromises the long-term stability and performance of the hybrid system. In addition, many QDs contain heavy metal components, and the gradual release of metal ions can induce cytotoxic effects, including oxidative stress, cell-cycle arrest, and even cell death.20 While surface modification has been employed to enhance the dispersion of QDs in aqueous solutions to inhibit aggregation, the ligand and penetration of cell membrane is also toxic to bacteria.54 Future research will focus on the development of core–shell materials to enhance biocompatibility, reduce biological toxicity, and further optimize quantum dot dispersion through more biocompatible surface modification techniques. The challenges associated with quantum-scale materials underscore the importance of exploring other material classes. Mesoscale materials, with greater structural stability, provide a natural next step for enhancing hybrid system performance.
Despite their broad potential, mesoscale materials used in NMHSs face several intrinsic challenges that constrain stability and solar-to-chemical conversion efficiency. First, photostability remains a key bottleneck. Metal-based semiconductors such as CdS are prone to photocorrosion under illumination, leading to the release of metal ions that can cause biological toxicity and disrupt long-term operation. Second, the rapid recombination of photoelectron–hole pairs limits carrier availability for microbial uptake, fundamentally restricting the energy conversion efficiency of NMHSs.84 Mesoporous heterojunctions (e.g., BiVO4/WO3) illustrate enhanced coupling light harvesting, charge separation, and transport within a spatially coordinated architecture promote directional electron flow toward microbial redox proteins such as cytochromes.85 We refer to this enzymatic example because molecular-level electron-transfer pathways are more explicitly resolved in enzyme–nanoparticle systems, providing mechanistic insights that are directly translatable to the design of biotic–abiotic interfaces in whole-cell NMHSs. This indicates the heterojunctions architecture facilitates directional electron flow toward biological membrane receptors, including cytochrome c6, driven by the interfacial potential difference. Limitations arise from synthesis precision, structural instability, and mismatches between pore sizes and biomolecules or whole cells, which can restrict reactions to surface interactions. Moreover, high specific surface areas often introduce surface defects, such as dangling bonds, that reduce catalytic activity or accelerate charge carrier recombination (e.g., via surface states in TiO2).86
Addressing these issues requires material designs that provide stable, well-defined heterojunctions and pore structures compatible with biological components, providing a pathway to more effective integration with mesoscale materials in NMHSs. Additionally, efforts could be directed towards refining the design of the material–biological interface to optimize electron transfer pathways, such as used the redox mediator for facilitating the interfacial electron transfer. Furthermore, surface passivation, interface engineering, and defect regulation can suppress recombination and improve biocompatibility.10,87 More broadly, integrating mesoscopic architectures with quantum-scale sensitizers or microscale porous networks may enable multiscale complementarity, enhancing charge generation, separation, and utilization across the biotic–abiotic interface.
In NMHSs, these advantages of micrometer-scale materials are exemplified across a variety of widely used photocatalysts, including carbon-based composites, MOFs, perovskite microcrystals, and semiconductor microspheres. For instance, MOFs-constructed from the self-assembly of metal ions or clusters with organic ligands—possess abundant surface oxygen vacancies that enhance photon capture and facilitate electron transport, while their micrometer-scale frameworks provide superior mechanical robustness against environmental perturbations.95 Similarly, strengthened interlayer interactions in micrometer-scale g-C3N4 improve its structural integrity and photostability.96 InP, another representative photosensitizer, benefit from inhibited aggregation and sustained long-term performance when uniformly dispersed within larger host matrices.97 Collectively, these examples highlight how micrometer-scale architecture not only enhances mechanical stability and defect tolerance but also enables more reliable optical and catalytic performance, reinforcing their essential role in constructing durable and high-efficiency NMHSs. Notably, the processability of micrometer-scale materials makes them suitable for large-scale industrial production, offering a significant advantage for the practical implementation of micrometer-scale materials based NHMSs. In industrial manufacturing, such systems often require uniform coverage across extensive carrier surfaces. Micrometer-scale materials can be evenly deposited onto diverse substrates through simple and efficient techniques such as spin-coating, spray-coating, and doctor-blading, thereby forming continuous functional coatings. For example, solution-based spin coating can be employed to produce NMHSs utilizing conjugated polymers like PPy/PFP/PDI, resulting in large-area uniform films. These coatings enhance overall light-to-energy conversion efficiency by closely integrating with biological processes while also offering remarkable mechanical strength and flexibility.72–74 The conjugated polymer chains contain multiple electron donor and acceptor units, which generate excited states that enhance charge separation and transport upon photoexcitation.
However, the application of micrometer-scale materials in NMHSs still presents several non-negligible limitations. The significantly reduced specific surface area presents a substantial challenge. The surface area is a significant factor influencing the interaction between catalysts and reactants. In NMHSs, an increased surface area leads to a higher number of active sites for charge transfer, thereby enhancing photocatalytic activity. As shown in Table 1, the proportion of surface atoms decreases with increasing material size into the micrometer range, resulting in a significant number of atoms being located in the bulk phase and thereby reducing the availability of accessible active sites for charge transfer. SiO2@MoS2 composites exhibit numerous surface-active sites at the quantum and mesoscopic scales; however, their activity diminishes at the micrometer scale due to a reduced availability of surface atoms.11 Extended charge transport distances represent a significant limitation. The reduced photocatalytic effectiveness is attributed to the increased distance that photogenerated carriers must traverse to reach the surface, thereby elevating the likelihood of recombination at defect or impurity sites.98 Direct electron transfer with intracellular receptors is often impractical because of size limitations, typically necessitating the involvement of redox mediators.
To overcome these limitations, multilevel porous structural designs can be employed for further optimization. Hierarchical porous structures refer to material systems that simultaneously contain micropores, mesopores, and macropores, and their rational design serves as an important strategy to enhance light-harvesting capabilities and improve photocatalytic efficiency. For example, in MOF-based NMHSs, hierarchical MOFs can be obtained by tuning synthetic conditions. Macropores function as transport channels for bulk reactant molecules and can also host photosensitizers such as porphyrin derivatives, thereby facilitating rapid diffusion of reactants into the mesoporous and microporous regions where catalytic active sites are located. In addition, the multiple scattering and reflection of light within hierarchical pores significantly strengthen the light-harvesting capacity of MOFs, promoting the generation and separation of photogenerated charge carriers and ultimately enhancing photocatalytic performance.99–101 Employing conductive biofilms to construct electron “highways” represents another important optimization strategy. Conductive biofilms, which are secreted by microorganisms and possess intrinsic electrical conductivity, can efficiently transport electrons and reduce the recombination probability of photogenerated charge carriers.102 Integrating conductive biofilms with microscale photocatalysts can provide efficient pathways for photogenerated electron transport. For photocatalysts such as PFP/PDI conjugated polymers and rRO, coupling with conductive biofilms significantly enhances their charge-transfer performance and overcomes the inherent limitation of long charge-migration distances at the microscale, thereby improving the overall efficiency.22–24 Together, these strategies enhance both mass diffusion and electron transport, thereby reinforcing the overall efficiency and robustness of micrometer-scale materials based NMHSs.
2.2 Material architecture and interfacial properties
Recent studies highlight that the three-dimensional (3D) topology of materials strongly affects photogenerated charge carrier dynamics, including separation, transport, and recombination. Quantum confinement and surface state distributions largely govern these effects. Material topology also influences the spatial organization and metabolic activity of biological components. This influence is mediated by interfacial chemical features (e.g., functional group type, charge distribution) and physical morphology (e.g., pore size, surface roughness).29 The structural parameters collectively influence the overall energy conversion efficiency of NMHSs. Hierarchically porous MOFs featuring integrated micro-, meso-, and macropores enhance light absorption via confinement effects. Additionally, surface-exposed carboxyl groups facilitate site-specific immobilization of carbon fixation enzymes, thereby significantly improving the coupling efficiency of CO2 reduction pathways.103 While this study was conducted on enzyme–nanoparticle hybrids, the underlying principle of utilizing surface carboxyl moieties to anchor catalytic proteins provides a valuable design strategy for NMHSs, potentially facilitating the binding of microbial outer membrane proteins to reduce interfacial transfer resistance. This section systematically examines how material morphology influences electron transfer across the material–biological interface, building on the previous analysis of size-dependent effects and interfacial structure in nanomaterials. We propose a multidimensional co-optimization framework that integrates recent research advances, coordinating topological design, surface chemistry, and biological compatibility to improve light-to-energy transduction in NMHSs.However, particulate architectures also present distinct challenges. Due to their irregular shape, attaching or uptake of particles can induce changes in membrane permeability, potentially leading to metabolic disturbances or cell cycle arrest.106,107 Additionally, the high surface energy of particles makes them prone to aggregation; for example, Au NPs tend to spontaneously aggregate into micron-scale clusters in solution, which significantly reduces the density of active sites for charge transfer.108 Therefore, surface chemical modifications and dispersion optimization are critical to ensuring the long-term stability and biocompatibility of particulate NMHSs. Certain researchers have employed protective coatings, such as SiO2 or polydopamine, on particulate structures to form core–shell structures, thereby addressing their limitations. The design of heterointerfaces and shell encapsulation effectively addresses issues related to aggregation, toxicity, and inadequate photostability commonly observed in traditional particles. Core–shell CdS@TPPA nanospheres were synthesized through the in situ growth of a covalent organic framework (COF) shell layer on the surface of CdS nanospheres. The confinement effect of the porous shell facilitates precise regulation of reactant transport pathways, thereby enhancing photocatalytic selectivity.109 In NMHSs, composite materials such as CdS@Mn3O4 are formed through the deposition of Mn3O4 onto the surface of CdS. Co-assembly with Thiobacillus denitrificans resulted in superior nitrogen fixation under light conditions. The Mn3O4 shell acts as a protective buffer layer, preventing photodegradation of the photocatalyst and mitigating the effects of reactive oxygen species (ROS) generated on the CdS surface. This significantly reduces oxidative damage and improves long-term system stability.25 The fabrication of core–shell structures typically requires careful multi-step management, and interfacial defects can increase charge carrier recombination rates. Additionally, excessively thick shells can hinder mass diffusion and light penetration. Improving shell porosity is essential to balance protective effects with mass transfer requirements. Future applications of dynamic self-assembly methods may enable the development of adaptive core–shell interfaces, enhancing both material stability and energy transfer efficiency at the material–biological interface.
Excessive epitaxial growth of 2D sheets may decrease the density of edge-active sites, thereby reducing the capacity for enzyme or bacterial loading. As shown in Fig. 1, robust interlayer van der Waals interactions frequently lead to aggregation, thereby constraining the effective surface area.116 This challenge can be addressed through surface functionalization, such as carboxylation, or intercalation strategies, including the introduction of carbon QDs to inhibit restacking.26 For example, graphene/MoS2 heterojunctions have been developed to address the low density of planar active sites, combining the high conductivity of graphene with the catalytic properties of MoS2.117 These combinations increase carrier lifetime while enhancing both efficiency and stability. Another promising method involves defect engineering, specifically the introduction of nitrogen-vacancy through plasma treatment, which enhances the density of edge-active sites and improves the catalytic performance of 2D materials.118 Besides, mechanical fragility presents a significant issue; ultrathin layers are vulnerable to structural damage during prolonged operation, and certain materials are prone to oxidative degradation in humid conditions.
Layered perovskites utilize organic cations, such as phenylethylamine, to adjust interlayer spacing, which enhances carrier transport and reduces the recombination of photogenerated charges.120 LDHs feature positively charged layers that electrostatically adsorb negatively charged microbial membranes, thereby facilitating direct extracellular electron injection into the microbial photosynthetic chain and promoting efficient coupling of light-driven CO2 reduction with microbial metabolism.121 A representative study involved the construction of a Z-scheme NMHSs utilizing Mo-doped BiVO4 modified with RuO2 (BiVO4:Mo|RuO2) as the light-absorbing component, combined with hydrogenase or formate dehydrogenase.105 The system effectively accomplished overall water splitting and CO2 reduction under light irradiation. The stepwise energy band alignment markedly improved interfacial electron transfer and decreased the time necessary for electron delivery into microbial cells. Materials with high biocompatibility, such as polydopamine (PDA), have also been utilized to improve the stability of material–biological interfaces. The catechol moieties of PDA demonstrate a specific binding affinity for microbial exopolysaccharides, resulting in the formation of a controllable bio-adhesive layer. NiO nanosheets coated with PDA (NiO@PDA) exhibit strong adhesion to nitrogen-fixing bacteria (Azotobacter vinelandii), facilitating uniform bacterial immobilization.122 This biohybrid system utilizes the PDA layer as a bio-adhesive, charge transport channel, and protective barrier, thereby preserving microbial viability in complex reaction environments, capabilities seldom realized with single-function materials. An electroconductive biofilm was developed through the genetic modification of Escherichia coli to secrete extracellular matrix components, which acted as a scaffold for the in situ oxidative polymerization of PPy.123 A self-supporting Z-scheme photocatalytic system was constructed by depositing photocatalysts onto glass substrates and conducting thermal annealing, thereby effectively integrating biological and inorganic components via a stable and conductive interface.
Despite their structural benefits, layered materials present significant implementation challenges. From a synthesis perspective, precise control over interlayer chemical bonding is often required, resulting in complex and costly fabrication processes. Operational stability is another concern; for example, layered perovskites tend to delaminate or undergo structural collapse when exposed to high humidity (>60%) or prolonged illumination. Therefore, developing effective surface passivation strategies is essential for improving their durability in aqueous biological environments.124 In addition to stability issues, mass transport and active site accessibility often restrict the performance of layered architectures. Excessive layer thickness in conjugated polymers (e.g., PPy, PFP) can inhibit microbial activity and undermine system stability, while tight interlayer stacking may limit substrate access to catalytic sites. For instance, the utilization efficiency of edge sites in LDHs is often less than 30%.125 To overcome these limitations, strategies such as intercalation, exfoliation, or biomimetic mineralization, which dynamically modulates interlayer spacing using biological molecules, have been proposed to maintain structural integrity while enhancing charge transfer at the material–biological interface.27
2.3 Research status and challenges
The energy transfer chain in NMHSs, which includes light harvesting, transmembrane transport, and metabolic conversion, demonstrates notable efficiency losses at the material–biological interface. In standard systems, the quantum efficiency of light capture can surpass 80%; however, the efficiency of transmembrane electron transfer significantly declines to only 15–30%, with the ultimate metabolic conversion efficiency frequently dropping below 10%.126 The energy loss cascade is mainly due to charge recombination at the material–biological interface. During the material synthesis process, gradient band engineering strategies, such as ZnO/TiO2/CdS heterojunctions, can significantly decrease interfacial charge recombination rates by 2–3 orders of magnitude.127 However, there is still no guidance for the rational design of cross-species electron transfer pathways to mitigate energy loss. Unlike enzyme-based hybrids where active sites are exposed and defined, whole-cell NMHSs face greater challenges due to the insulating nature of cell membranes and the complexity of transmembrane electron transport, necessitating more robust interface engineering strategies.From a materials-design perspective, the transition from quantum-scale to microscale structures introduces additional limitations. Enlarged particle size causes an exponential decline in specific surface area and active contact area, resulting in reduced light absorption, extended charge-carrier transport distances, and weakened interfacial coupling. For instance, QDs exhibit specific surface areas ranging from 300 to 500 m2 g−1, whereas micron-sized MOFs generally have surface areas under 100 m2 g−1.128 The diminished material–microbe contact area further impairs electron-transfer efficiency and increases interfacial impedance.129 To address these challenges, multiscale material-engineering strategies including hierarchical pore design and surface nano structuring, such as decorating micron-sized particles with quantum dot arrays, which partially compensate for surface area loss through mesoscopic-scale engineering.130 Nevertheless, such composite structures may introduce new issues, including additional interfacial defects and enhanced carrier recombination. Future efforts must integrate cross-scale simulations with dynamic interface engineering to rationally regulate the spatial distribution of active sites while ensuring mechanical robustness and manufacturing feasibility. Beyond these structural considerations, NMHSs also face significant cost and stability constraints. High-efficiency solar-assisted photoelectrochemical systems still rely predominantly on noble-metal-based light-harvesting components, where precious metal catalysts account for 60–80% of the total system cost. In terms of durability, NMHSs synthesized by solution-phase methods often exhibit marked performance degradation after 100 hours of continuous operation.131 Although techniques such as atomic layer deposition (ALD) can generate uniform protective coatings that mitigate photo-corrosion, their complex fabrication procedures may increase device costs by a factor of 4–5.132 Achieving a balanced integration of material stability, microbial viability, and scalable manufacturing therefore remains a major bottleneck to industrial deployment.
Despite these advances in multiscale material design, achieving a balanced integration of stability, microbial compatibility, and scalable manufacturing remains a central barrier to the practical deployment of NMHSs. These limitations highlight that improvements in material architecture alone are insufficient; rather, coordinated progress on both the materials and biological fronts is essential for further enhancing system performance. The synergistic integration of material engineering and microbial genetic engineering, involving the coordinated modulation of material composition, structural hierarchy, and interfacial organization, has already enabled certain NMHSs to achieve solar-to-chemical conversion efficiencies of 3–5%, representing a substantial improvement over the historical benchmark of ∼1%.11 Reaching efficiencies comparable to natural photosynthesis, however, requires breakthroughs in three key areas: (1) elucidating and engineering charge-transfer dynamics at the material–biological interface with atomic-scale precision, supported by cross-scale energy-transfer models; (2) developing photosensitizers that couple biomimetic functionality with environmental safety to overcome efficiency—toxicity trade-offs; and (3) establishing integrated R&D platforms that combine computational design, automated synthesis, and in situ characterization for the precise co-optimization of materials and living systems. Moving forward, the convergence of synthetic biology and artificial intelligence will require NMHSs research to transcend traditional structure–property paradigms. Future optimization frameworks must operate across quantum, mesoscopic, and microscale dimensions, while integrating knowledge from synthetic biology, computational materials science, and systems-level modeling. Such multidisciplinary strategies will be crucial for advancing NMHSs toward high efficiency, long-term durability, and scalable industrial application.
3 Microbial structural characteristics determine the efficiency of photoelectron utilization
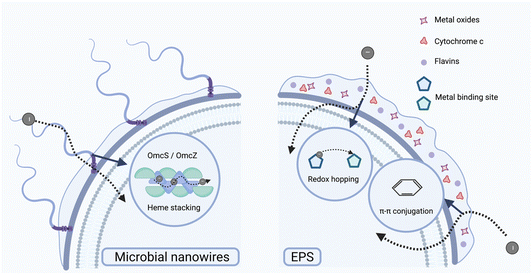
Photoelectron generation is a crucial characteristic of photocatalytic materials; however, their effective utilization is significantly influenced by the capacity of microorganisms to capture and convert these electrons. In heterologous systems that include photocatalysts and microorganisms, photoelectrons must surmount several barriers prior to being effectively acquired and utilized by microbial cells.19,133 This process is affected by various factors, such as the transfer efficiency of photoelectrons,134 the electron transport properties of microbial surfaces,135 and the intracellular capacity for exogenous electron assimilation via metabolic pathways.136 Variations in interfacial formation, electron transfer mechanisms, and metabolic conversion capabilities primarily account for the differences among microbial species in their ability to utilize photoelectrons. The characteristics offer essential theoretical foundations for the design of NMHSs. An in-depth comprehension of the structural characteristics of various microbial types can inspire the optimization of transfer pathways and enhance energy conversion efficiency substantially.In the construction of heterojunction electron transfer interfaces, microbial extracellular components and transmembrane structures exhibit synergistic roles that fundamentally affect the binding affinity between microbes and materials, along with the efficiency of interfacial electron transfer. Extracellular structures, including EPS, flagella, and outer membrane proteins, enhance microbial adhesion to material surfaces, facilitate the construction of stable conductive networks, and modulate the local microenvironment, thereby promoting sustained and efficient extracellular electron transfer. Functional groups in EPS, including hydroxyl, carboxyl, and amine moieties, are capable of forming hydrogen bonds, electrostatic interactions, or covalent bonds with material surfaces, which enhances the efficiency of cross-interface electron transfer.137–140 Transmembrane structures, including the Mtr complex (an outer-membrane cytochrome complex involved in extracellular electron transfer in Shewanella species), outer membrane vesicles (OMVs), and specific ion or metal transport channels, are crucial for facilitating efficient electron transfer across cellular membranes while preserving the electrochemical stability of the cells.141–143 Active transport processes (e.g., substrate uptake and secretion systems) and metal ion transport mechanisms can enhance microbial responsiveness to material stimuli and may facilitate the in situ synthesis of nanomaterials or the stabilization of heterogeneous interfaces. The interaction between extracellular and transmembrane mechanisms creates a functionally diverse and biologically adaptable material–biological hybrid interface, presenting a promising approach to improve photo-electronic coupling efficiency in NMHSs.
Heterologous electron transfer across the interface can be categorized into five distinct functional pathways: extracellular, transmembrane, periplasmic, inner membrane, and intracellular pathways. Each pathway has a specific function in electron transport and energy utilization, characterized by varying transfer potentials. The extracellular pathway primarily entails direct electron transfer (DET) or mediated electron transfer (MET) to enable electron export, with notable electroactive bacteria like Geobacter sulfurreducens and Shewanella oneidensis demonstrating exceptional performance.144,145 The transmembrane pathway relies on membrane-associated conductive complexes, such as MtrCAB cytochromes system, to facilitate electron transport across the membrane, thereby supporting respiration and maintaining the extracellular electron transfer chain. The periplasmic pathway, prevalent in Gram-negative bacteria, serves as an intermediary between the inner and outer membranes, utilizing cytochromes and other redox-active proteins to facilitate electron transfer.146 The inner membrane pathway encompasses essential reaction systems, including the respiratory chain and the photosynthetic electron transfer chain, which function as the metabolic engine for energy conversion.147 Finally, the intracellular pathway facilitates terminal reduction reactions essential for CO2 fixation, nitrogen cycling, and other assimilatory processes, allowing microorganisms to perform functional outputs. The efficiency of transfer and the mechanistic characteristics of each pathway dictate the ability of microbes to capture, transport, and utilize photoelectrons. A systematic understanding and regulation of electron transfer routes benefits to optimize electron flow between microbes and materials, enhancing energy conversion efficiency and operational stability in NMHSs.
3.1 Electron transfer carriers in the extracellular pathway
The electron transfer capabilities of various microorganisms in the extracellular pathway are significantly affected by their external structural features. EPS and flagella contribute to the performance of NMHSs through complementary mechanisms. EPS matrixes, often rich in redox mediators, can serve as the medium for electron exchange,139 whereas flagella facilitate the initial adhesion and subsequent biofilm stabilization on material surfaces via motility and scaffolding effects, rather than participating directly in electron conduction.148,1493.2 Transmembrane electron transfer pathways
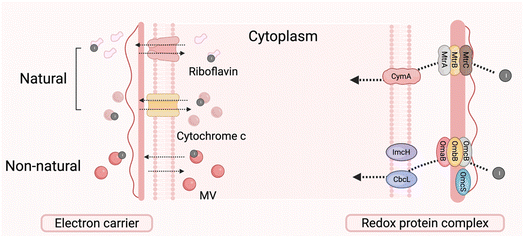
Extracellular interactions, along with the structural characteristics of microbial cells, significantly affect their response to environmental stimuli. The cell membrane is particularly crucial in regulating mass transport and facilitating extracellular electron transfer (EET).160 The cell membrane, consisting of a phospholipid bilayer with embedded transmembrane proteins, demonstrates selective permeability that regulates the exchange of ions, small molecules, and macromolecules.161 Extracellular electrons must cross the membrane via specific mechanisms during electron transfer processes before engaging in intracellular metabolic pathways. The process relies on the coordinated function of conductive proteins, transmembrane transfer proteins, and redox-active electron carriers.3.3 Periplasmic electron transfer relay pathways
The periplasmic space, unique to Gram-negative bacteria, functions as an essential electron transfer center located between the inner and outer membranes. It enables the uptake of extracellular electrons, mediates the linkage between transmembrane electron transport chains, and directs electrons into intracellular metabolic networks.169,170 This pathway involves various redox-active molecules, such as cytochromes and hydrogenases, which are essential for photoelectron transfer, providing a range of functional capabilities and potential applications.3.4 Electron transfer via the inner membrane pathway
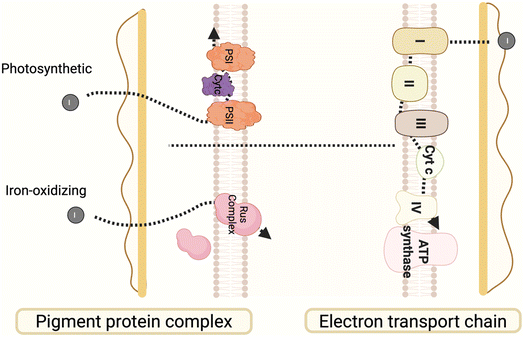
The inner membrane pathway involves various microbial oxidation–reduction chains and rhodopsins that are essential for photoelectron transfer, demonstrating significant application potential. The oxidation–reduction respiratory chain serves as the primary electron transfer system within microbial inner membranes, prevalent in both aerobic and anaerobic microorganisms. The components create an electron flow chain associated with proton pumps, establishing a transmembrane proton gradient that facilitates ATP synthesis.183 Rhodopsins act as light-driven proton pumps, absorbing photon energy and causing conformational changes that facilitate the transport of protons from the cytoplasm to the extracellular space, thereby creating membrane potential and proton gradients.184 By selecting or engineering microorganisms with effective oxidation–reduction chain components and proton pump activity-such as cyanobacteria, photosynthetic bacteria, and halophilic archaea-it is feasible to enhance the efficiency of photoelectron transfer and energy conversion, thereby supporting NMHSs.3.5 Intracellular electron transport pathway
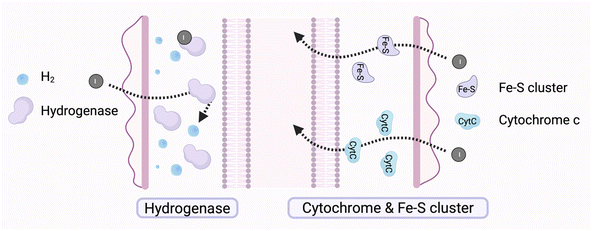
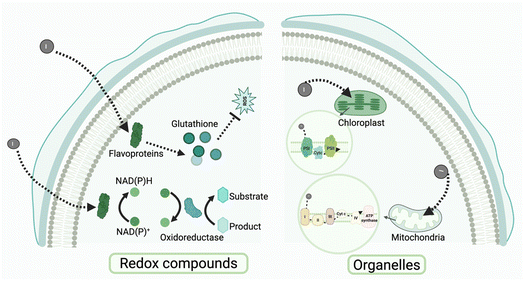
The intracellular environment of microorganism functions as a central hub for biochemical reactions, with its material composition and structural characteristics influencing pathways for exogenous electron transfer and utilization. In biologically mediated photoelectron reactions, the binding sites of intracellular materials and their chemical properties are essential for determining the pathways of electron transfer. Microorganisms contain multiple potential electron-binding sites, including redox compounds,196 protein complexes,58 intracellular membrane-associated systems,197 and soluble electron carriers.164 Together, these sites form heterogeneous intracellular pathways for electron transfer. The electron affinity, redox potential, and spatial arrangement of these pathways determine how photoelectrons are utilized once they enter the cell. Analyzing the characteristics of intracellular electron acceptors, optimizing electron transfer pathways, and modulating the redox environment can enhance the potential for photoelectron applications in biocatalysis and artificial photosynthesis.3.6 Research status and challenges
The rapid advancement of bioengineering and materials science has positioned the integration of microorganisms with exogenous materials and the optimization of photoelectron utilization pathways as critical research areas in solar energy conversion and biocatalysis. Prokaryotes and eukaryotes exhibit differences in cell structure, metabolic pathways, and engineering plasticity, providing unique research platforms for the capture, transfer, and utilization of photoelectrons.10 Cell surface engineering in prokaryotes can markedly improve the interaction between microorganisms and materials. For instance, microorganisms can precisely position photocatalytic nanomaterials or conductive polymers on their surfaces by expressing specific proteins or peptides, such as those that anchor to metal oxide surfaces, thus constructing efficient electron transfer pathway.204 This method enhances the binding capacity between microorganisms and materials while also improving the efficiency of photoelectron transfer. Eukaryotic microorganisms demonstrate distinct advantages in the utilization of photoelectrons and material binding. Their intricate intracellular structures and varied metabolic pathways facilitate the multi-pathway use of photoelectrons. The cell walls and membrane structures of eukaryotic microorganisms offer increased potential for material binding. Modifying surface proteins, such as by enhancing their affinity for metal ions or nanomaterials, allows functionalized materials to be targeted for anchoring at specific extracellular or intracellular locations. This method demonstrates significant potential for enhancing the capture and transfer efficiency of photoelectrons, especially through the targeted incorporation of light-active materials, such as QDs, into microalgae cells.205 The intricate metabolic networks of eukaryotes facilitate the distribution of photoelectrons across multiple pathways. For example, genetically engineering heterologous metabolic pathways allows for more efficient participation of photoelectrons in intracellular synthesis processes, such as the production of shikimic acid.206 This adaptable metabolic regulation increases the capabilities of eukaryotic microorganisms in photobiocatalysis and synthetic metabolism.Photosynthetic bacteria, such as Rhodobacter sphaeroides, are extensively utilized in NMHSs for solar energy conversion and the production of high-value-added compounds.207 Cyanobacteria, such as Synechococcus, engage in photosynthesis and contribute to system functionality via nitrogen fixation.63 Although these microorganisms possess significant potential for genetic manipulation, their capacity to adapt to environmental fluctuations necessitates further enhancement. Conversely, electroactive bacteria such as G. sulfurreducens and Sporomusa ovata exhibit the capability to efficiently accept electrons and interface with photoelectrodes, thereby utilizing photoelectrons for the reduction of CO2 or the synthesis of organic compounds, including acetic acid.208,209 Furthermore, the introduction of exogenous metabolic pathways has enabled engineered microorganisms, such as E. coli, to achieve photo-driven synthesis of chemicals, including lysine.66 Eukaryotic microorganisms, including Saccharomyces cerevisiae and green algae, can effectively produce hydrogen and other high-value products when integrated with photocatalytic materials.206,210 The microbial species utilized in NMHSs are transitioning from individual photosynthetic organisms to engineered, multi-species collaborations and interdisciplinary integrations. Future advancements will depend on interdisciplinary innovations in synthetic biology, materials science, and systems engineering to realize efficient, stable, and scalable clean energy production. These advancements will significantly enhance the application of microorganisms in energy conversion and environmental remediation.
4 Interface construction determines electron transfer efficiency and system compatibility
The interaction between microorganisms and materials governs energy and mass exchange in NMHSs and thus directly influences system efficiency. In these biohybrid systems, photoactive materials convert and supply energy, whereas microorganisms execute metabolic transformations. A poorly engineered interface can lead to photoelectron loss, mass-transfer inefficiencies, or biological incompatibility, highlighting the critical importance of rational interface design.18,19Effective interface engineering must consider several essential factors: the physiochemical properties of materials on separation and migration of photogenerated charges as well as cell coupling, the pathways for photoelectron utilization, and the physiological and metabolic characteristics of the microbial chassis. Microbial extracellular structures, including EPS, cell walls, and membrane-associated redox proteins, interact with material surfaces through physical adsorption, electrostatic interactions, and ligand-specific binding, thereby shaping the formation, stability, and conductivity of electron-transfer interfaces. For example, EPS widely mediates microbial adhesion and sorption, and can modulate the aggregation, mobility, and bioavailability of nanoparticles within biofilms.150,211 These interactions ultimately influence the chemical and electronic microenvironment at the abiotic–biotic interface and are therefore highly relevant to NMHSs design. Moreover, in systems where microorganisms and materials co-exist, EPS has been shown to mediate interaction with NPs: EPS can adsorb NPs, modulate their distribution and diffusion inside the biofilm matrix, and affect NP bioavailability and toxicity.212,213 These findings imply that EPS-material interactions can significantly modulate the stability and electronic/chemical environment at the biotic–abiotic interface, which is relevant for NMHSs design. On the other hand, the size, morphology, and surface chemistry of materials also critically determine the type and performance of the interface. Materials at different scales-quantum, mesoscopic, or micron-sized-and with diverse morphologies (e.g., particulate, layered, sheet-like) establish distinct spatial relationships with cells and consequently differ in their modes of electron delivery and biological compatibility. Accordingly, NMHSs interfaces can be broadly classified as extracellular, transmembrane, or intracellular, each exhibiting unique structural constraints, coupling strengths, and regulatory opportunities for optimizing photoelectron separation and utilization.
Given these material- and microbe-dependent constraints, selecting an appropriate strategy for constructing the abiotic–biotic interface becomes essential for achieving effective photoelectron delivery. Depending on the targeted interface type (extracellular, transmembrane, or intracellular) and the required level of electronic coupling, different assembly approaches can be employed to control material–cell proximity, adhesion strength, and electron-conduction continuity. Among these strategies, the most widely used and experimentally accessible method is the direct co-dispersion of microorganisms with photosensitizer materials. This approach is experimentally simple and requires minimal pretreatment, but weak adhesion between materials and cells can limit electron transfer efficiency. Many photocatalytic materials possess or can be modified with surface functional groups (e.g., –NH2, –OH) that engage in hydrogen bonding or electrostatic interactions with microbial surfaces, partially improving contact.214,215 Conductive additives or pathways formed by microbial redox proteins can further construct “electron highways” that enhance the transfer of photogenerated electrons to the cell surface. However, for many non-electroactive or eukaryotic microorganisms, achieving high-efficiency photoelectron transfer through surface contact alone remains difficult.
As an alternative, some studies have explored EPS-mediated synthesis and stabilization of metal nanoparticles by microorganisms, whereby EPS acts as a template or capping agent for particle formation/stabilization, altering material–microbe interactions and providing a route for material integration.211,216 Another approach involves facilitating the penetration of particles through the cell wall and membrane to shorten the electron transport distance. However, direct internalization of particles remains highly challenging, physical intrusion can disrupt membrane integrity and trigger intracellular ROS generation, both of which compromise cellular viability and long-term system stability.10,212 Overall, the construction of material–microbe interfaces is fundamentally shaped by material physicochemical properties and microbial electron-handling mechanisms. Understanding their interplay enables the rational selection of interface types and engineering strategies that maximize electron-transfer efficiency while maintaining system compatibility.
4.1 Synergistic interface construction between biological structures and multiscale materials
Microbial extracellular and transmembrane structures collectively enable adaptive, multiscale coupling with diverse material morphologies, forming the physical and electrochemical foundation for heterogeneous electron transfer in NMHSs. Through synergistic interactions involving EPS, membrane-bound proteins, vesicle secretion, and ion channels, microbes dynamically modulate material adhesion, electron transfer efficiency, and interfacial stability.129,141,213 Rationally engineering these bio-interfaces in coordination with material design unlocks new potential for constructing robust and efficient NMHSs.4.2 Construction and characteristics of extracellular interfaces
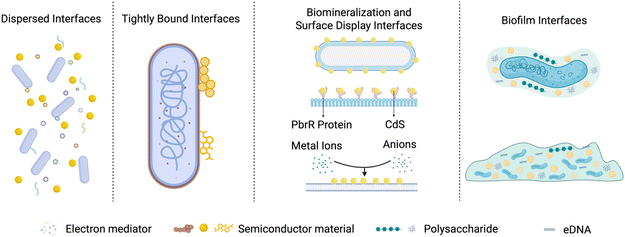
To complement the intracellular nanomaterial internalization pathways discussed above, it is equally essential to recognize that many NMHSs do not rely on materials entering the cytosol to achieve efficient electron or metabolite exchange. Instead, the majority of functional interactions occur at the extracellular material–cell interface, where microbes maintain intact membrane structures while receiving photoelectrons, metabolites, or signaling cues from externally associated materials. Therefore, beyond understanding cellular uptake routes, it becomes critical to examine how engineered extracellular interfaces are constructed and regulated to mediate selective adhesion, directional charge transfer, and stable yet reversible coupling between materials and microorganisms. The construction of extracellular interfaces in NMHSs is a crucial strategy in interface design, marked by the relative independence of materials and microbes at the individual level, without creating an inseparable entity. Interfaces are generally formed via physical or chemical interactions, including electrostatic forces, hydrogen bonding, or covalent bonding, to enable the transfer of matter and energy between photocatalytic materials and microorganisms, which typically divided into four types of interfaces: dispersed, tightly bound, biomineralization and surface display, and biofilm interfaces.The dispersed interface strategy facilitates large-scale preparation and application by eliminating the need for complex stable binding interactions. The lack of stringent specifications regarding material size allows for the utilization of diverse photocatalysts, including micron-sized materials. In these systems, the naturally occurring pores among particles offer both physical protection and effective energy acquisition routes. For example, microbes like S. ovata, which depend on specific electron donors, benefit from large pores that protect them from intense light while sustaining hydrogen utilization, leading to improved biocatalytic activity.208
Despite these advantages, the absence of stable contact between photocatalytic materials and cells constitutes a significant limitation. Electron transfer from photocatalytic materials to the microbial cytoplasm in dispersed systems relies heavily on diffusion, making the process susceptible to physical disturbances such as fluid flow and microbial motility. Consequently, this can lead to fluctuating transfer efficiencies and reduced overall system performance compared to tightly bound interfaces.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–H, and N–H moieties, which facilitate efficient energy transfer to associated microbes. Leveraging this property, a recent study incorporated E. coli into a PCN-222-based NMHSs, resulting in a threefold increase in lysine production.66
N–H, and N–H moieties, which facilitate efficient energy transfer to associated microbes. Leveraging this property, a recent study incorporated E. coli into a PCN-222-based NMHSs, resulting in a threefold increase in lysine production.66
Additionally, hydrogen bonding is another commonly utilized in contemporary research as a method for creating tightly bound interfaces (Fig. 8). Microbial cell walls are predominantly composed of peptidoglycan or glucan, resulting in surfaces abundant in functional groups (e.g., –NH2 and –CHO) capable of forming hydrogen bonds with specific compounds, including natural polyphenols and organic ligands of MOFs.239,240 Compounds containing –OH and –COOH groups typically exhibit negative charges in aqueous solutions because of their ability to donate protons, which facilitates coordination with metal ions and the formation of stable complexes.241 Natural polyphenols, such as tea polyphenols, tannic acid, and terephthalic acid, can function as broad-spectrum biological adhesives for the construction of material–biological interfaces. Tannic acid can form stable complexes with iron ions in alkaline conditions (pH > 8). Its abundant –OH groups enable the formation of hydrogen bonds with polysaccharides and proteins on biological surfaces, resulting in a dense coating layer that provides microbial protection.242–244 Additionally, metal-centered photocatalytic materials demonstrate effective binding to polyphenol complexes. A study utilized InP as photosensitizers material, which was applied to a tannic acid–iron coating layer, resulting in the development of a yeast-based NMHSs that markedly increased intracellular NADPH levels and enhanced product yields.206 Similarly, Zr-based MOFs demonstrate exceptional performance in interfacing with bacteria. Constructed via the coordination of Zr(OH)4 with 1,3,5-benzenetribenzoate (BTB), these frameworks bind tightly to M. thermoacetica, primarily anchored by the formation of (Zr)–O–P bonds with the phosphate moieties of teichoic acid on the bacterial cell wall. Furthermore, the dense architecture of the Zr-based MOF creates a protective barrier that effectively shields the anaerobic bacteria from oxygen exposure.68
Interfaces constructed via electrostatic interactions and hydrogen bonding offer several notable advantages. By promoting stable adhesion between materials and microbial surfaces, these interactions ensure close spatial proximity, which facilitates efficient electron and energy transfer while minimizing energy loss. At the same time, such non-covalent interactions preserve the integrity of the microbial cell envelope, avoiding the cytotoxic effects or structural disruption that might occur with direct internalization of photocatalytic particles. The resulting interfaces are generally robust, enabling sustained material–microbe coupling under moderate environmental fluctuations.
Despite these benefits, this strategy also has inherent limitations. The predominance of negatively charged microbial surfaces restricts the range of compatible materials to those with complementary surface charges or functional groups, limiting material diversity. Strong electrostatic or hydrogen-bonding interactions can also promote microbial aggregation, which may reduce interface uniformity and light penetration, ultimately affecting energy transfer efficiency and system stability. Careful optimization of material surface properties and functional group density is therefore necessary to maximize interface performance while minimizing adverse impacts on microbial physiology.
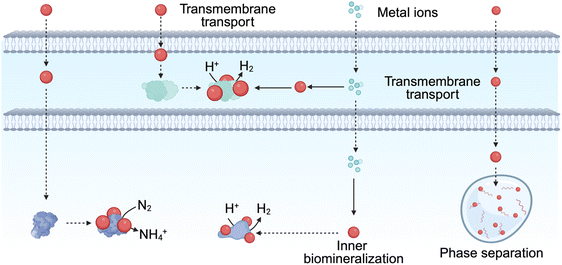
This biomineralization-based interface strategy leverages the intrinsic metabolic capabilities of microorganisms to autonomously generate photocatalytic materials, enabling the formation of robust, tightly coupled material–microbe interfaces with high biocompatibility. Such self-assembled interfaces enhance electron transfer efficiency by ensuring close spatial proximity and strong material–cell interactions without compromising cell integrity. However, the spontaneous nature of biomineralization poses challenges for precise control over material composition, size, and uniformity. In addition, the toxicity of certain metal ions can adversely affect microbial viability, limiting the range of usable ions and necessitating careful optimization of ion concentrations. Strategies to improve microbial tolerance and stabilize biomineralized biohybrid systems are therefore critical for achieving consistent performance and long-term operational stability.
While biofilm-based interfaces provide high biocompatibility, facilitate stable material–microbe coupling, and enable efficient extracellular electron transfer, they also present certain challenges. The dense matrix of extracellular polymeric substances can impede mass transport and limit light penetration, potentially reducing electron transfer efficiency and metabolic activity in the deeper layers of the biofilm. Additionally, the heterogeneity of biofilm formation and variability in thickness across cells can lead to inconsistent interface performance. Careful control of biofilm architecture, combined with material and microbial engineering strategies, is therefore essential to optimize energy transfer and maintain system stability in NMHSs.
In summary, the separation of photoelectrons and their transfer into microbial cells are critical factors in extracellular interface design. Non-electroactive microorganisms or those without transmembrane electron transfer chains, such as cytochrome complexes, can benefit from the addition of external electron mediators like methylene blue or neutral red, which significantly improve electron transfer efficiency.56,254,255 Furthermore, through the rational design of material types and microstructures, optimization of active sites on microbial surfaces, and utilization of microbial physiological and ecological characteristics, one can effectively reduce the transport distance of photoelectrons or electron mediators into the cells, thus enhancing the energy conversion efficiency of the NMHSs.
4.3 Construction and characteristics of transmembrane interfaces
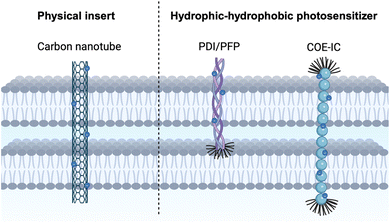
As the distance increases during the transfer of photoelectrons or electron mediators, the loss of electrons escalates. Thus, it is essential to complete electron transport via the most direct route. A feasible approach involves enabling photosensitizers materials to traverse the cell membrane and reach the target enzymatic sites directly. Recent research has investigated various approaches for the development of transmembrane interfaces, such as membrane intercalation, intracellular biomineralization, and the free diffusion of QDs. The following sections elaborate on these approaches.Membrane-intercalated interfaces offer significant advantages by enabling direct and rapid electron transfer into microbial cells. The close integration of materials with the lipid bilayer facilitates efficient energy and electron delivery, enhances metabolic activity. Additionally, the use of amphiphilic or functionalized photosensitizers allows fine-tuning of electron transfer pathways and photocatalytic efficiency. However, this strategy also presents limitations. The introduction of foreign materials into the membrane can potentially disrupt membrane fluidity or local protein function if not carefully optimized, and the design of compatible materials requires precise control over chemical structure, hydrophobicity, and amphiphilicity. Furthermore, material internalization may be restricted by cell type or size, limiting the general applicability of this approach across diverse microbial species.
4.4 Construction and characteristics of intracellular interfaces
The periplasmic interfaces minimize energy loss and reduce unintended interactions, such as ROS generation in cytoplasmic space, thereby preserving cellular integrity and enhancing metabolic activity. However, these interfaces also present challenges. The engineering of precise targeting mechanisms requires sophisticated genetic or chemical modifications, which may be strain-specific and difficult to generalize. Additionally, excessive accumulation of materials or reactive species in confined cellular compartments could potentially disrupt local homeostasis or impair enzymatic function, necessitating careful optimization to balance efficiency and cellular health.
Compartmentalization can similarly establish defined cytoplasmic interfaces by creating distinct functional regions within the cell through liquid–liquid phase separation. This process generates microenvironments that differ from the cytoplasm, thereby locally increasing the concentrations of substrates and enzymes.262–264 Liquid–liquid phase separation is influenced by local concentration gradients resulting from intrinsically disordered proteins and RNA. Modification of photocatalytic materials with peptide functionalities enables the achievement of cell-organelle-like compartmentalization driven by the materials themselves.265 This strategy facilitated the construction of a CdS-based compartmentalized structure in E. coli, which enhances the degradation of environmental pollutants.264
Cytoplasmic interfaces offer the advantage of bringing photosensitizers materials into close proximity with intracellular enzymes, thereby enabling highly efficient electron and energy transfer without the need to cross the cell membrane. This spatial precision enhances reaction specificity, local substrate concentrations, and metabolic efficiency, while also allowing the formation of microenvironments tailored for particular biochemical processes. Furthermore, the use of endogenous molecules and liquid–liquid phase separation can facilitate controlled material assembly and mimic organelle-like compartmentalization. However, these strategies also present challenges, the introduction or in situ formation of materials within the cytoplasm may disrupt cellular homeostasis or organelle function, and excessive accumulation of photocatalytic materials or reactive species could induce cytotoxicity. Achieving consistent intracellular distribution and maintaining long-term cellular viability thus require careful optimization of material properties, delivery methods, and metabolic conditions.
4.5 Intracellular functional modular interfaces based on genetic engineering
The engineering of intracellular functional interfaces in NMHSs relies on the direct modification of microbial cells at the genetic level. By integrating natural light-responsive systems, such as rhodopsins or chloroplast components, non-photosynthetic microorganisms can be endowed with the ability to capture and convert light energy.266 For example, exogenous expression of rhodopsin in E. coli facilitated ATP synthesis rates.267 Beyond single-gene integration, more complex strategies involve incorporating entire photosynthetic microorganisms, such as cyanobacteria, into non-photosynthetic hosts via cell fusion, mimicking the symbiotic relationship between chloroplasts and mitochondria. These approaches, combined with the reconstruction of metabolic networks, enable the establishment of stable intracellular energy-metabolism interfaces. Research has explored such symbiotic systems using model chassis organisms, including S. cerevisiae and cyanobacteria.268,269Genetically engineered intracellular interfaces provide precise control over energy capture and metabolic flux by integrating light-responsive modules directly into microbial cells. This approach enables non-photosynthetic organisms to harness solar energy, improve ATP generation, and establish stable intracellular energy-metabolism pathways, effectively converting them into functional NMHSs. However, such strategies also present challenges: the introduction of exogenous photosystems or metabolic modules may impose a metabolic burden, disrupt native cellular regulation, or provoke unintended interactions between engineered and endogenous pathways. Careful optimization of gene expression levels, module compatibility, and host physiology is therefore essential to maximize energy conversion efficiency while maintaining cell viability.
4.6 Current status and challenges
The material–biological interface in NMHSs is a significant element that has garnered considerable interest from researchers. Current research emphasizes the development of stable and efficient interfaces for long-term energy–matter exchange, utilizing the distinct physical, chemical, and metabolic characteristics of catalysts and microbial surfaces. This necessitates a comprehensive understanding of the catalytic mechanisms of photocatalysts, microbial metabolic pathways, and their interfacial interactions. Current technical limitations present numerous challenges in the study of such interfaces.The mechanism of “information communication” at the interface, specifically the pathways for signal and energy exchange from electron transfer to molecular interactions, is not well understood. Direct visual evidence concerning alterations in the redox state of intermediates and the behavior of redox protein complex during electron transfer is insufficient. Generally, electron transfer occurs via two main routes: the direct EET pathway, where photogenerated electrons from semiconductors are transferred directly to bacterial redox-active proteins (e.g., cytochromes and hydrogenases); and the indirect EET pathway, where electrons are shuttled into bacterial cells via soluble redox mediators (e.g., flavins or hydrogen). These pathways, along with the key proteins involved, have been comprehensively discussed in a recent review.18 Future advancements may involve the use of in situ techniques, including X-ray absorption spectroscopy (XAS), transient absorption spectroscopy (TAS) and other real-time observation methods, to monitor the dynamic process of photoelectron transfer from materials to biological interfaces. Furthermore, fluorescently labeled proteins—which emit specific signals when excited by appropriate wavelengths (e.g., 405 nm for PAmC1, 514 nm for mVenus, 561 nm for mC/PAmC1)—enable the precise tracking of electron transfer events. Notably, recent advances combining multi-channel fluorescence imaging with photoelectrochemical measurements have allowed for the real-time visualization of electron transport at the single-cell level. This integrated approach permits the simultaneous monitoring of protein spatiotemporal dynamics and photocurrent responses from individual cells. Utilizing this platform, Ralstonia eutropha was demonstrated to possess direct EET capability mediated by both soluble and membrane-bound hydrogenases, complementing its well-established H2 mediated indirect electron uptake mechanism.270
Secondly, in contrast to biological systems, photocatalytic materials lack the ability to self-replicate. Moreover, ongoing materials results in material degradation. Achieving long-term stability of material–biological interfaces presents a considerable challenge. A potential solution involves establishing microbial reliance on the energy supply from materials and facilitating microbial perception and regulation of abiotic interfaces. Through the screening of various precursor materials and the application of genetic engineering to the biological host, microorganisms can be engineered to detect precursor molecules in their environment and autonomously construct material–biological interfaces as required. This approach facilitates dynamic interface assembly while ensuring long-term stability.
Efficient interfacial electron transfer constitutes a significant limitation in NMHSs. Microorganisms have a comprehensive and self-regulating energy transfer and metabolic network, complicating the precise direction of external energy inputs to specific target sites. Through the application of genetic engineering, adaptive evolution, and machine learning in the design of materials and microbial chassis, it is feasible to create and manage material–biological interfaces that facilitate unidirectional intracellular energy flow. Furthermore, the development of “interface switches” that are inactive under standard conditions but can be activated by specific stimuli (e.g., temperature, pH, current, or light) may facilitate the regulated activation of NMHSs. This enables microbes to transition from natural growth modes to “production worker” states, facilitating efficient, high-yield, and customizable conversion of solar energy into high-value products.
5 Summary and outlook
NHMSs offer a promising blueprint for solar-to-chemical energy conversion by bridging synthetic materials with living organisms. As a biohybrid platform at the intersection of materials science and microbiology, their performance critically depends on the rational integration of components across scales and functions. Central to this integration is the design of the material–cell interface, which underpins both the stability and the efficiency of the system. Material properties such as size, morphology and surface chemistry dictate how interfaces are assembled, while cellular structures and metabolic capabilities govern interfacial electron transfer dynamics. The spatial configuration and mutual compatibility of materials and microbes collectively determine electron transport routes and, in turn, the yield of desired products. Maximizing the potential of these systems requires a bottom-up design strategy, in which materials and microorganisms are selected and tailored according to the functional demands of the hybrid construct. This entails not only matching interfacial properties but also coordinating the interaction landscape to support efficient and sustained energy flow.To address the complexity of interface engineering, a multi-dimensional optimization framework is essential. Key strategies include: (1) hierarchical assembly to establish efficient charge and mass transport pathways; (2) dynamic interfaces that respond adaptively to environmental cues; (3) biocompatible designs that preserve microbial activity and viability over time; (4) data-driven approaches, such as AI-assisted optimization, to accelerate the discovery and integration of functional material–microbe pairs; and (5) techno-economic analysis for practical application, considering the full energy chain from light harvesting to product formation. Together, these approaches form a roadmap toward constructing robust and high-performing NMHSs, paving the way for scalable and sustainable biohybrid technologies.
5.1 Precision assembly of hierarchical structures
For interface design, a progressive, integrated assembly strategy from photocatalytic materials to microorganisms can be employed, wherein the synergistic interaction between functionalized materials and specific microbes enables in situ energy supply and dynamic organization, thereby precisely regulating energy flow and enhancing the conversion efficiency of target products. Within this framework, the hierarchical design and optimization of materials play a pivotal role. For instance, wavelength-tunable quantum dots encapsulated within the pores of MOFs can achieve full-spectrum light harvesting from ultraviolet to near-infrared (300–850 nm), while the confinement effect of the MOFs matrix improves charge separation efficiency of the photocatalytic components.271 This strategy not only broadens the range of usable solar energy but also significantly enhances photocatalytic activity. Recent studies demonstrate that constructing atomic-level electron bridges at material–microbe interfaces can markedly enhance charge separation and reduce interfacial transfer barriers. For instance, in a C3N4/Ru–Shewanella hybrid, a single-atom Ru–N4 motif enables over a tenfold increase in direct electron uptake and leads to more than an order-of-magnitude improvement in solar-driven H2 production. This illustrates a precise hierarchical strategy in interface engineering governs energy flow and boosts conversion efficiency.272 Besides, applying this hierarchical assembly principle to NMHSs, future designs could utilize similar frameworks to co-localize distinct microbial strains or to couple microbial surface catalysts with photosensitizers, effectively reducing ROS damage and optimizing electron transfer pathways.Beyond material design, the photosynthetic performance of microorganisms is also strongly influenced by their structural distribution. By regulating microbial community composition, metabolic pathways, and cell surface properties, NMHSs can be further optimized. For instance, genetic engineering can be used to control the expression of surface proteins, enabling microorganisms to spontaneously form stable multilevel architectures that improve light utilization efficiency. Moreover, tuning extracellular matrix secretion can enhance microbial immobilization within defined spatial domains, thereby improving system stability and catalytic performance.273 In summary, by coupling material optimization for enhanced light capture and charge separation with microbial engineering strategies for controlled self-assembly and improved robustness, more efficient energy conversion can be achieved in NMHSs.
5.2 Design of stimuli-responsive interfaces
Stimuli-responsive materials have attracted growing attention in photocatalysis, bioelectronics, and artificial photosynthesis due to their inherent environmental adaptability and dynamic tunability.274 These materials are capable of undergoing reversible physical or chemical changes upon exposure to external stimuli such as light, heat, or pH. A prominent example is azobenzene derivatives, which undergo reversible trans–cis isomerization under ultraviolet irradiation or thermal activation. Leveraging this property, photo-responsive azobenzene units have been widely integrated into polymers, MOFs, and other nanostructured materials.275 In the context of NMHSs, introducing such dynamic materials into the light-harvesting module enables the real-time modulation of surface properties. For instance, light-induced conformational switching of azobenzene moieties can dynamically alter the surface zeta potential of the material, which may in turn influence the contact angle at the cell membrane interface. This tunability provides a mechanism for stimulus-triggered activation of electron transport pathways, enhancing transmembrane electron flux and thus improving energy conversion efficiency.Microorganisms inherently possess environmental sensing capabilities, enabling them to reprogram metabolic activity via signal transduction networks. Synthetic biology offers powerful tools to augment this capacity, allowing for the design of intelligent microbial chassis that dynamically adjust metabolic pathways in response to cues such as light intensity, nutrient availability, or redox state. For example, genetically encoded redox-sensitive switches can be used to activate photosynthetic pathways under specific intracellular redox conditions, thereby enhancing overall energy conversion efficiency.276 Specifically, redox-switchable polymer shells have been used to dynamically modulate the conductivity of microbe–semiconductor junctions, thereby optimizing intracellular electron allocation under variable illumination. At the interface level, genetic and metabolic engineering can rewire microbial behavior to preferentially utilize solar energy under light while maintaining viability in the absence of light. Simultaneously, light-responsive catalytic materials can be engineered to sense and respond to changes in the local photonic environment, enabling spatiotemporal control over material–microbe interactions. The integration of stimuli-responsive materials with synthetic biology strategies thus provides a versatile platform for the precise regulation of hybrid photosynthetic systems, offering enhanced control over electron transfer dynamics and energy conversion processes. This dynamic regulation strategy not only improves the stability and responsiveness of NMHSs but also lays the groundwork for future developments in sustainable bioenergy and programmable biohybrid technologies.
5.3 Innovative design of biocompatible interfaces
The selection and design of photocatalytic materials are central to achieving efficient energy conversion. However, many high-performance photocatalysts, such as CdS and CdTe quantum dots as well as organic dyes, while exhibiting excellent light absorption and charge separation capabilities, often suffer from intrinsic biotoxicity, which severely limits their applications in biological contexts. Under illumination, these materials may release toxic metal ions or generate ROS, thereby damaging microbial cells and compromising system stability and long-term functionality. Consequently, enhancing the biocompatibility of photocatalytic materials while maintaining their catalytic efficiency has emerged as a key challenge in this field. Various strategies such as surface modification and interfacial engineering can significantly reduce their adverse effects on microorganisms. For example, coating photocatalysts with biocompatible layers such as polydopamine, silica, or biomacromolecules not only minimizes direct contact with cells but also improves stability and reduces toxicity. Alternatively, modification with EPS secreted by microbes can further enhance material–microbe compatibility, decreasing membrane disruption and alleviating host rejection of foreign materials, thereby improving system robustness.22,277Strengthening microbial adaptability through genetic and metabolic engineering is crucial for the stability of NMHSs. For example, enhancing the expression of antioxidant enzymes can significantly reduce cellular damage caused by ROS generated during photocatalysis. Furthermore, engineering membrane proteins to display specific binding motifs can facilitate the efficient anchoring of photosensitizers, thereby improving electron transfer efficiency at the cellular boundary.204 Complementary to genetic modifications, structural and ecological strategies can further reinforce system robustness. Optimizing microbial community interactions—such as co-culturing strains with complementary metabolic functions—can establish cooperative networks that boost both efficiency and long-term stability.278 Additionally, physical encapsulation strategies can create protective microenvironments that shield microbes from harsh reaction conditions. Similarly, biocompatibility-oriented material engineering, such as introducing lipid-mimetic surface groups onto carbon nitride nanosheets, has also been proven effective in reducing membrane perturbation while sustaining long-term microbial activity. Therefore, integrating approaches such as material surface engineering, microbial adaptability enhancement, and synergistic community design holds great promise for simultaneously improving photocatalytic efficiency and reducing biotoxicity, thereby supporting the long-term stability of NMHSs. This not only expands the applicability of photocatalytic materials in biological contexts but also provides important theoretical and practical foundations for optimizing bio-material interfaces.
5.4 Artificial intelligence for rational design and dynamic control
Advances in computational power and big data analytics have catalyzed the rapid integration of artificial intelligence (AI) into interdisciplinary fields such as synthetic biology and materials science. In the context of NMHSs, AI is evolving from optimizing individual components to enabling end-to-end system-level design. For example, machine learning algorithms can be used to predict the compatibility between material band structures and microbial metabolic networks, or to identify optimal material–microbe pairings via high-throughput screening platforms.279 Data-driven high-throughput simulation methods are emerging as powerful tools in materials discovery, accelerating the selection of efficient photosensitizer–catalyst combinations. In a recent study, Xiong et al. employed a machine learning-assisted screening strategy to develop a high-efficiency molecular artificial photocatalytic system.280 Their approach used key descriptors-including CO2 adsorption energy, photosensitizer lifetime, and electronic coupling induced by intrinsic transition dipoles-to rapidly identify promising catalyst–photosensitizer pairs from thousands of potential combinations. Looking forward, the integration of modular synthetic biology with AI-powered dynamic control could enable real-time optimization of electron transfer pathways, facilitating the transition of NMHSs from laboratory prototypes to scalable applications.281 In microbial optimization, AI can support genome editing strategies, refine metabolic regulation models, and fine-tune culture conditions. By analyzing genomic and metabolomic datasets, machine learning models can predict microbial growth performance and photosynthetic output under defined conditions, enabling the selection of high-performing strains. Moreover, computational simulations-when coupled with synthetic biology tools-can direct metabolic rewiring to enhance light-to-chemical energy conversion efficiency.282AI also holds promise for real-time monitoring and feedback control of microbial consortia. By dynamically adjusting cultivation parameters in response to population-level fluctuations, AI can help maintain long-term system stability and performance. These capabilities not only enhance the compatibility between biological and material components, but also accelerate dynamic system optimization across time and environmental conditions. The deep integration of AI and synthetic biology is poised to expand the functional landscape of NMHSs, offering new strategies for renewable energy production and environmental remediation. For example, AI-guided structural analysis of target proteins can inform the design of materials that selectively interact with catalytic active sites. Combined with machine learning-based high-throughput evaluation of system compatibility, this approach could enable the efficient and low-cost construction of next-generation biohybrid photosynthetic platforms.
5.5 Techno-economic analysis for practical application
Evaluating the techno-economic potential of NMHSs is critical for assessing their scalability and real-world applicability. Techno-economic analysis (TEA) provides a systematic framework to quantify energy input, material costs, and product yields across the entire biohybrid workflow, from light harvesting to target chemical formation. By integrating data on material synthesis, microbial cultivation, and system assembly, TEA can identify cost-intensive steps, energy losses, and bottlenecks in electron and mass transport pathways. The practical potential of NMHSs can be further illustrated by recent examples of scalable solar-to-chemical biohybrids. In one study, semiconductor–microbe hybrids were constructed in situ by introducing an aerobic sulfate reduction pathway into Vibrio natriegens, enabling the utilization of heavy metal ions, sulfate, and organics in wastewater to biosynthesize functional semiconductor nanoparticles. The resulting biohybrids supported efficient solar-driven conversion of organics to 2,3-butanediol, achieving a titer of 13.09 g L−1 in a 5 L illuminated fermenter. Life-cycle assessment confirmed substantial sustainability gains relative to conventional production routes, highlighting both the scalability and environmental benefits of this approach.283The rational design of NMHSs should incorporate TEA to guide their potential practical application. Specifically, when materials were designed to enhance light capture and charge separation, their fabrication costs and long-term stability directly influence the overall economic feasibility. Similarly, microbial engineering strategies that improve self-assembly or metabolic efficiency must be balanced against cultivation complexity and operational expenses. Through iterative TEA-guided design, NMHSs can be optimized not only for maximum photocatalytic performance but also for energy- and cost-efficient implementation, thereby bridging the gap between laboratory-scale demonstrations and large-scale industrial or environmental applications.
Therefore, the future development of NMHSs will no longer be confined to single-dimensional performance enhancement, but will instead advance through precise multilevel structural assembly, dynamic regulation of intelligent responsive interfaces, continuous optimization of biocompatibility, and deep empowerment by artificial intelligence, driving the system toward greater complexity, adaptability, and sustainability. Hierarchical structural design not only maximizes full-spectrum solar energy capture and utilization, but also enables efficient coupling of solar-to-chemical conversion through self-organization and microbial community engineering. Intelligent responsive interfaces endow the system with dynamic “sensing–regulation–feedback” capabilities, maintaining optimal operation under diverse environmental conditions. Continuous breakthroughs in biocompatibility are expected to overcome the dual bottlenecks of material toxicity and microbial tolerance, thereby ensuring long-term stability and ecological safety. Moreover, the integration of artificial intelligence provides a new paradigm for system optimization, enabling data-driven predictions and control from the molecular to the community scale with unprecedented efficiency and precision. Importantly, evaluating system-level energy efficiency and economic viability—including catalyst cost, material scalability, and operational energy inputs—will be crucial for translating NMHSs from academic demonstrations to industrial deployment. Collectively, these cutting-edge strategies lay the scientific foundation for building more efficient, stable, and controllable hybrid systems.
A growing body of studies offers concrete examples of how these strategies can be operationalized. For hierarchical assembly, porphyrinic MOFs such as PCN-222 have been co-engineered with E. coli to form multiscale architectures in which macropores enhance photocarrier generation and directional electron delivery.66 In the domain of intelligent–responsive interfaces, redox-switchable polymer shells have been used to dynamically modulate the conductivity of microbe–semiconductor junctions, thereby optimizing intracellular electron allocation under variable illumination. Together, these representative cases illustrate practical routes for implementing the proposed multidimensional optimization framework.
With further progress, NMHSs are expected to bridge the critical energy-integration gap between modern photovoltaics, photoelectrocatalysis, and biomanufacturing, thereby achieving efficient delivery of exogenous energy into microbial cells, significantly improving the utilization efficiency of one-carbon resources (e.g., CO2, methanol, formate) in microbial factories, and accelerating the establishment of a third-generation green biomanufacturing paradigm. Beyond enabling the efficient transformation of “non-food resources” into “high-value products” under the carbon-neutrality framework, these systems also show broad potential in environmental remediation (CO2 capture and valorization), agricultural enhancement (light-driven biosynthesis of natural products), biomedicine (precise synthesis of pharmaceutical precursors), and intelligent materials (construction of living functional materials), thereby opening new pathways for cross-disciplinary applications. With deeper interdisciplinary collaboration and continuous integration of emerging technologies, NMHSs now stand at a pivotal transition from laboratory research to large-scale application, and are poised to become a key technological driver for green energy development and ecological civilization.
Author contributions
Hao Wang: writing – -original draft, writing – review & editing. Jialu Li: validation, writing – review & editing. Yuhua Feng: validation, writing – review & editing. Donghao He: validation. Xiaolei Fan: conceptualization. Bo Wang: conceptualization, formal analysis, validation. Zhonghua Cai: conceptualization, funding acquisition. Cuiping Zeng: conceptualization, funding acquisition. Kemeng Xiao: conceptualization, visualization, project administration, funding acquisition, writing – original draft, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review. All figures in this review were redrawn by the authors and therefore do not require permission from any journals.Acknowledgements
This work was supported by the National Key R&D Program of China (Grant no. 2022YFA0912800), Natural Science Foundation of China (Grant no. 22508406 and 22406192), Shenzhen Science and Technology Program (Grant No. RCYX20231211090115013 and ZDSYS20220606100606013), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2023A1515030212 and 2022B1111080005).References
- The solar generation, Nat. Synth., 2022, 1, 189 Search PubMed.
- N. Kornienko, J. Z. Zhang, K. K. Sakimoto, P. Yang and E. Reisner, Interfacing nature's catalytic machinery with synthetic materials for semi-artificial photosynthesis, Nat. Nanotechnol., 2018, 13, 890–899 CrossRef CAS.
- C. F. Shih, T. Zhang, J. Li and C. Bai, Powering the Future with Liquid Sunshine, Joule, 2018, 2, 1925–1949 CrossRef CAS.
- A. W. D. Larkum, Limitations and prospects of natural photosynthesis for bioenergy production, Curr. Opin. Biotechnol., 2010, 21, 271–276 CrossRef CAS PubMed.
- R. E. Blankenship, D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, M. R. Gunner, W. Junge, D. M. Kramer, A. Melis, T. A. Moore, C. C. Moser, D. G. Nocera, A. J. Nozik, D. R. Ort, W. W. Parson, R. C. Prince and R. T. Sayre, Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement, Science, 2011, 332, 805–809 CrossRef CAS PubMed.
- O. Khaselev and J. A. Turner, A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting, Science, 1998, 280, 425–427 CrossRef CAS.
- E. Verlage, S. Hu, R. Liu, R. J. R. Jones, K. Sun, C. Xiang, N. S. Lewis and H. A. Atwater, A monolithically integrated, intrinsically safe, 10% efficient, solar-driven water-splitting system based on active, stable earth-abundant electrocatalysts in conjunction with tandem III–V light absorbers protected by amorphous TiO2 films, Energy Environ. Sci., 2015, 8, 3166–3172 RSC.
- X. Fang, S. Kalathil and E. Reisner, Semi-biological approaches to solar-to-chemical conversion, Chem. Soc. Rev., 2020, 49, 4926–4952 RSC.
- J. Cui, H. Sun, R. Chen, J. Sun, G. Mo, G. Luan and X. Lu, Multiple routes toward engineering efficient cyanobacterial photosynthetic biomanufacturing technologies, Green Carbon, 2023, 1, 210–226 CrossRef CAS.
- J. Liang, K. Xiao, X. Wang, T. Hou, C. Zeng, X. Gao, B. Wang and C. Zhong, Revisiting Solar Energy Flow in Nanomaterial-Microorganism Hybrid Systems, Chem. Rev., 2024, 124, 9081–9112 CrossRef CAS PubMed.
- K. Xiao, J. Liang, X. Wang, T. Hou, X. Ren, P. Yin, Z. Ma, C. Zeng, X. Gao, T. Yu, T. Si, B. Wang, C. Zhong, Z. Jiang, C.-S. Lee, J. C.-M. Yu and P. K. Wong, Panoramic insights into semi-artificial photosynthesis: origin, development, and future perspective, Energy Environ. Sci., 2022, 15, 529–549 RSC.
- D. Kim, K. K. Sakimoto, D. Hong and P. Yang, Artificial Photosynthesis for Sustainable Fuel and Chemical Production, Angew. Chem., Int. Ed., 2015, 54, 3259–3266 CrossRef CAS PubMed.
- K. K. Sakimoto, A. B. Wong and P. Yang, Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production, Science, 2016, 351, 74–77 CrossRef CAS PubMed.
- X. Peng, S. G. Karakalos and W. E. Mustain, Preferentially Oriented Ag Nanocrystals with Extremely High Activity and Faradaic Efficiency for CO2 Electrochemical Reduction to CO, ACS Appl. Mater. Interfaces, 2018, 10, 1734–1742 CrossRef CAS PubMed.
- C. Liu, J. J. Gallagher, K. K. Sakimoto, E. M. Nichols, C. J. Chang, M. C. Y. Chang and P. Yang, Nanowire-Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals, Nano Lett., 2015, 15, 3634–3639 CrossRef CAS PubMed.
- E. M. Nichols, J. J. Gallagher, C. Liu, Y. Su, J. Resasco, Y. Yu, Y. Sun, P. Yang, M. C. Y. Chang and C. J. Chang, Hybrid bioinorganic approach to solar-to-chemical conversion, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11461–11466 CrossRef CAS PubMed.
- J. Zhang, F. Li, D. Liu, Q. Liu and H. Song, Engineering extracellular electron transfer pathways of electroactive microorganisms by synthetic biology for energy and chemicals production, Chem. Soc. Rev., 2024, 53, 1375–1446 RSC.
- W. Zhang, C. Xiong, P. Chen, B. Fu and X. Mao, Elucidating Energy Conversion Pathways at Biotic/Abiotic Interfaces in Microbe-Semiconductor Hybrids, J. Am. Chem. Soc., 2025, 5, 20171–20188 CrossRef PubMed.
- X. Guan, Y. Xie and C. Liu, Performance evaluation and multidisciplinary analysis of catalytic fixation reactions by material-microbe hybrids, Nat. Catal., 2024, 7, 475–482 CrossRef CAS.
- E. H. Edwards, J. Jelušić, R. M. Kosko, K. P. McClelland, S. S. Ngarnim, W. Chiang, S. Lampa-Pastirk, T. D. Krauss and K. L. Bren, Shewanella oneidensis MR-1 respires CdSe quantum dots for photocatalytic hydrogen evolution, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2206975120 CrossRef CAS PubMed.
- H. Zhang, Z. Zhao, A. T. Turley, L. Wang, P. R. McGonigal, Y. Tu, Y. Li, Z. Wang, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregate Science: From Structures to Properties, Adv. Mater., 2020, 32, 2001457 CrossRef CAS.
- X. Wang, J. Zhang, K. Li, B. An, Y. Wang and C. Zhong, Photocatalyst-mineralized biofilms as living bio-abiotic interfaces for single enzyme to whole-cell photocatalytic applications, Sci. Adv., 2022, 8, eabm7665 CrossRef CAS PubMed.
- L. Chen, M. A. Maigbay, M. Li and X. Qiu, Synthesis and modification strategies of g-C3N4 nanosheets for photocatalytic applications, Adv. Powder Mater., 2024, 3, 100150 CrossRef.
- Y. Liu, A. Bin Mohamad Annuar, S. Rodríguez-Jiménez, C. W. S. Yeung, Q. Wang, A. M. Coito, R. R. Manuel, I. A. C. Pereira and E. Reisner, Solar Fuel Synthesis Using a Semiartificial Colloidal Z-Scheme, J. Am. Chem. Soc., 2024, 146, 29865–29876 CrossRef CAS PubMed.
- D. Cui, J. Wang, H. Wang, Y. Yang and M. Zhao, The cytotoxicity of endogenous CdS and Cd2+ ions during CdS NPs biosynthesis, J. Hazard. Mater., 2021, 409, 124485 CrossRef CAS PubMed.
- A. Zeinedini and M. M. Shokrieh, Agglomeration phenomenon in graphene/polymer nanocomposites: Reasons, roles, and remedies, Appl. Phys. Rev., 2024, 11, 041301 CAS.
- S. u. Rehman, S. Kong, J. Zhang, H. Xia, R. Chen, Z. Guo, Z. Li, R. Ahmed, A. Rehman, H. Kazemian, Y. Jiang, S. Xu, Y. Jiang, K. Ma and J. Wang, Hydrophilic Metal-Organic Frameworks Regulated by Biomineralized Protein for Enhanced Stability and Drug Delivery, Nano Lett., 2024, 24, 15652–15661 CrossRef CAS PubMed.
- J. Ming, S.-Q. Ni, Z. Guo, Z.-B. Wang and L. Xie, Photocatalytic material-microorganism hybrid systems in water decontamination, Trends Biotechnol., 2025, 43, 1031–1047 CrossRef CAS PubMed.
- F. Chen, H. Zheng, Y. Yusran, H. Li, S. Qiu and Q. Fang, Exploring high-connectivity three-dimensional covalent organic frameworks: topologies, structures, and emerging applications, Chem. Soc. Rev., 2025, 54, 484–514 RSC.
- S. M. Strycharz-Glaven, R. M. Snider, A. Guiseppi-Elie and L. M. Tender, On the electrical conductivity of microbial nanowires and biofilms, Energy Environ. Sci., 2011, 4, 4366–4379 RSC.
- R. M. Snider, S. M. Strycharz-Glaven, S. D. Tsoi, J. S. Erickson and L. M. Tender, Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15467–15472 CrossRef CAS.
- D. J. Filman, S. F. Marino, J. E. Ward, L. Yang, Z. Mester, E. Bullitt, D. R. Lovley and M. Strauss, Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire, Commun. Biol., 2019, 2, 219 CrossRef PubMed.
- F. Wang, Y. Gu, J. P. O'Brien, S. M. Yi, S. E. Yalcin, V. Srikanth, C. Shen, D. Vu, N. L. Ing, A. I. Hochbaum, E. H. Egelman and N. S. Malvankar, Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers, Cell, 2019, 177, 361–369 CrossRef CAS PubMed.
- Y. Wu, X. Zhu, X. Wang, Z. Lin, J. R. Reinfelder, F. Li and T. Liu, A New Electron Shuttling Pathway Mediated by Lipophilic Phenoxazine via the Interaction with Periplasmic and Inner Membrane Proteins of Shewanella oneidensis MR-1, Environ. Sci. Technol., 2023, 57, 2636–2646 CrossRef CAS PubMed.
- N. M. Tefft, K. Ford and M. A. TerAvest, NADH dehydrogenases drive inward electron transfer in Shewanella oneidensis MR-1, Microb. Biotechnol., 2023, 16, 560–568 CrossRef CAS PubMed.
- G. Peltier, E.-M. Aro and T. Shikanai, NDH-1 and NDH-2 Plastoquinone Reductases in Oxygenic Photosynthesis, Annu. Rev. Plant Biol., 2016, 67, 55–80 CrossRef CAS PubMed.
- E. Brostedt, A. Lindblad, J. Jansson and S. Nordlund, Electron transport to nitrogenase in Rhodospirillum rubrum: the role of NAD(P)H as electron donor and the effect of fluoroacetate on nitrogenase activity, FEMS Microbiol. Lett., 1997, 150, 263–267 CrossRef CAS.
- M. Schmacht, E. Lorenz and M. Senz, Microbial production of glutathione, World J. Microbiol. Biotechnol., 2017, 33, 106 CrossRef PubMed.
- E. E. Barton, D. M. Rampulla and A. B. Bocarsly, Selective Solar-Driven Reduction of CO2 to Methanol Using a Catalyzed p-GaP Based Photoelectrochemical Cell, J. Am. Chem. Soc., 2008, 130, 6342–6344 CrossRef CAS PubMed.
- S. Cestellos-Blanco, H. Zhang, J. M. Kim, Y.-X. Shen and P. Yang, Photosynthetic semiconductor biohybrids for solar-driven biocatalysis, Nat. Catal., 2020, 3, 245–255 CrossRef CAS.
- X. Wang, F. Wang, Y. Sang and H. Liu, Full-Spectrum Solar-Light-Activated Photocatalysts for Light-Chemical Energy Conversion, Adv. Energy Mater., 2017, 7, 1700473 CrossRef.
- S. Chen, H. Yin, P. Liu, Y. Wang and H. Zhao, Stabilization and Performance Enhancement Strategies for Halide Perovskite Photocatalysts, Adv. Mater., 2023, 35, 2203836 CrossRef CAS PubMed.
- K. Miriyala, S. A. Shor Peled, D. Klotz and D. A. Grave, Quantification of mobile charge carrier yield and transport lengths in ultrathin film light-trapping ZnFe2O4 photoanodes, J. Mater. Chem. A, 2025, 13, 2965–2973 RSC.
- L. Hu, M. Liu, A. Mandelis, A. Melnikov and E. H. Sargent, Colloidal quantum dot solar cell power conversion efficiency optimization using analysis of current-voltage characteristics and electrode contact imaging by lock-in carrierography, Prog. Photovoltaics Res. Appl., 2017, 25, 1034–1050 CrossRef CAS.
- S. Yaghoubi, S. M. Mousavi, A. Babapoor, M. Binazadeh, C. W. Lai, R. H. Althomali, M. M. Rahman and W.-H. Chiang, Photocatalysts for solar energy conversion: Recent advances and environmental applications, Renewable Sustainable Energy Rev., 2024, 200, 114538 CrossRef CAS.
- Y. Takeda and T. Morikawa, How to supply more solar energy to reactive sites for highly efficient artificial photosynthesis, J. Phys.: Energy, 2025, 7, 012002 CAS.
- S. Kumar, N. Patra, I. Hossain, A. Thakur, T. Jaseetharan and N. G. Shimpi, Exponential developments of quantum dots ecosystem for solar energy conversion and photocatalytic reactions: From photoanode design to renewable energy applications, Mater. Res. Bull., 2025, 184, 113223 CrossRef CAS.
- N. Qin, H. Han, G. Guan and M.-Y. Han, Structurally altered size, composition, shape and interface-dependent optical properties of quantized nanomaterials, Nano Res., 2024, 17, 10543–10569 CrossRef.
- D. E. Westmoreland, R. López-Arteaga, L. P. Kantt, M. R. Wasielewski and E. A. Weiss, Dynamic Tuning of the Bandgap of CdSe Quantum Dots through Redox-Active Exciton-Delocalizing N-Heterocyclic Carbene Ligands, J. Am. Chem. Soc., 2022, 144, 4300–4304 CrossRef CAS PubMed.
- R. K. Goyal, S. Maharaj, P. Kumar and M. Chandrasekhar, Exploring quantum materials and applications: a review, J. Mater. Sci.: Mater. Eng., 2025, 20, 4 Search PubMed.
- Y. Zhang, Y. Li, X. Xin, Y. Wang, P. Guo, R. Wang, B. Wang, W. Huang, A. J. Sobrido and X. Li, Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for photocatalytic overall water splitting, Nat. Energy, 2023, 8, 504–514 CrossRef CAS.
- Y. Gan, T. Chai, J. Zhang, C. Gao, W. Song, J. Wu, L. Liu and X. Chen, Light-driven CO2 utilization for chemical production in bacterium biohybrids, Chin. J. Catal., 2024, 60, 294–303 CrossRef CAS.
- X. Guan, S. Erşan, Y. Xie, J. Park and C. Liu, Redox and Energy Homeostasis Enabled by Photocatalytic Material-Microbial Interfaces, ACS Nano, 2024, 18, 20567–20575 CrossRef CAS PubMed.
- M. T. Gamache, R. Charaf, L. Kurth, D. T. Filmon, M. Senger, N. Plumeré, L. Hammarström and G. Berggren, Elucidating Electron Transfer Kinetics and Optimizing System Performance for Escherichia coli-Based Semi-Artificial H2 Production, ACS Catal., 2023, 13, 9476–9486 CrossRef CAS.
- M. Lorenzi, M. T. Gamache, H. J. Redman, H. Land, M. Senger and G. Berggren, Light-Driven [FeFe] Hydrogenase Based H2 Production in E. coli: A Model Reaction for Exploring E. coli Based Semiartificial Photosynthetic Systems, ACS Sustainable Chem. Eng., 2022, 10, 10760–10767 CrossRef CAS.
- H. Li, X. Yu, Y. Qin, T. Jiang, J. Wang, Z. Cai, J. Xu, Y. Ge, H. Sun, Z. Qi and J. Liu, Synergistic Approaches for Enhanced Light-Driven Hydrogen Production: A Membrane-Anchoring Protein-Engineered Biohybrid System with Dual Photosensitizers Strategy, ACS Mater. Lett., 2024, 6, 1418–1428 CrossRef CAS.
- B. Wang, C. Zeng, K. H. Chu, D. Wu, H. Y. Yip, L. Ye and P. K. Wong, Enhanced Biological Hydrogen Production from Escherichia coli with Surface Precipitated Cadmium Sulfide Nanoparticles, Adv. Energy Mater., 2017, 7, 1700611 Search PubMed.
- X. Lv, W. Huang, Y. Gao, R. Chen, X. Chen, D. Liu, L. Weng, L. He and S. Liu, Boosting solar hydrogen production via electrostatic interaction mediated E. coli-TiO2−x biohybrid system, Nano Res., 2024, 17, 5390–5398 CrossRef CAS.
- X. Pan, W. Li, Y. Fang, H. Zhang, Y. Xiao, M. Molokeev, S. Ping Jiang, Y. Liu and B. Lei, Semi-artificial photosynthetic system based on TiO2/Chlorophyll composite and microalgae for N2 fixation, Chem. Eng. J., 2023, 475, 146179 CrossRef CAS.
- Y. Honda, M. Watanabe, H. Hagiwara, S. Ida and T. Ishihara, Inorganic/whole-cell biohybrid photocatalyst for highly efficient hydrogen production from water, Appl. Catal., B, 2017, 210, 400–406 CrossRef CAS.
- Y.-H. Kuo, M.-C. Hsu, W.-J. Wang, H.-H. Peng and W.-P. Li, Highly conductive riboflavin-based carbon quantum dot–embedded SiO2@MoS2 nanocomposite for enhancing bioelectricity generation through synergistic direct and indirect electron transport, Nano Energy, 2024, 121, 109251 Search PubMed.
- Y. Cong, X. Wang, H. Bai, C. Yao, J. Liu, Y. Wei, Y. Kang, S. Wang and L. Li, Intracellular Gold Nanocluster/Organic Semiconductor Heterostructure for Enhancing Photosynthesis, Angew. Chem., Int. Ed., 2024, 63, e202406527 CrossRef CAS PubMed.
- Q. Hu, H. Hu, L. Cui, Z. Li, D. Svedruzic, J. L. Blackburn, M. C. Beard, J. Ni, W. Xiong, X. Gao and X. Chen, Ultrafast Electron Transfer in Au-Cyanobacteria Hybrid for Solar to Chemical Production, ACS Energy Lett., 2023, 8, 677–684 CrossRef CAS.
- J. Kim, J.-A. Lin, J. Kim, I. Roh, S. Lee and P. Yang, A red-light-powered silicon nanowire biophotochemical diode for simultaneous CO2 reduction and glycerol valorization, Nat. Catal., 2024, 7, 977–986 Search PubMed.
- D. Li, H. Dong, X. Cao, W. Wang and C. Li, Enhancing photosynthetic CO2 fixation by assembling metal-organic frameworks on Chlorella pyrenoidosa, Nat. Commun., 2023, 14, 5337 CrossRef CAS PubMed.
- J. Li, J. Shen, T. Hou, H. Tang, C. Zeng, K. Xiao, Y. Hou and B. Wang, A Self-Assembled MOF-Escherichia Coli Hybrid System for Light-Driven Fuels and Valuable Chemicals Synthesis, Adv. Sci., 2024, 11, 2308597 CrossRef CAS PubMed.
- Q. Zhao, Y. Li, B. Shen, Q. Zhao, L. Zhu and L. Jiang, UiO-66-Mediated Light-Driven Regeneration of Intracellular NADH in Clostridium tyrobutyricum to Strengthen Butyrate Production, ACS Sustainable Chem. Eng., 2023, 11, 3405–3415 CrossRef CAS.
- Z. Ji, H. Zhang, H. Liu, O. M. Yaghi and P. Yang, Cytoprotective metal-organic frameworks for anaerobic bacteria, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10582–10587 CrossRef CAS PubMed.
- M. Simonazzi, S. Lemaire, A. Adamiano, M. Calvaresi, S. Cioffi, F. D. Giorgio, M. D. Giosia, P. Galletti, M. Malferrari, V. Papa, L. Pezzolesi, G. Ruani and C. Samorì, Single-walled carbon nanotubes for enhanced photosynthesis and high-value compound production in microalgae and cyanobacteria, Bioresour. Technol., 2025, 435, 132869 Search PubMed.
- K. Xiao, T. H. Tsang, D. Sun, J. Liang, H. Zhao, Z. Jiang, B. Wang, J. C. Yu and P. K. Wong, Interfacing Iodine-Doped Hydrothermally Carbonized Carbon with Escherichia coli through an “Add-on” Mode for Enhanced Light-Driven Hydrogen Production, Adv. Energy Mater., 2021, 11, 2100291 Search PubMed.
- W. Cheng, X. Wang, H. Hu, Y. Yang, X. Yu, W. X. Chao, M. Yu, J. Ding, Y. Lin, W. Zhao, Q. Zhao, R. Ledesma-Amaro, C. Zhong, L. Lu, X. Chen, J. Liu, C. Yang and X. Gao, Closed-loop enhancement of plant photosynthesis via biomass-derived carbon dots in biohybrids, Commun. Mater., 2025, 6, 40 CrossRef CAS.
- P. Gai, W. Yu, H. Zhao, R. Qi, F. Li, L. Liu, F. Lv and S. Wang, Solar-Powered Organic Semiconducto-Bacteria Biohybrids for CO2 Reduction into Acetic Acid, Angew. Chem., Int. Ed., 2020, 59, 7224–7229 CrossRef CAS PubMed.
- H. Li, Y. Liu, Z. Chen, Y. Yang, T. Lv and T. Chen, High voltage and healing flexible zinc ion battery based on ionogel electrolyte, J. Colloid Interface Sci., 2023, 639, 408–415 CrossRef CAS PubMed.
- X. Zhou, Y. Zeng, F. Lv, H. Bai and S. Wang, Organic Semiconductor-Organism Interfaces for Augmenting Natural and Artificial Photosynthesis, Acc. Chem. Res., 2022, 55, 156–170 Search PubMed.
- Y. Ding, J. R. Bertram, C. Eckert, R. R. Bommareddy, R. Patel, A. Conradie, S. Bryan and P. Nagpal, Nanorg Microbial Factories: Light-Driven Renewable Biochemical Synthesis Using Quantum Dot-Bacteria Nanobiohybrids, J. Am. Chem. Soc., 2019, 141, 10272–10282 Search PubMed.
- S. Jin, O. Allam, K. Lee, J. Lim, M. J. Lee, S. H. Loh, S. S. Jang and S. W. Lee, Carbon Quantum Dot Modified Reduced Graphene Oxide Framework for Improved Alkali Metal Ion Storage Performance, Small, 2022, 18, 2202898 CrossRef CAS PubMed.
- D. Koo, Y. Choi, U. Kim, J. Kim, J. Seo, E. Son, H. Min, J. Kang and H. Park, Mesoporous structured MoS2 as an electron transport layer for efficient and stable perovskite solar cells, Nat. Nanotechnol., 2025, 20, 75–82 Search PubMed.
- A. Bernheim-Groswasser, N. S. Gov, S. A. Safran and S. Tzlil, Living Matter: Mesoscopic Active Materials, Adv. Mater., 2018, 30, 1707028 CrossRef PubMed.
- Y. Estrin, B. Yan, K. Roman, G. Peter, F. Peter, Z. Yuntian and H. Hahn, Architecturing materials at mesoscale: some current trends, Mater. Res. Lett., 2021, 9, 399–421 CrossRef CAS.
- C. Wang, X.-G. Nie, Y. Shi, Y. Zhou, J.-J. Xu, X.-H. Xia and H.-Y. Chen, Direct Plasmon-Accelerated Electrochemical Reaction on Gold Nanoparticles, ACS Nano, 2017, 11, 5897–5905 CrossRef CAS PubMed.
- Y. Ye, C. Jo, I. Jeong and J. Lee, Functional mesoporous materials for energy applications: solar cells, fuel cells, and batteries, Nanoscale, 2013, 5, 4584–4605 RSC.
- W. Zhang, H. He, Y. Tian, K. Lan, Q. Liu, C. Wang, Y. Liu, A. Elzatahry, R. Che, W. Li and D. Zhao, Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting, Chem. Sci., 2019, 10, 1664–1670 RSC.
- C. Zhao, X. Li, M. Jia, Z. Xu, Z. Yang and W. Xiong, Polydopamine functionalized TiO2 with N doping, oxygen vacancies, and carbon layers for enhanced photoelectrocatalytic performance, Environ. Res., 2025, 267, 120592 CrossRef CAS PubMed.
- Z. Jiang, B. Wang, J. C. Yu, J. Wang, T. An, H. Zhao, H. Li, S. Yuan and P. K. Wong, AglnS2/In2S3 heterostructure sensitization of Escherichia coli for sustainable hydrogen production, Nano Energy, 2018, 46, 234–240 Search PubMed.
- L. Chen, X. An, S. Zhao, J. Tang, H. Liu and J. Qu, Multienergy Codriven Electron Transfer Across the Nano-Bio Interface for Efficient Photobiocatalysis, ACS Nano, 2025, 19, 11164–11175 CrossRef CAS PubMed.
- D. Kuranov, A. Grebenkina, A. Bogdanova, V. Platonov, S. Polomoshnov, V. Krivetskiy and M. Rumyantseva, Effect of Donor Nb(V) Doping on the Surface Reactivity, Electrical, Optical and Photocatalytic Properties of Nanocrystalline TiO2, Materials, 2024, 17, 375 Search PubMed.
- M. W. Fu and J. L. Wang, Size effects in multi-scale materials processing and manufacturing, Int. J. Mach. Tools Manuf., 2021, 167, 103755 Search PubMed.
- S. Walia, C. M. Shah, P. Gutruf, H. Nili, D. R. Chowdhury, W. Withayachumnankul, M. Bhaskaran and S. Sriram, Flexible metasurfaces and metamaterials: A review of materials and fabrication processes at micro- and nano-scales, Appl. Phys. Rev., 2015, 2, 011303 Search PubMed.
- G. Zhu, D. Chao, W. Xu, M. Wu and H. Zhang, Microscale Silicon-Based Anodes: Fundamental Understanding and Industrial Prospects for Practical High-Energy Lithium-Ion Batteries, ACS Nano, 2021, 15, 15567–15593 CrossRef CAS PubMed.
- D. Gu, X. Shi, R. Poprawe, D. L. Bourell, R. Setchi and J. Zhu, Material-structure-performance integrated laser-metal additive manufacturing, Science, 2021, 372, eabg1487 Search PubMed.
- G. Mo, Q. Wang, W. Lu, C. Wang and P. Li, Artificial and Semi-artificial Photosynthesis (AP and SAP) Systems Based on Metal-Organic Frameworks, Chin. J. Chem., 2023, 41, 335–354 CrossRef CAS.
- M. N. O'Brien, M. R. Jones and C. A. Mirkin, The nature and implications of uniformity in the hierarchical organization of nanomaterials, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11717–11725 CrossRef PubMed.
- D. Cheng, J. Zhang, J. Fu, H. Song and C. Yu, A hierarchical spatial assembly approach of silica-polymer composites leads to versatile silica/carbon nanoparticles, Sci. Adv., 2023, 9, eadi7502 CrossRef CAS PubMed.
- L. Wang, Y. Li, X. Y. Yang, B. B. Zhang, N. Ninane, H. J. Busscher, Z. Y. Hu, C. Delneuville, N. Jiang, H. Xie, G. Van Tendeloo, T. Hasan and B. L. Su, Single-cell yolk-shell nanoencapsulation for long-term viability with size-dependent permeability and molecular recognition, Natl. Sci. Rev., 2021, 8, nwaa097 Search PubMed.
- W.-J. Ong, L.-L. Tan, Y. H. Ng, S.-T. Yong and S.-P. Chai, Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability?, Chem. Rev., 2016, 116, 7159–7329 Search PubMed.
- J. Liang, Z. Chen, P. Yin, H. Hu, W. Cheng, J. Shang, Y. Yang, Z. Yuan, J. Pan, Y. Yin, W. Li, X. Chen, X. Gao, B. Qiu and B. Wang, Efficient Semi-Artificial Photosynthesis of Ethylene by a Self-Assembled InP-Cyanobacterial Biohybrid System, ChemSusChem, 2023, 16, e202300773 CrossRef CAS PubMed.
- X. Wang, B. Zhang, J. Zhang, X. Jiang, K. Liu, H. Wang, X. Yuan, H. Xu, Y. Zheng, G. Ma and C. Zhong, Conformal and conductive biofilm-bridged artificial Z-scheme system for visible light-driven overall water splitting, Sci. Adv., 2024, 10, eadn6211 CrossRef CAS PubMed.
- F. Wang, J. Y. Cheong, Q. He, G. Duan, S. He, L. Zhang, Y. Zhao, I.-D. Kim and S. Jiang, Phosphorus-doped thick carbon electrode for high-energy density and long-life supercapacitors, Chem. Eng. J., 2021, 414, 128767 CrossRef CAS.
- Y. Yao, X. Zhao, G. Chang, X. Yang and B. Chen, Hierarchically Porous Metal-Organic Frameworks: Synthetic Strategies and Applications, Small Struct., 2023, 4, 2200187 CrossRef CAS.
- Q. Li, Q. Li, Z. Wang, X. Zheng, S. Cai and J. Wu, Recent Advances in Hierarchical Porous Engineering of MOFs and Their Derived Materials for Catalytic and Battery: Methods and Application, Small, 2024, 20, 2303473 CrossRef CAS PubMed.
- Z. You, J. Li, Y. Wang, D. Wu, F. Li and H. Song, Advances in mechanisms and engineering of electroactive biofilms, Biotechnol. Adv., 2023, 66, 108170 CrossRef CAS PubMed.
- J. Neu, C. C. Shipps, M. J. Guberman-Pfeffer, C. Shen, V. Srikanth, J. A. Spies, N. D. Kirchhofer, S. E. Yalcin, G. W. Brudvig, V. S. Batista and N. S. Malvankar, Microbial biofilms as living photoconductors due to ultrafast electron transfer in cytochrome OmcS nanowires, Nat. Commun., 2022, 13, 5150 CrossRef CAS PubMed.
- C. Wang, H. Zhang, Y. Wang, J. Wu, K. O. Kirlikovali, P. Li, Y. Zhou and O. K. Farha, A General Strategy for the Synthesis of Hierarchically Ordered Metal-Organic Frameworks with Tunable Macro-, Meso-, and Micro-Pores, Small, 2023, 19, 2206116 Search PubMed.
- H. Li, X. Yu, Y. Wu, C. Li, Z. Xu, W. Liu, S. Chen, H. Sun, Y. Ge, Z. Qi and J. Liu, Membraneless organelles assembled by AuNPs-enzyme integration in non-photosynthetic bacteria: Achieving high specificity and selectivity for solar hydrogen production, Chem. Eng. J., 2024, 492, 152207 CrossRef CAS.
- B. Luo, Y.-Z. Wang, D. Li, H. Shen, L.-X. Xu, Z. Fang, Z. Xia, J. Ren, W. Shi and Y.-C. Yong, A Periplasmic Photosensitized Biohybrid System for Solar Hydrogen Production, Adv. Energy Mater., 2021, 11, 2100256 CrossRef CAS.
- A. Kumari, S. K. Khare and J. Kundu, Adverse effect of CdTe quantum dots on the cell membrane of Bacillus subtilis : Insight from microscopy, Nano-Struct. Nano-Objects, 2017, 12, 19–26 CrossRef CAS.
- S. J. Soenen, W. J. Parak, J. Rejman and B. Manshian, (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications, Chem. Rev., 2015, 115, 2109–2135 Search PubMed.
- J. K. Lee, D. Samanta, H. G. Nam and R. N. Zare, Spontaneous formation of gold nanostructures in aqueous microdroplets, Nat. Commun., 2018, 9, 1562 Search PubMed.
- Y. Chen, D. Yang, Y. Gao, R. Li, K. An, W. Wang, Z. Zhao, X. Xin, H. Ren and Z. Jiang, On-Surface Bottom-Up Construction of COF Nanoshells towards Photocatalytic H2 Production, Research, 2021, 2021, 9798564 Search PubMed.
- L. Liu, Z. Cai, S. Xue, H. Huang, S. Chen, S. Gou, Z. Zhang, Y. Guo, Y. Yao, W. Bao and P. Zhou, A mass transfer technology for high-density two-dimensional device integration, Nat. Electron., 2025, 8, 135–146 Search PubMed.
- S. Wan, S. Fang, L. Jiang, Q. Cheng and R. H. Baughman, Strong, Conductive, Foldable Graphene Sheets by Sequential Ionic and π Bridging, Adv. Mater., 2018, 30, 1802733 Search PubMed.
- H. Zhang, L. Yu, T. Chen, W. Zhou and X. W. Lou, Surface Modulation of Hierarchical MoS2 Nanosheets by Ni Single Atoms for Enhanced Electrocatalytic Hydrogen Evolution, Adv. Funct. Mater., 2018, 28, 1807086 CrossRef.
- M. Pourmadadi, M. Rajabzadeh-Khosroshahi, F. Saeidi Tabar, N. Ajalli, A. Samadi, M. Yazdani, F. Yazdian, A. Rahdar and A. M. Díez-Pascual, Two-Dimensional Graphitic Carbon Nitride (g-C3N4) Nanosheets and Their Derivatives for Diagnosis and Detection Applications, J. Funct. Biomater., 2022, 13, 204 CrossRef CAS PubMed.
- B. Lin, M. Xia, B. Xu, B. Chong, Z. Chen and G. Yang, Bio-inspired nanostructured g-C3N4-based photocatalysts: A comprehensive review, Chin. J. Catal., 2022, 43, 2141–2172 CrossRef CAS.
- X. Wen, Y.-N. Hou, J. Guo, Z. Liu, N. Ren, A.-J. Wang, W. Wei, B.-J. Ni and C. Huang, Mechanistic insight into enhanced methyl orange degradation by Raoultella planticola/MoS2 biohybrid: Implication for electron transfer and microbial metabolism, J. Cleaner Prod., 2024, 469, 143201 CrossRef CAS.
- B. Zhao, W. Zhong, F. Chen, P. Wang, C. Bie and H. Yu, High-crystalline g-C3N4 photocatalysts: Synthesis, structure modulation, and H2-evolution application, Chin. J. Catal., 2023, 52, 127–143 CrossRef CAS.
- M. Pan, X. Zhang, J. Li, J. W. Chew and B. Pan, Stimulating the Intrinsic Activities of the MoS2 Nanosheet Coated on S,N-Graphene for Efficient Membrane Electrofiltration, ACS ES&T Water, 2023, 3, 1963–1971 Search PubMed.
- X. Chen, X. Zhai, J. Hou, H. Cao, X. Yue, M. Li, L. Chen, Z. Liu, G. Ge and X. Guo, Tunable nitrogen-doped delaminated 2D MXene obtained by NH3/Ar plasma treatment as highly efficient hydrogen and oxygen evolution reaction electrocatalyst, Chem. Eng. J., 2021, 420, 129832 CrossRef CAS.
- M. Zhao, Y. Huang, Y. Peng, Z. Huang, Q. Ma and H. Zhang, Two-dimensional metal-organic framework nanosheets: synthesis and applications, Chem. Soc. Rev., 2018, 47, 6267–6295 RSC.
- X. Xiao, M. Wu, Z. Ni, S. Xu, S. Chen, J. Hu, P. N. Rudd, W. You and J. Huang, Ultrafast Exciton Transport with a Long Diffusion Length in Layered Perovskites with Organic Cation Functionalization, Adv. Mater., 2020, 32, 2004080 CrossRef CAS PubMed.
- X. Lu, H. Xue, H. Gong, M. Bai, D. Tang, R. Ma and T. Sasaki, 2D Layered Double Hydroxide Nanosheets and Their Derivatives Toward Efficient Oxygen Evolution Reaction, Nano-Micro Lett., 2020, 12, 86 Search PubMed.
- N. A. Pechnikova, K. Domvri, K. Porpodis, M. S. Istomina, A. V. Iaremenko and A. V. Yaremenko, Carbon Quantum Dots in Biomedical Applications: Advances, Challenges, and Future Prospects, Aggregate, 2025, 6, e707 Search PubMed.
- X. Zhou, L. Zhou, P. Zhang, F. Lv, L. Liu, R. Qi, Y. Wang, M.-Y. Shen, H.-H. Yu, G. Bazan and S. Wang, Conducting Polymers-Thylakoid Hybrid Materials for Water Oxidation and Photoelectric Conversion, Adv. Electron. Mater., 2019, 5, 1800789 CrossRef.
- Y. Zou, W. Yu, H. Guo, Q. Li, X. Li, L. Li, Y. Liu, H. Wang, Z. Tang, S. Yang, Y. Chen, B. Qu, Y. Gao, Z. Chen, S. Wang, D. Zhang, Y. Chen, Q. Chen, S. M. Zakeeruddin, Y. Peng, H. Zhou, Q. Gong, M. Wei, M. Grätzel and L. Xiao, A crystal capping layer for formation of black-phase FAPbI3 perovskite in humid air, Science, 2024, 385, 161–167 Search PubMed.
- S. He, Z. An, M. Wei, D. G. Evans and X. Duan, Layered double hydroxide-based catalysts: nanostructure design and catalytic performance, Chem. Commun., 2013, 49, 5912–5920 Search PubMed.
- K. Qin, Z. Zheng, J. Wang, H. Pan and R. Tang, Biomineralization strategy: from material manufacturing to biological regulation, Giant, 2024, 19, 100317 CrossRef CAS.
- A. Razzaq, M. Zafar, T. Saif, J. Y. Lee, J. K. Park and W. Y. Kim, Efficiency Enhancement by Insertion of ZnO Recombination Barrier Layer in CdS Quantum Dot-Sensitized Solar Cells, J. Nanosci. Nanotechnol., 2021, 21, 3800–3805 CrossRef CAS PubMed.
- F. P. García de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, M. Bayer and E. H. Sargent, Semiconductor quantum dots: Technological progress and future challenges, Science, 2021, 373, eaaz8541 CrossRef PubMed.
- I. L. Bishara Robertson, H. Zhang, E. Reisner, J. N. Butt and L. J. C. Jeuken, Engineering of bespoke photosensitiser-microbe interfaces for enhanced semi-artificial photosynthesis, Chem. Sci., 2024, 15, 9893–9914 RSC.
- Y. Xiang, L. Lu, A. G. P. Kottapalli and Y. Pei, Status and perspectives of hierarchical porous carbon materials in terms of high-performance lithium-sulfur batteries, Carbon Energy, 2022, 4, 346–398 Search PubMed.
- L. Chen, R. Luque and Y. Li, Controllable design of tunable nanostructures inside metal-organic frameworks, Chem. Soc. Rev., 2017, 46, 4614–4630 RSC.
- F. Yang, H. H. Xie, F. Du, X. Hou and S. F. Tang, Insight into the efficient loading and enhanced activity of enzymes immobilized on functionalized UiO-66, Int. J. Biol. Macromol., 2024, 279, 135557 Search PubMed.
- W. Ji, J. Liu, C. Sha, Y.-C. Yong, Y. Jiang and Z. Fang, Nanomaterial-biological hybrid systems: Advancements in solar-driven CO2-to-chemical conversion, Green Carbon, 2024, 2, 322–336 CrossRef CAS.
- J. Ye, C. Wang, C. Gao, T. Fu, C. Yang, G. Ren, J. Lü, S. Zhou and Y. Xiong, Solar-driven methanogenesis with ultrahigh selectivity by turning down H2 production at biotic-abiotic interface, Nat. Commun., 2022, 13, 6612 CrossRef CAS PubMed.
- E. Dumas, C. Gao, D. Suffern, S. E. Bradforth, N. M. Dimitrijevic and J. L. Nadeau, Interfacial Charge Transfer between CdTe Quantum Dots and Gram Negative Vs Gram Positive Bacteria, Environ. Sci. Technol., 2010, 44, 1464–1470 CrossRef CAS PubMed.
- S. Bassett, Y. Ding, M. K. Roy, J. A. Reisz, A. D'Alessandro, P. Nagpal and A. Chatterjee, Light-Driven Metabolic Pathways in Non-Photosynthetic Biohybrid Bacteria, ChemBioChem, 2024, 25, e202300572 Search PubMed.
- C. Qu, S. Yang, M. Mortimer, M. Zhang, J. Chen, Y. Wu, W. Chen, P. Cai and Q. Huang, Functional group diversity for the adsorption of lead(Pb) to bacterial cells and extracellular polymeric substances, Environ. Pollut., 2022, 295, 118651 CrossRef CAS PubMed.
- D. Strieth, J. Stiefelmaier, B. Wrabl, J. Schwing, A. Schmeckebier, S. Di Nonno, K. Muffler and R. Ulber, A new strategy for a combined isolation of EPS and pigments from cyanobacteria, J. Appl. Phycol., 2020, 32, 1729–1740 CrossRef CAS.
- Y. Xiao, E. Zhang, J. Zhang, Y. Dai, Z. Yang, H. E. M. Christensen, J. Ulstrup and F. Zhao, Extracellular polymeric substances are transient media for microbial extracellular electron transfer, Sci. Adv., 2017, 3, e1700623 CrossRef PubMed.
- S. Subramanian and D. B. Kearns, Functional Regulators of Bacterial Flagella, Annu. Rev. Microbiol., 2019, 73, 225–246 Search PubMed.
- A. Kulp and M. J. Kuehn, Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles, Annu. Rev. Microbiol., 2010, 64, 163–184 Search PubMed.
- L.-G. Wu, E. Hamid, W. Shin and H.-C. Chiang, Exocytosis and Endocytosis: Modes, Functions, and Coupling Mechanisms, Annu. Rev. Physiol., 2014, 76, 301–331 Search PubMed.
- S. J. Biller, F. Schubotz, S. E. Roggensack, A. W. Thompson, R. E. Summons and S. W. Chisholm, Bacterial Vesicles in Marine Ecosystems, Science, 2014, 343, 183–186 Search PubMed.
- L. Mao and W. S. Verwoerd, Theoretical exploration of optimal metabolic flux distributions for extracellular electron transfer by Shewanella oneidensis MR-1, Biotechnol. Biofuels, 2014, 7, 118 Search PubMed.
- L. Mao and W. S. Verwoerd, Model-driven elucidation of the inherent capacity of Geobacter sulfurreducens for electricity generation, J. Biol. Eng., 2013, 7, 14 CrossRef CAS PubMed.
- R. Kumar, L. Singh and A. W. Zularisam, Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications, Renewable Sustainable Energy Rev., 2016, 56, 1322–1336 Search PubMed.
- S. Scherer, Do photosynthetic and respiratory electron transport chains share redox proteins?, Trends Biochem. Sci., 1990, 15, 458–462 Search PubMed.
- A. Houry, R. Briandet, S. Aymerich and M. Gohar, Involvement of motility and flagella in Bacillus cereus biofilm formation, Microbiology, 2010, 156, 1009–1018 Search PubMed.
- G. Reguera, K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen and D. R. Lovley, Extracellular electron transfer via microbial nanowires, Nature, 2005, 435, 1098–1101 Search PubMed.
- H.-C. Flemming, E. D. van Hullebusch, B. J. Little, T. R. Neu, P. H. Nielsen, T. Seviour, P. Stoodley, J. Wingender and S. Wuertz, Microbial extracellular polymeric substances in the environment, technology and medicine, Nat. Rev. Microbiol., 2025, 23, 87–105 CrossRef CAS PubMed.
- H. C. Flemming and J. Wingender, The biofilm matrix, Nat. Rev. Microbiol., 2010, 8, 623–633 Search PubMed.
- M. Stöckl, N. C. Teubner, D. Holtmann, K.-M. Mangold and W. Sand, Extracellular Polymeric Substances from Geobacter sulfurreducens Biofilms in Microbial Fuel Cells, ACS Appl. Mater. Interfaces, 2019, 11, 8961–8968 Search PubMed.
- V. S. Grinev, K. V. Tregubova, A. A. Anis'kov, E. N. Sigida, A. A. Shirokov, Y. P. Fedonenko and I. V. Yegorenkova, Isolation, structure, and potential biotechnological applications of the exopolysaccharide from Paenibacillus polymyxa 92, Carbohydr. Polym., 2020, 232, 115780 Search PubMed.
- M. Hamidi, O. V. Okoro, G. Ianiri, H. Jafari, K. Rashidi, S. Ghasemi, R. Castoria, D. Palmieri, C. Delattre, G. Pierre, M. Mirzaei, L. Nie, H. Samadian and A. Shavandi, Exopolysaccharide from the yeast Papiliotrema terrestris PT22AV for skin wound healing, J. Adv. Res., 2023, 46, 61–74 CrossRef CAS PubMed.
- N. S. Malvankar, M. Vargas, K. P. Nevin, A. E. Franks, C. Leang, B.-C. Kim, K. Inoue, T. Mester, S. F. Covalla, J. P. Johnson, V. M. Rotello, M. T. Tuominen and D. R. Lovley, Tunable metallic-like conductivity in microbial nanowire networks, Nat. Nanotechnol., 2011, 6, 573–579 Search PubMed.
- X. Liu, S. Zhuo, X. Jing, Y. Yuan, C. Rensing and S. Zhou, Flagella act as Geobacter biofilm scaffolds to stabilize biofilm and facilitate extracellular electron transfer, Biosens. Bioelectron., 2019, 146, 111748 Search PubMed.
- Y. Gu, V. Srikanth, A. I. Salazar-Morales, R. Jain, J. P. O'Brien, S. M. Yi, R. K. Soni, F. A. Samatey, S. E. Yalcin and N. S. Malvankar, Structure of Geobacter pili reveals secretory rather than nanowire behaviour, Nature, 2021, 597, 430–434 Search PubMed.
- F. Wang, K. Mustafa, V. Suciu, K. Joshi, C. H. Chan, S. Choi, Z. Su, D. Si, A. I. Hochbaum, E. H. Egelman and D. R. Bond, Cryo-EM structure of an extracellular Geobacter OmcE cytochrome filament reveals tetrahaem packing, Nat. Microbiol., 2022, 7, 1291–1300 Search PubMed.
- S. E. Yalcin and N. S. Malvankar, The blind men and the filament: Understanding structures and functions of microbial nanowires, Curr. Opin. Chem. Biol., 2020, 59, 193–201 CrossRef CAS PubMed.
- M. Edidin, Lipids on the frontier: a century of cell-membrane bilayers, Nat. Rev. Mol. Cell Biol., 2003, 4, 414–418 CrossRef CAS PubMed.
- M. P. Stewart, A. Sharei, X. Ding, G. Sahay, R. Langer and K. F. Jensen, In vitro and ex vivo strategies for intracellular delivery, Nature, 2016, 538, 183–192 CrossRef CAS PubMed.
- A. I. Pimenta, C. M. Paquete, L. Morgado, M. J. Edwards, T. A. Clarke, C. A. Salgueiro, I. A. C. Pereira and A. G. Duarte, Characterization of the inner membrane cytochrome ImcH from Geobacter reveals its importance for extracellular electron transfer and energy conservation, Protein Sci., 2023, 32, e4796 CrossRef CAS PubMed.
- C. E. Levar, C. L. Hoffman, A. J. Dunshee, B. M. Toner and D. R. Bond, Redox potential as a master variable controlling pathways of metal reduction by Geobacter sulfurreducens, ISME J., 2017, 11, 741–752 CrossRef CAS PubMed.
- Y. Jia, D. Liu, Y. Chen and Y. Hu, Evidence for the feasibility of transmembrane proton gradient regulating oxytetracycline extracellular biodegradation mediated by biosynthesized palladium nanoparticles, J. Hazard. Mater., 2023, 455, 131544 Search PubMed.
- W. Yu, M. V. Pavliuk, A. Liu, Y. Zeng, S. Xia, Y. Huang, H. Bai, F. Lv, H. Tian and S. Wang, Photosynthetic Polymer Dots-Bacteria Biohybrid System Based on Transmembrane Electron Transport for Fixing CO2 into Poly-3-hydroxybutyrate, ACS Appl. Mater. Interfaces, 2023, 15, 2183–2191 CrossRef CAS PubMed.
- X. Li, X. Tian, X. Yan, N. Huo, X. Wu and F. Zhao, Lumichrome from the photolytic riboflavin acts as an electron shuttle in microbial photoelectrochemical systems, Bioelectrochemistry, 2023, 152, 108439 CrossRef CAS PubMed.
- X. Lin, F. Yang, L.-X. You, H. Wang and F. Zhao, Liposoluble quinone promotes the reduction of hydrophobic mineral and extracellular electron transfer of Shewanella oneidensis MR-1, Innovation, 2021, 2, 100104 CAS.
- J. J. Pierella Karlusich and N. Carrillo, Evolution of the acceptor side of photosystem I: ferredoxin, flavodoxin, and ferredoxin-NADP+ oxidoreductase, Photosynth. Res., 2017, 134, 235–250 CrossRef CAS PubMed.
- M. Mouhib, M. Reggente, L. Li, N. Schuergers and A. A. Boghossian, Extracellular electron transfer pathways to enhance the electroactivity of modified Escherichia coli, Joule, 2023, 7, 2092–2106 CrossRef CAS.
- Y. Li, S. Qiao, M. Guo, L. Zhang, G. Liu and J. Zhou, Biological Self-Assembled Transmembrane Electron Conduits for High-Efficiency Ammonia Production in Microbial Electrosynthesis, Environ. Sci. Technol., 2024, 58, 7457–7468 Search PubMed.
- J. H. van Wonderen, C. R. Hall, X. Jiang, K. Adamczyk, A. Carof, I. Heisler, S. E. H. Piper, T. A. Clarke, N. J. Watmough, I. V. Sazanovich, M. Towrie, S. R. Meech, J. Blumberger and J. N. Butt, Ultrafast Light-Driven Electron Transfer in a Ru(II)tris(bipyridine)-Labeled Multiheme Cytochrome, J. Am. Chem. Soc., 2019, 141, 15190–15200 CrossRef CAS PubMed.
- B. Xing, N. J. D. Graham, B. Zhao, X. Li, Y. Tang, A. Kappler, H. Dong, M. Winkler and W. Yu, Goethite Formed in the Periplasmic Space of Pseudomonas sp. JM-7 during Fe Cycling Enhances Its Denitrification in Water, Environ. Sci. Technol., 2023, 57, 11096–11107 CrossRef CAS PubMed.
- H.-C. Jung, K. Lim Jae, T.-J. Yang, G. Kang Sung and S. Lee Hyun, Direct Electron Transfer between the frhAGB-Encoded Hydrogenase and Thioredoxin Reductase in the Nonmethanogenic Archaeon Thermococcus onnurineus NA1, Appl. Environ. Microbiol., 2020, 86, e02630 Search PubMed.
- A. Parkin, L. Bowman, M. M. Roessler, R. A. Davies, T. Palmer, F. A. Armstrong and F. Sargent, How Salmonella oxidises H2 under aerobic conditions, FEBS Lett., 2012, 586, 536–544 Search PubMed.
- B.-E. Jugder, J. Welch, K.-F. Aguey-Zinsou and C. P. Marquis, Fundamentals and electrochemical applications of [Ni–Fe]-uptake hydrogenases, RSC Adv., 2013, 3, 8142–8159 RSC.
- J. Meyer, [FeFe] hydrogenases and their evolution: a genomic perspective, Cell. Mol. Life Sci., 2007, 64, 1063 CrossRef CAS PubMed.
- T. Palmer and B. C. Berks, The twin-arginine translocation (Tat) protein export pathway, Nat. Rev. Microbiol., 2012, 10, 483–496 CrossRef CAS PubMed.
- T. Schubert, O. Lenz, E. Krause, R. Volkmer and B. Friedrich, Chaperones specific for the membrane-bound [NiFe]-hydrogenase interact with the Tat signal peptide of the small subunit precursor in Ralstonia eutropha H16, Mol. Microbiol., 2007, 66, 453–467 CrossRef CAS PubMed.
- D. S. Horner, P. G. Foster and T. M. Embley, Iron Hydrogenases and the Evolution of Anaerobic Eukaryotes, Mol. Biol. Evol., 2000, 17, 1695–1709 CrossRef CAS PubMed.
- G. Eisbrenner, P. Roos and H. Bothe, The Number of Hydrogenases in Cyanobacteria, Microbiology, 1981, 125, 383–390 CAS.
- D. Tolleter, B. Ghysels, J. Alric, D. Petroutsos, I. Tolstygina, D. Krawietz, T. Happe, P. Auroy, J.-M. Adriano, A. Beyly, S. Cuiné, J. Plet, I. M. Reiter, B. Genty, L. Cournac, M. Hippler and G. Peltier, Control of Hydrogen Photoproduction by the Proton Gradient Generated by Cyclic Electron Flow in Chlamydomonas reinhardtii, Plant Cell, 2011, 23, 2619–2630 CrossRef CAS PubMed.
- R. van Lis, C. Baffert, Y. Couté, W. Nitschke and A. Atteia, Chlamydomonas reinhardtii Chloroplasts Contain a Homodimeric Pyruvate:Ferredoxin Oxidoreductase That Functions with FDX1, Plant Physiol., 2013, 161, 57–71 CrossRef CAS PubMed.
- G. Reguera, Biological electron transport goes the extra mile, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5632–5634 CrossRef CAS PubMed.
- K. Kojima and Y. Sudo, Convergent evolution of animal and microbial rhodopsins, RSC Adv., 2023, 13, 5367–5381 RSC.
- B. Liu, H. Wang, C. Su, S. ShangGuan, Y. Zhang, S. Nie, R. Wang, P. Li, J. Wang and J. Su, Reconfiguring the Escherichia coli Electron Transport Chain to Enhance trans-2-Decenoic Acid Production, ACS Synth. Biol., 2024, 13, 3646–3657 CrossRef CAS PubMed.
- K. Matsushita, M. Yamada, E. Shinagawa, O. Adachi and M. Ameyama, Membrane-bound respiratory chain of Pseudomonas aeruginosa grown aerobically, J. Bacteriol., 1980, 141, 389–392 CrossRef CAS PubMed.
- A. G. M. Tielens and J. J. Van Hellemond, The electron transport chain in anaerobically functioning eukaryotes, Biochim. Biophys. Acta, Bioenerg., 1998, 1365, 71–78 CrossRef CAS PubMed.
- B. Kraft, M. Strous and H. E. Tegetmeyer, Microbial nitrate respiration-Genes, enzymes and environmental distribution, J. Biotechnol., 2011, 155, 104–117 CrossRef CAS PubMed.
- C. Bücking, A. Piepenbrock, A. Kappler and J. Gescher, Outer-membrane cytochrome-independent reduction of extracellular electron acceptors in Shewanella oneidensis, Microbiology, 2012, 158, 2144–2157 CrossRef PubMed.
- Z. Peng, Z. Liu, Y. Jiang, Y. Dong and L. Shi, In vivo interactions between Cyc2 and Rus as well as Rus and Cyc1 of Acidithiobacillus ferrooxidans during extracellular oxidization of ferrous iron, Int. Biodeterior. Biodegrad., 2022, 173, 105453 CrossRef CAS.
- J. A. Mejía-Barajas, J. A. Martínez-Mora, R. Salgado-Garciglia, R. Noriega-Cisneros, O. Ortiz-Avila, C. Cortés-Rojo and A. Saavedra-Molina, Electron transport chain in a thermotolerant yeast, J. Bioenerg. Biomembr., 2017, 49, 195–203 CrossRef PubMed.
- M. Li, A. Calteau, D. A. Semchonok, T. A. Witt, J. T. Nguyen, N. Sassoon, E. J. Boekema, J. Whitelegge, M. Gugger and B. D. Bruce, Physiological and evolutionary implications of tetrameric photosystem I in cyanobacteria, Nat. Plants, 2019, 5, 1309–1319 CrossRef CAS PubMed.
- S. Khorobrykh, T. Tsurumaki, K. Tanaka, T. Tyystjärvi and E. Tyystjärvi, Measurement of the redox state of the plastoquinone pool in cyanobacteria, FEBS Lett., 2020, 594, 367–375 CrossRef CAS PubMed.
- V. Shevchenko, T. Mager, K. Kovalev, V. Polovinkin, A. Alekseev, J. Juettner, I. Chizhov, C. Bamann, C. Vavourakis, R. Ghai, I. Gushchin, V. Borshchevskiy, A. Rogachev, I. Melnikov, A. Popov, T. Balandin, F. Rodriguez-Valera, D. J. Manstein, G. Bueldt, E. Bamberg and V. Gordeliy, Inward H+ pump xenorhodopsin: Mechanism and alternative optogenetic approach, Sci. Adv., 2017, 3, e1603187 Search PubMed.
- M. Hasegawa-Takano, T. Hosaka, K. Kojima, Y. Nishimura, M. Kurihara, Y. Nakajima, Y. Ishizuka-Katsura, T. Kimura-Someya, M. Shirouzu, Y. Sudo and S. Yoshizawa, Cyanorhodopsin-II represents a yellow-absorbing proton-pumping rhodopsin clade within cyanobacteria, ISME J., 2024, 18, wrae175 CrossRef CAS PubMed.
- N. Chen, N. Du, R. Shen, T. He, J. Xi, J. Tan, G. Bian, Y. Yang, T. Liu, W. Tan, L. Yu and Q. Yuan, Redox signaling-driven modulation of microbial biosynthesis and biocatalysis, Nat. Commun., 2023, 14, 6800 CrossRef CAS PubMed.
- Y. Lin, J. Shi, W. Feng, J. Yue, Y. Luo, S. Chen, B. Yang, Y. Jiang, H. Hu, C. Zhou, F. Shi, A. Prominski, D. V. Talapin, W. Xiong, X. Gao and B. Tian, Periplasmic biomineralization for semi-artificial photosynthesis, Sci. Adv., 2023, 9, eadg5858 CrossRef CAS PubMed.
- Y. Liu, S. Liu, A. Tomar, F. S. Yen, G. Unlu, N. Ropek, R. A. Weber, Y. Wang, A. Khan, M. Gad, J. Peng, E. Terzi, H. Alwaseem, A. E. Pagano, S. Heissel, H. Molina, B. Allwein, T. C. Kenny, R. L. Possemato, L. Zhao, R. K. Hite, E. V. Vinogradova, S. S. Mansy and K. Birsoy, Autoregulatory control of mitochondrial glutathione homeostasis, Science, 2023, 382, 820–828 CrossRef CAS PubMed.
- G. Shimakawa, Electron transport in cyanobacterial thylakoid membranes: are cyanobacteria simple models for photosynthetic organisms?, J. Exp. Bot., 2023, 74, 3476–3487 Search PubMed.
- S. Lim and D. S. Clark, Phase-separated biomolecular condensates for biocatalysis, Trends Biotechnol., 2024, 42, 496–509 Search PubMed.
- S. S. Correa, J. Schultz, B. Zahodnik-Huntington, A. Naschberger and A. S. Rosado, Carboxysomes: The next frontier in biotechnology and sustainable solutions, Biotechnol. Adv., 2025, 79, 108511 CrossRef CAS PubMed.
- M. Ostermeier, A. Garibay-Hernández, V. J. C. Holzer, M. Schroda and J. Nickelsen, Structure, biogenesis, and evolution of thylakoid membranes, Plant Cell, 2024, 36, 4014–4035 CrossRef CAS PubMed.
- J. Liu, W. Lu, B. Shi, S. Klein and X. Su, Peroxisomal regulation of redox homeostasis and adipocyte metabolism, Redox Biol., 2019, 24, 101167 Search PubMed.
- W. Wei, P. Sun, Z. Li, K. Song, W. Su, B. Wang, Y. Liu and J. Zhao, A surface-display biohybrid approach to light-driven hydrogen production in air, Sci. Adv., 2018, 4, eaap9253 CrossRef PubMed.
- D. Li, S. Yao, X. Cao, Y. Zhang, W. Wang and C. Li, Enhancing Cyanobacterial Photosynthetic Carbon Fixation via Quenching Reactive Oxygen Species by Intracellular Gold Nanoparticles, ACS Sustainable Chem. Eng., 2023, 11, 11140–11148 Search PubMed.
- J. Guo, M. Suástegui, K. K. Sakimoto, V. M. Moody, G. Xiao, D. G. Nocera and N. S. Joshi, Light-driven fine chemical production in yeast biohybrids, Science, 2018, 362, 813–816 CrossRef CAS PubMed.
- Q. Jiang, Y. Li, M. Wang, W. Cao, X. Yang, S. Zhang and L. Guo, Light energy utilization and microbial catalysis for enhanced biohydrogen: Ternary coupling system of triethanolamine-mediated Fe@C-Rhodobacter sphaeroides, Bioresour. Technol., 2024, 401, 130733 CrossRef CAS PubMed.
- Q. Wang, S. Kalathil, C. Pornrungroj, C. D. Sahm and E. Reisner, Bacteria-photocatalyst sheet for sustainable carbon dioxide utilization, Nat. Catal., 2022, 5, 633–641 Search PubMed.
- S. F. Rowe, G. Le Gall, E. V. Ainsworth, J. A. Davies, C. W. J. Lockwood, L. Shi, A. Elliston, I. N. Roberts, K. W. Waldron, D. J. Richardson, T. A. Clarke, L. J. C. Jeuken, E. Reisner and J. N. Butt, Light-Driven H2 Evolution and C=C or C=O Bond Hydrogenation by Shewanella oneidensis: A Versatile Strategy for Photocatalysis by Nonphotosynthetic Microorganisms, ACS Catal., 2017, 7, 7558–7566 CrossRef CAS.
- Z. Xu, J. Qi, S. Wang, X. Liu, M. Li, S. Mann and X. Huang, Algal cell bionics as a step towards photosynthesis-independent hydrogen production, Nat. Commun., 2023, 14, 1872 CrossRef CAS PubMed.
- G. Sathiyanarayanan, K. Dineshkumar and Y. H. Yang, Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles, Crit. Rev. Microbiol., 2017, 43, 731–752 CrossRef CAS PubMed.
- K. Zhou, Y. Hu, L. Zhang, K. Yang and D. Lin, The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae, Sci. Rep., 2016, 6, 32998 CrossRef CAS PubMed.
- S. Fulaz, S. Vitale, L. Quinn and E. Casey, Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix, Trends Microbiol., 2019, 27, 915–926 Search PubMed.
- B. Lu, J. Zhang, G. Zhu, T. Liu, J. Chen and X. Liang, Highly hydrophilic and dispersed TiO2 nano-system with enhanced photocatalytic antibacterial activities and accelerated tissue regeneration under visible light, J. Nanobiotechnol., 2023, 21, 491 CrossRef CAS PubMed.
- E. Deiss-Yehiely, A. E. Dzordzorme, M. E. Loiselle, L. M. Yonker and P. T. Hammond, Carboxylated Nanoparticle Surfaces Enhance Association with Mucoid Pseudomonas aeruginosa Biofilms, ACS Appl. Mater. Interfaces, 2024, 16, 14573–14582 CrossRef CAS PubMed.
- R. Raj, K. Dalei, J. Chakraborty and S. Das, Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution, J. Colloid Interface Sci., 2016, 462, 166–175 Search PubMed.
- G. Yang, X. Xia, W. Nie, B. Qin, T. Hou, A. Lin, S. Yao and L. Zhuang, Bidirectional extracellular electron transfer pathways of Geobacter sulfurreducens biofilms: Molecular insights into extracellular polymeric substances, Environ. Res., 2024, 245, 118038 Search PubMed.
- L. Zou, F. Zhu, Z.-e. Long and Y. Huang, Bacterial extracellular electron transfer: a powerful route to the green biosynthesis of inorganic nanomaterials for multifunctional applications, J. Nanobiotechnol., 2021, 19, 120 Search PubMed.
- T.-O. Peulen and K. J. Wilkinson, Diffusion of Nanoparticles in a Biofilm, Environ. Sci. Technol., 2011, 45, 3367–3373 CrossRef CAS PubMed.
- N. Joshi, B. T. Ngwenya and C. E. French, Enhanced resistance to nanoparticle toxicity is conferred by overproduction of extracellular polymeric substances, J. Hazard. Mater., 2012, 241–242, 363–370 CrossRef CAS PubMed.
- X. Gao, H. Zhang, X. Zhang, C. Zhang, C. Mao, S. Shan, F. Wei, M. Mortimer and J. Fang, Interactions between extracellular polymeric substances and engineered nanoparticles in aquatic systems and their environmental effects: a comprehensive review, Environ. Sci.: Nano, 2025, 12, 2177–2192 RSC.
- K. Wu, S. Ouyang, Z. Tao, X. Hu and Q. Zhou, Algal extracellular polymeric substance compositions drive the binding characteristics, affinity, and phytotoxicity of graphene oxide in water, Water Res., 2024, 260, 121908 CrossRef CAS PubMed.
- M. Mu, S. Liu, W. DeFlorio, L. Hao, X. Wang, K. S. Salazar, M. Taylor, A. Castillo, L. Cisneros-Zevallos, J. K. Oh, Y. Min and M. Akbulut, Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion, Langmuir, 2023, 39, 5426–5439 CrossRef CAS PubMed.
- J. Li, H. Han, Y. Chang and B. Wang, The material-microorganism interface in microbial hybrid electrocatalysis systems, Nanoscale, 2023, 15, 6009–6024 RSC.
- C. Berne, A. Ducret, G. Hardy Gail and V. Brun Yves, Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria, Microbiol. Spectrum, 2015, 3, 1128 Search PubMed.
- S. Pirbadian, S. E. Barchinger, K. M. Leung, H. S. Byun, Y. Jangir, R. A. Bouhenni, S. B. Reed, M. F. Romine, D. A. Saffarini, L. Shi, Y. A. Gorby, J. H. Golbeck and M. Y. El-Naggar, Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12883–12888 CrossRef CAS PubMed.
- F. Wang, C. H. Chan, V. Suciu, K. Mustafa, M. Ammend, D. Si, A. I. Hochbaum, E. H. Egelman and D. R. Bond, Structure of Geobacter OmcZ filaments suggests extracellular cytochrome polymers evolved independently multiple times, eLife, 2022, 11, e81551 CrossRef CAS PubMed.
- Y. Yang, J. M. Mathieu, S. Chattopadhyay, J. T. Miller, T. Wu, T. Shibata, W. Guo and P. J. J. Alvarez, Defense Mechanisms of Pseudomonas aeruginosa PAO1 against Quantum Dots and Their Released Heavy Metals, ACS Nano, 2012, 6, 6091–6098 CrossRef CAS PubMed.
- J. F. Diffels, M. L. Seret, A. Goffeau and P. V. Baret, Heavy metal transporters in Hemiascomycete yeasts, Biochimie, 2006, 88, 1639–1649 CrossRef CAS PubMed.
- H. Chakdar, S. Thapa, A. Srivastava and P. Shukla, Genomic and proteomic insights into the heavy metal bioremediation by cyanobacteria, J. Hazard. Mater., 2022, 424, 127609 CrossRef CAS PubMed.
- T. S. Radniecki, L. Semprini and M. E. Dolan, Expression of merA, trxA, amoA, and hao in continuously cultured Nitrosomonas europaea cells exposed to cadmium sulfate additions, Biotechnol. Bioeng., 2009, 104, 1004–1011 CrossRef CAS PubMed.
- Y. Wang, V. Selvamani, I.-K. Yoo, T. W. Kim and S. H. Hong, A Novel Strategy for the Microbial Removal of Heavy Metals: Cell-surface Display of Peptides, Biotechnol. Bioprocess Eng., 2021, 26, 1–9 CrossRef.
- X. F. Liu and V. C. Culotta, Post-translation Control of Nramp Metal Transport in Yeast: Role of Metal Ions and The BSD2 Gene, J. Biol. Chem., 1999, 274, 4863–4868 Search PubMed.
- H. Wu, X. Feng, L. Wang, C. Chen, P. Wu, L. Li, J. Xu, F. Qi, S. Zhang, F. Huo and W. Zhang, Solar Energy for Value-Added Chemical Production by Light-Powered Microbial Factories, CCS Chem., 2024, 6, 1776–1788 Search PubMed.
- P. A. Davison, W. Tu, J. Xu, S. Della Valle, I. P. Thompson, C. N. Hunter and W. E. Huang, Engineering a Rhodopsin-Based Photo-Electrosynthetic System in Bacteria for CO2 Fixation, ACS Synth. Biol., 2022, 11, 3805–3816 CrossRef CAS PubMed.
- E. C. Hann, S. Overa, M. Harland-Dunaway, A. F. Narvaez, D. N. Le, M. L. Orozco-Cardenas, F. Jiao and R. E. Jinkerson, A hybrid inorganic-biological artificial photosynthesis system for energy-efficient food production, Nat. Food, 2022, 3, 461–471 CrossRef CAS PubMed.
- K. Zhang, R. Li, J. Chen, L. Chai, Z. Lin, L. Zou and Y. Shi, Biohybrids of twinning Cd0.8Zn0.2S nanoparticles and Sporomusa ovata for efficient solar-driven reduction of CO2 to acetate, Appl. Catal., B, 2024, 342, 123375 CrossRef CAS.
- Y. He, S. Wang, X. Han, J. Shen, Y. Lu, J. Zhao, C. Shen and L. Qiao, Photosynthesis of Acetate by Sporomusa ovata-CdS Biohybrid System, ACS Appl. Mater. Interfaces, 2022, 23364–23374 Search PubMed.
- T. Lithgow, C. J. Stubenrauch and M. P. H. Stumpf, Surveying membrane landscapes: a new look at the bacterial cell surface, Nat. Rev. Microbiol., 2023, 21, 502–518 Search PubMed.
- M. Toyofuku, S. Schild, M. Kaparakis-Liaskos and L. Eberl, Composition and functions of bacterial membrane vesicles, Nat. Rev. Microbiol., 2023, 21, 415–430 CrossRef CAS PubMed.
- S. Quideau, D. Deffieux, C. Douat-Casassus and L. Pouysegu, Plant polyphenols: chemical properties, biological activities, and synthesis, Angew Chem. Int. Ed. Engl., 2011, 50, 586–621 Search PubMed.
- J. Pan, G. Gong, Q. Wang, J. Shang, Y. He, C. Catania, D. Birnbaum, Y. Li, Z. Jia, Y. Zhang, N. S. Joshi and J. Guo, A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea, Nat. Commun., 2022, 13, 2117 CrossRef CAS PubMed.
- J. H. Park, K. Kim, J. Lee, J. Y. Choi, D. Hong, S. H. Yang, F. Caruso, Y. Lee and I. S. Choi, A cytoprotective and degradable metal-polyphenol nanoshell for single-cell encapsulation, Angew Chem. Int. Ed. Engl., 2014, 53, 12420–12425 CrossRef CAS PubMed.
- Y. Guo, Q. Sun, F. G. Wu, Y. Dai and X. Chen, Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery, Adv. Mater., 2021, 33, e2007356 CrossRef PubMed.
- H. Dong, L. Huang, L. Zhao, Q. Zeng, X. Liu, Y. Sheng, L. Shi, G. Wu, H. Jiang, F. Li, L. Zhang, D. Guo, G. Li, W. Hou and H. Chen, A critical review of mineral-microbe interaction and co-evolution: mechanisms and applications, Natl. Sci. Rev., 2022, 9, nwac128 CrossRef CAS PubMed.
- J. D. Holmes, P. R. Smith, R. Evans-Gowing, D. J. Richardson, D. A. Russell and J. R. Sodeau, Bacterial Photoprotection through Extracellular Cadmium Sulfide Crystallites, Photochem. Photobiol., 1995, 62, 1022–1026 CrossRef CAS.
- B. Wang, Z. Jiang, J. C. Yu, J. Wang and P. K. Wong, Enhanced CO2 reduction and valuable C2+ chemical production by a CdS-photosynthetic hybrid system, Nanoscale, 2019, 11, 9296–9301 RSC.
- P. Sun, K. Li, K. Lin, W. Wei and J. Zhao, Ag2S Quantum Dots for Use in Whole-Cell Biohybrid Catalyst for Visible-Light-Driven Photocatalytic Organic Pollutant Degradation, ACS Appl. Nano Mater., 2022, 5, 9754–9760 CrossRef CAS.
- B. Li, Z. Xu, R. Wang, R. Nie, Z. Tao and X. Huang, Mineralizing Biofilm towards Sustainable Conversion of Plastic Wastes to Hydrogen, Angew Chem. Int. Ed. Engl., 2024, e202416577 Search PubMed.
- S. Vaidya, D. Saha, D. K. H. Rode, G. Torrens, M. F. Hansen, P. K. Singh, E. Jelli, K. Nosho, H. Jeckel, S. Gottig, F. Cava and K. Drescher, Bacteria use exogenous peptidoglycan as a danger signal to trigger biofilm formation, Nat. Microbiol., 2025, 10, 144–157 CrossRef CAS PubMed.
- H. C. Flemming, T. R. Neu and D. J. Wozniak, The EPS matrix: the “house of biofilm cells”, J. Bacteriol., 2007, 189, 7945–7947 Search PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS PubMed.
- H. Yi, K. P. Nevin, B.-C. Kim, A. E. Franks, A. Klimes, L. M. Tender and D. R. Lovley, Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells, Biosens. Bioelectron., 2009, 24, 3498–3503 Search PubMed.
- R. Rivera-Lugo, S. Huang, F. Lee, R. Méheust, A. T. Iavarone, A. M. Sidebottom, E. Oldfield, D. A. Portnoy and S. H. Lightb, Distinct Energy-Coupling Factor Transporter Subunits Enable Flavin Acquisition and Extracytosolic Trafficking for Extracellular Electron Transfer in Listeria monocytogenes, mBio, 2023, 14, 14 CrossRef PubMed.
- X. Z. Fan, J. W. Rong, H. L. Wu, Q. Zhou, H. P. Deng, J. D. Tan, C. W. Xue, L. Z. Wu, H. R. Tao and J. Wu, Eosin Y as a Direct Hydrogen-Atom Transfer Photocatalyst for the Functionalization of C-H Bonds, Angew Chem. Int. Ed. Engl., 2018, 57, 8514–8518 CrossRef CAS PubMed.
- H. J. Gwon, G. Park, J. Yun, W. Ryu and H. S. Ahn, Prolonged hydrogen production by engineered green algae photovoltaic power stations, Nat. Commun., 2023, 14, 6768 CrossRef CAS PubMed.
- Z. Chen, G. Quek, J. Y. Zhu, S. J. W. Chan, S. J. Cox-Vazquez, F. Lopez-Garcia and G. C. Bazan, A Broad Light-Harvesting Conjugated Oligoelectrolyte Enables Photocatalytic Nitrogen Fixation in a Bacterial Biohybrid, Angew Chem. Int. Ed. Engl., 2023, 62, e202307101 Search PubMed.
- S. Koh, Y. Choi, I. Lee, G. M. Kim, J. Kim, Y. S. Park, S. Y. Lee and D. C. Lee, Light-Driven Ammonia Production by Azotobacter vinelandii Cultured in Medium Containing Colloidal Quantum Dots, J. Am. Chem. Soc., 2022, 144, 10798–10808 Search PubMed.
- S. Cui, Y. Si, X.-Z. Fu, H.-H. Li, X.-M. Wang, W.-Z. Du, L. Teng, R.-L. He, H.-Q. Liu, R. Ye and W.-W. Li, Intracellularly-photosensitized bio-hybrid with biogenic quantum dots for enhanced wastewater denitrification, Chem. Eng. J., 2023, 457, 141237 Search PubMed.
- P. Liu, Y. Chang, X. Ren, T. Liu, H. Meng, X. Ru, Z. Bai, L. Yang and X. Ma, Endowing cells with unnatural photocatalytic ability for sustainable chemicals production by bionic minerals-triggering, Green Chem., 2023, 25, 431–438 RSC.
- L.-J. Tian, Y. Min, W.-W. Li, J.-J. Chen, N.-Q. Zhou, T.-T. Zhu, D.-B. Li, J.-Y. Ma, P.-F. An, L.-R. Zheng, H. Huang, Y.-Z. Liu and H.-Q. Yu, Substrate Metabolism-Driven Assembly of High-Quality CdSxSe1–x Quantum Dots in Escherichia coli : Molecular Mechanisms and Bioimaging Application, ACS Nano, 2019, 13, 5841–5851 Search PubMed.
- Y. Shin and C. P. Brangwynne, Liquid phase condensation in cell physiology and disease, Science, 2017, 357, eaaf4382 CrossRef PubMed.
- A. S. Holehouse and B. B. Kragelund, The molecular basis for cellular function of intrinsically disordered protein regions, Nat. Rev. Mol. Cell Biol., 2024, 25, 187–211 CrossRef CAS PubMed.
- G. Liang, Y. Liu, Z. Gu, X. Chen, W. Song, W. Wei, J. Wu, G. Hu, J. Zhao, L. Liu and C. Gao, Shortening electron transfer distance to enhance chemicals and electric energy production in Escherichia coli, Chem. Eng. J., 2024, 497, 154932 CrossRef CAS.
- M. V. Garabedian, W. Wang, J. B. Dabdoub, M. Tong, R. M. Caldwell, W. Benman, B. S. Schuster, A. Deiters and M. C. Good, Designer membraneless organelles sequester native factors for control of cell behavior, Nat. Chem. Biol., 2021, 17, 998–1007 CrossRef CAS PubMed.
- X. Zhao, K. Palczewski and H. Ohguro, Mechanism of rhodopsin phosphorylation, Biophys. Chem., 1995, 56, 6 CrossRef PubMed.
- Y. Toya, Y. Hirono-Hara, H. Hirayama, K. Kamata, R. Tanaka, M. Sano, S. Kitamura, K. Otsuka, R. Abe-Yoshizumi, S. P. Tsunoda, H. Kikukawa, H. Kandori, H. Shimizu, F. Matsuda, J. Ishii and K. Y. Hara, Optogenetic reprogramming of carbon metabolism using light-powering microbial proton pump systems, Metab. Eng., 2022, 72, 227–236 CrossRef CAS PubMed.
- J. E. Cournoyer, S. D. Altman, Y. L. Gao, C. L. Wallace, D. Zhang, G. H. Lo, N. T. Haskin and A. P. Mehta, Engineering artificial photosynthetic life-forms through endosymbiosis, Nat. Commun., 2022, 13, 2254 CrossRef CAS PubMed.
- A. P. Mehta, L. Supekova, J. H. Chen, K. Pestonjamasp, P. Webster, Y. Ko, S. C. Henderson, G. McDermott, F. Supek and P. G. Schultz, Engineering yeast endosymbionts as a step toward the evolution of mitochondria, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11796–11801 Search PubMed.
- B. Fu, X. Mao, Y. Park, Z. Zhao, T. Yan, W. Jung, D. H. Francis, W. Li, B. Pian, F. Salimijazi, M. Suri, T. Hanrath, B. Barstow and P. Chen, Single-cell multimodal imaging uncovers energy conversion pathways in biohybrids, Nat. Chem., 2023, 15, 1400–1407 CrossRef CAS PubMed.
- D. Yan, Z. Wang and Z. Zhang, Stimuli-Responsive Crystalline Smart Materials: From Rational Design and Fabrication to Applications, Acc. Chem. Res., 2022, 55, 1047–1058 Search PubMed.
- W. Song, Y. Liu, Y. Wu, C. Wang, Z. Liu, Y. Liu, X. Zhang, L. Cao, B. Li, B. Song, B. Cao, Y. Yao, X. Mao, Q. He, Z. Zou and B. Liu, Single-atom bridges across biotic-abiotic interfaces facilitate direct electron transfer for solar-to-chemical conversion, Nat. Commun., 2025, 16, 6708 CrossRef CAS PubMed.
- D. Wu, B. Zhang, S. Shi, R. Tang, C. Qiao, T. Li, J. Jia, M. Yang, X. Si, Y. Wang, X. Sun, D. Xiao, F. Li and H. Song, Engineering extracellular electron transfer to promote simultaneous brewing wastewater treatment and chromium reduction, J. Hazard. Mater., 2024, 465, 133171 CrossRef CAS PubMed.
- Q. Zhang, H. Sun, G. Jiang and A. Zhang, Azophenyl Covalent Organic Frameworks for Efficient Photothermal Conversion and UV-Driven Soft Actuators, Ind. Eng. Chem. Res., 2024, 63, 9772–9778 CrossRef CAS.
- D. Samanta, J. Gemen, Z. Chu, Y. Diskin-Posner, L. J. W. Shimon and R. Klajn, Reversible photoswitching of encapsulated azobenzenes in water, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9379–9384 CrossRef CAS PubMed.
- H. Yu, F. Li, Y. Wang, C. Hu, B. Zhang, C. Qiao, Q. Liu, Z. You, J. Zhang, L. Shi, H. Gao, K. H. Nealson and H. Song, Electro-controlled distribution of reducing equivalents to boost isobutanol biosynthesis in microbial electro-fermentation of S. oneidensis, Joule, 2025, 9, 101773 CrossRef CAS.
- N. Xu, X. Zhang, P.-C. Guo, D.-H. Xie and G.-P. Sheng, Biological self-protection inspired engineering of nanomaterials to construct a robust bio-nano system for environmental applications, Sci. Adv., 2024, 10, eadp2179 Search PubMed.
- W. Yu, Y. Zeng, Z. Wang, S. Xia, Z. Yang, W. Chen, Y. Huang, F. Lv, H. Bai and S. Wang, Solar-powered multi-organism symbiont mimic system for beyond natural synthesis of polypeptides from CO2 and N2, Sci. Adv., 2023, 9, eadf6772 CrossRef CAS PubMed.
- M. K. Goshisht, Machine Learning and Deep Learning in Synthetic Biology: Key Architectures, Applications, and Challenges, ACS Omega, 2024, 9, 9921–9945 CrossRef CAS PubMed.
- Y. Hu, C. Yu, S. Wang, Q. Wang, M. Reinhard, G. Zhang, F. Zhan, H. Wang, D. Skoien, T. Kroll, P. Su, L. Li, A. Chen, G. Liu, H. Lv, D. Sokaras, C. Gao, J. Jiang, Y. Tao and Y. Xiong, Identifying a highly efficient molecular photocatalytic CO2 reduction system via descriptor-based high-throughput screening, Nat. Catal., 2025, 8, 126–136 CrossRef CAS.
- J. H. Mussgnug, S. Thomas-Hall, J. Rupprecht, A. Foo, V. Klassen, A. McDowall, P. M. Schenk, O. Kruse and B. Hankamer, Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion, Plant Biotechnol. J., 2007, 5, 802–814 Search PubMed.
- Y. Huang, Y. Wu, H. Hu, B. Tong, J. Wang, S. Zhang, Y. Wang, J. Zhang, Y. Yin, S. Dai, W. Zhao, B. An, J. Pu, Y. Wang, C. Peng, N. Li, J. Zhou, Y. Tan and C. Zhong, Accelerating the design of pili-enabled living materials using an integrative technological workflow, Nat. Chem. Biol., 2024, 20, 201–210 CrossRef CAS PubMed.
- S. Pi, W. Yang, W. Feng, R. Yang, W. Chao, W. Cheng, L. Cui, Z. Li, Y. Lin, N. Ren, C. Yang, L. Lu and X. Gao, Solar-driven waste-to-chemical conversion by wastewater-derived semiconductor biohybrids, Nat. Sustainability, 2023, 6, 1673–1684 Search PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |