Open Access Article
Open Access ArticleOrganic–inorganic perovskite ferroelectric catalytic selective alkyne coupling under ultrasound sonication
Jun-Chao
Qi†
,
Xiao-Gang
Chen†
 ,
Zhen-Yu
Wang
,
Yuan-Yuan
Tang
,
Zhen-Yu
Wang
,
Yuan-Yuan
Tang
 ,
Xian-Jiang
Song
,
Xian-Jiang
Song
 ,
Yan
Qin
,
Yan
Qin
 ,
Hui-Peng
Lv
,
Ren-Gen
Xiong
and
Wei-Qiang
Liao
,
Hui-Peng
Lv
,
Ren-Gen
Xiong
and
Wei-Qiang
Liao
 *
*
Ordered Matter Science Research Center, Nanchang University, Nanchang, 330031, People's Republic of China. E-mail: liaowq@ncu.edu.cn
First published on 18th November 2025
Abstract
Alkyne coupling represents a significant reaction in organic synthesis, while conventional methods rely on high-cost catalysts and harsh reaction conditions. Although ferroelectrics with spontaneous polarization facilitating charge separation have recently attracted great attention as emerging catalysts, their catalytic potential in alkyne coupling remains unexplored. Herein, we report a new organic–inorganic perovskite ferroelectric [4,4-difluoropiperidinium]2-CuCl4 ([DFPD]2-CuCl4), which exhibits a high phase transition temperature of 398 K and room-temperature ferroelectricity. Significantly, by ultrasonic sonication under ambient conditions, and by using [DFPD]2-CuCl4 and additive CuCl as the catalysts, a variety of terminal alkynes can selectively form 1,3-diynes with high yields, with the selectivity and yield up to 99% and 85%, respectively. The high yields enable the growth of crystals of products, three of which exhibit phase transitions. Compared with its inorganic ferroelectric and piezoelectric counterparts, the molecular ferroelectric [DFPD]2-CuCl4 shows significantly enhanced catalytic activity, being approximately 4.5 times that of BaTiO3 and 64 times that of ZnO. Moreover, the [DFPD]2-CuCl4 catalyst demonstrates robust recyclability, with its crystal phase and catalytic activity remaining unchanged over 10 cycles. Our findings provide an efficient and sustainable catalyst system for alkyne coupling and a new perspective on the exploration of ferroelectric catalysis.
Introduction
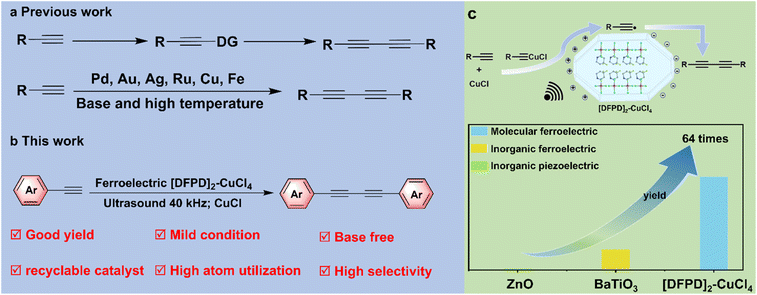
Carbon–carbon (C–C) bond formation is of critical importance in organic chemistry.1 1,3-Diynes have found widespread applications in synthesizing functional materials, natural products, and pharmaceuticals.2–6 Compared with the homocoupling of various alkynyl organometallics, alkynyl tellurides, or alkynyl iodides,7–9 Glaser coupling—a reaction that constructs C–C bonds via terminal alkynes—is widely recognized as the most convenient method for accessing 1,3-diyne products from alkynes in transition metal-catalyzed coupling reactions.5 Since Glaser first reported in 1869 that the oxidative coupling of copper acetylides could be utilized for diyne synthesis, a diverse range of metal-catalyzed systems has been explored for this reaction, including CoBr2, palladium reagents, [Ru(dppp)2(CH3CN)Cl][BPh4], gold nanoparticles, and Au(I)/Au(III) complexes.7,10–12 However, their widespread application has been constrained by the high cost of these catalysts or their instability in ambient air. Although significant advances have been made using these approaches, several limitations persist, such as the complex preparation procedures of the catalysts and harsh reaction conditions (e.g., the need for an excess of base or elevated temperatures of 110 °C).10,13–17 Therefore, there remains an urgent need to develop simpler, more efficient, and environmentally benign methods for the construction of symmetrical 1,3-diyne compounds via Glaser coupling.Ferroelectrics are a significant class of multifunctional electroactive materials with switchable spontaneous polarization, which have been studied for over a century and have found broad applications, such as in data storage, smart sensors, energy conversion, and mechanical actuation.18–23 In recent years, they have gained increasing attention as emerging catalysts, in which the polarization-induced built-in electric fields and polarization changes under external stimuli such as mechanical stress promote the separation of charge carriers, facilitating redox reactions.24–37 Their catalytic potentials in hydrogen production, organic synthesis, and environmental remediation under mild conditions of ultrasonic vibration have been intensively studied based on classical ferroelectrics like inorganic BaTiO3 (BTO).38–49 The inherent polarization electric fields of ferroelectrics also make them attractive for enhancing photocatalytic efficiency through the promotion of photogenerated charge carrier separation and migration dynamics.35 Molecular ferroelectrics, as represented by [Me3NCH2Cl]CdCl3, are ferroelectric materials with molecular constituents in their crystal lattices in which the ferroelectricity is related to the collective interactions and arrangements of the molecular components.50 Compared with the widely used atom-based inorganic ferroelectrics with rigid ionic/covalent bonds throughout the lattices, molecular ferroelectrics constructed from molecular constituents with intermolecular interactions (e.g., hydrogen bonds and van der Waals forces) possess many advantages and distinct properties, such as low-temperature solution processability, light weight, mechanical flexibility, lower acoustic impedance, bio-compatibility, chemical tunability, homochirality, and photoisomerization.50–63 Notably, the low acoustic impedance of molecular ferroelectrics can facilitate the mechanical energy transfer from solvent to catalysts, and their solvent solubility enables good catalyst recyclability, rendering them highly promising for efficient and sustainable ferroelectric catalysis under mild conditions.64–70 Among molecular ferroelectrics, organic–inorganic metal halide perovskite ferroelectrics represent a synergistic integration of structural and functional merits,50 such as the coexistence of ferroelectric and magnetic orders in copper(II)-based halide perovskite ferroelectrics.71–73 Nevertheless, molecular ferroelectric catalysis remains in its infancy, and ferroelectric catalytic alkyne coupling has never been explored.
Herein, we demonstrated a new Cu2+-based organic–inorganic perovskite ferroelectric [4,4-difluoropiperidinium]2-CuCl4 ([DFPD]2-CuCl4), which shows a high phase transition temperature of 398 K and room-temperature ferroelectricity. Significantly, by using molecular ferroelectric [DFPD]2-CuCl4 with the addition of CuCl as a catalyst, we achieved efficient construction of 1,3-diynes via terminal alkyne homocoupling under ultrasonic irradiation. Traditional methods typically require pre-functionalization, noble metal catalysts, an excess of base, or high temperature (Scheme 1a).7–17 This catalytic system exhibits several advantages, such as mild reaction conditions, high selectivity, and good yield (maximum 88%) (Scheme 1b). Furthermore, the molecular ferroelectric catalysts demonstrate robust recyclability, with their crystalline structure and catalytic activity remaining unchanged over multiple cycles—aligning with green chemistry principles. Moreover, the catalytic performance of the molecular ferroelectric [DFPD]2-CuCl4 with CuCl is significantly superior to that of inorganic ferroelectrics and piezoelectric materials with CuCl, that is, approximately 4.5 times that of BaTiO3 and 64 times that of ZnO (Scheme 1c). This work opens up a new avenue for catalytic alkyne coupling and provides a new perspective on the exploration of ferroelectric catalysis.
Results and discussion
Crystal structures and phase transitions
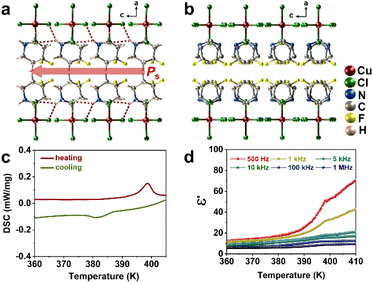
We prepared [DFPD]2-CuCl4 as crystals by evaporating stoichiometric amounts of 4,4-difluoropiperidine hydrochloride and copper dichloride in a hydrochloric acid solution at 323 K. Powder X-ray diffraction (PXRD) patterns of as-grown crystals match the simulated patterns, revealing the good phase purity of [DFPD]2-CuCl4 (Fig. S1). The thermogravimetric analysis (TGA) curve of [DFPD]2-CuCl4 demonstrates thermal stability up to about 512 K (Fig. S2). Single-crystal structure determination reveals that [DFPD]2-CuCl4 crystalizes in a non-centrosymmetric orthorhombic space group Cmc21 with a polar point group of mm2 at 300 K (Table S1), which is consistent with the previous report of the polar structure of [DFPD]2-CuCl4 by L.-N. Quan and co-workers.74 As shown in Fig. 1a and S3, [DFPD]2-CuCl4 adopts an A2BX4-type organic–inorganic perovskite structure, in which the two organic DFPD+ cationic layers alternate with the inorganic anionic CuCl42− framework. The presence of N–H⋯Cl hydrogen bonds between inorganic anions and organic cations with donor–acceptor distances of 3.269(3)–3.408(3) Å (Table S2) not only stabilizes the structure of [DFPD]2-CuCl4 but also promotes the ordered arrangement of DFPD+ cations along the c-axis, thereby inducing spontaneous polarization in this direction. At 410 K, the space group transitions to the centrosymmetric space group Cmcm with the point group mmm. At this point, the DFPD+ cations are located precisely at special symmetric sites on the mirror plane, changing from the initial ordered state to a two-fold disordered state, resulting in the cancellation of polarization (Fig. 1b), which is distinct from the displacive-type mechanism prevalent in inorganic ferroelectrics. According to the 88 types of ferroelectric phase transitions,20 [DFPD]2-CuCl4 undergoes a mmmFmm2-type ferroelectric–paraelectric phase transition.The variable-temperature PXRD patterns show that as the temperature increases, the peak positions exhibit a distinct shift to lower angles, indicating an increase in the 2D interlayer spacing (Fig. S4). Furthermore, the diffraction peak at around 23° at 410 K undergoes a significant change, providing further evidence of a structural phase transition. This structural phase transition was subsequently accurately confirmed by differential scanning calorimetry testing, with the Curie temperature (Tc) during the heating process being 398 K (Fig. 1c). The non-centrosymmetric to centrosymmetric transition of [DFPD]2-CuCl4 was further demonstrated by second-harmonic generation (SHG) spectroscopy. As the temperature increased to 410 K, the SHG signal vanished, consistent with the structural phase transition from non-centrosymmetric space group Cmc21 to centrosymmetric space group Cmcm. The SHG activity can be recovered when the crystal is cooled to 300 K, implying the reversibility of the phase transition (Fig. S5). As shown in Fig. 1d, the pronounced anomaly in the real part (ε′) of the dielectric constant near Tc further confirms the occurrence of the ferroelectric–paraelectric phase transition. The non-centrosymmetric crystal symmetry also indicates that [DFPD]2-CuCl4 has a piezoelectric response. We then measured its piezoelectric coefficient d33, one of the most useful piezoelectric coefficients, via the quasi-static method (Berlincourt method) on single crystals. A d33 value of 12 pC/N was obtained (Fig. S6).
Ferroelectricity
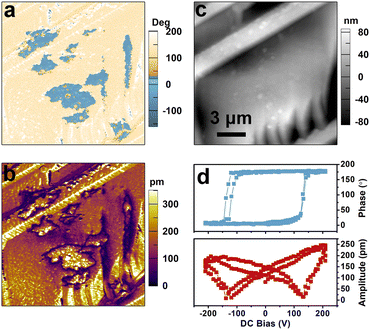
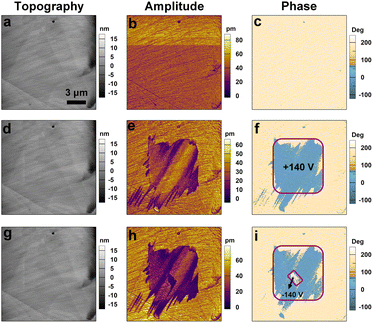
The polar point group mm2 of [DFPD]2-CuCl4 belongs to one of ten polar point groups for ferroelectrics. We then detected the ferroelectricity of [DFPD]2-CuCl4 by piezoresponse force microscopy (PFM), which is a promising technique for characterizing micro- to nanoscale structures, enabling direct probing and visualization of ferroelectric domain configurations. In the amplitude and phase images generated by PFM, distinct colors are employed to represent the local piezoelectric response and the polarization orientation of the material, respectively. To confirm the ferroelectric properties of [DFPD]2-CuCl4, PFM analysis was performed on its thin film, as shown in Fig. 2 and 3. The phase image reveals an approximate 180° contrast difference between two regions of distinct colors (Fig. 2a), indicating opposing polarization directions in these areas. In the amplitude image (Fig. 2b), the boundaries between these two regions exhibit a significant reduction in signal intensity, clearly indicating the locations of the domain walls. Moreover, the PFM signals appear to be independent of surface morphological features (Fig. 2c), thereby ruling out the influence of surface topography and further confirming the presence of ferroelectric domains. Meanwhile, switching measurements were conducted to verify the polarization reversal phenomenon in the film under the application of an electric field. As shown in Fig. 2d, the phase–bias curves display a clear hysteresis loop, and the amplitude–bias curves exhibit a characteristic butterfly-shaped pattern, demonstrating the domain switching.We also conducted domain switching measurements to directly visualize the polarization reversal behavior (Fig. 3). We initially select a single domain to serve as the starting state (Fig. 3a–c). Subsequently, a +140 V voltage was applied to the central region (Fig. 3d–f). It can be observed from Fig. 3f that a new color contrast emerges in the phase diagram, differing from the original by nearly 180°, which indicates the formation of a new domain. Furthermore, an opposite tip voltage of −140 V was applied to a smaller region within the newly formed domain, resulting in partial back-switching of the domain (Fig. 3g–i). These observations provide strong evidence for polarization switching occurring within the ferroelectric domains of compound [DFPD]2-CuCl4.
Ferroelectric catalysis
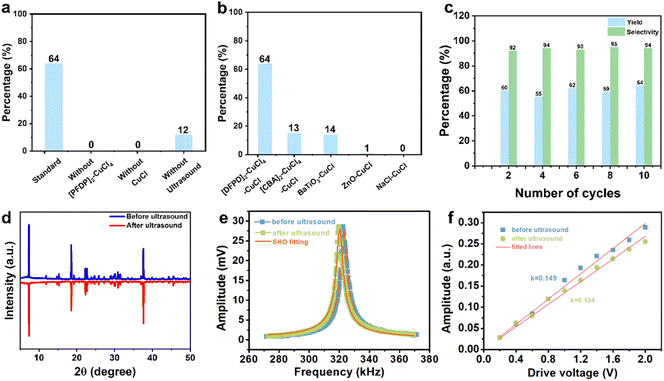
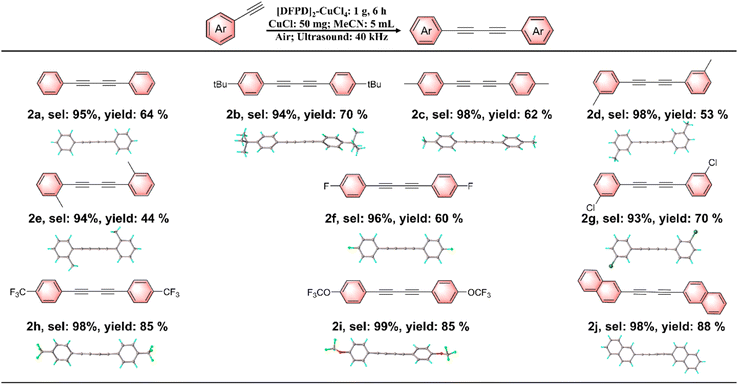
We next investigated the catalytic activity of [DFPD]2-CuCl4 in alkyne coupling reactions. Initially, the reaction was conducted using 1 mmol of phenylacetylene as the substrate, [DFPD]2-CuCl4 crystals as the catalyst, CuCl as the additive, and acetonitrile (MeCN) as the solvent, with ultrasonic irradiation at 40 kHz for 6 hours (Fig. 4 and 5). Following purification by column chromatography, product 2a was obtained in 64% yield. Notably, colorless and transparent crystals of 2a were further isolated via slow evaporation of the reaction solution in n-hexane (Fig. 5). Subsequently, the role of each catalytic component was examined (Fig. 4a and Table S3). In the absence of [DFPD]2-CuCl4, no 2a was detected; similarly, omitting CuCl also resulted in no formation of 2a. These results confirm that the reaction proceeds via the co-catalysis of [DFPD]2-CuCl4 and CuCl, in which CuCl reacts with phenylacetylene to form a cuprous phenylethynide intermediate,16,17 while the release of free charges from the surface of the [DFPD]2-CuCl4 ferroelectric catalyst under ultrasonic stimulation facilitates the conversion of the cuprous phenylethynide intermediate to the alkyne coupling product. We also evaluated the effect of ultrasound on reaction efficiency by testing the yield in the absence of ultrasonic irradiation. Only 12% of 2a was obtained under non-ultrasonic conditions, which is significantly lower than that achieved with ultrasound—clearly indicating that ultrasound promotes reaction progression. Next, the influence of different catalysts on the reaction was investigated (Fig. 4b and Table S4). When sodium chloride (NaCl), a centrosymmetric crystal with neither ferroelectric nor piezoelectric properties, was used as the catalyst; no 2a was detected. Employing piezoelectric ZnO crystals as the catalyst only yielded trace amounts of 2a (≈1%). Replacing [DFPD]2-CuCl4 with BTO afforded 2a in a 14% yield. Although both ZnO and BTO were capable of promoting product formation, their catalytic activities were substantially lower than that of [DFPD]2-CuCl4: specifically, the catalytic activity of [DFPD]2-CuCl4 is approximately 64 times that of ZnO and 4.5 times that of BTO. This reaction was also carried out using the non-ferroelectric centrosymmetric Cu2+-based organic–inorganic hybrid perovskite [cyclobutylammonium]2-CuCl4 ([CBA]2-CuCl4)65 as the catalyst. Under standard conditions, product 2a was obtained with a yield of only 13% (Fig. 4b and Table S4), which is significantly lower than that achieved when [DFPD]2-CuCl4 is used as the catalyst. This further demonstrates the essential role of molecular ferroelectrics in catalysis. Finally, the impact of different Cu+ sources was explored. When CuBr or CuI was used instead of CuCl, the yield of 2a decreased: CuCl afforded the highest yield (64%), whereas CuBr and CuI gave yields of 40% and 44%, respectively (Table S5). Based on the control experiment, a possible mechanism has been proposed. CuCl first reacts with phenylacetylene to form a cuprous phenylethynide intermediate. Under ultrasonic stimulation, free charges of electron–hole pairs are released from the molecular ferroelectric [DFPD]2-CuCl4 crystal surface. This facilitates the cuprous phenylethynide intermediate to lose an electron to form a phenylethynyl radical. Subsequently, the phenylethynyl radicals couple to generate product 2a.After optimizing the reaction conditions, we next investigated the recyclability of the [DFPD]2-CuCl4 catalyst. Following reaction completion, the solid [DFPD]2-CuCl4 catalyst was recovered via filtration and reused in subsequent cycles. Notably, the catalyst maintained consistent catalytic activity, yield, and selectivity over 10 consecutive cycles (Fig. 4c), confirming its good recyclability. As shown in Fig. 4d, the PXRD patterns of [DFPD]2-CuCl4 after 10 cycles of use are consistent with the original ones, indicating a stable crystal phase during the reaction. We also investigated the piezoelectric properties of the recycled catalyst (Fig. 4e and f). Since the samples were polycrystalline, we selected points from the PFM mapping that exhibited the highest amplitude signals for resonant measurements, using a driving voltage of 2 V. By sweeping the AC voltage frequency, typical resonant peaks in the amplitude response were observed, as shown in Fig. 4e. These curves were well fitted by a simple harmonic oscillator (SHO) model. Based on the resonance peaks, no significant variation was observed in the piezoresponse of the catalyst before cycling and after 10 cycles of recycling. From the SHO fitting, we extracted the quality factor, which reflects the degree of amplitude signal amplification. Dividing the measured amplitude signal by the quality factor yields the intrinsic amplitude signal, which is expected to be linearly proportional to the applied driving voltage, as illustrated in Fig. 4f. The slopes of these curves represent the magnitude of the piezoelectric response, confirming that the detected signals originate from the intrinsic piezoelectric effect. This further demonstrates that the piezoelectric properties of [DFPD]2-CuCl4 remain stable after 10 cycles of use.
Subsequently, the substrate scope of this molecular ferroelectric-catalyzed coupling of terminal alkynes has been evaluated (Fig. 5). First, a wide range of alkynes with electron-donating groups was investigated. Phenylacetylenes substituted with either a tert-butyl group (2b) or a methyl group (2c) at the para position are compatible with this strategy, and the corresponding products can be obtained in good yields of 70% and 62% with a selectivity of 94% and 98%, respectively. We further investigated the effect of electron-donating groups at different positions on the reaction efficiency, and found that phenylacetylenes with methyl substituents at the ortho and meta positions both exhibited a significant decrease in yield. Additionally, we explored the substrate scope of alkynes containing electron-withdrawing groups. Both 4-fluorophenylacetylene and 3-chlorophenylacetylene were able to generate the corresponding products in good yields of 60% and 70% with an excellent selectivity of 96% and 93% respectively. Notably, 4-trifluoromethylphenylethyne and 4-trifluoromethoxyphenylethyne—bearing strong electron-withdrawing groups—exhibit not only excellent compatibility with this protocol but also afford the corresponding products in outstanding yields of 85% and 85% with an excellent selectivity of 98% and 99% respectively. In addition to phenylacetyne derivatives, we also explored other aryl-acetylenes. 2-Ethynyl-naphthalene was also applicable to this protocol, providing the corresponding product in an excellent yield of 88% with an excellent selectivity of 98%. These results demonstrate that the current protocol has excellent substrate generality, suggesting great potential for practical applications. The structures of the target products were demonstrated by nuclear magnetic resonance (NMR) spectroscopy and single-crystal XRD (Fig. S7–S38).
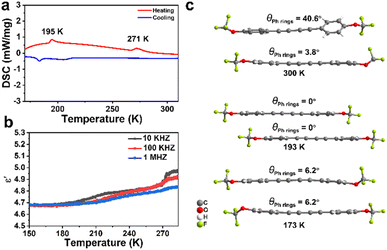
The high yield of obtained products enables them to yield the corresponding crystals through slow crystallization in n-hexane (Fig. 6, S30–S38, Tables S6 and S7). Notably, in these products, 2f, 2i, and 2j exhibit phase transition behavior (Fig. 6a, S35, and S38). As shown in Fig. 6a, DSC analyses indicate that compound 2i exhibits two pairs of reversible phase transitions with transition temperatures of Tc1 = 271 K and Tc2 = 195 K, respectively. The temperature-dependent real part ε′ of the dielectric constant shows anomalies near the two phase transition temperatures (Fig. 6b), further confirming the phase transition. The variable-temperature single-crystal XRD measurements of compound 2i show that it crystallized in the centrosymmetric space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) at 300 K. When cooling to the intermediate-temperature phase at 193 K and the low-temperature phase at 173 K, the space groups remain P
at 300 K. When cooling to the intermediate-temperature phase at 193 K and the low-temperature phase at 173 K, the space groups remain P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , while the cell volume reduces to approximately 1/4 and 1/2 that of the room-temperature phase, respectively (Table S7). As Fig. 6c shows, the change in the dihedral angle of two benzene rings in the 2i molecule is responsible for the phase transitions.
, while the cell volume reduces to approximately 1/4 and 1/2 that of the room-temperature phase, respectively (Table S7). As Fig. 6c shows, the change in the dihedral angle of two benzene rings in the 2i molecule is responsible for the phase transitions.
Conclusions
In summary, we demonstrated a Cu2+-based new organic–inorganic perovskite ferroelectric [DFPD]2-CuCl4 and its excellent catalytic activity in alkyne coupling. [DFPD]2-CuCl4 shows a high phase transition temperature of 398 K and room-temperature ferroelectricity. Under ultrasonic vibration, with [DFPD]2-CuCl4 and CuCl as the catalysts, a variety of terminal alkynes can selectively form 1,3-diynes with high yields. Specifically, for the electron-deficient alkyne (4-trifluoromethylphenylacetylene), the selectivity and yield reach up to 99% and 85%, respectively. Compared with inorganic ferroelectrics and piezoelectrics with CuCl, the molecular ferroelectric [DFPD]2-CuCl4 shows significantly enhanced catalytic activity, being approximately 4.5 times that of BaTiO3 and 64 times that of ZnO. Moreover, the molecular ferroelectric catalysts demonstrate robust recyclability, with their crystalline structure and catalytic activity remaining unchanged over multiple cycles. This work develops an efficient and sustainable catalyst system for alkyne coupling. It also advances the fundamental understanding of ferroelectric catalysis, paving the way for next-generation catalytic technologies in fine chemical synthesis and materials engineering.Experimental
Synthesis and growth of crystals
Materials were obtained from commercial suppliers and purified by standard procedures unless otherwise noted. Solvents for reactions were purchased from commercial suppliers. The molecular ferroelectric crystal [DFPD]2-CuCl4 was obtained using the following method. 4,4-Difluoropiperidine hydrochloride (20 mmol) and CuCl2 (10 mmol) were dissolved in hydrochloric acid to get a clear solution. Slow evaporation of solvent at 323 K results in the [DFPD]2-CuCl4 crystals.For the catalytic reaction, the [DFPD]2-CuCl4 crystals (1 g) were first ground into powder. Then, in a sealed tube, 1 mmol of phenylacetylene, 1 g of powder [DFPD]2-CuCl4, and 0.05 g CuCl were placed in 5 mL MeCN. The reaction was then placed in an ultrasonic environment with a frequency of 40 kHz for 6 h. During the experiment, circulating water was used. The organic layer was collected, dried over sodium sulfate, filtered, and concentrated. The crude product was subsequently purified by column chromatography on silica gel (200–300 meshes) using a mixture of PE/EA (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent. Slow evaporation of the n-hexane solution of the obtained products yields the corresponding crystals.
1) as the eluent. Slow evaporation of the n-hexane solution of the obtained products yields the corresponding crystals.
Characterization
Methods for powder and single-crystal XRD, TGA, DSC, dielectric constant, SHG, PFM, and NMR measurements were described in the SI.Author contributions
W.-Q. L. and R.-G. X. devised and developed the project. J.-C. Q. and X.-G. C. contributed equally to this work. J.-C. Q. synthesized the samples and carried out the catalytic experiments. X.-G. C. conducted XRD, DSC, and dielectric measurements. Y. Q. performed SHG measurements. Z.-Y. W. performed crystal structure analysis. H.-P. L. performed the d33 measurement. Y.-Y. T. and X.-J. S. performed PFM experiments. All the authors analyzed the data, discussed the results and contributed to the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc07784b.CCDC 2484549, 2484550, 2484236–2484244, and 2484246–2484250, respectively contain the supplementary crystallographic data for this paper.75a–p
Acknowledgements
This work was supported by the National Key R&D Program of China (2024YFA1509300) and the National Natural Science Foundation of China (22488101, 22175082, 22201120, and 22401132).Notes and references
- Y. Zhou and J. Zhao, Appl. Catal., B., 2022, 300, 120721 CrossRef CAS.
- J.-Q. Li, Z.-Q. Xie, Y. Xiong, Z.-Z. Li, Q.-X. Huang, S.-Q. Zhang, J.-Y. Zhou, R. Liu, X. Gao, C.-G. Chen, L.-M. Tong, J. Zhang and Z.-F. Liu, Adv. Mater., 2017, 29, 1700421 CrossRef.
- S. Eisler, A.-D. Slepkov, E. Elliott, T. Luu, R. McDonald, F.-A. Hegmann and R.-R. Tykwinski, J. Am. Chem. Soc., 2005, 127, 2666–2676 CrossRef CAS PubMed.
- B. Zhang, Y. Wang, S.-P. Yang, Y. Zhou, W.-B. Wu, W. Tang, J.-P. Zuo, Y. Li and J.-M. Yue, J. Am. Chem. Soc., 2012, 134, 20605–20608 CrossRef CAS PubMed.
- J.-Z. Liu, J.-W.-Y. Lam and B.-Z. Tang, Chem. Rev., 2009, 109, 5799–5867 CrossRef CAS.
- B. Saikia, T.-J. Devi and N.-C. Barua, Org. Biomol. Chem., 2013, 11, 23189–23193 RSC.
- F. Albrecht, D. Rey, S. Fatayer, F. Schulz, D. Pérez, D. Peña and L. Gross, Angew. Chem., Int. Ed., 2020, 59, 1780–1787 CrossRef.
- Y. Nishihara, K. Ikegashira, K. Hirabayashi, J. -I Ando, A. Mori and T. Hiyama, J. Org. Chem., 2000, 65, 1780 CrossRef CAS PubMed.
- H.-H. Kong, L. Viergutz, L.-C. Liu, A. Sandvoß, X.-C. Peng, H.-N. Klaasen, H. Fuchs and A. Studer, Adv. Mater., 2023, 35, 2210997 CrossRef CAS PubMed.
- H.-Y. Gao, H. Wagner, D.-Y. Zhong, J.-H. Franke, A. Studer and H. Fuchs, Angew. Chem., Int. Ed., 2013, 52, 4024–4028 CrossRef CAS.
- W. Shi, Y.-D. Luo, X.-C. Luo, L. Chao, H. Zhang, J. Wang and A.-W. Lei, J. Am. Chem. Soc., 2008, 130, 14713–14720 CrossRef CAS PubMed.
- A. Leyva-Pérez, A. Doménech-Carbó and A. Corma, Nat. Commun., 2015, 6, 6703 CrossRef.
- H. Plenio, Angew. Chem., 2008, 120, 7060–7063 CrossRef.
- P. Kuhn, A. Alix, M. Kumarraja, B. Louis, P. Pale and J. Sommer, Eur. J. Org. Chem., 2009, 3, 423–429 CrossRef.
- O. Vechorkin, D. Barmaz, V. Proust and X.-L. Hu, J. Am. Chem. Soc., 2009, 131, 12078–12079 CrossRef CAS PubMed.
- L.-B. Su, J.-Y. Dong, L. Liu, M.-L. Sun, R.-H. Qiu, Y.-B. Zhou and S.-F. Yin, J. Am. Chem. Soc., 2016, 138, 12348–12351 CrossRef CAS.
- Z.-H. Zhang, X.-Y. Dong, X.-Y. Du, Q.-S. Gu and X.-Y. Liu, Nat. Commun., 2019, 10, 5689 CrossRef.
- J. F. Scott, Science, 2007, 315, 954–959 CrossRef CAS.
- M. E. Lines and A. M. Glass, Principles and Applications of Ferroelectrics and Related Materials, Oxford University Press, Oxford, 2001 Search PubMed.
- H. Peng, J.-C. Qi, Y.-S. Liu, J.-M. Zhang, W.-Q. Liao and R.-G. Xiong, Chin. J. Chem., 2024, 42, 1133–1144 CrossRef CAS.
- J.-C. Qi, Y. Qin, H. Peng, H.-P. Lv, Y.-J. Bai, X. Shen, Z.-T. Xia and W.-Q. Liao, Sci. China: Chem., 2024, 67, 4167–4174 CrossRef CAS.
- F. Wang, L. Ju, B. Wu, S. Li, J. Peng, Y. Chen, M. G. Sendeku, K. Wang, Y. Cai, J. Yi, Y. Yang, Z. Wang and X. Sun, Angew. Chem., Int. Ed., 2024, 63, e202402033 CrossRef CAS PubMed.
- C.-F. Wang, Y. Yang, Y. Hu, C. Ma, H.-F. Ni, P.-G. Liu, H.-F. Lu, Z.-X. Zhang, J.-G. Wang, Y.-J. Zhang, D.-W. Fu, K. Zhao and Y. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202413726 CrossRef CAS PubMed.
- T. Choi, S. Lee, Y. J. Choi, V. Kiryukhin and S. W. Cheong, Science, 2009, 324, 63–66 CrossRef CAS PubMed.
- X.-L. Xu, L.-B. Xiao, J. Zhao, B.-K. Pan, J. Li, W.-Q. Liao, R.-G. Xiong and G.-F. Zou, Angew. Chem., Int. Ed., 2020, 59, 20149–20157 CrossRef.
- Y. Liu, S. Ye, H. Xie, J. Zhu, Q. Shi, N. Ta, R. Chen, Y. Gao, H. An, W. Nie, H. Jing, F. Fan and C. Li, Adv. Mater., 2020, 32, 1906513 CrossRef CAS.
- Y. Du, X. Zhao, F. He, H. Gong, J. Yang, L. Wu, X. Cui, S. Gai, P. Yang and J. Lin, Adv. Mater., 2024, 36, 2403253 CrossRef CAS PubMed.
- M. M. Yang and M. Alexe, Adv. Mater., 2018, 30, 1704908 CrossRef.
- H. You, Z. Wu, L. Zhang, Y. Ying, Y. Liu, L. Fei, X. Chen, Y. Jia, Y. Wang, F. Wang, S. Ju, J. Qiao, C.-H. Lam and H. Huang, Angew. Chem., Int. Ed., 2019, 58, 11905–11910 CrossRef.
- S. Li, Z. Zhao, J. Zhao, Z. Zhang, X. Li and J. Zhang, Appl. Nano Mater., 2020, 3, 1063–1079 CrossRef CAS.
- J. Huang, Y. Kang, J. Liu, T. Yao, J. Qiu, P. Du, B. Huang, W. Hu, Y. Liang, T. Xie, C. Chen, L.-C. Yin, L. Wang, H.-M. Cheng and G. Liu, Nat. Commun., 2023, 14, 7948 CrossRef CAS PubMed.
- L. Ju, Y. Ma, X. Tan and L. Kou, J. Am. Chem. Soc., 2023, 145, 26393–26402 CrossRef CAS.
- S.-L. Niu, W. Yuan, X. Gong, B. Bao, Z.-W. Wu, B. Xu, R. Zeng, Q.-W. Yang and Q. Ouyang, ACS Sustainable Chem. Eng., 2023, 11, 17816–17825 CrossRef CAS.
- X. Liao, H. Xie, B. Liao, S. Hou, Y. Yu and X. Fan, Nano Energy, 2022, 94, 106890 CrossRef CAS.
- G. Wan, L. Yin, X. Chen, X. Xu, J. Huang, C. Zhen, H. Zhu, B. Huang, W. Hu, Z. Ren, H. Tian, L. Wang, G. Liu and H.-M. Cheng, J. Am. Chem. Soc., 2022, 144, 20342–20350 CrossRef CAS PubMed.
- Z. Wang, J. Wang, J. Ayarza, T. Steeves, Z.-Y. Hu, S. Manna and A.-P. Esser-Kahn, Nat. Mater., 2021, 20, 869–874 CrossRef CAS.
- C. Schumacher, J. G. Hernández and C. Bolm, Angew. Chem., Int. Ed., 2020, 59, 16357–16360 CrossRef CAS.
- D.-X. Zhou, Z. Li, Y. Chen, H.-H. Dong, Y.-Y. Zhou, Z.-F. Bian and M.-S. Zhu, Angew. Chem., Int. Ed., 2025, 64, e202502751 CrossRef CAS PubMed.
- S. Tu, Y. Guo, Y. Zhang, C. Hu, T. Zhang, T. Ma and H. Huang, Adv. Funct. Mater., 2020, 30, 2005158 CrossRef CAS.
- K. Wang, C. Han, J.-Q. Li, J.-S. Qiu, J. Sunarso and S.-M. Liu, Angew. Chem., Int. Ed., 2022, 61, e202110429 CrossRef CAS PubMed.
- Z.-Y. Ren, Y.-H. Peng, H.-L. He, C.-H. Ding, J.-L. Wang, Z. Wang and Z.-B. Zhang, Chin. J. Chem., 2023, 41, 111–128 CrossRef.
- R. Su, H. A. Hsain, M. Wu, D. Zhang, X. Hu, Z. Wang, X. Wang, F.-T. Li, X. Chen, L. Zhu, Y. Yang, Y. Yang, X. Lou and S. J. Pennycook, Angew. Chem., Int. Ed., 2019, 58, 15076–15081 CrossRef CAS.
- Y. Zhang, Y.-M. Sun, X. Ren, J. Hu, H.-J. Yu, J.-G. Liu, H.-W. Huang and J. Han, Angew. Chem., Int. Ed., 2025, 64, e202416221 CrossRef CAS.
- P.-X. Zhang, Q. Li, R. Su, H.-Y. Wang, X.-M. Shi, S.-Q. Deng, Y. Bai, H. Zeng, X.-X. Yu, Z.-G. Li, H. Wu, F. Xue, M.-X. Lv, C.-Y. Yu, Y.-L. Cao, X. Chen, J.-X. Deng, J. Miao, K. Lin and X.-R. Xing, J. Am. Chem. Soc., 2025, 147, 12012–12023 CrossRef CAS PubMed.
- X.-H. Wang, X. Zhang, X. He, G. Guo, Q. Huang, F. You, Q. Wang, R. Qu, F. Zhou and Z. Lian, Angew. Chem., Int. Ed., 2024, 63, e202410334 CrossRef CAS PubMed.
- X. Wang, X. Zhang, L. Xue, Q. Wang, F. You, L. Dai, J. Wu, S. Kramer and Z. Lian, Angew. Chem., Int. Ed., 2023, 62, e202307054 CrossRef CAS PubMed.
- Y.-D. Pang, J.-W. Lee, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2020, 59, 22570–22576 CrossRef CAS.
- K. Kubota, Y. D. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500–1504 CrossRef CAS PubMed.
- R. Qu, S. Wan, X. Zhang, X. Wang, L. Xue, Q. Wang, G.-J. Cheng, L. Dai and Z. Lian, Angew. Chem., Int. Ed., 2024, 63, e202400645 CrossRef CAS.
- Q. Pan, Z.-X. Gu, R.-J. Zhou, Z.-J. Feng, Y.-A. Xiong, T.-T. Sha, Y.-M. You and R.-G. Xiong, Chem. Soc. Rev., 2024, 53, 5781–5861 RSC.
- H.-Y. Liu, H.-Y. Zhang, X.-G. Chen and R.-G. Xiong, J. Am. Chem. Soc., 2020, 142, 15205–15218 CrossRef CAS PubMed.
- C.-R. Huang, X. Luo, X.-G. Chen, X.-J. Song, Z.-X. Zhang and R.-G. Xiong, Natl. Sci. Rev., 2021, 8, nwaa232 CrossRef CAS PubMed.
- H.-Y. Zhang, Y.-Y. Tang, Z.-X. Gu, P. Wang, X.-G. Chen, H.-P. Lv, P.-F. Li, Q. Jiang, N. Gu, S. Ren and R.-G. Xiong, Science, 2024, 383, 1492–1498 CrossRef CAS.
- C. Shi, J.-J. Ma, J.-Y. Jiang, M.-M. Hua, Q. Xu, H. Yu, Y. Zhang and H.-Y. Ye, J. Am. Chem. Soc., 2020, 142, 9634–9641 CAS.
- J. Harada, H. Takahashi, R. Notsuka, M. Takehisa, Y. Takahashi, T. Usui and H. Taniguchi, Angew. Chem., Int. Ed., 2023, 62, e202215286 CrossRef CAS.
- Y.-H. Fang, Z. Liu, S. Zhou, P.-X. Fu, Y.-X. Wang, Z.-Y. Wang, Z.-M. Wang, S. Gao and S.-D. Jiang, J. Am. Chem. Soc., 2022, 144, 8605–8612 CrossRef CAS.
- W.-F. Deng, Y.-X. Li, Y.-X. Zhao, J.-S. Hu, Z.-S. Yao and J. Tao, J. Am. Chem. Soc., 2023, 145, 5545–5552 CrossRef CAS.
- S.-G. Han, J. Bie, W. Fa, S. Chen, L. W. Tang, W.-Q. Guo, H.-J. Xu, Y. Ma, Y. Liu, X.-T. Liu, Z.-H. Sun and J.-H. Luo, J. Am. Chem. Soc., 2024, 146, 8298–8307 CrossRef CAS PubMed.
- H.-H. Chen, H. Peng, Z.-K. Xu, X.-J. Song, X.-G. Chen, R.-G. Xiong and W.-Q. Liao, J. Am. Chem. Soc., 2025, 147, 10715–10723 CrossRef CAS PubMed.
- S. Sahoo, S. Mukherjee, V. B. Sharma, W. I. Hernández, A. C. Garcia-Castro, J. K. Zareba, D. Kabra, G. Vaitheeswaran and R. Boomishankar, Angew. Chem., Int. Ed., 2024, 63, e202400366 CrossRef CAS.
- Y. Hu, L. You, B. Xu, T. Li, S. A. Morris, Y. Li, Y. Zhang, X. Wang, P.-S. Lee, H.-J. Fan and J. Wang, Nat. Mater., 2021, 20, 612–617 CrossRef CAS.
- T.-M. Guo, F.-F. Gao, Y.-J. Gong, Z.-G. Li, F. Wei, W. Li and X.-H. Bu, J. Am. Chem. Soc., 2023, 145, 22475–22482 CrossRef CAS PubMed.
- J. Long, M. S. Ivanov, V. A. Khomchenko, E. Mamontova, J.-M. Thibaud, J. Rouquette, M. Beaudhuin, D. Granier, R. A. S. Ferreira, L. D. Carlos, B. Donnadieu, M. S. C. Henriques, J. A. Paixão, Y. Guari and J. Larionova, Science, 2020, 367, 671–676 CrossRef CAS.
- X.-J. Song, W. Sun, L.-X. Zhou, W.-X. Mao, H.-M. Xu, J.-F. Lan, Y. Zhang and H.-Y. Zhang, J. Am. Chem. Soc., 2024, 146, 32519–32528 CrossRef CAS.
- H.-H. Hu, Z.-Y. Jing, Q. Pan, T.-T. Sha, H.-R. Ji, X.-X. Cao, X.-J. Song, Z.-J. Feng, J. Yao, R.-J. Zhou, C. Wang, R.-G. Xiong and Y.-M. You, Adv. Mater., 2024, 36, 2413547 CrossRef CAS.
- J.-C. Qi, H. Peng, Z.-K. Xu, Z.-X. Wang, Y.-Y. Tang, W.-Q. Liao, G.-F. Zou, Y.-M. You and R.-G. Xiong, Nat. Commun., 2024, 15, 6738 CrossRef CAS.
- L.-T. Li, C. Wang, C.-R. Huang, W.-Q. Liao, X.-L. Xu, L.-B. Xiao, R.-N. Wang, W.-Y. Cheng, T.-W. He, S. Cong, Z.-H. Kang, R.-G. Xiong and G.-F. Zou, J. Am. Chem. Soc., 2025, 147, 12635–12643 CrossRef CAS.
- J.-C. Qi, X.-J. Song, H. Peng, X.-G. Chen, R.-G. Xiong and W.-Q. Liao, Adv. Funct. Mater., 2025, 35, 2502822 CrossRef CAS.
- H.-H. Hu, R. Liu, Y.-B. Zhu, T.-T. Sha, X.-X. Cao, Z.-J. Feng, H.-R. Ji, Q. Pan, R.-G. Xiong and Y.-M. You, Angew. Chem., Int. Ed., 2025, 64, e202500176 CrossRef CAS PubMed.
- Z.-N. Zhou, Q.-D. Chong, Y.-W. Yang, Z.-W. Hu, W. Ren, S.-S. Zhang and Q. Ye, Angew. Chem., Int. Ed., 2025, 64, e202510596 CrossRef CAS PubMed.
- H. Zheng, A. Ghosh, M. J. Swamynadhan, Q. Zhang, W. P. D. Wong, Z. Wu, R. Zhang, J. Chen, F. Cimpoesu, S. Ghosh, B. J. Campbell, K. Wang, A. Stroppa, R. Mahendiran and K. P. Loh, Nat. Commun., 2024, 15, 5556 CrossRef CAS.
- A.-O. Polyakov, A.-H. Arkenbout, J. Baas, G.-R. Blake, A. Meetsma, A. Caretta, P.-H. M. van Loosdrecht and T. T. M. Palstra, Chem. Mater., 2012, 24, 133–139 CrossRef CAS.
- B. Kundys, A. Lappas, M. Viret, V. Kapustianyk, V. Rudyk, S. Semak, Ch. Simon and I. Bakaimi, Phys. Rev. B, 2010, 81, 224434 CrossRef.
- Y.-X. Dou, X.-M. Wang, N. W. G. Smith, P. Behera, R.-H. Mudiyanselage, B. Guzelturk, D. A. Walko, Y. Pleimling, S.-H. Liu, N. Nici, C. Slebodnick, B. Dryzhakov, B. Hu, A. Raja, R. Ramesh, G. A. Khodaparast, Y.-F. Yan and L.-N. Quan, Nat. Commun., 2025, 16, 7230 CrossRef CAS PubMed.
- (a) CCDC 2484549: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pdcr7; (b) CCDC 2484550: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pdcs8; (c) CCDC 2484236: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1nt; (d) CCDC 2484237: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1pv; (e) CCDC 2484238: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1qw; (f) CCDC 2484239: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1rx; (g) CCDC 2484240: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1sy; (h) CCDC 2484241: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1tz; (i) CCDC 2484242: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1v0; (j) CCDC 2484243: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1w1; (k) CCDC 2484244: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1x2; (l) CCDC 2484246: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd1z4; (m) CCDC 2484247: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd206; (n) CCDC 2484248: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd217; (o) CCDC 2484249: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd228; (p) CCDC 2484250: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd239.
Footnote |
| † The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |