Open Access Article
Open Access ArticleModulation of nanoscale sinuosity in asymmetric nano-channels for high-resolution separation of trace xylene isomer impurities
Ming Xu†
 ,
Xiao-Yi Fu†,
Sha-Sha Meng†,
Shu-Rui Gao,
Yu Wang
,
Xiao-Yi Fu†,
Sha-Sha Meng†,
Shu-Rui Gao,
Yu Wang and
Zhi-Yuan Gu
and
Zhi-Yuan Gu *
*
State Key Laboratory of Microbial Technology, Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, College of Chemistry and Materials Science, School of Food Science and Pharmaceutical Engineering, Nanjing Normal University, Nanjing 210023, China. E-mail: guzhiyuan@njnu.edu.cn
First published on 29th December 2025
Abstract
The purification of para-xylene (pX) to ultra-high levels is critical for producing high-performance polyethylene terephthalate, yet trace-level quantification of its isomeric impurities, meta-xylene (mX) and ortho-xylene (oX), remains a formidable challenge due to their nearly identical boiling points and molecular dimensions. This study presents an aluminum-based metal–organic framework, Al-TCPB-Me2, featuring asymmetric one-dimensional channels with a high sinuosity ratio that coupled molecular sieving and shape matching to achieve the baseline separation of xylene isomers. Structural characterization confirmed a sinuous channel with an entrance size of ∼6.8 Å, which excluded oX while enabling selective recognition of pX over mX through asymmetric binding sites. Density functional theory calculations revealed that four methyl groups in the sinuous channel formed hydrophobic interactions with two methyl groups in pX molecules, while only three methyl groups interacted with methyl groups in mX molecules, resulting in stronger binding of pX than mX. The Al-TCPB-Me2 column achieved high separation resolutions (24.9 for mX/pX and 31.1 for oX/pX) and successfully quantified the impurities as low as 200 ppm in the real high-quality xylene sample, surpassing all conventional columns. Comparative tests with a straight-channel Al-TCPB variant emphasized the sinuous geometry's role in enabling tandem separation mechanisms. This work establishes asymmetric sinuous channels as an effective design principle for integrating tandem molecular sieving and shape matching, offering a powerful strategy for the quantification of trace-level structurally similar impurities in real sample analysis.
Introduction
para-Xylene (pX) is a crucial precursor in the production of terephthalic acid (PTA), which is essential for manufacturing polyethylene terephthalate (PET) plastics and polyester fibers widely used in packaging, textiles, and consumer goods.1–3 The production of high-performance polyester requires ultra-high-purity pX, placing stringent demands on the detection and quantification of impurities, particularly its isomeric counterparts. meta-Xylene (mX) and ortho-xylene (oX) are the primary isomeric impurities, and even trace amounts of them can adversely affect the properties and performance of downstream products.4 However, the quantification of impurities in pX presents severe analytical challenges, which arises from the isomers' minimal boiling point variations (0.7–6.0 K) and similar molecular dimensions (pX: 5.8 Å; mX: 6.8 Å; oX: 6.8 Å).5,6 Conventional gas chromatography (GC) stationary phases hardly achieve xylene isomer separation due to these physicochemical similarities, leading to compromised accuracy in the quantification of pX impurities, especially the isomeric impurities at trace levels.7,8 This underscores the urgent need for highly selective chromatographic stationary phases with enhanced separation efficiency and accuracy in trace impurity quantification.9Metal–organic frameworks (MOFs) have emerged as promising materials for the separation of structurally similar molecules due to their precisely tunable pore environments.10–12 Controlling pore architecture has enabled improved separation of aromatic isomers, alkane/alkene mixtures, electronic specialty gases, and cyclohexane/benzene azeotropes in adsorption and chromatographic systems.13,14 Despite these developments, current MOF-based separators remain insufficient for the analytical demands of industrial pX purification. Most reported systems focus on equimolar or simplified binary mixtures and aim to enhance adsorption selectivity under balanced laboratory conditions.15 However, industrial impurity analysis presents a fundamentally different challenge, where trace-level mX and oX must be detected in the presence of an overwhelmingly dominant pX matrix—often with concentration differences spanning several orders of magnitude. Under such extreme disparity, even MOFs with good inherent selectivity struggle because of competitive adsorption from the dominant component. A fundamentally new material design paradigm is required, one capable of selective recognition of minor impurities despite the intense competitive adsorption from the major component.
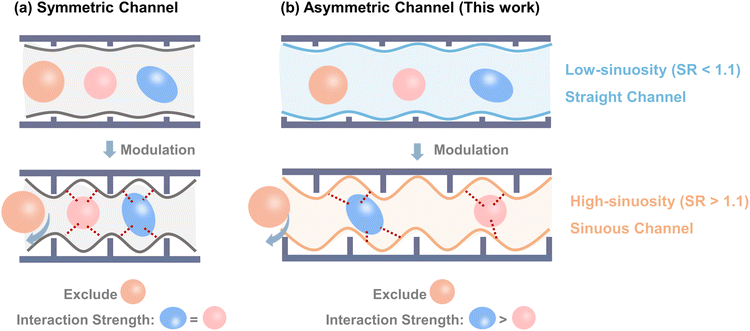
Classical separation mechanisms provided by MOF separators alone are insufficient to meet this requirement.16–21 Molecular sieving selectively adsorbs smaller molecules while excluding larger species,22–24 whereas shape matching relies on the geometric fit between adsorbates and pore environments to induce strong configuration-specific interactions.25–29 Recent studies have validated combining molecular sieving and shape matching as an efficient means to further enhance the separation performance of MOFs.30–32 In practice, however, these approaches have relied predominantly on size-based sieving, while the potential of shape matching has been less fully realized. In most cases, shape matching merely reinforces sieving or enhances adsorption capacity, rather than directly separating molecules after adsorption.13,14 This limitation arises largely from the prevailing use of symmetric channel architectures, in which separation is achieved by tuning channel windows to exclude larger molecules (Scheme 1a). Such an approach makes it difficult to modify the internal cavity geometry and thus restricts the precision of shape matching.30,31 From a geometric standpoint, asymmetric channels present a more versatile alternative because they are inherently sensitive to subtle differences in molecular geometry and provide greater opportunities for coupling sieving with shape recognition.33–35 Importantly, asymmetry can be introduced through chemical functionalization, which not only regulates channel window size but also modifies channel sinuosity, thereby enabling more precise discrimination of structural isomers (Scheme 1b). The sinuosity ratio (SR) of MOF channels can be defined analogously to the river sinuosity ratio, where SR values below 1.1 indicate straight channels and values above 1.1 indicate sinuous channels (Fig. S1). Applying this principle to the particularly demanding separation of xylene isomers requires delicate channel design, in which both asymmetry and sinuosity are finely tuned to achieve optimal molecular complementarity.
Herein, to construct the asymmetric sinuous channels, Al(OH)2O4 chains with zigzag coordination mode and TCPB-Me2 (H4TCPB-Me2 = 1,2,4,5-tetrakis(4-carboxyphenyl)-3,6-dimethyl-benzene) with two additional methyl groups as constraints were chosen to coordinate in a staggered manner. It was then developed as a high-performance stationary phase for baseline separation of trace xylene isomers from their main component through the synergistic molecular sieving and shape-matching mechanisms. The powder X-ray diffraction (PXRD) pattern and high-angle annular dark-field (HAADF) image confirmed that the Al-TCPB-Me2 framework possessed an frl topology with a one-dimensional (1D) sinuous channel (channel 1, SR = 1.14, Fig. 1) with the entrance size of approximately 6.8 Å. oX with the largest size was excluded from channel 1 and exhibited the fastest diffusion constant among the three isomers. At the same time, the sinuous geometry resulted in asymmetric binding sites inside the channel, leading to different shape-matching effects for pX and mX. Density functional theory (DFT) simulations demonstrated that pX exhibited the strongest binding energy to the channel.36,37 In detail, the alignment of the four methyl groups in the channel interacted with the two methyl groups in pX molecules, while only three methyl groups interacted with the two methyl groups in mX molecules, resulting in more negative binding energies of pX than mX. Moreover, the Al-TCPB-Me2 stationary phase achieved the separation resolution (Rs) values of 24.9 and 31.1 for mX/pX and oX/pX, respectively, which were much higher than those of the commercial column HP-5. Most importantly, the Al-TCPB-Me2 column can quantify the trace mX or oX isomer impurities at concentrations as low as 200 ppm in the pure pX sample. For comparison, an Al-TCPB stationary phase with straight channel 1 (SR = 1.05) was also developed.38 Although Al-TCPB demonstrated good performance in separating the para-isomer from chlorotoluene and dichlorobenzene, it failed to separate meta- and ortho-isomers, highlighting the crucial role of the asymmetric sinuous channel in providing tandem separation mechanisms and achieving superior separation performance. This work not only presented promising materials for the qualification of trace-level xylene impurity but also provided an effective design strategy for the development of separators with tandem separation mechanisms for challenging multi-component separations.
Results and discussion
Synthesis and characterization
The MOF Al-TCPB was synthesized through a one-pot method, and Al-TCPB-Me2 was obtained in a similar manner but by changing the ligand from H4TCPB to H4TCPB-Me2. The 1H nuclear magnetic resonance (NMR) spectra proved the structures of the two ligands (Fig. S2). The reported structural data revealed that the Al-TCPB framework adopts the frl topology, with the 3D coordination network consisting of infinite 1D Al(OH)2O4 chains and tetratopic carboxylic acid ligands.38 In the 1D Al(OH)2O4 chains, the aluminum ions exhibit an octahedral coordination environment, with two µ-OH groups and four carboxyl oxygen atoms from four distinct ligands participating in the coordination. The ligands coordinated with Al(OH)2O4 chains in a staggered manner along the b-axis, forming two types of 1D channels, referred to as channel 1 and channel 2 (Fig. 1). Channel 2 is a completely straight channel in which four benzene rings are oriented toward the pore interior, while channel 1 is nearly straight but exhibits slight sinuosity due to the presence of hydrogen atoms on the asymmetric organic ligands. The SR of Al-TCPB channel 1 was calculated as 1.05, confirming that it can reasonably be regarded as a straight channel in the subsequent discussion. The powder X-ray diffraction (PXRD) pattern of the synthesized Al-TCPB was recorded to confirm its structure and crystallinity. The sharp diffraction peaks of Al-TCPB showed minimal deviation from the simulated one, confirming its high crystallinity and the frl topology (Fig. 2a). The diffraction peaks at 5.23°, 8.00°, and 9.70° correspond to the (001), (200), and (201) planes of the Al-TCPB structure.Although the structural data for Al-TCPB-Me2 has not yet been reported, we hypothesized that Al-TCPB-Me2 shared an identical topology with Al-TCPB, with the only difference being the organic ligands. The introduction of additional methyl groups as constraints increased the sinuosity of channel 1 as well as functionalized the asymmetric methyl sites (Fig. 1). Consequently, the SR of channel 1 increased from 1.05 to 1.14, indicating the formation of an asymmetric sinuous channel in Al-TCPB-Me2. The solid-state 13C NMR spectra proved the presence of two methyl groups in the synthesized Al-TCPB-Me2 (Fig. S3). Al-TCPB-Me2 exhibited almost identical PXRD patterns to the simulated Al-TCPB, supporting their structural similarity. Notably, the diffraction peak for the (200) plane shifted from 8.00° to 9.29°, likely due to the introduction of the methyl groups, which reduced the interplanar spacing.39,40 Furthermore, the diffraction peaks of Al-TCPB-Me2 were broader compared to those of the synthesized Al-TCPB, suggesting relatively lower crystallinity.41,42
The scanning electron microscopy (SEM) images showed that Al-TCPB-Me2 possessed nanoplate morphology (Fig. S4). In order to investigate the topology of Al-TCPB-Me2, high-angle annular dark-field (HAADF) imaging was performed. The HAADF images revealed distinct lattice fringes (Fig. 2b), in which the Al chains were distinguishable as white lines against the background and other nonmetallic atoms. The simulated structure of Al-TCPB-Me2 viewed from the a-axis indicated that the interspacing between Al chains was 16.2 Å (Fig. 2c). The intensity profiles along the highlighted orange area in the HAADF image exhibited a spacing of 16.0 Å between Al chains, which coincides with the simulated interplanar spacing of the (001) plane. In addition, the calculated d-spacing of two adjacent points in the fast Fourier transform (FFT) pattern was also 16.0 Å, which was consistent with the interplanar spacing (16.2 Å) of the (001) plane. The above experimental findings further confirmed that Al-TCPB-Me2 has the same structure as Al-TCPB.
The N2 adsorption analysis was employed to analyze the pore structures of the two Al-MOFs (Fig. 2d). Both materials displayed type-I isotherms and steep slopes at ultra-low pressures, indicating the presence of intrinsic micropores and a strong binding affinity between N2 molecules and the MOF channels. The N2 adsorption capacity (p/p0 = 0.8) of Al-TCPB-Me2 (312 cm3 g−1) was slightly lower than that of Al-TCPB (353 cm3 g−1). Similarly, the BET surface area of Al-TCPB-Me2 (1141 m2 g−1) was smaller than that of Al-TCPB (1331 m2 g−1). These differences were attributed to the introduction of asymmetric methyl groups, which slightly changed the pore structure and hindered the N2 adsorption. The pore size distribution, calculated using the H–K (Saito–Foley) method, revealed that both Al-MOFs contained three distinct pores (Fig. 2e). Both Al-TCPB and Al-TCPB-Me2 had two types of pores with similar dimensions of 9.3 Å and 11.8 Å, respectively (Table S1). The smallest pore size shifted from 7.3 Å in Al-TCPB to 6.8 Å in Al-TCPB-Me2, with a noticeable reduction in the proportion of the smallest pores in Al-TCPB-Me2 (Fig. 2f). These observations further indicated that the introduction of the asymmetric methyl groups in the ligand partially reduced the pore volume, forming the sinuous channel with a reduction in the size of channel 1. However, the remaining channel 2 was not affected due to the similarity in topology.
The thermal stability of Al-TCPB and Al-TCPB-Me2 was evaluated by thermogravimetric analysis (TGA) under an oxygen atmosphere (Fig. S5). The results showed that due to differing amounts of residual solvent in the materials, the mass loss of Al-TCPB and Al-TCPB-Me2 before 250 °C was 5.60% and 3.64%, respectively. The sinuous channel of Al-TCPB-Me2 adsorbed less residual solvent compared to the straight channel of Al-TCPB. Both host frameworks maintained structural integrity up to 425 °C, indicating the high thermostability of the two Al-MOFs. This stability can be attributed to the strong aluminum–oxygen (Al–O) bonds and the stable coordination between the Al3+ center and the carboxylate ligands.
Molecular sieving provided by the asymmetric sinuous channel
After the successful synthesis of the two Al-MOFs with distinct channel architectures, they were measured in the inverse GC using xylene isomers as model analytes to evaluate the molecular sieving capabilities for isomer differentiation. The SEM images of the MOF-coated 15 meter columns in the cross-sectional view revealed that the dynamic coating process and temperature programming did not affect the morphology of MOFs (Fig. S6). Then, the kinetic parameters, including diffusion constants (Ds) and mass transfer coefficients (C-term) at 230 °C, were calculated for the comparison between Al-TCPB and Al-TCPB-Me2. Notably, the Ds values and C-terms for oX, mX, and pX showed minimal differences when tested with the Al-TCPB coated column. Similar Ds values and C-terms indicated that Al-TCPB cannot provide the molecular sieving effect to distinguish these isomers. The smallest pore window of Al-TCPB is 7.3 Å, which is still larger than all the xylene isomers, meaning that all the xylene isomers can enter channel 1 and channel 2 of Al-TCPB, resulting in no sieving effect.When it came to Al-TCPB-Me2, the phenomenon had changed. pX with the smallest kinetic diameter clearly exhibited the smallest Ds and the largest C-terms in the Al-TCPB-Me2 coated column, indicating the slowest diffusion of pX in Al-TCPB-Me2. This suggested that, unlike Al-TCPB, Al-TCPB-Me2, with its smallest pore window of 6.8 Å, was able to provide the molecular sieving effect, allowing the smallest pX to get into channel 1 while excluding larger isomers. Interestingly, oX and mX, which have almost identical kinetic diameters, displayed significantly different diffusion behaviors in Al-TCPB-Me2. The Ds value of oX was 2.4 times higher than that of mX, indicating that most oX molecules likely diffused through the stationary phase without entering the smallest channel 1 (Fig. S7 and Table S2). This behavior resulted from the unique sinuous shape of channel 1. The sinuous channel provided a tilted entrance for meta-positioned mX molecules, while the methyl group positioned at the channel entrance imposed additional steric hindrance on oX molecules with ortho-positioned dual methyl groups. During the fast adsorption and desorption processes, the large diffusion barrier prevented most oX molecules from entering channel 1, leading to molecular exclusion. Notably, this molecular exclusion of oX was weakened during adsorption at thermodynamic equilibrium. Single-component vapor adsorption experiments revealed that oX exhibited a slightly higher adsorption capacity in Al-TCPB-Me2 under saturated conditions, suggesting that oX was also able to access channel 1 at high molecular concentrations (Fig. S8). The equilibrium adsorption experiments allowed molecules sufficient time to explore accessible pore spaces. Under these conditions, oX might occupy the local adsorption pockets, and its adjacent methyl groups provided a larger hydrophobic contact area, resulting in slightly higher equilibrium uptake than mX. It is worth noting that since channel 2 of both Al-TCPB and Al-TCPB-Me2 was identical and larger than all the xylene isomers, the differing mass transfer behavior of isomers was primarily attributed to the shape of channel 1.
Shape matching with the asymmetric sinuous channel
To further investigate the shape-matching effects provided by the two Al-MOFs, the enthalpy of adsorption (ΔH), entropy of adsorption (ΔS), and Gibbs free energy of adsorption (ΔG) for xylene isomers were calculated. As shown in Fig. S9, the van't Hoff plot exhibited a linear relationship, indicating that the interaction mechanism between the MOFs and adsorbates remained consistent across the temperature range.For Al-TCPB, the ΔH values followed the order mX > oX > pX (−70.9 > −72.7 > −73.2 kJ mol−1, Table S3). pX exhibited the most negative ΔH value when tested with the Al-TCPB coated column, although the differences were not pronounced. Thus, pX, with the longest molecular length, was able to interact synergistically with both channel walls, leading to a stronger affinity for Al-TCPB. In contrast, oX, with two methyl groups in adjacent positions, was reported to interact simultaneously with the metal chains, resulting in intermediate interactions with the backbone.43 The shape of mX allowed it to use only one of its two methyl groups to interact with the channel wall, leading to the weakest interaction. These results suggested that Al-TCPB with a low SR can provide a shape-matching effect to distinguish the thermodynamic differences between the three xylene isomers, although this effect was not significant.
For Al-TCPB-Me2, the ΔH values for Al-TCPB-Me2 followed the order oX > mX > pX (−68.5 > −75.7 > −89.4 kJ mol−1). The difference in ΔH values was much more significant, indicating that Al-TCPB-Me2 offered greater distinguishability than Al-TCPB. Notably, the ΔH value for oX in Al-TCPB-Me2 was less negative than in Al-TCPB and also less negative than pX and mX in Al-TCPB-Me2, further supporting the speculation that oX molecules were predominantly excluded by sinuous channel 1, preventing them from accessing the adsorption sites within channel 1. The ΔH values for pX were much more negative than those for mX, suggesting a stronger interaction between pX and Al-TCPB-Me2. We supposed that the high sinuosity introduced by asymmetric methyl groups enhanced the shape-matching effect, thereby amplifying the geometric distinctions among xylene isomers. Within the sinuous channels, the methyl groups can closely interact with the linear pX molecules, forming the strongest interaction. In contrast, mX molecules with meta-positioned methyl groups were unable to form the same interactions, resulting in a weaker interaction. The ΔS and ΔG values for the three isomers on Al-TCPB-Me2 further supported this hypothesis (Table S3). These results proved that the sinuous channels of Al-TCPB-Me2 can provide an effective shape-matching effect to distinguish isomers.
To test the above hypothesis, density functional theory (DFT) simulations, conducted using VASP software, were employed to further explore the shape-matching phenomena between pX and mX within the asymmetric sinuous channel 1 of Al-TCPB-Me2 (see the SI for details). The adsorption positions of pX and mX molecules within the channel were optimized to minimize the binding energy before the comparison. The final calculated binding energies for pX and mX were −84.42 kJ mol−1 and −75.22 kJ mol−1, respectively. The observed trend in binding energies aligned with the experimental ΔH values obtained from the inverse GC method. As illustrated in Fig. 3d and e, both pX and mX could form C–H⋯π interactions with the Al-TCPB-Me2 framework. However, the dual methyl groups of linear pX were in closer proximity to the four methyl groups of the organic ligands, resulting in a more complete hydrophobic contact between its two methyl groups and the densely methyl-decorated channel walls, which maximized dispersive interactions. In contrast, the mX molecule was shorter, and its meta-positioned methyl groups interacted with only three methyl groups of the organic ligands, leading to an incomplete overlap with these hydrophobic regions. Thus, the asymmetric sinuous channel amplified the difference in the accessible hydrophobic surface area among isomers, directly translating geometric mismatches into measurable binding energy differences. DFT further proved that the asymmetric sinuous channels of Al-TCPB-Me2 can provide the shape-matching effect to distinguish pX and mX.
Separation performance of the asymmetric sinuous channel in Al-MOFs
Following the confirmation of the asymmetric sinuous channel's capacity for tandem molecular sieving and shape-matching effects in isomer discrimination, the Al-TCPB-Me2 material was implemented as a GC stationary phase for isomer separation. Comparative Al-TCPB coated columns and commercial HP-5 columns were tested to separate identical isomer mixtures (Fig. 4 and S10–S13).The concentrations of coating material were controlled as 1 mg mL−1 (1 mL) for each column, respectively, to avoid their influence (Fig. S13). Experimental results revealed that the Al-TCPB-Me2 column achieved baseline resolution for 10 groups of disubstituted benzene isomers and alkane compounds, demonstrating superior analytical scope and separation efficiency. For xylene isomer separation, Rs values reached 24.9 (mX/pX) and 31.1 (oX/pX), significantly outperforming state-of-the-art HP-5 columns and most of the reported stationary phases (Fig. 4c and Table S4). The elution sequence (oX > mX > pX) reflected differential molecular interactions. The oX's early elution aligned with the rapid kinetic diffusion through molecular sieves, while the delayed elution of pX corresponded to the enhanced thermodynamic binding through shape matching. This tandem mechanism facilitated consistent baseline separation across various disubstituted benzene isomers, confirming the strategic advantage of engineered sinuous channels in the synergistic size exclusion and molecular recognition for ortho-, meta-, and para-isomer differentiation (Fig. 4a and S11). The column also exhibited excellent operational and long-term stability, as confirmed by its consistent performance across multiple batches, repeated injections, thermal cycling, and seven-month storage (Fig. S14–S17).
The separation performance of Al-TCPB with straight channels was also evaluated. The Al-TCPB coated column baseline separated several isomers, such as xylene and ethyltoluene, although the separation performance was significantly lower than the Al-TCPB-Me2 coated column. For example, the Rs values of oX/pX and mX/pX were 5.3 and 9.1, respectively (Table S4). Notably, the elution order of xylenes on Al-TCPB was mX > oX > pX, differing from that observed in Al-TCPB-Me2. This elution sequence was consistent with the relative ΔH values, suggesting that the separation in Al-TCPB was primarily governed by shape-matching interactions without significant molecular sieving effects. The column efficiency of these two columns was measured by using oX as the target (Fig. S18). The column efficiency of the Al-TCPB coated column was 1895 plates/m, smaller than that of the Al-TCPB-Me2 coated column (3289 plates/m, Table S5). When separating chlorotoluene isomers and dichlorobenzene isomers, Al-TCPB can separate para-isomers from others but cannot distinguish the meta- and ortho-isomers (Fig. 4b and S10). These comparative results conclusively validated the superior performance of the asymmetric sinuous channel in Al-TCPB-Me2 with a tandem separation mechanism.
Quantification of isomer impurities in a high quality sample
To assess the quantification ability of the Al-TCPB-Me2 column, four xylene isomers (oX, mX, pX, and EB) were mixed in n-heptane at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. Their concentrations were adjusted by varying the volume of n-heptane. The solutions of each concentration were injected into the 30 meter Al-TCPB-Me2 column under the same programmed heating conditions, respectively. Chromatograms showed baseline separation of all isomers with no interference in peak area integration (Fig. S19). Each isomer's calibration curve had a goodness of fit above 0.99 (Fig. 4d and Table S6). Detection limits (LODs, S/N = 3) were 2.59 ± 0.22 pg for oX, 3.11 ± 0.22 pg for mX, 15.39 ± 1.99 pg for pX, and 3.08 ± 0.13 pg for EB (Table S7). It is worth noting that the above LODs could be even lower with proper analytical strategies, such as sample preparation and mass spectrometry (MS) or MS/MS detectors. These results confirmed the Al-TCPB-Me2 column's reliable quantification performance.
1 molar ratio. Their concentrations were adjusted by varying the volume of n-heptane. The solutions of each concentration were injected into the 30 meter Al-TCPB-Me2 column under the same programmed heating conditions, respectively. Chromatograms showed baseline separation of all isomers with no interference in peak area integration (Fig. S19). Each isomer's calibration curve had a goodness of fit above 0.99 (Fig. 4d and Table S6). Detection limits (LODs, S/N = 3) were 2.59 ± 0.22 pg for oX, 3.11 ± 0.22 pg for mX, 15.39 ± 1.99 pg for pX, and 3.08 ± 0.13 pg for EB (Table S7). It is worth noting that the above LODs could be even lower with proper analytical strategies, such as sample preparation and mass spectrometry (MS) or MS/MS detectors. These results confirmed the Al-TCPB-Me2 column's reliable quantification performance.
To further confirm the Al-TCPB-Me2 column's ability to quantify trace isomeric impurities, a real high-quality pX sample was analyzed. The three impurities (oX, mX, and EB) eluted between 4 and 6 minutes, while pure pX eluted after 12 minutes, ensuring the main pX peak broadening and trailing did not interfere with impurity detection (Fig. 4e). Using calibration curves, the impurities of oX, mX, and EB were measured as 200 ± 30 ppm, 800 ± 10 ppm, and 200 ± 20 ppm (w/w), respectively (Table S8), in the pure pX sample. It is worth noting that the values did not align with the LODs for the Al-TCPB-Me2 column. Theoretically, impurities as low as 6.3 ppm could be quantified, which is suitable for the analysis of ultrahigh-grade samples, such as electronic chemicals (>99.9994% w/w). For comparison, the conventional HP-5 column was used to analyze the same pX sample, but it failed to quantify impurities due to poor separation and interference from pX peak tailing (Fig. 4f and S20). This validates the Al-TCPB-Me2 column's superior performance in trace isomer impurity quantification.
Conclusions
In summary, we demonstrated the successful development of Al-TCPB-Me2 with asymmetric sinuous one-dimensional channels as a high-performance GC stationary phase for resolving persistent challenges in pX trace isomer separation and qualification. By synergistically integrating molecular sieving and shape matching mechanisms, the material's unique asymmetric sinuous channel architecture enabled exclusion of oX through size selectivity while leveraging asymmetric binding interactions to differentiate pX and mX. The separation mechanism was proved by both thermodynamic interaction and kinetic diffusion investigation. The Al-TCPB-Me2 column achieved exceptional separation resolutions and impurity quantification down to 200 ppm in a high-purity sample, significantly surpassing conventional columns. The comparison material Al-TCPB with straight channels showed lower separation resolution of all isomer mixtures than Al-TCPB-Me2, emphasizing the critical role of sinuous geometry in achieving the high performance. These findings advanced the precision of industrial xylene isomer analysis and established a versatile design strategy for engineering advanced separation materials targeting complex multi-component systems with near-identical physicochemical properties.Author contributions
Z.-Y. G. conceived the idea and supervised the research. M. X., X. Y. F. and S.-S. M. performed the synthesis, characterization, and GC experiments and discussed the results. S. R. G. and Y. W. performed the DFT simulation. Z.-Y. G. and M. X. discussed the experimental data and wrote the paper. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc07611k.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22374077 and 22474059) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. This work was carried out with the support of BL17B1 at the Shanghai Synchrotron Radiation Facility (proposal 2023-NFPS-PT-500772).Notes and references
- Y. Liu, C. Wang, Q. Yang, Q. Ren and Z. Bao, Coord. Chem. Rev., 2025, 523, 216229 CrossRef CAS.
- M. Xu, W.-Q. Tang, S.-S. Meng and Z.-Y. Gu, Chem. Soc. Rev., 2025, 54, 1613–1633 RSC.
- M. Rahmani, C. R. M. O. Matos, S.-Q. Wang, A. A. Bezrukov, A. C. Eaby, D. Sensharma, Y. Hjiej-Andaloussi, M. Vandichel and M. J. Zaworotko, J. Am. Chem. Soc., 2023, 145, 27316–27324 CrossRef CAS.
- L. Yu, J. Zhang, S. Ullah, J. Yao, H. Luo, J. Huang, Q. Xia, T. Thonhauser, J. Li and H. Wang, Angew. Chem., Int. Ed., 2023, 62, e202310672 CrossRef CAS PubMed.
- N. Zhu, J. Wu and D. Zhao, ACS Nano, 2025, 19, 2029–2046 CrossRef CAS PubMed.
- Y.-J. Zhao, W.-Q. Tang, X.-W. Wang, H.-F. Zhao, Z.-Y. Gu, Q. Yang and D. Liu, Chem. Sci., 2022, 13, 11896–11903 RSC.
- S. Halder, Z. Xie, M. H. Nantz and X.-A. Fu, J. Chromatogr. A, 2022, 1673, 463083 Search PubMed.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390–1393 CrossRef CAS.
- J. Wang, X. Lu, Z. Zhang, R. Gao, C. Pei and H. Wang, J. Chromatogr. A, 2024, 1718, 464718 CrossRef CAS.
- M. Chang, Z. Wang, R. Wang, M. Liu, Y. Wang and D. Liu, Angew. Chem., Int. Ed., 2025, 64, e202515496 CrossRef CAS.
- Z.-H. Guo, L.-Q. Yang, Q.-G. Zhai, G.-P. Yang and Y.-Y. Wang, Angew. Chem., Int. Ed., 2025, 64, e202519278 CrossRef CAS.
- C.-X. Chen, X. Cui, Y.-Y. Xiong, P. C. Lan, K. Tan, Z.-W. Wei, Z. Niu, C. Shan, L. Yang, S. Ma and C.-Y. Su, Nat. Synth., 2025 DOI:10.1038/s44160-025-00911-7.
- Y. Wang, N.-Y. Huang, X.-W. Zhang, H. He, R.-K. Huang, Z.-M. Ye, Y. Li, D.-D. Zhou, P.-Q. Liao, X.-M. Chen and J.-P. Zhang, Angew. Chem., Int. Ed., 2019, 58, 7692–7696 CrossRef CAS PubMed.
- Q. Ding, Z. Zhang, C. Yu, P. Zhang, J. Wang, X. Cui, C.-H. He, S. Deng and H. Xing, Sci. Adv., 2020, 6, eaaz4322 CrossRef CAS PubMed.
- S. Lee, A. Sharma, J. H. Lee, J. Lim, S. K. Min, H. Chun and M. S. Lah, Angew. Chem., Int. Ed., 2025, 64, e202512244 CrossRef CAS.
- S. A. Mohamed, R. Zheng, N. Zhu, D. Zhao and J. Jiang, J. Am. Chem. Soc., 2025, 147, 12251–12262 CrossRef CAS PubMed.
- L. Li, L. Guo, D. H. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren and J. Li, Science, 2022, 377, 335–339 CrossRef CAS.
- X.-J. Xie, H. Zeng, Y.-L. Huang, Y. Wang, Q.-Y. Cao, W. Lu and D. Li, Chem, 2025, 11, 102339 CAS.
- Q. Liu, Y. Miao, L. F. Villalobos, S. Li, H.-Y. Chi, C. Chen, M. T. Vahdat, S. Song, D. J. Babu, J. Hao, Y. Han, M. Tsapatsis and K. V. Agrawal, Nat. Mater., 2023, 22, 1387–1393 CrossRef CAS PubMed.
- X. Wu, X. Tian, W. Zhang, X. Peng, S. Zhou, P. J. S. Buenconsejo, Y. Li, S. Xiao, J. Tao, M. Zhang and H. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202410411 CrossRef CAS PubMed.
- X. Zhang, X. Tian, N. Wu, S. Zhao, Y. Qin, F. Pan, S. Yue, X. Ma, J. Qiao, W. Xu, W. Liu, J. Liu, M. Zhao, K. Ostrikov and Z. Zeng, Sci. Adv., 2024, 10, eadl6498 CrossRef CAS PubMed.
- H. Wang, Y. Liu and J. Li, Adv. Mater., 2020, 32, 2002603 CrossRef CAS PubMed.
- M. Xu, S.-S. Meng, P. Cai, W.-Q. Tang, Y.-D. Yin, J. A. Powell, H.-C. Zhou and Z.-Y. Gu, Chem. Sci., 2021, 12, 4104–4110 RSC.
- R. Lyndon, Y. Wang, I. M. Walton, Y. Ma, Y. Liu, Z. Yu, G. Zhu, S. Berens, Y.-S. Chen, S. G. Wang, S. Vasenkov, D. S. Sholl, K. S. Walton, S. H. Pang and R. P. Lively, Chem. Commun., 2022, 58, 12305–12308 RSC.
- Q. Wang, Y. Li, Z. Qiu, D. Zhou, L. Yang, X. Suo, X. Cui and H. Xing, Angew. Chem., Int. Ed., 2024, 63, e202408817 Search PubMed.
- X. Cui, Z. Niu, C. Shan, L. Yang, J. Hu, Q. Wang, P. C. Lan, Y. Li, L. Wojtas, S. Ma and H. Xing, Nat. Commun., 2020, 11, 5456 Search PubMed.
- J. Li, B. Sheng, R. Chen, H. Wu, W. Zhou, F. Zheng, B. Liu, Q. Yang, Z. Zhang, Y. Yang, Q. Ren and Z. Bao, Adv. Mater., 2025, 37, 2413506 CrossRef CAS.
- Z. Chu, J. Li, F. Chen, Y. Cao, L. Chen, F. Zhou, H. Ma, Q. Yang, Z. Zhang, K. Qiao, Q. Ren and Z. Bao, ACS Cent. Sci., 2024, 10, 1861–1870 Search PubMed.
- H. Yuan, K. Li, D. Shi, H. Yang, X. Yu, W. Fan, P. J. S. Buenconsejo and D. Zhao, Adv. Mater., 2023, 35, 2211859 CrossRef CAS.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Nature, 2021, 595, 542–548 CrossRef CAS PubMed.
- L. Li, Z. Yang, Q. Wang, L. Yang, X. Suo, X. Cui and H. Xing, Small, 2025, 21, 2412724 CrossRef CAS PubMed.
- M.-Y. Zhou, X.-W. Zhang, H. Yi, Z.-S. Wang, D.-D. Zhou, R.-B. Lin, J.-P. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2024, 146, 12969–12975 CrossRef CAS.
- Z.-J. Jiang, Y. Wang, D. Luo, R.-J. Wei, W. Lu and D. Li, Angew. Chem., Int. Ed., 2024, 63, e202403209 Search PubMed.
- X.-W. Zhang, H. He, Y.-W. Gan, Y. Wang, N.-Y. Huang, P.-Q. Liao, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2024, 63, e202317648 CrossRef CAS PubMed.
- F. Xie, L. Chen, E. M. Cedeño Morales, S. Ullah, Y. Fu, T. Thonhauser, K. Tan, Z. Bao and J. Li, Nat. Commun., 2024, 15, 2240 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B:Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- M. Krüger, R. Siegel, A. Dreischarf, H. Reinsch, J. Senker and N. Stock, Micropor. Mesopor. Mat., 2015, 216, 27–35 CrossRef.
- G. M. R. Dontireddy, S. P. Suman, J. L. Merino-Gardea, A. H. Javed, J. Wang, T. Chen, S. Kampouri and H. Banda, J. Mater. Chem. A, 2025, 13, 8734–8741 RSC.
- L. Yan, H.-T. Zheng, L. Song, Z.-W. Wei, J.-J. Jiang and C.-Y. Su, Chem. Eng. J., 2023, 472, 145145 Search PubMed.
- A. Gładysiak, A.-Y. Song, R. Vismara, M. Waite, N. M. Alghoraibi, A. H. Alahmed, M. Younes, H. Huang, J. A. Reimer and K. C. Stylianou, JACS Au, 2024, 4, 4527–4536 CrossRef.
- M. Lammert, H. Reinsch, C. A. Murray, M. T. Wharmby, H. Terraschke and N. Stock, Dalton Trans., 2016, 45, 18822–18826 RSC.
- Y. Yang, P. Bai and X. Guo, Ind. Eng. Chem. Res., 2017, 56, 14725–14753 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |