Open Access Article
Open Access ArticleThermal decomposition behavior and sustainable recycling of flexible perovskite solar modules
Xiaoyu Shi,
Yangyang Liu,
Tianxiao Liu,
Siwei Luo,
Feifei Wang and
Shangshang Chen *
*
State Key Laboratory of Coordination Chemistry, MOE Key Laboratory of High-Performance Polymer Materials & Technology, School of Chemistry, Nanjing University, Nanjing, Jiangsu 210023, P. R. China. E-mail: schen@nju.edu.cn
First published on 10th January 2026
Abstract
The environmental implications of all photovoltaic (PV) technologies should be rigorously assessed throughout their entire lifecycle. Perovskite PVs have emerged as a transformative innovation, challenging the dominance of conventional silicon PVs, with numerous companies now commercializing this promising technology. While efforts to enhance the efficiency and longevity of perovskite PVs are crucial, it is equally important to develop sustainable and cost-effective methods for disposing of waste perovskite solar panels, especially given their significant content of water-soluble lead ions. In this study, we explore the feasibility of employing incineration to process degraded flexible perovskite solar modules. We analyze the decomposition byproducts and their potential environmental impacts. By implementing careful management of hazardous decomposition products, we demonstrate that incineration can serve as a sustainable and economical solution for the disposal of waste perovskite solar modules, offering valuable insights for the future handling of these materials.
1 Introduction
Since the 1990s, solar photovoltaic (PV) power generation has experienced robust and steady growth with an annual rate of 37%, positioning solar energy as the fastest-growing renewable energy source.1–3 More intuitively, the cumulative PV installed capacity, which stood at a mere 1.28 GW in 2000, surged to a cumulative PV capacity of 709.67 GW by 2020.4 Meanwhile, the emergence of new thin-film PV technologies has also significantly contributed to the development of solar PVs, particularly perovskite PVs that exhibit advantages including low-cost manufacturing, light weight, and mechanical flexibility.5,6 Compared to rigid and heavy silicon PVs, perovskite PVs have huge application potential in building-integrated, foldable, and flexible PVs.As the scale of PV power installation continues to expand, the environmental implications of these PV panels must be rigorously assessed throughout their entire lifecycle. Global PV module waste is expected to surge to 60–78 million tons (equivalent to 630 GW) by 2050.7 The existing PV waste management strategies, which mainly focus on crystalline silicon modules, include landfilling and recycling.8,9 Among them, recycling, which aligns with the principles of environmental sustainability and resource recovery, stands as the most extensively researched disposal method. The recycling process for crystalline silicon modules involves physical separation, chemical treatment, and heat treatment, aiming to efficiently recover valuable materials such as silicon, Ag, and Cu while minimizing environmental pollution.10 However, the recycling approach faces multiple challenges. The first is the environmental challenge, which includes the release of extremely harmful gases, such as hydrofluoric acid, during chemical treatments, as well as the dust and noise generated during physical processes like high-pressure crushing.11–13 The second challenge is economic feasibility because the cost of establishing and operating recycling infrastructures is relatively high.7,14 Additionally, the economic viability of recycling is also constrained by the limited quantity and overall value of recoverable materials.15–18 The related studies have indicated that the recycled products of crystalline silicon modules are mainly composed of 68% glass and 15% base Al metal.9,19 The low value of these materials, combined with the high costs of recycling and disposal, results in a lack of economic sustainability for this recycling method.
Emerging as a transformative innovation challenging conventional silicon PVs, perovskite PV technology has attracted hundreds of industrial players pursuing its commercialization.20,21 While ongoing research focuses on enhancing device efficiency and operational stability, equal emphasis must be placed on developing sustainable and economically viable disposal methods for end-of-life perovskite modules, particularly given their substantial environmental risk from high concentrations of water-soluble lead compounds.22–24 Current recycling strategies primarily target the recovery of conductive substrates and PbI2 precursors, yet critical challenges persist: the economic feasibility of PbI2 reprocessing remains unproven (particularly as large amounts of solvents are involved), while flexible conductive substrates often suffer irreversible degradation from mechanical stress and environmental exposure during operational lifespan. These limitations underscore the urgent need for alternative disposal solutions to ensure the sustainable development of perovskite PVs.
Traditional landfill disposal of household waste presents unacceptable environmental hazards due to potential pollution to soil and groundwater systems.25–28 Modern waste management practice demonstrates that advanced incineration technologies, when equipped with proper emission control systems and ash treatment protocols, can achieve both economic viability and environmental safety.29,30 Drawing inspiration from these developments, this study systematically investigates the thermal decomposition pathways of degraded flexible perovskite solar modules. Through comprehensive analysis of incineration byproducts (including solid residues and gaseous emissions), we characterize the complete incineration profile and propose optimized post-treatment protocols for resultant materials, providing important guidance for the post-treatment of degraded perovskite modules. Our findings demonstrate that controlled incineration, when integrated with appropriate flue gas purification and ash stabilization techniques, presents a promising dual solution addressing both economic constraints and environmental concerns in perovskite PV waste management.
2 Results and discussion
2.1. Module incineration and ash analysis
The complete thermal degradation profile and analytical results are systematically presented in Fig. 1. For this investigation, we employed a representative flexible perovskite module architecture of polyethylene terephthalate (PET)/indium tin oxide (ITO)/poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA)/perovskite/C60/bathocuproine (BCP)/Cu. The perovskite active layer comprised Cs0.1FA0.9PbI3 (FA+: formamidinium) composition, widely adopted in commercial module fabrication due to its high efficiency and thermal stability.31,32 The pristine perovskite modules were able to realize a high power conversion efficiency (PCE) of 15.6% at an aperture area of 59.3 cm2, with an open-circuit voltage (VOC) of 16.2 V (1.16 V for each sub-cell), a short-circuit current (ISC) of 82.4 mA, and a fill factor (FF) of 0.691 (Fig. S1). Then the unencapsulated module was put under light soaking to accelerate the natural degradation. To simulate real-world disposal conditions while ensuring complete incineration, modules were subjected to controlled incineration in a chamber under continuous air supply. Post-incineration ash residues were first analyzed via X-ray photoelectron spectroscopy (XPS) to determine elemental composition, chemical speciation, and potential lead-containing byproducts. Note that the encapsulants like polyolefin elastomer (POE) or polyisobutylene (PIB) were not involved in this study, as their incineration products have been well studied previously.33,34 | ||
| Fig. 1 Schematic illustration of the incineration product analysis of degraded flexible perovskite solar modules. | ||
As shown in Fig. 2a, the C 1s spectrum of the ash displays three binding energies at 284.5, 285.6, and 288.9 eV, which can be assigned to C–C/C–H, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. The characteristic peak observed at 400.0 eV in the N 1s XPS spectrum (Fig. 2b) provides additional evidence for the presence of C–N bonds within the obtained ash,35 which could potentially originate from residual FA+ or insufficiently combusted BCP or PTAA compounds. The Pb 4f peak at 138.5 eV (Fig. 2c) is well assigned to that of PbI2 (138.5 eV),36 indicating a large amount of PbI2 remaining in the ash after incineration. In addition, the presence of a characteristic shoulder at 138.9 eV suggested the formation of a complex mixture rather than pure PbI2, potentially comprising various oxide phases and possibly carbonates. This can also be verified by the peak of corresponding β-PbO and/or PbCO3 with a binding energy of 531.7 eV in the O 1s XPS spectrum (see Fig. 2c and d, respectively).37,38 Furthermore, the XPS spectra of I 3d and Cs 3d are presented in Fig. 2e and f. The characteristic peaks of I 3d5/2 and I 3d3/2 are identified at binding energies of 619.2 eV and 630.7 eV, while Cs 3d5/2 and Cs 3d3/2 peaks are observed at 724.6 eV and 738.6 eV, respectively.39,40 These spectral features are consistent with the spectra of I− and Cs+ in the perovskite structure. Overall, following the incineration of waste flexible modules, the predominant chemical states of key elements in the residual solid ash are identified as Pb2+ (primarily existing in PbI2, PbO, and PbCO3 compounds), along with Cs+ and I−. The ash residue can be further recycled to reproduce the PbI2 precursor.
O, respectively. The characteristic peak observed at 400.0 eV in the N 1s XPS spectrum (Fig. 2b) provides additional evidence for the presence of C–N bonds within the obtained ash,35 which could potentially originate from residual FA+ or insufficiently combusted BCP or PTAA compounds. The Pb 4f peak at 138.5 eV (Fig. 2c) is well assigned to that of PbI2 (138.5 eV),36 indicating a large amount of PbI2 remaining in the ash after incineration. In addition, the presence of a characteristic shoulder at 138.9 eV suggested the formation of a complex mixture rather than pure PbI2, potentially comprising various oxide phases and possibly carbonates. This can also be verified by the peak of corresponding β-PbO and/or PbCO3 with a binding energy of 531.7 eV in the O 1s XPS spectrum (see Fig. 2c and d, respectively).37,38 Furthermore, the XPS spectra of I 3d and Cs 3d are presented in Fig. 2e and f. The characteristic peaks of I 3d5/2 and I 3d3/2 are identified at binding energies of 619.2 eV and 630.7 eV, while Cs 3d5/2 and Cs 3d3/2 peaks are observed at 724.6 eV and 738.6 eV, respectively.39,40 These spectral features are consistent with the spectra of I− and Cs+ in the perovskite structure. Overall, following the incineration of waste flexible modules, the predominant chemical states of key elements in the residual solid ash are identified as Pb2+ (primarily existing in PbI2, PbO, and PbCO3 compounds), along with Cs+ and I−. The ash residue can be further recycled to reproduce the PbI2 precursor.
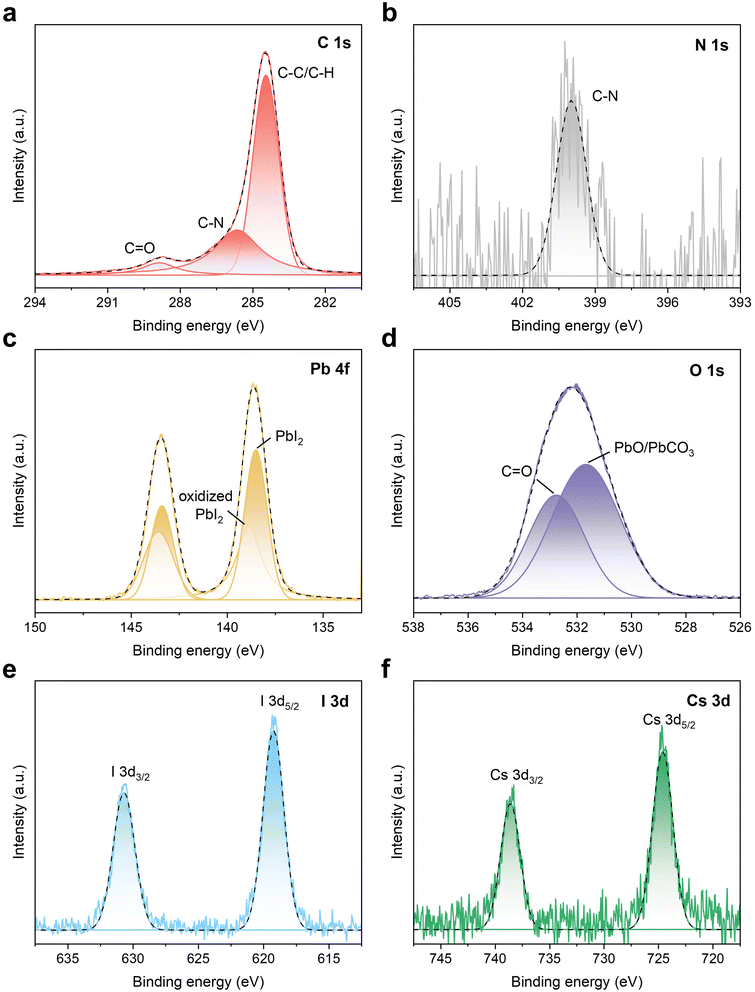 | ||
| Fig. 2 XPS analysis of the ash obtained from incineration: (a) C 1s, (b) N 1s, (c) Pb 4f, (d) O 1s, (e) I 3d, and (f) Cs 3d. | ||
Besides the elements present in the perovskite light-absorbing layer, we also analyzed the chemical states of electrode materials after incineration, including ITO and Cu. Similarly, XPS analysis was performed on key metallic elements including In, Sn, and Cu, within these incineration products (Fig. 3a–c). The results reveal that the In element primarily exists as In2O3, evidenced by the corresponding binding energy of 445.1 eV.41,42 Furthermore, the binding energy of Sn 3d5/2 located at 487.0 eV indicates the presence of Sn4+, thereby confirming the possibility of the existence of SnO2.43 As presented in Fig. 3c, the Cu 2p3/2 spectrum exhibits two distinct peaks located at 932.8 and 934.0 eV, respectively, demonstrating that the Cu element exists in the form of Cu+ and Cu2+ oxidation states within the incineration products of burned waste flexible perovskite modules.44,45 To verify the above conclusion, the PET-ITO flexible substrate coated with a 130-nm-thick Cu layer was subjected to identical incineration. Subsequently, as shown in Fig. 3d, X-ray diffraction (XRD) analysis was performed on the resulting residue. The diffraction pattern closely matched the standard card for In2O3 (PDF#06-0416), strongly supporting the presence of crystalline In2O3 in the pyrolyzed residue, consistent with the XPS results. However, it is noteworthy that no characteristic diffraction peaks associated with Cu were detected in the XRD pattern. Given that XPS identified Cu and its oxidation states, we infer that this absence is most likely attributable to the relatively low Cu content in the sample or its existence in an amorphous form, resulting in a diffraction signal below the XRD detection limit or indistinguishable from the background. Collectively, the above results elucidate the primary chemical speciation of key metallic elements within the pyrolyzed residue of electrodes (ITO/Cu) from waste flexible PV modules: In element predominantly exists as crystalline In2O3; Sn is stabilized in the Sn4+ oxidation state within the SnO2 matrix; and Cu co-exists in both Cu+ and Cu2+ oxidation states. This result directly confirms the elemental transformation pathways of electrode materials during incineration.
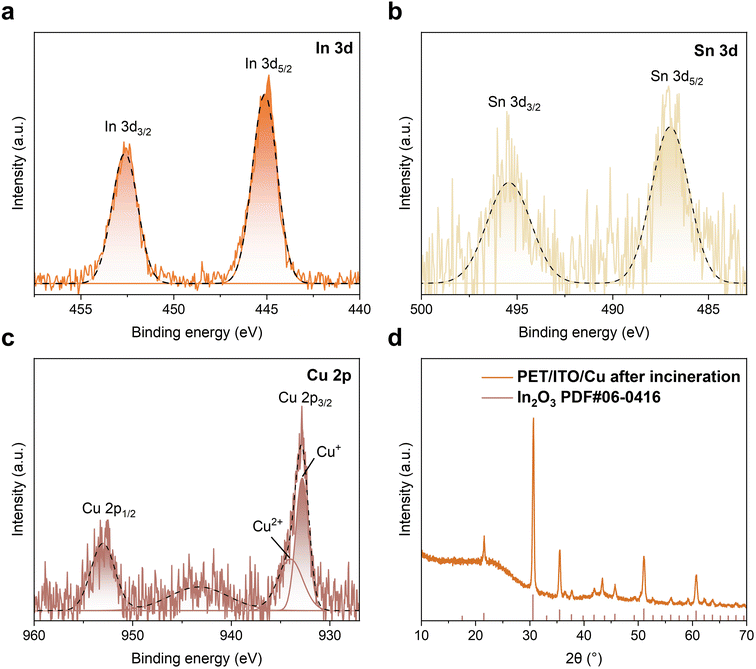 | ||
| Fig. 3 XPS analysis of the ash obtained from incineration: (a) In 3d, (b) Sn 3d, and (c) Cu 2p. (d) XRD pattern of the ash after incineration of the PET/ITO/Cu sample. | ||
2.2. Thermal decomposition and exhaust analysis
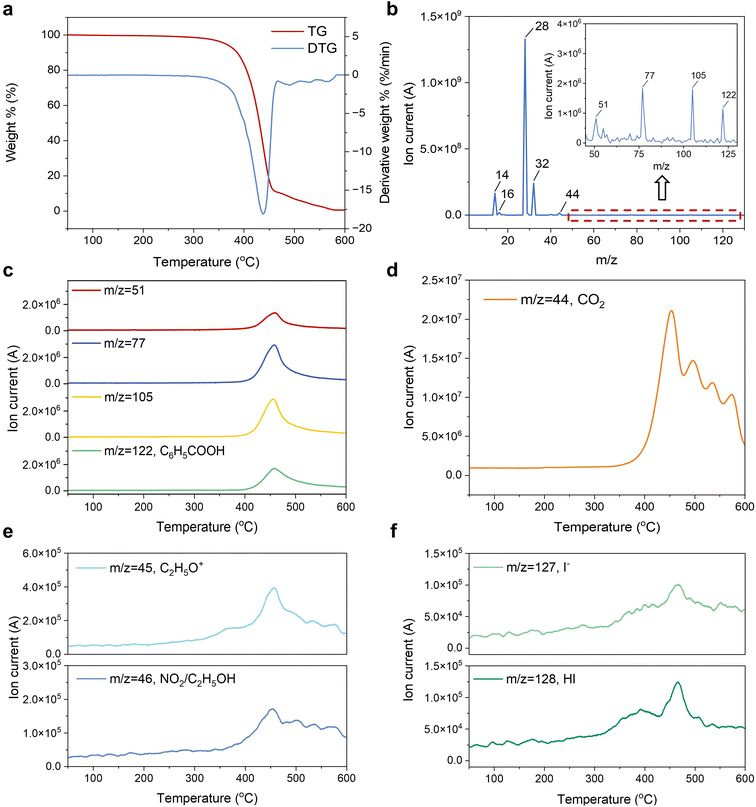
To systematically investigate the thermal decomposition behavior of the waste module, gas analysis was then conducted using TG-MS at a heating rate of 10 °C min−1 in ambient air. The experiment was conducted using a combination of TG analyzer and MS technology to enable real-time monitoring of mass loss dynamics and formation of gaseous species. As shown in Fig. 4a, TG curves reveal four distinct thermal decomposition stages. The flexible module demonstrates remarkable thermal stability below 300 °C with negligible mass loss (1.5%), indicating that the module has little moisture content and few low-temperature volatile components. Within the temperature range of 300–465 °C, the sample undergoes significant thermal decomposition, resulting in a mass loss of 89%. Furthermore, by analyzing the derivative TG curve, a prominent peak at 438 °C can be observed, signifying the maximum thermal decomposition rate of the sample at this temperature. This intensive mass loss is mainly attributed to the decomposition and volatilization of organic macromolecular substances into small molecular substances.46 In the temperature range of 465–600 °C, there are three small peaks in the derivative thermogravimetry (DTG) curve, corresponding to 491, 530, and 567 °C, respectively. This stage is mainly for the formation of carbon ash and decomposition of some inorganic substances.47 Beyond 600 °C, the rate of weight loss stabilizes, indicating that the thermal decomposition of the material is nearly complete. Since the thermal decomposition behavior of C60 has been extensively studied previously,48–50 we did not investigate this point in the present work.To systematically investigate the thermal decomposition behavior of the waste module, gas analysis was then conducted using TG-MS. Fig. 4b presents the mass spectrum acquired at the DTG peak temperature (438 °C), revealing predominant characteristic ions corresponding to N+ (the mass/charge ratio (m/z) = 14), O+ (m/z = 16), N2 (m/z = 28), O2 (m/z = 32), and CO2 (m/z = 44).47 Furthermore, the inset diagram in Fig. 4b presents an enlarged view of the m/z 45–130 region. The observed characteristic peaks at m/z = 51, 77, and 105 exhibit a strong correlation with the standard characteristic peaks of the benzoic acid molecule (C6H5COOH). Additionally, the molecular ion peak at m/z = 122 corresponds to the C6H5COOH from PET substrate, providing definitive evidence for its identification.51,52 Fig. 4c–f show the ionic current curves of typical gas fragments with temperature, and the signal intensity is positively correlated with the concentration of the corresponding substance. Specifically, the ion current curve of m/z = 44 (CO2) with temperature (Fig. 4d) exhibits multi-stage release characteristics: an initial emission peak at 453 °C followed by secondary peaks at 497, 536, and 574 °C, which correspond well with the peak points of the weight loss rate on the DTG curve. This multi-step release mechanism is due to the differential thermal stability of the multilayer structure of the device. The primary CO2 arises from the deacetylation reaction of ethylene-vinyl acetate, the incineration product of PET, while subsequent emissions originate from further cleavage of remaining organic substances and oxidation of organic molecules such as PTAA, BCP, and C60 at elevated temperatures.53 Notably, weak signals of the ion current are detected near a temperature of 453 °C (Fig. 4e), which correspond to the thermal decomposition products m/z = 45 (C2H5O+) and 46 (NO2). The generation of NO2 is primarily due to the oxidation of the PTAA and BCP layers in the components, as well as the FA+ in the active layer. In addition, trace signals of m/z = 127 and 128 are detected near 467 °C (Fig. 4f), which correspond to ion peaks of I− and HI, respectively, which are obtained by thermal decomposition of the perovskite layer. The low ionic current values of these peaks indicate that halogen ion products from decomposition of the perovskite active layer account for a very low proportion in the tail gas.
TG analysis indicates that the incineration of the waste flexible module primarily occurs between 300 and 465 °C, with complete incineration occurring around 600 °C. TG-MS analysis reveals that the principal gaseous products generated during the incineration of waste flexible modules include CO2, NO2, C2H5OH, C6H5COOH, and HI. Notably, I2 was hypothesized as a potential major decomposition product, but its detection proved challenging through conventional MS due to its solidification inside the channel before reaching the detector.
To verify whether iodine vapor is produced during the incineration of the waste module, we further set up a simple detection test as shown in Fig. S2a. First, we cut the discarded flexible module into small pieces and put them in a crucible, which was then positioned inside a stainless-steel pipe (painted as a glass tube in the schematic diagram for clarity). The polytetrafluoroethylene piston T-shaped three-way joint can be connected to both the balloon and the stainless-steel pipe separately by using polyurethane (PU) tubes. The exhaust outlet was connected via the PU tubing to a conical flask containing a starch indicator. Before starting the heating, the T-valve was opened to introduce O2 from the balloon into the system. Then, the bottom of the stainless-steel pipe was continuously heated using a butane air gun. After about 2 minutes, the starch indicator in the conical flask visibly changed from white to purple, as shown in Fig. S2b and S2c, indicating that iodine vapor was indeed produced during the incineration process of the waste module, thus confirming our hypothesis.
2.3. Sustainable management and recycling
Through systematic analysis of incineration byproducts, we demonstrate that thermal treatment can serve as a sustainable disposal method for waste flexible perovskite modules, provided that exhaust gases and residual ash are properly managed like the incineration of household waste. The acidic exhaust gases can be effectively treated using established alkaline scrubbing systems containing NH3·H2O or Ca(OH)2 – a technology already widely implemented in municipal solid waste incineration facilities.The resulting ash, containing valuable rare metals (In) and toxic heavy metals (Pb), requires centralized processing with particular attention to preventing Pb contamination. While the economic feasibility of metal recovery remains a consideration, we propose a comprehensive recycling protocol for incineration residues from perovskite modules (Fig. 5). Our approach addresses two critical challenges simultaneously: (1) safe containment of hazardous Pb and (2) recovery of strategically important elements (In and Cu). Notably, the combustion process generates primarily acidic off-gases, which can be efficiently neutralized through absorption in alkaline solutions (e.g., aqueous ammonia). This integrated treatment strategy not only ensures environmental safety but also enables resource recovery from end-of-life PV devices. For the most metal ions in the ash, they can first be treated with an acidic solution (such as HNO3) to dissolve the components to form soluble Pb2+ and Cu2+, which can subsequently be precipitated as PbI2 (Ksp = 1.39 × 10−8) and CuI (Ksp = 1.1 × 10−12) by adding KI. Since the solubility of PbI2 increases significantly above 80 °C, a temperature gradient method can be used to separate PbI2 from CuI. By heating, most of the PbI2 dissolves, and the CuI precipitate is filtered off while still hot. The solution is then cooled to 0–4 °C to obtain PbI2 precipitate.54 As for In2O3, it can first be dissolved in an acid solution to form InCl3. Then, ammonia water is added to it to generate In(OH)3. Finally, a dehydration reaction at high temperature is carried out to produce pure In2O3. A lab-scale trial was carried out to verify the feasibility of the proposed recycling scheme. The specific experimental procedures and the analysis of the resulting products are presented in the SI.
 | ||
| Fig. 5 Schematic illustration of a proposed management strategy for incineration products of degraded flexible perovskite modules. | ||
Building upon the preceding analysis, the integrated incineration process, synergistically coupled with the hydrometallurgical recovery pathway outlined earlier, presents a viable and promising technological solution for the future large-scale treatment of waste flexible perovskite PV modules. This combined approach offers a tripartite advantage: first, it effectively decomposes organic constituents such as the polymer substrate and organic charge transport layers; second, it concentrates valuable (e.g., In and Cu) and potentially toxic metals (notably Pb) within the residual ash; and third, it enables the stabilization of hazardous Pb while facilitating the efficient, sequential resource recovery of critical elements like In, Cu, and I through tailored wet-chemical techniques. It is worth mentioning that all chemicals in this process are aqueous solutions, and no organic solvents (e.g., DMF or alcohols) are involved. Consequently, this integrated strategy significantly mitigates environmental hazards associated with landfilling or improper disposal, while simultaneously ensuring economic feasibility through the recuperation of valuable materials, thereby offering a holistic and sustainable end-of-life management solution.
3 Conclusions
In summary, this study presents the first systematic characterization and analysis of incineration products from waste flexible perovskite modules, providing comprehensive insights into material transformations and dynamic compositional evolution during thermal decomposition. Our findings demonstrate that controlled incineration, when accompanied by rigorous control measures for harmful gas and toxic substance emissions, represents an environmentally sound and economically viable disposal strategy for waste flexible perovskite modules. These findings are largely extendable to rigid perovskite modules through appropriate process optimization. This investigation not only establishes an empirical foundation for understanding incineration mechanisms in next-generation PV waste, but also proposes practical strategies and fundamental scientific principles to guide industrial-scale recycling of perovskite solar modules.4 Experimental section
4.1 Materials
PTAA (average Mn 7000–10,000), BCP, PbI2 (99.999% trace metals), dimethylformamide (DMF), 1-methyl-2-pyrrolidinone (NMP), and toluene were purchased from Sigma-Aldrich and used without further purification. C60 was purchased from Nanjing Zhiyan Inc. Formamidinium iodide (FAI), methylammonium chloride (MACl), and cesium iodide (CsI) were purchased from Greatcell. Flexible PET/ITO substrates were purchased from Langu Electronic Technology Co., Ltd.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This solution was subsequently diluted to obtain a final concentration of 1.1 M. The precursor solution was then blade-coated on the substrates coated with PTAA, utilizing a gap of 300 µm and a movement speed of 20 mm s−1. The nitrogen knife was operated at a pressure of 30 psi and a movement speed of 5 mm s−1 after the coating process. Following this, the perovskite films were annealed at 120 °C for 30 minutes in ambient air. C60 (30 nm at 0.25 Å s−1), BCP (6 nm at 0.1 Å s−1), and Cu (35 nm at 0.5 Å s−1) were then evaporated onto the perovskite layer sequentially. P2 lines were scribed with a laser marker (345 nm), and then the second layer of Cu was evaporated with a thickness of 130 nm. The flexible modules were completed with P3 line scribed with the same laser marker.
1. This solution was subsequently diluted to obtain a final concentration of 1.1 M. The precursor solution was then blade-coated on the substrates coated with PTAA, utilizing a gap of 300 µm and a movement speed of 20 mm s−1. The nitrogen knife was operated at a pressure of 30 psi and a movement speed of 5 mm s−1 after the coating process. Following this, the perovskite films were annealed at 120 °C for 30 minutes in ambient air. C60 (30 nm at 0.25 Å s−1), BCP (6 nm at 0.1 Å s−1), and Cu (35 nm at 0.5 Å s−1) were then evaporated onto the perovskite layer sequentially. P2 lines were scribed with a laser marker (345 nm), and then the second layer of Cu was evaporated with a thickness of 130 nm. The flexible modules were completed with P3 line scribed with the same laser marker.Author contributions
S. C. conceived the idea for the study and designed the experiments. X. S. fabricated flexible perovskite solar modules, and performed characterization of incineration products. Y. L., T. L. and S. L. assisted with the module fabrication. F. W. assisted with the data analysis of mass spectrometry. X. S. and S. C. wrote the manuscript with inputs from all co-authors. All authors discussed the results and reviewed the manuscript.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this article have been included in this article or as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc07083j.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2023YFB3809700), National Natural Science Foundation of China (No. 52372196), and the open research fund of Suzhou Laboratory (SZLAB-1308-2024-TS007).Notes and references
- X. Xiao, N. Xu, X. Tian, T. Zhang, B. Wang, X. Wang, Y. Xian, C. Lu, X. Ou, Y. Yan, L. Sun, F. You and F. Gao, Nature, 2025, 638, 670–675 Search PubMed.
- N. Kannan and D. Vakeesan, Renewable Sustainable Energy Rev., 2016, 62, 1092–1105 CrossRef.
- H. H. Pourasl, R. V. Barenji and V. M. Khojastehnezhad, Energy Rep., 2023, 10, 3474–3493 Search PubMed.
- D. Sah, Chitra and S. Kumar, Sol. Energy Mater. Sol. Cells, 2022, 246, 111908 Search PubMed.
- M. S. J.-P. Correa-Baena, T. Buonassisi, M. Grätzel, A. Abate, W. Tress and A. Hagfeldt, Science, 2017, 358, 739–744 Search PubMed.
- Y. Rong, Y. Hu, A. Mei, H. Tan, M. I. Saidaminov, S. I. Seok, M. D. McGehee, E. H. Sargent and H. Han, Science, 2018, 361, 1214 CrossRef CAS PubMed.
- R. Deng, N. L. Chang, Z. Ouyang and C. M. Chong, Renewable Sustainable Energy Rev., 2019, 109, 532–550 Search PubMed.
- R. Vinayagamoorthi, P. B. Bhargav, N. Ahmed, C. Balaji, K. Aravinth, A. Krishnan, R. Govindaraj and P. Ramasamy, J. Environ. Chem. Eng., 2024, 12, 111715 Search PubMed.
- D. Sah and S. Kumar, Sol. Energy, 2024, 273, 112534 CrossRef CAS.
- Y. Xu, J. Li, Q. Tan, A. L. Peters and C. Yang, Waste Manage., 2018, 75, 450–458 CrossRef PubMed.
- S. Verma, T. Lee, E. Sahle-Demessie, M. Ateia and M. N. Nadagouda, Chem. Eng. J. Adv., 2023, 13, 100421 Search PubMed.
- J.-K. Lee, J.-S. Lee, Y.-S. Ahn, G.-H. Kang, H.-E. Song, J.-I. Lee, M.-G. Kang and C.-H. Cho, Sol. Energy Mater. Sol. Cells, 2017, 160, 301–306 CrossRef CAS.
- V. Savvilotidou, A. Antoniou and E. Gidarakos, Waste Manage., 2017, 59, 394–402 CrossRef CAS PubMed.
- P.-H. Chen, W.-S. Chen, C.-H. Lee and J.-Y. Wu, Sustainability, 2023, 16, 60 CrossRef.
- M. Tao, V. Fthenakis, B. Ebin, B. M. Steenari, E. Butler, P. Sinha, R. Corkish, K. Wambach and E. S. Simon, Prog. Photovoltaics Res. Appl., 2020, 28, 1077–1088 CrossRef CAS.
- J. Tao and S. Yu, Sol. Energy Mater. Sol. Cells, 2015, 141, 108–124 CrossRef CAS.
- F. Ardente, C. E. L. Latunussa and G. A. Blengini, Waste Manage., 2019, 91, 156–167 Search PubMed.
- P. R. Dias, L. Schmidt, N. L. Chang, M. Monteiro Lunardi, R. Deng, B. Trigger, L. Bonan Gomes, R. Egan and H. Veit, Renewable Sustainable Energy Rev., 2022, 169, 112900 CrossRef CAS.
- G. A. Heath, T. J. Silverman, M. Kempe, M. Deceglie, D. Ravikumar, T. Remo, H. Cui, P. Sinha, C. Libby, S. Shaw, K. Komoto, K. Wambach, E. Butler, T. Barnes and A. Wade, Nat. Energy, 2020, 5, 502–510 CrossRef CAS.
- T. Abzieher, D. T. Moore, M. Roß, S. Albrecht, J. Silvia, H. Tan, Q. Jeangros, C. Ballif, M. T. Hoerantner, B.-S. Kim, H. J. Bolink, P. Pistor, J. C. Goldschmidt, Y.-H. Chiang, S. D. Stranks, J. Borchert, M. D. McGehee, M. Morales-Masis, J. B. Patel, A. Bruno and U. W. Paetzold, Energy Environ. Sci., 2024, 17, 1645–1663 RSC.
- L. Duan, D. Walter, N. Chang, J. Bullock, D. Kang, S. P. Phang, K. Weber, T. White, D. Macdonald, K. Catchpole and H. Shen, Nat. Rev. Mater., 2023, 8, 261–281 CrossRef CAS.
- M. Tammaro, J. Rimauro, V. Fiandra and A. Salluzzo, Renewable Energy, 2015, 81, 103–112 CrossRef CAS.
- M. Akhter, A. Al Mansur, M. I. Islam, M. S. H. Lipu, T. F. Karim, M. G. M. Abdolrasol and T. A. H. Alghamdi, Sustainability, 2024, 16, 5785 CrossRef CAS.
- S. Chen, Y. Deng, H. Gu, S. Xu, S. Wang, Z. Yu, V. Blum and J. Huang, Nat. Energy, 2020, 5, 1003–1011 CrossRef.
- H. E. Al-Hazmi, G. K. Hassan, T. A. Kurniawan, B. Śniatała, T. M. Joseph, J. Majtacz, G. Piechota, X. Li, F. A. El-Gohary, M. R. Saeb and J. Mąkinia, J. Environ. Manage., 2024, 354, 120414 CrossRef CAS PubMed.
- X. Yu, Q. Sui, S. Lyu, W. Zhao, X. Cao, J. Wang and G. Yu, Environ. Int., 2020, 135, 105404 Search PubMed.
- H. I. Abdel-Shafy, A. M. Ibrahim, A. M. Al-Sulaiman and R. A. Okasha, Ain Shams Eng. J., 2024, 15, 102293 Search PubMed.
- R. Kumar, A. Verma, A. Shome, R. Sinha, S. Sinha, P. K. Jha, R. Kumar, P. Kumar, Shubham, S. Das, P. Sharma and P. V. Vara Prasad, Sustainability, 2021, 13, 9963 CrossRef CAS.
- D. Cudjoe and H. Wang, Fuel Process. Technol., 2022, 237, 107470 Search PubMed.
- X. Zhang and B. Liu, Sustainability, 2024, 16, 3579 CrossRef.
- X. Wang, J. Li, R. Guo, X. Yin, R. Luo, D. Guo, K. Ji, L. Dai, H. Liang, X. Jia, J. Chen, Z. Jia, Z. Shi, S. Liu, Y. Wang, Q. Zhou, T. Wang, G. Pan, P. Müller-Buschbaum, S. D. Stranks and Y. Hou, Nat. Photonics, 2024, 18, 1269–1275 CrossRef CAS.
- J. Chen, X. Wang, T. Wang, J. Li, H. Y. Chia, H. Liang, S. Xi, S. Liu, X. Guo, R. Guo, Z. Jia, X. Yin, Q. Zhou, Y. Wang, Z. Shi, H. Zhou, D. Lai, M. Zhang, Z. Xing, W. R. Leow, W. Yan and Y. Hou, Nat. Energy, 2024, 10, 181–190 Search PubMed.
- G. Wang, D. Xu, J. Tang, B. Liu, Z. Wang, Q. Xu, Y. Hu, J. Zhou and S. Wang, Combust. Flame, 2023, 255, 112909 Search PubMed.
- G. Guo, K. Fan, Z. Guo and W. Guo, Energy, 2023, 280, 128202 CrossRef CAS.
- J. Tang, L. Liu, Z. Yu, J. Du, X. Cai, M. Zhang, M. Zhao, L. Bai, Z. Gai, S. Cui, X. Li and T. Jiu, Adv. Sustainable Syst., 2022, 6, 2100510 CrossRef CAS.
- S. Rades, F. Oswald, S. Narbey, J. Radnik and V. D. Hodoroaba, Surf. Interface Anal., 2018, 50, 1234–1238 CrossRef CAS.
- C. Das, M. Wussler, T. Hellmann, T. Mayer and W. Jaegermann, Phys. Chem. Chem. Phys., 2018, 20, 17180–17187 RSC.
- W. Li, J. Fan, J. Li, Y. Mai and L. Wang, J. Am. Chem. Soc., 2015, 137, 10399–10405 CrossRef CAS PubMed.
- J. Zhang, S. Cao, L. Gong, H. He, Z. Fang, C. Zhou and J. Chen, Mater. Sci. Semicond. Process., 2023, 163, 107564 CrossRef CAS.
- X. Jiang, K. Dong, P. Li, L. Zheng, B. Zhang, Y. Yin, G. Yang, L. Wang, M. Wang, S. Li, L. Zhu, S. Niu, S. Yu, S. Liu, W. Tian, X. Guo, M. Wei, S. M. Zakeeruddin, L. Sun, S. Pang and M. Grätzel, Angew. Chem., Int. Ed., 2024, 64, e202414128 CrossRef PubMed.
- C. Nunes de Carvalho, A. M. Botelho do Rego, A. Amaral, P. Brogueira and G. Lavareda, Surf. Coat. Technol., 2000, 124, 70–75 CrossRef CAS.
- S. Lædre, L. Mendizabal, O. E. Kongstein, A. Oedegaard, H. Karoliussen and F. Seland, J. Electrochem. Soc., 2022, 169, 034504 CrossRef.
- Y. Li, G. Zhao, X. Zhi and T. Zhu, Surf. Interface Anal., 2007, 39, 756–760 Search PubMed.
- Y. Li, J. Zhao, J. Han and X. He, Mater. Res. Bull., 2005, 40, 981–989 Search PubMed.
- J. Yang, M. Huang, S. Wang, X. Mao, Y. Hu and X. Chen, Water, 2020, 12, 3583 Search PubMed.
- L. A. Carvalho, J. D. Ardisson, R. M. Lago, M. D. Vargas and M. H. Araujo, RSC Adv., 2015, 5, 97248–97255 RSC.
- M. Zhong, J. Li, L. Zhou, T. Wang, J. Liu, M. Mei and S. Chen, J. Anal. Appl. Pyrolysis, 2023, 172, 106002 Search PubMed.
- Z. Wu, X. Cai and Z. Yang, J. Nanopart. Res., 2015, 17, 329 CrossRef.
- H. Gaspar, L. Fernandes, P. Pereira and G. Bernardo, Polym. Bull., 2015, 72, 1775–1786 CrossRef CAS.
- S. Aghili, M. Panjepour and M. Ghiaci, Diamond Relat. Mater., 2022, 124, 108943 CrossRef CAS.
- A. Tadashi, I. Shoji, N. Hideaki and F. Nobuyuki, Thermochim. Acta, 1997, 319, 139–149 Search PubMed.
- T. Ma, R. Wang, W. Wang, W. Gu, Y. Yuan, A. Zhang and J. Wei, Polym. Degrad. Stab., 2022, 206, 110185 CrossRef CAS.
- Z. Sebestyén, E. Barta-Rajnai, J. Bozi, M. Blazsó, E. Jakab, N. Miskolczi and Z. Czégény, Energy Procedia, 2017, 105, 718–723 CrossRef.
- X. Wang, B. Dong, M. Feng, D.-J. Xue and S.-M. Wang, J. Mater. Chem. A, 2022, 10, 15861–15864 RSC.
| This journal is © The Royal Society of Chemistry 2026 |