Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Glycofullerene–corrole hybrids: a new class of multifunctional nanomaterials with potential in targeted photodynamic therapy
Jennifer Patino-Alonsoabc,
Carla I. M. Santos *bc,
Adriana F. Cruzd,
Sandra Pinto
*bc,
Adriana F. Cruzd,
Sandra Pinto d,
Justo Cabrera-González
d,
Justo Cabrera-González ae,
M. Amparo F. Faustino
ae,
M. Amparo F. Faustino c,
M. Graça P. M. S. Neves
c,
M. Graça P. M. S. Neves c,
Ermelinda M. S. Maçôas
c,
Ermelinda M. S. Maçôas *b,
Nazario Martín
*b,
Nazario Martín *af and
Beatriz M. Illescas
*af and
Beatriz M. Illescas *a
*a
aDepartamento de Química Orgánica, Facultad de Química, Universidad Complutense, E-28040 Madrid, Spain. E-mail: beti@ucm.es; nazmar@ucm.es
bCentro de Química Estrutural, Institute of Molecular Sciences, Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisboa, Portugal. E-mail: carla.santos@tecnico.ulisboa.pt
cLAQV-REQUIMTE and Department of Chemistry, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal
diBB-Institute for Bioengineering and Biosciences, Instituto Superior Técnico, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
eDepartamento de Química en Ciencias Farmacéuticas, Facultad de Farmacia, Universidad Complutense, E-28040 Madrid, Spain
fIMDEA-Nanoscience, Campus de Cantoblanco, C/ Faraday 9, 28049 Madrid, Spain
First published on 8th December 2025
Abstract
Corrole photosensitizers for photodynamic therapy (PDT) have garnered significant attention due to their promising potential in cancer treatment. Advances in understanding their photophysical and photochemical properties have facilitated the development of more efficient and targeted PDT strategies. This study reports the synthesis and characterization of a series of alkyne-substituted gallium(III) corrole complexes, as well as their conjugation to azide-functionalized glycofullerenes via copper-catalyzed azide–alkyne cycloaddition (CuAAC) reactions. The resulting glycofullerene–corrole conjugates were obtained and fully characterized using standard spectroscopic techniques. Their photodynamic efficacy was evaluated in vitro using HeLa cells. Among the series, the mono- and tris-alkyne-substituted corroles, as well as the monofunctionalized glycofullerene conjugate, exhibited the most potent PDT effects in cells, achieving IC50 values below 1.0 µM under blue irradiation at 420 nm with a total light dose as low as 5 J cm−2. These findings highlight the potential of gallium(III) corrole-based nanostructures as water-soluble and efficient photosensitizers for PDT applications.
1. Introduction
Cancer's high mortality rate makes it one of the most challenging and fatal diseases globally. According to the latest estimates from the International Agency for Research on Cancer GLOBOCAN 2022 database, approximately 20 million new cases of cancer and close to 10 million cancer deaths were estimated worldwide in 2022.1 As a result, there is an urgent need for novel diagnostic techniques and effective treatments to address this complex disease. In contrast to traditional therapies such as surgery, radiotherapy, and chemotherapy, photodynamic therapy (PDT) has garnered significant attention due to its ability to achieve precise spatiotemporal control, low invasiveness, and minimal side effects. PDT is based on the topical or systemic application of a dye known as a photosensitizer (PS), that, when locally activated by light of an appropriate wavelength in the presence of dioxygen (3O2), produces reactive oxygen species (ROS) that trigger a sequence of chemical and photobiological reactions, capable of inducing selective cell death.2–4 Ideally, the PS should exhibit absorption within the therapeutic window (620–850 nm), possess high photostability, and present selective uptake and accumulation in cancer cells with minimal dark toxicity.5 Corroles, porphyrinoids characterized by the absence of one methine (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) bridge compared to porphyrins, have emerged as promising PS for PDT.6–12 These contracted tetrapyrrolic macrocycles show unique properties, such as low symmetry, high molar extinction coefficients, fluorescence emission within the therapeutic window, and ability to generate singlet oxygen (1O2), which make them attractive therapeutic agents for PDT.13–15 Among them, gallium(III) corroles have demonstrated effectiveness in tumor detection and therapy, due to their relatively high fluorescence quantum yield and efficient ROS generation. The insertion of gallium(III) into the corrole core enhances the rate of intersystem crossing (ISC) and promotes the efficient generation of 1O2 (ref. 10). In early studies, developed by Gross et al., it was demonstrated that sulfonated gallium(III) corroles functionalized with protein carriers (HerGa) are efficient in PDT and fluorescence imaging. The resulting macrocycles exhibited good cytotoxicity against several cancer cell lines and generated superoxide, which led to disruption of the cytoskeleton and mitochondria.16,17 In 2018, Zeng et al. reported the synthesis of the gallium(III) complex of 5,10,15-tris(ethoxycarbonyl)corrole and its coupling with a monoclonal antibody (mAb) targeting CT83. The obtained conjugate displayed efficient photodynamic activity for the selective treatment of CT83-expressing cancer.18 Recently, the group of Liu et al. demonstrated that gallium(III) corroles with various substituents such as hydroxyl groups, coumarins and azides, could induce cancer cell apoptosis, due to the increased intracellular ROS and the disruption of the mitochondrial membrane potential.8,19,20 Despite the previously mentioned advances in the development of corroles as PS, some obstacles to their progress are still present, such as their low water solubility, as well as their lack of selective delivery and specificity to cancer cells. Conjugation of corroles to nanomaterials has been explored as a strategy to address these challenges.21–23 Soy et al. reported the synthesis and characterization of phosphorus(V) and gallium(III) complexes of an A3 triarylcorrole with 4-methylthiophenyl meso-groups, as well as the formation of new conjugates resulting from their coupling with gold nanoparticles (AuNPs). The photodynamic activities of the resulting hybrid nanomaterials were assessed in MCF-7 breast cancer cells. Upon conjugation to AuNPs, the fluorescence quantum yields of the phosphorus(V) and gallium(III) corroles decreased, while the 1O2 quantum yields increased due to an external heavy atom effect. In addition, the P(V) complex and their AuNP conjugates displayed more favorable PDT activity than gallium(III) derivatives.10 Conjugation of corroles with biocompatible carbon nanostructures, as graphene quantum dots or nanodiamonds, have also been studied. In these conjugates, the photoluminescence properties are additive with respect to the absorption and emission characteristics of the constituent moieties. The cellular uptake of the hybrids is confirmed and therefore they hold promise for different biological applications, as fluorescent labels, photodynamic and photothermal therapy agents or cancer theragnostic platforms for bioimaging.24,25
CH–) bridge compared to porphyrins, have emerged as promising PS for PDT.6–12 These contracted tetrapyrrolic macrocycles show unique properties, such as low symmetry, high molar extinction coefficients, fluorescence emission within the therapeutic window, and ability to generate singlet oxygen (1O2), which make them attractive therapeutic agents for PDT.13–15 Among them, gallium(III) corroles have demonstrated effectiveness in tumor detection and therapy, due to their relatively high fluorescence quantum yield and efficient ROS generation. The insertion of gallium(III) into the corrole core enhances the rate of intersystem crossing (ISC) and promotes the efficient generation of 1O2 (ref. 10). In early studies, developed by Gross et al., it was demonstrated that sulfonated gallium(III) corroles functionalized with protein carriers (HerGa) are efficient in PDT and fluorescence imaging. The resulting macrocycles exhibited good cytotoxicity against several cancer cell lines and generated superoxide, which led to disruption of the cytoskeleton and mitochondria.16,17 In 2018, Zeng et al. reported the synthesis of the gallium(III) complex of 5,10,15-tris(ethoxycarbonyl)corrole and its coupling with a monoclonal antibody (mAb) targeting CT83. The obtained conjugate displayed efficient photodynamic activity for the selective treatment of CT83-expressing cancer.18 Recently, the group of Liu et al. demonstrated that gallium(III) corroles with various substituents such as hydroxyl groups, coumarins and azides, could induce cancer cell apoptosis, due to the increased intracellular ROS and the disruption of the mitochondrial membrane potential.8,19,20 Despite the previously mentioned advances in the development of corroles as PS, some obstacles to their progress are still present, such as their low water solubility, as well as their lack of selective delivery and specificity to cancer cells. Conjugation of corroles to nanomaterials has been explored as a strategy to address these challenges.21–23 Soy et al. reported the synthesis and characterization of phosphorus(V) and gallium(III) complexes of an A3 triarylcorrole with 4-methylthiophenyl meso-groups, as well as the formation of new conjugates resulting from their coupling with gold nanoparticles (AuNPs). The photodynamic activities of the resulting hybrid nanomaterials were assessed in MCF-7 breast cancer cells. Upon conjugation to AuNPs, the fluorescence quantum yields of the phosphorus(V) and gallium(III) corroles decreased, while the 1O2 quantum yields increased due to an external heavy atom effect. In addition, the P(V) complex and their AuNP conjugates displayed more favorable PDT activity than gallium(III) derivatives.10 Conjugation of corroles with biocompatible carbon nanostructures, as graphene quantum dots or nanodiamonds, have also been studied. In these conjugates, the photoluminescence properties are additive with respect to the absorption and emission characteristics of the constituent moieties. The cellular uptake of the hybrids is confirmed and therefore they hold promise for different biological applications, as fluorescent labels, photodynamic and photothermal therapy agents or cancer theragnostic platforms for bioimaging.24,25
In the present study, the synthesis of alkyne-substituted corroles and their subsequent conjugation with glycofullerene derivatives through copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction is explored. While numerous glyco-conjugates of porphyrinoids with carbohydrates have been studied,26,27 examples involving glycocorroles remain scarce.28,29 The conjugation of corroles with glycofullerenes is anticipated to enhance their biocompatibility, water solubility and reduce cytotoxicity, making the resulting materials suitable for biomedical applications.30–32 Carbohydrates are not only expected to increase water solubility, as they also play important biological roles. In our previous work, porphyrins functionalized with four glycofullerene moieties showed an efficient inhibition of SARS-CoV-2 trans-infection process by competitively interacting with DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin).33 Glycofullerenes themselves have demonstrated significant anti-proliferative effects against various cancer cell lines.34,35 These sugar-functionalized fullerene derivatives exhibit enhanced selectivity toward malignant cells, potentially improving therapeutic efficacy while minimizing off-target effects. In some cases, they also enable targeted delivery of the fullerene core to tumor tissues. This work aims to develop a synthetic protocol of a series of novel glycofullerene–corrole conjugates and determine their photochemical and photophysical properties, as well as their PDT activity toward HeLa tumor cells.
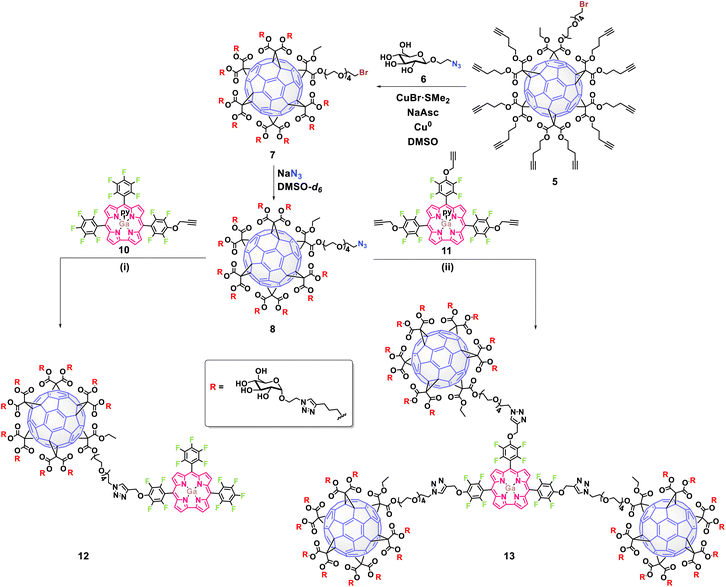
2. Results and discussion
The synthetic strategy to obtain the new glycofullerene–corrole conjugates 12 and 13 (Scheme 1) was based on a click-chemistry methodology, following our previously reported synthetic protocol.33 The approach aimed to create an asymmetric [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] fullerene hexakis-adduct, designed for orthogonal functionalization, which allowed for the initial attachment to carbohydrates followed by subsequent linkage to corroles (Scheme S1). The synthesis began with the esterification of monobromo-pentaethylene glycol (PEG 1) (Fig. S1 and S2) with ethyl malonyl chloride, followed by stepwise Bingel–Hirsch cyclopropanation reactions of the resulting malonate 2 (Fig. S3 and S4) and di(pent-4-yn-1-yl) malonate 4 with fullerene C60, affording the hexakis-adduct 5 in good yield (71%) (Fig. S5–S7). Glucose-azide derivative 6 was then attached to complementary alkynes in 5 through a CuAAC reaction, followed by the elimination of copper to prevent interference in biological studies. Finally, the nucleophilic substitution of terminal bromine in the resulting intermediate 7 (Fig. S8–S10) with sodium azide under microwave (MW) irradiation afforded the building block 8 in high yield (93%) (Fig. S8–S13).
1] fullerene hexakis-adduct, designed for orthogonal functionalization, which allowed for the initial attachment to carbohydrates followed by subsequent linkage to corroles (Scheme S1). The synthesis began with the esterification of monobromo-pentaethylene glycol (PEG 1) (Fig. S1 and S2) with ethyl malonyl chloride, followed by stepwise Bingel–Hirsch cyclopropanation reactions of the resulting malonate 2 (Fig. S3 and S4) and di(pent-4-yn-1-yl) malonate 4 with fullerene C60, affording the hexakis-adduct 5 in good yield (71%) (Fig. S5–S7). Glucose-azide derivative 6 was then attached to complementary alkynes in 5 through a CuAAC reaction, followed by the elimination of copper to prevent interference in biological studies. Finally, the nucleophilic substitution of terminal bromine in the resulting intermediate 7 (Fig. S8–S10) with sodium azide under microwave (MW) irradiation afforded the building block 8 in high yield (93%) (Fig. S8–S13).
The synthesis of alkyne-substituted corroles 10 and 11 involved the controlled nucleophilic substitution of the para-fluorine atoms of the gallium(III) complex of 5,10,15-tris(pentafluorophenyl)corrole (9) with propargyl alcohol (Scheme S2). By adjusting the equivalents of propargyl alcohol and the reaction conditions, the monosubstituted (10) or trisubstituted (11) products were selectively obtained. Thus, using only a slight excess of propargyl alcohol at 100 °C in DMSO for 3 h and K2CO3 as the base, the monosubstituted corroles 10 were preferentially obtained. Differently from our previous reported glycol substituted corroles,24 the alkyne functionalized corroles 10a and 10b were obtained as an inseparable mixture of isomers by flash chromatography or preparative TLC. However, both regioisomers could be distinguished by 1H NMR and 19F NMR spectroscopy (Fig. S14–S17) revealing that 10a and 10b are present in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively.
1 ratio, respectively.
To obtain the triply functionalized corrole 11, a slight excess of propargyl alcohol, previously treated with NaH, was added to the fluorinated corrole 9 and the reaction mixture was kept under reflux in THF for 1 h. After work-up and chromatographic purification, the desired derivative was isolated in 56% yield (Fig. S18–21).
The conjugation of alkyne-substituted Ga(III) corrole complexes to the azide-functionalized glycofullerene 8 was performed via CuAAC reactions (Scheme 1). Under these conditions, the glycofullerene–corrole conjugate 12 was obtained using the inseparable regioisomers 10a and 10b, while conjugate 13 was obtained in the presence of corrole 11. The resulting conjugates 12 and 13, which contain 10 and 30 glucose units, respectively, were isolated with yields exceeding 70% (Fig. S22–S29).
The characterization of these new nanostructures was carried out by standard spectroscopic techniques. Fullerene hexakis-adducts can be easily characterized using 13C NMR due to the high symmetry of the molecule with the appearance of only two signals corresponding to the sp2 carbons of the C60 core at ∼140 and 145 ppm (Fig. S6). In the case of derivative 7, its correct functionalization with glucose is unequivocally confirmed through 13C NMR by the appearance of the signal at ∼102 ppm assigned to the anomeric carbon of the glucose moiety and two new signals at ∼123 and 146 ppm corresponding to the new triazole rings formed during the click reaction (Fig. S9).
The substitution of bromine by azide to obtain compound 8 can also be tracked down by 13C NMR under the disappearance of the corresponding C–Br signal at ∼32 ppm and the appearance of the new C–N3 resonance at ∼50 ppm (Fig. S12). Additionally, using infrared spectroscopy, the appearance of the azido band at 2108 cm−1 can be detected (Fig. S13).
Regarding the characterization of the new glycofullerene–corrole conjugates 12 and 13, 19F NMR spectra show signals corresponding to fluorine atoms from the corrole moiety, indicating successful functionalization (Fig. 1, S24 and S28). Moreover, the 1H NMR spectra show new peaks in the aromatic region corresponding to the resonance of β-H of the corrole core and a broad singlet at ∼5.7 ppm corresponding to the CH2 of the propargyl linker (Fig. S22 and S26). Additionally, two signals corresponding to the two different triazole rings formed in the click reaction are also observed, one at δ ∼ 8.5 ppm integrating for 1H in compound 12 and 3H in compound 13, and another at δ ∼ 7.9 ppm integrating for 10H and 30H for compounds 12 and 13 respectively, as expected, considering the number of fullerene units in each hybrid. Once again, 13C NMR allows the complete characterization of the molecules. The disappearance of the C–N3 signal of the starting glycofullerene 8, accompanied by the emergence of two distinct signals corresponding to the resonance of each type of triazole ring in the structure (δ ∼ 146 ppm and 123 ppm for the triazole ring of the glycofullerene moiety and δ ∼ 146 ppm and 126 ppm for the triazole ring connecting the corrole with the glycofullerenes) as well as minor signals in the aromatic region, confirm both the presence and correct functionalization of the corroles (Fig. 1, S23 and S27). Despite comprehensive NMR characterization, the molecular ion peaks of compounds 12 and 13 could not be observed in their mass spectra, likely due to the extensive fragmentation typically associated with these compounds.30
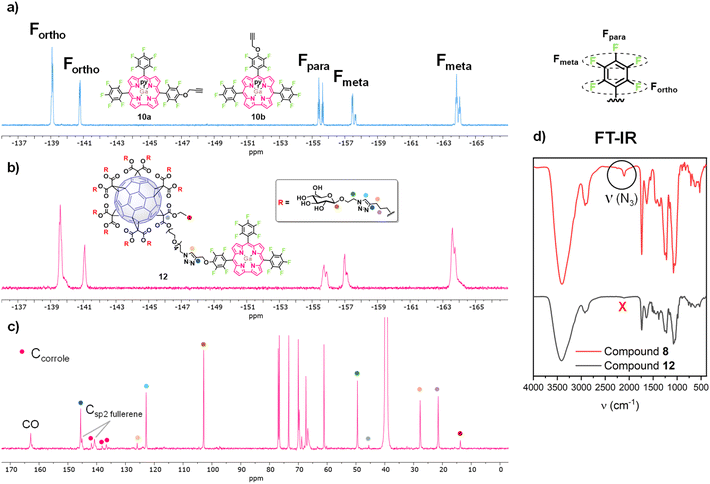 | ||
| Fig. 1 (a) 19F NMR spectrum of 10 (CD2Cl2, 471 MHz). (b) 19F NMR spectrum of 12 (CD2Cl2, 471 MHz). (c) Partial view of the 13C NMR spectrum of 12 (DMSO-d6, 176 MHz). (d) FT-IR of 8 and 12. | ||
DOSY (Diffusion-Ordered NMR Spectroscopy) NMR experiments (∼2 mg mL−1 in D2O, Fig. S30 and S31) and DLS (Dynamic Light Scattering) measurements (Fig. S32 and S33) were carried out to estimate the overall molecular size of conjugates 12 and 13. These techniques enable the calculation of the hydrodynamic radius (RH) of the molecules. Molecular weights (MW), diffusion coefficient (D) and hydrodynamic radius (RH) values are shown in Table 1. It can be observed that there is a correlation between the molecular weight and the diffusion coefficient, where an increase in the size of the molecule between 12 (RH = 38.9 Å) and 13 (RH = 42.1 Å) is observed as anticipated.
| Compound | Molecular weight (MW) | Diffusion coefficient (D, m2 s−1) | Hydrodynamic radii (RH, Å) |
|---|---|---|---|
| 12 | 5658.70 | 6.30 × 10−11 | 38.9 |
| 13 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249.75 249.75 |
5.82 × 10−11 | 42.1 |
2.1 Photophysical studies
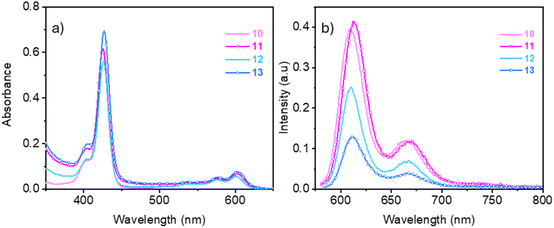
The photophysical characterization of the glycofullerene–corrole conjugates 12 and 13 was performed in water, DMF and DMSO at room temperature and the spectra of their precursors 10 and 11 were evaluated in DMSO and DMF. The absorption and emission spectra of all compounds in DMSO are shown in Fig. 2 and in the remaining solvents are depicted in Fig. S34 and S35. The recorded spectroscopic data are summarized in Table 2. No significant spectral differences were observed across the various solvents evaluated. All compounds exhibit the typical absorption spectrum of corrole, including a strong Soret band at ∼425 nm and several weak Q bands between 500–660 nm.36 The UV-Vis absorption spectra of the glycofullerene–corrole conjugates 12 and 13 appear dominated by the absorbance of the corrole unit with no major shifts observed upon conjugation to the glycofullerene. This observation suggests that there is no strong interaction between the two moieties in the ground state.| Comp. | Solvent | Soret/nm (ε/103 M−1 cm−1) | Q-Bands/nm (ε/103 M−1 cm−1) | λmaxem/nm | Stokes shift (nm) (Q2-λmaxem) | ϕFa (%) | ΦΔb (%) | τr (ns) |
|---|---|---|---|---|---|---|---|---|
| a Fluorescence quantum yields (ΦF) were measured by the reference method using a cresyl violet perchlorate solution in methanol (ΦF = 0.54) as ref. 45. The quantum yields in water are not reported because a reduction by two orders of magnitude was observed.b Singlet oxygen quantum yields (ΦΔ) were determined with TPP in DMF (ΦΔ = 0.65) as ref. 46. | ||||||||
| 10 | DMF | 423 (228.7) | 574 (17.7) | 605 | 7 | 8.9 | 60 | 1.8 |
| 598 (24.6) | 660 | |||||||
| DMSO | 425 (219.9) | 575 (17.7) | 608 | 9 | 7.8 | — | — | |
| 599 (20.1) | 665 | |||||||
| 11 | DMF | 424 (231.5) | 574 (17.6) | 608 | 8 | 9.9 | 65 | 1.8 |
| 600 (23.9) | 663 | |||||||
| DMSO | 427 (231.7) | 576 (18.7) | 612 | 9 | 8.7 | — | — | |
| 603 (22.8) | 666 | |||||||
| 12 | DMF | 424 (212.6) | 575 (15.6) | 609 | 10 | 2.3 | 61 | 1.7 |
| 599 (20.3) | 661 | |||||||
| DMSO | 426 (207.2) | 576 (16.6) | 610 | 9 | 1.6 | — | — | |
| 601 (19.9) | 668 | |||||||
| H2O | 423 (114.0) | 574 (8.2) | 606 | 11 | — | — | — | |
| 595 (12.2) | 657 | |||||||
| 13 | DMF | 425 (215.3) | 576 (17.1) | 611 | 9 | 1.2 | 30 | 0.7 |
| 602 (25.4) | 666 | |||||||
| DMSO | 427 (244.5) | 577 (20.3) | 612 | 8 | 1.4 | — | — | |
| 604 (26.5) | 666 | |||||||
| H2O | 425 (222.1) | 576 (22.9) | 606 | 8 | — | — | — | |
| 598 (29.0) | 660 | |||||||
The starting corroles 10 and 11, along with the glycofullerene conjugates derivatives 12 and 13 show fluorescence emission values peaking at ca 610 nm (Fig. 2b and Table 2) characteristic of the corrole unit. The glycofullerene moieties in conjugates 12 and 13 do not contribute to the observed emission spectra. This observation aligns with the non-emissive nature of glycofullerene 8, confirming that the fluorescence properties of the conjugates are primarily attributed to the corrole component. As shown in Table 2, glycofullerene–corrole conjugates 12 and 13 and the starting alkyne corroles 10 and 11 have significant Stokes shift which make them suitable for application in bioimaging where self-quenching is minimized and the signal-to-noise ratio is also reduced.37
The fluorescence quantum yield in DMF of the alkyne substituted corroles 10 and 11 (ΦF = 8.9 and 9.9%) are significantly higher than those of the corresponding glycofullerene–corrole conjugates 12 and 13 (ΦF = 2.3 and 1.2% respectively) (Table 2). The radiative lifetime (tr) of the corrole 10 (1.8 ns) is only slightly affected by conjugation with glycofullerene in the conjugate 12 (1.7 ns) (Fig. S36). Conversely, radiative lifetime of corrole 11 (1.8 ns) decreases in glycofullerene–corrole conjugate 13 (0.7 ns), concomitantly with the reduction in the emission quantum yield. From the radiative lifetime and the fluorescence emission quantum yield, we can estimate the radiative relaxation rate (kr = ΦF/tr) and the non-radiative relaxation rate (knr = (1/tr) − kr). The emission lifetimes are largely determined by the non-radiative relaxation rates (5 × 108 to 14 × 108 s−1) that are one order of magnitude higher than the radiative relaxation rate (1× 107–5 × 107 s−1). Conjugation of a single glycofullerene unit increased only slightly the non-radiative relaxation rate that changed from 5.2 × 108 s−1 in 10 to 5.7 × 108 s−1 in 12. Conversely, conjugating simultaneously three glycofullerene units to corrole 11 increases the non-radiative relaxation rate by a factor of three, from 5 × 108 (11) to 14 × 108 s−1 (13). Internal conversion followed by vibrational cooling and/or intersystem crossing followed by singlet oxygen (1O2) generation are two of the possible competing non-radiative relaxation pathways that could be enhanced in the hybrids.
The 1O2 quantum yield (ΦΔ) in DMF of 10, 11, 12, and 13 were measured as 60, 65, 61 and 30%, respectively. The singlet oxygen generation remains as efficient in conjugate 12, bearing a single glycofullerene unit, as in the corroles 10 and 11. Upon conjugation of three glycofullerene units in conjugate 13, the ΦΔ value decreases by half relative to the alkyne functionalized corroles. The high local concentration of glycofullerenes around the corrole seems to contribute to reduce the singlet oxygen generation yield in conjugate 13. Fullerenes are well-known ROS generators under irradiation, but they are also recognized for their radical-scavenging properties in the ground state.38–42 Alternatively, the simultaneous conjugation of three glycofullerenes to the corrole could affect the external heavy atom effect promoted by gallium(III). Gallium(III) is a relatively heavy atom that enhances the rate of ISC to the triplet state from where 1O2 is generated.43,44 The decrease in the ΦΔ value of conjugate 13 allows us to exclude singlet oxygen generation as one of the pathways contributing to the increased non-radiative relaxation rate in the hybrids. Alternatively, efficient internal conversion and vibrational cooling facilitated by the increased density of vibrational states arising from the glycofullerene units seems to be a more relevant non-radiative relaxation mechanism.
2.2 Biological assays
Compounds 10, 11, and 12 exhibit IC50 values of 4.16 µM, 4.96 µM, and 4.42 µM, respectively, under irradiation at 1 J cm−2 (Table S1). Under a higher light dose of 5 J cm−2, their IC50 values are below 1.0 µM.
3. Experimental
3.1 Materials and methods
Reagents and solvents were obtained from commercial suppliers and used without further purification. All work-up and purification procedures were carried out with HPLC-grade solvents in air. Microwave reactions were performed in an Anton-Parr Monowave 300 microwave reactor. Flash chromatography was performed using Scharlab silica gel (230–400 mesh, 0.040–0.063 mm) and for gel filtration Sephadex G-25 or G-50 were purchased from Cytiva. Analytical thin layer chromatography (TLC) was performed using E. Merck silica gel 60 F254 precoated plates (0.25 mm). The developed chromatogram was analyzed by UV lamp (λ = 254 nm) charring with potassium permanganate as development reagent.1H, 13C and 19F NMR spectra were performed on a Bruker AVIII HD 300 MHz BACS-60 (1H: 300 MHz, 13C: 75 MHz), Bruker NEO 500 MHz (1H: 500 MHz, 19F: 471 MHz) and Bruker AVIII 700 MHz (1H: 700 MHz, 13C: 176 MHz) at 298 K using partially deuterated solvents as internal standards. Coupling constants (J) are expressed in Hertz (Hz) and chemical shifts (δ) in parts per million (ppm) relative to the solvent. Data are reported as follows: s = singlet, t = triplet, q = quartet, m = multiplet. IR spectra (cm−1) were recorded on a Spectrum 3™ FT-IR spectrometer. Mass analyses were carried out in a MALDI TOF/TOF Bruker Ultraflex using trans-2-[3-(4-tbutylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) or 1,8-dihydroxi-9,10-dihydroanthracen-9-one (dithranol) as matrixes. Hydrodynamic diameter measurements were acquired on a Dynamic Light Scattering (DLS) Zetasizer Nano ZS Zen 3600 (Malvern), working with an He–Ne laser operating at λ = 633 nm. The samples were recorded with a scattering angle of 173°. Measurements were made in a 1.0 cm path-length quartz cell. Solution samples were filtered through nylon Acrodisc syringe filters (Pall Life Sciences) with a 0.2 µm pore size.
3.2 Photophysical measurements
The UV-vis absorption spectra were recorded with a JASCO V-540 spectrophotometer. The photoluminescence spectra were obtained on a Horiba Jobin Yvon Fluorolog 3-22 spectrofluorimeter equipped with a 450 W xenon lamp.Fluorescence microscopy images were acquired using a Leica TCS-SP5 laser scanning confocal fluorescence microscope. The system is equipped with two continuous-wave lasers: an argon laser providing excitation lines at 458, 488, 496, and 514 nm, and a HeNe laser offering excitation at 633 nm. Additionally, a Ti:Sapphire pulsed laser (Mai Tai, Spectra-Physics) delivers tunable excitation in the 730–990 nm range, with a pulse width of 100 fs and a repetition rate of 82 MHz.
3.3 Synthesis
Compounds 1 (ref. 47) and 2 (ref. 48) were previously reported, but a different synthetic methodology was used in this work. Compounds 3,48 4,49 6,50 and 9 (ref. 51) were prepared according to previously reported procedures (Schemes S1 and S2).1H NMR (300 MHz, CDCl3) δ (ppm) = 3.81 (t, 3JH,H = 6.3 Hz, 2H; CH2CH2Br), 3.75–3.70 (m, 2H; HOCH2CH2O), 3.70–3.64 (m, 12H; CH2O), 3.63–3.59 (m, 2H; HOCH2CH2O), 3.47 (t, 3JH,H = 6.3 Hz, 2H; CH2Br), 2.34 (br s, 1H; OH).
13C NMR (75 MHz, CDCl3) δ (ppm) = 72.7 (OHCH2CH2O), 71.3 (CH2CH2Br), 70.7, 70.6 (CH2O), 61.8 (OHCH2CH2O), 30.4 (CH2Br).
1H NMR (300 MHz, CDCl3) δ (ppm) = 4.31–4.24 (m, 2H; COOCH2CH2O), 4.18 (q, 3JH,H = 7.2 Hz, 2H; CH2CH3), 3.78 (t, 3JH,H = 6.3 Hz, 2H; CH2CH2Br), 3.71–3.67 (m, 2H; COOCH2CH2O), 3.66–3.60 (m, 12H; CH2O), 3.45 (t, 3JH,H = 6.3 Hz, 2H; CH2Br), 3.38 (s, 2H; COCH2CO), 1.26 (t, 3JH,H = 7.2 Hz, 3H; CH3).
13C NMR (75 MHz, CDCl3) δ (ppm) = 166.7, 166.5 (CO), 71.3 (CH2CH2Br), 70.7, 70.6 (CH2O), 68.9 (COOCH2CH2O), 64.6 (COOCH2CH2O), 61.6 (CH2CH3), 41.6 (COCH2CO), 30.4 (CH2Br), 14.2 (CH3).
1H NMR (700 MHz, CDCl3) δ (ppm) = 4.60–4.11 (m, 24H; COOCH2), 3.76 (t, 3JH,H = 6.3 Hz, 2H; CH2CH2Br), 3.74–3.66 (m, 2H; COOCH2CH2O), 3.65–3.54 (m, 12H; CH2OPEG), 3.43 (t, 3JH,H = 6.3 Hz, 2H; CH2Br), 2.45–2.14 (m, 20H; COOCH2CH2CH2), 2.06–1.59 (m, 30H; COOCH2CH2CH2 and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 1.40–1.20 (m, 3H; CH3).
CH), 1.40–1.20 (m, 3H; CH3).
13C NMR (176 MHz, CDCl3) δ (ppm) = 163.7 (CO), 145.8, 141.0 (Csp2 fullerene), 82.5 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 78.4 (C
CH), 78.4 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 71.2 (CH2CH2Br), 70.6, 70.5 (CH2OPEG), 69.8 (C
CH), 71.2 (CH2CH2Br), 70.6, 70.5 (CH2OPEG), 69.8 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 69.0 (Csp3 fullerene), 68.6 (COOCH2CH2O), 65.8 (COOCH2CH2O), 65.4 (COOCH2CH2CH2), 63.0 (CH2CH3), 45.3, 39.4 (Cq bridge), 30.4 (CH2Br), 27.1 (COOCH2CH2CH2), 16.4, 15.1 (COOCH2CH2CH2), 14.1 (CH3).
CH), 69.0 (Csp3 fullerene), 68.6 (COOCH2CH2O), 65.8 (COOCH2CH2O), 65.4 (COOCH2CH2CH2), 63.0 (CH2CH3), 45.3, 39.4 (Cq bridge), 30.4 (CH2Br), 27.1 (COOCH2CH2CH2), 16.4, 15.1 (COOCH2CH2CH2), 14.1 (CH3).
FTIR-ATR (ν cm−1): 3293 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H), 2958 (C–H), 1739 (C
C–H), 2958 (C–H), 1739 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
1H NMR (700 MHz, DMSO-d6) δ (ppm) = 8.05 (s, 10H; CHtriazole), 5.10 (s, 10H; OH), 4.99 (s, 10H; OH), 4.94 (s, 10H; OH), 4.61–4.48 (m, 30H; OCH2CH2Nglu and OH), 4.44–4.25 (m, 22H; CH2CH3 and COOCH2CH2CH2), 4.24–4.18 (m, 12H; CH1glu and COOCH2CH2O), 4.11–4.05 (m, 10H; OCH2CH2Nglu), 3.93–3.85 (m, 10H; OCH2CH2Nglu), 3.70–3.64 (m, 12H; CH2OHglu and CH2OPEG), 3.56–3.53 (m, 4H; CH2Br and CH2OPEG), 3.52–3.46 (m, 10H; CH2OPEG), 3.45–3.40 (m, 12H; CH2OHglu and CH2OPEG), 3.16–3.10 (m, 20H; CHglu), 3.06–3.01 (m, 10H; CHglu), 2.99–2.93 (m, 10H; CHglu), 2.83–2.59 (m, 20H; COOCH2CH2CH2), 2.10–1.84 (m, 20H; COOCH2CH2CH2), 1.28–1.13 (m, 3H; CH3).
13C NMR (176 MHz, DMSO-d6) δ (ppm) = 162.8 (CO), 145.9 (Ctriazole), 145.0, 140.7 (Csp2 fullerene), 123.5 (CHtriazole), 102.8 (CH1glu), 76.9, 76.5, 73.2 (CHglu), 70.3 (CH2OPEG), 70.0 (CHglu), 69.7, 69.5 (CH2OPEG), 68.7 (Csp3 fullerene), 67.9 (COOCH2CH2O), 67.1 (OCH2CH2Nglu), 66.6 (COOCH2CH2CH2), 63.3 (CH2CH3), 61.0 (CH2OHglu), 49.9 (OCH2CH2Nglu), 45.5 (Cq bridge), 32.2 (CH2Br), 27.6 (COOCH2CH2CH2), 21.5 (COOCH2CH2CH2), 13.7 (CH3).
IR (KBr) (ν cm−1): 3401 (OH), 2920 (C–H), 1741 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 8 as a brown glassy solid (138 mg, 93%) (Fig. S11–S13).
1) to obtain 8 as a brown glassy solid (138 mg, 93%) (Fig. S11–S13).1H NMR (700 MHz, DMSO-d6) δ (ppm) = 7.91 (s, 10H; CHtriazole), 5.10 (s, 10H; OH), 4.98 (s, 10H; OH), 4.93 (s, 10H; OH), 4.53 (s, 10H; OH), 4.52–4.44 (m, 20H; OCH2CH2N), 4.42–4.24 (m, 24H; CH2CH3, COOCH2CH2CH2 and COOCH2CH2O), 4.21 (d, 3JH,H = 7.7 Hz, 10H; CH1glu), 4.08–4.03 (m, 10H; OCH2CH2N), 3.89–3.83 (m, 10H; OCH2CH2N), 3.70–3.63 (m, 12H; CH2OHglu and CH2OPEG), 3.58–3.56 (m, 2H; CH2OPEG), 3.53–3.46 (m, 10H; CH2OPEG), 3.45–3.41 (m, 12H; CH2OHglu and CH2OPEG), 3.36 (m, 2H; CH2N3 overlaps with water), 3.16–3.10 (m, 20H; CHglu), 3.06–3.02 (m, 10H; CHglu), 2.99–2.93 (m, 10H; CHglu), 2.75–2.58 (m, 20H; COOCH2CH2CH2), 2.09–1.87 (m, 20H; COOCH2CH2CH2), 1.28–1.15 (m, 3H; CH3).
13C NMR (176 MHz, DMSO-d6) δ (ppm) = 162.9 (CO), 145.6 (Ctriazole), 145.1, 140.7 (Csp2 fullerene), 122.8 (CHtriazole), 102.8 (CH1glu), 77.0, 76.6, 73.3, 70.0 (CHglu), 69.8, 69.7, 69.3 (CH2OPEG), 68.7 (Csp3 fullerene), 68.0 (COOCH2CH2O), 67.3 (OCH2CH2Nglu), 66.7 (COOCH2CH2CH2), 63.4 (CH2CH3), 61.1 (CH2OHglu), 50.0 (CH2N3), 49.5 (OCH2CH2Nglu), 45.6 (Cq bridge), 27.7 (COOCH2CH2CH2), 21.4 (COOCH2CH2CH2), 13.8 (CH3).
IR (KBr) (ν cm−1): 3401 (OH), 2921 (C–H), 2108 (N3), 1741 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02) to obtain the monosubstituted derivatives 10 as a fuchsia solid (5 mg, 16%). These derivatives were obtained as a 2
0.02) to obtain the monosubstituted derivatives 10 as a fuchsia solid (5 mg, 16%). These derivatives were obtained as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the inseparable regioisomers 10a and 10b (Fig. S14–S17).
1 mixture of the inseparable regioisomers 10a and 10b (Fig. S14–S17).1H NMR (500 MHz, CD2Cl2) δ (ppm) = 9.28 [d, 3JH,H = 4.0 Hz, 3H; CHβ (2H, 10a; 1H, ½ 10b)], 8.95 (d, 3JH,H = 4.6 Hz, 1H; CHβ, ½ 10b), 8.91 (d, 3JH,H = 4.3 Hz, 2H; CHβ, 10a), 8.88 (d, 3JH,H = 4.0 Hz, 1H; CHβ, ½ 10b), 8.84 (d, 3JH,H = 4.0 Hz, 2H; CHβ, 10a), 8.74 (d, 3JH,H = 4.6 Hz, 1H; CHβ, ½ 10b), 8.70 (d, 3JH,H = 4.3 Hz, 2H; CHβ, 10a), 6.69–6.61 [m, 1.5H; CHpara py (1H, 10a; 0.5H, ½ 10b)], 5.89–5.80 [m, 3H; CHmeta py (2H, 10a; 1H, ½ 10b)], 5.20 (d, 3JH,H = 2.4 Hz, 2H; OCH2, 10a), 5.18 (d, 3JH,H = 2.4 Hz, 1H; OCH2, ½ 10b), 2.98–2.86 [m, 4H; CHortho py (2H, 10a; 1H, ½ 10b) and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, 10a], 2.85 (t, 3JH,H = 2.4 Hz, 0.5H; C
CH, 10a], 2.85 (t, 3JH,H = 2.4 Hz, 0.5H; C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, ½ 10b).
CH, ½ 10b).
19F{1H} NMR (471 MHz, CD2Cl2) δ (ppm) = −138.86–−139.33 [m; Fortho (10-Fortho, 15-Fortho, 10a; 5-Fortho, 15-Fortho, 10b)], −140.78 [dd, 3JF,F = 23.8, 8.9 Hz; Fortho, (5-Fortho, 10a; 10-Fortho, 10b)], −155.34–−155.39 (m; Fpara, 10a), −155.64 (t, 3JF,F = 20.6 Hz; Fpara, 10b), −157.46 (dd, 3JF,F = 23.1, 8.6 Hz; 5-Fmeta, 10a), −157.64 (dd, 3JF,F = 24.0, 8.3 Hz; 10-Fmeta, 10b), −163.73–−163.93 (m; 10-Fmeta and 15-Fmeta, 10a), −164.02 (dd, 3JF,F = 25.0, 5.0 Hz; 5-Fmeta and 15-Fmeta, 10b).
MALDI-TOF: m/z calcd for C40H11F14GaN4O: 898.0000, found: 898.0750 [M+].
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02) to obtain 11 as a fuchsia solid (6 mg, 52%) (Fig. S18–S21).
0.02) to obtain 11 as a fuchsia solid (6 mg, 52%) (Fig. S18–S21).1H NMR (500 MHz, CD2Cl2) δ (ppm) = 9.27 (d, 3JH,H = 4.0 Hz, 2H; CHβ), 8.94 (d, 3JH,H = 4.5 Hz, 2H; CHβ), 8.86 (d, 3JH,H = 4.0 Hz, 2H; CHβ), 8.72 (d, 3JH,H = 4.5 Hz, 2H; CHβ), 6.70–6.63 (m, 1H; CHpara py), 5.88–5.84 (m, 2H; CHmeta py), 5.20 (d, 3JH,H = 2.4 Hz, 4H; OCH2), 5.18 (d, 3JH,H = 2.4 Hz, 2H; OCH2), 2.94 (d, 3JH,H = 5.4 Hz, 2H; CHortho py), 2.87 (t, 3JH,H = 2.4 Hz, 2H; C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 2.85 (t, 3JH,H = 2.4 Hz, 1H; C
CH), 2.85 (t, 3JH,H = 2.4 Hz, 1H; C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH).
CH).
19F{1H} NMR (471 MHz, CD2Cl2) δ (ppm) = −140.69–−140.83 (m; Fortho), −157.52 (dd, 3JF,F = 23.6, 8.8 Hz; Fmeta), −158.71 (dd, 3JF,F = 24.2, 9.0 Hz; Fmeta).
MALDI-TOF: m/z calcd for C51H22F12GaN5O3: 1049.0787, found: 1049.1874 [M]+..
FTIR-ATR (ν cm−1): 3303 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H), 2929 (C–H), 1489 (C
C–H), 2929 (C–H), 1489 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 12 as a violet solid (60 mg, 74%) (Fig. S22–S25).
1) to obtain 12 as a violet solid (60 mg, 74%) (Fig. S22–S25).The product was obtained as a mixture of isomers indistinguishable by 1H and 13C NMR. For simplicity, only the major isomer (12a) is represented in Fig. 1.
1H NMR (700 MHz, DMSO-d6) δ (ppm) = 9.36–8.52 (m, 8H; CHβ corrole), 8.48 (s, 1H; CHtriazole), 7.91 (s, 10H; CHtriazole), 5.66 (m, 2H; OCH2 corrole), 5.12 (s, 10H; OH), 5.00 (s, 10H; OH), 4.95 (s, 10H; OH), 4.67–4.64 (m, 2H; OCH2CH2NPEG), 4.55 (s, 10H; OH), 4.52–4.46 (m, 20H; OCH2CH2Nglu), 4.41–4.27 (m, 24H; CH2CH3, COOCH2CH2CH2 and COOCH2CH2O), 4.21 (d, 3JH,H = 7.7 Hz, 10H; CH1glu), 4.08–4.04 (m, 10H; OCH2CH2Nglu), 3.89–3.84 (m, 12H; OCH2CH2Nglu and CH2OPEG), 3.69–3.65 (m, 12H; CH2OHglu and CH2OPEG), 3.56–3.54 (m, 2H; CH2OPEG), 3.48–3.42 (m, 22H; CH2OHglu and CH2OPEG), 3.15–3.11 (m, 20H; CHglu), 3.06–3.03 (m, 10H; CHglu), 2.98–2.95 (m, 10H; CHglu), 2.69–2.58 (m, 20H; COOCH2CH2CH2), 2.02–1.90 (m, 20H; COOCH2CH2CH2), 1.25–1.15 (m, 3H; CH3).
13C NMR (176 MHz, DMSO-d6) δ (ppm) = 162.9 (CO), 145.6 (Ctriazole), 145.1 (Csp2 fullerene), 141.8 (Ccorrole), 140.8 (Csp2 fullerene), 138.1, 136.7 (Ccorrole), 125.9, 122.8 (CHtriazole), 102.9 (CH1glu), 77.0, 76.6, 73.3, 70.0 (CHglu), 69.7 (CH2OPEG), 68.9 (Csp3 fullerene and OCH2 corrole), 68.0 (COOCH2CH2O), 67.4 (OCH2CH2Nglu), 66.8 (COOCH2CH2CH2), 63.4 (CH2CH3), 61.1 (CH2OHglu), 49.5 (OCH2CH2N), 45.7 (Cq bridge), 27.7 (COOCH2CH2CH2), 21.5 (COOCH2CH2CH2), 13.8 (CH3).
19F{1H} NMR (471 MHz, DMSO-d6) δ (ppm) = −139.27 to −140.25 [m; Fortho (10-Fortho, 15-Fortho, 12a; 5-Fortho, 15-Fortho, 12b)], −140.81 to −141.50 [m; Fortho (5-Fortho, 12a; 10-Fortho, 12b)], −155.37 to −156.21 (m; Fpara), −156.59 to −157.60 [m; Fmeta (5-Fmeta, 12a; 10-Fmeta, 12b)], −163.17 to −164.55 [m; Fmeta (10-Fmeta and 15-Fmeta, 12a; 5-Fmeta and 15-Fmeta, 12b)].
IR (KBr) (ν cm−1): 3414 (OH), 2926 (C–H), 1740 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 13 as a purple solid (137 mg, 81%) (Fig. S26–29).
1) to obtain 13 as a purple solid (137 mg, 81%) (Fig. S26–29).1H NMR (700 MHz, DMSO-d6) δ (ppm) = 9.34–8.60 (m, 8H; CHβ corrole), 8.48 (s, 3H; CHtriazole), 7.91 (s, 30H; CHtriazole), 5.66 (s, 6H; OCH2 corrole), 5.11 (s, 30H; OH), 4.99 (s, 30H; OH), 4.94 (s, 30H; OH), 4.67–4.63 (m, 6H; OCH2CH2NPEG), 4.54 (s, 30H; OH), 4.51–4.45 (m, 60H; OCH2CH2Nglu), 4.42–4.27 (m, 72H; CH2CH3, COOCH2CH2CH2 and COOCH2CH2O), 4.21 (d, 3JH,H = 8.0 Hz, 30H; CH1glu), 4.08–4.04 (m, 30H; OCH2CH2Nglu), 3.90–3.84 (m, 36H; OCH2CH2Nglu and CH2OPEG), 3.69–3.64 (m, 36H; CH2OHglu and CH2OPEG), 3.57–3.55 (m, 6H; CH2OPEG), 3.49–3.41 (m, 66H; CH2OHglu and CH2OPEG), 3.15–3.10 (m, 60H; CHglu), 3.06–3.02 (m, 30H; CHglu), 2.98–2.94 (m, 30H; CHglu), 2.72–2.59 (m, 60H; COOCH2CH2CH2), 2.04–1.89 (m, 60H; COOCH2CH2CH2), 1.28–1.16 (m, 9H; CH3).
13C NMR (176 MHz, DMSO-d6) δ (ppm) = 162.9 (CO), 145.6 (Ctriazole), 145.1 (Csp2 fullerene), 141.9 (Ccorrole), 140.8 (Csp2 fullerene), 136.6 (Ccorrole), 125.9, 122.8 (CHtriazole), 102.8 (CH1glu), 77.0, 76.6, 73.3, 70.0 (CHglu), 69.7 (CH2OPEG), 68.8 (Csp3 fullerene), 68.0 (COOCH2CH2O), 67.3 (OCH2CH2Nglu and OCH2 corrole), 66.7 (COOCH2CH2CH2), 63.4 (CH2CH3), 61.1 (CH2OHglu), 49.5 (OCH2CH2NPEG and OCH2CH2Nglu), 45.6 (Cq bridge), 27.7 (COOCH2CH2CH2), 21.4 (COOCH2CH2CH2), 13.8 (CH3).
19F{1H} NMR (471 MHz, DMSO-d6) δ (ppm) = −139.92 to −142.19 (m; Fortho), −156.21 to −158.23 (m; Fmeta).
IR (KBr) (ν cm−1): 3392 (OH), 2926 (C–H), 1740 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
3.4 Spectroscopic and photophysical characterization
The spectroscopic and photophysical characterization was performed in air-equilibrated dimethylsulfoxide (DMSO), dimethylformamide (DMF) or water using 0.5 cm path length quartz cells (Fig. S34 and S35). Fluorescence quantum yields were measured by the reference method using a solution of cresyl violet perchlorate in methanol as standard (ΦF = 0.54).45 Several solutions were prepared in µM concentration range. To avoid non-linear effects, the absorbances were kept bellow 0.1 at the excitation wavelengths. The fluorescence spectra were measured for each sample and standard at the same conditions. The slopes (m) obtained from the linear fit of the integrated fluorescence intensity as a function of the absorbance at the excitation wavelength were used to calculate the emission quantum yield (ΦF) according to eqn (1):
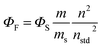 | (1) |
The singlet oxygen (1O2) generation efficiency of compounds 10–13 was evaluated in DMF using 9,10-dimethylanthracene (9,10-DMA) as a chemical probe for 1O2 detection.52,53 The absorbance of each PS sample was adjusted to 0.2 at 420 nm prior to irradiation. Experiments were carried out in quartz cuvettes, with irradiation provided by a Horiba Spex Fluoromax 4 Plus spectrofluorimeter equipped with an excitation source centered at 420 ± 5 nm. The oxidation of 9,10-DMA (∼50 µM) was monitored by tracking the decrease in absorbance at 378 nm every 120 s for a total of 600 s, using a UV-2501PC SHIMADZU spectrophotometer. For comparison, 5,10,15,20-tetraphenylporphyrin (TPP) was used as a reference standard due to its well-characterized photophysical properties.53 Control experiments confirmed that no significant photodegradation of 9,10-DMA occurred in the absence of a PS under identical irradiation conditions. All measurements were performed in triplicate to ensure reproducibility. 1O2 quantum yield (ΦΔ) was calculated using eqn (2).
 | (2) |
The fluorescence decays were measured in 5 mm quartz cuvettes by the single-photon timing technique under excitation at 570 nm by collecting the emission at 670 nm (Fig. S36).
3.5 Biological assays
Phototoxicity was evaluated following the same cell seeding and compound treatment protocol. After the 12 h pre-incubation with the compounds, cells were exposed to blue light (λ = 420 nm) using a Unilight irradiation system at energy doses of 1, 5, and 10 J cm−2. Cell viability was assessed 24 h post-irradiation.
Viability was determined using the PrestoBlue™ reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer's protocol. Fluorescence intensity was measured at 590 nm using a PolarStar Optima microplate reader (BMG Labtech). The percentage of metabolically active cells was calculated based on the reduction of resazurin and expressed relative to untreated control cells, with normalization to the negative control. For phototoxicity assays, irradiated samples were normalized to their respective dark (non-irradiated) controls. All experiments were performed in triplicate.
4. Conclusions
In this study, new alkyne-substituted Ga(III) corrole complexes were prepared from Ga(III) complex of 5,10,15-tris(pentafluorophenyl)corrole and propargylic alcohol via nucleophilic aromatic substitution under controlled conditions. These complexes were subsequently conjugated to an azide functionalized glycofullerene via copper-catalyzed azide alkyne cycloaddition reactions. The successful synthesis of the target nanostructures, was confirmed by a combination of standard spectroscopic techniques, including 1H NMR, 13C NMR, HRMS, FTIR, and UV-Vis spectroscopy, with the glycofullerene corrole conjugates obtained in appreciable yields exceeding 70%. All compounds exhibited the characteristic optical features of corrole, including a strong Soret band near 425 nm and several weaker Q bands between 500 and 660 nm. The UV-Vis absorption spectra of the conjugates were dominated by the corrole unit, with no significant spectral shifts observed upon conjugation to the glycofullerene, indicating minimal ground-state electronic interaction between the two moieties. Similarly, fluorescence measurements showed that both the precursor corroles and the mono- and tris-functionalized conjugates emitted around 610 nm, consistent with typical corrole fluorescence. The glycofullerene moiety did not contribute to the emission, in line with its non-emissive nature. These results confirm that the photophysical properties of the conjugates are primarily dominated by the corrole core. The PDT potential of compounds was evaluated through in vitro assays using HeLa cells. The HeLa cells were irradiated at 420 nm with varying light doses (1, 5, and 10 J cm−2) to assess the phototoxicity of the compounds under controlled light exposure. Among the tested compounds, the mono- and tris-alkyne-substituted corroles as well as the mono glycofullerene–corrole conjugate showed the most promising photosensitizing activity, with IC50 values below 1 µM at a light dose as low as 5 J cm−2. Importantly, none of those compounds exhibited observable dark toxicity at concentrations up to 20 µM, which was the highest concentration tested.Taken together, these results demonstrate the promise of glycofullerene hybrids bearing a corrole unit as efficient, water-soluble, and stable photosensitizers, paving the way for next-generation nanotherapeutics in cancer treatment. Future work should focus on synthesizing individual regioisomers in which only one C6F5 substituent serves as the coupling site to the fullerene, while the remaining meso positions bear distinct aryl groups. The potential role of the glycofullerene moiety in mediating interactions with lectins or sugar transporters could also be investigated to explore opportunities for enhanced cellular uptake or targeting of cancer cells. Such studies will clarify structure–activity relationships and further optimize photodynamic performance.
Author contributions
Jennifer Patino-Alonso: synthesis, photophysical studies, data analysis, writing – original draft. Carla I. M. Santos: synthesis, photophysical studies, data analysis, writing – original draft, writing – review & editing. Adriana F. Cruz: biological assays. Sandra Pinto: biological assays. Justo Cabrera-González: formal analysis, review and validation. M. Amparo F. Faustino: validation, analysis and review. M. Graça P. M. S. Neves: validation, data analysis and review. Ermelinda M. S. Maçôas: confocal microscopy, analysis, validation and review. Nazario Martín: design, formal analysis, supervision, funding acquisition. Beatriz M. Illescas: design, methodology, formal analysis, writing, review, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
All the data supporting this article are available in the supplementary information (SI). Additional datasets or information are available from the corresponding author upon reasonable request. Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc06977g.Acknowledgements
Financial support by the Spanish Ministry of Science and Innovation (MICIN) through project PID2023-148420NB-I00 and CM for the ‘(MAD2D-CM)-UCM’ project, funded by the Recovery, Transformation and Resilience Plan, and the Next Generation European Union, are acknowledged. Authors are grateful to University of Aveiro, Instituto Superior Técnico (IST, Portugal) and FCT/MCTES (Fundação para a Ciência e a Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) for the support of UID/50006 – Laboratório Associado para a Química Verde – Tecnologias e Processos Limpos, and CQE-IST to the project: 2022.05950.PTDC (https://doi.org/10.54499/2022.05950.PTDC) through national funds, where applicable, co-financed by European Union, FEDER, QREN, and COMPETE within the PT2020 Partnership Agreement, and to the Portuguese NMR Network. C. I. M. Santos is also grateful to her research contract (2023.08400.CEECIND; https://doi.org/10.54499/2023.08400.CEECIND/CP2830/CT0013).References
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229 Search PubMed.
- X. Wang, J. Peng, C. Meng and F. Feng, Chem. Sci., 2024, 15, 12234 Search PubMed.
- E. Nestoros, A. Sharma, E. Kim, J. S. Kim and M. Vendrel, Nat. Rev. Chem., 2025, 9, 46 Search PubMed.
- Y. Wang, K. Ma, M. Kang, D. Yan, N. Niu, S. Yan, P. Sun, L. Zhang, L. Sun, D. Wang, H. Tan and B. Z. Tang, Chem. Soc. Rev., 2024, 53, 12014 Search PubMed.
- H. Abrahamse and M. R. Hamblin, Biochem. J., 2016, 473, 347 Search PubMed.
- R. D. Teo, J. S. Hwang, J. Termini, Z. Gross and H. B. Gray, Chem. Rev., 2016, 117, 2711 Search PubMed.
- W. Deng, Z. Wei, Y. Xu, Z. Gong, F. Cai, Q. Shi, K. Guo, M. Jia, Y. Zhao, Y. Feng, J. Deng and B. Zhang, Small, 2024, 21, e2408975 Search PubMed.
- J.-H. Cen, Q.-H. Xie, G.-H. Guo, L.-J. Gao, Y.-H. Liao, X.-P. Zhong and H.-Y. Liu, Eur. J. Med. Chem., 2024, 265, 116102 Search PubMed.
- X. Jiang, R.-X. Liu, H.-Y. Liu and C. K. Chang, J. Chin. Chem. Soc., 2019, 66, 1090 Search PubMed.
- R. C. Soy, B. Babu, J. Mack and T. Nyokong, Dyes Pigm., 2021, 194, 109631 Search PubMed.
- W. Yang, G. Yang, W. Hu, M.-Y. Li, Z.-Y. Liu, D. J. Yu, Y.-H. Liao and H.-Y. Liu, J. Photochem. Photobiol., A, 2023, 438, 114580 Search PubMed.
- Z. Zhang, H.-J. Yu, H. Huang, H.-H. Wang, S. Wu, H.-Y. Liu and H.-T. Zhang, Eur. J. Med. Chem., 2019, 163, 779 Search PubMed.
- X. Zhan, D. Kim, Z. Ullah, W. Lee, Z. Gross and D. G. Churchill, Coord. Chem. Rev., 2023, 495, 215363 Search PubMed.
- J. F. B. Barata, M. G. P. M. S. Neves, M. A. F. Faustino, A. C. Tomé and J. A. S. Cavaleiro, Chem. Rev., 2017, 117, 3192 Search PubMed.
- V. K. Sharma, Y. G. Assaraf and Z. Gross, Drug Resistance Updates, 2023, 67, 100931 Search PubMed.
- J. Y. Hwang, Y. Lubow, D. Chu, J. Ma, H. Agadjanian, J. Sims, H. B. Gray, Z. Gross, D. L. Farkas and L. K. Medina, Mol. Pharm., 2011, 8, 2233 Search PubMed.
- P. Lim, A. Mahammed, Z. Okun, I. Saltsman, Z. Gross, H. B. Gray and J. Termini, Chem. Res. Toxicol., 2012, 25, 400 Search PubMed.
- Z. Ye, Y. Liang, Y. Ma, B. Lin, L. Cao, B. Wang, Z. Zhang, H. Yu, J. Li, M. Huang, K. Zhou, Q. Zhang, X. Liu and J. Zeng, Cancer Med., 2018, 7, 3057 Search PubMed.
- Y.-M. Sun, X. Jiang, Z.-Y. Liu, L.-G. Liu, Y.-H. Liao, L. Zeng, Y. Ye and H.-Y. Liu, Eur. J. Med. Chem., 2020, 208, 112794 Search PubMed.
- F. Cheng, H.-H. Wang, A. Ali, J. Kandhadi, H. Wang, X.-L. Wang and H.-Y. Liu, J. Porphyrins Phthalocyanines, 2018, 22, 886 Search PubMed.
- M. Soll, V. K. Sharma, S. Khoury, Y. G. Assaraf and Z. Gross, J. Med. Chem., 2023, 66, 766 Search PubMed.
- V. K. Sharma, A. Mahammed, M. Soll, B. Tumanskii and Z. Gross, Chem. Commun., 2019, 55, 12789 Search PubMed.
- K. Lewandowska, B. Barszcz, A. Graja, B. Bursa, A. Biadasz, D. Wróbel, W. Bednarski, S. Waplak, M. Grzybowski and D. T. Gryko, Synth. Met., 2013, 166, 70 Search PubMed.
- C. I. M. Santos, M. Cicuéndez, G. Gonçalves, L. Rodríguez-Pérez, M. T. Portolés, M. A. F. Faustino, M. Á. Herranz, M. G. P. M. S. Neves, J. M. G. Martinho, E. M. S. Maçôas and N. Martín, J. Mater. Chem. B, 2023, 11, 675 Search PubMed.
- C. I. M. Santos, L. Rodríguez-Pérez, G. Gonçalves, S. N. Pinto, M. Melle-Franco, P. A. A. P. Marques, M. A. F. Faustino, M. Á. Herranz, N. Martín, M. G. P. M. S. Neves, J. M. G. Martinho and E. M. S. Maçôas, Carbon, 2020, 166, 164 Search PubMed.
- S. Singh, A. Aggarwal, N. V. S. Dinesh, K. Bhupathiraju, G. Arianna, K. Tiwari and C. M. Drain, Chem. Rev., 2015, 115, 10261 Search PubMed.
- F. Yin, J.-J. Li, B. Shi, K. Zhang, X.-L. Li, K.-R. Wang and D.-S. Guo, Mater. Chem. Front., 2023, 7, 5263 Search PubMed.
- T. A. F. Cardote, J. F. B. Barata, M. A. F. Faustino, A. Preuß, M. G. P. M. S. Neves, J. A. S. Cavaleiro, C. I. V. Ramos, M. G. O. Santana-Marques and B. Röder, Tetrahedron Lett., 2012, 53, 6388 Search PubMed.
- R. Klingenburg, C. B. W. Stark and A. Wiehe, Org. Lett., 2019, 21, 5417 Search PubMed.
- A. Muñoz, D. Sigwalt, B. M. Illescas, J. Luczkowiak, L. Rodríguez-Pérez, I. Nierengarten, M. Holler, J.-S. Remy, K. Buffet, S. P. Vincent, J. Rojo, R. Delgado, J.-F. Nierengarten and N. Martín, Nat. Chem., 2016, 8, 50 Search PubMed.
- J. Ramos-Soriano, J. J. Reina, B. M. Illescas, N. de la Cruz, L. Rodríguez-Pérez, F. Lasala, J. Rojo, R. Delgado and N. Martí, J. Am. Chem. Soc., 2019, 141, 15403 Search PubMed.
- J. Ramos-Soriano, B. M. Illescas, A. Pérez-Sánchez, R. Sánchez-Bento, F. Lasala, J. Rojo, R. Delgado and N. Martín, Int. J. Mol. Sci., 2022, 23, 5083 Search PubMed.
- J. Patino-Alonso, J. Cabrera-González, J. Merino, G. Nieto-Ortiz, F. Lasala, J. Katati, C. H. B. da Cruz, A. K. Monnappa, P. Mateos-Gil, Á. Canales, I. López-Montero, B. M. Illescas, R. Delgado and N. Martín, Small, 2024, 20, e2307045 Search PubMed.
- X. Bai, T. Yang, X. Shao, B. Jia, H. Hao, F.-U. Rahman and Y. Zhang, Eur. J. Med. Chem., 2024, 281, 117009 Search PubMed.
- L. Tanzi, M. Terreni and Y. Zhang, Eur. J. Med. Chem., 2022, 230, 114104 Search PubMed.
- B. Bursa, B. Barszcz, W. Bednarski, J. P. Lewtak, D. Koszelewski, O. Vakuliuk, D. T. Gryko and D. Wróbel, Phys. Chem. Chem. Phys., 2015, 17, 7411 Search PubMed.
- C. Liu, X. Jiao, Q. Wang, K. Huang, S. He, L. Zhao and X. Zeng, Chem. Commun., 2017, 53, 10727 Search PubMed.
- C.-W. Lee, Y.-H. Su, Y.-C. Chiang, I.-T. Lee, S.-Y. Li, H.-C. Lee, L.-F. Hsu, Y.-L. Yan, H.-Y. Li and M.-C. Chen, Biomolecules, 2020, 10, 514 Search PubMed.
- M. R. Hamblin, Photochem. Photobiol. Sci., 2018, 17, 1515 Search PubMed.
- J. Ramos-Soriano, J. J. Reina, B. M. Illescas, J. Rojo and N. Martín, J. Org. Chem., 2018, 83, 1727 Search PubMed.
- C. N. McEwen, R. G. McKay and B. S. Larsen, J. Am. Chem. Soc., 1992, 114, 4412 Search PubMed.
- J. Ramos-Soriano, A. Pérez-Sánchez, S. Ramírez-Barroso, B. M. Illescas, K. Azmani, A. Rodríguez-Fortea, J. M. Poblet, C. Hally, S. Nonell, D. García-Fresnadillo, J. Rojo and N. Martín, Angew. Chem., 2021, 60, 16109 Search PubMed.
- H. Saigusa, T. Azumi, M. Sumitani and K. Yoshihara, J. Chem. Phys., 1980, 72, 1713 Search PubMed.
- A. Mahammed and Z. Gross, Coord. Chem. Rev., 2019, 379, 121 Search PubMed.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of Photochemistry, Taylor & Francis, 2006 Search PubMed.
- S. R. D. Gamelas, J. M. M. Rodrigues, J. P. C. Tomé and L. M. O Lourenço, J. Photochem. Photobiol., 2024, 454, 115742 Search PubMed.
- G. Tao, T. Ji, N. Wang, G. Yang, X. Lei, W. Zheng, R. Liu, X. Xu, L. Yang, G.-Q. Yin, X. Liao, X. Li, H.-m. Ding, X. Ding, J. Xu, H.-B. Yang and G. Chen, ACS Macro Lett., 2020, 9, 61 Search PubMed.
- T. M. N. Trinh, M. Holler, J. P. Schneider, M. I. Garcia-Moreno, J. M. Garcia Fernandez, A. Bodlenner, P. Compain, C. Ortiz Mellet and J.-F. Nierengarten, J. Mater. Chem. B, 2017, 5, 6546 Search PubMed.
- Y. Min, F. Leng, B. F. Machado, P. Lecante, P. Roblin, H. Martinez, T. Theussl, A. Casu, A. Falqui, M. Barcenilla, S. Coco, B. M. I. Martínez, N. Martin, M. R. Axet and P. Serp, Eur. J. Inorg. Chem., 2020, 43, 4069 Search PubMed.
- V. Yarlagadda, M. M. Konai, G. B. Manjunath, C. Ghosh and J. Haldar, J. Antibiot., 2015, 68, 302 Search PubMed.
- C. I. M. Santos, E. Oliveira, J. F. B. Barata, M. A. F. Faustino, J. A. S. Cavaleiro, M. G. P. M. S. Neves and C. Lodeiro, J. Mater. Chem., 2012, 22, 13811 Search PubMed.
- R. Schmidt, Photochem. Photobiol., 2017, 82, 1161 Search PubMed.
- E. Gross, B. Ehrenberg and F. M. Johnson, Photochem. Photobiol., 1993, 57, 808 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |