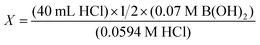Investigating the Gordian knot: how teaching assistants enact equitable and effective teaching during acid–base titrations
Cassandra
Miller
 and
Meng-Yang Matthew
Wu
*
and
Meng-Yang Matthew
Wu
*
Department of Chemistry & Biochemistry, The Ohio State University, Columbus, OH, USA. E-mail: wu.6250@osu.edu
First published on 16th October 2025
Abstract
Laboratory teaching assistants (TAs) crucially shape undergraduates' chemistry learning experiences. However, akin to the Gordian knot metaphor (i.e., an intractable problem near impossible to solve), TA pedagogy is intricate and difficult to support by conventional means. More attention is needed to discern the complexities of enacted TA pedagogies and their alignment with equitable and effective teaching. Using Teacher Noticing, Multidimensional Noticing, and teaching for Meaningful Learning, this study involved two focal TAs, Alexandra and Bred, as a comparative case study. We used video research principles and video-stimulated recall interviews to qualitatively investigate how participants' teach acid–base titrations. Our findings indicate that TA efforts related to equitable and effective instructional moves can be both complementary and conflicting. Surprisingly, TAs may actually be the ones meaningfully learning in place of their students. Implications include suggestions for long-term training programs (video club and instructional coaching) that invite TAs to analyse students’ learning via enacted pedagogies. We offer specific, accessible, and practical suggestions to foreground particulate-level interactions, sensemaking, local agriculture, nutrition, and university life when teaching acid–base chemistry. We thus invite our community to interrogate and reimagine what we want as evidence of learning and of teaching to inform shifts in instructional laboratory culture.
Introduction
According to the 2024 Science and Engineering Indicators, the U.S. has fallen behind other countries in published research articles, patent applications, and knowledge- and technology-intensive industry outputs (National Science Board and National Science Foundation, 2024). The report also notes national inconsistencies in science, technology, engineering, and math (STEM) degree attainment among racial, ethnic, gender, and geographic groups. To address these concerns, the National Academies of Science, Engineering, and Medicine (NASEM, 2025) have issued calls to transform undergraduate STEM education by incentivizing equitable and effective teaching. Such sentiments echo the Boyer 2030 Commission's equity/excellence imperative. To prepare undergraduates for world readiness, higher education institutions must recognize that,“Excellence and equity are inextricably entwined, such that excellence without equity (privilege reproducing privilege) is not true excellence, and equity (mere access) without excellence is unfulfilled promise.” (The Boyer 2030 Commission, 2022, p. 3).
One lever for such change is the instructional laboratory, which has been a widely accepted component of undergraduate chemistry (Agustian and Seery, 2017). Educators have argued for the laboratory's merits given its potential to engage students with hands-on experimentation (Reid and Shah, 2007). However, especially with increasing fiscal and labour costs, Bretz (2019) reminds our community that we can no longer assume the laboratory's affordances, advocating for more evidence of student learning. We the authors add that what, how, and why a chemistry student learns (and their variations) are also contingent on the instructor (Bussey et al., 2013). Given that pedagogy can shape students’ perceptions of course values and the larger chemistry discipline (Reynders et al., 2019; Ralph et al., 2022; Rosa et al., 2022), further explorations of instructor-student interactions are warranted. In other words, before evaluating evidence of laboratory learning, we must first determine the evidence of laboratory teaching.
Various avenues of research have expanded what is known about equitable and effective laboratory teaching, albeit separately. Scholarship on equity has identified how inequalities in prior preparation impact first-year undergraduates' laboratory learning (Jimenez and August, 2025) and proposed how experiments can be designed to be more physically accessible (D’Agostino, 2022; Egambaram et al., 2022). Sanders Johnson (2022) foregrounded cultural identities by spotlighting black individuals like Nearest Green (who influenced the Jack Daniels brand) when learning whiskey chemistry. In terms of effectiveness, Zotos et al. (2021) showed that varying content knowledge levels can affect how graduate teaching assistants and faculty instructors convey mechanistic information and discern gaps in student understanding. Integrating authentic research practices with laboratory curriculum can enhance engagement (Barr et al., 2022) while technological affordances such as virtual experiments produce equal, if not greater, learning outcomes compared to those of in-person experiments (Chan et al., 2021).
These two reform efforts should coalesce if the U.S. aims to achieve the equity/excellence imperative (The Boyer 2030 Commission, 2022) and respond to NASEM's (2025) calls for transforming undergraduate STEM education. We address this gap by analysing teaching assistant-student interactions within general chemistry instructional laboratories. Situated in acid–base titrations, this study aims to investigate the Gordian knot of laboratory instruction via its subtle, messy, and—oftentimes—unexpected happenings. While other scholarship has sought to “cut” (Esping-Andersen, 2004), “untie” (Hansson, 2009), or “disentangle” (Tune et al., 2020) the Gordian knot using clever (re)solutions, this study instead embraces the nuances of teaching assistant pedagogy by highlighting its breadth and depth. We hope our findings resonate both domestically and internationally, promoting further inquiry regarding the entanglements of equitable and effective teaching.
Background
We begin with a brief review of chemistry teaching assistant populations. Next, we address acid–base titrations incorporated throughout chemical education research. We then conclude by articulating how these two literature bases inform this study.Teaching assistants (TAs) are a population of student instructors who influence the learning outcomes and retention of undergraduate students in chemistry (O’Neal et al., 2007). Unlike learning assistants who are typically undergraduates trained to facilitate small group discussions (Donis et al., 2024), teaching assistants may possess a variety of roles and responsibilities and may be undergraduate students, graduate students, and/or staff. Particularly at large universities with high student enrolment, TAs in the laboratory can be regarded as the “first line of defence” given their extensive interactions with students (Nicklow et al., 2007). Because many TAs have little to no prior pedagogical preparation (Kurdziel et al., 2003), scholars have developed and evaluated a variety of novel training programs (Dragisich et al., 2016; Ruder and Stanford, 2018; Wan et al., 2020; Fantone et al., 2023). How TAs conceptualize their “teacher” role (Zotos et al., 2020), the professional relationships between TAs and their students (Roshandel et al., 2025), and their pedagogical content knowledge have also been documented (Connor and Shultz, 2018). Recently, researchers have endorsed prioritizing enacted teaching practices in conjunction with teacher beliefs and reflections (Carlson et al., 2019; Rodriguez and Towns, 2019). Especially for TA populations, there is much to learn regarding what they literally do moment to moment in the laboratory.
Acid–base chemistry has been a standard topic in instructional laboratories for decades (Strong, 1930). In addition, the titration experiment is almost ubiquitously present throughout various countries when students learn acid–base chemistry (Aydin-Gunbatar and Akin, 2022; Lau et al., 2023; Pentucci et al., 2025). To revamp acid–base titrations, studies have improved its relevance (Muhamad Hugerat et al., 2018; Winstead et al., 2022) and conceptual learning opportunities (Bandyopadhyay and Rathod, 2017; Mahaffey, 2021; Larkin et al., 2023). Such findings, however, predominantly stem from the student's perspective. Research related to the chemistry instructor typically involves beliefs and reflections (Alvarado et al., 2015; Baldwin and Orgill, 2019; Boothe et al., 2023) where enacted acid–base instruction is relatively underdiscussed. How acid–base titrations are taught in real time requires more attention, especially if we intend to overcome the prevailing culture of instructors and students' prioritizing experimental completion (DeKorver and Towns, 2016; Baldwin and Orgill, 2019).
In summary, TAs have the potential to greatly influence students' laboratory experiences. Nevertheless, even with experiments as prevalent as acid–base titrations, what transpires in terms of TA–student interactions largely remains a “black box.” To focus our attention on equitable and effective teaching, we leverage theories of Multidimensional Noticing and Meaningful Learning, respectively. We also employ video research and video-stimulated recall interviews to analyse real-time instruction and pedagogical decision making with greater resolution. By using a comparative case study of two TAs who taught acid–base chemistry across two sequences of general chemistry, we intend to offer specific, accessible, and practical guidelines to promote equitable and effective teaching (Rodriguez and Towns, 2019).
Conceptual framework
Equitable teaching via multidimensional noticing
Conceptualized in mathematics education research, noticing is how a teacher iteratively attends, interprets, and responds to students’ ideas (van Es and Sherin, 2002; Jacobs et al., 2010). Attend refers to what a teacher deems noteworthy, interpret consists of instructional decision making, and respond includes teachers’ resultant actions (see Table 1 with acid–base examples). When imported into chemistry education research, noticing has been used in various ways. Talanquer et al. (2015) described how prospective teachers superficially provide descriptive feedback when engaging in noticing via formative assessment. Murray et al. (2020) also characterized middle- and high-school teacher noticing to be combinations of authoritative/dialogic interpretation (evaluative vs. sense-making) and acting (directive vs. responsive). Others have investigated how TAs attend to and interpret students’ questions, engagement, and work pace (Geragosian et al., 2024), as well as shown how TA noticing exposes the tensions/resolution between teaching and learning objectives (Zaimi et al., 2024).| Attend | Hearing a student say the products of mixing HCl(aq) and NaOH(aq) is H2O(l) and NaCl(aq) |
| Interpret | Wondering whether the student understands that NaCl(aq) exists as solvated Na+(aq) cations and Cl−(aq) anions, not ionically bonded as a salt would be |
| Respond | Asking the students to further elaborate by drawing out how the spectator ions are represented in solution |
Shifting to more asset-based and justice-oriented framing, scholars have adapted the noticing framework for equity (e.g., Louie et al., 2021; van Es and Sherin, 2021). Typically understood as empowerment through confronting deficit perspectives and processes (Nasir and Shah, 2011; van Es et al., 2017), equitable pedagogy can include (1) fostering dialogic classroom interactions, (2) blending intellectual and cultural activities, and (3) re-positioning students’ and teachers’ cultural-historical, sociopolitical, and academic selves so students can “take up” more space (Hand, 2012). More specifically, we leverage Multidimensional Noticing (van Es et al., 2022), which incorporates dimensions of stretch and expanse, when operationalizing equitable teaching. Stretch describes what a teacher knows about the past and future of their students and of themselves. Expanse, like a camera's aperture, is the broadening of a teacher's selective attention during an instructional moment. Table 2 builds upon the acid–base scenario with examples of stretch and expanse. While Teacher Noticing has been used to frame chemistry TA experiences, research including stretch and expanse remains underexplored.
| Stretch | Remembering a student's aspiration to work in an art museum and fostering connections to how alkaline and acidic cleaners are related to art restoration |
| Expanse | Playfully designing a hypothetical titration with students by eliciting their favourite energy drinks, what they would anticipate in terms of pH, and what alternative titrants/indicators could be used |
Effective teaching for meaningful learning
Meaningful Learning, an interaction between teacher and student, involves leveraging prior knowledge, recognizing that new information is useful, and moving beyond rote memorization (Bretz, 2001). Given that three facets of experiences drive knowledge construction (Novak, 1998), we operationalize effective teaching to intentionally incorporate students’ cognitive (thinking), affective (feeling), and psychomotor (acting) domains. Within instructional laboratory contexts, the cognitive domain involves reasoning with chemicals at different representational levels (e.g., written equations, observable phenomena, and particulate illustrations) (Johnstone, 1982; Talanquer, 2011). The affective domain comprises students’ values, interest, and motivation (Flaherty, 2020). The psychomotor domain includes the handling of glassware, experimental techniques, and the writing of pre-laboratory protocols (Towns et al., 2015; Hensiek et al., 2016; Agustian and Seery, 2017; Hennah and Seery, 2017). Table 3 shows examples of effective teaching for Meaningful Learning in the context of acid–base chemistry.| Cognitive | Guiding students to conceptually discern the difference between weak acid/strong acid dissociation vs. molarity (i.e., relative ratio of acid to water molecules in solution) |
| Affective | Fostering a growth mindset when students over-titrate their solutions and produce a deep pink colour from phenolphthalein indicator |
| Psychomotor | Supporting students’ checking for air bubbles before titrating to increase accuracy or lowering the burette below eye-level when refilling the titrant to promote safety |
When teaching for Meaningful Learning, ignoring one domain may adversely affect the other two. For example, laboratory students, if not positioned to feel autonomous, may result in their completing the procedure correctly without cognitively exploring the experimental rationale or processes (Galloway et al., 2016). Gupte et al. (2021) conversely showed how students’ Meaningful Learning of thalidomide reactivity can be enhanced through psychomotor activities (i.e., Writing-to-Learn) that are situated in interesting and relevant contexts. Although thalidomide reactivity is different from acid–base titrations, Gupte et al.'s study provides theoretical evidence that blending different dimensions of Meaningful Learning can benefit students. Even in Course-Based Undergraduate Research Experiences, course design principles that emphasize discovery, relevance, collaboration, iteration, and science practices (Watts and Rodriguez, 2023) inherently address students’ cognitive, affective, and psychomotor domains. We note that research inspired by Meaningful Learning tends to foreground students' perspectives in lieu of that of teachers' (Galloway and Bretz, 2015; States et al., 2023). Because of the latter's influence on the former's experiences (Bretz, 2001), research on whether and how pedagogies can effectively promote Meaningful Learning requires commensurate attention.
Thus, we use Multidimensional Noticing and Meaningful Learning to substantiate our assumptions of equitable and effective teaching. Like interlocking gears depicted in Fig. 1, our conceptual framework assumes the intertwined nature of equitable and effective teaching. Cycles of teacher noticing (attend–interpret–respond) inform recurring learning opportunities for cognitive, psychomotor, and affective engagement, with stretch and expanse as essential cogs. To unpack the pedagogical nuances related to acid–base titrations, we ask the following research questions with attention towards two focal TAs: Alexandra and Bred.
 | ||
| Fig. 1 Conceptual framework of teacher noticing, multidimensional noticing (stretch and expanse), and teaching for meaningful learning. | ||
RQ1: How, if at all, do focal TAs equitably teach by enacting Multidimensional Noticing during acid–base titrations?
RQ2: How, if at all, do focal TAs effectively teach by creating opportunities for Meaningful Learning during acid–base titrations?
Methods
Context and participants
This study was conducted at a public, research-intensive university in the midwestern U.S. Approximately 4000 students enrol in General Chemistry 1 and 2 (GC1 and GC2, respectively) each year, requiring over 100 TAs to facilitate instructional laboratories. Because GC1 and GC2 are large service courses required by most STEM major programs, general chemistry laboratories may crucially inform students’ “first impressions” of chemistry and STEM at large.During the 2023 autumn semester (AU23), GC1 and GC2 TAs were recruited via convenience sampling (Merriam and Tisdell, 2016). An announcement was posted on the TA learning management system, and a brief solicitation was conducted at the beginning of several staff meetings. Participants (N = 21; n = 14 for GC1; n = 7 for GC2) comprised undergraduate, graduate, and staff laboratory TAs. To enhance confidentiality, the number of undergraduate, graduate, or staff TAs will not be disclosed. We will instead report semesters of teaching experience, with some participants being more experienced while a majority were teaching for the first time. A complete summary of participant pseudonyms, teaching experience, course, and experiment topic is included in the Appendix.
Ethical considerations
Procedures for collecting, storing, and analysing human subjects' data were approved by the research site's institutional review board (2023B0412). Informed consent was collected via individual meetings in which TAs could ask questions and clarify uncertainties. Participants were compensated $30 for the interview and $10 for the recording. We also present our positionality statements later in this section. Student utterances were paraphrased to maintain focus on the TA participant. Finally, to minimize risk in participant identification, screenshots of the videos were intentionally not included in this study. We instead provide in-depth details in the written narrative to better illustrate focal laboratory TA interactions.Data collection
Fig. 2 summarizes video research principles that guided our data collection and analytical procedures (Derry et al., 2010). Audio-video recordings and interview data were collected throughout the AU23 semester. Laboratories were recorded with a SONY FDR-AX700 HK HDR Video Camcorder, using a wide-angle lens. Audio was captured using a Bluetooth lapel microphone clipped to the participant's laboratory coat. Although not integral to the scope of this study, whether the instructional laboratory was renovated (GC1 and GC2) or unrenovated (only GC2) determined how the camera was placed and what was feasibly captured within frame (Fig. 3). | ||
| Fig. 2 Video research principles used for this project. We started with guiding questions and went clockwise, doubling back when appropriate. | ||
 | ||
| Fig. 3 Camera placement, student stations (circles), and TA desks (stars) in GC1 and GC2 instructional laboratories. | ||
The second author iteratively reviewed the collected footage and indexed noteworthy events of TA–student discourse. Because Derry et al. (2010) recommend choosing segments that are representative of a phenomenon within and across events, three to four TA–student interactions per each participant's recording were identified. These events revolved around learner uncertainty, confusion, and frustration, which warranted the TA's attention. In addition, such interactions often occurred multiple times throughout the recording, suggesting that these moments contained potentially common and persistent issues both TAs and students experienced.
Shortly after event selection, TA participants were invited for a 60-minute, semi-structured, video-stimulated recall interview (Calderhead, 1981; Gazdag et al., 2019). Audio/video playback functions as pedagogical amplifiers (Cummins et al., 2007), enabling more contextualized analysis and reflection of teaching practices situated in authentic events (Blomberg et al., 2013). The interview informally functioned as member checking, in which the interviewer elicited participant perspectives (“Why was the student confused?”), clarified enacted instruction (“How did you resolve the student's question?”), and unpacked pedagogical mechanisms and underlying rationale (“What was your teaching strategy and goal?”). Participants were also encouraged to identify additional events they recalled being noteworthy. Interviews were roughly transcribed by Otter.AI and then cleaned by the research team.
Data analysis
For this specific study, we used a subset of our data and conducted a comparative case study analysis (Merriam and Tisdell, 2016) between two focal TAs with similar teaching experience, Alexandra (GC2) and Bred (GC1). This subset consisted of six hours of recordings (three hours per TA) and two hours of interviews (one hour per TA). Juxtaposing their footage enables not only rich participant-specific insights but also informative pedagogical similarities and differences across GC1/GC2 via a cross-case analysis (Merriam and Tisdell, 2016). Because both TAs facilitated strong acid–strong base titrations, emergent findings may be more useful given this topic's presence in throughout domestic and international higher education chemistry curricula.After the interviews, the research team iteratively (re)viewed Bred and Alexandra's events, inductively attending to TAs’ spoken words, gestures, gaze, and proxemics. Key events were then deductively organized using the teacher noticing framework (i.e., attend–interpret–respond), where attend was denoted as the starting-up of the TA–student interaction and respond as the winding-down (Jordan and Henderson, 1995). We explored the effectiveness of enacted instruction based on how the TA positioned students to engage in Meaningful Learning. Instances included how TAs’ appealed to students’ feelings (affective), facilitated experimental practices (psychomotor), and facilitated the co-construction of chemistry knowledge (cognitive). The first and second authors independently analysed the events in which memos were extensively recorded. Informed by video research principles (Derry et al., 2010), weekly meetings involved dialogic discussions to elicit various interpretations and dialectic negotiations to identify which were most strongly evidenced.
During the second round of analysis, (1) interview transcripts were used to provide more context on how TAs interpret and (2) stretch and expanse were investigated to determine how laboratory teaching was equitable. Specifically, we considered instances when TAs shaped access to and resources for chemistry learning beyond those noted in staff notes and the laboratory manual. For example, a TA could elicit students’ prior course experiences or future career aspirations to contextualize the learning of acid–base concepts (i.e., stretch). A TA could also connect students’ everyday experiences to rationalize the experiment (i.e., expanse). Weekly meetings extended prior discussions to characterize the extent stretch and expanse emerged via TAs’ noticing.
To enhance trustworthiness and rigor (Lincoln and Guba, 1985), the research team adhered to the following criteria. Credibility was improved by triangulating data sources. Attend and respond were mapped onto visibly seen/audibly heard TA discourse captured in recordings, while interview transcripts helped clarify TAs’ interpret based on their externalized thought processes. Our emic perspectives, from previous and ongoing research at this site, also helped us detect underlying pedagogical norms. Another round of member checking with Alexandra and Bred was also conducted to ensure findings aligned with their perspectives. To ensure transferability, we used multimodality and memoing to generate thick descriptions (Geertz, 2008) of events. Our use of established video research protocols (Conlin and Scherr, 2018; Grinath and Southerland, 2019; Wu and Yezierski, 2023) further strengthened our findings’ dependability.
Finally, to improve confirmability, we engage in reflexivity to make transparent our sensemaking of the data. The first author (CM) leveraged her prior experiences as an undergraduate student and as an organic laboratory head TA when analysing TA participants’ noticing (attend–interpret–respond). CM stretched into her own past experiences as a general chemistry learner to identify evidence of instruction advancing chemistry learning and engagement. CM also expanded the range of possible laboratory pedagogies, drawing upon her experiences training organic laboratory TAs. Realizing that the teaching culture between general and organic chemistry laboratories are vastly different, CM used these gaps as levers to delve deeper into the unique ways TA noticing manifests in the former context.
The second author (MYW) similarly used his past experiences as a head TA and teacher educator of pre- and in-service teachers. MYW expanded what could be considered noteworthy by melding reform-based efforts in both chemistry and science education fields. Given his familiarity with the historicity of laboratory instruction as both a researcher and practitioner, MYW incorporated his subjectivity as a lens to discern the complexities of enacted instruction as a negotiation of what TAs may want to do vs. what they may be obligated to do.
Findings and discussion
The following section is organized with details that contextualize Alexandra's (GC2) and Bred's (GC1) vignettes, followed by supporting evidence and analyses of equitable and effective teaching. We have selected a series of acid–base titration events that not only typify teaching–learning interactions in Alexandra's and Bred's sections but also moments that other chemistry education researchers and practitioners have likely experienced. To prime readers' attention filter, key theoretical constructs are italicized.Calculations in acid–base titrations
Alexandra taught a titration experiment using a strong monoprotic acid and a strong diprotic base. Students were expected to calculate the volume of base needed to reach the equivalence point with a predetermined volume of acid. While students were shown how to compute this value using a Before–Change–After (BCA, analogous to an Initial–Change–Equilibrium table) table to track molar amounts of chemical species, Alexandra recognized that this procedure can be overwhelming. She opted for a simpler formula equating the concentration and volume of acid with those of base (i.e., MacidVacid = MbaseVbase). During her laboratory period, however, students polarized into two groups: they either understood or struggled with the calculations.Alexandra's event 1: pinpointing where the “2” goes
Alexandra's first event represents a commonly occurring difficulty among her students. The interaction began where Alexandra attended to a raised hand by turning and walking toward the student. Alexandra evaluated the student's calculating two moles of monoprotic acid reacting with one mole of diprotic base. As Alexandra walked away, the student raised his hand again to ask another question about the same calculation. Alexandra responded that the “2” should align with the OH− ion and supplemented with a hand gesture—she cupped her hands into parentheses and moved them down and to the right, mimicking the way OH− is included in the written formula (i.e., B(OH)2). The student suggested a ratio, referencing H+ and OH− (rather than acid and base). Agreeing with the student, Alexandra exclaimed “Exactly!”.During the interview, Alexandra shared that she tried to pre-emptively resolve student questions about calculations, likely relating to how she interpreted conversations like this one.
“I think [BCA tables] trip up students a lot. That definitely tripped me up when I was taking that course. So I tried to really hone in on that […] Depending on how you write the BCA table, I kind of found when I was teaching, I write mine a little bit differently than the students do. So there's kind of like a gap in that and trying to explain how I see it.” – Alexandra (6:16–7:01).
Despite mathematics in chemistry producing inequitable results via summative assessments and supplanting mechanistic reasoning (Ralph et al., 2022; Rosa et al., 2022), we recognize Alexandra's use of an alternative, more streamlined calculation approach as an attempt to make the cognitive dimension of learning more equitable (i.e., expanse). In addition, Alexandra had helped the student connect symbolic and particulate-level ideas (Talanquer, 2011; Taber, 2013) with the corresponding math. However, Alexandra's rationales involved stretching backwards to when she was a student in this course (e.g., “I think, tripped me up, when I was taking that course, I write mine,” emphasis added). Multidimensional Noticing ideally draws upon both instructor and students’ pasts (van Es et al., 2022), aligned with a Freire (2000) perspective that supports blurring the lines separating instructors and students for dialogic knowledge generation. While Alexandra expressed a desire for students’ success by “really [honing] in on” chemistry ideas that “trip up students a lot,” Alexandra appears to assume that what worked in her past will work for students presently. While TAs’ relying on their learner experiences can be productive (Zotos et al., 2020), eliciting students’ experiences and ideas as continuous feedback (Carless, 2019) should also be considered for fine-tuning instruction in real time.
Furthermore, Alexandra's interview revealed that she knows students typically use “acid and base” interchangeably with “H+ and OH−,” exemplifying how communicating chemistry can be confusing for new learners (Taber, 2019). Consequently, the student's ratio between acidic protons (H+) and basic anions (OH−) could imply an incorrectly balanced written equation (i.e., 1HA + 2B(OH)2 (incorrect) vs. 2HA + 1B(OH)2 (correct)). Alexandra's agreement may inadvertently raise future ambiguity between chemistry symbols and the molar amounts of ions. Although Alexandra possibly enacted expanse by recalling students’ prior knowledge, why she had not followed up and clarified the student's idea is unknown. Later in this experiment, where the “2” goes and what it conceptually means become fuzzier between Alexandra and a pair of students.
Alexandra's event 2: rationalizing numbers for a titration
Alexandra's second event involves her being at the chalkboard and addressing a question about equivalence point. We infer that this question is an extension of the ongoing confusion around acid and base vs. H+ and OH− ions.A student asked, “If it's OH2, do I divide the concentration by 2?” First, we note that dividing by “2” is reasonable if the volume was determined by using the balanced written equation (Table 4, Scenario 1). Alexandra, likely using her instructional plan of using the MacidVacid = MbaseVbase calculation, responded by first simulating a calculation that does not entail the number “2” (Table 4, Scenario 2; note that the volumes of acid and base are incorrectly swapped [sic]). She stated that calculating the volume of base in this manner would result in an impractical number (i.e., “our burettes don’t even go to 47 [millilitres]”). In this instance, Alexandra mistakenly swapped the volumes of acid and base, potentially indicating her own difficulty with this question and/or how cognitively overloaded Alexandra may have been in this moment. This likely unintentional error when showing students an intentionally inaccurate calculation was not rectified until the end of this event.
Sounds of Alexandra's typing on a calculator are interrupted by another student joining, saying that they also “got this [question] wrong.” Alexandra interjected this new conversation by showing how dividing the OH− molar concentration by “2” produces an answer of 23.56 mL (Table 4, Scenario 3; note that the volumes are still incorrectly swapped [sic]). Alexandra responded by describing how this value is unlikely because of experimental constraints (i.e., “we only can use 25 mL of our base”) and how students were expected to continue titrating beyond the equivalence point. Shortly after, Alexandra inputted what happens when multiplying by “2” (Table 4, Scenario 4; note that the volumes are now correctly shown). Alexandra concluded by stating how the final answer of 16.9 mL satisfies the burette's physical limits, the experimental instructions, and the requirement to titrate past the equivalence point.
Although such instruction likely connected cognitive and psychomotor dimensions, Alexandra's noticing again stemmed from her perspective. When asked why she referenced the burette, Alexandra shared how she encourages students to ponder questions like “what am I using?” and “what's my equipment?” because that is what Alexandra learned while working “in a research lab” (stretch). Interestingly, Alexandra did not inquire whether her students have analogous research experiences. Additionally, Alexandra provided sample questions and answers without waiting for students’ responses. Providing information that is external to students’ experiences provides an opportunity for but does not guarantee students’ finding relevance (Berg and Moon, 2022). Per Meaningful Learning, students must choose to engage with new information via their prior knowledge (Bretz, 2001). Despite possible intentions, Alexandra was likely engaging with her own cognitive and psychomotor domains as opposed to that of her students.
Alexandra's noticing also did not capitalize on students’ conversation about their shared mistake. Perhaps Alexandra was motivated to quickly resolve students’ questions. During the interview, Alexandra interpreted how students “think they’re not allowed to collaborate and […] are afraid to ask each other.” Working in groups can be unwelcoming, stressful, and unprofitable for students who perceive differences in their preparation and culture (Nardo et al., 2022). But, others argue that collaboration can advance understanding (Moon et al., 2018; Finkenstaedt-Quinn et al., 2019; Walker et al., 2023). Alexandra could have encouraged students’ sharing their mutual experiences, boosting their affective engagement. Instead, Alexandra maintained her own authority, reinforcing the “sage on the stage” motif in which answers flow from instructor to student (Freire, 2000; Schafer et al., 2023). Dialogic discourse could be an viable alternative strategy (Carlos et al., 2023). By pausing, asking, and listening for students’ perspectives (i.e., “How are your calculations similar/dissimilar with your peers, and why?”), TAs can encourage students to resolve their own answers collaboratively, thereby minimizing notions of “sage on the stage.” Table 5 summarizes Alexandra's equitable and effective instruction.
| Event # | Equitable instruction via multidimensional noticing | Effective instruction via teaching for meaningful learning | Teacher noticing (attend–interpret–respond) |
|---|---|---|---|
| 1 | • Stretch: Alexandra's experiences as a student | • Cognitive: chemistry symbols, molar amounts of chemical species, and acid–base calculations | • Addressed student's question about number placement |
| • Expanse: alternatives to the BCA table for calculations | • Contextualized using Alexandra's perspective and experiences | ||
| • Did not explicitly address how acid/base can be conflated with protons/hydroxides during instruction | |||
| 2 | • Stretch: Alexandra's experiences as a researcher | • Cognitive: acid–base calculations | • Discerned students' confusion with calculating moles |
| • Expanse: various scenarios of calculating MAVA = MBVB | • Psychomotor: the volume capacity of a burette and experimental restrictions on permitted use of reagents by volume | • Recognized own thought process as useful model for students to emulate | |
| • Reinforced TA as source of knowledge | |||
Experimentations in acid–base titrations
Bred's (GC1) lesson involved titrating a triprotic citric acid solution of unknown concentration with a standardized base solution. During the interview, Bred shared that the most important takeaway was “the neutralization process more like, chemically…one proton, like, reacts with one hydroxide atom, like OH−.” At first glance, Bred appeared to describe students’ particulate-level interactions. However, Bred then stated that “doing some mathematical calculations” is a prerequisite for understanding chemistry concepts, agreeing to the interviewer's clarification that calculations comprise the essential first step.Bred's event 1: clarifying an error, monoprotic vs. triprotic acids
During Bred's instruction, there were instances that invited students’ cognitive and affective learning. Bred attended to his student's confusion regarding the moles of citric acid. After Bred had incorrectly responded that citric acid is monoprotic, the student paused and then pointed to the laboratory manual to confirm that citric acid is triprotic. Abruptly standing up and stepping back, Bred loudly called for everyone's attention. He then apologized, announcing the relevant laboratory manual page to rectify his error. Bred also reified this message by writing the ratio of monoprotic base to triprotic acid on the whiteboard. Near the end of this event, Bred turned and publicly thanked the student by name for catching this mistake.Bred likely leveraged stretch by circumventing future errors and supported students’ cognitive learning by filtering out unnecessary uncertainty. Instructors’ minimizing distractors and extraneous information enables students to focus on concepts and their inherent complexity (Sweller, 2011). Additionally, Chen and Qiao (2020) highlight the importance of managing uncertainty (i.e., identifying, unpacking, and reducing) as students may not understand what information is distracting until indicated by an instructor.
Bred's later interpretation further supports his perceived teaching responsibility:
“I'm trying to, like, be a teacher like, who can just suggest them to the route […] there's like a big stem and they can, like, make a branch out of it to get into the other, like, concepts. So I'm trying to, like, build a stem […] for the students and make them—by themselves make out their branches.” – Bred (18:38–19:44).
Bred affirmed his role (“I’m trying to build”, emphasis added) in initiating the “stem” of a calculation, upon which students would then independently comprehend specific acid–base phenomena (“by themselves make their branches”, emphasis added). Bred's goal of providing the first step likely requires being accurate and precise, reinforced by prior findings in which TAs were trained and prescribed to disseminate instructions verbatim (Miller and Wu, 2025). Bred's interpretation also manifests more clearly given his gratitude (“You saved my life”) in preventing the loss of points. By giving the student credit for resolving a TA error, Bred may have created an affective opportunity for this student to momentarily became the source of correct information (Hand, 2012). Just as Galloway et al. (2016) showed that emotions can influence cognitive and psychomotor behaviors, Bred's interacting with the students' uncertainty about how proticity affects acid–base calculations (cognitive) and later thanking the student (affective) may have been an instance of expanse in which two dimensions of Meaningful Learning were linked.
However, Bred did not appear to use such uncertainty (or its resolution) as an invitation for all students to engage cognitively and affectively. Facilitating student exploration of discrepancies with each other can lead to deeper engagement with concepts, benefitting their overall comprehension (Haverly et al., 2020; Chen, 2022). Although Bred legitimized a single student's contribution, how Bred exhibited authority via the class-wide announcement (re)contextualizes his earlier quote. Bred may not have considered students’ collaborative branching as noteworthy, with the implication that learning occurs when the instructor deposits knowledge into the minds of learners (Freire, 2000). Bred may still be implicitly prioritizing individual completion as the metric for laboratory success (DeKorver and Towns, 2015).
Bred's event 2: manipulating a burette during a titration
Bred's second event involved the psychomotor and affective domains. Nearing the end of the session, Bred attended to a student's question about how much base solution to add. Bred then drew attention to the necessary colour change (i.e., pale pink), which would signal the end point. He then took a step backwards but remained nearby. After glancing around the laboratory and looking at the clock, Bred leaned in between the student and the experimental setup. Bred acknowledged that the student had only 30 minutes left and revealed the volume of base solution needed to finish the titration. Towards the end, Bred responded that he would help her get to the end point. The event concluded with Bred manipulating the burette and swirling the analyte flask.Bred's commandeering the titration, while potentially saving the student time and mollifying frustration, may be at the expense of the student's psychomotor learning. Prior chemistry education research has shown instructional laboratories providing opportunities for students to gain expertise in a variety of experimental techniques and science practices (Hensiek et al., 2016; Reynders et al., 2019; Walker et al., 2023). These studies collectively underscore that the psychomotor dimension is an integral component of chemistry laboratory learning. However, as Bred had demonstrated, TAs in general may be more obligated to prioritize the experimental outputs in lieu of the process when time is limited. Combined with Bred's interview, we see that his enacted instruction may have been further compounded by how stretch and expanse were noticed.
When asked to explain his instructional decision making, Bred interpreted that “[she] doesn’t know what she's doing chemically” and typically relies on nearby peers “to like teach [her] one by one.” Bred's remaining nearby to evaluate whether “[the student] can do the next procedure by [herself]” was likely motivated by his stretching into the student's past difficulties. Bred may have been accustomed to seeing the student do well and/or make progress when receiving direct instruction. Scholars have warned that helping via excessive direct instruction could dampen intellectual exploration and creativity (Kapur, 2016). Long-term learning is better supported by instructor moves that allow students to experience short-term struggles (Kapur and Bielaczyc, 2012; Kapur, 2016). What remains ambiguous is whether TAs understand how to strategically raise, maintain, and resolve short-term struggles (Chen and Techawitthayachinda, 2021) beyond just providing the answer or completing the experiment for students.
Bred's perception that this student would unproductively struggle without his assistance could be interpreted as expanse that interferes with equitable instruction. We recognize that, given how time was running out, both Bred and the student could have been overwhelmed (Flaherty, 2022), stressed (Miller and Lang, 2016), and anxious (Ural, 2016). Bred's commitment to the experimental “heavy lifting” may entail Bred assuming the saved time would foster the student's future cognitive and affective learning. By not recognizing student assets in this specific moment, Bred inadvertently strengthened correctness and completeness as dominant aspects of instructional laboratory culture. Louie and colleagues (2021) differentiated types of framing in which various values (discipline-first or student-first) transforms Teacher Noticing. Bred's event thus hints at a broader system obligating certain TA behaviours. Rather than identifying possibilities in which students’ assets can amplify teaching in difficult moments, TAs like Bred are likely conditioned to first notice the various ways they could do the experiment in lieu of their students. Table 6 summarizes Bred's equitable and effective instruction.
| Event # | Equitable instruction via multidimensional noticing | Effective instruction via teaching for meaningful learning | Teacher noticing (attend-interpret-respond) |
|---|---|---|---|
| 1 | • Stretch: minimization of future student errors in which Bred is at fault | • Cognitive: chemistry symbols, molar amounts of chemical species, acid–base calculations, and reduction of cognitive load | • Realized TA mistake, warranting class-wide announcement |
| • Expanse: different resources (announcement, laboratory manual, and whiteboard) for key information | • Affective: gratitude toward student | • Created opportunity for student to “take up space” by expressing gratitude | |
| • Focused on individual progress rather than class-wide sensemaking | |||
| 2 | • Stretch: student's past difficulties working independently | • Affective: student discomfort with uncertainty | • Identified that the student will continue to struggle without intervention |
| • Expanse: possible actions Bred can enact to ensure accurate, correct, and completed results for students | • Psychomotor: Bred's manipulation of burette and analyte flask during titration | • Recognized student's prior difficulties with laboratory experiments | |
| • Assisted student with titration due to time constraints | |||
Recapping Alexandra's and Bred's equitable and effective instruction
Our findings foregrounded the complicated nature of enacted laboratory instruction in terms of equity and effectiveness. In terms of RQ1, how TAs attended to stretch and expanse via Multidimensional Noticing varied. Alexandra stretched into her past experiences, rather than her students’, to inform how she shaped chemistry learning opportunities. Bred stretched into his student's past struggles, not as a resource for learning but as reason to take over the titration. What Alexandra and Bred both considered noteworthy as expanse was also nuanced but primarily connected with broader themes of laboratory norms and priorities (e.g., calculations, notions of epistemic authority, and efficiency) and their expected roles as TAs.In terms of RQ2, teaching for Meaningful Learning involved instances of the three dimensions but never all dimensions simultaneously. While Alexandra focused on the cognitive dimension by showcasing various approaches to calculate the volume of base in her second event, she did the calculations and most of the talking instead of her students. Bred, in some instances, appeared to bridge the cognitive and affective dimensions. But, he also minimized psychomotor opportunities by disclosing the volume needed to reach the end point and completing the remainder of the titration to help the student finish on time.
Limitations
We reflected on this study's limitations regarding study design, sample, and prompt (Rodriguez et al., 2024). While videos provide rich data, participant actions were sometimes not visible due to the camera's positioning. Addressing this limitation involved using multimodal analysis and data triangulation to develop a more holistic understanding of TAs' decision making and instruction. Including two TAs and excluding newly considered constructs (e.g., conative, social, and epistemic dimensions) in recent interpretations of Meaningful Learning (Agustian, 2022) may also limit our generalizability. Given how TA populations are historically new to teaching, our narrow scope intends to enhance feasibility of potential uptake for other institutions. By focusing on the experiences of new TAs, we hope to provide a foothold for TA stakeholders to have more advanced conversations about reformed-based instruction and its feasibility.Implications
We first recommend others to leverage our conceptual framework to better understand how TAs may use Multidimensional Noticing and teach for Meaningful Learning. The chemical education research community should consider new observation protocols (Velasco et al., 2016) that closely attend to the complexities of real time teaching and the extent instructional moves specifically align with equitable and effective teaching. Second, we invite scholars to deeply investigate the mechanisms of how TAs learn about pedagogy and translate their ideas into enacted practice. For example, one could explore the processes of TA inquiry via long-term training programs. A model such as video club (van Es and Sherin, 2010) or instructional coaching (Fantone et al., 2023) could be useful contexts for generating new insights of how TAs themselves make sense of the Gordian knots of their teaching practice. Lastly, to better externalize how TAs may dynamically undergo pedagogical decision making, digital surveys filled out periodically during classroom instruction could characterize how TAs' perceptions of what is noteworthy in their classroom changes over time (Shoshani, 2025).For practitioners, we share Rodriguez and Towns' (2019) sentiments that suggestions should be specific, accessible, and practical. When teaching acid–base concepts, we recommend less focus on calculations and more focus on particulate interactions and mechanistic reasoning to promote equity and effectiveness. Instructors can specifically draw inspiration from Pedagogical Chemistry Sensemaking, a lesson-planning framework that simultaneously uses the limitations/utilities of Johnstone's triangle when promoting students' conceptual understanding (Wu and Yezierski, 2022). To foreground potential conflation between H+/OH−(aq) ions and acid/base, respectively, TAs can use prompts such as, “How would rinsing the sides of my analyte flask containing 0.1 M H2SO4 with deionized water vs. tap water change my results and how molarity is visualized?” TAs and students could then evaluate how and why the titration curve would be altered as well as the visual changes when representing solvated ions, bulk water molecules, and the dissociation of H2SO4 in aqueous solution. Such discussions may require TAs to incorporate stretch to draw upon students’ prior coursework or reference future chemistry topics that students may encounter. Additionally, student uncertainty can be more intentionally harnessed for Multidimensional Noticing and teaching for Meaningful Learning. The Student Uncertainty as a Pedagogical Resource (SUPeR) framework (Chen and Jordan, 2024; Chen et al., 2025) could be adapted for higher education contexts to incentivize exploration, interconnecting psychomotor, cognitive, and affective dimensions. An example could include investigating how soil acidity impacts crop growth. TAs can incorporate a think-pair-share activity for students to experimentally plan how pH affects soil quality and crop output. Alternatively, a related experiment could deliberately incorporate experimental “errors” (e.g., adding too much acid or base) to rationalize the protocol. TAs may also use expanse to interconnect students’ acid–base knowledge with food (from field to plate) (Opara and Mazaud, 2001) and nutritional physiology (Manz, 2001). Teaching opportunities could involve engaging with acid–base impacts on agriculture (especially if there are nearby local farming industries); students’ awareness of food deserts, unhealthy eating behaviours, and body dissatisfaction associated with university life (Almoraie et al., 2024); and/or their food heritage and cultural knowledge (Kapelari et al., 2020) to contextualize acid–base consumption.
Conclusions
Like other TAs, Alexandra and Bred want to help their students learn chemistry (Herrington and Nakhleh, 2003). Nevertheless, TAs are sandwiched by various tensions that may obligate other instructional behaviours than intended. Efforts to improve equity may be at the expense of students’ cognitive, psychomotor, and affective learning. In addition, effective teaching may also be impeded if dimensions of Meaningful Learning are not simultaneously emphasized. Our findings raise an intriguing question: are extant TA practices essentially demonstrations of their own laboratory learning? If so, TAs may be the ones who are actually learning instead of their students. Staff meetings, experimental curricula, and instructional laboratory culture must consequently define the nature of teaching and how it unfolds moment to moment. Disrupting the status quo requires blurring the lines separating TAs from students (Freire, 2000) where epistemic authority is shared and knowledge can be equitably, as well as effectively, co-constructed (Raviv et al., 2003). Perhaps reforming how we train TAs (via video club or instructional coaching) as well as foregrounding particulate-level interactions, sensemaking, stretch (through prior and future coursework), and expanse (local agriculture, eating habits and nutrition at universities, and food heritage) can yield new opportunities to more meaningfully teach acid–base titrations.Author contributions
The last author recruited participants, conducted the interviews, and chose focal events from video data. The first and last authors collected the recordings, engaged in data analysis, and met weekly for dialectic negotiations. The first author was responsible for the original draft, while both first and last authors reviewed and edited the final draft.Conflicts of interest
There are no conflicts to declare.Data availability
To minimize a potential breach of participant confidentiality, the full data corpus supporting this work is not publicly available. However, focal TAs’ transcripts of events (gestures and utterances) and salient interview portions are included in the Appendix.Appendix
Table of participant information. Participants specific to this case study are bolded.| Course | Laboratory experiment | Pseudonym | Semester(s) of teaching experience |
|---|---|---|---|
| GC1 | Quantitative Analysis of the Citric Acid Content in Juices | Bred | 0 |
| Investigating heat transfer and calorimetry | Emily | 0 | |
| Paul | 0 | ||
| Sid | 0 | ||
| Eva | 2 | ||
| Kenny | 3 | ||
| Sophia | 4 | ||
| Emission of light and atomic models | Daniel | 0 | |
| Leyla | 0 | ||
| Ricky | 0 | ||
| Zach | 0 | ||
| Brutus | 2 | ||
| Determination of selected metal cations | Mark | 2 | |
| Wynn | >4 | ||
| GC2 | Strong acid and strong base titration | Alexandra | 0 |
| Finn | 0 | ||
| John | 0 | ||
| Jordan | 0 | ||
| Rain | 0 | ||
| Katie | 2 | ||
| Weak acid and strong base titration | Keira | 0 |
Alexandra's event 1 transcript, “Pinpointing where the “2” goes”
bolded = utterances, [italicized in square brackets] = gestures, {curly brackets} = inaudible utterances| Timestamp | Speaker | Utterance |
|---|---|---|
| 01:05:15 | TA | [Walks to student who is waiting with their hand raised] Yes? |
| 01:05:19 | Student | Can you see if my calculation correct? |
| 01:05:25 | TA | [Leans over student's lab notebook] Is this for your equivalence point? |
| 01:05:26 | Student | Yes. |
| 01:05:27 | TA | Yup, [stands up straight and turns to face student] that looks just around the right range. And then yeah, so once you get close to there, do the 0.1, or when you see the pH 3, whatever happens first. [turns away from student and takes a step] |
| 01:05:33 | Student | [Raises hand] Can I ask another quick question? |
| 01:05:34 | TA | [Turns and steps toward student] Yeah? |
| 01:05:35 | Student | Why does the 2 go on the bottom? I tried it on top, but it gave me a really big number. |
| 01:05:35 | TA | Looking at the calculation… [leans closer to student's lab notebook] The 2 corresponds to that OH−. So that's why the 2 is corresponding here [points to notebook]. It literally just lines up with our OH [stands up straight and faces student] because we see it as OH [cups hands like parentheses] two [moves hands downwards and to the right] in our formula, right? |
| 01:06:02 | Student | So for every one mole of H +, [TA leans in] there are two moles of OH−. |
| 01:06:10 | TA | [Stands up straight and turns toward student] Exactly, so it's a one to two ratio. [gestures with hands again, moving left to right] Exactly! That's exactly it. [walks away from student] |
Alexandra's event 2 transcript, “rationalizing numbers for a titration”
bolded = utterances, [italicized in square brackets] = gestures, {curly brackets} = inaudible utterances| Timestamp | Speaker | Utterance |
|---|---|---|
| 01:22:55 | Student A | Wait, do I divide the concentration by two because it's OH 2 ? |
| 01:22:59 | TA | [Standing at chalkboard, out of frame] So…if you divide it, it's gonna, it gives you a bigger number, which we know for this situation, it's gonna give you a funky number. So, here let me find a calculator. [rustles in bag] What was the umm base molarity? [Student B approaches] Do you remember off the top of your head? |
| 01:23:17 | Student A | It was zero point zero seven. |
| 01:23:18 | TA | [Typing into calculator] Zero…point zero seven. Okay so if we had forty, and we were to divide it by…sorry, I’m like putting you on the spot. Do you remember the molarity of the acid? Was it point zero five nine? |
| 01:23:30 | Student A | It was zero point zero five nine four. |
| 01:23:33 | Student B | Oh wait, I got this one wrong on the prelab too. |
| 01:23:36 | TA | Okay… |
| 01:23:37 | Student A | Which one did you get wrong? |
| 01:23:40 | Student B | It was one that was like this…with volumes and… {inaudible} |
| 01:23:43 | TA | Alright, we see when we just do this, the one to one, it just obviously gives us a crazy big number. Our burets don’t even go to forty-seven. So we know that can’t be right. Right? |
| 01:23:49 | Students A and B | Mm hmm. |
| 01:23:50 | TA | So, when we do [typing into calculator] point zero seven, if we were to divide that by two…let's plug in that number, just so we can see the actual kind of numbers it's gonna give us. Point zero five nine four divided by [trails off] and we see that being twenty-three point five six. And we know that's kind of, like logistically, we know that's not really realistic for our titration. We only can use twenty-five millilitres of our base, and we’re supposed to add enough where we see…let's see, where's that graph? This whole plateau. So just logistically, that number's not really gonna work. So, we multiply it by two because we have double the amount. It's kind of weird. |
| 01:24:36 | Student B | Do we divide by two at the end then? |
| 01:24:38 | TA | Let's see! [types into calculator] Sorry I need to like, look at the numbers to uhh. |
| 01:24:44 | Student B | No, you’re fine. |
| 01:24:45 | TA | So, we have forty… |
| 01:24:48 | Student B | My brain is not working. |
| 01:24:52 | TA | [Laughs while continuing to type on calculator] Okay…ahh! |
| 01:24:55 | Student B | So there's double? |
| 01:24:56 | TA | Oh, I’m like, yeah…blanking. |
| 01:25:00 | Student B | So for M A V A = MBVB…do we multiply one side by two or one-half? Is that how we do it? |
| 01:25:10 | TA | The M A V A … |
| 01:25:13 | Student A | I think we do the H + concentration times two. |
| 01:25:18 | TA | [Sighs] So…we’re looking specifically at our OH. ‘Cuz that's our OH, we have M(OH)2, right? Let me see. |
| 01:25:28 | Student B | I’m sweating up here. |
| 01:25:30 | TA | I know! It's—I always get real hot up in the front. So our HCl, [writing on chalkboard] we have M(OH)2. So when you guys got, did you guys get your, you guys got like sixteen point nine seven. Did you guys get that? |
| 01:25:48 | Student A | Yes. |
| 01:25:49 | TA | What were the steps you guys took to get there for that equivalence point? |
| 01:25:53 | Student A | Well, you multiply by…you can get it here— |
| 01:25:54 | Student B | Here? I didn’t get it here. |
Clips from Alexandra's interview
| Timestamp | Speaker | Utterance |
|---|---|---|
| Emphasizing acid–base calculations | ||
| 05:36 | Interviewer | I see, so speaking of your [pre-laboratory talk]—we're now in the during teaching or the interview phase—what would you say was the main purpose? Like, what did the, what did you want your students to take away from your [pre-laboratory talk]? |
| 05:50 | TA | Probably a better understanding of the calculations and hopefully having the calculations actually make sense with the content and what they're learning in lecture. Calculations are always, like, the most asked questions in my lab, so I really wanted to try to help kind of iron out some of those things before we get started. |
| 06:11 | Interviewer | Okay, and was there a specific calculation that you wanted to hone in on or was it just all the calculations? |
| 06:16 | TA | Um, it was probably the BCA tables. I think those, those trip up students a lot. That definitely tripped me up when I was taking that course. So, I tried to really hone in on that and push them to use that in their own calculations. |
| 06:32 | Interviewer | Okay, and if you don't mind elaborating, what aspect of the BCA table do you think trip students up or had tripped you up when you were a student? |
| 06:41 | TA | Yeah, I think it's the, that change line. So the coefficients in the balance equation kind of confused people. Depending on how you write the BCA table, I kind of found when I was teaching, I write mine a little bit differently than the students do. So there's kind of, like, a gap in that and trying to explain how I see it. But yeah, the change line is definitely the hardest. |
| Teaching in renovated vs unrenovated laboratories | ||
| 14:18 | Interviewer | How do you think your teaching would have changed if you taught in this [renovated laboratory] space, if at all? |
| 14:26 | TA | Based on the pictures, like, at least when I look at that, it looks like more of like a collaborative space. Just like, the seating. Like I think students would be more inclined to work with each other. So like, when I'm teaching [in the unrenovated laboratory space], they're all kind of in their individual stations. And I think they kind of think that, like, they're not allowed to collaborate. And so I get a big line of questions usually because I think students are afraid to ask each other…and this, I think, fosters that more working together environment. |
| Event 1 Discussion | ||
| 20:16 | Interviewer | So I wanted to revisit the whole moles of H+ and moles of OH−, right? To what extent do you think it's confusing for students in that, on one hand, in your balance reaction for this experiment they have it written like that 2 HCl and MOH2? But, on the other hand, when you're just comparing moles of acids and bases, it also makes intuitive sense to do it like that. So what are your, what's your takeaway from that? |
| 20:43 | TA | Yeah, I think it's definitely…for some, it kind of confuses them more than for some, it really helps them. So it's kind of, like, these polarized ends. When I was in [staff] meeting, and I can't remember who was presenting, but whoever presented had presented with this similar idea, and to me, it made sense. I was like, “Okay, perfect. That's what I'm gonna go with.” And then, when I got to that, then I kind of realized, like, that's not how they totally do it. So I tried to hone in on like, doing it either way, whatever way makes sense to them. |
| 21:23 | Interviewer | I'm curious, like, what about this method appeals to you? |
| 21:27 | TA | I think, it's just kind of a simplified, watered down version of this. I think, a lot of times when students see the products, they kind of get more overwhelmed, especially with, for whatever reason, like with H2O, and they see it as a liquid. They're like, “Is this really playing a role in things?” And then also just getting rid of like the Cl, and then whatever that “M” was in that situation, kind of also just helps them to, like, really hone in on this relationship and why that's important. |
| 21:57 | Interviewer | I see. So it sounds like you're gravitating to this method, because this one just has less distracting information…It drills down because of what you exactly are looking for. |
| 22:08 | TA | Yeah, and I think especially also, because for the equivalence point, they, you know, they stated as moles of H+ equals moles of OH−. So there's also confusion in the lab with people being like, “Well, is it, you know, HCl equals M(OH)2?” And it's, like, no…it's just this part. |
| Event 2 Discussion | ||
| 26:15 | Interviewer | A lot of the things that happened here are resonate with what you just described, you know, students being confused about the factor of two, do you multiply, do you divide it. I heard students talking about, like, the M1V1 = M2V2, which is, like, another way that you can approach this. It doesn't exactly have to be a BCA table, right? So what I am really curious about is…you talked about like logistics, you talked about how…your buret can't possibly fit that much volume, right? Why did you bring that up? |
| 26:48 | TA | It kind of is like, uhh kind of…I think back to, like, test taking strategies and how, you know, they say, you know, if you're taking an educated guess. Also, I work in a research lab, and I think from that, I've learned a lot about like, realistically, we can't see certain things, and how we can, you know, take our calculation and be like, “Okay, this doesn't make sense, because of what I'm actually going to see.” So I think it just kind of a different way to have them look at it and kind of build more like, like thinking reasonably, especially in lab settings. Umm, I think it also kind of helps to apply to other calculations, you know, so like, if they get really huge numbers, or really small numbers, they can think, “Okay, like, this doesn't feel right, let me think about why in settings of, what am I using? What's my equipment?” |
| 27:52 | Interviewer | So you talked about how, at least for you, especially working in a research lab, you're able to determine, you know, how realistic things are, and you can kind of compare with your expected values with your observed values, right? I'm curious, to what extent do you think your students are connecting the math with what they're actually doing in the lab? |
| 28:13 | Alexandra | Um, I think I think for [General Chemistry 2], it's like, kind of like, in the middle. I don't think it's like a great understanding. I don't think it's a bad understanding. I think it's hard because a lot of like, the [General Chemistry 2] calculations are very specific, you know, like seeing this, you know, the exact ratio, and I'm canceling out at the equivalence point. And then we look at their data, and they have a jump from three to nine in their pH. And so they're gonna get weird numbers, and they're not going to see this perfect, you know, cancellation like they're expecting. So I think, for them, it's hard to apply those ideas in lab, just because the data gets so funky with specific titrations. |
Bred's event 1 transcript, “clarifying an error, monoprotic vs. triprotic acids”
bolded = utterances, [italicized in square brackets] = gestures, {curly brackets} = inaudible utterances, redacted| Timestamp | Speaker | Utterance |
|---|---|---|
| 02:04:46 | Student | [lowers raised hand] I’m just confused on how to calculate the moles [points to notebook] in citric acid. |
| 02:04:50 | TA | So [raises hand and then drops it] |
| 02:04:51 | Student | Is it times three or is it divided by three? [looks up at TA] Because I know it's triprotic, but… |
| 02:04:55 | TA | Citric acid is monoprotic. |
| 02:04:57 | Student | It's mono? |
| 02:04:58 | TA | Yeah. |
| 02:04:58 | Student | Oh okay. [turns away from TA] |
| 02:04:58 | TA | So they would be the same right? |
| 02:04:59 | Student | Yeah. [nods head without looking back to TA] |
| 02:05:01 | TA | Did you look for the {inaudible} |
| 02:05:02 | Student | [brings head back, allowing TA to view lab manual] It says citric acid is triprotic right here. [points to lab manual] |
| 02:05:09 | TA | Oh my god… [whips head back to view the class with mouth agape] Ah… And I searched for the… |
| 02:05:14 | TA | Guys, I need to make an announcement. I’m so sorry. [holds up manual, pointing to page that states citric acid is triprotic] So followed by the notebook, it says citric acid is a triprotic acid. So you have to… How much you have to multiply? [gestures at student with raised hand] |
| 02:05:29 | Student | Three. |
| 02:05:30 | TA | Three, yeah. |
| 02:05:32 | Student | Okay, thanks. |
| 02:05:33 | TA | So mole of the citric acid would be 1/3 of the moles of NaOH right? |
| 02:05:40 | Student | Alright. |
| 02:05:41 | TA | The ratio would be 3 to 1. [walks away from students to whiteboard] See? [writing on whiteboard] Thanks, {Student Name}. [turns to look at student, then continues writing] So the moles of the NaOH would be triple of the citric acid. [pointing towards written information on whiteboard] That means the moles of citric acid should be divided by 3, with the moles of the NaOH, alright? [walks to TA desk, picks up then sets down lab manual] It was on the page 62 overview. [walks back to student] Thanks, {Student Name}, you saved my life. |
| 02:06:29 | Student | Okay so, just divide that by three? |
| 02:06:32 | TA | [Leans in towards student] Yeah, yeah, yeah. You did perfect. |
| 02:06:33 | Student | Sweet, thank you. |
Bred's event 2 transcript, “manipulating a burette during a titration”
bolded = utterances, [italicized in square brackets] = gestures, {curly brackets} = inaudible utterances| Timestamp | Speaker | Utterance |
|---|---|---|
| 01:40:18 | Student | Okay, umm my initial reading is zero point zero seven, right? |
| 01:40:23 | TA | Mm Hmm. |
| 01:40:24 | Student | And…now I add…this to here, correct? |
| 01:40:28 | TA | Yup, correct. |
| 01:40:29 | Student | But, how much am I supposed to add? |
| 01:40:32 | TA | How much? We don’t know that. |
| 01:40:34 | Student | We don’t know that? |
| 01:40:35 | TA | Yeah, we’re gonna put it in until we see the slight pink. |
| 01:40:39 | Student | Slight pink…okay. |
| 01:40:41 | TA | [Nods head] Did you put the uhh…color change— |
| 01:40:43 | Student | Yeah, I put the drops in it. |
| 01:40:45 | TA | And, when you put it in…you see the color change. |
| 01:40:47 | Student | Uh huh. |
| 01:40:48 | TA | When we see that, we’re gonna see it, like, totally. But you have to keep swirling it until we see it, okay? |
| 01:40:52 | Student | Yeah, okay. |
| 01:40:53 | TA | Alright, let's do it! [Nods and turns to walk away] |
| 01:40:55 | Student | Do I do it again? |
| 01:40:56 | TA | [Turns to face student] Yeah, you do it until you see total color change, like…eternal pink. |
| 01:41:02 | Student | Total color…okay… |
| 01:41:06 | TA | [Steps back, scans room, and looks at clock] I know you have 30 minutes left [leans in] so I’m gonna just tell you, like, it's going to be around, like, about 13–14. Then just put it in until like 13 and you’re gonna start dropping it until you see the total color change. |
| 01:41:22 | Student | Alright. |
| 01:41:22 | TA | So, I’m gonna help you get there. [grabs experimental setup and manipulates buret] |
| 01:41:24 | Student | Okay. |
| 01:41:50 | TA | [Stands up straight, still manipulating glassware] You see? It's turned to the yellow, [grabs rinse bottle] I mean the white. |
| 01:41:56 | Student | Yes. |
| 01:41:59 | TA | Now what you have to do is dropwise drop it like this. [manipulating flask and buret] Swirl it…Stop. Swirl it…Swirl it… |
| 01:43:14 | TA | [Sets flask down] It's starting to change really slowly, right? |
| 01:43:15 | Student | [murmurs in agreement] |
| 01:43:28 | TA | I’ll put in the drop, then you’re gonna see the color change. |
| 01:43:29 | Student | Alright. |
| 01:43:35 | TA | See? It totally changed to that slight pink. [Holds up flask to eye level] Your color is like really good because most of the students were like a deep pink. Anyway, I did it though! [chuckles] |
Clips from Bred's interview
| Timestamp | Speaker | Utterance |
|---|---|---|
| Important acid–base takeaways | ||
| 03:33 | Interviewer | What would you say is the most important thing for students to learn from the just totally from that lab? |
| 03:44 | TA | They need to know the neutralization process more like, chemically. Like, they feel like they’re just putting the same amount of the liquid and that it changed to the color like that. But as we’ve gone through the, like, diprotic and triprotric protons molecules, and then you need to do some like mathematical calculations…they then, they get into the concept like, “Oh, then that's like one proton like reacts with one hydroxide–hydroxide atom, like, OH minus.” Like that. And, that's the one they need to take on. |
| 04:25 | Interviewer | I see. So, the most important thing is that students need to know the calculations to get the concept because they need to realize there is a proton reacting with hydroxide anion, right? |
| 04:30 | TA | Yup. |
| Teaching strategies for chemistry | ||
| 18:34 | Interviewer | How would you just define a teaching strategy? |
| 18:39 | TA | Define a teaching strategy? When I study, I have to…like it's some kind of procedure to bring up the…uhh when we go to the answers. Like when you go near to the answers, you have to come up with the nearest concepts. So, I'm trying to, like, be a teacher like, who can just like suggest them to the route they can, they should be thinking—not like they should—but like they better be thinking, like from generalization to like more specific. Like this…And then they can reach out to the answer, and there's like a big stem, and they can like make a branch out of it to like get into the other like, concepts, I guess. So I'm trying to like build a stem…like we have gone through this semester—important sig figs and this chemical reactions. And in this titration, concepts like pH, like this. And, um, what I can do is teaching strategy is building a stand for the students and make them—by themselves make out their branches. |
| 20:12 | Interviewer | Sure, sure. So it sounds like the way that you define teaching strategy is…it's something that you do as well. You want to provide students the ideas about the pathway that they should take, right? That's the stem. And then eventually, you also want them to begin, once they have that pathway, they arrive to the answer to begin applying it and connecting it to different concepts. So it's the different branches basically. |
| 20:37 | TA | Mm hmm. |
| Related to Event 1 | ||
| 23:18 | Interviewer | Hypothetically, how would you respond to a student question that you did not know the answer to? |
| 23:24 | TA | I sometimes go to my desk and search for the question. Like my case, there was a lime juice just was a tri-… |
| 23:37 | Interviewer | Triprotic acid? |
| 23:38 | TA | Triprotic acid and it was written on the lab manuals, but I couldn't find it also. And I thought it was like simple like, my guess. I should look for it. And because I should not told them like, it's just monoprotic actually. I searched it. I don't know why but it appeared that it's a monoprotic acid. Actually, it was a triprotic acid. So I just after I searched it, and I looked through this lab manuals, I couldn't also find it but one of my students held them like, I think this is uh he was asking another question, but I found one, and I need to make this public like this is a triprotic acid we should like divide it by three like that. |
| 24:28 | Interviewer | Yeah, I remember that. Actually. It's funny that you mentioned because we'll revisit that particular event. |
| Event 1 Discussion | ||
| 51:13 | Interviewer | So the triprotic acid, okay. I actually think you can handled this really well. And like the way you did, it's totally fine! Like, it's actually not what I wanted to ask about. What I'm curious is, to what extent do you think your students understand what a triprotic acid means? And what's happening in terms of the neutralization reaction? |
| 51:37 | TA | I think I mentioned it on my [pre-laboratory talk]. And I also suggestion that this is diprotic—it wasn't a diprotic though—diprotic, like reaction process. It was sulfuric acid, I guess. Yeah, I expect them to understand if I say triprotic, and they would just realized that “Oh, there's three protons.” |
| Event 2 Discussion | ||
| 46:21 | Interviewer | So in this case, I think you're helping a student with the titration, right? There's not that much time left, and you roughly know, like, the volume needed to reach a equivalence point. So you're basically, like, helping them with a titration by doing, like, all the unnecessary parts first, but then you're giving it to the student in terms of doing it dropwise. Is that right? |
| 46:41 | TA | Mm hmm, mm hmm. |
| 46:43 | Interviewer | Okay. I'm just curious, like, what was your decision making process at this moment? |
| 46:46 | TA | Actually, like, every student's umm time, speed. I mean, that they're working, their velocity for titration was, was slow. Like this. Yeah, but it increased to the, after like second experiment for the titration of sodium hydroxide, and then just titration. Because in first step, they, like, stuck on to it, and there's a process—they have a realization, uhh thinking procedures. But for that student case, like she was like…doesn't know what they're doing, or what she's doing, actually. So I'm trying to teach her one by one, like, this is the one we expected, and this is why we need to titrate the sodium hydroxide, right? And then you can do it, next procedure by yourself. And then you can move on to the lemon juice titration. |
| 47:47 | Interviewer | To what extent do you think your students, like, kind of see the bigger picture of the lab? Like, do they see how, like, Part A is connected with Part B? |
| 48:01 | TA | I think they all know about that. But not every student can do it. Mostly they can think it, but I feel like…she's a [Student A] and she's the one who was like collaborating with [Student B]. And I know, [Student B] is really, really smart and productive student, and [Student B] tried to like teach [Student A] like one by one. And but this lab was an individual experiments. So, I feel like [Student A] was stuck onto the her own experiment. And the [Student C], next to her, not too much of a talkative guy. And so, I felt like I'm the one who has to like teach her during this lab. Yeah, that's what I would say. |
Acknowledgements
We would like to express gratitude for our TA participants and their willingness to let us investigate and understand their pedagogical practices and experiences. We also thank the laboratory supervisors and our undergraduate researchers, Mark Petzing and Kira Cheren, for supporting our research efforts throughout all stages.References
- Agustian H. Y., (2022) Considering the hexad of learning domains in the laboratory to address the overlooked aspects of chemistry education and fragmentary approach to assessment of student learning, Chem. Educ. Res. Pract., 23(3), 518–530 10.1039/D1RP00271F.
- Agustian H. Y. and Seery M. K., (2017), Reasserting the role of pre-laboratory activities in chemistry education: a proposed framework for their design, Chem. Educ. Res. Pract., 18(4), 518–532 10.1039/C7RP00140A.
- Almoraie N. M., Alothmani N. M., Alomari W. D. and Al-Amoudi A. H., (2024), Addressing nutritional issues and eating behaviours among university students: a narrative review, Nutr. Res. Rev., 38(1), 53–68 DOI:10.1017/S0954422424000088.
- Alvarado C., Cañada F., Garritz A. and Mellado V., (2015), Canonical pedagogical content knowledge by CoRes for teaching acid–base chemistry at high school, Chem. Educ. Res. Pract., 16(3), 603–618 10.1039/C4RP00125G.
- Aydin-Gunbatar S. and Akin F. N., (2022), Pre-service chemistry teachers’ use of pedagogical transformation competence to develop topic-specific pedagogical content knowledge for planning to teach acid–base equilibrium, Chem. Educ. Res. Pract., 23(1), 137–158 10.1039/D1RP00106J.
- Baldwin N. and Orgill M., (2019), Relationship between teaching assistants’ perceptions of student learning challenges and their use of external representations when teaching acid–base titrations in introductory chemistry laboratory courses, Chem. Educ. Res. Pract., 20(4), 821–836 10.1039/c9rp00013e.
- Bandyopadhyay S. and Rathod B. B., (2017), The sound and feel of titrations: a smartphone aid for color-blind and visually impaired students, J. Chem. Educ., 94(7), 946–949 DOI:10.1021/acs.jchemed.7b00027.
- Barr C. A., Brodeur D. R., Kumar U. and Heilman D. W., (2022), Integrating Authentic Research, Peer Learning, and High-Impact Project Work into the General Chemistry Laboratory, J. Chem. Educ., 99(12), 3899–3905 DOI:10.1021/acs.jchemed.2c00346.
- Berg S. A. and Moon A., (2022), Prompting hypothetical social comparisons to support chemistry students’ data analysis and interpretations, Chem. Educ. Res. Pract., 23(1), 124–136 10.1039/D1RP00213A.
- Blomberg G., Renkl A., Gamoran Sherin M., Borko H. and Seidel T., (2013), Five research-based heuristics for using video in pre-service teacher education, J. Educ. Res. Online, 5(1), 90–114 DOI:10.25656/01:8021.
- Boothe J. R., Zotos E. K. and Shultz G. V., (2023), Analysis of post-secondary instructors’ pedagogical content knowledge of organic acid–base chemistry using content representations, Chem. Educ. Res. Pract., 24(2), 577–598 10.1039/D2RP00253A.
- Bretz S. L., (2001), Novak's Theory of Education: Human Constructivism and Meaningful Learning, J. Chem. Educ., 78(8), 1107 DOI:10.1021/ed078p1107.6.
- Bretz S. L., (2019), Evidence for the Importance of Laboratory Courses, J. Chem. Educ., 96(2), 193–195 DOI:10.1021/acs.jchemed.8b00874.
- Bussey T. J., Orgill M. and Crippen K. J., (2013), Variation theory: A theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14(1), 9–22 10.1039/C2RP20145C.
- Calderhead J., (1981), Stimulated Recall: A Method for Research on Teaching, Br. J. Educ. Psychol., 51(2), 211–217 DOI:10.1111/j.2044-8279.1981.tb02474.x.
- Carless D., (2019), Feedback loops and the longer-term: towards feedback spirals, Assess. Eval. High. Educ., 44(5), 705–714 DOI:10.1080/02602938.2018.1531108.
- Carlos C. M. L., Maggiore N. M. and Dini V. et al.., (2023), Characterizing facilitation practices of learning assistants: an authoritative-to-dialogic spectrum, Int. J. STEM Educ., 10(1), 38 DOI:10.1186/s40594-023-00429-4.
- Carlson J., Daehler K. R., Alonzo A. C., Barendsen E., Berry A. and Borowski A. et al., (2019), The Refined Consensus Model of Pedagogical Content Knowledge in Science Education, in Hume A., Cooper R. and Borowski A. (ed.), Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science, Springer Nature, pp. 77–94 DOI:10.1007/978-981-13-5898-2_2.
- Chan P., Van Gerven T., Dubois J.-L. and Bernaerts K., (2021), Virtual chemical laboratories: a systematic literature review of research, technologies and instructional design, Comput. Educ. Open, 2, 100053 DOI:10.1016/j.caeo.2021.100053.
- Chen Y.-C., (2022), Epistemic uncertainty and the support of productive struggle during scientific modeling for knowledge co-development, J. Res. Sci. Teach., 59(3), 383–422 DOI:10.1002/tea.21732.
- Chen Y.-C. and Jordan M., (2024), Student Uncertainty as a Pedagogical Resource (SUPeR) approach for developing a new era of science literacy: practicing and thinking like a scientist, Sci. Act., 61(1), 1–15 DOI:10.1080/00368121.2023.2281694.
- Chen Y.-C., Park J. and Jordan M., (2025), Student Uncertainty as a Pedagogical Resource (SUPeR): an approach for phenomena-based science teaching, Sci. Act., 62(2), 105–119 DOI:10.1080/00368121.2024.2419086.
- Chen Y.-C. and Qiao X., (2020), Using students’ epistemic uncertainty as a pedagogical resource to develop knowledge in argumentation, Int. J. Sci. Educ., 42(13), 2145–2180 DOI:10.1080/09500693.2020.1813349.
- Chen Y.-C. and Techawitthayachinda R., (2021), Developing deep learning in science classrooms: Tactics to manage epistemic uncertainty during whole-class discussion, J. Res. Sci. Teach., 58(8), 1083–1116 DOI:10.1002/tea.21693.
- Conlin L. D. and Scherr R. E., (2018), Making Space to Sensemake: Epistemic Distancing in Small Group Physics Discussions, Cogn. Instruct., 36(4), 396–423 DOI:10.1080/07370008.2018.1496918.
- Connor M. C. and Shultz G. V., (2018), Teaching assistants’ topic-specific pedagogical content knowledge in 1H NMR spectroscopy, Chem. Educ. Res. Pract., 19(3), 653–669 10.1039/C7RP00204A.
- Cummins J., Brown K. and Sayers D., (2007), Literacy, technology, and diversity: Teaching for success in changing times, Pearson Boston, MA.
- D’Agostino A. T., (2022), Accessible Teaching and Learning in the Undergraduate Chemistry Course and Laboratory for Blind and Low-Vision Students, J. Chem. Educ., 99(1), 140–147 DOI:10.1021/acs.jchemed.1c00285.
- DeKorver B. K. and Towns M. H., (2015), General Chemistry Students’ Goals for Chemistry Laboratory Coursework, J. Chem. Educ., 92(12), 2031–2037 DOI:10.1021/acs.jchemed.5b00463.
- DeKorver B. K. and Towns M. H., (2016), Upper-level undergraduate chemistry students’ goals for their laboratory coursework, J. Res. Sci. Teach., 53(8), 1198–1215 DOI:10.1002/tea.21326.
- Derry S. J., Pea R. D., Barron B., Engle R. A., Erickson F. and Goldman R. et al., (2010), Conducting Video Research in the Learning Sciences: Guidance on Selection, Analysis, Technology, and Ethics, J. Learn. Sci., 19(1), 3–53 DOI:10.1080/10508400903452884.
- Donis K., Aikens M. L., Swamy U., Delgado M., Gillespie M., Graves P. and Eddy S. L., (2024), Learning Assistants and Instructors Provide Social Support That Influences Student Engagement Differently in Undergraduate Active Learning Chemistry Courses, J. Chem. Educ., 101(8), 2989–3002 DOI:10.1021/acs.jchemed.3c01137.
- Dragisich V., Keller V. and Zhao M., (2016), An Intensive Training Program for Effective Teaching Assistants in Chemistry, J. Chem. Educ., 93(7), 1204–1210 DOI:10.1021/acs.jchemed.5b00577.
- Egambaram O., Hilton K., Leigh J., Richardson R., Sarju J., Slater A. and Turner B., (2022), The Future of Laboratory Chemistry Learning and Teaching Must be Accessible, J. Chem. Educ., 99(12), 3814–3821 DOI:10.1021/acs.jchemed.2c00328.
- Esping-Andersen G., (2004). Untying the Gordian knot of social inheritance, Res. Soc. Stratif. Mobil., 21(1), 115–138 DOI:10.1016/S0276-5624(04)21007-1.
- Fantone R. C., Zaimi I., Meserve K., Geragosian E. K., Álvarez-Sánchez C. O., Spencer J. L. and Shultz G. V., (2023), Chemistry Instructional Coaching: Adapting a Peer-Led Professional Development Program for Chemistry Graduate Teaching Assistants, J. Chem. Educ., 100(11), 4343–4350 DOI:10.1021/acs.jchemed.3c00580.
- Finkenstaedt-Quinn S. A., Snyder-White E. P., Connor M. C., Gere A. R. and Shultz G. V., (2019), Characterizing Peer Review Comments and Revision from a Writing-to-Learn Assignment Focused on Lewis Structures, J. Chem. Educ., 96(2), 227–237 DOI:10.1021/acs.jchemed.8b00711.
- Flaherty A. A., (2020), A review of affective chemistry education research and its implications for future research, Chem. Educ. Res. Prac., 21(3), 698–713 10.1039/C9RP00200F.
- Flaherty A., (2022), The Chemistry Teaching Laboratory: A Sensory Overload Vortex for Students and Instructors? J. Chem. Educ., 99(4), 1775–1777 DOI:10.1021/acs.jchemed.2c00032.
- Freire P., (2000), Pedagogy of the Oppressed, 30th Anniversary. Continuum International Publishing.
- Galloway K. R. and Bretz S. L., (2015), Development of an Assessment Tool To Measure Students’ Meaningful Learning in the Undergraduate Chemistry Laboratory, J. Chem. Educ., 92(7), 1149–1158 DOI:10.1021/ed500881y.
- Galloway K. R., Malakpa Z. and Bretz S. L., (2016), Investigating Affective Experiences in the Undergraduate Chemistry Laboratory: Students’ Perceptions of Control and Responsibility, J. Chem. Educ., 93(2), 227–238 DOI:10.1021/acs.jchemed.5b00737.
- Gazdag E., Nagy K. and Szivák J., (2019), “I Spy with My Little Eyes…” The use of video stimulated recall methodology in teacher training – The exploration of aims, goals and methodological characteristics of VSR methodology through systematic literature review, Int. J. Educ. Res., 95, 60–75 DOI:10.1016/j.ijer.2019.02.015.
- Geertz C., (2008), Thick Description: Toward an Interpretive Theory of Culture, The Cultural Geography Reader, Routledge.
- Geragosian E. K., Zhu D., Skriloff M. and Shultz G. V., (2024), Chemistry graduate teaching assistants’ teacher noticing, Chem. Educ. Res. Pract., 25(1), 300–312 10.1039/D3RP00003F.
- Grinath A. S. and Southerland S. A., (2019), Applying the ambitious science teaching framework in undergraduate biology: responsive talk moves that support explanatory rigor, Sci. Educ., 103(1), 92–122 DOI:10.1002/sce.21484.
- Gupte T., Watts F. M., Schmidt-McCormack J. A., Zaimi I., Gere A. R. and Shultz G. V., (2021), Students’ meaningful learning experiences from participating in organic chemistry writing-to-learn activities, Chem. Educ. Res. Pract., 22(2), 396–414 10.1039/D0RP00266F.
- Hand V., (2012), Seeing culture and power in mathematical learning: toward a model of equitable instruction, Educ. Stud. Math., 80(1), 233–247 DOI:10.1007/s10649-012-9387-9.
- Hansson S. O., (2009). Cutting the Gordian knot of demarcation, Int. Stud. Philosophy Sci., 23(3), 237–243. DOI:10.1080/02698590903196007.
- Haverly C., Calabrese Barton A., Schwarz C. V. and Braaten M., (2020), “Making Space”: How Novice Teachers Create Opportunities for Equitable Sense-Making in Elementary Science, J. Teach. Educ., 71(1), 63–79 DOI:10.1177/0022487118800706.
- Hennah N. and Seery M. K., (2017), Using digital badges for developing high school chemistry laboratory skills, J. Chem. Educ., 94(7), 844–848 DOI:10.1021/acs.jchemed.7b00175.
- Hensiek S., DeKorver B. K., Harwood C. J., Fish J., O’Shea K. and Towns M., (2016), Improving and Assessing Student Hands-On Laboratory Skills through Digital Badging, J. Chem. Educ., 93(11), 1847–1854 DOI:10.1021/acs.jchemed.6b00234.
- Herrington D. G. and Nakhleh M. B., (2003), What Defines Effective Chemistry Laboratory Instruction? Teaching Assistant and Student Perspectives, J. Chem. Educ., 80(10), 1197 DOI:10.1021/ed080p1197.
- Hugerat M., Najami N., Abu-Much R., Khatib W. and Hofstein A., (2018), Making the Learning of Acid-Base Concepts More Relevant-A Research Study, J. Lab. Chem. Educ., 6(2), 36–45 DOI:10.5923/j.jlce.20180602.04.
- Jacobs V. R., Lamb L. L. C. and Philipp R. A., (2010), Professional Noticing of Children's Mathematical Thinking, JRME, 41(2), 169–202 DOI:10.5951/jresematheduc.41.2.0169.
- Jimenez A. G. and August D. P., (2025), Identifying skill inequalities in undergraduate chemistry laboratory teaching, Chem. Educ. Res. Pract., 26, 926–935 DOI:10.1186/s40594-023-00429-4.
- Johnstone A. H., (1982), Macro and microchemistry, Sch. Sci. Rev., 64, 377–379.
- Jordan B. and Henderson A., (1995), Interaction Analysis: Foundations and Practice, J. Learn. Sci., 4(1), 39–103 DOI:10.1207/s15327809jls0401_2.
- Kapelari S., Alexopoulos G., Moussouri T., Sagmeister K. J. and Stampfer F., (2020), Food heritage makes a difference: the importance of cultural knowledge for improving education for sustainable food choices, Sustainability, 12(4), 1509 DOI:10.3390/su12041509.
- Kapur M., (2016), Examining Productive Failure, Productive Success, Unproductive Failure, and Unproductive Success in Learning. Educ. Psychol., 51(2), 289–299 DOI:10.1080/00461520.2016.1155457.
- Kapur M. and Bielaczyc K., (2012), Designing for Productive Failure, J. Learn. Sci., 21(1), 45–83 DOI:10.1080/10508406.2011.591717.
- Kurdziel J. P., Turner J. A., Luft J. A. and Roehrig G. H., (2003), Graduate Teaching Assistants and Inquiry-Based Instruction: Implications for Graduate Teaching Assistant Training, J. Chem. Educ., 80(10), 1206 DOI:10.1021/ed080p1206.
- Larkin E., Ahn D., Ahn N., Alzarooni F., Busaibe Y., Cho S. Y., Lee S., Madani B., Mun J., Quirós-Canales K. and Rabeh N., (2023), Modernizing Titrations in the Undergraduate Laboratory: No More Burets and End Point Confusion, Just a Top-Loading Balance and a Smartphone, J. Chem. Educ., 101(2), 438–447 DOI:10.1021/acs.jchemed.3c00799.
- Lau P. N., Teow Y., Low X. T. T. and Tan S. T. B., (2023), Integrating chemistry laboratory–tutorial timetabling with instructional design and the impact on learner perceptions and outcomes, Chem. Educ. Res. Pract., 24(1), 12–35 10.1039/D2RP00055E.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, SAGE.
- Louie N., Adiredja A. P. and Jessup N., (2021), Teacher noticing from a sociopolitical perspective: the FAIR framework for anti-deficit noticing, ZDM Math. Educ., 53(1), 95–107 DOI:10.1007/s11858-021-01229-2.
- Mahaffey A. L., (2021), 1-2-3 Benchtop to Laptop: Teamwork of an Educator and Instructional Designer to Convert a Popular Ksp and Titration Lab to an Online Module, J. Chem. Educ., 98(6), 1928–1936 DOI:10.1021/acs.jchemed.0c01281.
- Manz F., (2001), History of nutrition and acid–base physiology, Eur. J. Nutr., 40(5), 189–199 DOI:10.1007/s394-001-8346-7.
- Merriam S. B. and Tisdell E. J., (2016), Qualitative Research: A Guide to Design and Implementation, 4th edn, Jossey-Bass.
- Miller D. K. and Lang P. L., (2016), Using the Universal Design for Learning Approach in Science Laboratories To Minimize Student Stress, J. Chem. Educ., 93(11), 1823–1828 DOI:10.1021/acs.jchemed.6b00108.
- Miller C. and Wu M.-Y. M., (2025), Signals from Staff Notes: Investigating Diachronous Messages for Being a Laboratory Teaching Assistant, J. Chem. Educ., 102(2), 495–507 DOI:10.1021/acs.jchemed.4c01210.
- Moon A., Zotos E., Finkenstaedt-Quinn S., Ruggles Gere A. and Shultz G., (2018), Investigation of the role of writing-to-learn in promoting student understanding of light–matter interactions, Chem. Educ. Res. Pract., 19(3), 807–818 10.1039/C8RP00090E.
- Murray S. A., Huie R., Lewis R., Balicki S., Clinchot M. and Banks G. et al., (2020), Teachers’ Noticing, Interpreting, and Acting on Students’ Chemical Ideas in Written Work, J. Chem. Educ., 97(10), 3478–3489 DOI:10.1021/acs.jchemed.9b01198.
- Nardo J. E., Chapman N. C., Shi E. Y., Wieman C. and Salehi S., (2022), Perspectives on Active Learning: Challenges for Equitable Active Learning Implementation, J. Chem. Educ., 99(4), 1691–1699 DOI:10.1021/acs.jchemed.1c01233.
- Nasir N. S. and Shah N., (2011), On Defense: African American Males Making Sense of Racialized Narratives in Mathematics Education, JAAME, 2(1), 24–45.
- National Academies of Sciences, Engineering, and Medicine, (2025), in Holmes A., Brenner K. and Gao J. (ed.), Transforming Undergraduate STEM Education: Supporting Equitable and Effective Teaching, National Academies Press DOI:10.17226/28268.
- National Science Board and National Science Foundation, (2024), Science and Engineering Indicators 2024: The State of U.S. Science and Engineering 2024.
- Nicklow J. W., Marikunte S. S. and Chevalier L. R., (2007), Balancing Pedagogical and Professional Practice Skills in the Training of Graduate Teaching Assistants, J. Profess. Issues Eng. Educ. Pract., 133(2), 89–93 DOI:10.1061/(ASCE)1052-3928(2007)133:2(89).
- Novak J. D., (1998), Learning, Creating, and Using Knowledge: Concept Maps(tm) As Facilitative Tools in Schools and Corporations, 1st edn, Mahwah, NJ: Routledge DOI:10.4324/9781410601629.
- O’Neal C., Wright M. and Cook C., (2007), The Impact of Teaching Assistants on Student Retention in the Sciences: Lessons for TA Training, J. College Sci. Teach., 36(5), 24–29.
- Opara L. U. and Mazaud F., (2001), Food traceability from field to plate, Outlook Agric., 30(4), 239–247 DOI:10.5367/000000001101293724.
- Pentucci M., Mascitti A., d’Alessandro N., Tonucci L. and Coccia F., (2025), Developing self-reflection in students: a case study in chemistry education, Chem. Educ. Res. Pract., 26, 834–845 10.1039/D4RP00368C.
- Ralph V. R., Scharlott L. J., Schafer A. G. L., Deshaye M. Y., Becker N. M. and Stowe R. L., (2022), Advancing Equity in STEM: The Impact Assessment Design Has on Who Succeeds in Undergraduate Introductory Chemistry, JACS Au, 2(8), 1869–1880 DOI:10.1021/jacsau.2c00221.
- Raviv A., Bar-Tal D., Raviv A., Biran B. and Sela Z., (2003), Teachers’ Epistemic Authority: Perceptions of Students and Teachers, Soc. Psychol. Educ., 6(1), 17–42 DOI:10.1023/A:1021724727505.
- Reid N. and Shah I., (2007), The role of laboratory work in university chemistry, Chem. Educ. Res. Pract., 8(2), 172–185 10.1039/b5rp90026c.
- Reynders G., Suh E., Cole R. S. and Sansom R. L., (2019), Developing Student Process Skills in a General Chemistry Laboratory, J. Chem. Educ., 96(10), 2109–2119 DOI:10.1021/acs.jchemed.9b00441.
- Rodriguez J.-M. G., Finkenstaedt-Quinn S. A., Watts F. M. and Nardo J. E., (2024), Self-Reported Limitations in Chemistry Education Research: Providing Specific and Contextualized Limitations Supports Researchers and Practitioners. J. Chem. Educ., 101(7), 2602–2607 DOI:10.1021/acs.jchemed.4c00217.
- Rodriguez J.-M. G. and Towns M. H., (2019), Alternative Use for the Refined Consensus Model of Pedagogical Content Knowledge: Suggestions for Contextualizing Chemistry Education Research, J. Chem. Educ., 96(9), 1797–1803 DOI:10.1021/acs.jchemed.9b00415.
- Rosa V., States N. E., Corrales A., Nguyen Y. and Atkinson M. B., (2022), Relevance and equity: should stoichiometry be the foundation of introductory chemistry courses? Chem. Educ. Res. Pract., 23(3), 662–685 10.1039/D1RP00333J.
- Roshandel M., Cote E. and Randles C., (2025), Exploring the Professional Relationships between Undergraduate Students and Their Graduate Teaching Assistants and Undergraduate Learning Assistants, J. Chem. Educ., 102(3), 1083–1096 DOI:10.1021/acs.jchemed.4c01172.
- Ruder S. M. and Stanford C., (2018), Strategies for Training Undergraduate Teaching Assistants To Facilitate Large Active-Learning Classrooms, J. Chem. Educ., 95(12), 2126–2133 DOI:10.1021/acs.jchemed.8b00167.
- Sanders Johnson S., (2022), Embracing Culturally Relevant Pedagogy to Engage Students in Chemistry: Celebrating Black Women in the Whiskey and Spirits Industry, J. Chem. Educ., 99(1), 428–434 DOI:10.1021/acs.jchemed.1c00504.
- Schafer A. G. L., Kuborn T. M., Schwarz C. E., Deshaye M. Y. and Stowe R. L., (2023), Messages about valued knowledge products and processes embedded within a suite of transformed high school chemistry curricular materials, Chem. Educ. Res. Pract., 24(1), 71–88 10.1039/D2RP00124A.
- Shoshani A., (2025), Teachers’ flow, emotional well-being, and optimal teaching and learning experiences: an experience sampling study, Teach. Teacher Educ., 165, 105138 DOI:10.1016/j.tate.2025.105138.
- States N., Stone E. and Cole R., (2023), Creating Meaningful Learning Opportunities through Incorporating Local Research into Chemistry Classroom Activities, Educ. Sci., 13(2), 192 DOI:10.3390/educsci13020192.
- Strong R. K., (1930), Laboratory examination in general chemistry, J. Chem. Educ., 7(5), 1125 DOI:10.1021/ed007p1125.
- Sweller J., (2011), CHAPTER TWO – Cognitive Load Theory, in Mestre J. P. and Ross B. H. (ed.), Psychology of Learning and Motivation, Academic Press, pp. 37–76 DOI:10.1016/B978-0-12-387691-1.00002-8.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168 10.1039/C3RP00012E.
- Taber K. S., (2019), The Nature of the Chemical Concept: Re-constructing Chemical Knowledge in Teaching and Learning, Royal Society of Chemistry.
- Talanquer V., (2011), Macro, Submicro, and Symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195 DOI:10.1080/09500690903386435.
- Talanquer V., Bolger M. and Tomanek D., (2015), Exploring prospective teachers’ assessment practices: noticing and interpreting student understanding in the assessment of written work, J. Res. Sci. Teach., 52(5), 585–609 DOI:10.1002/tea.21209.
- The Boyer 2030 Commission, (2022), The Equity-Excellence Imperative: A 2030 Blueprint for Undergraduate Education at U.S. Research Universities, The Association for Undergraduate Education at Research Universities.
- Towns M., Harwood C. J., Robertshaw M. B., Fish J. and O’Shea K., (2015), The digital pipetting badge: A method to improve student hands-on laboratory skills, J. Chem. Educ., 92(12), 2038–2044 DOI:10.1021/acs.jchemed.5b00464.
- Tune J. D., Goodwill A. G., Kiel A. M., Baker H. E., Bender S. B., Merkus D. and Duncker D. J., (2020), Disentangling the Gordian knot of local metabolic control of coronary blood flow, Am. J. Physiol. Heart Circ. Physiol., 318(1), H11–H24 DOI:10.1152/ajpheart.00325.2019.
- Ural E., (2016), The Effect of Guided-Inquiry Laboratory Experiments on Science Education Students’ Chemistry Laboratory Attitudes, Anxiety and Achievement, J. Educ. Train. Studies, 4(4), 217–227 DOI:10.11114/jets.v4i4.1395.
- van Es E. A., Hand V., Agarwal P. and Sandoval C., (2022), Multidimensional Noticing for Equity: Theorizing Mathematics Teachers’ Systems of Noticing to Disrupt Inequities, J. Res. Math. Educ., 53(2), 114–132 DOI:10.5951/jresematheduc-2019-0018.
- van Es E. A., Hand V. and Mercado J., (2017), Making Visible the Relationship Between Teachers’ Noticing for Equity and Equitable Teaching Practice, in Schack E. O., Fisher M. H. and Wilhelm J. A. (ed.), Teacher Noticing: Bridging and Broadening Perspectives, Contexts, and Frameworks, Springer International Publishing, pp. 251–270 DOI:10.1007/978-3-319-46753-5_15.
- van Es E. A. and Sherin M. G., (2002), Learning to Notice: Scaffolding New Teachers’ Interpretations of Classroom Interactions, J. Tech. Teach. Educ., 10(4), 571–596.
- van Es E. A. and Sherin M. G., (2010), The influence of video clubs on teachers’ thinking and practice, J. Math. Teach. Educ., 13(2), 155–176 DOI:10.1007/s10857-009-9130-3.
- van Es E. A. and Sherin M. G., (2021), Expanding on prior conceptualizations of teacher noticing, ZDM Math. Educ., 53(1), 17–27 DOI:10.1007/s11858-020-01211-4.
- Velasco J. B., Knedeisen A., Xue D., Vickrey T. L., Abebe M. and Stains M., (2016), Characterizing Instructional Practices in the Laboratory: The Laboratory Observation Protocol for Undergraduate STEM, J. Chem. Educ., 93(7), 1191–1203 DOI:10.1021/acs.jchemed.6b00062.
- Walker J. P., Allen W. E., Clevenger L., Hosbein K. N., Kennedy A. M., Vance-Chalcraft H. and Whiting B., (2023), Course-Based Undergraduate Research Experiences as a Community of Practice (CoP), J. Chem. Educ., 100(7), 2520–2528 DOI:10.1021/acs.jchemed.2c00844.
- Wan T., Geraets A. A., Doty C. M., Saitta E. K. H. and Chini J. J., (2020), Characterizing science graduate teaching assistants’ instructional practices in reformed laboratories and tutorials, IJ STEM Ed., 7(1), 30 DOI:10.1186/s40594-020-00229-0.
- Watts F. M. and Rodriguez J.-M. G., (2023), A Review of Course-Based Undergraduate Research Experiences in Chemistry, J. Chem. Educ., 100(9), 3261–3275 DOI:10.1021/acs.jchemed.3c00570.
- Winstead A. J., McCarthy P. C., Rice D. S. and Nyambura G. W., (2022), Linking chemistry to community: integration of culturally responsive teaching into General Chemistry I laboratory in a remote setting, J. Chem. Educ., 99(1), 402–408 DOI:10.1021/acs.jchemed.1c00494.
- Wu M.-Y. M. and Yezierski E. J., (2022), Pedagogical chemistry sensemaking: a novel conceptual framework to facilitate pedagogical sensemaking in model-based lesson planning, Chem. Educ. Res. Prac., 23(2), 287–299 10.1039/D1RP00282A.
- Wu M.-Y. M. and Yezierski E. J., (2023), Investigating the mangle of teaching oxidation–reduction with the VisChem approach: problematising symbolic traditions that undermine chemistry concept development, Chem. Educ. Res. Pract., 24(3), 807–827 10.1039/D2RP00321J.
- Zaimi I., Haas D. B., Silverstein M. J. and Shultz G. V., (2024), A case study on graduate teaching assistants’ teacher noticing when enacting a case-comparison activity in organic chemistry, Chem. Educ. Res. Pract., 25(4), 1268–1288 10.1039/D4RP00093E.
- Zotos E. K., Moon A. C. and Shultz G. V., (2020), Investigation of chemistry graduate teaching assistants’ teacher knowledge and teacher identity, J. Res. Sci. Teach., 57(6), 943–967 DOI:10.1002/tea.21618.
- Zotos E. K., Tyo J. J. and Shultz G. V., (2021), University instructors’ knowledge for teaching organic chemistry mechanisms, Chem. Educ. Res. Pract., 22, 715–732 10.1039/D0RP00300J.
| This journal is © The Royal Society of Chemistry 2026 |