Chemistry in early childhood: storytelling and hands-on water-based activities to foster scientific concepts and ideas
Carla Morais *a,
Ana Ferreira
*a,
Ana Ferreira a,
José Luís Araújo
a,
José Luís Araújo b and
Luciano Moreira
b and
Luciano Moreira cd
cd
aCIQUP – IMS, Science Teaching Unit, Faculty of Science, University of Porto, Rua do Campo Alegre, 687, 4169-007 Porto, Portugal. E-mail: cmorais@fc.up.pt
bCIDTFF, Department of Education and Psychology, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal
cCETAPS, Faculty of Arts and Humanities, University of Porto, Via Panorâmica s/n, 4150-564 Porto, Portugal
dCIQUP – IMS, University of Porto, Rua do Campo Alegre, 687, 4169-007 Porto, Portugal
First published on 5th December 2025
Abstract
In a societal context where chemistry is often perceived negatively, it is essential to promote educational approaches that foster meaningful and engaging learning experiences from an early age. This study aimed to explore how the integration of storytelling and hands-on activities can contribute to primary school children's engagement and understanding of scientific concepts, leading to a more meaningful chemistry learning. The research was conducted in four primary schools in the northern region of Portugal, involving 237 third- and fourth-grade students in non-formal educational initiatives. A qualitative methodology was employed, based on the analysis of students’ laboratory notebooks. The intervention consisted of a dramatized story from the book Histórias com Química [Stories with Chemistry], followed by three water-based hands-on activities addressing key chemical concepts: acid–base reactions, supersaturated solutions, and redox processes. Children's responses were analysed using framework theory, which enabled the categorization of their conceptual development. The findings showed that students' responses progressed from simple sensory observations and intuitive explanations to the construction of basic scientific models. High levels of participation, engagement, and enthusiasm were observed throughout the activities. These results suggest that combining storytelling with hands-on experimentation represents a promising pedagogical strategy for introducing chemistry concepts in early education and promoting a more positive and accessible image of this science.
Challenging “chemophobia” and promoting a positive image of chemistry
In current society, children grow up surrounded by stereotypes from the moment they are born. They are exposed to biased and preconceived situations with their family and, later, with school, their peers and society in general, and begin to form their own perceptions (Brussino and McBrien, 2022). An important subject of these stereotypes and prejudices is chemistry. In society, it is possible to identify a prevalent “chemophobia”. Some authors (Michaelis, 1995; Laszlo, 2006; Guerris et al., 2020; Hoover et al., 2020; Tarasova and Makarova, 2020) define it as “the fear of chemistry” in which chemistry is often associated with toxic and dangerous substances, chemical warfare, and environmental pollution, not to mention the frequent association with pseudoscience, alchemy, magic and even the stereotype of the mad scientist (Morais, 2015; Rollini et al., 2022). The root cause of “chemophobia” is often linked to a lack of understanding of chemistry and an overload of information. Although this might seem contradictory, it can be explained by the fact that an excess of information is frequently unreliable and, in many cases, biased or distorted to support chemophobic views. As a result, the public, including young children, is exposed to many sources that consistently convey messages that promote fear or a negative image toward chemistry, whether directly or indirectly (Sáez-Hernández and Ballesteros-Garrido, 2024). As emphasised by Rollini et al. (2022):One of the causes of the negative image of chemistry and chemicals, is the global media's sensationalization of chemical accidents while simultaneously marginalising major advances in science and technology. From this perspective, “chemophobia” seems to emerge and prosper from an insufficient, inefficient, and inaccurate chemistry communication (p. 2).
Studies on social representations of scientific topics have shown how public perceptions are often shaped by limited or superficial knowledge, reinforcing the need for more effective science communication strategies (Morais et al., 2022). In view of this societal reality, there is a need to combat these prejudices and misconceptions by promoting chemistry to society, presenting another view of this science. It is crucial to present chemistry as a science that has several positive contributions in the area of health, such as advances in medicine, improved food safety, production of cosmetics and hygiene products, advances in the pharmaceutical industry; in the areas of technology and the environment, such as the production of cutting-edge technology, the promotion of renewable energies, improvements in environmental protection measures, the development of more resistant materials, innovations in water treatment, the promotion of sustainable agriculture, among others (Francl, 2013; Morais, 2015, 2020; Rollini et al., 2022). According to the Stockholm Declaration on Chemistry for the Future (Welton et al., 2025), chemistry is essential for achieving the Sustainable Development Goals (SDGs), particularly those relating to health and well-being (SDG 3), quality education (SDG 4), drinking water and sanitation (SDG 6), industry, innovation and infrastructure (SDG 9), responsible consumption and production (SDG 12), climate action (SDG 13) and partnerships for the goals (SDG 17); and this is essential to be presented and addressed within society.
The literature suggests that children's science perceptions are constructed and deeply rooted by the age of nine or ten, remaining unaltered when these children reach secondary school and subsequently influencing their future career choices (Denessen et al., 2015; DeWitt and Archer, 2015). From an early age, some children start to exhibit negative perceptions of science in general and chemistry in particular, which are influenced by societal factors and the prevalence of “chemophobia” in their environment, for example, among teachers and family (Denessen et al., 2015; Guerris et al., 2020; Härmälä-Braskén et al., 2020; Rollini et al., 2022). Taking this into account, we consider necessary to equip the students with a broader vision and knowledge of chemistry and this starts early in schools with formal and non-formal activities (Whitcombe, 2019; Rulev, 2021; Chalupa and Nesměrák, 2023).
In this study, we aim to explore how the integration of storytelling and hands-on activities can contribute to primary school children's engagement and understanding of scientific concepts, leading to a more meaningful chemistry learning.
Chemistry in primary education
Research has revealed growing evidence that the development of students' knowledge and understanding of science content takes place in a variety of environments, both in and out of school, and that this knowledge and understanding accumulates over time through exposure to a wide range of educational experiences (OCDE, 2012; Bathgate et al., 2014; Berg et al., 2021).The foundations for many key scientific concepts are laid in primary education. Children arrive at school with their own ideas and explanations for everyday phenomena, and these initial understandings are further developed through formal and non-formal education (Härmälä-Braskén et al., 2020). Several studies indicate that learners who become disengaged from science in primary school often face increasing difficulty in reconnecting with the subject as they progress through their education (Denessen et al., 2015; DeWitt and Archer, 2015; Deehan et al., 2024). Early science education, including chemistry, is essential for equipping students with the tools they need as individuals, members of society, and global citizens. Even though chemistry is not yet a defined core subject at the primary school level, getting children familiar with basic topics related to chemistry is essential (Härmälä-Braskén et al., 2020, Saúde et al., 2025). By introducing young learners to these concepts early on, we equip them with the foundational understanding they need to tackle more advanced topics as they progress. This approach not only sets the stage for a deeper understanding of chemistry but also fosters a broader scientific literacy that is critical for their overall education. Moreover, learning experiences that involve collaboration with families and technology have also been shown to promote children's scientific understanding, particularly when grounded in an ecological framework that values shared knowledge-building (Paiva et al., 2017).
There has been some research on children's understanding of chemistry in the early years of schooling, particularly regarding concepts such as matter and water. Harrison and Treagust (2001) found that chemistry teaching should address changes in substance properties (e.g., taste, temperature) through a micro-level perspective. They identified three levels for teaching chemical concepts: sensory, atomic/molecular, and symbolic. They also emphasized the importance of using metaphors, analogies, and models in teaching, which can help increase students' interest and motivation. Tytler (2000) compared how children from different age groups (6–7 and 12–13 years old) understood evaporation and condensation. Younger children often explained water phenomena with associations, like linking them to the water cycle, while older children had more scientifically acceptable explanations. Tytler's study showed that even young children could grasp the concept of the water cycle. Åkerblom and colleagues (2019) investigated how 6-year-old children conceptualised water, molecules, and chemistry both before and after participating in a playfully dramatized chemistry activity. The children were divided into three categories based on their understanding: everyday concepts, experientially-based concepts, and generalized experiences. The results highlighted that, prior to the activity, most children could describe water in terms of everyday experiences, such as its function (e.g., “water is something you drink”). After the activity, some children expanded their understanding, using terms like “water molecules” and relating them to phenomena such as temperature and movement. The study found that children's conceptions evolved after the intervention, with some children able to provide more scientific explanations, such as understanding the connection between molecules and temperature. However, some children still conceptualised molecules in concrete terms or used metaphors, like associating water molecules with visible characteristics (e.g., “blue” or “soft”). This shift in understanding shows how children moved from everyday knowledge to more abstract scientific concepts, but the understanding was still evolving. In the study by Sundberg et al. (2025), the authors investigate how primary school students (age 8) use disciplinary drawings to navigate between everyday and scientific discourses of water. The findings show that most students used their drawings to bridge everyday experiences with scientific explanations. However, they often needed guidance from an adult to effectively transition between everyday and scientific reasoning. Some students, on the other hand, demonstrated the ability to independently use their drawings to move between the two discourses, suggesting a deepening of their understanding of the chemical properties of water. The authors suggest that disciplinary drawings can be a valuable tool for connecting everyday experiences to scientific concepts, fostering a richer understanding of the scientific nature of matter.
These findings highlight the importance of providing early and meaningful chemistry-related experiences that connect children's everyday observations with more formal scientific reasoning. When supported with developmentally appropriate and engaging activities, young learners can begin to make sense of scientific ideas in ways that feel relevant and accessible. By helping children move between what they already know from daily life and new ways of thinking about the world, educators can nurture curiosity and lay the foundations for learning and long-term engagement with science.
Acquisition of scientific knowledge
The learning process and cognitive development of children, as well as the acquisition of scientific concepts, is regarded as a transition from everyday concepts, derived from children's sensory observations of their environment to formal scientific concepts. As they engage with formal or non-formal educational activities, their cognitive frameworks evolve towards more scientifically rigorous models. This process can be framed by framework theory, which focuses on how children's cognitive models evolve as they encounter new knowledge. Framework theory is defined by the evolution of epistemological and ontological concepts, leading to the development of children's knowledge and the acquisition of scientific understanding (Carey, 1991; Vosniadou, 2013; Christodoulakis and Adbo, 2024a, b). Fig. 1 shows the framework theory approach, constituted by models and concepts, in which the thicker arrows illustrate the evolution of knowledge acquisition, and the thinner arrows indicate that this evolution is variable, progressing or regressing depending on the stage of development and maturation of the concepts and information. The framework theory aims to present the evolution of knowledge acquisition by recognising this process as dynamic and variable (Vosniadou, 2013; 2019). | ||
| Fig. 1 Framework theory approach, constituted by models and concepts (adapted from Christodoulakis and Adbo, 2024a, b). | ||
In the work of Christodoulakis and Adbo (2024a, b), children's scientific learning is explored through framework theory, emphasising the dynamic interaction between intuitive and counterintuitive concepts. Their research highlights that the transition to scientific models is a gradual process involving the formation of synthetic concepts, which serve as a foundation for understanding more complex scientific concepts. In Vosniadou's (2019) work, children's scientific learning is also explored through framework theory, highlighting how their initial intuitive concepts evolve into scientific models. The study emphasises that children's intuitive understandings coexist with emerging scientific concepts, and the learning process involves integrating these concepts to form more refined, synthetic models.
A plethora of initiatives have been implemented to explore different scientific subjects and promote knowledge acquisition. These include the use of storytelling to foster affective-motivational connections, as well as hands-on activities that involve the formulation of predictions and their testing, particularly in disciplines such as chemistry, and the conceptualisation of chemical processes.
Storytelling and hands-on activities
The use of storytelling is associated with cognitive and affective-motivational factors that can promote greater motivation and emotional development in children. In addition, exposure to stories about chemistry allows children to recognise the relevance of the area, thus increasing their commitment and general interest in this science (Barchas-Lichtenstein et al., 2023).Research on storytelling approaches has focused on pre-school, primary and elementary school children, with the aim of teaching and learning science and chemistry (Walan and Enochsson, 2019; Türkoguz and Ercan, 2022).
Walan and Enochsson (2019) analysed how 4 to 8-year-old children from pre-school and primary school recalled scientific concepts after listening to a story about immunology and viruses. After listening to the story, the children performed a play based on it and made drawings about it. The results showed that many children had learnt the names of concepts associated with the immune system, viruses, and colds, as well as how immune system cells work when someone has a cold. This study concluded that the storytelling approach has a positive potential for teaching science to children.
Türkoguz and Ercan (2022) demonstrated that using anthropomorphic stories in science activities organised according to the 5E model of instruction (engagement, exploration, explanation, elaboration, and evaluation) can help elementary school students to better understand microscopic particles and related concepts that are not visible in their daily lives, such as the particulate nature of matter. The findings revealed that teaching supported by visual anthropomorphic stories encouraged students to respond more positively to the evaluations, concluding that storytelling can contribute to the development of students' verbal-linguistic intelligence. Similarly, poems and caricatures have also been used to make abstract and invisible chemical concepts more approachable, such as atomic radius and ionization energy (Araújo et al., 2015), challenging the limits of specialisation and creating convergences between various areas and fields of knowledge (Paiva et al., 2013). Moreover, the hands-on activities allow children to experiment, test hypotheses, and actively engage in the learning process, reinforcing the gradual evolution of their knowledge acquisition (Singh, 2021; Chalupa and Nesměrák, 2023; Morais and Araújo, 2023). The implementation of hands-on activities allows children to actively participate in chemistry-related issues, promoting their involvement, interest and understanding of the real world. This approach has been shown to allow children to develop essential skills such as perseverance, adaptability, problem-solving, creativity, communication, collaboration, and critical thinking. (Singh, 2021). Fakaruddin and colleagues (2024) presented the premise that hands-on activities enable primary school children to process information more effectively and generate original ideas. Furthermore, students' imagination can be encouraged and developed through activities that stimulate creative thinking. This qualitative study aimed to explore the creative thinking patterns of six 11-year-old fifth-grade primary school students. The findings suggest that two cognitive patterns are involved in the creative process of primary school students: ideas stimulated by the students' existing information, and ideas generated through imagination and reasoning. The challenge of developing more abstract reasoning, however, is not exclusive to younger learners. As shown, for example, by Spitha et al. (2024), even university students often struggle to move beyond observable phenomena towards submicroscopic explanations, requiring explicit scaffolding to support this transition. Their findings underline that these cognitive difficulties persist throughout the educational trajectory, reinforcing the importance of introducing supportive strategies in primary education, when the foundations of scientific reasoning are still being laid.
Building on this perspective, the combination of storytelling and hands-on activities can generate interest in and engagement with science, scientific research, and scientific concepts and knowledge (Morais, 2015; 2020). This combination promotes advantages from both approaches, and according to Morais (2015): “The hands-on activities, prepared by the prior storytelling, may engage students through listening, reading, imagining, understanding, making, and explaining, and thus can generate interest in science and scientific research.” (p. 63).
This integration represents an approach that merges two distinct elements in a successful way that allows children to establish meaningful connections between theoretical knowledge and empirical evidence, which can contribute to the formation of positive perceptions regarding different topics such as chemistry; and is based on evidence that shows that children's interest in scientific-technological areas increases if they are exposed to stories before engaging in hands-on activities (Morais, 2015; 2020).
Walan (2019) analysed the perceptions and experiences of five pre-school teachers who combined storytelling and hands-on activities to teach science. They found that hands-on activities are a highly effective approach when implemented with pre-school children. However, the teachers demonstrated that combining stories and hands-on activities can be effective and that the practical application of stories can make a difference. In addition, Morais (2015, 2020); Morais et al. (2018) investigated the impact of combining storytelling and hands-on activities in non-formal educational settings with primary school students. The findings highlight that this integrated approach promotes interest, enjoyment, and engagement with chemistry, suggesting its pedagogical potential for combating “chemophobia” and fostering early scientific curiosity. In line with this, Morais and colleagues (2019) conducted a study based on the same premise of combining storytelling and hands-on activities. The study involved 37 pre-school students (aged 4–5) and 16 middle school students (aged 12–14). The intervention was conducted in elementary school chemistry classes and with pre-schoolers during the storytelling moment. It was found that, for elementary school students, using a storytelling approach combined with hands-on activities to teach acid–base content contributes to learning. The younger pre-school students understood the concepts, enjoyed the activity, and were motivated to learn science. This interaction also enabled them to acquire knowledge. This study reinforced the idea that the students had understood the concepts presented in the storytelling and hands-on activities and had enjoyed them, concluding that this combination is an effective strategy for motivating young children to learn chemistry.
In Portugal – the context in which the present study was conducted – the national science curriculum for early childhood education aims to foster children's familiarity with scientific concepts and ways of scientific thinking. While chemistry is not taught as a separate subject at the primary level, it's important to introduce children to fundamental concepts that chemistry deals with, such as the properties of different materials (colour, taste, smell, shine, buoyancy and solubility) associating these proprieties with their daily applications; learn about informational symbols of different products; establish the correspondence between the changes in physical states, such as evaporation, condensation, solidification, fusion, and the conditions that originate them; learn about the water cycle; predict the transformations caused by the heating and cooling of materials; understand the differences between solids, liquids and gases; identify the reversible transformations such as condensation, evaporation, solidification, dissolution and fusion (DGE, 2018).
Research objective and questions
The study aims to explore how integrating storytelling and hands-on activities enhances young children's engagement and promotes their understanding of scientific concepts and ideas involved. Based on the research objective, the following research question has been formulated:RQ. How does the integration of storytelling and hands-on activities promote young children's understanding of scientific concepts and ideas?
Methodology
Setting and participants
The initiatives were carried out, within the scope of qualitative research, in four primary schools in the North of Portugal, as complementary initiatives to formal education. A total of 237 children, aged 8–10 years old, participated in the initiatives during their 3rd and 4th years of primary education. Because of the pedagogically oriented nature of the intervention, no systematic data were collected about the socio-demographic characteristics of the participants (e.g., gender or academic success). However, every class was gender balanced.Description of the initiatives
Beyond the storybook, three hands-on activities related to the story were developed, with water playing a central role in each. Each activity focuses on a set of chemical concepts, allowing students to explore the properties and behaviour of water. The activities were designed to simulate and promote the scientific method and inquiry-based thinking: (i) making predictions based on their prior knowledge; (ii) observing and experimenting; (iii) registering and analysing the results; (iv) drawing conclusions and explanations about the scientific phenomena explored. A laboratory notebook was also developed for students to record their predictions, observations, and explanations of the chemical process in each hands-on activity. The notebook, which was given to each child at the beginning of the initiative, contained questions to support each phase, such as questions before the activity to encourage predictions, during the activity to guide observations, and after the activity to foster analysis and explanation of the chemical processes involved. Table 1 contains the identification and description of the hands-on activities, the chemical concepts involved, the explanation of the chemical processes that occur, and the focus of the questions in the laboratory notebook related to each hands-on activity. This structure ensured that the scientific ideas evoked in the story were not only embedded in a narrative context but also further explored and made explicit through the hands-on activities.
| Activities | Chemical concepts | Description | Explanation of the chemical process | Focus of questions in the lab notebook |
|---|---|---|---|---|
| Activity I – “Water that changes colour” | Acids and bases | In this experiment, three cups were employed: one containing water, one containing vinegar, and one containing glass cleaner. All the liquids were colourless to the human eye. | The water used in the cooking of red cabbage undergoes a colour change under the chemical composition of the ambient environment, thereby enabling the identification of acidity or alkalinity. | Establishing a connection to the story and set the context children were asked to think about the juice to accompany the cake that Mum made for Pepo and Lina in the story. |
| pH indicators | The children were presented with the three cups, without being informed of their respective contents, but considering that they can have water. | When the red cabbage cooking water was exposed to vinegar that is an acid, a pink colouration was observed in the beaker. | The children were presented with three cups containing liquids and were asked to identify the colours of each cup after adding red cabbage cooking water. They were then shown an image of the pH scale and the colours associated with each value, to help them identify the acid–base properties of the liquids in the cups. | |
| Concen-tration | Prior to the activity implementation, red cabbage was boiled, and the purple water that resulted from the boiling process was collected in a container. | Conversely, when it was exposed to glass cleaner, which is a base, a green colouration was observed. | We also asked the children about the process of making the coloured juice without fruit and how to change the concentration of a solution. | |
| In each cup, we added the red cabbage water and mix it with the content of the cup. | Finally, when the red cabbage cooking water was placed in the third beaker with water, it retained its initial purple colour, indicating that the chemical nature of the mixture had not changed. | |||
| Activity II – “Make ice in one second” | Supersa-turated solutions | In this activity, we pour the sodium acetate into the beaker until it reaches a volume of about 50 mL and then add about 8 coffee spoons of distilled water. Then heat the beaker containing the mixture of sodium acetate and water, stirring gently with a spatula until all the solids have dissolved. | The sodium acetate is very poorly soluble in water at room temperature. However, its solubility in water increases significantly as the temperature rises. It was therefore possible to dissolve all the sodium acetate in the beaker in such a small amount of water. | This activity is associated with the section of the story that details the physical transformation of water, where the characters Lina and Pepo use a freezer to create ice. Starting from this everyday idea of solidification, the experiment was designed to challenge children to consider whether all solids form by freezing. We showed the children a bottle and asked if it contained only water. Then we showed them a spoon containing some salt (sodium acetate). We asked the children what would happen if we poured the salt into the liquid, and then did it to demonstrate. The children saw a precipitate form in a few seconds. We then asked the children if the bottle contained only water and what the formed solid was. We also asked them to predict whether the bottle would be high or low temperature, before giving them the bottle to check. Through these questions, the children were encouraged to contrast the intuitive concept of freezing (from the story) with the scientific explanation of precipitation from a supersaturated solution, helping them differentiate physical and chemical processes. |
| Precipi-tate formation | Together with the children, we poured a spoon with a little of salt (sodium acetate) into the mix and the children saw the liquid of the bottle (supersaturated solution) turn in a precipitate solid in a few seconds. | On the other hand, as the mixture cooled, the solubility of the sodium acetate in water decreased, but the solid remained dissolved in water, unlike the initial situation. This solution is called supersaturated and is very unstable. | ||
| Therefore, when the “pinch” of salt is added, the unstable equilibrium state is changed and the sodium acetate precipitates again, forming the solid observed at the end of the experiment. | ||||
| Activity III – “Stirred water” | Redox reactions | Before the activity: | Methylene blue, the substance that gives the mixture its intense colour, turns into a colourless substance called leucomethylene when it reacts with glucose. So, when the mixture is at rest, the chemical reaction takes place, and the mixture appears colourless/white. However, when the mixture is stirred, the oxygen in the air dissolves in the mixture and chemically reacts with the leucomethylene to form methylene blue again. This means that when the mixture is stirred it turns blue and when at rest it is colourless or whitish. | The correlation between this activity and the story is predicated on the chemical properties involved. |
| Chemical equili-brium | In a beaker, dissolve 3,5 g of sodium hydroxide in 100 mL of water. | In the story, following a discussion on the various states of water, the question is posed whether “all water is equal inside”. The subsequent answer in the story delineates the chemical composition of water, as the elements oxygen and hydrogen. | ||
| In another beaker, dissolve 6 g of glucose in 100 mL of water. | In this activity, the chemical properties of mixtures are explored. | |||
| In another beaker, dissolve 0.1 g of methylene blue in 100 mL of water. | To end the hands-on activities, we developed an activity with a surprise effect; we asked the children to look at a bottle and predict whether it contained only water. Then we shook the bottle, and the substance turned blue, and at rest it became transparent again. We then asked them again if this substance was water. Through this sequence of questions, the children were encouraged to confront the intuitive idea that “all transparent liquids are water” with a more scientific explanation based on the presence of different substances and their transformations. This contrast helped them recognise that liquids with a similar appearance may behave differently, laying the groundwork for later understanding of chemical processes such as oxidation–reduction and equilibrium. | |||
| To the beaker containing the glucose, add the sodium hydroxide mixture and a few drops of methylene blue. | ||||
| Shake to homogenise the solution and store the mixture in a transparent glass bottle. | ||||
| Together with the children, shake the mixture vigorously in the bottle and observe what happens. Then leave the mixture to stand for a few seconds/minutes and see if anything happens again. | ||||
Prior to the storytelling, the children filled in the first page of the laboratory notebook with words associated with water that they remembered (free association task). Then, the story was told and theatricalised with the use of appropriate props to emphasise its content, taking nearly 20 minutes.
At the end, the students were asked questions about their understanding of the story and were asked to respond again to the words associated with water, to compare the associations mentioned in terms of concepts, everyday knowledge, and story.
After the story have been told and interpreted, the students were challenged to take part in a series of three hands-on activities, cross-referencing the story with the hands-on activities.
The hands-on activities were developed in the same environment, with kits set up by the monitors and allowing for the engagement of the children. The monitors oral communication were accompanied by a digital presentation that they used to explain the different activities to the children, introducing scientific concepts and make clear the chemistry behind the activities. Each activity had a duration of 20–25 minutes. During the hands-on activities, the children were asked to make predictions, compare them with the results, and explain the phenomena they observed. All their answers and notes were recorded in a laboratory notebook.
Ethical statement
Data were collected under school-approved conditions authorised by the standing parental consent that parents or legal guardians sign at enrolment, permitting educational activities and the use of anonymised learning data. Because the study was embedded in routine classroom work and posed only minimal risk, no additional individual written consent was requested. Parents/guardians were reminded of each invited chemistry session via the class teacher and could opt out, though no withdrawals occurred. Classroom teachers and school leadership approved the study, and every child gave voluntary oral assent after an age-appropriate explanation. All data were analysed only in anonymised form. These procedures adhered to local practice, the principles of the Declaration of Helsinki, and the Royal Society of Chemistry's ethical-publishing guidelines (Lawrie et al., 2021).Presentation and discussion of results
Words associated with water before and after the storytelling
The results showed that the words or ideas that the children associated with water before and after the story were different, as presented in Chart 1. The collected words were firstly categorised for comparison, with words or ideas of the same meaning grouped together and their singular and plural forms standardised. For instance, “solid/liquid/gaseous water” and “solid/liquid/gaseous state” were categorised as “liquid”, while “save water” and “don’t waste water” were categorised as “water conservation”. Singular and plural forms were also unified, such as “sea”, “ocean”, “river” and “lake”. | ||
| Chart 1 Frequency plot of words related to water before and after the storytelling activity. The children represented H2O as H2O, so it is written in the chart as such. | ||
Looking at the frequency plot of words before the activity (Chart 1), it is possible to see that certain words stand out more prominently because the children associated them more frequently with water. These words reflect the children's associations with water in terms of natural water bodies (e.g. sea, river, lake, and ocean), basic needs (e.g. drinking), water's state (e.g. ice, liquid, and gaseous), water's role in daily activities (e.g. shower and brushing teeth). There are other supporting words that add context to these ideas, such as “rain” and “clouds”, which reinforces the diverse forms and functions of water in nature.
The word “drops” appears less prominently but still contributes to the overall understanding of water's various manifestations. In addition to these, words like “watering plants”, “water conservation” reinforce environmental awareness and water's essential role in life. Overall, the frequency plot emphasises both functional and emotional connections to water.
During the storytelling activity, the children showed great interest and enthusiasm, listening attentively to the story of Lina and Pepo. Following the storytelling, a series of words were identified that were directly associated with the story itself. The frequency plot presented shows children's associations with water in terms of its forms (e.g., drops) and examples of water in different physical states in nature (e.g., clouds, ice, snow, liquid, solid, and gaseous). Children also refer to natural water bodies (e.g., river, sea, and ocean), representing water in its liquid state, and “rain”.
In terms of scientific concepts, children referred to the essential chemical elements present in water: “oxygen” and “hydrogen”. Additionally, the “water cycle” is represented, illustrating the natural flow of water and its processes of transformation (e.g., evaporation). The formula for water (H2O) is also mentioned, and the children expressed the name of this formula as “Agá-two-ó,” which is both the playful reading of H2O and the title of the story. There is also a focus on water's role in daily activities, such as “brushing teeth”, “water conservation” and “watering plants”, which reflects both practical water use and environmental awareness. Overall, the frequency plot emphasises both the functional and emotional connections to water, reflecting its role in the environment, its essential chemical elements, and its different physical states and transformations.
The frequency plots presented illustrate the evolution of children's associations with water, moving from everyday concepts to scientific concepts after the storytelling activity. Initially, children's associations with water were solely grounded in intuitive ideas, referring to natural water bodies (e.g., sea, river, lake, ocean) and basic needs (e.g., drinking, bathing/showering).
These findings align with Tytler (2000), who observed similar associations in younger children, where everyday experiences, such as linking water to the water states, were central to their initial understanding of water. These associations reflect children's sensory observations and practical interactions with water, as emphasised by Tytler (2000) in his research on young children's understanding of scientific concepts. Interestingly, while these everyday associations with water were referenced before and after the storytelling activity, their frequency decreased, suggesting that the activity prompted children to expand their understanding of water beyond familiar and concrete concepts. Following the storytelling activity, the frequency plot revealed a clear increase in scientific concepts such as oxygen, hydrogen, and the water cycle, as well as a use of more concepts connected to water's transformation processes, like evaporation. This finding is consistent with Åkerblom et al. (2019), who found that after a dramatized chemistry activity, children expanded their conceptualisation of water, incorporating scientific ideas like molecules and temperature. These additions reflect a transition from intuitive models to scientific models (Tytler, 2000), aligning with the findings of Åkerblom et al. (2019) and Sundberg et al. (2025), where children's conceptualisations expanded after structured educational interventions. Research has shown that storytelling can enhance motivation, emotional engagement, and the perception of science as relevant (Walan and Enochsson, 2019; Türkoguz and Ercan, 2022; Barchas-Lichtenstein et al., 2023), while also contributing to scientific literacy and promoting holistic perspectives that connect science with imagination and social reflection (Paiva et al., 2013). By introducing scientific concepts through a narrative, the children were encouraged to refine their scientific models and integrate them into their existing cognitive frameworks.
Hands-on activities on the properties of water
Following the storytelling activity and the word association exercise, the children actively participated in three hands-on activities, displaying curiosity, excitement, and keen engagement throughout each activity.The framework theory is applied to analyse the responses given by children in their laboratory notebooks during the intervention, specifically in the explanation questions of each activity displayed in the notebook. Based on the concepts and ideas they used in their answers, we classify the responses into five categories: sensory observations, intuitive concepts, counterintuitive concepts, synthetic models, and scientific models (Table 2). By categorising the concepts and ideas that children are expected to understand by the end of the intervention, resulting from the theatricalisation of the story, and the engagement with hands-on activities, we can observe the different levels of scientific knowledge acquisition (Carey, 1991; Vosniadou, 2013).
| Categories | Description of the scientific phenomenon observed… |
|---|---|
| Sensory observations | …without reference to any chemical concepts presented in the activity. |
| Intuitive concepts | … with concepts of their surrounding reality. |
| Counterintuitive concepts | … with concepts that contradict their reality. |
| Synthetic models | … reference to specific concepts of the activity. |
| Scientific models | … reference to chemical concepts. |
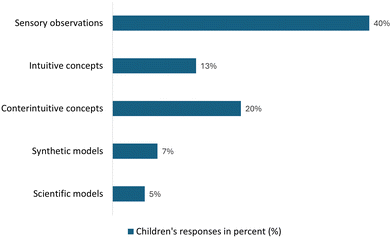 | ||
| Chart 2 Analysis of the children's answers to Activity I, showing different levels of knowledge acquisition. | ||
Children's explanations ranged from sensory observations, which often echoed everyday ideas first evoked in the story (e.g., the coloured “juice”), to more advanced descriptions involving concepts such as acidity, pH, and chemical reactions. The sensory observations consist of the children's ability to describe the result without explaining the process, and 40% of the children were able to do this.
About 13% of the children tried to explain the experience in terms of intuitive concepts derived from their everyday life and their general knowledge of reality. At the same time, 20% of the children were able to identify the experience as a contrast to their reality and an impossibility to explain the observed phenomenon with their everyday knowledge, resorting to counterintuitive concepts. In addition, 7% of the children tried to explain the observed phenomenon with counterintuitive concepts, but also by integrating concepts specific to the experience; for example, children focused on identifying the contents of the cups as liquids, specifically identifying which liquids they were, and describing the phenomenon of colour change. A more concrete example of the use of counterintuitive concepts can be the sentence “In each cup we had a transparent liquid, and we put in a purple cabbage water; when we mixed cup A, the colour changed to red, and the colour of cup B changed to green”, in which the use of the expression “transparent liquid” shows the careful reference to a substance rather than identifying it as water, and the sentence described the observed activity more rigorously. Finally, the fact that 5% of children formulated explanations within the framework of scientific models demonstrates that they were able to understand and explain the activity using simple scientific principles. The more detailed results are presented in Table 3, with examples of excerpts from children's answers.
| Categories | Explanation of the observed scientific phenomenon | Examples of excerpts from children's answers |
|---|---|---|
| Sensory observations | Description of the scientific phenomenon without reference to chemical concepts presented: children observed changes in the cups when different substances were added, without identification of the content of them. | “The colour changed; cup A became pink, and cup B became green” |
| “The colour changed” | ||
| “Cup A became pink, and cup B became green” | ||
| “Cup A changed from transparent to pink. Cup B changed from transparent to green” | ||
| Intuitive concepts | Description of the scientific phenomenon observed with concepts of their surrounding reality: children identified the contents of the cups as water and related changes to their everyday experiences. | “We had two cups of water, we added purple water, and they became different colours” |
| “Water was poured into two cups, then red cabbage water was added and the cups changed colour” | ||
| Counterintuitive concepts | Description of the scientific phenomenon observed with concepts that contradict their reality: children observed changes in the cups and identified the contents as liquid. | “When we added the red cabbage water to the transparent liquid in cup A it turned pink and when we added the red cabbage water to the liquid in cup B it turned green” |
| “In each cup we had a transparent liquid, and we put in a purple cabbage water; when we mixed cup A, the colour changed to red, and the colour of cup B changed to green” | ||
| Synthetic models | Description of the scientific phenomenon observed with reference to specific concepts of the activity: children identified the specific contents of each cup. | “In cup A, there was vinegar, and it turned pink. In cup B, there was glass cleaner, and it turned green” |
| “The liquid in the cups was not water” | ||
| Scientific models | Description of the scientific phenomenon observed with reference to chemical concepts: children identified the specific contents of each cup and articulated scientific concepts. | “When the red cabbage water was added to cup A, the chemical reaction formed a green liquid, and when it was added to cup B, it turned pink” |
| “The cups changed colour because water coloured with red cabbage was added and its pH measured” | ||
| “Cup A had the more acidic liquid” | ||
After this experience, the children were challenged one last time in this activity and asked to associate the concentration of a solution with the intensity of its colour; 97% of the children made the correct association. Most of the children accurately identified the colour changes when red cabbage cooking water was exposed to different liquids. This suggests that most children grasped the basic concept of pH indicators. However, some children struggled to explain the phenomenon beyond sensory observations, reflecting a reliance on intuitive concepts. These findings align with Tytler (2000), who observed that children's understanding of chemical concepts often begins with everyday experiences and sensory knowledge.
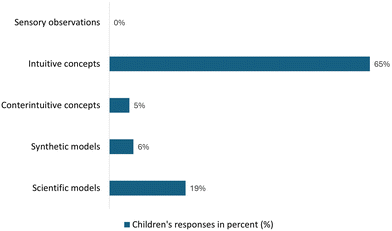 | ||
| Chart 3 Analysis of the children's answers to Activity II, showing different levels of knowledge acquisition. | ||
A significant proportion of children (65%) initially misidentified the solid formed as ice when asked to explain the phenomenon, suggesting a strong reliance on intuitive concepts associated with everyday freezing processes to develop an explanation. About 5% of the children were more careful in their explanation, recognising that the solid formed was different from ice, even if it looked like ice. Among the children, 6% were able to explain the observed phenomenon by referring to a specificity of the experiment, such as the increase in temperature. Lastly, 19% of the children could enunciate simple but accurate scientific concepts to explain the experience.
When asked to identify the solid formed on the bottle and the options were water, not water and other substance, 70% of the children correctly recognised that the solid was a different substance from water. Their justifications varied, with 25% of the children couldn't do so, with some attributing their reasoning to the temperature being too high for ice formation (37%), while others (3%) noted the rapid nature of the phase change as inconsistent with typical freezing and 4% of the children justified their choice on the presence of substances in the bottle.
The ability to challenge their own ideas and refine their explanations highlights the cognitive flexibility fostered through experimental learning (Table 4).
| Categories | Explanation of the observed scientific phenomenon | Examples of excerpts from children's answers |
|---|---|---|
| Intuitive concepts | Description of the scientific phenomenon observed with concepts of their surrounding reality: children identified the content of the bottle as water and the solid formed as ice, and the process as freezing, based on their everyday experiences with water changing state from liquid to solid. | “Became/transformed into ice” |
| “When pouring the powder into the bottle, the water froze into ice” | ||
| “The water transformed into ice” | ||
| “As the temperature of the crystal is very low, the water quickly turns to ice” | ||
| Counterintuitive concepts | Description of the scientific phenomenon observed with concepts that contradict their reality: children identified the formed solid as a substance that looked like ice, but struggled to reconcile it with their everyday understanding, as it had formed in an unusual way, contradicting their expectations of how ice should form. | “It has become ice-like/looking like ice” |
| “It turned into something that looked like ice but wasn't” | ||
| Synthetic models | Description of the scientific phenomenon observed with reference to specific concepts of the activity: children identified the phenomenon as a process that contributes to the increase in temperature. | “In a bottle with a liquid colourless like water, a powder was added, and the bottle became hot” |
| “When Lina put the crystal in the bottle, the temperature increased” | ||
| Scientific models | Description of the scientific phenomenon observed with reference to chemical concepts: children identified the process with the articulation of scientific concepts. | “We had a liquid in the bottle, we put in some form of crystal, and the liquid became solid” |
| “We had a transparent liquid, added a powder, and it solidified” | ||
| “The liquid mixed with the powder crystallised” | ||
Although most of the children incorrectly identified the solid as ice in the explanation of the chemical phenomenon, their answers did not rely on sensory observations, and the explanations revealed a coexistence of intuitive explanations and more scientific ones during the activity. Many relied on the everyday idea of freezing, but others articulated reasoning that distinguished this familiar image from the chemical process of precipitation in a supersaturated solution. This highlights the children's reliance on intuitive concepts, where they linked the solid formed to everyday freezing experiences, an association also evoked in the story, where ice was created in a freezer.
As the activity continued, children were able to describe the phenomenon and recognize the solid as something different from ice, pointing to a more counterintuitive and synthetic understanding. The ability to connect temperature change and the dissolution of sodium acetate suggests that the children were able to refine their explanations, moving closer to scientific models. These findings are consistent with Åkerblom et al. (2019), who observed that children, after engaging in hands-on activities, begin to refine their understanding and integrate new concepts.
During the experiment, 98% of the children observed and described the colour change upon agitation, while 97% recognized that the solution returned to its original state upon settling.
At the end of the experience, the children were asked again whether the content of the bottle was water, and 83% said it wasn't, justifying their answers in different ways, using different levels of knowledge acquisition, as shown in Chart 4. Chart 4 illustrates how these responses were distributed across categories, reflecting different ways of reasoning rather than a developmental sequence.
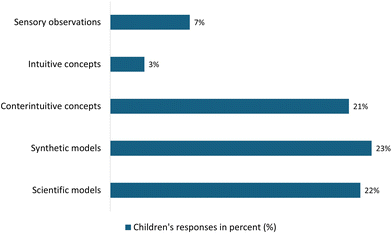 | ||
| Chart 4 Analysis of the children's answers to Activity III, showing different levels of knowledge acquisition. | ||
In this activity, a few children, about 7%, referred to the description of the observation to explain their point of view. Even fewer children (3%) used intuitive concepts to explain the content as water. A significant proportion (21%) were more careful in their explanation, excluding the content as water. A total of 23% of children were able to explain the observed phenomenon by referring to the properties of water. Finally, 22% of the children were able to use simple but accurate scientific concepts to explain their experience.
The more detailed results are presented in Table 5, with examples. of excerpts from children's answers.
| Categories | Explanation of the observed scientific phenomenon | Examples of excerpts from children's answers |
|---|---|---|
| Sensory observations | Description of the scientific phenomenon without reference to chemical concepts presented: children observed and described the colour changes of the bottle and the effect of the shaking process, without identification of the content of them. | “The bottle was shaken once more and then became colourless.” |
| Intuitive concepts | Description of the scientific phenomenon observed with concepts of their surrounding reality: children identified the content of the bottle as water, connecting it to their everyday experiences with the liquid form of water. | “The water is transparent and has become transparent again” |
| “It's purple water” | ||
| Counterintuitive concepts | Description of the scientific phenomenon observed with concepts that contradict their reality: children excluded the content of the bottle as water, as they observed unexpected changes that contradicted their usual understanding of water. | “It's not only water because it's changed colour” |
| “It's not only water because once you have stirred it, it changes colour” | ||
| “It becomes coloured when stirred and colourless when left to settle” | ||
| Synthetic models | Description of the scientific phenomenon observed with reference to specific concepts of the activity: children excluded the content of the bottle as water, recurring to the reference of properties of water. | “Water is always colourless, even when stirred” |
| “If it were only water, it would stay colourless” | ||
| “Water is transparent, but the liquid in the bottle changes colour” | ||
| “Water doesn’t have a colour or is colourless” | ||
| Scientific models | Description of the scientific phenomenon observed with reference to chemical concepts: children identified the process with the articulation of scientific concepts. | “A chemical reaction has occurred” |
| “The bottle also contained oxygen” | ||
| “The water contained other chemicals/substances” | ||
| “The water had dye and with the oxygen changed its colour.” | ||
When asked initially about the contents of the bottle, children were unsure, but upon observing the colour change, they were able to describe it accurately. This indicates that the activity successfully captured their attention and prompted them to engage in observation-based learning. As for the explanation of the process, most children concluded that the substance was not just water, suggesting an increasing awareness of the chemical nature of the solution. The presence of counterintuitive concepts (realizing that the substance was changing) instead of intuitive concepts (such as referring to the solution as “just water”) was evident. In the children's explanations, showing that alongside intuitive ideas some students also recognised phenomena that contradicted their everyday expectations. Furthermore, some of children articulated simple scientific concepts, referring to chemical reactions and oxidation. Children were able to describe chemical reactions using reasoning and concepts acquired through engagement with the hands-on activity, moving beyond the initial idea that “all water is equal inside” raised in the story and recognising that apparently similar liquids may behave differently due to underlying chemical processes. This aligns with Vosniadou (2019), who noted that children's early conceptions often evolve into more scientifically accurate models after engaging in structured learning experiences like this one.
Considering the results of Activities I, II, and III, a clear progression in children's understanding was observed, shifting from simple observations and intuitive concepts to more complex scientific explanations. In Activity I, the children initially connected changes in colour to their everyday experiences, relating them to familiar phenomena. However, as the activities progressed, the children began to identify chemical properties like solubility and precipitation, evolving to more sophisticated explanations. By Activity III, children were able to identify redox reactions and describe chemical properties more accurately.
Throughout the activities, children moved from intuitive concepts based on their reality to more structured explanations using scientific concepts. This shift reflects the dynamic interaction between intuitive and counterintuitive concepts, leading to the development of synthetic and scientific models, fostering critical thinking (Christodoulakis and Adbo, 2024a).
As they progressed, children's understanding evolved from everyday knowledge to scientifically accurate models, demonstrating the stages of epistemological and ontological development in science learning (Christodoulakis and Adbo, 2024b). This process aligns with framework theory, where children refine their concepts through engagement and cognitive conflict, evolving from initial synthetic models to more accurate scientific frameworks. The integration of storytelling and hands-on activities can support this development by activating prior knowledge, challenging misconceptions, and fostering deeper learning (Vosniadou, 2013; Christodoulakis and Adbo, 2024b). Although our study focused on primary school children, the process of moving from everyday ideas to more abstract scientific reasoning has been recognised as a challenge throughout science education. Research has documented that such transitions are not always straightforward, persisting even in secondary school and higher education (Tytler, 2000; Harrison and Treagust, 2001; Spitha et al., 2024). Placing our findings in this broader perspective reinforces the importance of introducing supportive strategies in primary education, such as storytelling and hands-on activities, where the foundations of scientific reasoning can be established from the outset.
Conclusions
This study explored the integration of storytelling and hands-on activities as a way to engage young children with chemistry and promote their understanding of scientific concepts. Regarding the research question, the study showed progression in children's reasoning. Their explanations developed from simple descriptions and everyday ideas to more structured reasoning based on synthetic and scientific models. The children were able to articulate the properties of water that they had learnt through storytelling, as well as the scientific concepts presented in the hands-on activities. Throughout the activities, the children were challenged to develop their imagination and reasoning skills, as well as their ability to formulate arguments about chemistry-related phenomena. These differences cannot be seen as a simple linear sequence. The content of the stories and the nature of the hands-on tasks also shaped how children responded. Activity I, which revolved around colour changes, was closer to everyday experience and therefore elicited mostly intuitive explanations, while Activity III, with its surprise effect and less familiar transformations, invited more reflection and supported the articulation of more scientific ideas. In the transition from Activity I to Activity III, the children were prompted to extend their understanding of the properties of water and to cultivate their chemical thinking by progressing from physical properties to chemical properties. Framework theory helped illustrate how children moved from informal knowledge to more formal scientific understanding, particularly in relation to acid–base reactions, supersaturation, and redox processes. By linking abstract chemical ideas to concrete experiences, this approach supports concept development and encourages reasoning and reflection. It also shows the importance of engaging both cognitively and emotionally in the learning process. The results point to the relevance of early contact with science that connects with children's imagination, emotions, and prior knowledge, instead of presenting chemistry as distant or complex.In addition to the children's reasoning, the monitor's observations during implementation reinforce the value of this combined approach. The integration of storytelling and hands-on activities created a learning environment that sustained children's attention, promoted participation, and encouraged emotional and cognitive engagement. The children were consistently curious and attentive, showing interest throughout both the narrative and experimental moments.
This study focused on short-term learning outcomes. The long-term retention and stability of scientific understanding remain open questions. Future research should consider longitudinal approaches to explore how children's ideas change over time and with further interaction with their peers.
In sum, combining storytelling and hands-on activities offers a practical educational strategy, one that supports learning in chemistry and helps address chemophobia by presenting chemistry as approachable and relevant. It contributes to building a foundation for scientific literacy and reflective thinking in the early years of science education. Our findings also suggest that introducing such supportive strategies at the primary level can help establish the foundations of scientific reasoning at an early stage, addressing challenges that are known to persist throughout science education.
Conflicts of interest
There are no conflicts to declare.Data availability
An anonymised transcription of the children's workbook responses (in Portuguese), together with a codebook and an English translation on request, is available from the corresponding author for bona-fide research purposes. Original notebooks remain in secure custody in accordance with the conditions of the standing parental consent and are not publicly available.An English translation of the story “Agá-dois-ó: uma gota de água”, from the Portuguese book “Histórias com Química”, which was used in the storytelling activity of this study, has been included as part of the supplementary information (SI). See DOI: https://doi.org/10.1039/d5rp00271k.
Acknowledgements
The authors thank all the students who participated in the study. We greatly appreciate your willingness to contribute and share your insights. We also extend our thanks to Cidália André, Joana Alves and Sofia Oliveira for their contribution. The study was supported by national funds through FCT – Fundação para a Ciência e a Tecnologia, I. P., under the projects UIDB/00081/2025 (CIQUP, Faculty of Science, University of Porto), LA/P/0056/2020 (IMS, Institute of Molecular Sciences), UID/00194/2025 (CIDTFF, Department of Education and Psychology, University of Aveiro), UIDP/04097/2020 and UID/4097/2025 (CETAPS, Centre for English, Translation, and Anglo-Portuguese Studies) and PhD Grant 2024.03553.BDANA (co-author AF).References
- Åkerblom A., Součková D. and Pramling N., (2019), Preschool children's conceptions of water, molecule, and chemistry before and after participating in a playfully dramatized early childhood education activity, Cult. Stud. Sci. Educ., 14(4), 879–895 DOI:10.1007/s11422-018-9894-9.
- Araújo J. L., Morais C. and Paiva J. C., (2015), Poetry and alkali metals: building bridges to the study of atomic radius and ionization energy, Chem. Educ. Res. Pract., 16(4), 893–900 10.1039/C5RP00115C.
- Barchas-Lichtenstein J., Sherman M., Voiklis J. and Clapman L., (2023), Science through storytelling or storytelling about science? Identifying cognitive task demands and expert strategies in cross-curricular STEM education, Front. Educ., 8, 1279861 DOI:10.3389/feduc.2023.1279861.
- Bathgate M. E., Schunn C. D. and Correnti R. J., (2014), Children's Motivation Toward Science Across Contexts, Manner of Interaction, and Topic, Sci. Educ., 98(2), 189–215 DOI:10.1002/sce.21095.
- Berg T. B., Achiam M., Poulsen K. M., Sanderhoff L. B. and Tøttrup A. P., (2021), The Role and Value of Out-of-School Environments in Science Education for 21st Century Skills, Front. Educ., 6, 674541 DOI:10.3389/feduc.2021.674541.
- Brussino O. and McBrien J., (2022), Gender stereotypes in education: Policies and practices to address gender stereotyping across OECD education systems, OECD Education Working Papers, No. 271, OECD Publishing, Paris DOI:10.1787/a46ae056-en.
- Carey S., (1991), Knowledge Acquisition: Enrichment or Conceptual Change? in Carey S. and Gelman R. (ed.), The epigenesis of mind: Essays on biology and cognition, Lawrence Erlbaum Associates, Inc., pp. 257–291.
- Chalupa R. and Nesměrák K., (2023), Chemophobia and practical chemistry: the laboratory as a place of origin or, on the contrary, suppression of the fear of chemistry? Monatshefte Für Chemie – Chemical Monthly, 154(9), 957–965 DOI:10.1007/s00706-023-03098-9.
- Christodoulakis N. and Adbo K., (2024a). An Analysis of the Development of Preschoolers’ Natural Science Concepts from the Perspective of Framework Theory, Educ. Sci., 14(2), 126 DOI:10.3390/educsci14020126.
- Christodoulakis N. and Adbo K., (2024b), Exploring the Emergence of Chemistry in Preschool Education: A Qualitative Perspective, Educ. Sci., 14(9), 1033 DOI:10.3390/educsci14091033.
- Deehan J., MacDonald A. and Morris C., (2024), A scoping review of interventions in primary science education, Stud. Sci. Educ., 60(1), 1–43 DOI:10.1080/03057267.2022.2154997.
- Denessen E., Vos N., Hasselman F. and Louws M., (2015), The Relationship between Primary School Teacher and Student Attitudes towards Science and Technology, Educ. Res. Int., 2015, 34690 DOI:10.1155/2015/534690.
- DeWitt J. and Archer L., (2015), Who Aspires to a Science Career? A comparison of survey responses from primary and secondary school students, Int. J. Sci. Educ., 37(13), 2170–2192 DOI:10.1080/09500693.2015.1071899.
- DGE, (2018), Aprendizagens Essenciais – Ensino Básico [Essential Learnings – Basic Education]. Retrieved 16th July 2025 from https://www.dge.mec.pt/aprendizagens-essenciais-ensino-basico.
- Fakaruddin F. J., Shahali E. H. M. and Saat R. M., (2024), Creative thinking patterns in primary school students’ hands-on science activities involving robotic as learning tools, Asia Pac. Educ. Rev., 25(1), 171–186 DOI:10.1007/s12564-023-09825-5.
- Francl M., (2013), How to counteract chemophobia, Nat. Chem., 5(6), 439–440 DOI:10.1038/nchem.1661.
- Guerris M., Cuadros J., González-Sabaté L. and Serrano V., (2020), Describing the public perception of chemistry on twitter, Chem. Educ. Res. Pract., 21(3), 989–999 10.1039/C9RP00282K.
- Härmälä-Braskén A.-S., Hemmi K. and Kurtén B., (2020), Misconceptions in chemistry among Finnish prospective primary school teachers – a long-term study, Int. J. Sci. Educ., 42(9), 1447–1464 DOI:10.1080/09500693.2020.1765046.
- Harrison A. and Treagust G., (2001), Conceptual change using multiple interpretive perspectives: Two case studies in secondary school chemistry, Instruct. Sci., 29(1), 45–85 DOI:10.1023/A:1026456101444.
- Hoover J. M., Lee J. and Hamrick T., (2020), Community Engagement in Science Through Art (CESTA) Summer Program, J. Chem. Educ., 97(8), 2153–2159 DOI:10.1021/acs.jchemed.9b01101.
- Laszlo P., (2006), On the Self-Image of Chemists, 1950–2000, Int. J. Philos. Chem., 12(1), 99–130.
- Lawrie G. A., Graulich N., Kahveci A. and Lewis S. E., (2021), Ethical statements: a refresher of the minimum requirements for publication of chemistry education research and practice articles Check for updates, Chem. Educ. Res. Pract., 22, 234–236 10.1039/D1RP90003J.
- Michaelis A. R., (1995), Stop – chemophobia, Interdiscip. Sci. Rev., 20(4), 130–139 DOI:10.1179/isr.1995.20.4.130.
- Morais C., (2015), Storytelling with Chemistry and Related Hands-On Activities: Informal Learning Experiences To Prevent “Chemophobia” and Promote Young Children's Scientific Literacy, J. Chem. Educ., 92(1), 58–65 DOI:10.1021/ed5002416.
- Morais C., (2020), Storytelling and hands-on activities boosting young children's awareness and understanding of chemistry, L'Actualité Chimique, 447, 43–47.
- Morais C. and Araújo J. L., (2023), An alternative experimental procedure to determine the solubility of potassium nitrate in water with automatic data acquisition using Arduino for secondary school: development and validation with pre-service chemistry teachers, J. Chem. Educ., 100(2), 774–781 DOI:10.1021/acs.jchemed.2c00615.
- Morais C., Araújo J. L., Oliveira S. and Moreira L., (2018), Chemistry in a primary school: storytelling and hand-on activities about water, Book of Abstracts of the ECRICE 2018 – 14th European Conference on Research in Chemistry Education, EuCheMS, European Chemical Sciences, p. 17.
- Morais C., Araújo J. L. and Saúde I., (2019), Awakening to chemistry through storytelling and practical activities: middle school students interacting with pre-school children, Chem. Educ. Res. Pract., 20(1), 302–315 10.1039/C8RP00096D.
- Morais C., Moreira L., Teixeira A. and Aguiar T., (2022), No waves from surface knowledge: diving into the social representations of the deep sea, Int. J. Sci. Educ., Part B: Commun. Public Engage., 12(1), 22–41 DOI:10.1080/21548455.2021.2017507.
- Morais C. and Teixeira P. M., (2012), Histórias com Química [Stories with Chemistry], QuidNovi.
- OCDE, (2012), Education at a Glance 2012: OECD Indicators, OECD Publishing.
- Paiva J. C., Morais C. and Moreira L., (2013). Specialization, chemistry and poetry: challenging chemistry boundaries, J. Chem. Educ., 90(12), 1577–1579 DOI:10.1021/ed4003089.
- Paiva J. C., Morais C. and Moreira L., (2017), Activities with Parents on the Computer: an ecological framework, Educ. Technol. Soc., 20(2), 1–14.
- Rollini R., Falciola L. and Tortorella S., (2022), Chemophobia: a systematic review, Tetrahedron, 113, 132758 DOI:10.1016/j.tet.2022.132758.
- Rulev A., (2021), Chemical Education contra Chemophobia, Chimia, 75(1–2), 98 DOI:10.2533/chimia.2021.98.
- Sáez-Hernández R. and Ballesteros-Garrido R., (2024), Effect of Science Outreach Activities on Chemophobic Conceptions at the High School Level, J. Chem. Educ., 101(4), 1635–1641 DOI:10.1021/acs.jchemed.4c00074.
- Saúde I., Araújo J. L. and Morais C., (2025). The underrepresentation of Chemistry in the preferences of primary school teachers in Portugal: challenges and perspectives from a continuing professional development programme, Res. Sci. Technol. Educ., 1–21 DOI:10.1080/02635143.2025.2586596.
- Singh M. N., (2021), Inroad of Digital Technology in Education: Age of Digital Classroom, High. Educ. Future, 8, 20–30 DOI:10.1177/2347631120980272.
- Spitha N., Zhang Y., Pazicni S., Fullington S., Morais C., Buchberger A. R. and Doolittle P., (2024). Supporting Submicroscopic Reasoning in Students’ Explanations of Absorption Phenomena using a Simulation-Based Activity, Chem. Educ. Res. Pract., 25, 133–150 10.1039/D3RP00153A.
- Sundberg B., Andersson J., Areljung S., Hermansson C. and Skoog M., (2025), How primary school students use their disciplinary drawings to navigate between everyday and scientific discourses of water, Chem. Educ. Res. Pract., 26, 631–646 10.1039/D4RP00080C.
- Tarasova N. P. and Makarova A. S., (2020), Green Chemistry and Chemophobia, Herald Russian Acad. Sci., 90(2), 245–250 DOI:10.1134/S1019331620020161.
- Türkoguz S. and Ercan İ., (2022), Effect of visual anthropomorphic stories on students’ understanding of the particulate nature of matter and anthropomorphic discourse, Chem. Educ. Res. Pract., 23(1), 206–225 10.1039/d1rp00057h.
- Tytler R., (2000), A comparison of year 1 and year 6 students’ conceptions of evaporation and condensation: dimensions of conceptual progression, Int. J. Sci. Educ., 22(5), 447–467 DOI:10.1080/095006900289723.
- Vosniadou S., (2013), International Handbook of Research on Conceptual Change, 2nd edn, Routledge DOI:10.4324/9780203154472.
- Vosniadou S., (2019), The Development of Students’ Understanding of Science, Front. Educ., 4, 32 DOI:10.3389/feduc.2019.00032.
- Walan S., (2019), Teaching children science through storytelling combined with hands-on activities – a successful instructional strategy? Education, 47(1), 34–46 DOI:10.1080/03004279.2017.1386228.
- Walan S. and Enochsson A.-B., (2019), The potential of using a combination of storytelling and drama, when teaching young children science, Eur. Early Childhood Educ. Res. J., 27(6), 821–836 DOI:10.1080/1350293x.2019.1678923.
- Welton T., Cole-Hamilton D. J., Kerton F., Liu H., Liu Z., Nyamori V. O., Pozo-Gonzalo C., Prechtl M. H. G., Sun Z. and Sutton M., (2025), Stockholm Declaration on Chemistry for the Future, RSC Sustainability, 3, 4187–4189 10.1039/D5SU90041G.
- Whitcombe T., (2019), Why Communicating Chemistry Can Be Complicated, ACS Symp. Ser., 205–2013 DOI:10.1021/bk-2019-1327.ch014.
| This journal is © The Royal Society of Chemistry 2026 |
