Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparative photochemical flow synthesis of ketenimines via Hg-lamp and UV-C LED irradiation
Adam Cruise a,
Rémy Broersmab,
Frouke van den Bergb and
Marcus Baumann
a,
Rémy Broersmab,
Frouke van den Bergb and
Marcus Baumann *a
*a
aSchool of Chemistry, University College Dublin, Dublin 4, Ireland. E-mail: marcus.baumann@ucd.ie
bSignify Research, High Tech Campus 7, 5656 AE Eindhoven, The Netherlands
First published on 12th December 2025
Abstract
The use of novel UV-C LEDs emitting monochromatic light at 265 nm is reported for the continuous flow synthesis of ketenimines from isoxazoles. The resulting flow process is compared to the use of a traditional Hg-lamp showing that substantial improvements in reaction performance and efficiency are obtained demonstrating the value of these high-power UV-C LED modules (up to 40 W of input power).
The photochemistry of isoxazoles has a long history resulting in the discovery of several important chemical transformations. For instance, oxazoles and azirines can be generated from isoxazoles upon photochemical cleavage of the labile O–N single bond.1 The underlying radical process requires the use of UV light due to the inefficient light absorbance of the isoxazole substrate at higher wavelengths. While several mechanistic studies2 have been published since the first reports on these transformations in the 1960s,3 the exploitation of the salient features of continuous flow technology4 as well as the general synthetic value of these heterocycle transposition strategies resulted in renewed interest over the past years.5 Crucially, these efforts have also led to the discovery of new reactivity modes allowing for the selective generation of reactive entities such as ketenimines from isoxazoles.6
One remaining bottleneck in this context is the lack of efficient light sources to selectively trigger these photochemical processes. Most studies rely on medium-pressure Hg-lamps that emit light not only across the entire UV and visible range but moreover generate substantial amounts of heat due to the poor energy efficiency of these lamps. As a consequence, active cooling strategies as well as appropriate filters are typically required to minimise secondary photoreactions and related side-products.
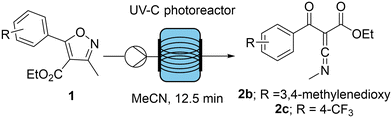
Our group has recently demonstrated the superior performance of a novel UV-B LED module (emitting at 330 nm) over classical medium-pressure Hg-lamps allowing for the effective generation of oxazoles from isoxazolones in flow mode.7 Encouraged by the success of this study, we wished to evaluate whether an analogous UV-C LED module (emitting at 265 nm) would enable the generation of ketenimines from isoxazoles in a manner that would increase the practicality of this intriguing transformation (Scheme 1).
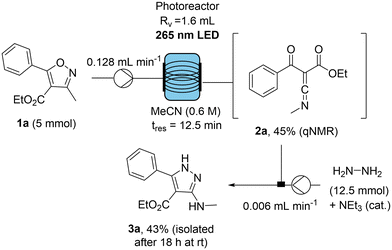
We commenced our study with the development of a continuous flow platform suitable for the low-wavelength LED-system. This was achieved through a 3D-printed reactor frame and coiled PFA (perfluoroalkoxy) tubing to afford a chip-like reactor enabling the effective irradiation of the substrate solution with UV light (see SI for details of the set-up). The two light sources explored in this work were a medium-pressure Hg-lamp housed in a UV-150 flow reactor from Vapourtec,8 and a LED module developed by Signify.9 Both systems allowed for the adjustment of the light intensity which was crucial to optimise the respective photochemical flow processes. As prior work from our group6 had already reported an optimised process for the photo-isomerisation of isoxazoles to ketenimines using the medium-pressure Hg-lamp in combination with the Vapourtec reactor system, only the new UV-C LED module and reactor required optimisation.
At the beginning a DoE optimisation was conducted and the variables selected to assess the conversion of isoxazole 1a (R = H) into ketenimine were residence time and concentration. As this is a unimolecular reaction, it is anticipated to follow first order kinetics, and the rate of reaction is anticipated to strongly correlate with the number of photons absorbed by the reaction. A central composite design (CCD) was thereby chosen as it was anticipated that the residence time and concentration would have a strong correlation with one another, which could be explored efficiently using a CCD initialisation. As the decomposition of the resulting ketenimine 2a via rapid hydrolysis is problematic for accurate quantification, qNMR studies using anhydrous CDCl3 were exploited allowing for the generation of a surface response model of the DoE approach (Fig. 1).
As shown in Fig. 1, the UV-C LED system did not surpass the previous best yield of 54% when using the Hg-lamp for the same photochemical reaction. However, using the UV-C LED module gave clear signs of reduced decomposition and an overall higher tolerance for increased reaction concentrations in comparison to when this reaction was conducted utilising a medium-pressure Hg-lamp. Consequentially, subsequent efforts concentrated on throughput as the optimisation metric rather than conversion to ketenimine (Fig. 2).
 | ||
| Fig. 2 DoE optimisation evaluating throughput as the optimising metric when employing the UV-C LED set-up. | ||
This re-evaluation demonstrated that high throughputs can be achieved at concentrations up to 700 mM marking a clear improvement over the medium-pressure Hg-lamp where maximum concentrations ranged between 10 and 20 mM. To validate these findings a comparative study was conducted exploring conditions for both the highest yield and the highest throughput for each lamp (at 25 °C). Given the differences in reactor volume, the space–time yield (mmol h−1 mL−1) was selected as the comparative metric (Table 1). Furthermore, as this is a unimolecular reaction the difference in flow rates to achieve the same residence time in each instance is not significant.
| Lamp | Conc. (M) | tRes (min) | Yield (qNMR) | STY (mmol h−1 mL−1) | |
|---|---|---|---|---|---|
| 1 | Hg | 0.02 | 12 | 54% | 0.052 |
| 2 | UV-C | 0.02 | 12 | 52% | 0.049 |
| 3 | Hg | 0.6 | 13 | 9% | 0.168 |
| 4 | UV-C | 0.6 | 13 | 47% | 0.900 |
The data in Table 1 clearly shows that using the UV-C LED module does not surpass the yield of the target ketenimine 2a (entry 1 vs. 2), however, it greatly improves the reaction throughput by a factor of ca. 18 when compared to the original conditions reported using the Hg-lamp (entry 1 vs. 4).6 These promising results highlight the suitability of this flow process for reaction telescoping which is advantageous given the challenges in isolating the reactive ketenimine species (vide infra).
Having demonstrated the higher throughput capabilities and improved reaction efficiency utilising the 265 nm LED module, an energy efficiency comparison was conducted. Hg-based lamps often suffer from a significant thermal output and reduced conversion of input power to optical power whereas LEDs typically demonstrate excellent efficiency in this context. This study was conducted by evaluating the external quantum yield of the photochemical process which describes how much energy is required to convert one mole of substrate. This was selected in preference to other common metrics such as quantum efficiency or quantum yield as it allows for consideration of inefficient energy usage. Secondly external quantum yield was preferable as suboptimal irradiation can often elicit a chemical reaction, therefore any calculations utilising quantum flux at a specific wavelength would disregard adjacent wavelengths which also promote photochemical reactions. In this instance conversion of the isoxazole was the target metric being evaluated as this study aimed for a comparison of the efficiency of both light sources, rather than the efficiency of the photoisomerisation of isoxazoles to ketenimines.
As shown in Fig. 3, both lamps perform very similar to one another when using a concentration of 0.1 M despite the different photochemical reactor designs. Next the external quantum yields were calculated for each lamp (see SI for details). This shows that the energy efficiency of the 265 nm LED is approximately 14 times greater than the medium-pressure Hg-lamp despite the similar rate of consumption (Table 2). Additionally, by calculating the external quantum yield (in mol J−1), the cost of light as a reagent can be calculated. This can be done by converting the energy (in J) required to form a mole of product into kWh. The increased wattage of the Hg-lamp thereby carries an increased cost with a significant price of €79 per mole of isoxazole consumed, equating to a cost increase by a factor of 14. In fact, the cost of operating the Hg-lamp is even greater when other factors are considered such as the lifetime of the lamp and the cost of cooling the Hg-lamp.
 | ||
| Fig. 3 Consumption of isoxazole 1a for comparison of light efficiency (blue: 265 nm LED; slope = 3.63, orange: Hg lamp; slope = 3.60). | ||
In addition to the increased energy efficiency of the UV-C LED module, a notable reduction in side-product formation was observed, indicating that the monochromatic LED source provides for superior selectivity. To further probe these advantages, we decided to explore additional isoxazole substrates that were known to be challenging due to their altered light absorbance. Using the original Hg-lamp for such substrates possessing either electron-donating or withdrawing groups had given modest ketenimine yields (R = 3,4-methylenedioxy: 25%; R = 4-CF3: 57%) which was ascribed to the poor match of the emitted wavelengths with the substrate absorbance along with thermal effects leading to side reactions despite the applied cooling.
After recording the UV-vis absorption spectrum of each compound a UV-LED closely matching the maximum absorbance was evaluated using the previously optimised conditions at 20 mM and 600 mM (Scheme 2).
When isoxazole 1b (λmax = 309 nm) was examined, the availability of a LED emitting at 308 nm proved particularly advantageous due to the close alignment of emitted light with absorbance. The yield of ketenimine product was thereby more than doubled (at 308 nm: 25% to 68%; at 265 nm: 53%) and the higher concentration enabled an increased throughput by a factor of ca. 80. On the other hand, isoxazole 1c has a much lower λmax (260 nm) which did not allow for further improvements as the LED emitting at 265 nm provided the lowest wavelength available for this study and higher wavelengths were not better (i.e., 24% and 32% at 280 nm) (Fig. 4).10
 | ||
| Fig. 4 Performance of isoxazoles 1b and 1c using different light sources at different concentrations. | ||
Lastly to validate the amenability of the newly optimised flow procedure for the scaled preparation of ketenimine 2a, its formation was coupled with a subsequent reaction with hydrazine to yield pyrazole 3a in a telescoped manner. As shown in Scheme 3, the clean formation of 2a was achieved at a high substrate concentration of 0.6 M giving a qNMR yield of 45% in only 12.5 min residence time and allowing for the recovery of ca. 50% of isoxazole 1a (5 mmol scale). Crucially, the high purity of the ketenimine intermediate enabled the effective production of pyrazole 3a which was isolated after stirring the reaction product with hydrazine in the collection vessel for 18 hours at ambient temperature. Moreover, this photochemical process is attractive as it used only 9 mL of MeCN, while consuming 0.039 kWh of electricity, whereas the analogous reaction using Hg-lamp irradiation would require 250 mL of solvent, consume 0.69 kWh of electricity and require an operating time of 312 minutes.
In conclusion, this work demonstrates the clear advantages of using a new high-power UV-C LED module emitting at 265 nm over the use of traditional Hg-lamps for the preparation of ketenimines from readily available isoxazoles. Highlights include the significantly improved purity profile of the reaction mixture as well as the ability to employ high substrate concentrations that are not feasible otherwise. Therefore, substantial increases in reaction throughput along with a significant reduction in solvent usage are possible using these UV-C LEDs. Critical for the success of this approach is the matching of the emitted wavelength with the maximum UV light absorbance of the substrate, which can be facilitated by using appropriate UV-LED modules. Under optimised conditions this approach can also be exploited for the scaled synthesis of pyrazoles from isoxazoles via reaction telescoping. Lastly, this study clearly shows the impact of the presented UV-LED module to improve the energy efficiency of photochemical transformations in view of developing sustainable processes utilising light.
Conflicts of interest
There are no conflicts to declare.Data availability
All data relating to this study (i.e., details on reaction optimisation, calculations, experimental procedures and spectroscopic data) can be found in the supplementary information (SI) file.Supplementary information is available. See DOI: https://doi.org/10.1039/d5re00484e.
Acknowledgements
We are grateful to Research Ireland for providing a PhD scholarship (GOIPG/2021/974, to AC). Support by the School of Chemistry at UCD in providing access to spectroscopic facilities (with infrastructure upgrades via University College Dublin and a Science Foundation Ireland Infrastructure Award 18/RI/5702) is acknowledged. Open access funding provided by IReL.Notes and references
- B. Roure, M. Alonso, G. Lonardi, D. Berna Yildiz, C. S. Buettner, T. dos Santos, Y. Xu, M. Bossart, V. Derdau, M. Méndez, J. Llaveria, A. Ruffoni and D. Leonori, Nature, 2025, 637, 860 Search PubMed; E. E. Galenko, A. F. Khlebnikov and M. S. Novikov, Chem. Heterocycl. Compd., 2016, 52, 637 Search PubMed; A. Padwa, Chem. Rev., 1977, 77, 37 CrossRef CAS; G. L'Abbe, Heterocycl. Chem., 1984, 21, 627 CrossRef; F. Hu and M. Szostak, Adv. Synth. Catal., 2015, 357, 2583 CrossRef; C. Lefebvre, L. Fortier and N. Hoffmann, Eur. J. Org. Chem., 2020, 2020, 1393 CrossRef.
- M.-D. Su, J. Phys. Chem. A, 2015, 119, 9666 CrossRef CAS; C. M. Nunes, I. Reva and R. Fausto, J. Org. Chem., 2013, 78, 10657 CrossRef PubMed; K. H. Grellmann and E. Tauer, J. Photochem., 1977, 6, 365 CrossRef.
- E. F. Ullman and B. Singh, J. Am. Chem. Soc., 1966, 88, 1844 CrossRef CAS; E. F. Ullman and B. Singh, J. Am. Chem. Soc., 1967, 89, 6911 Search PubMed.
- S. E. Raby-Buck, J. Devlin, P. Gupta, C. Battilocchio, M. Baumann, A. Polyzos, A. G. Slater and D. L. Browne, Nat. Rev. Methods Primers, 2025, 5, 44 CrossRef CAS; L. Capaldo, Z. Wen and T. Noël, Chem. Sci., 2023, 14, 4230 RSC; G. Lyall-Brookes, A. C. Padgham and A. G. Slater, Digital Discovery, 2025, 4, 2364 RSC; A. I. Alfano, J. García-Lacuna, O. M. Griffiths, S. V. Ley and M. Baumann, Chem. Sci., 2024, 15, 4618 RSC; S. B. Ötvös and C. Oliver Kappe, Green Chem., 2021, 23, 6117 RSC.
- C. Bracken and M. Baumann, J. Org. Chem., 2020, 85, 2607 CrossRef CAS; A. V. Serebryannikova, E. E. Galenko, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2019, 84, 15567 CrossRef; U. Devi Newar, S. Borra and R. Awatar Maurya, Org. Lett., 2022, 24, 4454 Search PubMed; A. Saha, E. Casali, A. Ghosh and D. Maiti, Org. Lett., 2025, 27, 2811 CrossRef PubMed.
- C. Bracken and M. Baumann, Org. Lett., 2023, 25, 6593 CrossRef CAS.
- R. Crawford, R. Broersma, F. van den Berg and M. Baumann, Org. Lett., 2025, 27(36), 10152–10156 CrossRef CAS PubMed.
- For details, please see: https://www.vapourtec.com/.
- For details on the used UV spot modules, please see: https://www.assets.signify.com/is/content/Signify/Assets/signify/global/20250520-signify-photochemistry-spot-module-specification-sheet.pdf.
- Furthermore, a reduced extinction coefficient of 9798 L mol−1 cm−1 was recorded for isoxazole 1c, whereas 1a had an extinction coefficient of 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732 L mol−1 cm−1 and 1b 11
732 L mol−1 cm−1 and 1b 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703 L mol−1 cm−1 (all in MeCN at 265 nm).
703 L mol−1 cm−1 (all in MeCN at 265 nm).
| This journal is © The Royal Society of Chemistry 2026 |