Open Access Article
Open Access ArticleMerging dynamic and static chirality in propeller-shaped carbazole oligomers via chiral amine substitution
Yuma Taniokaa,
Ryosuke Omasaa,
Tetsuo Okujima ab,
Hidemitsu Unoa and
Masayoshi Takase
ab,
Hidemitsu Unoa and
Masayoshi Takase *ab
*ab
aGraduate School of Science and Engineering, Ehime University, Matsuyama 790-8577, Japan. E-mail: takase.masayoshi.ry@ehime-u.ac.jp
bResearch Unit on Molecular Materials Science for Toroidal π-Electron Systems, Ehime University, Matsuyama 790-8577, Japan
First published on 7th January 2026
Abstract
Organic emissive materials exhibiting circularly polarized luminescence (CPL) have attracted considerable attention as promising candidates for next-generation photonic and electronic devices. However, most CPL-active molecules require laborious procedures such as asymmetric synthesis or optical resolution. In this study, chiral propeller-shaped molecules were conveniently synthesized by introducing chiral amines possessing static chirality into a dynamically chiral propeller framework. The molecule was obtained via a two-step aromatic nucleophilic substitution (SNAr) sequence and exhibited distinct CPL activity (|glum| ≈ 2.0 × 10−3 at room temperature) without optical resolution. Furthermore, a bifunctionalized derivative employing a chiral diamine with a similar substructure was synthesized, but no enhancement in CPL anisotropy was observed. These results indicate that precise control over the relative orientation of the magnetic and electric transition dipole moments is crucial for improving CPL performance.
Introduction
Circularly polarized luminescence (CPL)-active organic materials have attracted considerable attention owing to their promising applications in emerging technologies such as 3D displays,1 quantum communication,2,3 and bioimaging.4 A wide variety of CPL-active molecules have been developed to date, typically represented by helicenes,5,6 twistacenes,7 and distorted polycyclic aromatic hydrocarbons.8 Their chirality originates from twisted molecular frameworks arising from steric strain. When the inversion barrier is sufficiently low, interconversion between the two enantiomeric conformations becomes feasible, leading to so-called “dynamic chirality”.9 Although dynamic chirality has been exploited in the development of molecular chirality switches, achieving both reversible interconversion and structural stability remains a persistent challenge.10–12To address this issue, various molecular design strategies have been investigated. These include extending helical π-conjugated systems to increase the inversion barrier,13–15 and introducing bulky substituents into polycyclic aromatic frameworks to sterically lock the chiral conformation.16–18 However, such approaches typically require laborious procedures involving asymmetric synthesis or the separation of racemates.14,19 Recent studies have demonstrated that these challenges can be more efficiently overcome by coupling dynamic chiral units with conformationally rigid, static chiral moieties.10,20–26 Despite its conceptual simplicity, this approach has remained surprisingly underexplored.
Propeller-shaped molecules such as hexaarylbenzenes (HABs) represent an attractive platform for such molecular design. Owing to the steric congestion among the six peripheral aryl blades, HABs adopt a propeller-like geometry. In the idealized HAB structure, each blade is twisted by approximately 60° relative to the central benzene core, generating two atropisomers (Fig. 1). In parent HABs, both conformers are energetically equivalent, rendering the molecule achiral. In contrast, the introduction of chiral auxiliaries can bias the torsional sense of the blades through steric interactions, thereby giving rise to distinct propeller chirality.27–30 Thus, the HAB skeleton serves as a versatile scaffold that exhibits controllable dynamic chirality.
 | ||
| Fig. 1 Schematic illustrations of propeller chirality, and MA (monoamine)-functionalized propeller and DA (diamine)-functionalized propeller dimer studied in this work. | ||
In this context, we previously developed a propeller-shaped molecule, Bz-6PDI, composed of a benzene core bearing six perylene diimide blades, in which all blades are equipped with conformationally stable chiral units.30 Bz-6PDI was conveniently synthesized via an aromatic nucleophilic substitution (SNAr) reaction with hexafluorobenzene and exhibited solvent-induced chirality inversion along with CPL activity at room temperature. Despite its relative simplicity, this approach still required the pre-synthesis of chiral blades bearing stable chiral units, and therefore remained somewhat limited in practicality.
In this work, we aimed to achieve facile fixation of molecular chirality by merging dynamic and static chiral elements within an HAB framework (Fig. 1). To this end, chiral amines were introduced into the blade positions of the HAB core, employing carbazole—a well-known and versatile building block—as the aryl component.31–33 Readily available chiral amines served as the source of static chirality. The target monoamine (MA)-functionalized molecule 1 was synthesized through a two-step SNAr sequence using enantiopure amine. As expected, (R/S)-1 exhibited both circular dichroism (CD) and CPL activity (|glum| = 2.0 × 10−3 at room temperature) without optical resolution. Furthermore, the dimeric derivative (R,R/S,S)-2, in which two carbazole oligomers were connected via a diamine linker, also displayed CD and CPL responses with a slightly reduced anisotropy factor (|glum| = 1.0 × 10−3 at room temperature). Taken together, these findings demonstrate that the covalent coupling of dynamic and static chiral units represents an effective and synthetically simple strategy for achieving stable molecular chirality and pronounced CPL activity.
Results and discussion
Synthesis
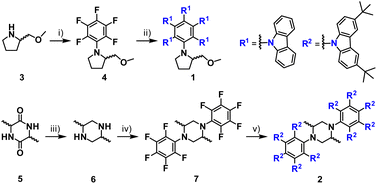
To construct propeller-shaped chiral molecules, we selected 2-(methoxymethyl)pyrrolidine (3) as a chiral auxiliary owing to its commercial availability and high nucleophilicity. Both enantiomers, (R)-1 and (S)-1, were obtained in moderate yields through a two-step SNAr sequence (Scheme 1).34 The first step involved the SNAr reaction of chiral amine 3 with hexafluorobenzene to afford the mono-substituted intermediate 4. Subsequent SNAr substitution under basic conditions using calcium hydride as the base35,36 furnished the desired product 1. In the 1H NMR spectrum of 1 in C2D2Cl4, the aromatic proton signals appeared broadened at room temperature, reflecting both low molecular symmetry and internal motion within the propeller framework. Upon heating, these signals sharpened and became more resolved in C2D2Cl4, indicating a thermally activated conformational process (Fig. S3a).For the synthesis of the dimeric derivative, cis-2,5-dimethylpiperazine (6) was chosen as a linker because it is readily accessible and provides a chiral framework analogous to that of monomeric 3. According to a reported procedure,37 the piperazine precursor, diketopiperazine 5, was prepared, and subsequently reduced with LiAlH4 to yield the chiral diamine 6. Attempts to synthesize the dimer 2 using parent carbazole, following a similar SNAr strategy to that used for 1, were unsuccessful due to the extremely poor solubility of the resulting product, which hindered both the reaction progress and purification. To overcome this limitation, solubilizing substituents were introduced at the 3,6-positions of the carbazole units. The resulting dimer 2, in which two carbazole pentamers were linked via the chiral piperazine-based linker, was successfully obtained through the same two-step SNAr sequence employed for 1. In the aromatic region of the 1H NMR spectra of 2 in CDCl3 and C2D2Cl4, pronounced signal broadening was observed, and even upon heating, no significant improvement in resolution occurred in C2D2Cl4 (Fig. S3b). In contrast, the signals corresponding to the piperazine framework remained sharp, suggesting the formation of a single, well-defined product. Furthermore, the absence of any detectable fluorine signals in the 19F NMR spectrum confirmed complete substitution of all fluorine atoms by carbazole units, verifying the formation of the fully substituted derivative 2.
Optical and chiroptical properties
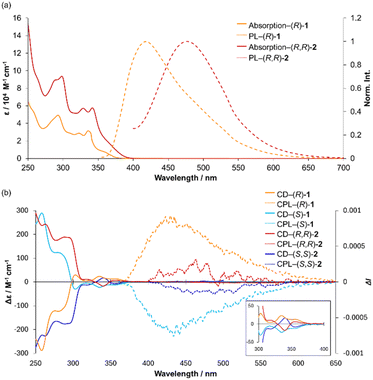
The optical properties of 1 and 2 were evaluated in various solvents, including cyclohexane, dichloromethane (DCM), MeOH, and DMF (Fig. S4a, b and 2a). The UV-vis absorption spectra showed little dependence on solvent polarity, exhibiting a main absorption band around 340 nm with a shoulder peak near 360 nm. In contrast, the emission spectra displayed distinct solvent-dependent behavior, characteristic of carbazole-based architectures,38,39 suggesting the involvement of an intramolecular charge-transfer (ICT) process. The absorption spectrum of 2 exhibited no appreciable red shift compared to that of 1 (Fig. 2a), indicating that π-conjugation was not significantly extended upon dimerization. However, the emission maxima of 2 were consistently red-shifted in all solvents examined (Fig. S4b), which can be attributed to increased structural flexibility arising from the linkage of two carbazole units via a diamine linker.Next, the influence of the chiral auxiliary on the chiroptical properties of these propeller-type molecules was examined by CD and CPL spectroscopy in DCM. Both 1 and 2 bearing the (R)-amine moiety displayed positive Cotton effects, whereas the corresponding (S)-enantiomers exhibited mirror-image negative cotton effects (Fig. 2b). In CPL measurements, 1 showed clear CPL activity with a |glum| value of 2.0 × 10−3. Dimer 2 also exhibited CPL signals, albeit with a smaller anisotropy factor of |glum| = 1.0 × 10−3. Interestingly, the |glum| value of (R,R)-2 was not simply additive relative to that of (R)-1; instead, its CPL intensity was reduced. This behavior can be rationalized from a structural perspective. In (R,R)-2, the two methyl groups on the central piperazine core adopt axial and equatorial orientations, respectively, leading to the loss of bilateral symmetry between the two propeller units. This asymmetry likely prevents the propeller helicities from aligning in the same sense, as observed for (R)-1, thereby diminishing the overall anisotropy of the system.
Solvent and temperature dependence of chiroptical response
The solvent- and temperature-dependent CD behaviors of (R)-1 and (R,R)-2 were subsequently investigated (Fig. 3 and S6c, d). Such analyses are highly informative for elucidating the dynamic nature of chiral propeller molecules.30,40 As observed for previously reported propeller-type systems,27,28,30 the CD intensity of (R)-1 gradually decreased across the entire wavelength region upon heating. In contrast, no appreciable wavelength shift was detected within the examined temperature range. The absence of spectral shifts suggests that conformational changes involving torsional motion within the propeller framework are restricted, even under thermal perturbation.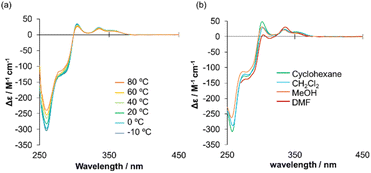 | ||
| Fig. 3 CD spectra of (R)-1 (a) at various temperatures in tetrachloroethane and (b) in various solvents at rt. | ||
With respect to solvent effects, (R)-1 exhibited comparable CD intensities in cyclohexane, DCM, MeOH, and DMF. This observation indicates that the chiroptical response is not governed solely by solvent polarity. Instead, it is likely influenced by a combination of factors such as solvent size, shape, and viscosity, all of which can modulate the conformational freedom and dynamic equilibrium of the propeller scaffold.
DFT calculations
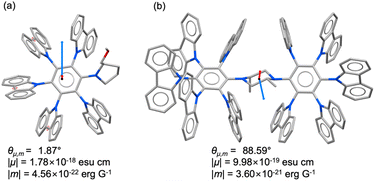
To gain deeper insight into the above hypothesis, DFT calculations were carried out (Fig. 4). The molecular geometries of (R)-1 and (R,R)-2 were optimized at the B3LYP/6-31G(d) and B3LYP/6-31G levels, respectively. The CD spectra were then evaluated by TD-DFT at the B3LYP/6-31+G(d) level. The dissymmetry factor (g value) is defined as the vector product of the electric and magnetic transition dipole moments. For (R)-1, the angle between these two moments was calculated to be 1.9°, giving a cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ value close to 1, which indicates that the two vectors are nearly parallel. In contrast, (R,R)-2 exhibited an angle of 88.6°, implying that the two moments are almost orthogonal, thereby accounting for its smaller g value.
θ value close to 1, which indicates that the two vectors are nearly parallel. In contrast, (R,R)-2 exhibited an angle of 88.6°, implying that the two moments are almost orthogonal, thereby accounting for its smaller g value.
The calculated CD spectra further clarify the relationship between molecular geometry and chiroptical properties. In (R)-1, the rotational strength associated with the HOMO → LUMO transition is large (Rvel = 8.02), whereas in (R,R)-2, the longest-wavelength band, corresponding to the HOMO−1 → LUMO transition, shows a much smaller rotational strength (Rvel = 0.83). Moreover, both (R)-1 and (R,R)-2 display a characteristic pattern in which a large negative rotational strength at shorter wavelengths is followed by a positive one at longer wavelength (Fig. S5b). Accordingly, the absence of a negative CD signal in the 300–400 nm region for (R)-1 can be ascribed to the dominant HOMO → LUMO transition with large rotational strength, whereas the relatively weak HOMO−1 → LUMO transition in (R,R)-2 results in a CD spectrum featuring a distinct negative component in this region.
Conclusions
Propeller-shaped molecules 1 and 2 were readily synthesized through a two-step SNAr sequence using chiral amines. Both molecules exhibited distinct CD and CPL activities without any optical resolution, demonstrating that the intrinsic chirality of the molecular framework is sufficient to induce pronounced chiroptical responses. Although the introduction of a bifunctional linker was expected to enhance the anisotropy factor, no significant improvement was observed experimentally. DFT calculations clarified that the difference in glum values between 1 and 2 originates from the distinct orientations of their electric and magnetic transition dipole moments. Overall, this study demonstrates that the incorporation of chiral auxiliaries into a propeller-type π-conjugated scaffold effectively stabilizes its dynamic chirality. These findings offer valuable design principles for the rational development of next-generation chiroptical molecular materials.Author contributions
M. T. and H. U. designed the project. Y. T. and R. O. performed most of the experimental works. M. T. and Y. T. prepared the overall manuscript. All authors, including T. O. and H. U., contributed by commenting on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this manuscript can be found in its supplementary information (SI). Supplementary information: detailed synthetic procedures, NMR spectra for all new compounds, and computational details. See DOI: https://doi.org/10.1039/d5ra09136e.Acknowledgements
The authors are grateful to Dr Kosuke Oki and Kohei Hamada for their contributions to the preliminary synthetic experiments. The authors further acknowledge Prof. Masashi Hasegawa (Kitasato Univ.) for his advice on the DFT calculations of the transition dipole moments. This work was supported by JSPS KAKENHI (JP24K01470 (M. T.), JP23H03964 (M. T.), JP20H02725 (M. T.), and JP19K05422 (H. U.)). M. T. acknowledge the Nagase Science Technology Foundation. Y. T. acknowledges JSPS Research Fellowship for Young Scientists.Notes and references
- D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 Search PubMed
.
- J. F. Sherson, H. Krauter, R. K. Olsson, B. Julsgaard, K. Hammerer, I. Cirac and E. S. Polzik, Nature, 2006, 443, 557–560 Search PubMed
.
- D. Zhu, W. Jiang, Z. Ma, J. Feng, X. Zhan, C. Lu, J. Liu, J. Liu, Y. Hu, D. Wang, Y. S. Zhao, J. Wang, Z. Wang and L. Jiang, Nat. Commun., 2022, 13, 3454 CrossRef CAS PubMed
.
- P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed
.
- Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463–1535 Search PubMed
.
- M. Rickhaus, M. Mayor and M. Juríček, Chem. Soc. Rev., 2016, 45, 1542–1556 Search PubMed
.
- A. Bedi and O. Gidron, Acc. Chem. Res., 2019, 52, 2482–2490 Search PubMed
.
- M. A. Majewski and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 86–116 CrossRef CAS
.
- J. M. Fernández-García, P. Izquierdo-García, M. Buendía, S. Filippone and N. Martín, Commun. Chem., 2022, 58, 2634–2645 Search PubMed
.
- S. T. Bao, S. Louie, H. Jiang, Q. Jiang, S. Sun, M. L. Steigerwald, C. Nuckolls and Z. Jin, J. Am. Chem. Soc., 2024, 146, 51–56 Search PubMed
.
- I. Shioukhi, H. Batchu, G. Schwartz, L. Minion, Y. Deree, B. Bogoslavsky, L. J. W. Shimon, J. Wade, R. Hoffman, M. J. Fuchter, G. Markovich and O. Gidron, Angew. Chem., Int. Ed., 2024, 63, e202319318 CrossRef CAS PubMed
.
- N. Saleh, B. Moore II, M. Srebro, N. Vanthuyne, L. Toupet, J. A. G. Williams, C. Roussel, K. K. Deol, G. Muller, J. Autschbach and J. Crassous, Chem.–Eur. J., 2015, 21, 1673–1681 CrossRef CAS
.
- Y. Matsuo, M. Gon, K. Tanaka, S. Seki and T. Tanaka, J. Am. Chem. Soc., 2024, 146, 17428–17437 CrossRef CAS
.
- F. Morita, Y. Kishida, Y. Sato, H. Sugiyama, M. Abekura, J. Nogami, N. Toriumi, Y. Nagashima, T. Kinoshita, G. Fukuhara, M. Uchiyama, H. Uekusa and K. Tanaka, Nat. Synth., 2024, 3, 774–786 Search PubMed
.
- Y. Nakakuki, T. Hirose, H. Sotome, M. Gao, D. Shimizu, R. Li, J.-Y. Hasegawa, H. Miyasaka and K. Matsuda, Nat. Commun., 2022, 13, 1475 Search PubMed
.
- B. Mahlmeister, M. Mahl, H. Reichelt, K. Shoyama, M. Stolte and F. Würthner, J. Am. Chem. Soc., 2022, 144, 10507–10514 Search PubMed
.
- X. Liu, Z. Jin, F. Qiu, Y. Guo, Y. Chen, Z. Sun and L. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202407547 Search PubMed
.
- Y. Dong, Z. Zhang, Y. Hashikawa, H. Meng, F. Bai, K. Itami and Chaolumen, Angew. Chem., Int. Ed., 2024, 63, e202406927 CrossRef CAS PubMed
.
- M. Gingras, G. Félix and R. Peresutti, Chem. Soc. Rev., 2013, 42, 1007–1050 Search PubMed
.
- J. Lorkowski, D. Bouetard, P. Yorkgitis, M. Gembicky, T. Roisnel, N. Vanthuyne, D. Munz, L. Favereau, G. Bertrand, M. Mauduit and R. Jazzar, Angew. Chem., Int. Ed., 2023, 62, e202305404 CrossRef CAS
.
- P. Jiang, A. S. Mikherdov, H. Ito and M. Jin, J. Am. Chem. Soc., 2024, 146, 12463–12472 Search PubMed
.
- S. Feuillastre, M. Pauton, L. Gao, A. Desmarchelier, A. J. Riives, D. Prim, D. Tondelier, B. Geffroy, G. Muller, G. Clavier and G. Pieters, J. Am. Chem. Soc., 2016, 138, 3990–3993 CrossRef CAS
.
- M. Coehlo, L. Frédéric, L. Poulard, N. Ferdi, L. Estaque, A. Desmarchelier, G. Clavier, J.-P. Dognon, L. Favereau, M. Giorgi, J.-V. Naubron and G. Pieters, Angew. Chem., Int. Ed., 2024, e202414490 Search PubMed
.
- A. Swain, K. Radacki, H. Braunschweig and P. Ravat, Chem. Sci., 2024, 15, 11737–11747 Search PubMed
.
- G. Ouyang, J. Rühe, Y. Zhang, M.-J. Lin, M. Liu and F. Würthner, Angew. Chem., Int. Ed., 2022, 61, e202206706 Search PubMed
.
- N. Saleh, V. Zullo, E. Sucre-Rosales, L. Arrico, F. Zinna, G. Pescitelli, C. Besnard, E. Vauthey and J. Lacour, Chemistry, 2025, 31, e202500490 CrossRef CAS PubMed
.
- T. Kosaka, S. Iwai, Y. Inoue, T. Moriuchi and T. Mori, J. Phys. Chem. A, 2018, 122, 7455–7463 Search PubMed
.
- T. Kosaka, Y. Inoue and T. Mori, J. Phys. Chem. Lett., 2016, 7, 783–788 Search PubMed
.
- M. Toyoda, Y. Imai and T. Mori, J. Phys. Chem. Lett., 2017, 8, 42–48 CrossRef CAS
.
- Y. Tanioka, M. Takase, M. Hamasu, S. Hata, K. Hashimoto, S. Mori, Y. Ishibashi, Y. Nukumi, M. Higashi, H. Sato, T. Okujima and H. Uno, Angew. Chem., Int. Ed., 2025, 64, e202509190 CrossRef CAS PubMed
.
- K. Shizu, J. Lee, H. Tanaka, H. Nomura, T. Yasuda, H. Kaji and C. Adachi, Pure Appl. Chem., 2015, 87, 627–638 CrossRef CAS
.
- N. Blouin and M. Leclerc, Acc. Chem. Res., 2008, 41, 1110–1119 Search PubMed
.
- M. L. S. Beaupréa, J. Mater. Chem. A, 2013, 1, 11097–11105 RSC
.
- H. Uno, M. Ishiwata, K. Muramatsu, M. Takase, S. Mori and T. Okujima, Bull. Chem. Soc. Jpn., 2019, 92, 973–981 CrossRef CAS
.
- Y. Sasaki, M. Takase, N. Kobayashi, S. Mori, K. Ohara, T. Okujima and H. Uno, J. Org. Chem., 2021, 86, 4290–4295 CrossRef CAS PubMed
.
- T. Narita, K. Fujiwara, H. Uno, M. S. Asano, T. Nishinaga and M. Takase, J. Porphyrins Phthalocyanines, 2023, 27, 1357–1363 CrossRef CAS
.
- J. Chen, J. Li, L. Zhu, X. Peng, Y. Feng, Y. Lu, X. Hu, J. Liang, Q. Zhao and Z. Wang, Org. Chem. Front., 2018, 5, 3402–3405 RSC
.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS
.
- M. Mamada, S. Yada, M. Hayakawa, R. Uchida, H. Katagiri, T. Hatakeyama and C. Adachi, Commun. Chem., 2024, 7, 212 CrossRef CAS
.
- S. T. Bao, H. Jiang, C. Schaack, S. Louie, M. L. Steigerwald, C. Nuckolls and Z. Jin, J. Am. Chem. Soc., 2022, 144, 18772–18777 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |