 Open Access Article
Open Access ArticleBoosting the adsorption and sensing performance of MoS2 for SF6 decomposition gases by non-metal atom doping:a DFT study
Mamutjan Tursun *,
Yifan Liu and
Abulimiti Yumaier*
*,
Yifan Liu and
Abulimiti Yumaier*
Xinjiang Key Laboratory of Novel Functional Materials Chemistry, College of Chemistry and Environmental Sciences, Kashi University, Kashi 844000, PR China. E-mail: mmtj15@stu.xjtu.edu.cn
First published on 14th January 2026
Abstract
In electrical power systems, SF6 in Gas Insulated Switchgear (GIS) decomposes under partial discharge, yielding toxic products such as H2S, SO2, SOF2, SO2F2. Molybdenum disulfide (MoS2), a promising two-dimensional material, exhibits potential in gas sensing but its pristine form suffers from weak adsorption capacity for gas molecules. Herein, we carry out a systematic exploration of the gas-sensing capabilities of eight non-metal (NM)-doped MoS2 (NM@MoS2) materials toward SF6 decomposition gases by leveraging first-principles calculations. The results reveal that all NM@MoS2 substrates exhibit thermodynamic stability with negative binding energies ranging from −0.84 to −7.11 eV. Pristine MoS2 shows weak physisorption of target gases, accompanied by low adsorption energies (−0.21 to −0.33 eV), large adsorption distances (2.90 to 3.70 Å), minimal charge transfer and limited sensitivity. In contrast, the NM@MoS2 substrates demonstrate distinct adsorption behaviors: O@MoS2, Se@MoS2, and Te@MoS2 retain physical adsorption (adsorption energies: −0.14 to −0.37 eV; distances: 2.73 to 4.02 Å), whereas B@MoS2, C@MoS2, N@MoS2, P@MoS2, and Si@MoS2 demonstrate enhanced adsorption (adsorption energies: −0.39 to −1.67 eV; distances: 1.60 to 3.24 Å), accompanied by significant charge transfer and enhanced sensing-response. Of these substrates, Si@MoS2 demonstrates moderate recovery times at ambient temperature (2.82 s) and demonstrates significant sensing-response to SF6 decomposition components, highlighting its potential for practical gas sensing applications. This study demonstrates that non-metal doping can effectively enhance the gas-detection efficacy of MoS2 towards SF6 decomposition products, providing theoretical support for developing high-efficiency gas sensors.
1. Introduction
In the realm of power systems, Gas Insulated Switchgear (GIS) has become increasingly prevalent as power grids expand, and its safe and reliable operation is of utmost importance.1,2 A key component of GIS is sulfur hexafluoride (SF6), which serves as a vital gas-insulating medium, renowned for its remarkable electrical insulation and arc-quenching properties. However, during extended periods of operation, local discharge events can trigger the breakdown of SF6 gas, producing byproducts such as hydrogen sulfide (H2S), sulfur dioxide (SO2), sulfuryl fluoride (SOF2), and thionyl difluoride (SO2F2).3,4 These byproducts have significantly poorer insulating properties than SF6, which weakens equipment insulation, intensifies local discharge incidents and poses a severe threat to system stability.5,6 In light of these challenges, it is of the utmost importance to develop effective technologies that can detect and remove SF6 decomposition gases. Such advancements are essential for enhancing the safety and reliability of power systems, as well as for mitigating potential environmental and health risks.7,8In recent years, two-dimensional transition metal dichalcogenides (TMDs) have garnered significant attention due to their ultra-high specific surface area and exceptional electronic transport properties. Taking MoS2 as an example, chemical vapor deposition (CVD) enables the fabrication of atomic-thickness monolayer films.9 When thickness is reduced to the atomic level, the specific surface area increases significantly, expanding the interface area for gas molecule contact. Simultaneously, the bandgap transitions from the bulk indirect bandgap (1.2 eV) to a direct bandgap (1.8 eV), substantially enhancing electron mobility and accelerating rapid charge transfer between gas molecules and the surface.10 These attributes have propelled their extensive utilization in gas sensing applications.11–14 Additionally, atomically functionalized TMDs have been employed for detecting SF6 decomposition gases. For instance, Li et al. demonstrated the potential of platinum-doped HfS2 (Pt-HfS2) for dual gas detection of H2S and SO2.15 Jiang et al. investigated the adsorption behavior of iridium-embedded hafnium disulfide (Ir-HfS2) monolayers toward three sulfur hexafluoride decomposition products (H2S, SOF2, and SO2F2) and their sensing prospects.16 TMDs are characterized by a layered two-dimensional structure, where each layer is composed of alternating transition metal and chalcogen atoms. This unique structural arrangement imparts distinctive properties to TMDs, unlocking a wide range of potential applications.17,18 Currently, extensive research efforts have been directed toward exploring two-dimensional materials, particularly compounds such as MoS2, MoSe2, and SnS2.19–22 Investigations have revealed that TMDs monolayer exhibits favorable characteristics, including narrow band gaps and high carrier mobility. These features render them highly suitable for applications in optoelectronics and nanoelectronics.23–25
Among TMDs materials, MoS2 has attracted the most research interest in gas detection, particularly for the sensing of toxic gases. This is due to its favourable semiconductor properties, which include a substantial band gap, a large surface-to-volume ratio, an abundance of sites for redox reactions, and high carrier mobility.26,27 MoS2-based gas sensing materials achieve significant gas sensitivity through charge transfer between gas molecules and the surface. For instance, Late et al. utilised ab initio calculations to ascertain that the observed reduction in resistance of monolayer and bilayer MoS2 under an applied magnetic field is attributable to charge transfer.28 Dong et al. fabricated an Au/MoS2/Au photoelectrochemical gas sensor exhibiting sensitivity as high as S = 4.9%/ppb (4900%/ppm) upon exposure to ppb-level NO2.29 However, chemical adsorption impedes recovery processes, and MoS2 still faces bottlenecks such as poor selectivity and insufficient sensitivity.30 Therefore, new strategies are urgently needed to overcome performance limitations.
In recent decades, a vast body of research has concentrated on modifying the MoS2 monolayer to improve its gas-sensing performance. These modification strategies primarily include doping with metal (both precious and non-precious metals) and non-metal (NM) atoms, as well as compositing with other materials.27,31–39 Such modifications are credited with significantly improving the electronic properties and structural stability of MoS2 monolayers, thereby optimizing their gas-sensing capabilities.31,39Therefore, doping has a significant influence on the electronic structure and gas-detection capability of MoS2, including the adsorption strength, interfacial charge displacement and adsorbate–substrate interaction. For example, Fan et al. studied how the doping of transition metals (including several precious and non-precious metals) affects the gas-detecting performance of MoS2 monolayer when exposed to various gas molecules (CO, NO, O2, NO2 and NH3).37 The results demonstrated that doping significantly enhanced the sensing performance. Luo et al. selected Al, Si, and P atoms as dopants due to the close similarity of their covalent radii to that of the S atom.38 Doping MoS2 with these atoms has demonstrated promising potential for NO2 sensing despite of hazardous gasses sensing, the MoS2 doped with metal (both precious and non-precious metals) atom materials also used detection of SF6 decomposition gases.3,36,40,41
Similarly, extensive research has been conducted on utilizing NM atoms to modify MoS2 for detecting sulfur-containing gases.42,43 For instance, Szary et al. systematically investigated adsorption selectivity by adsorbing H2S, N2, and O2 molecules on pristine MoS2 films and MoS2 films doped with P, Cl and Ge.42 Piosik et al. used doping strategies involving Si, P, Cl, Ge and Se to significantly improve the sensing performance of MoS2 for SO2.43 It should be emphasized that MoS2 substrates doped with NM atoms are renowned for the simplicity of their preparation. For instance, through density functional theory (DFT) calculations, Ma and his colleagues showed that common gases such CO, NO, NO2, and O2 are capable of occupying S vacancies in MoS2 at room temperature. In this way, doping with C, N, and O can be realized.44 This implies that MoS2 doped with NM atoms can be realized under gentle conditions. Moreover, Song and his colleagues managed to synthesize P-doped MoS2 through a simple pyrolysis procedure.45 Xie and their team successfully fabricated MoS2 materials with different concentrations of oxygen doping by controlling the preparation temperature.46 Zhang and his colleagues fabricated Se-doped MoS2 materials employing a hydrothermal process, and Song et al. produced O-doped MoS2 materials by means of pyrolysis.47,48 In a similar vein, multiple experimental studies have emphasized that B-doped MoS2 materials obtained via the hydrothermal route display outstanding performance in electrocatalytic reactions.49–51 Consequently, these research outcomes provide strong theoretical backing for the synthesizability and stability of NM@MoS2 materials.
In this study, we performed a comprehensive analysis of the adsorption and detection characteristics of NM@MoS2 materials (where NM = B, C, N, O, P, Si, Se and Te) using DFT calculations, with regard to four SF6 decomposition products: H2S, SO2, SOF2 and SO2F2. Firstly, we calculated the formation energies of NM@MoS2 to evaluate the thermodynamic stability of the different substitution sites. Next, we computed and analyzed the adsorption characteristics of the four SF6 decomposition gases on NM@MoS2 substrates, making comparisons with intrinsic MoS2. Finally, we explored their gas-sensing potential further via electronic structure calculations and sensitivity analysis.
2. Computational methods
We performed spin-polarized DFT calculations using the Vienna Ab Initio Simulation Package (VASP).52 The electronic exchange and correlation terms were characterised using the generalized gradient approximation (GGA) and the Perdew–Burke–Ernzerhof (PBE) functional.53 The GGA-PBE functional is one of the most widely used exchange-correlation approximations for evaluating electronic properties. Compared to the Local Density Approximation (LDA), meta-GGA, and hybrid functions, it strikes a favourable balance between computational cost and accuracy, and it has also been extensively studied for calculations involving two-dimensional materials.3,41,44 Ion–electron interactions were characterized using the Projector Augmented Wave (PAW) approach, and the energy cutoff was fixed at 450 eV.54 Complete relaxation was achieved for every atom, with standard convergence parameters of 1 × 10−4 eV and 0.02 eV Å−1 established for energies and forces, respectively. The DFT-D3 approach was then employed to strengthen the weak van der Waals forces between the adsorbates and surfaces.55 The limited capability of traditional functions in describing dispersion effects makes the introduction of empirical dispersion correction terms the most viable solution currently available.56 Compared to methods such as DFT-D2 and optPBE, the DFT-D3 correction technique offers greater accuracy, broader elemental coverage (H–Pu) and improved computational efficiency.57 It is particularly well-suited to large-scale or rapidly iterative research systems, and has been widely adopted in chemistry, materials science and related fields.58,59 During the geometry optimization process, a 4 × 4 × 1 Monkhorst–Pack k-point grid was adopted to sample the Brillouin zone.60 A single-layer supercell with dimensions of 4 × 4 × 1 was built to serve as a model for the substrate surface. To eradicate the possible effect of periodicity on computational results, a 15 Å-thick vacuum layer was established in the lattice's Z-direction. To examine the possibility of doping MoS2 with NM atom, the formation energies (Ef) of NM@MoS2 substrates were computed in line with eqn (1):| Ef = ENM@MoS2 − EMoS2 − µNM + µsubstitute-atom | (1) |
The adsorption energies (Ead) of adsorbed gases are calculated according to eqn (2):
| Ead = ENM@MoS2+adsorbed gas − ENM@MoS2 − Egas molecule | (2) |
3. Results and discussion
3.1. Structural stability of NM@MoS2
Starting from the 2H–MoS2 unit cell, the geometric structure was first optimized to obtain lattice parameters. The optimized lattice constant was a = 3.16 Å, the S–Mo bond length was 2.41 Å, and the S–Mo–S bond angle was 80.61°, which are highly consistent with literature reports.61,62 Given that MoS2 monolayer features two substitutional sites (i.e., S and Mo), this study systematically investigated the substitution of B, C, N, O, P, Si, Se, and Te at both sites. The corresponding configurations are presented in Fig. 1a and S1a. To quantitatively evaluate the synthetic feasibility of these NM@MoS2 materials, we initially computed their formation energies (Ef). As illustrated in Fig. 1b and S1b, the Ef values of NM atoms substituting S sites in MoS2 monolayer range from −3.86 to 2.32 eV, whereas those substituting Mo sites span from −0.22 to 9.52 eV. The substantially lower energy barrier for S-site substitution highlights a distinct preference of NM atoms for occupying the S positions. It is noteworthy that although the Ef value for N atom replacing the S site is relatively large, this result stems from setting the chemical potential of the N atom to half that of nitrogen gas in this work. Nevertheless, the Ef value for N atom replacing S site remains significantly lower than the corresponding value for replacing the Mo site, indicating that the N atom preferentially substitutes the S site. Experimental synthesis of N@MoS2 via S-site substitution has already been achieved,63,64 thereby confirming its experimental feasibility and stability. Besides, the majority of other NM@MoS2 materials have been successfully synthesized experimentally, which further validating their stability.44–48,51 Huang et al. successfully synthesized ultrathin P-doped MoS2 nanosheets via pyrolysis, achieving a surface phosphorus content of 4.7 at%.45 Xie et al. used hydrothermal synthesis to produce oxygen-doped MoS2 ultrathin nanosheets (O–MoS2), with oxygen atom content ranging from 1.92 to 4.18 at%.46 Huang et al. thermally decomposed MoS2 ultrathin nanosheets synthesized using ammonium molybdate, thiourea, and layered g-C3N4 as templates. Following simple H2O2 treatment, they achieved controlled oxygen atom introduction at defect sites (including edges), yielding an oxygen content of 6.2 at%.47 Yuan et al. synthesized N-doped MoS2 photocatalysts via a two-step hydrothermal calcination process, achieving a nitrogen content of approximately 18.39 at%.65 | ||
| Fig. 1 (a) Structure of NM atoms replacing S sites in a monolayer MoS2; (b) formation energies of NM atom substitutions at S sites in a monolayer MoS2. | ||
On the basis of the analysis mentioned above, we have gone for the S-site substitution of NM@MoS2.
3.2. Adsorption performance analysis
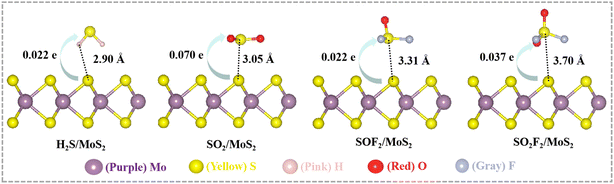 | ||
| Fig. 2 Optimized structure of adsorbed gases on pristine MoS2. Green directional lines show the number of transferred charges, and dashed lines represent the adsorption distance. | ||
| Adsorbed gases | Ead (eV) | d (Å) | Q (e) |
|---|---|---|---|
| H2S | −0.21 | 2.90 | 0.022 |
| SO2 | −0.33 | 3.05 | 0.070 |
| SOF2 | −0.29 | 3.31 | 0.022 |
| SO2F2 | −0.33 | 3.70 | 0.037 |
Since adsorption configurations vary with surface structure, “d” must be explicitly defined for each system. In the case of SO2F2 bonding to the sulfur site on the pristine MoS2 surface via its sulfur atom, and the corresponding d is defined as the nearest interatomic distance from the SO2F2–S bond to the surface S atom. In NM@MoS2 system, SO2F2 couples to the NM site via oxygen atom. Here d is defined as the shortest distance from SO2F2–O bond to the NM atom. H2S adsorbs onto the surface chalcogen site via the hydrogen atom, with d being defined as the distance from H2S–H to the corresponding chalcogen atom. SO2 and SOF2 both adsorb onto the surface sulfur site or dopant site via the sulfur atom, with d defined as the shortest distance from adsorbate–S to the sulfur or NM atom.
Based on Fig. 2 and Table 1, the adsorption efficiency of pristine MoS2 towards the four gases is comparatively low (Ead ≈ −0.30 eV), with the corresponding d-values range from 2.9 to 3.7 Å, suggesting that the adsorption process is of a physisorptive in nature. In this scenario, charges consistently transfer from the MoS2 substrate to the gas molecules, with corresponding Q values of merely 0.022, 0.070, 0.022, and 0.037 e. van der Waals forces are weak intermolecular interactions originating from transient charge fluctuations, with strengths significantly lower than those of covalent or ionic bonds.66 Following adsorption onto the pristine MoS2 surface, the d values of the four gases are significantly greater than the sum of the S–S covalent radii. Consequently, no chemical bonds are formed or broken. The interaction between these four gas molecules and the pristine MoS2 surface is primarily dominated by van der Waals forces. Given the relatively low Ead, greater adsorption distances, and smaller quantities of charge transfer, it can be inferred that pristine MoS2 exhibits limited adsorption capability toward H2S, SO2, SOF2, and SO2F2.
| Substrates | Ead (eV) | |||
|---|---|---|---|---|
| H2S | SO2 | SOF2 | SO2F2 | |
| O@MoS2 | −0.32 | −0.37 | −0.33 | −0.31 |
| Se@MoS2 | −0.15 | −0.26 | −0.23 | −0.26 |
| Te@MoS2 | −0.14 | −0.20 | −0.19 | −0.24 |
| Substrates | Ead (eV) | |||
|---|---|---|---|---|
| H2S | SO2 | SOF2 | SO2F2 | |
| B@MoS2 | −1.66 | −1.22 | −0.98 | −0.49 |
| C@MoS2 | −1.62 | −1.15 | −1.00 | −0.44 |
| N@MoS2 | −0.46 | −0.68 | −0.51 | −0.39 |
| P@MoS2 | −0.55 | −0.75 | −0.44 | −0.39 |
| Si@MoS2 | −1.67 | −0.93 | −0.64 | −0.74 |
This study categorises dopant atoms as either chalcogens (O, Se and Te) or non-chalcogens (B, C, N, P and Si). Non-chalcogen doping significantly enhances the gas adsorption capacity, as evidenced by the Ead values of the four gases adsorbed on the NM@MoS2 substrate. As shown in Table 2, the Ead values of O@MoS2, Se@MoS2, and Te@MoS2 exhibit range from −0.14 to −0.37 eV, indicating a lack of significant adsorption enhancement compared to pristine MoS2 (−0.21 to −0.33 eV).
As shown in Table 3, the Ead values of B@MoS2, C@MoS2, P@MoS2, N@MoS2 and Si@MoS2 range from −0.39 to −1.67 eV, indicating enhanced adsorption. This adsorption enhancement is reflected by the significant reduction in the intermolecular distance between the substrate and the molecules. It can be concluded that a shorter adsorption distance generally implies a closer interaction between the gas molecule and the NM@MoS2 substrate. The relatively short distance of adsorption implies a more intimate contact, aligns with its higher adsorption energy. In contrast, the longer distance of adsorption may be related to its relatively weaker interaction with NM@MoS2 substrate. In terms of the transferred charge (Q), the amount of charge transfer reflects the degree of electron interaction during adsorption. The adsorption process shows a significant charge transfer, indicating a strong electron – donating or – accepting behaviour in the adsorption process, which associated with its relatively high adsorption energy as well.
We systematically compared the calculated adsorption energies with those of previously reported doping strategies (see Table S1), which further confirms the significant application potential of single-layer NM@MoS2 (where NM = B, C, N, P or Si) in gas sensing. The study also reveals that MoS2 substituted with transition metal atoms has a significantly higher adsorption capacity for SF6 decomposition components.3,40,67,68 However, the excessively strong adsorption strength makes the adsorption sites difficult to regenerate, which severely hinders sensor recovery. In contrast, H2S, SO2, SOF2, and SO2F2 exhibit insufficient adsorption stability on the PtSe2 surface and readily desorbing at room temperature due to thermal agitation.69 Compared to these materials, SF6 decomposition gases show moderately reduced adsorption strength on the NM@MoS2 monolayer, thereby avoiding the regeneration hindrance caused by excessively strong chemisorption.15,16,70–72
In summary, the adsorption of SF6 decomposition gases onto eight NM@MoS2 substrates can be categorized into two group: substitution systems involving chalcogen elements (O, Se, Te) retain the original surface characteristics of MoS2 and have no significant impact on the adsorption behavior of SF6 decomposition products. In contrast, substitution systems involving non-chalcogen elements (B, C, N, P and Si) exhibit distinct adsorption properties, indicating that surface modification significantly alters gas adsorption behaviour.
3.3. Recovery time
A high-performance gas sensor demands strong sensitivity toward the sensing gas, as well as a moderate recovery (desorption) time after adsorption. As previously confirmed, B@MoS2, C@MoS2, N@MoS2, P@MoS2 and Si@MoS2 substrates exhibit excellent adsorption efficiency for H2S, SO2, SOF2 and SO2F2 molecules. This section examines the scientific feasibility of using these materials in practice by exploring the specifics of recovery time during the desorption process. Recovery time is can be calculated using eqn (3):4
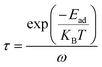 | (3) |
A moderate recovery period is essential for gas-sensing materials and is correlated with adsorption strength.4,6 Stronger adsorption of gases enhances the influence of individual molecule–substrate interactions, leading to a prompt and clear response. However, excessively strong adsorption can also slow down the recovery process. Therefore, the optimal adsorption strength must strike a balance between recovery behaviour and response capability. Intrinsic MoS2 exhibits weak adsorption for SF6 decomposition gas, resulting in an extremely short recovery time and limited response capability. However, doping with non-halogen elements (B, C, N, P and Si) enhances adsorption strength to a moderate level, thereby optimizing the recovery time. Recovery times of H2S, SO2, SOF2 and SO2F2 gases at 298 K, 398 K and 498 K were summarized in Table 4.
| System | Gas | 298 K | 398 K | 498 K |
|---|---|---|---|---|
| B@MoS2 | H2S | 1.27 × 1016 | 1.11 × 109 | 6.85 × 104 |
| SO2 | 4.04× 108 | 2.68 × 103 | 2.14 | |
| SOF2 | 3.11 × 104 | 2.23 | 7.40 × 10−3 | |
| SO2F2 | 1.97 × 10−4 | 1.62 × 10−6 | 9.18 × 10−8 | |
| C@MoS2 | H2S | 2.99 × 1015 | 3.73 × 108 | 2.79 × 104 |
| SO2 | 3.56 × 107 | 4.35 × 102 | 5.0 × 10−1 | |
| SOF2 | 9.45 × 104 | 5.13 | 1.44 × 10−2 | |
| SO2F2 | 3.38 × 10−5 | 4.33 × 10−7 | 3.20 × 10−8 | |
| N@MoS2 | H2S | 6.61 × 10−5 | 7.17 × 10−7 | 4.78 × 10−8 |
| SO2 | 3.28 × 10−1 | 4.18 × 10−4 | 7.78 × 10−6 | |
| SOF2 | 5.50 × 10−4 | 3.50 × 10−6 | 1.70 × 10−7 | |
| SO2F2 | 4.22 × 10−6 | 9.13 × 10−8 | 9.21 × 10−9 | |
| P@MoS2 | H2S | 1.73 × 10−3 | 8.27 × 10−6 | 3.38 × 10−7 |
| SO2 | 3.39 | 3.41 × 10−3 | 4.15 × 10−5 | |
| SOF2 | 2.67 × 10−5 | 3.64 × 10−7 | 2.78 × 10−8 | |
| SO2F2 | 1.03 × 10−6 | 3.19 × 10−8 | 3.79 × 10−9 | |
| Si@MoS2 | H2S | 1.73 × 1016 | 1.39 × 109 | 4.51 × 104 |
| SO2 | 5.62 × 103 | 5.92 × 10−1 | 2.55 × 10−3 | |
| SOF2 | 6.11 × 10−2 | 1.19 × 10−4 | 2.84 × 10−6 | |
| SO2F2 | 2.82 | 2.10 × 10−3 | 2.81 × 10−5 |
As shown in Table 4, a rise in temperature leads to an enhancement in the intensity of molecular Brownian motion, thereby shortening the recovery time. Therefore, controlling temperature is an effective way to adjust the system's recovery time. However, the B@MoS2 and C@MoS2 substrates have prolonged recovery times because they have high adsorption energies for H2S, SO2 and SOF2 molecules. Meanwhile, the Si@MoS2 substrate exhibits a prolonged recovery time for H2S and SO2 molecules at room temperature. Even at an elevated temperature of 498 K, the recovery times for the H2S/B@MoS2, H2S/C@MoS2 and H2S/Si@MoS2 systems are still extremely long at 6.85 × 104 s, 2.79 × 104 s and 4.51 × 104 s, respectively. Therefore, for systems with high adsorption energies, integrating with heating or UV irradiation is essential for accelerating the gas desorption process and improving the recycling of the B@MoS2, C@MoS2 and Si@MoS2 substrates. Nevertheless, it is feasible to reduce the recovery time for the SOF2/B@MoS2, SO2/C@MoS2, SOF2/C@MoS2 and SO2/C@MoS2 adsorption systems by increasing the temperature. For example, at 398 K, the desorption times for the SOF2/B@MoS2 and SOF2/C@MoS2 systems are reduced to 2.23 and 5.13 seconds, respectively. Notably, the recovery times for the P@MoS2 and Si@MoS2 substrates are relatively short: at 298 K, the recovery times are 3.39 and 2.82 s for SO2 and SO2F2 gases, respectively. This suggests that both the P@MoS2 and Si@MoS2 substrates can rapidly desorb SO2 and SO2F2 at ambient temperature, indicating excellent recyclability.
3.4. Response capability
The work function is defined as the minimum energy required to move an electron from the surface of a material into a vacuum.76 As a key parameter characterizing the surface electronic structure of sensing materials, its variation can quantitatively reflect the degree of charge transfer between gas molecules and the material surface, thereby providing an important indication of the potential response capability of the sensing material. Thus,we systematically calculated the work functions (Φ) and the percentage change in work function (ΔΦ%) of four gases before and after adsorption on pristine MoS2 and NM@MoS2 substrates. Φ and ΔΦ% can be calculated using eqn (4) and (5):| Φ = VVacuum − VFermi | (4) |
| ΔΦ% = (|ΦUnadsorbed − Φadsorbed|/ΦUnadsorbed) × 100% | (5) |
 | ||
| Fig. 6 Work function of four gases before and after adsorption on MoS2, O@MoS2, Se@MoS2, Te@MoS2, B@MoS2, C@MoS2, N@MoS2, P@MoS2, and Si@MoS2 substrates, respectively. | ||
 | ||
| Fig. 7 The percentage change in work function of four gases after adsorption on MoS2, O@MoS2, Se@MoS2, Te@MoS2, B@MoS2, C@MoS2, N@MoS2, P@MoS2, and Si@MoS2 substrates, respectively. | ||
Although the work function of the material increases slightly with the substitution of B, C, N, P and Si atoms, it remains lower than that of h-BN (5.986 eV) and Ti3C2O2 MXene (5.97 eV).77,78 It is noteworthy that when the four gases adsorb onto the pristine MoS2 surface, ΔΦ% ranges from 0.17% to 0.64%, indicating that monolayer MoS2 exhibits low response to these gases. Similar observations were made in the case of the SO2F2-chalcogen substituted systems, where ΔΦ% ranged from 0.03% to 0.29%. The response of the other three adsorbed gases in the chalcogen substitution system increased slightly, with ΔΦ% ranging from 1.16% to 4.53%. In non-chalcogen-substituted systems, particularly in the cases of Si@MoS2 and B@MoS2, the ΔΦ% range of was found to be 4.74% to 10.85% and 2.91% to 5.71%, respectively. This demonstrates that the substrate exhibits a high response to all four of these gases. While the C@MoS2, N@MoS2 and P@MoS2 substrates cannot maintain high response for all four gases simultaneously, they do significantly enhance the response of one or tow of these four gases. If it were possible, combining N@MoS2 and C@MoS2 materials achieves concurrent high-response of all four gases.
In summary, the theoretical analysis of work function variation and gas–solid adsorption interactions suggests that B@MoS2 and Si@MoS2 substrates both have high potential for responding to SF6 decomposition gases. Therefore, they can be considered promising candidate sensing materials for all four target gases. Furthermore, combining N@MoS2 and C@MoS2 substrates enhances the theoretical response potential towards the four gases even further, suggesting a feasible strategy for optimising the sensing performance of MoS2-based materials.
However, the boundaries between theoretical predictions and practical sensing performance must be clarified, as must the limitations of the current computational model. This is necessary in order to contextualize the significance of these results. Although work function modulation indicates the potential sensing response, it differs from actual sensitivity, which depends on various factors, such as surface coverage, doping concentrations and humidity. The current model makes idealized assumptions (uniform doping, single-gas saturated adsorption and ignoring humidity), which deviate from reality. Future experimental work in this field should therefore consider controllable synthesis, testing in humid conditions, and optimizing the model with realistic parameters.
4. Conclusion
In the present study, DFT calculations were utilized to systematically explore the adsorption characteristics of NM@MoS2 in relation to four SF6 decomposition gases (H2S, SO2, SOF2, SO2F2). The aim was to enhance the gas-detection efficacy of MoS2. The key findings are summarized below:(1) According to the formation energy calculations for NM@MoS2, the results suggest that substitution at the S site is more feasible than substitution at the Mo site, as the former exhibits a lower (more negative) formation energy.
(2) Pristine MoS2 shows weak physisorption for the target gases, with low adsorption energies (−0.21 to −0.33 eV), large adsorption distances (2.90 to 3.70 Å) and minimal charge transfer, which limits its sensing efficiency.
(3) The substitution systems involving chalcogen elements (O, Se and Te) retain the original surface characteristics of MoS2 and have no significant impact on the adsorption behavior of SF6 decomposition products. In contrast, substitution systems involving non-chalcogen elements (B, C, N, P and Si) exhibit distinct adsorption properties, indicating that surface modification significantly alters gas adsorption behaviour.
(4) Recovery time analysis indicates that temperature control can optimize reusability. In particular, P@MoS2 and Si@MoS2 exhibit moderate recovery times for SO2 and SO2F2 at ambient temperature, demonstrating their potential for repeated use in gas sensing.
(5) Work functional analysis confirms that NM atomic substitution significantly enhances the response capability of the intrinsic MoS2 surface's response to SF6 decomposition gas. The B@MoS2 and Si@MoS2 surfaces demonstrate a potential gas-sensing response to these gases.
Overall, non-metal doping is a viable strategy for enhancing the gas-sensing response of MoS2 towards SF6 decomposition products. This study provides valuable theoretical insights for designing high-sensitivity, reusable gas sensors for monitoring the safety of GIS operation.
Author contributions
Mamutjan Tursun: conceptualization, data curation, writing – original draft, Funding acquisition; Yifan Liu: data curation, formal analysis; Abulimiti Yumaier: data curation, formal analysis.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and as its supplementary information (SI). Supplementary information: structure of NM atoms replacing Mo sites in monolayer MoS2; formation energies of NM atoms substitution at Mo sites in monolayer MoS2; comparison of adsorption energies for SF6 decomposition gases; references. See DOI: https://doi.org/10.1039/d5ra08522e.Acknowledgements
We thank the financial support from the “Tianchi Talented young Doctors Program of Xinjiang Uygur Autonomous Region”, “Fundamental Research Grants for Universities in the Autonomous Region (Grant No. XJEDU2024P114)”, “Tianshan Innovation Team Plan of Xinjiang Uygur Autonomous Region (2023D14002)”, and the “Research Initiation Fund for High-level Talents at Kashi University (Grant No. GCC2023ZK-008)”.References
- A. Dong and M. Liu, A DFT study on the adsorption properties of Ti3C2O2 MXene towards SF6 decomposition gases, Surf. Sci., 2023, 734, 122317 CrossRef CAS.
- E. Mohammadi, Z. K. Horastani and A. K. Horestani, DFT Study of SO2F2 and SOF2 Adsorption on (6,0) AlN Nanotube: Adsorbent and Gas Detector Toward SF6 Decomposition Products, IEEE Sens. J., 2025, 25, 14646–14657 Search PubMed.
- H. Liu, F. Wang, K. Hu, T. Li, Y. Yan and J. Li, The Adsorption and Sensing Performances of Ir-modified MoS2 Monolayer toward SF6 Decomposition Products: A DFT Study, Nanomaterials, 2021, 11, 100 CrossRef CAS.
- W. Zhou, Z. Li, L. Li, W. Zeng and Q. Zhou, Adsorption and detection of SF6 decomposed toxic gases (H2S, SO2, SOF2, SO2F2) on transition metal (Fe, Ru, Os) modified WTe2 monolayer: A DFT investigation, J. Environ. Chem. Eng., 2025, 13, 115545 CrossRef CAS.
- Z. Shi, Y. Zhang, W. Zeng and Q. Zhou, A DFT study on adsorption of SF6 decomposition gases (H2S, SO2, SO2F2 and SOF2) on Sc-MoTe2 monolayer, Sens. Actuators. A Phys., 2023, 360, 114548 Search PubMed.
- S.-Y. Xia, L.-Q. Tao, T. Jiang, H. Sun and J. Li, Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: A DFT study, Appl. Surf. Sci., 2021, 536, 147965 CrossRef CAS.
- M. Wang, J. Cao, P. Jia, Y. Zhang, J. Liu, M. Xu and D. Chen, Research on high-performance materials for adsorption and monitoring of SF6 and its decomposed gases: First principle DFT calculations, Mater. Chem. Phys., 2025, 335, 130533 CrossRef CAS.
- Y. Yang, L. Huang, W. Zeng and Q. Zhou, Metal clusters (Pt3 and Pd3) modified InSe monolayer: An adsorbent and gas sensor for SF6 decomposition gases (SO2, H2S, SOF2, SO2F2) based on density functional theory, J. Environ. Chem. Eng., 2025, 13, 117095 CrossRef CAS.
- A. K. Pandey and A. K. Mishra, Angle-dependent growth of 2D MoS2 monolayer, bilayer by chemical vapor deposition method, Phys. Scr., 2025, 100, 075938 CrossRef CAS.
- P. Prajapat, A. A. Chaudhary, A. Yadav, V. Kandwal, P. Vashishtha, M. A. M. Ali, S. Walia and G. Gupta, Enhancement in hazardous gas detection capabilities of MoS2 monolayer-based devices through defect engineering and photonic activation, Sci. Rep., 2025, 15, 39174 CrossRef CAS PubMed.
- J. He, T. Yao, Y. Xiong and F. Xie, High gas-sensing performance of SF6 decomposition gases on PdSe2/MoS2 heterojunction: A DFT study, Comput. Theor. Chem., 2025, 1251, 115330 CrossRef CAS.
- C. Xue, L. Lin, K. Xie, Z. Zhang and P. Wang, Adsorption of toxic gases by Janus MoSeTe monolayers doped with transition metals and surface defects: A first-principles study, Colloids Surf. A, 2024, 694, 134131 CrossRef CAS.
- G. Sanyal, A. Vaidyanathan, C. S. Rout and B. Chakraborty, Recent developments in two-dimensional layered tungsten dichalcogenides based materials for gas sensing applications, Mater. Today Commun., 2021, 28, 102717 CrossRef CAS.
- W. Zheng, X. Liu, J. Xie, G. Lu and J. Zhang, Emerging van der Waals junctions based on TMDs materials for advanced gas sensors, Coord. Chem. Rev., 2021, 447, 214151 CrossRef CAS.
- F. Li, H. Wu and H. Cui, Favorable adsorption and sensing properties of the HfS2 monolayer upon H2S and SOF2 gases by Pt-doping: A first-principles study, Comput. Theor. Chem., 2025, 1244, 115031 Search PubMed.
- S. Jiang, F. Li and H. Cui, First-Principles Insight Into Adsorption Characteristics of Ir-Embedded HfS2 Monolayers for Gas-Sensing of H2S, SOF2, and SO2F2, ChemistrySelect, 2025, 10, e01393 CrossRef CAS.
- L. Wang, D. Xu, L. Jiang, J. Gao, Z. Tang, Y. Xu, X. Chen and H. Zhang, Transition Metal Dichalcogenides for Sensing and Oncotherapy: Status, Challenges, and Perspective, Adv. Funct. Mater., 2021, 31, 2004408 Search PubMed.
- S. A. Kadam, Advancements in monolayer TMD-based gas sensors: Synthesis, mechanisms, electronic structure engineering, and flexible wearable sensors for real-world applications and future prospects, Chem. Eng. J., 2025, 517, 164223 Search PubMed.
- F. Jiang, W.-S. Zhao and J. Zhang, Mini-review: Recent progress in the development of MoSe2 based chemical sensors and biosensors, Microelectron. Eng., 2020, 225, 111279 Search PubMed.
- R. K. Mishra, H. J. Choi, J. W. Ryu, G. J. Choi, V. Kumar, P. Kumar, J. Singh, S. Kumar and J. S. Gwag, Recent progress in gas sensing based on 2D SnS2 and its heterostructure platforms: A review, Sens. Actuators. A Phys., 2024, 365, 114860 CrossRef CAS.
- X. Wang and J. Wang, Effects of Pt and Au adsorption on the gas sensing performance of SnS2 monolayers: A DFT study, Mater. Sci. Semicond. Process., 2021, 121, 105416 CrossRef CAS.
- S. Kumar, A. Mirzaei, A. Kumar, M. Hoon Lee, Z. Ghahremani, T.-U. Kim, J.-Y. Kim, M. Kwoka, M. Kumar, S. Sub Kim and H. Woo Kim, Nanoparticles anchored strategy to develop 2D MoS2 and MoSe2 based room temperature chemiresistive gas sensors, Coord. Chem. Rev., 2024, 503, 215657 CrossRef CAS.
- I. Shahbaz, M. Tahir, L. Li and Y. Song, Advancements in 2D transition metal dichalcogenides (TMDs) inks for printed optoelectronics: A comprehensive review, Mater. Today, 2024, 77, 142–184 CrossRef CAS.
- S. Susarla, A. Kutana, J. A. Hachtel, V. Kochat, A. Apte, R. Vajtai, J. C. Idrobo, B. I. Yakobson, C. S. Tiwary and P. M. Ajayan, 2D Materials: Quaternary 2D Transition Metal Dichalcogenides (TMDs) with Tunable Bandgap, Adv. Mater., 2017, 29, 1702457 Search PubMed.
- H. Schmidt, F. Giustiniano and G. Eda, Electronic transport properties of transition metal dichalcogenide field-effect devices: surface and interface effects, Chem. Soc. Rev., 2015, 44, 7715–7736 Search PubMed.
- M. Schleicher and M. Fyta, Lateral MoS2 Heterostructure for Sensing Small Gas Molecules, ACS Appl. Electron. Mater., 2020, 2, 74–83 CrossRef CAS.
- R. Du and W. Wu, Adsorption of gas molecule on Rh, Ru doped monolayer MoS2 for gas sensing applications: A DFT study, Chem. Phys. Lett., 2022, 789, 139300 CrossRef.
- D. J. Late, Y.-K. Huang, B. Liu, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, Sensing Behavior of Atomically Thin-Layered MoS2 Transistors, ACS Nano, 2013, 7, 4879–4891 CrossRef CAS PubMed.
- T. Pham, G. Li, E. Bekyarova, M. E. Itkis and A. Mulchandani, MoS2-Based Optoelectronic Gas Sensor with Sub-parts-per-billion Limit of NO2 Gas Detection, ACS Nano, 2019, 13, 3196–3205 CrossRef CAS PubMed.
- J. Cha, K.-A. Min, D. Sung and S. Hong, Ab initio study of adsorption behaviors of molecular adsorbates on the surface and at the edge of MoS2, Curr. Appl. Phys., 2018, 18, 1013–1019 CrossRef.
- J. Zhu, H. Zhang, Y. Tong, L. Zhao, Y. Zhang, Y. Qiu and X. Lin, First-principles investigations of metal (V, Nb, Ta)-doped monolayer MoS2: Structural stability, electronic properties and adsorption of gas molecules, Appl. Surf. Sci., 2017, 419, 522–530 Search PubMed.
- M. Yin, K. Wang, C. Gao, R. Yang, Y. Huang and L. Yu, Synthesis and insights into the gas sensing mechanisms of N-doped MoS2 hierarchical structures with superior gas sensing properties at room temperature, Mater. Res. Bull., 2024, 179, 112943 CrossRef CAS.
- R. Zhang, D. Fu, J. Ni, C. Sun and S. Song, Adsorption for SO2 gas molecules on B, N, P and Al doped MoS2: The DFT study, Chem. Phys. Lett., 2019, 715, 273–277 Search PubMed.
- N. N. Viet, L. V. Thong, T. K. Dang, P. H. Phuoc, N. H. Chien, C. M. Hung, N. D. Hoa, N. Van Duy, N. Van Toan, N. T. Son and N. Van Hieu, MoS2 nanosheets-decorated SnO2 nanofibers for enhanced SO2 gas sensing performance and classification of CO, NH3 and H2 gases, Anal. Chim. Acta, 2021, 1167, 338576 CrossRef CAS PubMed.
- W. Guo, K. Chen, S. Wang, H. Zhang and D. Wu, Dual functionalized flower-like MoS2 nanospheres with Pd and g-C3N4 for triethylamine gas sensing performance, Sens. Actuators. B Chem., 2025, 433, 137490 CrossRef CAS.
- Y. Gui, J. Shi, P. Yang, T. Li, C. Tang and L. Xu, Platinum modified MoS2 monolayer for adsorption and gas sensing of SF6 decomposition products: a DFT study, High Voltage, 2020, 5, 454–462 CrossRef.
- Y. Fan, J. Zhang, Y. Qiu, J. Zhu, Y. Zhang and G. Hu, A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption, Comput. Mater. Sci., 2017, 138, 255–266 CrossRef CAS.
- H. Luo, Y. Cao, J. Zhou, J. Feng, J. Cao and H. Guo, Adsorption of NO2, NH3 on monolayer MoS2 doped with Al, Si, and P: A first-principles study, Chem. Phys. Lett., 2016, 643, 27–33 Search PubMed.
- A. V. Agrawal, N. Kumar and M. Kumar, Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide, Nanomicro. Lett., 2021, 13, 38 Search PubMed.
- Z. Cui, X. Zhang, Y. Li, D. Chen, Y. Li and H. Xiao, Theoretical study of SF6 decomposition on the MoS2 monolayer doped with Ag, Ni, Au, Pt: a first-principles study, Adsorption, 2019, 25, 225–233 CrossRef CAS.
- B. Li, Q. Zhou, R. Peng, Y. Liao and W. Zeng, Adsorption of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2) on Sc-doped MoS2 surface: A DFT study, Appl. Surf. Sci., 2021, 549, 149271 Search PubMed.
- M. J. Szary, MoS2 doping for enhanced H2S detection, Appl. Surf. Sci., 2021, 547, 149026 CrossRef CAS.
- E. Piosik and M. J. Szary, Development of MoS2 doping strategy for enhanced SO2 detection at room temperature, Appl. Surf. Sci., 2023, 638, 158013 CrossRef CAS.
- D. Ma, Q. Wang, T. Li, C. He, B. Ma, Y. Tang, Z. Lu and Z. Yang, Repairing sulfur vacancies in the MoS2 monolayer by using CO, NO and NO2 molecules, J. Mater. Chem. C Mater., 2016, 4, 7093–7101 Search PubMed.
- H. Huang, X. Feng, C. Du and W. Song, High-quality phosphorus-doped MoS2 ultrathin nanosheets with amenable ORR catalytic activity, Chem. Commun., 2015, 51, 7903–7906 Search PubMed.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS.
- H. Huang, X. Feng, C. Du, S. Wu and W. Song, Incorporated oxygen in MoS2 ultrathin nanosheets for efficient ORR catalysis, J. Mater. Chem. A Mater., 2015, 3, 16050–16056 Search PubMed.
- M. Zhu, Y. Zhang, S. Xu, X. Yan, Y. Song, M. Wang, Y. Dong and J. Zhang, Enhanced lithium-sulfur battery eilectrochemistry via Se-doped MoS2/rGO ultrathin sheets as sulfur hosts, Appl. Surf. Sci., 2025, 682, 161718 CrossRef CAS.
- Y. Luo, K. Chen, P. Shen, X. Li, X. Li, Y. Li and K. Chu, B-doped MoS2 for nitrate electroreduction to ammonia, J. Colloid Interface Sci., 2023, 629, 950–957 CrossRef CAS.
- X. Chen, S. Lu, Y. Wei, M. Sun, X. Wang, M. Ma and J. Tian, Basal Plane-Activated Boron-Doped MoS2 Nanosheets for Efficient Electrochemical Ammonia Synthesis, ChemSusChem, 2023, 16, e202202265 CrossRef CAS.
- S. Chen, D. Fang, Z. Zhou, Z. Zhao, Y. Yang, Z. Dai and J. Shi, B-doped MoS2/MoO2 heterostructure catalyst for the electrocatalytic reduction of N2 to NH3, Catal. Lett., 2024, 154, 4055–4064 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- L. Goerigk, A Comprehensive Overview of the DFT-D3 London-Dispersion Correction, in: Non-Covalent Interactions in Quantum Chemistry and Physics, Elsevier, 2017, pp. 195–219 Search PubMed.
- T. Lu and F. Chen, Revealing the nature of intermolecular interaction and configurational preference of the nonpolar molecular dimers (H2)2, (N2)2, and (H2)(N2), J. Mol. Model., 2013, 19, 5387–5395 CrossRef CAS PubMed.
- J. B. A. Davis, F. Baletto and R. L. Johnston, The Effect of Dispersion Correction on the Adsorption of CO on Metallic Nanoparticles, J. Phys. Chem. A, 2015, 119, 9703–9709 CrossRef CAS.
- P. P. Mkhonto, X. Zhang, L. Lu, W. Xiong, Y. Zhu, L. Han and P. E. Ngoepe, Design, synthesis and investigating the interaction of novel s-triazine collector with pyrite surface: A DFT-D3+U and experimental studies, Surf. Interfaces, 2023, 38, 102820 CrossRef CAS.
- K. Boezar, A. Reisi-Vanani and M. Dehkhodaei, Modification of graphenylene nanostructure with transition metals (Fe, Sc and Ti) to promote hydrogen storage ability: A DFT-D3 study, Int. J. Hydrogen Energy, 2021, 46, 38370–38380 CrossRef CAS.
- D. J. Chadi and M. L. Cohen, Special Points in the Brillouin Zone, Phys. Rev. B: Condens. Matter Mater. Phys., 1973, 8, 5747–5753 CrossRef.
- Y. Fu, X. Feng, M.-F. Yan, K. Wang and S. Wang, First principle study on electronic structure and optical phonon properties of 2H-MoS2, Phys. B Condens. Matter, 2013, 426, 103–107 CrossRef CAS.
- Y. Zhao, Y. Chen, P. Ou and J. Song, Basal Plane Activation via Grain Boundaries in Monolayer MoS2 for Carbon Dioxide Reduction, ACS Catal., 2023, 13, 12941–12951 CrossRef CAS.
- W. Xiao, P. Liu, J. Zhang, W. Song, Y. P. Feng, D. Gao and J. Ding, Dual-Functional N Dopants in Edges and Basal Plane of MoS2 Nanosheets Toward Efficient and Durable Hydrogen Evolution, Adv. Energy Mater., 2017, 7 DOI:10.1002/aenm.201602086.
- P. Tao, J. He, T. Shen, Y. Hao, J. Yan, Z. Huang, X. Xu, M. Li and Y. Chen, Nitrogen-Doped MoS2 Foam for Fast Sodium Ion Storage, Adv. Mater. Interfaces, 2019, 6, 1900460 CrossRef.
- D. Yuan, C. Guo, Y. Ning, X. Fu, X. Li, X. Xu, C. Wang, Y. Kou and J. Cui, N-Doped Modified MoS2 for Piezoelectric–Photocatalytic Removal of Tetracycline: Simultaneous Improvement of Photocatalytic and Piezoelectric Properties, Water, 2025, 17, 1296 Search PubMed.
- L. Song, M. Song, Z. Lu, G. Yu, Z. Liang, W. Hou, Q. Liao and Y. Song, Recent Advances of Preparation and Application of Two-Dimension van der Waals Heterostructure, Coatings, 2022, 12, 1152 CrossRef CAS.
- Y. Gui, J. Chen, W. Wang, Y. Zhu, C. Tang and L. Xu, Adsorption mechanism of hydrogen sulfide and sulfur dioxide on Au–MoS2 monolayer, Superlattices Microstruct., 2019, 135, 106280 Search PubMed.
- H. Wei, Y. Gui, J. Kang, W. Wang and C. Tang, A DFT Study on the Adsorption of H2S and SO2 on Ni Doped MoS2 Monolayer, Nanomaterials, 2018, 8, 646 CrossRef PubMed.
- D. Chen, X. Zhang, J. Tang, Z. Cui, H. Cui and S. Pi, Theoretical Study of Monolayer PtSe2 as Outstanding Gas Sensor to Detect SF6 Decompositions, IEEE Electr. Device L., 2018, 39, 1405–1408 CAS.
- T.-Y. Sang, T. Li, Y. Yang, Y. Song, H. Tian, R. Song, C. Wang, X. Hu, Z. Yang, Y. Lu and W. Chen, Pd, Rh-decorated Se-vacancy MoSe2 monolayer: A promising candidate for sensing and detecting SO2F2, SOF2, H2S and SO2, Surf. Interfaces, 2022, 33, 102269 CrossRef CAS.
- X. Zhou, J. Bai, H. Cui, T. Tian, Y. Luo and L. Tian, Outstanding sensing property of Cu-substituted MoTe2 monolayer upon SF6 decomposed species from first-principles calculations, Comput. Theor. Chem., 2023, 1228, 114273 Search PubMed.
- Z. Xu, H. Cui and G. Zhang, Pd-Decorated WTe2 Monolayer as a Favorable Sensing Material toward SF6 Decomposed Species: A DFT Study, ACS Omega, 2023, 8, 4244–4250 CrossRef CAS PubMed.
- Z. Wang, M. Wang and X. Hu, Adsorption and sensing performances of greenhouse gases (CO2, CH4, N2O, and SF6) on pristine and Pd-doped GeSe monolayer: A DFT study, Sens. Actuators. A Phys., 2024, 370, 115222 CrossRef CAS.
- Z. Wang, T. Xia and X. Hu, Metal Oxide (Ag2O, ZnO)-Doped MoSe2 Monolayer as a Highly Sensitive Gas Sensor for Greenhouse Gases (CO2, CH4, N2O, SF6) Detection, ACS Appl. Nano Mater., 2024, 7, 20994–21004 CrossRef CAS.
- M. Mohammadi and E. Pakizeh, SiB Monolayers-Based Gas Sensor: Work Function and Conductometric Type Gas Sensors, Adv. Theory. Simul., 2025, 8, 2401127 Search PubMed.
- P. Hurdax, M. Hollerer, C. S. Kern, P. Puschnig, M. Sterrer and M. G. Ramsey, Integer Charge Transfer Model–PTCDA on MgO(001)/Ag(001) Probing the Transition from Single to Double Integer Charge Transfer, J. Phys. Chem. C, 2025, 129, 1553–1561 CrossRef CAS.
- B. Yu, H. Ren and X. Piao, Towards Adsorptive Enrichment of Flavonoids from Honey Using h-BN Monolayer, ChemPhysChem, 2022, 23, e202100828 CrossRef CAS.
- R. Li, W. Sun, C. Zhan, P. R. C. Kent and D. Jiang, Interfacial and electronic properties of heterostructures of MXene and graphene, Phys. Rev. B: Condens. Matter Mater. Phys., 2019, 99, 085429 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |



