Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Class-II-aldolase-mimicking polyfunctional Lewis acid/azolium–aryloxide catalysts in direct enantioselective nitro-aldol additions
Khushbu Jangid†
a,
Lisa A. Schmoltzi† b,
Alexander Allgaierc,
Radhika Katariac,
Daniel M. Wannera,
Justin Herrmanna,
Janis Gschwinda,
Silvio Münchc,
Joris van Slageren
b,
Alexander Allgaierc,
Radhika Katariac,
Daniel M. Wannera,
Justin Herrmanna,
Janis Gschwinda,
Silvio Münchc,
Joris van Slageren c,
Johannes Kästner
c,
Johannes Kästner b and
René Peters
b and
René Peters *a
*a
aUniversität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany. E-mail: rene.peters@oc.uni-stuttgart.de
bUniversität Stuttgart, Institut für Theoretische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
cUniversität Stuttgart, Institut für Physikalische Chemie, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
First published on 23rd December 2025
Abstract
The catalytic asymmetric nitroaldol reaction is a powerful tool for accessing chiral 1,2-difunctionalized motifs, and numerous catalytic systems have been reported. Despite considerable progress in achieving high levels of stereocontrol, key challenges persist. In particular, optimal selectivity often requires cryogenic conditions, resulting in prolonged reaction times and limited practicality. In this article, a novel concept for asymmetric nitroaldol additions is introduced through a polyfunctional catalyst. This system integrates a Lewis acid, Co(II), with an azolium–aryloxide betaine, and exhibits mechanistic features reminiscent of class-II aldolases. The proposed mode of action is supported by comprehensive DFT calculations, microkinetic simulations, and detailed spectroscopic analyses. By the unique synergistic interplay of the Lewis acidic metal center, the aryloxide as Brønsted base and the corresponding aromatic alcohol serving as both hydrogen bond donor and Brønsted acid, high stereoselectivity was accomplished even at slightly elevated temperature for MeNO2. Like in class-II-aldolases, the aldehyde is activated by H-bonding to the aromatic alcohol and not by the Lewis acid. The latter serves to stabilize and direct the nitronate. The computational studies further demonstrate that the catalyst‘s key functional groups precisely orchestrate all accompanying transformations. As a result of the mild reaction conditions, not necessitating the use of an external base, the method also proved to be applicable to readily enolizable aliphatic aldehydes, such as phenylacetaldehyde.
Introduction
The catalytic nitroaldol- or Henry-reaction is a well-established way for forming C,C bonds between nitroalkanes and aldehydes or ketones.1 The resulting β-nitroalcohols are versatile intermediates that can undergo a wide range of synthetically valuable transformations. These include for instance dehydrations to form conjugated nitroalkenes,2 reductions to β-amino alcohols,3 denitrations,3 oxidation reactions to α-nitroketones4 and Nef reactions to give α-hydroxy carbonyl derivatives.5Highly enantioenriched β-nitroalcohols have been widely employed in the synthesis of key intermediates for accessing biologically active compounds, including natural products, insecticides, fungicides, and antibiotics.6–15 Moreover, highly enantioenriched β-amino alcohols, resulting from reduction of the nitro group, are found in numerous active pharmaceutical ingredients,16–30 underscoring the importance of the nitroaldol reaction as a valuable source of chiral building blocks.3
Since the first reports of an asymmetric version of this reaction using chiral lanthanum and heterobimetallic lanthanum/lithium complexes,31–35 the development of efficient catalytic systems and the synthetic applications of nitroaldol products have attracted substantial attention.8,36 Different synthetic challenges were addressed through the design of various catalyst types. In particular, chiral Lewis acid catalysts based on copper,37–58 zinc,59–64 chromium,65–68cobalt69–74 and rare-earth metal centers75–79 making use of ligands such as amino acids,80 amino alcohols,81–88 oxazolines,89–95 diamines,96,97 Schiff bases98,99and salen ligands60,100–103 were reported. Additionally, a range of chiral organocatalyst types such as guanidines, thioureas, cinchona alkaloid derivatives and quaternary ammonium salts have proven effective for enantioselective methods.36,104,105
The first reported cobalt-catalyzed asymmetric nitroaldol reactions were developed by Yamada et al. using salen complexes in combination with an external base.73 Following this, several other research groups reported highly enantioselective cobalt–salen catalysts.67,70–72
These methods typically required extended reaction times and cryogenic temperatures for satisfactory yields and high enantioselectivity. Although other cobalt-based catalysts, such as BOX69and amino acid complexes,74 have been reported, their catalytic performance has generally been inferior.
Here, we report a different concept making use of a new polyfunctional Co(II)-/1,2,3-triazolium–aryloxide catalyst system combining Lewis acid, Brønsted base and hydrogen-bonding activation. Very detailed DFT studies show that the Co(II) center does not activate the aldehyde, but stabilizes the nitronate, which is formed by deprotonation of the nitroalkane by the catalyst's Co-naphthoxide moiety. The thus formed naphthol OH group then activates the aldehyde by hydrogen bonding106 in close proximity to the Co-nitronate, resulting in a quasi intramolecular C,C-bond forming transition state. The naphthol finally delivers the proton to the alcoholate intermediate formed during the 1,2-addition step (Scheme 1, bottom).
The polyfunctional catalyst thus promotes each individual step of this reaction, avoiding the need for an external base. The mode of action is highly reminiscent of class-II aldolases, which operate in an analogous way: the combination of a Lewis acid (including Co(II))107 and a Brønsted base (typically glutamate) enable the formation of a metal-stabilized enolate. Meanwhile a tyrosine's aromatic alcohol function is typically responsible for simultaneous activation of the aldehyde substrate by hydrogen bonding and protonation of the alcoholate generated during the C,C-bond forming step (Scheme 1, bottom).107 Using a class-II-aldolase-mimicking approach, the title reaction proceeds efficiently without the need for low temperatures using nitromethane.
Results and discussion
Development of the method
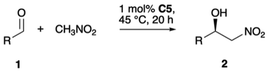
The investigation began with our previously reported Cu(II)/imidazolium–naphthoxide catalyst C1 (Table 1),108–112 choosing the 1,2-addition of nitromethane to 2-naphthylcarbaldehyde (1a) as model reaction. With 5 mol% C1 in THF at 25 °C for 20 h, nearly racemic product 2a was formed in small quantities (9%, entry 1). Changing to the corresponding Co(II) catalyst C2,113 the product yield was significantly increased, while enantioselectivity was still poor (entry 2). Our previously reported Cu(II) and Ni(II)/1,2,3-triazolium–naphthoxide catalysts C3 (entry 3)114 and C4113 (entry 4) performed on a similar level. The related new Co(II) catalyst C5 also featuring a 1,2,3-triazolium unit allowed for improved productivity (60% yield, entry 5), while its diastereomer C6, differing in the configuration of the iminosulfonamide ligand motif, provided slightly inferior results (entry 6). Note that all yields reported in Table 1 are identical to the observed conversions, and no side products were thus detected.| # | C | X (mol%) | Solvent (c/mol L−1) | T/°C | Yielda/% | eeb/% |
|---|---|---|---|---|---|---|
| a Determined by 1H-NMR using mesitylene as internal standard.b Determined by HPLC.c Reaction time is 5 h. | ||||||
| 1 | C1 | 5 | THF (0.2) | 25 | 09 | 11 |
| 2 | C2 | 5 | THF (0.2) | 25 | 41 | 19 |
| 3 | C3 | 5 | THF (0.2) | 25 | 15 | 19 |
| 4 | C4 | 5 | THF (0.2) | 25 | 22 | 14 |
| 5 | C5 | 5 | THF (0.2) | 25 | 60 | 20 |
| 6 | C6 | 5 | THF (0.2) | 25 | 49 | 15 |
| 7 | C5 | 5 | THF (1.0) | 25 | 80 | 91 |
| 8 | C5 | 5 | Toluene (1.0) | 25 | 66 | 60 |
| 9 | C5 | 5 | MeCN (1.0) | 25 | 43 | 69 |
| 10 | C5 | 5 | DCM (1.0) | 25 | 25 | 55 |
| 11 | C5 | 5 | CH3NO2 (1.0) | 25 | 86 | 93 |
| 12 | C5 | 5 | CH3NO2 (1.0) | 45 | 80 | 97 |
| 13c | C5 | 1 | CH3NO2 (1.0) | 45 | 74 | 96 |
| 14c | C6 | 1 | CH3NO2 (1.0) | 45 | 62 | 69 |
C5 was then used for further optimization. It was found that enantioselectivity is strongly increased with increased aldehyde concentration as the ee value increased to 91% using a 1M THF solution. Under otherwise identical conditions, in solvents such as toluene, acetonitrile and dichloromethane the reaction efficiency was considerably lower (entries 8–10). Improvements in terms of yield and enantioselectivity were possible using neat nitromethane, still 1M in aldehyde (entry 11). Surprisingly, the enantioselectivity further increased to 97% ee at 45 °C (entry 12). Lowering the catalyst loading from to 5 to 1 mol% and the reaction time from 20 to 5 h only slightly affected productivity and enantioselectivity (entry 13).
Diastereomeric catalyst C6 was also studied under these optimized conditions (Table 1, entry 14), but was again found to be inferior (ee = 69%, yield = 62%). This mismatch situation also indicates that the axial chirality element is the most important control factor for the stereochemical outcome, as the major enantiomer's absolute configuration stays the same.
Scope
With the optimized conditions, the applicability to other substrates was probed (Table 2). It was found that there is no obvious trend regarding the electronic and steric properties of the substituents on aromatic aldehydes. Independently of ortho-, meta- and para-substitution, high enantioselectivity was attained (entries 4–13). Similarly, σ-donors, π-donors and σ-acceptors were well tolerated. A limitation was found for a π-acceptor substituent reported in entry 14. In this case the yield was high, but the ee value was only 70%. Next to extended π-systems within the aldehyde (entries 1 and 2), also electron rich 2- and 3-furyl moieties were well accommodated (entries 15 & 16).| # | R | 1/2 | Yielda/% | eeb/% |
|---|---|---|---|---|
| a Yield of isolated product.b Determined by HPLC. | ||||
| 1 | 2-Naphthyl | a | 74 | 96 |
| 2 | 1-Naphthyl | b | 71 | 89 |
| 3 | Ph | c | 78 | 86 |
| 4 | 2-Me-C6H4 | d | 84 | 92 |
| 5 | 3-Me-C6H4 | e | 91 | 94 |
| 6 | 4-Me-C6H4 | f | 51 | 90 |
| 7 | 2-MeO-C6H4 | g | 92 | 94 |
| 8 | 3-MeO-C6H4 | h | 80 | 90 |
| 9 | 4-MeO-C6H4 | i | 64 | 94 |
| 10 | 4-F-C6H4 | j | 71 | 87 |
| 11 | 4-Cl-C6H4 | k | 69 | 80 |
| 12 | 4-Br-C6H4 | l | 87 | 89 |
| 13 | 4-I-C6H4 | m | 91 | 95 |
| 14 | 4-NC-C6H4 | n | 94 | 70 |
| 15 | 2-Furyl | o | 79 | 90 |
| 16 | 3-Furyl | p | 83 | 93 |
| 17 | tBu | q | 72 | 81 |
| 18 | nPr | r | 87 | 83 |
| 19 | PhCH2 | s | 93 | 94 |
Of particular note is the applicability to aliphatic sterically hindered aldehydes like pivalic aldehyde (entry 17)51,115 but also to enolizable, small linear aldehydes such as butanal (entry 18),31–33,35,51,115 which are to prone to side reactions like aldol condensations. Even more challenging is phenylacetaldehyde 1s (entry 19),84 due to the double activated α-position. Under the mild conditions provided by the Co(II)-triazolium/naphthoxide catalyst C5, it also underwent clean nitroaldol addition without observable side reactions. This underscores the functional group compatibility of the catalyst system.
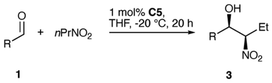
In addition, we studied the use of 1-nitropropane in order to generate two stereocenters (Table 3). Useful to high yields of isolated 3 were attained using 1 mol% of C5 at −20 °C for 20 h, with moderate preference for the syn-diastereomer (69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31–81
31–81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) which was formed with good to high enantioselectivity (ee 80–94%).116,117
19) which was formed with good to high enantioselectivity (ee 80–94%).116,117
Mechanistic studies
| # | CC | External base | Conversiona/% | Yielda/% | eeb/% |
|---|---|---|---|---|---|
| a Determined by 1H-NMR using mesitylene as internal standard.b Determined by HPLC. | |||||
| 1 | CC1 | — | <2 | <2 | — |
| 2 | CC1 | Cs2CO3 | 90 | 90 | rac |
| 3 | CC2 | Cs2CO3 | 71 | 71 | rac |
| 4 | CC7 | — | 96 | 82 | rac |
| 5 | CC2 + CC7 | — | 92 | 92 | rac |
| 6 | CC3 | — | 70 | 67 | 21 |
| 7 | CC4 | Cs2CO3 | 80 | 80 | rac |
| 8 | CC5 | — | 83 | 83 | −7 |
| 9 | CC6 | — | 32 | 32 | 19 |
In CC1, the naphthoxide base is missing and formally replaced by a methyl ether unit (entry 1). In that case, no product was found. By addition of catalytic amounts of Cs2CO3 as external base, activity was regained, but racemic product was formed. Notably, when only Cs2CO3 was used without any catalyst, considerable background reactivity was found, leading to 33% conversion under the same conditions. Similarly, catalyst CC2 lacking an azolium side arm,118–120 gave racemic product in the presence of the external base. The organocatalytic betaine control system CC7 also formed racemic product in good yield, however in contrast to all other cases shown here, there was a significant difference between conversion and yield. Combining CC2 and CC7 to a binary catalyst system resulted in improved yield and a clean transformation, but also here a racemate was obtained.
Like our standard catalyst C5, complex CC3 is a polyfunctional Lewis acid/betaine catalyst. In that case the axially chiral binaphthyl unit is missing, causing a strong loss of enantioselectivity. CC4 contains a binaphthyl unit, but the azolium is formally replaced by an ether bridge. For activity, the base was necessary again and the product was racemic.
CC5 and CC6 are both betaine catalysts featuring triazolium moieties and axial chirality in the aryloxide part, in which the conformational flexibility is increased compared to C5 by methylene and methylene ether linkers between the azolium and the binaphthyl fragments. In addition, in CC5, the triazolium is connected by a C,C-bond to the phenoxyimine ligand. In both case enantioselectivity was found to be low.
The results of the control experiments are suggesting that a well-balanced spatial fit between all the key functional groups of the catalyst, i.e., chiral Lewis acid sphere, azolium and aryloxide and the functional units of the activated substrates is required for high enantioselectivity.
| r = kobs [C5]0.90 [1a]1.08 [MeNO2]0.85 | (1) |
The results thus show a first-order dependence on the catalyst concentration, suggesting that a single catalyst molecule is likely involved in the turnover-determining step(s). A non-linear effect (NLE)124,125study (see SI, chapter 3.3) revealed a linear relationship between the enantiomeric excess (ee) of catalyst C5 and that of the product 2a, supporting that higher-order catalyst aggregates are not involved.
The reaction rate also showed a first-order dependence on the aldehyde (1a) substrate and a nearly first-order dependence on nitromethane (NM) (reaction order = 0.85). This rate law is in good agreement with the energy profile of the calculated mechanism (see below).
The properties of paramagnetic catalyst C5 and its precatalyst (featuring the neutral naphthol unit) were investigated using magnetometry and spectroscopy in both the solid state and in solution, to gain insights into their electronic structure and derive implications regarding the catalytic mechanism.
SQUID (superconducting quantum interference device) magnetometry measurements were conducted on powder samples of C5 and its precatalyst C5*HPF6. The room temperature χmT value for the precatalyst C5*HPF6 was 2.3 cm3 mol−1 K (Fig. S15), which agrees well with the value expected for a spin S = 3/2 and giso = 2.2. Below 70 K, the χmT value decreases and reaches 1.5 cm3 mol−1 K at the lowest measurement temperature. This decrease is attributed to sizable zero-field splitting. The temperature dependence of the χmT value for C5 is qualitatively very similar, but the absolute values are reproducibly lower by 30% (Fig. S16). To assess if this discrepancy is due to lower g-values, and to further characterize the compounds, X-band electron paramagnetic resonance (EPR) spectra were recorded on powder samples of the precatalyst C5*HPF6 (Fig. S17) and C5 (Fig. S18) at 4 K. The spectra are qualitatively very similar and consistent with excitations within the lowest Kramers doublet, where the combined action of g-value anisotropy and zero-field splitting leads to a rhombic shape. The combined fit of EPR and SQUID data gave the spin Hamiltonian parameters reported in Table 5. The g-tensor values for C5 and its precatalyst are very similar and the discrepancy between the χmT values of C5 and its precatalyst can therefore not be due to differences in g-values. Therefore, we attribute the difference to the presence of a small amount of a diamagnetic cobalt(III) species formed in the activation process.
| Spin Hamiltonian parameters | C5*HPF6 | C5 |
|---|---|---|
| g1 | 2.295(2) | 2.54(3) |
| g2 | 2.228(3) | 2.22(5) |
| g3 | 2.098(3) | 2.21(2) |
| D (cm−1) | 28.0(5) | 28.2(6) |
| E/D | 0.250(8) | 0.258(9) |
UV-Vis measurements on C5 (2 mM) were carried out to investigate the dependence of the ligand field splitting of the Co(II) center on solvents and substrates. The room temperature UV/Vis spectrum of C5 in non-coordinating DCM displays two overlapping low-energy bands centered at around at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 and 18
740 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, respectively, that are assigned to dd-transitions (Fig. S11). The spectrum in pure nitromethane displays slightly lower intensities of the above bands, with slightly changed intensity ratio in favour of the high-energy component. Interestingly, the spectrum in pure THF displays an additional band at the lower energy of 14
000 cm−1, respectively, that are assigned to dd-transitions (Fig. S11). The spectrum in pure nitromethane displays slightly lower intensities of the above bands, with slightly changed intensity ratio in favour of the high-energy component. Interestingly, the spectrum in pure THF displays an additional band at the lower energy of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 188 cm−1. Addition of the aldehyde 1a to the last solution results in insignificant changes, demonstrating that 1a coordinates less strongly than THF (and the other ligands). However, addition of 1000 eq. of nitromethane to a THF solution causes the disappearance of the lowest-energy absorption band, indicating that nitromethane displaces THF in this case. Coordination of nitromethane to metal centers is known and the ligand field strength is similar to that of water.126,127
188 cm−1. Addition of the aldehyde 1a to the last solution results in insignificant changes, demonstrating that 1a coordinates less strongly than THF (and the other ligands). However, addition of 1000 eq. of nitromethane to a THF solution causes the disappearance of the lowest-energy absorption band, indicating that nitromethane displaces THF in this case. Coordination of nitromethane to metal centers is known and the ligand field strength is similar to that of water.126,127
By means of circular dichroism CD spectroscopy, it is possible to probe the influence of chirality on electronic transitions that involve the metal center. CD-spectra (Fig. S12–S14) recorded in the area of 380 nm (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) at room temperature reveal negligible intensities for the precatalyst and for the free binaphthol ligand. In contrast, clear CD intensity was observed for C5 (0.2 mM in DCM), indicating that the relevant electronic transition must involve both the metal and the (chiral) ligand. Therefore, the absorption band is assigned to a charge transfer transition. Addition of nitromethane leads to a small, but significant change in intensity, which could point toward partial dissociation of the naphthoxide moiety from the metal center. Addition of aldehyde does not lead to a measurable change in intensity of the relevant band, but the situation is complicated by strong absorption of the substrate at slightly shorter wavelengths.
000 cm−1) at room temperature reveal negligible intensities for the precatalyst and for the free binaphthol ligand. In contrast, clear CD intensity was observed for C5 (0.2 mM in DCM), indicating that the relevant electronic transition must involve both the metal and the (chiral) ligand. Therefore, the absorption band is assigned to a charge transfer transition. Addition of nitromethane leads to a small, but significant change in intensity, which could point toward partial dissociation of the naphthoxide moiety from the metal center. Addition of aldehyde does not lead to a measurable change in intensity of the relevant band, but the situation is complicated by strong absorption of the substrate at slightly shorter wavelengths.
As no crystal structure of C5 is available, a model was constructed manually based on calculated geometries of a similar catalytic system.108,109,113,114 That structure was optimized and served as starting point for all subsequent calculations. In accordance with magnetic measurements identifying the active species as a high-spin Co(II) complex, the reaction pathway was computed with a spin multiplicity of 4.
To investigate the following proposed reaction mechanisms, we computed all relevant stationary points along the reaction pathway, including minima and transition state structures. Building on previous investigations, we propose two possible catalytic cycles, illustrated in Schemes 2 and 3.
 | ||
| Scheme 2 Proposed catalytic cycle for the standard mechanism. Like in Scheme 1, red circles indicate a Lewis acid, green circles a Brønsted base, and blue circles a H bond donor participating in the individual steps. | ||
 | ||
| Scheme 3 Proposed catalytic cycle of the inverse mechanism. Like in Scheme 1, red circles indicate a Lewis acid, green circles a Brønsted base, and blue circles a H bond donor participating in the individual steps. | ||
The first mechanism, here referred to as the standard mechanism, follows the common pathway for polyfunctional Lewis acid/azolium–aryloxide betaine catalysts.108,113,114 Here, the pronucleophile is deprotonated by the naphthoxide base and the resulting nucleophile stabilized by a metal center, in our case cobalt(II). In this mechanism, the electrophile is activated by the resulting azolium naphthol moiety via H-bonding. Based on the pKa values of nitromethane (pKa = 10.2 at 25 °C)139 and 2-naphthol (pKa = 9.5 at 25 °C)139,140 in water, the deprotonation step is expected to be slightly endergonic, a finding that is also consistent with the DFT calculations presented in Fig. 1.
 | ||
| Fig. 1 Free energy profile of the standard mechanism. The catalyst C5/nitromethane adduct I was chosen as reference state. | ||
In the proposed inverse mechanism, the naphthoxide base also deprotonates the nitromethane leading to a nitronate which is further stabilized by coordination to the metal center and by hydrogen bonding to the naphthol OH generated during the proton transfer. In that case, the aldehyde also binds to the metal center, which distinguishes the inverse from the standard pathway.
In neither mechanism does the catalyst exist in its bare activated form, but it is generally interacting with a nitromethane molecule as depicted in I in Scheme 2.
The interaction is further facilitated by the use of nitromethane as solvent, but the association is also energetically favourable, as the binding of nitromethane to the catalyst is exothermic. It involves a H-bond between the azolium and the nitro group, which further acidifies the nitromethane, which, at the same time, is exposing its methyl unit to the basic naphtholate unit. In the energetically lowest starting structure I visualized in Fig. 2, the cobalt center adopts a highly distorted tetrahedral coordination geometry, as indicated by the O–Co–N angle of 124° between the binaphthyl-O, the Co(II) and the adjacent N atom.
The catalytic cycle of the standard mechanism is depicted in Scheme 2 and the Gibbs energy along the cycle as obtained by DFT is shown in Fig. 1. The cycle begins with the deprotonation of nitromethane by the binaphtholate. The resulting nitronate anion is stabilized by a H-bond to the binaphthyl-OH, which was generated during the proton transfer, yielding intermediate IIs. During the deprotonation, the bond between the Co center and the naphthoxide-O is broken, which is supported by UV/VIS spectroscopy. After adding nitromethane to the catalyst, the charge-transfer band associated with the Co–O bonding diminishes by 20%, indicating a cleavage of this bond. The barrier for the deprotonation from I to IIs was found to be 92.0 kJ mol−1 with respect to I, see Fig. 1. The coordination geometry of Co remains largely unchanged except for replacing the naphthoxide-O by a nitronate-O atom.
In the subsequent step of the catalytic cycle, from IIs to IIIs, the aldehyde undergoes a H-bond formation with the naphthol moiety, thus cleaving the H-bond between naphthol and nitronate. Instead, both oxygen atoms of the nitronate now function as a bidentate ligand to the Co center, forming intermediate IIIs. At the same time, the coordination environment of Co shifts from a distorted tetrahedral geometry to a very distorted square pyramidal arrangement.
The following C–C bond formation step from IIIs to IVs is stereo-determining as the corresponding transition state has the highest energy in the catalytic cycle, 102.8 kJ mol−1 (see Fig. 1 and Fig. 3, top). The transition state forming the unfavored (S)-enantiomer is visualized in Fig. 3 (bottom) and found at a significantly higher energy of 115.2 kJ mol−1, resulting in a computed ee of 98%, which is in close agreement with the experimental value of 97%. The higher energy of the (S)-transition state may be attributed to a weaker H-bond between the aldehyde substrate and the naphthol, with a distance of 1.55 Å compared to 1.46 Å in the transition state leading to the favored (R)-enantiomer.
In addition, the hydrogen bonding atoms in the (S)-transition state deviate more from a linear geometry, showing an angle of 169.2° versus 173.5° for the (R)-transition state (see Fig. 3). Simultaneously, upon the C–C bond formation, the resulting alkoxide is protonated intramolecularly by the naphthol moiety. In intermediate IVs, the cobalt coordination sphere returns to a distorted tetrahedral geometry, similar to I. In the second to last step, i.e., from IVs to V, the bond between Co and the naphtholate-O is formed again to yield intermediate V. Finally, the neutral product is released from the complex and a new nitromethane molecule binds to the catalyst, formally completing the catalytic cycle. Although no transition state for the last step was identified, relaxed surface scans suggest a low barrier, consistent with the predictions from microkinetic modelling which will be further explained below.
In the inverse mechanism, the first intermediate (I) is identical to that of the standard mechanism (Scheme 3). The initial step is the coordination of the aldehyde to the Co center to form intermediate IIi, which is in contrast to the standard pathway where it binds to the binaphthyl-OH moiety. In IIi, an octahedral coordination is found, as the nitroolefin also coordinates to the metal center. The following step is the deprotonation of the coordinating nitromethane, yielding intermediate IIIai via a transition state with an energy of 110.5 kJ mol−1 (Fig. 4). This is followed by the formation of a H-bond between the nitronate and the naphthol unit in IIIbi with a relative energy of 102.2 kJ mol−1. In structures IIIai and IIIbi, the cobalt has a square pyramidal coordination environment, as the bond between the binaphthyl-O and Co becomes elongated, while the bond to the nitronate is shortened. In IIIbi and the subsequent intermediate IVi, the key difference between the two mechanisms becomes evident: in this alternative option, the aldehyde coordinates to the Co center rather than forming a H-bond to the naphthol's OH that is observed in the standard mechanism. Furthermore, the nitronate is simultaneously binding to both the Co center and the binaphthyl-OH in the inverse mechanism.
The C–C bond formation from IIIbi to IVi proceeds through the turnover determining transition state (TDTS, Fig. 5) with the highest energy of the catalytic cycle (117.0 kJ mol−1, see Fig. 4). Since the turnover determining intermediate is I with a relative energy of 0 kJ mol−1, the energetic span is also 117.0 kJ mol−1.
 | ||
| Fig. 5 Transition state of the C–C bond formation from IIIbi to IVi for the inverse mechanism. Most H atoms are omitted for clarity. | ||
In addition to the C–C bond formation, the shift of a H-bond from stabilizing the nitronate to stabilizing the newly formed alkoxide occurs in the same step, thereby changing the coordination sphere of Co to a trigonal bipyramidal geometry.
Subsequently, the alkoxide is protonated by the naphthol while the resulting naphthoxide binds to Co again and the product's OH is released from the metal center to give intermediate V. Product release and association of nitromethane to the catalyst closes the catalytic cycle.
To conclude, the rate-limiting barrier of the standard mechanism is calculated to be 14.2 kJ mol−1 lower than that of the inverse mechanism. Thus, a preference for the standard pathway is likely and in good agreement with the microkinetic modelling (see below).
| Elementary reaction | ΔG‡ forward (kJ mol−1) | ΔG‡ backward (kJ mol−1) |
|---|---|---|
| I ↔ IIs | 82.0 | 61.5 |
| IIs + RCHO ↔ IIIs | 40.8 | 13.8 |
| IIIs ↔ V | 42.2 | 70.0 |
| V + H3CNO2 ↔ I + product | 14.8 | 33.8 |
| IIs → side product | 85.2 | — |
The concentration profiles of the aldehyde and product were measured experimentally under four different conditions. The initial concentrations (Table 1, entry 13) of the reaction were varied by increasing either the aldehyde or the catalyst concentrations. In an additional experiment, the catalyst was pre-stirred in nitromethane for 5 h hours before the increased amount of aldehyde was added. The pre-stirring experiment was designed to investigate the possibility of catalyst decay. Comparing the concentration profiles with and without pre-stirring shows that pre-stirring significantly slows down the reaction, which suggests that the catalyst decays over time. Consequently, catalyst decay was included as an elementary step in the reaction network (Table 6). Simulations indicate that 41% of the catalyst decays after 5 h of pre-stirring. The decay product has not yet been identified.
However, it should be noted that the model is not unique, and that alternative parameters and reaction networks can also fit the experimental data equally well for the standard mechanism (Fig. 6, gray). Only the sensitive barriers (I to IIs and IIIs to V) can be precisely determined, while others vary widely without affecting the fit quality. Nevertheless, the model is in close agreement with the experimental and DFT data, suggesting that the reaction network is plausible.
Conclusions
The development of polyfunctional catalysts opens new opportunities for mimicking the efficiency of enzymes in asymmetric transformations. In this context, we presented a novel approach to the nitroaldol reaction that relies on a catalyst system combining cobalt(II) as a Lewis acid with an azolium–aryloxide betaine. This catalyst displays mechanistic features reminiscent of class-II aldolases, where several functional elements cooperate to achieve high stereocontrol.DFT calculations, microkinetic simulations, and spectroscopic studies provided deep insight into how the catalyst most likely operates. The cobalt(II) center contributes Lewis acidity (like in class-II-aldolases Zn(II), Co(II) etc.), the aryloxide moiety functions as a Brønsted base (like in class-II-aldolases usually glutamate), while the corresponding aromatic alcohol derived from it plays a dual role: as a hydrogen-bond donor and as a Brønsted acid (like in class-II-aldolases usually tyrosine). Together, these functions create a chiral highly orchestrated reaction environment, in which bond activation, stabilization of intermediates and transition states, and stereochemical control are precisely balanced. Remarkably, for nitromethane this cooperative interplay allows the reaction to proceed with excellent stereoselectivity even at slightly elevated temperatures.
Beyond its mechanistic mode of action, the practical advantages of this system are noteworthy. Because no external base is required and the participating functional groups of the catalyst are only weakly acidic and basic, the conditions are very mild minimizing competing side reactions.144–146 As a result, the scope of the reaction extends to readily enolizable aldehydes, including substrates such as phenylacetaldehyde, which are problematic in classical nitroaldol protocols.
Thus, this polyfunctional catalyst design not only provides a new route to stereoselective nitroaldol reactions, but also demonstrates carefully integrated catalytic elements matching the multifaceted strategies of enzymatic systems.
Author contributions
K. J. planned and conducted the catalytic and kinetic experiments and carried out the catalyst syntheses. L. A. S. performed all DFT calculations and the microkinetic modeling. A. A. and R. K. planned the spectroscopic studies. A. A., R. K. and S. M. performed the spectroscopic investigations. J. v. S. directed the spectroscopic studies. J. K. directed the DFT calculations and the microkinetic modelling. R. P. conceptualized and directed the project. R. P., K. J., L. A. S., A. A. and R. K. wrote the manuscript. All authors contributed to the discussion.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the supplementary information (SI) of this article.Supplementary information is available. See DOI: https://doi.org/10.1039/d5qo01558h.
Acknowledgements
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG, project ID 310990893 – PE 818/7-2; project ID 358283783 – CRC 1333/2, project ID 516238647 – CRC 1667/1 - ATLAS). The authors acknowledge support by the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster). K. J. acknowledges financial support in the form of a PhD BMBF scholarship by the Friedrich-Naumann-Foundation (FNF). L. A. S. acknowledges financial support in the form of a Ph.D. scholarship from the Studienstiftung des Deutschen Volkes (German National Academic Foundation).References
- L. Henry, Formation synthétique d'alcools nitrés, Bull. Soc. Chim. Fr., 1895, 13, 999–1002 Search PubMed.
- R. S. Varma, R. Dahiya and S. Kumar, Microwave-assisted Henry reaction: solventless synthesis of conjugated nitroalkenes, Tetrahedron Lett., 1997, 38, 5131–5134 Search PubMed.
- N. Ono, The Nitro Group in Organic Synthesis, John Wiley & Sons, 2003 Search PubMed.
- R. Ballini, New and convenient synthesis of (Z)-heneicos-6-en-11-one, the douglas fir tussock moth (Orgyia pseudotsugata) sex pheromone, and (Z)-non-6-en-2-one, the immediate precursor for the synthesis of brevicomin, the sex attractant of the western pine beetle Dentroctonus brevicomis, J. Chem. Soc., 1991, 1419–1421 Search PubMed.
- W. E. Noland, The NEF reaction, Chem. Rev., 1955, 55, 137–155 Search PubMed.
- P. B. Kisanga and J. G. Verkade, P(RNCH2CH2)3N: An Efficient promoter for the nitroaldol (Henry) reaction, J. Org. Chem., 1999, 64, 4298–4303 Search PubMed.
- R. J. Heffner, J. Jiang and M. M. Joullie, Total synthesis of (-)-nummularine F, J. Am. Chem. Soc., 1992, 114, 10181–10189 Search PubMed.
- C. Palomo, M. Oiarbide and A. Laso, Eur. J. Org. Chem., 2007, 2561–2574 Search PubMed.
- O. Sakanaka, T. Ohmori, S. Kozaki and T. Suami, Recent advances in the catalytic asymmetric nitroaldol (Henry) reaction, Bull. Chem. Soc. Jpn., 1986, 59, 3523–3528 Search PubMed.
- T. Suami, H. Sasai and K. Matsuno, Synthesis of methyl hexaacetyl-tunicaminyl uracil, Chem. Lett., 1983, 12, 819–822 Search PubMed.
- G. Mikite, E. Jakucs, A. Kis-Tamás, F. Darvas and A. Lopata, Synthesis and antifungal activity of new nitro–alcohol derivatives, Pestic. Sci., 1982, 13, 557–562 Search PubMed.
- T. Yasuhara, N. Zaima, S. Hashimoto, M. Yamazaki and O. Muraoka, First total synthesis of crispin B by nitro Aldol and the Bischler-Napieralski reaction, Heterocycles, 2009, 77, 1397 Search PubMed.
- K. Sato, S. Akai, H. Shoji, N. Sugita, S. Yoshida, Y. Nagai, K. Suzuki, Y. Nakamura, Y. Kajihara and M. Funabashi, Stereoselective and efficient total synthesis of optically active Tetrodotoxin from d-glucose, J. Org. Chem., 2008, 73, 1234–1242 Search PubMed.
- Y. Li, J. P. Feng, W. H. Wang, J. Chen and X. P. Cao, Total synthesis and correct absolute configuration of malyngamide U, J. Org. Chem., 2007, 72, 2344–2350 Search PubMed.
- J. Boruwa and N. C. Barua, Stereoselective total synthesis of (+)-boronolide, Tetrahedron, 2006, 62, 1193–1198 Search PubMed.
- M. E. Bunnage, S. G. Davies, C. J. Goodwin and O. Ichihara, An expeditious asymmetric synthesis of allophenylnorstatine, Tetrahedron, 1994, 50, 3975–3986 Search PubMed.
- U. Veith, O. Schwardt and V. Jäger, Concise synthesis of deacetylanisomycin and 2-substituted analogues from N, 2-O-Dibenzyl-L-threose imine1, Synlett, 1996, 1181–1183 Search PubMed.
- P. M. Koskinen and A. M. P. Koskinen, Sphingosine, an enigmatic lipid: a review of recent literature syntheses, Synthesis, 1998, 1075–1091 Search PubMed.
- D. Lednicer and L. A. Mitscher, The Organic Chemistry of Drug Synthesis, John Wiley & Sons, 1980, vol. 2 Search PubMed.
- N. Shibata, T. Katoh and S. Terashima, An expeditious synthesis of (2R, 3S)-3-tert-butoxycarbonylamino-1-isobutylamino-4-phenyl-2-butanol, a key building block of HIV protease inhibitors, Tetrahedron Lett., 1997, 38, 619–620 Search PubMed.
- T. Kumamoto, N. Aoyama, S. Nakano, T. Ishikawa and S. Narimatsu, Synthesis of enantiomeric 4-hydroxypropranolols from 1, 4-dihydroxynaphthalene, Tetrahedron: Asymmetry, 2001, 12, 791–795 Search PubMed.
- J. B. Hickie, Alprenolol (“APTIN”) in Angina pectoris a double-bund multicentre trial, Med. J. Aust., 1970, 2, 268–272 Search PubMed.
- M. R. Candelore, L. Deng, L. Tota, X. M. Guan, A. Amend, Y. Liu, R. Newbold, M. A. Cascieri and A. E. Weber, Potent and selective human β3-adrenergic receptor antagonists, J. Pharmacol. Exp. Ther., 1999, 290, 649–655 Search PubMed.
- M. J. Kendall and V. A. John, Oxprenolol: Clinical pharmacology, pharmacokinetics, and pharmacodynamics, Am. J. Cardiol, 1983, 52, D27–D33 Search PubMed.
- H. M. Krumholz, β-blockers for mild to moderate heart failure, Lancet, 1999, 353, 2–3 Search PubMed.
- K. Tekes, P. Szegi, F. Hashemi, R. Laufer, H. Kalász, A. Siddiq and C. Ertsey, Medicinal chemistry of antimigraine drugs, Curr. Med. Chem., 2013, 20, 3300–3316 Search PubMed.
- V. A. Cullum, J. B. Farmer, D. Jack and G. P. Levy, Salbutamol: a new, selective β–adrenoceptive receptor stimulant, Br. J. Pharmacol., 1969, 35, 141–151 Search PubMed.
- K. K. Chen and C. F. Schmidt, The action of ephedrine, the active principle of the Chinese drug Ma Huang, J. Pharmacol. Exp. Ther., 1924, 24, 339–357 Search PubMed.
- A. J. Coleman, W. P. Leary and A. C. Asmal, The cardiovascular effects of etilefrine, Eur. J. Clin. Pharmacol., 1975, 8, 41–45 Search PubMed.
- D. C. Harrison, C. A. Chidsey and E. Braunwald, Studies on the mechanism of action of metaraminol (Aramine), Ann. Intern. Med., 1963, 59, 297–305 Search PubMed.
- M. Shibasaki and N. Yoshikawa, Lanthanide complexes in multifunctional asymmetric catalysis, Chem. Rev., 2002, 102, 2187–2210 Search PubMed.
- H. Sasai, T. Suzuki, S. Arai, T. Arai and M. Shibasaki, Basic character of rare earth metal alkoxides. Utilization in catalytic carbon-carbon bond-forming reactions and catalytic asymmetric nitroaldol reactions, J. Am. Chem. Soc., 1992, 114, 4418–4420 Search PubMed.
- M. Shibasaki, H. Sasai and T. Arai, Asymmetric catalysis with heterobimetallic compounds, Angew. Chem., Int. Ed. Engl., 1997, 36, 1236–1256 Search PubMed.
- T. Arai, Y. M. A. Yamada, N. Yamamoto, H. Sasai and M. Shibasaki, Self-Assembly of heterobimetallic complexes and reactive nucleophiles: a general strategy for the activation of asymmetric reactions promoted by heterobimetallic catalysts, Chem. – Eur. J., 1996, 2, 1368–1372 Search PubMed.
- H. Sasai, T. Tokunaga, S. Watanabe, T. Suzuki, N. Itoh and M. Shibasaki, Efficient diastereoselective and enantioselective nitroaldol reactions from prochiral starting materials: utilization of La-Li-6,6′-disubstituted BINOL complexes as asymmetric catalysts, J. Org. Chem., 1995, 60, 7388–7389 Search PubMed.
- J. Boruwa, N. Gogoi, P. P. Saikia and N. C. Barua, Catalytic asymmetric Henry reaction, Tetrahedron: Asymmetry, 2006, 17, 3315–3326 Search PubMed.
- C. Christensen, K. Juhl, R. G. Hazell and K. A. Jørgensen, Copper-catalyzed enantioselective Henry reactions of α-keto esters: An easy entry to optically active β-nitro-α-hydroxy esters and β-amino-α-hydroxy esters, J. Org. Chem., 2002, 67, 4875–4881 Search PubMed.
- K. Ma and J. You, Rational design of sterically and electronically easily tunable chiral bisimidazolines and their applications in dual Lewis acid/Brønsted base catalysis for highly enantioselective nitroaldol (Henry) reactions, Chem. – Eur. J., 2007, 13, 1863–1871 Search PubMed.
- B. Qin, X. Xiao, X. Liu, J. Huang, Y. Wen and X. Feng, Highly enantioselective Henry (nitroaldol) reaction of aldehydes and α-ketoesters catalyzed by N, N’-dioxide-copper(I) complexes, J. Org. Chem., 2007, 72, 9323–9328 Search PubMed.
- M. Bandini, M. Benaglia, R. Sinisi, S. Tommasi and A. Umani-Ronchi, Recoverable PEG-supported copper catalyst for highly stereocontrolled nitroaldol condensation, Org. Lett., 2007, 9, 2151–2153 Search PubMed.
- Y. Xiong, F. Wang, X. Huang, Y. Wen and X. Feng, A new copper(I)–tetrahydrosalen–catalyzed asymmetric Henry reaction and Its extension to the synthesis of (S)–norphenylephrine, Chem. – Eur. J., 2007, 13, 829–833 Search PubMed.
- T. Arai, M. Watanabe and A. Yanagisawa, Practical asymmetric Henry reaction catalyzed by a chiral diamine-Cu(OAc)2 complex, Org. Lett., 2007, 9, 3595–3597 Search PubMed.
- G. Blay, E. Climent, I. Fernández, V. Hernández-Olmos and J. R. Pedro, Enantioselective Henry reaction catalyzed with copper(II)–iminopyridine complexes, Tetrahedron: Asymmetry, 2007, 18, 1603–1612 Search PubMed.
- K. Y. Spangler and C. Wolf, Asymmetric copper(I)-catalyzed Henry reaction with an aminoindanol-derived bisoxazolidine ligand, Org. Lett., 2009, 11, 4724–4727 Search PubMed.
- G. Blay, L. R. Domingo, V. Hernández-Olmos and J. R. Pedro, New highly asymmetric Henry reaction catalyzed by Cu II and a C1-symmetric aminopyridine ligand, and Its application to the synthesis of miconazole, Chem. – Eur. J., 2008, 14, 4725–4730 Search PubMed.
- G. Zhang, E. Yashima and W. Woggon, Versatile supramolecular copper(II) complexes for Henry and Aza–Henry reactions, Adv. Synth. Catal., 2009, 351, 1255–1262 Search PubMed.
- M. Breuning, D. Hein, M. Steiner, V. H. Gessner and C. Strohmann, Chiral 2−endo–substituted 9−oxabispidines: novel ligands for enantioselective copper(II)–catalyzed Henry reactions, Chem. – Eur. J., 2009, 15, 12764–12769 Search PubMed.
- A. Noole, K. Lippur, A. Metsala, M. Lopp and T. Kanger, Enantioselective Henry reaction catalyzed by CuII salt and bipiperidine, J. Org. Chem., 2010, 75, 1313–1316 Search PubMed.
- M. Steurer and C. Bolm, Synthesis of amino-functionalized sulfonimidamides and their application in the enantioselective Henry reaction, J. Org. Chem., 2010, 75, 3301–3310 Search PubMed.
- L. Cheng, J. Dong, J. You, G. Gao and J. Lan, Copper(II)-catalyzed syn-selective Henry reaction, Chem. – Eur. J., 2010, 16, 6761–6765 Search PubMed.
- I. Panov, P. Drabina, Z. Padelkova, P. Šimůnek and M. Sedlak, Highly enantioselective nitroaldol reactions catalyzed by copper(II) complexes derived from substituted 2-(pyridin-2-yl) imidazolidin-4-one ligands, J. Org. Chem., 2011, 76, 4787–4793 Search PubMed.
- X. Wang, W. Zhao, G. Li, J. Wang, G. Liu, L. Liu, R. Zhao and M. Wang, Enantioselective copper(II)–catalyzed Henry reaction utilizing chiral aziridinyl alcohols, Appl. Organomet. Chem., 2014, 28, 892–899 Search PubMed.
- D. Didier, C. Magnier-Bouvier and E. Schulz, Charge–transfer interactions: an efficient tool for recycling bis (oxazoline)–copper complexes in asymmetric Henry reactions, Adv. Synth. Catal., 2011, 353, 1087–1095 Search PubMed.
- G. Lai, F. Guo, Y. Zheng, Y. Fang, H. Song, K. Xu, S. Wang, Z. Zha and Z. Wang, Highly enantioselective Henry reactions in water catalyzed by a copper tertiary amine complex and applied in the synthesis of (S)-N-trans-Feruloyl octopamine, Chem. – Eur. J., 2011, 17, 1114–1117 Search PubMed.
- Q. J. Yao, Q. Gao and M. A. J. Zaher, Efficient asymmetric copper(I)–catalyzed Henry reaction using chiral N–alkyl–C1−tetrahydro–1,1′–bisisoquinolines, Eur. J. Org. Chem., 2011, 4892–4898 Search PubMed.
- K. Xu, G. Lai, Z. Zha, S. Pan, H. Chen and Z. Wang, A Highly anti-selective asymmetric Henry reaction catalyzed by a chiral copper complex: applications to the syntheses of (+)-spisulosine and a pyrroloisoquinoline derivative, Chem. – Eur. J., 2012, 18, 12357–12362 Search PubMed.
- S. Hazra, A. Karmakar, C. Maria de Fátima, L. Dlháň, R. Boča and A. J. L. Pombeiro, Sulfonated Schiff base dinuclear and polymeric copper(II) complexes: crystal structures, magnetic properties and catalytic application in Henry reaction, New J. Chem., 2015, 39, 3424–3434 Search PubMed.
- D. A. Evans, D. Seidel, M. Rueping, H. W. Lam, J. T. Shaw and C. W. Downey, A new copper acetate-bis (oxazoline)-catalyzed, enantioselective Henry reaction, J. Am. Chem. Soc., 2003, 125, 12692–12693 Search PubMed.
- S. Saranya, N. A. Harry, S. M. Ujwaldev and G. Anilkumar, Recent advances and perspectives on the zinc–catalyzed nitroaldol (Henry) reaction, Asian J. Org. Chem., 2017, 6, 1349–1360 Search PubMed.
- B. Zheng, M. Wang, Z. Li, Q. Bian, J. Mao, S. Li, S. Liu, M. Wang, J. Zhong and H. Guo, Asymmetric Henry reaction catalyzed by a Zn–amino alcohol system, Tetrahedron: Asymmetry, 2011, 22, 1156–1160 Search PubMed.
- A. Bulut, A. Aslan and O. Dogan, Catalytic asymmetric nitroaldol (Henry) reaction with a zinc-Fam catalyst, J. Org. Chem., 2008, 73, 7373–7375 Search PubMed.
- S. Liu and C. Wolf, Asymmetric nitroaldol reaction catalyzed by a C2-symmetric bisoxazolidine ligand, Org. Lett., 2008, 10, 1831–1834 Search PubMed.
- C. Palomo, M. Oiarbide and A. Laso, Enantioselective Henry reactions under dual Lewis acid/amine catalysis using chiral amino alcohol ligands, Angew. Chem., Int. Ed., 2005, 44, 3881–3884 Search PubMed.
- B. M. Trost and V. S. C. Yeh, A dinuclear Zn catalyst for the asymmetric nitroaldol (Henry) reaction, Angew. Chem., 2002, 114, 889–891 Search PubMed.
- R. Kowalczyk, Ł. Sidorowicz and J. Skarżewski, Asymmetric nitroaldol reaction catalyzed by a chromium(III)–salen system, Tetrahedron: Asymmetry, 2007, 18, 2581–2586 Search PubMed.
- A. Zulauf, M. Mellah and E. Schulz, New chiral thiophene− salen chromium complexes for the asymmetric Henry reaction, J. Org. Chem., 2009, 74, 2242–2245 Search PubMed.
- F. Ibrahim, N. Jaber, V. Guerineau, A. Hachem, G. Ibrahim, M. Mellah and E. Schulz, Recoverable salen-based macrocyclic chiral complexes; catalysts for enantioselective Henry reactions, Tetrahedron: Asymmetry, 2013, 24, 1395–1401 Search PubMed.
- M. M. Islam, P. Bhanja, M. Halder, A. Das, A. Bhaumik and S. M. Islam, Chiral Cr(III)-salen complex embedded over sulfonic acid functionalized mesoporous SBA-15 material as an efficient catalyst for the asymmetric Henry reaction, Mol. Catal., 2019, 475, 110489 Search PubMed.
- K. Ishihara, Y. Kato, N. Takeuchi, Y. Hayashi, Y. Hagiwara, S. Shibuya, T. Natsume and M. Matsugi, Asymmetric Henry Reaction Using Cobalt Complexes with Bisoxazoline Ligands Bearing Two Fluorous Tags, Molecules, 2023, 28, 7632 Search PubMed.
- Y. L. Wei, K. F. Yang, F. Li, Z. J. Zheng, Z. Xu and L. W. Xu, Probing the evolution of an Ar-BINMOL-derived salen–Co(III) complex for asymmetric Henry reactions of aromatic aldehydes: salan–Cu(II) versus salen–Co(III) catalysis, RSC Adv., 2014, 4, 37859 Search PubMed.
- K. Lang, J. Park and S. Hong, Urea/transition–metal cooperative catalyst for anti–selective asymmetric nitroaldol reactions, Angew. Chem., Int. Ed., 2012, 51, 1620–1624 Search PubMed.
- J. Park, K. Lang, K. A. Abboud and S. Hong, Self-assembled dinuclear cobalt(II)-salen catalyst through hydrogen-bonding and its application to enantioselective nitro-aldol (Henry) reaction, J. Am. Chem. Soc., 2008, 130, 16484–16485 Search PubMed.
- Y. Kogami, T. Nakajima, T. Ikeno and T. Yamada, Enantioselective Henry reaction catalyzed by salen-cobalt complexes, Synthesis, 2004, 1947–1950 Search PubMed.
- N. Ananthi, Chiral cobalt complexes synthesised from l-valine: as potential catalysts for asymmetric Henry reaction, MOJ Biorg. Org. Chem., 2017, 1, 00038 Search PubMed.
- T. Karasawa, N. Kumagai and M. Shibasaki, Heterogeneous heterobimetallic catalysis enabling expeditious access to CF3-containing vic-amino alcohols, Org. Lett., 2018, 20, 308–311 Search PubMed.
- T. Karasawa, R. Oriez, N. Kumagai and M. Shibasaki, Anti-selective catalytic asymmetric nitroaldol reaction of α-keto esters: intriguing solvent effect, flow reaction, and synthesis of active pharmaceutical ingredients, J. Am. Chem. Soc., 2018, 140, 12290–12295 Search PubMed.
- A. Nonoyama, K. Hashimoto, A. Saito, N. Kumagai and M. Shibasaki, preparation of Nd/Na heterogeneous catalyst from bench-stable and inexpensive Nd salt for an anti-selective catalytic asymmetric nitroaldol reaction, Tetrahedron Lett., 2016, 57, 1815–1819 Search PubMed.
- T. Ogawa, N. Kumagai and M. Shibasaki, Self-assembling neodymium/sodium heterobimetallic asymmetric catalyst confined in a carbon nanotube network, Angew. Chem., Int. Ed., 2013, 52, 6196–6201 Search PubMed.
- T. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai and M. Shibasaki, Anti-selective catalytic asymmetric nitroaldol reaction via a heterobimetallic heterogeneous catalyst, J. Am. Chem. Soc., 2009, 131, 13860–13869 Search PubMed.
- D. Xu, Q. Sun, Z. Quan, W. Sun and X. Wang, The synthesis of chiral tridentate ligands from L-proline and their application in the copper(II)-catalyzed enantioselective Henry reaction, Tetrahedron: Asymmetry, 2017, 28, 954–963 Search PubMed.
- G. Lu, F. Zheng, L. Wang, Y. Guo, X. Li, X. Cao, C. Wang, H. Chi, Y. Dong and Z. Zhang, Asymmetric Henry reaction catalyzed by Cu(II)-based chiral amino alcohol complexes with C2-symmetry, Tetrahedron: Asymmetry, 2016, 27, 732–739 Search PubMed.
- F. Xu, L. Yan, C. Lei, H. Zhao and G. Li, Asymmetric Cu-catalyzed Henry reaction promoted by chiral camphor-derived β-amino alcohols with a thiophene moiety, Tetrahedron: Asymmetry, 2015, 26, 338–343 Search PubMed.
- F. Xu, C. Lei, L. Yan, J. Tu and G. Li, Copper–chiral camphor β–amino alcohol complex catalyzed asymmetric Henry reaction, Chirality, 2015, 27, 761–765 Search PubMed.
- W. Jin, X. Li and B. Wan, A highly diastereo-and enantioselective copper(I)-catalyzed Henry reaction using a bis (sulfonamide)− diamine ligand, J. Org. Chem., 2011, 76, 484–491 Search PubMed.
- Z. L. Guo, S. Zhong, Y. B. Li and G. Lu, Chiral 1, 1′-binaphthylazepine derived amino alcohol catalyzed asymmetric Henry reaction, Tetrahedron: Asymmetry, 2011, 22, 238–245 Search PubMed.
- D. Xin, Y. Ma and F. He, Synthesis of new planar chiral [2.2] paracyclophane Schiff base ligands and their application in the asymmetric Henry reaction, Tetrahedron: Asymmetry, 2010, 21, 333–338 Search PubMed.
- H. Y. Kim and K. Oh, Brucine-derived amino alcohol catalyzed asymmetric Henry reaction: an orthogonal enantioselectivity approach, Org. Lett., 2009, 11, 5682–5685 Search PubMed.
- M. Çolak, T. Aral, H. Hoşgören and N. Demirel, Synthesis of novel chiral Schiff-base ligands and their application in asymmetric nitro aldol (Henry) reaction, Tetrahedron: Asymmetry, 2007, 18, 1129–1133 Search PubMed.
- H. Cruz, G. Aguirre, D. Madrigal, D. Chávez and R. Somanathan, Enantioselective nitromethane addition to brominated and fluorinated benzaldehydes (Henry reaction) catalyzed by chiral bisoxazoline–copper(II) complexes, Tetrahedron: Asymmetry, 2016, 27, 1217–1221 Search PubMed.
- L. W. Tang, X. Dong, Z. M. Zhou, Y. Q. Liu, L. Dai and M. Zhang, The first 4, 4′-imidazolium-tagged C 2-symmetric bis (oxazolines): application in the asymmetric Henry reaction, RSC Adv., 2015, 5, 4758–4765 Search PubMed.
- E. Wolińska, Chiral oxazoline ligands containing a 1, 2, 4-triazine ring and their application in the Cu-catalyzed asymmetric Henry reaction, Tetrahedron, 2013, 69, 7269–7278 Search PubMed.
- K. Balaraman, R. Vasanthan and V. Kesavan, Asymmetric Henry reaction catalyzed by novel chiral bioxazolines from tartaric acid, Synthesis, 2012, 2455–2462 Search PubMed.
- W. Yang and D. Du, Highly Enantioselective Henry reaction catalyzed by C2−symmetric modular BINOL–oxazoline Schiff base copper(II) complexes generated in situ, Eur. J. Org. Chem., 2011, 1552–1556 Search PubMed.
- K. Lang, J. Park and S. Hong, Development of bifunctional aza-bis (oxazoline) copper catalysts for enantioselective henry reaction, J. Org. Chem., 2010, 75, 6424–6435 Search PubMed.
- S. K. Ginotra and V. K. Singh, Enantioselective Henry reaction catalyzed by a C 2-symmetric bis (oxazoline)–Cu(OAc)2·H2O complex, Org. Biomol. Chem., 2007, 5, 3932 Search PubMed.
- F. Liu, S. Gou and L. Li, Symmetric Henry reactions of aldehydes with various nitroalkanes catalyzed by copper(II) complexes of novel chiral N–monoalkyl cyclohexane–1,2−diamines, Appl. Organomet. Chem., 2014, 28, 186–193 Search PubMed.
- R. Kowalczyk, Ł. Sidorowicz and J. Skarżewski, Asymmetric Henry reaction catalyzed by chiral secondary diamine-copper(II) complexes, Tetrahedron: Asymmetry, 2008, 19, 2310–2315 Search PubMed.
- K. Mondal and S. Mistri, Schiff base based metal complexes: A review of their catalytic activity on aldol and Henry reaction, Comments Inorg. Chem., 2023, 43, 77–105 Search PubMed.
- M. Sharma, B. Das, G. V. Karunakar, L. Satyanarayana and K. K. Bania, Chiral Ni-Schiff base complexes inside zeolite-Y and their application in asymmetric Henry reaction: effect of initial activation with microwave irradiation, J. Phys. Chem., 2016, 120, 13563–13573 Search PubMed.
- G. Ouyang, Y. He and Q. Fan, Podand-based dimeric chromium(III)–salen complex for asymmetric Henry reaction: cooperative catalysis promoted by complexation of alkali metal ions, Chem. – Eur. J., 2014, 20, 16454–16457 Search PubMed.
- J. D. White and S. Shaw, A new catalyst for the asymmetric Henry reaction: synthesis of β-nitroethanols in high enantiomeric excess, Org. Lett., 2012, 14, 6270–6273 Search PubMed.
- R. Kowalczyk, P. Kwiatkowski, J. Skarżewski and J. Jurczak, Enantioselective nitroaldol reaction catalyzed by sterically modified salen−chromium complexes, J. Org. Chem., 2009, 74, 753–756 Search PubMed.
- J. Guo and J. Mao, Asymmetric Henry reaction catalyzed by bifunctional copper–based catalysts, Chirality, 2009, 21, 619–627 Search PubMed.
- Y. A. Casao, E. M. Lopez and R. P. Herrera, Organocatalytic enantioselective Henry reactions, Symmetry, 2011, 3, 220–245 Search PubMed.
- T. Marcelli, R. N. S. van der Haas, J. H. van Maarseveen and H. Hiemstra, Asymmetric organocatalytic Henry reaction, Angew. Chem., Int. Ed., 2006, 45, 929–931 Search PubMed.
- Aldehyde activation by H-bonding with water molecules preorganized by a Cu(II) center was suggested by Larionov, Belokon, et al.: V. A. Larionov, L. V. Yashkina, M. G. Mendvedev, A. F. Smol'yakov, A. S. Peregudov, A. A. Pavlov, D. B. Eremin, T. F. Savel'yeva, V. I. Maleev and Y. N. Belokon, Henry reaction revisited. Crucial role of water in an asymmetric Henry reaction catalyzed by chiral NNO-type copper(II) complexes, Inorg. Chem., 2019, 58, 11051–11065 Search PubMed.
- P. Clapés, W. D. Fessner, G. A. Sprenger and A. K. Samland, Recent progress in stereoselective synthesis with aldolases, Curr. Opin. Chem. Biol., 2010, 14, 154–167 Search PubMed.
- F. Willig, J. Lang, A. C. Hans, M. R. Ringenberg, D. Pfeffer, W. Frey and R. Peters, Polyfunctional imidazolium aryloxide betaine/Lewis acid catalysts as tools for the asymmetric synthesis of disfavored diastereomers, J. Am. Chem. Soc., 2019, 141, 12029–12043 Search PubMed.
- V. Miskov-Pajic, F. Willig, D. M. Wanner, W. Frey and R. Peters, Enantiodivergent [4 + 2] cycloaddition of dienolates by polyfunctional Lewis acid/zwitterion catalysis, Angew. Chem., 2020, 132, 20045–20049 Search PubMed.
- D. Uraguchi and T. Ooi, Chemistry of ammonium betaines: application to ion-pair catalysis for selective organic transformations, J. Synth. Org. Chem., Jpn., 2018, 76, 1144–1153 Search PubMed.
- X. Ye and C.-H. Tan, Enantioselective transition metal catalysis directed by chiral cations, Chem. Sci., 2021, 12, 533–539 Search PubMed.
- H. K. Adams, M. Kadarauch, N. J. Hodson, A. R. Lit and R. J. Phipps, Design approaches that utilize ionic interactions to control selectivity in transition metal catalysis, Chem. Rev., 2025, 125, 2846–2907 Search PubMed.
- A. Bürstner, P. M. Becker, A. Allgaier, L. Pfitzer, D. M. Wanner, J. Dollinger, F. Willig, J. Herrmann, V. Miskov-Pajic, A. C. Hans, W. Frey, J. van Slageren, J. Kästner and R. Peters, Tunable endo/exo selectivity in direct catalytic asymmetric 1,3−dipolar cycloadditions with polyfunctional lewis acid/azolium–aryloxide catalysts, Angew. Chem., Int. Ed., 2025, 64, e202508024 Search PubMed.
- D. M. Wanner, P. M. Becker, S. Suhr, N. Wannenmacher, S. Ziegler, J. Herrmann, F. Willig, J. Gabler, K. Jangid, J. Schmid, A. C. Hans, W. Frey, B. Sarkar, J. Kästner and R. Peters, Cooperative Lewis acid–1, 2, 3−triazolium–aryloxide catalysis: pyrazolone addition to nitroolefins as entry to diaminoamides, Angew. Chem., 2023, 135, e202307317 Search PubMed.
- Y. Zhou, J. Dong, F. Zhang and Y. Gong, Synthesis of C1-symmetric chiral secondary diamines and their applications in the asymmetric copper(II)-catalyzed Henry (nitroaldol) reactions, J. Org. Chem., 2011, 76, 588–600 Search PubMed.
- A. Toussaint and A. Pfaltz, Asymmetric Henry reactions catalyzed by metal complexes of chiral boron-bridged bisoxazoline (borabox) ligands, Eur. J. Org. Chem., 2008, 4591–4597 Search PubMed.
- A. Chougnet, G. Zhang, K. Liu, D. Haussinger, A. Kagi, T. Allmendinger and W. D. Woggon, Diastereoselective and Highly Enantioselective Henry Reactions using C1-Symmetrical Copper(II) Complexes, Adv. Synth. Catal., 2011, 353, 1797–1806 Search PubMed.
- A. C. Hans, P. M. Becker, J. Haußmann, S. Suhr, D. M. Wanner, V. Lederer, F. Willig, W. Frey, B. Sarkar, J. Kästner and R. Peters, A Practical and robust zwitterionic cooperative lewis acid/acetate/benzimidazolium catalyst for direct 1,4-additions, Angew. Chem., Int. Ed., 2023, 62, e202217519 Search PubMed.
- J. Schmid, T. Junge, J. Lang, W. Frey and R. Peters, Polyfunctional bis-Lewis-acid-/bis-triazolium catalysts for stereo-selective 1,4-additions of 2-oxindoles to maleimides, Angew. Chem., Int. Ed., 2019, 58, 5447–5451 Search PubMed.
- M. Mechler and R. Peters, Diastereodivergent asymmetric 1,4-addition of oxindoles to nitroolefins by using polyfunctional nickel-hydrogen-bond-azolium catalysts, Angew. Chem., Int. Ed., 2015, 54, 10303–10307 Search PubMed.
- J. Burés, A simple graphical method to determine the order in catalyst, Angew. Chem., Int. Ed., 2016, 55, 2028–2031 Search PubMed.
- J. Burés, Variable time normalization analysis: general graphical elucidation of reaction orders from concentration profiles, Angew. Chem., Int. Ed., 2016, 55, 16084–16087 Search PubMed.
- C. D. T. Nielsen and J. Burés, Visual kinetic analysis, Chem. Sci., 2019, 10, 348–353 Search PubMed.
- T. Satyanarayana, S. Abraham and H. B. Kagan, Nonlinear effects in asymmetric catalysis, Angew. Chem., Int. Ed., 2009, 48, 456–494 Search PubMed.
- H. B. Kagan, Practical consequences of non–linear effects in asymmetric synthesis, Adv. Synth. Catal., 2001, 343, 227–233 Search PubMed.
- W. L. Driessen and W. L. Groeneveld, Nitromethane as a ligand. Part I: complexes with some divalent metals and the hexachloroantimonate anion, Recl. Trav. Chim. Pays-Bas, 1969, 88, 491–498 Search PubMed.
- A. M. Wright, G. Wu and T. W. Hayton, Late-metal nitrosyl cations: synthesis and reactivity of [Ni(NO)(MeNO2)3][PF6], Inorg. Chem., 2011, 50, 11746–11753 Search PubMed.
- A. Klamt and G. Schüürmann, COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 Search PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 Search PubMed.
- C. Bannwarth, S. Ehlert and S. Grimme, GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions, J. Chem. Theory Comput., 2019, 15, 1652–1671 Search PubMed.
- P. Pracht, F. Bohle and S. Grimme, Automated exploration of the low-energy chemical space with fast quantum chemical methods, Phys. Chem. Chem. Phys., 2020, 22, 7169–7192 Search PubMed.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297 Search PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 Search PubMed.
- A. D. Becke, Phys. Rev., 1988, 38, 3098–3100 Search PubMed.
- S. Grimme, J. G. Brandenburg, C. Bannwarth and A. Hansen, Density-functional exchange-energy approximation with correct asymptotic behavior, J. Chem. Phys., 2015, 143, 3098 Search PubMed.
- J. Kästner, J. M. Carr, T. W. Keal, W. Thiel, A. Wander and P. Sherwood, DL-FIND: an open-source geometry optimizer for atomistic simulations, J. Phys. Chem. A, 2009, 113, 11856–11865 Search PubMed.
- P. Sherwood, A. H. de Vries, M. F. Guest, G. Schreckenbach, C. R. A. Catlow, S. A. French, A. A. Sokol, S. T. Bromley, W. Thiel, A. J. Turner, S. Billeter, F. Terstegen, S. Thiel, J. Kendrick, S. C. Rogers, J. Casci, M. Watson, F. King, E. Karlsen, M. Sjøvoll, A. Fahmi, A. Schäfer and C. Lennartz, QUASI: A general purpose implementation of the QM/MM approach and its application to problems in catalysis, J. Mol. Struct.: THEOCHEM, 2003, 632, 1–28 Search PubMed.
- TURBOMOLE V7.7.1 2022, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007, available from https://www.turbomole.org Search PubMed.
- D. Bhattacharyya, C. E. Hamrin Jr and R. P. Northey, Oxidation of hazardous organics in a two-phase fluorocarbon-water system, Hazard. Waste Hazard. Mater., 1986, 3, 405–427 Search PubMed.
- D. Turnbull and S. H. Maron, The ionization constants of aci and nitro forms of some nitroparaffins, J. Am. Chem. Soc., 1943, 65, 212–218 Search PubMed.
- M. Besora and F. Maseras, Microkinetic modeling in homogeneous catalysis, Wiley Interdiscip. Rev.:Comput. Mol. Sci., 2018, 8, e1372 Search PubMed.
- S. Persson, F. Fröhlich, S. Grein, T. Loman, D. Ognissanti, V. Hasselgren, J. Hasenauer and M. Cvijovic, PEtab.jl: advancing the efficiency and utility of dynamic modelling, Bioinformatics, 2025, 41, btaf497 Search PubMed.
- T. E. Loman, Y. Ma, V. Ilin, S. Gowda, N. Korsbo, N. Yewale, C. Rackauckas and S. A. Isaacson, Catalyst: Fast and flexible modeling of reaction networks, PLoS Comput. Biol., 2023, 19, e1011530 Search PubMed.
- O. V. Khromova, L. V. Yashkina, N. V. Stoletova, V. I. Maleev, Y. N. Belokon and V. A. Larionov, Selectivity control in nitroaldol (Henry) reaction by changing the basic anion in a chiral copper(II) complex based on (S)-2-aminomethylpyrrolidine and 3,5-di-tert-butylsalicylaldehyde, Molecules, 2024, 29, 5207 Search PubMed.
- M. A. Ei-Atawy, K. D. Khalil and A. H. Bashal, Chitosan capped copper oxide nanocomposite: efficient, recyclable, heterogeneous base catalyst for synthesis of nitroolefins, Catalysts, 2022, 12, 964 Search PubMed.
- T. Jose, J. Ftouni and P. C. A. Bruijnincx, Structured hydroxyapatite composites as efficient solid base catalysts for condensation reactions, Catal. Sci. Technol., 2021, 11, 3428 Search PubMed.
Footnote |
| † Both authors contributed equally. |
| This journal is © the Partner Organisations 2026 |