Electroreductive thiocarboxylation of alkenes with cyclosulfonium salts and CO2: access to thioether acids
Yong-Fei
Huang†
,
Li-Jie
Wang†
,
Mu-Jiang
Luo
 ,
Chaozhihui
Cheng
,
Chaozhihui
Cheng
 * and
Qiang
Xiao
* and
Qiang
Xiao
 *
*
Jiangxi Provincial Key Laboratory of Organic Functional Molecules, Institute of Organic Chemistry, Jiangxi Science and Technology Normal University, Nanchang, 330013, China. E-mail: czhcheng@hnu.edu.cn; xiaoqiang@tsinghua.org.cn
First published on 20th October 2025
Abstract
Electroreductive carboxylation has emerged as a prominent strategy in modern organic synthesis, demonstrating well-established applications in two-component coupling reactions. However, multi-component reactions for selective mono-carboxylation remain underdeveloped with only a limited number of examples reported. We herein report a novel electroreductive intermolecular 1,2-thiocarboxylation of alkenes with cyclosulfonium salts and carbon dioxide (CO2) via selective C–S bond cleavage for the construction of thioether acids. This transformation enables the single-step installation of both distal aryl thioether-functionalized alkyl chains and electrophiles into C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of alkenes via a radical relay cascade mechanism, achieving sequential covalent bond formation through controlled radical insertion. With demonstrated broad substrate adaptability and excellent functional group tolerance, this method paves a straightforward and selective pathway for the modular synthesis of thiocarboxylation derivatives.
C bonds of alkenes via a radical relay cascade mechanism, achieving sequential covalent bond formation through controlled radical insertion. With demonstrated broad substrate adaptability and excellent functional group tolerance, this method paves a straightforward and selective pathway for the modular synthesis of thiocarboxylation derivatives.
Introduction
Thioether acid is an essential structural unit in many biologically active compounds, drugs, and natural products, such as glutamyl-S-allyl-mercapto-L-cysteine, γ-L-glutamyl-S-trans-1-propenyl-L-cysteine, N-acetyl-S-allylcysteine, and so on (Fig. 1),1 which have the ability to significantly inhibit atherosclerosis through antioxidant, lipid-lowering, anti-inflammatory, and antithrombotic mechanisms.2,3 Due to the significant importance, synthesizing innovative and efficient molecules containing thioether acids has an important place in the toolbox strategies of researchers. The strategic utilization of CO2 as a versatile C1 building block for carboxylic acid synthesis has emerged as a frontier research area, driven by its inherent advantages of sustainability, abundance, cost-effectiveness, and environmental friendliness.4–10 In the past decade, the difunctional carboxylation strategy employing CO2 has emerged as an effective approach in synthetic chemistry, enabling concurrent incorporation of carboxyl and other functional moieties through single-step protocols.11–24 For example, Martin and co-workers, Wu and co-workers, Yu and co-workers, Han and Jing reported carbocarboxylation, aminocarboxylation, arylcarboxylation, silacarboxylation, phosphonocarboxylation, and alkynylcarboxylation of alkenes with CO2via visible-light photoredox or electroreduction. Given that there are only a limited number of examples of synthesis of thioether-containing carboxylic acids, we seek to develop the first late-stage thiocarboxylation reaction employing organosulfur compounds with CO2 and alkenes by using environmentally benign and sustainable strategies.Sulfonium salts, as a representative class of organosulfur compounds, demonstrate significant potential in organic synthesis owing to their distinctive structural architecture and tunable reactivity profiles.25–37 Considerable progress has been achieved in selective difunctionalization for the synthesis of both C–C and C–X (X = Br, S, N, etc.) bonds by using sulfonium salts as the radical precursors with alkenes and nucleophiles over the past few decades (Scheme 1a).38–41 Unfortunately, to date, only a few successful strategies have been reported for the use of sulfonium salts to react with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and electrophiles, for example, Xie and co-workers reported a visible-light-driven protocol for achieving the elegant arylcarboxylation of alkenes with CO2 and aryl thianthrenium salts (Scheme 1b).42 Although novel, in conventional sulfonium salt-based methods, sulfur-containing moieties are always discarded as sacrificial leaving groups, leading to low atom economy and severely limiting further applications.
C bonds and electrophiles, for example, Xie and co-workers reported a visible-light-driven protocol for achieving the elegant arylcarboxylation of alkenes with CO2 and aryl thianthrenium salts (Scheme 1b).42 Although novel, in conventional sulfonium salt-based methods, sulfur-containing moieties are always discarded as sacrificial leaving groups, leading to low atom economy and severely limiting further applications.
To address such a problem, our aim was to further develop the electroreductive thiocarboxylation of alkenes with various cyclothionium salts and CO2 as an electrophile to synthesize highly valuable thioether acids (Scheme 1c). However, several challenges may impede the success. (1) How to avoid the C(sp2)–S bond cleavage of cyclothionium salts and selectively enable the radical ring-opening pathway; (2) how to preferentially reduce cyclothionium salts compared to alkenes without affecting any sensitive functional groups, as well as avoiding overreduction of thioether-containing alkyl radicals; and (3) how to achieve late-stage functionalization43–46 with benign and selective installation of a thioether-containing alkyl group on the desired structural motifs of bio-relevant compounds. Once successfully implemented, this strategy will provide a more sustainable and environmentally friendly alternative to previous methods and generate valuable thioether acids, which serve as crucial structural motifs in various naturally occurring complex compounds and drug derivatives.
Results and discussion
We first employed 1,1-diphenyl ethylene (1a) and a cyclosulfonium salt (2a) as model substrates and examined various reaction conditions with CO2 (1 atm) for the electroreductive alkylcarboxylation of alkenes (Table 1). After systematic screening of various reaction parameters, 1a and 2a treated with CO2, Me4NPF6, dimethyl terephthalate (DMTP) and 10 mA constant current in an undivided cell with a magnesium flake anode and a C felt cathode generated the desired thioether acid 3aa in 72% yield (entry 1). Controlled experiments showed that constant current is essential for the present transformation (entry 2). Electrode systems, such as Zn(+)/C felt(−), Fe(+)/C felt(−), Ni(+)/C felt(−), C(+)/C felt(−), Mg(+)/Nb(−) and Mg(+)/Pt(−), were investigated, while Mg(+)/C felt(−) was proven to be the best electrode system (entries 3–8). It is a remarkable fact that no reaction was observed without the Me4NPF6 electrolyte (entry 9). When the Me4NPF6 electrolyte was replaced with tetrabutylammonium iodide (TBAI), nBu4NBF4, nBu4NOAc or LiClO4, the yield was not improved (entries 10–13). However, employing N,N-dimethylformamide (DMF) and MeCN as solvent led to lower conversion of 1a (entries 14 and 15). The desired product 3aa was obtained in 47% yield without the additive DMTP, providing strong support for its ability to facilitate the reaction (entry 16). To our delight, the optimal conditions are compatible to a scale up to 5.0 mmol of 1a, giving 3aa in good yield (entry 17).| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
a Standard reaction conditions: undivided cell, magnesium flake anode (10 × 15 × 0.5, mm), C felt cathode (10 × 15 × 3, mm), constant current = 10 mA, 1a (0.2 mmol), 2a (0.3 mmol), CO2 (1 atm; balloon), Me4NPF6 (0.1 M), DMSO (2.0 mL), room temperature and 3.5 h.
b ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a (5.0 mmol). 1a (5.0 mmol).
|
||
| 1 | None | 72 |
| 2 | Without current | 0 |
| 3 | Zn(+)/C felt(−) instead of Mg(+)/C felt(−) | Trace |
| 4 | Fe(+)/C felt(−) instead of Mg(+)/C felt(−) | 37 |
| 5 | Ni(+)/C felt(−) instead of Mg(+)/C felt(−) | 42 |
| 6 | C rod(+)/C felt(−) instead of Mg(+)/C felt(−) | 26 |
| 7 | Mg(+)/Nb(−) instead of Mg(+)/C felt(−) | 53 |
| 8 | Mg(+)/Pt(−) instead of Mg(+)/C felt(−) | 19 |
| 9 | Without Me4NI | Trace |
| 10 | n Bu4NI instead of Me4NPF6 | 35 |
| 11 | n Bu4NBF4 instead of Me4NPF6 | 43 |
| 12 | n Bu4NOAc instead of Me4NPF6 | <10 |
| 13 | LiClO4 instead of Me4NPF6 | Trace |
| 14 | DMF instead of DMSO | 43 |
| 15 | MeCN instead of DMSO | Trace |
| 16 | Without DMTP | 47 |
| 17b | None | 58 |
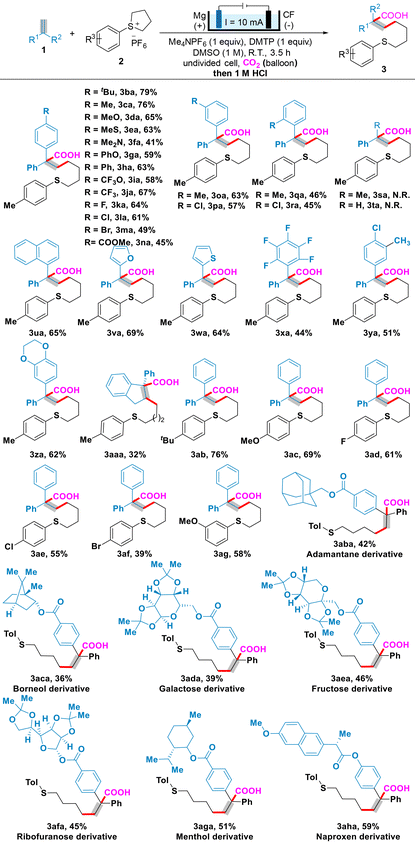
After identifying optimal reaction parameters, we set out to explore the scope of alkenes and cyclosulfonium salts (Table 2). In the presence of CO2, Me4NPF6, DMTP, DMSO and 10 mA constant current, the scope of alkenes 1b–aa was competent for the electroreductive thiocarboxylation reaction with cyclosulfonium salt 2a. To our delight, a wide range of functional groups, such as tBu, Me, MeO, MeS, Me2N, PhO, Ph, CF3O, CF3, F, Cl, Br and CO2Me, on the aryl ring were well tolerated. Substituted substrates at different positions on the aryl ring of the alkene exhibit different yields. For example, 1,1-diaryl ethylene 1c bearing a Me group at the para position was efficiently converted to 3ca in 76% yield. In contrast, the 1,1-diaryl ethylene compound with meta and ortho substituents produced 3oa and 3qa in decreasing yields (63% and 46%, respectively), likely due to the higher steric hindrance leading to lower yield. 1,1Diaryl ethylenes 1i andj and 1n with a strongly electron-withdrawing CF3O, CF3 or CO2Me group could also undergo thiocarboxylation with CO2 to generate 3ia andja and 3na in good yields. To our regret, α-methylstyrene 1s and styrene 1t were not suitable for the three-component coupling reaction. The desired product 3ua was isolated in a 65% yield using 2-(1-phenylvinyl)naphthalene 1u. Heteroarylethylenes, such as 2-(1-phenylvinyl)furan 1v and 2-(1-phenylvinyl)thiophene 1w, were competent substrates (3va andwa). Moreover, 1,1-diaryl ethylenes bearing multiple substituents, such as perfluorinated groups, a Me group and a Cl group, and an ethyl dioxygen group, were also suitable substrates and could be converted smoothly to 3xa–za. Furthermore, 3-phenyl-1H-indene 1aa was used to enable facile access to the corresponding thiocarboxylation product 3aaa in a 32% yield.
| a Standard reaction conditions: undivided cell, magnesium flake anode (10 × 15 × 0.5, mm), C felt cathode (10 × 15 × 3, mm), constant current = 10 mA, 1 (0.2 mmol), 2 (0.3 mmol), CO2 (1 atm; balloon), Me4NPF6 (0.1 M), DMSO (2 mL), room temperature and 3.5 h. |
|---|
|
Next, using 1,1-diphenyl ethylene (1a) as a model substrate, we investigated a variety of cyclosulfonium salts (2b–g). A number of substituents, including methyl, methoxyl, fluoro, chloro, and bromo, were compatible with the optimized conditions (3ab–ag). Encouraged by the wide scope of the alkenes, we then applied substrates derived from several natural products and drug molecules in the procedure. Various natural product-based or drug-based 1,1-diaryl ethylenes, such as adamantane, borneol, galactose, fructose, ribofuranose, menthol, and naproxen derivatives, underwent the late-stage modification to deliver the highly valuable natural-product-based or drug-based thioether acids 3aba–aha, which highlights the applicability of the protocol in synthesis.
As shown in Scheme 2A, synthetic utilizations of the resulting thioether acid 3aa were examined. In the presence of LiAlH4, reduction of 3aa occurred to afford 2,2-diphenyl-7-(p-tolylthio)heptan-1-ol 4 in 81% yield. Interestingly, treatment of 3aa with 2 equiv. of TMSCHN2 was successful and delivered the highly valuable methyl 2,2-diphenyl-7-(p-tolylthio)heptanoate 5. The reaction of 3aa with methyl glycylglycinate hydrochloride in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI), 1-hydroxybenzotriazole (HOBt), and N,N-diisopropylethylamine (DIPEA) proceeded to furnish product 6 in 61% yield.
We performed several mechanistic experiments to gain insight into the mechanism of this transformation. First, when the model reaction was conducted using 3.0 equiv. of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or butylated hydroxytoluene (BHT) as a radical trapping reagent, only 18% yield of product 3aa was isolated (Scheme 2B, 1 and 2). Next, the deuteration experiment of 1a with 2a and 10.0 equiv. of D2O under the optimal conditions gave the hydrogenation product (3aa′) in 29% yield with 59%-D (Scheme 2B, 3), suggesting that the carbanion intermediate might be involved in the transformation. Electricity on/off experiments indicated that electrocatalysis is crucial for the reaction, as no reaction is observed in the absence of electricity (Fig. S2; SI). Thereafter, cyclic voltammetry (CV) analysis of 1a and 2a under an argon (Ar) atmosphere revealed onset reduction potentials of −2.43 V and −1.65 V versus SCE, respectively, and under a CO2 atmosphere, the reductive potential was retained (Fig. S3; SI). These observations imply that 2a is more readily reduced than 1a and CO2. Furthermore, when CV experiments of DMTP were performed under the condition of increasing equivalents of 2a, a reduction in the oxidation current of the radical anion of DMTP was observed (Fig. S4; SI), indicating that DMTP potentially served as a mediator for the single-electron reduction of 2a.
Based on the above experiments and previous reports, a plausible reaction mechanism is proposed for this electroreductive thiocarboxylation protocol (Scheme 3).47–49 Initially, the cyclosulfonium salt 2a is reduced at the cathode to generate the radical anion intermediate I. Simultaneously, DMTP undergoes reduction at the cathode to form DMTP˙−, which subsequently mediates SET reduction of the remaining portion of cyclosulfonium salt 2a, enabling the formation of key intermediate I. This intermediate subsequently undergoes ring-opening processes, resulting in the generation of the alkyl radical II, which then undergoes sequential addition across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of alkene 1a and goes through SET reduction at the cathode, producing the alkyl anion IV. The carboxylation of IV culminates in the formation of the desired product 3aa.
C bond of alkene 1a and goes through SET reduction at the cathode, producing the alkyl anion IV. The carboxylation of IV culminates in the formation of the desired product 3aa.
Conclusions
In summary, we have developed a novel transition-metal-free electroreductive thiocarboxylation of alkenes with CO2 and cyclosulfonium salts to afford valuable thioether acids. This protocol enables the direct incorporation of the thioether group and the carboxyl group to achieve 1,2-dicarbonyl functionalization, and its mild conditions, good selectivity and exceptional compatibility toward functional groups are highlighted. Most importantly, the successful thiocarboxylation of structurally complex natural products and pharmaceutically relevant derivatives conclusively demonstrates the operational versatility and drug discovery applicability of the method. Further applications of the electroreductive ring-opening protocols of cyclosulfonium salts are currently underway in our laboratory.Author contributions
Q. Xiao designed the experiments and directed the project. Y. F. Huang performed the chemical experiments. C. Z. H. Cheng wrote the manuscript. M. J. Luo and L. J. Wang analysed the data. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5qo01313e.Acknowledgements
We thank the National Natural Science Foundation of China (no. 22261019), the PhD start-up fund of Jiangxi Science & Technology Normal University (no. 2023BSQD14) and the Jiangxi Provincial Key Laboratory of Organic Functional Molecules (no: 2024SSY05141) for financial support.References
-
P. Ahmad, S. S. Alvi and M. S. Khan, Functioning of organosulfur compounds from garlic (allium sativum linn) in targeting risk factor-mediated atherosclerosis: A cross talk between alternative and modern medicine, in Natural Bio-active Compounds: Volume 1: Production and Applications, 2019, pp. 561–585 Search PubMed
.
- H. Amagase, B. L. Petesch, H. Matsuura, S. Kasuga and Y. Itakura, Intake of garlic and its bioactive components, J. Nutr., 2001, 131, 955S–962S CrossRef CAS PubMed
.
- G. Schafer and C. H. Kaschula, The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention, Anti-Cancer Agents Med. Chem., 2014, 14, 233–240 CrossRef CAS PubMed
.
- T. Sakakura, J.-C. Choi and H. Yasuda, Transformation of Carbon Dioxide, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS
.
- Y. Cao, X. He, N. Wang, H.-R. Li and L.-N. He, Photochemical and Electrochemical Carbon Dioxide Utilization with Organic Compounds, Chin. J. Chem., 2018, 36, 644–659 CrossRef CAS
.
- S. Wang and C. Xi, Recent advances in nucleophile-triggered CO2-incorporated cyclization leading to heterocycles, Chem. Soc. Rev., 2019, 48, 382–404 RSC
.
- C.-K. Ran, L.-L. Liao, T.-Y. Gao, Y.-Y. Gui and D.-G. Yu, Recent progress and challenges in carboxylation with CO2, Curr. Opin. Green Sustainable Chem., 2021, 32, 100525 CrossRef CAS
.
- H. Senboku, Electrochemical Fixation of Carbon Dioxide: Synthesis of Carboxylic Acids, Chem. Rec., 2021, 21, 2354–2374 CrossRef CAS PubMed
.
- Q. Wang, Y.-W. Wang, M. Liu, G.-H. Chu and Y.-A. Qiu, Recent Advances in Photochemical /Electrochemical Carboxylation of Olefins with CO2, Chin. J. Chem., 2024, 42, 2249–2266 CrossRef CAS
.
- G.-Q. Sun, L.-L. Liao, C.-K. Ran, J.-H. Ye and D.-G. Yu, Recent advances in electrochemical carboxylation with CO2, Acc. Chem. Res., 2024, 57, 2728–2745 CrossRef CAS PubMed
.
- V. Yatham, Y. Shen and R. Martin, Catalytic Intermolecular Dicarbofunctionalization of Styrenes with CO2 and Radical Precursors, Angew. Chem., Int. Ed., 2017, 56, 10915–10919 CrossRef CAS
.
- L. Chen, Q. Qu, C.-K. Ran, W. Wang, W. Zhang, Y. He, L.-L. Liao, J.-H. Ye and D.-G. Yu, Photocatalytic Carboxylation of C–N Bonds in Cyclic Amines with CO2 by Consecutive Visible-Light-Induced Electron Transfer, Angew. Chem., Int. Ed., 2023, 62, e202217918 CrossRef CAS PubMed
.
- Z. Yang, Q. Du, Y. Jiang and J. Wang, Asymmetric Access of γ-Amino Acids and γ-Amino Phosphonic Acid Derivatives via Copper-Catalyzed Enantioselective and Regioselective Hydroamination, CCS Chem., 2022, 4, 1901–1911 CrossRef CAS
.
- X. Wang, Y. Chen, P. Liang, J.-Q. Chen and J. Wu, Synthesis of γ-Amino Acids via Photocatalyzed Intermolecular Carboimination of Alkenes, Org. Chem. Front., 2022, 9, 4328–4333 RSC
.
- H. Hou, M. Luo, S. Zhai, T. Yuan, M. Zheng and S. Wang, Metal-Free Semiconductors for Visible-Light-Induced Carbocarboxylation of Styrenes with Aliphatic Redox-Active Esters and CO2, Green Chem., 2024, 26, 1317–1321 RSC
.
- K. Vega, A. Oliveira, B. König and M. Paixão, Visible-Light-Induced Synthesis of 1,2-Dicarboxyl Compounds from Carbon Dioxide, Carbamoyl-dihydropyridine, and Styrene, Org. Lett., 2024, 26, 860–865 CrossRef CAS
.
- L. Song, D.-M. Fu, L. Chen, Y.-X. Jiang, J.-H. Ye, L. Zhu, Y. Lan, Q. Fu and D.-G. Yu, Visible-Light Photoredox-Catalyzed Remote Difunctionalizing Carboxylation of Unactivated Alkenes with CO2, Angew. Chem., Int. Ed., 2020, 59, 21121–21128 CrossRef CAS PubMed
.
- L.-L. Liao, G.-M. Cao, Y.-X. Jiang, X.-H. Jin, X.-L. Hu, J. Chruma, G.-Q. Sun, Y.-Y. Gui and D.-G. Yu, α-Amino Acids and Peptides as Bifunctional Reagents: Carbocarboxylation of Activated Alkenes via Recycling CO2, J. Am. Chem. Soc., 2021, 143, 2812–2821 CrossRef CAS PubMed
.
- L. X. Lu, Y. Wang, W. D. Zhang, K. A. See and S. Lin, Three-component cross-electrophile coupling: regioselective electrochemical dialkylation of alkenes, J. Am. Chem. Soc., 2023, 145, 22298–22304 CrossRef CAS PubMed
.
- J. Hou, A. Ee, H. Cao, H. W. Ong, J. H. Xu and J. Wu, Metal-Free Difunctionalization of Alkenes with CO2 and Silanes or C(sp3)–H Alkanes, Angew. Chem., Int. Ed., 2018, 57, 17220–17224 CrossRef CAS PubMed
.
- T. Yuan, Z. Wu, S. Zhai, R. Wang, S. Wu, J. Cheng, M. Zheng and X. Wang, Photosynthetic Fixation of CO2 in Alkenes by Heterogeneous Photoredox Catalysis with Visible Light, Angew. Chem., Int. Ed., 2023, 62, e202304861 CrossRef CAS PubMed
.
- H. Huang, J.-H. Ye, L. Zhu, C.-K. Ran, M. Miao, W. Wang, H. Chen, W.-J. Zhou, Y. Lan, B. Yu and D.-G. Yu, Visible-Light-Driven Anti-Markovnikov Hydrocarboxylation of Acrylates and Styrenes with CO2, CCS Chem., 2020, 2, 1746–1756 Search PubMed
.
- B. Zhang, T.-T. Li, Z.-C. Mao, M. Jiang, Z. Zhang, K. Zhao, W.-Y. Qu, W.-J. Xiao and J.-R. Chen, Enantioselective Cyanofunctionalization of Aromatic Alkenes via Radical Anions, J. Am. Chem. Soc., 2024, 146, 1410–1422 CrossRef CAS
.
- X.-T. Gao, Z. Zhang and X. Wang, Direct Electrochemical Defluorinative Carboxylation of α-CF3 Alkenes with Carbon Dioxide, Chem. Sci., 2020, 11, 10414–10420 RSC
.
- S. Ni, J. Yan, S. Tewari, E. J. Reijerse, T. Ritter and J. Cornella, Nickel Meets Aryl Thianthrenium Salts: Ni(I)-Catalyzed Halogenation of Arenes, J. Am. Chem. Soc., 2023, 145, 9988–9993 CrossRef CAS
.
- F. Berger, M. B. Plutschack, J. Riegger, W. Yu, S. Speicher, M. Ho, N. Frank and T. Ritter, Site-Selective and Versatile Aromatic C–H Functionalization by Thianthrenation, Nature, 2019, 567, 223–228 CrossRef CAS PubMed
.
- P. S. Engl, A. P. Häring, F. Berger, G. Berger, A. Pérez-Bitrián and T. Ritter, C–N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts, J. Am. Chem. Soc., 2019, 141, 13346–13351 CrossRef CAS PubMed
.
- E. M. Alvarez, M. B. Plutschack, F. Berger and T. Ritter, Site-Selective C–H Functionalization–sulfination Sequence to Access Aryl Sulfonamides, Org. Lett., 2020, 22, 4593–4596 CrossRef CAS
.
- J. Li, J. Chen, R. Sang, W.-S. Ham, M. B. Plutschack, F. Berger, S. Chabbra, A. Schnegg, C. Genicot and T. Ritter, Photoredox Catalysis with Aryl Sulfonium Salts Enables Site-Selective Late-Stage Fluorination, Nat. Chem., 2020, 12, 56–62 CrossRef CAS
.
- W. W. Cui, X. F. Li, G. J. Guo, X. Y. Song, J. Lv and D. S. Yang, Radial Type Ring Opening of Sulfonium Salts with Dichalcogenides by Visible Light and Copper Catalysis, Org. Lett., 2022, 24, 5391–5396 CrossRef CAS
.
- W. W. Cui, G. J. Guo, Y. F. Wang, X. Y. Song, J. Lv and D. S. Yang, Visible light/copper catalysis enabled alkylation of silyl enol ethers with arylsulfonium salts, Chem. Commun., 2023, 59, 6367–6370 RSC
.
- X. Li, M. Jiang, J. Z. Zuo, X. Y. Song, J. Lv and D. S. Yang, Anti-Markovnikov ring-opening of sulfonium salts with alkynes by visible light/copper catalysis, Sci. China: Chem., 2023, 66, 791–798 CAS
.
- X. Li, X. F. Li, W. W. Cui, Q. L. Wu, L. Y. Wang, J. Lv and D. S. Yang, Visible-Light Copper Catalysis for the Synthesis of α-Alkyl-Acetophenones by the Radical-Type Ring Opening of Sulfonium Salts and Oxidative Alkylation of Alkenes, Org. Lett., 2023, 25, 3260–3265 CrossRef CAS PubMed
.
- X. Q. Liu, X. F. Li, L. Y. Wang, Y. J. Shi, J. Lv and D. S. Yang, Visible light/copper catalysis enabled Heck-like coupling between alkenes and cyclic sulfonium salts via selective C–S bond cleavage, Org. Chem. Front., 2024, 11, 2195–2200 RSC
.
- J. Ma, X. F. Li, Y. Q. Chen, Y. J. Shi, J. Lv and D. S. Yang, Annulation Cascades of Cyclosulfonium Salts and Alkenes towards Sulfur-Containing N-Heterocycles by Visible Light/Copper Catalysis, Chin. J. Chem., 2024, 42, 1637–1643 CrossRef CAS
.
- L. J. Zhong, H. Chen, X. Shang, J. H. Fan, K. W. Tang, Y. Liu and J. H. Li, Photoredox Ring Opening 1,2-Alkylarylation of Alkenes with Sulfonium Salts Toward Thioether-Substituted Oxindoles, J. Org. Chem., 2024, 89, 11233–11243 CrossRef PubMed
.
- M. J. Humbrías, G. J. J. Garrido, U. K. Medrano, G. Pelosi, L. Laze and L. Dell'Amico, Microfluidic Photocatalytic Ring Expansion of Sulfonium Salts for the Synthesis of Cyclic Sulfide, ACS Catal., 2025, 15, 6507–6513 CrossRef
.
- Y. Cai and T. Ritter, Meerwein-type Bromoarylation with Arylthianthrenium Salts, Angew. Chem., Int. Ed., 2022, 61, e202209882 CrossRef CAS PubMed
.
- Y. Cai, S. Chatterjee and T. Ritter, Photoinduced Copper-Catalyzed Late-Stage Azidoarylation of Alkenes via Arylthianthrenium Salts, J. Am. Chem. Soc., 2023, 145, 13542–13548 CrossRef CAS PubMed
.
- W. Zhang, T. Liu, H. T. Ang, P. Luo, Z. Lei, X. Luo, M. J. Koh and J. Wu, Modular and Practical 1,2-Aryl (Alkenyl) Heteroatom Functionalization of Alkenes through Iron/Photoredox Dual Catalysis, Angew. Chem., Int. Ed., 2023, 62, e202310978 CrossRef CAS PubMed
.
- H. Chen, X. Shang, N. Jiang, Q. Zhou, K. W. Tang, L. J. Zhong and Y. Liu, Photoinduced copper-catalyzed three-component alkylarylation of alkenes involving C–S bond cleavage of sulfonium salts, Org. Chem. Front., 2025, 10, 1498–1505 RSC
.
- W. Qi, S. Gu and L. G. Xie, Reductive Radical-Polar Crossover Enabled Carboxylative Alkylation of Aryl Thianthrenium Salts with CO2 and Styrenes, Org. Lett., 2024, 26, 728–733 CrossRef CAS PubMed
.
- K. Liu, M. Schwenzer and A. Studer, Radical NHC catalysis, ACS Catal., 2022, 12, 11984–11999 CrossRef CAS
.
- J. Q. Sun, L. H. Wang, G. F. Zheng and Q. Zhang, Recent advances in three-component radical acylative difunctionalization of unsaturated carbon–carbon bonds, Org. Chem. Front., 2023, 10, 4488–4515 RSC
.
- M. R. Li, Y. T. Wu, X. Song, J. Q. Sun, Z. X. Zhang, G. F. Zheng and Q. Zhang, Visible light-mediated organocatalyzed 1, 3-aminoacylation of cyclopropane employing N-benzoyl saccharin as bifunctional reagent, Nat. Commun., 2024, 15, 8930 CrossRef CAS PubMed
.
- M. R. Li, X. Song, X. Y. Lu, J. L. Xia, G. F. Zheng and Q. Zhang, Visible light-mediated 1,3-acylative chlorination of cyclopropanes employing benzoyl chloride as bifunctional reagents in NHC catalysis, Sci. China: Chem., 2025, 68, 3628–3635 CrossRef CAS
.
- Y. W. Wang, S. Y. Tang, G. Q. Yang, S. Y. Wang, D. K. Ma and Y. A. Qiu, Electrocarboxylation of Aryl Epoxides with CO2 for the Facile and Selective Synthesis of β–Hydroxy Acids, Angew. Chem., Int. Ed., 2022, 61, e202207746 CrossRef CAS
.
- K. Zhang, B.-H. Ren, X.-F. Liu, L.-L. Wang, M. Zhang, W.-M. Ren, X.-B. Lu and W.-Z. Zhang, Direct and Selective Electrocarboxylation of Styrene Oxides with CO2 for Accessing β–Hydroxy Acids, Angew. Chem., Int. Ed., 2022, 61, e202207660 CrossRef CAS PubMed
.
- F. F. Xie, F. Han, Q. Su, Y. L. Peng, L. H. Jing and P. Han, Electroreductive Arylcarboxylation of Styrenes with CO2 and Aryl Halides via a Radical–Polar Crossover Mechanism, Org. Lett., 2024, 26, 4427–4432 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2026 |





