Open Access Article
Open Access ArticlePerimidine directed Rh(III)-catalyzed [4 + 1] and [4 + 2] annulations: synthesis of perimidine linked spiro-succinimides and isoquinolines
Vidya
Kumari
and
Lokman H.
Choudhury
 *
*
Department of Chemistry, Indian Institute of Technology Patna, Bihta, 801106, Bihar, India. E-mail: lokman@iitp.ac.in; lokman.iitp@gmail.com
First published on 10th November 2025
Abstract
We report a novel Rh(III)-catalyzed perimidine-directed C–H activation strategy for the synthesis of perimidine-fused heterocycles using various types of alkenes. The reaction of 2-aryl perimidines with maleimides in the presence of the [RhCp*Cl2]2 catalyst facilitates a cascade [4 + 1] spiro-annulation, affording perimidine-linked isoindoles spiro-fused with succinimides. Conversely, vinylene carbonates provided a fused six-membered ring via a [4 + 2] cyclization, yielding perimidine-fused isoquinolines. This methodology is useful for synthesizing a library of polycyclic perimidine-fused heterocycles.
Introduction
In modern organic synthesis, site-selective C–H activation is considered an efficient strategy for the synthesis of complex molecules. Utilizing biologically relevant scaffolds as intrinsic directing groups has gained considerable interest as it circumvents the need for installing and removing external auxiliaries, thereby providing an efficient synthetic route.1Perimidine, a unique fused pyrimidine derivative, holds significant promise across diverse fields including medicine, life sciences, and industrial chemistry.2 Perimidine derivatives demonstrate a wide spectrum of biological activities, exhibiting antimicrobial,3 antibacterial,4 anticancer,5 antitumor,6 and anti-inflammatory7 properties, as well as being useful chemosensors8 and coloring agents.9 Perimidine exhibits distinctive electronic features arising from delocalization of the nitrogen lone pair into the naphthalene ring, imparting a unique combination of π-rich and π-deficient character that enhances its reactivity in diverse transformations.2 This characteristic electronic structure enables perimidine to coordinate with metals by accepting electrons into its low-lying vacant π-orbitals. This coordination also enhances the electropositive character of the metal centre, thereby promoting the coordination of alkenes.10
In recent years, pyrimidine has been extensively utilized as a removable auxiliary and transient directing group for directed C–H activation.10–13 In 2022, Chatani and co-workers reported the activation of the ortho-C–H bond of pyrimidine-protected aniline for the oxidative ortho-alkylation with secondary allyl alcohols to synthesize β-aryl ketones (eqn (a), Scheme 1).12 Similarly, in 2021, Maiti and co-workers reported meta-olefination of complex biaryls using a palladium catalyst and pyrimidine as a transient directing group via reversible imine formation (eqn (b), Scheme 1).13 Despite the enormous applications in drugs and the synthetic utility of pyrimidines, using it as an intrinsic directing group is still limited. On the other hand, to the best of our knowledge, perimidine-directed C–H activation remains largely unexplored. The only related studies were reported by Li and co-workers, in which 2-aryl-2,3-dihydro-1H-perimidines underwent Rh(III)-catalyzed annulation to furnish cationic azaperylene derivatives (eqn (c), Scheme 1).14
Furthermore, intrinsic group directed C–H annulation and spiro-annulation have gathered considerable attention recently.15–20 Motivated by the directing properties of pyrimidine and the growing interest in intrinsic group-directed annulation and spiro-annulation, and in continuation of our work on C–H functionalization of heterocycles,21 we turned our attention towards C–H activation using perimidine as an intrinsic directing group (eqn (d), Scheme 1). Herein, we report for the first time an efficient Rh(III)-catalyzed C–H activation strategy utilizing 2-aryl-1H-perimidine as an intrinsic directing group for the synthesis of structurally diverse polycyclic spiro-succinimides and isoquinoline-fused perimidines.
Results and discussion
We initiated our study with the reaction between 2-phenyl-1H-perimidine (1a) and N-phenylmaleimide (2a) in the presence of [RhCp*Cl2]2 (5 mol%), NaOAc (1 equiv.), and AcOH (2 equiv.) in 1 mL of MeOH at 80 °C, which afforded the desired product 3a in 84% yield (Table 1, entry 1). Notably, alternative C–H activation catalysts such as [Ru(p-cymene)Cl2]2, Pd(OAc)2, and [CoCp*(CO)I2] failed to afford the desired product (Table 1, entries 2–4). Other additives such as AgOAc and Cu(OAc)2·H2O were ineffective, with no improvement in yield (Table 1, entry 5). Subsequently, various solvents (e.g., 1,2-DCE, ACN, THF, and acetone) were screened to optimize the yield (Table 1, entry 6). These results established MeOH as the most suitable solvent. Substituting AcOH with Na2CO3 resulted in a decreased yield of 3a (52%) (Table 1, entry 7). Similarly, altering the acid source was ineffective, affording a comparable yield (Table 1, entry 8). Conducting the reaction under a N2 atmosphere resulted in no improvement in yield, with 24% of the starting material remaining, suggesting that air serves as a key oxidant (Table 1, entry 9). No product formation was observed without [RhCp*Cl2]2 or NaOAc, while omission of AcOH led to a moderate 47% yield (Table 1, entries 10–12).| Entry | Deviation from standard conditions | Yieldb |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), catalyst (5 mol%), additive 1 (1.0 equiv.), and additive 2 (2.0 equiv.) in solvent (1.0 mL) at 80 °C for 1 h. b Isolated yield. c Under a N2 atmosphere; 24% of 1a was recovered; NR = no reaction. | ||
| 1 | None | 84 |
| 2 | [Ru(p-cymene)Cl2]2 instead of [RhCp*Cl2]2 | NR |
| 3 | Pd(OAc)2 instead of [RhCp*Cl2]2 | NR |
| 4 | [CoCp*(CO)I2] instead of [RhCp*Cl2]2 | NR |
| 5 | AgOAc/Cu(OAc)2·H2O instead of NaOAc | 65/45 |
| 6 | 1,2-DCE/ACN/THF/acetone instead of MeOH | 43/27/NR/55 |
| 7 | Na2CO3 instead of AcOH | 52 |
| 8 | PivOH instead of AcOH | 71 |
| 9 | Under N2 gas | 43c |
| 10 | No [RhCp*Cl2]2 | NR |
| 11 | No NaOAc | NR |
| 12 | No AcOH | 47 |
With the optimized reaction conditions established, we next explored the substrate scope by varying perimidine derivatives while keeping N-phenylmaleimide (2a) constant (Scheme 2). Under standard conditions, unsubstituted 2-phenyl-1H-perimidine (1a) reacted with N-phenylmaleimide (2a) to afford polycyclic fused succinimide 3a in 84% yield. We then examined the effect of para-substitution on the phenyl ring of perimidine. Electron-donating (1b, 1f) and halogen-substituted (1c, 1d) compounds reacted efficiently with 2a, affording the corresponding products in yields of 80–88%. In contrast, the fluoro-substituted derivative (1e) gave a reduced yield of 62%, while the strongly electron-withdrawing cyano-substituted perimidine (1g), i.e., 4-(2,3-dihydro-1H-perimidin-2-yl) benzonitrile, failed to produce the desired product. So, we performed the reaction under the modified conditions using 20 mol% AgOAc instead of NaOAc; the reaction proceeded slowly to provide a low yield of the product 3g in 24 h. The 4-phenyl-substituted perimidine (1h) afforded spirocyclic product 3h in 79% yield, while naphthyl perimidine (1i) delivered 3i in 77% yield. Notably, ortho-substituted derivatives (1j and 1k) were also tolerated, furnishing 3j and 3k in moderate yields. All the products were fully characterized by recording 1H, 13C NMR and HRMS spectra. For unambiguous confirmation of the structures, the single crystal X-ray structure of the product 3b (CCDC no. 2279344) was obtained. The crystal was grown in EtOAc as a solvent by a slow evaporation method. The details of the XRD structure of 3b are available in the SI. In the case of meta-substituted perimidine 1l, selective activation of the less hindered ortho C–H bond afforded product 3l in 76% yield.15,17 The structure was further confirmed by performing single crystal-XRD of 3l (CCDC no. 2495811). The crystal was grown in EtOAc as a solvent by a slow evaporation method (for crystal details, please see the SI). The carbazole-linked perimidine 1m also underwent smooth transformation with 2a, delivering 3m in 62% yield, demonstrating the protocol's compatibility with heterocycle-linked frameworks. Next, we performed the reaction with 2-(thiophen-2-yl)-1H-perimidine 1n with N-phenylmaleimide 2a under the standard reaction conditions. Interestingly, this reaction did not provide the desired spiro-succinimides, instead it provided the [4 + 2] annulated product 3n in only 38% yield even after a longer reaction time (3 h), and the structure was confirmed by recording 1H, 13C NMR and HRMS spectra. This could be due to the slow reactivity of the 2-(thiophen-2-yl)-1H-perimidine 1n towards the aza-Michael addition step. On the other hand, 2-(pyridin-4-yl)-1H-perimidine 1o was found to be not suitable for this reaction and the corresponding expected product 3o could not be prepared using this methodology.
Encouraged by these results, next, we explored the substrate scope with diverse maleimides and alkenes as summarized in Scheme 3. Perimidine 1b reacted with N-benzyl (2b) and N-cyclohexyl maleimide (2c) to afford spirocycles 4a and 4b in excellent yields (86% and 90%, respectively). The 4-bromo-substituted maleimide 2d provided 4c in 69% yield, while the strongly electron-withdrawing 4-nitro derivative 2e gave 4d in a good yield of 78%. The meta-substituted maleimide 2f reacted efficiently with perimidine 1b to afford spirocycle 4e in a good yield of 71%. Similarly, 3,4-dimethoxy-substituted maleimide 2g gave 4f in 72% yield. Notably, the heteroaryl-linked N-methyl thiophene maleimide 2h also reacted smoothly, furnishing 4g in 80% yield. The applicability of this methodology was further demonstrated with N-alkyl-substituted maleimides 2i and 2j, which reacted efficiently with perimidine 1b to afford 4h and 4i in 76% and 84% yields, respectively. Likewise, N-oleyl maleimide 2k reacted with perimidine 1b to afford the spirocyclized product 4j in 76% yield. The unsubstituted maleimide 2l also reacted with 1b, furnishing 4k in a moderate yield of 62%. Reaction of 1b with carbazole-linked maleimide 2m provided product 4l in 77% yield. Naphthoquinone 2n, a cyclic electron-deficient alkene, also afforded the [4 + 1] spirocyclized product 4m in 82% yield. Acyclic electron-deficient alkenes like ethyl acrylate 2o and acrylonitrile 2p reacted with 1b to provide [4 + 1] annulated perimidines 4n and 4o in excellent yields of 93% and 84%, respectively. In contrast, symmetric alkene trans-stilbene 2q remained unreactive under the optimized conditions. Subsequently, 4-chlorophenyl perimidine 1c was reacted with various maleimides, including N-benzyl 2b, N-cyclohexyl 2c, and N-ethyl 2i. In all instances, the reaction proceeded efficiently, demonstrating the broad scope of this methodology.
After obtaining these encouraging results, we next explored vinylene carbonate as a reaction partner, replacing maleimide. Under the optimized conditions, no reaction occurred. Consequently, we optimized reaction conditions for the reaction of 2-phenyl-1H-perimidine 1a with vinylene carbonate 5a. After a series of reactions (summarized in Table 2), we found that vinylene carbonates can be reacted with 1a. Interestingly, the resulting product is a fused six-membered ring formed via a [4 + 2] annulation. Among all the screened conditions, entry 1 conditions in Table 2 provided the best result and were therefore considered the optimal reaction conditions.
| Entry | Deviation from standard conditions | Yieldb |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 5a (0.2 mmol), catalyst (5 mol%), additive 1 (1.0 equiv.), and additive 2 (2.0 equiv.) in solvent (1.0 mL) at 110 °C for 4 h under N2. b Isolated yield. c MeOH instead of 1,2-DCE; NR = no reaction. | ||
| 1 | None | 60 |
| 2 | AgSbF6/AgOTf/AgNTf2/KPF6/NaOAcc Instead of Zn(OAc)2 | 45/45/41/30/trace |
| 3 | Toluene/DCM/ACN instead of 1,2-DCE | 41/37/22 |
| 4 | 80 °C/130 °C instead of 110 °C | 41/45 |
| 5 | Air instead of N2 gas | Trace |
| 6 | No [RhCp*Cl2]2 | NR |
| 7 | No Zn(OAc)2 | NR |
| 8 | No AcOH | 30 |
After establishing the optimal conditions, next we studied the generality of this methodology, and the results are summarized in Scheme 4. We initially reacted 2-phenyl-1H-perimidine (1a) with vinylene carbonate (5a) under the optimal conditions, yielding product 6a in 60%. Subsequent reactions with para-substituted perimidines (1a–1f) generally gave good yields, except for the 4-fluoro derivative (1e), which produced 6e in only 42% yield under the standard conditions. We then treated naphthyl-1H-perimidine (1i) with vinylene carbonate (5a), yielding 50% of the [4 + 2] cyclized product 6g. The substituent at the ortho position of 2-phenyl-1H-perimidine provided 6h in a moderate yield of 50%, whereas the meta substituted perimidine resulted in the selective activation of the less hindered C–H bond, furnishing a 54% yield of 6i.15,17 Next, we treated the heteroaromatic 2-(thiophen-2-yl)-1H-perimidine 1n. The reaction proceeded to furnish the [4 + 2] annulated product 6j in 6 h.
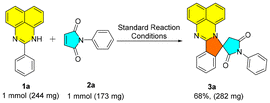
To demonstrate scalability, the reaction was performed on a 1 mmol scale with perimidine (1a) and N-phenylmaleimide (2a), providing 68% yield of compound 3a under standard conditions as shown in Scheme 5.
Subsequently, to illustrate late-stage modifications, an additional reaction was carried out using the reported method as shown in Scheme 6.22 We conducted a ring-opening reaction of spiro-succinimide 3a with NaOMe, followed by acid work-up yielding product 7 in 85% yield.
To understand the mechanism, we conducted a few control experiments (Scheme 7). Reacting 1e and 1f (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with N-phenyl maleimide 2a under standard conditions (eqn (a), Scheme 7) yielded 3e and 3f in a 1
1) with N-phenyl maleimide 2a under standard conditions (eqn (a), Scheme 7) yielded 3e and 3f in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 ratio, suggesting an electrophilic cyclo-rhodation. The deuterium exchange with perimidine 1f under the optimum reaction conditions for [4 + 1] and [4 + 2] cyclizations resulted in 25% and 78% deuteration, respectively, at both the ortho positions, suggesting a reversible C–H activation (eqn (b), Scheme 7).23,24 The radical trapping experiment with 2 equivalents of BHT under standard reaction conditions proceeded without any significant abatement of yield, i.e., 72% and 48%, respectively, ruling out the probability of any radical being involved in the reaction pathway (eqn (c), Scheme 7). The competitive kinetic isotope effect studies afforded kH/kD values of 2.70 and 5.25, respectively, in both cases. This delineates that C–H bond breaking is involved in the rate-determining step (eqn (d) and (e), Scheme 7).
1.3 ratio, suggesting an electrophilic cyclo-rhodation. The deuterium exchange with perimidine 1f under the optimum reaction conditions for [4 + 1] and [4 + 2] cyclizations resulted in 25% and 78% deuteration, respectively, at both the ortho positions, suggesting a reversible C–H activation (eqn (b), Scheme 7).23,24 The radical trapping experiment with 2 equivalents of BHT under standard reaction conditions proceeded without any significant abatement of yield, i.e., 72% and 48%, respectively, ruling out the probability of any radical being involved in the reaction pathway (eqn (c), Scheme 7). The competitive kinetic isotope effect studies afforded kH/kD values of 2.70 and 5.25, respectively, in both cases. This delineates that C–H bond breaking is involved in the rate-determining step (eqn (d) and (e), Scheme 7).
On the basis of our control experiments and previously reported studies25–32 and the obtained mass spectra, we have proposed a plausible mechanism accounting for the cascade annulations (Schemes 8 and 9). Initially, the [RhCp*Cl2]2 is activated upon reaction with NaOAc, resulting in the monomeric active catalyst [RhCp*(OAc)2] A, which undergoes reversible C–H activation with 2-aryl-1H-perimidine, leading to the formation of intermediate B. The maleimide 2 then coordinates with B, yielding complex C, which, upon migratory insertion, forms the seven-membered rhodacycle D. A β-hydride elimination generates the alkenylated intermediate E along with regeneration of the active catalyst A. Intermediate E then undergoes a rapid aza-Michael addition to form the spiro-annulated product 3/4.
In the catalytic cycle with vinylene carbonate (Scheme 9), [RhCp*Cl2]2 is activated by Zn(OAc)2 to form monomeric Rh(III) complex A followed by C–H activation with 2-aryl-1H-perimidine to form rhodacycle B′. Coordination with vinylene carbonate 5a forms complex C′, which undergoes migratory insertion to yield the seven-membered intermediate D′. A β-oxygen elimination and decarboxylation from D′ generates the eight-membered ring E′. The intermediate E′ undergoes protonation with AcOH, producing intermediate F′ and regenerates the active catalyst A. Intermediate F′ undergoes intramolecular cyclization and dehydration to afford the [4 + 2] cyclized product 6.
Conclusions
In conclusion, we have developed an operationally simple and efficient strategy involving cascade C–H activation of 1-aryl-1H-perimidines for the synthesis of perimidine-linked spiro-succinimides and isoquinolines. The use of various alkenes, including maleimides, naphthoquinones, ethyl acrylate, and acrylonitrile, predominantly led to the [4 + 1] annulated products via selective C–H activation. In contrast, vinylene carbonates exhibited divergent reactivity, furnishing [4 + 2] annulated products. This methodology demonstrates high regioselectivity, broad functional group tolerance, moderate to excellent yields, and scalability, thus offering a valuable approach for the synthesis of structurally diverse heterocycles.Author contributions
Vidya Kumari: concept building, performing experiments, data acquisition, data analysis and manuscript writing. Lokman H. Choudhury: concept building, supervision, fund acquisition and manuscript writing.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included in the manuscript and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5qo01292a.CCDC 2279344 and 2495811 contain the supplementary crystallographic data for this paper.33a,b
Acknowledgements
L. H. C. gratefully acknowledges the financial support from the ANRF, Government of India (Sanction No. CRG/2021/003716). The authors also express their sincere gratitude to IIT Patna for providing essential research facilities, and to SAIF IIT Patna, IIT (ISM) Dhanbad and BHU, Varanasi, for NMR, HRMS and SC-XRD analyses. Special thanks are extended to Mr Kamlesh Satpute (IIT Ropar) for his assistance with single-crystal X-ray diffraction analysis.References
- (a) G. Meng, N. Y. S. Lam, E. L. Lucas, T. G. Saint-Denis, P. Verma, N. Chekshin and J. Q. Yu, Achieving Site-Selectivity for C–H Activation Processes Based on Distance and Geometry: A Carpenter's Approach, J. Am. Chem. Soc., 2020, 142, 10571–10591 CrossRef CAS; (b) L. Zhang and T. Ritter, A Perspective on Late-Stage Aromatic C–H Bond Functionalization, J. Am. Chem. Soc., 2022, 144, 2399–2414 CrossRef CAS PubMed; (c) C. M. Josephitis, H. M. Nguyen and A. McNally, Late-Stage C–H Functionalization of Azines, Chem. Rev., 2023, 123, 7655–7691 CrossRef CAS PubMed; (d) Y. Wang, S. Dana, H. Long, Y. Xu, Y. Li, N. Kaplaneris and L. Ackermann, Electrochemical Late-Stage Functionalization, Chem. Rev., 2023, 123, 11269–11335 CrossRef CAS.
- N. Sahiba and S. Agarwal, Recent Advances in the Synthesis of Perimidines and Their Applications, Top. Curr. Chem., 2020, 378, 44 CrossRef CAS.
- M. Azam, I. Warad, S. I. Al-Resayes, N. Alzaqri, M. R. Khan, R. Pallepogu, S. Dwivedi, J. Musarrat and M. Shakir, Synthesis and Structural Characterization of Pd(II) Complexes Derived from Perimidine Ligand and Their in vitro Antimicrobial Studies, J. Mol. Struct., 2013, 1047, 48–54 CrossRef CAS.
- T. A. Farghaly and H. K. Mahmoud, Site–and Regioselectivity of the Reaction of Hydrazonoyl Chlorides with Perimidine Ketene Aminal. Antimicrobial Evaluation of the Products, J. Heterocycl. Chem., 2015, 52, 86–91 CrossRef CAS.
- H. A. Eldeab and A. F. A. Eweas, A Greener Approach Synthesis and Docking Studies of Perimidine Derivatives as Potential Anticancer Agents, J. Heterocycl. Chem., 2018, 55, 431–439 CrossRef CAS.
- T. A. Farghaly, E. M. Abbas, K. M. Dawood and T. B. El-Naggar, Synthesis of 2-phenylazonaphtho [1,8-ef,][1,4] diazepines and 9-(3-arylhydrazono)pyrrolo[1,2-a] Perimidines as Antitumor Agents, Molecules, 2014, 19, 740–755 CrossRef PubMed.
- F. A. Bassyouni, S. M. Abu-Bakr, K. H. Hegab, W. El-Eraky, A. A. El Beih and M. E. A. Rehim, Synthesis of New Transition Metal Complexes of 1H-perimidine Derivatives Having Antimicrobial and Anti-Inflammatory Activities, Res. Chem. Intermed., 2012, 38, 1527–1550 CrossRef CAS.
- N. Chakraborty, S. Banik, A. Chakraborty, S. K. Bhattacharya and S. Das, Synthesis of a Novel Pyrene Derived Perimidine and Exploration of its Aggregation Induced Emission, Aqueous Copper Ion Sensing, Effective Antioxidant and BSA Interaction Properties, J. Photochem. Photobiol., A, 2019, 377, 236–246 CrossRef CAS.
- P. Kalle, M. A. Kiseleva, S. V. Tatarin, D. E. Smirnov, A. Y. Zakharov, V. V. Emets, A. V. Churakov and S. I. Bezzubov, A Panchromatic Cyclometalated Iridium Dye Based on 2-Thienyl-perimidine, Molecules, 2022, 27, 3201 CrossRef CAS PubMed.
- S. Bag, K. Surya, A. Mondal, R. Jayarajan, U. Dutta, S. Porey, R. B. Sunoj and D. Maiti, Palladium-Catalyzed meta-C–H Allylation of Arenes: A Unique Combination of a Pyrimidine-Based Template and Hexafluoroisopropanol, J. Am. Chem. Soc., 2020, 142, 12453–12466 CrossRef CAS.
- (a) J. A. Leitch, C. L. McMullin, A. J. Paterson, M. F. Mahon, Y. Bhonoah and C. G. Frost, Ruthenium–Catalyzed para–Selective C–H Alkylation of Aniline Derivatives, Angew. Chem., Int. Ed., 2017, 56, 15131–15135 CrossRef CAS PubMed; (b) S. Bag, R. Jayarajan, U. Dutta, R. Chowdhury, R. Mondal and D. Maiti, Remote meta–C–H Cyanation of Arenes Enabled by a Pyrimidine–Based Auxiliary, Angew. Chem., Int. Ed., 2017, 56, 12538–12542 CrossRef CAS PubMed; (c) T. K. Achar, X. Zhang, R. Mondal, M. S. Shanavas, S. Maiti, S. Maity, N. Pal, R. S. Paton and D. Maiti, Palladium–Catalyzed Directed meta–Selective C–H Allylation of Arenes: Unactivated Internal Olefins as Allyl Surrogates, Angew. Chem., 2019, 131, 10461–10468 CrossRef; (d) S. M. Khake and N. Chatani, The Direct Rh(III)-Catalyzed C–H Amidation of Aniline Derivatives Using a Pyrimidine Directing Group: The Selective Solvent Controlled Synthesis of 1,2-Diaminobenzenes and Benzimidazoles, Org. Lett., 2020, 22, 3655–3660 CrossRef CAS.
- S. M. Khake and N. Chatani, Rhodium(III)-Catalyzed Oxidative C–H Alkylation of Aniline Derivatives with Allylic Alcohols to Produce β-Aryl Ketones, ACS Catal., 2022, 12, 4394–4401 CrossRef CAS.
- S. Bag, S. Jana, S. Pradhan, S. Bhowmick, N. Goswami, S. K. Sinha and D. Maiti, Imine as a Linchpin Approach for meta-C–H Functionalization, Nat. Commun., 2021, 12, 1393 CrossRef CAS PubMed.
- (a) H. Li, Y. Zeng, F. Liang, Y. Yang, K. Li, F. Pang and S. Li, Programmable Synthesis of Cationic Azaperylenes via Rh(III)-Catalyzed Multiple C–H/N–H Bonds Activation and Annulation, Org. Lett., 2024, 26, 11179–11183 CrossRef CAS; (b) Y. Zeng, H. Li, F. Liang and S. Li, Nitrogen Source-Tailored Multicomponent C–H Di/Triannulation of α-Oxocarboxylic Acids and Alkynes to Diverse Cationic N-Doped PAHs, ACS Catal., 2025, 15, 12094–12102 CrossRef CAS.
- B. Hu, G. Chen, J. Zhao, L. Xue, Y. Jiang, X. Zhang and X. Fan, Synthesis of Succinimide Spiro-Fused Sultams from the Reaction of N-(Phenylsulfonyl)acetamides with Maleimides via C (sp2)–H Activation, J. Org. Chem., 2021, 86, 10330–10342 CrossRef CAS.
- N. Li, B. Hu, X. Zhang and X. Fan, Selective Construction of Spiro or Fused Heterocyclic Scaffolds via One-Pot Cascade Reactions of 1-Arylpyrazolidinones with Maleimides, J. Org. Chem., 2023, 88, 60–74 CrossRef CAS PubMed.
- W. Y. Pu, X. Y. Chen and L. Dong, Rh(III)-Catalyzed [5 + 1] Spirocyclization to Produce Novel Benzimidazole-incorporated Spirosuccinimides, Green Synth. Catal., 2023, 4, 338–341 CAS.
- Q. Zhou, B. Li, X. Zhang and X. Fan, C–H Activation-Initiated Spiroannulation Reactions and Their Applications in the Synthesis of Spirocyclic Compounds, Org. Biomol. Chem., 2024, 22, 2324–2338 RSC.
- R. N. Patel, D. M. Patel, D. G. Thakur, N. B. Rathod, S. D. Patel, M. A. Sonawane and S. C. Ghosh, Synthesis of Benzo [d,e] Quinoline-Spiro-Succinimides via Rhodium-Catalyzed C–H Activation/Annulation of 1-Naphthylamides with Maleimides, Org. Biomol. Chem., 2025, 23, 6897–6902 RSC.
- C. Yang, B. Li, P. Shi, H. Xu, X. Zhang and X. Fan, Synthesis of Benzoisochromene Derivatives via C–H Activation-Initiated Cascade Formal [4 + 2] and [2 + 4] Annulation of Aryl Enaminone with Vinyl-1,3-dioxolan-2-one, Org. Lett., 2025, 27, 2964–2969 CrossRef CAS.
- (a) D. Ali, N. Mondal and L. H. Choudhury, I2/PIDA Mediated Regioselective Metal-Free Synthesis of Aryl Selenoether-Linked Trisubstituted Thiazoles, J. Org. Chem., 2025, 90, 8903–8916 CrossRef CAS; (b) V. Kumari, S. S. Acharya, N. Mondal and L. H. Choudhury, Maleimide-Dependent Rh(III)-Catalyzed Site-Selective Mono and Dual C–H Functionalization of 2-Arylbenzo[d]thiazole and Oxazole Derivatives, J. Org. Chem., 2024, 89, 18003–18018 CrossRef CAS PubMed.
- A. K. Gola, A. Dubey and S. K. Pandey, Mn(I)-Catalyzed Site-Selective C–H Activation: Unlocking Access to 3-Arylated Succinimides from 2-Arylpyridines and Maleimides, J. Org. Chem., 2024, 89, 15020–15025 CrossRef CAS PubMed.
- D. H. Dethe, V. Kumar and R. Das, Ru(II)-Catalyzed C–H Activation/[4 + 2] Annulation of Sulfoxonium Ylide with Maleimide: Access to Fused Benzo[e]isoindole-1,3,5-trione, Org. Lett., 2024, 26, 6830–6834 CrossRef CAS PubMed.
- (a) A. Mandal, S. Dana, H. Sahoo, G. S. Grandhi and M. Baidya, Ruthenium(II)-catalyzed ortho-C–H Chalcogenation of Benzoic Acids via Weak O-Coordination: Synthesis of Chalcogenoxanthones, Org. Lett., 2017, 19, 2430–2433 CrossRef CAS; (b) Z. J. Zhang, M. M. Simon, S. Yu, S. W. Li, X. Chen, S. Cattani, X. Hong and L. Ackermann, Nickel-Catalyzed Atroposelective C–H Alkylation Enabled by Bimetallic Catalysis with Air-Stable Heteroatom-Substituted Secondary Phosphine Oxide Preligands, J. Am. Chem. Soc., 2024, 146, 9172–9180 CrossRef CAS PubMed; (c) S. Devkota, H. J. Lee, S. H. Kim and Y. R. Lee, Direct Construction of Diverse Polyheterocycles Bearing Pyrrolidinediones via Rh(III)–Catalyzed Cascade C–H Activation/Spirocyclization, Adv. Synth. Catal., 2019, 361, 5587–5595 CrossRef CAS.
- M. Aslam, S. Mohandoss and Y. R. Lee, Chemoselective Installation of Diverse Succinimides on Fused Benzimidazoles via Rhodium-Catalyzed C–H Activation/Annulation: Chemosensor for Heavy Metals, Org. Lett., 2021, 23, 6206–6211 CrossRef CAS.
- K. Seal and B. Banerji, Ru(II) Catalyzed Oxidative Dehydrogenative Annulation and Spirocyclization of Isoquinolones with N–Substituted Maleimides, Adv. Synth. Catal., 2024, 366, 1788–1808 CrossRef CAS.
- J. Y. Kang, W. An, S. Kim, N. Y. Kwon, T. Jeong, P. Ghosh, H. S. Kim, N. K. Mishra and I. S. Kim, Synthesis of Spirosuccinimides via Annulative Cyclization Between N-Aryl Indazolols and Maleimides Under Rhodium(III) Catalysis, Chem. Commun., 2021, 57, 10947–10950 RSC.
- K. Ghosh, Y. Nishii and M. Miura, Rhodium-Catalyzed Annulative Coupling Using Vinylene Carbonate as an Oxidizing Acetylene Surrogate, ACS Catal., 2019, 9, 11455–11460 CrossRef CAS.
- L. Wang, K. C. Jiang, N. Zhang and Z. H. Zhang, Rhodium–Catalyzed Synthesis of Isoquinolino [1,2–b] Quinazolines via C– H Annulation in Biomass–Derived γ–Valerolactone, Asian J. Org. Chem., 2021, 10, 1671–1674 CrossRef CAS.
- M. Kato, K. Ghosh, Y. Nishii and M. Miura, Rhodium-Catalysed Direct Formylmethylation using Vinylene Carbonate and Sequential Dehydrogenative Esterification, Chem. Commun., 2021, 57, 8280–8283 RSC.
- Z. H. Wang, H. Wang, H. Wang, L. Li and M. D. Zhou, Ruthenium(II)-Catalyzed C–C/C–N Coupling of 2-arylquinazolinones with Vinylene Carbonate: Access to Fused Quinazolinones, Org. Lett., 2021, 23, 995–999 CrossRef CAS PubMed.
- G. Huang, J. T. Yu and C. Pan, Rhodium–Catalyzed C–H Activation/Annulation of N–Aryl–pyrazolidinones with Vinylene Carbonate, Eur. J. Org. Chem., 2022, e202200279 CrossRef CAS.
- (a) CCDC 2279344: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ghv72; (b) CCDC 2495811: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ps31n.
| This journal is © the Partner Organisations 2026 |