Open Access Article
Open Access ArticleHomoleptic biisoquinolinedioxide lanthanide complexes for near-infrared circularly polarized luminescence
Douglas Kariuki Mundia a,
Ashley Schmidt
a,
Ashley Schmidt b,
Jerome R. Robinson
b,
Jerome R. Robinson c,
Nathan D. Schley
c,
Nathan D. Schley d and
Gaël Ung
d and
Gaël Ung *a
*a
aDepartment of Chemistry, University of Connecticut, Storrs, Connecticut 06269, USA. E-mail: gael.ung@uconn.edu
bBrucker AXS LLC, Madison, Wisconsin 53711-5373, USA
cDepartment of Chemistry, Brown University, Providence, RI 02912, USA
dDepartment of Chemistry, Vanderbilt University, Nashville, Tennessee 37235, USA
First published on 15th December 2025
Abstract
Near-infrared circularly polarized luminescence (CPL) emitters are of growing interest due to their potential applications in optical telecommunications, bioimaging, and secure information technologies. We report the synthesis and chiroptical properties of lanthanide complexes supported by axially chiral 1,1′-biisoquinoline-N,N′-dioxide ligands. 8-Coordinate homoleptic complexes composed of a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand
1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal stoichiometry were obtained. Emissions in the near-infrared I and II windows (900–1700 nm) were observed for both ytterbium and erbium complexes. Although modest quantum yields (6.2% and 3.1%, respectively), and moderate circularly polarized luminescence emission metrics (glum = ±0.07 and ±0.12, respectively and BCPL = 16.1 M−1 cm−1 and 3.1 M−1 cm−1, respectively) were obtained, the polarization information contained in the CPL spectra combined with a solid-state structure allowed us to assign the symmetry of the complex in solution.
metal stoichiometry were obtained. Emissions in the near-infrared I and II windows (900–1700 nm) were observed for both ytterbium and erbium complexes. Although modest quantum yields (6.2% and 3.1%, respectively), and moderate circularly polarized luminescence emission metrics (glum = ±0.07 and ±0.12, respectively and BCPL = 16.1 M−1 cm−1 and 3.1 M−1 cm−1, respectively) were obtained, the polarization information contained in the CPL spectra combined with a solid-state structure allowed us to assign the symmetry of the complex in solution.
Introduction
The emission of circularly polarized light has garnered recent attention due to promising potential applications in 3D display,1 encryption,2 sensing,3 and telecommunication technologies.4 The generation of circularly polarized light is straightforward but energy inefficient through optical filtration. The development of direct circularly polarized luminescence (CPL) emitters is thus key to unlock practical applications.The efficiency of CPL emitters can be quantified by two figures of merit: a dissymmetry factor, glum, that indicates the degree of polarization of light; and a brightness factor, BCPL, that adds information on the light emitting capabilities. The former can be calculated from experimental data using the following equation: glum = ½ × (IL − IR)/(IL + IR) (where IL and IR are the intensities of the left and right circularly polarized light emitted, respectively); while the latter is calculated through the formula: BCPL = ε × Φ × glum (where ε is the molar absorptivity and Φ is the quantum yield of the species of interest).5 Whilst many types of CPL emitters have recently emerged,6 lanthanide-based CPL emitters have been the most consistent at delivering high metrics.7
To date, in lanthanide emitters, high metrics seem to correlate with high symmetry systems: for example, the highest dissymmetry factor (−1.54) at 594 nm from a C4 symmetrical tetrakis(camphorate) europium(III) complex,8 and the highest brightness, BCPL (3760 M−1 cm−1) at 545 nm from a D3 symmetrical tris(spinolate) terbium complex.9 Other high symmetry complexes also exhibit a combination of high metrics.7 Notably, a D4 symmetrical dilanthanide complex recently featured high metrics for near-infrared (NIR) CPL.10 However, in this example, the local C4 symmetry at the metal centre makes it difficult to discern which symmetry element (local at the metal vs. extended for the complex) is responsible for the high metrics. We were interested in investigating this symmetry effect and thus targeted a monometallic D4 symmetrical species.
Aiming to achieve high dissymmetry factors, we took inspiration from the studies employing axially chiral ligands for CPL.11–14 Additionally, we were motivated by the work utilizing N-oxide ligands for high NIR quantum yields to obtain higher brightness.15 Owing to the advantages offered by NIR light (reduced scattering, deeper tissue penetration, use in telecommunication) and the scarcity of NIR CPL emitters,7c we targeted the axially chiral 1,1′-biisoquinoline-N,N′-dioxide ligand that we hypothesized could yield an 8-coordinate D4 symmetrical complex16 emitting in the NIR region.
We show herein the coordination of axially chiral 1,1′-biisoquinoline-N,N′-dioxide to lanthanide ions and the (chir)optical properties of the corresponding complexes. We used a combination of solution and solid-state analyses to conclude on the symmetry of the complexes obtained and correlate the chiroptical metrics with structure.
Synthesis and characterization
The targeted 1,1′-biisoquinoline-N,N′-dioxide (BIQNO) ligand was synthesized from a route adapted from previous reported procedures.17–19 In summary, racemic BIQNO was obtained by a base-promoted homocoupling of isoquinoline, followed by oxidation using meta-chloroperbenzoic acid. Separation of the enantiomers was achieved by generating diastereomeric hydrogen-bonded complexes using enantiopure 1,1′-bi-2-naphthol (Binol). Preferential crystallization of the S-BIQNO/R-Binol adduct followed by removal of R-Binol on a silica column yielded S-BIQNO in high enantiopurity (99.4% ee). The enantiomer R-BIQNO was isolated using a similar procedure using S-Binol (see SI for details).Coordination to the lanthanides was achieved by reacting 4.05 equivalents of R-BIQNO with a lanthanide trifluoromethanesulfonate salt in tetrahydrofuran (THF) (Fig. 1). The complexes were purified by washing off the slight excess of free ligand with cold THF followed by recrystallization in acetonitrile. Complexes of Y, Yb, and Er, were obtained in high yields (91–95%). Purity and stoichiometry were confirmed by combustion analyses (see SI).
 | ||
| Fig. 1 Synthesis of [Ln(R-BIQNO)4][OTf]3 (Ln = Y, Er, Yb). The synthesis of the enantiomeric [Ln(S-BIQNO)4][OTf]3 is performed the same way using S-BIQNO. | ||
In the 1H NMR spectrum of the non-emissive but diamagnetic yttrium complex [Y(R-BIQNO)4][OTf]3, a single set of six resonances were observed, consistent with either D4- and D2-symmetry in solution. Those resonances are significantly shifted from the free ligand (Fig. 2), confirming binding to yttrium. As expected, the 1H NMR spectrum of the ytterbium and erbium complexes display paramagnetically shifted resonances, ranging from 12.7 to −22.3 ppm and 19.4 to −59.7 ppm, respectively (see Fig. S5 and S8).
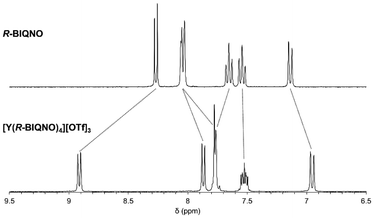 | ||
| Fig. 2 Aromatic region of the 1H NMR spectra of R-BIQNO and [Y(R-BIQNO)4][OTf]3 in CD3CN. See SI for full spectra. | ||
Optical and chiroptical properties
The absorption spectrum of the complex displayed a broad band from 300 to 400 nm attributed to a π–π* transition (Fig. 3, dashed blue). The circular dichroism (CD) spectrum of the Yb and Er complexes featured a unique band at 365 nm with a gabs ∼ 0.001, contrasting with the CD spectrum of the free ligand exhibiting two bands at 327 and 377 nm (Fig. S27–S29). The excitation maximum, recorded for the Yb emission at 980 nm (Fig. 3, dotted black), was red-shifted compared to the maximum absorbance, consistent with a sensitization mechanism.20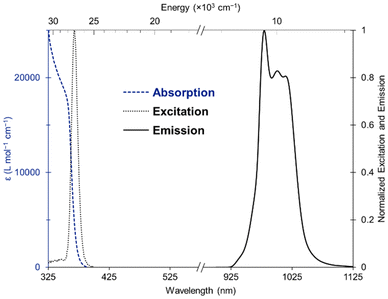 | ||
| Fig. 3 Absorption (blue dashed), excitation (black dotted), emission (black solid) spectra of [Yb(R-BIQNO)4][OTf]3 (160 µM) in 1,2-difluorobenzene. | ||
Upon excitation at 365 nm, the complex [Yb(R-BIQNO)4][OTf]3 displayed a luminescence response with a maximum emission peak at 978 nm characteristic of a 2F5/2 → 2F7/2 transition (Fig. 3, solid black). Several features due to crystal field splitting are observed, although the signal is relatively narrow compared to other emitters (FWHM = 61.5 nm/∼610 cm−1) indicating relatively weak crystal field strength from the ligands. The complex [Er(R-BIQNO)4][OTf]3 is also emissive, displaying a typical signal centered at 1535 nm attributed to the 4I13/2 → 4I15/2 transition (Fig. S21). Several shoulders arising from crystal field splitting can be observed, albeit with low resolution. The quantum yields were 6.2% and 3.1% for the ytterbium and erbium complexes, respectively.
The singlet and triplet energy levels were determined by synthesizing the analogous gadolinium [Gd(R-BIQNO)4][OTf]3 complex. The singlet and triplet levels at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 and 18
970 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cm−1, respectively, are relatively higher than the emissive levels of both ytterbium and erbium, which is consistent with the low quantum yields observed.
300 cm−1, respectively, are relatively higher than the emissive levels of both ytterbium and erbium, which is consistent with the low quantum yields observed.
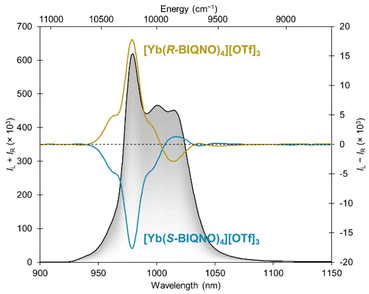
Circularly polarized luminescence spectra of [Yb(R/S-BIQNO)4][OTf]3 were collected in dilute solutions in 1,2-difluorobenzene, a solvent that we found more adapted to the long exposure times required to acquire CPL data. Fairly well resolved features are seen in the spectra, including a sign inversion at a hot band (attributed to the 2′ → 0 transition) around 925 nm and a multisignate pattern between 1000 and 1075 nm (Fig. 4). We note that the 1′ → 0 transition and 0′ → 0 transition have the same sign, which is unusual compared to other well resolved CPL spectra of high symmetry (C3, D3, C4), which all show an inversion of sign between those transitions. The maximum dissymmetry factor of ±0.07 is obtained at 959 nm (Fig. S25), a significantly lower value than other systems (Table 1 and Table S3), as a result, the CPL brightness BCPL = 16.1 M−1 cm−1 is also relatively low.
| Complex | Symmetry | ε (M−1 cm−1) | Φ (%) | τ (μs) | glumb (nm) | BCPL (M−1 cm−1) | LnBCPL (M−1 cm−1) |
|---|---|---|---|---|---|---|---|
| a See Table S3 for a complete overview.b Values reported in the literature, head-to-head comparisons may not be perfectly accurate due to bandpass differences.11c,21c C4 at metal. | |||||||
[Yb(Binol)3][Na]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11b 11b |
D3 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17 | — | ±0.17 (975) | 379 | 160 |
[Yb(Sphenol)3][Na]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11e 11e |
D3 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
7.5 | 11.6 | ±0.22 (973) | 146.3 | 66.0 |
| [Yb(hfbc)4][Cs]13a | C4 | — | — | — | ±0.38 (988) | — | — |
| [Yb(iPrPyBox)(TTA)3]21 | C1 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.69 | — | ±0.029 (972) | 5.2 | — |
| [Yb(pydac)(Binol)2][Na]14 | C2 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 2.49 | ±0.01 (980) | — | — |
| 2.06 | |||||||
| [Yb2(BTHP)4]10 | D4c | — | 6.5 | 14 | ±0.81 (980) | 821 | — |
| [Yb(BIQNO)4][OTf]3 | D2 | 7474 | 6.2 | 35.7 | ±0.07 (959) | 16.1 | 12.6 |
[Er(Binol)3][Na]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11b 11b |
D3 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.58 | — | ±0.47 (1540) | 57.3 | 22.1 |
[Er(Sphenol)3][Na]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11e 11e |
D3 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
0.28 | 2.8 | ±0.77 (1540) | 20.7 | 16.5 |
[Er(F12-Binol)3][K]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11d 11d |
D3 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.5 | 10 | ±0.17 (1526) | 184 | 33.8 |
| [Er(hfbc)4][Cs]13b | C4 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
0.07 | 6.6 | +0.83 (1510) | 0.23 | — |
[Er(pybam)3][OTf]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
D3 | 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 0.21 | ±0.66 (1519) | — | — |
[Er(pybox)2][OTf]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
C2 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.04 | 4 | ±0.33 (1539) | 0.7 | — |
| [Er(BIQNO)4][OTf]3 | D2 | 1689 | 3.1 | — | ±0.12 (1535) | 3.1 | 1.3 |
The [Er(R/S-BIQNO)4][OTf]3 complexes also displayed circularly polarized luminescence (Fig. 5). A trisignate pattern was observed with a maximum glum of ±0.12 at 1535 nm (Fig. S26). As in the case of the ytterbium complexes, these dissymmetry factors represent relatively small polarization compared to other emitters (Tables 1 and S3). Relatively low brightness were also obtained (BCPL = 3.1 M−1 cm−1).
Solid-state studies
Single crystals were grown from vapor diffusion of ether into a solution of [Ln(R-BIQNO)4][OTf]3 in acetonitrile. Large clear blocks were obtained, which, despite their apparent quality, diffracted poorly on standard instruments. Using a 2nd generation microfocus source, diffraction patterns at lower angles with brighter spots were obtained. The structure was solved in the non-centrosymmetric P212121 space group with an unusually large unit cell (14.0 × 25.5 × 44.7 Å). The structures for [Yb(R-BIQNO)4][OTf]3 and [Yb(S-BIQNO)4][OTf]3 were clearly modulated showing satellite reflections in the diffraction pattern. As a result, the solution given from an average structure displayed smeared ellipsoids, and difficulties in locating the electron density of all of the trifluoromethanesulfonate counterions were encountered. The structure of [Er(S-BIQNO)4][OTf]3 could be solved as an average structure, although signs of minor modulation were observed resulting in some elongated ellipsoids. One of the two complexes in the asymmetric unit displayed ellipsoids that we deemed satisfactory to provide reliable bond metrics and angles (Fig. 6).An 8-coordinate geometry is obtained, intermediate between square antiprism and triangular dodecahedron. The ligands are arranged around the metal in a way that shows only C2 axes, confirming the D2 symmetry of the complex. The average M–O bond distance (2.34 Å) is typical for neutral ligands’ Ln–Oneut bonds but longer than those of anionic ligands (Ln–Oan ∼ 2.24 Å). This is consistent with the weaker crystal field splitting observed in luminescence, indicative of the weaker binding of the neutral N-oxide ligand. Due to the structural similarities between the BIQNO and Binol ligands, we were interested in comparing some of the structural features of the corresponding complexes. The average bite angle O–M–O (72.9°) is smaller compared to that of Binol (81.07°) consistent with both the longer Ln–O bond distances and a more sterically demanding 8-coordinate geometry. A wider torsion angle between the two aryl planes of the BIQNO ligand (67.5°) was also observed relative to that of Binol (62.4°).11b
Discussion
Understanding the speciation in solution is key to providing accurate structure/CPL activity relationships that would inform the design of more efficient emitters. Whilst NMR spectroscopy and standard optical spectroscopies were insufficient to determine the symmetry of the [Ln(R/S-BIQNO)4][OTf]3 complexes in solution (either D4 or D2), we leveraged the added resolution provided by CPL spectroscopy to help assign the speciation. The added polarization information (given by the sign of CPL signal) allows for better deconvolution of the transitions between crystal field splitting states (Stark levels). The selection rules of those transitions may thus differ with symmetry (yielding a possible sign change). Examining and comparing the signs of transitions between crystal field splitting states should thus provide clues about the symmetry of the complex.As mentioned above, the sign of the hot bands in the [Yb(R/S-BIQNO)4][OTf]3 complexes are inconsistent with the data from other high symmetry complexes. Additionally, the sign and CPL pattern for the [Er(R/S-BIQNO)4][OTf]3 complexes are reminiscent of a low symmetry C2 complex.23 Lastly, the dissymmetry factors are lower than those of high symmetry complexes. Overall, these results suggest that the D2 symmetry for the [Ln(R/S-BIQNO)4][OTf]3 complexes is retained in solution, which correlates with the solid-state structure.
We recognize that our comparisons are made with the only few examples available in the literature, which highlights the need for the community to explore more complexes with well-defined symmetries and high-resolution CPL data.
Conclusions
We have successfully synthesized homoleptic lanthanide complexes supported by four enantiopure 1,1′-biisoquinoline-N,N′-dioxide ligands. Whilst we originally hypothesized the ligands to be suitable to generate D4 symmetrical complexes, a combination of single crystal XRD analysis, NMR spectroscopy, combustion analyses, and chiroptical spectroscopy allowed us to determine that the complexes were, in fact, D2 symmetrical. The added resolution provided by CPL spectroscopy was key to assign its symmetry in solution. Whilst the overall CPL metrics are modest, this study provides additional data necessary for a complete understanding of the factors necessary to obtain higher CPL metrics.Author contributions
DKM: investigation: synthesis, spectroscopy – data curation – writing: original draft; AS: investigation: X-ray diffraction, JRR: investigation: X-ray diffraction; NDS: investigation: X-ray diffraction; GU: conceptualization – funding acquisition – writing: review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, spectra. See DOI: https://doi.org/10.1039/d5qi02406d.CCDC 2503942 contains the supplementary crystallographic data for this paper.24
Acknowledgements
We thank the University of Connecticut for partial support of this work. This material is based upon work supported by the National Science Foundation under grant no. CHE-2041084.References
- M. Schadt, Liquid crystal materials and liquid crystal displays, Annu. Rev. Mater. Sci., 1997, 27, 305–379 CrossRef CAS.
- L. E. MacKenzie and R. Pal, Circularly polarized lanthanide luminescence for advanced security inks, Nat. Rev. Chem., 2021, 5, 109–124 CrossRef CAS PubMed.
- S. Shuvaev, M. A. Fox and D. Parker, Monitoring of the ADP/ATP ratio by induced circularly polarised europium luminescence, Angew. Chem., 2018, 130, 7610–7614 CrossRef.
- X. Li, P. L. Voss, J. E. Sharping and P. Kumar, Optical-fiber source of polarization-entangled photons in the 1550 nm telecom band, Phys. Rev. Lett., 2005, 94, 053601 CrossRef.
- (a) Y. Nagata and T. Mori, Irreverent nature of dissymmetry factor and quantum yield in circularly polarized luminescence of small organic molecules, Front. Chem., 2020, 8, 448 CrossRef CAS; (b) L. Arrico, L. Di Bari and F. Zinna, Quantifying the overall efficiency of circularly polarized emitters, Chem. – Eur. J., 2021, 27, 2920–2934 CrossRef CAS.
- (a) Y. Zhao, J. Xie, Y. Tian, S. Mourdikoudis, N. Fiuza-Maneiro, Y. Du, L. Polavarapu and G. Zheng, Colloidal chiral carbon dots: an emerging system for chiroptical applications, Adv. Sci., 2024, 11, 2305797 Search PubMed; (b) Y. Liu, X. Gao, B. Zhao and J. Deng, Circularly polarized luminescence in quantum dot-based materials, Nanoscale, 2024, 16, 6853–6875 RSC; (c) T. Zhang, Y. Zhang, Z. He, T. Yang, X. Hu, T. Zhu, Y. Zhang, Y. Tang and J. Jiao, Recent advances of chiral isolated and small organic molecules: structure and properties for circularly polarized luminescence, Chem. – Asian J., 2024, 19, e202400049 CrossRef CAS PubMed; (d) J. Chen, Q. Gao, B. Shi, Y. Zhang, T. Wai and Q. Lin, Recent advances in circularly polarized luminescence of planar chiral organic compounds, Chem. Commun., 2024, 60, 6728–6740 RSC; (e) Y. Huang, Y. Zhou, X. Guo, Z. Tong and T. Zhuang, Near-infrared circularly polarized luminescence enabled by chiral inorganic nanomaterials, Nanoscale, 2025, 17, 1922–1931 RSC; (f) X. Zou, N. Gan, Y. Gao, L. Gu and W. Huang, Organic circularly polarized room-temperature phosphorescence: strategies, applications and challenges, Angew. Chem., Int. Ed., 2025, 64, e202417906 CrossRef CAS; (g) X. Wang, W. Yan, D. W. Pang and J. Cai, From synthesis to chiroptical activities: advancements in circularly polarized luminescent inorganic quantum dots, Nanoscale, 2025, 17, 158–186 RSC; (h) C. Maeda and T. Ema, Recent development of azahelicenes showing circularly polarized luminescence, Chem. Commun., 2025, 61, 4757–4773 RSC; (i) V. Kumar, J. L. Páez, S. Míguez-Lago, J. M. Cuerva, C. M. Cruz and A. G. Campaña, Chiral nanographenes exhibiting circularly polarized luminescence, Chem. Soc. Rev., 2025, 54, 4922–4947 Search PubMed; (j) M. Ikeshita and T. Tsuno, Recent advances in circularly polarized luminescence (CPL) of chiral boron difluoride complexes, Phys. Chem. Chem. Phys., 2025, 27, 17116–17129 RSC; (k) P. Zhao, H. Y. Lu and C. F. Chen, Recent advances in preparation and applications of white circularly polarized luminescent materials, Chem. Soc. Rev., 2025, 54, 8534–8554 RSC.
- (a) F. Zinna and L. Di Bari, Lanthanide circularly polarized luminescence: bases and applications, Chirality, 2015, 27, 1–13 Search PubMed; (b) H. Y. Wong, W. S. Lo, K. H. Yim and G. L. Law, Chirality and chiroptics of lanthanide molecular and supramolecular assemblies, Chem, 2019, 5, 3058–3095 CrossRef CAS; (c) O. G. Willis, F. Zinna and L. Di Bari, NIR-circularly polarized luminescence from chiral complexes of lanthanides and d-metals, Angew. Chem., Int. Ed., 2023, 62, e202302358 CrossRef CAS; (d) A. G. Bispo Jr, N. A. Oliveira, I. M. Diogenis and F. A. Sigoli, Perspectives and challenges in circularly polarized luminescence of lanthanide (III) complexes: from solution-based systems to solid-state applications, Coord. Chem. Rev., 2025, 523, 216279 CrossRef CAS.
- M. Tsurui, R. Takizawa, Y. Kitagawa, M. Wang, M. Kobayashi, T. Taketsugu and Y. Hasegawa, Chiral tetrakis Eu(III) complexes with ammonium cations for improved circularly polarized luminescence, Angew. Chem., Int. Ed., 2024, 63, e202405584 CrossRef CAS PubMed.
- B. A. N. Willis, D. Schnable, N. D. Schley and G. Ung, Spinolate lanthanide complexes for high circularly polarized luminescence metrics in the visible and near-infrared, J. Am. Chem. Soc., 2022, 144, 22421–22425 CrossRef CAS PubMed.
- L. Wang, Z. Yao, W. Huang, T. Gao, P. Yan, Y. Zhou and H. Li, Remarkable 980 nm circularly polarized luminescence from dinuclear Yb(III) helicates with a D4 symmetry, Inorg. Chem. Front., 2023, 10, 3664–3674 RSC.
- (a) M. Deng, N. D. Schley and G. Ung, High circularly polarized luminescence brightness from analogues of Shibasaki's lanthanide complexes, Chem. Commun., 2020, 56, 14813–14816 RSC; (b) N. F. Mukthar, N. D. Schley and G. Ung, Strong circularly polarized luminescence at 1550 nm from enantiopure molecular erbium complexes, J. Am. Chem. Soc., 2022, 144, 6148–6153 CrossRef CAS PubMed; (c) J. A. Adewuyi, N. D. Schley and G. Ung, Vanol-supported lanthanide complexes for strong circularly polarized luminescence at 1550 nm, Chem. – Eur. J., 2023, 29, e202300800 CrossRef CAS PubMed; (d) J. A. Adewuyi and G. Ung, High quantum yields from perfluorinated binolate erbium complexes and their circularly polarized luminescence, J. Am. Chem. Soc., 2024, 146, 7097–7104 CrossRef CAS; (e) D. Schnable and G. Ung, Inorg. Chem., 2024, 63, 7378–7385 Search PubMed.
- T. Feng, R. Cai, Z. Zhu, Q. Zhou, A. Sickinger, O. Maury, Y. Guyot, A. Bensalah-Ledoux, S. Guy, B. Baguenard, B. Le Guennic and J. Tang, Enhanced near-infrared circularly polarized luminescence activity in fluorinated binolate ytterbium complexes, Chem. – Eur. J., 2025, 31, e202500910 CrossRef CAS PubMed.
- (a) O. G. Willis, F. Zinna, G. Pescitelli, C. Micheletti and L. Di Bari, Remarkable near-infrared chiroptical properties of chiral Yb, Tm and Er complexes, Dalton Trans., 2022, 51, 518–523 RSC; (b) O. G. Willis, A. Pucci, E. Cavalli, F. Zinna and L. Di Bari, Intense 1400–1600 nm circularly polarised luminescence from homo- and heteroleptic chiral erbium complexes, J. Mater. Chem. C, 2023, 11, 5290–5296 RSC.
- S. Ruggieri, O. G. Willis, S. Mizzoni, E. Cavalli, M. Sanadar, A. Melchior, F. Zinna, L. Di Bari, G. D. Bisag, M. Fochi, L. Bernardi and F. Piccinelli, Near infrared-circularly polarized luminescence/circular dichroism active Yb(III) complexes bearing both central and axial chirality, Inorg. Chem., 2025, 64, 5505–5512 Search PubMed.
- C. Doffek and M. Seitz, The radiative lifetime in near-IR-luminescent ytterbium cryptates: the key to extremely high quantum yields, Angew. Chem., Int. Ed., 2015, 54, 9719–9721 CrossRef CAS PubMed.
- (a) T. Saraidarov, R. Reisfeld and M. Pietraszkiewicz, Luminescent properties of silica and zirconia xerogels doped with europium(III) salts and europium(III) cryptate incorporating 3,3′-biisoquinoline-2,2′-dioxide, Chem. Phys. Lett., 2000, 330, 515–520 Search PubMed; (b) P. Gawryszewska, O. L. Malta, R. L. Longo, F. R. Goncalves e Silva, S. Alves Jr., K. Mierzwicki, Z. Latajka, M. Pietraszkiewicz and J. Legendziewicz, Experimental and theoretical study of the photophysics and structures of europium cryptates incorporating 3,3′-bi-isoquinoline-2,2′-dioxide, ChemPhysChem, 2004, 5, 1577–1584 Search PubMed; (c) J.-M. Lehn, M. Pietraszkiewicz and J. Karpiuk, Synthesis and properties of acyclic and cryptate europium(III) complexes incorporating the 3,3′-biisoquinoline 2,2′-dioxide unit, Helv. Chim. Acta, 1990, 73, 106–111 CrossRef CAS; (d) J.-M. Lehn and C. O. Roth, Synthesis and properties of sodium and europium(III) cryptates incorporating the 2,2′-bipyridine 1,1′-dioxide and 3,3′-biisoquinoline 2,2′-dioxide units, Helv. Chim. Acta, 1991, 74, 572–578 CrossRef CAS; (e) R. O. Freire, M. E. Mesquita, M. A. C. dos Santos and N. B. da Costa Jr., Sparkle model and photophysical studies of europium BiqO2-cryptate, Chem. Phys. Lett., 2007, 442, 488–491 CrossRef CAS; (f) R. Reisfeld, T. Saraidarov, M. Gaft and M. Pietraszkiewicz, Luminescence of cryptate-type Eu3+ complexes incorporated in inorganic and ormocer sol–gel matrices, Opt. Mater., 2007, 29, 521–527 Search PubMed; (g) P. P. Gawryszewska, M. Pietraszkiewicz, J. P. Riehl and J. Legendziewicz, Investigations of the spectral properties of lanthanide(III) complexes with 3,3′-bi-isoquinoline-2,2′-dioxide (biqO2) and a biqO2-cryptate in solution, solids, and gels, J. Alloys Compd., 2000, 300–301, 283–288 CrossRef CAS; (h) M. Pietraszkiewicz, J. Karpiuk and O. Pietraszkiewicz, Luminescent lanthanide complexes with macrocyclic N-oxides, Spectrochim. Acta, Part A, 1998, 54, 2229–2236 CrossRef; (i) P. Gawryszewska, L. Jerzykiewicz, M. Pietraszkiewicz, J. Legendziewicz and J. P. Riehl, Photophysics and crystal structure of a europium(III) cryptate incorporating 3,3′-biisoquinoline-2,2′-dioxide, Inorg. Chem., 2000, 39, 5365–5372 CrossRef CAS; (j) M. Pietraszkiewicz, J. Karpiuk and A. K. Rout, A 3,3′-biisoquinoline-2,2′-dioxide with two dibenzoylmethane side-arms, and its luminescent europium(III) complexes, J. Coord. Chem., 1997, 42, 207–210 CrossRef CAS; (k) M. Pietraszkiewicz, J. Karpiuk, R. Gasiorowski and A. K. Rout, Synthesis and photochemical properties of luminescent Eu(III) complexes with macrocyclic and macropolycyclic ligands incorporating 3,3′-biisoquinoline-2,2-dioxide units, J. Inclusion Phenom. Mol. Recognit. Chem., 1997, 28, 325–334 CrossRef CAS; (l) M. Pietraszkiewicz, J. Karpiuk, R. Gąsiorowski, O. Pietraszkiewicz and A. K. Rout, Luminescent macrocyclic lanthanide complexes bearing N-oxides: potential fluorescent labels for modern medical diagnostics, Acta Phys. Pol., A, 1996, 90, 207–213 CrossRef CAS.
- W.-W. Xie, Y. Liu, R. Yuan, D. Zhao, T.-Z. Yu, J. Zhang and C.-S. Da, Transition metal-free homocoupling of unactivated electron-deficient azaarenes, Adv. Synth. Catal., 2016, 358, 994–1002 CrossRef CAS.
- C. Reep, P. Morgante, R. Peverati and N. Takenaka, Axial-chiral biisoquinoline N,N′-dioxides bearing polar aromatic C-H bonds as catalysts in Sakurai-Hosomi-Denmark allylation, Org. Lett., 2018, 20, 5757–5761 CrossRef CAS.
- M. Nakajima, M. Saito, M. Shiro and S. I. Hashimoto, (S,)-3,3′-dimethyl-2,2′-biquinoline N,N′-dioxide as an efficient catalyst for enantioselective addition of allyltrichlorosilanes to aldehydes, J. Am. Chem. Soc., 1998, 120, 6419–6420 CrossRef CAS.
- A. de Bettencourt-Dias, P. S. Barber, S. Viswanathan, D. T. de Lill, A. Rollett, G. Ling and S. Altun, Para-derivatized Pybox ligands as sensitizers in highly luminescent Ln(III) complexes, Inorg. Chem., 2010, 49, 8848–8861 CrossRef CAS PubMed.
- F. Zinna, L. Arrico and L. Di Bari, Near-infrared circularly polarized luminescence from chiral Yb(III)-diketonates, Chem. Commun., 2019, 55, 6607–6609 RSC.
- A. Sickinger, B. Baguenard, A. Bensalah-Ledoux, Y. Guyot, L. Guy, F. Pointillart, O. Cador, M. Grasser, B. Le Guennic, F. Riobé, O. Maury and S. Guy, Impact of the experimental bandwidth on circularly polarized luminescence measurements of lanthanide complexes: the case of erbium(III), J. Mater. Chem. C, 2024, 12, 4253–4260 RSC.
- O. G. Willis, F. Petri, G. Pescitelli, A. Pucci, E. Cavalli, A. Mandoli, F. Zinna and L. Di Bari, Efficient 1400–1600 nm circularly polarized luminescence from a tuned chiral erbium complex, Angew. Chem., Int. Ed., 2022, 61, e202208326 CrossRef CAS.
- CCDC 2503942: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2q1kbp.
| This journal is © the Partner Organisations 2026 |