Open Access Article
Open Access ArticleSpatial construction of Pd and MnOx on a facet-engineered BiVO4 photocatalyst for efficient removal of glyphosate pesticide
Nengcong
Yang†
a,
Lulu
Liu†
a,
Yue
Chen†
a,
Mengmeng
Liu
a,
Zhian
Chen
a,
Ruyan
Hou
 *a,
Sheng
Ye
*a,
Sheng
Ye
 *a and
Rengui
Li
*a and
Rengui
Li
 *b
*b
aState Key Laboratory of Tea Plant Germplasm Innovation and Resource Utilization, Agricultural Photocatalysis Laboratory, School of Materials and Chemistry, Anhui Agricultural University, Hefei 230036, China
bState Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China
First published on 14th November 2025
Abstract
As a widely used organophosphorus pesticide, glyphosate in aquatic environments and soil is causing increasing risks to public health and the environment, and feasible solutions to eliminate glyphosate contaminants are still in demand. Herein, we report on the photocatalytic removal of glyphosate using a decahedron bismuth vanadate (BiVO4) photocatalyst by incorporating Pd and MnOx cocatalysts on different exposed facets. It is elucidated that the selective deposition of Pd on the electron-accumulating {010} facets of BiVO4 substantially enhances the oxygen reduction reaction, thereby accelerating the formation of ˙O2− radicals. Concurrently, the construction of MnOx on the hole-accumulating {110} facets promotes the water oxidation reaction, augmenting the production of ˙OH radicals. The synergistic integration of Pd and MnOx dual cocatalysts onto different facets of BiVO4 results in a striking enhancement in glyphosate degradation rates, surpassing those of pristine BiVO4 by two orders of magnitude. Furthermore, the optimized BiVO4 was assembled on cellulose fabric and shows effective degradation capability in glyphosate-polluted soil. This study underscores the significance of rationally designing semiconductor photocatalysts at the nano-/micrometer scale for application in the removal of persistent environmental contaminants.
Introduction
Organophosphorus contaminants (OCs) have been emerging as one kind of universal pollutant due to their widespread presence in agricultural environments, of which glyphosate (GP) stands out as the most prevalently used organophosphorus pesticide renowned for its broad-spectrum applicability and effectiveness in the control of weeds.1–3 However, the extensive utilization of GP leads to residues in water and the soil, resulting in a potentially serious risk to public health and the ecological environment.4 Currently, various treatment techniques, including adsorption, chemical oxidation, biodegradation, and photo-degradation, are employed to remove GP.5–12 However, these methods possess some disadvantages, including a low capacity to capture GP, high energy consumption or the requirement of oxidants such as persulfates.13–16Photocatalysis is emerging as a promising approach for environmental remediation owing to its various merits, including complete mineralization, no secondary pollution, low cost, and mild operating temperature and pressure conditions.17–19 Photogenerated charge separation is a key process that determines the efficiency of semiconductor-based photocatalysis.20–23 Charge separation among different facets of photocatalysts has gained more attention for its feasibility in promoting charge separation and the rational assembly of cocatalysts on particulate photocatalysts.24,25 Meanwhile, the decoration of cocatalysts or an interfacial layer is a universal strategy for optimizing photocatalytic reaction.26,27 Facet engineering has also been shown to activate the contaminant molecules due to the unique surface structure.28–30 In particular, BiVO4 is one of the most representative photocatalysts with the definite spatial separation of photogenerated electrons and holes among the {010} and {110} facets.31 The ideal behavior of the charge carriers is driven by the variance of the surface energy among different facets that function as facet heterojunctions. The distinct spatial separation of charge carriers is favorable to the regulation of specific reactions for the particular reaction sites on different facets. Furthermore, it was reported that selective decoration of cocatalysts on specific crystal facets of semiconductors could modify the reaction path due to the uniform electronic and structural properties.25,32 One of the key challenges is how to accomplish the high generation rate of reactive oxygen species (ROS), which are the crucial species acting as oxidizing reagents for degradation, and the formation mechanism of ROS involves the participation of photogenerated electrons or holes.33–35 Nevertheless, how the crystal facets of semiconductor-based photocatalysts affect the formation of ROS is still elusive. Moreover, unraveling the role of cocatalysts in oxidized degradation reactions on particulate photocatalysts is crucial for the efficient removal of persistent organic contaminants from polluted water and soil.
Herein, we report the photocatalytic removal of glyphosate using a decahedron BiVO4 photocatalyst with the rational assembly of Pd and MnOx cocatalysts spatially on different facets. Facet-selective deposition of Pd nanoparticles as a cocatalyst onto the {010} facets results in a remarkable promotion of charge separation and acceleration of the oxygen reduction reaction, leading to the generation of ˙O2− radicals, which serve as the main reactive species for glyphosate degradation. Further construction of MnOx on the hole-accumulating {110} facets facilitates the water oxidation reaction to promote the production of ˙OH radicals. The synergistic integration of the Pd and MnOx dual cocatalysts gives a remarkable augmentation of the glyphosate degradation rates compared to BiVO4. Cellulose non-woven fabric is an eco-friendly material derived from natural cellulose fibers, renowned for its biodegradability, lightweight, and excellent hydrophilic properties,36 making it an ideal support for the BiVO4 photocatalyst in wastewater treatment. Moreover, the optimized BiVO4-coated cellulose fabric can be implemented in flow panel systems for agricultural contaminant degradation in tea plantation soil, and exhibits superior activity for glyphosate with an average removal rate over 80%. This universal strategy holds great significance in addressing pesticide pollution and advancing the goal of green agriculture.
Experimental
Chemicals
Glyphosate (Innochem, 95%), Bi(NO3)3·5H2O (Sinopharm Chemical, AR), NH4VO3 (Sinopharm Chemical, AR), NH3·H2O (Sinopharm Chemical, AR), PdCl2 (Sinopharm Chemical, 99.9%), AgNO3 (Sinopharm Chemical, 99.8%), H2PtCl6 (Sinopharm Chemical, AR), Ni(NO3)2·6H2O (Sinopharm Chemical, AR, 98%), Co(NO3)2·6H2O (Sinopharm Chemical, AR, 98.5%), MnSO4 (Sinopharm Chemical, AR, 98%), dihydroethidium (DHE, Yeasen, ≥90%), nitrotetrazolium blue chloride (NTB, Macklin, 98%), and 3,3′,5,5′-tetramethylbenzidine (TMB, Macklin, 99%) were used. All reagents were used as received without further purification.Synthesis of decahedron BiVO4 crystals
Decahedron BiVO4 crystals were synthesized as previously reported.31 Typically, the precursors NH4VO3 (36 mmol) and Bi(NO3)3·5H2O (36 mmol) were dissolved in 300 mL of 2.0 M nitric acid solution, and the pH value of the solution was then adjusted to 2.0 with ammonia solution under stirring until the formation of an orange precipitate. After about 2 h of aging, the orange precipitate at the bottom of the beaker was transferred to a Teflon-lined stainless-steel autoclave with a capacity of 100 mL and hydrothermally treated at 200 °C for 24 h. After the autoclave was cooled to room temperature, a vivid yellow powder was separated by filtration, washed with deionized water more than 3 times, and then dried at 60 °C in air overnight.Facet-selective photo-deposition of metals and/or oxides
Three different deposition routes (single photo-reduction, single photo-oxidation and simultaneous photo-reduction and photo-oxidation) were adopted, and all the photo-depositions were similarly carried out at room temperature without adjusting the pH value. Typically, 0.50 g of BiVO4 powder and a calculated amount of deposition precursor (1 wt% noble metal or 0.5 wt% metal oxide) were mixed in 100 mL of deionized water, and the suspension was then irradiated by a 300 W Xe lamp (λ ≥420 nm) under continuous stirring. After 5 h photo-deposition, the suspension was filtered, washed with deionized water more than 3 times, and finally dried at 60 °C overnight. The as-obtained powder was used for characterizations and/or activity tests. As for the photo-reduction of the single Au, Pd or Ag particles, HAuCl4, PdCl2 and AgNO3 were chosen as precursors, respectively, and the as-synthesized samples were denoted as Au (Pd, or Ag)/BiVO4, respectively. To ensure the counterpart oxidation reaction, 20% methanol as a hole scavenger was also used as a sacrificial reagent for comparison. The photo-oxidation of the single MnOx, CoOx or NiOx was achieved with MnSO4, Co(NO3)2 or Ni(NO3)2 as a precursor, and NaIO3 was employed as the electron acceptor. The as-prepared samples were denoted as MnOx/BiVO4, CoOx/BiVO4 and NiOx/BiVO4, respectively. For Pd/MnOx/BiVO4, the deposition process is the same as that of MnOx/BiVO4 except for the replacement of BiVO4 with Pd/BiVO4. For the impregnated sample, MnOx was impregnated from MnSO4 solution by post-calcination at 350 °C for 1 h, and the as-prepared sample was denoted as MnOx(imp)/BiVO4.Characterization
The as-prepared BiVO4 samples were characterized using X-ray powder diffraction (XRD) on a Rigaku SmartLab powder diffractometer. The scan rate of 20° min−1 was applied to record the XRD patterns in the range of 10–60° at a step size of 0.01°. UV–visible (UV–vis) diffuse reflectance spectra were recorded on a UV–vis spectrophotometer (UV-2600, Shimadzu) equipped with an integrating sphere. The morphologies and particle sizes were examined by scanning electron microscopy (SEM, S4800, Hitachi) and high-resolution scanning electron microscopy (HRSEM, S5500, HITACHI).Photocatalytic degradation experiments
The photocatalytic degradation performance of the BiVO4 system was evaluated in an in-house-fabricated photoreactor (250 mL) using a 300 W Xe lamp (Beijing Perfectlight Technology Co., Ltd). Normally, 20 mg photocatalyst was dispersed in the 20 ppm glyphosate solution and stirred in the dark for 2 h, followed by visible light irradiation (λ >420 nm). After different times, 5 mL suspensions were taken out and extracted using a 0.22 μm membrane filter for later detection. The concentration of glyphosate was detected by high-performance liquid chromatography (HPLC system, Agilent 1260). An Agilent C18 column (250 × 4.6 mm, 5 μm) with the mobile phases of 20 mM PBS (A: 90%) and methanol (B: 10%) and a flow rate of 1.0 mL min−1 was employed.Preparation of the Pd/MnOx/BiVO4-loaded Cellulose fabric
500 mg of Pd/MnOx/BiVO4 powder was dispersed in 100 mL of 0.5 wt% Nafion isopropanol and ultrasonicated for 2 minutes to obtain a homogeneous suspension. The homogeneous suspension was dropped onto the cellulose-based non-woven fabric (25 cm × 25 cm), and the non-woven fabric was dried in a fume hood for 1 h to evaporate the solvent. Finally, the Pd/MnOx/BiVO4-loaded fabric was washed in deionized water to remove the loosely adhered Pd/MnOx/BiVO4.Results and discussion
The decahedron BiVO4 photocatalyst was synthesized using a previously reported hydrothermal process.31 The as-prepared BiVO4 shows a smooth surface with a regular decahedron morphology and a size of 1–2 μm, and the crystal structure is in accordance with the pure monoclinic scheelite phase (Fig. S1–S4). Meanwhile, the crystallite size is calculated to be 430 nm according to the corresponding XRD peak of the (010) crystallographic plane. As depicted in Fig. 1a, the deposition of the cocatalyst was carried out using an in situ photo-deposition method (the sample is denoted as X/BiVO4, X = Au, Ag, Pd, CoOx, NiOx, MnOx). It can be clearly observed that selectively reduced deposition of metallic nanoparticles (Au, Ag, Pd) occurs on the (010) facet of the BiVO4 decahedron particles, while photo-oxidation of metal ions to metal oxide species (CoOx, NiOx, MnOx) only occurs on the (110) facets (Fig. S5 and S6). These results indicate that spatial charge separation and selective photo-deposition between the (010) and (110) facets can be well maintained in our prepared BiVO4 crystals. Thereafter, dual cocatalysts (Pd and MnOx) were sequentially deposited as cocatalysts on specific facets. The SEM images show the morphology of the dual cocatalyst-deposited BiVO4, where it can be seen that the oxidation and reduction cocatalysts are successfully deposited on the (110) and (010) facets of BiVO4, respectively (Fig. 1b and S7). The deposited Pd nanoparticles are highly dispersed on the (010) facet with a size of 2–10 nm, and the particle density is about 8000 per μm2 (Fig. S8). Their existence is further confirmed by high-resolution TEM (Fig. 1c and d), in which the planar spacing of 0.22 nm well matches the (111) plane of metallic Pd.37 The oxidation cocatalyst MnOx can also be validated on the hole-rich (110) facets of BiVO4, as shown by the TEM and HRTEM images (Fig. 1e and f) and the elemental mapping (Fig. 1g and S8).38 The optical properties remain invariable for the samples with cocatalyst deposition (Fig. S9). Furthermore, EDX imaging for the different facets of Pd/MnOx/BiVO4 also shows that the different substances on the different facets represent MnOx and Pd, respectively (Fig. 1g).Thermogravimetric analysis (TGA) of MnOx/BiVO4 and Pd/MnOx/BiVO4 exhibits high thermal stability with negligible weight loss step at 25–500 °C (Fig. S10). Pd/MnOx/BiVO4 shows an increase in surface area (5.77 m2 g−1) versus MnOx/BiVO4 (4.03 m2 g−1) by BET measurement, confirming enhanced active site exposure for the highly dispersed Pd cocatalyst (Fig. S11).
The photocatalytic degradation performance of glyphosate by BiVO4 photocatalysts with different reduction cocatalysts was evaluated first. As observed in Fig. 2a, the bare BiVO4 with a simple crystal facet effect just presents poor activity for photocatalytic decomposition of glyphosate, indicating that the pristine decahedron BiVO4 is unable to accomplish the effective degradation of glyphosate. On implementing the selective deposition of Ag nanoparticles, the degradation activity of Ag/BiVO4 exhibits a negligible increase, and superior performance is realized when the Ag is replaced by Au, with the degradation efficiency of 50% in 250 min. While the glyphosate degradation is significantly promoted by Pd cocatalyst-decorated BiVO4, the efficiency distinctly increases to 80% within 120 min. The kinetic constant is also significantly enhanced with metal cocatalyst deposition, especially for the Pd cocatalyst (Fig. 2b). As noble metals with a relatively large work function on BiVO4 promote the electron transfer from BiVO4 to metal nanoparticles, the decoration of Pd could enhance charge separation. The choice of suitable reduction cocatalysts is substantial for constructing efficient photocatalytic degradation systems of glyphosate. As Pd is generally utilized in O2 reduction reactions as a cocatalyst due to its excellent performance, the enhanced mechanism is very likely the result of O2 reduction to produce a sufficient amount of ˙O2−, thus leading to fast glyphosate degradation through a free radical-related reaction process.
Correspondingly, different oxidation cocatalysts were also employed in BiVO4, and Fig. 2c shows the photocatalytic degradation performance of different metal oxides (MnOx, CoOx, and NiOx) as oxidation cocatalysts. It can be seen that the loading of the single oxidation cocatalyst can only achieve slightly better activities for the photocatalytic degradation of glyphosate, and better performances were all obtained for different oxide cocatalyst-loaded BiVO4 than bare BiVO4. The activity order is MnOx > CoOx > NiOx, which is more evident as indicated by the degradation kinetics (Fig. 2d). The results indicate that the mere loading of MnOx on BiVO4 can lead to relatively low enhancement of photocatalytic degradation. Specifically, when only Pd was deposited as the cocatalyst, the degradation activity of glyphosate was increased much more obviously than that with only MnOx as the cocatalyst. It seems that the reduction reaction (generating ˙O2−) is the possible rate-determining step for the degradation of glyphosate. However, when only the reduction cocatalyst was deposited on BiVO4, the reaction activity was still unsatisfactory.
In this case, the dual cocatalysts were selectively decorated on BiVO4 subsequently. Naturally, the degradation performance of glyphosate in the dual cocatalyst system (Pd/MnOx/BiVO4) is obviously superior to that of the single cocatalyst system (Fig. 2e). The possible reason is that dual cocatalysts substantially facilitate the extraction of photogenerated carriers owing to the selective deposition on specific facets appropriately, and the proper location of the Pd cocatalyst subsequently provides abundant active sites for O2 reduction to ˙O2−. Meanwhile, the dual cocatalysts have a synergistic effect on charge separation, thus resulting in distinguished degradation for glyphosate. The degradation curve obeys the pseudo-first-order kinetic equation, and the rate constant is determined to be 0.024 min−1 for Pd/MnOx/BiVO4, which is 2.4 times higher than that of Pd/BiVO4 (0.010 min−1), while the degradation constants by sole BiVO4 and MnOx/BiVO4 are very slow (Fig. 2f). The evident synergetic effect of dual cocatalysts is amply validated by comparison of BiVO4 with a single cocatalyst or dual cocatalysts selectively deposited on the corresponding correct facets. Furthermore, these results indicate that the Pd cocatalyst is particularly vital for the removal of glyphosate and verify the importance of the simultaneous deposition of dual cocatalysts. Moreover, the stability tests over 3 cycles indicate satisfactory stability of Pd/MnOx/BiVO4 (Fig. S12). The XRD and SEM analyses indicated that the morphology and crystalline structure remained well after the stability test (Fig. S13 and S14). The ICP analysis of the post-reaction solution demonstrated that the dissolution of the cocatalysts is negligible (Table S1). These results highlight the stability of the Pd/MnOx/BiVO4 photocatalyst after multiple reaction cycles.
The valence states of MnOx/BiVO4 and Pd/MnOx/BiVO4 were examined using X-ray photoelectron spectroscopy (XPS). The XPS results confirm the Mn3+ and Mn4+ oxidation state for both MnOx/BiVO4 and Pd/MnOx/BiVO4, which is consistent with the previous literature39 (Fig. S15, S16 and Tables S2, S3). The binding energy of Pd 4f reveals that the deposited Pd element on the BiVO4 exists in metallic form for the Pd/BiVO4 sample (Fig. S16 and Table S3). The valence state of the deposited Pd, including the positive chemical state of Pd2+, may result from self-oxidation of metallic Pd on exposure to air.40 The states of Pd/MnOx/BiVO4 were also explored after the stability test, and the XPS results indicate partial oxidation of MnOx species as the role of the oxidation cocatalyst, while the states of Pd are nearly the same as those of the prepared Pd/MnOx/BiVO4 (Fig. S16 and Tables S3, S4).
The electrochemical performance of O2 reduction was explored in O2-saturated electrolyte solution for the above BiVO4 samples with different noble metals (Au, Ag, Pd). The current density curves all exhibit the typical characteristic of O2 reduction for the three metals (Fig. 3a).41 Notably, the performance is remarkably increased for Pd/BiVO4 as compared with the others, indicating the excellent activity of Pd nanoparticles in the oxygen reduction reaction. Mott–Schottky analysis quantitatively determines the conduction band (CB) edge of BiVO4 at 0.05 V vs. RHE (Fig. S17a), and thus the valence band (VB) is established at 2.45 V vs. RHE according to the bandgap of 2.4 eV derived from UV–vis absorption.42 The valence band edge of BiVO4 (2.45 V vs. RHE) exceeds the ˙OH/H2O potential (2.31 V vs. RHE), and thus is favorable to produce ˙OH.43 The band alignment diagrams for various BiVO4 samples show negligible variance, which indicates the nearly invariable energy band structure (Fig. S17b). Moreover, the in situ EPR signals using DMPO as a probe molecule of ˙OH demonstrate that MnOx/BiVO4 exhibits substantial intensity compared to BiVO4, indicating that the MnOx can promote the generation of ˙OH (Fig. S18). Photoelectrochemical measurement was also conducted on BiVO4 and MnOx/BiVO4, as only the oxidation half-reaction is evaluated, and the charge extraction driving force provided by the loaded Pd is relatively weak compared to the applied bias. The results verified the significance of selective deposition of MnOx on BiVO4, as the photocurrent response of MnOx/BiVO4 is superior to the impregnated sample MnOx(imp)/BiVO4 (Fig. S19). Photoelectrochemical impedance spectroscopy (PEIS) of Pd/MnOx/BiVO4 exhibits an evidently smaller charge-transfer resistance compared to pristine BiVO4, Pd/BiVO4 and MnOx/BiVO4, confirming accelerated charge separation (Fig. S20a). Furthermore, the decay of photoluminescence (PL) of Pd/MnOx/BiVO4 shows a prolonged carrier lifetime (τ = 13.5 ns vs. 5.2 ns for BiVO4 and the others), indicating suppressed electron–hole recombination due to dual cocatalyst-induced separation (Fig. S20b). When the MnOx cocatalyst was randomly deposited on the (110) facets, the photocurrent was obviously increased as compared with the bare BiVO4 electrode. This demonstrates that the photocurrent can be efficiently promoted even when the oxidation cocatalyst is randomly distributed on the BiVO4. In addition, the increased photocurrent of Pd/MnOx/BiVO4 is 3 times higher than that of the BiVO4 electrode with MnOx randomly deposited on all of the facets. This indicates that the MnOx can function efficiently for synchronously accelerating charge separation and surface reaction, which is in good agreement with the results of photocatalytic degradation experiments.
The role of O2 was examined to identify the determining factor during glyphosate degradation. As shown in Fig. 3b, the degradation efficiency is further increased for all of the BiVO4 samples as the glyphosate degradation proceeds in streaming pure O2 conditions, and complete degradation is accomplished within 60 min for Pd/MnOx/BiVO4. The corresponding pseudo-first-order rate constants (kobs) are calculated to be 0.05 min−1, demonstrating that the degradation process of glyphosate is mainly accompanied by O2 participation (Fig. S21a). Furthermore, from another control experiment in N2 streaming, the results show that the degradation efficiency of glyphosate in an anaerobic system is only 70% within 120 min, and the degradation rate constants decrease to 0.0092 min−1 (Fig. 3b and Fig. S21b). Obviously, O2 reduction to ˙O2− plays a pivotal role in the aerobic system, while the decomposition of glyphosate in anaerobic conditions is severely hindered due to the lack of ˙O2−. The degradation is hindered because the generation of ˙O2− is forbidden under anaerobic conditions, implying that O2 is essential for glyphosate degradation by the BiVO4 system. Furthermore, the appropriate accumulation of photogenerated electrons on the (010) facet is of great benefit to the subsequent O2 reduction on Pd nanoparticles, which is reasonable to maximize the synergistic effect of spatial charge separation on specific facets and the rational construction of reactive sites for rapid production of ˙O2− with adequate O2 supply. The blank experiment was conducted to examine the possible mechanism of direct reduction of p-benzoquinone to hydroquinone by excited electrons,44 and the results showed negligible production of hydroquinone in the ultraviolet absorption spectra (Fig. S22). The argon-degassed system exhibits significant attenuation of glyphosate degradation by 78% (Fig. S23), while O2-saturated solutions evidently enhanced the degradation efficiency. These experiments conclusively validate the photocatalytic role of ˙O2− and exclude the BQ/HQ side reaction.
Interestingly, the degradation products under O2 obtained by liquid chromatography–mass spectrometry (LCMS) indicated that Pd/MnOx/BiVO4 selectively decomposes glyphosate to aminomethylphosphonic acid (AMPA), and the formation of AMPA is well in agreement with the removal of glyphosate (Fig. 3c). To evaluate the toxicity of the degradation products, we further monitored the concentration of PO43− over a long period of 8 h as organophosphate derivatives are the main toxic substances (Fig. 3d). The combined results of AMPA and PO43− indicates that glyphosate decomposes to AMPA initially (within 2 h), and then the AMPA is further cleaved, releasing PO43− anions as the degradation of glyphosate is completed.
To verify the primary active spices responsible for glyphosate degradation by BiVO4, we employed benzoquinone (BQ), methanol (CH3OH), isopropyl alcohol (IPA), and potassium dichromate (K2Cr2O7) to trap ˙O2−, h+, ˙OH, and e−, respectively (Fig. S24). To prominently distinguish the performance differences in capturing different radicals, we normalized the degradation efficiency against a normal control sample in Fig. 4. As shown in Fig. 4a, the normalized degradation efficiencies of glyphosate exhibit irregularity for bare BiVO4 upon adding any scavengers due to the sluggish degradation kinetics. The performances are both enhanced upon adding CH3OH and IPA for Pd/BiVO4, which results from the inhibited photoexcited carrier recombination due to the rapid consumption of h+ and ˙OH by CH3OH and IPA, allowing more photogenerated electrons to migrate to the Pd surface for ˙O2− generation. However, after adding BQ, the degradation rate by Pd/BiVO4 decreased significantly, suggesting that ˙O2− is dominant in the glyphosate degradation reaction. The capture result of K2Cr2O7 further demonstrates the role of ˙O2− as the degradation activity decreases faster via the impediment of O2 reduction (Fig. 4b). As for MnOx/BiVO4, the photocatalytic degradation performance is slightly promoted with the addition of CH3OH (Fig. 4c). Meanwhile, the degradation activity of the MnOx/BiVO4 system decreases when IPA and K2Cr2O7 are added as scavengers for ˙OH and e−. This results from the fact that the MnOx is responsible for accelerating charge separation, and that the main active radicals are ˙OH and e−-induced ˙O2−. Additionally, we found a similar tendency for the photocatalytic activity of the Pd/MnOx/BiVO4 system with Pd/BiVO4 after adding different scavengers (Fig. 4d). This indicates that ˙O2− radicals play a dominant role in the photocatalytic degradation of glyphosate by Pd/MnOx/BiVO4.
The production of ROS was semi-quantitatively measured by visible light absorption spectroscopy. The nitroblue tetrazolium (NBT) was used as a probe molecule to measure the production of ˙O2−.45 The increased absorption intensity of NBT also suggests the increase of ˙O2− with continuous irradiation, as shown in Fig. 4e. The yield of ˙O2− on dual cocatalyst-decorated BiVO4 rises dramatically compared to the one produced by single cocatalyst-decorated BiVO4 and is six times higher than that of bare BiVO4, suggesting that the combination of BiVO4 and cocatalysts enhanced the yield of ˙O2−. The production of ˙OH could be measured by 3,3′,5,5′-tetramethylbenzidine (TMB).46 A slight enhancement in absorption intensity is observed for all of the samples, indicating that photo-induced production of ˙OH is more favorable after the deposition of MnOx (Fig. 4f). These results suggest that both ˙O2− and ˙OH radicals are promoted on the Pd/MnOx/BiVO4 photocatalyst under visible light, which is responsible for the greatly enhanced photocatalytic degradation rates. Comparative analysis with published photocatalytic glyphosate degradation studies demonstrates that our system achieves predominant degradation performance with nearly complete efficiency within 120 min and further mineralization of organophosphorus (Table S5). Moreover, our work reveals that the selective decoration of dual cocatalysts on facet-engineered BiVO4 can strengthen the generation of specific radicals, which highlights the significant scientific merit and potential applications in the degradation of other pollutants and selectivity regulation.
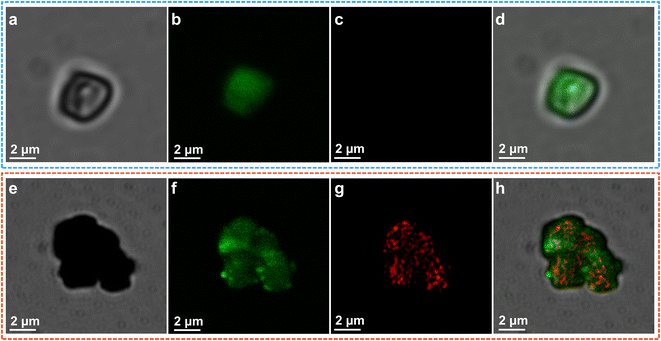
To further map the crystal facet-dependent photocatalytic reaction sites of O2 reduction, laser scanning confocal fluorescence microscopy (LSCM) was used to investigate the population of ˙O2− on individual BiVO4 particles (Fig. 5a). The dihydroethidium (DHE) oxidation induced by ˙O2− is taken as the probe reaction, which produces fluorescence at 610 nm.47 Fluorescence mapping for the BiVO4 crystals was implemented in an air-saturated solution containing fluorescent probe molecules under a 488 nm laser. As shown in Fig. 5b, evident green fluorescence was observed during irradiation, and the locations entirely overlapped on the surface of the BiVO4 crystals. The fluorescence signal can be ascribed to the intrinsic photoluminescence of excited BiVO4.48 Moreover, the fluorescence magnitude of the oxidized probe molecules is too faint to be detected, suggesting that electron-induced oxygen reduction nearly does not occur on the bare BiVO4 (Fig. 5c and d). A similar appearance of intrinsic photoluminescence is also observed on dual cocatalyst-decorated BiVO4 (Fig. 5e and f). Interestingly, the bright red fluorescence clearly indicates strong enhancement on the generation of O2− for Pd/MnOx/BiVO4, indicating that the activity of electron-induced O2 reduction is dominated synergistically by charge separation and catalytic processes (Fig. 5g). Surprisingly, red fluorescence spots of the DHE probe on the green background were found to be remarkable and preferentially located on the lateral (010) facet of the BiVO4 decahedrons due to the selective deposition of Pd nanoparticles on the (010) facet (Fig. 5h). The above results validate that the reaction of photocatalytic O2 reduction to ˙O2− is related to the accumulation of photogenerated electrons and Pd cocatalyst decoration simultaneously, which results from the spatial charge separation and related reduction reaction. In addition to the photogenerated holes and electrons preferentially distributing at the different facets of the BiVO4 microcrystal photocatalysts, we further reveal that the reduction of O2 tends to occur at the Pd sites on the (010) facet.
As glyphosate is one kind of common pesticide residue in agricultural soil, the photocatalytic degradation of glyphosate in soil was conducted by Pd/MnOx/BiVO4. As the traditional configuration that combines a slurry of photocatalyst powder and soil dispersion is unpractical as soil is an effective shield against light, we assembled a continuous flow reactor to examine the photocatalytic degradation capability for polluted soil remediation (Fig. 6a). Thus, a separated configuration with two parallel individual chambers was developed to ensure the light absorption by the photocatalyst powder while keeping it separate from the soil sample. As presented in Fig. 6b, the reactor is composed of a water inlet system, a photocatalyst chamber, a soil chamber, a water outlet system, and a water circulation system. To ensure the light absorption efficiency, the upper chamber is covered by Pd/MnOx/BiVO4-loaded cellulose membrane for adequate light incidence, followed by the soil layer being placed in the bottom chamber. A continuous water stream flows from the photocatalyst chamber to the soil layer through the membrane, and thus, the eluted free glyphosate molecules from the soil can be taken to the photocatalyst chamber for degradation.
To validate the degradation practicability for polluted soil samples of our photocatalytic reactor, the degradation experiment was carried out under simulated solar irradiation. The degradation efficiency is up to 82% for the illumination time of 10 h. The average light intensity is about 60 mW cm−2, which is fundamentally enough to guarantee the photocatalytic removal of glyphosate. The average removal efficiencies of glyphosate in soil were maintained above 70% after 5 cycles (Fig. 6c). The aforementioned results indicate that the glyphosate in soil can be effectively removed through consecutive flow by appropriately decorating the BiVO4 system. Thus, the integrated photocatalytic degradation device based on dual cocatalyst BiVO4 has promising prospects in soil recovery applications under natural conditions.
Conclusions
In summary, we successfully constructed a photocatalyst system with dual cocatalysts rationally decorated to achieve highly efficient degradation of glyphosate. Remarkably enhanced photocatalytic activities were observed for the rationally assembled photocatalysts. Systemic investigations show that the enhanced photocatalytic performances are ascribed to the intrinsic nature of spatial charge separation among different facets of BiVO4 and the induced synergistic effect of dual cocatalyst selective deposition on specific facets of BiVO4. This unique effect endows excellent performance for fast generation of ˙O2− on selectively deposited Pd nanoparticles by accumulated photogenerated electrons. Concurrently, the construction of MnOx on the hole-accumulating {110} facets promotes the water oxidation reaction, augmenting the production of ˙OH radicals. These radicals serve as the primary reactive species responsible for the photocatalytic degradation of glyphosate. The synergistic integration of Pd and MnOx dual cocatalysts onto different facets of BiVO4 results in a striking enhancement of the glyphosate degradation rates, surpassing those of pristine BiVO4 by two orders of magnitude. Furthermore, we assembled a Pd/MnOx/BiVO4-loaded cellulose fabric that shows effective remediation capability on glyphosate-polluted soil, suggesting potential in agricultural remediation. This work further demonstrates the distinctive property that spatial charge separation and selective deposition of dual cocatalysts on facet-engineered BiVO4 can be expanded to the application of photocatalytic agricultural contaminant removal and other environmental remediation processes.Author contributions
Nengcong Yang was responsible for conducting all the experiments, performing data analysis, and drafting the original manuscript. Lulu Liu and Yue Chen performed the synthesis and characterization. Mengmeng Liu and Zhian Chen were involved in the experiments and data analysis. Ruyan Hou and Sheng Ye conducted a theoretical study. Rengui Li was responsible for review and editing. All the authors discussed the results and contributed to the writing of the paper.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5qi01894c.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC, 22372001, 22502002), Starting Fund for Scientific Research of High-Level Talents, Anhui Agricultural University (rc382108), and Postdoctoral Fellowship Program of CPSF (GZB20240003). We thank Dr Qin Wang and Dr Yang Xu of the Biotechnology Center of Anhui Agricultural University for their help with material characterization in this work.References
- E. G. Jaworski, Mode of action of N-phosphonomethylglycine. Inhibition of aromatic amino acid biosynthsis, J. Agric. Food Chem., 1972, 20, 1195–1198 CrossRef CAS.
- T. Funke, H. Han, M. L. Healy-Fried, M. Fischer and E. Schönbrunn, Molecular basis for the herbicide resistance of Roundup Ready crops, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13010–13015 CrossRef CAS.
- S. O. Duke, Taking stock of herbicide-resistant crops ten years after introduction, Pest Manage. Sci., 2005, 61, 211–218 CrossRef CAS.
- L. P. Agostini, R. S. Dettogni, R. S. dos Reis, E. Stur, E. V. W. dos Santos, D. P. Ventorim, F. M. Garcia, R. C. Cardoso, J. B. Graceli and I. D. Louro, Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies, Sci. Total Environ., 2020, 705, 135808 CrossRef CAS PubMed.
- A. Pankajakshan, M. Sinha, A. A. Ojha and S. Mandal, Water-Stable Nanoscale Zirconium-Based Metal–Organic Frameworks for the Effective Removal of Glyphosate from Aqueous Media, ACS Omega, 2018, 3, 7832–7839 CrossRef CAS.
- X. Zhu, B. Li, J. Yang, Y. Li, W. Zhao, J. Shi and J. Gu, Effective Adsorption and Enhanced Removal of Organophosphorus Pesticides from Aqueous Solution by Zr-Based MOFs of UiO-67, ACS Appl. Mater. Interfaces, 2015, 7, 223–231 CrossRef CAS PubMed.
- M. Cycoń, A. Żmijowska, M. Wójcik and Z. Piotrowska-Seget, Biodegradation and bioremediation potential of diazinon-degrading Serratia marcescens to remove other organophosphorus pesticides from soils, J. Environ. Manage., 2013, 117, 7–16 CrossRef.
- H. Hossaini, G. Moussavi and M. Farrokhi, The investigation of the LED-activated FeFNS-TiO2 nanocatalyst for photocatalytic degradation and mineralization of organophosphate pesticides in water, Water Res., 2014, 59, 130–144 CrossRef CAS.
- N. Negishi, T. Sano, T. Hirakawa, F. Koiwa, C. Chawengkijwanich, N. Pimpha and G.-R. M. Echavia, Photocatalytic detoxification of aqueous organophosphorus by TiO2 immobilized silica gel, Appl. Catal., B, 2012, 128, 105–118 CrossRef CAS.
- H. Dong, Q. Xu, L. Lian, Y. Li, S. Wang, C. Li and X. Guan, Degradation of Organic Contaminants in the Fe(II)/Peroxymonosulfate Process under Acidic Conditions: The Overlooked Rapid Oxidation Stage, Environ. Sci. Technol., 2021, 55, 15390–15399 CrossRef CAS.
- B. Balci, M. A. Oturan, N. Oturan and I. Sirés, Decontamination of Aqueous Glyphosate, (Aminomethyl)phosphonic Acid, and Glufosinate Solutions by Electro-Fenton-like Process with Mn2+ as the Catalyst, J. Agric. Food Chem., 2009, 57, 4888–4894 CrossRef CAS.
- D. Feng, A. Soric and O. Boutin, Treatment technologies and degradation pathways of glyphosate: A critical review, Sci. Total Environ., 2020, 742, 140559 CrossRef CAS.
- X. Zha, X. Hou, Q. Li, H. Nan, F. Ge, Y. Liu, F. Li, D. Zhang and J. Tian, Loading Glyphosate in Attapulgite and Sodium Alginate Hydrogels to Construct pH-Responsive Controlled Release Microsphere for Enhanced Soil Sustained Release, ACS Agric. Sci. Technol., 2022, 2, 1090–1100 CrossRef CAS.
- D. P. Jaisi, H. Li, A. F. Wallace, P. Paudel, M. Sun, A. Balakrishna and R. N. Lerch, Mechanisms of Bond Cleavage during Manganese Oxide and UV Degradation of Glyphosate: Results from Phosphate Oxygen Isotopes and Molecular Simulations, J. Agric. Food Chem., 2016, 64, 8474–8482 CrossRef CAS.
- L. Jin, Y. Huang, H. Liu, L. Ye, X. Liu and D. Huang, Efficient treatment of actual glyphosate wastewater via non-radical Fenton-like oxidation, J. Hazard. Mater., 2024, 463, 132904 CrossRef CAS.
- V. E. C. da Silva, Y. S. Tadayozzi, F. F. Putti, F. A. Santos and J. C. Forti, Degradation of commercial glyphosate-based herbicide via advanced oxidative processes in aqueous media and phytotoxicity evaluation using maize seeds, Sci. Total Environ., 2022, 840, 156656 CrossRef CAS PubMed.
- L. Chen, J. Hu, A. G. L. Borthwick, W. Sun, H. Zhang, D. Jia and W. Liu, Solar-light-activated periodate for degradation and detoxification of highly toxic 6PPD-quinone at environmental levels, Nat. Water, 2024, 2, 453–463 CrossRef CAS.
- Y. Guan, Z. Liu, N. Yang, S. Yang, L. E. Quispe-Cardenas, J. Liu and Y. Yang, Near-complete destruction of PFAS in aqueous film-forming foam by integrated photo-electrochemical processes, Nat. Water, 2024, 2, 443–452 CrossRef CAS.
- H. Wang, Q. Zhao, D. Li, Z. Zhang, Y. Liu, X. Guo, X. Li, Z. Liu, L. Wang, J. Ma and H. He, Boosting Photothermocatalytic Oxidation of Toluene Over Pt/N-TiO2: The Gear Effect of Light and Heat, Environ. Sci. Technol., 2024, 58, 7662–7671 CrossRef CAS PubMed.
- Z. Tahmasebi, M. M. Momeni, A. N. Chermahini and B.-K. Lee, The improvement of photocatalytic activity of BiVO4 modified with Co(OH)2 nanoflake arrays for photocatalytic and photo-electrocatalytic desulfurization, J. Alloys Compd., 2024, 976, 173295 CrossRef CAS.
- M. M. Momeni and Z. Tahmasebi, Etching time effect on morphological, optical and photoelectrochemical properties of BiVO4 electrodeposited films, Inorg. Chem. Commun., 2022, 139, 109300 CrossRef CAS.
- Z. Tahmasebi, M. M. Momeni and A. N. Chermahini, Enhanced BiVO4 photocatalytic and photoelectrocatalytic oxidative desulfurization performance via electrodeposited copper oxide nanoparticles, Appl. Phys. A, 2023, 129, 212 CrossRef CAS.
- N. Yang, Z. Yin, Z. Chen, C. Gao, Z. Cao, Y. Zheng, Z. Pan, H. Cao, S. Ye and Y. Xiong, Solar-Driven Massive Production of Dimerized Imine in Aqueous Phase via an Atomically Engineered Photocatalyst, Angew. Chem., Int. Ed., 2025, e202502202 CAS.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Photocatalytic water splitting with a quantum efficiency of almost unity, Nature, 2020, 581, 411–414 CrossRef CAS PubMed.
- R. Li, H. Han, F. Zhang, D. Wang and C. Li, Highly efficient photocatalysts constructed by rational assembly of dual-cocatalysts separately on different facets of BiVO4, Energy Environ. Sci., 2014, 7, 1369–1376 RSC.
- A. S. Correa, L. G. Rabelo, W. S. Rosa, N. Khan, S. Krishnamurthy, S. Khan and R. V. Gonçalves, Interfacial band alignment and photoelectrochemical properties of all-sputtered BiVO4/FeNiOx and BiVO4/FeMnOx p–n heterojunctions, Energy Adv., 2023, 2, 123–136 RSC.
- N. Khan, A. Koche, H. A. Centurion, L. Rabelo, J. Bettini, G. T. dos Santos, F. L. Souza, R. V. Gonçalves and S. Khan, Triggering Synergy between p-Type Sputter-Deposited FeMnOx or FeNiOx and W-Doped BiVO4 for Enhanced Oxygen Evolution, ACS Appl. Energy Mater., 2024, 7, 2129–2141 CrossRef CAS.
- X. Li, J. Zhu and H. Li, Influence of crystal facets and F-modification on the photocatalytic performance of anatase TiO2, Catal. Commun., 2012, 24, 20–24 CrossRef CAS.
- J. Jiang, K. Zhao, X. Xiao and L. Zhang, Synthesis and Facet-Dependent Photoreactivity of BiOCl Single-Crystalline Nanosheets, J. Am. Chem. Soc., 2012, 134, 4473–4476 CrossRef CAS PubMed.
- L. Wang, J.-C. Zhou, Z.-H. Li, X. Zhang, K. M. Y. Leung, L. Yuan and G.-P. Sheng, Facet-Specific Photocatalytic Degradation of Extracellular Antibiotic Resistance Genes by Hematite Nanoparticles in Aquatic Environments, Environ. Sci. Technol., 2023, 57, 21835–21845 CrossRef CAS PubMed.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- Y. Hermans, C. Olivier, H. Junge, A. Klein, W. Jaegermann and T. Toupance, Sunlight Selective Photodeposition of CoOx(OH)y and NiOx(OH)y on Truncated Bipyramidal BiVO4 for Highly Efficient Photocatalysis, ACS Appl. Mater. Interfaces, 2020, 12, 53910–53920 CrossRef CAS PubMed.
- T. Guo, L. Jiang, K. Wang, Y. Li, H. Huang, X. Wu and G. Zhang, Efficient persulfate activation by hematite nanocrystals for degradation of organic pollutants under visible light irradiation: Facet-dependent catalytic performance and degradation mechanism, Appl. Catal., B, 2021, 286, 119883 CrossRef CAS.
- C. Wang, W. Li, Z. Zhang, D. Lei, G. Che, C. Gou, J. Zhang and Z. Hao, A novel iron sulfide phase with remarkable hydroxyl radical generation capability for contaminants degradation, Water Res., 2024, 251, 121166 CrossRef CAS PubMed.
- L. Yang, Z. Chen, Q. Cao, H. Liao, J. Gao, L. Zhang, W. Wei, H. Li and J. Lu, Structural Regulation of Photocatalyst to Optimize Hydroxyl Radical Production Pathways for Highly Efficient Photocatalytic Oxidation, Adv. Mater., 2024, 36, 2306758 CrossRef CAS PubMed.
- A. S. Santos, P. J. T. Ferreira and T. Maloney, Bio-based materials for nonwovens, Cellulose, 2021, 28, 8939–8969 CrossRef CAS.
- J. Deng, F. Li, Z. Qi, W. Huang, Z. Wan, L. Zhang, D. Zheng, G. Li and F. Zhang, A novel redox synergistic mechanism of peroxymonosulfate activation using Pd-Fe3O4 for ultra-fast chlorinated hydrocarbon degradation, Appl. Catal., B, 2024, 359, 124499 CrossRef CAS.
- M. H. Urgesa, D.-H. Kuo, T. N. Gemeda, Q.-N. Ha and N. S. Gultom, Ambient NN bond weakening hydrogenation by utilizing the highly defective Cu3BiS3/MnO2 electrocatalyst for ammonia yield above 3 mg/h cm2: The N2-nano dipole interaction micromechanism, Appl. Catal., B, 2024, 355, 124194 CrossRef CAS.
- E. S. Ilton, J. E. Post, P. J. Heaney, F. T. Ling and S. N. Kerisit, XPS determination of Mn oxidation states in Mn (hydr)oxides, Appl. Surf. Sci., 2016, 366, 475–485 CrossRef CAS.
- T. Liu, Z. Pan, J. J. M. Vequizo, K. Kato, B. Wu, A. Yamakata, K. Katayama, B. Chen, C. Chu and K. Domen, Overall photosynthesis of H2O2 by an inorganic semiconductor, Nat. Commun., 2022, 13, 1034 CrossRef CAS.
- T. Ghosh, M. B. Vukmirovic, F. J. DiSalvo and R. R. Adzic, Intermetallics as Novel Supports for Pt Monolayer O2 Reduction Electrocatalysts: Potential for Significantly Improving Properties, J. Am. Chem. Soc., 2010, 132, 906–907 CrossRef CAS PubMed.
- N. Yang, S. Zhang, Y. Xiao, Y. Qi, Y. Bao, P. Xu, S. Jin and F. Zhang, Insight into the Key Restriction of BiVO4 Photoanodes Prepared by Pyrolysis Method for Scalable Preparation, Angew. Chem., Int. Ed., 2023, 62, e202308729 CrossRef CAS PubMed.
- Y. Sheng, I. A. Abreu, D. E. Cabelli, M. J. Maroney, A.-F. Miller, M. Teixeira and J. S. Valentine, Superoxide Dismutases and Superoxide Reductases, Chem. Rev., 2014, 114, 3854–3918 CrossRef CAS PubMed.
- F. Puga, J. A. Navío and M. C. Hidalgo, A critical view about use of scavengers for reactive species in heterogeneous photocatalysis, Appl. Catal., A, 2024, 685, 119879 CrossRef CAS.
- J. Li, X. Liu, Y. Zheng, Z. Cui, H. Jiang, Z. Li, S. Zhu and S. Wu, Achieving Fast Charge Separation by Ferroelectric Ultrasonic Interfacial Engineering for Rapid Sonotherapy of Bacteria-Infected Osteomyelitis, Adv. Mater., 2023, 35, 2210296 CrossRef CAS.
- Y. Wang, R. Zhang, P. Zang, R. Zhao, L. Wu, Y. Zhu, D. Yang, S. Gai and P. Yang, Synergizing Pyroelectric Catalysis and Enzyme Catalysis: Establishing a Reciprocal and Synergistic Model to Enhance Anti-Tumor Activity, Adv. Mater., 2024, 36, 2401111 CrossRef CAS.
- C. Cao, H. Zou, N. Yang, H. Li, Y. Cai, X. Song, J. Shao, P. Chen, X. Mou, W. Wang and X. Dong, Fe3O4/Ag/Bi2MoO6 Photoactivatable Nanozyme for Self-Replenishing and Sustainable Cascaded Nanocatalytic Cancer Therapy, Adv. Mater., 2021, 33, 2106996 CrossRef CAS.
- Y. Qi, B. Zhang, G. Zhang, Z. Zheng, T. Xie, S. Chen, G. Ma, C. Li, K. Domen and F. Zhang, Efficient overall water splitting of a suspended photocatalyst boosted by metal-support interaction, Joule, 2024, 8, 193–203 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2026 |