Construction of higher-ordered structures in alkylpyridinium-based polymers via π-conjugated anion pairing and humidity annealing
Erika
Saito
a,
Taeka
Yonekura
a,
Shuji
Okada
 a,
Shotaro
Nishitsuji
a,
Shotaro
Nishitsuji
 a,
Jun
Matsui
a,
Jun
Matsui
 *b and
Ryohei
Yamakado
*b and
Ryohei
Yamakado
 *a
*a
aDepartment of Organic Materials Science, Graduate School of Organic Materials Science, Yamagata University, Yonezawa 992-8510, Japan. E-mail: yamakado@yz.yamagata-u.ac.jp
bFaculty of Science, Yamagata University, Yamagata 990-8560, Japan. E-mail: jun_m@sci.kj.yamagata-u.ac.jp
First published on 5th November 2025
Abstract
In this study, π-conjugated anions were used as counterions in poly(4-vinyl-N-alkylpyridinium) (P4VCxP+) to construct highly ordered ionic microstructures. Nuclear magnetic resonance, Fourier transform infrared, and ultraviolet–visible spectroscopies, and differential scanning calorimetry were employed to characterize the resulting ion-pair polymers. Furthermore, thin films were fabricated and subjected to humidity annealing above the glass transition temperature. Grazing-incidence small-angle X-ray scattering showed that humidity annealing promoted the formation of hexagonally packed cylindrical microdomains in P4VCxP+ with π-conjugated anions. The π-conjugated anions increased the effective hydrophilic volume and facilitated the structural alignment via π–π and dipole–dipole interactions. Optical absorption analysis indicated that both pyridinium and π-conjugated anions exhibited end-on orientations and revealed the dynamic behavior of 7,7,8,8-tetracyanoquinodimethane radical anion, including dimerization and charge-transfer complex formation. Thus, π-conjugated ion pairs show significant promise for the controlled self-assembly and functional optical properties of polymeric materials.
Introduction
Microstructures composed of multiple chemical species are widely employed in materials for ionic conduction, gas separation, and photonic applications, depending on the combination of their constituent elements.1,2 Intermolecular interactions, including π–π interactions, electrostatic interactions, and van der Waals interactions, are effective for controlling the assembled structure of such multi-component systems. In polymer chemistry, microstructures are constructed by the segregation between polymer chains in block copolymers. By controlling the volume ratio of each block, self-assembled structures such as spheres, cylinders, gyroids and lamellae can be formed.3 Notably, our group has recently reported a higher-order structure through humidity annealing of comb-shaped homo(co)polymers composed of polar main chains with non-polar alkyl chains.4 Water is adsorbed onto the amide and ester main chains, thereby increasing the segregation force between the polar main chains and non-polar side chains, which leads to the formation of a lamellar structure. Subsequently, Terashima et al. reported that water annealing can be used to form higher-order structures in cationic polymers with nonpolar alkyl chains.5,6 The counter anion plays a key role in enhancing the hydrophilicity of these polymers.However, the influence of the interactions between counter anions or anions and cations on the higher-order structure of polymers has not been explored. In addition, the majority of higher-order structures reported to be induced by humid annealing are lamellar structures,4–9 with only a few studies reporting the formation of more highly ordered cylindrical structures.10,11 Ion-pair materials offer a notable advantage because different combinations can impart diverse functions and properties. We previously reported the synthesis of poly(4-vinyl-N-dodecylpyridinium) (P4VC12P+) and introduced a fan-shaped anion as a counter anion to form a hexagonal columnar structure.12 Furthermore, by incorporating a photo-responsive counter anion enabled photoisomerization and mass transfer upon ultraviolet (UV) irradiation.
Based on this background, the present study investigated two types of π-conjugated anions, 1,3-(bisdicyanomethylidene)indan anion (In−)13 and 7,7,8,8-tetracyanoquinodimethane radical anion (TCNQ˙−),14–19 which are expected to promote the formation of higher-order structures and impart functional properties. The stable π-conjugated anion, In−, is produced through the deprotonation of indane. As evidenced in prior research, its significant dipole moment allows it to overcome electrostatic repulsion between identical charges, thereby facilitating the formation of dimers.20,21 Similarly, TCNQ˙− is known to facilitate structural control and modulate electrical conductivity when paired with suitable cations, as reported by Akutagawa et al.22 In this study, we aimed to construct highly ordered microstructures and assess the optoelectronic properties of In− and TCNQ˙− by introducing them as counter anions in cationic polymers. We synthesized poly(4-vinyl-N-decylpyridinium) (P4VC10P+) and poly(4-vinyl-N-tetradecylpyridinium) (P4VC14P+), incorporating In− and TCNQ˙− as counter anions and evaluated their higher-order structures and optical properties. Consequently, we successfully constructed a hexagonal columnar structure of ionic polymers comprising π-conjugated anions via humidity annealing for the first time.
Experimental
Materials and methods
The reagents were purchased from FUJIFILM Wako Pure Chemical, Kanto Chemical, TCI, MITSUBISHI Chemical, and Sigma-Aldrich, and used without further purification unless otherwise stated. The solvents, chloroform (CHCl3, ≥99.0%), n-hexane, methanol (MeOH, ≥99.8%), ethanol (≥99.5%), tetrahydrofuran (THF, ≥99.0%) and N,N-dimethylformamide (DMF, ≥99.9%), were used without further purification. 1H and 13C nuclear magnetic resonance (NMR) spectroscopies were investigated on JEOL ECX-500 500 MHz spectrometers using CDCl3 and DMSO-d6 as the internal standards. The phase transitions were measured on a differential scanning calorimetry using Hitachi SII EXSTAR DSC6220. UV-visible (vis)-near infrared (NIR) diffuse reflectance spectra were recorded on a JASCO V-750 spectrometer using a quartz plate.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively, onto the glass substrates under ambient conditions (600 rpm for 10 s, followed by 1000 rpm for 60 s). To remove the solvent, the films were annealed in vacuum at 50 °C for 1 h. Humidity annealing was performed in a sealed glass container with pure water at 60 or 65 °C in an oven for 0.5, 1, 3, 6, 12, and 24 h.
1), respectively, onto the glass substrates under ambient conditions (600 rpm for 10 s, followed by 1000 rpm for 60 s). To remove the solvent, the films were annealed in vacuum at 50 °C for 1 h. Humidity annealing was performed in a sealed glass container with pure water at 60 or 65 °C in an oven for 0.5, 1, 3, 6, 12, and 24 h.
Synthesis of ionic polymer
Poly(4-vinyl-N-decylpyridinium bromide) (P4VC10P+–Br−). Pale yellow powder, 82% yield. 1H NMR (500 MHz, CDCl3, δ): 8.91 (2H, Ar–H), 7.99 (2H, Ar–H), 4.66 (2H, NCH2), 2.04 (2H, NCH2CH2), 1.59–1.00 (14H, C2H4(CH2)7), 0.88 (3H, C9H18CH3). IR (ATR, cm−1): 3745, 3648, 3392, 3116, 3043, 2922, 2360, 2337, 2046, 1995, 1944, 1869, 1844, 1792, 1772, 1749, 1716, 1637, 1558, 1541, 1516, 1466, 1417, 1375, 1230, 1173.
Poly(4-vinyl-N-tetradecylpyridinium bromide) (P4VC14P+–Br−). White powder, 80% yield. 1H NMR (500 MHz, CDCl3, δ): 8.98 (2H, Ar–H), 8.29 (2H, Ar–H), 4.79 (2H, NCH2), 2.06 (2H, NCH2CH2), 1.46–1.09 (22H, C2H4(CH2)11), 0.88 (3H, C13H26CH3). IR (ATR, cm−1): 3735, 3648, 3566, 3523, 2920, 2850, 2363, 2163, 2331, 1992, 1942, 1869, 1844, 1792, 1749, 1716, 1697, 1647, 1558, 1541, 1518, 1508, 1420, 1375, 1338, 1174.
Poly[4-vinyl-N-decylpyridinium 1,3-(bisdicyanomethylidene)indan anion] (P4VC10P+–In−). Deep blue powder, 78% yield. 1H NMR (500 MHz, DMSO-d6, δ): 8.91 (2H, Ar–H), 7.99 (2H, Ar–H), 7.91–7.88 (m, 2H), 7.41–7.39 (m, 2H), 5.65 (1H, s), 4.45 (2H, NCH2), 2.09 (2H, NCH2CH2), 1.48–1.04 (14H, C2H4(CH2)7), 0.84 (3H, C9H18CH3). IR (ATR, cm−1): 3735, 3689, 3649, 3568, 3545, 3523, 2981, 2922, 2852, 2362, 2330, 2185, 1992, 1967, 1869, 1844, 1792, 1772, 1749, 1716, 1647, 1541, 1516, 1508, 1473, 1456, 1417, 1396, 1362, 1338, 1178, 690, 669, 617.
Poly[4-vinyl-N-tetradecylpyridinium 1,3-(bisdicyanomethylidene)indan anion] (P4VC14P+–In−). Deep blue powder, 66% yield. 1H NMR (500 MHz, DMSO-d6, δ):8.90 (2H, Ar–H), 8.30 (2H, Ar–H), 7.90–7.88 (m, 2H), 7.41–7.38 (m, 2H), 5.66 (1H, s), 4.50 (2H, NCH2), 2.08 (2H, NCH2CH2), 1.48–1.05 (22H, C2H4(CH2)11), 0.84 (3H, C13H26CH3). IR (ATR, cm−1): 3735, 3689, 3649, 3566, 3545, 3523, 2981, 2920, 2850, 2360, 2332, 2183, 1967, 1869, 1844, 1792, 1772, 1749, 1716, 1697, 1647, 1541, 1522, 1508, 1473, 1458, 1419, 1396, 1362, 1338, 1177, 688, 669, 617.
Poly(4-vinyl-N-decylpyridinium 7,7,8,8-tetracyanoquinodimethane radical anion) (P4VC10P+–TCNQ˙−). Deep blue powder, yield: 81%, IR (ATR, cm−1): 3520, 3391, 3123, 3045, 2952, 2233, 2175, 2122, 1988, 1942, 1919, 1868, 1844, 1772, 1749, 1697, 1684, 1647, 1558, 1540, 1506, 1458, 1417, 1361, 1338, 1319, 1270, 1170, 1102, 1017, 827, 659.
Poly(4-vinyl-N-tetradecylpyridinium 7,7,8,8-tetracyanoquinodimethane radical anion) (P4VC14P+–TCNQ˙−). Deep blue solid, yield: 65%, IR (ATR, cm−1): 3523, 3124, 3051, 2952, 2921, 2852, 2168, 2123, 1942, 1919, 1869, 1844, 1792, 1772, 1749, 1704, 1684, 1637, 1593, 1541, 1506, 1466, 1437, 1362, 1319, 1271, 1171, 1103, 1047, 1022, 827, 769, 665.
Results and discussion
Synthesis
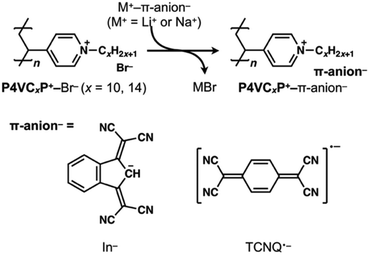
Ion pairs of poly(4-vinyl-N-alkylpyridinium) cations (P4VCxP+, x = 10 or 14) with either In−or TCNQ˙− were prepared via an ion exchange reaction using the bromide salt of P4VCxP+ (P4VCxP+–Br−) and either the sodium salt of In− (Na+–In−) or lithium salt of TCNQ˙− (Li+–TCNQ˙−) (Fig. 1). 1H and 13C NMR, along with FT-IR spectroscopy, were used to characterize the synthesized ion-pair polymers. UV-vis absorption spectra were recorded in MeOH and DMF to confirm the successful ion exchange. The absorption spectra of P4VCxP+–Br− (x = 10 or 14) in MeOH showed a band at approximately 230–240 nm and 260 nm, which were attributed to the absorption from the pyridine group (Fig. 2(a)). In contrast, the ion-exchanged polymers exhibited absorption bands corresponding to π-conjugated anions at 582 nm for In− (Fig. 2(b)) and 410 and 850 nm for TCNQ˙− (Fig. 2(c)). Furthermore, the degree of anion incorporation was determined by method-specific spectroscopy: for In−, incorporation was quantified from the 1H NMR integral ratio between the CH proton at the 2-position of the indan anion and the methylene protons adjacent to the quaternary nitrogen of the polymer backbone (Fig. S4 and 5); for TCNQ˙−, incorporation was estimated from UV–vis absorption analysis because the radical anion is not observable by NMR (Table S1). Using these procedures, the incorporation ratios were 58% (P4VC10P+–In−), 64% (P4VC14P+–In−), 59% (P4VC10P+–TCNQ˙−), and 49% (P4VC14P+–TCNQ˙−).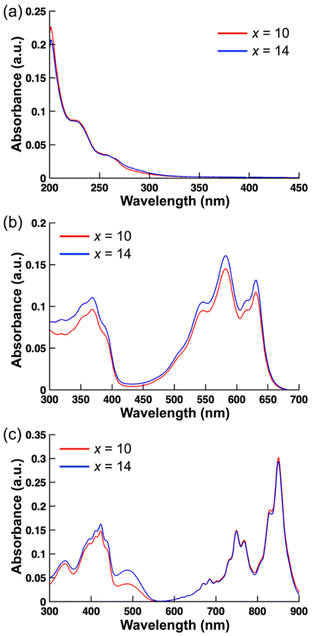 | ||
| Fig. 2 UV–vis absorption spectra of (a) P4VCxP+–Br− in MeOH, (b) P4VCxP+–In− in DMF, and (c) P4VC10P+–TCNQ˙− in DMF (10−5 M). | ||
Thermal properties
DSC was employed to examine the thermal properties of ion pairs in their bulk states. The DSC thermograms show baseline shifts corresponding to glass transition temperatures (Tg) of 50, 45, 57, 56, 58, and 49 °C for P4VC10P+–Br−, P4VC14P+–Br−, P4VC10P+–In−, P4VC14P+–In−, P4VC10P+–TCNQ˙−, and P4VC14P+–TCNQ˙−, respectively (Fig. S7). The incorporation of π-conjugated anions restricted the mobility of the polymer chains, resulting in a slight increase in the Tg. The absence of melting points for the long alkyl chains suggests that they did not crystallize within the measured temperature range, which is consistent with previous studies.4,23 On the other hands, the TG–DTA curves showed no significant weight loss below 100 °C, confirming the absence of residual solvent molecules (Fig. S8).X-ray diffraction measurement
Thin films of P4VCxP+–Br−, P4VCxP+–In−, and P4VCxP+–TCNQ˙− (x = 10, 14) were prepared by spin-coating using chloroform, THF, and a DMF/THF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
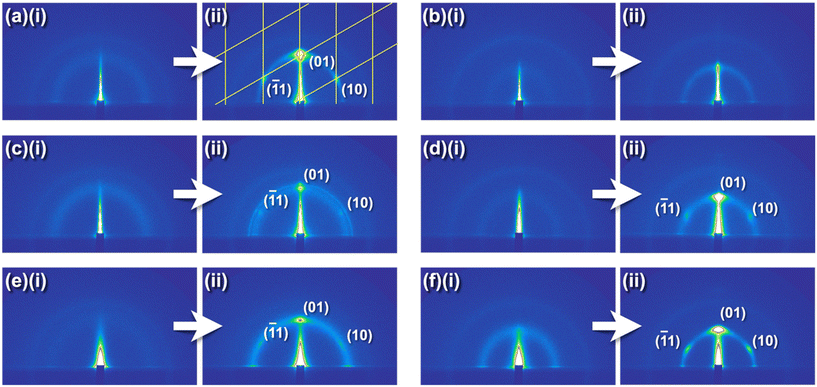
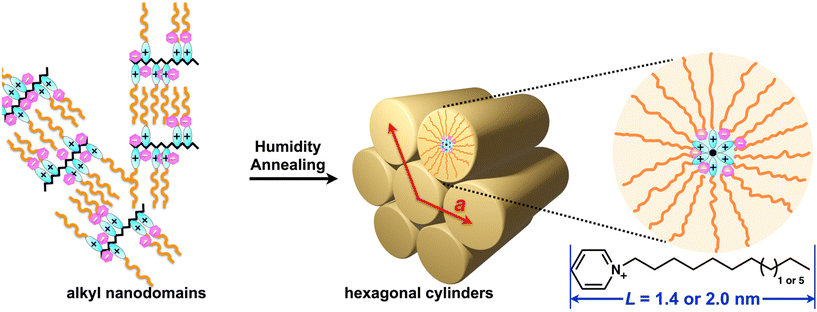
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution for P4VCxP+–Br−, P4VCxP+–In−, and P4VCxP+–TCNQ˙−, respectively. The films were subjected to annealing for durations of 0, 0.5, 1, 3, 6, 12, and 24 h under conditions of saturated humidity at 60 °C (for P4VCxP+–Br− and P4VCxP+–TCNQ˙−) or 65 °C (for P4VCxP+–In−), which is above the Tg. Subsequently, the structural characteristics of the films were analyzed using GI-SAXS (Fig. 3 and Table 1). Before humidity annealing, P4VC10P+–Br− and P4VC14P+–Br− exhibited Debye–Scherrer rings in the 2D diffraction profiles at d = 3.0 and 4.0 nm, respectively (Fig. 3(a)(i) and (b)(i)). This scattering can be attributed to aggregation of the alkyl side chains.23 In contrast, a humidity-annealed film of P4VC10P+–Br− exhibited spot-like diffraction peaks at q = 2.55 nm−1 (d = 2.46 nm) in two distinct directions: the out-of plane direction and a direction inclined approximately 60° from the surface normal. These peaks indicated the formation of hexagonally packed cylindrical structures oriented parallel to the substrate plane.24 The lattice constant was determined to be a = 2.8 nm, which is approximately twice the extended chain model of N-decylpyridinium (L = 1.4 nm). These findings suggested the formation of a columnar structure, in which the alkyl side chains on the pyridinium groups were oriented outward from the polymer backbone (Fig. 4). The phase morphology and domain spacing of P4VC10P+–Br− was further observed by scanning transmission electron microscopy (STEM, Fig. S22). In contrast, humidity annealing had no effect on the diffraction peak in P4VC14P+–Br− (Fig. 3(b)(ii)). Strong hydrophilic-hydrophilic interactions have been reported to occur in long side chains, which hinder the segregation force.4 Therefore, the tetradecyl side chains likely remained in the aggregated structure of the humidity-annealed film. These results indicated that the length of the alkyl groups plays a crucial role in the formation of the microphase-separated structure of P4VCxP+. By utilizing the higher-order structures of these cationic polymers, π-conjugated anions such as In− and TCNQ˙− are anticipated to be spatially organized. In the GI-SAXS measurements of P4VC10P+–In− thin films, a Debye–Scherrer ring (q = 2.34 nm−1) was observed before humidity annealing, whereas distinct diffraction peaks at q = 2.69 nm−1 appeared after humidity annealing. These findings indicated a structural transition from alkyl nanodomains to a hexagonal columnar structure induced by humidity annealing, which is similar to that observed for P4VC10P+–Br−. A similar transition was observed for P4VC10P+–TCNQ˙−. These finding indicate that even when the counter anion was altered from Br− to bulkier species such as In− or TCNQ˙−, the formation of higher-order structures in P4VC10P+ was not impeded.
1) solution for P4VCxP+–Br−, P4VCxP+–In−, and P4VCxP+–TCNQ˙−, respectively. The films were subjected to annealing for durations of 0, 0.5, 1, 3, 6, 12, and 24 h under conditions of saturated humidity at 60 °C (for P4VCxP+–Br− and P4VCxP+–TCNQ˙−) or 65 °C (for P4VCxP+–In−), which is above the Tg. Subsequently, the structural characteristics of the films were analyzed using GI-SAXS (Fig. 3 and Table 1). Before humidity annealing, P4VC10P+–Br− and P4VC14P+–Br− exhibited Debye–Scherrer rings in the 2D diffraction profiles at d = 3.0 and 4.0 nm, respectively (Fig. 3(a)(i) and (b)(i)). This scattering can be attributed to aggregation of the alkyl side chains.23 In contrast, a humidity-annealed film of P4VC10P+–Br− exhibited spot-like diffraction peaks at q = 2.55 nm−1 (d = 2.46 nm) in two distinct directions: the out-of plane direction and a direction inclined approximately 60° from the surface normal. These peaks indicated the formation of hexagonally packed cylindrical structures oriented parallel to the substrate plane.24 The lattice constant was determined to be a = 2.8 nm, which is approximately twice the extended chain model of N-decylpyridinium (L = 1.4 nm). These findings suggested the formation of a columnar structure, in which the alkyl side chains on the pyridinium groups were oriented outward from the polymer backbone (Fig. 4). The phase morphology and domain spacing of P4VC10P+–Br− was further observed by scanning transmission electron microscopy (STEM, Fig. S22). In contrast, humidity annealing had no effect on the diffraction peak in P4VC14P+–Br− (Fig. 3(b)(ii)). Strong hydrophilic-hydrophilic interactions have been reported to occur in long side chains, which hinder the segregation force.4 Therefore, the tetradecyl side chains likely remained in the aggregated structure of the humidity-annealed film. These results indicated that the length of the alkyl groups plays a crucial role in the formation of the microphase-separated structure of P4VCxP+. By utilizing the higher-order structures of these cationic polymers, π-conjugated anions such as In− and TCNQ˙− are anticipated to be spatially organized. In the GI-SAXS measurements of P4VC10P+–In− thin films, a Debye–Scherrer ring (q = 2.34 nm−1) was observed before humidity annealing, whereas distinct diffraction peaks at q = 2.69 nm−1 appeared after humidity annealing. These findings indicated a structural transition from alkyl nanodomains to a hexagonal columnar structure induced by humidity annealing, which is similar to that observed for P4VC10P+–Br−. A similar transition was observed for P4VC10P+–TCNQ˙−. These finding indicate that even when the counter anion was altered from Br− to bulkier species such as In− or TCNQ˙−, the formation of higher-order structures in P4VC10P+ was not impeded.
| Samples | Annealing | q/nm−1 (d/nm) | Morphology |
|---|---|---|---|
| q values correspond to the peak positions observed in the GI-SAXS patterns shown in Fig. 3. | |||
| P4VC10P+–Br− | 0 h | 2.09 (3.01) | |
| 6.55 (0.95) | |||
| 24 h | 2.55 (2.46) | Hexagonal (a = 2.8 nm) | |
| 7.35 (0.85) | |||
| P4VC14P+–Br− | 0 h | 1.55 (4.04) | |
| 6.49 (0.97) | |||
| 24 h | 1.88 (3.34) | ||
| 7.41 (0.85) | |||
| P4VC10P+–In− | 0 h | 2.34 (2.68) | |
| 24 h | 2.69 (2.40) | Hexagonal (a = 2.7 nm) | |
| P4VC14P+–In− | 0 h | 2.00 (3.15) | |
| 24 h | 2.11 (2.98) | Hexagonal (a = 3.4 nm) | |
| P4VC10P+–TCNQ˙− | 0 h | 2.03 (3.09) | |
| 6.55 (0.96) | |||
| 24 h | 2.48 (2.53) | Hexagonal (a = 2.9 nm) | |
| 16.13 (0.39) | |||
| P4VC14P+–TCNQ˙− | 0 h | 1.73 (3.63) | |
| 6.72 (0.93) | |||
| 24 h | 2.02 (3.12) | Hexagonal (a = 3.6 nm) | |
| 15.60 (0.40) | |||
Notably, P4VC14P+, which did not exhibit higher-order structure formation under humidity annealing with Br−, successfully formed a hexagonal columnar structure when In− or TCNQ˙− was used as the counter anion. The large π-conjugated anions increase the hydrophilic volume. Simple calculations of Br− and π-conjugated anions indicated that the size of the π-conjugated anions was approximately four times larger than that of Br− (Fig. S23). Consequently, P4VC14P+ polymers self-assembled into hexagonal cylinders, similar to P4VC10P+, due to the segregation between the tetradecyl side chains and the hydrophilic main chain, in combination with π-conjugated anions. In addition, π-conjugated anions are expected to exhibit attractive forces owing to dipole–dipole and π–π interactions. Indeed, the 1D GI-WAXS profile exhibited a diffraction peak at q = 14–16 nm−1 for the TCNQ˙− salt, which can be attributed to the π–π stacking of TCNQ˙− (Table 1, Fig. S17 and S19). In summary, the GI-SAXS data revealed that all polymers initially exhibited random structures with the formation of alkyl nanodomains. Following humidity annealing, P4VC10P+ with a decyl alkyl chain forms a horizontally oriented hexagonal columnar structure on the substrate, regardless of the counter anions. In contrast, P4VC14P+ with a tetradecyl alkyl chain formed a hexagonal structure by humidity annealing only when it had a relatively large and highly hydrophilic π-conjugated anion. Interestingly, the lattice constants (a) of P4VC14P+–In− (a = 3.4 nm) and P4VC14P+–TCNQ˙− (a = 3.6 nm) were greater than those of P4VC10P+–In− (a = 2.7 nm) and P4VC10P+–TCNQ˙− (a = 2.9 nm), reflecting the influence of the alkyl chain length.
Optical properties in film state
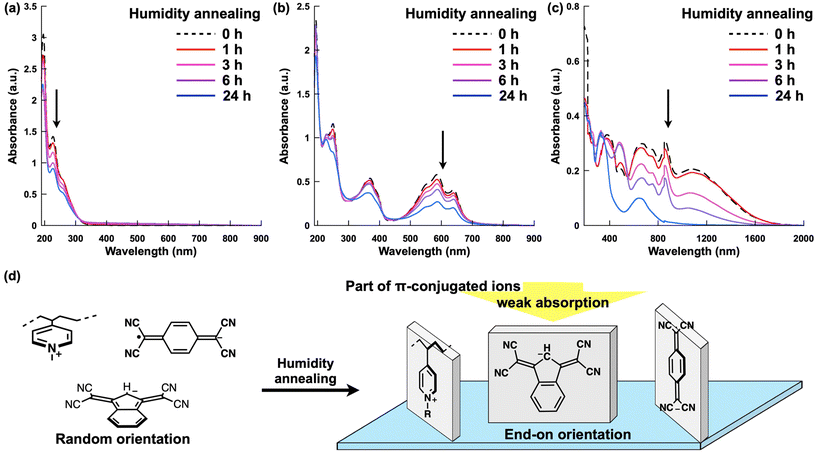
UV–vis absorption spectroscopy was used to characterize the optical properties of the structured films and investigated the orientation of the polymer and π-conjugated anions (Fig. 5 and S27–30). In P4VC10P+–Br−, an absorption band at approximately 230–280 nm from pyridine groups decreased with humidity annealing, which suggests that a part of pyridine rings adopted an end-on orientation relative to the substrate by formation of cylinders (Fig. 5a and d). This orientation is consistent with the proposed structure in which main chains are oriented parallel to the substrate plane. Although P4VC14P+–Br− did not form a hexagonal structure, similar changes in the absorption spectrum were observed, indicating an end-on orientation of the pyridine groups relative to the substrate. Notably, similar trends were observed for the absorption bands associated with the π-conjugated anions. For P4VCxP+–In−, the absorption peak position did not change, however, the intensity decreased following the formation of hexagonally aligned cylindrical structures (Fig. 5b). In addition, these decreases in the absorption bands were recovered by collapsing the cylindrical structure into a randomly oriented film through vapor annealing using THF (Fig. S29). These results indicated that the π-conjugated anions also adopted an end-on conformation within the cylinders (Fig. 5d). In addition, the spectrum of P4VCxP+–TCNQ˙− exhibited complicated changes following humidity annealing (Fig. 5c). Prior to annealing, the films exhibited multiple absorption peaks across a wide range from the visible to the NIR region. The absorption bands at 400–410 nm and 750–950 nm correspond to the characteristic transitions of TCNQ˙−, while the bands at 370 nm and 600–700 nm can be attributed to the transitions of the TCNQ˙− dimer.14 Furthermore, the broad absorption band at 1120 nm toward the NIR region suggests the formation of a charge-transfer (CT) state between P4VCxP+ and TCNQ˙−. These results indicated that TCNQ˙− coexisted in three distinct states in the pre-annealed film: monomeric, dimeric, and CT complexes. Annealing led to an overall decrease in absorption, with the absorption band at 680 nm derived from the TCNQ dimers remaining after 24 h. This behavior can be attributed to the dissociation of CT complexes and enhanced dimer formation, both of which resulted from changes in the higher-order structure caused by annealing. The overall decrease in the absorption can be explained by the end-on orientation of the π-conjugated systems. In addition, the transient increase followed by a decrease in absorption at 480 nm during annealing can be attributed to the partial oxidation of TCNQ˙− and subsequent formation of the α,α-dicyano-p-toluoylcyanide anion (DCTC−) (Fig. S31).25 The generation of DCTC− was also confirmed by NMR. However, this unexpected oxidation did not affect the ion balance. Thus, we conclude that the π-conjugated anions also adopted an end-on conformation within the cylinders.Conclusions
P4VCxP+–Br− with linear alkyl chains (x = 10 or 14) were successfully synthesized. Subsequently, ion exchange between Br− and In− or TCNQ˙− was performed to prepare P4VCxP+–In− and P4VCxP+–TCNQ˙−, incorporating In− and TCNQ˙− as the counter anions, respectively. Humidity annealing was used to form higher-order structures of these ionic polymers. GI-SAXS analysis revealed microphase-separated structures, indicating that both the alkyl chain length of the cationic polymers and counter anion species influenced the resulting morphologies of the ionic polymers. For the P4VCxP+–Br− films, a hexagonal columnar structure was formed following humidity annealing for x = 10, whereas no significant structural change was observed for x = 14. Notably, P4VCxP+–In− and P4VCxP+–TCNQ˙−, which contained In− and TCNQ˙− as counter anions, respectively, exhibited well-aligned hexagonal structures parallel to the glass substrate following humidity annealing. Furthermore, the π-conjugated anions adopted the end-on conformation within the cylinders. These results suggest that In− and TCNQ˙−, which have larger in volumes than Br− and exhibit π–π interactions, affect the higher-order structure of P4VCxP+. Therefore, the microphase-separated morphology of ionic polymers can be effectively tuned by simply varying the alkyl chain length and the counter anion. Although still speculative, the detailed mechanism by which π-conjugated anions facilitate the rapid transformation from a random aggregate to a hexagonal columnar structure during humidity annealing requires elucidation in future studies.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: synthetic procedures, optical properties, thermal properties, XRD patterns, DFT calculations. See DOI: https://doi.org/10.1039/d5py00879d.Data for this article, including synthetic procedures, analytical data, optical properties, and theoretical calculations, are available at https://doi.org/10.1039/x0xx00000x.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP21K14606, JP24K01292, JP24K21669, and JP24K08523, and JSPS Fellows Grant Numbers JP21J22405 and JP22KJ0332. The computation was performed using the Research Center for Computational Science, Okazaki, Japan (Project: 24-IMS-C093 and 25-IMS-C096). We thank Dr Hiromi Sekiguchi (ThermoFisher Scientific) for STEM measurements, and Prf. Seigo Kawaguchi and Dr Moriya Kikuchi (Yamagata University) for GPC measurements. The NMR analysis was performed using a JEOL JNM-EC500, JEOL Ltd introduced by the subsidy program for development of advanced research infrastructure. This work was partly supported by Program for Forming Japan's Peak Research Universities (J-PEAKS).References
- Z. Wang, C. L. C. Chan, T. H. Zhao, R. M. Parker and S. Vignolini, Adv. Opt. Mater., 2021, 9, 2100519, DOI:10.1002/adom.202100519.
- J. Zhu, Z. Zhang, S. Zhao, A. S. Westover, I. Belharouak and P. F. Cao, Adv. Energy Mater., 2021, 11, 2003836, DOI:10.1002/aenm.202003836.
- H. Feng, X. Lu, W. Wang, N. G. Kang and J. W. Mays, Polymers, 2017, 9, 494, DOI:10.3390/polym9100494.
- K. Ebata, Y. Hashimoto, S. Yamamoto, M. Mitsuishi, S. Nagano and J. Matsui, Macromolecules, 2019, 52, 9773–9780, DOI:10.1021/acs.macromol.9b01817.
- S. Imai, M. Arakawa, Y. Nakanishi, M. Takenaka, H. Aoki, M. Ouchi and T. Terashima, Macromolecules, 2022, 55, 9113–9125, DOI:10.1021/acs.macromol.2c01287.
- R. Sujita, S. Imai, M. Ouchi, H. Aoki and T. Terashima, Macromolecules, 2023, 56, 9738–9749, DOI:10.1021/acs.macromol.3c01722.
- T. Ikami, Y. Watanabe, H. Ogawa, M. Takenaka, N. L. Yamada, M. Ouchi, H. Aoki and T. Terashima, ACS Macro Lett., 2021, 10, 1524–1528, DOI:10.1021/acsmacrolett.1c00571.
- M. Kikuchi, N. Saito, M. Ohke, S. Nagano, S. Nishitsuji and J. Matsui, Soft Matter, 2023, 19, 3058–3068, 10.1039/d3sm00265a.
- M. Hara, A. Kodama, S. Washiyama, Y. Fujii, S. Nagano and T. Seki, Macromolecules, 2022, 55, 4313–4319, DOI:10.1021/acs.macromol.2c00404.
- Z. K. Zhang, S. P. Ding, D. L. Xia and J. T. Xu, ACS Macro Lett., 2023, 12, 1005–1011, DOI:10.1021/acsmacrolett.3c00277.
- S. Weng, Z. Lu, J. Cai, L. Xiao, K. Wang, R. Zhao, X. Fu and L. Hou, Polym. Chem., 2025, 16, 2506–2513, 10.1039/d5py00135h.
- R. Yamakado, I. Kitamura, M. Hara, S. Nagano, T. Seki and H. Maeda, Chem. Commun., 2021, 57, 4287–4290, 10.1039/d0cc07640f.
- Y. Son and S. Kim, Dyes Pigm., 2005, 64, 153–155, DOI:10.1016/j.dyepig.2004.05.006.
- Y. Iida, Bull. Chem. Soc. Jpn., 1969, 42, 71–75, DOI:10.1246/bcsj.42.71.
- J. M. Lu, S. V. Rosokha and J. K. Kochi, J. Am. Chem. Soc., 2003, 125, 12161–12171, DOI:10.1021/ja0364928.
- A. L. Sutton, B. F. Abrahams, D. M. D'Alessandro, R. W. Elliott, T. A. Hudson, R. Robson and P. M. Usov, CrystEngComm, 2014, 16, 5234–5243, 10.1039/c4ce00289j.
- A. L. Sutton, B. F. Abrahams, D. M. D'Alessandro, T. A. Hudson, R. Robson and P. M. Usov, CrystEngComm, 2016, 18, 8906–8914, 10.1039/c6ce02015a.
- L. Ma, P. Hu, H. Jiang, C. Kloc, H. Sun, C. Soci and A. A. Voityuk, Sci. Rep., 2016, 6, 28510, DOI:10.1038/srep28510.
- Y. Irie, L. Li, H. Imoto, M. Komada, T. Nishino and K. Naka, RSC Adv., 2016, 6, 114513–114518, 10.1039/c6ra21636f.
- Y. Tanaka, K. Ichijo, S. Kodama, S. Aoyama, T. Yoshida, R. Yamakado and S. Okada, Cryst. Growth Des., 2019, 19, 5811–5818, DOI:10.1021/acs.cgd.9b00841.
- E. Saito, R. Yamakado, T. Yasuhara, H. Yamaguchi, S. Okada and T. Yoshida, RSC Adv., 2023, 13, 32039–32044, 10.1039/d3ra06782c.
- K. Sambe, N. Hoshino, T. Takeda, T. Nakamura and T. Akutagawa, Cryst. Growth Des., 2020, 20, 3625–3634, DOI:10.1021/acs.cgd.0c00169.
- M. Beiner and H. Huth, Nat. Mater., 2003, 2, 595–599, DOI:10.1038/nmat966.
- M. Hara, N. Wakitani, A. Kodama, S. Nagano and T. Seki, ACS Appl. Polym. Mater., 2020, 2, 2284–2290, DOI:10.1021/acsapm.0c00253.
- C. A. M. Weir, T. Hadizad, A. M. R. Beaudin and Z. Y. Wang, Tetrahedron Lett., 2003, 44, 4697–4700, DOI:10.1016/s0040-4039(03)01054-2.
| This journal is © The Royal Society of Chemistry 2026 |