Thermoresponsive simple coacervation of copolymers composed of LCST-type and hydrophilic monomers
Haowei
Sun
a,
Takafumi
Enomoto
 *a,
Shota
Michida
b,
Takuya
Katashima
b and
Ryo
Yoshida
*a,
Shota
Michida
b,
Takuya
Katashima
b and
Ryo
Yoshida
 *a
*a
aDepartment of Materials Engineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: enomoto@cross.t.u-tokyo.ac.jp; ryo@cross.t.u-tokyo.ac.jp
bDepartment of Bioengineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 10th December 2025
Abstract
Coacervates have emerged as promising systems for achieving dynamic compartmentalization. In particular, synthetic polymeric complex coacervates represent a well-established class. However, they often disassemble at high ionic strength condition due to charge screening effects. In contrast, simple coacervates, which form via phase separation of a single polymer species, serve as complementary systems that can exist under such conditions. Despite this advantage, the design and understanding of synthetic simple coacervates remains limited. Here, we report the thermoresponsive simple coacervation behavior of synthetic copolymers compose of lower critical solution temperature (LCST)-type and hydrophilic monomers. Through systematic investigations, we revealed that a wide variety of combinations of LCST-type monomers and hydrophilic monomers enables thermoresponsive simple coacervation in water in the presence of salts. Our findings provide a guideline for designing thermoresponsive simple coacervate systems based on synthetic LCST-type copolymers. We also highlight the importance of carefully characterizing the microphases that emerge upon phase separation of thermoresponsive copolymers under each specific usage condition.
Introduction
Coacervates, membraneless condensed droplets formed via liquid–liquid phase separation, have attracted considerable attention because they combine compartmentalization with an open-system nature, thereby enabling dynamic molecular exchange with their surroundings.1–4 In particular, polymer-based coacervates have emerged as versatile platforms, inherently possessing both the tunability of molecular design and the dynamic, liquid-like properties of coacervates.5,6 This unique combination affords precise control over chemical functionality, responsiveness, and phase behavior, making polymer coacervates highly adaptable for diverse applications, including artificial cell models,2,6–9 the development of chemical reaction networks,10–14 and drug delivery systems.15–19Complex coacervates, constituting a major class of polymer coacervates, form through electrostatic interactions between oppositely charged polyelectrolytes, resulting in the separation of a dense coacervate phase from the surrounding dilute phase.3,7,11,20 The formation and stability of complex coacervates are highly dependent on environmental factors such as ionic strength, pH, and polymer concentration.21–24 At high ionic strength condition, charge shielding weakens electrostatic interactions between the polyelectrolytes, destabilizing the coacervate structure and ultimately leading to their dissolution.24–26 In contrast, simple coacervates, which form via coacervation of a single type of polymer, serve as complementary systems to complex coacervates because they are able to exist under high ionic strength conditions.27,28 Many studies have focused on developing simple coacervation systems and elucidating the various mechanisms that contribute to simple coacervation.29 In this context, thermoresponsive peptide systems represent an important class of simple coacervation.1,17,30 However, the corresponding systems based on thermoresponsive synthetic polymers remain largely unexplored. For example, only a few studies have reported simple coacervation using synthetic copolymers composed of thermoresponsive monomer units.28,31–35 A systematic investigation of thermoresponsive simple coacervation using synthetic polymers is still lacking, and a general design principle for forming such coacervates has yet to be established.
In this study, we report the thermoresponsive simple coacervation behavior of a series of synthetic copolymers composed of lower critical solution temperature (LCST)-type and hydrophilic monomer units (Fig. 1). We found that a wide variety of combinations of these monomers exhibit LCST-type simple coacervation in water in the presence of salts. Our results offer a guideline for designing LCST-type simple coacervates using synthetic copolymers. We emphasize the need for careful characterization of the microphase properties of copolymers composed of LCST-type and hydrophilic monomers under each specific usage condition.
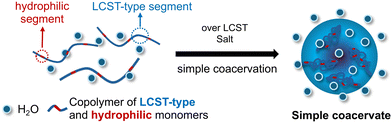 | ||
| Fig. 1 Simple coacervation of copolymers of LCST-type and hydrophilic monomers in presence of salts. | ||
Experimental
Materials
N-Isopropylacrylaminde (NIPAAm) was kindly provided by KJ Chemicals (Tokyo, Japan) and purified by recrystallization from toluene/n-hexane. N-(3-Aminopropyl) methacrylamide (NAPMAm) hydrochloride was purchased from Polyscience (Warrington, PA, USA) and purified by reprecipitation from methanol/tetrahydrofuran. 2-[[(2-Carboxyethyl) sulfanylthiocarbonyl]-sulfanyl]propanoic acid (CSTSP) was purchased from ALDRICH (Burlington, MA, United States). N,N-Dimethyl acrylamide (DMAAm), acrylamide (AAm), [3-(methacryloylamino)propyl]trimethylammonium chloride (MAPTAC), 2-acrylamido-2-methylpropane sulfonic acid (AMPS), N-(3-aminopropyl) methacrylamide hydrochloride (NAPMAm), 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044), N-propylacryamide (N-n-PAAm), N-vinylcaprolactam (NVCL), 2-(dimethylamino)ethyl methacrylate (DMAEMA), poly(ethylene glycol) methyl ether methacrylate (PEGMEM) were purchased from Wako Pure Chemical Industries (Osaka, Japan).Preparation and characterization of copolymers of LCST-type and hydrophilic monomers
We synthesized copolymers of LCST-type and hydrophilic monomers to study their phase separation behavior. The reaction conditions for the copolymerizations are summarized in Tables S1–S5. These copolymers were prepared by a reversible addition fragmentation chain transfer (RAFT) copolymerization. Firstly, a LCST-type monomer, a hydrophilic monomer, and a chain transfer reagent (CTA) CSTSP were placed in a round-bottom flask and methanol was added to dissolve all chemicals. VA-044 was then added to the solution. Dimethylformamide (DMF) was put in the flask when synthesizing DMAEMA and NVCL series polymers to trace the conversion ratio. The solution was degassed by Ar bubbling for 30 minutes in an ice bath. The RAFT polymerization was carried out at 50 °C for 1.5 hours. 1H nuclear magnetic resonance (1H-NMR) spectroscopy was performed to calculate the monomer conversion ratio and to confirm the polymer structure (Fig. S2–S34) using a JLM-LA400WB spectrometer (JEOL, Tokyo, Japan). From the 1H-NMR analysis, polymer compositions and number average molecular weights (Mn(NMR)) were determined respectively. The reaction mixture was dialyzed against deionized water for three days. The synthesized polymers were collected after lyophilization. The final product was characterized by 1H-NMR. The polydispersity indexes (PDIs) of synthesized polymers were determined from the GPC traces collected by a gel permeation chromatography (GPC) system (Jasco, Tokyo, Japan). The GPC traces are shown in Fig. S36–S40 and Table 1.| LCST-type monomers | Hydrophilic monomers | Polymer structure | M n(1H-NMR) | M w/Mn |
|---|---|---|---|---|
| a NVCL structure was broken after polymerization (Fig. S35). | ||||
| NIPAAm | DMAAm | Poly(NIPAAm220.1-co-DMAAm12.9)-CTA | 26.2 kDa | 1.302 |
| AAm | Poly(NIPAAm220.1-co-AAm12.4)-CTA | 25.8 kDa | 1.182 | |
| MAPTAC | Poly(NIPAAm203.6-co-MAPTAC11.9)-CTA | 25.7 kDa | 1.324 | |
| AMPS | Poly(NIPAAm249.0-co-AMPS14.1)-CTA | 31.1 kDa | 1.428 | |
| NAPMAm | Poly(NIPAAm194.6-co-NAPMAm12.7)-CTA | 24.3 kDa | 1.527 | |
| N-n-PAAm | DMAAm | Poly(N-n-PAAm119.0-co-DMAAm11.0)-CTA | 14.6 kDa | 1.499 |
| MAPTAC | Poly(N-n-PAAm98.2-co-MAPTAC11.3)-CTA | 13.6 kDa | 1.503 | |
| AMPS | Poly(N-n-PAAm205.5-co-AMPS12.6)-CTA | 25.9 kDa | 1.503 | |
| PEGMEM | DMAAm | Poly(PEGMEM132.4-co-DMAAm8.2)-CTA | 67.0 kDa | 3.769 |
| MAPTAC | Poly(PEGMEM165.4-co-MAPTAC10.9)-CTA | 85.1 kDa | 2.692 | |
| AMPS | Poly(PEGMEM167.6-co-AMPS13.3)-CTA | 85.4 kDa | 2.287 | |
| DMAEMA | DMAAm | Poly(DMAEMA242.2-co-DMAAm11.7)-CTA | 38.1 kDa | 1.721 |
| MAPTAC | Poly(DMAEMA235.0-co-MAPTAC6.9)-CTA | 37.1 kDa | 1.725 | |
| AMPS | Poly(DMAEMA227.8-co-AMPS5.5)-CTA | 36.0 kDa | 1.982 | |
| NVCL | DMAAm | Poly(NVCL208.6-co-DMAAm12.9)-CTA | 30.3 kDa | 1.747 |
| MAPTAC | Poly(NVCL205.3-co-MAPTAC12.9)-CTA | 31.4 kDa | 4.949 | |
| Poly(NVCL204.9-co-MAPTAC25.8)-CTA | 33.9 kDa | 3.553 | ||
| AMPS | Sample abandoned due to synthesis limitationa | — | — | |
LCSTs determination of copolymers of NIPAAm and hydrophilic monomers
We characterized the LCSTs of copolymers of NIPAAm and hydrophilic monomers based on an established method.36,37 We utilized ultraviolet-visible (UV-vis) absorption spectroscopy to determine the LCSTs. The optical transmittance of the sample solutions was recorded using a UV-1900i spectrometer (SHIMADZU, Kyoto, Japan). The polymer sample was prepared at 0.1 wt% in water under varying concentrations of NaCl. Each time, [NaCl] was increased by 100 mM for next characterization. The transmittance-temperature curve for the polymer solutions in the equilibrium states was observed by increasing the temperature from 25 °C to 55 °C in 150 minutes. LCSTs were determined as the temperature point where transmittance decreased by 1% (Fig. S49–S53).Coacervate observation and by optical microscopy
The separated microphase was observed using optical microscope (Keyence VHF970, Osaka, Japan). After preparing the sample solution, the solution was placed on a tiny glass slide, then grease was applied around it and finally it was clamped with another piece of glass to seal the sample. The sample temperature was set to the target temperature, and the separated phases were observed using the optical microscope. The coalescence behavior was recorded by video (SI Movie 1).Determination of separated phases
Polymer solutions were equilibrated at the target temperatures for 24 hours prior to observation. When a bulk, liquid-like layer accumulated at the bottom of the sample, the separated phase was assigned as a coacervate phase. When an opaque suspension persisted without macroscopic phase separation, the phase is classified as unidentified.Dynamic light scattering (DLS) characterization
The hydrodynamic diameter (Dh) of the separated phase of synthesized poly(NIPAAm-co-DMAAm) in water was determined by DLS (detailed information in Fig. S41). We prepared two samples, one is 0.1 wt% poly(NIPAAm-co-DMAAm) dissolved in DI water, and the other is 0.1 wt% poly(NIPAAm-co-DMAAm) dissolved in DI water containing 500 mM NaCl. The DLS measurement was performed at 50 °C.Rheological measurements
Macroscopic rheological measurements were carried out using a stress-controlled rheometer (MCR 301, Anton Paar, Graz, Austria) equipped with a parallel-plate jig (diameter: 50 mm; gap: 0.6–1.0 mm) operated within the linear viscoelastic regime (strain amplitude: 0.1–100%). Frequency sweep tests were conducted over an angular frequency range of 0.1–100 rad s−1, and steady shear measurements were performed over a shear rate range of 0.1–100 s−1. All measurements were carried out at various temperatures (from 35 to 50 °C). Prior to the measurements, bulk separated phases were formed by settling polymer solution at target temperatures for 24 hours and were equilibrated at each temperature for more than 30 min before testing. After loading the samples onto the rheometer, they were maintained at the target temperature and equilibrated for an additional 5 min before starting the measurements.Results and discussion
Synthesis of copolymers of LCST-type and hydrophilic monomers
To study the phase separation behavior, we synthesized a series of copolymers by RAFT copolymerization. As previously reported, the partial dehydration is necessary for simple coacervation for the NIPAAm-based polymer chains.28,31 Based on this finding, we hypothesized the copolymerization of LCST-type and hydrophilic monomers is a key to inducing the simple coacervation behavior. To verify our hypothesis, we selected several well-studied LCST-type monomers (acrylamide type monomers NIPAAm and N-n-PAAm, PEG-type monomer PEGMEM, pH responsive DMAEMA, and cyclic NVCL),38–44 and combined them with various hydrophilic monomers (neutral DMAAm and AAm, positively charged MAPTAC, negatively charged AMPS, and NAPMAm, which can attract protons). The copolymerizations were carried out with LCST-type monomers containing 5 mol% of hydrophilic comonomers. According to 1H-NMR results shown in Fig. S1–S34, we calculated the monomer conversion ratio (see details in SI form page S7 to page S23) and Mn (1H-NMR) of each synthesized polymer. The 1H-NMR spectra of the dialyzed copolymers confirmed the successful synthesis of the target structures in most samples. However, upon the synthesis of poly(NVCL-co-AMPS), the decomposition of NVCL monomer was suggested by the 1H-NMR spectrum shown in Fig. S35. In addition, poly(NVCL-co-MAPTAC) and poly(PEGMEM-co-DMAAm) displayed relatively high PDIs (PDIs > 3) as shown in Table 1. These results highlight limitations in copolymerization for specific monomer combinations, however, detailed optimization of polymerization conditions was not pursued, as it falls outside the primary scope of this study. The polymer compositions, Mn, and polydispersity index values of the synthesized polymers were summarized in Table 1.Thermoresponsive phase separation behavior of poly(NIPAAm-co-DMAAm) in aqueous media
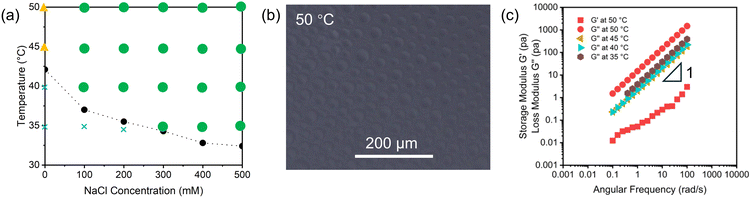
To characterize the detailed phase separation behavior of our synthesized polymers, we started from characterization of poly(NIPAAm-co-DMAAm) in aqueous media. Fig. 2a represents many spherical droplets formed in aqueous solution containing poly(NIPAAm-co-DMAAm) at 50 °C in the presence of NaCl ([NaCl] = 500 mM), which is a signature of coacervate formation.2,3 Additionally, we captured coalescence behavior by optical microscopy as shown in Fig. 2b. The coalescence behavior originates from the liquid-like nature and open system characteristic of coacervates, leading to a gradual increase in their size over time.6,45,46 This is also supported by results shown in Fig. S41a, which demonstrated an increase in Dh of the separated phase observed in the aqueous solution containing poly(NIPAAm-co-DMAAm) and 500 mM NaCl over time at 50 °C. We consider that the increasing Dh was contributed by autonomous coacervate growth based on coalescence. On the other hand, in pure water, Dh of the separated phase maintained a stable and small value even after 4 hours (Fig. S41b). Based on this phenomenon, we also investigated the phase separation behavior from a bulk perspective. We prepared 1 ml of each solution and heated them at 50 °C for 24 hours in a glass bottle (Fig. 2c and d). Upon heating, both polymer solutions immediately became opaque due to light scattering caused by microphase separation. After 24 hours, the polymer dispersion remained opaque in the absence of NaCl (Fig. 2c). In contrast, the polymer solution with 500 mM NaCl resulted in bulk condensation, which supported our finding of simple coacervation and coacervate growth behavior. According to some previous research,28,31,47 adding salt contributes to partial dehydration rather than complete dehydration of the polymer chain. The partial dehydration contributes to intermolecular interaction, leading to the formation of polymer coacervates.Phase diagram of poly(NIPAAm-co-DMAAm) in aqueous media with varied NaCl concentration and temperatures
Aforementioned results suggest that the thermoresponsive phase separation behavior of the poly(NIPAAm-co-DMAAm) solution switches upon the addition of NaCl. Therefore, we investigated the effect of NaCl concentration on the phase separation behavior of the copolymer in aqueous media. After equilibrating the polymer solution for 24 h, we examined the separated phase by optical microscopy under varying the NaCl concentration and the temperature. Fig. 3a shows the NaCl concentration–temperature phase diagram of the poly(NIPAAm-co-DMAAm) solution after 24 h of equilibration. The polymer solutions exhibited LCST-type phase separation across the NaCl concentration range from 0 to 500 mM when the temperature exceeded their respective LCSTs. Moreover, in most cases, we observed spherical condensates at the bottom of each sample following incubation above the corresponding LCST (Fig. 3b). The morphology is characteristic of liquid–liquid phase separation, which causes simple coacervation in the early stage and subsequently coalesce into a bulk condensate.2,3 The decrease in LCST with increasing [NaCl] is likely due to the salting-out effect.48–50 Notably, in the absence of NaCl, no bulk condensates were observed, and the dispersion remained opaque after the incubation. We classified this state as an unidentified phase. To quantitatively characterize these separated bulk phases, we also performed rheology measurements. Fig. 3c shows the storage (G′) and the loss (G″) moduli of the bulk phases plotted against angular frequency. At 50 °C in dispersion containing 500 mM NaCl, under small-amplitude oscillatory shear, G″ increases linearly with angular frequency, exhibiting a log–log slope near unity across the probed range, while G′ remains substantially smaller. These results clearly demonstrate the viscoelastic nature of the condensate under this condition. Moreover, for condensates formed below 50 °C, only the G″ was observed, suggesting a diminished elastic component at lower temperature. These results imply that the coacervates become more fluid-like as the temperature decreases. Based on these investigations, we conclude that the condensates are liquid, demonstrating that poly(NIPAAm-co-DMAAm) undergoes coacervation by liquid–liquid phase separation under these conditions.After confirming that poly(NIPAAm-co-DMAAm) exhibits coacervation in the presence of NaCl, we further investigated the effect of different salts in aqueous media. As shown in Fig. S48, this polymer exhibited simple coacervation in the presence of several salts including NaNO3, Na2SO4, and CaCl2. Although salting-out effect depends on salt species,51 this phenomenon exhibits salt-triggered simple coacervation of our synthesized polymers is not limited to specific salt species.
Phase diagrams of copolymers containing NIPAAm and hydrophilic monomers in aqueous media
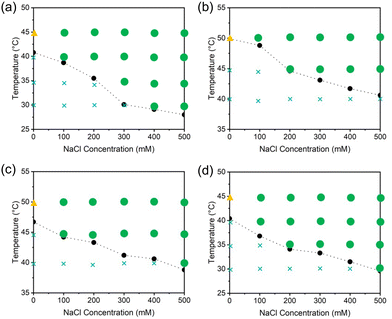
To assess the generality of simple coacervation of NIPAAm-based copolymers, we investigated the phase separation behavior of copolymers composed of NIPAAm and various hydrophilic monomers in aqueous media containing different concentrations of NaCl by optical microscopy after 24 h of equilibration. The corresponding phase diagrams are presented in Fig. 4. Under the conditions indicated in Fig. 4, bulk condensates were observed for all copolymers of NIPAAm and hydrophilic monomers prepared in this study (Fig. S43). The LCSTs of copolymers containing ionic monomer units were higher than those of other copolymers, reflecting the increased hydrophilicity imparted by the ionic units. These results strongly support versatility of LCST-type simple coacervation across poly(NIPAAm) series.Thermoresponsive phase separation of copolymers containing LCST-type and hydrophilic monomers in aqueous media
We next investigated whether this simple coacervation phenomenon could be extended to LCST-type monomers other than NIPAAm. Here we selected several well-studied LCST-type monomers: NIPAAm and N-n-PAAm (acrylamide-type), PEGMEM (PEG-type), DMAEMA (pH responsive), and NVCL (cyclic). Although some of these monomers exhibit stimuli responsive behavior like pH responsiveness of poly(DMAEMA),43 we only focused on the effect of NaCl concentration on phase behavior, in line with the scope of this study. Solutions of various copolymers listed in Table 2 were prepared, and their separated phases were observed by optical microscopy at 50 °C under different NaCl concentrations (Fig. S42 and S44–S47). These observations revealed that most samples exhibited the ability of simple coacervation at 50 °C in the presence of the NaCl over LCSTs. One of the exceptions was poly(NVCL-co-MAPTAC), which did not exhibit simple coacervation in aqueous media even at a high NaCl concentration of 2 M. To determine whether insufficient MAPTAC content in the polymer chain was responsible, we doubled the MAPTAC feed ratio during synthesis. However, the product still did not exhibit thermoresponsive simple coacervation. We note that our synthesized poly(NVCL-co-MAPTAC), likely due to polydispersity limitations in polymer synthesis (Table 1). Overcoming such synthetic limitations will be essential for further investigation of such exceptions, and efforts to address this issue are currently underway. Although a few exceptions were observed, these results support the generality of LCST-type simple coacervation induced by copolymers composed of LCST-type and hydrophilic monomers.| Coacervates were confirmed by optical microscopy mentioned in method characterizing samples with 0.1 wt% polymer concentration with various NaCl concentrations at 50 °C (Fig. S42, S44–S47).a We synthesized two independent poly(NVCL-co-MAPTAC) with different MAPTAC containing ratio (Table S5 and Fig. S32, S34). These two samples do not exhibit appearance of coacervates even [NaCl] was increased to 2 M in aqueous media over LCST.b Decomposition of NVCL monomer was suggested by the 1H-NMR (Fig. S35). |
|---|

|
Conclusions
We revealed the thermoresponsive simple coacervation behavior of synthetic copolymers composed of LCST-type and hydrophilic monomers. Systematic investigation indicates that most copolymers exhibited simple coacervation in the presence of salts. Notably, most copolymers examined in this study follow the same trend, highlighting the generality of this phase separation behavior. In contrast to complex coacervate systems, which typically undergo phase separation only at low ionic strength condition due to the shielding of electrostatic interactions between oppositely charged polymers at high ionic strength,24–26 the copolymers of LCST-type and hydrophilic monomers exhibited simple coacervation in high ionic strength environments. We consider this unique behavior expands the applicability of polymer coacervates under conditions where traditional complex coacervates are destabilized. We believe that our findings expand the avenue for studying polymer coacervation in such high ionic aqueous environments, addressing a previously unexplored aspect of polymer coacervate research. Overall, we believe that our findings provide a guideline for the design and application of thermoresponsive simple coacervate systems based on synthetic LCST-type copolymers.Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5py00758e.Acknowledgements
This work was partially supported by Grants-in-Aid for Scientific Research Grant Numbers 24H00471, JP20H00388 and JP20K20563 to R. Y. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.References
- M. Abbas, W. P. Lipin, J. Wang and E. Spruijt, Chem. Soc. Rev., 2021, 50, 3690–3705 RSC.
- N. A. Yewdall, A. A. M. André, T. M. Lu and E. Spruijt, Curr. Opin. Colloid Interface Sci., 2021, 52, 101416 CrossRef CAS.
- C. E. Sing and S. L. Perry, Soft Matter, 2020, 16, 2885–2914 RSC.
- C. D. Crowe and C. D. Keating, Interface Focus, 2018, 8, 20180032 CrossRef PubMed.
- S. P. Moulik, A. K. Rakshit, A. Pan and B. Naskar, Colloids Interfaces, 2022, 6, 45 CrossRef CAS.
- Z. Lin, T. Beneyton, J. C. Baret and N. Martin, Small Methods, 2023, 7, 2300496 CrossRef CAS.
- A. B. Cook, S. Novosedlik and J. C. M. van Hest, Acc. Mater. Res., 2023, 4, 287–298 CrossRef CAS.
- K. S. Nair, S. Radhakrishnan and H. Bajaj, ACS Synth. Biol., 2023, 12, 2168–2177 CrossRef CAS.
- W. T. Jiang, Z. Y. Wu, Z. Gao, M. M. Wan, M. Zhou, C. Mao and J. Shen, ACS Nano, 2022, 16, 15705–15733 CrossRef CAS PubMed.
- R. Harris, N. Berman and A. Lampel, Chem. Soc. Rev., 2025, 54, 4183–4199 RSC.
- N. Gao and S. Mann, Acc. Chem. Res., 2023, 56, 297–307 CrossRef CAS.
- R. Booth, Y. Qiao, M. Li and S. Mann, Angew. Chem., Int. Ed., 2019, 58, 9120–9124 CrossRef CAS PubMed.
- Y. F. Chen, M. Yuan, Y. W. Zhang, S. Y. Liu, X. H. Yang, K. M. Wang and J. B. Liu, Chem. Sci., 2020, 11, 8617–8625 RSC.
- I. B. A. Smokers, B. S. Visser, A. D. Slootbeek, W. T. S. Huck and E. Spruijt, Acc. Chem. Res., 2024, 57, 1885–1895 CrossRef CAS.
- C. Lim and W. C. B. McTigue, ACS Biomater. Sci. Eng., 2024, 10, 6766–6789 CrossRef CAS.
- S. J. Chen, Q. Guo and J. Yu, Aggregate, 2022, 3, e293 CrossRef CAS.
- L. L. S. Ma, X. C. Fang and C. Wang, Front. Bioeng. Biotechnol., 2023, 10, 1100365 CrossRef PubMed.
- N. R. Johnson and Y. D. Wang, Expert Opin. Drug Delivery, 2014, 11, 1829–1832 CrossRef CAS PubMed.
- K. Mirlohi and W. C. B. McTigue, Soft Matter, 2024, 21, 8–26 RSC.
- A. M. Rumyantsev, N. E. Jackson and J. J. de Pablo, Annu. Rev. Condens. Matter Phys., 2021, 12, 155–176 CrossRef.
- G. Y. Li, Q. H. Chen, C. R. Su, H. Wang, S. He, J. Liu, A. Nag and Y. Yuan, Innovative Food Sci. Emerging Technol., 2021, 68, 102612 CrossRef CAS.
- X. W. Zhu, C. Y. Wei, F. Zhang, Q. Q. Tang and Q. Zhao, Macromol. Rapid Commun., 2019, 40, 1800758 CrossRef.
- C. Fu, H. M. Fares and J. B. Schlenoff, Macromolecules, 2017, 50, 1066–1074 CrossRef.
- S. L. Perry, Y. Li, D. Priftis, L. Leon and M. Tirrell, Polymers, 2014, 6, 1756–1772 CrossRef.
- J. van der Gucht, E. Spruijt, M. Lemmers and M. A. C. Stuart, J. Colloid Interface Sci., 2011, 361, 407–422 CrossRef CAS PubMed.
- D. Priftis, N. Laugel and M. Tirrell, Langmuir, 2012, 28, 15947–15957 CrossRef CAS PubMed.
- S. Kim, H. Y. Yoo, J. Huang, Y. Lee, S. Park, Y. Park, S. Jin, Y. M. Jung, H. B. Zeng, D. S. Hwang and Y. Jho, ACS Nano, 2017, 11, 6764–6772 CrossRef CAS PubMed.
- T. Maeda, M. Takenouchi, K. Yamamoto and T. Aoyagi, Biomacromolecules, 2006, 7, 2230–2236 CrossRef CAS.
- Q. Y. Peng, T. Wang, D. L. Yang, X. W. Peng, H. Zhang and H. B. Zeng, Prog. Polym. Sci., 2024, 153, 101827 CrossRef CAS.
- L. L. Jiang, Y. W. Zeng, H. Li, Z. X. Lin, H. Liu, J. J. Richardson, Z. S. Gao, D. D. Wu, L. Liu, F. Caruso and J. J. Zhou, J. Am. Chem. Soc., 2023, 145, 24108–24115 CrossRef CAS.
- T. Maeda, M. Takenouchi, K. Yamamoto and T. Aoyagi, Polym. J., 2009, 41, 181–188 CrossRef CAS.
- X. C. Yin and H. D. H. Stöver, Macromolecules, 2003, 36, 9817–9822 CrossRef CAS.
- K. Yamamoto, T. Serizawa and M. Akashi, Macromol. Chem. Phys., 2003, 204, 1027–1033 Search PubMed.
- A. Narayanan, J. R. Menefee, Q. H. Liu, A. Dhinojwala and A. Joy, ACS Nano, 2020, 14, 8359–8367 Search PubMed.
- J. P. Swanson, M. R. Martinez, M. A. Cruz, S. G. Mankoci, P. J. Costanzo and A. Joy, Polym. Chem., 2016, 7, 4693–4702 RSC.
- H. W. Sun, T. Enomoto, W. S. Lee, A. M. Akimoto and R. Yoshida, ACS Macro Lett., 2024, 13, 1503–1508 CrossRef CAS.
- T. Masuda, A. M. Akimoto, K. Nagase, T. Okano and R. Yoshida, Chem. Mater., 2015, 27, 7395–7402 CrossRef CAS.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342–15367 CrossRef CAS.
- T. E. de Oliveira, C. M. Marques and P. A. Netz, Phys. Chem. Chem. Phys., 2018, 20, 10100–10107 RSC.
- Y. Koda, T. Terashima and M. Sawamoto, ACS Macro Lett., 2015, 4, 1366–1369 CrossRef CAS.
- S. Zhuo, E. Halligan, B. S. H. Tie, C. Breheny and L. M. Geever, Polymers, 2022, 14, 3155 CrossRef CAS PubMed.
- N. A. Cortez-Lemus and A. Licea-Claverie, Prog. Polym. Sci., 2016, 53, 1–51 CrossRef CAS.
- A. R. Hernández-Martínez and E. Bucio, Des. Monomers Polym., 2009, 12, 543–552 CrossRef.
- S. H. Yuk, S. H. Cho and S. H. Lee, Macromolecules, 1997, 30, 6856–6859 CrossRef CAS.
- J. P. Douliez, A. Perro and L. Béven, ChemBioChem, 2019, 20, 2546–2552 CrossRef CAS PubMed.
- A. D. Slootbeek, M. H. I. van Haren, I. B. A. Smokers and E. Spruijt, Chem. Commun., 2022, 58, 11183–11200 Search PubMed.
- T. Maeda, T. Kanda, Y. Yonekura, K. Yamamoto and T. Aoyagi, Biomacromolecules, 2006, 7, 545–549 CrossRef CAS PubMed.
- H. B. Du, R. Wickramasinghe and X. H. Qian, J. Phys. Chem. B, 2010, 114, 16594–16604 CrossRef CAS.
- Y. J. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- Y. Zhang, S. Furyk, L. B. Sagle, Y. Cho, D. E. Bergbreiter and P. S. Cremer, J. Phys. Chem. C, 2007, 111, 8916–8924 CrossRef CAS.
- S. Z. Moghaddam and E. Thormann, J. Colloid Interface Sci., 2019, 555, 615–635 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |