Open Access Article
Open Access ArticleQbD-driven solid dispersion development for enhanced solubility and dissolution of the BCS Class II drug voriconazole as a model drug
Bhaskar Daravath *,
Shasidhar Goud Pendlimadugula,
Uday Kiran Kummari,
Ravi Teja C and
Ajay Reddy K
*,
Shasidhar Goud Pendlimadugula,
Uday Kiran Kummari,
Ravi Teja C and
Ajay Reddy K
Department of Pharmaceutics, GITAM School of Pharmacy, GITAM Deemed to be University, Rudraram, Patancheru, Sangareddy, Hyderabad, Telangana, India – 502329. E-mail: bdaravat@gitam.edu
First published on 3rd December 2025
Abstract
Voriconazole (VC), a second-generation triazole antifungal, suffers from low aqueous solubility (∼0.5 mg mL−1), which restricts its oral bioavailability. The present study sought to enhance the solubility and dissolution profile of VC through solid dispersion (SD) technology, employing PEG 4000 and PEG 6000 as hydrophilic carriers. A systematic Quality by Design (QbD) framework was applied to optimize the formulation. A Central Composite Design (CCD) was implemented to evaluate the influence of PEG concentrations on solubility (Y1) and cumulative drug release (Y2). Phase solubility studies confirmed the formation of VC–PEG complexes exhibiting AL-type profiles. Numerical optimization, supported by graphical analysis, identified the optimized formulation consisting of 600 mg of PEG 4000 and 600 mg of PEG 6000. The optimized solid dispersion showed a marked improvement in solubility (30.68 mg mL−1) and drug release (cumulative drug release = 89.88%), achieving a desirability score of 0.901, thereby validating the robustness of the QbD-based optimization strategy. Model validation confirmed excellent predictive performance (adjusted R2 and predicted R2 within acceptable limits). Solid-state characterization (FTIR, DSC, XRD and SEM) indicated complete amorphization and uniform molecular dispersion of VC. Stability studies over six months confirmed the stability of the formulation (f2 = 78.27). PEG-based solid dispersions offer a promising and stable approach to improve the solubility and dissolution of poorly water-soluble antifungal agents such as voriconazole.
1. Introduction
Oral drug delivery is the most commonly preferred route for therapeutic administration owing to its ease of use, affordability, and excellent patient compliance. However, a major challenge in oral formulation development lies in overcoming poor aqueous solubility and erratic bioavailability, which severely limit the clinical utility of many promising drug candidates. The Biopharmaceutics Classification System (BCS) classifies drugs according to water solubility and permeability through the gastrointestinal membrane. Approximately 40% of currently marketed formulations and nearly 90% of drug candidates under development are BCS Class II. These drugs show dissolution-rate limited absorption and variable therapeutic responses.1,2To address these challenges, various solubility enhancement techniques have been developed, including micronization, nanonization, salt formation, co-solvent systems, surfactant use, lipid-based formulations, inclusion complexation with cyclodextrins, and use of non-volatile solvents.3–6 These techniques have demonstrated varying degrees of success, but each has specific drawbacks such as limited scalability, chemical stability issues, and carrier incompatibilities. Accordingly, the demand for a robust, scalable, and widely applicable formulation strategy has drawn considerable attention toward solid dispersion (SD) systems.
Solid dispersion (SD) technology involves dispersing a poorly water-soluble drug within a hydrophilic carrier at the molecular level. This strategy increases the effective surface area of the drug, decreases its crystallinity, and enhances wettability, thereby improving the dissolution rate and ultimately oral bioavailability.7,8 Compared with other solubility enhancement techniques, SDs exhibit superior scalability, reproducibility, and long-term stability, particularly when developed through a systematic formulation approach. Among the available preparation techniques, solvent evaporation is considered especially advantageous because it minimizes thermal degradation, provides favorable processing conditions, and is amenable to large-scale manufacturing.9 In contrast to melting or fusion methods, solvent evaporation facilitates the formation of a uniform amorphous dispersion with improved physical stability.
Voriconazole, classified as a second-generation triazole antifungal, is prescribed for serious systemic fungal infections, such as invasive aspergillosis and candidemia; however, it suffers from solubility and dissolution limitations characteristic of BCS Class II drugs. With a reported aqueous solubility of approximately 0.5 mg mL−1,10 voriconazole exhibits highly variable oral absorption, leading to inconsistent therapeutic outcomes. Although commercial formulations exist, these products often suffer from inter- and intra-patient variability due to poor dissolution and erratic bioavailability. Consequently, enhancing the solubility and dissolution profile of voriconazole through SD technology presents a promising strategy to achieve more consistent therapeutic levels. The novelty of this work lies in addressing voriconazole's poor solubility and inconsistent absorption through a targeted dissolution enhancement strategy. By improving its biopharmaceutical performance, the study aims to achieve more consistent therapeutic outcomes for severe systemic fungal infections.
While SD-based solubility enhancement has been extensively studied for related azole antifungals such as itraconazole and posaconazole,11,12 the development of voriconazole SDs utilizing a Quality by Design (QbD)-guided solvent evaporation method remains relatively unexplored. Quality by Design (QbD) emphasizes a thorough understanding of processes and control strategies to ensure consistent product quality.13
Central to QbD is the Design of Experiments (DoE) methodology, particularly Central Composite Design (CCD), which allows systematic evaluation of multiple variables and their interactions in a limited number of experimental runs. This statistical tool enables the formulation scientist to define a “design space” where robust and optimized product quality can be consistently achieved. Such an approach not only improves product efficacy and safety but also enhances regulatory flexibility during post-approval changes.14–16
The present study proposes the development and optimization of voriconazole-loaded SDs using a solvent evaporation technique under a QbD framework with CCD design. The novelty lies in the integration of a statistically driven formulation design process specifically for voriconazole, which has not been widely documented in the literature. Recent research has demonstrated the successful application of SDs in increasing the solubility of other poorly soluble drugs like curcumin, lumefantrine, flurbiprofen, and artemether.17–20 These findings collectively reinforce the potential of SD-based formulations to transform the oral delivery profiles of BCS Class II drugs.
This work contributes to the advancement of pharmaceutical formulation science by presenting a rational, reproducible, and scalable method for enhancing the solubility and bioavailability of a clinically important antifungal agent. The use of QbD-guided formulation design offers significant advantages in achieving consistent quality and improved therapeutic performance, with broad applicability across a wide range of poorly soluble drug candidates like voriconazole.
2. Materials and methods
2.1 Materials
Voriconazole was generously provided by MSN Laboratories Pvt. Ltd (Hyderabad, Telangana, India), while all other chemicals were obtained from SD Fine Chemicals (Mumbai, India).2.2 Phase solubility study
Phase solubility studies were performed to assess the effect of polymer concentration on the aqueous solubility of voriconazole. Excess amounts of voriconazole were introduced into 10 mL of 0, 1, 2, 3, 4, and 5% w/v aqueous solutions of PEG 4000 and PEG 6000 separately.21 The resulting suspensions were stirred continuously using a magnetic stirrer for 48 hours at room temperature to attain equilibrium. The mixtures were filtered through a 0.45 µm membrane filter to eliminate any undissolved drug. The clear filtrates were appropriately diluted, and the drug concentration was determined spectrophotometrically at 250 nm. The solubility data were then plotted to generate phase solubility diagrams for both PEG 4000 and PEG 6000 systems.2.3 Mathematical model and experimental design
To systematically optimize the variables and evaluate their impact on responses, a Central Composite Design (CCD) was used by employing Design-Expert® software (version 13). This experimental design targeted the optimization of two independent variables, such as PEG 4000 (A) and PEG 6000 (B), each studied at five levels (−α, −1, 0, +1 and +α), and the corresponding values are presented in Table 1. The dependent responses selected for the study included solubility (Y1) and percentage cumulative drug release (%CDR, Y2). According to the CCD matrix, 13 experiments were conducted (Table 2). The resulting data were analyzed using a second-order polynomial equation, and the relationship between the variables and the responses was determined.| Factors (independent variables) | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| PEG 4000 (mg) | 117.157 | 200 | 400 | 600 | 682.843 |
| PEG 6000 (mg) | 117.157 | 200 | 400 | 600 | 682.843 |
| Run | Factor A: PEG 4000 | Factor B: PEG 6000 | Solubility (mg ml−1) | %CDR |
|---|---|---|---|---|
| 1 | 0 | 0 | 18.82 | 77.59 |
| 2 | 0 | 1.414 | 34.35 | 92.49 |
| 3 | 0 | −1.414 | 8.71 | 43.43 |
| 4 | 0 | 0 | 18.37 | 71.36 |
| 5 | 1.414 | 0 | 25.47 | 72.33 |
| 6 | 0 | 0 | 20.55 | 69.74 |
| 7 | 0 | 0 | 17.59 | 71.59 |
| 8 | −1.414 | 0 | 12.73 | 52.17 |
| 9 | −1 | −1 | 10.66 | 56.27 |
| 10 | −1 | 1 | 18.49 | 76.35 |
| 11 | 1 | −1 | 16.59 | 65.36 |
| 12 | 0 | 0 | 16.47 | 71.67 |
| 13 | 1 | 1 | 29.77 | 87.49 |
The second-order polynomial model equation is:
| Y = β0 + β1A + β2B + β11A2 + β22B2 + β12AB. | (1) |
In the polynomial equation, Y denotes the dependent responses, either solubility (Y1) or cumulative percentage drug release (%CDR, Y2). The term β0 represents the intercept; β1 and β2 are the linear coefficients for the independent variables A (PEG 4000) and B (PEG 6000), respectively; β11 and β22 are the quadratic (square) coefficients; and β12 denotes the interaction coefficient between A and B.
Statistical analysis, along with p-values, F-values, and coefficients, is given in Table 3, highlighting the significance of individual model terms. The model's predictive accuracy was confirmed by comparing the experimental (observed) and predicted values, as shown in Table 4. A robust and flexible design space was established using the desirability function and predefined optimization criteria. An optimized formulation was then developed within this design space by selecting the most appropriate levels of PEG 4000 and PEG 6000.
| Source | Y1: solubility | Y3: cumulative % drug release | ||||
|---|---|---|---|---|---|---|
| F-Value | p-Value | Coefficient estimate | F-Value | p-Value | Coefficient estimate | |
| Model p < 0.05: statistically significant lack of fit; p > 0.05: lack of fit if not significant. | ||||||
| Model | 46.92 | <0.0001 | 30.66 | <0.0001 | ||
| Intercept | 19.12 | 69.83 | ||||
| A-PEG 4000 | 25.76 | 0.0005 | 4.40 | 9.82 | 0.0106 | 6.09 |
| B-PEG 6000 | 68.08 | <0.0001 | 7.16 | 51.49 | <0.0001 | 13.95 |
| AB | ||||||
| A2 | ||||||
| B2 | ||||||
| Lack of fit | 3.71 | 0.1125 | 4.89 | 0.0732 | ||
| Check points | Independent variables | Dependent responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor A: | Factor B: | Y1: solubility | Y4: cumulative % drug release | |||||
| PEG 4000 (mg) | PEG 6000 (mg) | Predicted value (mg ml−1) | Experimental value (mg ml−1) | Standard error | Predicted value (%) | Experimental value (%) | Standard error | |
| R4 | 400 | 400 | 19.12 | 18.37 | 2.454 | 69.83 | 71.36 | 5.498 |
| R9 | 200 | 200 | 7.59 | 10.66 | 2.454 | 49.79 | 56.27 | 5.498 |
| R11 | 600 | 200 | 16.37 | 16.59 | 2.454 | 61.98 | 65.36 | 5.498 |
| Optimized | 600 | 600 | 30.68 | 29.77 | 2.454 | 89.88 | 87.49 | 5.498 |
2.4 Formulation of the VC solid dispersions
SDs of voriconazole (VC) were formulated using the solvent evaporation method with PEG 4000 and PEG 6000 as hydrophilic carriers in varying weight ratios, as detailed in Table 1. The drug and polymers were co-dissolved in 10 mL of ethanol within a round-bottom flask. Solvent removal was carried out under vacuum at a controlled temperature not more than 45 °C. The resulting solid mass was pulverized with a mortar and pestle, transferred into glass vials, and stored in a desiccator for further analysis.21 For comparative purposes, a physical mixture (PM) was prepared by thoroughly blending VC and the respective PEGs with a spatula for 5 minutes.2.5 Solubility studies of the VC solid dispersions
The solubility of the formulated SDs was evaluated using the method described by Daravath et al., using pH 7.2 phosphate buffer as the medium.22 The amount of solubilized voriconazole was quantified using a UV–Visible spectrophotometer at a wavelength of 250 nm.2.6 Cumulative percentage drug release studies of the VC solid dispersions
Dissolution profiles of VC, the physical mixture, and the SDs were obtained using a USP Type II dissolution apparatus in a pH 7.2 phosphate buffer at 37 ± 0.5 °C with a rotation speed of 50 rpm. The aliquot samples were withdrawn at predetermined time intervals and analyzed using a UV spectrophotometer at 250 nm. The initial dissolution rate (IDR) and relative dissolution rate (RDR) were calculated and compared with the pure drug.232.7 Characterization of the VC solid dispersions
To confirm the formation of SDs and assess potential interactions between voriconazole and the polymeric carriers, the following characterization methods were used.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi for 3 minutes.24
000 psi for 3 minutes.242.8 Stability studies of the VC solid dispersions
The optimized solid dispersion was evaluated for accelerated stability over a six-month period under controlled temperature conditions of 40 ± 2 °C and 75 ± 5% relative humidity. The assay and dissolution profiles were re-examined after the storage, and the similarity factor (f2) was calculated to observe potential alterations in drug release behaviour.63. Results and discussion
Voriconazole (VC) is a lipophilic antifungal agent with poor aqueous solubility of approximately 0.5 mg mL−1 and a moderate log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value of 1.65.10 These properties classify VC as a suitable candidate for solubility enhancement through solid dispersion, particularly with hydrophilic carriers such as PEG 4000 and PEG 6000.
P value of 1.65.10 These properties classify VC as a suitable candidate for solubility enhancement through solid dispersion, particularly with hydrophilic carriers such as PEG 4000 and PEG 6000.
3.1 Phase solubility study
The phase solubility profile of VC in PEGs (0–5% w/v) is shown in Fig. 1. As PEG concentrations increase, the solubility of VC increases linearly, indicating an AL-type complex formation.27 This linear relationship between VC concentration and PEG 4000 concentration showed a slope of 0.7152 and a high correlation coefficient (R2 = 0.9943), and PEG 6000 concentration showed a slope of 0.7012 and a high correlation coefficient (R2 = 0.9952).These findings confirm the successful formation of solid dispersions, wherein a single PEG molecule effectively interacts with a single VC molecule to enhance its apparent aqueous solubility. This finding is similar to previous reports, as reported in the study by Suresh et al.,28 which showed similar AL-type solubility profiles for hydrophobic drugs incorporated into PEG-based matrices.
3.2 Mathematical model development
Experimental design techniques were employed to develop a mathematical model that describes the relationship between formulation variables and responses. In this study, a Central Composite Design (CCD) was systematically applied to evaluate the influence of PEG 4000 (factor A) and PEG 6000 (factor B) concentrations on key responses, namely solubility (Y1) and percentage cumulative drug release (%CDR, Y2). The independent factors were assessed at five coded levels (–α, −1, 0, +1, and +α), facilitating the development of a robust quadratic model for response surface analysis.Statistical analyses, including analysis of variance (ANOVA), were performed using Design-Expert® software (version 13). The significance of regression models was assessed according to the F-value and p-value criteria, where a model was deemed statistically significant if the F-value exceeded 1 and the corresponding p-value was less than 0.05. Additionally, a non-significant lack-of-fit (p > 0.05) was essential to ensure the model's adequacy.
The model's predictive capability was further confirmed by verifying that the difference between the predicted R2 and adjusted R2 should be less than 0.2, and that the adequate precision value was exceeded by 4, showing an acceptable signal-to-noise ratio. The detailed model statistics are presented in Table 3.
3.3 Solubility studies of the prepared VC solid dispersions
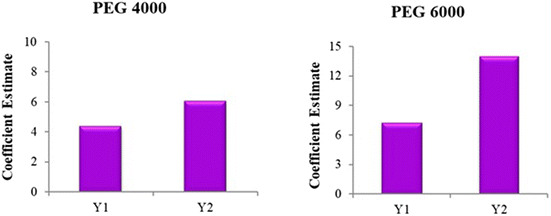
The solubility of the formulated VC solid dispersions ranged from 8.71 mg mL−1 to 34.35 mg mL−1, as presented in Table 2. Statistical analysis confirmed that a linear regression model best described the influence of formulation factors on solubility (Y1). The F-value was 46.92, and the p-value was less than 0.0001, indicating that the model terms were highly significant and the model exhibited strong statistical significance. Furthermore, a non-significant lack-of-fit (p = 0.1125) confirmed the adequacy and reliability of the model (Table 3).Both PEG 4000 (A) and PEG 6000 (B) were identified as significant contributors to solubility enhancement, with p-values of 0.0005 and <0.0001. The regression coefficients for PEG 4000 and PEG 6000 were +4.40 and +7.16, respectively, indicating a positive and synergistic effect on solubility as the concentration of each PEG increased. Notably, PEG 6000 exhibited a more pronounced effect on solubility, as indicated by its higher regression coefficient value.
Model reliability was further confirmed with a difference between the adjusted R2 (0.8844) and predicted R2 (0.8074) that was well below the acceptable threshold of 0.2. The adequate precision value was 19.62, significantly exceeding the minimum requirement of 4.
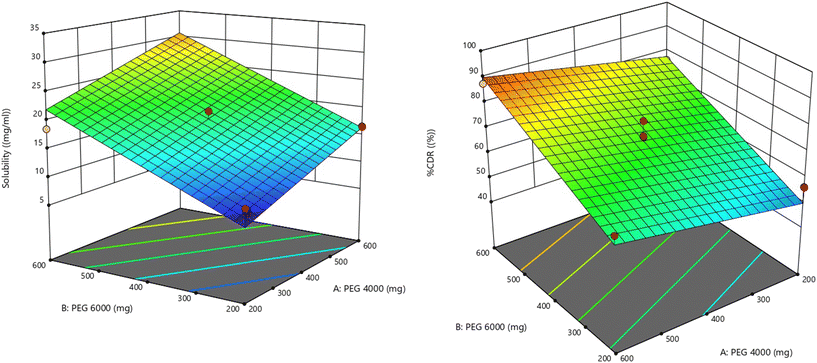
The effect of formulation variables was also visualized using 2D contour and 3D surface response plots (Fig. 2 and 3), clearly illustrating the increasing trend in solubility with increasing PEG concentrations. The regression model for solubility is represented in coded form as:
| Y1 = 19.12 + 4.40A + 7.16B | (2) |
where Y1 = predicted solubility (mg mL−1); A = concentration of PEG 4000; and B = concentration of PEG 6000.
This equation confirms that both PEG 4000 and PEG 6000 positively influence the solubility of VC, with PEG 6000 exerting a more pronounced effect. Accordingly, solubility enhancement in the VC solid dispersions was directly proportional to the level of PEGs incorporated (Fig. 4i and ii).
3.4 %CDR (cumulative percentage drug release) of the VC solid dispersions
Fig. 5 presents the dissolution profiles of the prepared solid dispersions, PM, and pure voriconazole (VC). The solid dispersions exhibited a markedly improved drug release compared to both the physical mixtures and plain VC, highlighting the effectiveness of PEG-based dispersion systems in enhancing dissolution.As detailed in Table 2, the cumulative percentage drug release (%CDR) for various formulations ranged from 43.43% to 92.49%. Statistical analysis confirmed that a linear regression model describes the influence of PEGs on %CDR (Y2).
The F-value was 30.66, and the p-value was less than 0.0001, indicating that the model terms were highly significant and the model exhibited strong statistical significance. Furthermore, a non-significant lack-of-fit (p = 0.0732) confirmed the adequacy and reliability of the model (Table 3).
Both PEG 4000 (A) and PEG 6000 (B) were identified as significant contributors to %CDR enhancement (p-values of 0.0106 and <0.0001). The regression coefficients for PEG 4000 and PEG 6000 were +6.09 and +13.95, respectively, indicating a positive and synergistic effect on %CDR as the concentration of each PEG increased. Notably, PEG 6000 exerted a stronger effect on %CDR, as evidenced by its higher coefficient.
Model reliability was further confirmed with a difference between the adjusted R2 (0.8317) and predicted R2 (0.7235) that was well below the acceptable threshold of 0.2. The adequate precision value was 15.16, significantly exceeding the minimum requirement of 4.
The effects of formulation variables on %CDR were also visualized through 2D contour and 3D response surface plots (Fig. 2 and 3), confirming the increasing trend in drug release with increasing PEG levels. The regression equation in coded terms is as follows:
| Y2 = 69.83 + 6.09A + 13.95B | (3) |
This equation clearly indicates that both PEG 4000 and PEG 6000 positively affect the cumulative drug release, with PEG 6000 contributing more significantly. Therefore, increasing the concentration of either PEG improves the dissolution profile of the VC solid dispersions, as shown in Fig. 4i and ii.
3.5 Multiple response optimization study
Multiple-response optimization strategy was used to enhance the solubility (Y1) and cumulative drug release (%CDR, Y2) of voriconazole (VC) solid dispersions by modulating the concentrations of PEG 4000 (A) and PEG 6000 (B). This approach combined statistical modeling with desirability and numerical optimization algorithms to identify a robust and well-balanced formulation.The desirability, which ranges from ‘0’ (undesirable) to ‘1’ (highly desirable), was utilized to optimize both Y1 and Y2 concurrently. Numerical optimization was conducted within the defined experimental domain for PEG 4000 and PEG 6000, aiming to maximize both dependent variables. Graphical optimization through overlay plots allowed the visual delineation of the design space, identifying factor combinations yielding acceptable response outcomes.29
The optimal formulation identified through this process consisted of 600 mg each of PEG 4000 and PEG 6000, achieving a desirability score of 0.901, which is close to 1, indicating that the model is highly desirable. Similar results were reported by Mundada et al.29 The optimized solid dispersion showed a solubility of 30.68 mg mL−1 and a %CDR of 89.88%, as shown in Fig. 6. These were very much higher compared to the pure drug (0.5 mg ml−1 and 35.99%). The initial dissolution rate (IDR) and relative dissolution rate (RDR) for the optimized formulation were found to be 0.77 percent per minute and 2.57, respectively.
The yellow region in the overlay plot represents the design space wherein the formulation criteria were met. The optimized batch was further subjected to physicochemical characterization.
3.6 Model adequacy verification
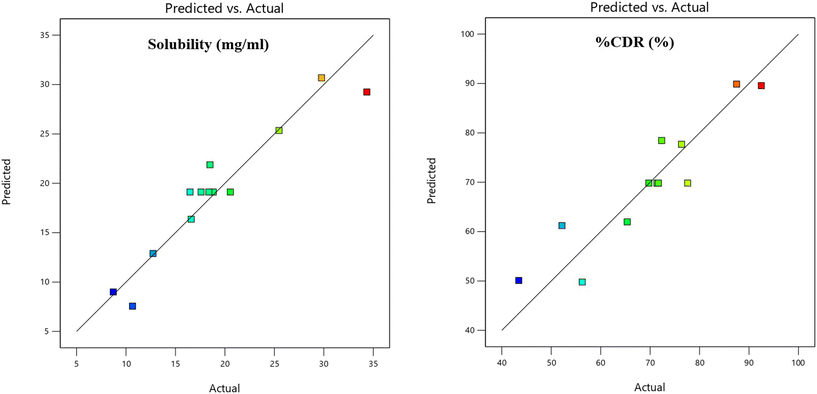
The adequacy of the predictive model for solubility (Y1) and %CDR (Y2) was confirmed through comparisons of predicted versus actual values (Table 4 and Fig. 7). The close alignment between the experimental responses and the predicted values validated the linear regression model, indicating its reliability in anticipating formulation performance.303.7 Characterization of the VC solid dispersions
Solid-state characterization of the optimized formulation provided further evidence of successful dispersion.3.8 Stability studies
Stability testing of the optimized solid dispersion over a six-month period revealed no significant difference between the before and after storage conditions (Table 5). A similarity factor (f2) of 78.27 indicated no change in the drug release profile and confirmed the formulation's stability under storage conditions.34| Time (min) | Before storage | After 6 months | Similarity factor (f2) |
|---|---|---|---|
| 0 | 0 | 0 | 78.27 |
| 5 | 32.15 ± 1.51 | 31.54 ± 1.34 | |
| 10 | 42.06 ± 1.35 | 40.68 ± 1.56 | |
| 15 | 48.31 ± 1.43 | 47.25 ± 1.54 | |
| 30 | 60.65 ± 1.72 | 59.57 ± 1.48 | |
| 45 | 68.60 ± 1.26 | 66.34 ± 1.62 | |
| 60 | 74.12 ± 1.34 | 73.61 ± 1.83 | |
| 75 | 78.93 ± 1.83 | 77.85 ± 1.62 | |
| 90 | 85.55 ± 1.75 | 82.26 ± 1.28 | |
| 120 | 92.49 ± 1.64 | 90.57 ± 1.37 | |
| % Assay | 99.14 ± 1.18 | 98.46 ± 1.53 | Not significant |
4. Conclusion
The present study demonstrates the effective use of polyethylene glycols (PEG 4000 and PEG 6000) in enhancing the aqueous solubility and dissolution of voriconazole (VC). Phase solubility studies confirmed the formation of AL-type inclusion complexes, while optimization through a Central Composite Design (CCD) identified the optimal formulation parameters. The optimized solid dispersion, comprising 600 mg each of PEG 4000 and PEG 6000, exhibited markedly improved solubility (30.68 mg mL−1) and dissolution efficiency (89.88%), supported by a high desirability value of 0.901.Physicochemical characterization studies, including FTIR, DSC, PXRD, and SEM, demonstrated the successful development of a molecularly dispersed system, as evidenced by spectral modifications, disappearance of the crystalline melting peak, reduced crystallinity, and distinct morphological changes. Moreover, the optimized formulation maintained its stability over six months under accelerated conditions, with no significant variation in dissolution performance.
Collectively, these results highlight the potential of PEG-based solid dispersions as a reliable strategy to overcome solubility-related challenges in BCS Class II antifungal agents. The approach provides a robust and scalable formulation platform capable of enhancing both the solubility and dissolution of voriconazole.
Author contributions
Bhaskar Daravath: conceptualization, experimental work, manuscript drafting, and critical revision. Shasidhar Goud Pendlimadugula, Uday Kiran Kummari, Ravi Teja C and Ajay Reddy K: experimental work and drafting of the original manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting the findings of the study are available from the corresponding author upon request.Acknowledgements
The authors did not receive any funding from any agencies for this research. The authors acknowledge the GITAM School of Pharmacy, GITAM (Deemed to be University), Hyderabad, for providing research facilities. The authors also extend sincere thanks to MSN Laboratories Pvt. Ltd, Hyderabad, Telangana, India, for supplying the voriconazole sample for the study.References
- G. L. Amidon, H. Lennernäs, V. P. Shah and J. R. Crison, A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm. Res., 1995, 12(3), 413–420 CrossRef CAS.
- S. Kalepu and V. Nekkanti, Insoluble drug delivery strategies: review of recent advances and business prospects, Acta Pharm. Sin. B, 2015, 5(5), 442–453 CrossRef.
- T. Loftsson and M. E. Brewster, Pharmaceutical applications of cyclodextrins: basic science and product development, J. Pharm. Pharmacol., 2010, 62(11), 1607–1621 CrossRef CAS PubMed.
- A. T. M. Serajuddin, Salt formation to improve drug solubility, Adv. Drug Delivery Rev., 2007, 59(7), 603–616 CrossRef CAS PubMed.
- B. Daravath and S. Somalanka, Enhancement of dissolution rate of racecadotril by liquisolid compact technology, Braz. J. Pharm. Sci., 2022, 58, e21044 CrossRef CAS.
- B. Daravath, S. K. Vemula and N. Chella, Optimizing Nateglinide Liquisolid Compacts: Achieving Formulation Excellence Through the Quality by Design Approach, J. Pharm. Innovation, 2024, 19(64) DOI:10.1007/s12247-024-09873-3.
- T. Vasconcelos, S. Marques, J. D. Neves and B. Sarmento, Amorphous solid dispersions: rational selection of a manufacturing process, Adv. Drug Delivery Rev., 2016, 100, 85–101 Search PubMed.
- R. Chokshi, H. Zia, H. Sandhu, N. H. Shah and W. Malick, Improving the dissolution rate of poorly water-soluble drug by solid dispersion and solid solution–pros and cons, Drug Delivery, 2007, 14(1), 33–45 CrossRef CAS PubMed.
- M. M. Patel and N. M. Patel, A comprehensive review on solid dispersion technology for solubility enhancement of poorly water-soluble drugs, Asian J. Pharm., 2020, 14(1), 10–22 Search PubMed.
- H. M. S. H. Soe, K. Kerdpol, T. Rungrotmongkol, P. Pruksakorn, R. Autthateinchai and S. Wet-osot, et al., Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes, Int. J. Mol. Sci., 2023, 24(3), 2343 CrossRef CAS PubMed.
- B. B. Patel, J. K. Patel, S. Chakraborty and D. Shukla, Revealing facts behind spray dried solid dispersion technology used for solubility enhancement, Saudi Pharm. J., 2015, 23(4), 352–365 Search PubMed.
- S. Raina, R. Singh, A. Sharma and S. Arora, Development and characterization of solid dispersions of posaconazole for enhancement of oral bioavailability, AAPS PharmSciTech, 2020, 21(7), 268 CrossRef PubMed.
- F. E. Maha, A. E. Ahmed and M. M. Nadia, et al., Optimization of Meloxicam Solid Dispersion Formulations for Dissolution Enhancement and Storage Stability Using 33 Full Factorial Design Based on Response Surface Methodology, AAPS PharmSciTech, 2022, 23(7), 248 CrossRef.
- D. C. Montgomery, Design and Analysis of Experiments, Wiley, 9th edn, 2017 Search PubMed.
- L. X. Yu, Pharmaceutical quality by design: product and process development, understanding, and control, Pharm. Res., 2008, 25(4), 781–791 Search PubMed.
- S. K. Vemula, B. Daravath and M. Repka, Quality by design (QbD) approach to develop fast-dissolving tablets using melt-dispersion paired with surface-adsorption method: formulation and pharmacokinetics of flurbiprofen melt-dispersion granules, Drug Delivery Transl. Res., 2023, 13(12), 3204–3222 Search PubMed.
- S. Jain, N. Patel and S. Lin, Formulation and optimization of curcumin solid dispersion using polymer blends and DoE approach, J. Drug Delivery Sci. Technol., 2021, 61, 102095 Search PubMed.
- B. Shah, D. Khunt and M. Misra, Development of solid lipid nanoparticles of artemether for parenteral administration: optimization, in vitro and in vivo studies, Biomed. Pharmacother., 2014, 68(4), 439–445 CrossRef PubMed.
- B. Daravath, R. R. Tadikonda and S. K. Vemula, Formulation and pharmacokinetics of gelucire solid dispersions of flurbiprofen, Drug Dev. Ind. Pharm., 2015, 41(8), 1254–1262 CrossRef CAS PubMed.
- H. V. Chavda and C. N. Patel, Preparation and characterization of lumefantrine solid dispersions for dissolution enhancement, J. Pharm. Innovation, 2011, 6(4), 265–275 Search PubMed.
- B. Daravath, C. Naveen, S. K. Vemula and R. R. Tadikonda, Solubility and dissolution enhancement of flurbiprofen by solid dispersion using hydrophilic carriers, Braz. J. Pharm. Sci., 2017, 53(4), e00233 Search PubMed.
- B. Daravath, Surface solid dispersion: A novel method for improving in vitro dissolution and in vivo pharmacokinetics of meclizine hydrochloride, Res. J. Pharm. Technol., 2021, 14(2), 685–693 CrossRef.
- S. K. Vemula and P. R. Veerareddy, Fast Disintegrating Tablets of Flurbiprofen: Formulation and Characterization, Lat, Am. J. Pharm., 2011, 30(6), 1135–1141 CAS.
- N. Chella, B. Daravath, D. Kumar and R. R. Tadikonda, Formulation and Pharmacokinetic Evaluation of Polymeric Dispersions Containing Valsartan, Eur. J. Drug Metab. Pharmacokinet., 2016, 41(5), 517–526 CrossRef CAS PubMed.
- B. Daravath and G. Kumari, Improvement of bioavailability of poorly soluble racecadotril by solid dispersion with surface adsorption method: A case study, J. Rep. Pharm. Sci., 2021, 10(1), 77 Search PubMed.
- B. A. Kondoros, O. Berkesi, Z. Tóth, Z. Aigner, R. Ambrus and I. Csóka, Cyclodextrin Complexation of Fenofibrate by Co-Grinding Method and Monitoring the Process Using Complementary Analytical Tools, Pharmaceutics, 2022, 14(7), 1329 Search PubMed.
- M. L. Manca, M. Zaru, G. Ennas, D. Valenti, C. Sinico and G. Loy, et al., Diclofenac-β-cyclodextrin binary systems: Physicochemical characterization and in vitro dissolution and diffusion studies, AAPS PharmSciTech, 2005, 6(3), E464–E472 CrossRef.
- B. Suresh, J. Subash, B. E. Basanth, D. Rajeshri, J. Raju and R. V. Prabhakar, Physicochemical characterization and dissolution enhancement of loratadine by solid dispersion technique, Korean J. Chem. Eng., 2013, 30(1), 238–244 CrossRef.
- P. K. Mundada, K. K. Sawant and V. P. Mundada, Formulation and optimization of controlled release powder for reconstitution for metoprolol succinate multi unit particulate formulation using risk based QbD approach, J. Drug Delivery Sci. Technol., 2017, 41, 462–474 Search PubMed.
- M. A. Bezerra, R. E. Santelli, E. P. Oliveira, L. S. Villar and L. A. Escaleira, Response surface methodology (RSM) as a tool for optimization in analytical chemistry, Talanta, 2008, 76(5), 965–977 CrossRef CAS.
- T. Vasconcelos, B. Sarmento and P. Costa, Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs, Drug Discovery Today, 2007, 12(23–24), 1068–1075 Search PubMed.
- D. Sharma, M. Soni, S. Kumar and G. D. Gupta, Solubility enhancement—Eminent role in poorly soluble drugs, Res. J. Pharm. Technol., 2009, 2(2), 220–224 CAS.
- A. Paradkar, A. A. Ambike, B. K. Jadhav and K. R. Mahadik, Characterization of curcumin–PVP solid dispersion obtained by spray drying, Int. J. Pharm., 2004, 271(1–2), 281–286 Search PubMed.
- T. Kaur, A. Kaur and I. P. Kaur, A comprehensive study of PEGylated solid dispersion of itraconazole: physicochemical characterization, in vitro dissolution and stability evaluation, J. Drug Delivery Sci. Technol., 2021, 61, 102320 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |