Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent-free reactions at work towards densely functionalized targets: synthesis of 3-amino(azido)-3-deoxy-D-galactose, a key structural motif of galectin ligands
Serena Traboni a,
Emiliano Bedini
a,
Emiliano Bedini a,
Fabiana Espositoa,
Marcello Ziaco
a,
Fabiana Espositoa,
Marcello Ziaco b and
Alfonso Iadonisi
b and
Alfonso Iadonisi *a
*a
aDepartment of Chemical Sciences, University of Naples Federico II, Via Cinthia 4, I-80126 Naples, Italy. E-mail: iadonisi@unina.it
bInstitute of Biomolecular Chemistry (ICB), National Research Council (CNR), Via Campi Flegrei 34, 80078 Pozzuoli, Italy
First published on 9th December 2025
Abstract
A straightforward approach is reported for the synthesis of 3-deoxy-3-amino(azido)-D-galactose, a key intermediate for the synthesis of high-affinity therapeutic inhibitors of galectins. The synthetic route highlights the viable applicability of experimentally simple solvent-free reactions in nearly half of the steps in the sequence, including a procedure specifically developed for a key epoxidation step. Unlike other known synthetic approaches, the current strategy stands out for the fast and efficient SN2 steps necessary for setting the nitrogen at C-3 with the correct configuration, achieved by taking advantage of intramolecular reactions.
Introduction
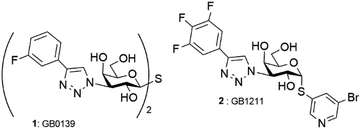
3-Amino-3-deoxy-galactose and its 3-azido analogue became very important targets in organic synthesis since the discovery that symmetrical sulfide or thioglycoside derivatives thereof can be excellent ligands of galectins,1 which are important β-galactoside receptors playing a pivotal role in numerous biological processes with therapeutic implications.2 A remarkable effort is being currently devoted to searching for inhibitors of galectin, as exemplified by derivatives falling into this class that are advancing in clinical trial stages (Fig. 1).3In addition, this saccharide residue was also unveiled as a component of a high-affinity inhibitor of glycosyl transferase.4
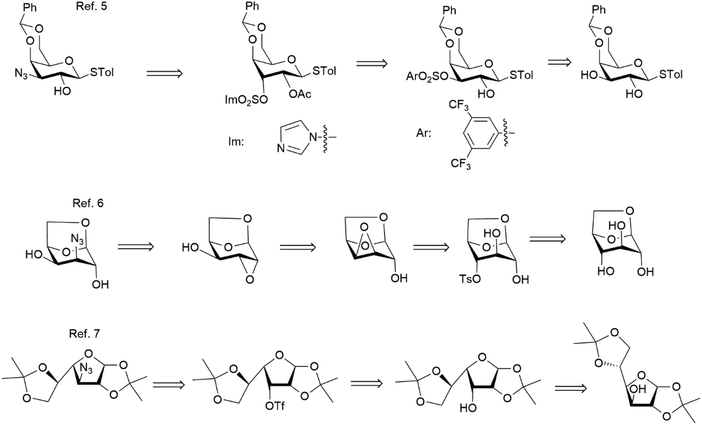
Synthesis of 3-amino-3-deoxy-galactose is not trivial and all the described routes entail difficult SN2 reactions at secondary sites of the saccharide precursors. In fact, D-glucose and D-galactose derivatives have been reported as precursors of this target, and each of the known synthetic schemes requires at least two steps involving inverting the configuration.
Scheme 1 shows a summary of the most significant intermediates of three representative approaches to 3-deoxy-3-azido-D-galactose described so far.5–7 The first route5 is based on the attachment of the nitrogenated functionality at the C-3 position of a galacto-precursor, with an overall retention of configuration at this position implying a sequence of two SN2 processes. The viability of this route is critically dependent on the efficient regioselective installation of a suitable leaving group at C-3 prior to each substitution process, and relies on a favourable outcome of the SN2 steps. In pursuing this general strategy, Nilsson and co-workers initially attached a 3,5-(trifluoromethyl)benzensulfonyl group onto the O-3 position of a 4,6-O-benzylidene–protected thiogalactoside, in order to perform with cesium acetate the first substitution process leading to galacto-to-gulo epimerization;5 subsequently, a sufonylimidazolide was adopted as the leaving group precursor for the subsequent azidation step, restoring the galacto-configuration while introducing a nitrogen at C-3.
In the second approach,6 the inexpensive 1,6-anhydro-β-D-glucose (levoglucosan) was selected in place of a galacto-precursor. Here, access to 3-azido galactose was achieved with an initial 4-O-tosylation, followed by a Payne rearrangement involving initial generation of a 3,4-epoxide then becoming a 2,3-epoxide; diaxial opening of the latter epoxide with nucleophilic azide, ultimately led to azidation at C-3 of 1,6-anhydro-β-D-galactose.
In the third approach,7 a protected glucofuranose precursor was employed, and five steps were needed to carry out the inversion of configuration at both C-3 and C-4 (via an unsaturated intermediate submitted to a syn-hydrogenation), and azidation at C-3 was conducted in the final steps through the expensive triflate activation.7 These synthetic approaches are plagued by several drawbacks such as the need for especially extended reaction times for both the regioselective sulfonylation steps and the substitution steps. In addition, use of highly expensive and/or sensitive reagents—such as the arylsulfonating agent adopted in the first synthesis for the first SN2 step (about 1000 times as expensive as the corresponding tosylating reagent), and such as cesium acetate, tetramethylpiperidide, and triflic anhydride—has a profound impact on their scalability.
Also, an interesting strategy was recently reported for the synthesis of 3-nitrogenated galacto-building-blocks starting from 1,2-galactal. The strategy takes advantage of a sequence of reactions, namely a Ferrier rearrangement and an intramolecular aza-Wacker step, with the latter being, however, a rather time-consuming step (48–72 hours).8 In addition, adaptation of this strategy towards the synthesis of galectin ligands would require additional steps (not optimized to date) for the removal of the N-tosyl-carbamate functionality and the introduction of a suitable anomeric leaving group.
Results and discussion
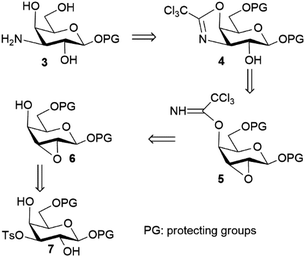
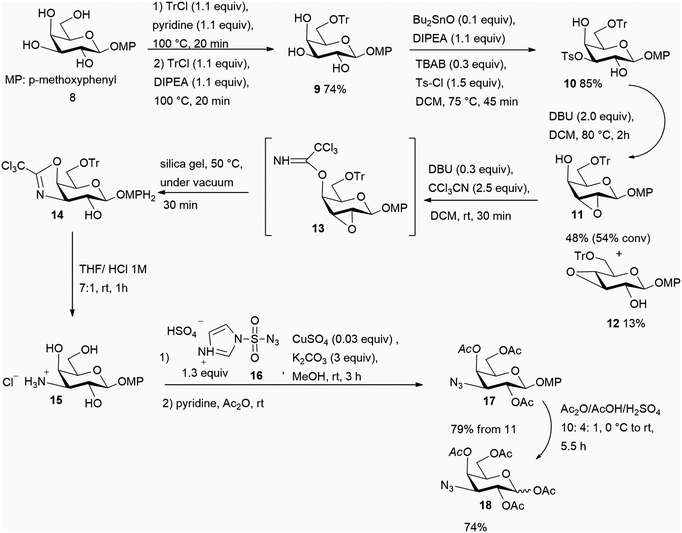
In order to implement a more practical synthesis requiring less experimental effort, an alternative strategy herein described was devised, starting from the general idea that intramolecular processes might be fruitfully exploited for all the requisite SN2 steps, with an expected beneficial effect on both the reaction rates and the corresponding yields. As shown in the retrosynthesis in Scheme 2, some inspiration was drawn from a synthetic strategy described some years ago to access useful building blocks of 3-deoxy-3-fucosamine;9 one of the key steps in that route was an intramolecular attack of a trichloroacetimidate nitrogen (axially placed at C-4) against a suitably oriented 2,3-epoxide of a gulo-configured sugar (conversion of 5 to 4 in Scheme 2). In order to avoid the demanding triflate activation exploited in that scheme,9 a critical goal of the initial steps here was the regioselective installation of a practically more convenient leaving group at C-3 to carry out the subsequent generation of the 2,3-epoxide functionality. For this purpose, we relied on a recently reported approach allowing a fast 3-O-tosylation of galactosides under tin-catalyzed solvent-free reaction conditions,10 and also demonstrated in the current work that solvent-free conditions can be further usefully exploited in a new application, namely in the subsequent 2,3-epoxidation step, as well as in other key steps of the scheme. In fact, the overall scheme demonstrated that combining established solvent-free approaches11 with newly-developed ones can provide a powerful strategy to conveniently access high–added-value targets featuring a dense array of functional groups and stereocentres.The synthetic sequence (Scheme 3) started from aryl glycoside 8, which is commercially available or easily obtained from commercial peracetylated galactose under solvent-free conditions.12 In order to prevent undesired side reactions, the reactive primary 6-OH was preliminarily protected with the bulky and acid-labile trityl group. Following a recently reported protocol based on the exclusive use of a slight excess of pyridine and trityl chloride (2.5 and 1.1 equiv., respectively) at 100 °C,13 only a partial conversion of 8 to 9 (ca. 50%) was observed after 45 minutes, with no evidence of significant further evolution upon increasing the reaction time. This peculiar behaviour of galacto-precursors was already noticed when the procedure was developed,13 in sharp contrast to gluco- and manno-precursors, which were 6-O-tritylated in much higher yields under similar conditions. A substantial improvement in the yield of 9 (up to 74%) was achieved upon further addition, at 20 minutes after the start, of DIPEA (2 equiv.) and another aliquot of trityl chloride (1.1 equiv.) (Scheme 3); the reaction was much less efficient when the entire amounts of all employed reagents were added at the start of the reaction. After being chromatographically isolated, compound 9 was submitted to regioselective tosylation at O-3 by taking advantage of a recent solvent-free approach based on the catalytic generation of a 3,4-O-stannylene acetal intermediate from the cis-diol motif present in the galactoside substrate.10 Very interestingly, adding a small amount of dichloromethane to the initial reaction mixture had a beneficial impact, with desired product 10 obtained in 85% isolated yield after only 45 minutes at 75 °C, whereas about a 70% yield was recorded in its absence. This result can be accounted for by the ability of dichloromethane to create a homogenous reaction medium prior to its distillation under the thermal conditions required by the reaction. Note that regioselective attachment of alternative sulfonyl moieties derived from agents more reactive than tosyl chloride (triflic anhydride or mesyl chloride) did not prove viable in our hands. Likewise, applicability of halide leaving groups is here affected by the practical need for extended reaction times and harsh conditions for the halogenation of secondary alcohols as well as the difficult stereocontrol requested.14
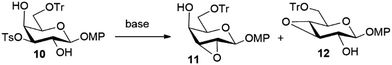
Achieving the following epoxidation step of the synthesis (Scheme 3) was not trivial. Initial attempts were made under described basic conditions such as NaH in DMF,6 sodium methoxide in a dichloromethane/methanol mixture,6 or DBU in dichloromethane.15 The latter condition resulted in a very sluggish reaction, whereas the yield under the other conditions was strongly affected by the rate of generation of the undesired 3,4-epoxide 12 (arising from the Payne rearrangement of desired epoxide 11) and the recovery of the starting tosylated compound 10 (Scheme 4).
According to NMR analysis of the respective crude mixtures, use of NaH in DMF led to a complete consumption of 10 and the generation of an almost equimolar mixture of epoxides 11 and 12, whereas use of sodium methoxide in a dichloromethane/methanol mixture provided a more favourable 11/12 ratio (ca. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), but a substantial recovery of starting compound 10 (ca. 40%). In an attempt to achieve synthetically satisfying results with a minimally demanding procedure, some experimentation was conducted, specifically testing solvent-free conditions for this step; compound 10 was thus treated with a large set of bases in the absence of solvent and at different temperatures. DBU proved to be the only tested base that afforded an appreciable extent of epoxidation (Scheme 3), the most satisfying results being recorded at 80 °C. Interestingly, when 1 equiv. of DBU was adopted at this temperature, an approximate 50% consumption of 10 was observed with prevalent conversion to 11. Upon doubling the amount of base, an appreciable prevalence of desired epoxide 11 was observed after 2 hours in an NMR analysis of the crude product. Silica gel purification afforded epoxide 11 in 48% isolated yield, together with a ca. 20% recovery of 10, and a ca. 15% recovery of epoxide 12, corresponding to a conversion higher than 50% (Scheme 3). Structural identification of the obtained epoxides 11 and 12 was based on the observation of the typical high-field 1H and 13C NMR shifts for the protons (δ lower than 3.5 ppm) and carbons (δ at 50–55 ppm) of the epoxide moiety.
1), but a substantial recovery of starting compound 10 (ca. 40%). In an attempt to achieve synthetically satisfying results with a minimally demanding procedure, some experimentation was conducted, specifically testing solvent-free conditions for this step; compound 10 was thus treated with a large set of bases in the absence of solvent and at different temperatures. DBU proved to be the only tested base that afforded an appreciable extent of epoxidation (Scheme 3), the most satisfying results being recorded at 80 °C. Interestingly, when 1 equiv. of DBU was adopted at this temperature, an approximate 50% consumption of 10 was observed with prevalent conversion to 11. Upon doubling the amount of base, an appreciable prevalence of desired epoxide 11 was observed after 2 hours in an NMR analysis of the crude product. Silica gel purification afforded epoxide 11 in 48% isolated yield, together with a ca. 20% recovery of 10, and a ca. 15% recovery of epoxide 12, corresponding to a conversion higher than 50% (Scheme 3). Structural identification of the obtained epoxides 11 and 12 was based on the observation of the typical high-field 1H and 13C NMR shifts for the protons (δ lower than 3.5 ppm) and carbons (δ at 50–55 ppm) of the epoxide moiety.
Interestingly, this new solvent-free DBU-based epoxidation procedure was discovered after a screening of alternative bases, but it could have in hindsight been inferred more directly by the reagent requested in the above-mentioned known procedure in solution (DBU in dichloromethane),15 which proved to be much less efficient in this synthetic project. That the best result was, remarkably, recorded with the solvent-free version of the epoxidation step suggests a generalizable optimization strategy for whatever organic transformation planned in a synthetic project, by attempting the adaptation of known procedures in solution to a solvent-less version, even when those in solution are not effective for the specific application under scrutiny. The potential of this methodological option is indeed also supported by some previous examples of selective manipulation of carbohydrates in which the solvent-free conditions permitted the occurrence of reactions otherwise ineffective in solution.16
As to the synthetic scope, the epoxidation procedure herein reported represents a new example, in addition to the recently described carbodiimide-based esterification and amidation,17 of a solvent-free reaction unveiled on saccharide substrates which appears of general applicability in organic synthesis.
After being chromatographically purified, epoxide 11 was submitted to a sequence of short steps performed without intermediate purifications, aimed at introducing the nitrogen at C-3 with the correct configuration. This sequence began with the attachment of the requested trichloroacetimidate functionality to O-4 (to yield intermediate 13), achieved by exposing 11 to trichloroacetonitrile and a sub-stoichiometric amount of DBU for 45 min at rt (Scheme 3). Direct addition of silica gel to the mixture and then heating the resulting mixture at 50 °C under vacuum for 30 minutes led to a crude product mixture mostly containing imidate 14 (NMR spectra of crude 13 and 14 are shown in the SI).9 Treatment of 14 with a 1 M aq HCl in THF smoothly promoted simultaneous hydrolysis of the cyclic imidate and 6-O-detritylation, yielding 3-amino-3-deoxy-galactose hydrochloride 15 in 1 h (Scheme 3). As expected, the 1H NMR spectrum of this product exhibited the typical profile of coupling constants related to β-galactosides, providing evidence for an overall retention of configuration at C-3. In addition, the presence of the nitrogen attached at C-3 was confirmed by the relatively shielded signal for H-3 at ca. 3.40 ppm, and the appearance of a shielded 13C signal at ca. 55 ppm.
Product 15 can be usefully exploited for the synthesis of potential galectin ligands bearing a N-acylated derivatization at C-3. On the other hand, well-established galectin ligands (such as those in Fig. 1) feature a triazole moiety resulting from a Huisgen cyclo-addition occurring on a 3-azido-galactoside, so the amino-to-azide conversion at C-3 of 15 was performed with the diazotransfer agent 16.18 The resulting mixture was then exposed to pyridine and acetic anhydride in order to achieve the peracetylation of free saccharide alcohols. Compound 17 was isolated in 79% yield from epoxide 11 (yield over 5 steps). In order to make the anomeric position activatable for further useful elaborations towards galectin ligands (such as the synthesis of symmetrical sulfides), the anomeric para-methoxy-phenyl group was acetolyzed with a mixture of acetic anhydride, acetic acid and sulfuric acid,19 to yield 1-O-acetylated 18 as an anomeric mixture in a bit less than six hours (this reaction took the longest of all the reactions of our overall synthesis, though still much less time than did several steps found in the routes summarized in Scheme 1). This compound represents the key intermediate that can be converted with already described procedures1b,f,6 into a variety of high-affinity galectin ligands, through its conversion into the corresponding glycosyl bromide. The procedure herein reported therefore represents a straightforward formal synthesis of therapeutically valuable inhibitors of galectin.
Conclusions
In conclusion, here we have reported a straightforward synthetic route to 3-amino-3-deoxy-galactose and the corresponding azido analogue with a suitable functionalization permitting their conversion into therapeutically useful galectin ligands. The strategy was designed in such a way to avoid kinetically disfavoured intermolecular reactions in the two SN2 steps employed to set the nitrogenated functionality with the correct stereochemistry. The nine-step synthetic pathway provides the key intermediate 18 in almost 20% overall yield, and features numerous distinctive advantages such as application of short and efficient reactions, use of inexpensive reagents, experimental simplicity, and the need for fewer chromatographic purifications. Quite remarkably, nearly half of the steps (four out of the nine steps) were conducted with especially simple solvent-free procedures, including an original epoxidation procedure that was specifically developed for one of the critical steps of the sequence.Apart from the unprecedented extended application of solvent-free reactions towards a target with such a dense array of functional groups and stereocentres, another significant conceptual contribution of this paper lies in the viability of a generalizable optimization strategy for whatever organic transformation, starting from the adaptation of known procedures in solution to a solvent-less version, even when those in solution are not very effective for the specific step under optimization. Overall, the synthetic scheme highlights the powerful innovative potential of solvent-less reactions in synthetic carbohydrate chemistry and, more generally, in the synthesis of highly functionalized targets.11,20,21
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included in the supplementary information (SI), including experimental procedures, spectroscopic data, and NMR spectra of all intermediates and the final product of the synthesis. See DOI: https://doi.org/10.1039/d5ob01710f.Acknowledgements
S. T. thanks PON project “Ricerca e Innovazione” 2014-2020 (PON R&I) – AZIONE IV.4 e “Contratti di Ricerca su tematiche dell'Innovazione” for supporting a researcher position. The University of Naples Federico II (FRA-2022-C grant) is acknowledged for funding. E. B. acknowledges the support of the Italian Ministry for University and Research (grant number PRIN2022-PNR_P20224T45H).References
- (a) I. Cumpstey, A. Sundin, H. Leffler and U. J. Nilsson, C2 -Symmetrical Thiodigalactoside Bis-Benzamido Derivatives as High-Affinity Inhibitors of Galectin-3: Efficient Lectin Inhibition Through Double Arginine-Arene Interactions, Angew. Chem., Int. Ed., 2005, 44, 5110 Search PubMed; (b) M. van Scherpenzeel, E. E. Moret, B. Lluis, R. M. J. Liskamp, U. J. Nilsson, H. Leffler and R. J. Pieters, Synthesis and Evaluation of New Thiodigalactoside-Based Chemical Probes to Label Galectin-3, ChemBioChem, 2009, 10, 1724 Search PubMed; (c) I. Cumpstey, E. Salomonsson, A. Sundin, H. Leffler and U. J. Nilsson, Double Affinity Amplification of Galectin-Ligand Interactions Through Arginine-Arene Interactions: Synthetic, Thermodynamic, and Computational Studies with Aromatic Diamido Thiodigalactosides, Chem. – Eur. J., 2008, 14, 4233 Search PubMed; (d) B. A. Salameh, I. Cumpstey, A. Sundin, H. Leffler and U. J. Nilsson, 1H-1,2,3-Triazol-1-yl Thiodigalactoside Derivatives as High Affinity Galectin-3 Inhibitors, Bioorg. Med. Chem., 2010, 18, 5367 Search PubMed; (e) A.-L. Noresson, O. Aurelius, C. T. Öberg, O. Engström, A. P. Sundin, M. Hakansson, O. Stenström, M. Akke, D. T. Logan, H. Leffler and U. J. Nilsson, Designing Interactions by Control of Protein–Ligand Complex Conformation: Tuning Arginine–Arene Interaction Geometry for Enhanced Electrostatic Protein–Ligand Interactions, Chem. Sci., 2018, 9, 1014 Search PubMed; (f) H. Zhang, D. Laaf, L. Elling and R. J. Pieters, Thiodigalactoside-Bovine Serum Albumin Conjugates as High-Potency Inhibitors of Galectin-3: an Outstanding Example of Multivalent Presentation of Small Molecule Inhibitors, Bioconjugate Chem., 2018, 29, 1266 Search PubMed.
- F. T. Liu and G. A. Rabinovich, Galectins as Modulators of Tumour Progression, Nat. Rev. Cancer, 2005, 5, 529 Search PubMed.
- (a) F. R. Zetterberg, K. Peterson, U. J. Nilsson, K. Andréasson Dahlgren, C. Diehl, I. Holyer, M. Håkansson, A. Khabut, B. Kahl-Knutson, H. Leffler, A. C. MacKinnon, J. A. Roper, R. J. Slack, R. Zarrizi and A. Pedersen, Discovery of the Selective and Orally Available Galectin-1 Inhibitor GB1908 as a Potential Treatment for Lung Cancer, J. Med. Chem., 2024, 67, 9374 Search PubMed; (b) F. R. Zetterberg, C. Diehl, M. Håkansson, B. Kahl-Knutson, H. Leffler, U. J. Nilsson, K. Peterson, J. A. Roper and R. J. Slack, Discovery of Selective and Orally Available Galectin-1 Inhibitors, J. Med. Chem., 2023, 66, 16980 Search PubMed; (c) F. R. Zetterberg, A. MacKinnon, T. Brimert, L. Gravelle, R. E. Johnsson, B. Kahl-Knutson, H. Leffler, A. Pedersen, K. Peterson, J. A. Roper, H. Schamby, R. J. Slack and S. Tantawi, Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease, J. Med. Chem., 2022, 65, 12626 Search PubMed; (d) C. Liu, P. R. Jalagam, J. Feng, W. Wang, T. Raja, M. R. Sura, R. K. V. L. P. Manepalli, B. R. Aliphedi, S. Medavarapu, S. K. Nair, V. Muthalagu, R. Natesan, A. Gupta, B. Beno, M. Panda, K. Ghosh, J. K. Shukla, H. Sale, P. Haldar, N. Kalidindi, D. Shah, D. Patel, A. Mathur, B. A. Ellsworth, D. Cheng and A. Regueiro-Ren, Identification of Monosaccharide Derivatives as Potent, Selective, and Orally Bioavailable Inhibitors of Human and Mouse galectin-3, J. Med. Chem., 2022, 65, 11084 Search PubMed.
- T. L. Lowary and O. Hindsgaul, Recognition of Synthetic O-Methyl, Epimeric, and Amino Analogues of the Acceptor α-L-Fucp-(1→2)-β-D-Galp-OR Glycosyltransferase, Carbohydr. Res., 1994, 251, 33 Search PubMed.
- (a) C. Öberg, A. L. Noresson, T. Delaine, A. Larumbe, J. Tejler, H. von Wachenfeldt and U. J. Nilsson, Synthesis of 3-Azido-3-Deoxy-β-D-Galactopyranosides, Carbohydr. Res., 2009, 344, 1282 Search PubMed; (b) K. Peterson, A. Weymouth-Wilson and U. J. Nilsson, Aryl Sulfonates in Inversions at Secondary Carbohydrate Hydroxyl Groups: a New and Improved Route Toward 3-Azido-3-Deoxy-β-D-Galactopyranosides, J. Carbohydr. Chem., 2015, 34, 490 Search PubMed.
- J. St-Gelais, V. Denavit and D. Giguère, Efficient Synthesis of a Galectin Inhibitor Clinical Candidate (TD139) Using a Payne Rearrangement/Azidation Reaction Cascade, Org. Biomol. Chem., 2020, 18, 3903 Search PubMed.
- (a) R. U. Lemieux, R. Sweda, E. Paszkiewicz-Hnatiw and U. Spohr, The Effect of Substituting Key Hydroxyl-Groups by Amino Groups on the Binding of the Lewis-B Tetrasaccharide by a Lectin and a Monoclonal Antibody, Carbohydr. Res., 1990, 205, C12 Search PubMed; (b) A. Hoffmann-Röder and M. Johannes, Chem. Commun., 2011, 47, 9903 Search PubMed.
- A. Geulin, Y. Bourne-Branchu, K. Ben Ayed, T. Lecourt and A. Joosten, Ferrier/Aza-Wacker/Epoxidation/Glycosylation (FAW8EG) Sequence to Access 1,2-Trans 3-Amino-3-Deoxyglycosides, Chem. – Eur. J., 2023, 29, e202203987 Search PubMed.
- E. Bedini, A. Iadonisi, A. Carabellese and M. Parrilli, First Preparative Synthesis of a 3-Acetamido-3,6-Dideoxy-D-Galactopyranose Glycosyl Donor Via Intramolecular Cyclization of an Epoxytrichloroacetimidate, Tetrahedron Lett., 2004, 45, 4445 Search PubMed.
- S. Traboni, E. Bedini, A. Landolfi, G. Vessella and A. Iadonisi, Catalytic, Regioselective Sulfonylation of Carbohydrates with Dibutyltin Oxide Under Solvent-Free Conditions, Catalysts, 2021, 11, 202 Search PubMed.
- For the first review of solvent-free approaches in synthetic carbohydrate chemistry: S. Traboni, E. Bedini, G. Vessella and A. Iadonisi, Solvent-Free Approaches in Carbohydrate Synthetic Chemistry: Role of Catalysis in Reactivity and Selectivity, Catalysts, 2020, 10, 1142 Search PubMed.
- S. Traboni, E. Bedini, A. Silipo, G. Vessella and A. Iadonisi, Solvent-Free Glycosylation from Per-O-Acylated Donors Catalyzed by Methanesulfonic Acid, Eur. J. Org. Chem., 2021, 5669 Search PubMed.
- S. Traboni, E. Bedini and A. Iadonisi, Solvent-Free One-Pot Diversified Protection of Saccharide Polyols Via Regioselective Tritylations, ChemistrySelect, 2017, 2, 4906 Search PubMed.
- O. Lessard, D. Lainé and D. Giguère, Polyhalogenated Carbohydrates: Synthesis and Applications of Sugar Halides from Fluorine to Iodine, Eur. J. Org. Chem., 2024, 27, e202400120 Search PubMed.
- F. Shui, J. Jia, X. Yang, Q. Zhou, Y. Jiang and X. Chen, Synthesis of (+)-Epoxydon, (-)-Phyllostine, (-)-RKTS 33, and (-)-Parasitenone Featuring Selective Sulfonylation and Oxirane Ring Closure of Aldol Cyclization Products, Eur. J. Org. Chem., 2020, 3981 Search PubMed.
- (a) M. Giordano and A. Iadonisi, Tin-Mediated Regioselective Benzylation and Allylation of Polyols: Applicability of a Catalytic Approach Under Solvent-Free Conditions, J. Org. Chem., 2014, 79, 213 Search PubMed; (b) S. Traboni, E. Bedini, M. Giordano and A. Iadonisi, Three Solvent-Free Catalytic Approaches to the Acetal Functionalization of Carbohydrates and Their Applicability to One-Pot Generation of Orthogonally Protected Building Blocks, Adv. Synth. Catal., 2015, 357, 3562 Search PubMed.
- (a) S. Traboni, F. Esposito, M. Ziaco, E. Bedini and A. Iadonisi, A Comprehensive Solvent-Free Approach for the Esterification and Amidation of Carboxylic Acids Mediated by Carbodiimides, Tetrahedron, 2023, 133, 133291 Search PubMed; (b) S. Traboni, F. Esposito, M. Ziaco, N. De Cesare, E. Bedini and A. Iadonisi, Catalytic Cleavage of the 9-Fluorenylmethoxycarbonyl (Fmoc) Protecting Group Under Neat Conditions, Org. Lett., 2024, 26, 3284 Search PubMed.
- (a) E. D. Goddard-Borger and R. V. Stick, An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-Sulfonyl Azide Hydrochloride, Org. Lett., 2007, 19, 3797 Search PubMed; (b) G. T. Potter, G. C. Jayson, G. J. Miller and J. M. Gardiner, An Updated Synthesis of the Diazo-Transfer Reagent Imidazole-1-Sulfonyl Azide Hydrogen Sulfate, J. Org. Chem., 2016, 81, 3443 Search PubMed.
- M. Adinolfi, G. Barone, A. Iadonisi, L. Mangoni and M. Schiattarella, Activation of Disarmed 2-O-Alkoxycarbonylated Glycosyl Trichloroacetimidates with Lanthanide Triflates: an Efficient Approach for the Synthesis of 1,2-Trans Glycosides, Tetrahedron Lett., 2001, 42, 5967 Search PubMed.
- For an unusual large-scale application of a solvent-free step in the synthesis of a potential therapeutic agent serving as a vaccine adjuvant: M. Ziaco, L. Fioretto, G. Nuzzo, A. Fontana and E. Manzo, Short Gram-Scale Synthesis of Sulfavant A, Org. Process Res. Dev., 2020, 24, 2728 Search PubMed.
- For a review on the application of mechanochemistry with pharmaceuticals: J. Templ and L. Borchardt, Mechanochemical Strategies Applied to the Late-Stage Modifications of Pharmaceutically Active Compounds, Angew. Chem., Int. Ed., 2025, e202503061 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |