Deformable metal–organic nanosheets@SiO2 core–shell for heterogeneous tandem catalytic transformations
Houting
Wang†
,
Yongjie
Wang†
,
Zeyang
Liu†
,
Yuanli
Zhu
,
Cheng
Wang
,
Leyi
Wang
,
Rui
Liu
 *,
Guohua
Liu
*,
Guohua
Liu
 * and
Chunxia
Tan
* and
Chunxia
Tan
 *
*
Shanghai Frontiers Science Center of Biomimetic Catalysis, Joint Laboratory of International Cooperation of Resource Chemistry of Ministry of Education, Shanghai Normal University, Shanghai 200234, China. E-mail: rliu@shnu.edu.cn; tanchx@shnu.edu.cn; ghliu@shnu.edu.cn
First published on 8th December 2025
Abstract
Overcoming mass transport limitations imposed by stagnant boundary layers is critical for advancing heterogeneous catalysis. Building upon strategies utilizing deformable metal–organic nanosheets (MONs) to enhance diffusion, we report the synthesis of well-defined core–shell microspheres that integrate flexible and functional two-dimensional MONs. Nonporous carboxyl-terminated SiO2 nanoparticle cores are seamlessly enveloped by ultrathin Zr-MON shells through a facile bottom-up approach. The resulting MON@SiO2 architecture exposes abundant coordinatively unsaturated Zr(IV) Lewis acid sites on its deformable nanosheets. Further introduction of the triethylenediamine (DABCO) moieties into the MON produces MON-DABCO@SiO2, enabling the co-existence of isolated Lewis acid and base sites amenable to promoting challenging reactions that are unachievable by homogeneous systems. These dynamic core–shell structures significantly enhance molecular diffusion to the active sites, as evidenced by ultra-efficient catalysis (>99% yield) in the one-pot hydrolysis-Knoevenagel tandem reactions across broad-scope substrates. Importantly, the SiO2 core confers exceptional structural durability, enabling great catalytic recyclability for at least 5 consecutive cycles without any degradation of the performances, which is in stark contrast to the unsupported MONs. This work therefore establishes core–shell engineering of deformable MONs as a versatile approach for architecting high-performance and durable heterogeneous catalysts by synergistically combining enhanced mass transport with nanoconfinement effects.
Introduction
In biological systems, flexible primary cilia function as dynamic sensory antennae, significantly enhancing mass transport across cell surfaces through their ability to deform in response to fluid flow.1–3 This natural paradigm inspires the design of anchored, deformable catalytic nanostructures on core–shell materials, aiming to mimic the cilia's capacity to overcome diffusion barriers and achieve high catalytic efficiency.4–8 Crucially, such an “antenna-like” shell must integrate abundant accessible active sites with inherent deformability to actively promote substrate/product diffusion and collision kinetics.9,10Ultrathin two-dimensional metal–organic nanosheets (MONs), derived from conventional bulk MOF crystals by linking metal nodes with organic ligands, represent an emerging class of 2D materials.11–17 Distinguished from their bulk counterparts, MONs offers distinct advantages including abundant open metal sites, ultrahigh surface areas, adjustable surface chemistry, and enhanced accessibility of active sites for target molecules.11–14,18–20 Additionally, their single-unit thickness facilitates molecular transport pathways and enables their use as nanoplatforms for constructing hybrids and carbon nanosheets.21–23 These properties, coupled with their crystalline yet flexible nature, excellent synthetic tunability, and robust chemical stability, establish MONs as an ideal platform for constructing dynamic catalytic interfaces.24–27 Their inherent flexibility allows the creation of soft, responsive shells capable of adapting to fluid dynamics within the reaction medium.28,29 Therefore, our interest in constructing “antenna-like” shell catalyst promoted us to consider whether integrate these deformable MONs onto a solid core could concentrate reactants and promote molecular diffusion on the surface of the flexible nanosheets.30–36
Building upon this design principle, we report herein the synthesis of well-defined core–shell microspheres featuring nonporous, carboxyl-terminated SiO2 nanoparticle cores seamlessly enveloped by ultrathin MON shells. Among the most reported MONs, NUS-8(Zr) was an 2D nanosheet with thickness of 10–20 nm and planar porous structure with highly exposed Lewis acid sites which can be synthesized directly under modulated hydrothermal approach in aqueous media near 100 °C and 1 bar.37 Therefore, based on the bottom-up approach38 and utilizing NUS-8(Zr) as MON shells, the resulting MON@SiO2 architecture was constructed and the obtained “antenna-like” shell exposes abundant coordinatively unsaturated Zr(IV) Lewis acid sites on its highly accessible and deformable nanosheet surface. Extending this platform, functionalization of the H3BTB linker with triethylenediamine (DABCO) yields MON-DABCO@SiO2, which incorporates synergistic Lewis acid–base pairs within its shell structure (Fig. 1a). This core–shell engineering enabled us to tune the deformability of the MONs and thereby increase local mass transport than pure MONs, similar to flexible primary cilia function as dynamic sensory antennae, significantly enhancing mass transport across cell surfaces through their ability to deform in response to fluid flow (Fig. 1b). And the deformable MON shells actively enhance molecular diffusion to active sites, as confirmed by ultra-efficient catalytic performance. The rigid SiO2 core provides exceptional structural stability, enabling facile recovery and recyclability–a defining advancement over unsupported MONs. Collectively, this work establishes a bioinspired materials platform where engineered deformability at the nanoscale overcomes fundamental mass transport limitations in heterogeneous catalysis, paving the way for advanced multifunctional catalyst design.
Experimental
Materials
Tetraethyl orthosilicate, 3-aminopropyltriethoxysilane, succinic anhydride, 1,3,5-tri bromobenzene, 2,4,6-tribromotoluene, zirconium tetrachloride, and other commercially available solvents were purchased in high purity from Energy Chemical, China. All reagents were used without further treatment unless otherwise referred.Characterization
Zr loading amount in the catalysts was analyzed using an inductively coupled plasma optical emission spectrometer (ICP, Varian VISTA-MPX). Fourier transforms infrared (FTIR) spectra were collected on a Nicolet Magna 550 spectrometer using the KBr method. X-ray powder diffraction (XRD) was carried out on a Rigaku D/Max-RB diffractometer with CuKα radiation. Scanning electron microscopy (SEM) images were obtained using a JEOL JSM-6380LV microscope operating at 20 kV. Transmission electron microscopy (TEM) images were performed on a JEOL JEM2010 electron microscope at an acceleration voltage of 220 kV. Nitrogen adsorption isotherms were measured at 77 K with a Quantachrome Nova 4000 analyzer. The samples were measured after being outgassed at 423 K overnight.Synthesis
H3L1 was synthesized as described in the literature.39 And H3L2 was synthesized from 1,2,3,5-tetra bromobenzene after four steps of reactions including Suzuki-coupling, bromination, substitution by DABCO, and hydrolysis. The overall yield of the H3L2 is 39%. The details of the synthesis are in the supporting.Synthesis of MON@SiO2 and MON-DABCO@SiO2
Adding the carboxyl-terminated SiO2 nanoparticles40,41 (10 mg), ZrCl4 (20 mg, 0.08 mmol) into a mixed solvent of dimethylformamide (DMF) (6.9 mL), formic acid (0.9 mL) and H2O (0.2 mL). After vigorously shaking at 120 °C for 24 h, H3L1 (17.5 mg, 0.04 mmol) or H3L2 (22 mg, 0.04 mmol) was added and the mixture keep shaking for another 48 h afforded white solid of MON@SiO2 or MON-DABCO@SiO2 core–shell composite material. The products were stable in air and insoluble in water and common organic solvents. They were tested on powder X-ray diffraction, ICP, IR spectra, and thermogravimetric analysis (TGA).Catalysis test
A typical procedure of MON@SiO2-catalyzed Friedel–Crafts acylation was conducted under an ambient atmosphere. The 2-methoxy naphthalene (138 mg, 1.0 mmol) in neat acetic anhydride (1.0 mL) was added into a 10 mL tube, and 17 mg MON@SiO2 (1mol% Zr based on the ICP test) was added. The reaction mixture was stirred at 25 °C for 2 h, then the solid catalyst was removed by centrifugation. The residue was further washed with ethyl acetate (3 × 3.0 mL) and NaHCO3 to remove the products and excess reactants. The organic was combined and washed with H2O and NaHCO3 before being purified by silica gel chromatography (EA/PE = 1/8). The solid catalyst was collected and subsequently reused in the next run with the same reaction condition.In a typical procedure, benzaldehyde dimethyl acetal (0.1 mmol) and malononitrile (0.12 mmol) were well dispersed into 1 mL of CD3CN followed by adding catalysts (10 mg, 5.4 mol% Zr). After sonication for 5 min, the obtained solution was magnetically stirred in a stainless-steel autoclave at 80 °C for a period to finish the decentralization and Knoevenagel reactions. After the reaction was finished, the target conversion of the substrate was authenticated by 1H NMR. For recycling tests, the catalyst was isolated and washed with toluene and MeOH in sequence after each reaction and subsequently reused in the next run with the same reaction conditions.
Results and discussion
As a proof of concept, NUS-8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42,43 nanosheets was selected as the pristine matrix owing to its facile synthesis via a kinetically controlled bottom-up process. The abundant unsaturated Zr4+ and –OH groups functioning as Lewis and Brønsted acid sites, and the H3BTB ligand can immobilized base sites by grafting DABCO group. Therefore, the NUS-8 nanosheet can construct a bifunctional cascade catalyst. Especially, the exceptional stability of Zr-based metal–organic nanosheets under harsh conditions enables the support to maintain its structural integrity during the following post-synthetic treatment. As illustrated in Fig. 1, by regulating the ratio of carboxyl-terminated SiO2 nanoparticles and Zr4+ ions, we succeed in immobilizing Zr4+ ions on the SiO2 surface via coordination with carboxyl groups. And then under the same solvothermal conditions, a specific proportion of ligands (H3BTB–DABCO or H3BTB) and ZrCl4 can be added in situ to achieve uniform growth of the ultra-thin NUS-8 nanosheets on SiO2 nanoparticles. Besides, considering the highly ordered arrangement maybe facilitate the formation of the NUS-8 MOFs, during the solvothermal process, the reaction solution remains in the oscillator at all times.
42,43 nanosheets was selected as the pristine matrix owing to its facile synthesis via a kinetically controlled bottom-up process. The abundant unsaturated Zr4+ and –OH groups functioning as Lewis and Brønsted acid sites, and the H3BTB ligand can immobilized base sites by grafting DABCO group. Therefore, the NUS-8 nanosheet can construct a bifunctional cascade catalyst. Especially, the exceptional stability of Zr-based metal–organic nanosheets under harsh conditions enables the support to maintain its structural integrity during the following post-synthetic treatment. As illustrated in Fig. 1, by regulating the ratio of carboxyl-terminated SiO2 nanoparticles and Zr4+ ions, we succeed in immobilizing Zr4+ ions on the SiO2 surface via coordination with carboxyl groups. And then under the same solvothermal conditions, a specific proportion of ligands (H3BTB–DABCO or H3BTB) and ZrCl4 can be added in situ to achieve uniform growth of the ultra-thin NUS-8 nanosheets on SiO2 nanoparticles. Besides, considering the highly ordered arrangement maybe facilitate the formation of the NUS-8 MOFs, during the solvothermal process, the reaction solution remains in the oscillator at all times.
The phase purity, structural stability, and porosity profiles of MON@SiO2 and MON-DABCO@SiO2 were systematically investigated through powder X-ray diffraction (XRD), thermogravimetric analysis (TGA), and Brunauer–Emmett–Teller (BET) surface area measurements. As shown in Fig. 2a, the PXRD patterns of both core–shell architectures exhibit characteristic reflections identical to those of pristine NUS-8, confirming retention of the parent MOF's crystalline framework. Critically, no detectable peak broadening or phase impurities were observed following either the epitaxial growth of Zr-MONs on SiO2 nanoparticles or post-synthetic functionalization with DABCO moieties. This demonstrates the robustness of the synthetic protocol in preserving long-range crystallinity during multi-step fabrication. The surface areas and pore volume of the MON-DABCO@SiO2 (236.4 m2 g−1) decreased relative to MON@SiO2 (315.3 m2 g−1) (Fig. 2b) measured by N2 adsorption at 77 K, indicating partial occupation of cavities by well dispersed DABCO groups. Consistently, TGA profiles (Fig. S1) under air flow demonstrated high thermal stability up to 400 °C.
The residual mass analysis indicated MON loadings of 58.3 wt% (MON@SiO2) and 62.2 wt% (MON-DABCO@SiO2), corroborated by inductively coupled plasma optical emission spectroscopy (ICP-OES, SI). Measured Zr contents of 0.5895 mmol g−1 (5.38 g Zr/100 g, ∼60.3 wt% of nanosheets) and 0.5424 mmol g−1 (4.95 g Zr/100 g, ∼61.4 wt% of nanosheets) further confirmed successful MON immobilization. The slight mass increase in MON-DABCO@SiO2 despite reduced Zr density reflects the incorporation of nitrogen-rich DABCO ligands. To further elucidate the interaction between SiO2 and MONs, Fourier transform infrared (FT-IR) spectra were recorded (Fig. 2c). The spectrum of the SiO2 nanoparticles exhibits a strong band around 1710 cm−1, attributed to free carboxyl (–COOH) stretching. In contrast, for MON@SiO2, this band weakens significantly, while new asymmetric and symmetric carboxylate stretching vibrations appear around 1401–1416 cm−1. These changes suggest coordination bonding between Zr4+ and the carboxyl groups on the SiO2 nanoparticle surface. Inaddition, MON-DABCO@SiO2 exhibits distinct C–N stretches at 1093 cm−1 and N–H bends at 1630 cm−1, which absent in MON@SiO2. The broad band at 3164 cm−1 corresponds to μ3-OH vibrations in Zr6O4(OH)4 clusters, unaffected by functionalization—demonstrating cluster stability. Notably, the retention of framework linker vibrations (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1500–1600 cm−1; C–H at 2900 cm−1) further evidences minimal structural perturbation during DABCO grafting.
C at 1500–1600 cm−1; C–H at 2900 cm−1) further evidences minimal structural perturbation during DABCO grafting.
Morphological characterization by SEM (Fig. 3b, c and Fig. S2, S3) confirmed the nanosheet coating, where MON layers were anchored onto the SiO2 surface through coordination between terminal carboxyl groups and Zr clusters, and the NUS-8 MONs remained the initial nanosheet shape for both MON@SiO2 and MON-DABCO@SiO2. High-resolution transmission electron microscopy (HRTEM, Fig. 3d & i) imaging reveals that MON shell coated on the SiO2 nanoparticle with thicknesses of 100–200 nm consistent with the size of individual nanosheets. In addition, the successful coating of MONs on the SiO2 nanoparticle could also be confirmed by Energy-dispersive X-ray spectroscopy (EDS) mapping. As shown in Fig. 3e–h & j–m, uniform Si signals localized exclusively in the core region, Zr and C distributions precisely co-localized at the periphery, N elements (in MON-DABCO@SiO2) spatially correlated with Zr/C. This elemental segregation confirms covalent anchoring via Zr-carboxyl coordination rather than physical adsorption. The absence of interfacial voids suggests strong bonding between SiO2–COOH terminal groups and Zr-oxo clusters.
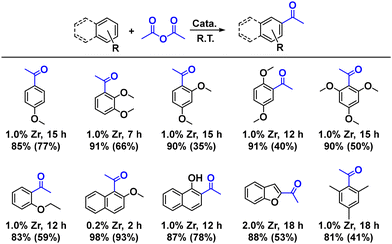
Having established the successful fabrication of the “antenna” core–shell catalysts, we further verify that the deformable nanosheets increased mass exchange and facilitating reactant accessibility and accelerating product diffusion. First, as the surface of the nanosheets contains a large number of acidic exposed sites, the Friedel–Crafts acylation was choosen as a model reaction to test the activity of MON@SiO2. Traditional homogeneous Lewis acids catalysts are effective for this reaction, including metal halides (e.g., ZnCl2, AlCl3, TiCl4) and metal triflates (e.g., Sc(OTf)3, Hf(OTf)4).44–46 however, stoichiometric amount of metal halides are usually required in the reactions as the coordination between the Lewis acid and the produced aromatic ketone. Especially, the recovery of homogeneous Lewis acidic metal salts and the generation of large amounts of wastes are long-standing challenges. Recently, heterogeneous Lewis acid catalyst for Friedel–Crafts acylation, such as modified zeolites,47,48 metal oxides,49 heteropoly acids,50 hybrid materials51,52 and MOFs have been developed and showed moderate to good activity. In our study, as shown in Fig. 4, although the MONs and MON@SiO2 had similar accessible active sites, the 1.0 mol% Zr loading of MON@SiO2 can catalyze the F–C acylation of 1,2-dimethoxylbenzene into the target 3,4-dimethoxy acetophenone with 85% isolated yield in 18 h. While the neat MON as a catalyst, the yield of the product was only 65%. The higher catalytic efficiency of MON@SiO2 as compared to the bulk-type NUS-8 film is attributed to faster mass diffusion via disturbance of the diffusion layer. We also monitored the dynamic process during the catalytic synthesis of the 3,4-dimethoxy acetophenone by MON, and MON@SiO2, which exhibited quite different reaction processes. As seen in Fig. 4, the two catalysts showed high reaction speed at first and experienced a period of 18 h to reach a stable yield. However, due to the MONs being concentrated on the SiO2 core, the activity of the MON@SiO2 gave relatively high mass diffusion transport rates indeed exhibited much higher catalytic activity (91%) than did the bulk-type MONs (65%) and the physical mixture of MON and SiO2 (76%), while the pure SiO2 core can only got the target product with 11% yield (Table S5). This result further confirmed in Friedel–Crafts acylation of various aromatic ethers. As shown in Table 1, in the presence of 0.2–2 mol% MON@SiO2 at room temperature afforded the desired acylated product in 81%–98% yields. This level of activity is much higher than the corresponding MONs (35–93%).
 | ||
| Fig. 4 Kinetic profiles for Friedel–Crafts acylation of 1,2-dimethoxylbenzene with 1 mol% (based on the content of Zr) of MON, and MON@SiO2. | ||
Encouraged by the high activity of MON@SiO2 in Friedel–Crafts acylation catalyzed by the abundant Lewis and/or Brønsted acid sites, we further functionalized the H3BTB ligands of MONs with triethylenediamine (DABCO) alkaline tails. Therefore, the resultant MON-DABCO@SiO2 contains both acidic and basic catalytic sites. We have explored the catalytic potential of the new catalysts for the acid–base catalyzed one-pot hydrolysis-Knoevenagel Condensation tandem reactions which involves hydrolysis of the acetal catalyzed by the acidic sites of Zr4+ and –OH groups followed by Knoevenagel condensation catalyzed by the adjacent basic sites of amine bonds. Typically, we study the one-pot hydrolysis-Knoevenagel Condensation tandem process through the use of the model reaction of 3a and 4a (Table 2), and the catalytic reaction was performed with 1 mmol of each reactant in CD3CN (1.0 mL) with MON-DABCO@SiO2 (0.2 mol%) at 60 °C for 17 h. Reaction progress and product distribution were quantified by 1H NMR spectroscopy relative to the starting materials. A control reaction employing bare SiO2 particles proved ineffective, yielding only trace amounts of benzaldehyde (Table S6). Strikingly, MON@SiO2 – possessing exclusively Lewis acidic sites – catalyzed the hydrolysis of benzaldehyde dimethyl acetal to benzaldehyde as the sole product with ∼100% conversion within 1 h. Critically, MON-DABCO@SiO2 enabled a tandem reaction pathway, achieving near-quantitative yield (>99%) of product 6a within 17 h while the physical mixture of MON and SiO2 got 6a with 90% yield when the reaction time prolonged to 24 h (Table S6). This distinct performance highlights a synergistic effect between catalytic sites in the tandem process, potentially facilitated by enhanced local dynamics within the soft MON framework.
| Entry | R1 | R2 | Cat. | Yieldb | |
|---|---|---|---|---|---|
| 5a | 6a | ||||
| a For reaction condition: acetal (1 equiv., 1.0 mmol), propylene glycol (2.7 mmol), H2O (2.4 mmol), Cat. (20 mg, 1.0 mol%) in 6.0 mL CD3CN, 60 °C. b The isolated yield. | |||||
| 1 | H | Me | SiO2-COOH | 31 | — |
| 2 | H | Me | MON@SiO2 | 98 | — |
| 3 | H | Me | MOF-DABCO | — | 82 |
| 4 | H | Me | MON-DABCO | — | 84 |
| 5 | 4-OMe | Me | MON-DABCO | — | 86 |
| 6 | 4-Br | Me | MON-DABCO | — | 82 |
| 7 | 4-Br | Et | MON-DABCO | — | 85 |
| 8 | 4-(MeO2)CH | Me | MON-DABCO | — | 87 |
| 9 | H | Me | MON-DABCO@SiO2 | — | 97 |
| 10 | 4-OMe | Me | MON-DABCO@SiO2 | — | 97 |
| 11 | 4-Br | Me | MON-DABCO@SiO2 | — | 96 |
| 12 | 4-Br | Et | MON-DABCO@SiO2 | — | 99 |
| 13 | 4-(MeO2)CH | Me | MON-DABCO@SiO2 | — | 99 |
Leveraging optimal conditions, we evaluated the substrate scope using diverse benzaldehyde acetals. Employing only 0.2 mol% MON-DABCO@SiO2 at 60 °C consistently delivered benzylmalononitriles in excellent yields (96–99%). This catalytic efficiency markedly surpasses that of the conventional, rigid MON-DABCO catalyst (15–87% yields across most substrates). The superior performance of the soft, deformable MON@SiO2 architecture strongly supports the proposed mechanism wherein framework flexibility significantly enhances mass transport to active sites – a critical factor for optimal catalytic activity, analogous to the mass transport enhancement demonstrated in deformable MON@SiO2 systems.
Therefore, the superior catalytic performance of MON@SiO2 and MON-DABCO@SiO2 stems from the synergistic interplay between substrate molecules and the deformable ultrathin MON layers. This architecture facilitates enhanced substrate diffusion to active sites within the confined nanostructure–a critical mass transport advantage inaccessible to homogeneous catalysts due to the absence of confinement effects, and significantly diminished in bulk MOFs owing to their limited external surface area and inherent rigidity. Crucially, the MON@SiO2 core–shell design uniquely combines the beneficial flexibility of ultrathin MONs with enhanced structural stability. This is evidenced by recyclability studies: after each catalytic cycle, mass recovery measurements revealed that MON-DABCO@SiO2 maintained a recovery rate of >85% even after five cycles (Fig. 2d & Tables S3, S4). After five cycles, the PXRD patterns of both MON@SiO2 and MON-DABCO@SiO2 remained nearly identical to those of the pristine samples (Fig. 2a). However, the BET surface areas of both composites decreased, possibly due to detachment of nanosheets (Fig. 2b). This substantial difference arises directly from the ultrathin, fragile nature of the unsupported MON nanosheets, which are prone to loss during processing. We also tested the ICP to test the Zr leaching after recycle, but almost none Zr4+ was found in the reaction mixture, this may due to the robust of the Zr–O bond and high chemical stability of the MONs. However, we can see from the SEM images (Fig. S4) that the nanosheets loaded on the SiO2 becomes sparse compared with the MON@SiO2 as synthesized after recycle under the mechanical force. Collectively, these findings demonstrate that MON-DABCO@SiO2 represents a novel class of heterogeneous catalysts, engineered by integrating the dynamic, mass-transport-enhancing properties of soft MON nanostructures with the robust confinement and stability characteristic of framework materials.
Conclusions
In summary, a novel core–shell catalyst, MON@SiO2, was developed by anchoring ultrathin MONs onto SiO2 microspheres, forming a uniform ∼100 nm coating with preserved crystallinity. Functionalization with DABCO yielded bifunctional MON-DABCO@SiO2. MON@SiO2 exhibited superior activity in Friedel–Crafts acylation (up to 98% yield) and broad substrate scope (81–98% yields). Bifunctional MON-DABCO@SiO2 enabled efficient one-pot deacetalization–Knoevenagel reactions (96–99% yields), outperforming the SiO2-free DABCO-MONs catalyst (15–87%). Both catalysts showed excellent stability and recyclability for at least five cycles. This design integrates the accessibility of MONs with the robustness of SiO2, offering a versatile platform for architecting highly efficient heterogeneous catalysts.Author contributions
Y. W., H. W. and Z. L. contributed equally. C. Tan initiated the concept. C. T., Y. W., H. W., Z. L., and Y. Z. performed the experiments and collected the data. C. T., Y. W., H. W., Z. L., and R. L. analysed the data. R. L., G. L. and C. T. provided the main funding for this work. G. L. and C. T. wrote and edited the paper.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information including general procedure for catalysis, TGA curve, additional SEM images, additional catalytic results, and NMR is available. See DOI: https://doi.org/10.1039/d5nr04441c.Acknowledgements
We are grateful to the China National Natural Science Foundation (22001171, 22001170 and 22071154), the Shanghai Rising-Star Program (23QA1407200), the Sailing Program (2020YF1435200), the Shanghai STDF (20070502600), and the Shanghai Frontiers Science Center of Biomimetic Catalysis for financial support.References
- M. Delling, A. Indzhykulian, X. Liu, Y. Li, T. Xie, D. Corey and D. Clapham, Primary cilia are not calcium-responsive mechanosensors, Nature, 2016, 531, 656–660 CrossRef CAS.
- M. B. Short, C. A. Solari, S. Ganguly, T. R. Powers, J. O. Kessler and R. E. Goldstein, Flows driven by flagella of multicellular organisms enhance long-range molecular transport, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8315–8319 CrossRef CAS.
- X. Meng, C. Xu, J. Li, B. Qiu, J. Luo, Q. Hong, Y. Tong, C. Fang, Y. Feng, R. Ma, X. Shi, C. Lin, C. Pan, X. Zhu, X. Yan and Y. Cong, Multi-scale structures of the mammalian radial spoke and divergence of axonemal complexes in ependymal cilia, Nat. Commun., 2024, 362(15), 1–17 Search PubMed.
- C. Huang, Z. Guo, X. Zheng, X. Chen, Z. Xue, S. Zhang, X. Li, B. Li, X. Guan, G. Hu and T. Wang, Deformable Metal-Organic Framework Nanosheets for Heterogeneous Catalytic Reactions, J. Am. Chem. Soc., 2020, 142, 9408–9414 CrossRef CAS PubMed.
- G. Zuo, Y. Wang, W. L. Teo, Q. Xian and Y. Zhao, Direct Z-scheme TiO2−ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting, Appl. Catal., B, 2021, 291, 120126–120133 CrossRef CAS.
- J. M. Wu, W. E. Chang, Y. T. Chang and C.-K. Chang, Piezo-Catalytic Effect on the Enhancement of the Ultra-High Degradation Activity in the Dark by Single- and Few-Layers MoS2 Nanoflowers, Adv. Mater., 2016, 28, 3718–3725 CrossRef CAS.
- J.-C. Gan, Z.-F. Jiang, K.-M. Fang, X.-S. Li, L. Zhang, J.-J. Feng and A.-J. Wang, Low Rh doping accelerated HER/OER bifunctional catalytic activities of nanoflower-like Ni-Co sulfide for greatly boosting overall water splitting, J. Colloid Interface Sci., 2025, 677, 221–231 CrossRef CAS PubMed.
- Y. Wei, Y. Wu, J. Wang, Y.-H. Wu, Z. Weng, W.-Y. Huang, K. Yang, J.-L. Zhang, Q. Li, K.-Q. Lu and B. Han, Rationally designed dual cocatalysts on ZnIn2S4 nanoflowers for photoredox coupling of benzyl alcohol oxidation with H2 evolution, J. Mater. Chem. A, 2024, 12, 18986–18992 RSC.
- C. Li, L. Fan, T. Hu, Q.-P. Qin and X. Zhang, Two-Dimensional Nanoporous {Tb2}–Organic Framework with Acid–Base for CO2-Epoxide Cycloaddition and Deacetalization–Knoevenagel Condensation, ACS Appl. Nano Mater., 2025, 8, 15288–15298 CrossRef CAS.
- J. Tan and J. A. Foster, Metal-Organic Nanosheet Gels: Hierarchically Porous Materials for Selective Loading and Differential Release, Adv. Funct. Mater., 2025, 7474–7482 Search PubMed.
- Y. Peng and W. Yang, Metal-organic framework nanosheets: a class of glamorous low-dimensional materials with distinct structural and chemical natures, Sci. China:Chem., 2019, 62, 1561–1575 CrossRef CAS.
- M. Zhao, Y. Huang, Y. Peng, Z. Huang, Q. Ma and H. Zhang, Two-dimensional metal–organic framework nanosheets: synthesis and applications, Chem. Soc. Rev., 2018, 47, 6267–6295 RSC.
- L. Zhuang, L. Ge, H. Liu, Z. Jiang, Y. Jia, Z. Li, D. Yang, R. K. Hocking, M. Li, L. Zhang, X. Wang, X. Yao and Z. Zhu, Surfactant-Free and Scalable General Strategy for Synthesizing Ultrathin Two-Dimensional Metal–Organic Framework Nanosheets for the Oxygen Evolution Reaction, Angew. Chem., Int. Ed., 2019, 58, 13565–13572 CrossRef CAS PubMed.
- W. Shi, L. Cao, H. Zhang, X. Zhou, B. An, Z. Lin, R. Dai, J. Li, C. Wang and W. Lin, Surface Modification of Two-Dimensional Metal–Organic Layers Creates Biomimetic Catalytic Microenvironments for Selective Oxidation, Angew. Chem., Int. Ed., 2017, 56, 9704–9709 CrossRef CAS.
- C. Tan, G. Liu, H. Li, Y. Cui and Y. Liu, Ultrathin two-dimensional metal–organic framework nanosheets—an emerging class of catalytic nanomaterials, Dalton Trans., 2020, 49, 11073–11084 RSC.
- P. V. Alekseevskiy, X. Yu, A. S. Efimova, N. A. Zhestkij, Y. A. Mezenov, Y. A. Kenzhebayeva, S. A. Povarov, A. Lubimova, S. V. Bachinin, E. A. Stepanidenko, V. Dyachuk, N. Li, V. P. Fedin and A. S. Potapov, Ultrathin Lanthanide-Based Metal-Organic Nanosheets with Thickness- and Temperature-Driven Light Emission, Laser Photonics Rev., 2025, 19, 2401912 CrossRef CAS.
- X. Zhang, X. Tian, N. Wu, S. Zhao, Y. Qin, F. Pan, S. Yue, X. Ma, J. Qiao, W. Xu, J. Liu, M. Zhao, K. Ostrikov and Z. Zeng, Metal-organic frameworks with fine-tuned interlayer spacing for microwave absorption, Sci. Adv., 2025, 10, 6498–6508 CrossRef.
- U. R. Gandra, R. P. Pandey, L. Palanikumar, A. Irfan, M. Magzoub, Y. Belmabkhout, S. W. Hasan and M. I. H. Mohideen, Cu-TCPP Metal–Organic Nanosheets Embedded Thin-Film Composite Membranes for Enhanced Cyanide Detection and Removal: A Multifunctional Approach to Water Treatment and Environmental Safety, ACS Appl. Mater. Interfaces, 2025, 17, 9563–9574 CrossRef CAS.
- B. Shao, X.-L. He, D. Huang, Y.-L. Xiang, Y. Luo, Y.-M. Wei, L. Jiang, R.-K. Huang, M. Dong and J. Huang, Oriented Exfoliating 3D Metal–Organic Frameworks into Ultrathin Metal–Organic Nanosheets with Different Crystal Faces, Adv. Funct. Mater., 2024, 34, 2315911–2315919 CrossRef CAS.
- X. Sun, L. Shi, H. Huang, X. Song and T. Ma, Surface engineered 2D materials for photocatalysis, Chem. Commun., 2020, 56, 11000–11013 RSC.
- C. Tan, K. Yang, J. Dong, Y. Liu, Y. Liu, J. Jiang and Y. Cui, Boosting Enantioselectivity of Chiral Organocatalysts with Ultrathin Two-Dimensional Metal–Organic Framework Nanosheets, J. Am. Chem. Soc., 2019, 141, 17685–17695 CrossRef CAS.
- L.-M. Cao, C.-G. Hu, H.-H. Li, H.-B. Huang, L.-W. Ding, J. Zhang, J.-X. Wu, Z.-Y. Du, C.-T. He and X.-M. Chen, Molecule-Enhanced Electrocatalysis of Sustainable Oxygen Evolution Using Organoselenium Functionalized Metal–Organic Nanosheets, J. Am. Chem. Soc., 2023, 145, 1144–1154 CrossRef CAS.
- J. Wang, Y. Shen, G. Wei, W. Xi, X. Ma, W. Zhang, P. Zhu and C. An, Synthesis of ultrathin Co2AlO4 nanosheets with oxygen vacancies for enhanced electrocatalytic oxygen evolution, Sci. China Mater., 2020, 63, 91–99 CrossRef CAS.
- Y. Guo, S. Li, W. Abebe, J. Wang, L. Shi, D. Liu and S. Zhao, Non-derivatized metal-organic framework nanosheets for water electrolysis: Fundamentals, regulation strategies and recent advances, Chin. J. Catal., 2024, 67, 21–53 CrossRef.
- D. J. Ashworth and J. A. Foster, Metal–organic framework nanosheets (MONs): a new dimension in materials chemistry, J. Mater. Chem. A, 2018, 6, 16292–16307 RSC.
- T. Riant, D. Rebiscoul, J. Maynadié and D. Meyer, Surfactant-assisted lamellar structuration of tunable Co-based hybrid nanosheets, New J. Chem., 2023, 47, 20109–20116 RSC.
- J. Hong, L. Kang, X. Shi, R. Wei, X. Mai, D. Pan, N. Naik and Z. Guo, Highly efficient removal of trace lead(II) from wastewater by 1,4-dicarboxybenzene modified Fe/Co metal organic nanosheets, J. Mater. Sci. Technol., 2022, 98, 212–218 CrossRef CAS.
- C. Cui, G. Li and Z. Tang, Metal-organic framework nanosheets and their composites for heterogeneous thermal catalysis: Recent progresses and challenges, Chin. Chem. Lett., 2021, 32, 3307–3321 CrossRef CAS.
- H. Qin, X. Li, C. Zhou, S. Zhang, Y. Zhao, M. Zhang, P. Guo, H. Li and R. Wang, Porous polyacrylonitrile nanofiber membranes modified with UiO-66-NH2 nanosheets for excellent detoxification of nerve agent simulant, Sep. Purif. Technol., 2025, 362, 131721–131730 CrossRef CAS.
- B. Konkena, J. Masa, A. J. R. Botz, I. Sinev, W. Xia, J. Koßmann, R. Drautz, M. Muhler and W. Schuhmann, Metallic NiPS3@NiOOH Core–Shell Heterostructures as Highly Efficient and Stable Electrocatalyst for the Oxygen Evolution Reaction, ACS Catal., 2017, 7, 229–237 CrossRef CAS.
- B. Shao, X. Chen, Y.-T. Xu, G.-Q. Li, J.-P. Zhong, T. Meng, Z. Zhang, F.-P. Huang and J. Huang, Low-potential-driven electrocatalytic reduction of CO2 to hydrocarbons by cobalt-based metal-organic nanosheets, J. Catal., 2022, 413, 168–175 CrossRef CAS.
- S. Wang, D. Zhang, X. Pu, L. Zhang, D. Zhang and J. Jiang, Photothermal-Enhanced S-Scheme Heterojunction of Hollow Core–Shell FeNi2S4@ZnIn2S4 toward Photocatalytic Hydrogen Evolution, Small, 2024, 20, 2311504–2311516 CrossRef CAS.
- R. Sakamoto, N. Fukui, H. Maeda, R. Toyoda, S. Takaishi, T. Tanabe, J. Komeda, P. Amo-Ochoa, F. Zamora and H. Nishihara, Layered metal-organic frameworks and metal-organic nanosheets as functional materials, Coord. Chem. Rev., 2022, 472, 214787–214813 CrossRef CAS.
- W. Pang, B. Shao, X. Chen, Q.-X. Gu, F.-J. Yang, S. Li and J. Huang, Enhancing the activity of metal-organic nanosheets for oxygen evolution reaction by substituent effects, J. Colloid Interface Sci., 2022, 608, 306–312 CrossRef CAS.
- H. Huang, Y. He, X. Du, P. K. Chu and Y. Zhang, General and Facile Approach to Heterostructured Core/Shell BiVO4/BiOI p–n Junction: Room-Temperature in Situ Assembly and Highly Boosted Visible-Light Photocatalysis, ACS Sustainable Chem. Eng., 2015, 3, 3262–3273 CrossRef CAS.
- K. Sasitharan, R. C. Kilbride, E. L. K. Spooner, J. Clark, A. Iraqi, D. G. Lidzey and J. A. Foster, Metal-Organic Framework Nanosheets as Templates to Enhance Performance in Semi-Crystalline Organic Photovoltaic Cells, Adv. Sci., 2022, 9, 2200366–2200376 CrossRef CAS PubMed.
- D. Rodríguez-San-Miguel, C. Montoro and F. Zamora, Covalent organic framework nanosheets: preparation, properties and applications, Chem. Soc. Rev., 2020, 49, 2291–2302 RSC.
- Y. Wang, L. Li, L. Yan, X. Gu, P. Dai, D. Liu, J. G. Bell, G. Zhao, X. Zhao and K. M. Thomas, Bottom-Up Fabrication of Ultrathin 2D Zr Metal–Organic Framework Nanosheets through a Facile Continuous Microdroplet Flow Reaction, Chem. Mater., 2018, 30, 3048–3059 CrossRef CAS.
- J.-Y. Tan, J.-X. Shi, P.-H. Cui, Y.-Y. Zhang, N. Zhang, J.-Y. Zhang and W. Deng, Ni3(OH)(COO)6−based MOF from C3 symmetric ligands: Structure and heterogeneous catalytic activities in one-pot synthesis of imine, Microporous Mesoporous Mater., 2019, 287, 152–158 CrossRef CAS.
- L. Zhang, F. Song, Y. Wu, L. Cheng, J. Qian, S. Wang, Q. Chen and Y. Li, A Novel Amino and Carboxyl Functionalized Mesoporous Silica as an Efficient Adsorbent for Nickel(II), J. Chem. Eng. Data, 2019, 64, 176–188 CrossRef CAS.
- Z. Li and H. C. Zeng, Armored MOFs: enforcing soft microporous MOF nanocrystals with hard mesoporous silica, J. Am. Chem. Soc., 2014, 136(15), 5631–5639 CrossRef CAS PubMed.
- Z. Hu, E. M. Mahdi, Y. Peng, Y. Qian, B. Zhang, N. Yan, D. Yuan, J.-C. Tan and D. Zhao, Kinetically controlled synthesis of two-dimensional Zr/Hf metal–organic framework nanosheets via a modulated hydrothermal approach, J. Mater. Chem. A, 2017, 5, 8954–8963 RSC.
- W. Zhang, X. Wu, X. Peng, L. Zhu, H. Wang, H. Liu and H. Yuan, Construction of Solution Processable NUS-8/PANI Nanosheets via Template-Directed Polymerization for Ultratrace Gas Sensing, Small, 2024, 20, 2405636–2405645 CrossRef CAS PubMed.
- I. Hachiya, M. Moriwaki and S. Kobayashi, Hafnium(IV) Trifluoromethanesulfonate, An Efficient Catalyst for the Friedel-Crafts Acylation and Alkylation Reactions, Bull. Chem. Soc. Jpn., 1995, 68, 2053–2060 CrossRef CAS.
- A. Kawada, S. Mitamura, J.-I. Matsuo, T. Tsuchiya and S. Kobayashi, Friedel-Crafts Reactions Catalyzed by Rare Earth Metal Trifluoromethanesulfonates, Bull. Chem. Soc. Jpn., 2000, 73, 2325–2333 CrossRef CAS.
- A. Dzudza and T. J. Marks, Lanthanide Triflate-Catalyzed Arene Acylation, Relation to Classical Friedel-Crafts Acylation, J. Org. Chem., 2008, 73(11), 4004–4016 CrossRef CAS.
- M. L. Kantam, K. V. S. Ranganath, M. Sateesh, K. B. S. Kumar and B. M. Choudary, Friedel-Crafts acylation of aromatics and heteroaromatics by beta zeolite, J. Mol. Catal. A: Chem., 2005, 225, 15–20 CrossRef CAS.
- K. Gaare and D. Akporiaye, Modified zeolites as catalysts in the Friedel-Crafts acylation, J. Mol. Catal. A: Chem., 1996, 109, 177–187 CrossRef CAS.
- V. Quaschning, J. Deutsch, P. Druska, H. J. Niclas and E. Kemnitz, Properties of modified zirconia used as friedel-crafts-acylation catalysts, J. Catal., 1998, 177, 164–174 CrossRef CAS.
- I. V. Kozhevnikov, Friedel-Crafts acylation and related reactions catalysed by heteropoly acids, Appl. Catal., A, 2003, 256, 3–18 CrossRef CAS.
- L. T. L. Nguyen, C. V. Nguyen, G. H. Dang, K. K. A. Le and N. T. S. Phan, Towards applications of metal-organic frameworks in catalysis: Friedel-Crafts acylation reaction over IRMOF-8 as an efficient heterogeneous catalyst, J. Mol. Catal. A: Chem., 2011, 349, 28–35 CrossRef CAS.
- L. Kurfiřtovǎ, Y.-K. Seo, Y. K. Hwang, J.-S. Chang and J. Čejka, High activity of iron containing metal-organic-framework in acylation of p-xylene with benzoyl chloride, Catal. Today, 2012, 179, 85–90 CrossRef.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |