Novel iron–nickel bimetallic nanozyme with peroxidase-like activity for ultrasensitive uric acid detection and hyperuricaemia therapy evaluation
Hanbing
Yao
ab,
Yifu
Li
ab,
Yujuan
Zhan
ab,
Binyu
Xiao
ab,
Jiayi
Yan
ab,
Shuangshuang
Liu
ab,
Zimo
Chen
ab and
Chang
Shu
 *ab
*ab
aDepartment of Pharmaceutical Analysis, School of Pharmacy, China Pharmaceutical University, Nanjing 211198, China. E-mail: shuchang@cpu.edu.cn
bKey Laboratory of Drug Quality Control and Pharmacovigilance (China Pharmaceutical University), Ministry of Education, Nanjing 210009, China
First published on 5th December 2025
Abstract
Hyperuricemia (HUA) associated with a range of metabolic disorders has become a risk factor for many chronic diseases. Nanozymes, which mimic enzymatic activities, are prized for their high activity, low cost, and robust stability. Investigating the peroxidase (POD)-like activity of nanozymes is crucial for advancing biosensing and biocatalysis. In this work, we synthesized a series of FexNiy-NFs with POD-like activity, featuring varying mass ratios of iron to nickel. Among these, the Fe4Ni-NFs, which exhibited the highest catalytic activity, were selected to develop a user-friendly point-of-care (POC) detection method for the colorimetric quantification of uric acid (UA). This method achieved a detection limit of 1.13 µM and a linear range of 2–500 µM, enabling rapid, visual detection of UA in serum. Furthermore, we assessed serum UA levels in hyperuricemic rats treated with allopurinol and benzbromarone, demonstrating rapid drug efficacy evaluation. Our findings highlight the potential of Fe4Ni-NFs in UA detection and hyperuricemia management, suggesting broad applications in drug development and precision medicine. This work provided mechanistic insights into bimetallic nanozymes’ POD-like activity and underscores their potential for biomedical applications, offering a new strategy for hyperuricemia diagnosis and treatment.
1. Introduction
In recent years, the improvement in living standards has led to significant changes in dietary patterns, characterized by increased intake of sugars, proteins, and fats. This shift has had a profound impact on public health. Specifically, a diet high in purines can lead to the accumulation of uric acid (UA) in the body, resulting in hyperuricemia.1 Hyperuricemia, a condition caused by excessive production or impaired excretion of UA, is associated with a range of metabolic disorders, including gout, hypertension, and hyperlipidemia.2,3 Under normal physiological conditions, the serum UA concentration ranges from 120 to 450 μM, while the urinary UA concentration ranges from 1.4 to 4.4 mM.4 Elevated serum UA levels can trigger the deposition of UA in the kidneys and joints, leading to acute inflammatory responses, joint deformities, nephropathy, and acute gouty arthritis.5,6 The incidence of hyperuricemia is on the rise, posing a substantial threat to human health as a metabolic disorder.6–8 Given the strong correlation between UA levels in body fluids and various metabolic diseases, the detection of UA holds significant importance in medical and pharmaceutical research.9 To date, a variety of uric acid (UA) detection techniques have been developed, including chemiluminescence,10 high-performance liquid chromatography (HPLC),11 electrochemical methods,12 ultraviolet–visible absorption,13 colorimetry,14 capillary electrophoresis,15 and enzymatic assays. Each of these methods possesses distinct advantages and limitations. For instance, electrochemical detection is characterized by its simplicity and high sensitivity. However, the redox potential of UA is similar to that of many biomolecules in living organisms, rendering it susceptible to interferences and lacking specificity.16 Capillary electrophoresis is advantageous for its straightforward operation and low cost, but it still faces limitations in sensitivity and reproducibility of results.17 Chromatographic methods such as HPLC offer excellent separation and quantification capabilities but typically require expensive instrumentation, complex sample pre-treatment, and lengthy detection times.18 Many of these techniques encounter challenges when analyzing real samples, such as complicated sample pre-treatment procedures, inability to directly measure UA in real samples, and lack of real-time analysis capabilities. These limitations have driven the continuous search for improved methods. Therefore, there is an urgent need to develop convenient, efficient, and highly sensitive UA detection methods that are applicable to real samples to meet the demands of clinical medicine and routine monitoring.Colorimetric assays are highly intuitive and convenient methods that offer distinct advantages in the detection of biomolecules. Traditional colorimetric analyses typically employ natural enzymes to catalyze substrate chromogenesis. While natural enzymes are characterized by their high specificity and efficient catalysis, their practical applications are limited by several drawbacks, including poor thermal stability, narrow pH range, susceptibility to inactivation, and high costs.19 With the rapid development of nanotechnology and catalytic science, nanomaterials that function as enzyme mimics have garnered increasing attention.20 In 2004, Scrimin and colleagues introduced the concept of “nanozymes”.21 In 2007, Yan et al. discovered that Fe3O4 nanoparticles exhibited significant peroxidase-like activity. This finding sparked extensive research into the catalytic kinetic mechanisms of nanozymes and ignited a surge of exploration into nanozyme design and activity regulation.22 Since then, a wide range of nanozyme materials, including metal nanoparticles,23 metal oxides,24 metal–organic frameworks,25 non-metal compounds,26 and carbon-based nanomaterials,27 have emerged. The excellent catalytic activity, superior stability, and unique optical properties of nanozymes have laid the foundation for their broad applications across multiple fields.28 The advent of nanozymes has provided an effective solution to the poor stability of natural enzymes, thereby expanding the scope of applications for nanozymes.29
In recent years, bimetallic nanozymes have attracted extensive research attention. Liu et al. fabricated Pt-MoS2/SiO2 nanocomposites with excellent peroxidase activity via a one-step hydrothermal reduction method. They further developed a rapid and sensitive colorimetric sensing device for hydroquinone, which was successfully applied to the detection of hydroquinone in tap water and river water samples.30 In addition, they synthesized a novel nanozyme with a CuFeS2 hollow sphere structure and outstanding peroxidase activity. By introducing cholesterol oxidase, a three-stage sequential sensing platform was constructed, enabling the visual and sensitive detection of H2O2 and cholesterol over a wide concentration range.31 Bimetallic metal–organic frameworks (MOFs) have emerged as promising candidates for peroxidase-like (POD) nanozymes, characterized by their porous three-dimensional structures and large specific surface areas.32 Bimetallic MOFs possess several advantages that are not found in monometallic MOFs. The synergistic interactions between bimetallic active sites can enhance the stability of the material framework and improve catalytic activity, thereby conferring higher POD-like activity.33–35 Researchers can also design the properties of bimetallic MOF materials by adjusting the ratios of the two metals to optimize their performance.36,37 For instance, Yang et al. synthesized copper–cobalt bimetallic MOFs (Cu@Co-MOFs) with peroxidase-like activity via a one-pot hydrothermal method and applied them to the colorimetric detection of glutathione (GSH).32 Wang et al. reported a low-cost and facilely synthesized iron–nickel bimetallic MOF nanozyme (Fe4Ni-MOF) and demonstrated its high sensitivity for the detection of glucose and H2O2.38 These studies highlight the broad recognition of bimetallic MOFs as a highly promising class of materials for various applications.
Inspired by these advancements, in this study, we successfully synthesized a novel peroxidase-like nanoflower structured metal organic framework (Fe4Ni-NFs) using hexahydrated ferric chloride and hexahydrated nickel nitrate as metal precursors and pyrazine-2,3-dicarboxylic acid as the organic ligand. The Fe4Ni-NFs were successfully fabricated via a one-step solvothermal method. The introduction of nickel into the iron-based metal–organic framework (Fe-NFs) significantly enhanced the electron transfer capability from the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) to H2O2, thereby markedly improving the peroxidase-like activity of the Fe4Ni-NFs. Leveraging the above characteristics, we developed a rapid, simple, and highly specific colorimetric method for the detection of uric acid (UA). Uric acid was enzymatically converted to H2O2 by uricase, which was subsequently catalyzed by the peroxidase-like Fe4Ni-NFs to generate hydroxyl radicals (˙OH). Under these conditions, the TMB was oxidized to its blue-colored product (oxTMB), resulting in a significant color change from colorless to blue and a corresponding increase in absorbance at 652 nm. This change established a quantitative relationship between the detection signal and UA concentration, enabling the rapid and visual detection of uric acid in biological samples such as serum. Furthermore, we applied the established method to evaluate the uric acid levels in the serum of hyperuricemic rats during short-term and long-term oral administration of allopurinol and benzbromarone (Fig. 1). This approach allowed for the rapid assessment of drug efficacy and uric acid levels, providing a technical reference for the precise treatment and post-treatment monitoring of hyperuricemia in clinical settings. Additionally, this method offered a valuable tool for the development, screening, and efficacy evaluation of drugs targeting hyperuricemia.
 | ||
| Fig. 1 Illustration of novel iron–nickel bimetallic nanozyme with peroxidase-like activity for ultrasensitive uric acid detection and hyperuricaemia therapy evaluation. | ||
2. Experimental
2.1 Synthesis
The synthesize of procedure Fe4Ni-NFs was follows: ferric chloride hexahydrate (0.5319 g, 2 mM) was dissolved in 3 mL of N,N-dimethylformamide (DMF) to form Solution A. Nickel nitrate hexahydrate (0.1431 g, 0.5 mM) was dissolved in 2 mL of DMF to form Solution B. Pyrazine-2,3-dicarboxylic acid (0.208 g) was dissolved in 10 mL of DMF to form Solution C. Solutions A and B were subsequently added to Solution C, and the mixture was stirred thoroughly. The reaction was carried out at 110 °C for 20 hours, followed by slow cooling to room temperature over a period of 6 hours. The resulting precipitate was collected by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037g for 10 minutes, washed three times with hot ethanol, and then re-centrifuged at 5794g for 10 minutes. The final precipitate was redispersed in anhydrous ethanol and dried under vacuum at 60 °C overnight to obtain the powder. By adjusting the molar ratios of ferric chloride hexahydrate and nickel nitrate hexahydrate, a series of FexNiy-NFs with different Fe
037g for 10 minutes, washed three times with hot ethanol, and then re-centrifuged at 5794g for 10 minutes. The final precipitate was redispersed in anhydrous ethanol and dried under vacuum at 60 °C overnight to obtain the powder. By adjusting the molar ratios of ferric chloride hexahydrate and nickel nitrate hexahydrate, a series of FexNiy-NFs with different Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni mole ratios (1
Ni mole ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 5
1, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were synthesized.
1) were synthesized.
2.2 Peroxidase-like activity
The peroxidase-like activity of Fe4Ni-NFs was evaluated using TMB and H2O2 as substrates. The TMB (5 mM, 30 μL), Fe4Ni-NFs dispersion (0.1 mg mL−1, 40 μL), and H2O2 (1 mM, 30 μL) were added into a sodium acetate–acetic acid buffer solution (pH 4.0, 200 mM, 200 μL). After reaction for 5 minutes at room temperature, the UV-Vis absorption spectra were recorded in the range of 500–800 nm, and the absorbance at 652 nm was measured. The POD-like activity of FexNiy-NFs with different Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratios were compared based on the intensity of the absorbance at 652 nm.
Ni ratios were compared based on the intensity of the absorbance at 652 nm.
2.3 Enzyme kinetics
The catalytic efficiency of Fe4Ni-NFs was evaluated through steady-state kinetic analysis. To elucidate the kinetic behavior of the peroxidase-like activity of Fe4Ni-NFs, we varied the concentrations of either TMB or H2O2 while keeping the other substrate constant. Initially, TMB (5 mM) was mixed with different concentrations of H2O2 (0.02–1.0 mM). Subsequently, H2O2 (1 mM) was mixed with varying concentrations of TMB (0.02–1.5 mM). The reaction mixture was prepared by adding FexNiy-NFs dispersion (0.1 mg mL−1, 40 μL) and sodium acetate–acetic acid buffer solution (pH4.0, 200 mM, 200 μL) to each substrate mixture. After reaction for 5 minutes at room temperature, the UV-Vis absorption spectra were recorded in the range of 500–800 nm, with particular attention to the absorbance at 652 nm. The apparent kinetic parameters (Vmax and Km) were determined using the Michaelis–Menten equation (eqn (1)) and the Lineweaver–Burk equation (eqn (2)).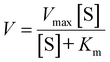 | (1) |
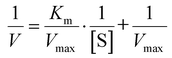 | (2) |
2.4 Stability
To evaluate the pH stability of Fe4Ni-NFs, the TMB (5 mM, 30 µL), a dispersion of Fe4Ni-NFs in anhydrous ethanol (0.1 mg mL−1, 40 µL), and H2O2 (1 mM, 30 µL) were sequentially added to 200 µL of NaAc–HAc buffer solutions with varying pH values. After a reaction period of 5 minutes at room temperature, the absorbance at 652 nm was measured. The stability of Fe4Ni-NFs in various organic solvents was also investigated. Fe4Ni-NFs were dispersed in acetone (ACN), ethyl acetate (EA), cyclohexane (CYH), N,N-dimethylformamide (DMF), anhydrous ethanol (ET), isopropanol (IPA) and dimethyl sulfoxide (DMSO) to prepare dispersions (0.1 mg mL−1). At room temperature, TMB (30 µL, 5 mM), Fe4Ni-NFs dispersion (0.1 mg mL−1, 40 µL), and H2O2 (1 mM, 30 µL) were added to 200 µL of NaAc–HAc buffer solution (pH 3.5). The absorbance at 652 nm was measured after 5 minutes to determine the most suitable organic solvent for Fe4Ni-NFs. The long-term stability of Fe4Ni-NFs was assessed by monitoring their activity over 40 days when stored as a powder at 4 °C. Every two days, Fe4Ni-NFs powder was weighed and dispersed in anhydrous ethanol to prepare a dispersion (0.1 mg mL−1). At room temperature, the TMB (5 mM, 30 µL), Fe4Ni-NFs dispersion (0.1 mg mL−1, 40 µL), and H2O2 (1 mM, 30 µL) were added to 200 µL of NaAc–HAc buffer solution (pH 3.5). The absorbance at 652 nm was measured after 5 minutes. The stability of Fe4Ni-NFs dispersions in anhydrous ethanol was evaluated over 7 days at room temperature. Daily, TMB (5 mM, 30 µL), Fe4Ni-NFs dispersion (0.1 mg mL−1, 40 µL), and H2O2 (1 mM, 30 µL) were added to 200 µL of NaAc–HAc buffer solution (pH 3.5). The absorbance at 652 nm was measured after 5 minutes. The absorbance value measured on the first day was used as a reference to calculate and compare the relative activity over the 7-day period.2.5 UA detection and optimization
To develop a method for detecting UA, different concentrations of UA solutions (25 µL, ranging from 5 µM to 500 µM) were mixed with uricase (1.38 U mL−1, 15 µL) and sodium acetate (200 mM, 15 µL). The mixtures were incubated at 37.5 °C for 15 minutes. Subsequently, NaAc–HAc buffer solution (pH 3.5, 200 mM, 90 µL), Fe4Ni-NFs dispersion (0.1 mg mL−1, 15 µL), and TMB (5 mM, 15 µL) were added. After incubation at 35 °C for 20 minutes, the absorbance at 652 nm and the UV-Vis absorption spectra in the range of 500–800 nm were measured. To enhance the sensitivity and accuracy of UA detection, we optimized the incubation temperature, buffer pH, and reaction time. Uric acid (250 µM, 50 µL) was mixed with uricase solution (1.38 U mL−1, 30 µL) and sodium acetate solution (200 mM, 30 µL) for 15 minutes at 37.5 °C. NaAc–HAc buffer solution (pH 3.5, 200 mM, 180 µL), Fe4Ni-NFs dispersion (0.1 mg mL−1, 30 µL) and TMB (5 mM, 30 µL) were added and mixed thoroughly. The mixtures were then incubated at various temperatures (25 °C, 30 °C, 35 °C, 40 °C, 45 °C, and 50 °C) for 20 minutes, and the absorbance at 652 nm was measured. Following the same initial steps as above, different pH values of NaAc–HAc buffer solution (200 mM, 180 µL, pH 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0) were used. The mixtures were incubated at 35 °C for 20 minutes, and the absorbance at 652 nm was measured. Again, following the same initial steps, the mixtures were incubated at 35 °C, and the absorbance at 652 nm was measured every 2 minutes over a 20-minute period to determine the optimal reaction time.2.6 Specificity
To evaluate the specificity of UA detection method, we examined the potential interference from various common compounds and ions, including glucose (Glu), dopamine (DA), glutathione (GSH), urea, ascorbic acid (AA), lysine (Gly), histidine (His), and several inorganic ions (K+, Mn2+, Mg2+, Ca2+, and NH4+). To simulate real-world conditions, each of these compounds and ions was added to the UA detection system at a concentration 10-fold higher than that of UA (500 µM). The specificity of UA detection was then assessed in the presence of these potential interferents. The above experiments were performed in triplicate (n = 3) to ensure reproducibility and reliability.2.7 Detection of UA in human serum
Human plasma samples were provided by the First Hospital of Nanjing (Ethics Approval No.: KY20240902-KS-03). To assess the applicability of the established method in biological samples, we conducted experiments using serum samples spiked with different concentrations of UA (50 µM, 40 µM, 30 µM, 20 µM, 10 µM, 5 µM, and 2 µM). Specifically, 25 µL of serum was spiked with the corresponding concentration of UA, followed by the addition of uricase solution (1.38 U mL−1, 15 µL) and sodium acetate solution (15 µL). The mixture was incubated at 37.5 °C for 15 minutes. Subsequently, NaAc–HAc buffer solution (pH 3.5, 200 mM, 90 µL), Fe4Ni-NFs dispersion (0.1 mg mL−1, 15 µL), and TMB (5 mM, 15 µL) were added. After incubation at 35 °C for 20 minutes, the absorbance at 652 nm and the UV-Vis absorption spectra in the range of 500–800 nm were measured.2.8 Pharmacodynamics study
A hyperuricemia rat model was established through a high-purine diet, resulting in serum uric acid levels exceeding 200 µM in the rats. The animals were maintained under controlled conditions with a constant temperature of 24 ± 1 °C, relative humidity of 50 ± 10%, and a 12-hour light–dark cycle, with free access to food and water. The animal experiments were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 8023, revised 1978) and were approved by the Animal Ethics Committee of China Pharmaceutical University (Approval No. 2023-11-002). The rats were randomly divided into two groups, with three rats per group. One group was administered allopurinol (AP group), and the other group received benzbromarone (BZ group). The drugs were formulated in a solution containing 5% DMSO and 95% CMC-Na, and the dosing regimen was 6 mg kg−1. Prior to drug administration, blood samples were collected from the orbital venous plexus of the rats to serve as the 0-hour baseline. For the short-term efficacy study, blood samples were collected at 1, 2, 3, 5, 7 and 9 hours post-administration. For the long-term efficacy study, blood samples were collected before and 3 hours after daily drug administration. The blood samples were centrifuged at 814g for 5 minutes, and the supernatant serum was analyzed. The established method was employed to measure the changes in serum UA levels of the rats, thereby evaluating the efficacy of allopurinol and benzbromarone.3. Results and discussion
3.1 Characterization
We successfully synthesized a series of FexNiy-NFs via a one-step solvothermal method, using ferric chloride hexahydrate and nickel nitrate hexahydrate as the metal precursors and pyrazine-2,3-dicarboxylic acid as the organic ligand. When dispersed in anhydrous ethanol, the solution appears black. The color of the anhydrous ethanol solutions of FexNiy-NFs with different molar ratios gradually transitions from orange to brown (Fig. S1). As shown in the scanning electron microscopy (SEM) images, Fe-NFs exhibited a uniform lamellar mesh structure (Fig. S2A). The introduction of Ni altered the microstructure of Fe-NFs to some extent. Specifically, FeNi-NFs displayed a dendritic morphology (Fig. S2B), while Fe2Ni-NFs (Fig. S2C) and Fe3Ni-NFs (Fig. S2D) exhibited a flower-like layered structure. In contrast, Fe4Ni-NFs (Fig. S2E) and Fe5Ni-NFs (Fig. S2F) formed large flake-like aggregates. As the proportion of Fe increased, the overall morphology evolved from a lamellar to a clustered structure. To compare the POD-like activity of FexNiy-NFs with different Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni molar ratios, we employed TMB as the peroxidase substrate. As shown in Fig. S3, the solutions turned blue in the presence of FexNiy-NFs, TMB, and H2O2. The weakest POD-like activity was observed when the Fe
Ni molar ratios, we employed TMB as the peroxidase substrate. As shown in Fig. S3, the solutions turned blue in the presence of FexNiy-NFs, TMB, and H2O2. The weakest POD-like activity was observed when the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio was 1
Ni ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The activity increased with the increasing molar ratio of Fe, reaching its maximum when the Fe
1. The activity increased with the increasing molar ratio of Fe, reaching its maximum when the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio was 4
Ni ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Like most iron-based MOFs, Fe-NFs exhibited inherent POD-like activity, primarily driven by Fe3+, which induced the single-electron oxidation of TMB. Upon the introduction of Ni, the catalytic activity of the bimetallic MOFs was significantly enhanced, likely due to improved electron transfer efficiency. Ultimately, we selected Fe4Ni-NFs, which demonstrated the optimal POD-like activity, as the most effective nanozyme for further analytical applications. As shown in Fig. 2A, Fe4Ni-NFs exhibited a uniform microstructure with a typical cauliflower-like surface morphology (Fig. 2B). The transmission electron microscopy (TEM) results were consistent with the SEM observations (Fig. 2C). EDS mapping images of Fe4Ni-NFs analysis indicated that C, O, Fe, Ni, and N were homogeneously dispersed throughout Fe4Ni-NFs (Fig. 2D). FexNiy-NFs displayed good crystallinity, characterized by strong and sharp diffraction peaks. The diffraction peaks at 10.0°, 16.4°, and 18° correspond to the (101), (103), and (200) planes, respectively, confirming the successful synthesis of FexNiy-NFs.39 Compared to Fe-NFs, Fe4Ni-NFs and Fe5Ni-NFs exhibited significantly enhanced intensity of the 10.0° diffraction peak. Additionally, the peaks at 16.4° and 18° were sharper and more distinct in Fe4Ni-NFs and Fe5Ni-NFs compared to FeNi-NFs with higher Ni content, indicating superior crystallinity and crystal structure (Fig. 2E).
1. Like most iron-based MOFs, Fe-NFs exhibited inherent POD-like activity, primarily driven by Fe3+, which induced the single-electron oxidation of TMB. Upon the introduction of Ni, the catalytic activity of the bimetallic MOFs was significantly enhanced, likely due to improved electron transfer efficiency. Ultimately, we selected Fe4Ni-NFs, which demonstrated the optimal POD-like activity, as the most effective nanozyme for further analytical applications. As shown in Fig. 2A, Fe4Ni-NFs exhibited a uniform microstructure with a typical cauliflower-like surface morphology (Fig. 2B). The transmission electron microscopy (TEM) results were consistent with the SEM observations (Fig. 2C). EDS mapping images of Fe4Ni-NFs analysis indicated that C, O, Fe, Ni, and N were homogeneously dispersed throughout Fe4Ni-NFs (Fig. 2D). FexNiy-NFs displayed good crystallinity, characterized by strong and sharp diffraction peaks. The diffraction peaks at 10.0°, 16.4°, and 18° correspond to the (101), (103), and (200) planes, respectively, confirming the successful synthesis of FexNiy-NFs.39 Compared to Fe-NFs, Fe4Ni-NFs and Fe5Ni-NFs exhibited significantly enhanced intensity of the 10.0° diffraction peak. Additionally, the peaks at 16.4° and 18° were sharper and more distinct in Fe4Ni-NFs and Fe5Ni-NFs compared to FeNi-NFs with higher Ni content, indicating superior crystallinity and crystal structure (Fig. 2E).
The thermal decomposition characteristics and chemical structure of Fe4Ni-NFs were investigated using thermogravimetric analysis (TGA) (Fig. 3A). The initial slight weight loss around 100 °C was likely attributed to the cleavage of Fe–O bonds within the chemical structure of Fe4Ni-NFs. The second weight loss observed between 260 °C and 280 °C was ascribed to the decomposition of the pyrazine nitrogen and carboxyl groups on the surface of the pyrazine-2,3-dicarboxylate organic ligand in Fe4Ni-NFs. The final weight loss occurring in the range of 280 °C to 400 °C may be due to the cleavage of Ni–O bonds or the pyrolysis of crosslinked carbon networks within the material's chemical structure.40 FT-IR spectroscopy was employed to analyze the functional group structures of Fe4Ni-NFs and Fe-NFs. As shown in Fig. 3B, Fe4Ni-NFs and Fe-NFs exhibited highly similar infrared spectra. The strong vibrational bands at 1630 cm−1 and 1385 cm−1 were attributed to the asymmetric and symmetric vibrations of carboxyl groups, respectively. The intense vibrational band at 753 cm−1 corresponded to C–H bond vibrations, confirmed the presence of the pyrazine-2,3-dicarboxylate organic ligand in Fe4Ni-NFs. Additionally, the band at 541 cm−1 was assignable to Fe–O and Ni–O vibrations, corresponding to the bimetallic sites of Fe and Ni in Fe4Ni-NFs.41 The zeta potential measurements (Fig. 3C) revealed that Fe-NFs exhibited a negative charge, while Fe4Ni-NFs showed a slight increase in potential. The presence of Ni2+ ions enhanced the positive charge of the nanoparticles, further confirmed the successful incorporation of Ni into the structure. XPS analysis corroborated these findings. As shown in Fig. 3D, the synthesized Fe4Ni-NFs were composed of five elements: C 1s (284.8 eV), N 1s (399.6 eV), O 1s (530.9 eV), Fe 2p (710.0 eV), and Ni 2p (855.6 eV). The high-resolution XPS spectra of C 1s (Fig. 3E), N 1s (Fig. 3F), and O 1s (Fig. 3G) were attributed to the C, N, and O components of the organic ligand. The high-resolution Ni 2p spectrum of Fe4Ni-NFs displayed fitted peaks for Ni 2p1/2 (873.1 eV) and Ni 2p3/2 (855.6 eV), each accompanied by two satellite peaks (Fig. 3H). The splitting energy between these peaks indicated the presence of Ni in the +2 oxidation state. The relatively weaker peak intensities suggested a lower Ni content. Similarly, the Fe 2p spectrum showed peaks for Fe 2p1/2 (723.4 eV) and Fe 2p3/2 (710.0 eV), along with satellite peaks (Fig. 3I), confirming the presence of Fe2+ in the synthesized Fe4Ni-NFs. These data collectively confirmed the successful synthesis of the Fe–Ni bimetallic nanozyme, with partial substitution of Fe sites by Ni, thereby retaining the intrinsic characteristics of Fe-NFs.
3.2 Mechanistic studies
To further elucidate the POD-like activity of Fe4Ni-NFs, as shown in Fig. 4A, we employed three typical chromogenic substrates: 3,3′,5,5′-tetramethylbenzidine (TMB), o-phenylenediamine (OPD), and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). In the presence of Fe4Ni-NFs, TMB, and H2O2, a characteristic absorption peak emerged at 652 nm. The absorbance increased progressively with the concentration of H2O2, and the solution color shifted from colorless to blue (Fig. 4B). When Fe4Ni-NFs, ABTS, and H2O2 were combined, a characteristic peak appeared at 420 nm. The absorbance rose with increasing H2O2 concentration, and the solution turned green (Fig. 4C). With Fe4Ni-NFs, OPD, and H2O2, a peak at 450 nm was observed. The absorbance increased with H2O2 concentration, and the solution color changed from colorless to orange-yellow (Fig. 4D). These results demonstrated that Fe4Ni-NFs could effectively catalyze the oxidation of all three chromogenic substrates (ABTS, OPD, and TMB) in the presence of H2O2. Notably, Fe4Ni-NFs exhibited the highest sensitivity towards TMB, with no interference from end-point absorption. Therefore, TMB was selected as the chromogenic substrate for subsequent experiments in this study. As shown in Fig. 4E, no significant color changed or characteristic absorption peak was observed, indicating that Fe4Ni-NFs alone did not induce a colorimetric response with TMB. Similarly, blue color or absorption peak at 652 nm was not detected, demonstrating that TMB and H2O2 alone did not produce a detectable reaction. A pronounced blue color and a characteristic absorption peak for oxidized TMB (oxTMB) at 652 nm were evident. This result confirmed that the presence of both Fe4Ni-NFs and H2O2 was essential for the catalytic oxidation of TMB to oxTMB. The presence of H2O2 was essential for Fe4Ni-NFs to catalyze the generation of radicals, which subsequently oxidized TMB to oxTMB. This finding further confirmed that the POD-like activity of Fe4Ni-NFs was significantly higher than their potential oxidase-like activity, which was negligible. The absence of blue color in the control groups without H2O2 ruled out any background interference from Fe4Ni-NFs alone or TMB alone. This conclusion provided a solid foundation for the subsequent detection of uric acid, ensuring that the observed colorimetric response was specifically due to the catalytic activity of Fe4Ni-NFs in the presence of H2O2.To elucidate the types of radicals generated during the catalytic reactions of Fe4Ni-NFs, we conducted radical scavenging experiments using isopropanol (IPA) and superoxide dismutase (SOD) as scavengers for hydroxyl radicals (˙OH) and superoxide radicals (O2˙−), respectively. As shown in Fig. 4F and G, the absorbance at 652 nm progressively decreased with increasing concentrations of isopropanol IPA and SOD, indicating reduced formation of oxidized TMB (oxTMB) and enhanced suppression of the oxidation effect. The results suggested that the ˙OH and O2˙− radical generated during the catalytic process were effectively scavenged, thereby inhibiting the colorimetric reaction of TMB. Further investigation into the impact of ˙OH and superoxide anion radicals on the enhanced POD-like activity of Fe4Ni-NFs was conducted using electron spin resonance (ESR) spectroscopy (Fig. 4H). In the presence of Fe4Ni-NFs and H2O2, the ESR spectrum of hydroxyl radicals exhibited characteristic quartet peaks with relative signal intensities of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4I). Similarly, the ESR spectrum of superoxide anion radicals displayed characteristic sextet peaks with relative signal intensities of 1
1 (Fig. 4I). Similarly, the ESR spectrum of superoxide anion radicals displayed characteristic sextet peaks with relative signal intensities of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4J). These results confirmed that both ˙OH and superoxide anion radicals could be trapped by DMPO to form spin adducts with distinct signal patterns, thereby verifying that the radicals generated during the catalytic reactions of Fe4Ni-NFs and H2O2 were indeed hydroxyl and superoxide anion radicals.
1 (Fig. 4J). These results confirmed that both ˙OH and superoxide anion radicals could be trapped by DMPO to form spin adducts with distinct signal patterns, thereby verifying that the radicals generated during the catalytic reactions of Fe4Ni-NFs and H2O2 were indeed hydroxyl and superoxide anion radicals.
Given that the pH of the catalytic system and the organic solvent used for material dispersion significantly influence the POD-like activity, we investigated the activity of Fe4Ni-NFs across a range of pH values (3 to 8). As shown in Fig. S4A, Fe4Ni-NFs exhibited optimal peroxidase-like activity under slightly acidic conditions (pH < 5), which was consistent with the pH preferences of most peroxidase-like nanozymes reported to date. Our research also explored the dispersion of Fe4Ni-NFs in various organic solvents, including anhydrous ethanol, isopropanol, DMSO and so on. As depicted in Fig. S4B, Fe4Ni-NFs demonstrated higher peroxidase-like activity when dispersed in anhydrous ethanol, isopropanol, and DMSO. Considering safety and environmental concerns, we selected anhydrous ethanol as the dispersion solvent for Fe4Ni-NFs. To assess the long-term stability of Fe4Ni-NFs, we stored the synthesized Fe4Ni-NFs in anhydrous ethanol at room temperature and monitored their activity over a period of 7 days. As shown in Fig. S4C, the Fe4Ni-NFs maintained over 90% of their initial activity, demonstrating excellent recyclability and reusability. Furthermore, we examined the stability of Fe4Ni-NFs when stored as a powder at 4 °C for 40 days. As illustrated in Fig. S4D, the activity of Fe4Ni-NFs remained at a high level throughout this period, highlighting their robust environmental tolerance and a significant advantage over natural enzymes.
3.3 Feasibility
To achieve the colorimetric detection of UA using Fe4Ni-NFs, we employed uricase (UOX), which catalyzed the oxidation of UA to allantoin and H2O2 in the presence of O2. Fe4Ni-NFs then catalyzed the decomposition of H2O2, linking the two catalytic processes through H2O2 as a bridge (Fig. 5A). To further investigate the peroxidase-like activity of Fe4Ni-NFs, we compared their steady-state kinetic parameters with those of horseradish peroxidase (HRP), a natural peroxidase. The results were consistent with typical Michaelis–Menten kinetics, yielding characteristic Michaelis–Menten curves. As shown in Fig. 5B, Fig. S5A, S6A, S6B and Table S1, when TMB was used as the substrate, the Km value of Fe4Ni-NFs was 0.59 mM and the Vmax value was 1.045 × 10−7 M s−1, while the Km and Vmax values of HRP were 0.0829 mM and 3.04 × 10−8 M s−1, respectively. When H2O2 was used as the substrate (Fig. 5B, Fig. S5B, S6C, S6D and Table S1), the Km value of Fe4Ni-NFs was 0.109 mM and the Vmax value was 8.49 × 10−8 M s−1, whereas the Km and Vmax values of HRP were 1.074 mM and 1.02 × 10−7 M s−1, respectively. Compared with HRP, Fe4Ni-NFs exhibited lower affinity for the substrate TMB. However, Fe4Ni-NFs had a lower Km value for H2O2 than HRP—and a lower Km value indicates higher affinity of the catalyst for the substrate. Specifically, the affinity of Fe4Ni-NFs for H2O2 was more than 10 times that of HRP. Therefore, during the catalytic reaction of Fe4Ni-NFs, only a small amount of H2O2 is required to achieve excellent catalytic activity. These results demonstrate that Fe4Ni-NFs possess superior peroxidase-like activity, which is comparable to that of the commonly used natural enzyme HRP. Fe4Ni-NFs could be used for the colorimetric detection of H2O2 in the presence of TMB. As shown in Fig. 5D, the absorbance at 652 nm increased with the concentration of H2O2, and the reaction solution changed color from colorless to blue. A good linear relationship was observed between absorbance and H2O2 concentration in the range of 2.5 to 200 µM (A652 nm = 0.3432 [H2O2] + 0.08885, R2 = 0.9901, Fig. 5E), with a detection limit of 0.21 µM. These findings demonstrated that Fe4Ni-NFs offered a broader linear range and an ultra-sensitive detection limit for H2O2, outperforming many previously reported nanozymes (Table S2).3.4 Linearity
Using TMB as the chromogenic substrate, we developed a highly sensitive colorimetric method for the visualization and detection of uric acid. As shown in Fig. 5F and G, the absorbance at 652 nm increased progressively with the concentration of UA. The method exhibited good linearity in two concentration ranges: 2–150 µM (A652 nm = 0.002645 [UA] + 0.1293, R2 = 0.9931, Fig. S7A) and 150–500 µM (A652 nm = 0.0006482 [UA] + 0.4187, R2 = 0.9904, Fig. S7B). The detection limit for UA was determined to be 1.13 µM (S/N = 3), indicating the high sensitivity of this method. Table 1 summarized the accuracy and precision for the detection of UA. The accuracy ranged from 91.3% to 103.8%, with relative standard deviations (RSD) all below 12.1%. These results demonstrated the reliability and accuracy of the proposed method for UA detection. To enhance the sensitivity of uric acid (UA) detection, we optimized the detection conditions. We selected NaAc–HAc buffer solutions with pH values ranging from 3.0 to 6.0 for the catalytic incubation of Fe4Ni-NFs. As shown in Fig. S8A, a pH of 3.5 was identified as the optimal condition for the UA detection system, which was consistent with the pH stability studies of Fe4Ni-NFs. We also investigated the optimal incubation temperature for the UA detection system by testing temperatures ranging from 25 °C to 50 °C. As depicted in Fig. S8B, the signal at 652 nm remained stable within the temperature range of 25 °C to 40 °C. This finding further demonstrated the good thermal tolerance of Fe4Ni-NFs. For convenience, we chose room temperature (25 °C–30 °C) as the incubation temperature for the UA detection system. Lastly, we optimized the incubation time for UA detection by measuring the signal at 652 nm every 2 minutes. As shown in Fig. S8C, the incubation reached a plateau within 18 minutes. To ensure stable detection, we selected 20 minutes as the incubation time for the UA detection system. Under the optimized detection conditions, we constructed a smartphone-based RGB detection platform for the visual quantification of uric acid (UA) concentration. As shown in Fig. 5H, the intensity of the blue color deepens with increasing UA concentration. By photographing a 96-well plate with a smartphone and subsequently extracting and analyzing the red (R), green (G), and blue (B) values, a linear relationship was established within the range of 2–200 µM, as described by the equation (G + B)/R = 0.003260 × [UA] + 2.070 (R2 = 0.9913, Fig. 5I). The detection limit for UA was determined to be 1.06 µM (S/N = 3). Thus, the method we developed enabled sensitive and convenient quantitative analysis of uric acid levels.| Conc. (μM) | Measured (μM) | Accuracy (%) | RSD (%) |
|---|---|---|---|
| Conc.: concentration; RSD: relative standard deviation; n: number of replicates. | |||
| 5 | 5.2 ± 0.6 | 103.8 | 12.1 |
| 80 | 73.1 ± 2.4 | 91.3 | 1.3 |
| 250 | 240.3 ± 6.3 | 96.1 | 2.6 |
| 450 | 456.5 ± 12.7 | 101.8 | 2.8 |
3.5 Biological analysis and specificity
To validate the applicability of the developed method for detecting UA in complex biological matrices, we applied it to human serum samples. As shown in Fig. 6A, the absorbance at 652 nm increased with the concentration of UA in the serum, exhibiting a good linear relationship within the range of 2–150 µM (A652 nm = 0.0006235 [UA] + 0.1048, R2 = 0.9931, Fig. 6B). The accuracy for UA detection, summarized in Table 2, ranged from 90.6% to 111.90%, with RSD all below 10.8%. These results demonstrated the accuracy and reliability of the method for UA detection in human serum. Considering the presence of multiple potential interferents in biological samples, we evaluated the specificity of the developed method using common compounds, including glucose (Glu), dopamine (DA), glutathione (GSH), urea, ascorbic acid (AA), lysine (Gly), histidine (His), and various inorganic ions (K+, Mn2+, Mg2+, Ca2+, and NH4+). As shown in Fig. 6C, the method exhibited no significant signals for these potential interferents. Furthermore, when UA was co-incubated with these interferents, the detection signal for UA remained unaffected (Fig. 6D). These findings indicated that the developed method was highly specific and sensitive for detecting UA in complex biological matrices, such as human serum, and could accurately and reliably quantify UA levels in real biological samples.| Conc. (μM) | Measured (μM) | Accuracy (%) | RSD (%) |
|---|---|---|---|
| Conc.: concentration; RSD: relative standard deviation; n: number of replicates. | |||
| 5 | 4.5 ± 0.3 | 90.6 | 5.6 |
| 15 | 14.6 ± 1.6 | 97.2 | 10.8 |
| 25 | 28.0 ± 1.7 | 111.9 | 5.9 |
| 45 | 46.3 ± 0.8 | 102.8 | 1.8 |
3.6 Pharmacodynamics study
We applied the developed method to investigate the pharmacodynamics of drugs used for the treatment of hyperuricemia. Allopurinol, a structural analog of xanthine, is a potent inhibitor of xanthine oxidase. Xanthine oxidase catalyzes the oxidation of hypoxanthine to xanthine and subsequently to uric acid. By inhibiting this enzyme, allopurinol effectively reduces serum and urinary uric acid levels, making it a highly efficient uric acid-lowering drug.42,43 Benzbromarone, on the other hand, is a potent uricosuric agent that has been proven to be highly effective and well-tolerated in the treatment of hyperuricemia and gout-related diseases.44,45 Therefore, we selected allopurinol and benzbromarone to evaluate their therapeutic effects through short-term (within 9 hours) and long-term (7 days) continuous oral administration in hyperuricemia (HUA) rats (Fig. 6E). In the short-term efficacy study, we first analyzed the allopurinol-treated group (AP group). The mean serum UA level in HUA rats of the AP group was 220 µM. Following drug administration, the serum UA levels progressively decreased. At 2 hours post-administration, the reduction rate of serum UA was 55.3%. By 9 hours, the serum UA level had dropped to 21.5 µM, representing a reduction rate of 90.23% (Fig. 6F and H). For the benzbromarone-treated group (BZ group), the mean serum UA level in HUA rats was 168 µM prior to drug administration. After treatment, the serum UA levels also decreased throughout the day. At 2 hours post-administration, the reduction rate of serum UA was 48.4%. By 9 hours, the serum UA level had decreased to 18 µM, with a reduction rate of 89.28% (Fig. 6G and H). Compared to the AP group, the reduction rate of serum UA in the BZ group was slightly lower. Based on the serum UA levels and reduction rates observed within the day, the therapeutic efficacy of allopurinol was comparable to that of benzbromarone in the short term.Given that clinical treatment for hyperuricemia typically spans at least 7 days, we further investigated the long-term efficacy of both drugs by monitoring the dynamic changes in serum UA levels in HUA rats before and 3 hours after daily administration over a 7-day period. As shown in Fig. 6I and K, during the 7-day treatment period, the serum UA levels measured before daily dosing consistently decreased compared to the previous day. Starting from day 3, the differences in serum UA levels before dosing became statistically significant compared to the previous day. On day 4, the reduction rate of serum UA compared to day 1 exceeded 50%, and by day 7, the reduction rate reached 89.1%, with serum UA levels returning to normal. Additionally, the serum UA levels measured 3 hours after dosing each day showed significant decreases compared to pre-dosing levels, especially during the first three days of treatment. This significant reduction was attributed to the high initial UA levels in the HUA rats, which were rapidly lowered by the drug. On day 7, the serum UA level 3 hours post-dosing was 18 µM, which was not significantly different from the pre-dosing level on the same day (23 µM). These results indicated that allopurinol effectively reduced serum UA levels over a 7-day treatment period. A similar trend was observed in the benzbromarone-treated group (BZ group) (Fig. 6J and K). During the 7-day treatment period, the serum uric acid (UA) levels measured before daily dosing consistently decreased compared to the previous day. Starting from day 4, the differences in serum UA levels before dosing became statistically significant compared to the previous day. On day 4, the reduction rate of serum UA compared to day 1 was 47.1%, and by day 7, the reduction rate reached 86.4%. These reduction rates were lower than those observed in the allopurinol-treated group (AP group), consistent with the results from the short-term efficacy study. On day 7, the serum UA level before dosing was 23 µM, and 3 hours post-dosing, it was 16 µM, with no significant difference between the two. The dynamic changes in serum UA levels over the 7-day period in the BZ group were consistent with those in the AP group. The reduction rates of serum UA levels indicated that both drugs are highly effective in treating hyperuricemia, with allopurinol showing slightly superior efficacy compared to benzbromarone. The findings demonstrate that the Fe4Ni-NFs nanozyme could rapidly, conveniently, and efficiently detect serum UA levels following drug administration. This method was suitable for clinical detection of UA-related diseases, assessment of drug efficacy, and screening of new drugs for the treatment of hyperuricemia. The consistent results from both short-term and long-term studies highlight the robustness and reliability of Fe4Ni-NFs as a diagnostic and evaluative tool in hyperuricemia management.
4. Conclusions
In summary, we have developed a simple, cost-effective solvothermal method for one-step synthesis of novel iron-nickel bimetallic metal–organic frameworks (Fe4Ni-NFs) exhibiting high peroxidase-mimicking activity. This method was characterized by high sensitivity, strong specificity, high throughput, simplicity, ease of sample pre-treatment, and low cost, making it suitable for detecting UA in human serum. Utilizing the Fe4Ni-NFs -based UA sensing strategy, we performed high-throughput determination of UA levels in hyperuricemia (HUA) rats during short-term and long-term oral administration of allopurinol and benzbromarone. These results demonstrate that the prepared Fe4Ni-NFs can rapidly evaluate drug efficacy and changes in uric acid levels. The biosensing platform constructed using Fe4Ni-NFs exhibits great application potential in the clinical detection and diagnosis of hyperuricemia, gout, and other related diseases. It can provide technical support for the development and screening of new drugs for treating these diseases, and also holds broad application prospects in the fields of precise treatment and post-treatment monitoring of hyperuricemia.Author contributions
Hanbing Yao (first author): conceptualization, methodology, investigation, writing – original draft. Yifu Li: software, writing – original draft, formal analysis. Yujuan Zhan: visualization, investigation; Binyu Xiao: data curation; Jiayi Yan: investigation. Shuangshuang Liu: investigation. Zimo Chen: investigation. Chang Shu (Corresponding Author): conceptualization, funding acquisition, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available on request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5nr04096e.
Acknowledgements
This study was supported by the Open Project Program of MOE Key Laboratory of Drug Quality Control and Pharmacovigilance (No. DQCP20/21PQ02).References
- J. Miake, I. Hisatome, K. Tomita, T. Isoyama, S. Sugihara, M. Kuwabara, K. Ogino and H. Ninomiya, Biomedicines, 2023, 11, 1258 CrossRef CAS PubMed.
- X. L. Guo, Y. Y. Gao, Y. X. Yang, Q. F. Zhu, H. Y. Guan, X. He, C. L. Zhang, Y. Wang, G. B. Xu, S. H. Zou, M. C. Wei, J. Zhang, J. J. Zhang and S. G. Liao, Phytomedicine, 2023, 116, 154868 CrossRef CAS PubMed.
- B. Wei-Yun and Z. Cailin, Food Agric. Immunol., 2021, 32, 778–797 CrossRef.
- X. Wang, Q. Yao, X. Tang, H. Zhong, P. Qiu and X. Wang, Anal. Bioanal. Chem., 2019, 411, 943–952 CrossRef CAS PubMed.
- Y.-B. Cheng and Y. Li, Hypertension, 2018, 71, 66–67 CrossRef CAS PubMed.
- T. J. Major, N. Dalbeth, E. A. Stahl and T. R. Merriman, Nat. Rev. Rheumatol., 2018, 14, 341–353 CrossRef CAS.
- N. Dalbeth, T. R. Merriman and L. K. Stamp, Lancet, 2016, 388, 2039–2052 CrossRef CAS.
- L. Liu, X. Li, Y. Chen, J. Gao, Y. Jiang, Y. Ye, P. Wang, F. Peng and Y. Tu, Nat. Commun., 2025, 16, 2339 CrossRef CAS.
- K. Iseki, S. Oshiro, M. Tozawa, C. Iseki, Y. Ikemiya and S. Takishita, Hypertens. Res., 2001, 24, 691–697 CrossRef CAS PubMed.
- Y. Sheng, H. Yang, Y. Wang, L. Han, Y. Zhao and A. Fan, Talanta, 2017, 166, 268–274 CrossRef CAS.
- A. Matsumoto, S. Hosoyama, K. Higashi and T. Toida, Carbohydr. Res., 2012, 358, 82–88 CrossRef CAS.
- Z. Xu, M.-q. Zhang, H.-q. Zou, J.-s. Liu, D.-z. Wang, J. Wang and L.-d. Wang, J. Electroanal. Chem., 2019, 841, 129–134 CrossRef CAS.
- C. Donadio, D. Calia, S. Ghimenti, M. Onor, E. Colombini, R. Fuoco and F. Di Francesco, J. Nephrol., 2014, 27, 331–337 CAS.
- J. Cao, C. Xie, Y. Zeng and Y. Wu, Microchem. J., 2024, 204, 111079 CrossRef CAS.
- J. C. Fanguy and C. S. Henry, Electrophoresis, 2002, 23, 767–773 CrossRef CAS.
- X. Xie, D. P. Wang, C. Guo, Y. Liu, Q. Rao, F. Lou, Q. Li, Y. Dong, Q. Li, H. B. Yang and F. X. Hu, Anal. Chem., 2021, 93, 4916–4923 CrossRef CAS.
- X. Yao, Y. Wang and G. Chen, Biomed. Chromatogr., 2007, 21, 520–526 CrossRef CAS PubMed.
- J. Buckberry, R. Telford, L. Castells Navarro, J. Snaith, D. Swinson, A. Healey and M. B. Brickley, Am. J. Biol. Anthropol., 2024, 184, e24938 CrossRef PubMed.
- R. Zhang, X. Yan and K. Fan, Acc. Mater. Res., 2021, 2, 534–547 CrossRef CAS.
- M. Bilal, N. Khaliq, M. Ashraf, N. Hussain, Z. Baqar, J. Zdarta, T. Jesionowski and H. M. N. Iqbal, Colloids Surf., B, 2023, 221, 112950 CrossRef CAS.
- F. Manea, F. B. Houillon, L. Pasquato and P. Scrimin, Angew. Chem., Int. Ed., 2004, 43, 6165–6169 CrossRef CAS.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- J. Chen, Q. Ma, M. Li, D. Chao, L. Huang, W. Wu, Y. Fang and S. Dong, Nat. Commun., 2021, 12, 3375 CrossRef CAS PubMed.
- S. Li, H. Ding, J. Chang, S. Dong, B. Shao, Y. Dong, S. Gai, F. He and P. Yang, J. Colloid Interface Sci., 2022, 623, 787–798 CrossRef CAS PubMed.
- Z. Chen, S. Song, H. Zeng, Z. Ge, B. Liu and Z. Fan, Chem. Eng. J., 2023, 471, 144649 CrossRef CAS.
- B. Marciniec, C. Pietraszuk, P. Pawluć and H. Maciejewski, Chem. Rev., 2022, 122, 3996–4090 CrossRef CAS.
- S. Feng, Y. Xiao, J. Lu, Z. Chen, Z. Jiang, Q. Xu, W. Gu, S. Wang and Q. Zhao, J. Colloid Interface Sci., 2024, 663, 577–590 CrossRef CAS PubMed.
- J. Sheng, Y. Wu, H. Ding, K. Feng, Y. Shen, Y. Zhang and N. Gu, Adv. Mater., 2024, 36, e2211210 CrossRef PubMed.
- L. Yang, X. Xu, Y. Song, J. Huang and H. Xu, Chem. Eng. J., 2024, 487, 150612 CrossRef CAS.
- X. Zhu, Y. Xue, S. Hou, P. Song, T. Wu, H. Zhao, N. A. Osman, A. K. Alanazi, Y. Gao, H. M. Abo-Dief, H. Li, B. B. Xu, P. Wasnik and Q. Liu, Adv. Compos. Hybrid Mater., 2023, 6, 142 CrossRef CAS.
- X. Zhu, H. Li, S. Hou, P. Song, J. Zheng, T. Wu, H. Zhao and Q. Liu, Chem. Eng. J., 2024, 482, 148589 CrossRef CAS.
- Y. Gu, J. Han, N. Zhang, W. Yan, Y. Guo, H. Tan, C. Yang, F. Wang and H. Yao, ACS Appl. Nano Mater., 2024, 7, 24683–24696 CrossRef CAS.
- S. Li, Y. Gao, N. Li, L. Ge, X. Bu and P. Feng, Energy Environ. Sci., 2021, 14, 1897–1927 RSC.
- Z. Mu, J. Mu, M. Zhao and Y. Wang, Sens. Actuators, B, 2023, 394, 134422 CrossRef CAS.
- Y. Zhang, Y.-S. Feng, X.-H. Ren, X.-W. He, W.-Y. Li and Y.-K. Zhang, Biosens. Bioelectron., 2022, 196, 113718 CrossRef CAS PubMed.
- X. He, D.-R. Chen and W.-N. Wang, Chem. Eng. J., 2020, 382, 122825 CrossRef CAS.
- Y. Zhu, R. Zhao, L. Feng, C. Wang, S. Dong, M. V. Zyuzin, A. Timin, N. Hu, B. Liu and P. Yang, ACS Nano, 2023, 17, 6833–6848 CrossRef CAS.
- Z. Mu, S. Wu, J. Guo, M. Zhao and Y. Wang, ACS Sustainable Chem. Eng., 2022, 10, 2984–2993 CrossRef CAS.
- J. Li, J. Zhao, S. Li, Y. Chen, W. Lv, J. Zhang, L. Zhang, Z. Zhang and X. Lu, Nano Res., 2021, 14, 4689–4695 CrossRef CAS.
- H. Zhang, J. Luo and F. Gan, Anal. Chim. Acta, 2023, 1279, 341788 CrossRef CAS.
- C. Fang, Z. Deng, G. Cao, Q. Chu, Y. Wu, X. Li, X. Peng and G. Han, Adv. Funct. Mater., 2020, 30, 1910085 CrossRef CAS.
- M. Barrientos, R. A. Macabeo and R. A. Ragasa, Eur. Heart J., 2020, 41, ehz872.097 CrossRef.
- O. N. Okafor, K. Farrington and D. A. Gorog, Pharmacol. Ther., 2017, 172, 139–150 CrossRef CAS.
- E. H. Kang, E. H. Park, A. Shin, J. S. Song and S. C. Kim, Eur. Heart J., 2021, 42, 4578–4588 CrossRef CAS PubMed.
- F. Yan, X. Xue, J. Lu, N. Dalbeth, H. Qi, Q. Yu, C. Wang, M. Sun, L. Cui, Z. Liu, Y. He, X. Yuan, Y. Chen, X. Cheng, L. Ma, H. Li, A. Ji, S. Hu, Z. Ran, R. Terkeltaub and C. Li, Arthritis Rheumatol., 2022, 74, 2015–2023 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





