Open Access Article
Open Access ArticleEmerging nano-delivery systems for targeting mitochondria
Agata N.
Burska
 ab,
Kristina E.
Raish
ab,
Kristina E.
Raish
 a,
Dinmukhamet
Bayandy
a,
Dinmukhamet
Bayandy
 c and
Vesselin N.
Paunov
c and
Vesselin N.
Paunov
 *c
*c
aNational Laboratory Astana, Nazarbayev University, Astana, Kazakhstan
bDepartment of Biomedical Science, School of Medicine, Nazarbayev University, Astana, Kazakhstan
cDepartment of Chemistry, School of Sciences and Humanities, Nazarbayev University, Astana, Kazakhstan. E-mail: vesselin.paunov@nu.edu.kz
First published on 22nd December 2025
Abstract
This review aims to provide a comprehensive analysis of the potential of mitochondria-targeting nanosystems as a novel therapeutic approach for treating a wide range of diseases. It explores the underlying mechanisms of mitochondrial dysfunction in disease progression and shows how nanotechnology offers an innovative platform for delivering targeted therapies directly to mitochondria. We also highlight the role of mitochondria in cellular function and disease pathology particularly in cancer, followed by a consideration of the therapeutic potential of targeting these organelles. We explore the recent development and design principles of mitochondria-targeting nanosystems, assessing their applications and challenges and finally outline future research directions, emphasizing the importance of overcoming current limitations to expand the use of these nanosystems in medicine. This is intended to provide valuable insights into the promising connection of mitochondrial biology and nanotechnology, with the goal of advancing innovative treatments for various diseases.
1. Introduction
Mitochondria are complex organelles that play a crucial role in cellular energy production and signalling. They are known as “cell powerhouses” and are responsible for generating the majority of cellular ATP through oxidative phosphorylation. Over 1.45 billion years ago, through a process known as endosymbiosis, a prokaryotic organism established a symbiotic relationship within a eukaryotic cell, eventually evolving into mitochondrion. Both mitochondria and prokaryotes possessing their own circular genome (mtDNA),1 and their membranes share the presence of structural phospholipid cardiolipin.2 The word “mitochondrion” is derived from two Greek terms: “mitos” and “chondrion” meaning “thread” and “granule, respectively”.3 In 1952, advancements in high-resolution electron microscopy enabled scientists to observe the detailed structure of mitochondria for the first time.4 Mitochondria function as highly efficient and adaptable bioenergetic systems, powering biosynthesis in eukaryotic cells.5Mitochondria harbour two distinct membranes, the outer and inner membranes, and two functionally diverse compartments, the intermembrane space and the matrix. The outer membrane acts as a barrier and mediates transport, while the inner membrane houses the electron transport chain (ETC) and is highly convoluted to increase surface area for efficient oxidative phosphorylation. The intermembrane space contains enzymes involved in protein import and apoptosis signalling, while the matrix contains the mitochondrial DNA and enzymes for metabolism.6 In addition, mitochondria interact with other cell organelles, such as the endoplasmic reticulum, via membrane contact sites. These sites mediate the exchange of lipids, ions, and metabolites and regulate mitochondrial dynamics, mtDNA replication, and quality control.7 Mitochondria display remarkable functional and compositional diversity even within an individual organism, despite being derived from a common bacterial ancestor. As mitochondria have lost most of their ancestral genome, many of these genes were transferred to the nucleus. The vast majority (99%) of the mitochondrial proteome in humans is encoded by the nuclear genome. The small 16.6 kb mitochondrial genome encodes only 13 proteins devoted to producing ATP via oxidative phosphorylation.8 During this process, NADH and FADH2 donate electrons, which fuel oxidative phosphorylation (OXPHOS), ultimately producing ATP. This mechanism is essential for maintaining cellular energy supply and overall function.10,11
Here, emerging nano-delivery systems for targeting mitochondria refers to the development of novel, ultra-small carrier particles designed to transport therapeutic or diagnostic agents directly to the mitochondria within cells. The core function of these nano-systems is to overcome biological barriers and achieve precise subcellular delivery, thereby enabling the direct treatment of mitochondrial dysfunction or the exploitation of the organelle's role in critical cellular processes like apoptosis.
This review evaluates the latest approaches and developments in nanoparticles-based delivery of actives to mitochondria for targeting a range of conditions. This is a new and emerging area of interdisciplinary research that bridges materials science with cancer research, nanotechnology, pharmacology and molecular biology.
The review will also outline the newest ideas in nano-formulations of commonly used mitochondria targeting drugs which boost their efficacy. The review will benefit a wide range of researchers working on novel biomaterials and biomedical applications, bio-nanotechnology, anticancer and anti-aging research, and improved chemotherapies.
Mitochondrial dysfunction is implicated in a wide range of diseases, including neurodegenerative disorders, cardiovascular diseases, metabolic disorders, and cancer. Apart from their energy production function, mitochondria play crucial roles as key regulators of cellular metabolism, programmed cell death (apoptosis), and reactive oxygen species (ROS) production, all of which can be effectively “hijacked” by cancer cells to support their growth and survival.9 In eukaryotic cells, mitochondria generate energy by oxidizing nutrients through metabolic pathways such as glycolysis, the tricarboxylic acid (TCA) cycle, and fatty acid β-oxidation.
Tumour cells frequently alter these processes to maximize their survival and enhance their resistance to the treatment. Dysregulation of mitochondria significantly contributes to tumour development, cancer cell anabolism, uncontrolled replication, resistance to anticancer treatments, and apoptosis avoidance. Under normoxic conditions, most cells generate energy primarily through mitochondrial oxidative phosphorylation. Cancer cells, however, often reprogram their metabolism toward increased glycolysis, even when oxygen is sufficient in order to support rapid biomass production. This metabolic shift is known as the Warburg effect.12 Otto Warburg's research highlighted that cancer cells exhibit increased lactate production and a glycolytic shift even in the presence of oxygen, a process termed “aerobic glycolysis”. He suggested that all cancer cells have impaired respiration, potentially due to oxygen deficiency, which he believed was a key factor in cancer development.13,14 Subsequent research on growth factors in oncogenesis showed that targeting both glycolysis and mitochondrial metabolism is crucial for halting tumour progression.12 Mitochondrial glycolysis and fatty acid oxidation supply the ATP necessary for tumour cell survival, while OXPHOS supports cancer cell invasion and metastasis. Increased mitochondrial energy metabolism also boosts ROS production, activating protein tyrosine kinases and stimulating metastasis.15 As cancer cells proliferate uncontrollably, they often adapt mitochondrial functions and to meet their increasing demands for energy and biosynthetic precursors. During this metabolic reprogramming, they shift energy production from OXPHOS toward glycolysis, leading to extensive glucose consumption. Glucose is rapidly converted to pyruvate and subsequently to lactate, which is released into the cytosol and contributes to extracellular acidification. This acidic environment limits oxygen availability, disrupting its transfer and causing a breakdown of the mitochondrial electron transport chain (ETC), ultimately leading to decreased ATP production.11,16 This is known as metabolic reprogramming, resistance to cell death, and adaptation to environmental stressors like hypoxia and nutrient deprivation. Moreover, cancer cells tend to have a more hyperpolarized mitochondrial membrane potential (ΨIM) than normal cells (−220 mV vs. −140 mV), and this effect becomes even more pronounced in more aggressive tumors.17,18 In some cases, they can have nearly double value of the ΨIM than that of healthy cells as shown in Neu4145 cancer cells where ΨIM reached around −210 mV. This highlighting a key difference in their metabolism and energy dynamics.18–20 In cancer cells, the balance between ROS and antioxidant systems is often disrupted, leading to increased oxidative stress or, in some cases, enhanced antioxidant capacity to support tumour survival. Elevated levels of mitochondria-derived ROS in cancer cells enhance tumour-promoting traits and drive genetic mutations, facilitating metastasis. To counteract this, cancer cells often upregulate antioxidant defences, such as glutathione and superoxide dismutase, allowing them to adapt to hostile conditions and evade oxidative stress-induced apoptosis. This altered redox homeostasis plays a crucial role in cancer development, metastasis, and treatment resistance.21,22 Moreover, mitochondrial dynamics, the balance between fission and fusion, play a particularly important role in cancer. A shift towards mitochondrial fission is characteristic to many cancer cells and is associated with increased metabolic flexibility and resistance to therapy. This adaptability helps the cells maintain energy production under stress conditions such as hypoxia, nutrient deprivation or chemotherapy. By interfering with mitochondrial dynamics, treatments can directly weaken the cancer cells’ ability to survive under adverse conditions.
2. Discussion
2.1 Mitochondria as attractive targets in cancer and other therapies
Mitochondrial dysfunction is a key driver of cellular damage and disease progression. In cancer, mitochondria can become highly efficient, allowing cancer cells to survive and grow even in harsh conditions like hypoxia (e.g. in a hypoxic core)23 while in neurodegenerative diseases like Alzheimer's or Parkinson's, damaged mitochondria contribute to cell death, accelerating disease symptoms.24 In heart diseases, improper mitochondrial function can lead to poor energy production and increased oxidative stress, weakening heart muscle cells.25 Since mitochondria are at the heart of these processes, targeting them directly can be a powerful way to not only manage symptoms but also to intervene in the disease process itself.By targeting mitochondrial pathways, it is possible to selectively induce cell death in these cells. Therapies aimed at restoring normal mitochondrial function can potentially ameliorate these conditions. Targeting mitochondrial ROS production or enhancing mitochondrial antioxidant defences can help mitigate oxidative stress-related damage in various diseases.15
Mitochondria are an attractive target for therapy due to their central role in numerous physiological and pathological processes, mainly maintaining cellular energy homeostasis, regulating apoptosis, and influencing metabolic and signalling pathways.26 Because of this and several other unique characteristics of this organelle, they are particularly compelling in therapeutic development. These cellular structures have unique features that can be specifically targeted without affecting the rest of the cell, allowing for the development of therapies with potentially fewer side effects.
Mitochondrial DNA (mtDNA) is highly prone to mutations27 which may contribute to multiple diseases and other conditions, from rare mitochondrial diseases to common conditions like cancer and aging.28 Therapies targeting mtDNA or its replication and repair mechanisms hold promise for treating such diseases at their genetic root. Moreover, mitochondria are key regulators of apoptosis. They mediate intrinsic apoptotic pathway via releasing cytochrome c as well as other pro-apoptotic factors, which further activate downstream effector caspases. Drugs with mitochondria targeting properties can have dual effect on apoptosis, they can induce it, which is essential for targeting cancer cells programmed to avoid apoptosis29 or, contrary, inhibit mitochondria-induced cell death in certain neurodegenerative or ischemic conditions when excessive apoptosis is a problem.30
Mitochondria are also a significant source of ROS that are mediators of oxidative stress and important signalling molecules. Elevated mitochondrial ROS levels contribute to cellular damage in neurodegenerative and cardiovascular disorders and aging. Similarly, to apoptosis targeting mitochondrial ROS might go both ways. Direct suppression or quenching of ROS can mitigate oxidative stress but alternatively, exacerbation of oxidative stress can induce significant cytotoxic stress and kill ROS-vulnerable cancer cells.9,31,32 In addition, mitochondria regulate intracellular calcium homeostasis and signalling, processes vital for metabolism, muscle contraction, and neural function. Dysregulated mitochondrial calcium handling is an attractive therapeutic target for cardiac and neurodegenerative diseases.
Mitochondrial metabolism is highly plastic, transitioning between catabolic oxidative burners and anabolic modes to support growth and repair. This metabolic plasticity is linked to structural plasticity, with changes in mitochondrial shape and distribution resulting from the combined actions of the mitochondrial division, fusion, and motility machines.5 Mitochondrial dynamics refers to the changing processes of fission, fusion, mitophagy, and transport that are critical for maintaining mitochondrial and cellular health and by changing; they allow adapting to different cellular stresses and metabolic needs. Fragmented mitochondria are linked to increased energy demands, cell proliferation, and apoptosis, while fused mitochondria support metabolic efficiency and cell differentiation. Mitophagy, in contrast, selectively removes damaged mitochondria through autophagy. Mitochondrial transport is facilitated by motor proteins like kinesin and dynein that bind to the organelle via adaptor proteins.5 Balanced mitochondrial dynamic is essential for numerous cellular processes such as immune cell function, cell cycle progression, and cellular senescence, hence an imbalance in mitochondrial dynamics can disrupt mitochondrial function and lead to cancer progression, neurodegeneration, metabolic complications and range of other diseases. Drugs targeting these processes in mitochondria can restore mitochondrial quality control, thereby improving cellular function and resilience. Various pharmacological and genetic approaches have been explored to target mitochondrial dynamics for therapeutic benefit. Fission inhibitors like Mdivi-1, P110, and Dynasore or compounds that promote fusion, such as melatonin and paeonol, have also demonstrated therapeutic potential.33–35
Finally, mitochondria's role in inflammation and immune signalling has also an important therapeutic relevance. Dysfunctional mitochondria drive chronic inflammation in autoimmune diseases, sepsis, and metabolic syndromes as they release damage-associated molecular patterns (DAMPs) and activate inflammasomes. Drugs targeting of these aspects of mitochondrial activity can help control inflammatory responses and promote tissue repair.
2.2 Subcellular-targeted nanocarrier drug delivery
The drug nanocarriers systems have been considered as a “magic bullet” concept of developing drugs that can selectively and efficiently reach their intended cellular targets.36 This approach has paved the way for a development of targeted therapies using variety of therapeutic compounds. Nanotechnology brings new possibilities for implementation of this concept by designing nanocarrier systems that can deliver targeted drugs with high organelle-level precision.37 The drug delivery nanosystems are exceptionally small, often well under 100 nm and can be engineered to transport therapeutic agents, including drugs, proteins, or oligonucleotides, directly to targeted locations within the body, including specific cells and subcellular organelles (see Scheme 1A).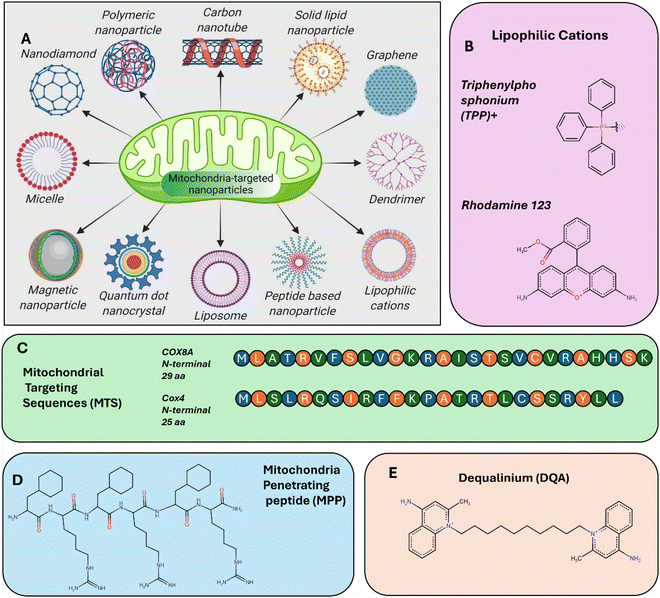 | ||
| Scheme 1 (A) Types of nanocarriers for targeting mitochondria.153 Adapted and reprinted with permission from ref. 153; copyright 2021 Elsevier. (B)–(E) Typical mitochondria targeting ligands. | ||
Nanosystems have unique properties, like enhanced permeability and retention (EPR) effect and can accumulate effectively in target tissues, such as tumours. This targeted accumulation enhances treatment precision and reduces side effects commonly associated with more widespread drug distribution, maximizing the therapeutic potential while minimizing systemic exposure and toxicity. To target cancer cells, nanoparticles must overcome multiple biological barriers, including cell membranes and the mitochondrial membrane itself. Since mitochondrial dysfunction varies between diseases, these systems can be fine-tuned for specific conditions, such as selectively killing cancer cells, rejuvenating mitochondria in degenerative diseases or delivering mitochondrial genes in mitochondrial diseases therapies and improving mitochondrial function post ischemic stroke.38–40
In the case of mitochondria-targeting nanosystems, these particles are engineered to find and interact specifically with this organelle, delivering their therapeutic “payload” precisely where it's needed. Mitochondria-targeting nanosystems are carefully designed to recognize and enter mitochondria themselves.41 This is often mediated by mitochondria-penetrating peptides, which naturally seek out and bind to mitochondria or other targeting molecules that “guide” the nanoparticles to their destination. Once inside, the nanosystems can deliver drugs, antioxidants, or genetic materials in order to: (i) restore mitochondrial function by repairing damaged mitochondria, or (ii) induce cell death for example in cancer cells by disrupting their mitochondrial energy production, or (iii) to reduce oxidative stress by delivering antioxidants that neutralize harmful ROS inside the mitochondria.42 In cancer, drug nano-delivery systems can deliver chemotherapy drugs to the mitochondria of tumour cells where can trigger cancer cell death more effectively.43–45 The versatility of nanosystems allows loading them with different types of therapeutics, from small molecules to large proteins or even gene-editing tools. Regulatory hurdles and a long development timeline are significant challenges. Other targeted cancer therapies, like antibody–drug conjugates or CAR-T cell therapies, have recently seen significant success and investment, potentially drawing resources away from nanoparticle approaches. Manufacturing challenges include producing consistent, high-quality nanoparticles at scale for clinical use, which can be technically challenging and expensive. Designing nanodelivery systems with a long enough shelf-life once loaded with the therapeutic agent also represents a major challenge. Developing novel nanoparticle therapies requires significant financial investment, and concerns about long-term safety data may have led to cautious backing until more robust preclinical data is available. Some mitochondria-targeting approaches may be more effective for specific cancer types, narrowing the focus and potentially slowing broader clinical development.46 Combination therapies may also be explored, which can take longer to develop and move into clinical trials. While still in the early stages of development, mitochondria-targeting nanosystems hold enormous potential.47 They offer a new way to treat a wide range of diseases where mitochondria play a critical role.
2.3 Design strategies for mitochondria targeting nanosystems
Research in nanomedicine has predominantly centred on the creation of novel nanoparticle (NP) systems and the exploration of their physicochemical characteristics in connection to their biological behaviour and applications, especially in the fields of cancer diagnosis and treatment.The integration of nanotechnology with pharmacology and physiology has driven the development of advanced drug delivery systems that harness the interplay between these disciplines. A significant advancement in this field is the emergence of “smart nanoparticles (NPs)”, which represent a promising alternative to conventional nanoparticles for cancer therapy. The nanocarriers structure gives a range of tuneable physico-chemical properties, e.g. very high surface areas, specific surface charge, increased mechanical strength, and unique morphology.110 Nanoparticles can be used to directly bind with a target to induce the therapeutic effect,111 or act as a carrier for other therapeutic agents which can physically or chemically interact with the target.114,115 A range of natural nanomaterials, as blood constituents117 and viral capsids116 can be used as well as synthetic NPs (Scheme 1A). Both can be surface-functionalized to achieve targeted delivery or protection of the therapeutic agent from external physiological conditions as low pH environment,118 or improve their encapsulation efficiency and prevent undesired targeting or inactivation.119 Stimuli responsive NPs can also allow encapsulated therapeutic agents to be released when specific conditions are present, including saline-sensitivity, thermo-sensitivity, or light-sensitively.120 The use of nanotechnology can lead to increase the efficacy of antibiotics which may to extend their functional lifespan and to counter antimicrobial resistance. Smart NPs can also be engineered to respond to specific stimuli, such as changes in pH, oxidative stress, or other physiological triggers, enabling precise drug delivery to targeted sites.48–51 Upon modification or activation by these factors, smart NPs can efficiently accumulate at the desired location and release their therapeutic payloads, creating a highly controlled and effective treatment approach.10,52 These innovative systems offer the unique ability to co-deliver therapeutic agents and diagnostic reagents, significantly advancing the field of theranostics. This dual functionality not only enhances the precision of cancer therapy but also addresses challenges such as multidrug resistance and off-target effects. By a combination of targeted drug delivery with diagnostic know-hows, smart nanoparticles are revolutionizing cancer treatment, maximizing therapeutic efficacy while minimizing side effects.
Nanoparticles targeting mitochondria can enter cells through different mechanisms, primarily (i) endocytosis or (ii) direct membrane fusion. The pathway chosen depends on the nanoparticle's size, surface charge, and functionalization. Most nanoparticles enter cells via clathrin- or caveolin-mediated endocytosis, macropinocytosis, or lipid raft-mediated uptake and once internalized, they are trafficked through endosomes and lysosomes, which may degrade some nanoparticles before they can even reach mitochondria. To escape endosomal entrapment and removal, nanoparticles can be designed with pH-sensitive or fusogenic peptides to facilitate endosomal escape. Direct membrane fusion entry of nanoparticles applies to cationic liposomes, dendrimers, and other positively charged nanoparticles, that can interact with negatively charged cell membranes, leading to direct fusion and cytoplasmic release. This method bypasses lysosomal degradation, which allows more efficient mitochondrial targeting (see Scheme 1A and Table 1). Mitochondria can be effectively targeted by nanoformulations through the strategic design of nanoparticles that exploit the unique characteristics of mitochondria. Mitochondria-Targeting Nanosystems (MTNS) have two general modes of action which are (i) passive and (ii) active targeting.53 Passive targeting relies on the physicochemical properties of nanoparticles, such as size, shape, and charge, lipophilicity to enhance their accumulation in mitochondria.54 The examples are liposomes, polymersomes and polymeric micelles. These nanocarriers use the natural cellular uptake mechanisms to deliver drugs to mitochondria without specific targeting ligands.55 Specifically, cationic nanoparticles exploit the negative membrane potential of mitochondria, allowing them to bind to and penetrate the mitochondrial membrane.56,57 Passive targeting can be further upgraded to enhance permeability and retention. The active targeting involves the functionalization58 of NPs with specific mitochondrial-targeting moieties, such as lipophilic cations, peptides, or other ligands that selectively bind to receptors or molecular structures on the mitochondrial membrane (Table 2).
| Uptake mechanism | Nanoparticle types | Pros | Cons | Mitochondrial targeting strategies |
|---|---|---|---|---|
| Endocytosis (clathrin/caveolae-mediated) | Liposomes, polymeric NPs, metallic NPs | Efficient internalization | May lead to lysosomal degradation | Surface modifications (e.g., MTS, fusogenic peptides) for escape |
| Macropinocytosis | Larger polymeric NPs, dendrimers | Allows uptake of large particles | Nonspecific, potential for degradation | Fusogenic peptides to escape vesicles |
| Direct membrane fusion | Lipophilic NPs, TPP + -modified micelles | Bypasses endosomes/lysosomes | Requires optimization of NP surface properties | Lipophilic cations (TPP+, rhodamine) for direct mitochondrial localization |
| Lipid raft-mediated uptake | Gold NPs, lipid-based NPs | Avoids lysosomal degradation | Limited by raft composition | Functionalization with hydrophobic ligands for raft interaction |
| Ligand type | Mechanism of mitochondrial targeting | Advantages | Disadvantages |
|---|---|---|---|
| Triphenylphosphonium (TPP)+ | Exploits negative mitochondrial membrane potential (ΔΨm) | High mitochondrial specificity, widely used | Can cause mitochondrial toxicity in high doses |
| Mitochondria-penetrating peptides (MPPs) | Amphiphilic peptides that cross membranes via direct insertion | High efficiency in bypassing cellular and mitochondrial membranes | Peptide stability can be an issue |
| Mitochondrial translocation sequences (MTS) | Recognized by mitochondrial import machinery (TOM/TIM complexes) | Specific delivery to mitochondrial matrix | Limited by the need for cellular uptake first |
| Dequalinium (DQA) | Cationic ligand that localizes to mitochondria due to electrostatic interactions | Effective in targeting mitochondria in cancer cells | Can accumulate in lysosomes |
| Lipophilic cations (e.g., rhodamine B, rhodamine 123) | Accumulate in mitochondria due to membrane potential | Simple and effective | Can induce oxidative stress |
| mitochondria-targeted small molecules (e.g., MitoQ, SkQ1) | Antioxidant-based targeting to mitochondrial ROS sites | Combines drug and targeting function | Limited clinical translation so far |
One of the active strategies to facilitate targeting mitochondria is the functionalization with peptides known as mitochondrial translocation signals (MTS) derived from mitochondrial precursor proteins. They can guide nanoparticles to mitochondria after cytosolic release. Once in the cytoplasm, MTS-functionalized nanoparticles are recognized by the translocase of the outer/inner mitochondrial membrane (TOM/TIM) complexes, enabling mitochondrial import. Other enhancing strategies involve the use of targeting ligands such as triphenylphosphonium (TPP)59 and mitochondria-penetrating peptides (MPPs)60 which leverage the high negative membrane potential of mitochondria to facilitate selective uptake. These ligands are often attached to the surface of nanoparticles, allowing them to specifically accumulate in mitochondria once inside the cell (Scheme 1B–E). A notable advancement in mitochondrial targeting involves the development of modified PAMAM dendrimers. Researchers engineered these dendrimers by conjugating them with varying amounts of triphenylphosphonium (TPP), resulting in gene delivery systems designated as G5-TPP12, G5-TPP23, G5-TPP35, and G5-TPP99. These modified dendrimers demonstrated enhanced transfection efficiency compared to both unmodified versions and commercial reagents. Among them, G5-TPP23 and G5-TPP35 exhibited low cytotoxicity while achieving transfection levels comparable to Lipofectamine 2000. Confocal microscopy confirmed effective mitochondrial targeting of G5-TPP/DNA polyplexes, indicating improved intracellular trafficking and endosomal escape (Fig. 2).61 These strategies are frequently integrated with other therapeutic approaches, such as photothermal therapy (PTT) and photodynamic therapy (PDT), to enhance their precision and efficacy.62 This synergistic approach not only improves the specificity of the therapy but also amplifies its therapeutic impact. By concentrating the effects of PTT and PDT within mitochondria researchers can achieve more efficient tumour cell destruction while sparing healthy tissues.
Another option is so called bio-responsive targeting. For both primary and secondary mitochondrial diseases, trait specific (such as cardiolipin oxidation or impaired mitochondrial DNA repair) formulations can be designed to release therapeutic agents in response to intracellular signals/stimuli that are present in the dysfunctional mitochondrial environment, such as: ROS,63,67 pH changes,64 redox imbalances (glutathione (GSH),65,66 hypoxia68 and adenosine-5′-triphosphate (ATP)).69 Such tumour cell responsive release of anticancer drugs enhance therapeutic efficacies. For example, they can induce ROS upon exposure to specific stimuli, such as light or heat. The production of ROS further induces oxidative stress within the targeted cells, leading to cellular damage and apoptosis, which is particularly effective in cancer treatment. Furthermore, the ability to precisely control the ROS generation through external stimuli, such as laser irradiation, allows for spatiotemporal regulation of the treatment, minimizing off-target effects and enhancing overall therapeutic outcomes. ROS-responsive linkers, for example, can undergo cleavage in the presence of high ROS levels, which are often elevated in dysfunctional mitochondria associated with conditions like cancer,70 neurodegenerative diseases,71 and cardiovascular disorders.72 By incorporating these stimuli or bio-responsive nanoformulations, controlled, on-demand release of drugs can be achieved and currently, a wide net of materials and strategies in designing nanosystems are employed.73 Tan et al.50 designed pH-sensitive micelles (CTPP-CSOSA-Cela) that release drugs in the alkaline environment of cancer cell mitochondria. These micelles achieved over 80% tumour inhibition rate, outperforming standard Celastrol (Cela) treatment. They released the drug faster at pH 8 than at pH 5 or 7, preventing premature leakage and ensuring targeted delivery to mitochondria. This process also boosted ROS production, triggering oxidative stress and cytochrome c release, which led to apoptosis. Overall, the micelles improved drug effectiveness against cancer cells.50 This innovative combination of active targeting and mitochondrial localization represents a significant advancement in the development of next-generation cancer therapies as a Smart Nanocarriers platform. It offers high flexibility based on materials’ tunability, relative biocompatibility, and the possibility of controlled release in a few practical settings. At the same time, the current understanding of the internal nature of the mitochondria, and the changes observed in mitochondrial dysfunction because of pathological changes74 opens several possibilities for mitochondria targeting nanosystems.
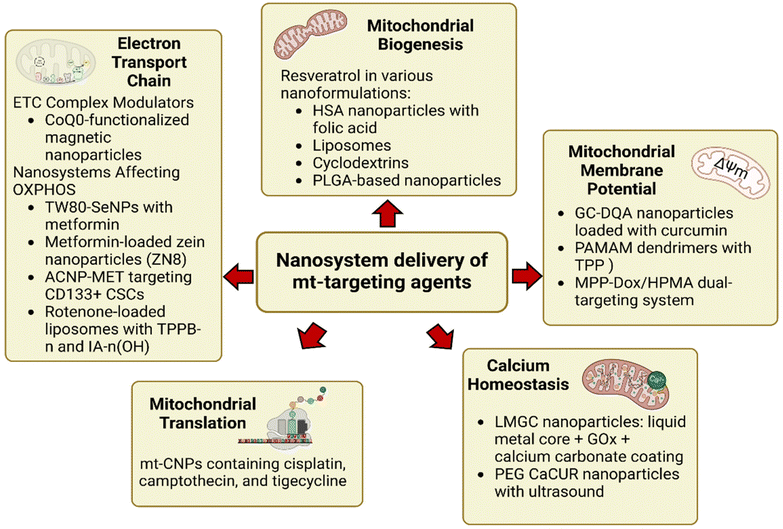
2.4 Targeting specific elements of mitochondria using therapeutic agents delivered via nanosystems
Several approaches have been developed to target mitochondria in cancer cells, aiming to disrupt their function and ultimately kill the cancer cells. Fig. 1 show typical pathways impacted by specific mitochondria targeting agents delivered via nanoparticle systems, which involve (i) electron transport chain, (ii) mitochondrial biogenesis, (iii) membrane potential, (iv) calcium homeostasis, and (v) translation. Specific nanodelivery systems for mitochondrial drugs presented in Fig. 1 are discussed further in the review. Some therapies work by deliberately causing damage to mitochondria, thereby triggering cell death pathways. For example, drugs that increase ROS production within mitochondria can cause oxidative stress, leading to mitochondrial dysfunction and cell death.75 While ROS are often detrimental to cells, cancer cells develop ways to manage excessive oxidative stress. Targeting mitochondrial antioxidants can disrupt this balance, either by overwhelming the cell with ROS or by preventing cancer cells from neutralizing the ROS they generate, leading to cell death e.g. vitamin C. Since cancer cells often rely heavily on mitochondrial energy production, they can be more vulnerable to such strategies than normal cells. Cancer cells usually alter their metabolism by switching between glycolysis and OXPHOS, depending on their energy needs and environmental conditions.13 Drugs like metformin, traditionally used in diabetes treatment, have been found to inhibit mitochondrial respiration, selectively affecting cancer cells that rely on OXPHOS for survival.76 Moreover, the significant electrical potential difference across the mitochondrial membrane offers a pathway to selectively deliver therapeutic agents to cancer cells. Positively charged drugs, such as some chemotherapeutic agents, can accumulate in mitochondria due to this membrane potential, leading to enhanced drug delivery and increased toxicity to cancer cells. As mitochondrial fission and fusion are critical for cancer cell survival, targeting the proteins involved in these processes—such as DRP1 (a key regulator of fission)—can disrupt mitochondrial homeostasis and push cancer cells toward cell death. | ||
| Fig. 2 Mitochondria-targeting nanoparticles modulating oxidative stress. (A) Schematic representation of the design and mechanism of cytochrome c–coated carbonized single-walled carbon nanotubes (cyt C@cSWCNT).82 (B) ROS-scavenging capability of cyt C@cSWCNT.82 Reproduced from ref. 82 with permission; copyright 2022 John Wiley and Sons. (C) Illustration of hollow Prussian blue nanoparticles applied for combined photodynamic and photothermal tumour therapy, accompanied by fluorescence microscopy images showing elevated ROS levels.62 (D) Concept of three-mode ROS burst generation and assessment of the GMCP construct for ˙OH production under different H2O2 concentrations.83 Reprinted with permission from ref. 62 and 83; copyright 2024 Elsevier. | ||
Therapies that inhibit fission, for example, could limit the flexibility cancer cells have to cope with treatment-induced stress, making them more vulnerable to therapy.77,78
As cancer cells evolve to resist conventional therapies like chemotherapy, they often modify mitochondrial functions to withstand treatment. Cancer cells often develop resistance to treatment through several mechanisms that allow them to evade the effects of chemotherapy, targeted therapy, immunotherapy, and even novel treatments like ferroptosis inducers. These mechanisms are diverse and often involve genetic, epigenetic, metabolic, and environmental changes within the tumour microenvironment. One of these is an avoidance of apoptosis. Cancer cells can inhibit apoptosis by upregulating the anti-apoptotic proteins such as Bcl-2 or Bcl-xL which can prevent the release of cytochrome c from mitochondria, blocking the activation of caspases and apoptosis. This can induce downregulation of pro-apoptotic proteins such as Bax and Bak, which are crucial for mitochondrial outer membrane permeabilization (MOMP), can further make cancer cells resistant to apoptosis.79 One of the most compelling reasons to focus on mitochondria in cancer therapy is their role in drug resistance. For example, mitochondrial fission has been linked to resistance to chemotherapy drugs like cisplatin in ovarian cancer. By targeting mitochondrial dynamics, it is possible to counteract these resistance mechanisms and make cancer cells more susceptible to this treatment. The interest in targeting mitochondria to overcome drug resistance in cancer treatment has grown significantly, as researchers have recognized their critical role across various cancer types. As tumours develop, mitochondria help maintain cellular balance and enable cells to manage the stress caused by treatments. For instance, in ovarian cancer, mitochondrial fission provides cisplatin-resistant cells with an edge under low-oxygen conditions compared to non-resistant ones. These adaptations in mitochondrial function directly influence metabolism, ultimately contributing to drug resistance.80,81 Given their dynamic nature and complexity, it's becoming increasingly important to explore new strategies and treatments aimed at targeting mitochondria in cancer therapy. Moreover, mitochondrial metabolism is often altered in drug-resistant cells, which may switch to OXPHOS or glycolysis depending on the availability of nutrients and oxygen. Inhibiting these metabolic pathways can disrupt the cells’ energy supply and sensitize them to other forms of therapy, such as radiotherapy or immunotherapy.
2.5 ROS scavengers
Shukla et al.82 investigated the application of single-walled carbon nanotubes (SWCNTs) conjugated with cytochrome c (Cyt C) as a nano-catalytic medicine designed to specifically target mitochondria and alleviate oxidative stress. The SWCNTs were functionalized with carboxyl groups (–COOH) through acid treatment, enabling strong electrostatic interactions with the positively charged lysine residues of Cyt C. This non-covalent conjugation preserved the native structure and catalytic activity of Cyt C, enhancing its stability and functionality within the cellular environment. Upon cellular uptake via endocytosis, the Cyt C@cSWCNT complex localized at mitochondria, where it released Cyt C influenced by pH, ionic conditions, and thermal environment.This strategic release allowed the nano-assembly to maintain mitochondrial membrane potential (ΔΨm) and reduce mitochondrial ROS generation. The conjugated SWCNTs facilitated Cyt C's enzymatic activity, mimicking catalase and peroxidase functions in neutralizing ROS. The study demonstrated that the SWCNTs provided a high surface area for Cyt C loading, improved biocompatibility, and protected Cyt C from rapid degradation, addressing a major limitation of Cyt C's short half-life in vivo. The sustained release from SWCNTs supported ongoing ROS scavenging, enhanced mitochondrial function, and offered significant protective effects against cellular damage, highlighting the nanotube's potential as a robust and targeted nanocarrier for mitochondrial therapies (Fig. 2A and B).
2.6 Calcium overload and ROS generation
In another study, manganese-doped hollow mesopores Prussian Blue (MMPB) nanocarriers were employed in a synthesis of the composite GOx@MMPB@CaP-PEG nanoparticles (GMCP), effectively inducing calcium overload in cancer cells while synergizing three modes of reactive oxygen species (ROS) generation.62,83 The calcification of cancer cells occurred as the calcium phosphate (CaP)-coated mineralized layer dissolved in the acidic tumor microenvironment (TME), releasing calcium ions (Ca2+) and resulting in calcium overload. Once the protective CaP shell dissolved, the exposed hollow mesoporous MMPB decomposed into Fe2+/Mn2+, facilitating a Fenton/Fenton-like reaction. Additionally, glutathione (GSH) was depleted by oxidized Fe3+/Mn3+, sustaining the Fenton/Fenton-like reaction through a self-cycling mechanism. MMPB also provided a significant amount of oxygen due to its catalase-like catalytic ability, attributed to its unique organometallic chemical structure. In well-oxygenated conditions, the loaded glucose oxidase (GOx) efficiently converted glucose into hydrogen peroxide (H2O2), which further promoted the Fenton reaction. Concurrently, GOx inhibited the energy supply to tumour cells, inducing cell starvation. Collectively, these mechanisms formed a three-mode ROS generation strategy. The inherent photothermal properties of MMPB, activated by absorption of an 808 nm near-infrared (NIR) laser, not only enabled localized warming but also enhanced the efficiency of the catalytic reactions (Fig. 2C and D).2.7 Mitochondrial membrane potential modulators
Mitochondria-targeting organic nanoparticles (DPP2 + NPs)84 achieved enhanced photodynamic and photothermal therapy by utilizing a diketopyrrolopyrrole-based photosensitizer modified with two imidazole groups, which specifically directed the nanoparticles to mitochondria.This structural modification significantly increased cellular uptake and mitochondrial localization, where DPP2+ NPs produced thermal energy and singlet oxygen under 635 nm laser irradiation, reaching a photothermal conversion efficiency of 35%. In vitro tests showed that DPP2+ NPs induced a 90% inhibition of cervical carcinoma cells (HeLa) at a 5 μM concentration and 0.3 W cm−2 laser intensity. The nanoparticles triggered mitochondrial membrane depolarization, as evidenced by JC-1 staining, leading to apoptosis. Confocal microscopy confirmed their effective colocalization with mitochondrial markers, demonstrating their precise targeting capability. In vivo experiments involved the encapsulation of DPP2+ NPs in F127 to form DPP2+@F127, enhancing their solubility and stability in the bloodstream. Upon intravenous or intra-tumoral administration in tumour-bearing mice, the nanoparticles raised the temperature of the tumour site by up to 15 °C under laser exposure, leading to significant tumour regression. Histological analysis showed minimal side effects on major organs, highlighting the nanoparticles’ biocompatibility. This approach effectively maximizes the phototherapeutic effects of photothermal and photodynamic therapies by leveraging the specific targeting of mitochondria within cancer cells. (Fig. 3A)84 An amphiphilic polymer system composed of glycol chitosan (GC) and dequalinium (DQA) allowed the successful formation of self-assembled nanoparticles for mitochondria-targeted drug delivery. In comparison to the free drug, curcumin-loaded GC-DQA nanoparticles demonstrated increased cytotoxicity in cancer cells, showing effective cellular uptake and mitochondrial accumulation. The selection of DQA as the mitochondria-targeting moiety was particularly significant due to both a mitochondria-targeting capability and facilitated nanoparticle self-assembly in aqueous solutions because of its lipophilic component. This approach effectively overcomes previous challenges in mitochondrial targeting using lipophilic cations, as they had low stability at low temperatures and high salt concentrations (Fig. 3B).85
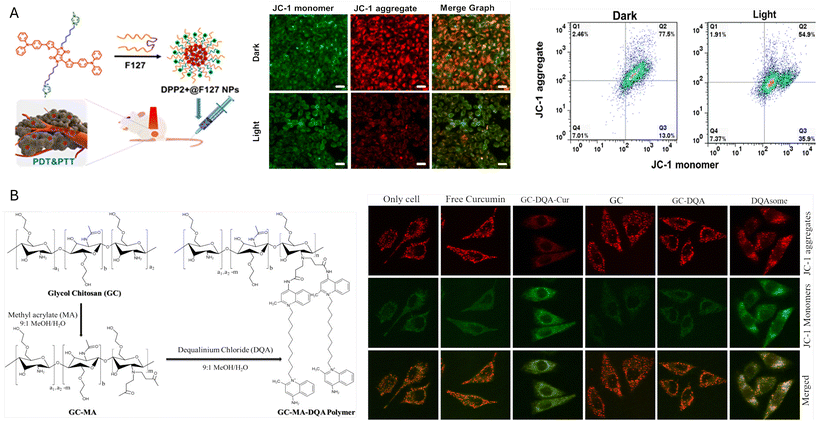 | ||
| Fig. 3 Mitochondrial membrane potential modulators. (A) Preparation of DPP2+@F127 NPs and mitochondrial membrane depolarization.84 Reprinted with permission from ref. 84. Copyright 2020 American Chemical Society. (B) Stepwise synthesis procedure of GC-DQA polymer and the effect of curcumin loaded GC-DQA on MMP.85 Reprinted with permission from ref. 85; copyright 2019 Elsevier. | ||
2.8 Nanosystems affecting electron transport chain (ETC) complex and OXPHOS
Several modulators of the ETC have been explored as anticancer agents due to their ability to disrupt mitochondrial energy production, induce oxidative stress, or modulate metabolic dependencies in cancer cells. Some have been delivered as nanoformulations to improve their therapeutic efficacy, stability, and targeted delivery.Metformin is a well-known drug for diabetes type 2 and complex I inhibitor of ETC. Recently, functionalized selenium nanoparticles (SeNPs) loaded with metformin were developed using different surface modifiers. Functionalization of SeNPs with Tween 80 (TW80) showed most effective synergistic action against breast cancer cells.
By initially depleting antioxidant selenoproteins in cancer it elevates the oxidative stress, further causing DNA damage and modulating other therapeutic pathways via combination of cell cycle arrest the in the S phase through the upregulation of key proteins, including p-ATM, p-ATR, and p38, while simultaneously activating the AMPK signalling pathway (Fig. 4A).91 Another group loaded metformin to zein nanoparticles (ZNs) and demonstrated improved antitumor activity against Ehrlich carcinoma. Their optimized formulation (ZN8) showed stability over three months while providing high entrapment efficiency and small particle size. Improved anticancer effects were observed In vivo when compared to conventional metformin treatment through multiple molecular mechanisms such as upregulating tumour suppressor gene P53 and miRNA-543, while downregulating NF-κB and miRNA-191-5p.92 Metformin was also tested in the activated carbon nanoparticles combined with ACNP-MET, to target hepatocellular cancer stem cells (CSCs). This system proved efficient in the inhibition of proliferation and self-renewal capacity of CD133+ CSC isolated from the Huh-7 hepatocellular cancer cell line. This ACNP-MET system is promising in targeting the CSC (CD133+) population rather than common cancer cells (CD133-). This suggests a potential for preventing tumour relapse and metastasis (Fig. 4B).93 A mitochondrial poison Rotenone, another complex I ETC inhibitor was also loaded into liposome formulated nanoparticles targeting mitochondria. This surfactant-modified liposomes with a delocalized charge composed of soy phosphatidylcholine (PC), cholesterol (Chol), and cationic surfactants incorporating triphenylphosphonium (TPPB-n) and imidazolium (IA-n(OH)) head groups were loaded with Rotenone. Further liposome cationization significantly enhanced their cellular uptake and mitochondrial targeting capabilities. Compared to free rotenone treatment, this NPs were effective in targeting pancreatic carcinoma (PANC-1) and duodenal adenocarcinoma (HuTu 80) cells with significantly increased cytotoxicity (Fig. 4C).94
 | ||
| Fig. 4 Nanosystems affecting OXPHOS: Metformin and Rotenone (A) anti-invasion effects of Metformin loaded TW-SeNPs treatment.91 (B) The in vivo inhibitory impact of ACNP-MET on the self-renewal ability of hepatocellular cancer stem cells.93 Reprinted with permission from ref. 93. Copyright 2020 Dovepress 2023. (C) (a–c) Cytotoxic effect of ROT-loaded cationic mitochondria-targeted liposomes compared to free ROT.94 | ||
Wang et al.151 used 3D human hepatic tumour cell culture model to assess in vitro the efficacy of “active” metformin-loaded nanoparticles (NPs) as anticancer therapeutics. The metformin nanocarrier design was repurposed from previous studies targeting bacterial and fungal biofilms with antimicrobials loaded in protease-coated nanoparticles. This approach has been used before in active nanocarriers of antibiotics to clear bacterial and fungal biofilms.128,129,132 These active nanocarriers were constructed with shellac cores loaded with metformin as anticancer agent and featuring a surface coating of the cationic protease lysozyme (Fig. 5A) which yields oval shaped particles of size under 100 nm and positive zeta-potential (Fig. 5B and C). The lysozyme role as a nanocarrier surface coating is to partially digest the extracellular matrix (ECM) of the 3D tumour cell culture, which increases its porosity and the nanocarrier penetration. Hep-G2 hepatic 3D clusteroids were formed using a water-in-water Pickering emulsion based on aqueous two-phase system (ATPS). The specific metformin nano-formulation, comprising 0.2 wt% lysozyme-coated, 0.2 wt% metformin-loaded 0.2 wt% shellac NPs sterically stabilized by 0.25 wt% Poloxamer 407, demonstrated significantly enhanced anticancer efficiency on 3D hepatic tumour cell clusteroids. The authors examined the role of the lysozyme surface functionality of the metformin nanocarriers on their ability to kill both 2D and 3D hepatic tumour cell cultures (Fig. 5D and E). The anticancer efficiency at high metformin payloads was compared to this at high concentration of nanocarriers at lower metformin payload. It was discovered that the high metformin payload NPs were more efficient than higher nanocarrier concentration at lower metformin payload.
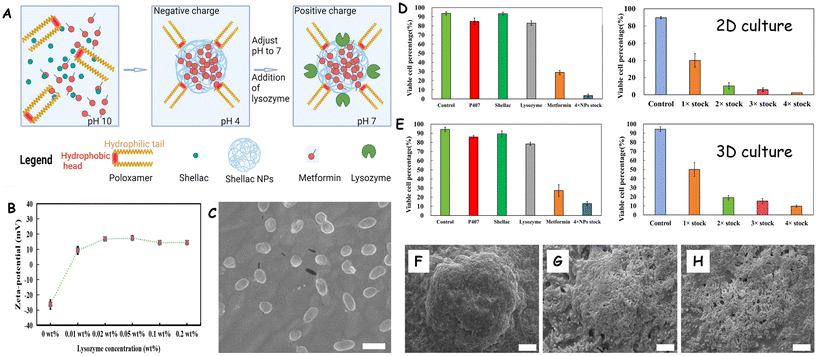 | ||
| Fig. 5 (A) Schematics of preparation of the lysozyme-coated metformin loaded NPs. (B) Zeta-potential versus the lysozyme concentration. (C) SEM image of a Ly-NPs. (D and E) Anti-cancer effect of the lysozyme-coated metformin-loaded stabilized shellac nanoparticles and their individual components and at different concentrations of the treatment corresponding to multiples of the original stock solution (1×) (D) on 2D culture (E) or the 3D cell Hep-G2 clusteroids and (C and D) for 2D Hep-G2 cell culture tested after 48 h. (F–H) SEM images of Hep-G2 clusteroids showing the degradation of the 3D cell culture after (F) 0 h (G) 24 h, (H) 48 h. The scale bar is 50 μm. Reprinted with permission from ref. 151. Copyright 2024 Royal Society of Chemistry. | ||
This study demonstrated the ability of the nanoparticles to degrade the ECM in 3D Hep-G2 cell culture (Fig. 5F–H) and introduces a potentially reliable in vitro model for potential targeting of solid tumours with smart nano-therapeutics, presenting a viable alternative to animal testing for evaluating anticancer nanotechnologies.151
Magnetic nanoparticles (silicated Fe3O4 (Fe3O4-SiO2-Q0)) functionalized with Coenzyme Q0 (CoQ0) displayed increased antiproliferative effects against multiple cancer cell lines.99 CoQ0 is a redox-active ubiquinone that accumulates in mitochondria.95 Previous studies have shown that CoQ0 has many biological effects on cancer cells, such as cell cycle arrest, apoptosis, and anti-tumorigenic activity.96 The proposed mechanism of action involves inhibiting mitochondrial respiratory complex I, preventing mitochondrial permeability transition pore opening, and induction of oxidative stress.97,98 At the same time, these NPs still maintained relatively lower toxicity towards normal fibroblasts, being highly effective in apoptosis induction in breast cancer cells. The sensitivity of different cancer cell lines to the treatment varied, with cervical cancer cells requiring higher concentrations (IC50 ∼ 500 μM) compared to other cancer types. These findings indicate the potential of CoQ0-functionalised magnetic nanoparticles as a promising therapeutic approach for cancer treatment. The main reason for that is their selective cytotoxicity towards cancer cells and their ability to target mitochondrial function99
The drug phenformin, a biguanide antidiabetic drug and more potent complex I inhibitor, can also eliminate cancer stem cells; however, it is considered cytotoxic to normal cells. When loaded into micelles via self-assembly using a mixture of a diblock copolymer of PEG- and urea- functionalized polycarbonate and a diblock copolymer of PEG and acid-functionalized polycarbonate through hydrogen bonding, it was not cytotoxic towards non-cancerous cells. Within 96 h the phenformin loaded micelles released over 90% of the payload. Treatment of the H460 human lung cancer cell line with free phenformin and micelles loaded with phenformin showed higher effectiveness of micelles the free in inhibiting the growth of cancer cells. Moreover, they showed greater efficacy in in vivo human lung cancer mouse model reducing tumour cells population in the tumour tissues without liver damage, a known side-effect of phenformin when used in clinical settings.100 Phenformin was also loaded to graphene-based drug nanocarriers, PEGylated graphene nanosheets (PGNS) and oxidized graphene GO. Using covalent PEGylation, PE-CVD graphene sheets can be modified to overcome its hydrophobicity and provide better colloidal stability in the presence of the metabolic drug phenformin. Furthermore, phenformin adsorption capacity was increased in the PEG-functionalized graphene compared to GO because of the higher pi- and PEG-mediated interaction possibilities. pH-responsive phenformin release was enhanced in PGNS showing that it might be a better carrier than oxidized graphene for phenformin delivery.101
2.9 Drugs targeting mitochondrial translation
Cholesterol-based chimeric nanoparticles that target mitochondria (mt-CNPs) has shown great promise for cancer treatment.86 These nanoparticles were used to incorporate and deliver cisplatin, camptothecin, and tigecycline to target mitochondrial transcriptional and translational machinery. As fast as six hours post administration, Mt-CNPs showed effective mitochondrial localization, along with alterations in mitochondrial morphology and elevated ROS production. The therapeutic efficacy of mt-CNPs was evaluated across multiple cancer cell lines, including lung adenocarcinoma, cervical carcinoma, and breast cancer cell lines, where enhanced cancer cell death was observed compared to free-drug combinations. This advanced therapeutic effect can be due to the simultaneous targeting of multiple mitochondrial functions, suggesting mt-CNPs as a promising platform for targeted cancer therapy.862.10 Drugs enhancing mitochondrial biogenesis
Several drugs and bioactive compounds that promote mitochondrial biogenesis have been successfully incorporated into nanotechnology-based delivery systems, which improve stability, bioavailability, and targeted delivery.Preclinical research has demonstrated that several compounds with mitochondrial-protective properties—such as the antioxidants coenzyme Q10 and glutathione, the mitochondrial permeability transition pore (mPTP) inhibitor cyclosporin A, the SIRT1 activator resveratrol, and the iron-chelating agent deferoxamine—can help prevent the onset and progression of certain diseases like CVD and cancer by preserving mitochondrial structure and function.87 Resveratrol, Coenzyme Q10 (CoQ10), mitochondrial-targeted antioxidants (e.g., MitoQ, SkQ1) and others inhibit cancer initiation and progression via direct or indirect enhancement of mitochondrial biogenesis in tissues such as liver, muscle, and blood vessels. This increase in mitochondrial content may reduce ROS production by distributing electron flow across more mitochondria. Resveratrol, a non-flavonoid polyphenol found in various plants and foods, has gained attention for its anticancer and health-promoting effects. These effects are largely mediated through SIRT1- and nitric oxide–dependent pathways.88 Despite its promising anticancer properties, resveratrol's clinical utility is limited by poor solubility, rapid metabolism, and low bioavailability.89 Various antioxidant nanoformulations-including liposomes, cyclodextrins, and polymeric nanoparticles—have shown enhanced therapeutic efficacy. For example, folic acid-conjugated resveratrol-loaded HSA nanoparticles improve tumour accumulation, while liposomal forms enhance brain cancer cytotoxicity and skin penetration. These systems act via mitochondrial apoptosis, ROS generation, and cell cycle arrest. Notably, mitochondrial-targeted formulations, such as those modified with dequalinium-PEG conjugates, help overcome drug resistance. Most formulations show low toxicity in normal cells, with selective effects in cancer models. Sustained-release systems like PLGA nanoparticles further boost resveratrol's therapeutic potential, underscoring the value of nanocarriers in advancing its application in oncology.90
2.11 One drug, multiple paths: the way different nano-delivery strategies change the game
Doxorubicin (DOX) is an FDA approved chemotherapeutic drug used to treat various cancers, including solid tumours and haematological malignancies. However, its use is limited by severe side effects and the development of drug resistance in the cancer cells. DOX enters cells via passive diffusion and interacts with DNA in multiple ways, leading to DNA damage and disruption of DNA repair. It can induce the production of free radicals and ROS, causing oxidative stress and various cellular damage including DNA damage.102,103 One key mechanism of DOX action involves the quinone structure of DOX, which can be oxidized by mitochondrial complexes (complex I and NADPH-Oxidases) to form a semiquinone radical. This semiquinone radical then reacts with oxygen to produce superoxide anions, which can further generate other ROS like hydrogen peroxide, peroxynitrite, and hydroxyl radicals. The cycling of the quinone–semiquinone forms enables DOX to produce these ROS continuously.102,104 DOX also affects iron metabolism by interacting with Iron Regulatory Proteins (IRPs) and ferritin, leading to the release of free iron. This free iron can then participate in Fenton reactions, generating more ROS and contributing to cell damage and death. The topoisomerase II enzyme involved in DNA replication can also produce ROS upon DOX administration.105,106 Furthermore, DOX disrupts calcium homeostasis by inhibiting calcium-conducting enzymes like the Adenosine Nucleotide Translocase (ANT). This leads to increased mitochondrial permeability, calcium imbalance, and further ROS generation, which can impair muscle function and contribute to cardiotoxicity.103Various cell death pathways are induced by DOX, such as apoptosis, autophagy, senescence, and necrosis. The specific pathways activated depend on cancer type, patient genotype, and the DOX dose. Despite DOX's efficacy, cancer cells can develop resistance utilising number of mechanisms. This can activate signalling pathways such as PI3K/Akt and MAPK/ERK which help cancer cells to overcome cell cycle checkpoints that further promote cell cycle progression, activate replication and prevent apoptosis and autophagic cell death. These pathways are often dysregulated in cancer cells.
Overexpression and upregulation of ATP-binding cassette (ABC) membrane transporters, such as P-glycoprotein (MDR1), multidrug resistance proteins (MRPs), and breast cancer resistance protein (BCRP), can lead to increased efflux of DOX from the cell, reducing its intracellular concentration and efficacy. The MDR1, MRP1, MRP2, MRP3, MRP5, MRP6, and BCRP all contribute to DOX resistance in various cancer types. However, the precise molecular mechanisms by which they transport drugs and how to effectively inhibit their function remain areas of ongoing research. They discovered that the natural product glycyrrhetinic acid (GA) is an effective mitochondria-targeting ligand. They functionalized graphene oxide (GO) with GA (GA-GO) to create a nanomaterial that can specifically deliver DOX to mitochondria. To avoid the problem of chemoresistance to the DOX, Zhang et al.107 have developed a nanomaterial that targets mitochondria. This nanomaterial demonstrated enhanced apoptosis induction and anti-cancer efficacy compared to non-GA-functionalized nanocarriers and free doxorubicin. In vivo studies confirmed the ability of GA-GO@DOX to disrupt the Bax/Bcl-2 balance, activate caspases, and induce mitochondria-mediated apoptosis in tumour tissue. The GA-GO@DOX also showed improved tumour accumulation and lower toxicity, suggesting it could be a useful tool for targeted drug delivery to mitochondria for cancer treatment (Fig. 6A). Buondonno et al.108 utilized chemically modified doxorubicin (mtDox) with a mitochondrial tropism against Dox-sensitive and Dox-resistant osteosarcoma cells. Osteosarcoma is an aggressive bone cancer with a poor prognosis, especially in cases where the cancer becomes resistant to the standard chemotherapy drug doxorubicin. Resistance often arises due to overexpression of the drug efflux transporter P-glycoprotein (Pgp), which pumps Dox out of the cancer cells. The modified mtDox is selectively delivered into the mitochondria due to the conjugation of the anthracycline moiety with a peptide containing cationic and hydrophobic residues that deliver cargoes into mitochondria (Fig. 6B and C). This limits the availability of Dox for the P-glycoprotein (Pgp) on the plasma membrane, reducing the efflux of the drug from tumour cells. Yang et al.109 designed a dual-targeting system combining mitochondria-penetrating peptide-modified doxorubicin (MPP-Dox) loaded N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer conjugates (PM) with nucleus-targeting HPMA copolymer Dox conjugates (PN). This innovative approach (PMN) achieved better efficiency in both; tumour growth suppression and metastasis inhibition by simultaneously targeting nuclear and mitochondrial functions.
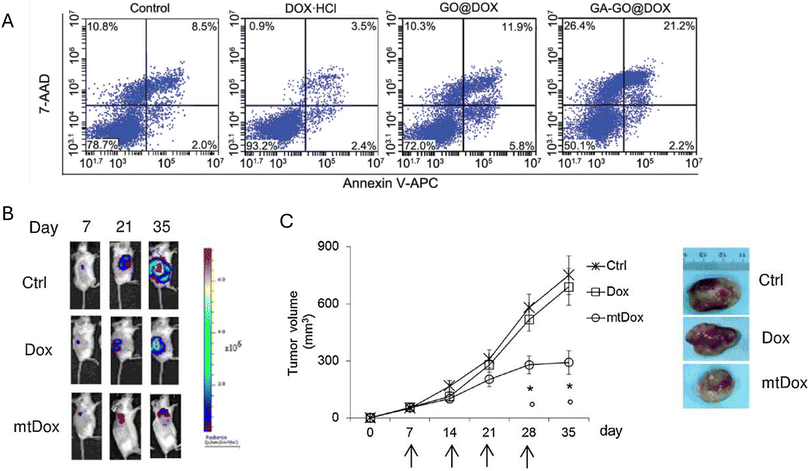 | ||
| Fig. 6 The enhanced effect of nanoparticle packaging of doxorubicin. (A) Cell death analysis of doxorubicin loaded nanoparticles107 Reproduced from ref. 107 with permission; copyright 2017 John Wiley and Sons. (B) and (C) The effect of mitochondria-targeted doxorubicin against drug-resistant osteosarcoma in vivo.108 | ||
Such efficiency of the system was achieved because of the nucleus-targeting PN component's contribution to tumour growth suppression and the mitochondria-targeting PM component played a crucial role in inhibiting tumour metastasis. Both cellular compartments were impaired concurrently, resulting in reduced ATP production via depleting the energy supply required for tumour cell migration as a result. The system increased the reactive oxygen species (ROS) levels, triggering intrinsic apoptosis pathways and suppressing the expression of MMP-9 and other metastasis-related proteins. In vivo studies confirmed that compared to individual treatments, the PMN combination increased efficacy in both tumour growth suppression and reduction of lung metastasis.109
Colorectal cancer (CRC) mutations drive resistance and poor prognosis, underscoring the need for more effective therapies. The oxidative drug therapy combining arsenic trioxide (ATO) and D-vitamin C (D-VC) has demonstrated promising efficacy by targeting mitochondrial functions and depleting antioxidant defences to induce apoptosis in CRC cells. Mun et al.152 evaluated the cytotoxic effects of ATO/D-VC in 2D and 3D cell models, known as clusteroids, generated from CRC cell lines HCT116 and SW620 (see Fig. 7A–C). In 2D, the ATO/D-VC combination significantly reduced cell proliferation to 40–60% and viability to below 30% of control levels. In contrast, clusteroids showed a more limited response, with proliferation reduced to 60–80% and viability to 80–90%, highlighting the impact of extracellular matrix (ECM) and cell–cell interactions in limiting drug diffusion within structured tumour microenvironments (see Fig. 7D). To overcome these diffusion barriers, ATO and D-VC were individually encapsulated in poloxamer-stabilized shellac-based nanoparticles (NPs) functionalized with Savinase, a protease known to degrade ECM components. Savinase-coated ATO/DV-C nanocarriers possessed good colloidal stability and a positive surface charge to enhance electrostatic adhesion with the negatively charged cancer cells confirmed by scanning electron microscopy of clusteroid surfaces. Dual treatment of Savinase-coated ATO- and D-VC-loaded NPs caused pronounced disruption of clusteroid morphology and substantially reduced both viability and proliferation to approximately 30–40% of untreated control levels. Compared to the free drug and uncoated nanoparticle formulations, the savinase-functionalized nanoparticle formulation achieved nearly twice the reduction in viability and proliferation, as well as enhanced cancer cell apoptosis, indicating a marked improvement in therapeutic effect (Fig. 7E, F and H).
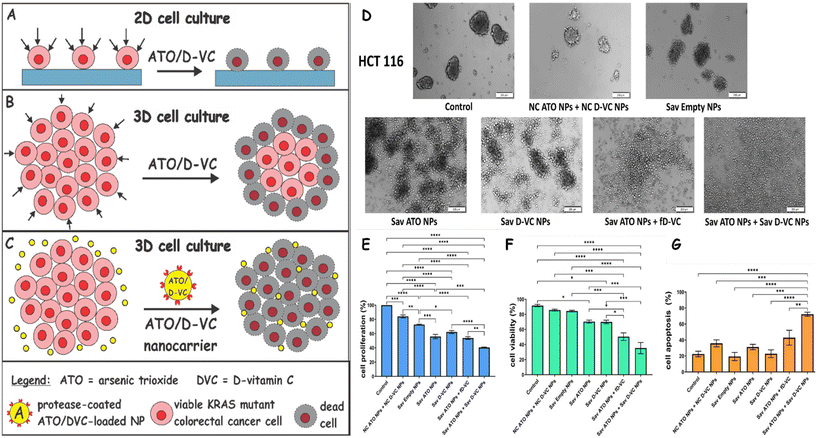 | ||
| Fig. 7 (A)–(C) Both ATO and D-VC independently elevate ROS levels and D-VC plays a significant chemo sensitizing role and their combined use generates an amplified oxidative burst that exceeds the antioxidant capacity of cancer cells, leading to suicidal ROS production by mitochondria followed by irreversible damage and apoptosis. The ATO/D-VC combination exhibits reduced efficacy in 3D CRC culture models (B) compared to its action on 2D cultures (A) due to limited drug penetration through the extracellular matrix (ECM). (C) Principle of action of the Savinase-coated ATO/D-VC nanocarrier on 3D cell clusters and solid tumors for enhanced drug delivery. (D) HCT116 clusteroids following 48 h treatment with ATO (7.5 μM) and D-VC (1.5 mM) delivered in Sav NPs formulations: untreated control, Savinase-coated non-loaded shellac nanoparticles (Sav-NPs), Sav-coated nanoparticles loaded with ATO (Sav-ATO NPs), Savinase-coated nanoparticles loaded with D-VC (Sav-D-VC NPs), Sav-ATO NPs combined with free D-VC, and combined treatment with both Sav-ATO NPs and Sav-D-VC NPs. (E) Quantitative analysis of cell proliferation in clusteroids after treatment, measured by MTS assay and expressed relative to untreated controls. (F) Cell viability represented as the percentage of viable cells for each treatment group. (G) Apoptosis assay performed by flow cytometry using annexin V and PI staining. The percentage of early and late apoptotic cells is shown for each treatment group, revealing increased apoptosis in response to the drug-loaded NP treatments, especially in combination groups. Reprinted with permission from ref. 152. Copyright 2024 Royal Society of Chemistry. | ||
2.12 Mitochondria targeting nanosystems to restore cellular homeostasis in ageing stem cells
A recent study introduced energy metabolism–engaged nanomedicines (EM-eNMs) as an innovative platform that directly targets and remodels mitochondria to restore cellular homeostasis in ageing stem cells.121 These nanoscale therapeutics are inspired by natural nanointerfaces and are designed to regulate mitochondrial function at the molecular level. EM-eNMs are constructed from ultra-small black phosphorus quantum dots (BP QDs) whose surfaces are selectively oxidized via contact-electro-catalysis (Fig. 8A). This controlled surface engineering transforms the QDs into structural analogues of inorganic polyphosphates (polyP), biomolecules deeply integrated into energy metabolism. The self-assembly process yields uniform, biocompatible nanoparticles optimized for mitochondrial uptake. Through this molecular mimicry, mesenchymal stem cells (MSCs) recognize EM-eNMs as endogenous metabolic substrates, enabling preferential trafficking into mitochondria rather than nonspecific sequestration in other organelles. Physicochemical characterization confirmed their nanoscale size, surface charge, and stability, while functional assays demonstrated selective binding to the β-subunit of mitochondrial ATP synthase (ATP5B).121 Once internalized by bone marrow mesenchymal stem cells (BMMSCs), EM-eNMs exert profound effects on mitochondrial structure, dynamics, and bioenergetics (Fig. 8B). By binding ATP5B, the nanoparticles modulate ATP synthase activity and promote mitochondrial fission through up-regulation of dynamin-related protein 1 (DRP1). This inhibition of overactive ATP synthase shifts the balance of mitochondrial morphology toward a more fragmented yet functionally healthier network (Fig. 8C), in contrast to the enlarged and dysfunctional mitochondria typical of aged cells. Importantly, EM-eNMs also stimulate mitophagy, as shown by increased colocalization of mitochondria with lysosomal and autophagy markers (Fig. 8D), thereby enhancing the removal of damaged organelles and maintaining mitochondrial quality. Through these combined actions, EM-eNMs restore mitochondrial quality control and prevent the accumulation of swollen, inefficient mitochondria in aged MSCs. At the functional level, EM-eNMs reprogram cellular metabolism (Fig. 8E). In aged BMMSCs, treatment partially redirected energy flux toward glycolysis (Fig. 8F) while simultaneously normalizing mitochondrial ATP production, reducing oxidative stress, and preventing the metabolic collapse associated with senescence. These metabolic modifications support the restoration of “stemness” properties, including proliferation and multipotency. In vivo, systemic administration of EM-eNMs in mice demonstrated preferential accumulation in bone, where they rejuvenated BMMSCs and alleviated age-related bone loss through mitochondrial remodeling. This work highlights how engineered nanodots can act as molecular surrogates for natural metabolic cofactors, directly engaging mitochondrial proteins and reshaping organelle dynamics. Such rational nanoscale designs, combined with rigorous validation, provide a strong foundation for next generation nanotherapeutics that target mitochondrial dynamics, mitophagy, and energy metabolism to combat ageing and related diseases.121 | ||
| Fig. 8 (A) Schematic illustration of contact-electro-catalytic preparation of EM-eNMs. US, ultrasound. (B) Schematic illustration of the role of EM-eNMs on glycolysis and OXPHOS. (C) Mitochondrial morphological changes observed by a super-resolution microscope. Following EM-eNMs treatment, mitochondria shifted from elongated strips to shorter, round shapes. TEM observation of the mitochondrial morphology following EM-eNMs treatment. TEM images show mitophagy, indicated by arrow (n = 5). (D) Schematic illustration showing the role of EM-eNMs on mitochondria. (E) (LHS) Oxygen consumption rates (OCR) represent mitochondrial oxidative phosphorylation metabolism. FCCP, carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone. (RHS) extracellular acidification rate (ECAR) rates (a biological measurement of cell metabolism) representing shift towards glycolytic metabolism. Rot/AA, rotenone/antimycin A; 2-DG, 2-deoxy-D-glucose. (F) Co-staining with Mito-Tracker (red) and Lyso-Tracker (green) to label mitochondria and lysosomes, respectively. Yellow regions indicate fusion between mitochondria and lysosomes, representing mitophagy. Reprinted with permission from ref. 121; copyright 2025 Springer Nature. | ||
2.13 Restoring mitochondrial functions with nanodelivery systems for treatment of neurodegenerative and cardiovascular diseases
Nanodelivery systems that can restore mitochondrial function are rapidly evolving into translational strategies for both neurodegenerative and cardiovascular disease. They combine targeted organelle accumulation with payloads that either remove pathological reactive oxygen species (ROS), can stimulate mitochondrial quality control (mitophagy/biogenesis) or can directly support the bioenergetic function. Mitochondria-targeted nanozymes and engineered nanocarriers have been shown to accumulate at or within mitochondria and to catalytically scavenge ROS, thereby limiting oxidative damage to mitochondrial membranes, preserving membrane potential and preventing downstream apoptotic signalling; for example, a ceria-based nanozyme platform that was engineered to target mitochondria reduced reperfusion-associated oxidative injury and improved outcomes in ischemic stroke models, demonstrating that organelle-localized catalytic scavenging is an effective route to restore mitochondrial homeostasis after acute ischemic insult.154 Delivery of mitochondria-biogenesis-stimulating cargos has been used to reprogram mitochondrial turnover and restore respiratory competence in degenerating neurons: biomimetic, mitochondria-targeted nanoparticles that promote the NAD+/SIRT1/PGC-1α axis recovered mitochondrial membrane potential and respiratory-chain integrity in dopaminergic neurons and improved behavioural outcomes in Parkinson's disease models, underlining the potential for nanocarriers to deliver signalling cargoes that actively rebuild mitochondrial function rather than merely buffering ROS.155 Beyond small molecules and signalling modulators, engineered “mitochondria-repair” constructs that combine exogenous mitochondrial augmentation with nanoparticle-mediated control of mitophagy can increase the load of functional mitochondria and restore population-level mitochondrial homeostasis. This strategy has been recently illustrated by mitophagy-enhanced, nanoparticle-engineered mitochondria that synchronized delivery and removal of dysfunctional organelles, improving mitochondrial pool quality and tissue function in disease models.156 For cardiovascular indications, single-atom and engineered nanozymes optimized for potent, rapid ROS elimination has demonstrated striking cardioprotective effects. For example, atomically dispersed Pt-anchored nanozymes and related antioxidant nano-architectures were found to reduce infarct size and cardiomyocyte apoptosis after ischemia–reperfusion. They also restore mitochondrial redox balance and support survival signalling in myocardium, providing strong proof-of-concept that catalytic nanodelivery can rapidly recover mitochondrial function in acute cardiac injury.157 These different strategies: (i) catalytic nanozymes, (ii) biogenesis-stimulating biomimetic nanoparticles, and (iii) mitophagy-enhancing mitochondria assemblies are underpinned by the following three engineering principles. (1) Ensuring efficient mitochondrial accumulation by using lipophilic cations such as triphenylphosphonium, peptide targeting motifs, or membrane-coating/biomimetic strategies. (2) Adjusting the release kinetics so that active agents become available at the organelle without provoking overflow toxicity. (3) Designing the particle size, surface charge and hydrophobicity to favour endosomal escape and cytosolic diffusion toward mitochondria. Nanozyme catalytic profile in terms of substrate scope, turnover, and stability, together with targeting specificity and biocompatibility, are the most important translational variables, the potency and organelle selectivity determine whether the mitochondrial function is restored in vivo.158 Acute injuries (stroke, myocardial infarction) may benefit most from fast-acting catalytic nanozymes that rapidly neutralize ROS and stabilize mitochondrial membranes, whereas chronic neurodegenerative disorders (Parkinson's, Alzheimer's) are more likely to respond to repeated or sustained delivery of biogenesis-stimulating or mitophagy-modulating nanocarriers that rebuild the organelle populations and restore respiratory function. Several recent studies also expose common translational bottlenecks as the reproducibility of targeted nanozymes, long-term persistence and clearance of catalytic nanoparticles, as well as the potential interference with physiological ROS signalling. Therefore, standardized in vivo mitochondrial endpoints and head-to-head comparisons with non-targeted formulations are required to prioritize candidates for clinical development. Examples of both cardiovascular and neurodegenerative models now show that properly engineered nanocarriers can be designed to restore mitochondrial function directly, via a catalytic ROS control, organelle population remodelling, or signalling-axis restoration.154–1582.14 Methods for fabrication of nano-delivery systems for mitochondria
Mitochondria-targeted nanomedicine relies fundamentally on preparation methods that encode subcellular routing, because mitochondria possess unique electrochemical polarity, double membrane architecture, and metabolic biomolecule densities. Scheme 1A shows the typical materials used for mitochondria targeting NPs with more details summarized in Table 3. Nanocarriers must be fabricated in such a way that particle assembly, morphology, surface charge anisotropy and external ligand accessibility collectively produce a net electrophoretic and organelle-biased trajectory after endosomal escape, cytosolic diffusion and cytoplasmic viscosity navigation.15,26| Nanocarrier material | Payload/therapeutic intent | Ref numbers |
|---|---|---|
| Glycolipid-like micelles (polymer) | celastrol (mitochondrial alkaline pH–triggered release) | 50 |
| Polymeric micelles/polymer NPs | doxorubicin, nucleic acids, other chemotherapeutics | 48, 61 |
| Ceria nanozymes | redox modulation, reperfusion injury | 40 |
| Hollow Prussian blue nanocarriers | biomacromolecules/ROS engineering | 62 |
| Graphene oxide nanocarriers | glycyrrhetinic acid functionalization for cancer therapy | 107 |
| Activated carbon nanoparticles | metformin metabolic control | 93 |
| chimeric carbon nanocarriers | anticancer payloads (various) | 86 |
| lysozyme-functionalized shellac nanoparticles | metabolic agents (anticancer) | 151 |
| savinase-functionalized nanocarriers | oxidative drugs for solid tumors | 152 |
| mitochondria-targeted liposomes | resveratrol + berberine co-delivery | 149 |
| CRISPR/Cas9 lipid nanoparticles | mitochondrial genome editing | 148 |
| glutathione-responsive nanovehicles | intracellular reductive-triggered payloads | 65 |
| mixed charge nanocarriers | dye payloads/mitochondrial entry switching | 38 |
| PB-derived nanocomposites | ROS outbreak for oncotherapy | 83 |
| “Mito-bomb” nanoplatform | ferroptosis-boosted sonodynamic therapy | 75 |
Preparation methods for the nanocarriers reflect the mitochondria charge density, amphiphilicity, hydrophobic depth, local chain mobility, proton scattering behavior and oxidative responsivity.
Polymer-based nanocarriers are generally formed by nanoprecipitation, emulsion–solvent evaporation, solvent displacement or aqueous self-assembly.10 For example, glycolipid-like micelles that release celastrol selectively at mitochondrial alkaline pH are fabricated by solution self-assembly under mild aqueous conditions to preserve pharmacophore integrity (Fig. 9A and B).50 Dendrimers can be prepared and loaded with nucleic acids through electrostatic complexation under controlled ionic strength, forming mitochondria-addressed gene-delivery nanocarriers when functional groups are selected appropriately (Fig. 9C and D).61 Polymeric micelles loaded with doxorubicin or other chemotherapeutics are typically nano-precipitated from solvent systems tuned to generate narrow hydrodynamic size distributions, and preparation steps define ligand availability and electrostatic geometry at the particle exterior.48 Glutathione-responsive polymeric nano-vehicles incorporate disulfide motifs that are stable extracellularly but reductively cleave in cytosol, bringing the payload into proximity with mitochondria.65 The sensitivities of the polymer assembly to solvents, ionic strength, relative amphiphile ratios and mixing kinetics directly define the obtained mitochondrial directionality. Lipid-based nanocarriers are fabricated using different strategies. Liposomes for mitochondrial co-delivery of resveratrol and berberine are usually formed by thin-film hydration and membrane extrusion.149 The thin-film technique gives lamellar vesicles, and extrusion yields narrow size distributions suitable for in vivo reliability. Microfluidic fabrication of lipid nanoparticles can be used for mitochondria-directed CRISPR/Cas9 assemblies.148 Microfluidic mixing produces kinetically trapped lipid–nucleic acid complexes where the packing density determines whether mitochondrial genome editing reaches stoichiometric effectiveness. In lipid-based nano-systems, assembly must consider the formation of protein corona, membrane fusion probability, cardiolipin affinity and outer-membrane contact frequency, which are also preparation-specific. Inorganic nanocarriers are typically prepared by (i) controlled precipitation, (ii) hydrothermal growth, (iii) surface ligand exchange and (iv) sol–gel-like templating. Preparation methods for Ceria NPs preserves Ce3+/Ce4+ switch-ability which allow them to act as redox-switched mitochondria-targeted enzyme (Fig. 9E and F).40 Prussian blue NPs are fabricated through co-precipitation and templated hollowing approaches, allowing internal loading of biomacromolecules.62 Organic nanostructures designed for intrinsic photophysical targeting must be prepared with solvent control and surfactant-free assemblies that preserve the photodynamic and photothermal signatures required for mitochondrial localization.84 Selenium NPs have been prepared via aqueous reducing conditions and can be surface-functionalized to synergize with metabolic therapeutics like metformin.91 Each of these preparation routes defines the NP surface oxidation state, its ability to generate/quench ROS, and its navigation to mitochondria. Carbon-based nanostructures are formed via oxidative exfoliation, graphitic defect engineering, carbonization tuning and sequential functionalization.
 | ||
| Fig. 9 (A) TEM images of mitochondria targeted conjugates CTPP-CSOSA produced by (4-Carboxybutyl) triphenylphosphonium bromide (CTPP), a lipophilic cation, conjugated with glucolipid-like conjugates (CSOSA). (B) Co-localization of the CTPP-CSOSA micelles within mitochondrial compartments (the yellow spots) in MCF-7 cells after incubation.50 Reproduced with permission from ref. 50, copyright 2018 Elsevier. (C) Synthetic route of TPP-functionalized G5 PAMAM dendrimers used for gene delivery. (D) CLSM images of HeLa cells incubated with G5-TPP35/YOYO-1 labelled DNA polyplexes.61 Reproduced with permission from ref. 61, copyright 2014 Royal Society of Chemistry. (E) Scheme of the synthetic process of TPP@(CeO2 + ROF). (F) High-resolution TEM images of the CeNZs.40 Reproduced with permission from ref. 40; copyright 2024 American Chemical Society. (G) Schematic illustration of acid-activated self-assembly of Prussian blue nanoparticles bearing polyoxometalate quantum dots.62 Reproduced with permission from ref. 62, copyright 2024 Elsevier. (H) mitochondria-targeting cholesterol-based chimeric nanoparticles (mt-CNPs) consisting of cisplatin, camptothecin, and tigecycline.86 Copyright 2022 Royal Society of Chemistry. | ||
Graphene oxide (GO) NPs functionalized with glycyrrhetinic acid are prepared from graphite oxidation followed by surface grafting.107 Activated carbon NPs are prepared precursor pyrolysis or hydrothermal approaches, which yield mesopores capable of loading metabolic therapeutics.93 Chimeric carbon nanostructures created by blending carbon building blocks and engineering new emergent surface functionality can enable mitochondrial targeting.86Protein-based nanocarriers require preparation strategies that preserve the native tertiary or quaternary structure of the biomacromolecular moiety. Lysozyme-functionalized shellac NPs have been formed under conditions that retain enzymatic activity while enabling drug loading and mitochondrial access.151 Savinase-functionalized NPs similarly rely on pH induced aqueous co-precipitation of shellac and oxidative drugs followed by surface coating with the enzyme, avoiding its denaturation.152 The crucial detail here is that preparation must preserve the biological activity that participates in mitochondrial docking, membrane destabilization, or metabolic catalysis. Stimuli-responsive NPs are prepared by embedding chemical trigger into their nanostructure. Glutathione cleavage handles, ROS-sensitive groups, photodynamic monomer units, proton-driven linker chemistries and calcium-loading regimes have been incorporated into the NPs design.57,65,67,83 “Mito-bomb” sonodynamic NPs for ferroptosis boosting have been prepared via sonosensitizer-loading methodology that intentionally encodes ferroptotic acceleration.75
The preparation method directly determines whether the nano-assembly executes mitochondrial ferroptosis amplification. Hence it is not a neutral step, but it is mapping the nanomaterial structure to the organelle addressability. The solvent composition, water/organic mixing rate, amphiphile proportion, surfactant concentration, pH, ionic strength, or rate of nanoprecipitation, determine not only the NPs size, but also in which other organelles inside the cell the nanocarrier prefers to accumulate. Mixed-charge NPs, for example, demonstrate that mitochondrial targeting can be achieved without any explicit mitochondrial ligand.38 This is also why the emerging “precision nanotechnology” implies that material synthesis and pharmacology cannot be separated, material identity, corona identity, metabolic compatibility and organelle tropism arise from the same synthetic step.10,51 This is also why preparation now intersects directly with regulation. Regulatory analyses for nano-enabled medical products explicitly state that nano-preparation determines degradation products, impurity profile, immunogenic signatures, accumulation risk, metabolic disassembly and biological interaction profile.42,55,63 Therefore, the methods used to prepare mitochondria-targeted nano-delivery systems are not merely manufacturing logistics. They determine whether nano-objects can cross cytoplasm, escape endosomes, penetrate mitochondrial membranes, avoid mitophagic clearance, activate mitochondrial signaling nodes, bias ROS flux, bias iron homeostasis, commit cells to apoptosis, or bias cells toward survival.64,73,143,144
2.15 Toxicity of mitochondria-targeting nanosystems
Ensuring the safety and biocompatibility of mitochondria-targeting nanosystems is essential for their successful clinical translation, requiring a critical examination of both immediate and long-term interactions with complex biological systems. The nanocarrier composition is a critical factor which makes biocompatible materials like lipids, PLGA, and PEG to be preferred for their biodegradability and stealth properties. However, even these materials can elicit unexpected immune responses or exhibit batch-to-batch variability in purity that influences toxicity.122,123 In addition, the nanoparticles, size, shape, and surface charge, are also intrinsically linked to their safety profile. Smaller nanoparticles (<10 nm) may exhibit enhanced tissue penetration but can raise significant concerns regarding renal accumulation and potential filtration issues, while larger sizes can lead to different clearance pathways and organ burden.124 The particle surface charge is particularly critical as although cationic surfaces enhance cellular uptake and mitochondrial targeting via electrostatic interactions with the negatively charged mitochondrial membrane, they are also strongly correlated with membrane disruption, lysosomal permeabilization, and the induction of oxidative stress, posing a clear trade-off between efficacy and cytotoxicity.123,125,127–133 A critical analysis toxicity extends beyond these initial interactions to long-term fate, as the potential for nanoparticle accumulation in off-target organs and the incomplete understanding of their degradation products and subsequent inflammatory responses remain major hurdles.126 Moreover, the very mechanism of mitochondrial targeting can be a double-edged sword. Although designed to disrupt mitochondrial function for therapy, these nanosystems can inadvertently cause severe bioenergetic failure and trigger apoptosis in healthy cells if their activity is not precisely controlled, a risk highlighted in critical reviews of the field.112 This underscores the necessity for advanced in vitro models, such as 3D organoids and micro-physiological systems, which can provide more predictive toxicity profiles than conventional 2D cultures by better mimicking tissue complexity and biological barriers.113 Hence, a holistic safety assessment must balance optimized nanocarrier physicochemical parameters for targeting with a thorough investigation of subcellular and systemic biocompatibility to advance these promising nanosystems toward clinical use.2.16 Biodegrability of mitochondria-targeting nanosystems
Nanocarrier biodegradability and clearance pathways are crucial for minimizing toxicity. Used nanomaterials should ideally be biodegradable, breaking down into non-toxic components that the body can easily eliminate. Non-biodegradable materials can accumulate and cause long-term toxicity. While conventional biodegradable polymers like PLGA are effective for cytoplasmic delivery, their degradation kinetics may be too slow within the mitochondrial compartment, potentially leading to undesirable organelle accumulation and impaired function. To address this, advanced strategies are employing mitochondriotropic linkers, such as thioketal or arylazide groups, that are specifically cleaved by the elevated ROS or the unique proteolytic environment within mitochondria, ensuring triggered payload release and subsequent nanocarrier breakdown.129 The emerging use of endogenous biomimetic materials, like heme or cardiolipin, allows for the construction of nanosystems that are not only inherently targeted but are also seamlessly processed by the organelle's own enzymatic pathways, minimizing the risk of residual debris.130 A critical analysis of the field emphasizes that true mitochondrial biocompatibility requires quantitative tracking of degradation fragments and their efflux from the organelle, moving beyond simple proof-of-concept studies to ensure that these sophisticated systems do not inadvertently contribute to mitochondrial toxicity through their breakdown products.131 Understanding how nanoparticles are cleared from the body (e.g., renal or hepatic clearance) helps in designing safer nanoparticles, as rapid clearance can reduce potential toxicity.135,136Evaluating toxicity involves both in vitro and in vivo studies.137,138In vitro studies include cytotoxicity assays to assess the effects of nanoparticles on various cell lines, measuring oxidative stress by examining the generation of ROS and related oxidative damage in cells,139 and assessing mitochondrial function by examining mitochondrial membrane potential, ATP production, and other indicators of mitochondrial health.140In vivo studies involve evaluating acute and chronic toxicity in animal models to observe any adverse effects over short and long durations, studying organ distribution and accumulation to understand how nanoparticles distribute across organs and their potential to accumulate in tissues, which could lead to toxicity, and assessing immunogenicity to determine whether nanoparticles provoke an immune response that could lead to inflammation or other immune-related issues.141 Histopathological analysis using histological techniques to examine tissues for signs of damage, inflammation, or other pathological changes caused by nanoparticles is also important.142 A comprehensive safety profile is essential and can be built by combining data from in vitro, in vivo, and computational studies. Adhering to regulatory guidelines and standards set by agencies such as the USFDA or EMA, which require thorough safety testing and validation, is crucial for ensuring the safety and efficacy of nanoparticles.143,144
2.17 Controlled release of mitochondria-targeting nanosystems
Developing controlled release systems for mitochondria-targeting nanosystems is crucial for maximizing therapeutic effects and minimizing potential side effects.145 Controlled release refers to the ability of a delivery system to release its therapeutic payload in a predetermined, sustained, and site-specific manner. The importance of controlled release lies in several key areas. First, it maximizes therapeutic efficacy by releasing the drug directly within the mitochondria, allowing the therapeutic agent to exert its effects precisely where it is needed. For example, anti-cancer drugs can be more effective when they directly target the mitochondria of cancer cells, inducing apoptosis more efficiently. Second, controlled release minimizes side effects by reducing the exposure of non-target cells and tissues to the therapeutic agent, which is particularly important for potent drugs that can cause significant damage if released in non-target areas. Third, a sustained release profile ensures that the drug is released over an extended period, maintaining therapeutic levels within the mitochondria and reducing the need for frequent dosing, which improves patient compliance and treatment outcomes.145Several mechanisms can be employed to achieve controlled release in mitochondria-targeting nanosystems. One approach is stimuli-responsive release, where the release of the drug is triggered by specific conditions found within the mitochondria. For example, pH-sensitive materials can be used to release the drug in response to the acidic environment of the mitochondria, or redox-sensitive materials can respond to the high levels of ROS present in dysfunctional mitochondria.50,134,150 These stimuli-responsive systems ensure that the drug is released only when it reaches the target site, enhancing specificity and reducing off-target effects. Another approach is the use of biodegradable polymers, which gradually degrade within the mitochondria, releasing the drug over time. Polymers such as PLGA (poly(lactic-co-glycolic acid)) are commonly used for this purpose, as they are biocompatible and can be engineered to degrade at specific rates. The degradation rate can be tailored to match the desired release profile, ensuring a sustained and controlled release of the therapeutic agent.146,147
Lipid-based nanocarriers, such as liposomes, and some micelles, can also be engineered for controlled release. These carriers can be designed to fuse with mitochondrial membranes, releasing their payload in a controlled manner. Additionally, the use of lipid-based carriers can enhance the stability of the encapsulated drug, protecting it from degradation before it reaches the target site.148,149 Controlled release can also be achieved by using of targeting ligands that enhance the specificity of the delivery system. Ligands such as triphenylphosphonium (TPP) cations or mitochondria-penetrating peptides (MPPs) can be conjugated to the surface of nanoparticles, directing them to the mitochondria and ensuring that the drug is released near the target organelle.150 This targeted approach reduces the likelihood of off-target effects and enhances the therapeutic efficacy of the drug. Nanocarrier systems of controlled release that actively target mitochondria are an efficient route for enhancing cancer therapies by delivering cytotoxic or adjuvant agents directly to the cell's powerhouse and releasing them in response to local stimuli. For example, Yang et al.164 developed a porphyrinic MOF (PCN-224) platform that uses photo-generated ROS to trigger on-site CO release and sensitize ferroptosis in mitochondria. Another team, led by Qu and coworkers165 used triphenylphosphonium (TPP)-functionalized mesoporous silica nanoparticles to escort doxorubicin preferentially to mitochondria and ensure controlled intracellular drug delivery causing mitochondrial dysfunction.163 Zhang et al.166 demonstrated ROS-responsive polyprodrug nanoreactors (DT-PNs) for mitochondria-specific, self-amplifying drug release: low endogenous mitochondrial ROS triggers initial camptothecin release in mitochondria, which boosts mtROS and drives further drug liberation, producing a sustained ROS burst and enhanced cell death.
2.18 Future perspectives
To further the field of nano therapeutics targeting mitochondria, it is necessary to use advanced high throughput screening methods. Developing new screening algorithms could facilitate testing a wide variety of ligands and nanoparticle designs quickly. Novel bioinformatics tools and computational modelling would allow researchers to design predictive models of the behaviour of nanoparticles in biological systems, supporting the design of more effective targeting strategies.167 Dynamic simulation strategies that combine membrane-specific molecular dynamics (MD) with enhanced-sampling methods are now central to deciphering how ligands approach, insert into, and detach from mitochondrial membranes. Coarse-grained MD with inner membrane lipid compositions, particularly cardiolipin, has been used to quantify binding energetics and membrane ordering and therefore provides a realistic target matrix for probing mitochondria-active ligands.169 Enhanced-sampling MD strategies such as metadynamics or milestoning allow direct access to slow association/dissociation pathways and permit reconstruction of free-energy profiles that cannot be obtained with brute-force MD.170 Metadynamics provides an efficient, smoothly converging scheme that stabilises sampling of rare transitions and is therefore directly suitable for ligand–membrane interaction free-energy estimation.171 Using innovative in vivo models such as 3D clusteroids, organoids and advanced animal models to study the biodistribution and targeting efficiency of nanoparticles in a living system would be desirable.152,168,172 For the clinical utility the work should focus on developing scalable manufacturing processes for nanoparticles that maintain targeting efficiency without compromised quality. Moreover, regulatory approvals and guidelines for rigorous preclinical and clinical testing for nanoparticles should be enforced to ensure that the nanoparticles are safe and effective.1433 Conclusions
In summary, developing controlled release systems for mitochondria-targeting nanosystems involves several strategies to ensure that the therapeutic agent is released in a controlled, sustained, and site-specific manner. By employing stimuli-responsive materials, biodegradable polymers, lipid-based nanocarriers, and targeting ligands, researchers can enhance the specificity, efficacy, and safety of mitochondria-targeting therapies. These advancements hold great promise for improving the treatment of a wide range of diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases.Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
All data regarding this manuscript are already presented in reviewed articles and there is no additional data enclosed in the review.Acknowledgements
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP19677474, Principal Investigator: A. N. B.).References
- M. M. K. Nass and S. Nass, Intramitochondrial Fibers with DNA Characteristics, J. Cell Biol., 1963, 19, 593–611, DOI:10.1083/jcb.19.3.593.
- E. Mileykovskaya and W. Dowhan, Cardiolipin membrane domains in prokaryotes and eukaryotes, Biochim. Biophys. Acta, Bioenerg., 2009, 1788, 2084–2091, DOI:10.1016/j.bbamem.2009.04.003.
- C. Benda, Ueber die spermatogenese der vertebraten und höherer evertebraten, II. Theil: Die histiogenese der spermien, Acta Physiol., 1898, 73, 393–398 Search PubMed.
- L. Ernster and G. Schatz, Mitochondria: a historical review, J. Cell Biol., 1981, 91, 227s–255s, DOI:10.1083/jcb.91.3.227s.
- W. Chen, H. Zhao and Y. Li, Mitochondrial dynamics in health and disease: mechanisms and potential targets, Signal Transduct. Target. Ther., 2023, 8, 333, DOI:10.1038/s41392-023-01547-9.
- W. Kühlbrandt, Structure and function of mitochondrial membrane protein complexes, BMC Biol., 2015, 13, 89, DOI:10.1186/s12915-015-0201-x.
- A. Suomalainen and J. Nunnari, Mitochondria at the crossroads of health and disease, Cell, 2024, 187, 2601–2627 Search PubMed.
- N. Lane and W. Martin, The energetics of genome complexity, Nature, 2010, 467, 929–934, DOI:10.1038/nature09486.
- H. Yu, F. Jin, D. Liu, G. Shu, X. Wang, J. Qi, M. Sun, P. Yang, S. Jiang, X. Ying and Y. Du, ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury, Theranostics, 2020, 10, 2342–2357, DOI:10.7150/thno.40395.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Engineering precision nanoparticles for drug delivery, Nat. Rev. Drug Discovery, 2021, 20, 101–124, DOI:10.1038/s41573-020-0090-8.
- C. Schiliro and B. L. Firestein, Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation, Cells, 2021, 10, 1056, DOI:10.3390/cells10051056.
- M. V. Liberti and J. W. Locasale, The Warburg Effect: How Does it Benefit Cancer Cells?, Trends Biochem. Sci., 2016, 41, 211–218, DOI:10.1016/j.tibs.2015.12.001.
- O. Warburg, The Metabolism of Carcinoma Cells, J. Cancer Res., 1925, 9, 148–163, DOI:10.1158/jcr.1925.148.
- O. Warburg, On the Origin of Cancer Cells, Science, 1956, 123, 309–314, DOI:10.1126/science.123.3191.309.
- C. Ganji, V. Muppala, M. Khan, G. P. Nagaraju and B. Farran, Mitochondrial-targeted nanoparticles: Delivery and therapeutic agents in cancer, Drug Discov. Today, 2023, 28, 103469, DOI:10.1016/j.drudis.2022.103469.
- D. J. Hanson, S. Nakamura, R. Amachi, M. Hiasa, A. Oda, D. Tsuji, K. Itoh, T. Harada, K. Horikawa, J. Teramachi, H. Miki, T. Matsumoto and M. Abe, Effective impairment of myeloma cells and their progenitors by blockade of monocarboxylate transportation, Oncotarget, 2015, 6, 33568–33586, DOI:10.18632/oncotarget.5598.
- M. A. Houston, L. H. Augenlicht and B. G. Heerdt, Stable differences in intrinsic mitochondrial membrane potential of tumor cell subpopulations reflect phenotypic heterogeneity, Int. J. Cell Biol., 2011, 2011, 978583, DOI:10.1155/2011/978583.
- M. D. Forrest, Why cancer cells have a more hyperpolarised mitochondrial membrane potential and emergent prospects for therapy, Biorxiv, 2015, 1–42, DOI:10.1101/025197 , preprint.
- V. R. Fantin, J. St-Pierre and P. Leder, Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance, Cancer Cell, 2006, 9, 425–434, DOI:10.1016/j.ccr.2006.04.023.
- S. Bonnet, S. L. Archer, J. Allalunis-Turner, A. Haromy, C. Beaulieu, R. Thompson, C. T. Lee, G. D. Lopaschuk, L. Puttagunta, S. Bonnet, G. Harry, K. Hashimoto, C. J. Porter, M. A. Andrade, B. Thebaud and E. D. Michelakis, A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth, Cancer Cell, 2007, 11, 37–51, DOI:10.1016/j.ccr.2006.10.020.
- S. S. Sabharwal and P. T. Schumacker, Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel?, Nat. Rev. Cancer, 2014, 14, 709–721, DOI:10.1038/nrc3803.
- B. Niu, K. Liao, Y. Zhou, T. Wen, G. Quan, X. Pan and C. Wu, Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy, Biomaterials, 2021, 277, 121110, DOI:10.1016/j.biomaterials.2021.121110.
- I. Genovese, M. Carinci, L. Modesti, G. Aguiari, P. Pinton and C. Giorgi, Mitochondria: Insights into Crucial Features to Overcome Cancer Chemoresistance, Int. J. Mol. Sci., 2021, 22, 4770, DOI:10.3390/ijms22094770.
- G. Monzio Compagnoni, A. Di Fonzo, S. Corti, G. P. Comi, N. Bresolin and E. Masliah, The Role of Mitochondria in Neurodegenerative Diseases: the Lesson from Alzheimer's Disease and Parkinson's Disease, Mol. Neurobiol., 2020, 57, 2959–2980, DOI:10.1007/s12035-020-01926-1.
- A. S. Manolis, A. A. Manolis, T. A. Manolis, N. E. Apostolaki, E. J. Apostolopoulos, H. Melita and N. Katsiki, Mitochondrial dysfunction in cardiovascular disease: Current status of translational research/clinical and therapeutic implications, Med. Res. Rev., 2021, 41, 275–313, DOI:10.1002/med.21732.
- D. Choudhary, H. Goykar, T. Karanwad, S. Kannaujia, V. Gadekar and M. Misra, An understanding of mitochondria and its role in targeting nanocarriers for diagnosis and treatment of cancer, Asian J. Pharm. Sci., 2021, 16, 397–418, DOI:10.1016/j.ajps.2020.10.002.
- Y. S. Ju, L. B. Alexandrov, M. Gerstung, I. Martincorena, S. Nik-Zainal, M. Ramakrishna, H. R. Davies, E. Papaemmanuil, G. Gundem, A. Shlien, N. Bolli, S. Behjati, P. S. Tarpey, J. Nangalia, C. E. Massie, A. P. Butler, J. W. Teague, G. S. Vassiliou, A. R. Green, M.-Q. Du, A. Unnikrishnan, J. E. Pimanda, B. T. Teh, N. Munshi, M. Greaves, P. Vyas, A. K. El-Naggar, T. Santarius, V. P. Collins, R. Grundy, J. A. Taylor, D. N. Hayes, D. Malkin, ICGC Breast Cancer Group, ICGC Chronic Myeloid Disorders Group, ICGC Prostate Cancer Group, C. S. Foster, A. Y. Warren, H. C. Whitaker, D. Brewer, R. Eeles, C. Cooper, D. Neal, T. Visakorpi, W. B. Isaacs, G. S. Bova, A. M. Flanagan, P. A. Futreal, A. G. Lynch, P. F. Chinnery, U. McDermott, M. R. Stratton and P. J. Campbell, Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer, eLife, 2014, 3, e02935, DOI:10.7554/eLife.02935.
- S. Wu, M. Akhtari and H. Alachkar, Characterization of Mutations in the Mitochondrial Encoded Electron Transport Chain Complexes in Acute Myeloid Leukemia, Sci. Rep., 2018, 8, 13301, DOI:10.1038/s41598-018-31489-0.
- S. N. Dijk, M. Protasoni, M. Elpidorou, A. M. Kroon and J.-W. Taanman, Mitochondria as target to inhibit proliferation and induce apoptosis of cancer cells: the effects of doxycycline and gemcitabine, Sci. Rep., 2020, 10, 4363, DOI:10.1038/s41598-020-61381-9.
- Y. Li, J. Sun, R. Wu, J. Bai, Y. Hou, Y. Zeng, Y. Zhang, X. Wang, Z. Wang and X. Meng, Mitochondrial MPTP: A Novel Target of Ethnomedicine for Stroke Treatment by Apoptosis Inhibition, Front. Pharmacol., 2020, 11, 352, DOI:10.3389/fphar.2020.00352.
- D. Begimbetova, A. Kukanova, F. Fazyl, K. Manekenova, T. Omarov, A. N. Burska, M. Khamijan, A. Gulyayev, B. Yermekbayeva, A. Makishev, T. Saliev, K. Batyrbekov, C. Aitbayev, Z. Spatayev and D. Sarbassov, The Oxidative Drug Combination for Suppressing KRAS G12D Inducible Tumour Growth, BioMed. Res. Int., 2022, 2022, 9426623, DOI:10.1155/2022/9426623.
- A. N. Burska, B. Ilyassova, A. Dildabek, M. Khamijan, D. Begimbetova, F. Molnár and D. D. Sarbassov, Enhancing an Oxidative ‘Trojan Horse’ Action of Vitamin C with Arsenic Trioxide for Effective Suppression of KRAS-Mutant Cancers: A Promising Path at the Bedside, Cells, 2022, 11, 3454, DOI:10.3390/cells11213454.
- X. Qi, N. Qvit, Y.-C. Su and D. Mochly-Rosen, Novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity, J. Cell Sci., 2013, 126, 789–802, DOI:10.1242/jcs.114439.
- W. Qian, J. Wang, V. Roginskaya, L. A. McDermott, R. P. Edwards, D. B. Stolz, F. Llambi, D. R. Green and B. Van Houten, Novel combination of mitochondrial division inhibitor 1 (mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells, Oncotarget, 2014, 5, 4180–4194, DOI:10.18632/oncotarget.1944.
- G. Preta, J. G. Cronin and I. M. Sheldon, Dynasore - not just a dynamin inhibitor, Cell Commun. Signaling, 2015, 13, 24, DOI:10.1186/s12964-015-0102-1.
- G. G. M. D'Souza and V. Weissig, Subcellular targeting: a new frontier for drug-loaded pharmaceutical nanocarriers and the concept of the magic bullet, Expert Opin. Drug Delivery, 2009, 6, 1135–1148, DOI:10.1517/17425240903236101.
- A. Tewabe, A. Abate, M. Tamrie, A. Seyfu and E. A. Siraj, Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives, J. Multidiscip. Healthc., 2021, 14, 1711–1724, DOI:10.2147/JMDH.S313968.
- D. V. Kolygina, M. Siek, M. Borkowska, G. Ahumada, P. Barski, D. Witt, A.-Y. Jee, H. Miao, J. C. Ahumada, S. Granick, K. Kandere-Grzybowska and B. A. Grzybowski, Mixed-Charge Nanocarriers Allow for Selective Targeting of Mitochondria by Otherwise Nonselective Dyes, ACS Nano, 2021, 15, 11470–11490, DOI:10.1021/acsnano.1c01232.
- R. J. Novorolsky, G. D. S. Kasheke, A. Hakim, M. Foldvari, G. G. Dorighello, I. Sekler, V. Vuligonda, M. E. Sanders, R. B. Renden, J. J. Wilson and G. S. Robertson, Preserving and enhancing mitochondrial function after stroke to protect and repair the neurovascular unit: novel opportunities for nanoparticle-based drug delivery, Front. Cell Neurosci., 2023, 17, 1226630, DOI:10.3389/fncel.2023.1226630.
- J. Liao, Y. Li, L. Fan, Y. Sun, Z. Gu, Q.-Q. Xu, Y. Wang, L. Xiong, K. Xiao, Z. Chen, Z. Ma, C. Zhang, T. Wang and Y. Lu, Bioactive Ceria Nanoenzymes Target Mitochondria in Reperfusion Injury to Treat Ischemic Stroke, ACS Nano, 2024, 18, 5510–5529, DOI:10.1021/acsnano.3c10982.
- A. Jhaveri and V. Torchilin, Intracellular delivery of nanocarriers and targeting to subcellular organelles, Expert Opin. Drug Delivery, 2016, 13, 49–70, DOI:10.1517/17425247.2015.1086745.
- Y. Li, X.-M. Li, L.-S. Wei and J.-F. Ye, Advancements in mitochondrial-targeted nanotherapeutics: overcoming biological obstacles and optimizing drug delivery, Front. Immunol., 2024, 15, 1451989, DOI:10.3389/fimmu.2024.1451989.
- M. S. Chan, L. S. Liu, H. M. Leung and P. K. Lo, Cancer-Cell-Specific Mitochondria-Targeted Drug Delivery by Dual-Ligand-Functionalized Nanodiamonds Circumvent Drug Resistance, ACS Appl. Mater. Interfaces, 2017, 9, 11780–11789 CrossRef.
- W.-Q. Li, Z. Wang, S. Hao, H. He, Y. Wan, C. Zhu, L.-P. Sun, G. Cheng and S.-Y. Zheng, Mitochondria-Targeting Polydopamine Nanoparticles To Deliver Doxorubicin for Overcoming Drug Resistance, ACS Appl. Mater. Interfaces, 2017, 9, 16793–16802, DOI:10.1021/acsami.6b15954.
- N. Peng, H. Yu, W. Yu, M. Yang, H. Chen, T. Zou, K. Deng, S. Huang and Y. Liu, Sequential-targeting nanocarriers with pH-controlled charge reversal for enhanced mitochondria-located photodynamic-immunotherapy of cancer, Acta Biomater., 2020, 105, 223–238, DOI:10.1016/j.actbio.2020.01.005.
- A. Ashokan, M. Birnhak, B. Surnar, F. Nguyen, U. Basu, S. Guin and S. Dhar, Cell specific mitochondria targeted metabolic alteration for precision medicine, Nanoscale, 2025, 17, 1260–1269, 10.1039/D4NR01450B.
- T. Hu, Z. Qin, C. Shen, H.-L. Gong and Z.-Y. He, Multifunctional Mitochondria-Targeting Nanosystems for Enhanced Anticancer Efficacy, Front. Bioeng. Biotechnol., 2021, 9, 786621, DOI:10.3389/fbioe.2021.786621.
- X. Li, X. Yang, Z. Lin, D. Wang, D. Mei, B. He, X. Wang, X. Wang, Q. Zhang and W. Gao, A folate modified pH sensitive targeted polymeric micelle alleviated systemic toxicity of doxorubicin (DOX) in multi-drug resistant tumor bearing mice, Eur. J. Pharm. Sci., 2015, 76, 95–101, DOI:10.1016/j.ejps.2015.04.018.
- W. Gao, G. Ye, X. Duan, X. Yang and V. C. Yang, Transferrin receptor-targeted pH-sensitive micellar system for diminution of drug resistance and targetable delivery in multidrug-resistant breast cancer, Int. J. Nanomed., 2017, 12, 1047–1064, DOI:10.2147/IJN.S115215.
- Y. Tan, Y. Zhu, Y. Zhao, L. Wen, T. Meng, X. Liu, X. Yang, S. Dai, H. Yuan and F. Hu, Mitochondrial alkaline pH-responsive drug release mediated by Celastrol loaded glycolipid-like micelles for cancer therapy, Biomaterials, 2018, 154, 169–181, DOI:10.1016/j.biomaterials.2017.07.036.
- R. X. Zhang, J. Li, T. Zhang, M. A. Amini, C. He, B. Lu, T. Ahmed, H. Lip, A. M. Rauth and X. Y. Wu, Importance of integrating nanotechnology with pharmacology and physiology for innovative drug delivery and therapy – an illustration with firsthand examples, Acta Pharmacol. Sin., 2018, 39, 825–844, DOI:10.1038/aps.2018.33.
- J. Liu, Z. Liu, Y. Pang and H. Zhou, The interaction between nanoparticles and immune system: application in the treatment of inflammatory diseases, J. Nanobiotechnol., 2022, 20, 127, DOI:10.1186/s12951-022-01343-7.
- T. A. Tabish and M. R. Hamblin, Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer, Biomater. Biosys., 2021, 3, 100023, DOI:10.1016/j.bbiosy.2021.100023.
- Y. Gao, H. Tong, J. Li, J. Li, D. Huang, J. Shi and B. Xia, Mitochondria-Targeted Nanomedicine for Enhanced Efficacy of Cancer Therapy, Front. Bioeng. Biotechnol., 2021, 9, 720508, DOI:10.3389/fbioe.2021.720508.
- S. Dhanasekaran, D. Venugopal, N. Al-Dayan, V. Ravinayagam and A. A. Mohammed, Emerging insights into mitochondria-specific targeting and drug delivering strategies: Recent milestones and therapeutic implications, Saudi J. Biol. Sci., 2020, 27, 3581–3592, DOI:10.1016/j.sjbs.2020.07.030.
- S. Bhattacharjee, D. Ershov, M. A. Islam, A. M. Kämpfer, K. A. Maslowska, J. van der Gucht, G. M. Alink, A. T. M. Marcelis, H. Zuilhof and I. M. C. M. Rietjens, Role of membrane disturbance and oxidative stress in the mode of action underlying the toxicity of differently charged polystyrene nanoparticles, RSC Adv., 2014, 4, 19321–19330, 10.1039/C3RA46869K.
- Z. Sun, W. Chen, J. Liu, B. Yu, C. Jiang and L. Lu, Mitochondria-Targeting Enhanced Phototherapy by Intrinsic Characteristics Engineered “One-for-All” Nanoparticles, ACS Appl. Mater. Interfaces, 2021, 13, 35568–35578, DOI:10.1021/acsami.1c10850.
- G. Sanità, B. Carrese and A. Lamberti, Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization, Front. Mol. Biosci., 2020, 7, 587012, DOI:10.3389/fmolb.2020.587012.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications, Chem. Rev., 2017, 117, 10043–10120, DOI:10.1021/acs.chemrev.7b00042.
- Q. Li and Y. Huang, Mitochondrial targeted strategies and their application for cancer and other diseases treatment, J. Pharm. Investig., 2020, 50, 271–293, DOI:10.1007/s40005-020-00481-0.
- X. Wang, N. Shao, Q. Zhang and Y. Cheng, Mitochondrial targeting dendrimer allows efficient and safe gene delivery, J. Mater. Chem. B, 2014, 2, 2546–2553, 10.1039/C3TB21348J.
- M. Zhu, P. Wang, B. Chen, L. Shi, R. Long, S. Wang and Y. Liu, Active-oxygenating hollow Prussian blue nanosystems loaded with biomacromolecules for photodynamic/photothermal therapy of cancer and alleviating hypoxic tumors, Mater. Des., 2024, 237, 112618, DOI:10.1016/j.matdes.2023.112618.
- H. Wang, B. Fang, B. Peng, L. Wang, Y. Xue, H. Bai, S. Lu, N. H. Voelcker, L. Li, L. Fu and W. Huang, Recent Advances in Chemical Biology of Mitochondria Targeting, Front. Chem., 2021, 9, 683220, DOI:10.3389/fchem.2021.683220.
- J. Xu, J. Shamul, E. Kwizera and X. He, Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy, Nanomaterials, 2022, 12, 743, DOI:10.3390/nano12050743.
- R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery, J. Controlled Release, 2011, 152, 2–12, DOI:10.1016/j.jconrel.2011.01.030.
- F. Ciccarese, V. Raimondi, E. Sharova, M. Silic-Benussi and V. Ciminale, Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells, Antioxidants, 2020, 9, 211, DOI:10.3390/antiox9030211.
- C. Tapeinos and A. Pandit, Physical, Chemical, and Biological Structures based on ROS–Sensitive Moieties that are Able to Respond to Oxidative Microenvironments, Adv. Mater., 2016, 28, 5553–5585, DOI:10.1002/adma.201505376.
- J. Yu, Y. Zhang, X. Hu, G. Wright and Z. Gu, Hypoxia-Sensitive Materials for Biomedical Applications, Ann. Biomed. Eng., 2016, 44, 1931–1945, DOI:10.1007/s10439-016-1578-6.
- C. Qian, Y. Chen, S. Zhu, J. Yu, L. Zhang, P. Feng, X. Tang, Q. Hu, W. Sun, Y. Lu, X. Xiao, Q.-D. Shen and Z. Gu, ATP-Responsive and Near-Infrared-Emissive Nanocarriers for Anticancer Drug Delivery and Real-Time Imaging, Theranostics, 2016, 6, 1053–1064, DOI:10.7150/thno.14843.
- Y. Luo, J. Ma and W. Lu, The Significance of Mitochondrial Dysfunction in Cancer, Int. J. Mol. Sci., 2020, 21, 5598, DOI:10.3390/ijms21165598.
- J. Johnson, E. Mercado-Ayon, Y. Mercado-Ayon, Y. N. Dong, S. Halawani, L. Ngaba and D. R. Lynch, Mitochondrial dysfunction in the development and progression of neurodegenerative diseases, Arch. Biochem. Biophys., 2021, 702, 108698, DOI:10.1016/j.abb.2020.108698.
- A. V. Poznyak, E. A. Ivanova, I. A. Sobenin, S.-F. Yet and A. N. Orekhov, The Role of Mitochondria in Cardiovascular Diseases, Biology, 2020, 9, 137, DOI:10.3390/biology9060137.
- S. S. Liew, X. Qin, J. Zhou, L. Li, W. Huang and S. Q. Yao, Smart Design of Nanomaterials for Mitochondria–Targeted Nanotherapeutics, Angew. Chem., Int. Ed., 2021, 60, 2232–2256, DOI:10.1002/anie.201915826.
- S. Buchke, M. Sharma, A. Bora, M. Relekar, P. Bhanu and J. Kumar, Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders, Life, 2022, 12, 657, DOI:10.3390/life12050657.
- J. Wang, Z. Zhao, Y. Liu, X. Cao, F. Li, H. Ran, Y. Cao and C. Wu, ‘Mito-Bomb’: a novel mitochondria-targeting nanosystem for ferroptosis-boosted sonodynamic antitumor therapy, Drug Delivery, 2022, 29, 3111–3122, DOI:10.1080/10717544.2022.2126027.
- M. Ko, J. Kim, R. Lazim, J. Y. Lee, J. Y. Kim, V. Gosu, Y. Lee, S. Choi and H. J. Kwon, The anticancer effect of metformin targets VDAC1 via ER-mitochondria interactions-mediated autophagy in HCC, Exp. Mol. Med., 2024, 56, 2714–2725, DOI:10.1038/s12276-024-01357-1.
- M. Peiris-Pagès, G. Bonuccelli, F. Sotgia and M. P. Lisanti, Mitochondrial fission as a driver of stemness in tumor cells: mDIVI1 inhibits mitochondrial function, cell migration and cancer stem cell (CSC) signalling, Oncotarget, 2018, 9, 13254–13275, DOI:10.18632/oncotarget.24285.
- S. Nagdas, J. A. Kashatus, A. Nascimento, S. S. Hussain, R. E. Trainor, S. R. Pollock, S. J. Adair, A. D. Michaels, H. Sesaki, E. B. Stelow, T. W. Bauer and D. F. Kashatus, Drp1 Promotes KRas-Driven Metabolic Changes to Drive Pancreatic Tumor Growth, Cell Rep., 2019, 28, 1845–1859, DOI:10.1016/j.celrep.2019.07.031.
- A. Chota, B. P. George and H. Abrahamse, Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death, Oncotarget, 2021, 12, 1615–1626, DOI:10.18632/oncotarget.28031.
- Y. Han, B. Kim, U. Cho, I. S. Park, S. I. Kim, D. N. Dhanasekaran, B. K. Tsang and Y. S. Song, Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells, Oncogene, 2019, 38, 7089–7105, DOI:10.1038/s41388-019-0949-5.
- L. Xie, F. Shi, Y. Li, W. Li, X. Yu, L. Zhao, M. Zhou, J. Hu, X. Luo, M. Tang, J. Fan, J. Zhou, Q. Gao, W. Wu, X. Zhang, W. Liao, A. M. Bode and Y. Cao, Drp1-dependent remodeling of mitochondrial morphology triggered by EBV-LMP1 increases cisplatin resistance, Signal Transduct. Target. Ther., 2020, 5, 56, DOI:10.1038/s41392-020-0151-9.
- A. K. Shukla, S. M. S. Abidi, C. Sharma, T. C. Saini and A. Acharya, Single-walled carbon nanotube conjugated cytochrome c as exogenous nano catalytic medicine to combat intracellular oxidative stress, Free Radicals Biol. Med., 2022, 193, 238–252, DOI:10.1016/j.freeradbiomed.2022.10.276.
- W. Xu, H. Zhou, B. Hu, X. Liang, Y. Tang, S. Ning, H. Ding, P. Yang and C. Wang, Prussian Blue–Derived Nanocomposite Synergized with Calcium Overload for Three–Mode ROS Outbreak Generation to Enhance Oncotherapy, Adv. Healthc. Mater., 2024, 13, e240059, DOI:10.1002/adhm.202400591.
- C. Li, W. Zhang, S. Liu, X. Hu and Z. Xie, Mitochondria-Targeting Organic Nanoparticles for Enhanced Photodynamic/Photothermal Therapy, ACS Appl. Mater. Interfaces, 2020, 12, 30077–30084, DOI:10.1021/acsami.0c06144.
- S. Mallick, S. J. Song, Y. Bae and J. S. Choi, Self-assembled nanoparticles composed of glycol chitosan-dequalinium for mitochondria-targeted drug delivery, Int. J. Biol. Macromol., 2019, 132, 451–460, DOI:10.1016/j.ijbiomac.2019.03.215.
- A. Bajpai, N. N. Desai, S. Pandey, C. Shukla, B. Datta and S. Basu, Chimeric nanoparticles for targeting mitochondria in cancer cells, Nanoscale Adv., 2022, 4, 1112–1118, 10.1039/d1na00644d.
- X. Long, M. Liu, Y. Nan, Q. Chen, Z. Xiao, Y. Xiang, X. Ying, J. Sun, Q. Huang and K. Ai, Revitalizing Ancient Mitochondria with Nano–Strategies: Mitochondria–Remedying Nanodrugs Concentrate on Disease Control, Adv. Mater., 2024, 36, 2308239, DOI:10.1002/adma.202308239.
- A. Csiszar, N. Labinskyy, J. T. Pinto, P. Ballabh, H. Zhang, G. Losonczy, K. Pearson, R. de Cabo, P. Pacher, C. Zhang and Z. Ungvari, Resveratrol induces mitochondrial biogenesis in endothelial cells, Am. J. Physiol.: Heart Circ. Physiol., 2009, 297, H13–H20, DOI:10.1152/ajpheart.00368.2009.
- I. A. Siddiqui, V. Sanna, N. Ahmad, M. Sechi and H. Mukhtar, Resveratrol nanoformulation for cancer prevention and therapy, Ann. N. Y. Acad. Sci., 2015, 1348, 20–31, DOI:10.1111/nyas.12811.
- J. Sharifi-Rad, C. Quispe, Z. Mukazhanova, E. Knut, A. Turgumbayeva, A. Kipchakbayeva, G. Seitimova, M. F. Mahomoodally, D. Lobine, A. Koay, J. Wang, H. Sheridan, G. Leyva-Gómez, M. L. Del Prado-Audelo, H. Cortes, A. Rescigno, P. Zucca, O. Sytar, M. Imran, C. F. Rodrigues, N. Cruz-Martins, H. Ekiert, M. Kumar, A. F. A. Razis, U. Sunusi, R. M. Kamal and A. Szopa, Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer, Front. Mol. Biosci., 2021, 8, 649395, DOI:10.3389/fmolb.2021.649395.
- Y. Yang, Z. Zhang, Q. Chen, Y. You, X. Li and T. Chen, Functionalized Selenium Nanoparticles Synergizes With Metformin to Treat Breast Cancer Cells Through Regulation of Selenoproteins, Front. Bioeng. Biotechnol., 2021, 9, 758482, DOI:10.3389/fbioe.2021.758482.
- Y. Elmahboub, R. Albash, M. M. William, A. H. Rayan, N. O. Hamed, M. S. Ousman, N. A. Raslan and S. Mosallam, Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study, Molecules, 2024, 29, 1614, DOI:10.3390/molecules29071614.
- L. Sun, H.-J. Yao, J.-C. Li, B.-Q. Zhao, Y.-A. Wang and Y.-G. Zhang, Activated Carbon nanoparticles Loaded with Metformin for Effective Against Hepatocellular Cancer Stem Cells, Int. J. Nanomed., 2023, 18, 2891–2910, DOI:10.2147/IJN.S382519.
- L. Vasileva, G. Gaynanova, D. Kuznetsova, F. Valeeva, A. Lyubina, S. Amerhanova, A. Voloshina, G. Sibgatullina, D. Samigullin, K. Petrov and L. Zakharova, Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases, Molecules, 2023, 28, 7229, DOI:10.3390/molecules28207229.
- G. Lenaz, R. Fato, G. Formiggini and M. L. Genova, The role of Coenzyme Q in mitochondrial electron transport, Mitochondrion, 2007, 7, S8–S33, DOI:10.1016/j.mito.2007.03.009.
- T. J. Somers-Edgar and R. J. Rosengren, Coenzyme Q0 induces apoptosis and modulates the cell cycle in estrogen receptor negative breast cancer cells, Anticancer Drugs, 2009, 20, 33–40, DOI:10.1097/CAD.0b013e328314b5c5.
- M. Turunen, J. Olsson and G. Dallner, Metabolism and function of coenzyme Q, Biochim. Biophys. Acta, Bioenerg., 2004, 1660, 171–199, DOI:10.1016/j.bbamem.2003.11.012.
- F. Devun, L. Walter, J. Belliere, C. Cottet-Rousselle, X. Leverve and E. Fontaine, Ubiquinone analogs: a mitochondrial permeability transition pore-dependent pathway to selective cell death, PLoS One, 2010, 5, e11792, DOI:10.1371/journal.pone.0011792.
- M. A. Amiri, M. R. Aghamaali, H. Parsian and H. Tashakkorian, Coenzyme Q0 immobilized on Magnetic Nanoparticle: Synthesis and Antitumoral Effect on Saos, MCF7 and Hela Cell Lines, Iran J. Pharm. Res., 2020, 19, 394–409, DOI:10.22037/ijpr.2020.112680.13890.
- S. Krishnamurthy, V. W. L. Ng, S. Gao, M.-H. Tan and Y. Y. Yang, Phenformin-loaded polymeric micelles for targeting both cancer cells and cancer stem cells in vitro and in vivo, Biomaterials, 2014, 35, 9177–9186, DOI:10.1016/j.biomaterials.2014.07.018.
- A. Alhourani, J.-L. Førde, L. A. Eichacker, L. Herfindal and H. R. Hagland, Improved pH-Responsive Release of Phenformin from Low-Defect Graphene Compared to Graphene Oxide, ACS Omega, 2021, 6, 24619–24629, DOI:10.1021/acsomega.1c03283.
- A.-M. Meredith and C. R. Dass, Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism, J. Pharm. Pharmacol., 2016, 68, 729–741 Search PubMed.
- I. Micallef and B. Baron, Doxorubicin: An overview of the anti-cancer and chemoresistance mechanisms, Ann. Clin. Toxicol., 2020, 3, 1031, DOI:10.1111/jphp.12539.
- M. Kciuk, A. Gielecińska, S. Mujwar, D. Kołat, Ż Kałuzińska-Kołat, I. Celik and R. Kontek, Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity, Cells, 2023, 12, 659, DOI:10.3390/cells12040659.
- R. Chen, M. Niu, X. Hu and Y. He, Targeting mitochondrial dynamics proteins for the treatment of doxorubicin-induced cardiotoxicity, Front. Mol. Biosci., 2023, 10, 1241225, DOI:10.3389/fmolb.2023.1241225.
- T. Ali, D. Li, T. N. F. Ponnamperumage, A. K. Peterson, J. Pandey, K. Fatima, J. Brzezinski, J. A. R. Jakusz, H. Gao, G. E. Koelsch, D. S. Murugan and X. Peng, Generation of Hydrogen Peroxide in Cancer Cells: Advancing Therapeutic Approaches for Cancer Treatment, Cancers, 2024, 16, 2171, DOI:10.3390/cancers16122171.
- C. Zhang, Z. Liu, Y. Zheng, Y. Geng, C. Han, Y. Shi, H. Sun, C. Zhang, Y. Chen, L. Zhang, Q. Guo, L. Yang, X. Zhou and L. Kong, Glycyrrhetinic Acid Functionalized Graphene Oxide for Mitochondria Targeting and Cancer Treatment In Vivo, Small, 2017, 14, 1703306, DOI:10.1002/smll.201703306.
- I. Buondonno, E. Gazzano, S. R. Jean, V. Audrito, J. Kopecka, M. Fanelli, I. C. Salaroglio, C. Costamagna, I. Roato, E. Mungo, C. M. Hattinger, S. Deaglio, S. O. Kelley, M. Serra and C. Riganti, Mitochondria-Targeted Doxorubicin: A New Therapeutic Strategy against Doxorubicin-Resistant Osteosarcoma, Mol. Cancer Ther., 2016, 15, 2640–2652, DOI:10.1158/1535-7163.MCT-16-0048.
- J. Yang, Q. Li, M. Zhou, X. Li, Y. Huang, N. Yang and Z. Zhou, Concurrent impairment of nucleus and mitochondria for synergistic inhibition of cancer metastasis, Int. J. Pharm., 2021, 608, 121077, DOI:10.1016/j.ijpharm.2021.121077.
- M. A. Gatoo, S. Naseem, M. Y. Arfat, A. M. Dar, K. Qasim and S. Zubair, Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations, Biomed. Res. Int., 2014, 2014, 498420, DOI:10.1155/2014/498420.
- X. Feng, A. Chen, Y. Zhang, J. Wang, L. Shao and L. Wei, Central nervous system toxicity of metallic nanoparticles, Int. J. Nanomed., 2015, 10, 4321–4340, DOI:10.2147/IJN.S78308 , eCollection 2015.
- S. R. Pandya, R. Mehta and M. Mani, “Circumventing challenges in mitochondrial targeting for cancer therapy: engineered nanomaterials, toxicity & unintended organelle stress”, Mater. Adv., 2024, 5, 2437–2460, 10.1039/d3ma00629h.
- S. N. Bhatia and D. E. Ingber, Microfluidic Organs-on-Chip, Nat. Biotechnol., 2014, 32, 760–772, DOI:10.1038/nbt.2989.
- P. J. Weldrick, S. Iveson, M. J. Hardman and V. N. Paunov, Breathing new life into old antibiotics: overcoming antibacterial resistance by antibiotic-loaded nanogel carriers with cationic surface functionality, Nanoscale, 2019, 11, 10472–10485, 10.1039/C8NR10022E.
- P. J. Weldrick, M. J. Hardman and V. N. Paunov, Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers, ACS Appl. Mater. Interfaces, 2019, 11, 43902–43919, DOI:10.1021/acsami.9b16119.
- K. J. Koudelka, A. S. Pitek, M. Manchester and N. F. Steinmetz, Virus-Based Nanoparticles as Versatile Nanomachines, Annu. Rev. Virol., 2015, 2, 379–401, DOI:10.1146/annurev-virology-100114-055141.
- T. T. Niemi, R. Miyashita and M. Yamakage, Colloid solutions: a clinical update, J. Anesth., 2010, 24, 913–925, DOI:10.1007/s00540-010-1034-y.
- R. Mout, D. F. Moyano, S. Rana and V. M. Rotello, Surface functionalization of nanoparticles for nanomedicine, Chem. Soc. Rev., 2012, 41, 2539–2544, 10.1039/C2CS15294K.
- C. G. Da Silva, G. J. Peters, F. Ossendorp and L. J. Cruz, The potential of multi-compound nanoparticles to bypass drug resistance in cancer, Cancer Chemother. Pharmacol., 2017, 80, 881–894, DOI:10.1007/s00280-017-3427-1.
- L. L. Tayo, Stimuli-responsive nanocarriers for intracellular delivery, Biophys. Rev., 2017, 9, 931–940, DOI:10.1007/s12551-017-0341-z.
- L. Chen, Y. Fan, N. Jiang, X. Huang, M. Yu, H. Zhang, Z. Xu, D. He, Y. Wang, C. Ding, X. Wu, C. Li, S. Zhang, H. Liu, X. Shi, F. Zhang, T. Zhang, D. Luo, C. Wang and Y. Liu, An energy metabolism-engaged nanomedicine maintains mitochondrial homeostasis to alleviate cellular ageing, Nat. Nanotechnol., 2025, 20, 1332–1344, DOI:10.1038/s41565-025-01972-7.
- C. V. Rocha, V. Gonçalves, M. C. da Silva, M. Bañobre-López and J. Gallo, PLGA-Based Composites for Various Biomedical Applications, Int. J. Mol. Sci., 2022, 23, 2034, DOI:10.3390/ijms23042034.
- P. Trucillo, Biomaterials for Drug Delivery and Human Applications, Materials, 2024, 17, 456, DOI:10.3390/ma17020456.
- W. Najahi-Missaoui, R. D. Arnold and B. S. Cummings, Safe Nanoparticles: Are We There Yet?, Int. J. Mol. Sci., 2020, 22, 385, DOI:10.3390/ijms22010385.
- A. Gallud, K. Klöditz, J. Ytterberg, N. Östberg, S. Katayama, T. Skoog, V. Gogvadze, Y.-Z. Chen, D. Xue, S. Moya, J. Ruiz, D. Astruc, R. Zubarev, J. Kere and B. Fadeel, Cationic gold nanoparticles elicit mitochondrial dysfunction and autophagy in human monocytic cells, Sci. Rep., 2019, 9, 7668, DOI:10.1038/s41598-019-40579-6.
- N. Feliu, D. Docter, M. Heine, P. del Pino, S. Ashraf, J. Kolosnjaj-Tabi, P. Macchiarini, P. Nielsen, D. Alloyeau, F. Gazeau, R. H. Stauber and W. J. Parak, In vivo degeneration and the fate of inorganic nanoparticles, Chem. Soc. Rev., 2016, 45, 2440–2457, 10.1039/C5CS00699F.
- A. B. Buya, A. Beloqui, P. B. Memvanga and V. Préat, Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery, Pharmaceutics, 2020, 12, 1194, DOI:10.3390/pharmaceutics12121194.
- S. S. M. Al-Obaidy, G. M. Greenway and V. N. Paunov, Dual surface functionalised curcumin-shellac nano-delivery system with enhanced antimicrobial action, J. Mater. Chem. B, 2019, 7, 3119–3133, 10.1039/D5RA03212A.
- A. Wang, P. J. Weldrick, M. J. Hardman and V. N. Paunov, Mater. Chem. Front., 2021, 5, 961–972A RSC; Wang, P. J. Weldrick, L. A. Madden and V. N. Paunov, Biomater. Sci., 2021, 9, 6927, 10.1039/D1BM01035B.
- P. J. Weldrick, A. Wang, A. F. Halbus and V. N. Paunov, Emerging nanotechnologies for targeting antimicrobial resistance, Nanoscale, 2022, 14, 4018–4041, 10.1039/D1NR08157H.
- E. O. Asare, E. A. Mun, E. Marsili and V. N. Paunov, Nanotechnologies for control of pathogenic microbial biofilms, J. Mater. Chem. B, 2022, 10, 5129–5153, 10.1039/d2tb00233g.
- A. Wang, P. J. Weldrick, L. A. Madden and V. N. Paunov, Biofilm-Infected Human Clusteroid Three-Dimensional Coculture Platform to Replace Animal Models in Testing Antimicrobial Nanotechnologies, ACS Appl. Nano Mater., 2021, 13, 22182–22194, DOI:10.1021/acsami.1c02679.
- E. O. Asare, A. Seidakhanova, D. Amangeldinova, E. Marsili, V. N. Paunov and S. Targeting, epidermidis Biofilms by the Tetracycline-Loaded Nanogel Surface Functionalized with Savinase, DNase, and Cellulase, ACS Appl. Nano Mater., 2023, 6, 22792–22806, DOI:10.1021/acsanm.3c05410.
- A. D. Ergin, Nanotechnological Approaches for Mitochondrial Targeting in Neurodegenerative Diseases, Curr. Top. Med. Chem., 2025, 25, 3251–3266, DOI:10.2174/0115680266397447250723073446.
- M. Yu and J. Zheng, Clearance Pathways and Tumor Targeting of Imaging Nanoparticles, ACS Nano, 2015, 9, 6655–6674, DOI:10.1021/acsnano.5b01320.
- G. Agnese, G. Elena, V. Eeda, F. Massimo, B. Stefania, A. Vibhudutta and C. Donato, Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors, Front. Pharmacol., 2021, 12, 601626, DOI:10.3389/fphar.2021.601626.
- D. T. Savage, J. Z. Hilt and T. D. Dziubla, In Vitro Methods for Assessing Nanoparticle Toxicity, Methods Mol. Biol., 2019, 1894, 1–29, DOI:10.1007/978-1-4939-8916-4_1.
- M. G. Tirumala, P. Anchi, S. Raja, M. Rachamalla and C. Godugu, Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways, Front. Pharmacol., 2021, 12, 612659, DOI:10.3389/fphar.2021.612659.
- M. Awashra and P. Młynarz, The toxicity of nanoparticles and their interaction with cells: an in vitro metabolomic perspective, Nanoscale Adv., 2023, 5, 2674–2723, 10.1039/d2na00534d.
- M. Akter, M. T. Sikder, M. M. Rahman, A. K. M. A. Ullah, K. F. B. Hossain, S. Banik, T. Hosokawa, T. Saito and M. Kurasaki, A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives, J. Adv. Res., 2017, 9, 1–16, DOI:10.1016/j.jare.2017.10.008.
- T. M. Gayathri, A. Pratibha, R. Susmitha, R. Mahesh and G. Chandraiah, Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways, Front. Pharmacol., 2021, 12, 612659, DOI:10.3389/fphar.2021.612659.
- A. L. Taka, C. M. Tata, M. J. Klink, X. Y. Mbianda, F. M. Mtunzi and E. B. Naidoo, A Review on Conventional and Advanced Methods for Nanotoxicology Evaluation of Engineered Nanomaterials, Molecules, 2021, 26, 6536, DOI:10.3390/molecules26216536.
- F. D. Rodríguez-Gómez, D. Monferrer, O. Penon and P. Rivera-Gil, Regulatory pathways and guidelines for nanotechnology-enabled health products: a comparative review of EU and US frameworks, Front. Med., 2025, 12, 1544393, DOI:10.3389/fmed.2025.1544393.
- W. Oualikene-Gonin, V. Sautou, E. Ezan, H. Bastos, E. Bellissant, L. Belgodère, P. Maison and J. Ankri, Scientific Advisory Board of the ANSM. Regulatory assessment of nano-enabled health products in public health interest. Position of the scientific advisory board of the French National Agency for the Safety of Medicines and Health Products, Front. Public Health, 2023, 11, 1125577, DOI:10.3389/fpubh.2023.1125577.
- L. Yang, L. Xiao-meng, W. Li-si and Y. Jun-feng, Advancements in mitochondrial-targeted nanotherapeutics: overcoming biological obstacles and optimizing drug delivery, Front. Immunol., 2024, 15, 1451989, DOI:10.3389/fimmu.2024.1451989.
- D. Gol and O. Gok, Development of mitochondria-targeted and quercetin-loaded PLGA nanoparticles for reducing ROS production, J. Drug Delivery Sci. Technol., 2025, 104, 106577, DOI:10.1016/j.jddst.2024.106577.
- Z. Wang, Y. Qin, X. Wang, T. Zhang, Y. Hu, D. Wang, L. Zhang and Y. Zhu, Glutathione Programmed Mitochondria Targeted Delivery of Lonidamine for Effective Against Triple Negative Breast Cancer, Int. J. Nanomed., 2023, 18, 4023–4042, DOI:10.2147/IJN.S413217.
- K. Norota, S. Ishizuka, M. Hirose, Y. Sato, M. Maeki, M. Tokeshi, S. M. Ibrahim, H. Harashima and Y. Yamada, Lipid nanoparticle delivery of the CRISPR/Cas9 system directly into the mitochondria of cells carrying m.7778G > T mutation in MtDNA (mt-Atp8), Sci. Rep., 2025, 15, 18717, DOI:10.1038/s41598-025-03671-8.
- L. Vasileva, G. Gaynanova, D. Kuznetsova, F. Valeeva, A. Lyubina, S. Amerhanova, A. Voloshina, G. Sibgatullina, D. Samigullin, K. Petrov and L. Zakharova, Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases, Molecules, 2023, 28, 7229, DOI:10.3390/molecules28207229.
- S. Buchke, M. Sharma, A. Bora, M. Relekar, P. Bhanu and J. Kumar, Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders, Life, 2022, 12, 657, DOI:10.3390/life12050657.
- A. Wang, L. A. Madden and V. N. Paunov, Enhanced anticancer effect of lysozyme-functionalized metformin-loaded shellac nanoparticles on a 3D cell model: role of the nanoparticle and payload concentrations, Biomater. Sci., 2024, 12, 4735–4746, 10.1039/D4BM00692E.
- A. G. Mun, N. Nurlankyzy, S. Kalmagambetova, D. Sarbassov, A. Baumuratov, V. N. Paunov and A. N. Burska, Savinase-Functionalised Oxidative Drug-Loaded Nanocarriers Enhance the Treatment of Solid Colorectal Tumours in 3D Cell Culture Model, J. Mater. Chem. B, 2025, 13, 14101–14118, 10.1039/D5TB01882J.
- S. Mani, G. Swargiary, S. Tyagi, M. Singh, N. K. Jha and K. K. Singh, Nanotherapeutic approaches to target mitochondria in cancer, Life Sci., 2021, 281, 119773, DOI:10.1016/j.lfs.2021.119773.
- J. Liao, Y. Li, L. Fan, Y. Sun, Z. Gu, Q.-Q. Xu, Y. Wang, L. Xiong, K. Xiao, Z.-S. Chen, Z. Ma, C. Zhang, T. Wang and Y. Lu, Bioactive Ceria Nanoenzymes Target Mitochondria in Reperfusion Injury to Treat Ischemic Stroke, ACS Nano, 2024, 18, 5510–5529, DOI:10.1021/acsnano.3c10982.
- Q. Zheng, H. Liu, H. Zhang, Y. Han, J. Yuan, T. Wang, Y. Gao and Z. Li, Ameliorating Mitochondrial Dysfunction of Neurons by Biomimetic Targeting Nanoparticles Mediated Mitochondrial Biogenesis to Boost the Therapy of Parkinson's Disease, Adv. Sci., 2023, 10, e2300758, DOI:10.1002/advs.202300758.
- Y. Wang, L.-F. Hu, N.-H. Liu, J.-S. Yang, L. Xing, J.-H. Jeong and H.-L. Jiang, Mitophagy-Enhanced Nanoparticle-Engineered Mitochondria Restore Homeostasis of Mitochondrial Pool for Alleviating Pulmonary Fibrosis, ACS Nano, 2024, 18, 32705–32722, DOI:10.1021/acsnano.4c10328.
- T. Ye, C. Chen, D. Wang, C. Huang, Z. Yan, Y. Chen, X. Jin, X. Wang, X. Ding and C. Shen, Protective Effects of Pt-N-C Single-Atom Nanozymes against Myocardial Ischemia–Reperfusion Injury, Nat. Commun., 2024, 15, 1682, DOI:10.1038/s41467-024-45927-3.
- G. Jiang, Q. Xu, J. Xie, Y. You, L. Cai, L. Zhao, X. Tang, H. Yang and Y. Yong, Emerging Nanozymes in Neurological Disorder Therapeutics: Bridging Oxidoreductase Mimicry and Antioxidant Chemistry, Adv. Funct. Mater., 2024, 34, 2405190, DOI:10.1002/adfm.202405190.
- C. Wirth, U. Brandt, C. Hunte and V. Zickermann, Structure and function of mitochondrial complex I, Biochim. Biophys. Acta, Bioenerg., 2016, 1857, 902–914, DOI:10.1016/j.bbabio.2016.02.013.
- N. M. C. Connolly, P. Theurey, V. Adam-Vizi, N. G. Bazan, P. Bernardi, J. P. Bolaños, C. Culmsee, V. L. Dawson, M. Deshmukh, M. R. Duchen, H. Düssmann, G. Fiskum, M. F. Galindo, G. E. Hardingham, J. M. Hardwick, M. B. Jekabsons, E. A. Jonas, J. Jordán, S. A. Lipton, G. Manfredi, M. P. Mattson, B. McLaughlin, A. Methner, A. N. Murphy, M. P. Murphy, D. G. Nicholls, B. M. Polster, T. Pozzan, R. Rizzuto, J. Satrústegui, R. S. Slack, R. A. Swanson, R. H. Swerdlow, Y. Will, Z. Ying, A. Joselin, A. Gioran, C. M. Pinho, O. Watters, M. Salvucci, I. Llorente-Folch, D. S. Park, D. Bano, M. Ankarcrona, P. Pizzo and J. H. M. Prehn, Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases, Cell Death Differ., 2018, 25, 542–572, DOI:10.1038/s41418-017-0020-4.
- Z.-A. Chen, A. Jawhari, L. Fischer, C. Buchen, S. Tahir, T. Kamenski, M. Rasmussen, L. Lariviere, n. Bukowski-Wills, M. Nilges, P. Cramer and J. Rappsilber, Architecture of the RNA polymerase II–TFIIF complex revealed by cross-linking and mass spectrometry, EMBO J., 2010, 29, 717–726, DOI:10.1038/emboj.2009.401.
- A. S. Divakaruni, A. Paradyse, D. A. Ferrick, A. N. Murphy and M. Jastroch, Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data, Methods Enzymol., 2014, 547, 309–354, DOI:10.1016/B978-0-12-801415-8.00016-3.
- M. Y. Berezin and S. Achilefu, Fluorescence Lifetime Measurements and Biological Imaging, Chem. Rev., 2010, 110, 2641–2684, DOI:10.1021/cr900343z.
- F. Yang, W. Yu, Q. Yu, X. Liu, C. Liu, C. Lu, X. Liao, Y. Liu and N. Peng, Mitochondria-Targeted Nanosystem with Reactive Oxygen Species-Controlled Release of CO to Enhance Photodynamic Therapy of PCN-224 by Sensitizing Ferroptosis, Small, 2023, 19, e2206124, DOI:10.1002/smll.202206124.
- Q. Qu, X. Ma and Y. Zhao, Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers, Nanoscale, 2015, 7, 16677–16686, 10.1039/C5NR05139H.
- W. Zhang, X. Hu, Q. Shen and D. Xing, Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy, Nat. Commun., 2019, 10, 1704, DOI:10.1038/s41467-019-09566-3.
- Y. Shamay, J. Shah, M. Işık, A. Mizrachi, J. Leibold, D. F. Tschaharganeh, D. Roxbury, J. Budhathoki-Uprety, K. Nawaly, J. L. Sugarman, E. Baut, M. R. Neiman, M. Dacek, K. S. Ganesh, D. C. Johnson, R. Sridharan, E. L. Chu, V. K. Rajasekhar, S. W. Lowe, J. D. Chodera and D. A. Heller, Quantitative self-assembly prediction yields targeted nanomedicines, Nat. Mater., 2018, 17, 362–368, DOI:10.1038/s41563-017-0007-z.
- A. K. F. Dyab and V. N. Paunov, Fabrication of 3D structured human cell networks using capillary cell suspensions from aqueous two-phase systems, Mater. Chem. Front., 2025, 9, 1882–1895, 10.1039/D5QM00196J.
- C. Arnarez, S. J. Marrink and X. Periole, Molecular mechanism of cardiolipin-mediated assembly of respiratory chain supercomplexes, Chem. Sci., 2016, 7, 324–332, 10.1039/C5SC04664E.
- K. Ahmad, A. Rizzi, R. Capelli and P. Carloni, Enhanced-Sampling Simulations for the Estimation of Ligand Binding Kinetics: Current Status and Perspective, Front. Mol. Biosci., 2022, 9, 899805, DOI:10.3389/fmolb.2022.899805.
- A. Barducci, G. Bussi and M. Parrinello, Well-Tempered Metadynamics: A Smoothly Converging and Tunable Free-Energy Method, Phys. Rev. Lett., 2008, 100, 020603, DOI:10.1103/PhysRevLett.100.020603.
- A. K. F. Dyab, A. N. Burska and V. N. Paunov, Aqueous two-phase emulsion systems in 3D cell culture, Curr. Opin. Colloid Interface Sci., 2025, 78, 101933, DOI:10.1016/j.cocis.2025.101933.
| This journal is © The Royal Society of Chemistry 2026 |





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 citations.
500 citations.