 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
MXene quantum lands: emerging trends and breakthroughs
Mahdi
Hasanzadeh Azar
 *ab,
Fatemeh
Etehadi
cm,
Nima
Mohamadbeigi
d,
Hessam
Shahbazi
*ab,
Fatemeh
Etehadi
cm,
Nima
Mohamadbeigi
d,
Hessam
Shahbazi
 ef,
Sara
Salehi Siouki
g,
Ali
Mirsepah
h,
Mohammad Reza
Rahmani Taji Boyuk
i,
Ahmad
Alem
g,
Amir
Hatamie
j,
Abdolreza
Simchi
*k and
Shayan
Angizi
*l
ef,
Sara
Salehi Siouki
g,
Ali
Mirsepah
h,
Mohammad Reza
Rahmani Taji Boyuk
i,
Ahmad
Alem
g,
Amir
Hatamie
j,
Abdolreza
Simchi
*k and
Shayan
Angizi
*l
aDepartment of Mechanical and Mechatronics Engineering, University of Waterloo, Waterloo, Ontario N2L 3G1, Canada. E-mail: mhasanza@uwaterloo.ca
bWaterloo Institute for Nanotechnology, University of Waterloo, Waterloo, Ontario, Canada
cLeibniz Institute for Composite Materials GmbH (IVW), Erwin-Schrödinger Str. 58, 67663 Kaiserslautern, Germany
dDepartment of Materials Science and Engineering, Imam Khomeini International University (IKIU), Qazvin 3414916818, Iran
eDepartment of Mechanical and Industrial Engineering, University of Illinois Chicago, Chicago, IL 60607, USA
fDepartment of Electrical and Computer Engineering, University of Illinois Chicago, Chicago, IL 60607, USA
gDepartment of Polymer Engineering and Science, Technical University of Leoben, Otto-Glöckel-Strasse 2, A-8700 Leoben, Austria
hDepartment of Physics, Isfahan University of Technology, Isfahan 84156-83111, Iran
iSchool of Metallurgy and Materials Engineering, College of Engineering, University of Tehran, P.O. Box: 11155-4563, Tehran, Iran
jDepartment of Chemistry, Institute for Advanced Studies in Basic Science (IASBS), No. 444, Prof. Yousef Sobouti Boulevard, Zanjan 45137-66731, Iran
kFraunhofer Institute for Manufacturing Technology and Advanced Materials, 28359 Bremen, Germany. E-mail: abdolreza.simchi@ifam.fraunhofer.de
lDepartment of Mechanical and Industrial Engineering, University of Toronto, 5 King's College Road, Toronto, Ontario M5S 3G8, Canada. E-mail: shayan.angizi@utoronto.ca
mInstitute for Automation and Applied Informatics, Karlsruhe Institute of Technology, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 19th December 2025
Abstract
Chemically stable two-dimensional MXene quantum dots (MQDs) have gained significant attention owing to their exceptional optical properties, tunable surface chemistry, and promising biocompatibility. Leveraging these properties, MQDs have found broad applicability across diverse domains, including optoelectronics (LEDs, lasers, detectors, and solar cells), energy storage (batteries and supercapacitors) and energy conversion (CO2 reduction and hydrogen evolution), sensing, and biomedicine. This review provides a comprehensive overview of recent advancements in eco-friendly synthesis and surface modification strategies aimed at enhancing the radiative recombination efficiency of fluorescent MQDs. Furthermore, we critically assess the wide-ranging practical applications of MQDs and evaluate the progress achieved through both experimental and computational approaches. Special emphasis is placed on the most promising avenues for improving their optical performance and integration into high-efficiency devices. Finally, we outline key challenges and offer insights into future research directions. This review bridges fundamental understanding with technological development, reinforcing the transformative potential of MQDs in next-generation applications.
1. Introduction
MXenes, a groundbreaking class of two-dimensional transition metal carbides (TMCs) and nitrides, have gained remarkable attention for their chemical stability, tuneable surface functionalities, and exceptional electrical conductivity.1–3 These materials have been extensively studied and successfully integrated into diverse applications, including energy storage,4–6 catalysis, optoelectronics,7–9 and biomedical devices.8,10 Despite the wealth of research on MXenes, their versatility continues to unveil new opportunities with new areas, such as nanoscale and quantum-sized materials.1,2,11 Therefore, there is yet untapped potential for scientific exploration and technological advancement.Among emerging frontiers, MXene Quantum Dots (MQDs) stand out as promising yet underexplored materials. By downsizing MXenes to quantum scales, MQDs retain the advantageous properties of their parent material, such as structural stability and chemical tunability, while gaining unique features like enhanced photoluminescence (PL), superior dispersibility, and improved biocompatibility.1,8,9,12,13 These characteristics make MQDs well-suited for a wide range of applications, from biosensing and bioimaging to energy and optoelectronic and conversion/storage devices (Fig. 1), with the latter including thousands of studies on MQDs and other nanostructured materials in less than two decades (Fig. S1).14–17 However, their synthesis, properties, and full application potential remain in the early stages of investigation, leaving ample room for breakthroughs in both fundamental science and practical implementations. Although some review papers have covered specific aspects of MQDs,3,18–21 there is a noticeable lack of comprehensive review covering the recent advances in the synthesis, properties, and applications of MQDs.
This review presents a comprehensive and conceptually unified framework that systematically integrates the rapidly expanding body of knowledge on MQDs. It aims to elucidate how the electronic structure and surface chemistry of MQDs govern their functional performance across diverse applications, while identifying key challenges and outlining prospective research opportunities. The discussion is organized into three interrelated domains, synthesis methodologies, structure–property relationships, and emerging applications, to provide a coherent connection between fundamental understanding and technological relevance. This article first introduces the structural diversity and compositional tunability of MXene materials, highlighting their vast potential as a versatile family for next-generation technologies. It then explores various synthesis techniques, including both top-down and bottom-up approaches, to emphasize how synthesis parameters critically influence particle size, surface states, and the resulting physicochemical properties.1 The subsequent sections delve into the optical, electronic, and biocompatible characteristics of MQDs, followed by an in-depth examination of their implementation in key application areas such as sensing,2,5–7,11 optoelectronics,8 energy conversion and storage,22 and biomedicine.10,13,23,24 Finally, through the introduction of a challenges–solutions–outlook framework, this review integrates the discussed aspects into a single analytical perspective, linking nanoscale design strategies to macroscopic device performance. This structured and comparative approach provides a comprehensive and forward-looking overview of the MQDs landscape, offering valuable insights into future research directions and underscoring the transformative potential of MQDs in advanced technologies.
2. Structural features
MAX phases, consisting of transition metal carbides or nitrides, serve as three-dimensional precursors to two-dimensional MXenes. They are represented by the formula Mn+1AXn (n = 1 to 4), where ‘M’ refers to an early transition metal (mostly from groups 3–6 (Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W)), ‘A’ denotes an element from groups 13–15 (such as Al, Ga, In, Si, Ge, Sn, P, As, etc.), and ‘X’ is either carbon or nitrogen.25,26 The distinct bonding structure of MAX phases features relatively weaker and more chemically reactive metallic M–A bonds compared to mixed covalent/ionic M–X bonds. Therefore, the A-layer in the MAX phase is selectively removed by a controlled etching process to form MXenes with the formula of Mn+1XnTx (n = 1 to 4). Here, Tx denotes the type and number of surface terminations (–F, –OH, –Cl, or –O).27,28 Typically, n + 1 layers of transition metal enclose n layers of carbon or nitrogen. When n = 1, the resulting structure, M2X, adopts a hexagonal close-packed structure with the ABABAB stacking sequence. For n = 2 and n = 3, the crystal structures of M3X2 and M4X3 are face-centered cubic (FCC) with the ABCABC stacking arrangement. It is distinct that the properties of MXene are profoundly influenced by its crystal structure and surface terminations.27,28Over the last decade, computational simulations have been instrumental in understanding the MXenes crystal structures and identifying novel, stable compounds, offering critical insights to experimental advancements. These simulations suggest that MXenes can manifest in five distinct structures (Fig. 2a): (i) mono-transition metal: structures like Ti2C, which consist of a single type of transition metal uniformly distributed within the M-layers (first row); (ii) solid solutions, like (Ti,V)3C2, where two different metals are randomly arranged within the M-layers (second row); (iii) ordered double-transition metals, including Mo2TiC2, with a well-organized arrangement of two distinct metals within the M-layers (third row); (iv) ordered divacancy, such as Cr4/3C, featuring systematically arranged two vacancy sites within the M-layers (fourth row); (v) high-entropy, like TiVNbMoC3, an analogous to the high-entropy oxide of similar chemistry with multiple transition metals randomly distributed within the M-layers (fifth row).29,30 Density functional theory (DFT) calculations further reveal that certain ordered MXenes are energetically more stable than their solid-solution counterparts. These findings have led to predictions of over 25 different ordered MXenes, highlighting the structural diversity and stability potential within this material class (third row).21
 | ||
| Fig. 2 (a) MXene structures and compositions: experimentally studied compositions are marked in orange and theoretical compositions in gray. Surface terminations are excluded. The schemes also include phases synthesized through bottom-up methods or phase transformations, such as W2N, V2N, and Mo2N.31,32 (b) Cross-sectional and (c) planar view of the available low-symmetry sites on an M3X2-type MXene surface. The four primary sites include HCP (purple), bridge (red), FCC (green), and atop (orange), with the vertical positioning of the terminating species being virtual and variable depending on the species (reprinted with permission from Elsevier, copyright © 2019).27 | ||
Regarding functional groups, the reactive M-element surfaces are rapidly functionalized by terminating species introduced during the etching process. These surface terminations occur at various symmetry sites, including FCC, HCP, atop positions directly above surface M elements, bridging sites between two M elements (Fig. 2b and c). Experimentally, synthesized MXenes predominantly exhibit –O, –OH, and –F terminations due to their greater thermodynamic stability compared to pristine MXenes.19 However, the hierarchy of functional group stability remains compound-specific and highly dependent on the synthesis method. Theoretical studies suggest that the stability of MXenes increases in the order of –OH < –F < –O for Ti4N3Tx, Ti3C2Tx, and Nb4C3Tx.30 The lower stability of OH terminations is attributed to hydrogen atom replacement or its conversion to –O at elevated temperatures. In practice, however, the stability order is also influenced by the etching method. For instance, in HF-based methods, the stability follows the order of –F > –O > –OH, whereas thermal treatments or HCl–LiF methods result in the order of –O > –F > –OH.27,29 However, most simulations overlook the influence of the etching solution, causing significant discrepancies in predictions regarding the functionalization order.27,29
When the lateral size of MXenes is reduced below 10 nm, quantum confinement effect may come into play, resulting in the formation of MQDs.19 While MQDs retain the essential structural attributes of 2D MXenes, they benefit from abundant, tunable functional groups, enhanced optical properties, and greater dispersibility. As a result, MQDs are exceptionally well-suited for sensing, energy storage, optoelectronics, and biomedical applications. The superior properties of MQDs provide a significant edge over their 2D counterparts, making them a versatile platform for cutting-edge research and technological advancements.20
3. Synthesis of MQDs
The synthesis of MQDs can be broadly categorized into two main strategies: top-down and bottom-up approaches. This section provides a general discussion of these methods. Table 1 presents details on the methods used for synthesizing various types of MQDs.| MQD | Method | Chemicals | Morphology | Size | Mechanism | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| NS | QD | l (nm) | t (nm) | |||||
| Average lateral size (l), thickness (t), 2,3-diaminophenazine (DAP), 2-methylimidazole (MIM), 2,2′-diethanolamine (DETA), 3-aminopropyltriethoxysilane (APTES), bovine serum albumin (BSA), carbon nanotube (CNT), dimethyl sulfoxide (DMSO), dimethylformamide (DMF), glutathione (GSH), methanol quantum dots (MQDs), nanosheet (NS), polyethyleneimine (PEI), poly(vinylpyrrolidone) (PVP), quantum dot (QD), tetramethylammonium hydroxide (TMAOH), tetrapropylammonium hydroxide (TPAOH), uric acid (UA), zeolitic imidazolate frameworks (ZIF).a MXene QDs functionalized with the thiol group. | ||||||||
| Ti2N | Sonication | NSs: Ti2AlN, HCl, KF | (1) 12 h etching of Ti2AlN | (1) 24 h ice bath sonication | 4.83 | 1.81 | — | 19 |
| QDs: NMP, ice bath, PBS, Ti2N, ethanol, soybean phospholipid, chloroform | (2) 2 h sonication at 45 °C | (2) 20 h tip sonication | ||||||
| (3) Centrifugations at 3500 rpm | (3) Centrifugation at 9000 rpm | |||||||
| (4) Decantation | (4) Centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm 000 rpm |
|||||||
| (5) Rinse by PBS or DI water | ||||||||
| (6) 10 min sonication | ||||||||
| (7) Rotary evaporator at 60 °C | ||||||||
| Nb2C | Sonication | NSs: Nb2AlC, HF, ethanol | (1) 2 days etching of Nb2AlC | (1) Stirring in TPAOH | 1–5 | 1.2 | — | 33 |
| QDs: TPAOH | (2) 15 min centrifugation | (2) 10 h pulsed ultra-sonication in ice bath | ||||||
| (3) Wash with ethanol | (3) Filtration | |||||||
| (4) Freeze-drying | ||||||||
| Ti3C2 | Sonication | NSs: Ti3AlC2, HF | (1) 20 h etching of Ti3AlC2 | (1) 24 h stirring | 3.4 | 1.65 | — | 31 |
| QDs: Ti3C2Tx, TMAOH | (2) Centrifugation | (2) Centrifugation | ||||||
| (3) Wash (pH = 5–6) | (3) 20 h sonication | |||||||
| (4) 10 min centrifugation | ||||||||
| Ti3C2OH | Sonication | NSs: Ti3AlC2, HF | (1) Ti3AlC2 + HF | (1) Sonication | 2.4–3.2 | 1.48–1.99 | — | 34 |
| QDs: Ti3C2Tx, NaOH | (2) Washing | (2) Agitation | ||||||
| (3) Freeze-drying | (3) Centrifugation | |||||||
| (4) Dialysis | ||||||||
| (5) Freeze-drying | ||||||||
| Ti3C2Tx | Sonication | NSs: Ti3AlC2, LiF | (1) 48 h at 40 °C in oil bath | (1) Ultrasonication | 10 | — | — | 35 |
| QDs: | (2) Washing | (2) Centrifugation | ||||||
| (3) Vacuum freeze-drying | ||||||||
| Ti3C2Tx | Centrifugation + stirring | NSs: Ti3AlC2, LiF, HCl, Ti3C2(OH)2, Ti3C2F2 | (1) 24 h mixing | (1) 1 h stirring | 2–6 | 5 | — | 36 |
| QDs: — | (2) Washing | (2) 1 h centrifugation | ||||||
| (3) Filter membrane | ||||||||
| (4) Drying | ||||||||
| N-doped Ti3C2 | Solvothermal + sonication | NSs: — | — | (1) 1 h sonication | 3.09 | — | — | 37 |
| QDs: Ti3C2, DMF, ammonium hydroxide | (2) Ammonium hydroxide → pH ∼ 9 | |||||||
| (3) 6 h heating | ||||||||
| (4) Centrifugation | ||||||||
| Ti3C2Tx | Sonication | NSs: Ti3AlC2, Ag wire, Pt wire, [EMIM][PF6], MeCN | (1) Electrochemical cell, V = 3–7 V, 5 h | (1) 10 h ultrasonication | 5.34 | 4 | Strong binding capability of F− with Al → etching | 38 |
| QDs: MeCN | (2) Centrifugation | (2) Centrifugation | ||||||
| Ti3C2 QDs-AuNPs | Sonication | NSs: Ti3AlC2, LiF | (1) 24 h stirring | (1) 10 h sonication | 6.5 | — | — | 39 |
| QDs: — | (2) 3 times centrifugation | (2) Centrifugation | ||||||
| (3) Filtration | ||||||||
| Ti3C2 | Sonication | NSs: Ti3AlC2, HF | (1) 24 h stirring at 45 °C | (1) 24 h stirring | ∼1.75 | 1.2 | — | 40 |
| QDs: Ti3C2 | (2) Centrifugation | (2) Centrifugation | ||||||
| (3) Washing (pH = 6) | (3) Washing | |||||||
| (4) Drying | (4) 10 h sonication | |||||||
| (5) Centrifugation | ||||||||
| (6) Filtration | ||||||||
| Ti3C2/watermelon peel aerogels | Sonication | NSs: Ti3AlC2, LiF, HCl, H2SO4 | (1) 48 h stirring at 30 °C | (1) 10 min purging by N2 | <10 | — | Immersing freeze-drying-dried fresh watermelon peel into QD dispersion | 41 |
| QDs: — | (2) Washing by H2SO4 | (2) 5 h ultrasonic | ||||||
| (3) 5–10 min stirring | (3) Centrifugation | |||||||
| (4) Centrifugation | (4) Filtration | |||||||
| (5) Washing | ||||||||
| (6) Stirring | ||||||||
| (7) Centrifugation | ||||||||
| Mo2C@NG | Sonication | NSs: — | — | (1) 5 min stirring | 1.8 (MQD) | 11.5 µm (MQD@NG) | — | 42 |
| QDs: (NH4)6Mo7O24·4H2O (AHM), graphene oxide, poly(oxypropylene) | (2) 1 min heating at 90 °C | |||||||
| Diamines | (3) Freeze-drying | |||||||
| (4) 1 h heating at 500 °C in quartz tube | ||||||||
| (5) 4 h heating at 700 °C under Ar. | ||||||||
| Ti3C2 | Sonication + hydrothermal | NSs: Ti3AlC2, NaF, HCl, DMSO | (1) 12 h etching of 1 g Ti3AlC2 | (1) 10 h sonication | Diameter: 3.96 | — | — | 32 |
| QDs: | (2) Centrifugation in HCl | (2) 12 h heating | ||||||
| (3) Washing | (3) Filtration | |||||||
| (4) Drying | (4) Dialysis | |||||||
| (5) 24 h stirring of Ti3C2 | ||||||||
| (6) Centrifugation | ||||||||
| (7) Sonication | ||||||||
| Ti3C2 | Hydrothermal | NSs: Ti3AlC2, HF, DMSO | (1) 64 h etching of 2 g Ti3AlC2 | (1) 12 h heating | — | — | — | 43 |
| QDs: PEI | (2) Washing | (2) Filtration | ||||||
| (3) Under vacuum | (3) Dialysis | |||||||
| (4) 24 h stirring | ||||||||
| (5) Centrifugation | ||||||||
| (6) Sonication | ||||||||
| V2C | Hydrothermal | NSs: V2AlC, HF | (1) V2AlC 24 h stirring | (1) 6 h at 120 °C | 4.13 | 2–3 | — | 44 |
| QDs: ammonium hydroxide | (2) 48 h stirring | (2) Filtration | ||||||
| (3) Freeze-drying | (3) Dialysis | |||||||
| (4) Sonication | ||||||||
| Ti3C2 | Hydrothermal | NSs: HF, Ti3AlC2, TMAOH | (1) HF etching of Ti3AlC2 | (1) 6 h heating at 100 °C at autoclave | 4.2 | 1.2–2.0 | — | 45 |
| QDs: Ti3C2, ammonia | (2) TMAOH intercalating | (2) Cooling | ||||||
| (3) Filtration | ||||||||
| (4) Freeze-drying | ||||||||
| Ti3C2 | Hydrothermal | NSs: — | Chemical exfoliation | (1) 5 h sonication | 4 | — | — | 1 |
| QDs: Ti3C2 NSs, ethylenediamine | (2) 12 h heating at 160 °C | |||||||
| (3) 220 nm mesh filter | ||||||||
| (4) 12 h dialysis | ||||||||
| BSA@Ti3C2 | Hydrothermal | NSs: Ti3AlC2, HF | (1) 20 h etching of 0.5 g Ti3AlC2 | (1) 50 mg Ti3C2 NS 6 h heating at 120 °C | 2 | — | — | 30 |
| QDs: Ti3C2, BSA | (2) 15 min centrifugation | (2) 15 min sonication | ||||||
| (3) Drying under vacuum | (3) pH = 9 by ammonia | |||||||
| (4) Centrifugation | ||||||||
| (5) Filter | ||||||||
| Ta4C3Tx | Hydrothermal | NSs: Ta4AlC3, HCl, NaF | (1) 48 h etching | (1) 12 h heating of Ta4C3Tx NSs | Diameter: 3.5 | — | (1) HCl/NaF etchant: fewer surface defects compared to HF | 20 |
| QDs: Ta4C3Tx | (2) Centrifugation | (2) ToxicityNaF < toxicityLiF | ||||||
| (3) Washing | (3) Mechanical vibration and/or sonication: intercalation and interlayer spacing increment | |||||||
| (4) Freeze-drying | ||||||||
| (5) Drying at 60 °C | ||||||||
| (6) Sonication | ||||||||
| (7) Homogenizer | ||||||||
| Nitrogen-doped-Ti3C2 | Hydrothermal | NSs: Ti3AlC2, HF | (1) 48 h etching of 1.5 g Ti3AlC2 | (1) 36 h mixing | 3.8 | 1.24 | — | 46 |
| QDs: Ti3C2, HNO3, H2SO4, NaOH, ethylenediamine | (2) Centrifugation | (2) Cooling to 25 °C | ||||||
| (3) pH tuning (∼7) | (3) pH tuning (∼7) | |||||||
| (4) Vacuum-drying | (4) 25 min ultrasonication | |||||||
| (5) 12 h heating at 180 °C | ||||||||
| (6) Filtration | ||||||||
| (7) Dialysis | ||||||||
| S and N doped-Ti3C2Tx | Hydrothermal | NSs: Ti2AlC, TiC, HF | (1) 16 h ball milling | (1) 2 h heating at 100 °C | N-MQDs: 50 | 14.15 | — | 9 |
| QDs: HNO3, H2SO4, Ti3C2, NaOH, Na2S2O3, NH3·H2O | (2) 2 h at 1350 °C | (2) NaOH → pH = 7 | S-MQDs: 15–35 | 20.41 | ||||
| (3) Crushing | (3) 12 h heating | |||||||
| (4) Washing | (4) Dialysis | |||||||
| (5) Centrifugation | ||||||||
| (6) Drying | ||||||||
| Ti3C2Tx | Hydrothermal | NSs: Ti3AlC2, HF | (1) 72 h stirring | (1) 6 h heating | <10 nm | 1.98 | — | 47 |
| QDs: ammonia | (2) Washing | (2) Centrifugation | ||||||
| (3) Drying | ||||||||
| (4) Sonication | ||||||||
| Ti3C2 QDs decorated TiO2/Nb2O5 composite | Hydrothermal | NSs: Ti3AlC2, HF | (1) 18 h etch | (1) Ammonia → pH = 9 | 3.8 | — | — | 48 |
| QDs: ammonia | (2) Rinsing | (2) 6 h heating | ||||||
| (3) Centrifugation | (3) Filtration | |||||||
| (4) Drying | (4) Drying | |||||||
| (5) Ultrasonication | ||||||||
| Na2Ti3O7/Ti3C2Tx QDs | Hydrothermal | NSs: Ti3AlC2, HF | (1) 24 h stirring | (1) 4 h vibration | — | — | — | 49 |
| QDs: Ti3C2Tx, dimethyl sulfoxide, NH3·H2O | (2) Washing | (2) Washing | ||||||
| (3) Drying | (3) 6 h heating | |||||||
| Ni–Co LDH@Ti3C2 QDs | Hydrothermal | NSs: Ti3AlC2, LiF, HCl | (1) 5 min stirring | (1) +Ammonia → pH = 9 | 3.06 ± 0.78 | — | — | 50 |
| QDs: — | (2) 24 h stirring | (2) 6 h heating at 100 °C | ||||||
| (3) Washing | (3) Centrifugation | |||||||
| (4) 1 h sonication | ||||||||
| N-doped Ti3C2 | Hydrothermal | NSs: Ti3AlC2, HF | (1) 24 h stirring | (1) 6 h heating at 100 °C | 7.5 | — | — | 51 |
| QDs: Ti3C2, H2SO4, HNO3, NaOH, methanol | (2) Centrifugation | (2) Decant to ice water | ||||||
| (3) Washing | (3) +NaOH (pH = 7) | |||||||
| (4) Drying | (4) Centrifugation | |||||||
| (5) 6 h drying | ||||||||
| (6) 5 h heating at 160 °C | ||||||||
| (7) Centrifugation | ||||||||
| (8) Evaporating | ||||||||
| AgPt@N-Ti3C2 QDs | Hydrothermal | NSs: Ti3C2, nitric acid | (1) 24 h in oil bath at 100 °C | (1) 12 h heating at 160 °C | 2–5 | — | — | 52 |
| QDs: ethanediamine | (2) +Ice water + NaOH → pH = 7 | (2) Filtration | ||||||
| (3) Centrifugation | ||||||||
| N-Ta4C3 | Hydrothermal | — | (1) Etch | (2) Hydrothermal | 1.9–3.3 (avg: 2.6) | 0.8–3.2 (avg: 1.9) | — | 53 |
| Eu doped-Ti3C2 | Hydrothermal | NSs: Ti3AlC2, HF, NH3·H2O | (1) 20 h stirring | (1) 6 h heating at 120 °C | 2.81 | 4.83 | — | 54 |
| QDs: — | (2) Washing | (2) 2 h heating at 90 °C | ||||||
| (3) Centrifugation | (3) Centrifugation | |||||||
| (4) 48 h drying | ||||||||
| (5) 30 min sonication | ||||||||
| TiCN (titanium carbonitride, MXene-like) | Hydrothermal | NSs: — | — | (1) 5 min stirring | 2.7 ± 0.2 | 2.7–4 (avg: 3.2 ± 0.3) | — | 55 |
| QDs: TiCN | (2) 10 h heating at 120 °C | |||||||
| (3) Centrifugation | ||||||||
| N, P-Ti3C2 | Hydrothermal | NSs: Ti3C2, HCl, nitric acid | (1) 12 h at 100 °C | (1) 12 h at 120 °C | N,P-MQDs: 1–8 (2.73 ± 0.50) | 0.85 ± 0.02 | — | 56 |
| QDs: diammonium phosphate (DAP) | (2) 100 mL ice + NaOH → pH = 7 | (2) Filtration | N-MQDs: 3.14 | 0.93 | ||||
| (3) 2 days dialysis | P-MQDs: 2.97 | 0.71 | ||||||
| (4) Freeze-drying | ||||||||
| S, N-Nb2C | Hydrothermal | NSs: Nb2C, sulphuric acid, nitric acid | (1) 12 h at 100 °C | (1) 12 h heating at 160 °C | S, N-Nb2C: 2.6–4.7 (avg: 3.56) | S, N-Nb2C: 1.74 | — | 57 |
| QDs: L-cysteine | (2) +NaOH → pH = 7 | (2) Filtration | Nb2C: 2.4 | Nb2C: 5.01 | ||||
| (3) 2 days dialysis | N-Nb2C: 2.66 | N-Nb2C: 2.80 | ||||||
| N-doped Ti3C2 QDs@DAP | Hydrothermal | NSs: Ti3C2, NH3·H2O | (1) 30 min ultrasonic | (1) 6 h heating at 120 °C | 3.4 | 0.5–1.3 | — | 58 |
| QDs: — | (2) +NH3·H2O (under N2) → pH = 9 | (2) Centrifugation | ||||||
| (3) Filtration | ||||||||
| Amino-functionalized Ti3C2 | Hydrothermal | NSs: Ti3AlC2, HF, NH3·H2O | (1) 24 h stirring at 60 °C | (1) 6 h heating at 120 °C | 2.73 | 2.76 | — | 59 |
| QDs: — | (2) Rinse by DI | (2) 1 h dialysis | ||||||
| (3) Centrifugation | (3) Centrifugation | |||||||
| (4) 20 h drying | (4) Filtration | |||||||
| (5) 30 min sonication | (5) Freeze-drying | |||||||
| N-doped Ti3C2 | Hydrothermal | NSs: Ti3AlC2, LiF, HCl, APTES, ethanol | (1) 10 min stirring | (1) 12 h heating at 120 °C | 7–10 (avg: 8.63) | — | — | 60 |
| QDs: — | (2) 24 h stirring at 35 °C | (2) Filter | ||||||
| (3) Centrifugal washing | (3) Dialysis | |||||||
| (4) Vacuum-drying | (4) Freeze-drying | |||||||
| (5) 1 h ultrasonication | ||||||||
| (6) Centrifugation | ||||||||
| (7) Ultrasonication | ||||||||
| (8) 24 h stirring | ||||||||
| (9) Centrifugation | ||||||||
| TiO2/Ti3C2 | Hydrothermal | NSs: Ti3AlC2, LiF, HCl | (1) Etch | (1) Heating | Diameter: ∼8.2 | 1.02 | — | 61 |
| QDs: — | (2) Sonication | |||||||
| GSH-Ti3C2 QDs | Hydrothermal | NSs: Ti3AlC2, HF | (1) 24 h stirring at 40 °C | (1) 12 h heating at 120 °C | 2.5 | — | Sonication: Ti3C2 film cutting + hydrothermal | 62 |
| QDs: Ti3C2 NP, GSH, NaOH | (2) Washing | (2) Filtration | ||||||
| (3) Centrifugation | (3) 12 h vacuum drying | |||||||
| (4) 12 h drying | ||||||||
| (5) 5 h sonication | ||||||||
| N, B-Ti3C2 | Hydrothermal | NSs: — | — | (1) 12 h heating at 100 °C | 2.25 | — | — | 63 |
| QDs: Ti3C2, boric acid, ammonia hydroxide | (2) Filtration | |||||||
| (3) Freeze-drying | ||||||||
| N-Ti3C2 | Hydrothermal | NSs: — | — | (1) 24 h at 100 °C in oil bath | Hydrothermal: 120 °C: 3.93 | Hydrothermal: 120 °C: 0.7 | — | 64 |
| QDs: Ti3C2, nitric acid, ethanediamine | (2) In beaker containing 100 mL ice, +NaOH → pH = 7. | 160 °C: 3.7 | 160 °C: 1.4 | |||||
| (3) 12 h heating at 160 °C | 200 °C: 5.76 | 200 °C: 3.8 | ||||||
| (4) Filtration | ||||||||
| (5) 2 days dialysis | ||||||||
| Nb2C | Solvothermal | NSs: — | — | (1) 0.5 h stirring of 15 mg Nb2C | — | — | — | 65 |
| QDs: Nb2C, ammonia | (2) pH = 6 by ammonia | |||||||
| (3) 6 h heating | ||||||||
| (4) Filtration | ||||||||
| (5) Drying | ||||||||
| Nb2C-SHa | Sonication | NSs: — | — | (1) 30 min sonication | 2.3–5.4 | — | — | |
| QDs: Nb2C, n-octadecyl mercaptan | (2) Stirring overnight | |||||||
| (3) Filtration | ||||||||
| Carbide-derived graphene QDs | Solvothermal | NSs: Ti3C2Tx, HF | (1) 5 h etching | (1) Centrifugation | 4–10 | 1.36 | (1) Solvothermal → C–Ti bonds break | 66 |
| QDs: DMF | (2) Wash | (2) 20 h heating | (2) C atoms self-assemble into GQDs | |||||
| (3) Centrifugation | (3) Centrifugation | |||||||
| (4) 3 h sonication | (4) Dialysis | |||||||
| Nb2C | Solvothermal | NSs: HF, Nb2AlC, TPAOH | (1) HF etching of Nb2AlC for 5 days | (1) 30 min sonication | 5 nm | — | — | 29 |
| QDs: 20 mg Nb2C NS, 20 mL TPAOH | (2) Stirring TPAOH + Nb2AlC for 5 days | (2) 24 h solvothermal at 110 °C | ||||||
| (3) Sonication | (3) 120 h dialysis in water | |||||||
| (4) Freeze-drying | ||||||||
| Ti3C2 | Solvothermal | NSs: Ti3AlC2, HF | (1) 24 h etching of Ti3AlC2 | (1) Centrifuging | 3 | — | — | 67 |
| QDs: DMSO, ammonia | (2) Drying | (2) Washing | ||||||
| (3) Sonication | (3) pH tuning (∼9) by ammonia | |||||||
| (4) Sonication | ||||||||
| (5) 6 h heating | ||||||||
| (6) Filtration | ||||||||
| Ti3C2Tx | Solvothermal | NSs: Ti3AlC2, HF | (1) 12 h etching of Ti3AlC2 | (1) 24 h shaking of as-prepared Ti3C2Tx | 4.99 | — | — | 68 |
| QDs: Ti3C2Tx, TMAOH | (2) Thermal annealing | (2) Centrifugation | ||||||
| (3) Sonication | ||||||||
| (4) Centrifugation | ||||||||
| (5) Filtration | ||||||||
| (6) Dialysis | ||||||||
| Ti3C2Tx | Solvothermal | NSs: HF | (1) Etch | (1) Disperse | s-MQDs: 1.8 | 1–2.5 | (1) Higher polarity solvent → stronger interaction → easier exfoliation of NSs to QDs | 69 |
| QDs: DMF, ethanol, DMSO | (2) 6 h heating | e-MQDs: 2.5 | (2) Lower boiling point solvent → higher pressure → stronger exfoliation efficiency | |||||
| (3) Centrifugation | f-MQDs: 3.3 | |||||||
| N-doped Ti3C2 | Solvothermal | NSs: Ti3C2, KOH | (1) 3 h sonication | (1) 12 h heating at 140 °C | 2.3 | — | — | 2 |
| QDs: Ti3C2, DMF | (2) Centrifugation | (2) Centrifugation | ||||||
| N-Ti3C2 | Solvothermal | NSs: DMF | (1) Sonication (12 h at 750 W) | (1) 8 h heating at 150 °C | 6.2 | 1 | — | 70 |
| QDs: DETA, DMF | (2) Centrifugation | (2) 5 centrifugation re-suspension cycles | ||||||
| (3) Drying | (3) Drying | |||||||
| Ti3C2 | Solvothermal | NSs: Ti3AlC2, HCl, LiF | (1) 5 min stirring | (1) 24 h heating at 120 °C under N2 | 10.2 | ∼1.0 | 71 | |
| QDs: PEI | (2) 24 h stirring at 40 °C | (2) Filtration | ||||||
| (3) Wash by DI | (3) Dialysis | |||||||
| (4) Centrifugation | ||||||||
| (5) Decanting → pH = 6 | ||||||||
| (6) 10 min purging with N2 | ||||||||
| (7) 5 h ultrasonication | ||||||||
| (8) Centrifugation | ||||||||
| PLL-protected Ti3C2 | Solvothermal | NSs: Ti3AlC2, HF | (1) 24 h stirring at 40 °C | (1) 24 h heating at 100 °C under N2 | 3 | — | — | 72 |
| QDs: Ti3C2 NP, ε-poly-L-lysine (PLL), NaOH | (2) Wash | (2) Filtration | ||||||
| (3) Centrifugation | (3) Dialysis | |||||||
| (4) Purging with N2 → film | (4) Drying | |||||||
| (5) 5 h sonication | (5) 12 h vacuum drying at 60 °C | |||||||
| (6) Centrifugation | ||||||||
| Ti3C2 | Reflux | NSs: Ti3AlC2, HF, TMAOH | (1) 5 h etching Ti3AlC2 | (1) 1 day reflux | 8 | — | — | 73 |
| QDs: TMAOH | (2) Wash | (2) Centrifugation | ||||||
| (3) Centrifugation | (3) Vacuum drying. | |||||||
| (4) Vacuum-dry | ||||||||
| (5) Intercalation by TMAOH | ||||||||
| (6) Centrifugation | ||||||||
| (7) Vacuum filtration | ||||||||
| (8) Vacuum drying | ||||||||
| N-Ti3C2Tx | Reflux | NSs: Ti3AlC2 | (1) 24 h stirring at 60 °C | (1) 3 days oil bath refluxing at 120 °C | 2.7 | — | — | 74 |
| QDs: Ti3C2Tx, TMAOH | (2) Centrifugation | (2) Centrifugation | ||||||
| (3) Wash → pH = 7 | (3) Overnight vacuum drying at 200 °C | |||||||
| (4) Overnight desiccation under vacuum at 80 °C | ||||||||
| Ti3C2Tx QDs/TiO2/FTO | Reflux | NSs: Ti3AlC2, HF, TMAOH | (1) 12 h stirring | (1) 1 day reflux at 110 °C | — | Avg: ∼1.0 | — | 75 |
| QDs: TMAOH | (2) Centrifugation | (2) Centrifugation | ||||||
| (3) Drying at 200 °C under vacuum | (3) Vacuum drying at 200 °C. | |||||||
| (4) 12 h stirring | ||||||||
| (5) Centrifugation | ||||||||
| (6) Vacuum drying | ||||||||
| Ti3C2Tx | Microwave + reflux | NSs: Ti3AlC2, HF | (1) 24 h stirring | (1) 30 min microwave irradiation | 3.3 | 2.5 | — | 76 |
| QDs: Ti3C2Tx | (2) Washing | (2) Filtration | ||||||
| (3) 12 h drying | (3) Reflux | |||||||
| UA@Ti3C2 QDs | Microwave | NSs: Ti3C2, HNO3, NaOH, HCl | (1) 24 h heating at 100 °C | (1) Microwave irradiation | 6.4 ± 0.5 | — | — | 77 |
| QDs: UA | (2) In beaker → pH = 7 | (2) Centrifugation | ||||||
| (3) Centrifugation | ||||||||
| N, P-Ti3C2 QDs | Microwave | NSs: HF | (1) Etch | (1) Microwave | 3.11 ± 0.86 | — | — | 78 |
| QDs: Ti3C2, formamide, ammonia | (2) Centrifugation | |||||||
| (3) Filtration | ||||||||
| (4) Vacuum drying | ||||||||
| Ti3C2 | Microwave | NSs: Ti3AlC2, ammonia | (1) 24 h stirring at 40 °C | (1) Microwave (800 W, 5 min, 90 °C) | Diameter: 2 ± 0.33 | — | — | 63 |
| QDs: — | (2) Washing | (2) Filtration | ||||||
| (3) Centrifugation | (3) 12 h vacuum drying at 60 °C | |||||||
| (4) Drying at 60 °C under vacuum | ||||||||
| (5) 3 min ultrasonication | ||||||||
| Cl, N doped-Ti3C2 | Potential static | NSs: — | — | (1) V = 0.1 V, 1 h | 3.45 | — | (1) Cl− promotes N doping in graphene | 79 |
| QDs: Ti3AlC2, Pt wire, TMAOH, NH4Cl | (2) Centrifugation | (2) Cl− breaks Ti–Al bonds | ||||||
| (3) Filtration | (3) NH4OH is intercalated | |||||||
| (4) Dialysis | ||||||||
| Ti3C2Tz | Acoustomicrofluidic | NSs: Ti3AlC2, HF | (1) 24 h stirring | (1) Cycles of centrifugation | 7.8 ± 5.2 | 1.1 ± 0.2 | Nebulization of sample driven by high frequency acoustic waves | 80 |
| QDs: — | (2) Washing | (2) Membrane vacuum-filtering | ||||||
| (3) 24 h drying | ||||||||
| Ti3C2Tx | Micro-explosion | NSs: Ti3AlC2, HF | (1) 3days stirring at 40 °C | (1) +L-N2 → evaporation | 7.23 | 5 | Temperature difference of L-N2 and hot deionized water | 81 |
| QDs: Ti3C2Tx | (2) Centrifugation | (2) 24 h stirring | ||||||
| (3) Washing | (3) Filtration | |||||||
| (4) 24 h drying | (4) Centrifugation | |||||||
| (5) Freeze-drying | ||||||||
| WO3/Ti3C2 QDs/In2S3 | Freezing-and-thawing + sonication | NSs: Ti3AlC2, LiF | (1) 24 h stirring | (1) 5 min centrifugation | 1.66 ± 0.04 | 0.5–2 | — | 82 |
| QDs: — | (2) Centrifugation | (2) 3 h at 4 °C | ||||||
| (3) 3 h at −38 °C | ||||||||
| (4) 4 times freezing-and-thawing | ||||||||
| (5) 2 h sonication | ||||||||
| (6) Filtration | ||||||||
| Mo2C QDs embedded in carbon NSs | Molten salt | NSs: — | — | (1) 15 min stirring at 70 °C | 2–3 | 3.5 (Mo2C/C NS) | — | 83 |
| QDs: molybdenum acetylacetonate, sucrose, ethanol, NaCl | (2) +NaCl | |||||||
| (3) 2 h calcination at 800 °C under Ar | ||||||||
| (4) 3 times washing | ||||||||
| (5) Centrifugation | ||||||||
| Mo2C nanoparticle-decorated carbon polyhedrons | Solvothermal + pyrolysis | NSs: — | — | (1) 48 h heating at 160 °C | <5 | — | — | 84 |
| QDs: Zn(CH3COO)2·2H2O, MIM, PVP, DMF, Mo/ZIF-8, H2SO4 | (2) Centrifugation | |||||||
| (3) Washing | ||||||||
| (4) Drying at RT | ||||||||
| (5) 2 h pyrolysis | ||||||||
| (6) Treating in 0.5 M H2SO4 aqueous | ||||||||
| Mo2C QDs-decorated CNT networks | Spray-drying | NSs: — | — | (1) 1 h ultrasonication | <3 | — | Mo2C QDs: polar and conductive → provide active sites to absorb + Mo2C QDs welding spots for CNT | 85 |
| QDs: ammonium molybdate tetrahydrate, CNT | (2) Spray drying | |||||||
| (3) 1 h carbonization at 500 °C | ||||||||
| (4) 4 h at 700 °C under Ar | ||||||||
| Mo2C/C nanoflowers (ultrathin carbon NSs decorated with Mo2C QDs) | In situ synthesis + calcination | NSs: — | — | (1) 30 min stirring | 2–4 | — | — | 86 |
| QDs: dopamine hydrochloride, ammonium molybdate, PEG, ethanol, NH3·H2O | (2) Injecting of NH3·H2O | |||||||
| (3) 6 h stirring | ||||||||
| (4) Centrifugation | ||||||||
| (5) Washing | ||||||||
| (6) 2 h stabilizing at 450 °C | ||||||||
| (7) 3 h calcining at 900 °C under Ar | ||||||||
3.1. Top-down approaches
The top-down procedure is generally comprised of three main steps, including (i) preparation of MXene by etching layer “A” from the MAX phase, (ii) intercalation and exfoliation of layers to produce MXene nanosheets, and (iii) treatment to convert the nanosheets to QDs.In the first step, the layer “A” is removed with either a chemical etching or electrochemical etching. Etchants like HF are commonly used to target MAX powders, for example, Nb2AlC and Ti3AlC2, to produce MXene phases.39,50,82 However, HF is a hazardous material, and even time-consuming multiple centrifugation processes have failed to remove HF completely.24 Numerous attempts have been performed to use alternative chemical etchants. For instance, mixtures of HCl/KF, HCl/sodium fluoride (NaF), and LiF/HCl have proven effective for removing Al from Ti2AlN,23 Ta4AlC3 or Ti3AlC2,35,38,41,43,60,64,71 and Ti3AlC2.87 The acid (HCl) and fluorine components react with the MAX phases to remove Al by the formation of AlCl3 and other products, such as metal hexafluoroaluminate, resulting in the formation of multilayered MXene. Alternatively, electrochemical etching can be conducted in a 3-electrode cell (Fig. 3a), consisting of a working electrode (e.g., Ti3AlC2), an Ag quasi-reference electrode, and a Pt counter-electrode. An electrolyte (e.g., [EMIM][PF6]/MeCN) is used to remove the A-layer (Al) to prepare the MXene phase (Ti3C2Tx).77
 | ||
| Fig. 3 (a) Electrochemical etching of Ti3AlC2 to produce Ti3C2Tx MQDs (reprinted with permission from American Chemical Society, copyright © 2020).77 (b–d) Size distribution, (e–g) AFM images, and (h–j) thickness of QDs depending on the processing temperature: (b, e and h) 120 °C; (c, f and i); 160 °C; (d, g and j) 200 °C (reprinted with permission from Royal Society of Chemistry, copyright © 2022).36 | ||
For the preparation of MXene nanosheets, studies have determined that stirring multilayer MXenes in certain solvents like tetramethylammonium hydroxide (TMAOH) and sonication in tetrapropylammonium hydroxide (TPAOH),50 dimethyl sulfoxide (DMSO),33 and potassium hydroxide (KOH)2 enable synthesizing MXene nanosheets.34 While converting MXene multilayers to MXene nanosheets can be accompanied by various methods, a following procedure is required to synthesis MQDs.88 A broad spectrum of approaches to synthesize MQDs are briefly presented below.
Although various parameters influence the hydrothermal processing of MQDs, temperature manifests the most determining effect on their properties. For instance, Xu et al. synthesized MQDs with different sizes by varying the hydrothermal temperature of MXene nanosheets from 120 to 200 °C. After heating the solution of Ti3C2 nanosheets in nitric acid at 100 °C for 24 h and adjusting the pH to 7, 2 mL ethanediamine was added to 20 mL Ti3C2 nanosheets in a stainless-steel autoclave, followed by heating at 120, 160, and 200 °C for 12 h to synthesize nitrogen-doped Ti3C2 QDs. Taking the atomic force microscopy (AFM) and transmission electron microscopy (TEM) results into account, the lateral size/thickness of the N-Ti3C2 QDs changed from 3.93/0.7 nm at 120 °C to 3.7/1.4 nm at 160 °C and 5.76/3.8 nm at 200 °C, respectively (Fig. 3b–j). These results showed that the QDs treated at 120 °C, 160 °C, and 200 °C correspond to monolayer, bilayer, and multilayer structures, respectively, revealing the effect of temperature on the number of layers. Despite revealing the effect, no mechanism was proposed to explain this trend, which should be addressed in future investigations. The XPS characterization of N-MQDs shows that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds was dominant at 200 °C stating that overcoming the energy barrier requires higher temperature. The life-time investigation demonstrates that temperature increment up to 160 °C increases life-time to a maximum, however, at higher temperature life-time decreases possibly owing to chemical state change.36
N bonds was dominant at 200 °C stating that overcoming the energy barrier requires higher temperature. The life-time investigation demonstrates that temperature increment up to 160 °C increases life-time to a maximum, however, at higher temperature life-time decreases possibly owing to chemical state change.36
Both doping and surface functionalization of MQDs can be performed during hydrothermal synthesis. In the former case, studies have shown that adding HNO3, H2SO4, and ethylenediamine results in nitrogen (N)-doping of Ti3C2 QDs.81 As a result of doping, the size of MQDs can be altered. For example, during N-doping of Nb2C, the thickness decreased from 5.0 to 2.8 nm with a marginal effect on the lateral size.90 Regarding the latter case, Al-Duais et al.39 have indicated that sonication-assisted hydrothermal treatment of aqueous solution of Ti3C2 and bovine serum albumin (BSA) forms BSA-functionalized Ti3C2 QDs (BSA@Ti3C2), serving as sensitive fluorescent probes.39
An alternative benefit of hydrothermal treatments is the fabrication of MQD/semiconductor heterostructures. Ding et al.81 have prepared N-Ti3C2 QDs/CdS nanorods heterostructure through a self-assembly strategy by mixing the CdS nanorods (length = 1–2 µm and D = 30 nm) with N-Ti3C2 (D = 3.8 nm and t = 1.24 nm) in DI water for two days. Using a similar strategy, g-C3N4@Ti3C2 QD composites were prepared by mixing the g-C3N4 NSs and Ti3C2 QDs for 24 h. Ti3C2 QDs assisted g-C3N4 in: (i) boosting the density of the active sites, (ii) increasing the specific surface area, and (iii) improving carrier transfer efficiency due to excellent electronic conductivity in view of metallic characteristic.33
The type of the solvent plays an important role in altering the properties of MQDs. Fig. 4a–f clearly shows that solvothermal treatment of MXene nanosheets in DMF, DMSO, and ethanol provides MQDs with a different lateral size. The higher polarity of the solvent increases the interaction between solvent and nanosheets, leading to exfoliation facilitation and further size reduction. Therefore, DMSO with the highest polarity and strongest oxidation ability formed the smallest particles. In contrast, DMF with higher polarity than ethanol shows larger particles, corresponding to the effect of boiling point. The lower the boiling point, the higher the vessel pressure, paving the way for preparing smaller particles. In this case, the boiling point of ethanol (74.8 °C) is lower than DMF and the solvothermal temperature (120 °C), thereby producing more evaporated gas molecules and higher pressure, enhancing the exfoliation and size reduction.67
 | ||
| Fig. 4 TEM images and size distribution of (a and d) DMSO-MQDs, (b and e) ethanol-MQDs and (c and f) DMF-MQDs (reprinted with permission from Wiley, copyright © 2022).67 | ||
 | ||
| Fig. 5 (a) Synthesis of Ti3C2Tx MQDs by the micro-explosion method (reprinted with permission from Wiley, copyright © 2020).92 (b) Synthesis of Ti3C2Tx and N-Ti3C2Tx MQDs by reflux methods (reprinted with permission from The Royal Society of Chemistry, copyright © 2022).93 | ||
The freezing-and-thawing method is one of the newest methods that is commonly applied in the exfoliation of boron nitride nanosheets,94 metal–organic nanosheets,94 graphene oxide (GO),94 and antimony triselenide (Sb2Se3).94 To prepare Ti3C2 QDs with 1.7 nm average lateral size, the solution of Ti3C2 experiences freezing (at −38 °C for 3 hours) and thawing (at room temperature) cycles, followed by sonication to convert MXene flakes to QDs. By repeating freezing and thawing cycles, water is solidified and expanded multiple times, creating a honeycomb structure. This structure boosts van der Waals breakage by exerting extrusion force, thus promoting nanosheets peeling and QDs formation.35
3.2. Bottom-up approaches
Compared to top-down methods, which offer a wide range of synthesis designs for MQDs preparation, only a few bottom-up processes have been proposed for producing MQDs, primarily Mo2C QDs. Various studies have demonstrated the great potential of molten salts to synthesis various QDs, such as polymeric carbon nitride,85 In1−xGaxP, In1−xGaxAs, and Mo2C. In the case of the latter, NaCl is mixed with Mo-containing precursor and sucrose in ethanol/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by continuous NaCl nanocubic crystal precipitation and stacking growth. Subsequently, sucrose and the Mo-containing precursor are confined within the interstices of the NaCl crystals. Finally, heat treatment at 800 °C for 2 hours in argon results in the formation of molybdenum carbide nanodots embedded in ultrathin carbon nanosheets (Mo2C/C NSs with an average size of 2–3 nm) (Fig. 6a).37
1), followed by continuous NaCl nanocubic crystal precipitation and stacking growth. Subsequently, sucrose and the Mo-containing precursor are confined within the interstices of the NaCl crystals. Finally, heat treatment at 800 °C for 2 hours in argon results in the formation of molybdenum carbide nanodots embedded in ultrathin carbon nanosheets (Mo2C/C NSs with an average size of 2–3 nm) (Fig. 6a).37
 | ||
| Fig. 6 (a) Molten salt synthesis of Mo2C/C nanosheets (reprinted with permission from Wiley, copyright © 2018).37 (b) Pyrolysis synthesis of Mo2C-decorated carbon polyhedrons (reprinted with permission from American Chemical Society, copyright © 2018).42 (c) Spray-drying synthesis of Mo2C QDs-decorated CNT networks (reprinted with permission from Wiley, copyright © 2021).98 | ||
Pyrolysis is a temperature-induced method, which creates QDs by chemical decomposition of organic materials in the absence of oxygen.40 To prepare Mo2C nanoparticle-decorated carbon polyhedrons, Mo/ZIF-8, formed by the hydrothermal treatment of the mixture of precursors, is heated for 2 hours in an argon-filled tube furnace at 700 °C. As a result of pyrolysis, Mo/ZIF-8 framework is transformed into a carbon matrix, simultaneously forming Mo2C NPs less than 5 nm (Fig. 6b).42
Spray-drying is another process for preparing particles, in which a solution of raw materials is pressurized to form droplets and then dried at high temperatures to form particles.44 This method was used to prepare Mo2C QDs-decorated CNT networks.98 As illustrated in Fig. 6c, an ultrasonication-treated ammonium molybdate tetrahydrate in CNT solution is spray-dried at a flow rate of 1 L h−1 at 170 °C, followed by carbonization in the quartz tube to complete the procedure.98
4. Properties
MQDs possess key properties, including high colloidal, chemical, and photostability, making them promising candidates for various applications, including biomedicine, sensing, and optoelectronics. We aim to highlight two interesting properties biocompatibility/cytotoxicity and optical behavior which highly influence the performance of biomedical and optoelectronic devices.4.1. Biocompatibility and cytotoxicity
MQD with excellent in vitro and in vivo biocompatibility have unlocked new opportunities in biomedical engineering across various fields, including transplantation medicine, cancer photo-thermal therapy, and bio-imaging.47 This section addresses recent advancements in bioinertness, bioactivity, biodistribution, cytotoxicity, and cellular dysregulation caused by oxidative stress, collectively referred to under the broad term biocompatibility.Materials selection is a crucial factor influencing the biocompatibility of MQDs. For instance, Ti3C2Tx can elevate intracellular reactive oxygen species (ROS), leading to oxidative stress with both beneficial and detrimental effects. While it can be used to treat tumors and bacterial infections,52 it also has the potential to cause cellular damage.53 In contrast, Ti2N QDs demonstrate no cytotoxic effects on human embryonic kidney cells, U87 human malignant glioma cells, or 4T1 murine mammary carcinoma cells (in vitro) in a standard 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) cytotoxicity assay.23 The destruction of cancer cells using Ti2N QDs only occurs when they are exposed to near-infrared (NIR) laser irradiation for a short duration, a result that could not be achieved with either MQDs or NIR irradiation alone. This phenomenon positions Ti2N as a promising candidate for photothermal cancer therapy. Fig. 7a shows the confocal fluorescence images after the treatment with calcein AM (green) and PI (red) for live and dead cells, respectively.23
 | ||
| Fig. 7 (a) Confocal images showing calcein-AM staining for live cells (green) and PI staining for dead cells (red). The scale bar is 100 µm. Injection (reprinted with permission from Elsevier, copyright © 2020).23 (b) H&E-stained images of major organs of the Ti2N QDs treated mice at 20 days post-injection (reprinted with permission from Elsevier, copyright © 2020).23 | ||
In addition to materials selection, surface functional groups significantly influence the biocompatibility and bioactivity of MQDs. For instance, the surface of Ta4C3Tx MQDs, enriched with negatively charged functional groups, enhanced bioactivity by facilitating interactions between the QDs and cells.24,54 In another study, it has been demonstrated that the surface charge of Ta4C3Tx MQDs varies with pH, enabling these QDs to escape endosomes shortly after cellular uptake, and localize near the nucleus.24,55
The concentration of MQD colloidal solution is another important factor that greatly influences its biocompatibility. In one study, various concentrations of Ta4C3Tx MQDs, ranging from 2 μg mL−1 to 100 μg mL−1 in PBS, were applied in cultures of human umbilical vein endothelial cells (HUVECs). Using CellROX green fluorescent dye and the CellEvent fluorescence-based apoptosis detection kit, the researchers found that these concentrations neither induced intracellular oxidative stress nor activated caspase-3 and caspase-7, which are key mediators of the cell death.24 No significant changes in cellular cytotoxicity or cell growth were observed over a seven-day period.24 It is noteworthy that most of the aforementioned findings are predominantly based on laboratory in vitro experiments; hence, in vivo investigations using diverse disease models and real clinical conditions are needed to fully understand the performance of MQDs in living organisms.
Biodistribution is also crucial for MQD applications in living organisms. Encapsulation of MQDs within stable substances like albumin has been explored,56 along with other techniques; for example, Ti3C2 MQDs, used as photothermal agents for cancer treatment, gain improved dispersion and biocompatibility by ultrasonic oxidation, introducing hydrophilic groups without surface modification.58 Biodistribution studies on Ti2N QDs for photothermal cancer therapy determined accumulation of the MQDs in tumors, the liver, and kidneys. The enhanced permeability and retention effect considerably promoted the uptake of MQDs in cancerous areas, improving the PTT efficacy. The high content of Ti2N within the liver primarily resulted from the clearance activity of the reticuloendothelial system, whereas the kidney absorption might be associated with potential renal elimination. The amounts of Ti2N QDs detected in the feces and urine of mice were similar to those accumulated in the kidney that were metabolized, showing a high clearance efficiency. Therefore, it was concluded that the long-term toxicity concerns for Ti2N QDs, as inorganic PTT nanoagents, are less significant.23
4.2. Optical properties
MXenes have garnered significant attention for their exceptional electrical conductivity (up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–24
000–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1 (ref. 59)). However, their PL quantum yield remains extremely low.61 This raises the question: Can the transition from a 2D structure to a QD morphology enhance the optical properties of MXenes? Through what mechanisms?
000 S cm−1 (ref. 59)). However, their PL quantum yield remains extremely low.61 This raises the question: Can the transition from a 2D structure to a QD morphology enhance the optical properties of MXenes? Through what mechanisms?
The answer is yes. The 2D to QD transformation induces various fascinating optical characteristics. First, QDs exhibit a strong quantum confinement effect, especially when their size falls below the Bohr radius.62 In this case, the photogenerated excitons, i.e., a pair of electron and hole, are tightly confined within the QD, increasing the probability of radiative recombination and enhancing photoluminescence quantum yield (PLQY). Second, QDs feature tunable bandgap energy.62 As the lateral size of QDs decreases, the highest occupied molecular orbital (HOMO)–the lowest unoccupied molecular orbital (HOMO–LUMO) gap gradually widens, the absorption edge is blue-shifted, and the continuous spectral bands change to sharp spectral peaks, resulting in a stronger quantum confinement effect.62 Beyond the quantum confinement effect, surface states and edge effects with various F–, OH–, and O– functional groups play an important role in tuning the optical properties of QDs.63
To quantitatively assess the optical properties of MQDs, ultraviolet-visible spectroscopy (UV-Vis) and PL spectrometry have widely been employed, providing valuable insights into the absorption edge, emission peak, and bandgap energy (Eg). Regarding absorbance, Ti3C2 MQDs have an absorption band between 245–275 nm related to π–π* electronic transitions.65 In some cases, an absorption peak between 300–320 nm was also observed, corresponded to the n–π* transition.65 In terms of PL, the strongest emission peak typically appears at ∼460 nm when QDs are optically excited at 365 nm (Fig. 8a).65 This emission may be controlled by the quantum confinement effect or edge defects,65 leading to excitation-dependent or excitation-independent PL, respectively. While increasing the excitation wavelength shifts the emission peak to longer wavelengths in the former case, there is no shift in the emission peak in the latter case. Although the underlying mechanisms of this phenomenon have thoroughly been explored in other QDs like SiC, there is a lack of in-depth investigation into this behavior for MQDs. In terms of bandgap energy, studies have revealed that by changing the lateral size of MQD, their bandgap energy can be tuned between 0.1–3.52 eV (Table 2).46
 | ||
| Fig. 8 (a) UV–vis absorption spectrum of Ti3C2 MQDs. The inset shows the emission and photographs of fluorescence spectra (reprinted with permission from Elsevier, copyright © 2022).99 (b) UV-Vis absorption spectrum and PL spectrum of N-doped MQDs (reprinted with permission from Elsevier, copyright © 2021).100 (c) The emission spectra of Ti3C2 MQDs prepared in different solvents (reprinted with permission from Elsevier, copyright © 2022).2 (d) Normalized ECL emission spectrum (red curve) of N-Ti3C2 QDs in 10 mM S2O82− solution (reprinted with permission from Elsevier, copyright © 2022).101 The charge density difference of (e) Ti3C2 MQDs, (f) N-functionalized Ti3C2 MQDs, (g) P-functionalized Ti3C2 MQDs, and (h) N, P functionalized MQDs. (i) PL decay spectra of doped MQDs (reprinted with permission from Royal Society of Chemistry, copyright © 2019).102 (j) UV-vis and PL spectra of SN-MQDs (reprinted with permission from Elsevier, copyright © 2019).9 | ||
| MXene QDs | Size* | λ abs (nm) | λ exc (nm) | λ em (nm) | PLQY (%) | τ (ns) | Ref. |
|---|---|---|---|---|---|---|---|
| l (nm) and t (nm) | |||||||
| * Average lateral size (l), thickness (t), quantum dot (QD), absorption wavelength (λabs), excitation wavelength (λexc), emission wavelength (λem), excitation wavelength (λexc), carrier lifetime (τ), photoluminescence quantum yield (PLQY), glutathione (GSH), ε-poly-L-lysine (PLL). | |||||||
| TiCN | l = 2.7 | 232 | 310 | 400 | — | — | 103 |
| t = 3.2 | |||||||
| Ti3C2 | l = 2.75 | — | 365 | 445 | 4.5 | — | 68 |
| Ti3C2 | l = 1.75 | — | 320 | 410 | 7.7 | — | 70 |
| N-Ti3C2 | l = 1.75 | 239, 293, 427 | 420 | 580 | 5.42 | — | 100 |
| (1) N-Ti3C2 | (1) l = 3.14, t = 0.93 | 234, 295 | — | — | (1) 10.3 | (1) 6.02 | 102 |
| (2) P-Ti3C2 | (2) l = 2.97, t = 0.85 | — | — | (2) 2.4 | (2) 4.99 | ||
| (3) N, P-Ti3C2 | (3) l = 2.73, t = 0.71 | 480 | 560 | (3) 20.1 | (3) 8.54 | ||
| N-Ti3C2 QDs | l = 3.4 | 229, 290 | 369 | 448 | DMF = 11.3, ethanol = 1.09, H2O = 0.34 | — | 104 |
| t = 0.5–1.3 | |||||||
| N-Ti3C2 | l = 2.3 | — | 335 | 420 | 14.46 | 5.24 | 61 |
| N-Ti3C2 | l = 8.63 | — | 330 | 427 | N-Ti3C2 (APTES) = 15.4 | 6.86 | 64 |
| Ti3C2 = 6.7 | |||||||
| S, N co-doped Nb2C | l = 3.8 | — | 390 | 520 | 17.25 | 7.12 | 2 |
| t = 1.2 | |||||||
| N-doped Ti3C2 | l = 3.4 | — | 360 | 470 | 18.7 | 7.06 | 36 |
| N, B-Ti3C2 | l = 2.25 | 220, 280 | 335 | 448 | 18.9 | 4.39 | 101 |
| GSH-Ti3C2 | l = 2.5 | 300 | 337 | 430 | 21 | — | 105 |
| PLL-protected Ti3C2 | l = 3 | — | 330 | 415 | 22 | — | 64 |
| N-doped Ta4C3 | l = 2.6 | 234, 293 | 380 | 450 | 23.4 | 7.92 | 106 |
There are several factors that play a crucial role in optoelectronic properties of MQDs, with the synthesis method being one of the most important. For example, the ultrasonication-assisted HF process in various organic solvents was among the earliest approaches used for synthesizing fluorescent MQDs. While MQDs synthesized in dimethylformamide (DMF) had an average size of 2.75 nm with a PLQY of 4.5%,68 using DMSO reduced the MQD size to less than 2 nm and enhanced the PLQY to 7.7%.70,72 Compared with the ultrasonication method, the solvothermal process in organic solvents exhibited different results: a brownish solution of Ti3C2Tx QDs in DMSO (D = 1.8 nm) exhibited an emission wavelength and PLQY of 570 nm and 4.1%, respectively; a yellowish QDs in DMF (D = 3.3 nm) and colorless QDs in ethanol (D = 2.5 nm) indicated lower emission wavelengths at 436 and 370 nm but higher PLQYs at 10.7% and 6.9%, respectively.67 These size and optical property differences were attributed to the polarity, boiling temperature, and oxidation capability of the solvents. Solvents with higher polarity and lower boiling temperature normally paves the way for 2D to QD transformation, due to the strong 2D/solvent interaction and higher gas pressure, leading to smaller QDs with higher PLQY. However, Ti3C2Tx QDs showed a reverse trend in terms of PLQY since DMSO formed the smallest QDs with the lowest PLQY because the solvent had the highest intensity of oxygen-containing functional groups (e.g., C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and Ti–O). This oxidation degraded Ti3C2Tx into anatase or rutile TiO2 NCs, leading to a decrease in PLQY.67
O, and Ti–O). This oxidation degraded Ti3C2Tx into anatase or rutile TiO2 NCs, leading to a decrease in PLQY.67
Beyond the type of solvents, the processing time and temperature significantly influence the optical properties of MQDs. Studies have shown that extending the solvothermal duration from 1 hour to 8 hours results in a blue shift of the emission peak and an increase in emission intensity of nitrogen-doped Ti3C2, attributed to the quantum size effect.74 Another study indicated that decreasing the hydrothermal temperature from 150 °C to 100 could increase the PLQY of the Ti3C2 MQDs from 7.9% to 9.9%, showing the optimized temperature value is dependent on type of MQDs.86 However, no mechanism was provided for this trend. Anyway, despite the capability of synthesis methods in preparing fluorescent MQDs, the PLQY yet remained quite low.
Doping has been introduced as an efficient way to modify the electronic structure and properties of MQDs. Here, nitrogen is the commonly used dopant, found remarkably effective in improving various properties.100 It has revealed that N-doping of Ti3C2 QDs changes the absorption spectrum by adding a new absorption peak at 427 nm which corresponds to the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (Fig. 8b). Consequently, a new yellow fluorescence peak at 580 nm appears. Besides, the fluorescence intensity of N-Ti3C2 QDs is highly stable in the presence of various compounds (e.g., 1.0 M NaCl)107 due to the abundant hydrophilic groups, providing excellent water solubility.107
N (Fig. 8b). Consequently, a new yellow fluorescence peak at 580 nm appears. Besides, the fluorescence intensity of N-Ti3C2 QDs is highly stable in the presence of various compounds (e.g., 1.0 M NaCl)107 due to the abundant hydrophilic groups, providing excellent water solubility.107
More importantly, nitrogen doping can remarkably boost the PLQY which is dependent on many factors. First of all, not all dopants are capable of improving the PLQY. For instance, by N-doping of MQDs, the PLQY and carrier lifetime could reach 10.3% and 6.02 ns, respectively, significantly higher than the phosphorous (P)-doped MQDs (2.40% and 4.99 ns).2,90,102,108 Regarding N-doping, the selected solvent plays an important role. Using DMF as both a nitrogen doping source and a medium for the solvothermal process, the PLQY of N-Ti3C2 QDs reached 11.13%.104 This improvement was related to the reaction of dimethylamine, derived from DMF decomposition, and carboxylic groups of QDs. In another study, a combination of DMF and KOH was used to synthesize N-Ti3C2 QDs with a PLQY of 14.46%.2 This value is significantly higher than that of QDs prepared by HNO3 and KOH (less than ∼2% for both) (Fig. 8c), owing to the nitrogen doping confirmed by the detection of C–N–C and N–H bonds in X-ray photoelectron spectroscopy (XPS). 3-Aminopropyltriethoxysilane (APTES) was another N-containing source that succeeded in enhancing the PLQY to 15.4%, attributed to the surface-passivating amino groups, which exert a strong electron-donating effect at the edges of the MQDs.64 To further enhance the PLQY of nitrogen-doped Ti3C2 QDs to 16.9%, MQDs were solvothermally synthesized by adding diethylenetriamine to DMF.74 Compared to diethylenetriamine, ethylenediamine was more effective in enhancing PLQY to 18.7% by prolonging the carrier lifetime to 7.06 ns due to the gap states formed close to LUMO.36 High-resolution N 1s XPS spectrum determined four new peaks at 397.9, 399.5, 400.2, and 401.2 eV, corresponding to the Ti–N band, pyrrole-like nitrogen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), N–H, and graphitic nitrogen C–N, respectively. Ethylenediamine also demonstrated electro-chemiluminescent (ECL) properties, exhibiting a ECL emission peak at 612 nm with a quantum yield of 1.58%. This emission was attributed to electronic injection into surface traps within the QDs’ band gap (Fig. 8d).101 Similar to Ti3C2, ethylenediamine enhanced the optical properties of Ta4C3 by forming –NH2 and C–N bands, resulting in a PLQY of 23.4%.106 Additionally, the formation of –NH groups on the surface of N-MQDs improved pH resistance.106
N), N–H, and graphitic nitrogen C–N, respectively. Ethylenediamine also demonstrated electro-chemiluminescent (ECL) properties, exhibiting a ECL emission peak at 612 nm with a quantum yield of 1.58%. This emission was attributed to electronic injection into surface traps within the QDs’ band gap (Fig. 8d).101 Similar to Ti3C2, ethylenediamine enhanced the optical properties of Ta4C3 by forming –NH2 and C–N bands, resulting in a PLQY of 23.4%.106 Additionally, the formation of –NH groups on the surface of N-MQDs improved pH resistance.106
Co-doping offers a greater potential for enhancing the optical properties of Ti3C2 QDs. For instance, Q. Guan et al.102 have shown that simultaneous doping of monolayer Ti3C2 QDs by nitrogen and phosphorus enhance PLQY to 20.1%. High-resolution N 1s and P 2p XPS spectra have indicated the characteristic peaks of NO3− (407.2 eV), N–Ti (396.1 eV), C–N (398.2 eV), N–H (400.7 eV), P–O (133.9 eV), and PO-C (134.8 eV). The electron density difference (EDD) distribution underscores the electron transfer between bonding atoms (Fig. 8e–h). In this image, the blue and red isosurfaces represent electron-depleted and electron-accumulated regions, respectively. Oxygen sites, with high electronegativity, appear red, while phosphorus atoms show blue electron-depleted regions. The enhanced blue intensity at P atoms in P, N-doped Ti3C2 QDs indicates electron transfer from P to N, leading to improved radiative recombination and optical performance.102 The trend of carrier lifetime in N, P-Ti3C2 QDs, N-Ti3C2 QDs, and P-Ti3C2 QDs (8.54 ns, 6.02 ns, and 4.99 ns, respectively) also confirm the photoluminescent enhancement (Fig. 8i).
Besides the P and N dopant pair, other dopants have also been explored to improve the optical properties of Ti3C2 QDs. For instance, nitrogen and boron co-doped Ti3C2 MQDs (N, B-Ti3C2 MQDs) were hydrothermally prepared from ammonia and boric acid, exhibiting good water solubility, strong stability, and high optical characteristics.109 Co-doped S, N Ti3C2 MQDs showed the most redshifted PL emission of 580 nm as well as the highest PLQY of 28.12%.9 Notably, the PLQY value was higher than that of N-Ti3C2 MQDs (8.33%) and S-Ti3C2 MQDs (7.78%), which correlated with the carrier lifetime enhancement from 4.67 ns for S-doped MQDs and 5.81 ns for N-doped MQDs to 7.74 ns for S, N-doped MQDs (Fig. 8j). Co-doping was also found effective in tuning the optical properties of other MQDs. For instance, co-doped Nb2C QDs (S, N-doped Nb2C MQDs) with green excitation-dependent emission at 520 nm indicated a high PLQY of 17.25%.2 Based on the S 2p XPS spectra, the peaks located at 168.2 eV and 169.3 eV, correspond to different oxidized sulfur forms of SOx (x = 2–4) bond, typically associated with the surface of the Nb2C layers.
Co-doped MQDs also offer higher PLQY combined with improved pH resistivity. Studies indicated that the fluorescence intensity of N, B-Ti3C2 QDs remained constant when pH was in the range of 3–13, showing high pH-resistive behavior of co-doped QDs.109 In contrast, the N-doped MQDs exhibited emission stability only within the pH range of 4–9, attributed to the presence of both amino and hydroxyl groups on the surface of QDs.108 Beyond this range, the intensity quenched out in both acidic and basic solutions. In alkaline solutions, deprotonation of hydroxyl groups forms O− groups on the MQDs surface. Consequently, oxidation of MQDs is accelerated and the PL intensity is reduced.109
Surface functionalization of MQDs with various molecules was also found effective in enhancing the PLQY of MQDs. This improvement is highly dependent on the synthesis procedure and type of molecules. In terms of synthesis methods, the hydrothermal reaction plays a critical role in the synthesis of fluorescent MQDs, as simple stirring of a mixture of molecules with Ti3C2 has been shown to be ineffective.39 Regarding the type of molecules, while the PLQY of BSA@MQDs and polyethyleneimine (PEI)-MQDs were 8% and 7.13%, respectively, an aqueous solution of GSH-Ti3C2 MQDs demonstrated a much higher PLQY of 21%. Although PEI successfully functionalized the surface of QDs by forming amide and N–H bonds,43 GSH increased the surface defect sites and enhanced the PLQY more effectively.105
It is noteworthy that molecule-passivated MQDs exhibit intriguing differences in their optical responses to pH tuning. Studies have revealed that the optical properties of BSA@MQDs and PEI@MQDs are highly sensitive to pH changes.39 The highest emission of BSA@MQDs is attained at pH = 2, and increasing the pH drastically reduces the PL intensity. At the low pH value, the full protonation of BSA amine groups enhances vibrational coupling with hydroxyl functional groups, while deprotonation at higher pH levels leads to the formation of nonradiative recombination centers associated with increased surface defects.39 A similar trend has been observed in PEI-functionalized QDs, as confirmed by time-resolved photoluminescence (TRPL) analysis.43 While the slow decay time constant (τ1), associated with intrinsic transitions of Ti3C2 MQDs, remains unchanged across different pH levels, the fast decay time constant (τ2), related to emissions from surface defect sites, decreases with increasing pH. This reduction is explained by the deprotonation of surface defects, which transforms luminescent surface defects into non-luminescent ones, thereby diminishing the emission intensity of Ti3C2 MQDs. In contrast, GSH-Ti3C2 MQDs,105 UA@Ti3C2 MQDs,88 and PLL-protected Ti3C2 MQDs exhibit stable PL intensity across a wide pH range of 1–13. This stability is attributed to the superior surface passivation provided by the functional molecules, effectively shielding the QDs from pH-induced surface changes.105,110
5. Applications
Leveraging the exceptional optical properties and inherent biocompatibility of MQDs, their utility has been demonstrated across a diverse range of applications. In this section, we will cover a wide range of applications, commencing with sensing technologies and highlighting the effective detection of various analytes. Subsequently, we will evaluate the significant impact of MQDs on the performance enhancement of optoelectronic devices, energy conversion systems, and biomedical applications.5.1. Sensing
• MechanismsQDs have revolutionized nanotechnology, offering tunable optical and electronic properties, including broad excitation spectra, adjustable emission properties, and intense fluorescence with long-term photostability driven by quantum confinement effects. This versatility has propelled advancements in various fields, such as optoelectronics, bioimaging, and sensing.6,107,111 Despite these merits, QDs face several limitations, particularly in sensing applications. First, their applicability in fluorescence detection is hindered by inherent biological toxicity, particularly in Pb- and Cd-based QDs.2,5,6,11 Second, many QDs are soluble only in toxic, nonpolar organic solvents, restricting their direct use in aqueous systems. Additionally, certain QDs, such as CdS, degrade under acidic conditions (pH < 7.0), resulting in the loss of their fluorescence properties over time.5 Therefore, MQDs with strong pH resistance in certain wavelengths and stable optical properties in aqueous solutions have gained remarkable attention in recent years.35 By taking advantage of various optical, electrochemical, and electrochemiluminescence (ECL) sensing techniques, MQDs have manifested great potential for use in sensors.88
So far, optical sensing by fluorescence quenching has been the one of the most used and efficient techniques.7 Fluorescence quenching refers to the fluorescence intensity reduction of a fluorophore due to different intermolecular interactions, such as excited state reactions, molecular rearrangements, Förster resonance energy transfer (FRET), photoinduced electron transfer (PET), inner filter effect (IFE), ground state complex formation, and collisional quenching.7 IFE, FRET, and PET are the mechanisms that have been widely utilized for sensing. The IFE is a non-specific mechanism (no probe-analyte interactions) that occurs when there is an overlap between the absorption spectrum of the quencher (analyte) and the excitation or emission spectra of the luminescent probe. This phenomenon reduces the amount of light available to excite the probe or diminishes the intensity of the emitted light, resulting in decreased luminescence.7 FRET is a non-radiative energy transfer process that occurs when a donor molecule (the luminescent probe) in an excited state transfers energy to an acceptor molecule. This phenomenon occurs when there is an overlap between the emission spectrum of the donor and the absorption spectrum of the acceptor. Therefore, the efficiency of FRET highly depends on the donor–acceptor distance.7 PET, which occurs over relatively longer distances than FRET, involves the transfer of an electron from the donor to the acceptor, forming a non-emissive complex and effectively quenching the probe's luminescence.7 Therefore, the PET efficiency is influenced by the redox properties as well as the HOMO and LUMO of the donor and acceptor.7
Compared to single-emission, dual-emission reverse change ratio PL offers higher sensitivity and accuracy due to intrinsic signal amplification.88 In dual-emission, the fluorescence of one component decreases while another's fluorescence significantly increases in the presence of the analyte. The ratio of the fluorescence intensities of the two components is then determined for quantitative analysis. For instance, curcumin (CUR) caused the fluorescence emission of Ti3C2 MQDs at 430 nm to be suppressed by the FRET, whereas CUR's fluorescence emission at 540 nm increased.88
Colorimetric sensors are another group of optical sensors that exhibit a distinct color shift in response to the analyte's reaction.112 This color transition is detected by the naked eye or specific instrumentation to determine the change in intensity at a certain wavelength within the visible (400–800 mm) range.112 For example, N, P-Ti3C2 MQDs showed a transparent-to-orange color change upon Fe2+ detection, demonstrating potential for portable sensors.84 ECL has been widely used in analytical chemistry and bioassays due to its high sensitivity, low background noise, and compatibility with miniaturized devices.72 In ECL, a voltage is applied to an electrode, which causes a redox reaction to occur and generates excited-state species. Then, these species emit light after returning to their ground state.101 To assess the redox activity, cyclic voltammetry (CV) is used by sweeping the potential of the working electrode linearly with time and measuring the resulting current, providing information about redox potentials, electron transfer kinetics, and surface area.113 By leveraging advanced sensing techniques and MQDs, researchers have successfully detected various types of targets. Table 3 provides a summary of the most recent advances in this field. The following sections offer a critical review on the application of MQDs in sensing.
| Application | Platform | Features | Mechanisms | Ref. |
|---|---|---|---|---|
| MXene quantum dots (MQDs), electrochemiluminescence (ECL), fluorescence (IFE), photoelectrochemical (PEC), ascorbic acid (AA), glucose (GLU), uric acid (UA), diaminopyridine (DAP), photoinduced electron transfer (PET), limit of detection (LOD), dopamine (DA), glucose oxidase (GOD), alkaline phosphatase (ALP), transferrin (Trf), lysozyme (Lyz), ovalbumin (Ova), chymotrypsin (Chy), immunoglobulin G (IgG), colony-forming unit (Cfu), kanamycin (KAN), cefixime (CFX), chloramphenicol (CHL), norfloxacin (NOR), oxytetracycline (OTC), doxorubicin (DOX), aspartic acid (Asp), serine (Ser), lysine (Lys), glycine (Gly), glutathione (GSH), nitrobenzoic acid (NBA), benzoic acid (BA), benzidine (BD), nitrobenzene (NB), 2,4-dinitrophenylhydrazine (DNPH), p-nitrophenyl phosphate (PNPP), tryptophan (Try), cysteine (Cys), Förster resonance energy transfer (FRET), electron spin resonance (ESR), hydrogen evolution reaction (HER), nitrate oxidation reduction reaction (NORR), nitrogen reduction reaction (NRR), covalent organic framework based on pyridine and dihydroxybenzidine (PY-DHBD-COF). | ||||
| miRNA sensing | Ti3C2 MQDs@Au nanobones heterostructure | • Target: miRNA-26a | ECL (short-distance electron transfer) | 72 |
| • LOD: 1.7 fM | ||||
| • Linear range: 5 fM–10 nM | ||||
| • Selectivity: miRNA-126, miRNA-155, miRNA-221 | ||||
| • Sensitivity in real conditions: human serum samples | ||||
| Proteins sensing | Amino-functionalized Ti3C2 MQDs | • Target: histidine | FL (IFE) | 114 |
| • LOD: 2.1 nM | ||||
| • Linear range: 100–1000 nM | ||||
| • Selectivity (Ni+): Li+, K+, Ag+, Na+, Mn2+, Zn2+, Mg2+, Ca2+, and Al3+ | ||||
| • Sensitivity in real conditions: human serum samples | ||||
| Organic acid sensing | Ti3C2 MQDs/TiO2 inverse opal heterojunction | • Target: glutathione | PEC | 115 |
| • LOD: 9.0 nM | ||||
| • Linear range: 0.1–1000 μM and 1 μM to 200 μM | ||||
| • Selectivity: AA, GLU, UA, Fe3+ | ||||
| Other targets sensing | N-doped Ti3C2 MQDs@DAP | • Target: H2O2 | Sensing: ratiometric fluorescence | 92 |
| • LOD: 0.57 μM | Quenching: PET | |||
| • Linear range: 2–50 μM | ||||
| • Selectivity: DA, AA, Phe, Glu, Lys, L-Cys, Tyr, GSH, GSSG, K+, Mg2+, Ca2+, Na+, Zn2+, Cd2+, Fe2+, Fe3+, Ag+, I−, and Br− | ||||
| • Target: xanthine | ||||
| • LOD: 0.24 μM | ||||
| • Linear range: 1–50 μM | ||||
| • Selectivity: adenosine, BSA, DA, AA, UA, cytidine, urea, Glu, K+, and Ca2+ | ||||
| • Sensitivity in real conditions: human serum | ||||
| Proteins sensing | Ti3C2 MQDs-AuNPs | • Target: polynucleotide kinase | ECL | 71 |
| • LOD: 2.7 × 10−5 U mL−1 | ||||
| • Linear range: 0.0001 to 10 U mL−1 | ||||
| • Selectivity: glucose oxidase (GOD), alkaline phosphatase (ALP), thrombin and lysozyme | ||||
| • Sensitivity in real conditions: HeLa cell lysate | ||||
| miRNA sensing sensing | TiO2 nanosheet arrays/Ti3C2 MQDs | • Target: MicroRNA-155 | PEC | 71 |
| • LOD: 0.025 pM | ||||
| • Linear range: 0.1 pM–10 nM | ||||
| • Selectivity: miRNA-122, miRNA-141, miRNA-21 | ||||
| Proteins and amino acids sensing | ε-Poly-L-lysine-protected Ti3C2 MQDs | • Target: cytochrome c | Sensing: FL | 110 |
| • LOD: 20.5 nM | Quenching: IFE | |||
| • Linear range: 0.2 to 40 μM | ||||
| • Selectivity: Trf, Lyz, Ova, Chy and BSA | ||||
| • Target: trypsin | ||||
| • LOD: 0.1 μg mL−1 | ||||
| • Linear range: 0.5 to 80 μg mL−1 | ||||
| • Selectivity: ALP, lysozyme, BSA, pepsin, thrombin and IgG | ||||
| • Sensitivity in real conditions: 50-fold diluted serum samples | ||||
| Environmental pollutants sensing | Aptamer modified polyhedral oligomeric silsesquioxane-perovskite quantum dots (POSS-PQDs-Apt)/Ti3C2 | • Target: Vibrio parahaemolyticus (VP) | Sensing: FL | 116 |
| • LOD: 30 cfu mL−1 | Quenching: FRET | |||
| • Linear range: 102 to 106 cfu mL−1 | ||||
| • Selectivity: common bacteria, including Listeria monocytogenes, Salmonella typhimurium, Escherichia coli, and Staphylococcus aureus (all concentrations were at 105 cfu mL−1) common ions and pesticide residues in water on the test results, including Na+, K+, Mg2+, Ca2+, and dimethoate. | ||||
| • Sensitivity in real conditions: real seawater samples | ||||
| Environmental pollutants sensing | N, B-doped Ti3C2 MQDs | • Target: tetracycline | Sensing: ratiometric fluorescence | 109 |
| • LOD: 20 nM (using the smartphone: 45 nM) | Quenching: IFE | |||
| • Linear range: 0.2–20 μM (using the smartphone: 0.4–20 μM) | ||||
| • Selectivity: other antibiotics (KAN, CFX, CHL, NOR, OTC, and DOX) and some potentially coexisting substances (Na+, Ba2+, Ca2+, Zn2+, Fe3+, BSA, Asp, Ser, Lys, Gys, His, GSH, GLU) | ||||
| • Sensitivity in real conditions: milk | ||||
| Environmental pollutants sensing | Eu-doped Ti3C2 MQDs | • Target: tetracycline | Sensing: ratiometric fluorescence | 65 |
| • LOD: 48.79 nM (using colorimetric method: 11.36 nM) | Quenching: FRET | |||
| • Linear range: 0–1000 μM | ||||
| • Selectivity: K+, Ag+, Li+, Na+, Mn2+, Zn2+, Ba2+, Mg2+, Ca2+, Al3+ and antibiotics (Kana, Azi, Chl) | ||||
| • Sensitivity in real conditions: milk, and soil | ||||
| • Target: oxytetracycline (OTc) | ||||
| • LOD: 3.86 nM | ||||
| • Linear range: 100–1000 μM | ||||
| • Target: chlortetracycline (CTc) | ||||
| • LOD: 64.73 nM | ||||
| • Linear range: 100–1000 μM | ||||
| Environmental pollutants sensing | N-doped Ti3C2 MQDs | • Target: Alizarin red | Sensing: FL | 100 |
| • LOD: 1.21 μM | Quenching: IFE | |||
| • Linear range: 0–80 μM | ||||
| • Sensitivity in real conditions: actual water samples | ||||
| Environmental pollutants sensing | Uric acid-capped Ti3C2 MQDs | • Target: 2,4,6-trinitrophenol | Sensing: FL | 88 |
| • LOD: 9.58 nM | Quenching: IFE | |||
| • Linear range: 0.01–40 μM | ||||
| • Selectivity: NBA, BA, BD, NB, DNPH; PNPP, K+, Cl−, Ca2+, Ba2+, Cr3+, Al3+, NH4+, S2−, SO32−, PO43−, Mg2+, Zn2+, Mn2+, Fe2+, and NO3−, Cu2+, Pb2+, and Fe3+, Co2+ and Cd2+ | ||||
| • Sensitivity in real conditions: in lake and tap water | ||||
| Other targets sensing | Ti3C2 MQDs | • Target: dopamine | — | 113 |
| • LOD: 3 nM | ||||
| • Linear range: 0.01–20 μM | ||||
| • Selectivity: ascorbic acid, uric acid, glucose, NaCl, KCl and urea | ||||
| • Sensitivity in real conditions: DA hydrochloride injection purchased from a drugstore | ||||
| Proteins and amino acids sensing | N-doped Ti3C2 MQDs | • Target: mucin 1 | Sensing: ECL | 101 |
| • LOD: 0.31 fg mL−1 | ||||
| • Selectivity: carcinoem-bryonic (CEA), α-1-fetoprotein (AFP), human serum albumin (HSA) and hemoglobin (Hb) | ||||
| • Sensitivity in real conditions: serum samples | ||||
| Ions/organic acids sensing | N-doped Ti3C2 MQDs | • Target: chromium(VI) | Sensing: FL | 107 |
| • LOD: 0.012 μM | Quenching: IFE | |||
| • Linear range: 0.1–500.0 μM | ||||
| • Selectivity: NH4+, K+, Na+, Ag+, Mg2+, Ca2+, Mn2+, Zn2+, Cu2+, Cd2+, Co2+, Hg2+, Fe2+, Fe3+, MnO4 | ||||
| • Sensitivity in real conditions: in tap and Yudai Lake water | ||||
| • Target: ascorbic acid | ||||
| • LOD: 0.02 μM | ||||
| • Linear range: 0.1–500.0 μM | ||||
| • Selectivity: common amino acids (L-phenylalanine; L-tyrosine; L-/D-tryptophan; L-arginine; L-glutamate) and other potentially biomolecules (glutathione; glucose; uric acid; urea) | ||||
| • Sensitivity in real conditions: vitamin C tablets and lemon juice samples | ||||
| Organic acids sensing | GSH-Ti3C2 MQDs | • Target: uric acid | Sensing: ratiometric fluorescence colorimetric (absorption) | 105 |
| • LOD: 125 nM (ratiometric fluorescence), 200 nM (absorption) | Quenching: FRET | |||
| • Linear range: 1.2–75 μM (ratiometric fluorescence), 1.2–100 μM (absorption) | ||||
| • Selectivity: Try, Cys, urea, Nap, Kp, GSH, DA, GLU, NO3−, SO4−, Cl−, UA | ||||
| • Sensitivity in real conditions: serum and saliva | ||||
| Other targets/ions sensing | Ti3C2 MQDs | • Target: curcumin | Sensing: ratiometric fluorescence | 117 |
| • LOD: 20 nM | Quenching: FRET | |||
| • Linear range: 0.05–10 μM | ||||
| • Selectivity: interfering ions (K+, Na+, Fe3+, Al3+, Ca2+, Mg2+, SO4−, Fe2+, Zn2+, Cl−, and HPO4−), various amino acids, GSH, folic acid, and L-ascorbic acid, two carbohydrates (fructose and glucose). | ||||
| • Target: hypochlorite | ||||
| • LOD: 5 μM | ||||
| • Linear range: 25 to 150 μM and 150 to 275 μM | ||||
| • Selectivity: ClO−: various oxidized anions and free radicals | ||||
| Other targets sensing | N-doped Ti3C2 MQDs | • Target: H2O2 | Sensing: FL | 118 |
| • LOD: 1.2 nM | ||||
| • Linear range: 5 to 100 nM | ||||
| • Selectivity: Na+, K+, Mg2+, Ca2+, Fe2+, Zn2+, and Al3+ ions | ||||
| Other targets sensing | Ti3C2 MQDs | • Target: H2O2 | — | 97 |
| • LOD: 5 nM | ||||
| Ions sensing | N-doped Ti3C2 MQDs | • Target: Fe3+ | Sensing: FL | 119 |
| • LOD: 2 μM | ||||
| • Linear range: 2 to 5000 μM | ||||
| • Selectivity: Na+, Mg2+, Cu2+, K+, Fe3+, Mn2+, Zn2+, Ca2+, Al3+, Ce3+, Cu+ and Ni2+ | ||||
| Ions sensing | N-doped Ti3C2 MQDs | • Target: Cu2+ | Sensing: FL | 74 |
| • Selectivity: Na+, K+, Mg2+, Ca2+, Fe2+, Fe3+, Co2+, Ni2+, Zn2+, Cd2+, Pb2+, Hg2+, and Ag+ | ||||
| Ions sensing | Ti3C2 N, P-MQDs | • Target: Cu2+ | Sensing: FL | 102 |
| • LOD: 2 μM | Quenching: IFE | |||
| • Linear range: 2–100 μM and 250–5000 μM | ||||
| • Selectivity: Zn2+, Pb2+, Na+, La3+, Mn2+, Ni2+, Ce3+, Fe3+, Ca2+, K+, Ag+ | ||||
| Ions sensing | N-doped Ti3C2 MQDs | • Target: Cu2+ | Sensing: FL | 120 |
| • LOD: 0.15 μM | Quenching: IFE | |||
| • Linear range: 0.5–100 μM | ||||
| • Selectivity: Hg2+, Mn2+, Ag+, Cd2+, Al3+, Zn2+, Mg2+, Ca2+, K+, and Na+ | ||||
| • Sensitivity in real conditions: water sample | ||||
| • Target: Fe3+ | ||||
| • LOD: 0.17 μM | ||||
| • Linear range: 0.5–100 μM | ||||
| • Selectivity: Hg2+, Mn2+, Ag+, Cd2+, Al3+, Zn2+, Mg2+, Ca2+, K+, Na+ | ||||
| • Sensitivity in real conditions: water sample | ||||
| Ions sensing | TiCN MQDs | • Target: Fe3+ | Sensing: FL | 103 |
| • LOD: 1.0 μM, 2.4 ± 0.1 μM (tap water) | Quenching: IFE | |||
| • Linear range: 2–400 μM and 500–800 μM | ||||
| • Selectivity: K+, Cd2+, Cu2+, Fe2+, Hg2+, Mg2+, Ni2+, Pb2+, Ce3+, Mn2+, Co2+, Al3+, and Zn2+ | ||||
| • Sensitivity in real conditions: tap water | ||||
| Ions sensing | Ti3C2 MQDs | • Target: Fe3+ | Sensing: FL | 70 |
| • LOD: 310 nM | Quenching: IFE | |||
| • Selectivity: cysteine, serine, arginine, ascorbic acid, dopamine, H2O2, Mn2+, Fe2+, Cu2+, Ca2+, Al3+, Cd2+, Co2+, Cr3+, Mg2+, Na+, Ni2+, Pb2+, Sn2+, and Zn2+ | ||||
| • Sensitivity in real conditions: serum and seawater samples | ||||
| Ions/physical parameters sensing | N-doped Ta4C3 MQDs | • Target: Fe3+ | Sensing: FL | 106 |
| • LOD: 2 μM | ||||
| • Selectivity: NO3−, SO4−, Cl−, Hg+, Mg+, Ce+, Na+, Ca+, K+, Fe+, Cu+, Ni+, Ba2+ | ||||
| Ions sensing | BSA@Ti3C2 MQDs | • Target: Fe3+ | Sensing: FL | 121 |
| • LOD: 1.25 nM | Quenching: IFE | |||
| • Linear range: 0–150 μM | ||||
| • Selectivity: ascorbic acid, Sn2+, Ca2+, Ni2+, Cd2+, Pb2+, Al3+, Zn2+, Ag+, Cr3+, Cr6+, Cu2+, Na+, and K+ | ||||
| • Sensitivity in real conditions: tap water samples | ||||
| Ions/pH sensing | Amino-functionalized Ti3C2Tx MQDs | • Target: Fe3+/pH | Sensing: ratiometric | 108 |
| • LOD: 2 nM | Fluorescence | |||
| • Selectivity: K+, Ca2+, Na+, Al3+, Zn2+, La3+, Co2+, Ni2+, Cu2+, Cd2+, Ce4+, Fe2+ | ||||
| • Sensitivity in real conditions: tap water samples | ||||
| Ions sensing | N-doped Ti3C2 MQDs | • Target: Co2+ | Sensing: FL | 2 |
| • LOD: 0.21 μM | Quenching: IFE | |||
| • Linear range: 0.5–100 μM | ||||
| • Selectivity: K+, Ca2+, Mg2+, Al3+, Zn2+, Fe3+, Cr3+, CO32−, SO42−, SO32−, PO43−, NH4+, Fe2+, Pb2+ and Cu2+ | ||||
| • Sensitivity in real conditions: tap water, river water | ||||
| • Target: Ag+ | ||||
| • LOD: 0.10 μM | ||||
| • Linear range: 0.5–150 μM | ||||
| • Selectivity: K+, Ca2+, Mg2+, Al3+, Zn2+, Fe3+, Cr3+, CO32−, SO42−, SO32−, PO43−, S2O32−, NH4+, Pb2+, and Cu2+ | ||||
| • Sensitivity in real conditions: tap water, river water | ||||
| Ions sensing | Ti3C2 MQDs | • Target: Cr3+ | Sensing: FL | 5 |
| • LOD: 30 mM | ||||
| • Selectivity: Cu2+, Na+, Ca2+, Fe3+ | ||||
| Ions sensing | N, P-doped Ti3C2 MQDs | • Target: NO2− | Sensing: colorimetry (absorption) fluorometric dual-modal | 84 |
| • LOD: FL intensity ratio: 0.25 μM absorbance ratio: 0.71 μM | Quenching: IFE | |||
| • Linear range: FL intensity ratio: 1.5–80 μM, absorbance ratio: 4–85 μM | ||||
| • Selectivity: anions (F−, Cl−, Br−, I−, NO3−, CO32−, SO32−, SO42−, PO43−, HPO42−), cations (Ca2+, Mg2+, Fe3+, K+, Zn2+, Ba2+) and coexisting substances (Arg, Try, Gly, Lys, Asp, Ser, His, Cys, Ala) | ||||
| • Sensitivity in real conditions: six types of sausage samples | ||||
| Ions sensing | Fe3O4/Ti3C2 MQDs | • Target: Cr(VI) | Sensing: colorimetry | 122 |
| • LOD: 0.26 μM | ||||
| • Linear range: 0–60 μM | ||||
| • Selectivity: Na+, Ni2+, K+, Ca2+, Mg2+, Co2+, Cu2+, Mn2+, Al3+, Fe3+, Pb2+ and Cr3+ | ||||
| • Sensitivity in real conditions: tap water samples | ||||
| pH sensing | Ti3C2 MQDs | • Target: intracellular pH | Sensing: ratiometric fluorescence | 43 |
| • Linear range: 6–8/5–9 | ||||
| Physical parameters sensing | MQD within natural 3D watermelon peel matrix | • Target: pressure sensor | — | 38 |
| • Sensitivity: 323 kPa−1 for 0–0.4 kPa and 51.38 kPa−1 for 0.4–20 kPa | ||||
| Others sensing | Ti3C2Tx MQD thin film on quartz bulk acoustic wave | • Target: infrared radiation | Sensing: electromechanical (admittance and frequency shift of the sensor throughout infrared radiation absorption) | 106 |
| Ions sensing | Ti3C2 MQDs | • Target: Ag+ | Sensing: FL | 123 |
| • LOD: 0.45 μM | ||||
| • Linear range: 0.5–40 μM | ||||
| • Selectivity: Na+, Au3+, Mg2+, Ca2+, Zn2+, Cu2+, Ce3+, Mn2+, Cd2+, Ni2+, Al3+, Fe3+, Cr3+, and Hg2+ | ||||
| • Sensitivity in real conditions: tap and river water samples | ||||
| Others sensing | Ti3C2 MQDs–EDTA–Eu3+ | • Target: 2,6-dipicolinic acid (DPA) | Sensing: colorimetry (absorption) fluorometric dual-modal | 124 |
| • LOD: 0.26 nM | Quenching: absorbance energy-transfer effect | |||
| • Linear range: 0–11 μM | ||||
| • Selectivity: BA, PH, PA, NA, ISA, Cys, Leu, Arg, UA, Phe | ||||
| • Sensitivity in real conditions: river water samples | ||||
| Environmental pollutants sensing | Cu@Ti3C2 MQDs-aerogel | • Target: pirimiphos-methyl | Sensing: colorimetry (absorption) | 125 |
| • LOD: 0.65 nM | ||||
| • Linear range: 100–3200 nM | ||||
| • Sensitivity in real conditions: seawater, tap water, pear juice, orange juice, milk, tomato juice and corn juice | ||||
| Others sensing | N-doped Ti3C2 MQDs | • Target: quercetin (QC) | Sensing: FL | 126 |
| • LOD: 1.35 nM | Quenching: static quenching/radiation-free complex formation and IFE | |||
| • Linear range: 25–600 nM | ||||
| • Selectivity: rutin, gallic acid, chrysin, morin, kaempferol, phloroglucinol, myricetin, galangin, dopamine, uric acid, serotonin, cysteine, methionine, glutathione, resorcinol, catechol, hydroquinone, lysine, Na+, Cu2+, Mg2+, K+ | ||||
| • Sensitivity in real conditions: onions, oranges, and red wine | ||||
| WLED | S-doped, N-doped, and S–N-doped Ti3C2 MQDs | • CIE coordinates of (0.31, 0.35) | Yellow, blue, and orange emissions to make white LED | 9 |
| • High stability under UV irradiation for a month | ||||
| Single-component WLED | PDMS + Ti3C2 MQDs nanocomposite | • Tuning the white emission temperature from cool to warm | Strong two-photon white fluorescence, originating from both carbon core and OLA-passivated surface states. | 127 |
| • Piezochromic effect | ||||
| White laser | V2C MQDs | • Enhancement in the PL intensity, covering the entire visible range | — | 128 |
| Solar cell | Ti3C2Clx MQDs modified-perovskite | • PCE: 21.31% | (1) Interaction between the Cl− and Pb2+ ions induce the preferred grain orientation with smaller residual tensile strain. | 129 |
| • Increase in Jsc and Voc | (2) Ti3C2Clx QDs near the bottom substrate improve the perovskite film crystallinity. | |||
| • Decrease in normal hysteresis | ||||
| • Maintaining over 84% of PCE after 1000 h | ||||
| Solar cell | Modified perovskite and TiO2 with Ti3C2 MQDs and nanosheets. | • PCE improvement from 12.0% to 17.1% | (1) Positive effect of nanosheets on electron mobility improvement of TiO2 | 34 |
| • Reduced hysteresis effect | (2) Reduced defects concentration via perovskite film passivation and increased perovskite crystallinity | |||
| Solar cell | Introduction of Nb2C MQDs into SnO2 | • PCE improvement by 3%, reaching high value of 22.86% | (1) Grains enlargement | 130 |
| • Maintaining 98% of PCE after 40 days | (2) Modifying the roughness and surface energy | |||
| (3) Lowering defects content | ||||
| (4) Enhanced perovskite crystallinity and effective carrier transport | ||||
| Solar cell | Ti3C2Tx MQDs-modified SnO2 | • Enhanced absorption in visible range | Improving the crystal quality and phase stability of the perovskite | 131 |
| • PCE: 23.3% | ||||
| • Outstanding stability against humidity and light soaking | ||||
| Solar cell | Nb2O5 and Ti3C2 MQDs into the TiO2 | • Increase in both photocurrent and efficiency (7.24%) | (1) Tuning the energy level alignment of the photoanode through the introduction of Nb2O5 and Ti3C2 QDs into the TiO2 photoanode | 132 |
| • Higher and wider absorption range for photoanodes in the visible region and better charge transfer | (2) Suppressing electron–hole recombination at the photoanode interface | |||
| UV detector | Ti3C2Tx MQDs into perovskite | • UV detector −1100% increase in spectral responsivity (264 A W−1 at 310 nm) | Enhancement of charge carriers and light absorption | 8 |
| Ultrafast photonics | Ytterbium-doped fiber laser/Ti3C2Tx MQDs | • Broadband saturable absorption from 540 to 1550 nm | Switching the polarization state in the cavity resulted in tunable mode-locked optical spectra | 77 |
| • Stable 357 ps mode | ||||
| • Locked pulses centered at 1069.17 nm under a low threshold power of 54 mW | ||||
| Ultrafast photonics | The photodeposition method to transfer Ti3C2Tx MQDs to the tapered area to fabricate the SA | • Ultrafast photonics – tapered fiber saturable absorber (SA) device for an Er3+-doped fiber laser (EDFL) | — | 46 |
| • Low saturation intensity (1.983 GW cm−2) and high modulation depth (11.6%) | ||||
| • Ultrashort pulses of 466 fs at a wavelength of 1566.57 nm with a fundamental frequency of 22.78 MHz | ||||
| Nonvolatile memory device | PVP + MQDs nanocomposite | • Stable operation during a retention test (1.2 × 104 s), with an on/off current ratio of up to 100 | (1) MQD charge trapping, originated from quantum confinement and the dissolvability of memristive components | 133 |
| (2) Tuning the electrical conductance of an ITO/MQD-PVP/Au structure from insulator behavior to irreversible resistive switching, reversible resistive switching, and conductor behavior | ||||
| Li–O2 battery | Ti3C2 MQDs/N-doped carbon nanosheets (E'lyte: 1 M LiTFSI/TEGDME) | • Discharge capacity: 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mAh g−1 000 mAh g−1 |
Enhanced electrocatalytic activity, through adsorption of Li+ to C side of the edge-rich of MQDs, and charge accumulation for Li2O2 production on cathode | 134 |
| • Cycling stability: 240 cycles at 200 mA g−1 | ||||
| • Voltage gap: 0.62 V (O2 consumption/evolution overpotential) | ||||
| • Coulombic efficiency: 93.8% | ||||
| Zn–air battery | Ti3C2 MQDs/Ni, Fe-layered dihydrate (Ni, Fe-LDH)/NG (E'lyte: 0.1 M KOH) | • Higher OER activity than pristine LDH/NG | Improved electrical conductivity associated with MQDs (metallic behavior), and provided a higher carrier density compared to LDH sample | 135 |
| • Faster OER kinetics with smaller Tafel slope of 57 mV dec−1 compared to 120 mV dec−1 for Pt/C | ||||
| • 98.8% current retention after 10 h of operation, exceeding 84.3% for the commercial Pt/C | ||||
| Li–S battery | g-C3N4@MQDs (E'lyte: 1 M LiTFSI@DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOL v/v 1 DOL v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2 wt% LiNO3) 1 + 2 wt% LiNO3) |
• Confinement of polysulfide shuttle | Improved capture–confinement–conversion through electrostatic adsorption of protonated g-C3N4 and MQDs to improve capturability of polysulfides | 136 |
| • Capacity = 1433 mA h g−1 at 0.1C | ||||
| • Capacity decay of 0.024% per cycle after 1000 cycles at 2C | ||||
| • Retention rate of nearly 70%, higher than Li–S batteries with GC separator (58%) | ||||
| Supercapacitor | Integrated Ti3C2Tx MQDs onto MXene nanosheets (PVA/H2SO4 gel electrolyte) | • Areal capacitance: 2202 mF cm−2 at 3 mA cm−2 | (1) Heterodimensional structure: 0D TCQDs + 2D TCNs → improved electron transport & ion accessibility | 137 |
| • Areal energy density: 90.33 μWh cm−2 at 450 mW cm−2 | (2) Strong interface interactions: polar functional groups enhance structural integrity & stability | |||
• Capacitance retention: 84% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles 000 charge–discharge cycles |
||||
| Supercapacitor | MQD-Ni(OH)2 composite (1 M KOH) | • Specific capacitance: 1660 F g−1 at 1 A g−1 | (1) Quantum confinement of QDs → enhanced conductivity → faster electron transport, and boosted electrochemical reactivity | 138 |
| • 98 F g−1 for asymmetric supercapacitor | (2) Thin Ni(OH)2 nanosheets → increased active sites | |||
| • Energy density: 30.6 Wh kg−1 (at 750 W kg−1 power density) | ||||
• Capacitance retention: 84% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
||||
| • Rct: 6.7 Ω (lower than Ni(OH)2-only) | ||||
| Supercapacitor | MQDs/Laser Reduced Graphene Oxide (LRGO) (PVA/H2SO4 gel electrolyte) | • Areal capacitance: 10.42 mF cm−2 (91% transmittance) | Enhanced surface area & edge states → improved charge storage synergistic MQD/LRGO interface → higher electron/ion transport | 12 |
| • 64.6 mF cm−2 (53% transmittance) | ||||
| • Energy density: 2.04 × 10−3 mWh cm−2 | ||||
| • Power density: 129.4 μWh cm−2 | ||||
• Cycle life: 97.6% retention after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
||||
| • Transparency: over 90% | ||||
| • Conductivity: 760.4 S m−1 | ||||
| Supercapacitor | Ti3C2 MQDs/large Ti3C2Tx (L-Ti3C2Tx) fiber (PVA/H2SO4 gel electrolyte) | • Electrode capacitance: 1560 F cm−3 at 1 A cm−3 | (1) Large L-Ti3C2Tx NSs → improved mechanical strength | 139 |
| • Capacity retention: 79% at 20 A cm−3 | (2) Synergistic interaction of Ti3C2Tx QDs & L-Ti3C2Tx → higher efficiency | |||
| • Mechanical strength: 130 MPa | ||||
| • Supercapacitor capacitance: 413 F cm−3 at 0.5 A cm−3 | ||||
• Cycle stability: 97% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
||||
| • Energy density: 36.7 mWh cm−3 at 311 mW cm−3 | ||||
| • Temperature range: −40 to 60 °C | ||||
| Photocatalytic HER | Ti3C2 MQDs/g-C3N4 (0.5 M Na2SO4) | • Co-catalysts for photocatalytic H2 evolution | Increased H2 reduction rate due to high aqueous solubility of MQDs and more abundant active edge sites | 140 |
| Photocatalytic HER | N-doped Ti3C2/1D-CdS (0.1 M Na2SO4) | • HER rate: 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094 μmol g−1 h−1 (14.79× higher than pure CdS) 094 μmol g−1 h−1 (14.79× higher than pure CdS) |
Facilitated separation and charge carriers transfer from photoexcitation of CdS QDs, and supply Lewis acid sites for adsorption of 1,2-BM to accelerate H2 evolution | 138 |
| • Rct: 3.57 Ω (significantly reduced) | ||||
| • Electron lifetime: 6.65× longer than pure CdS | ||||
| • Photostability: no significant loss after 4 cycles | ||||
| Photocatalytic HER | MQDs/Co2(OH)3Cl/CF (0.1 M KOH) | • Overpotential: 91 mV@10 mA cm−2 | (1) Interface charge transfer: induces Co2+δ high-valence sites for better H2O activation | 141 |
| • Tafel slope: 67 mV dec−1 | (2) Lattice strain effect: improves structural integrity | |||
| • Low charge transfer resistance (Rct): 4.514 Ω | (3) Lowering the kinetic barrier for H2O dissociation (Volmer step): tuning the H* adsorption Gibbs free energy toward the thermoneutral ideal. | |||
| • Electrochemical Surface Area (ECSA): 240 cm−2 | ||||
| • Stability: 82.6% retention after 30 h | ||||
| Photocatalytic HER | Ti3C2 MQDs modified 3D/2D TiO2/g-C3N4 (0.1 M Na2SO4) | • Hydrogen production rate of 5540.2 μmol g−1 h−1, 1.6 times higher than that of TiO2/g-C3N4 and 33 times higher than that of pure g-C3N4 | (1) Promoting strong interaction between cocatalysts due to MQDs’ functional groups | 142 |
| (2) Stronger performance due to reducing electron–hole recombination | ||||
| (3) Accelerating electron migration from the conduction band of g-C3N4 | ||||
| Nitrogen Reduction Reaction (NRR) | Ti3C2 MQDs/2D Ni-MOF (0.5 M Na2SO4) | • Ammonia yield: 88.79 μmol g−1 h−1 (4× higher than pristine Ni-MOF) | (1) MQD excitation → electrons transfer to Ni-MOF | 138 |
| • Band gap: 3.6 eV (Ni-MOF), 4.92 eV (Ti3C2-QD) | (2) Ni sites act as active centers → adsorption & activation of N2 MQDs with abundant active sites enable efficient NRR | |||
| NRR | Ti3C2 MQDs decorated on the 2D Ni-MOF (3 M KOH) | • Photo-reduction N2 activity of 88.79 μmol g−1 h−1, which was 4 times higher than that of the pristine 2D Ni-MOF | (1) Interfacial area of the Ti3C2 MQD/Ni-MOF composite to facilitate the photocarrier's direction migration | 4 |
| (2) Reducing the free-energy corresponding N–N bond and length of the N2 fixation pathways | ||||
| NORR | Ti3C2 MQDs/Cu NWs (0.1 M K2SO4) | • Electrochemical reduction of NO into NH3 high NH3 yield of 5346.3 μg h−1 mg−1 | NO into NH3 reaction pathway (*NO → *HNO → *N → *NH → *NH2 → *NH3) and potential determination step (*N → *NH) for the NORR on Ti3C2 QDs/Cu NWs | 143 |
| • Faradaic efficiency of 95.5% in 0.1 M K2SO4 solution at a much lower potential of −0.4 V vs. RHE | ||||
| • Zn–NO battery with Ti3C2 QDs/Cu NWs as the cathode showed power density of 3.03 mW cm−2 and an NH3 yield of 925.2 μg h−1 mg−1 | ||||
| NOx oxidation | Ti3C2Tx MQDs/g-C3N4/bismuth oxyiodide (BiOI) (0.2 M Na2SO4) | • NO removal rate: 42.23% (highest among tested catalysts) | p–n heterojunction led to enhanced charge separation and transfer, as well as promoting gas absorption and activization, further achieving an excellent photocatalytic performance. | 138 |
| • NO2 production: 31.52 ppb (lowest among tested catalysts) | ||||
| • NO2 selectivity: 0.17 (indicates efficient NO oxidation) | ||||
| • Stability: 5-cycle test shows minimal degradation | ||||
| CO2 photoreduction | Ti3C2Tx MQDs/g-C3N4/BiOI 0.5 M Na2SO4 | • CO production rate: 57.8 μmol g−1 h−1 (highest among tested catalysts) | Electron-withdrawing ability of MQDs, further promotes the generation of more active species involved in reactions | 144 |
| • CH4 production rate: 3.6 μmol g−1 h−1 | ||||
| • Adsorption capacity: 4.53 cm3 g−1 for CO2 | ||||
| • CN/MQDs/BOI showed CO production rate of 57.8 μmol g−1 h−1 and CH4 production rate of 3.6 μmol g−1 h−1 | ||||
| CO2RR | Ti3C2 MQDs decorated Cu2O NWs on Cu mesh (0.5 M Na2SO4) | • Methanol production | (1) Improved the stability of Cu2O NWs, enhanced charge transfer, carrier density, light adsorption, and decreasing band bending edge and charge recombination | 145 |
| • Photocatalytic reduction of CO2 by Ti3C2 QDs/Cu2O NWs/Cu is 8.25 times and 2.15 times of that from Cu2O NWs/Cu and Ti3C2 sheets/Cu2O NWs/Cu | (2) Ti3C2 QDs favorably chemisorbed CO2 instead of H2O and further facilitated CO2 reduction by promoting H+ attachment and donating electrons | |||
| Photocatalysis | Ti3C2 MQDs/Cu2O NWs/Cu (0.5 M Na2SO4) | • Photoelectrochemical CO2 reduction | Light absorption improvement, facilitated charge separation, and transport | 146 |
| H2O2 production | Nb2C MQDs@PY-DHBD-COF (pyrene-based) | • Achieved a H2O2 yield record of 3560 μmol g−1 h−1, and AQY of 12.8% at 400 nm together with SCC efficiency of 0.27% | • Adsorption of O2 on the MQDs to form *O2 | 147 |
| • Transfer of e− to the *O2 to be reduced to *OOH | ||||
| • Combination of H+ with *OOH to form *HOOH | ||||
| • Hydrogenation of *HOOH to form H2O2 | ||||
| Nucleus-target low-temperature photothermal therapy | V2C MQDs modified with the cell nucleus-target TAT peptides and cell target Arg-Gly-Asp | • Tumor clearance at low temperature | Processed proteins facilitated drug nuclear-targeting of cancer cells. Under NIR-II laser irradiation, V2C-PEG-TAT's temperature raised, leading to genetic material damage. | 148 |
| • Without side effects non-toxic | ||||
| Antitumor activity in vivo | Non-oxidizing N-MQDs-Ti3C2Tx | • Tumor inhibition rate of 91.9% | (1) By intratumorally MQDs administering in HeLa tumor xenografts, the Ti3+ bonded to cancer cells in H2O2, catalyzing its production of highly toxic species led to cancer cell death. | 92 |
| • No significant toxicity during the therapeutic treatment | (2) Tumor microvascular permeability caused hemorrhage. | |||
| • Excreted by the kidneys of animals 48 h after administration | ||||
| Photothermal cancer therapy | Fluorine-free Ti3C2 MQDs | • Photothermal conversion efficiency of 52.2% | Al(OH)4 on the surface of MQDs caused strong absorption in the NIR region. Irradiation of 808 nm with a power of 0.5 W cm−2 led to a tumor destruction rate of 100% with no significant change in the bodyweight of mice. | 149 |
| • Extinction coefficient of 52.8 L g−1 cm−1 | ||||
| • High photostability | ||||
| • No biotoxicity at a concentration of 100 PTT (in vitro) on HeLa, MCF-7, U251, and HEK 293 cells. | ||||
| • No toxic effects and side effects of the QDs on mouse cells | ||||
| PA imaging guided photothermal therapy | Ti2N MQDs | • Applicable in NIR-I and NIR-II region | Mice injected with Ti2N QDs. After, 10 min laser irradiation at 1.0 W cm−2 power density in NIR-I and NIR-II, the temperature increased from 37 °C to more than 60 °C and 69 °C. | 148 |
| • High absorption | ||||
| • High photothermal conversion efficiency | ||||
| • Photothermal stability | ||||
| PA/photothermal imaging-guided PTT/intracellular microRNA imaging | Mo2C MQDs | • Photothermal conversion efficiency of 42.9% at low concentration (100 µg mL−1) | After intravenous administration of Mo2C QDs in mouse bearing B16-10F tumor, an 808 nm laser (0.64 W cm−2, 10 min) irradiated. This increased the tumor temperatures from 27.9 °C to 63 °C. | 92 |
| • Biocompatibility | ||||
| • Photothermal stability | ||||
| • Efficient tumor accumulation through EPR | ||||
| • Providing photoacoustic imaging signal | ||||
| Photothermal immunoassay/protein point-of-care (POC) testing and diagnostics | Ti3C2 MQDs-encapsulated liposomes | • High photothermal efficiency | PSA detected at 808 nm with 1.5 W cm−2. NIR laser energy was converted into heat using Ti3C2 QDs and the temperature shift was assessed. | 149 |
| • Ability to detect infinitesimal concentrations of prostate-specific antigen | ||||
| Multicolor cellular imaging | Different morphologies of Ti3C2 MQDs with RAW264.7 cells | • Strong photoluminescence | Within 4 h incubation, the MQDs were incorporated into the cells by endocytosis. Then the images were taken with confocal fluorescence microscopy. | 82 |
| • High biocompatibility | ||||
| • Multicolor excitations at 405, 488, and 543 nm | ||||
| • Penetrate easily into the cell without damaging the nucleus | ||||
| Imaging and biolabeling abilities | Ratiometric fluorescent pH sensors based on Ti3C2 MQDs | • Excellent performance for living cells | Images taken with confocal fluorescence microscopy of MCF-7 cells after endocytosis of Ti3C2 QDs. As pH decreases from 8 to 6, fluorescence from the Ti3C2 QDs increases. | 150 |
| Fluorescence probes applicable for the cellular study | Nb2C MQDs | • Highly chemically stable | MQDs were cocultured with cells for 4 h. The cytoplasm of the cells was selectively stained. | 82 |
| • Biocompatible | ||||
| • Photobleach resistant | ||||
| • Selectively stained the cytoplasm of the cells | ||||
| Cell imaging/detecting Cu2+ ions/diagnosis of cytophagy-related diseases | N, P-MQDs | • The tests are performed at nontoxic concentrations | The N, P-MQDs were taken up by the THP-1 macrophages in 24 h and emitted green colors when exposed to 488 nm light source. | 151 |
| • Photoluminescence quantum yield (PLQY) of 20.01% (by doping) | ||||
| • Constant fluorescence intensity in a wide pH and temperature range | ||||
| • Good stability and stable expression in complex organisms | ||||
 | ||
| Fig. 9 (a) PL emission spectra of Cu2+-(N, P-MQD) depending on the Cu2+ concentration (reprinted with permission from The Royal Society of Chemistry, copyright © 2019).102 (b) Fluorescence-based detection of Fe3+ ions by BSA@MQDs (reprinted with permission from Wiley, copyright © 2022).39 (c) Fluorescence quenching and recovery of Ti3C2 MQDs by metal ions and EDTA addition, respectively (reprinted with permission from Elsevier, copyright © 2022).5 (d) UV-Vis spectra of Fe3O4@Ti3C2 MQDs depending on the Cr(VI) concentration (reprinted with permission from Elsevier, copyright © 2023).122 (e) ECL curves of MUC1 immunosensor (reprinted with permission from Elsevier, copyright © 2022).101 (f) ECL detection of miRNA-26a with various concentrations by Ti3C2 MQD@Au nanobones (reprinted with permission from the American Chemical Society, copyright © 2021).72 (g) Changes in the fluorescence spectra of N-Ti3C2 MQDs with increasing AA concentrations (reprinted with permission from Elsevier, copyright © 2021).107 (h) Variations in the fluorescence spectra of N-MQDs with ARS concentrations. The inset shows the colloidal samples under daylight (left) and 365 nm UV (right) (reprinted with permission from Elsevier, copyright © 2021) images.100 (i) The florescence spectra of UA@Ti3C2 MQDs fluorescence depending on the TNP concentrations (reprinted with permission from The Royal Society of Chemistry, copyright © 2020).88 | ||
Fe3+ is another critical element that has widely been detected by MQDs via fluorescence technique because iron deficiency can lead to anemia, while excessive iron levels are associated with conditions such as hyperferremia and cancer.121 Gao et al.68 used fluorescence quenching of Ti3C2 MQDs to detect Fe3+ with a high sensitivity (0.6377 mM−1) and low LOD (1.4 μM) through the electrostatic-induced aggregation mechanism.68 Amino-rich Ti3C2 MQDs demonstrated even greater sensitivity, achieving a LOD of 0.17 μM with excellent linearity for Fe3+ detection, through binding of the metal ions with the amino groups.64 The lowest reported LOD for Fe3+ detection (1.25 nM) was attained when BSA@Ti3C2 MQDs were used.70,121 The detection mechanism involved electron and energy transfer processes where the metal ions facilitated electron–hole recombination annihilation and altered the electronic state of the MQDs (Fig. 9b).
Cobalt and silver ions are of particular concern due to their environmental risks153 and potential to cause cellular diseases,2 making their sensitive and simultaneous detection highly significant. Cr3+ plays an essential role in biological processes and metabolism; however, elevated concentrations of Cr3+ can adversely affect cellular structure scan.2 N-Ti3C2 MQDs enabled real-time and simultaneous detection of Co2+ and Ag+ with a LOD of 0.21 μM and 0.10 μM, respectively.2 The primary detection mechanism was attributed to a combination of the IFE and the static quenching effect, resulting from the overlap between the absorption spectra of Co2+/Ag+ ions and the emission and excitation spectra of the N-Ti3C2 QDs.2 In another study, by taking advantage of Ti3C2 MQDs, Cr3+ was detected with a low limit of detection of 30 mM with high selectivity in the presence of interfering ions.5 Although all Cr3+, Fe2+, Co3+, and Cu2+ quenched the emission of QDs, the addition of ethylenediaminetetraacetic acid recovered the PL response for all interfering elements except Cr3+, indicating selective detection of the target ion (Fig. 9c).5 Another recent progress is colorimetric detection of Cr(VI) ions by Fe3O4@Ti3C2 MQDs since an exceeding concentration of Cr(VI) in drinking water causes various diseases.122 This colorimetric method includes a catalytic oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) by Fe3O4@Ti3C2 MQDs in the presence of H2O2, resulting in the formation of a blue-colored product. Then, introducing 8-Hydroxyquinoline (8-HQ) reduces the blue color intensity of oxidized TMB. Introducing Cr(VI) with various concentrations up to 60 μM leads to a linear increase in the absorbance and recovery of the blue color (Fig. 9d), allowing sensitive and selective detection of Cr(VI) with a LOD of 0.26 μM.122 Ti3C2 MQDs have also been utilized for the detection of manganese (Mn(VII)) with a low LOD of 5.2 nM, which is superior to carbon dots with a LOD of 230 nM. This exceptional sensitivity was associated to the inherent reducibility of the MQDs, which facilitated a redox reaction with Mn(VII), resulting in pronounced fluorescence quenching. In contrast, the fluorescence quenching mechanism in carbon dots involved a synergistic IFE and static quenching due to the overlap between the absorption peaks of Mn(VII) and the excitation/emission spectra of the CDs. Further studies underscored the potential of these MQDs to serve as a dual-functional platform for simultaneous detection and scavenging of Mn(VII) in plant leaves, showcasing their impeding application in environmental safety and biological monitoring.154
Researchers have developed a novel MXene-based biosensor for detecting polynucleotide kinase (PNK).71 This enzyme plays a crucial role in phosphorylation-related DNA repair and nucleic acid metabolism. This biosensor, which operates via an ECL mechanism (eqn (1)–(5)) utilizes a hybrid Ti3C2 MQD/Au NPs in the presence of a co-reactant (K2S2O8). The hybrid NPs offer significant advantages over Ti3C2 MQDs, including a threefold enhancement in the ECL signal due to surface plasmon resonance (SPR), superior electrocatalytic performance, and charge transfer capabilities. PNK interacts with the DNA probe immobilized on the modified electrode surface, triggering a series of reactions that amplify the ECL signal. Under optimal conditions, a linear relationship between ECL intensity and PNK concentration is attained, making this biosensor a powerful tool for PNK analysis in comparison to other sensors.71
| S2O82− + e− → SO42− + SO4˙− | (1) |
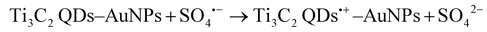 | (2) |
| Ti3C2 QDs–AuNPs + e− → Ti3C2 QDs˙−–AuNPs | (3) |
 | (4) |
| Ti3C2 QDs*–AuNPs → Ti3C2 QDs–AuNPs + hv | (5) |
Jiang et al.101 detected Mucin 1 (MUC1), a key molecule involved in cell signal transduction,155 by an ECL biosensor based on N-Ti3C2 MQDs.101 The MQDs exhibited fluorescence and ECL emissions at 455 nm and 612 nm, respectively. To fabricate the biosensor, Au NPs were electrodeposited onto a glassy carbon electrode (GCE) and functionalized with anti-MUC1 antibodies, followed by BSA treatment to prevent nonspecific binding. Next, the MUC1 antigen was introduced to this surface. The biosensor platform was then promoted with adding an AgPt@N-Ti3C2 QDs-anti-MUC1-BSA bioconjugate to enhance ECL sensitivity. The AgPt@N-Ti3C2 QDs nanocomposites played a crucial role in developing the ECL immunosensor for MUC1 detection through interacting with the co-reactant S2O82− in phosphate-buffered saline (PBS). As the concentration of MUC1 increased from 1 fg mL−1 to 1 ng mL−1, the ECL signal was linearly enhanced, providing a low LOD of 0.31 fg mL−1 (Fig. 9e).101
To detect VP with a LOD of 30 cfu mL−1, a composite probe comprised of modified polyhedral oligomeric silsesquioxane-perovskite quantum dots (POSS-PQDs) with aptamers (as the signal probe) and Ti3C2 MQDs (as the quencher) was used. The fluorescence emission of the probe was quenched through FRET in the absence of the VP. Introducing VP recovered the PL signal and amplified it in a concentration-dependent manner.116
ARS is a commonly used dyeing agent for textiles; however, its toxicity poses a significant risk to human health and ecological stability.100 Recently, N-Ti3C2 MQDs were found effective in the successful detection of ARS, achieving a LOD of 1.21 μM in the linear range of <80 (Fig. 9h).100 The quenching mechanism was identified as IFE due to three key observations. First, the absorption spectra of ARS overlapped with the excited emission spectra of N-Ti3C2 MQDs, thereby reducing the emission of N-Ti3C2 QDs. Second, the fluorescence lifetimes of N-Ti3C2 MQDs remained nearly unchanged before and after the addition of ARS, suggesting that the quenching is not due to static quenching but rather to IFE. Third, no new absorption peaks were observed in the absorption curves after adding ARS, indicating that there are no chemical interactions.100
TNP is a versatile chemical compound widely used in various industrial applications, including explosives, matches, dyes, and leather products.88 TNP is highly toxic, and prolonged exposure can cause adverse symptoms such as headaches, dizziness, and nausea. Wang et al.88 successfully detected TNP by UA-modified Ti3C2 (UA@Ti3C2 MQDs). A significant overlap was observed between the absorption spectrum of TNP and the emission spectrum of UA@Ti3C2 MQDs, suggesting the potential involvement of FRET or IFE. Further analysis using UV absorption and the average fluorescence lifetime of UA@Ti3C2 MQDs revealed that the quenching occurred via the IFE mechanism. The fluorescence intensity decreased with increasing TNP concentration, enabling the detection of TNP within a range of 0.01 to 300 μM, with a LOD of 9.58 nM (Fig. 9i). The detection limit was lower than the maximum residue limit of TNP in drinking water, as set by the US Environmental Protection Agency.88 In another study, Ding et al.125 used Cu@Ti3C2 MQDs-aerogel to detect pirimiphos-methyl, a kind of organophosphorus pesticides. The detection mechanism was based on the peroxidase-like activity of Cu@Ti3C2 MQDs-aerogel, which catalyzed the decomposition of hydrogen peroxide (H2O2) into reactive hydroxyl radicals (˙OH). This process enabled an effective colorimetric assay by measuring the peak intensity at 652 nm, corresponding to the oxidation of TMB. As the concentration of pirimiphos-methyl increased, the catalytic activity decreased, allowing for its precise detection with a LOD of 0.65 nM.125
Tc is a versatile antibiotic in animal care, but its misuse poses health risks, including allergic reactions and ecological accumulation.109 Therefore, there is an urgent need for efficient detection of Tc residues in food and water.109 A ratiometric fluorescent sensor based on N, B co-doped Ti3C2 MQDs and Eu3+ was found effective in Tc detection with a LOD of 20 nM. When Tc was added to a N, B co-doped Ti3C2 MQDs solution, the emission intensity at 448 nm was slightly reduced due to the IFE mechanism. As the Tc concentration increased, the 448 nm emission peak was gradually weakened, while another peak that appeared at 616 nm appeared, underscoring the prominent effect of Eu3+ in Tc detection. The successful performance of Eu3+/N, B-Ti3C2 MQDs in the detection of Tc in the milk samples demonstrated their strong potential for practical use.109
There is also an urgent need to advance sensitive and non-toxic detection methods for H2O2.118 As an example of potential application, the tumor microenvironment in cancer cells often exhibits an overproduction of H2O2, leading to the formation of highly reactive and toxic hydroxyl radicals (˙OH). These hydroxyl radicals, in turn, induce oxidative stress and damage to cancer cells, prompting processes such as apoptosis (programmed cell death) and inhibition of tumor growth.92 Hence, detecting H2O2 levels accurately is critical for understanding its role in diseases and for the development of sensitive, non-toxic detection methods.118 Recently, N-Ti3C2 MQDs were utilized for H2O2 sensing with a LOD of 1.2 nM which is the lowest reported value to date.118 In another study, it was shown that Ti3C2 MQDs synthesized via the acoustic method exhibit more H2O2 sensing capability compared to those prepared by the hydrothermal method.97 The sensor platform was based on Ti3C2 MQDs/glassy carbon (Naf/MQD/GCE).97 The square wave voltammetry revealed a LOD of 5 nM (Fig. 10a and b), with a higher linear regression slope of the current response plots.97 H2O2 was also detected by N-Ti3C2Tx MQDs via a colorimetric approach using TMB.92 Here, the breakdown of the O–O bond in H2O2 into ˙OH, which oxidized TMB, resulting in two distinctive absorbances at 370 and 652 nm. This enabled the detection and quantification of hydroxyl radicals and H2O2.92
 | ||
| Fig. 10 (a and b) Square wave voltammetry curves of Naf/MQD/GCE and Naf/HMQD/GCE at varying H2O2 concentrations (reprinted with permission from the American Chemical Society, copyright © 2021).97 (c) CV curves of Ti3C2-MQDs@3DE in PBS for DA detection (reprinted with permission from Elsevier, copyright © 2023).113 (d) Normalized PL spectra of [Ru(dpp)3]Cl2 and Ti3C2 MQDs at different pH levels (reprinted with permission from The Royal Society of Chemistry, copyright © 2019).43 (e) Quartz BAW infrared detector response to NIR irradiation before/after coating (reprinted with permission from Optica Publishing Group, copyright © 2022).157 | ||
N-Ti3C2 MQDs functionalized with oxOPD (N-Ti3C2 QDs@DAP) have proven highly effective for detecting substances like xanthine, which generates H2O2 through specific enzymatic reactions.92 The operation of this nanoprobe involves a multi-step enzymatic process. Xanthine is oxidized by xanthine oxidase (XOD) to produce H2O2, which subsequently reacts with OPD in the presence of HRP. This reaction results in the formation of DAP. The presence of DAP not only quenches the fluorescence of N-Ti3C2 MQDs but also provides a new emission peak at 560 nm, establishing a dual-emission sensor. Here, N-Ti3C2 MQDs serve as electron donors, while DAP acts as an electron acceptor. Consequently, a complex is formed on the functionalized surface of the QDs, quenching the fluorescence intensity. This dual-emission system provides a sensitive and reliable method for detecting xanthine and H2O2, showcasing the versatility and efficacy of N-MQDs@DAP in diagnostic applications.92
Ti3C2 MQDs have also been utilized for the ratiometric fluorescence detection of a Bacillus anthracis biomarker, 2,6-dipicolinic acid (DPA).124 Ti3C2 MQDs are endowed with abundant hydroxyl and amino groups, which are utilized for coordination with EDTA and Eu3+ to form a composite probe, named Ti3C2 QDs–EDTA–Eu3+. While Ti3C2 QDs emit stable blue color as a reference signal, the fluorescence of Eu3+ is quenched by surrounding water molecules. The introduction of DPA replaces the water molecules and enhances the Eu3+ fluorescence intensity at 616 nm due to the absorbance energy-transfer effect (AETE) from DPA to Eu3+. A visible fluorescence shift from blue to red with the DPA concentration enables quantitative detection by a smartphone equipped with color recognition software.124
In a recent study, Ti3C2-MQDs have been utilized to detect CUR, which has antioxidant, anti-inflammatory, antibacterial, and anticancer properties.62 It is known that excessive amounts of CUR may boost DNA oxidative activity, lower Adenosine triphosphate (ATP) levels, and trigger necrotic processes.62 Detection of CUR was achieved by leveraging the overlap between the emission peak of Ti3C2 MQDs at 430 nm and the absorption spectrum of CUR. When CUR is introduced, the fluorescence emission of Ti3C2 MQDs is quenched via FRET to CUR, while CUR fluorescence emission increases. Following the addition of ClO− to this solution, the fluorescence emission of Ti3C2 MQDs is recovered, as CUR's phenol and methoxy groups are oxidized to quinones in the presence of ClO−. The linear detection range of ClO− has been found to be 25–150 μM and 150–275 μM, with a detection limit of 5 μM. For CUR, the linear detection range is 0.05–10 μM, with a LOD of 20 nM. The MQDs also exhibit a colorimetric response to CUR, which causes a visible color change from colorless to yellow.99
In addition to CUR, quercetin, a well-known antioxidant bioflavonoid, was detected by bright bluish-green emissive N-doped Ti3C2 MQDs.126 The introduction of quercetin resulted in a significant fluorescence quenching due to static quenching/radiative-free complex formation and IFE. Consequently, the quantification of quercetin over a linear range of 25–600 nM with a low LOD of 1.35 nM was performed. The sensor demonstrated exceptional specificity for quercetin in real food samples, such as orange, onion, and red wine, with a quenching efficiency exceeding 95% compared to less than 10% for potential interferents. This finding demonstrates the significant potential of MQDs in various applications, including food safety, pharmacological assays, and the detection of various biological markers.126
Ti3C2 MQDs was also utilized to detect dopamine (DA), a catecholamine released by the adrenal medulla.113 A Ti3C2 QDs@3D-printed electrode sensor enabled DA monitoring with a LOD of 3 nM over a linear range of 0.01 to 20 μM. CV determined an oxidative potential of 0.29 V for DA with an increasing trend in the current density with its concentration (Fig. 10c).113
Recently, a nanosurface molecularly imprinted polymer (Ti3C2 MQD@MIP) resonance Rayleigh scattering (RRS) spectral probe has been developed for the sensitive and selective detection of thiocyanate (SCN−).158 In this platform, Ti3C2 MQDs serve as the matrix, while SCN− act as the template during the imprinting process. The functional monomer, (3-aminopropyl) triethoxysilane, and the cross-linker (tetraethoxysilane) facilitate the formation of the polymer network, with ammonia serving as the polymerization initiator. The detection mechanism is based on changes in RRS intensity, where the interaction between SCN− and the Ti3C2 MQD@MIP probe results in a concentration-dependent reduction in scattering intensity. This allows for accurate quantification of SCN− with a linear detection range of 0.87–5.22 μg L−1 and a LOD of 0.37 μg L−1.158 In another study, NO2− (nitrite), a crucial additive in food preservation, was successfully detected by the dual colorimetric/fluorometric method using N, P-Ti3C2 MQDs and the Phen-Fe2+ complex.84 The fluorescence emission of N, P-Ti3C2 QDs was quenched by the Phen-Fe2+ complex via the IFE, accompanied by the intensified orange color. Upon the addition of NO2−, the redox reaction between NO2− and Fe2+ generated Fe3+, reducing IFE by fluorescence recovery as well as a gradual change from orange to colorless. Using a smartphone-assisted colorimetric filter paper based on the N, P-Ti3C2 QDs/Phen-Fe2+ system, a linear relationship between the red, green, blue (RGB) ratio and NO2− concentration (up to 80 μM) was attained. More recently, N, B-doped Ti3C2 MQDs incorporated onto a functionalized paper were developed for the efficient adsorption and detection of dichromate ions (Cr2O72−).159 The adsorption mechanism was attributed to a combination of electrostatic interactions and chemical bonding. The sensing mechanism involved fluorescence quenching via IFE with an efficiency of 99.9%, a response time of 10 s, and a LOD of 1.2 μM. The incorporation of green and renewable wood pulp fibers as raw materials further emphasized the potential of this innovative material for large-scale production and industrial application in water pollution management.159
The pH sensing potential of Ti3C2 MQDs, as effective ratiometric pH sensors, was also demonstrated when combined with the pH-insensitive [Ru(dpp)3]Cl2 dye.43 This setup enabled precise intracellular pH monitoring via fluorescence intensity ratios. The ratiometric fluorescence change due to pH variations was characterized by a decrease in the intensity of the Ti3C2 MQD emission at 460 nm, while the emission of reference dye remained stable. A linear decrease in the fluorescence intensity ratio (I460/I615) with the pH in the range of 5 to 9 was noticed (Fig. 10d). This linear calibration curve for the fluorescence ratio (I460/I615) enabled accurate pH differentiation in the physiological range of 6.0 to 8.0. The sensors also demonstrated profound imaging and bio-labeling capabilities for reliable, long-term pH monitoring in biological environments.43
5.2. Optoelectronics
One of the earliest MQDs-based WLED with Commission Internationale de l’Éclairage (CIE) coordinates of (0.31, 0.35) was fabricated by Xu Quan et al.9 in 2019. To fabricate this device, which was stable at a voltage of 3 V after a month (Fig. 11a), Ti3C2Tx-MQDs were hydrothermally co-processed with sodium thiosulfate (S-doped), an ammonia solution (N-doped), and a combination of these two (SN-doped) to emit blue (440 nm), yellow (540 nm), and orange (580 nm), respectively, followed by mixing with polyvinylpyrrolidone (PVP) and casting on a blue chip. Similar to lead-free double perovskites and SiC QDs, Ti3C2 MQDs also manifested great potential to be used for single-component white LEDs (Fig. 11b).127,163 In this case, a hybrid nanocomposite was prepared by polymerizing Ti3C2 MQDs, having strong two-photon white fluorescence with the full width at half maximum (FWHM) and PLQY of 220 nm and 9.36%, respectively, in a polydimethylsiloxane solution. This working LED could emit white light with the color coordinates of (0.30, 0.34). Moreover, the fluorescence of the Ti3C2 MQDs under high pressure (2.53 GPa) enabled tuning the white emission temperature from cool to warm, (Fig. 11c) which is a fascinating phenomenon for the next generation of single-component warm WLEDs.127
 | ||
| Fig. 11 (a) CIE coordinates of WLED based on MQDs/PVP under 360 nm excitation. The inset showing fluorescence images of the W-MQDs/PVP composite under 365 nm emission (reprinted with permission from Elsevier, copyright © 2019).9 (b) PL spectra of the visible-light-emitting MQDs under 360 nm emission (reprinted with permission from Elsevier, copyright © 2019).9 (c) PL spectra of Ti3C2 QDs depending on the applied pressure (reprinted with permission from Wiley, copyright © 2019).127 (d) Images showing different excitation wavelengths for V2C MQDs (xenon lamp) (reprinted with permission from Wiley, copyright © 2019).128 | ||
White lasers are increasingly important in fields such as laser display technology, communication technology, biomedicine, and environmental detection.164–167 To fabricate white lasers, a wide range of materials, including dye mixtures168 semiconductors,169 laser or nonlinear optical crystals,166 and QDs128 have been used.170–172 However, constrained by compactness, difficulty in growth or wavelength control, and environmentally unfriendly chemicals, white lasers are still underdeveloped, and the available materials and approaches are still being extensively sought. One of the most promising white lasers was fabricated by Huang et al.128 using V2C MQDs. By employing passivation treatments, the PL intensity of V2C MQDs, spanning the entire visible spectrum, was significantly enhanced. Fig. 11d illustrates the light emission images of passivated V2C QDs under various excitation wavelengths. The localized nonlinear random scattering of the passivated V2C MQDs has been achieved through the generation of excitation-power-dependent solvent bubbles. This phenomenon plays a critical role in producing a white laser with multiple color outputs. With optimized excitation conditions, the amplification and simultaneous lasing of blue (490 nm), green (545 nm), yellow (587 nm), and red (613 nm) light has been demonstrated.
For instance, incorporation of Cl-terminated Ti3C2 MQDs into the FAxMA1−xPbI3 perovskite could increase the PCE from 19.53% to 21.31%. Not only short-circuit current density (Jsc) and open-circuit voltage (Voc) of the cells increased, but also the difference between the forward and reverse scan curves, called normal hysteresis, decreased (Fig. 12a and b). This performance improvement can be attributed to two key factors. First, the strong interactions between the Cl terminations of Ti3C2Clx and Pb2+ ions promote a preferred grain orientation with reduced residual tensile strain by slowing the crystallization rate, thereby improving the crystallinity of the perovskite film. Second, the natural formation of a top-down gradient distribution leads to a higher concentration of Ti3C2Clx MQD additives near the bottom substrate. These additives serve as nucleation sites for the growth of high-quality perovskite crystals to facilitate efficient charge extraction between the SnO2 electron transport layer (ETL) and the perovskite layer. Besides, Cl-terminated Ti3C2Tx MQDs avoid possible deprotonation of protonated organic amine in perovskite. Consequently, the solar cell exhibits outstanding long-term stability by maintaining over 84% of its initial PCE after being aged for 1000 h under 40% relative humidity.129 In another study, the MAPbI3 perovskite and TiO2 layers were modified by Ti3C2 MQDs and MXene nanosheets, respectively. Apart from the positive effect of nanosheets on electron mobility improvement of the ETL, 0.8 mg MQDs reduced defects concentration via successful perovskite film passivation and increased perovskite crystallinity. These claims could be supported by an enhancement in the PL emission intensity and carrier lifetime, as shown in Fig. 12c–e. As a result of this modification, the PCE improved from 12.0% to 17.1%, along with the reduced hysteresis effect.34
 | ||
| Fig. 12 (a) Schematic illustration of the Ti3C2Clx MQDs-treated FAxMA1−xPbI3 PSCs with colored cross-sectional scanning electron microscopy (reprinted with permission from Elsevier, copyright © 2022).129 (b) Reverse and forward scans for the pristine and 0.2 mg mL−1 Ti3C2Clx MQDs-treated solar cells (reprinted with permission from Elsevier, copyright © 2022).129 (c) UV-Vis absorption spectra, (d) PL spectra, and (e) J–V curve of perovskite films with different content of Ti3C2 MQDs (reprinted with permission from Elsevier, copyright © 2020).34 | ||
Similar to MXene nanosheets, the ETL modification by MQDs manifested great potential to boost both PCE and stability. For instance, it was demonstrated that the introduction of Nb2C MQDs into SnO2 (as the ETL) was effective in enlarging the grains, modifying the roughness and surface energy, and reducing defect sites.130 Subsequent deposition of Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 perovskite films on the SnO2–Nb2C ETL enhanced perovskite crystallinity and effective carrier transport, leading to a PCE of 22.86%. The device maintained 98% of its as-prepared efficiency after 40 days holding at 25 °C under 40–60% humidity (Fig. 13a and b).130 In another survey, an in situ synchrotron-based two-dimensional grazing-incidence X-ray diffraction (GIXRD) technique was used to explore the effect of Ti3C2Tx MQDs-modified SnO2 (MQDs-SnO2) on the perovskite crystallization kinetics.131 It was shown that the modified structure facilitated perovskite nucleation from the precursor solution, forming an intermediate perovskite phase upon anti-solvent treatment. As a result, a substantial improvement in the crystal quality and phase stability of the perovskite film was achieved. As shown in Fig. 13c–e, the more uniform and larger grain sizes, the more light absorption capacity, the shorter lifetime in TRPL, and the much brighter scattered rings in GIXRD patterns underscore the better crystallinity and charge extraction in the target layer compared to the reference cells. The steady-state PCE of up to 23.3% as well as outstanding stability against humidity and light soaking was achieved for the corresponding PSCs due to the superior charge extraction properties of the MQDs-SnO2 layer.131 Xu Chen et al.186 prepared high-performance Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 PSCs with simultaneously improved performance and stability via incorporating Ti3C2Tx MQDs into the ETL and active layer. The incorporation of MQDs in the mesoporous TiO2 reduced the interfacial defects and improved the electron extraction and injection from the perovskite absorber into the mesoporous TiO2. On the other hand, introducing MQDs into the perovskite active layer increased the crystallization size and conductivity of the perovskite, while reducing the grain boundaries and the intrinsic defect density, by passivating the charge recombination centers. The MQD-modified PSCs exhibited a hysteresis-free response with a PCE of 21.64%, which was remarkably higher than that of the reference device (18.31%).186
 | ||
| Fig. 13 High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM) images of (a) SnO2 and (b) SnO2–Nb2C MQDs (reprinted with permission from Elsevier, copyright © 2021).130 The SEM images of the perovskite films deposited on (c) SnO2 and (d) SnO2–Nb2C MQDs ETL basement.130 (e) UV-vis absorption and PL spectra of the reference and target perovskite films (reprinted with permission from Royal Society of Chemistry, copyright © 2021).131 | ||
MQDs have also been used for structural modification of dye-sensitized solar cells (DSSCs) towards higher efficiencies.132 The PCE of DSSCs still requires further improvement despite their apparent benefits such as low-cost preparation, multicolor transparency, and a wide range of practical applications.132 Recently, substantial increase in the photocurrent and efficiency of DSSCs has been achieved by successfully altering the energy level alignment of TiO2 photoanode through the introduction of niobium oxide (Nb2O5) and Ti3C2 MQDs. The modified photoanode exhibits a distinct mesoporous structure, offering high dye adsorption and with a broader absorption range in the visible region. An improved charge transfer suppressed electron–hole recombination at the photoanode interface, and enhanced PCE (7.24%) relative to the reference group (4.60%) has been demonstrated.132
Similar to MQD-modification of ETL in solar cells, MQDs have been used to enhance UV detector performance. For instance, Yiqiang Zheng et al.8 used Ti3C2Tx MQDs to enhance charge carriers and light absorption of 2D perovskite Ca2Nb3O10. The results showed a 1100% increase in spectral responsivity (264 A W−1 at 310 nm) compared to the reference photodetector without MQDs. The flexible MQDs/perovskite photodetector was then integrated with a data collector to simulate a wearable visible-blind UV monitoring system for skin and plant protection. The ambient UV radiation information in various weather conditions could be trained and recognized by an artificial neural network to alert users.8 Similar to MQD-modification of ETL in solar cells, MQDs have been used to enhance UV detector performance. For instance, Yiqiang Zheng et al.8 used Ti3C2Tx MQDs to enhance charge carriers and light absorption of 2D perovskite Ca2Nb3O10. The results showed a 1100% increase in spectral responsivity (264 A W−1 at 310 nm) compared to the reference photodetector without MQDs. The flexible MQDs/perovskite photodetector was then integrated with a data collector to simulate a wearable visible-blind UV monitoring system for skin and plant protection. The ambient UV radiation information in various weather conditions could be trained and recognized by an artificial neural network to alert users.8
 | ||
| Fig. 14 Memory unit and I–V characteristics of MQDs-PVP memory devices containing (a and b) Cd, (c and d) Cc, (e and f) Cb, and (g and h) Ca (reprinted with permission from Wiley, Copyright © 2019).133 | ||
5.3. Energy
Incorporation of MQDs can play a key role in enhancing energy storage efficiency in batteries and supercapacitors by improving electrochemical reaction kinetics and thermodynamics. This results in higher capacitance and energy density, facilitated by improved ion transport, species adsorption, and surface reactions to develop more efficient, high-capacity, and long-lasting energy storage devices. In the following section, we will explore various types of energy storage devices, highlighting the mechanisms behind the performance improvements when MQDs are incorporated.To fully understand the role of MQDs in energy storage and conversion devices, several key parameters must be considered. Specific capacitance (F g−1) measures the ability of the material to store charge per unit mass, which is a crucial parameter for energy storage systems. Cycling stability refers to the number of charge–discharge cycles an electrode material can undergo while maintaining performance. Overpotential (V) is the extra voltage required for electrocatalytic reactions, such as redox reactions, which should be minimized to improve energy efficiency.208 Power density (W kg−1) and energy density (Wh kg−1) define how much power and energy a material can deliver, particularly for supercapacitors.208–212 Charge carrier lifetime represents the duration of photogenerated charge carriers remaining separated before recombination, a critical factor in photocatalysis. Lastly, coulombic efficiency (CE) and faradaic efficiency (FE), and apparent quantum yield (AQY) are quantifying indicators of the cycle-to-cycle efficiency and ratio of the actual amount of product formed to the theoretical amount, and performance metric of photocatalysis that measures the fraction of absorbed photons, respectively.213,214 In the following sections we will delve into each application and explain recent achievements in each area to have a deeper understanding of energy storage applications of MQDs.
• Lithium–oxygen batteries
Li–O2 batteries, leveraging oxygen redox reactions, offer a high theoretical energy density of 3000 Wh kg−1.215 Electrocatalysts play a crucial role in these systems by accelerating reaction kinetics, minimizing energy losses, and enhancing battery stability, making them key to improving performance and cyclability.12,216 Among various electrocatalysts for electrochemical applications, the unique properties of MXenes such as good electrical conductivity,133 edge active catalytic sites,135 tunable structure,136 and dispersibility146 make them highly suitable for oxygen reduction/evolution reactions.217 However, literature reports on 2D materials, particularly MXenes, still lacks a systematic framework for the rational design of highly active electrocatalysts for such an application.
To address this challenge, various strategies, ranging from defect engineering to doping, have been explored.218–220 Defect-rich structure of MQDs, owing to higher edge-to-basal plane surface area ratio, can effectively improve electrochemical performance of carbon nanosheets (CNSs), while maintaining the same chemistry as their MXene counterparts.221 Ti3C2 MQD/CNSs catalysts demonstrated a remarkably low voltage gap of 0.62 V, which is substantially lower than 1.29 V for Ti3C2 nanosheets/CNSs (Fig. 15a). Besides, Ti3C2 QDs/CNSs cathode delivered a high discharge capacity of ∼16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mAh g−1 with a CE of 93.8%, outperforming Ti3C2 nanosheets/CNSs (∼11
000 mAh g−1 with a CE of 93.8%, outperforming Ti3C2 nanosheets/CNSs (∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mAh g−1, 70.9%). In terms of stability, the former was stable during 240 cycles at 200 mA g−1 compared to 65 cycles for the latter. DFT calculations unveiled the relation between electron rearrangement over the grain boundaries and edge sites, helping charge transfer between intermediates and active sites during redox reactions. While for Ti3C2 sheets, charge accumulation was restricted to oxygen-containing clusters, confirming the inactivity of basal planes, MQDs showed stronger chemical affinity to LiO2 intermediates. This finding was attributed to the fact that Li+ and oxygen atoms could attach to edge rich C-side and functional groups of the edge-rich MQDs as catalytically active sites. Edge defects altered local charge distribution, modulated intermediate adsorption behavior of oxygen species needed for storage of products, and reduced redox energy barriers during Li2O2 formation/decomposition.222
000 mAh g−1, 70.9%). In terms of stability, the former was stable during 240 cycles at 200 mA g−1 compared to 65 cycles for the latter. DFT calculations unveiled the relation between electron rearrangement over the grain boundaries and edge sites, helping charge transfer between intermediates and active sites during redox reactions. While for Ti3C2 sheets, charge accumulation was restricted to oxygen-containing clusters, confirming the inactivity of basal planes, MQDs showed stronger chemical affinity to LiO2 intermediates. This finding was attributed to the fact that Li+ and oxygen atoms could attach to edge rich C-side and functional groups of the edge-rich MQDs as catalytically active sites. Edge defects altered local charge distribution, modulated intermediate adsorption behavior of oxygen species needed for storage of products, and reduced redox energy barriers during Li2O2 formation/decomposition.222
 | ||
| Fig. 15 MQDs as a key component of different type of batteries. (a) Li–O2 battery: the discharge–charge curves of electrodes at a fixed capacity of 1000 mAh g−1 and current density of 200 mA g−1. QDs as cathode material showed lowest charge overpotential compared to nanosheets (0.62 V) (reprinted with permission from Wiley, copyright © 2021).222 (b) Zn–air battery: the energy density of rechargeable ZABs with LDH/Ti3C2-MQDs/NG hybrids, LDH/NG hybrids, LDH/NG + MQDs, bare NG, commercial Pt/C, and IrO2 + Pt/C air cathodes at a discharge current density of 5 mA cm−2 demonstrating a higher energy density for QDs containing sample (∼700 Wh kg−1) (reprinted with permission from Wiley, copyright © 2021).223 (c) Li–S battery: long-term cycling of the battery with various separators (red: GMC, blue: GC, green: Celgard) at 0.2C (reprinted with permission from Royal Society of Chemistry, copyright © 2023).224 | ||
• Zinc–air batteries
Zinc–air battery (ZAB) is a promising energy storage system which benefits from vast utilization of aqueous electrolytes and high theoretical energy density of 1086 Wh kg−1 (five times higher than that of Li-ion).225 Although traditional noble metal-based electrocatalysts, such as Pt- and Ir/Ru-based materials perform well in electrocatalytic reactions, they still suffer from high costs and poor long-term stability. A major constraint to novel materials in zinc–air batteries is their sensitivity to the CO2 concentration in air. Among different methods to overcome this issue, such as electrolyte optimization and electrode engineering,226 developing highly active electrocatalysts for oxygen redox reactions has gained significant attention.227,228 Considering that layered double hydroxide (LDH) nanosheets have limited conductivity and sluggish catalytic kinetics,229 the introduction of MQDs to LDH/N-doped graphene (NG) is effective in boosting kinetics of oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) during cycling.229 Confirmed by Mott–Schottky analysis and DFT calculations, LDH/Ti3C2 MQD/NG hybrid exhibits higher carrier density and a deeper Fermi level while pristine LDH suffers from poor charge transport. The LDH/Ti3C2-MQD hybrid also shows a lower Tafel slope (57 mV dec−1) than Pt/C (120 mV dec−1), indicating faster reaction kinetics. Furthermore, the MQD-enhanced hybrid offers a high energy density of 700 Wh kg−1, matching commercial Pt/C and far exceeding IrO2 (580 Wh kg−1) (Fig. 15b), and low potential difference of 0.81 V, significantly outperformed LDH/NG (0.92 V), commercial Pt/C (1.01 V), and IrO2 + Pt/C (0.91 V). The material's structural integrity during prolonged operation (150 h at 5 mA cm−2) and its mechanical flexibility, even in a bent state, highlights its potential for applications beyond batteries, such as wearable electronics.223
• Lithium–sulfur batteries
Li–sulfur (Li–S) batteries stand out among many candidates as the next generation of energy storage devices due to their high theoretical capacity (1675 mAh g−1) and the abundance of sulfur.230 However, industrialization of this type of battery is hindered by the shuttle effect of lithium polysulfides (LiPSs) and irreversible energy loss, leading to poor performance and cyclability.231 Strategies to mitigate this include engineering the sulfur host, such as using free-standing N-doped carbon/Ti3C2Tx MQDs (NC-S/Ti3C2Tx) films; this design uses polar N-doped carbon to anchor LiPSs while the Ti3C2Tx network provides conductivity and a physical barrier, enabling high areal capacities up to 3.41 mAh cm−2.215,232
A game-changing solution emerges with the introduction of an ion selective layer in electrolyte to capture LiPS. Through local confinement enabled by the small size of MQDs and their abundant active sites for polysulfide adsorption, the g-C3N4@Ti3C2Tx MQDs-modified Celgard (GMC) separator forms a highly functional modification layer.224 This approach leverages the strong interactions of polar materials to gradually trap and accumulate polysulfides, effectively creating a shuttle barrier between the cathode and anode. As a result, a significant improvement in the efficiency of polysulfide conversion is attained. A long-term stability with only 0.024% capacity decay per cycle after 1000 cycles at 2C and the retention rate of nearly 70% at 0.2C after 100 cycles are also obtained. The retention rate is higher than that of the Li–S batteries assembled with unmodified g-C3N4 Celgard separator (58%), ensuring significantly extended lifespan compared to traditional designs (Fig. 15c). The modified separator also enhances the stability of Li2S deposition (final discharge product), as evidenced by longer and more stable plateau regions in discharge curves.224 By efficiently capturing, confining, and catalyzing polysulfide conversion, MQDs eliminate the shuttle effect bottleneck in Li–S batteries.
• Supercapacitors
A flexible power supply is an essential component of portable and wearable electronic devices. Supercapacitors with high power density, long cycle life, and fast charging capabilities have garnered considerable attention in this field. They are lightweight, compact, and operate efficiently across a wide temperature range, making them suitable for portable, energy-efficient devices. The key to providing such engineering products to the market is how to increase their volume energy densities without sacrificing their volume power densities, life cycles, and other performance parameters.233 To achieve high volumetric energy density, recent research has focused on new strategies, such as fabrication of hybrid capacitors,234 utilizing high-voltage ionic liquid electrolytes,235 preparing high surface area electrodes,221,236 and all-solid-state systems.233
Among other flexible electrodes, integrating 2–4 nm Ti3C2Tx MQDs into RGO/Ti3C2Tx fiber electrodes (Q3M7) overcame a long-standing challenge in solid-state electrolyte supercapacitors by providing a trade-off between high capacitance and mechanical flexibility.237 Using MQDs as pillar agents, a highly conductive, structurally stable fiber was obtained. An exceptional volumetric capacitance of 1560 F cm−3 at 1 A cm−3 in 1 M H2SO4 (solution-based electrolyte) was also achieved, which is far surpassing other MXene-based fibers such as Ti3C2Tx/PEDOT:PSS (poly(3,4-ethylenedioxythiophene)polystyrene sulfonate) (614 F cm−3) and Ti3C2Tx/RGO (542 F cm−3). The Nyquist plots revealed a lower charge transfer resistance, confirming MQD pillars create expanded interlayer channels, which minimize ion transport tortuosity and facilitate rapid pseudocapacitive intercalation and ion diffusion and electrolyte accessibility by adding MQDs.215,238 Even a 20× higher current densities (20 A cm−3) gave rise to a capacity retention of 79%, compared to the 61% in Ti3C2Tx/PEDOT:PSS fibers.239 When assembled into an all-solid-state supercapacitor, the MQD-enhanced electrode demonstrated a volumetric capacitance of 413 F cm−3 at 0.5 A cm−3, an energy density of 36.7 mWh cm−3, and an impressive 97% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which hardly changed over time. Additionally, the Q3M7 fibers boast a remarkable mechanical strength of 130 MPa, ensuring their suitability for wearable and flexible electronics. Moreover, no change observed to the capacitive behavior of the fibers after bending, confirming the flexibility of this system under bending test (Fig. 16a).237 The volumetric energy density exceeded that of commercial supercapacitors (e.g., 2.75 V/44 mF, and 5.5 V/100 mF), but also outperformed previously reported MXene- and RGO-based designs (e.g., MnO2/Ti3C2Tx/RGO, Ti3C2Tx/PET fiber, Ti3C2Tx/CNT fiber, V2O5/single-walled carbon nanotube) (Fig. 16b).237
000 cycles, which hardly changed over time. Additionally, the Q3M7 fibers boast a remarkable mechanical strength of 130 MPa, ensuring their suitability for wearable and flexible electronics. Moreover, no change observed to the capacitive behavior of the fibers after bending, confirming the flexibility of this system under bending test (Fig. 16a).237 The volumetric energy density exceeded that of commercial supercapacitors (e.g., 2.75 V/44 mF, and 5.5 V/100 mF), but also outperformed previously reported MXene- and RGO-based designs (e.g., MnO2/Ti3C2Tx/RGO, Ti3C2Tx/PET fiber, Ti3C2Tx/CNT fiber, V2O5/single-walled carbon nanotube) (Fig. 16b).237
 | ||
Fig. 16 (a) Mechanical bending test showing the flexibility of solid-state supercapacitor system based on Ti3C2Tx MQDs/L-Ti3C2Tx fibers made with MQDs confirming flexibility after multiple bending steps (reprinted with permission from Wiley, Copyright © 2023).237 (b) Ragone plot indicating the potential of the MQD containing fibers at top right corner of energy density vs. power density plot (highest compared to other works) (reprinted with permission from Wiley, copyright © 2022).236 (c) Capacitance retention and CE of the 0D/2D MQD/MXene symmetric solid-state pseudocapacitor after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles showing a high retention of ∼84%. The first cycle and the last cycle are shown as an inset confirming minimal changes (reprinted with permission from Wiley, copyright © 2023).237 (d) Ti3C2Tx MQD/LRG laser fabrication process, image of exfoliated materials, SEM (scale bar, 20 nm) confirming size distribution below 5 nm (reprinted with permission from Wiley, copyright © 2022).236 000 cycles showing a high retention of ∼84%. The first cycle and the last cycle are shown as an inset confirming minimal changes (reprinted with permission from Wiley, copyright © 2023).237 (d) Ti3C2Tx MQD/LRG laser fabrication process, image of exfoliated materials, SEM (scale bar, 20 nm) confirming size distribution below 5 nm (reprinted with permission from Wiley, copyright © 2022).236 | ||
While flexibility is important, in the pursuit of transparent high-performance energy storage devices, researchers have faced a persistent challenge in balancing high transparency with efficient energy storage. The key to this issue lies in utilization of temporally and spatially shaped femtosecond laser to uniformly attach MQDs to laser-reduced graphene oxide (LRGO).236 The presence of nanosized Ti3C2Tx-MQDs and higher density of edges compared to basal sites provides a high-power density and areal capacitance of 2.04 × 10−3 mWh cm−2 and 10.42 mF cm−2, respectively. Well-distributed MQDs prevented restacking of graphene nanosheets (Fig. 16d), while providing more active sites for charge storage and transfer, thereby offering a flexible, ultrahigh transparency supercapacitor (areal capacitances = 10.42 mF cm−2 and transmittance = 91%) with high durability, i.e., maintaining 97.6% of its capacitance after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.236 In another study,137 Ti3C2Tx-MQDs were introduced into the Ti3C2Tx nanosheet matrix to create a heterodimensional hybrid structure (0D/2D) through a microwave hydrothermal method that altered charge storage behavior. Electrochemical impedance spectroscopy (EIS) indicated that the ohmic resistance (1.439 Ω) and charge transfer resistance (0.04078 Ω) of MQD/MXene nanosheet electrodes are smaller than those of Ti3C2Tx nanosheets electrodes (Rs = 1.637 Ω, Rct = 0.10032 Ω). An areal capacitance of 2202 mF cm−2 at 3 mA cm−2 was attained, which significantly outperformed conventional MXene-based electrodes. The material retained 84% of its capacity after 10
000 cycles.236 In another study,137 Ti3C2Tx-MQDs were introduced into the Ti3C2Tx nanosheet matrix to create a heterodimensional hybrid structure (0D/2D) through a microwave hydrothermal method that altered charge storage behavior. Electrochemical impedance spectroscopy (EIS) indicated that the ohmic resistance (1.439 Ω) and charge transfer resistance (0.04078 Ω) of MQD/MXene nanosheet electrodes are smaller than those of Ti3C2Tx nanosheets electrodes (Rs = 1.637 Ω, Rct = 0.10032 Ω). An areal capacitance of 2202 mF cm−2 at 3 mA cm−2 was attained, which significantly outperformed conventional MXene-based electrodes. The material retained 84% of its capacity after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and delivered an aerial energy density of 90.33 μWh cm−2 at a power density of 450 mW cm−2. No efficiency loss due to mechanical deformations was also noticed, highlighting their high flexibility and robust mechanical stability (Fig. 16c).137,236
000 cycles and delivered an aerial energy density of 90.33 μWh cm−2 at a power density of 450 mW cm−2. No efficiency loss due to mechanical deformations was also noticed, highlighting their high flexibility and robust mechanical stability (Fig. 16c).137,236
Beyond flexibility and mechanical properties, supercapacitors have long struggled with the trade-off between capacitance and stability.240 Despite the high theoretical capacitance of various classes of active materials, most of the structures suffer from low utilization of active sites due to limited electron mobility.241 Recently, J. Wang et al.205 have demonstrated that by incorporating Ti3C2Tx-MQDs through a microwave-assisted DMF exfoliation process, the Ni(OH)2 structure undergoes a remarkable transformation. As a result, a higher electroactive surface area, higher ion diffusion rate, and significantly improved charge transport efficiency are attained. The MQD-NH electrode on nickel foam offers a record-high specific capacitance of 1660 F g−1 at 1 A g−1 in 2 M KOH, far surpassing conventional Ni(OH)2 composites such as Ni(OH)2/rGO (802 F g−1) and Ni(OH)2 (1087.1 F g−1).205
• CO2reduction reaction
Electrochemical conversion can serve future energy demands by storing renewable energy and reducing anthropogenic emissions such as CO2. For this aim, photocatalysis has long been regarded as a promising technology for tackling environmental pollution and converting CO2 into valuable fuels.242 Among many photocatalytic materials, g-C3N4 stands out for its stability, non-toxicity, and visible-light activity. Yet, despite its potential, a persistent challenge is its tendency to lose charge carriers too quickly before they can drive meaningful chemical reactions. Among different methods to improve the efficiency of CO2 reduction electrodes, strategies such as reducing the activation energy of CO2 redox reactions,243 introducing novel electrocatalysts to enhance selectivity for CO2 reduction,244 and minimizing parasitic reactions like HER245 have been reported in literature.242 One recent approach is the formation of heterojunctions with bismuth oxyiodide (BiOI), a p-type semiconductor known for its strong light absorption and complementary band structure.246 While this combination does improve charge separation to some extent, it still lacks the efficiency needed for high-performance photocatalysis. The real challenge is not just preventing recombination but actively driving charge migration to ensure efficient and prolonged reaction activity. To improve charge separation, a heterojunction of g-C3N4 with BiOI has recently been investigated.247 Although this combination enhances charge transfer to some extent, it remains insufficient for achieving optimal performance. The missing piece in this system is a mechanism to accelerate charge migration and suppress recombination more efficiently.247
Another strategy to accelerate CO2 conversion is decreasing the activation energy of reaction. As an example, Ti3C2Tx-MQD heterostructure with g-C3N4/BiOI enhances the CO2 photoreduction properties of the device.247 While the absorption band edge of g-C3N4 shows a strong UV absorption and BiOI displays a broad UV to visible light absorption, modifying with the MQDs will tune the bandgap, and extend the photo-response to NIR range (Fig. 17a). The CN/MQDs/BOI offers an observable photocurrent response of 2.3 μA and a CO production rate of 57.8 μmol g−1 h−1, which is more than 30 times higher than C3N4/TiO2. The g-C3N4/MQDs/BOI exhibits a Nyquist semicircle, confirming enhanced charge transfer efficiency. However, it is noteworthy that NOx reduction is susceptible to occur during the reduction reaction process, which affects CO2 reduction yield and the corresponding production of CO and CH4 (Fig. 17b).247
 | ||
| Fig. 17 (a) UV–vis diffuse reflectance absorption spectra of CN/MQDs/BOI confirming the effectiveness of hybrid structure to tune adsorption edge at 440 nm for CN/MQDs and ∼520 nm for CN/MQDs/BOI (reprinted with permission from Elsevier, copyright © 2025).247 (b) Schematic of the reactions based on potential vs. NHE (reprinted with permission from Elsevier, copyright © 2025).247 | ||
While metal chalcogenides, such as Bi2S3, have shown potential for CO2 reduction due to their narrow bandgap and strong visible-light absorption, their efficiency is compromised by rapid recombination of carriers and insufficient adsorption sites for CO2.248 To address these issues, 3D porous Ti3C2-MQDs-OH/Bi2S3 structure that leverages the synergy between protonated Bi2S3, and alkalized MQDs (MQDs-OH) were investigated for CO2 reduction.249 The strong electron-withdrawing properties and abundant hydroxyl functional groups of MQDs-OH led to efficient charge carriers’ separation and retarded recombination, as evidenced by EIS. PL and photocurrent response measurements determined a high photocurrent density of 22 µA with 15-fold enhancement in the CO2 adsorption capacity, i.e., from 1.6 mg CO2 per g for pristine Bi2S3 to 23.0 mg CO2 per g for MQDs-OH/Bi2S3. This massive improvement resulted in a record-high methanol yield of 694.7 µmol g−1, more than double the output of unmodified Bi2S3-based catalysts (321.8 µmol g−1). The electron spin resonance (ESR) measurements showed a significant increase in ROS (O2˙−) generation, affirming that the presence of MQDs enhanced the activation of CO2 molecules for more efficient reduction reactions.249
• Hydrogen evolution reaction
The generation of hydrogen from H2O through semiconductor photocatalysis has garnered significant attention in recent years.250 Among various photocatalysts, conventional TiO2/g-C3N4 composites without MQDs have found of great interest for photocatalytic hydrogen evolution.251 It is noteworthy that the catalytic activity of TiO2/g-C3N4 heterostructures is constrained by rapid charge carrier recombination and limited active sites. While the S-scheme heterostructure of TiO2/g-C3N4 provides a foundation for improved charge separation, it is still not enough to sustain high reaction rates.145 The modification of S-scheme TiO2/g-C3N4 heterojunction with Ti3C2 MQDs is considered as an effective strategy for extending optical absorption into the visible range. Diffuse reflectance spectroscopy (DRS) measurements determine the bandgap energy of TiO2 (T), g-C3N4 (CN), TiO2/g-C3N4 (T-CN), and TiO2/g-C3N4/Ti3C2 QDs (T-CN-TCQD) as 3.22 eV, 2.86 eV, 2.57 eV, and 2.45 eV, respectively (Fig. 18a). By serving as electron acceptors and facilitating charge separation, the MQDs address the critical challenge of rapid recombination of photoinduced carriers in g-C3N4, resulting in a hydrogen production rate of 5111.8 μmol g−1 h−1, which is nearly 26 times higher than pristine g-C3N4 nanosheets (196.8 μmol g−1 h−1), 3 times higher than Pt/g-C3N4 (1896.4 μmol g−1 h−1), and 10 times higher than Ti3C2 MXene sheet/g-C3N4 (524.3 μmol g−1 h−1). This drastic improvement is attributed to the superior electronic conductivity of Ti3C2 MQDs, which not only facilitated efficient carrier transfer but also increased the specific surface area of g-C3N4 from 27.575 m2 g−1 to 40.149 m2 g−1. Time-resolved photoluminescence measurements indicate a longer lifetime, i.e., 10.1242 μs for g-C3N4@Ti3C2 QDs compared to 9.6841 μs for g-C3N4. The enhanced photocurrent response of g-C3N4@Ti3C2 QDs, along with a reduced EIS arc radius compared to pristine g-C3N4, further demonstrate the superior charge transport and separation efficiency introduced by MQDs.145
 | ||
| Fig. 18 (a) Flat-band potential (Efb) estimated by Mott–Schottky curve obtained from DRS test showing the extended bandgap energy of g-C3N4@Ti3C2 MQDs have been expanded to visible region compared to other candidates without MQDs (reprinted with permission from American Chemical Society, copyright © 2019).142 (b) The Tafel slope and overpotential of Co(OH)2-based catalysts at 10 mA cm−2 for HER in an alkaline 1 M KOH solution (reprinted with permission from Elsevier, copyright © 2025).141 (c) H2 evolution reaction as a function of time for (■) Ti3C2 QDs; (▼) Nb2C QDs; (●) Ti2C QDs; and (◊) V2C MQDs (reprinted with permission from Wiley, copyright © 2023).252 (d) Energy band positions of Ti3C2-MQD/Ni-MOF (reprinted with permission from American Chemical Society, copyright © 2020).4 (e) Energy level diagram of Ti3C2 MQDs/Cu2O NWs/Cu and Ti3C2 sheets/Cu2O NWs/Cu heterostructure (reprinted with permission from Wiley, copyright © 2018).145 | ||
Hydrogen evolution reaction (HER) efficiency also depends on the H+ reduction barriers. M. Y. Solangi et al.253 demonstrated that an assembly of Co(OH)2 and Ti2C MQDs, accelerated the adsorption of H2O and decreased the reduction barrier for H+. The presence of Co(OH)2 provided active sites for hydroxyl (OH) adsorption, while Co sites with electron-deficiency protected the Co(OH)2 from deteriorating under alkaline environment. The MQD introduction reduced the overall energy from −251.29 to −672.51 eV, while enhancing the structural stability of Co(OH)2. The Gibbs free energy of water dissociation was also substantially decreased from 1.52 to 0.14 eV by the incorporation of MQDs. Additionally, a low overpotential of 91 mV at the current density of 10 mA cm−2, which surpasses the performance of the CF (344 mV), Pt/C (111 mV), and the Co2(OH)3Cl/CF-R (268 mV) catalysts, was noticed (Fig. 18b).253
By utilizing HF-free laser ablation in an aqueous medium, not only can the need for HF be eliminated but also control over quantum confinement effects, surface functionalization, and bandgap modulation can be adopted. For instance, significantly enhanced photocatalytic performance of Ti3C2-MQDs for HER has been reported by this technique.252 Here, a hydrogen production rate of 2.02 mmol g−1 h−1, which is comparable to noble-metal-based catalysts like Pt/g-C3N4 (2.5 mmol g−1 h−1), has been reported (Fig. 18c). It has been shown that not only fine-tuning of the valence band (VB) and conduction band (CB) positions can achieved by laser ablation,254 but also the oxygenated surface terminations (OH, and O) can improve stability and catalytic activity of MQDs.252
• NOxreduction
NOx reduction is critical for environmental protection because it aims to convert hazardous materials to value added products, while reducing nitrogen oxide emissions from industrial processes. However, the large bond energy (940.95 kJ mol−1) of the nitrogen–nitrogen bond makes the nitrogen reduction reaction (NRR) difficult.255 Thus far different approaches, such as electrolyte engineering,256 crystallographic phase transformation engineering,257 transition metal dual-atom catalysts,258 transition metal oxides,259 covalent/metal organic frameworks, perovskite oxides, doping with transition metals to hybridizing with carbon-based materials like graphene,260 and incorporation of MQDs as a co-catalyst to tune NRR activity have been investigated.261 Among various methods to develop efficient and selective electrocatalysts, utilization of MQDs with different surface terminations have found of interest in nitrogen reduction to produce ammonia at room temperature.262,263 MQDs provide high density of edge sites, which are more catalytically active than basal planes, thus enhancing the adsorption and activation of nitrogenous molecules. Additionally, the surface chemistry of MQDs can be tailored with functional groups such as hydroxyl, or fluorine, optimizing their electronic structure and adsorption characteristics to lower energy barriers for nitrogen reduction.143 For instance, J. Qin et al.4 demonstrated that incorporating surface-functionalized Ti3C2 MQDs to Ni-MOF led to photocatalytic NH3 production rate of 88.79 μmol g−1 h−1, which was more than four times higher than pristine Ni-MOF (21.39 μmol g−1 h−1).139,264 EIS indicated a low charge transfer resistance for the coupled structure, while transient PL decay determined an extended carrier lifetime of 5.636 ns, compared to 2.685 ns for the pristine Ni-MOF. DFT calculations also affirmed that the MQD incorporation decreased the free-energy barrier for N2 activation, making the reaction more energetically favorable. Specifically, the initial N2 adsorption step becomes exothermic, releasing 0.74 eV, while the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond elongated from 1.098 Å to 1.118 Å, facilitating subsequent reduction steps. Details of the heterostructure and reaction mechanism, shown in Fig. 18d, proposed that the synergistic MQD–MOF system altered the reaction pathway, making nitrogen fixation significantly more efficient.4
N bond elongated from 1.098 Å to 1.118 Å, facilitating subsequent reduction steps. Details of the heterostructure and reaction mechanism, shown in Fig. 18d, proposed that the synergistic MQD–MOF system altered the reaction pathway, making nitrogen fixation significantly more efficient.4
Another product of nitrogen containing compounds reduction is ammonia which is mostly obtained from NORR using copper-based electrocatalysts.143 Ti3C2 MQDs have been proven to effectively boost the co-catalytic performance of NORR ammonia production performance of copper nanowires (Cu NWs).143 An NH3 yield of 5346.3 μg h−1 mg−1 and FE of 95.5%, which are much higher than those of unmodified Cu NWs (NH3 yield of 3313.1 μg h−1 mg−1 and FE of 89.5%) have been reported (Fig. 19a). The onset potential of reaction (−0.4 V vs. reversible hydrogen electrode (RHE)) is also significantly less than that of Cu foam (−0.9 V vs. RHE). A record-high NH3 yield, outperforming many well-performing catalysts previously reported in the literature like MoS2,139 MoC,265 Bi/C,266 and Ni2P nanosheet array on carbon paper (Ni2P/CP),267,268 have been demonstrated. An assembled Zn–NO battery with Ti3C2 MQDs/Cu NWs offers a power density of 3.03 mW cm−2 and a high NH3 yield of 925.2 μg h−1 mg−1.143
 | ||
| Fig. 19 (a) Linear Sweep Voltammetry (LSV) of Cu NWs and Ti3C2 MQDs/Cu NWs in Ar-saturated and NO-saturated 0.1 M K2SO4 exhibiting a high activity of MQDs modified Cu nanowires toward NO reduction (reprinted with the permission of Royal Society of Chemistry, copyright © 2023).143 (b) The proposed reaction mechanism for photocatalytic phthalide synthesis and H2 evolution by MQDs incorporation and electronic structure modification as a successful strategy to boost reactivity (reprinted with the permission of American Chemical Society, copyright © 2024).269 (c) Photocurrent vs. irradiation time for CdS, CdS/Ti3C2Tx, and Ni/CdS/Ti3C2Tx photocatalysts (reprinted with the permission of American Chemical Society, copyright © 2024).269 (d) H2O2 yield for Nb2C MQDs/COF as compared to other photocatalysts, demonstrating the promising potential of MQDs to modulate the H2O2 production (reprinted with the permission of Elsevier, copyright © 2025).147 | ||
• Photocatalyst
Copper oxide nanowires with p-type semiconductor properties and favorable band structure have been considered promising photocatalyst. Traditional approaches, such as integrating carbon nanomaterials,270 noble metals,271 and other semiconductors,272,273 have yielded only limited improvements on charge recombination without making the fabrication processes complex with reduced costs.145 Recent studies have demonstrated that MQDs can serve as electron shuttles to accelerate charge transport, while suppress recombination losses.145 For instance, by integrating Ti3C2 MQDs onto Cu2O nanowires (NWs), photocatalytic CO2 conversion results in a high rate of methanol production (153.38 ppm cm−2), which is 8.25 times higher than pristine Cu2O NWs (18.58 ppm cm−2) and 2.15 times higher than Ti3C2 nanosheets/Cu2O NWs (71.39 ppm cm−2).145 Mott–Schottky analysis reveals that carrier density (ND) increases dramatically from 1.09 × 1019 cm−3 in Cu2O NWs to 2.75 × 1019 cm−3 in MQD-modified Cu2O NWs, with prolonging photocarrier lifetimes. Additionally, DRS demonstrates significantly enhanced light absorption efficiency and shifts the bandgap from 2.2 eV (Cu2O) to 2.02 eV (Ti3C2 MQDs/Cu2O NWs) (Fig. 18e). This bandgap engineering facilitates a broader spectrum of light absorption and reduces the charge transfer resistance, as evidenced by EIS. Photostability studies of Ti3C2 MQDs/Cu2O NWs/Cu over a wide range of potential also affirm the potential of the MQDs to prevent Cu2O NWs from oxidation.145
• Radical scavengers
Radical scavenging plays a pivotal role in mitigating oxidative stress and preserving functional electrode stability and preventing electrode degradation,274 stabilizing intermediates in reactions like ORR/CO2RR.275,276 Benefiting from a large surface-to-volume ratio, Cl, N-doped Ti3C2 MQDs (D = ∼3.4 nm) synthesized via electrochemical etching demonstrated outstanding hydroxyl (˙OH) radical scavenging activity (93.3%) at a concentration of 12.5 μg mL−1, surpassing the performance of previously reported graphene-based nanoparticles.277 The scavenging mechanism is attributed to the electron transfer ability of the Ti3C2 structure and the electron donation of the double dopants.75 In another study, nitrogen-doped Ti3C2 MQDs synthesized by a hydrothermal method exhibited exceptional scavenging capability against multiple ROS, including ˙OH, 1O2 (singlet oxygen), and O2˙− (superoxide anion) multi-ROS scavenging capabilities, achieving a hydroxyl radical scavenging efficiency of 93.2% at a concentration of 12.5 μg mL−1, significantly outperforming graphene oxide (37.3%), and graphene QDs (53.3%) under comparable conditions. Detailed DFT simulations and voltammetry measurements revealed that nitrogen doping not only enhanced the electron density and created additional active sites but also dramatically strengthened the adsorption and charge-transfer processes with OH radical species, thereby underpinning the superior antioxidative activity of the N-Ti3C2 MQDs.118
• Oxidation of alcohols
Semiconductor photocatalysts often face the challenge of photoinduced decomposition, which hampers their efficiency and stability in selective oxidation of alcohols.278 To address this issue, recent studies have developed a robust photocatalyst by integrating nickel-decorated cadmium sulfide quantum dots (Ni/CdS QDs) with Ti3C2Tx MXene.269 This composite leverages the high conductivity and unique surface chemistry of Ti3C2Tx MXene to enhance charge separation and transfer, while the Ni decoration provides active sites that facilitate the oxidation process. This strategic combination not only mitigates the phot-corrosion of CdS but also significantly improves the efficiency and selectivity of alcohol oxidation reactions under visible light irradiation.269 Lactonization of 1,2-benzenedimethanol (1,2-BM) to phthalide is also integrated with concomitant H2 production, as Ni clusters in CdS accelerates H2 evolution (Fig. 19b). The anchored Ni as a single atom on Ti3C2Tx can also influence efficient adsorption and cyclization of diols. This composite material exhibits remarkably enhanced activity for lactone synthesis, which is 80.4 times higher than that of blank CdS, along with excellent selectivity of 91.6% for composite structure (Ni/CdS QDs) although blank CdS QDs had a much lower selectivity of only 48.4% under the same reaction conditions. While the blank CdS shows a significant decrease (43.6%) in the phthalide production after four repeated trials, no obvious deactivation phenomenon of phthalide and H2 production could be observed over Ni/CdS/Ti3C2T MQDs. The transient photocurrent response of the samples increases in the following order: Ni/CdS/Ti3C2Tx > CdS/Ti3C2Tx > CdS, suggesting the higher charge separation efficiency in Ni/CdS/Ti3C2Tx composite (Fig. 19c).269
• H2O2production reaction
Pyrene-based covalent organic frameworks (COFs) hold significant promise in photocatalytic applications; however, their practical use in hydrogen peroxide (H2O2) production is hindered by low charge-generation and charge-transfer efficiencies, as well as rapid charge recombination rate.208,279 To address these challenges, recent research has focused on integrating MQDs as active sites within pyrene-based COFs through a self-assembly method.147 This strategic combination enhances photocatalytic performance by improving charge separation and transfer, thereby facilitating efficient H2O2 photosynthesis from abundant seawater resources.147 For instance, a H2O2 production rate of 3560 μmol g−1 h−1 was reported by integration of Nb2C MQDs into high-crystalline COF structure.147 An AQY of 12.8% at 400 nm together with SCC (Solar-to-Chemical Conversion) efficiency of 0.27% was also reported (Fig. 19d). From the mechanistic point of view, femtosecond transient absorption spectroscopy (fs-TAS) determined electronic coupling between Nb2C MQDs and COF. Further supported by DFT simulations of the planar-averaged charge density difference along with Z direction it was proposed that the electrons mainly transferred from COF to Nb2C MQDs through intermediates governed by a strong electron–phonon coupling at the interface, which promotes directional charge migration over thermal relaxation. This process is further amplified by the significant spin–orbit coupling of the heavy Nb atoms, which modifies the electronic band structure to favor rapid carrier extraction at the MQD active sites and corresponding free energy changes.147,280
5.4. Biomedical applications
Transplantation immunology is critically important as it addresses the complex interplay between the immune system and transplanted tissues, biomaterials, and cells determining the success and safety of organ transplantation.281 Graft rejection occurs when the recipient's immune system recognizes the transplanted organ as foreign and begins the expansion of effector immune cells and inflammation.281 Inflammation, vital for the body's response to injury and infection to maintain the physiological balance, hinders the clinical use of tissue therapies in regenerative medicine by causing the rejection of transplanted biomaterials and stem cells.282,283 Since a number of studies have indicated that transplanted tissue constructs frequently trigger pronounced proinflammatory responses leading to rejection, it is needed to control this response to realize the advantages of allografts.284
MQDs with anti-inflammatory properties can be applied for immunomodulation. As an example, Ti3C2 MQDs with intrinsic immunomodulatory properties were shown to decrease human CD4+ IFN-γ+ T-lymphocytes activation by about 20%.284 The QDs also encouraged a 3% increase in the proliferation of immunosuppressive CD4+ CD25+ FoxP3+ regulatory T-cells within a stimulated lymphocyte population. Human naïve CD4+ T-lymphocytes were activated and turned into proinflammatory Th1 cells over 7 days with and without Ti3C2 MQDs. Flow cytometry indicated decreased expression of lymphocyte proliferation and upregulation of the regulatory T-cells. The mechanism behind the immunomodulatory effects of these MQDs is still not well-understood.51 On the other hand, Szuplewska et al.53 have proposed that the ROS produced by Ti3C2Tx MXenes induces the release of proinflammatory cytokines from resident tissue macrophages and interferes with anti-inflammatory and immunomodulatory properties of implanted biomaterials in the body.21
Rafieerad et al.24 have studied the immunomodulatory mechanism of Ta4C3Tx MQDs by activated HUVECs, human peripheral blood mononuclear cells, and Th1 cells. Activated HUVECs, PBMCs, and Th1 cells were selected as they represent key mediators of immune responses. Endothelial cells function as antigen-presenting cells during transplant rejection, PBMCs comprise essential immune effectors, and Th1 cells play a central role in proinflammatory responses and allograft rejection.285,286 A 3.3-fold increase in the PD-L1 expression and a simultaneous 1.3-fold decrease in the CD86 expression in activated HUVECs treated with Ta4C3Tx.287 MQDs can elucidate the immunomodulatory mechanism of these MQDs. Both PD-L1 and CD86 play important roles in T-cell activation via antigen-presenting cells. PD-L1 inhibits T-cell activation, while CD86 activates it.288 Ta4C3Tx MQDs hold the potential to diminish host inflammation against allogeneic organs and tissues by modifying the PD-L1 and CD86 equilibrium within antigen-presenting endothelial cells (Fig. 20a).24
 | ||
| Fig. 20 (a) Schematic representation of the immunomodulatory mechanisms of Ta4C3Tx MQDs. (b) H&E-staining of explanted abdominal aortic segments (reprinted with permission from Wiley, copyright © 2021).24 (c) Typical photographs of 4T1 tumor-bearing mice and tumors after different treatments for 16 days (reprinted with permission from Elsevier, copyright © 2020).23 | ||
• In vivo immunomodulation
To investigate the immunomodulatory potential of Ta4C3Tx MQDs within a living organism, Rafieerad et al.287 utilized a rat model of allograft vasculopathy, which is a significant factor in the rejection of transplanted organs.289 Following transplantation, Ta4C3Tx MQDs were promptly administered at a specific dose. No adverse effects were observed either over the course of a week or after that in blood and tissue samples. However, histologic examination of the abdominal aorta in transplanted animals revealed apparent inflammatory changes compared to sham animals (Fig. 20b). Nevertheless, animals treated with Ta4C3Tx MQDs showed reduced endothelial injury and immune cell infiltration when compared to those injected with saline, while both were different from control animals. Immunohistochemistry, flow cytometric analysis also revealed a decrease in alpha-smooth muscle actin to assess vascular injury. This protein is an early contributor to rejection.290 This reduction was ameliorated in animals treated with Ta4C3Tx MQDs. Tregs, which play a crucial role in immunologic tolerance after transplantation,291 were reduced in transplanted animals but restored by Ta4C3Tx MQD treatment. The results of the in vivo examinations revealed the potential of Ta4C3Tx MQDs in reducing allograft vasculopathy and improving transplantation outcomes.287
Recent studies have expanded the range of MQDs applied in bioimaging beyond conventional Ti3C2 composition. For example, niobium carbide (Nb2C) MQDs have attracted particular attention owing to their bright fluorescence and excellent biocompatibility. Notably, at concentrations as high as 100 µg mL−1, levels that are cytotoxic to Ti3C2 QDs, Nb2C MQDs exhibited no detectable toxicity toward HUVEC cells and even induced protective autophagy, highlighting their superior biological tolerance.292 Likewise, albumin-stabilized Ti3C2 MQDs have been engineered for targeted imaging of MDA-MB-231 breast cancer cells, producing strong and stable intracellular fluorescence signals in vitro.293
The structural versatility of MXene-based hybrid nanostructures further highlights their potential for advanced imaging applications. For example, Co–Mn Prussian-blue nanoparticles functionalized with Ti3C2 MQDs enabled in situ fluorescence sensing and spatial mapping of specific microRNAs within living cancer cells.294 Collectively, these studies underscore that rationally engineered MQDs, particularly those derived from non-Ti compositions, can serve as bright, stable, and biocompatible nanofluorophores suitable for live-cell imaging with minimal cytotoxicity. Overall, MQDs represent a newly emerging and promising material class of bioimaging materials. However, further research is required to achieve a deeper understanding of their behavior and potential in bioimaging.
Some cations demonstrate effective reactivity with H2O2 within the specific acidic microenvironment of tumors.298 An efficient generation of highly toxic ˙OH leads to the destruction of tumor blood vessel integrity and causing cancer cell death. This process can be facilitated in the presence of MQDs. For example, it has been reported that Ti3+ in Ti3C2Tx MQDs, or Mn2+ in a complex Ti3C2Tx-based system, effectively suppress HeLa tumor xenografts.298 Due to the biocompatibility of Ti3C2Tx MQDs, no side effects on normal tissues and organs have been observed, offering a safe and efficient strategy for tumor therapy.298
6. Conclusions and outlook
In this review, the emergence of MQDs as a promising class of nanomaterials for various applications is comprehensively discussed. To unlock their potential in advancing nanoscience and technology, recent progress in their synthesis, including strategies for quantum state engineering and surface passivation, has been reviewed. Through surface functionalization, doping, co-doping, and molecular conjugation, MQDs with high PLQY, exceeding ∼28%, can successfully be synthesized. Taking advantage of intrinsic biocompatibility and exceptional optical properties, MQDs have been integrated into a wide range of applications, from sensing (various ions and molecules) and biomedicine (cancer therapy and cell imaging) to optoelectronics (LEDs, lasers, solar cells, and photonics). Their high surface area, layered structure, and abundant active sites male MQDs highly promising for applications in energy storage (batteries and supercapacitors) and energy conversion (hydrogen evolution and CO2 reduction) systems.Despite significant advancements in the properties of MQDs and their widespread applications, many promising opportunities remain unexplored, necessitating further comprehensive research. Some key areas for future perspectives are outlined below.
• Current fabrication methods suffer from low yields, temporal inefficiency, inconsistent quality, and high costs.20 Scaling up production while maintaining uniformity in size, structure, and functionality remains a major challenge. One of the most recent techniques capable of synthesizing narrow-sized QDs from organic and inorganic semiconductors in a very short time scale (in less than hour) is pulsed laser ablation. This can be considered either top-down or bottom-up based on the pulse time, forming ultrasmall QDs. Using various additives, doped or surface functionalized MQDs can be synthesized. Further, the possibility of the hot injection method, according to which organometallic reagents rapidly inject into a hot solvent and produce homogeneous nuclei,299 that had been applied for other QDs, can be investigated for synthesizing MQDs.
• Regarding their optical properties, the precise contributions of quantum confinement (size-dependent behavior) and surface functional groups to enhanced radiative recombination by passivation of non-radiative trap states in MQDs are not yet clearly distinguished through in-depth investigation. To elucidate this, it is strongly recommended to combine DFT with PL, PLE, UV-Vis, and ultraviolet photoelectron spectroscopy to not only deepen interpretation about the electronic band structure but also find the excitation dependent or independent PL behavior of modified MQDs.300
• Heterojunctions have been capable of enhancing the sensing process. However, a few heterostructures based on MQDs and 2D structures have been fabricated. Transition metal dichalcogenides (TMDs) are chemically stable semiconductors showing ambipolar behavior.301 Therefore, they can be a great career for transportation of the transferred electrons or holes between MQDs and analytes towards contacts, increasing the sensitivity. Moreover, decorated MQDs combined with tungsten disulfide (WS2) or molybdenum disulfide (MoS2) on a flexible polymer substrate (PTE) offer a potentially advantageous architecture for the development of future flexible wearable sensors.
• While the fabrication of single-component WLEDs and lasers based on MQDs has been demonstrated, their performances currently lag behind those achieved with lead-free perovskite QDs exhibiting PLQYs of 70%. Although surface-modified MQDs have reached PLQYs of about 30%, further enhancements via systematic investigations into the effects of size, dopant incorporation, and surface functionalization are necessary to compete with other materials.
• Given the recent significant advancements in PLQY enhancement through surface modification of MQDs, the absence, to our knowledge, of fabricated electroluminescent LED devices based on MQDs represents a notable research opportunity.
• MQDs have modified perovskite and ETL in solar cells, but their effects on hole transport layers like Spiro remain unexplored. Engineering the band structure of MQDs can be helpful to use it as a dopant or sensitizer for the conductivity improvement of Spiro-OMeTAD.
• MQDs show great promise in batteries, supercapacitors, and CO2/N2 reduction applications due to their high surface area, quantum confinement, and abundant edge sites.302 A major challenge lies in controlling surface terminations, which strongly influence conductivity, ion intercalation, and long-term cycling stability. Current synthesis methods often yield a mixture of –O, –F, and –OH groups, limiting reproducibility and the ability to engineer their work function and surface catalytic energetics. Future strategies may include post-synthetic plasma treatments, thermal annealing in controlled environments, or selective chemical etching to achieve atomically precise surface control.
• While MQDs have shown promise in electrocatalytic systems such as Li–O2 batteries, their application in intercalation-based batteries (e.g., Li-ion, Na-ion) remains limited. The quantum-confined size of MQDs facilitates ion diffusion and access to active sites but falls short in practical performance. Current MQD-based electrodes reach only ∼200 mA g−1 and fewer than 300 cycles, compared to >5000 mA g−1 and over 700 cycles in leading Li–air systems.22 To bridge this gap, efforts should focus on embedding MQDs into conductive, porous frameworks to improve electron transport and accommodate discharge products. Stabilizing surface terminations, controlling aggregation, and designing efficient catalytic interfaces will be critical. Incorporating redox mediators or solid electrolytes may also lower the overpotentials and enhance the kinetics of reversibility and cycling life.303,304
• For photocatalytic and optical applications, most recent studies have focused on nanocomposites of MQDs with well-known catalysts such as TiO2, Cu nanowires, and g-C3N4 to leverage interfacial charge-transfer mechanisms.305 Future research should explore less conventional co-catalysts, such as conductive or semiconducting MOFs and COFs, to expand the functional landscape of MQD-based systems. In parallel, the intrinsic quantum confinement effects of MQDs, which can independently tune bandgap and electronic transitions, remain underexplored and should be systematically studied. A deeper understanding of how surface terminations modulate exciton dynamics and charge separation efficiency is also needed. Moreover, integrating plasmonic materials or defect-engineered semiconductors could induce plasmonic resonance energy transfer (PRET) or broaden the light-absorption cross-section.
• In energy conversion systems, stabilizing MQDs against aggregation and surface oxidation is critical for long-term electrode stability. This can be achieved through encapsulation in conductive matrices or by anchoring MQDs onto chemically compatible, high-surface-area supports such as carbonaceous materials or transition metal oxides. These strategies not only prevent degradation but also form ohmic contacts and reduce interfacial Schottky in charge transfer mechanistic. Additionally, operando spectroscopy and electrochemical impedance analysis should be used to monitor catalyst evolution and reveal degradation mechanisms. Long-term studies under realistic conditions, such as continuous electrolysis, variable gas concentrations, and pH, are still lacking.69,306 Future research must address these gaps to better evaluate and increase the activation energy of degradation of MQD stability in real-world environments.
• Selectivity in electrocatalytic reactions involving MQDs has received less attention than in other nanomaterials. Altering the adsorption energetics of competing reaction intermediates and selectivity is essential to reduce energy consumption and lower the free energy barrier for the desired efficiency in processes like CO2 and N2 reduction. Approaches such as heteroatom doping (e.g., N, S, B), anchoring single-metal atoms, or constructing well-defined active sites could offer greater control over catalytic pathways. Tailoring surface terminations can also steer intermediate adsorption energetics. Modulating the structural and electronic properties of MQDs, through strain engineering or controlled synthesis, could further enhance charge carrier mobility and reaction specificity.215 These directions require combined experimental and computational studies to uncover structure–property relationships.
• Drug delivery is a key area of interest, particularly targeted smart drug delivery systems where drug-loaded QDs enter cell nuclei and release drugs for treatment.
• The intrinsic properties of MQDs, demonstrated in vitro and in vivo, require deeper investigation to understand underlying mechanisms.
• Research is needed to explore how functional groups influence reactive oxygen species generation to optimize medical applications. This could lead to innovative solutions in regenerative medicine and immune-related challenges, including transplantation.
• Fig. 21 gives a clear and comprehensive overview of the current progress and future directions of MQDs. It is organized into three main categories of synthesis, properties, and applications. Each part contains three sections of challenges, solutions, and outlook. This structure helps to show how different experimental methods and material designs influence MQD performance and practical applications. For example, it illustrates how controlling surface terminations can tune the photoluminescence behavior, or how adjusting synthesis routes can improve device stability and energy efficiency.
• In the synthesis section, the Fig. 21 compares several key approaches, including top-down etching, laser ablation, bottom-up solvothermal synthesis, and surface engineering. It summarizes their main issues including oxidation, size distribution, and low yield and lists the corresponding solutions and improvements like HF-free synthesis, heteroatom doping, and ligand passivation. The properties section highlights how these strategies affect MQD optical response, charge transport, surface stability, and energy-related performance. Finally, the applications section outlines how MQDs are being developed for optoelectronics, energy storage, biomedical imaging, and environmental sensing.
• Overall, the scheme serves not only as a summary but also as a roadmap that guides readers to the next stage of MQD research. The outlook column points out key opportunities for progress, including precise surface functionalization, hybrid material design, environmentally friendly scalable production, and cross-disciplinary integration into energy, environmental, and biomedical technologies. Through this structured format, the figure helps readers understand the current state of the field while also identifying the challenges and emerging directions that will shape future MQD development.
Conflicts of interest
The authors declare no competing financial interest.Abbreviations
| AA | Ascorbic acid |
| AFM | Atomic force microscopy |
| APTES | 3-Aminopropyltriethoxysilane |
| AQY | Apparent quantum yield |
| ARS | Alizarin red S |
| ATP | Adenosine triphosphate |
| BAW | Bulk acoustic wave |
| BiOI | Bismuth oxyiodide |
| BSA | Bovine serum albumin |
| CE | Coulombic efficiency |
| CIE | Commission Internationale de l’Éclairage |
| COF | Covalent organic framework |
| CRI | Color rendering index |
| Cu NWs | Copper nanowires |
| CUR | Curcumin |
| CV | Cyclic voltammetry |
| DA | Dopamine |
| DFT | Density functional theory |
| DMSO | Dimethyl sulfoxide |
| DMF | Dimethylformamide |
| DOX | Doxorubicin |
| DPA | 2,6-Dipicolinic acid |
| DRS | Diffuse reflectance spectroscopy |
| DSSCs | Dye-sensitized solar cells |
| ECL | Electrochemiluminescence |
| EDD | Electron density difference |
| EDTA | Ethylenediaminetetraacetic acid |
| EIS | Electrochemical impedance spectroscopy |
| ESR | Electron spin resonance |
| ETL | Electron transport layer |
| FE | Faradaic efficiency |
| FRET | Förster resonance energy transfer |
| FWHM | Full width at half maximum |
| GCE | Glassy carbon electrode |
| GIXRD | Grazing-incidence X-ray diffraction |
| GSH | Glutathione |
| HAADF-STEM | High-angle annular dark-field scanning transmission electron microscopy |
| HER | Hydrogen evolution reaction |
| HOMO | Highest occupied molecular orbital |
| HRP | Horseradish peroxidase |
| IFE | Inner filter effect |
| iPSCs | Induced pluripotent stem cells |
| ITO | Indium tin oxide |
| KOH | Potassium hydroxide |
| LiPSs | Lithium polysulfides |
| LOD | Limit of detection |
| LRGO | Laser-reduced graphene oxide |
| LUMO | Lowest unoccupied molecular orbital |
| LSV | Linear sweep voltammetry |
| MQDs | MXene quantum dots |
| MSCs | Mesenchymal stem cells |
| MTT | 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| MUC1 | Mucin 1 |
| NaF | Sodium fluoride |
| NDC | Nitrogen-doped carbon |
| NG | N-doped graphene |
| NMP | N-Methylpyrrolidone |
| NRR | Nitrogen reduction reaction |
| OER | Oxygen evolution reaction |
| OPD | O-Phenylenediamine |
| ORR | Oxygen reduction reaction |
| oxOPD | 2,3-Diaminophenazine |
| PEC | Photoelectrochemical |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate |
| PEI | Polyethyleneimine |
| PET | Photoinduced electron transfer |
| PL | Photoluminescence |
| PLQY | Photoluminescence quantum yield |
| PLL | ε-Poly-L-lysine |
| PNK | Polynucleotide kinase |
| POSS-PQDs | Silsesquioxane-perovskite quantum dots |
| PQDs | Perovskite quantum dots |
| PSCs | Perovskite solar cells |
| PTT | Photothermal therapy |
| PVP | Polyvinylpyrrolidone |
| RHE | Reversible hydrogen electrode |
| RES | Reticuloendothelial system |
| RGB | Red, green, blue |
| RRS | Resonance Rayleigh scattering |
| ROS | Reactive oxygen species |
| SCC | Solar-to-chemical conversion |
| SCN− | Thiocyanate |
| SEM | Scanning electron microscopy |
| SWV | Square wave voltammetry |
| TC | Tetracycline |
| TMAOH | Tetramethylammonium hydroxide |
| TMCs | Transition metal carbides |
| TNP | Trinitrophenol |
| TPAOH | Tetrapropylammonium hydroxide |
| UA | Uric acid |
| UV-vis | Ultraviolet-visible spectroscopy |
| VP | Vibrio parahaemolyticus |
| WLED | White light-emitting diode |
| XPS | X-ray photoelectron spectroscopy |
| ZAB | Zinc–air battery |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5nr03616j.
References
- W. Luo, H. Liu, X. Liu, L. Liu and W. Zhao, Colloids Surf., B, 2021, 201, 111631 CrossRef CAS PubMed.
- X. Wang, X. Zhang, H. Cao and Y. Huang, Microchem. J., 2022, 180, 107629 CrossRef CAS.
- C. Guan, X. Yue, J. Fan and Q. Xiang, Chin. J. Catal., 2022, 43, 2484–2499 CrossRef CAS.
- J. Qin, B. Liu, K.-H. Lam, S. Song, X. Li and X. Hu, ACS Sustainable Chem. Eng., 2020, 8, 17791–17799 CrossRef CAS.
- S. Zhang, Z. Qi and Y. Li, Ceram. Int., 2022, 48, 21118–21124 CrossRef CAS.
- A. Kalkal, S. Kadian, S. Kumar, G. Manik, P. Sen, S. Kumar and G. Packirisamy, Biosens. Bioelectron., 2022, 195, 113620 CrossRef CAS PubMed.
- D. V. Tsyupka, Y. A. Podkolodnaya, E. A. Khudina, D. G. Koganova, O. A. Goryacheva, A. M. Abramova and I. Y. Goryacheva, TrAC, Trends Anal. Chem., 2024, 177, 117774 CrossRef CAS.
- Y. Zheng, Y. Wang, Z. Li, Z. Yuan, S. Guo, Z. Lou, W. Han, G. Shen and L. Wang, Matter, 2023, 6, 506–520 CrossRef CAS.
- Q. Xu, W. Yang, Y. Wen, S. Liu, Z. Liu, W.-J. Ong and N. Li, Appl. Mater. Today, 2019, 16, 90–101 CrossRef.
- A. Rafieerad, W. Yan, A. Amiri and S. Dhingra, Mater. Des., 2020, 196, 109091 CrossRef CAS.
- S. Jin, H. Chen, K. Pan, R. Li, X. Ma, R. Yuan, X. Meng and H. He, Talanta, 2024, 270, 125557 CrossRef CAS PubMed.
- S. I. G. P. Mohamed, S. Namvar, T. Zhang, H. Shahbazi, Z. Jiang, A. M. Rappe, A. Salehi-Khojin and S. Nejati, Adv. Mater., 2024, 36, 2309302 CrossRef CAS.
- W. Dai, H. Dong and X. Zhang, Materials, 2018, 11, 1776 CrossRef PubMed.
- P. Das, L. Biswal and K. Parida, Catal. Sci. Technol., 2025, 15, 6976–7003 RSC.
- Q. Xu, Y. Niu, J. Li, Z. Yang, J. Gao, L. Ding, H. Ni, P. Zhu, Y. Liu, Y. Tang, Z.-P. Lv, B. Peng, T. S. Hu, H. Zhou and C. Xu, Carbon Neutrality, 2022, 1, 13 CrossRef CAS.
- K. Kannan, K. K. Sadasivuni, A. M. Abdullah and B. Kumar, Catalysts, 2020, 10, 495 CrossRef CAS.
- S. Gokul Eswaran, M. Rashad, A. Santhana Krishna Kumar and A. F. M. El-Mahdy, Chem. – Asian J., 2025, 20, e202401181 CrossRef CAS PubMed.
- Y. Cheng, B. Jiang, S. Chaemchuen, F. Verpoort and Z. Kou, Carbon Neutralization, 2023, 2, 213–234 CrossRef CAS.
- C. Zhou, K. B. Tan, W. Han, L. Wang and M. Lu, Particuology, 2024, 91, 50–71 CrossRef CAS.
- Sariga, A. M. Babu, S. Kumar, R. Rajeev, D. A. Thadathil and A. Varghese, Adv. Mater. Interfaces, 2023, 10, 2202139 CrossRef CAS.
- B. Shao, Z. Liu, G. Zeng, H. Wang, Q. Liang, Q. He, M. Cheng, C. Zhou, L. Jiang and B. Song, J. Mater. Chem. A, 2020, 8, 7508–7535 RSC.
- J. Lu, Y. Jung Lee, X. Luo, K. Chun Lau, M. Asadi, H.-H. Wang, S. Brombosz, J. Wen, D. Zhai, Z. Chen, D. J. Miller, Y. Sub Jeong, J.-B. Park, Z. Zak Fang, B. Kumar, A. Salehi-Khojin, Y.-K. Sun, L. A. Curtiss and K. Amine, Nature, 2016, 529, 377–382 CrossRef CAS.
- J. Shao, J. Zhang, C. Jiang, J. Lin and P. Huang, Chem. Eng. J., 2020, 400, 126009 CrossRef CAS.
- A. Rafieerad, W. Yan, K. N. Alagarsamy, A. Srivastava, N. Sareen, R. C. Arora and S. Dhingra, Adv. Funct. Mater., 2021, 31, 2106786 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- D. Lei, N. Liu, T. Su, Q. Zhang, L. Wang, Z. Ren and Y. Gao, Adv. Mater., 2022, 34, 2110608 CrossRef CAS PubMed.
- P. O. Å. Persson and J. Rosen, Curr. Opin. Solid State Mater. Sci., 2019, 23, 100774 CrossRef CAS.
- G. Murali, J. K. Reddy Modigunta, Y. H. Park, J.-H. Lee, J. Rawal, S.-Y. Lee, I. In and S.-J. Park, ACS Nano, 2022, 16, 13370–13429 CrossRef CAS PubMed.
- R. M. Ronchi, J. T. Arantes and S. F. Santos, Ceram. Int., 2019, 45, 18167–18188 CrossRef CAS.
- J. Halim, J. Palisaitis, J. Lu, J. Thörnberg, E. J. Moon, M. Precner, P. Eklund, P. O. Å. Persson, M. W. Barsoum and J. Rosen, ACS Appl. Nano Mater., 2018, 1, 2455–2460 CrossRef CAS.
- B. Anasori and Y. Gogotsi, Graphene 2D Mater., 2022, 7, 75–79 CrossRef.
- K. Khan, A. K. Tareen, M. Iqbal, I. Hussain, A. Mahmood, U. Khan, M. F. Khan, H. Zhang and Z. Xie, J. Mater. Chem. A, 2023, 11, 19764–19811 RSC.
- Y. Li, L. Ding, Y. Guo, Z. Liang, H. Cui and J. Tian, ACS Appl. Mater. Interfaces, 2019, 11, 41440–41447 CrossRef CAS PubMed.
- J. Ge, W. Li, X. He, H. Chen, W. Fang, X. Du, Y. Li and L. Zhao, Mater. Today Energy, 2020, 18, 100562 CrossRef CAS.
- Z. Yuan, H. Huang, N. Li, D. Chen, Q. Xu, H. Li, J. He and J. Lu, J. Hazard. Mater., 2021, 409, 125027 CrossRef CAS PubMed.
- Q. Xu, L. Ding, Y. Wen, W. Yang, H. Zhou, X. Chen, J. Street, A. Zhou, W.-J. Ong and N. Li, J. Mater. Chem. C, 2018, 6, 6360–6369 RSC.
- H. Cheng, L. Ding, G. Chen, L. Zhang, J. Xue and H. Wang, Adv. Mater., 2018, 30, 1803694 CrossRef PubMed.
- J. Sun, H. Du, Z. Chen, L. Wang and G. Shen, Nano Res., 2022, 15, 3653–3659 CrossRef CAS.
- M. A. Al-Duais, Z. M. Mohammedsaleh, H. S. Al-Shehri, Y. S. Al-Awthan, S. A. Bani-Atta, A. A. Keshk, S. K. Mustafa, A. D. Althaqafy, J. N. Al-Tweher, H. A. Al-Aoh and C. Panneerselvam, Luminescence, 2022, 37, 633–641 CrossRef CAS PubMed.
- M. Devi, S. Rawat and S. Sharma, Oxford Open Mater. Sci., 2021, 1, itab014 CrossRef CAS.
- C. Lai, Z. An, H. Yi, X. Huo, L. Qin, X. Liu, B. Li, M. Zhang, S. Liu, L. Li, Y. Fu, X. Zhou, Z. Wang, N. An and X. Shi, J. Colloid Interface Sci., 2021, 600, 161–173 CrossRef CAS PubMed.
- Y. Wang, C. Li, X. Han, D. Liu, H. Zhao, Z. Li, P. Xu and Y. Du, ACS Appl. Nano Mater., 2018, 1, 5366–5376 CrossRef CAS.
- X. Chen, X. Sun, W. Xu, G. Pan, D. Zhou, J. Zhu, H. Wang, X. Bai, B. Dong and H. Song, Nanoscale, 2018, 10, 1111–1118 RSC.
- R. Liu, B. Zhang, L. Fu, Z. Fu, H. Xie, Y. Tang, H. Wang and D. Sun, Mater. Today Chem., 2024, 35, 101903 CrossRef CAS.
- G. Yang, J. Zhao, S. Yi, X. Wan and J. Tang, Sens. Actuators, B, 2020, 309, 127735 CrossRef CAS.
- J. Liu, S. Chen, J. He, R. Huang, L. Tao, Y. Zhao and Y. Yang, Nanomaterials, 2022, 12, 2043 CrossRef CAS PubMed.
- S. M. Mousavi, M. Y. Kalashgrani, M. Binazadeh, Y. Mazaheri, N. Omidifar, V. Rahmanian, M. Riazi, C. W. Lai, R. H. Althomali, M. M. Rahman, A. Gholami and W.-H. Chiang, Mater. Today Chem., 2024, 38, 102097 CrossRef CAS.
- T. S. de Windt, L. A. Vonk, I. C. M. Slaper-Cortenbach, R. Nizak, M. H. P. van Rijen and D. B. F. Saris, Stem Cells, 2017, 35, 1984–1993 CrossRef CAS PubMed.
- A. Trounson and C. McDonald, Cell Stem Cell, 2015, 17, 11–22 CrossRef CAS PubMed.
- Y. Zhang, M. Li, X. Zhang, P. Zhang, Z. Liu, M. Feng, G. Ren and J. Liu, Colloids Surf., B, 2023, 221, 113005 CrossRef CAS PubMed.
- A. Rafieerad, W. Yan, G. L. Sequiera, N. Sareen, E. Abu-El-Rub, M. Moudgil and S. Dhingra, Adv. Healthcare Mater., 2019, 8, 1900569 CrossRef PubMed.
- D. Xiao, C. Wu, B. Liang, S. Jiang, J. Ma and Y. Li, J. Mater. Chem. A, 2024, 12, 31655–31661 RSC.
- A. M. Jastrzębska, A. Szuplewska, T. Wojciechowski, M. Chudy, W. Ziemkowska, L. Chlubny, A. Rozmysłowska and A. Olszyna, J. Hazard. Mater., 2017, 339, 1–8 CrossRef PubMed.
- B. C. Bejgum and M. D. Donovan, Mol. Pharm., 2021, 18, 429–440 CrossRef CAS PubMed.
- L. Rueda-Gensini, J. Cifuentes, M. C. Castellanos, P. R. Puentes, J. A. Serna, C. Muñoz-Camargo and J. C. Cruz, Nanomaterials, 2020, 10, 1816 CrossRef CAS PubMed.
- S. Y. Won, R. Singhmar, S. Sahoo, H. Kim, C. M. Kim, S. M. Choi, A. Sood and S. S. Han, Colloids Surf., B, 2025, 245, 114207 CrossRef CAS PubMed.
- G. Yang and S.-J. Park, Materials, 2019, 12, 1177 CrossRef CAS PubMed.
- B. Hu, J. Chen, Z. Gao, L. Chen, T. Cao, H. Li, Q. Yu, C. Wang and Z. Gan, ACS Appl. Bio Mater., 2024, 7, 4339–4351 CrossRef CAS PubMed.
- L. Jia, S. Zhou, A. Ahmed, Z. Yang, S. Liu, H. Wang, F. Li, M. Zhang, Y. Zhang and L. Sun, Chem. Eng. J., 2023, 475, 146361 CrossRef CAS.
- L. Song, S. Zhu, L. Tong, W. Wang, C. Ouyang, F. Xu and Y. Wang, Mater. Adv., 2021, 2, 5622–5628 RSC.
- F. Shahzad, A. Iqbal, H. Kim and C. M. Koo, Adv. Mater., 2020, 32, 2002159 CrossRef CAS PubMed.
- K. Jin and X. Liu, Surf. Interfaces, 2024, 44, 103790 CrossRef CAS.
- X. Jiang, A. V. Kuklin, A. Baev, Y. Ge, H. Ågren, H. Zhang and P. N. Prasad, Phys. Rep., 2020, 848, 1–58 CrossRef CAS.
- M. Wan, J. Zhou, H. Yang, X. Dai, Y. Zheng, Z. Xia and L. Wang, ACS Appl. Nano Mater., 2022, 5, 11715–11722 CrossRef CAS.
- C. Fu, F. Ai, J. Huang, Z. Shi, X. Yan and X. Zheng, Spectrochim. Acta, Part A, 2022, 272, 120956 CrossRef CAS PubMed.
- Z. Guo, X. Zhu, S. Wang, C. Lei, Y. Huang, Z. Nie and S. Yao, Nanoscale, 2018, 10, 19579–19585 RSC.
- G. Xu, Y. Niu, X. Yang, Z. Jin, Y. Wang, Y. Xu and H. Niu, Adv. Opt. Mater., 2018, 6, 1800951 CrossRef.
- X. Gao, X. Shao, L. Qin, Y. Li, S. Huang and L. Deng, Nanoscale Res. Lett., 2021, 16, 160 CrossRef CAS PubMed.
- Z. Jin, C. Liu, Z. Liu, J. Han, Y. Fang, Y. Han, Y. Niu, Y. Wu, C. Sun and Y. Xu, Adv. Energy Mater., 2020, 10, 2000797 CrossRef CAS.
- Q. Zhang, Y. Sun, M. Liu and Y. Liu, Nanoscale, 2020, 12, 1826–1832 RSC.
- H. Zhang, L. Wang, T. Zhuang, Z. Wei, J. Xia and Z. Wang, Anal. Bioanal. Chem., 2022, 414, 6753–6760 CrossRef CAS PubMed.
- Y. Nie, Z. Liang, P. Wang, Q. Ma and X. Su, Anal. Chem., 2021, 93, 17086–17093 CrossRef CAS PubMed.
- Y. Liu, N. Ye, X. Li, X. Li, H. Liu, E. Wang, C. Liang and X. Peng, J. Solid State Chem., 2021, 293, 121781 CrossRef CAS.
- Y. Feng, F. Zhou, Q. Deng and C. Peng, Ceram. Int., 2020, 46, 8320–8327 CrossRef CAS.
- L. Zhao, Z. Wang, Y. Li, S. Wang, L. Wang, Z. Qi, Q. Ge, X. Liu and J. Z. Zhang, J. Mater. Sci. Technol., 2021, 78, 30–37 CrossRef CAS.
- X. Ding, L. Qu, R. Yang, Y. Zhou and J. Li, Luminescence, 2015, 30, 465–471 CrossRef CAS PubMed.
- F. Yang, Y. Ge, T. Yin, J. Guo, F. Zhang, X. Tang, M. Qiu, W. Liang, N. Xu, C. Wang, Y. Song, S. Xu and S. Xiao, ACS Appl. Nano Mater., 2020, 3, 11850–11860 CrossRef CAS.
- L. Gnanasekaran, R. Hemamalini and K. Ravichandran, J. Saudi Chem. Soc., 2015, 19, 589–594 CrossRef.
- F. He, B. Zhu, B. Cheng, J. Yu, W. Ho and W. Macyk, Appl. Catal., B, 2020, 272, 119006 CrossRef CAS.
- N. Prudhvi Raju, D. Tripathi, S. Lahiri and R. Thangavel, Sol. Energy, 2023, 259, 107–118 CrossRef CAS.
- L. Ding, S. Zeng, W. Zhang, C. Guo, X. Chen, B. Peng, Z. Lv, H. Zhou and Q. Xu, ACS Appl. Energy Mater., 2022, 5, 11540–11552 CrossRef CAS.
- G. Cai, Z. Yu, P. Tong and D. Tang, Nanoscale, 2019, 11, 15659–15667 RSC.
- H. Wang, R. Zhao, H. Hu, X. Fan, D. Zhang and D. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 40176–40185 CrossRef CAS.
- Y. Bai, Y. He, M. Wang and G. Song, Sens. Actuators, B, 2022, 357, 131410 CrossRef CAS.
- Y. Wang, J. Wang, P. Ma, H. Yao, L. Zhang and Z. Li, New J. Chem., 2017, 41, 14918–14923 RSC.
- Q. Xue, H. Zhang, M. Zhu, Z. Pei, H. Li, Z. Wang, Y. Huang, Y. Huang, Q. Deng, J. Zhou, S. Du, Q. Huang and C. Zhi, Adv. Mater., 2017, 29, 1604847 CrossRef PubMed.
- K. Gao, L. Hou, X. An, D. Huang and Y. Yang, Appl. Catal., B, 2023, 323, 122150 CrossRef CAS.
- X. Wang, X. Zhang, H. Cao and Y. Huang, J. Mater. Chem. B, 2020, 8, 10837–10844 RSC.
- G. P. Neupane, B. Wang, M. Tebyetekerwa, H. T. Nguyen, M. Taheri, B. Liu, M. Nauman and R. Basnet, Small, 2021, 17, 2006309 CrossRef CAS PubMed.
- Q. Xu, J. Ma, W. Khan, X. Zeng, N. Li, Y. Cao, X. Zhao and M. Xu, Chem. Commun., 2020, 56, 6648–6651 RSC.
- L. Zhou, F. Wu, J. Yu, Q. Deng, F. Zhang and G. Wang, Carbon, 2017, 118, 50–57 CrossRef CAS.
- X. Li, F. Liu, D. Huang, N. Xue, Y. Dang, M. Zhang, L. Zhang, B. Li, D. Liu, L. Wang, H. Liu and X. Tao, Adv. Funct. Mater., 2020, 30, 2000308 CrossRef CAS.
- Z. Wang, Y. Zhu, Y. Wu, W. Ding and X. Li, Nanoscale, 2022, 14, 9498–9506 RSC.
- X. Huang and P. Wu, Adv. Funct. Mater., 2020, 30, 1910048 CrossRef CAS.
- B. Yan, Z. Cheng, C. Lai, B. Qiao, R. Yuan, C. Zhang, H. Pei, J. Tu and Q. Wu, Nanomaterials, 2022, 12, 3557 CrossRef CAS PubMed.
- W. Han, X. Wen, Y. Ding, Z. Li, M. Lu, H. Zhu, G. Wang, J. Yan and X. Hong, Appl. Surf. Sci., 2022, 595, 153563 CrossRef CAS.
- H. Alijani, A. R. Rezk, M. M. Khosravi Farsani, H. Ahmed, J. Halim, P. Reineck, B. J. Murdoch, A. El-Ghazaly, J. Rosen and L. Y. Yeo, ACS Nano, 2021, 15, 12099–12108 CrossRef CAS PubMed.
- B. Yu, A. Huang, D. Chen, K. Srinivas, X. Zhang, X. Wang, B. Wang, F. Ma, C. Liu, W. Zhang, J. He, Z. Wang and Y. Chen, Small, 2021, 17, 2100460 CrossRef CAS PubMed.
- M. Liu, Y. Bai, Y. He, J. Zhou, Y. Ge, J. Zhou and G. Song, Microchim. Acta, 2021, 188, 15 CrossRef CAS PubMed.
- F. Yan, J. Sun, Y. Zang, Z. Sun, H. Zhang, J. Xu and X. Wang, Dyes Pigm., 2021, 195, 109720 CrossRef CAS.
- X. Jiang, H. Wang, Y. Shen, N. Hu and W. Shi, Sens. Actuators, B, 2022, 350, 130891 CrossRef CAS.
- Q. Guan, J. Ma, W. Yang, R. Zhang, X. Zhang, X. Dong, Y. Fan, L. Cai, Y. Cao, Y. Zhang, N. Li and Q. Xu, Nanoscale, 2019, 11, 14123–14133 RSC.
- W. Kong, Y. Niu, M. Liu, K. Zhang, G. Xu, Y. Wang, X. Wang, Y. Xu and J. Li, Inorg. Chem. Commun., 2019, 105, 151–157 CrossRef CAS.
- Q. Lu, J. Wang, B. Li, C. Weng, X. Li, W. Yang, X. Yan, J. Hong, W. Zhu and X. Zhou, Anal. Chem., 2020, 92, 7770–7777 CrossRef CAS PubMed.
- M. Liu, Y. He, J. Zhou, Y. Ge, J. Zhou and G. Song, Anal. Chim. Acta, 2020, 1103, 134–142 CrossRef CAS PubMed.
- S. Li, J. Ma, X. Zhao, P. Zhu, M. Xu, Y. Niu, D. Luo and Q. Xu, Chin. Chem. Lett., 2022, 33, 1850–1854 CrossRef CAS.
- D. Huang, Y. Wu, F. Ai, X. Zhou and G. Zhu, Sens. Actuators, B, 2021, 342, 130074 CrossRef CAS.
- Z. Wang, Y. Zhu, Y. Wu, W. Ding and X. Li, Nanoscale, 2022, 14, 9498–9506 RSC.
- Y. Bai, Y. He, Y. Wang and G. Song, Microchim. Acta, 2021, 188, 401 CrossRef CAS PubMed.
- M. Liu, J. Zhou, Y. He, Z. Cai, Y. Ge, J. Zhou and G. Song, Microchim. Acta, 2019, 186, 770 CrossRef CAS PubMed.
- N. Mohamadbeigi, L. Shooshtari, S. Fardindoost, M. Vafaiee, A. Iraji zad and R. Mohammadpour, Sci. Rep., 2024, 14, 1562 CrossRef CAS PubMed.
- I. I. Ebralidze, N. O. Laschuk, J. Poisson and O. V. Zenkina, in Nanomaterials Design for Sensing Applications, Elsevier, 2019, pp. 1–39 Search PubMed.
- M. Wan, A. Jimu, H. Yang, J. Zhou, X. Dai, Y. Zheng, J. Ou, Y. Yang, J. Liu and L. Wang, Microchem. J., 2023, 184, 108180 CrossRef CAS.
- F. Ai, C. Fu, G. Cheng, H. Zhang, Y. Feng, X. Yan and X. Zheng, ACS Appl. Nano Mater, 2021, 4, 8192–8199 CrossRef CAS.
- X. Chen, J. Li, G. Pan, W. Xu, J. Zhu, D. Zhou, D. Li, C. Chen, G. Lu and H. Song, Sens. Actuators, B, 2019, 289, 131–137 CrossRef CAS.
- J. Hong, W. Wang, J. Wang, X. Wang, H. Xie, T. Li and N. Gan, Microchimica Acta, 2021, 188, 45 CrossRef CAS PubMed.
- M. Liu, Y. Bai, Y. He, J. Zhou, Y. Ge, J. Zhou and G. Song, Microchem. J., 2024, 207, 112068 CrossRef.
- L. Wang, N. Zhang, Y. Li, W. Kong, J. Gou, Y. Zhang, L. N. Wang, G. Yu, P. Zhang, H. Cheng and L. Qu, ACS Appl. Mater. Interfaces, 2021, 13, 42442–42450 CrossRef CAS PubMed.
- Q. Xu, L. Ding, Y. Wen, W. Yang, H. Zhou, X. Chen, J. Street, A. Zhou, W. J. Ong and N. Li, J. Mater. Chem. C, 2018, 6, 6360–6369 RSC.
- M. Wan, J. Zhou, H. Yang, X. Dai, Y. Zheng, Z. Xia and L. Wang, ACS Appl. Nano Mater., 2022, 5, 11715–11722 CrossRef CAS.
- M. A. Al-Duais, Z. M. Mohammedsaleh, H. S. Al-Shehri, Y. S. Al-Awthan, S. A. Bani-Atta, A. A. Keshk, S. K. Mustafa, A. D. Althaqafy, J. N. Al-Tweher, H. A. Al-Aoh and C. Panneerselvam, Luminescence, 2022, 37, 633–641 CrossRef CAS PubMed.
- Y. Cheng, P. Shen, X. Li, X. Li, K. Chu and Y. Guo, Sens. Actuators, B, 2023, 376, 132979 CrossRef CAS.
- P. Jin, P. Wan, C. Zhang, X. Li, Y. Wang, J. Luo and K. Li, Anal. Chim. Acta, 2024, 1303, 342517 CrossRef CAS PubMed.
- C. Lin, C. Qiu, Y. Wang, Y. Liu, M. Rong and L. Niu, Sens. Diagn., 2024, 3, 431–439 RSC.
- Z. Ding, X. Zhan, Y. Zhang, K. Chu and Y. Guo, Microchem. J., 2024, 207, 111951 CrossRef CAS.
- R. Rajamanikandan, K. Sasikumar and H. Ju, Anal. Chim. Acta, 2024, 1322, 343069 CrossRef CAS PubMed.
- M. H. Azar, H. Abdollahi, S. Arabloo, N. Mohamadbeigi, A. F. Sohi, A. Simchi and K. Musselman, Prog. Mater. Sci., 2025, 158, 101624 CrossRef.
- D. Huang, Y. Xie, D. Lu, Z. Wang, J. Wang, H. Yu and H. Zhang, Adv. Mater., 2019, 31, 1901117 CrossRef PubMed.
- X. (Xiao) Liu, Z. Zhang, J. Jiang, C. Tian, X. Wang, L. Wang, Z. Zhang, X. Wu, Y. Zheng, J. Liang and C.-C. Chen, Chem. Eng. J., 2022, 432, 134382 CrossRef.
- Y. Niu, C. Tian, J. Gao, F. Fan, Y. Zhang, Y. Mi, X. Ouyang, L. Li, J. Li, S. Chen, Y. Liu, H.-L. Lu, X. Zhao, L. Yang, H. Ju, Y. Yang, C.-F. Ding, M. Xu and Q. Xu, Nano Energy, 2021, 89, 106455 CrossRef CAS.
- Y. Yang, H. Lu, S. Feng, L. Yang, H. Dong, J. Wang, C. Tian, L. Li, H. Lu, J. Jeong, S. M. Zakeeruddin, Y. Liu, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2021, 14, 3447–3454 RSC.
- H. Yan, M. Chen, W. Liu, P. Wang, M. Liu, Y. Liu, L. Ye and M. Gu, Opt. Mater., 2023, 140, 113902 CrossRef CAS.
- H. Mao, C. Gu, S. Yan, Q. Xin, S. Cheng, P. Tan, X. Wang, F. Xiu, X. Liu, J. Liu, W. Huang and L. Sun, Adv. Electron. Mater., 2020, 6, 1900493 CrossRef CAS.
- C. Qiao, H. Wu, X. Xu, Z. Guan and W. Ou-Yang, Adv. Mater. Interfaces, 2021, 8, 2100903 CrossRef CAS.
- Q. Zhu, Y. Cui, Y. Zhang, Z. Cao, Y. Shi, J. Gu, Z. Du, B. Li and S. Yang, Mater. Today Nano, 2021, 13, 100104 CrossRef CAS.
- S. Xiao, Y. Zheng, X. Wu, M. Zhou, X. Rong, L. Wang, Y. Tang, X. Liu, L. Qiu and C. Cheng, Small, 2022, 18, 2203281 CrossRef CAS PubMed.
- P. Liu, H. Liu, T. Zhang, L. Chen, W. Guo, T. Gu, F. Yu, Y. Liu and G. Wang, Chem. Eng. J., 2023, 477, 146913 CrossRef CAS.
- X. Li, C. Wen, M. Yuan, Z. Sun, Y. Wei, L. Ma, H. Li and G. Sun, J. Alloys Compd., 2020, 824, 153803 CrossRef CAS.
- L. Zhang, X. Ji, X. Ren, Y. Ma, X. Shi, Z. Tian, A. M. Asiri, L. Chen, B. Tang and X. Sun, Adv. Mater., 2018, 30, 1800191 CrossRef PubMed.
- M. A. K. Purbayanto, M. Chandel, M. Birowska, A. Rosenkranz and A. M. Jastrzębska, Adv. Mater., 2023, 35, 2301850 CrossRef CAS PubMed.
- Y. Liu, W. Zhang, X. Zou, Y. Yan, Q. Liang, F. Liu, W. Li, K. Song, X. Zhou, Z. Chen and W. Zheng, Acta Mater., 2025, 283, 120507 CrossRef CAS.
- G. Dong, Y. Zhang, Y. Wang, Q. Deng, C. Qin, Y. Hu, Y. Zhou and G. Tian, ACS Appl. Energy Mater., 2021, 4, 14342–14351 CrossRef CAS.
- B. Li, D. Shen, Z. Xiao, Q. Li, S. Yao, W. Wang and L. Liu, Inorg. Chem. Front., 2023, 10, 5927–5936 RSC.
- J. Nie, X. Zhang, M. Wang, Y. Ou, S. Li, P. Zhong, W. Wang, G. Zhu and X. Ma, 2024, preprint, DOI:10.2139/ssrn.4829261.
- Z. Zeng, Y. Yan, J. Chen, P. Zan, Q. Tian and P. Chen, Adv. Funct. Mater., 2019, 29, 1806500 CrossRef.
- S. Shen, T. Ke, K. Rajavel, K. Yang and D. Lin, Small, 2020, 16, 2002433 CrossRef CAS PubMed.
- Y. Huang, X. Wang, H. Zhang, L. Gao, J. Meng, Y. Liao, Q. Zhou, Y. Wei, B. Zong, H. Li and W.-L. Dai, 2025, preprint, DOI:10.2139/ssrn.5095895.
- Y. Cao, T. Wu, K. Zhang, X. Meng, W. Dai, D. Wang, H. Dong and X. Zhang, ACS Nano, 2019, 13, 1499–1510 CAS.
- X. Yu, X. Cai, H. Cui, S.-W. Lee, X.-F. Yu and B. Liu, Nanoscale, 2017, 9, 17859–17864 RSC.
- W. Dai, H. Dong and X. Zhang, Materials, 2018, 11, 1776 CrossRef PubMed.
- Q. Xue, H. Zhang, M. Zhu, Z. Pei, H. Li, Z. Wang and Y. Huang, J. Mater. Chem. A, 2017, 5, 20818–20823 RSC.
- X. Zhou, J. Zhang, D. Huang, Y. Yi, K. Wu and G. Zhu, Spectrochim. Acta, Part A, 2023, 293, 122484 CrossRef CAS PubMed.
- M. K. Schnizler, R. Bogdan, A. Bennert, N. R. Bury, M. Fronius and W. Clauss, Biochim. Biophys. Acta, Biomembr., 2007, 1768, 317–323 CrossRef CAS.
- J. Gu, X. Lu, G. Li, B. Shan, J. Liu, Y. Qu, H. Ye, K. Xi and H. Wu, Chem. Eng. J., 2023, 467, 143445 CrossRef CAS.
- X. Song, R. D. Airan, D. R. Arifin, A. Bar-Shir, D. K. Kadayakkara, G. Liu, A. A. Gilad, P. C. M. van Zijl, M. T. McMahon and J. W. M. Bulte, Nat. Commun., 2015, 6, 6719 CrossRef CAS PubMed.
- N. Mohamadbeigi, N. Rafiefard, F. Ejehi, R. Mohammadpour and A. Iraji zad, Energy Technol., 2024, 12, 2301136 CrossRef CAS.
- L. Feng, J. Luo, X. Ma, J. Cui, Y. Chen, J. Lu, L. Zhang and Z. Pei, Opt. Express, 2022, 30, 34129 CrossRef CAS PubMed.
- Z. Li, Q. Chen, G. Wen and Z. Jiang, Langmuir, 2024, 40, 17358–17366 CrossRef CAS PubMed.
- Z. Yu, C. Deng, S. Jiang, Y. Liu, C. Liu, F. Seidi, X. Zhang, Y. Huang, W. Wu, J. Han, Q. Yong and H. Xiao, J. Colloid Interface Sci., 2025, 679, 510–520 CrossRef CAS PubMed.
- P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnik, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Nat. Mater., 2014, 13, 891–896 CrossRef CAS PubMed.
- H.-Y. Lin, C.-W. Sher, C.-H. Lin, H.-H. Tu, X. Y. Chen, Y.-C. Lai, C.-C. Lin, H.-M. Chen, P. Yu, H.-F. Meng, G.-C. Chi, K. Honjo, T.-M. Chen and H.-C. Kuo, ACS Appl. Mater. Interfaces, 2017, 9, 35279–35286 CrossRef CAS PubMed.
- M. H. Azar, M. Mohammadi, N. T. Rezaei, S. Aynehband and A. Simchi, J. Alloys Compd., 2022, 907, 164465 CrossRef CAS.
- M. Hasanzadeh Azar, Z. Ji, J. Jahanzamin and A. Kitai, in Silicon Carbide - Materials, Devices and Emerging Applications, IntechOpen, 2024, DOI:10.5772/intechopen.1007535.
- M. Hasanzadeh Azar, J. Jahanzamin, Z. Ji, A. Kitai, D. Beke and A. Gali, Small Sci., 2025, 5, 2500013 CrossRef CAS PubMed.
- S.-W. Chang, W.-C. Liao, Y.-M. Liao, H.-I. Lin, H.-Y. Lin, W.-J. Lin, S.-Y. Lin, P. Perumal, G. Haider, C.-T. Tai, K.-C. Shen, C.-H. Chang, Y.-F. Huang, T.-Y. Lin and Y.-F. Chen, Sci. Rep., 2018, 8, 2720 CrossRef PubMed.
- G. Haider, H.-I. Lin, K. Yadav, K.-C. Shen, Y.-M. Liao, H.-W. Hu, P. K. Roy, K. P. Bera, K.-H. Lin, H.-M. Lee, Y.-T. Chen, F.-R. Chen and Y.-F. Chen, ACS Nano, 2018, 12, 11847–11859 CrossRef CAS PubMed.
- M. L. Pascu, N. Moise and A. Staicu, J. Mol. Struct., 2001, 598, 57–64 CrossRef CAS.
- A. Szukalska, A. Szukalski, M. Adaszynski and J. Mysliwiec, Adv. Opt. Mater., 2023, 11, 2300266 CrossRef CAS.
- F. Fan, S. Turkdogan, Z. Liu and C.-Z. Ning, in 2016 IEEE Photonics Conference (IPC), IEEE, 2016, pp. 90–90.
- K. Yamashita, N. Takeuchi, K. Oe and H. Yanagi, Opt. Lett., 2010, 35, 2451 CrossRef CAS PubMed.
- C. Dang, J. Lee, C. Breen, J. S. Steckel, S. Coe-Sullivan and A. Nurmikko, Nat. Nanotechnol., 2012, 7, 335–339 CrossRef CAS PubMed.
- X. P. Hu, G. Zhao, Z. Yan, X. Wang, Z. D. Gao, H. Liu, J. L. He and S. N. Zhu, Opt. Lett., 2008, 33, 408 CrossRef CAS PubMed.
- S. Liu, V. P. Biju, Y. Qi, W. Chen and Z. Liu, NPG Asia Mater., 2023, 15, 27 CrossRef CAS.
- M. Hasanzadeh Azar, S. Aynehband, H. Abdollahi, H. Alimohammadi, N. Rajabi, S. Angizi, V. Kamraninejad, R. Teimouri, R. Mohammadpour and A. Simchi, Photonics, 2023, 10, 271 CrossRef CAS.
- S. Aynehband, M. H. Azar, J.-M. Nunzi and A. Simchi, Mater. Lett., 2025, 397, 138810 CrossRef CAS.
- M. Hasanzadeh Azar, M. Mohammadi, N. T. Rezaei, S. Aynehband, L. Shooshtari, R. Mohammadpour and A. Simchi, ACS Appl. Nano Mater., 2021, 4, 7788–7799 CrossRef CAS.
- M. Hasanzadeh Azar, H. Abdollahi, S. Arabloo and A. Simchi, J. Mater. Chem. C, 2025, 13, 9061–9071 RSC.
- Q.-Q. Chu, Z. Sun, D. Wang, B. Cheng, H. Wang, C.-P. Wong and B. Fang, Matter, 2023, 6, 3838–3863 CrossRef CAS.
- S. Shao and M. A. Loi, Adv. Mater. Interfaces, 2020, 7, 1901469 CrossRef CAS.
- S. Palei, G. Murali, C.-H. Kim, I. In, S.-Y. Lee and S.-J. Park, Nano-Micro Lett., 2023, 15, 123 CrossRef CAS PubMed.
- M. S. K T and P. Karupppanan, Chem. Phys. Impact, 2024, 8, 100610 CrossRef.
- S. Qamar, K. Fatima, N. Ullah, Z. Akhter, A. Waseem and M. Sultan, Nanoscale, 2022, 14, 13018–13039 RSC.
- Z. Shi, R. Khaledialidusti, M. Malaki and H. Zhang, Nanomaterials, 2021, 11, 3170 CrossRef CAS PubMed.
- L. Yang, P. Li, J. Ma, X. Zhang, X.-F. Wang and Y. Liu, J. Energy Chem., 2023, 81, 443–461 CrossRef CAS.
- M. A. Saeed, A. Shahzad, K. Rasool, F. Mateen, J. Oh and J. W. Shim, Adv. Sci., 2022, 9, 2104743 CrossRef CAS PubMed.
- X. Chen, W. Xu, N. Ding, Y. Ji, G. Pan, J. Zhu, D. Zhou, Y. Wu, C. Chen and H. Song, Adv. Funct. Mater., 2020, 30, 2003295 CrossRef CAS.
- D. L. Narayanan, R. N. Saladi and J. L. Fox, Int. J. Dermatol., 2010, 49, 978–986 CrossRef PubMed.
- S. Aynehband, M. Mohammadi, R. Poushimin, M. H. Azar, J.-M. Nunzi and A. Simchi, Mater. Res. Bull., 2022, 147, 111648 CrossRef CAS.
- Q. Wang, H. Wang, R. Xue, M. Ning, S. Li, P. Chen, M. Sun and Z. Li, J. Alloys Compd., 2023, 965, 171399 CrossRef CAS.
- A. Singh, S. Mahapatra, R. Prasad, S. K. Singh and P. Chandra, Nanoscale, 2025, 17, 15554–15591 RSC.
- A. S. Sharbirin, R. E. Kong, W. B. Mato, T. T. Tran, E. Lee, J. W. P. Khor, A. L. Fadli and J. Kim, Opto-Electron. Adv., 2024, 7, 240029–240029 CAS.
- L. Thyda, K. Naresh, J. K. Joseph, S. Suneetha, C. E. Jeyanthi, P. Amaladass, C. Selvaraju and K. Thangaraju, Thin Solid Films, 2024, 790, 140221 CrossRef CAS.
- H. Lu, J. He, Z. Hu, X. Hu, P. He, Y. Zhao, M. Hao and L. Tao, Infrared Phys. Technol., 2021, 119, 103962 CrossRef CAS.
- J. Du, M. Zhang, Z. Guo, J. Chen, X. Zhu, G. Hu, P. Peng, Z. Zheng and H. Zhang, Sci. Rep., 2017, 7, 42357 CrossRef CAS PubMed.
- N. Xu, H. Li, Y. Gan, H. Chen, W. Li, F. Zhang, X. Jiang, Y. Shi, J. Liu, Q. Wen and H. Zhang, Adv. Sci., 2020, 7, 2002209 CrossRef CAS PubMed.
- Y. Shi, H. Long, S. Liu, Y. H. Tsang and Q. Wen, J. Mater. Chem. C, 2018, 6, 12638–12642 RSC.
- J. S. Meena, S. M. Sze, U. Chand and T.-Y. Tseng, Nanoscale Res. Lett., 2014, 9, 526 CrossRef PubMed.
- S. Tappertzhofen, in Metal Oxides for Non-volatile Memory, Elsevier, 2022, pp. 1–32 Search PubMed.
- S. Gokul Eswaran, M. Rashad, A. Santhana Krishna Kumar and A. F. M. El-Mahdy, Chem. – Asian J., 2025, 20, e202401181 CrossRef CAS PubMed.
- B. Vénosová and F. Karlický, Nanoscale Adv., 2023, 5, 7067–7076 RSC.
- J. Zhang, R. Jia, K. B. Tan, J. Li, S. Xu, G. Ying, W. Han and M. Lu, Nano-Micro Lett., 2025, 17, 173 CrossRef PubMed.
- Z. Wang, Y. Wang, Q. Gu, C. Zhao, J. Zhang, S. Xu, M. Lu and B. Zhang, Particuology, 2023, 72, 10–16 CrossRef CAS.
- Z. Fan, Y. Li, J. Pan, Z. Zhou, W. Li, T. Yang, H. Zhang, C. Shu, W. Hua, Y. Wu and W. Tang, EES Batteries, 2025, 1, 100–118 RSC.
- J. Sun, B. S. Shengping Zhang, M. Alomar, A. S. Alqarni, M. S. Najla Alotaibi, M. S. Badriah Alshahrani, A. A. Alghamdi, Z. Kou, W. Shen, Y. Chen and J. Zhang, Chem. Rec., 2023, 23, e202200268 CrossRef CAS PubMed.
- J. Wang, Y. Hong, Y. Pan, J. Zhu, X. Xu, W. M. Choi and J. Yang, Mater. Chem. Phys., 2024, 312, 128634 CrossRef CAS.
- S. Liu, H. Zhang, J. Chen, X. Peng, Y. Chai, X. Shao, Y. He, X. Wang and B. Ding, Energies, 2025, 18, 1223 CrossRef CAS.
- C.-M. Fung, B.-J. Ng, Y.-H. Chew, C.-C. Er, J. Low, X. Guo, X. Y. Kong, L.-L. Tan, H. Onishi, A. R. Mohamed and S.-P. Chai, Cell Rep. Phys. Sci., 2024, 5, 102296 CrossRef CAS.
- T. Ramachandran, F. Hamed, Y. A. Kumar, R. K. Raji and H. H. Hegazy, J. Energy Storage, 2023, 73, 109299 CrossRef.
- A. M. Bogale, T. Ramachandran, M. E. Suk, B. B. Badassa, M. M. Solomon, J. He, A. Yusuf, R. K. Raji, B. A. Zenebe, N. K. Amare and F. B. Tesema, J. Phys. Chem. Solids, 2026, 208, 113079 CrossRef CAS.
- T. Ramachandran, F. Hamed, R. K. Raji, S. M. Majhi, D. Barik, Y. A. Kumar, R. O. M. U. Jauhar, M. P. Pachamuthu, L. Vijayalakshmi and S. Ansar, J. Phys. Chem. Solids, 2023, 180, 111467 CrossRef CAS.
- C. Choi, D. S. Ashby and D. M. Butts, et al. , Nat Rev Mater., 2020, 5, 5–19 CrossRef.
- T. Ramachandran, R. K. Raji, S. Palanisamy, N. Renuka and K. Karuppasamy, J. Ind. Eng. Chem., 2025, 145, 144–168 CrossRef CAS.
- P. Naskar, D. Kundu, A. Maiti, P. Chakraborty, B. Biswas and A. Banerjee, ChemElectroChem, 2021, 8, 1393–1429 CrossRef CAS.
- D. G. Gibson, J. I. Glass, C. Lartigue, V. N. Noskov, R.-Y. Chuang, M. A. Algire, G. A. Benders, M. G. Montague, L. Ma, M. M. Moodie, C. Merryman, S. Vashee, R. Krishnakumar, N. Assad-Garcia, C. Andrews-Pfannkoch, E. A. Denisova, L. Young, Z.-Q. Qi, T. H. Segall-Shapiro, C. H. Calvey, P. P. Parmar, C. A. Hutchison, H. O. Smith and J. C. Venter, Science, 2010, 329, 52–56 CrossRef CAS PubMed.
- P. Seraji, H. Shahbazi, M. K. Ncube, N. Shan, F. Lagunas, I. Papailias, P. Navabi, C. Zhang, A. Jaradat, S. Kadkhodaei, K. D. Glusac, R. F. Klie, A. T. Ngo, L. A. Curtiss and A. Salehi-Khojin, Nano Energy, 2025, 134, 110510 CrossRef CAS.
- A. Jaradat, C. Zhang, S. K. Singh, J. Ahmed, A. Ahmadiparidari, L. Majidi, S. Rastegar, Z. Hemmat, S. Wang, A. T. Ngo, L. A. Curtiss, M. Daly, A. Subramanian and A. Salehi-khojin, Small, 2021, 17, 2102072 CrossRef CAS PubMed.
- X. Zheng, M. Yuan, D. Guo, C. Wen, X. Li, X. Huang, H. Li and G. Sun, ACS Nano, 2022, 16, 4487–4499 CrossRef CAS PubMed.
- J. Gan, F. Li and Q. Tang, J. Phys. Chem. Lett., 2021, 12, 4805–4813 CrossRef CAS PubMed.
- H. Chen, A. D. Handoko, T. Wang, J. Qu, J. Xiao, X. Liu, D. Legut, Z. Wei Seh and Q. Zhang, ChemSusChem, 2020, 13, 5690–5698 CrossRef CAS PubMed.
- K. Eid, Q. Lu, S. Abdel-Azeim, A. Soliman, A. M. Abdullah, A. M. Abdelgwad, R. P. Forbes, K. I. Ozoemena, R. S. Varma and M. F. Shibl, J. Mater. Chem. A, 2022, 10, 1965–1975 RSC.
- H. Shahbazi, M. Kazemzadeh, A. Malek Khachatourian, P. Seraji, M. Dehghani Mohammad Abadi and M. Golmohammad, Electrochim. Acta, 2024, 507, 145193 CrossRef CAS.
- P. Wang, D. Zhao, X. Hui, Z. Qian, P. Zhang, Y. Ren, Y. Lin, Z. Zhang and L. Yin, Adv. Energy Mater., 2021, 11, 2003069 CrossRef CAS.
- X. Han, N. Li, P. Xiong, M. G. Jung, Y. Kang, Q. Dou, Q. Liu, J. Y. Lee and H. S. Park, InfoMat, 2021, 3, 1134–1144 CrossRef CAS.
- K. Yang, C. Li, H. Qi, Y. Dai, Y. Cui and Y. He, J. Mater. Chem. A, 2023, 11, 10425–10434 RSC.
- D. Deckenbach and J. J. Schneider, Adv. Mater. Interfaces, 2023, 10, 2202494 CrossRef CAS.
- W. Sun, F. Wang, B. Zhang, M. Zhang, V. Küpers, X. Ji, C. Theile, P. Bieker, K. Xu, C. Wang and M. Winter, Science, 2021, 371, 46–51 CrossRef CAS PubMed.
- Z. Zeng, G. Fu, H. Bin Yang, Y. Yan, J. Chen, Z. Yu, J. Gao, L. Y. Gan, B. Liu and P. Chen, ACS Mater. Lett., 2019, 1, 432–439 CrossRef CAS.
- H. Zhang, M. Zhu, H. Tang, Q. Lu, T. Yang, X. Wang, B. Chen, Z. Qu, X. Wang, M. Yu, D. Karnaushenko, D. D. Karnaushenko, Y. Huang, O. G. Schmidt and K. Zhang, Energy Storage Mater., 2023, 59, 102791 CrossRef.
- X. Cai, L. Lai, J. Lin and Z. Shen, Mater. Horiz., 2017, 4, 945–976 RSC.
- J.-Q. Huang, Q. Zhang, H.-J. Peng, X.-Y. Liu, W.-Z. Qian and F. Wei, Energy Environ. Sci., 2014, 7, 347–353 RSC.
- D. Zalka, A. Vizintin, A. Maximenko, Z. Pászti, Z. Dankházi, K. Hegedüs, L. S. Shankar, R. Kun, K. Saksl, A. S. Fedorková and P. Jóvári, Commun. Mater., 2025, 6, 17 CrossRef CAS.
- R. Hou, H. Bai, X. Zhong, J. Cheng, J. Gao, J. Tang and B. Xu, RSC Adv., 2025, 15, 15443–15449 RSC.
- D. P. Dubal, N. R. Chodankar, D.-H. Kim and P. Gomez-Romero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- L. Pradhan, B. Mohanty, G. Padhy, R. Kumar Trivedi, D. Prasad Das, B. Chakraborty and B. Kumar Jena, Chem. Eng. J., 2024, 497, 154587 CrossRef CAS.
- Z. Bo, X. Zhang, Z. Huang, Y. Huang, J. Yan, K. Cen and H. Yang, RSC Adv., 2023, 13, 15762–15771 RSC.
- Y. Yuan, L. Jiang, X. Li, P. Zuo, X. Zhang, Y. Lian, Y. Ma, M. Liang, Y. Zhao and L. Qu, Adv. Mater., 2022, 34, 2110013 CrossRef CAS PubMed.
- J. He, F. Ma, W. Xu, X. He, Q. Li, J. Sun, R. Jiang, Z. Lei and Z. Liu, Adv. Sci., 2024, 11, 2305991 CrossRef CAS PubMed.
- E. Q. Chong, D. B. Lingerfelt, A. Petrone and X. Li, J. Phys. Chem. C, 2016, 120, 19434–19441 CrossRef CAS.
- X. Zhang, Z. Zhang and Z. Zhou, J. Energy Chem., 2018, 27, 73–85 CrossRef.
- Z. Ren, Y. Li and J. Yu, iScience, 2018, 9, 138–148 CrossRef CAS PubMed.
- J. W. Graydon, M. Panjehshahi and D. W. Kirk, J. Power Sources, 2014, 245, 822–829 CrossRef CAS.
- Z. Li, N. H. Attanayake, J. L. Blackburn and E. M. Miller, Energy Environ. Sci., 2021, 14, 6242–6286 RSC.
- J. Zhang, C. Guo, S. Fang, X. Zhao, L. Li, H. Jiang, Z. Liu, Z. Fan, W. Xu, J. Xiao and M. Zhong, Nat. Commun., 2023, 14, 1298 CrossRef CAS PubMed.
- N. H. Solangi, L. P. Lingamdinne, R. R. Karri, N. M. Mubarak, S. A. Mazari and J. R. Koduru, Carbon, 2025, 232, 119758 CrossRef CAS.
- M. Khalil, G. T. M. Kadja, F. A. A. Nugroho, L. G. Sutanto, P. K. Jiwanti, F. F. Abdi, F. Hussin and M. K. Aroua, Renewable Sustainable Energy Rev., 2024, 206, 114869 CrossRef CAS.
- A. A. Abuelwafa, R. M. Matiur, A. A. Putri and T. Soga, Opt. Mater., 2020, 109, 110413 CrossRef CAS.
- J. Nie, X. Zhang, M. Wang, Y. Ou, S. Li, P. Zhong, W. Wang, G. Zhu and X. Ma, Sep. Purif. Technol., 2025, 354, 128961 CrossRef CAS.
- R. Kim, J. Kim, J. Y. Do, M. W. Seo and M. Kang, Catalysts, 2019, 9, 998 CrossRef CAS.
- S. Zhang, J. Zhou, Q. Jia, X. Ma, C. Guo, L. Li and L. Gong, 2023, preprint, DOI:10.2139/ssrn.4625162.
- B. Abhishek, A. Jayarama, A. S. Rao, S. S. Nagarkar, A. Dutta, S. P. Duttagupta, S. S. Prabhu and R. Pinto, Int. J. Hydrogen Energy, 2024, 81, 1442–1466 CrossRef.
- H. Yan and H. Yang, J. Alloys Compd., 2011, 509, L26–L29 CrossRef CAS.
- R. Ramírez, A. Melillo, S. Osella, A. M. Asiri, H. Garcia and A. Primo, Small Methods, 2023, 7, 2300063 CrossRef PubMed.
- M. Y. Solangi, A. A. Lakhair, F. Z. Dayo, R. A. Qureshi, A. Alhazaa, M. A. Shar, A. J. Laghari, I. A. Soomro, M. N. Lakhan, A. Hanan and U. Aftab, RSC Sustainability, 2024, 2, 3424–3435 RSC.
- N. G. Semaltianos, Crit. Rev. Solid State Mater. Sci., 2010, 35, 105–124 CrossRef CAS.
- B. Chang, H. Zhang, S. Sun and G. Zhang, Carbon Energy, 2024, 6, e491 CrossRef CAS.
- H. Yun, C. Lim, M. Kwon, D. Lee, Y. Yun, D. Seo and K. Yong, Adv. Mater., 2024, 36, 2408280 CrossRef CAS PubMed.
- R. Zhang, Y. Zhang, B. Xiao, S. Zhang, Y. Wang, H. Cui, C. Li, Y. Hou, Y. Guo, T. Yang, J. Fan and C. Zhi, Angew. Chem., Int. Ed., 2024, 136, e202407589 CrossRef.
- Z. Shu, H. Chen, X. Liu, H. Jia, H. Yan and Y. Cai, Adv. Funct. Mater., 2023, 33, 2301493 CrossRef CAS.
- G. Zhang, Q. Ji, K. Zhang, Y. Chen, Z. Li, H. Liu, J. Li and J. Qu, Nano Energy, 2019, 59, 10–16 CrossRef CAS.
- W. Bi, X. Li, R. You, M. Chen, R. Yuan, W. Huang, X. Wu, W. Chu, C. Wu and Y. Xie, Adv. Mater., 2018, 30, 1706617 CrossRef PubMed.
- Q. Jiang, Y. Lei, H. Liang, K. Xi, C. Xia and H. N. Alshareef, Energy Storage Mater., 2020, 27, 78–95 CrossRef.
- S. Zhang, J. Wu, M. Zheng, X. Jin, Z. Shen, Z. Li, Y. Wang, Q. Wang, X. Wang, H. Wei, J. Zhang, P. Wang, S. Zhang, L. Yu, L. Dong, Q. Zhu, H. Zhang and J. Lu, Nat. Commun., 2023, 14, 3634 CrossRef CAS PubMed.
- Z. Han, D. Tranca, F. Rodríguez-Hernández, K. Jiang, J. Zhang, M. He, F. Wang, S. Han, P. Wu and X. Zhuang, Small, 2023, 19, 2208102 CrossRef CAS PubMed.
- J. Shah, T. Wu, J. Lucero, M. A. Carreon and M. L. Carreon, ACS Sustainable Chem. Eng., 2019, 7, 377–383 CrossRef CAS.
- G. Meng, M. Jin, T. Wei, Q. Liu, S. Zhang, X. Peng, J. Luo and X. Liu, Nano Res., 2022, 15, 8890–8896 CrossRef CAS.
- Y. Li, J. Liu, Z. Sun, R. Li, L. Guo, X. Zhang, Y. Wang, Y. Wang, Z. Yu and C. Fan, Green Chem., 2022, 24, 9253–9262 RSC.
- L. Zhang, Q. Zhou, J. Liang, L. Yue, T. Li, Y. Luo, Q. Liu, N. Li, B. Tang, F. Gong, X. Guo and X. Sun, Inorg. Chem., 2022, 61, 8096–8102 CrossRef CAS PubMed.
- T. Mou, J. Liang, Z. Ma, L. Zhang, Y. Lin, T. Li, Q. Liu, Y. Luo, Y. Liu, S. Gao, H. Zhao, A. M. Asiri, D. Ma and X. Sun, J. Mater. Chem. A, 2021, 9, 24268–24275 RSC.
- M.-Y. Qi, W.-Y. Xiao, M. Conte, Z.-R. Tang and Y.-J. Xu, ACS Catal., 2025, 15, 129–138 CrossRef CAS.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- D. Sun, W. Liu, Y. Fu, Z. Fang, F. Sun, X. Fu, Y. Zhang and Z. Li, Chem. – Eur. J., 2014, 20, 4780–4788 CrossRef CAS PubMed.
- J. Wang, X. Niu, R. Wang, K. Zhang, X. Shi, H. Y. Yang, J. Ye and Y. Wu, Appl. Catal., B, 2025, 362, 124763 CrossRef CAS.
- H. Ling, H. Sun, L. Lu, J. Zhang, L. Liao, J. Wang, X. Zhang, Y. Lan, R. Li, W. Lu, L. Cai, X. Bai and W. Wang, Nat. Commun., 2024, 15, 9505 CrossRef CAS PubMed.
- H. Zhang, H. Hu, Y. Li, J. Wang and L. Ma, Biosens. Bioelectron., 2024, 248, 115997 CrossRef CAS PubMed.
- K. S. Docherty and P. J. Ziemann, Aerosol Sci. Technol., 2003, 37, 877–891 CrossRef CAS.
- Y. Ma, K. Chen, J. Ma, G. Xu, S. Dong, B. Chen, J. Li, Z. Chen, X. Zhou and G. Cui, Energy Environ. Sci., 2019, 12, 273–280 RSC.
- H.-S. Hsieh and R. G. Zepp, Environ. Sci.: Nano, 2019, 6, 3734–3744 RSC.
- B. Weng, M.-Y. Qi, C. Han, Z.-R. Tang and Y.-J. Xu, ACS Catal., 2019, 9, 4642–4687 CrossRef CAS.
- M. Gryszel, A. Markov, M. Vagin and E. D. Głowacki, J. Mater. Chem. A, 2018, 6, 24709–24716 RSC.
- F. Kurtz, T. N. Dauwe, S. V. Yalunin, G. Storeck, J. G. Horstmann, H. Böckmann and C. Ropers, Nat. Mater., 2024, 23, 890–897 CrossRef CAS PubMed.
- Z. Zhang, L. Ding, X. Zuo, H. Feng and Q. Xia, Med, 2023, 4, 404–431 CrossRef CAS PubMed.
- R. Swijnenburg, S. Schrepfer, J. A. Govaert, F. Cao, K. Ransohoff, A. Y. Sheikh, M. Haddad, A. J. Connolly, M. M. Davis, R. C. Robbins and J. C. Wu, Proc. Natl. Acad. Sci., 2008, 105, 12991–12996 CrossRef CAS PubMed.
- K. S. Jones, Semin. Immunol., 2008, 20, 130–136 Search PubMed.
- A. Khademhosseini and R. Langer, Nat. Protoc., 2016, 11, 1775–1781 CrossRef CAS PubMed.
- S. Short, G. Lewik and F. Issa, Transplantation, 2023, 107, 2341–2352 CrossRef CAS PubMed.
- G. Piotti, A. Palmisano, U. Maggiore and C. Buzio, Front. Immunol., 2014, 5, 505 Search PubMed.
- A. Rafieerad, W. Yan, K. N. Alagarsamy, A. Srivastava, N. Sareen, R. C. Arora and S. Dhingra, Adv. Healthcare Mater., 2021, 1–17 Search PubMed.
- M. Bresinsky and A. Goepferich, Eur. J. Pharm. Biopharm., 2025, 208, 114634 CrossRef CAS PubMed.
- J. Merola, D. D. Jane-wit, J. S. Pober, N. Haven, N. Haven and N. Haven, JCI Insight, 2018, 3, e97881 CrossRef PubMed.
- T. Fan, L. Yan, S. He, Q. Hong, F. Ai, S. He, T. Ji, X. Hu, E. Ha, B. Zhang, Z. Li, H. Zhang, X. Chen and J. Hu, Chem. Soc. Rev., 2022, 51, 7732–7751 RSC.
- Q. Xu, S. Zhao, L. Deng, J. Ouyang, M. Wen, K. Zeng, W. Chen, L. Zhang and Y.-N. Liu, Chem. Commun., 2019, 55, 9471–9474 RSC.
- V. G. Gayathri, B. Richard, J. T. Chacko, J. Bayry and P. A. Rasheed, J. Mater. Chem. B, 2025, 13, 1212–1228 RSC.
- S. Y. Won, R. Singhmar, S. Sahoo, H. Kim, C. M. Kim, S. M. Choi, A. Sood and S. S. Han, Colloids Surf., B, 2025, 245, 114207 CrossRef CAS PubMed.
- Q. You, P. Wang, T. Zhu, Z. Jia, Z. Chang, L. Li and W.-F. Dong, Mater. Today Bio, 2025, 32, 101747 CrossRef CAS PubMed.
- J. Shao, H. Xie, H. Huang, Z. Li, Z. Sun, Y. Xu, Q. Xiao, X.-F. Yu, Y. Zhao, H. Zhang, H. Wang and P. K. Chu, Nat. Commun., 2016, 7, 12967 CrossRef CAS PubMed.
- A. Yilmazer, K. N. Alagarsamy, C. Gokce, G. Y. Summak, A. Rafieerad, F. Bayrakdar, B. I. Ozturk, S. Aktuna, L. G. Delogu, M. A. Unal and S. Dhingra, Small Methods, 2023, 7, 2300044 CrossRef CAS PubMed.
- F. Zhang, G. Sun, R. Zhao, F. Yang, X. Jiang, S. Song, J. Zhang, H. Shen and J. Shen, Langmuir, 2024, 40, 11381–11389 CrossRef CAS PubMed.
- L. Li, Z. Xing, T. Liao, J. Wang, Z. Xu, Y. Kuang and C. Li, Mater. Today Chem., 2024, 39, 102171 CrossRef CAS.
- J. Chang and E. R. Waclawik, RSC Adv., 2014, 4, 23505–23527 RSC.
- T. Zhang, Z. Xu, H. Chen, J. Liu, D. Luo, N. Liu, Y. Zhang and Z. Chen, Chem. Eng. J., 2024, 488, 150886 CrossRef CAS.
- H. Shahbazi, P. Seraji, H. Farraj, T. Yang, A. Kim, S. Fattahpour, I. Papailias, M. Diamond, S. Namvar, A. Ahmadiparidari, S. Wang, Z. Liu, S. Feng, K. Kumar, M. Ahart, J. Cabana, S. Kadkhodaei, J. Wang, Z. Huang, R. J. Hemley and A. Salehi-Khojin, Science, 2025, 388, 950–956 CrossRef CAS PubMed.
- B. Mohanty, L. Giri and B. K. Jena, Energy Fuels, 2021, 35, 14304–14324 CrossRef CAS.
- K. Wang, Q. Ren, Z. Gu, C. Duan, J. Wang, F. Zhu, Y. Fu, J. Hao, J. Zhu, L. He, C.-W. Wang, Y. Lu, J. Ma and C. Ma, Nat. Commun., 2021, 12, 4410 CrossRef CAS PubMed.
- H. Wan, Z. Wang, S. Liu, B. Zhang, X. He, W. Zhang and C. Wang, Nat. Energy, 2023, 8, 431–432 CrossRef.
- Z. Lu, L. Zeng, W. Song, Z. Qin, D. Zeng and C. Xie, Appl. Catal., B, 2017, 202, 489–499 CrossRef CAS.
- M. Abdinejad, A. Farzi, R. Möller-Gulland, F. Mulder, C. Liu, J. Shao, J. Biemolt, M. Robert, A. Seifitokaldani and T. Burdyny, Nat. Catal., 2024, 7, 1109–1119 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |








