An etching–templating dual strategy for the in situ synthesis of carbon-supported iron metaphosphate and its application as an electrocatalyst
Jingbo
Huang
a,
Junzheng
Wei
a,
Wei
Sang
b,
Qifu
Zhang
a,
Yongda
Guo
a and
Yating
Hu
 *a
*a
aGuangzhou Key Laboratory of Low-Dimensional Materials and Energy Storage Devices, School of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China. E-mail: yatinghu@gdut.edu.cn
bState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China
First published on 27th November 2025
Abstract
Carbon-supported transition-metal phosphates have emerged as promising non-noble electrocatalysts, but their poor conductivity and structural instability hinder their practical application. Here, we report a novel etching–templating dual strategy that enables the structural directing construction of carbon-supported iron metaphosphate nanocatalysts. The process is driven by n-hexylphosphonic acid (HPA), which simultaneously serves as the phosphorus source, soft template, and carbon precursor. In situ etching and templating yields a lamellar Fe–HPA intermediate with pre-organized Fe2+, phosphate, and alkyl components. The subsequent two-step heat treatment carbonizes the alkyl chains into a conductive matrix, enhances graphitization, and ensures uniform dispersion of Fe(PO3)2 nanocatalysts. The obtained carbon flake supported Fe-metaphosphate nanodots demonstrate a high oxygen–reduction reaction (ORR) onset potential of 0.85 V (vs. RHE) and a pseudo-four-electron transfer pathway. This work introduces a scalable templating strategy for one-pot integration of active metal phosphates and conductive carbon, offering a new platform for designing cost-effective electrocatalysts and beyond.
Alkaline fuel cells (AFCs) are emerging as efficient and environmentally friendly energy conversion technologies with significant commercial potential. Compared to proton exchange membrane fuel cells (PEMFCs), AFCs offer several advantages. Firstly, the ORR is significantly faster in alkaline media than in acidic environments.1 Furthermore, AFCs have lower cost potential because of non-noble metal catalysts (e.g., Fe, Co or Ni-based), much cheaper than PEMFCs, which rely heavily on Pt.2 Notably, AFCs can also achieve higher energy conversion efficiency, primarily due to the faster ORR kinetics and higher onset potentials enabled by alkaline-compatible catalysts. Moreover, the less corrosive environment of alkaline electrolytes allows for the use of more diverse and less expensive materials, further improving the system durability and economic viability.3,4
Significant progress has been made in designing cost-effective ORR catalysts based on transition-metal oxides,5,6 transition-metal phosphides,7–9 and metal–nitrogen–carbon (M–N–C) nanocomposites.10–12 Compared with these materials, transition metal phosphate-based catalysts are less explored, despite their potential advantages in structural tunability and cost-effectiveness. Among them, metal metaphosphates are particularly interesting due to their unique [PO3]− repeating units,13 which offer high surface hydrophilicity and flexible coordination environments. These intrinsic properties of metaphosphates are beneficial for stabilizing metal active sites and facilitating the adsorption of O2/OH− species during the ORR.14,15 However, their poor electrical conductivity and the tendency of active species to aggregate remain major barriers to practical application.
To address these limitations, integrating metal phosphate species with electrically conductive carbon supports has become a common strategy.8,16,17 The carbon matrix not only enhances electron transport, but also serves to stabilize small, highly dispersed active sites.18,19 Conventional synthesis methods, however, often involve multiple steps, such as independent carbon support fabrication from MOFs or agricultural residuals, post-synthesis modifications, or the use of binders and templates.20,21 These complex procedures limit structural control and reproducibility and hinder scalability. In some cases, achieving good dispersion and controlled crystallinity of the metal-phosphate is especially challenging, affecting mass transportation and ORR kinetics.16
In recent years, etching–templating strategies have been investigated for the synthesis of Fe-based electrocatalysts. For example, the hard-template method employs mesoporous silica as a removable scaffold, which is subsequently etched with an acid or alkali to yield porous Fe–N–C structures with enhanced catalytic activity.22 Meanwhile, soft-template approaches, such as those derived from (Fe,Ni)-nitrogen-doped porous carbon based on ZIF-8, achieve template removal through high-temperature pyrolysis, allowing better control over the internal pore architecture and active site accessibility.23 However, the use of phosphonic acid as both an etchant and structure-directing agent to coordinate with Fe and guide the self-assembly of active ORR catalysts via soft-template strategies remains largely unexplored.
Herein, we propose an etching–templating dual strategy for synthesizing carbon-supported iron metaphosphate nanocatalysts. The key to this method is HPA, a multifunctional structural-directing agent that simultaneously serves as a phosphorus source, a soft template and a carbon source via thermal decomposition and condensation of its alkyl chains. This integrated approach enables the in situ formation of both the active Fe(PO3)2 phase and the conductive carbon support, eliminating the need for additional carbon additives or complex multi-step processing. In reacting with Fe3O4 nanoparticles as a precursor, HPA deprotonates and etches Fe3O4 into Fe2+/Fe3+ ions that coordinate with phosphate groups to form a lamellar Fe–HPA micelle. Pyrolysis condenses alkyl chains into a carbon matrix anchoring Fe(PO3)2 nanodots, followed by annealing for crystallization and graphitization. The resulting hybrid catalysts exhibit higher conductivity and structural stability, leading to much enhanced ORR performance in alkaline media. Overall, a micelle assembly of all components is achieved through this dual-function etching and templating, enabling scalable and controllable fabrication of phosphate-based hybrid electrocatalysts without external carbon additives or post-synthetic modification.
Transformation from Fe3O4 to Fe–HPA
HPA played multiple roles in the synthesis of Fe–HPA: it acted as an etchant to generate Fe2+/Fe3+ ions, a soft template to direct the assembly of Fe and PO32− into micellar structures, and a carbon source that, upon pyrolysis, formed a conductive support through the decomposition and carbonization of its alkyl chain. Octahedral Fe3O4 nanoparticles were synthesized as precursors following the method reported by Zong et al.24 and characterized by X-ray diffraction (XRD) (Fig. 1b). Octahedral crystals are purposely chosen as they have eight identically exposed (111) facets, so that the following etching reaction could take place uniformly around all exposed facets of the oxide precursor. For the synthesis of Fe-HPA, 8 mmol HPA was dissolved in 30 g of lauric acid, followed by the addition of 1 mmol Fe3O4. The mixture was then heated to 295 °C under a nitrogen atmosphere with vigorous stirring. In lauric acid, HPA undergoes deprotonation to yield H+ and hexylphosphonate anions (HPA−). The generated H+ etches the Fe3O4 nanoparticles, dissolving them into Fe2+ and Fe3+ ions. Excessive HPA is used to make sure all the Fe2+ and Fe3+ ions will be etched out. These metal ions subsequently coordinate with HPA− through strong phosphate-metal ionocovalent bonds,25 as illustrated in the scheme in Fig. 1a. This strong affinity leads to a monolayer formed around the Fe3O4 nano-octahedra, with the hydrophobic alkyl tails of HPA pointing outward. These tails attract additional HPA molecules, promoting bilayer formation. Continued layer-by-layer assembly results in the formation of the final laminar Fe–HPA micelle structure (Fig. 1a). Low-angle XRD revealed distinct diffraction peaks at a 2θ of 4.7, 9.4, and 14.1°, corresponding to the (200), (300), and (400) planes of the laminar Fe–HPA micelles, respectively (Fig. 1c). XRD of Fe–HPA showed that the original octahedral Fe3O4 nanoparticles had dissolved in HPA with the help of lauric acid (Fig. S1a) and self-assembled into micelle-like sheets (Fig. S2a). While lauric acid can slowly dissolve Fe3O4 at high temperature (i.e., 290 °C), no precipitates are formed due to its long carbon chain and lack of phosphonic groups to coordinate Fe ions. Transmission electron microscopy (TEM) coupled with energy-dispersive X-ray spectroscopy (EDS) confirmed a homogeneous distribution of Fe, P, and O within the Fe–HPA flakes (Fig. 1e). This uniform elemental dispersion provides a pre-assembled platform for the growth of subsequent phosphate nanoparticles on carbon substrates.Transformation from Fe–HPA to carbon supported iron metaphosphate
To investigate the decomposition behaviour of alkyl chains within Fe–HPA, thermogravimetric analysis (TGA) was performed at a heating rate of 5 °C min−1 from room temperature to 600 °C. The TGA curve exhibited two significant weight losses around 200 °C and 520 °C, as indicated by the corresponding peaks in the derivative thermogravimetry (DTG) profile (Fig. S3). The first peak is attributed to the decomposition of residual solvent components (i.e., lauric acid), while the second peak corresponds to the thermal decomposition of HPA's alkyl chains, indicating the condensation of the laminar micelle into carbon. Guided by the TGA findings, we designed a series of pyrolysis/annealing pathways. The nomenclature and heat-treatment conditions for various samples are listed in Table S1. To evaluate the influence of heat treatment on the structure and crystallinity, we compared a conventional single-step process with an optimized two-step protocol. The single-step process involved direct heat treatment under a N2 atmosphere at temperatures ranging from 450 °C to 750 °C for 3 hours. In contrast, the two-step process separates decomposition and crystallization into sequential stages, targeting at the organic and inorganic parts of the Fe–HPA micelles, respectively: first, vacuum pyrolysis at 450 °C for 3 hours under high vacuum (10−4 mbar) to convert the alkyl chain in the Fe–HPA precursors into carbon-based composites; then, a brief annealing step under N2 at 600–750 °C for 30 minutes to crystallize the inorganic metal phosphate. A series of carbon-supported samples were synthesized using these conditions and designated accordingly: V450 (450 °C under vacuum), V450-N600 to V450-N750 (vacuum pyrolysis followed by N2 annealing at 600–750 °C), and N450 to N750 (direct N2 treatment). XRD analysis (Fig. 1d) revealed that the N700 and N750 samples exhibited high crystallinity of Fe(PO3)2 (PDF #30-0660). XPS of Fe 2p for the N700 sample agrees well with the Fe(PO3)2 material (Fig. S4).26 With increasing temperature, a new peak at 30.3° corresponding to Fe7(PO4)6 (PDF #49-1088) starts to appear, which is most obvious for N750. The TEM image (Fig. 2f) revealed that the resulting nanodots, with uniform size distributions of 5 to 10 nm, were well-dispersed across the carbon support. The crystallite sizes estimated from the Scherrer equation (Table S2) indicate that V450-N700 and V450-N750 (113 and 119 nm) possess smaller crystallites than N700 and N750 (176 and 197 nm), respectively. This result suggests that the two-step approach promotes more controlled crystallization, yielding smaller Fe-based nanocrystals that may be better dispersed within the carbon matrix, consistent with the TEM observations (Fig. 2). High-resolution TEM confirmed a lattice fringe spacing of 0.3 nm, corresponding to the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 13) plane of Fe(PO3)2. EDS elemental mapping (Fig. S5 and Table S3) further confirmed the homogeneous distribution of Fe, P, O, and C elements throughout the hybrid structure. The elemental atomic ratios of Fe
13) plane of Fe(PO3)2. EDS elemental mapping (Fig. S5 and Table S3) further confirmed the homogeneous distribution of Fe, P, O, and C elements throughout the hybrid structure. The elemental atomic ratios of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O are approximately 1
O are approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, agrees well with the iron-metaphosphate structure. Therefore, we confirm the successful formation of a carbon-supported Fe(PO3)2 nanocomposite with uniform particle size and good dispersion.
6, agrees well with the iron-metaphosphate structure. Therefore, we confirm the successful formation of a carbon-supported Fe(PO3)2 nanocomposite with uniform particle size and good dispersion.
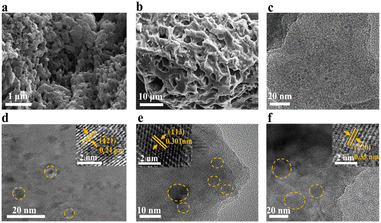 | ||
| Fig. 2 SEM images of (a) V450 and (b) V450-N700. TEM images of (c) V450, (d) V450-N650, (e) V450-N700 and (f) N650. | ||
Formation of 3D carbon–phosphate hybrids via the two-step processing
The products obtained via a two-step process (heat treatment under high vacuum followed by a N2 atmosphere) exhibited a hierarchical structure, wherein 2D nanoflakes wove into a 3D network with a large accessible surface area (Fig. 2a, b and Fig. S2). N2 adsorption shows a closed hysteresis loop, although the specific surface area is rather low (Fig. S6b). Mass loss data revealed that pyrolysis of the HPA alkyl chains in Fe–HPA was more complete under vacuum conditions (Table S4). This enhanced pyrolysis may be attributed to the effect of low pressure in promoting carbon chain breakdown.27 In contrast, under a N2 atmosphere, accumulation and reflux of pyrolysis gases are likely to occur,28 hindering the decomposition process. The brief pre-annealing vacuum pyrolysis created a 3D interconnected structure of 2D nanoflakes through alkyl chain condensation, which might create more catalytically active sites for subsequent catalysis. Subsequently, the Fe–PO3−–carbon composite nanoflakes undergo controlled crystal growth through short-term high-temperature annealing in the second step, leading to carbon nanoflake supported iron-metaphosphate nanodots (as shown in the scheme in Fig. 1a).TEM images (Fig. 2c–f) further supported the morphological distinctions. Upon thermal pyrolysis at 450 °C, the Fe-metaphosphate is not crystallized yet (Fig. 2c). V450-N650 (Fig. 2d) subjected to brief high-temperature annealing exhibited uniform nanocrystal dispersion, while prolonged N2 annealing led to uncontrolled crystal growth (Fig. 2f). The size increase observed agrees well with the trend in the degree of crystallinity shown by XRD results (Table S2). Thus, our two-step heat treatment effectively mitigates the limitations of extended high-temperature treatment in N2, which often results in large crystals and the reduction in catalytically active sites.
The chemical nature of the carbon flakes in these hybrid materials dominates their electrical conductivity and directly influences their electrocatalytic performance. Therefore, the degree of graphitization plays a crucial role in determining the efficiency of electron transfer during the ORR catalytic process. The structural transformation of the carbon during pyrolysis and carbonization was analysed by Raman spectroscopy (Fig. 3a and b), where all samples exhibited two characteristic peaks: the D-band (∼1350 cm−1), corresponding to disordered or amorphous carbon, induced by the growth of Fe(PO3)2 nanoparticles; the G-band (∼1580 cm−1), as a result of the condensation and carbonization of the alkyl chains. With increasing carbonization temperature under the same conditions (N2 or vacuum), the area ratio of the G band to D band (AG/AD) consistently rises, indicating a reduction in carbon defects or an enhancement in graphitic ordering.29
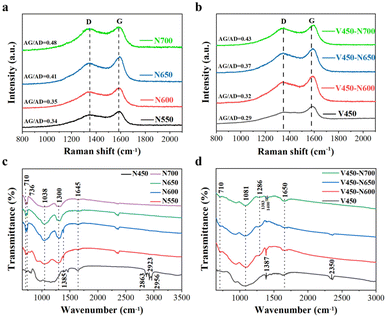 | ||
| Fig. 3 Characterization studies of various samples: (a and b) Raman spectra and (c and d) Fourier transform infrared spectroscopy (FT-IR) spectra. | ||
This improved graphitization enhances the intrinsic electrical conductivity, thereby boosting electron transport and overall catalytic activity. To further elucidate the evolution from the alkyl chain to carbon nanoflakes during thermal treatment, Fourier transform infrared (FTIR) spectroscopy was conducted (Fig. 3c and d). Compared to the Fe–HPA, the absorption bands between 2850 and 2960 cm−1 (C–H stretching vibrations) disappear after high-temperature annealing or vacuum treatment, suggesting complete decomposition of alkyl chains under these conditions (Fig. S7). In the mid-infrared region, several vibrational features indicate the formation of metaphosphate structures. The bands at 1645 and 1300 cm−1 correspond to P–OH bending vibrations and asymmetric P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, respectively,30,31 and those at 1038 and 1081 cm−1 correspond to P–O− groups.32 Bands at 710 and 736 cm−1 correspond to the C–H out-of-plane bending vibrations of mono-substituted benzene rings within the graphene framework.33 Therefore, these bands become more prominent with increased graphitization, as shown by the increased intensity for samples subjected to higher temperature annealing (Fig. 3c). By comparing the single-step and two-step pyrolysis methods (Fig. 3cvs.Fig. 3d), samples obtained via vacuum pyrolysis exhibit significantly weaker C–H bending bands. This suggests that vacuum pyrolysis at 450 °C more effectively removes hydrogen from alkyl chain fragments, consistent with the greater mass loss and the different colour of the samples observed after pyrolysis (Table S4 and Fig. S8). In addition, the atmospheric pressure spiked significantly during the vacuum pyrolysis (Fig. S6a), indicating a large amount of gaseous product released through the pyrolysis of HPA.
O stretching, respectively,30,31 and those at 1038 and 1081 cm−1 correspond to P–O− groups.32 Bands at 710 and 736 cm−1 correspond to the C–H out-of-plane bending vibrations of mono-substituted benzene rings within the graphene framework.33 Therefore, these bands become more prominent with increased graphitization, as shown by the increased intensity for samples subjected to higher temperature annealing (Fig. 3c). By comparing the single-step and two-step pyrolysis methods (Fig. 3cvs.Fig. 3d), samples obtained via vacuum pyrolysis exhibit significantly weaker C–H bending bands. This suggests that vacuum pyrolysis at 450 °C more effectively removes hydrogen from alkyl chain fragments, consistent with the greater mass loss and the different colour of the samples observed after pyrolysis (Table S4 and Fig. S8). In addition, the atmospheric pressure spiked significantly during the vacuum pyrolysis (Fig. S6a), indicating a large amount of gaseous product released through the pyrolysis of HPA.
Demonstration as electrocatalysts for ORR
Transition-metal metaphosphates have recently emerged as promising candidates for electrochemical catalysis, where the nature of the carbon support plays a critical performance of our catalysts. Initial cyclic voltammetry (CV) measurements were conducted in 0.1 M KOH using a rotating disk electrode (RDE) system with inert gas (N2) purging. The CV cycle simultaneously served as the activation process for the catalysts. Subsequent CV scans in an O2-saturated electrolyte revealed distinct cathodic peaks associated with oxygen reduction, whereas no significant features were observed under inert conditions. Notably, the V450-N700 sample exhibited a cathodic peak at a more positive potential (0.79 V vs. RHE) compared to other catalysts (Fig. 4a), indicating its enhanced ORR catalytic activity. Further evaluation was done via linear sweep voltammetry (LSV) at 1600 rpm and a scan rate of 10 mV s−1. The V450-N700 sample exhibited a higher onset potential (0.85 V vs. RHE) and a larger limiting current density relative to the other samples (Fig. 4b and Fig. S9), largely due to its balanced graphitization degree and small Fe(PO3)2 crystal size. The onset-potential of the V450-N700 is comparable to the reported values of transition-metal phosphates and most other non-noble metal ones,7,15,34 except that the Fe–N–C based material demonstrates an onset-potential higher than 0.9 V (as summarized in Table S5).35The electron transfer kinetics of the V450-N700 sample was investigated under rotation speeds ranging from 400 to 2000 rpm. The limiting current increased proportionally with rotation speed (Fig. 4c), indicating diffusion-controlled kinetics. The corresponding Koutecky–Levich (K–L) plots showed high linearity in the 0.2–0.6 V range (Fig. 4d), suggesting a first-order reaction kinetics on dissolved O2. By calculating the average slope of K–L plots obtained at different applied potentials, the average electron transfer number was determined to be 3.84. To further validate the electron transfer number and assess the formation of by-products (e.g., H2O2), rotating ring-disk electrode (RRDE) measurements were carried out for both V450-N700 and N700 at 1600 rpm (Fig. 4e and Fig. S10). Compared to V450-N700, N700 exhibited a broader range of two-electron reduction behavior and a higher H2O2 yield.36 Notably, both samples show two distinct redox waves, implying a mixed mechanism involving both 2e− and 4e− processes.37 Combined with our K–L plots, these results suggest that the ORR on V450-N700 follows a sequential pathway: it initiates with a 2e− reduction at potentials >0.32 V to produce H2O2, followed by further reduction to OH− at higher overpotentials (<0.32 V). This two-step process accounts for the observed pseudo-four-electron pathway during the overall ORR process.37 Nyquist plots from the electrochemical impedance spectroscopy (EIS) test revealed differences in charge transport behaviour. Firstly, the phosphate-based catalysts show much improved electrical conductivity over their oxide starting materials. Secondly, the increased graphitization degree of carbon supports induced by elevated annealing temperatures contributes to improved charge transport properties (Fig. 4f).
Conclusions
In this work, we developed a simple and effective synthesis strategy for carbon-supported iron (II) metaphosphate nanocatalysts by combining acid-etching and structure-directing templating. A key feature of this method is the use of HPA, which serves not only as the phosphorus and structure-directing agent, but also as the carbon precursor. During pyrolysis, the alkyl chains of HPA undergo condensation, directly forming the carbon support without the need for external carbon additives. By applying a two-step thermal treatment—initial vacuum pyrolysis followed by N2 annealing—we optimized the decomposition of organic chains, enhanced graphitization, and achieved better control over crystal growth. The resulting Fe(PO3)2 nanodots are uniformly distributed on carbon nanoflakes with a hierarchical 3D structure, offering high conductivity and abundant active sites. Among the synthesized samples, V450-N700 showed the best ORR performances, with a promising onset potential, high limiting current, and a pseudo-four-electron pathway. This study not only demonstrates the catalytic potential of iron metaphosphates in alkaline media, but also introduces an organic–inorganic hybrid design strategy, where organic precursors are leveraged to direct the synthesis and integration of inorganic nanocatalysts within conductive carbon frameworks.Author contributions
J. Huang and J. Wei conceived the idea of the project. J. Huang synthesized the materials and performed most material characterization tests. J. Wei performed the TEM and Raman tests. Q. Zhang performed the ORR catalytic and electrochemical tests. Y. Guo performed SEM tests. J. Huang wrote the manuscript. Y. Hu supervised the whole work and revised the manuscript. W. Sang supervised the ORR result analysis and the writing. All the authors participated in the discussion of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. Synthesis of Fe3O4 nano-size octahedra, Fe-HPA and Fe(PO3)2; Material Characterizations; Electrochemical measurements and calculations; All the supporting figures and tables. See DOI: https://doi.org/10.1039/d5nr03401a.Acknowledgements
This work is supported by the Department of Science and Technology of Guangdong Province, China (2022A1515110465).References
- G. F. McLean, T. Niet, S. Prince-Richard and N. Djilali, Int. J. Hydrogen Energy, 2002, 27, 507–526 CrossRef CAS.
- O. O. Fashedemi, A. Bello, T. Adebusuyi and S. Bindir, Curr. Opin. Electrochem., 2022, 36, 101132 CrossRef CAS.
- A. T. Hamada, M. F. Orhan and A. M. Kannan, Energy Rep., 2023, 9, 6396–6418 CrossRef.
- W. Zeng, B. Guan, Z. Zhuang, J. Chen, L. Zhu, Z. Ma, X. Hu, C. Zhu, S. Zhao, K. Shu, H. Dang, T. Zhu and Z. Huang, Int. J. Hydrogen Energy, 2025, 102, 222–246 CrossRef CAS.
- D. Mladenović, A. Mladenović, D. M. F. Santos, A. B. Yurtcan, Š. Miljanić, S. Mentus and B. Šljukić, J. Electroanal. Chem., 2023, 946, 117709 CrossRef.
- Y. Wang, J. Li and Z. Wei, J. Mater. Chem. A, 2018, 6, 8194–8209 RSC.
- L. Xu, P. Che, X. Zhang, S. Cosnier and D. Shan, Appl. Surf. Sci., 2023, 620, 156770 CrossRef CAS.
- R. Zhao, B. Ni, L. Wu, P. Sun and T. Chen, Colloids Surf., A, 2022, 635, 128118 CrossRef CAS.
- Y. Su, H. Du, J. Xu, J. Zhao, N. Zhang, B. Lu, H. Yan, K. Qu and X. Zhang, Fuel, 2025, 387, 134349–134349 CrossRef CAS.
- W. Xu, R. Zeng, M. Rebarchik, A. Posada-Borbón, H. Li, C. J. Pollock, M. Mavrikakis and H. D. Abruña, J. Am. Chem. Soc., 2024, 146, 2593–2603 CrossRef CAS PubMed.
- Y. Jiang, H. Xu, B. Ma, Z. Zhang and Y. Zhou, Fuel, 2024, 366, 131404 CrossRef CAS.
- J. Wang, L. Zhao, L. Lin, X. Du, X. He, X. Zeng, W. Li, H. Chen, W. Fang, D. Wang and Z. Chen, Surf. Interfaces, 2024, 50, 104518 CrossRef CAS.
- H. Zhao and Z. Yuan, ChemCatChem, 2020, 12, 3797–3810 CrossRef CAS.
- Y. Zhan, M. Lu, S. Yang, C. Xu, Z. Liu and J. Y. Lee, ChemCatChem, 2016, 8, 372–379 CrossRef CAS.
- M. Zhou, J. Guo, R. Lu, J. Li, S. Lee, C. Han, X. Liao, P. Luo, Y. Zhao and Z. Wang, Interdiscip. Mater., 2024, 4, 309–320 Search PubMed.
- X. Zhang, Q. Hou, S. Cao, X. Lin, X. Chen, Z. Wang, S. Wei, S. Liu, F. Dai and X. Lu, Green Chem., 2023, 25, 7883–7903 RSC.
- H. Gao, Y. Wang, S. Zhou, S. Song, X. Tian, W. Li, Y. Yuan and J. Zang, Chem. Eng. J., 2021, 426, 130854 CrossRef CAS.
- Y. Wang, H. Li, Q. Yao, R. Li, Z. Guo, H. Chen, K. Qu and R. Li, Int. J. Hydrogen Energy, 2020, 46, 6513–6521 CrossRef.
- I. C. Gerber and P. Serp, Chem. Rev., 2019, 120, 1250–1349 CrossRef.
- J. Zhang, Z. Chen, T.-C. Yang, J. Zhang, H. Zheng, C.-H. Yeh, Z. Jiang, C.-M. Yang, L. Liu and N.-C. Lai, Electrochim. Acta, 2024, 485, 144098 CrossRef.
- Y. Lv, Y. Lin, L. Yang, Z. Cai, B. Jing, J. Yu, X. Jiang, Z. Xing and J. Zou, Chem. Eng. J., 2018, 342, 228–237 CrossRef CAS.
- S. Kellner, M. Wang, Y. Wang, J. Barrio, G. Yang, J. Feng, S. E. Heutz, I. E. Stephens and M.-M. Titirici, RSC Appl. Interfaces, 2025, 2, 704–714 RSC.
- Z. Zhou, H. Fang, Y. Liu, L. He, J. Zhao, M. Liu and W. Yang, Isr. J. Chem., 2023, 63, e202200058 CrossRef CAS.
- Y. Zong, H. Xin, J. Zhang, X. Li, J. Feng, X. Deng, Y. Sun and X. Zheng, J. Magn. Magn. Mater., 2017, 423, 321–326 CrossRef CAS.
- C. Queffelec, M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777–3807 CrossRef CAS PubMed.
- H. Zhou, F. Yu, J. Sun, R. He, S. Chen, C.-W. Chu and Z. Ren, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5607–5611 CrossRef CAS PubMed.
- A. A. Koptelov and S. V. Karyazov, Dokl. Phys. Chem., 2003, 389, 101–105 CrossRef CAS.
- J. Guo, J. Huang, H. Huang, J. Yao and X. Jiang, J. Appl. Polym. Sci., 2021, 139, 51726 CrossRef.
- D. Geng, Y. Huang, S. Yuan, Y. Jiang, H. Ren, S. Zhang, Z. Liu, J. Feng, T. Wei and Z. Fan, Small, 2023, 19, 2207227 CrossRef CAS PubMed.
- Y. Lai, X. Liang, S. Yang, P. Liu, Y. Zeng and C. Hu, J. Alloys Compd., 2014, 617, 597–601 CrossRef CAS.
- M. Shi, Y. Liang, L. Chai, X. Min, Z. Zhao and S. Yang, J. Mol. Struct., 2015, 1081, 389–394 CrossRef CAS.
- S. Rani, S. Sanghi, A. Agarwal and V. P. Seth, Spectrochim. Acta, Part A, 2009, 74, 673–677 CrossRef CAS PubMed.
- B. Smith, Spectroscopy, 2016, 31(5), 36–39 CAS.
- L. An, W. Huang, N. Zhang, X. Chen and D. Xia, J. Mater. Chem. A, 2014, 62–65 RSC.
- B. Hou, C. C. Wang, R. Tang, Q. Zhang and X. Cui, Mater. Res. Express, 2020, 7, 025506–025506 CrossRef CAS.
- G. Zhang, Q. Wei, X. Yang, A. C. Tavares and S. Sun, Appl. Catal., B, 2017, 206, 115–126 CrossRef CAS.
- S. A. Gopal, A. E. Poulose, C. Sudakar and A. Muthukrishnan, ACS Appl. Mater. Interfaces, 2021, 13, 44195–44206 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |


