Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanistic elucidation of irreversible chemodosimetric sensing of hydrazine through structural, computational, and bioimaging analyses
Priyanka
Avala
ab,
Malavika S
Kumar
ab,
Avijit Kumar
Das
 *ab,
Ankan
Sardar
c,
Tilak Raj
Maity
d,
Aveek
Samanta
e and
Malay
Dolai
*ab,
Ankan
Sardar
c,
Tilak Raj
Maity
d,
Aveek
Samanta
e and
Malay
Dolai
 *f
*f
aDepartment of Chemistry, Christ University, Hosur Road, Bangalore, Karnataka 560029, India. E-mail: avijitkumar.das@christuniversity.in; sanjuavi.das@gmail.com
bCentre for Renewable Energy and Environmental Sustainability, Christ University, Karnataka 560029, India
cDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur 741246, India
dDepartment of Biotechnology, Haldia Institute of Technology, Haldia, Purba Medinipur–721657, West Bengal, India
eDepartment of Botany, Prabhat Kumar College, Contai, Purba Medinipur 721404, W.B., India
fDepartment of Chemistry, Prabhat Kumar College, Purba Medinipur 721404, West Bengal, India. E-mail: dolaimalay@yahoo.in
First published on 25th November 2025
Abstract
A fluorescent chemodosimeter, DBA, has been developed for the selective detection of hydrazine via an irreversible reaction pathway that leads to fluorescence enhancement. The crystal structure of the ligand has been successfully determined. The sensing mechanism involves the conversion of a hydrazide derivative into a hydrazone derivative, as confirmed by both 1H NMR and mass spectrometry. Upon interaction of DBA with hydrazine, the probe exhibits a significant decrease in absorbance at 366 nm and 279 nm, along with a three fold enhancement in fluorescence intensity at 423 nm, achieving a detection limit of 0.37 µM. The detection mechanism has also been supported by theoretical analysis using density functional theory (DFT) calculations. For practical applications, DBA has been employed in plant-based cell imaging to monitor hydrazine accumulation in Lathyrus sativus L. (grass pea). Overall, DBA is a simple, effective, and promising fluorescent probe for hydrazine detection in diverse fields such as environmental monitoring, food safety, and biological risk assessment.
1. Introduction
Fluorescent chemosensors are compounds that integrate a fluorescent unit (fluorophore), a specific binding site for the target analyte, and a mechanism that involves the interaction between these two components. The indicators are referred to as fluorescent chemodosimeters if the binding sites have irreversible chemical processes.1–6 The concept of chemodosimeters was first introduced by Chae and Czarnik in 1992.7–9 In chemodosimetric sensing, the presence of the target analyte triggers both the breaking and forming of chemical bonds, resulting in products structurally distinct from the original sensor. The creation of chemodosimeters for both the identification and quantification of various substances is a relatively recent development in scientific research. Techniques based on colour changes (colorimetric) and fluorescence (fluorometric) are widely used because they are affordable, highly selective, sensitive, and efficient and provide rapid results for detecting different analytes.10–14 In particular, colorimetric and fluorescence-based sensors that allow naked-eye detection have gained significant attention for their simplicity, rapid operation, and capability for real-time analysis. Chemosensors are now widely employed for monitoring hazardous substances and environmental contaminants in a variety of samples, because of their outstanding sensitivity, broad detection range, adaptable design, and strong resistance to interference.15–18Hydrazine (N2H4), a colourless, fuming liquid with strong reducing properties, is a highly reactive compound crucial to industrial and technological applications. It is a highly energetic compound, a hexatomic molecule comprising two nitrogen atoms and four highly reactive hydrogen atoms, which easily reacts with oxygen and other elements during chemical combustion or transformation. Globally, its production reached around 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons annually a decade ago, driven by its versatile uses.19 Hydrazine and its derivatives remain key fuels in bipropellant rocket engines due to their ability to release substantial energy through exothermic reactions.20 It serves as an effective fungicide and is also used in the synthesis of pesticides like maleic hydrazide, manufacturing plastic foams, and controlling grass growth in residential areas.21 Hydrazine derivatives treat conditions such as hypertension,22 urinary tract infections,23 and tuberculosis.24 It acts as an oxygen scavenger in boiler feedwater to prevent corrosion. Dual-injection of hydrogen and hydrazine into reactor water is regarded as a novel approach to minimize stress corrosion cracking (SCC) in boiling water reactors (BWRs).25 This compound's adaptability across aerospace, agriculture, medicine, and energy sectors underscores its industrial significance despite handling challenges posed by its reactivity. The U.S. Environmental Protection Agency has established a 10-ppb threshold limit value (TLV) for hydrazine exposure, derived from animal as well as human studies.26 Therefore, the lower limit of detection of hydrazine is very much important. In this respect, use of fluorescent sensors has emerged as a superior method for detecting hydrazine compared to traditional analytical techniques like liquid chromatography,27 mass spectrometry,28,29 potentiometry,30 and titrimetry.31 These conventional methods face limitations such as complex buffer preparation, time-consuming protocols, low sensitivity, and destructive sample processing unsuitable for live biological systems.32 Fluorescent probes address these challenges through enhanced features like straightforward operation, exceptional molecular selectivity, and ultra-low detection limits, reaching submicromolar concentrations.33
000 metric tons annually a decade ago, driven by its versatile uses.19 Hydrazine and its derivatives remain key fuels in bipropellant rocket engines due to their ability to release substantial energy through exothermic reactions.20 It serves as an effective fungicide and is also used in the synthesis of pesticides like maleic hydrazide, manufacturing plastic foams, and controlling grass growth in residential areas.21 Hydrazine derivatives treat conditions such as hypertension,22 urinary tract infections,23 and tuberculosis.24 It acts as an oxygen scavenger in boiler feedwater to prevent corrosion. Dual-injection of hydrogen and hydrazine into reactor water is regarded as a novel approach to minimize stress corrosion cracking (SCC) in boiling water reactors (BWRs).25 This compound's adaptability across aerospace, agriculture, medicine, and energy sectors underscores its industrial significance despite handling challenges posed by its reactivity. The U.S. Environmental Protection Agency has established a 10-ppb threshold limit value (TLV) for hydrazine exposure, derived from animal as well as human studies.26 Therefore, the lower limit of detection of hydrazine is very much important. In this respect, use of fluorescent sensors has emerged as a superior method for detecting hydrazine compared to traditional analytical techniques like liquid chromatography,27 mass spectrometry,28,29 potentiometry,30 and titrimetry.31 These conventional methods face limitations such as complex buffer preparation, time-consuming protocols, low sensitivity, and destructive sample processing unsuitable for live biological systems.32 Fluorescent probes address these challenges through enhanced features like straightforward operation, exceptional molecular selectivity, and ultra-low detection limits, reaching submicromolar concentrations.33
Recent progress in the development of fluorescent chemosensors for selective hydrazine detection holds considerable importance due to their wide-ranging applications in medical and environmental research.34–37 In recent years, the development of small-molecule fluorescent probes has accelerated significantly, employing a variety of molecular recognition approaches such as protecting-group removal, nucleophilic substitution, and chemodosimetric reactions that transform non-fluorescent precursors into strongly emissive products. Several recent reviews have comprehensively summarized these strategies and their relevance to environmental and biological hydrazine detection.38–40 Current trends in probe design focus on achieving ratiometric and near-infrared (NIR) emission responses, which enhance quantitative accuracy in complex systems and support applications in deep-tissue imaging and on-site sensing.41 Despite these advances, many existing sensors continue to exhibit drawbacks such as limited solubility in aqueous media, cross-reactivity with other nucleophiles, and challenging synthetic routes. Thus, there remains a strong demand for straightforward, highly selective chemodosimeters capable of reliable hydrazine detection in real samples—an objective that the present study seeks to fulfil. Colorimetric and fluorimetric sensors for the naked-eye detection have attracted considerable interest because of their simple and fast implementation as well as their high sensitivity. Herein, we have developed a chemodosimeter by mixing 2,4-di-tert-butyl-5,6-catechol-1-aldehyde with aceto-hydrazide in ethanol medium to produce target ligand DBA, which has been characterized by 1H-NMR, 13C-NMR, mass spectroscopy (Fig. S3–S5) and X-ray crystal analysis. Although several reports are available on the selective detection of hydrazine, we have included a comparison table in the SI that summarizes recent studies on related ligands (Table S1). The comparison table indicates that although hydrazine is the common analyte across all reported systems, our probe (DBA) achieves a substantially lower LOD than previously reported complexes. A key strength of this study is the structural characterization of the ligand through single-crystal X-ray diffraction, which provided an accurate molecular model for DFT calculations and ensured excellent agreement between experimental and theoretical results. Overall, the comparison highlights the superior performance of DBA across multiple parameters, underscoring its strong potential for diverse applications.
2. Results and discussion
2.1. Synthesis and characterization of DBA
The solution of 4,6-di-tert-butyl-2,3-dihydroxybenzaldehyde (226 mg, 1 mmol) in methanol and the solution of aceto-hydrazide (74 mg, 1 mmol) in methanol was mixed together (Scheme 1). Then the mixture was stirred for another 1 hour. A bright yellowish colored solution appeared. It was stirred for 2 hours more to complete the reaction by TLC monitoring. The yellow precipitate of (E)-N′-(4,6-di-tert-butyl-2,3-dihydroxybenzylidene) acetohydrazide (DBA) was separated out by filtration and the residue was washed with cold methanol and dried. The block type yellow crystals were obtained after 4 days by slow evaporation of methanol solvent. Mp: 110–120 °C, yield: 200 mg, 72%. 1H NMR (DMSO-d6, 400 MHz): 12.77 (s, 1H, –OH), 10.67 (s, 1H, –OH), 8.75 (s, 1H), 6.82 (s, 1H), 1.44 (s, 9H), 1.36 (s, 12H). 13C NMR (DMSO-d6, 100 MHz): 197.4, 152.6, 142.4, 142.3, 141.7, 116.1, 114.7, 35.4, 35.3, 33.3, 28.8. Mass (m/z, %): M+ calculated for chemical formula: C17H26N2O3 is 306.4060; found: 307.5123 (M+H)+.2.2. X-ray crystal analysis of DBA
Several spectroscopic methods are employed to determine the structure and chemical formula of the probe DBA. The crystal structure of DBA is determined by the single crystal X-ray diffraction (SCXRD) technique (CCDC no. 2472615). The crystal X-ray study indicates that the sensor DBA crystallizes in a monoclinic system with the space group P21/c. The structure in Fig. 1a shows that the aldehyde and carbohydrazide parts are connected through an imine bond (–C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N1) with a bond length of 1.276 Å, which is comparable to that of previously reported Schiff base ligands. DBA gains stability through the formation of intermolecular H-bonding interactions (O3⋯H5 = 2.033 Å) between adjacent units, allowing them to form a H-bonded 1D tape along the crystallographic b axis (Fig. 1b).
N1) with a bond length of 1.276 Å, which is comparable to that of previously reported Schiff base ligands. DBA gains stability through the formation of intermolecular H-bonding interactions (O3⋯H5 = 2.033 Å) between adjacent units, allowing them to form a H-bonded 1D tape along the crystallographic b axis (Fig. 1b).
2.3. Interference study
The selectivity of DBA for N2H4 over other interfering analytes like Cl−, Br−, I−, F−, NO2−, AsO23−, HSO42−, C2O4−, H2O2, NO3−, H2S, triethylamine (TEA), NH3, ethylenediamine (EDA), piperidine (Bpy), hypochlorous acid (HClO), and OH− was evaluated in CH3CN/HEPES buffer solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). Upon addition of N2H4, DBA exhibited a distinct increase in emission intensity at 423 nm. In contrast, no significant spectral changes were observed with other anions, confirming the high selectivity of DBA towards N2H4 (Fig. 2a). In the bar diagram, the brown bar with the highest intensity indicates the fluorescence response of DBA toward N2H4, while the green bars represent the negligible interference of DBA with other analytes (Fig. 2b).
1, v/v, pH 7.4). Upon addition of N2H4, DBA exhibited a distinct increase in emission intensity at 423 nm. In contrast, no significant spectral changes were observed with other anions, confirming the high selectivity of DBA towards N2H4 (Fig. 2a). In the bar diagram, the brown bar with the highest intensity indicates the fluorescence response of DBA toward N2H4, while the green bars represent the negligible interference of DBA with other analytes (Fig. 2b).
2.4. Spectroscopic response of DBA toward N2H4
The interaction between DBA and N2H4 was examined via spectrophotometric titration in CH3CN/HEPES buffer solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). The ligand DBA initially exhibited strong absorption signals at wavelengths 366 nm and 279 nm, which were progressively decreased upon incremental addition of N2H4 (Fig. 3a).
1, v/v, pH 7.4). The ligand DBA initially exhibited strong absorption signals at wavelengths 366 nm and 279 nm, which were progressively decreased upon incremental addition of N2H4 (Fig. 3a).
Similarly, the fluorescence sensing property of DBA towards N2H4 was systematically investigated using a fluorescence spectrophotometer in CH3CN/HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution at an excitation wavelength of 315 nm. The fluorescence intensity of DBA remains stable even at high proportions of water (Fig. S11); however, hydrazine sensing in water-rich media such as CH3CN/HEPES buffer (5
1, v/v, pH 7.4) solution at an excitation wavelength of 315 nm. The fluorescence intensity of DBA remains stable even at high proportions of water (Fig. S11); however, hydrazine sensing in water-rich media such as CH3CN/HEPES buffer (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) is challenging. This difficulty arises from the small size, high polarity, and strong hydration of hydrazine, which hinder effective interactions with sensing probes like DBA. Moreover, extensive solvation and hydrogen bonding around N2H4 further reduce its binding availability, resulting in lower sensitivity and selectivity (Fig. S12). Fluorescence studies across pH 2–14 reveal that DBA exhibits minimal emission at extreme pH values under both strong acidic and basic conditions but strong hydrazine-induced fluorescence near physiological pH, demonstrating optimal sensing performance under biologically relevant conditions (Fig. S9). Initially, DBA exhibited two emission signals centered at 350 nm with lower intensity and at 423 nm with high intensity, having a fluorescence quantum yield of Φ = 0.0735. Upon incremental addition of N2H4, three fold enhancement in fluorescence intensity was observed with a high fluorescence quantum yield (Φ = 0.21), accompanied by a hypsochromic shift (Δλ = 5 nm) of the emission signal at 418 nm up to a saturation level (Fig. 4a).
5, v/v) is challenging. This difficulty arises from the small size, high polarity, and strong hydration of hydrazine, which hinder effective interactions with sensing probes like DBA. Moreover, extensive solvation and hydrogen bonding around N2H4 further reduce its binding availability, resulting in lower sensitivity and selectivity (Fig. S12). Fluorescence studies across pH 2–14 reveal that DBA exhibits minimal emission at extreme pH values under both strong acidic and basic conditions but strong hydrazine-induced fluorescence near physiological pH, demonstrating optimal sensing performance under biologically relevant conditions (Fig. S9). Initially, DBA exhibited two emission signals centered at 350 nm with lower intensity and at 423 nm with high intensity, having a fluorescence quantum yield of Φ = 0.0735. Upon incremental addition of N2H4, three fold enhancement in fluorescence intensity was observed with a high fluorescence quantum yield (Φ = 0.21), accompanied by a hypsochromic shift (Δλ = 5 nm) of the emission signal at 418 nm up to a saturation level (Fig. 4a).
The detection limit of DBA toward N2H4 was evaluated at 0.37 µM through fluorometric analysis utilizing the established relationship DL = K × Sb1/S, where K = 3, Sb1 represents the standard deviation of blank measurements, and S denotes the calibration curve slope (Fig. S1). Based on the variations in DBA fluorescence intensity upon the addition of N2H4 at different time intervals, the rate constant was calculated as 2.705 s−1, using a first-order rate equation, indicating the rapid response of DBA toward N2H4 (Fig. S2).
2.5. Competition experiment
The competitive sensing ability of DBA toward N2H4 was evaluated in the presence of various interfering anions in a CH3CN/HEPES buffer solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). The fluorescence enhancement observed upon N2H4 binding remained unaffected by the presence of other competing analytes, demonstrating the high sensitivity and selectivity of DBA for N2H4 even in complex analyte environments (Fig. 5).
1, v/v, pH 7.4). The fluorescence enhancement observed upon N2H4 binding remained unaffected by the presence of other competing analytes, demonstrating the high sensitivity and selectivity of DBA for N2H4 even in complex analyte environments (Fig. 5).
2.6. Probable N2H4 sensing mechanism in the presence of DBA
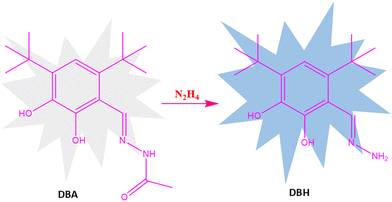
The probable sensing mechanism behind the improved fluorescence emission of the ligand DBA in the presence of N2H4 involves the transformation of the hydrazide derivative (DBA) into a hydrazone derivative (DBH) (Scheme 2 and Scheme S1).42 Although DBA contains electron-donating hydroxyl groups at the ortho positions, which promote intramolecular charge transfer (ICT) by increasing electron density across the aromatic system, the presence of the hydrazide group hinders efficient ICT, thereby reducing fluorescence. Moreover, in the solvatochromic analysis, DBA exhibited negligible fluorescence enhancement/shifting in most of the tested solvents, namely CH3CN, CH3OH, acetone, DMSO, H2O, toluene, and THF. Notably, weak fluorescence was detected in toluene, with comparatively lower intensities in DMSO and THF, showing two distinct emission bands at 350 nm and 423 nm (Fig. S10). Upon reaction with hydrazine, the ligand DBA has been converted to a hydrazone derivative DBH featuring an extended conjugated system comprising the aromatic ring, the hydrazone linkage (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH2), and two electron-donating hydroxyl groups. This transformation significantly improves ICT within the aromatic ring, which results in an increase in fluorescence upon reacting with N2H4.43 Moreover, the bulky tert-butyl groups at the 4 and 6 positions additionally suppress non-radiative decay pathways by restricting rotational and vibrational motions, stabilizing the excited state and resulting in enhanced fluorescence.
N–NH2), and two electron-donating hydroxyl groups. This transformation significantly improves ICT within the aromatic ring, which results in an increase in fluorescence upon reacting with N2H4.43 Moreover, the bulky tert-butyl groups at the 4 and 6 positions additionally suppress non-radiative decay pathways by restricting rotational and vibrational motions, stabilizing the excited state and resulting in enhanced fluorescence.
Moreover, the conversion of DBA to DBH was also confirmed through 1H-NMR and mass spectra (Fig. S6 and S7). Upon transformation of the hydrazide group in DBA to a hydrazone group in DBH, significant shifts in the proton signals were observed, particularly for the two hydroxyl protons attached to the benzene ring and the imine proton. In DBA, the hydroxyl proton signals appeared at 12.78 ppm (Ha) and 10.68 ppm (Hb), attributed to strong deshielding effects from the adjacent hydrazide group. In DBH, these signals shifted downfield to 12.63 ppm (Ha) and 9.49 ppm (Hb), consistent with the replacement of the hydrazide group by a hydrazone moiety. Additionally, the imine and aromatic protons also showed slight shifts: from 8.77 ppm (Hc) and 6.83 ppm (Hd) in DBA to 8.46 ppm (Hc) and 6.85 ppm (Hd) in DBH (Fig. S6). The transformation of DBA into DBH was additionally confirmed by the emergence of a distinctive molecular ion peak at m/z. = 265.4202 (M + H)+, which is close to the theoretical mass peak for C15H24N2O2 at 264.3690 for DBH (Fig. S7).
2.7 Theoretical calculation
To elucidate the sensing mechanism of DBA toward N2H4 through the formation of DBH, structural optimizations were carried out using density functional theory (DFT) at the B3LYP level (Fig. 6a). Additionally, we examined the spatial electron cloud distribution and calculated the frontier molecular orbital energies—specifically the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO)—for both DBA and DBH. For DBA, the energy gap between the HOMO (−8.34 eV) and LUMO (−0.45 eV) is 7.89 eV. In contrast, DBH exhibits a slightly larger HOMO–LUMO energy gap of 8.18 eV, with HOMO and LUMO energies of −8.39 eV and −0.206 eV, respectively (Fig. 6b). Notably, the stabilization of the HOMO level in DBH and the observed increase in the HOMO–LUMO energy gap from 7.89 eV (DBA) to 8.18 eV (DBH) suggest the thermodynamic feasibility of the conversion from DBA to DBH. The production of DBH is indicated by one absorption band at 366 nm in methanolic solution at 25 °C, and the theoretically calculated absorbance is 370 nm, which is well supported by experimental results. This spectrum is caused by the S0 → S8 transitions that occur during DBH formation. | ||
| Fig. 6 (a) Geometry optimized molecular structure of (i) DBA and (ii) DBH (DBA + N2H4). (b) Frontier molecular orbital with the energy difference of (i) DBA and (ii) DBH (DBA + N2H4). | ||
2.8. Root imaging study
In this study, we have demonstrated the potential of a fluorescent probe, DBA, for detecting hydrazine accumulation using a plant-based cell imaging approach. The legume Lathyrus sativus L. (grass pea) was employed as a cost-effective, convenient, and readily available model system for primary screening.44,45 Transverse sections of treated roots displayed clear fluorescence signals, indicating the successful accumulation and interaction of DBA with N2H4 (Fig. 7IB and D). Moreover, root extracts subjected to centrifugation and observed under UV light revealed strong blue fluorescence in the DBA and hydrazine-treated samples. The higher fluorescent zone is observed inside xylem upon treatment. This signifies that the chemical DBA is absorbed by the root and transported with water throughout the plant in contrast to the non-fluorescent control (Fig. 7IA and C). These results validate the efficacy of DBA as a reliable indicator of hydrazine presence in plant tissues. We proposed DBA as an advanced fluorescent probe in this study, which offers a promising, simple, and effective approach for hydrazine detection across various domains, including environmental monitoring, food safety assessment, and biological risk evaluation.The antioxidant activity of DBA was assessed using the DPPH radical scavenging assay. As shown in Fig. 7(I), the control containing only the DPPH reagent retained a strong purple coloration, suggestive of unreacted DPPH radicals. In contrast, the DBA treated samples demonstrated a significant reduction in the intensity of the purple color, signifying the presence of active antioxidant compounds capable of neutralizing free radicals. 50% reduction was seen at 10 µM concentration of DBA. The degree of decolorization visually suggested a concentration-dependent effect, where higher sample concentrations of DBA led to more pronounced fading. At higher concentrations (30–50 µM), the extent of fading appeared to be similar across treatments, indicating that the scavenging activity reached a plateau. Based on the visual inspection of color intensities, there is a significant difference between the control and all DBA treated samples. However, no significant difference is visually detectable among the 30 µM, 40 µM, and 50 µM treatments, suggesting that DPPH radicals were maximally scavenged at 30 µM and additional increases in concentration did not further enhance activity. Overall, the data indicate that DBA possesses strong antioxidant potential, with significant activity evident from 10 µM onward (Fig. 7II). This color transition from deep purple to lighter hues confirms the radical scavenging ability of DBA, underscoring its potential as a source of potential antioxidant compounds.46 Hydrazine (N2H4) is a volatile and highly toxic compound known to cause significant harm to water, soil, air, and living organisms. Numerous earlier studies have reported its carcinogenic potential following toxic or even subtoxic exposure.47,48 Given its wide industrial use in pharmaceuticals, pesticides, and textile dye production, hydrazine must be detected in real time and monitored in the environment for an extended period of time in order to preserve the environment and the health of humanity. Once absorbed by plants, hydrazine can infiltrate the food chain, exacerbate oxidative stress, induce lipid peroxidation, stimulate reactive oxygen species (ROS) formation, and ultimately damage essential biomolecules such as DNA, proteins, and cellular structures.
2.9. Soil analysis
Chemicals used in warfare are most commonly found in the soil of areas that once served as battlefields, where they have been released into the environment.49 So, we have performed real-time field soil analysis, to understand the selectivity for hydrazine. 1 g of field soil was placed in separate vials along with the ligand DBA (c = 5.0 × 10−5 M), in the presence of hydrazine (c = 1.0 × 10−5 M). In the fluorescence study, as the concentration of hydrazine increased, the corresponding emission intensity progressively increased. And from this titration, the detection limit was calculated and found to be 0.029 µM (Fig S8, SI). These findings confirm that the DBA probe is suitable for real-time hydrazine detection in environmental samples, especially soil.3. Conclusion
In conclusion, the fluorescent chemodosimeter DBA offers a highly efficient, selective, and sensitive platform for hydrazine detection via an irreversible reaction pathway that produces a pronounced enhancement in fluorescence intensity. The sensing mechanism, based on the transformation of the hydrazide group into its corresponding hydrazone form, is strongly supported by 1H NMR and mass spectrometric analyses and theoretical studies, confirming both structural changes and the mechanistic reliability of the system. DBA exhibits excellent photostability, rapid response behavior, and an exceptionally low detection limit, establishing it as a robust probe for diverse analytical applications. Its high biocompatibility and low cytotoxicity further enable effective plant-based cellular imaging, successfully visualizing hydrazine accumulation in Lathyrus sativus L. (grass pea). Additionally, DBA was used to detect hydrazine in environmental samples through soil analysis experiments, i.e., real environmental analysis. These results establish DBA as a promising candidate for biological, environmental, and analytical sensing applications. Future improvements in its molecular design could enhance solubility, spectral properties, and sensing performance, broadening its suitability for in vivo imaging. Coupling DBA-based probes with portable devices, microfluidic platforms, or nanomaterial interfaces may further expand their use in environmental monitoring, food quality analysis, and biomedical diagnostics. Overall, DBA shows strong promise as a versatile fluorescent sensor for advanced analytical and biosensing technologies.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5nj04044b.CCDC 2472615 contains the supplementary crystallographic data for this paper.50
Acknowledgements
The authors gratefully acknowledge Christ University, Bangalore, for providing the necessary research facilities, and the Centre for Research, Christ University, for the seed money grant (Grant Approval Number: CU-ORS-SM-24/09). Avijit Kumar Das extends special thanks to the State University Research Excellence (SERB-SURE) program of the Science and Engineering Research Board (SERB) (File Number: SUR/2022/002461), under the Anusandhan National Research Foundation (ANRF) and the Department of Science and Technology (DST), Government of India, for financial support through the research grant. Malavika S. Kumar expresses her sincere appreciation to the University Grants Commission (UGC), Government of India, for awarding the Savitribai Jyotirao Phule Fellowship for Single Girl Child (SJSGC), F. No. 82-7/2022(SA-III).References
- A. W. Czarnik, Acc. Chem. Res., 1994, 27, 302–308 CrossRef CAS.
- J. Yi, W. H. Hsieh, J. L. Chir and A. T. Wu, J. Fluoresc., 2014, 24(6), 1723–1726 CrossRef PubMed.
- L. Chaolong, M. Qin, S. Xu, Y. Yuan, K. Li and J. Tang, Sens. Actuators, B, 2024, 407, 135509 CrossRef.
- M. Shellaiah, H. C. Chen, K. Awasthi, B. Aazaad, K. W. Sun, N. Ohta and M. C. Lin, J. Mol. Struct., 2024, 1301, 137347 CrossRef CAS.
- Y. W. Liu, C. H. Chen and A. T. Wu, Analyst, 2012, 137(22), 5201–5203 RSC.
- S. Muthaiah, B. Aazaad, M. C. Lin, K. W. Sun, A. Murugan, K. Anandan, M. Bhushan, E. Manikandan and W. T. Li, J. Photochem. Photobiol., A, 2026, 472, 116786 CrossRef.
- K. Kaur, R. Saini, A. Kumar, V. Luxami, N. Kaur, P. Singh and S. Kumar, Coord. Chem. Rev., 2012, 256, 1992–2028 CrossRef CAS.
- H. Shinziya, A. K. Das, M. S. Kumar, A. Nag and M. Dolai, Sens. Diagn., 2025, 4, 622–630 RSC.
- M. S. Kumar, A. K. Das, Y. Bylappa and A. Nag, RSC Adv., 2025, 15, 6708–6717 RSC.
- S. Vishnu, Y. bylappa, A. Nag, M. Dolai and A. K. Das, Anal. Methods, 2024, 16, 8164–8178 RSC.
- M. S. Kumar and A. K. Das, New J. Chem., 2024, 48, 13776–13782 RSC.
- A. K. Das and S. Goswami, Sens. Actuators, B, 2017, 2045, 1062–1125 CrossRef.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- M. S. Kumar, M. Dolai and A. K. Das, New J. Chem., 2024, 48, 9103–9109 RSC.
- A. Pundi and C.-J. Chang, J. Environ. Chem. Eng., 2023, 11, 110346 CrossRef CAS.
- S. Pakrashy, M. Das, S. Manna, S. M. Choudhury, H. Shinziya, B. Das, M. Dolai and A. K. Das, Anal. Methods, 2025, 17, 6160–6169 RSC.
- S. Vishnu, A. K. Das, G. Karan and S. M. Choudhury, Mater. Adv., 2025, 6, 4499–4512 RSC.
- S. Vishnu, S. Maity, A. C. Maity, M. S. Kumar, M. Dolai, A. Nag, Y. bylappa, G. Dutta, B. Mukherjee and A. K. Das, Spectrochim. Acta, Part A, 2024, 315, 124249 CrossRef CAS.
- A. D. Sutton, A. K. Burrell, D. A. Dixon, E. B. Garner 3rd, J. C. Gordon, T. Nakagawa, K. C. Ott, J. P. Robinson and M. Vasiliu, Science, 2011, 331, 1426–1429 CrossRef CAS.
- S. Huang, X. Qi, T. Liu, K. Wang, W. Zhang, J. Li and Q. Zhang, Chemistry, 2016, 22, 10187–10193 CrossRef CAS.
- Y.-R. Wang, L. Yang, D.-T. Wang, A.-P. Li, S.-Y. Zhang, L.-L. Qin, Q. Bian, Z.-J. Zhang, Y.-Y. Ding, H. Zhou, D. Peng, G.-H. Wang and Y.-Q. Liu, Pest Manag. Sci., 2025, 81, 170–184 CrossRef CAS.
- M. R. Kandler, G. T. Mah, A. M. Tejani, S. N. Stabler and D. M. Salzwedel, Cochrane Database Syst. Rev., 2011, CD004934 Search PubMed.
- D. Poulain, Crit. Rev. Microbiol., 2015, 41, 208–217 CrossRef CAS.
- R. Dai, D. J. Wilson, T. W. Geders, C. C. Aldrich and B. C. Finzel, ChemBioChem, 2014, 15, 575–586 CAS.
- K. Ishida, Y. Wada, M. Tachibana, M. Aizawa, M. Fuse, E. Kadoi and H. Takiguchi, J. Nucl. Sci. Technol., 2007, 44, 222–232 CAS.
- A. Umar, M. M. Rahman, S. H. Kim and Y.-B. Hahn, Chem. Commun., 2008, 166–168 CAS.
- L. Song, D. Gao, S. Li, Y. Wang, H. Liu and Y. Jiang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1063, 189–195 CAS.
- E. Lattová and H. Perreault, Mass Spectrom. Rev., 2013, 32, 366–385 Search PubMed.
- W. E. Davis 2nd and Y. Li, Anal. Chem., 2008, 80, 5449–5453 CrossRef PubMed.
- S. Ganesh, F. Khan, M. K. Ahmed and S. K. Pandey, Talanta, 2011, 85, 958–963 CAS.
- J. D. Clark and J. R. Smith, Anal. Chem., 1961, 33, 1186–1187 CAS.
- R. Tyśkiewicz, M. Fedorowicz, A. Nakonieczna, P. Zielińska, M. Kwiatek and L. Mizak, Anal. Biochem., 2023, 675, 115215 Search PubMed.
- S. Goswami, A. K. Das, U. Saha, S. Maity, K. Khanra and N. Bhattacharyya, Org. Biomol. Chem., 2015, 13, 2134–2139 CAS.
- M. Uchiyama, N. Norberto, S. Gonçalves and I. A. Bagatin, Spectrochim. Acta, Part A, 2025, 327, 125356 CrossRef PubMed.
- M. Xing, K. Wang, X. Wu, S. Ma, D. Cao, R. Guan and Z. Liu, Chem. Commun., 2019, 55, 14980–14983 RSC.
- J. Borah, A. Chetry, A. Pegu, P. Dutta, A. Baruah and P. Khakhlary, Environ. Sci.: Adv., 2025, 7, 1054–1064 Search PubMed.
- X. Y. Zhang, Y. S. Yang, W. Wang, Q. C. Jiao and H. L. Zhu, Coord. Chem. Rev., 2020, 417, 213367 CrossRef CAS.
- K. Semwal and A. K. Das, RSC Adv., 2025, 15, 10005–10021 RSC.
- Q. Yi, J. He, X. Fu, J. Ying, L. Gong, J. Shen and X. He, Dyes Pigm., 2021, 196, 109816 CrossRef CAS.
- N. H. Oza, D. Kasundra, A. G. Deshmukh, N. Borane, R. Boddula and P. N. Patel, Environ. Sci.: Adv., 2025, 4, 235–244 CAS.
- M. Xing, Y. Han and Y. Zhu, Anal. Chem., 2022, 94, 12836–12844 CrossRef CAS PubMed.
- S. Singh and J. Kandasamy, Asian J. Org. Chem., 2023, 12, e202300115 CrossRef CAS.
- T. Wei, J. Wang, Y. Chen and Y. Han, RSC Adv., 2015, 5, 57141–57146 RSC.
- A. Samanta, S. Banerjee, T. R. Maity, J. Jahnavi and S. Datta, Protoplasma, 2022, 259, 1455–1466 CrossRef CAS PubMed.
- G. C. Das, A. Kumar Das, D. Das, T. Raj Maity, A. Samanta, F. Ali Alasmary, A. Salem Almalki, A. Iqbal and M. Dolai, J. Photochem. Photobiol. A Chem., 2023, 440, 114663 CrossRef CAS.
- S. Baliyan, R. Mukherjee, A. Priyadarshini, A. Vibhuti, A. Gupta, R. P. Pandey and C.-M. Chang, Molecules, 2022, 27, 1326 CrossRef CAS PubMed.
- D. Steinhoff and U. Mohr, Exp. Pathol., 1988, 33, 133–143 CrossRef CAS PubMed.
- X. Sun, X. Jiang, Z. Wang, Y. Li, J. Ren, K. Zhong, X. Li, L. Tang and J. Li, J. Hazard Mater, 2025, 483, 121442 CrossRef PubMed.
- C. Giacomo, R. Scalenghe and W. I. Woods, Earth-Sci. Rev., 2013, 127, 1–15 CrossRef.
- CCDC 2472615: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2nzysd.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |