Comparative mechanistic study of nitrogen- and oxygen-functionalized activated carbons for dual-phase adsorption
Chandresh
Bari
ab,
Sagnik
Mukherjee
a,
Harshal
Kulkarni
ab,
Rahulbhai
Parmar
ab and
Govind
Sethia
 *ab
*ab
aInorganic Material and Catalysis Division, CSIR-Central Salt and Marine Chemicals Research Institute, Bhavnagar 364002, Gujarat, India. E-mail: govinds@csmcri.res.in; Tel: +917718892365
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India
First published on 3rd December 2025
Abstract
The development of functional adsorbents for the removal of pollutants from both liquid and gas phases is a critical challenge for environmental remediation in the 21st century. Materials that exhibit dual phase adsorption are rare, and less attention has been given to elucidating the molecular level mechanism governing their performance. In the present study, the adsorption of methylene blue (liquid phase) and CO2 (gas phase) was investigated using aniline/phenol-functionalized activated carbon (AC). Both the materials exhibit complete MB adsorption in the liquid phase under ambient conditions. In contrast, aniline-loaded AC demonstrated nearly 2-fold enhanced CO2 uptake (42.8 cm3 g−1 at 298 K, 1 bar) as compared to phenol-loaded AC. X-ray photoelectron spectroscopy analysis reveals that aniline loading on AC preserves the local electronic environment around the –NH2 groups of aniline. This enables stronger quadrupolar interactions with CO2, resulting in higher adsorption capacity. In contrast, phenol loading on AC perturbs the local electronic environment around the –OH groups of phenol. This weakens its interaction with CO2 and lowers the uptake capacity. In the liquid phase, MB adsorption mainly proceeds through π–π stacking. Therefore, the influence of electronic factors is less significant. This study demonstrates the importance of the local electronic environment of loaded functional groups on a solid support in achieving dual-phase pollutant removal. The findings provide molecular-level insight useful for designing next-generation multifunctional sorbents.
1. Introduction
The rapid industrialization has led to widespread contamination of water bodies by synthetic organic dyes and atmospheric CO2 emissions, both posing severe environmental and health risks.1,2 Adsorption using activated carbon remains a critical, scalable first step for rapid dye removal, with high regenerability minimizing secondary pollution when coupled with thermal or solvent-based desorption.3,4 This work focuses on surface-engineered activated carbon to achieve efficient dual-phase adsorption of methylene blue (liquid) and CO2 (gas) via tailored nitrogen and oxygen functionalities. Adsorption offers a promising alternative for post-combustion CO2 capture due to its simplicity, cost-effectiveness, regenerability, and low environmental impact.5 Shao et al. reported N, S co-doped porous carbons from coconut shell using thiourea and KOH activation, achieving 4.38 mmol g−1 CO2 uptake at 25 °C and 1 bar, with detailed evaluation of humidity tolerance, contaminant resistance, and thermal stability under simulated flue gas conditions.3 Similarly, Wang et al. developed B-doped porous carbons using eco-friendly potassium and sodium metaborate activators, demonstrating dynamic CO2 capture (up to 0.80 mmol g−1) under mixed gas streams, variable temperatures, and humid environments, highlighting practical flue gas applicability.5,6 Sethia et al. reported ultra-microporous nitrogen-doped activated carbons with exceptional stability, under steam, pure CO2, dry air, and dry SO2 at 130 °C, underscoring the robustness of carbon-based sorbents for long-term flue gas applications.4These considerations have motivated efforts to develop solid adsorbents capable of removing pollutants across both aqueous and gaseous phases.7 A wide spectrum of porous materials have been explored for such dual-phase remediation, including activated carbons (ACs),8 zeolites,9 mesoporous silicas,10 metal oxides,11 metal–organic frameworks (MOFs),12 and covalent–organic frameworks (COFs).13 Among them, AC has retained a leading role, owing to its high surface area, structural robustness, chemical inertness, and long history of large-scale use in environmental remediation.14–16 Importantly, AC is highly amenable to surface modification, making it an adaptable platform for designing next-generation sorbents.
Considerable research has been devoted to tailoring the surface properties of ACs through thermal, chemical, or biological modifications.17–19 Among these strategies, chemical functionalization is particularly effective because it introduces heteroatom functionalities that modulate polarity, surface heterogeneity, and interaction strength with adsorbates.17,18 Nitrogen- and oxygen-containing groups have been the most widely investigated, as both can enhance pollutant adsorption by increasing adsorbate–adsorbent interactions.
Despite extensive studies on heteroatom-doped (N- and O-) carbons, a major knowledge gap persists regarding how nitrogen- and oxygen-based functionalities distinctly regulate adsorption mechanisms across liquid and gas phases. Most reported works address either dye removal from water or CO2 capture from flue gas independently, without exploring whether a single surface-engineered platform can simultaneously achieve high performance in both domains.20,21 Furthermore, while heteroatom doping is known to influence surface polarity and local electronic environment, the binding configuration, that is, whether functional groups preserve or perturb their native electronic environments upon anchoring, remains largely unexplored.
The present work introduces a distinct mechanistic pathway by correlating the binding mode of functional groups, electronic structure, and phase-specific adsorption within a single comparative system. To the best of our knowledge, this is the first direct experimental comparison of nitrogen- and oxygen-functionalized activated carbons that combines XPS and NMR characterization with dual-phase adsorption analysis. The results demonstrate how subtle variations in functional group anchoring dictate surface polarity, the local electronic environment around functional groups, and, consequently, the phase-selective adsorption efficiency. This integrated approach provides a new molecular-level perspective for designing next-generation functionalized activated carbons that unify aqueous dye removal and CO2 capture, two of the most urgent environmental challenges of the 21st century.22,23
2. Experimental
2.1. Materials
All chemicals were of analytical grade and used without further purification. Commercial activated carbon (AC) was procured from Bhagyoday Industries. Hydrochloric acid (HCl), aniline, and phenol were obtained from SD Fine-Chem Ltd, while methylene blue (MB) was purchased from Loba Chemie. Deionized water was used for all aqueous preparations. High-purity CO2 and N2 gases (≥99.996%) were employed for adsorption measurements.2.2. Preparation of phenol and aniline-adsorbed ACs
Pristine AC was pre-heated at 120 °C for 24 h to remove moisture and volatile impurities. For surface modification, 1 g of dried AC was separately dispersed in 100 mL of aqueous solution of aniline (0.86 g, 9.3 mmol) or phenol (0.87 g, 9.3 mmol) in 150 mL round-bottom flasks. To aid dissolution, 3 mL of dilute HCl (1 M) was added to the aniline solution, and dilute NaOH was added to the phenol solution. Each suspension was stirred at 250 rpm for 2 h at room temperature. The solids were filtered, washed with deionized water (2 × 25 mL), and dried overnight at 70 °C, affording aniline- and phenol-functionalized carbons, denoted N-AC and O-AC, respectively (Scheme 1). The overall recovery of the carbon material after functionalization was nearly 98%, with only minor handling losses. The conversion efficiency of the surface-loaded precursors was 54.4% for N-AC (with respect to aniline) and 65.0% for O-AC (with respect to phenol). Notably, the filtrate obtained after separation can be directly reused for preparing subsequent batches of N-AC and O-AC by simply replenishing the required amounts of aniline and phenol.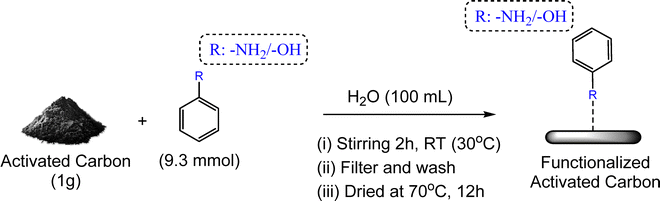 | ||
| Scheme 1 Schematic representation of the preparation of aniline- and phenol-functionalized activated carbons. | ||
Unlike conventional nitrogen/oxygen doping routes that typically require multistep oxidation–reduction or high-temperature carbonization (>800 °C) in ammonia or urea vapour,20,24 the present method employs a single-step aqueous adsorption-anchoring strategy under green and environmentally benign conditions. This mild functionalization process preserves the intrinsic textural properties of activated carbon and also ensures the retention of the electronic environment of –NH2 and –OH groups, which are often partially degraded during pyrolysis-based doping.
2.3. Characterizations
Physicochemical characterization of AC, N-AC, and O-AC included CHNS elemental analysis (Elementar Vario Microcube), powder X-ray diffraction (XRD, Philips X’Pert MPD, Cu Kα, λ = 1.5406 Å, 2θ = 2–80°), Fourier-transform infrared spectroscopy (FT-IR, ATR-equipped PerkinElmer GX, 4000–400 cm−1), N2 adsorption–desorption at 77 K (Micromeritics ASAP 2020) for BET surface area and BJH pore distribution, thermogravimetric analysis (TGA-5000/2960, TA Instruments, heating from RT to 700 °C at 10 °C min−1 under N2), X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250 Xi, Al Kα excitation), and field-emission scanning electron microscopy (FE-SEM, JSM-7100F). 1H NMR spectra were recorded on a Bruker Avance-II 500 MHz spectrometer. The samples (N-AC and O-AC) for 1H NMR analysis were prepared by dissolving the soluble fraction in DMSO-d6 (99.96%, Sigma-Aldrich). For each measurement, 20 mg of sample was dispersed in 1 mL of DMSO-d6 to ensure dissolution of the soluble components prior to analysis.2.4. Adsorption performance of N-AC and O-AC materials
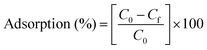 | (1) |
2.4.2.1. CO2 and N2 adsorption isotherm measurement. Adsorption isotherms were recorded at 298 K using a static volumetric system (Micromeritics ASAP 2020). Samples were degassed at 383 K under vacuum for 2 h prior to measurements to ensure reproducible surface conditions.
2.4.2.2. CO2/N2 selectivity calculation under flue gas conditions. Selectivity was evaluated using Ideal Adsorbed Solution Theory (IAST) for a 15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 CO2:N2 mixture, representative of post-combustion flue gas. Pure-component isotherms were fitted to the extended Freundlich model, and parameters were used for IAST calculations. Model fits showed excellent agreement with the experimental isotherm (R2 > 0.999).
85 CO2:N2 mixture, representative of post-combustion flue gas. Pure-component isotherms were fitted to the extended Freundlich model, and parameters were used for IAST calculations. Model fits showed excellent agreement with the experimental isotherm (R2 > 0.999).
The Freundlich model equation (eqn (2)) is identified as:
| q = KPn | (2) |
The CO2/N2 selectivity was determined using eqn (3):
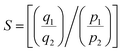 | (3) |
3. Results and discussion
3.1. Characterization of the prepared N-AC and O-AC materials
We systematically investigated surface-modified activated carbons (ACs) to elucidate the mechanistic pathways of aniline and phenol uptake and to understand the role of N- and O-functionality in MB and CO2 adsorption. Elemental analysis shows increased %C and %N for N-AC relative to pristine AC, evidencing aniline anchoring, while an increase in %C for O-AC indicates phenol adsorption (Table 1). Concomitant decreases in BET area and pore volume for both N-AC and O-AC further support surface coverage by the respective aromatics (Table 1). Notably, the larger surface-area loss for O-AC than N-AC suggests higher phenol loading than aniline.The high BET surface areas of the prepared materials (Table 1) are due to the presence of a large amount of micropores and mesopores (Fig. 1a and c). In pristine AC, micropores account for 88.5% and mesopores 11.4% of the total surface area. Nitrogen sorption (Fig. 1a) shows Type-I isotherms with a sharp low-pressure uptake (p/p0 < 0.05), characteristic of dominant microporosity.25 A gradual rise beyond the knee into the saturation region indicates a transition from mono- to multilayer adsorption. A distinct H4 hysteresis loop is observed, characteristic of mesoporous materials.12 The SEM image shows the surface texture, indicating the presence of large pores on the surface (Fig. 1b). The NL-DFT pore size distribution remains nearly identical for N-AC and O-AC (Fig. 1c), indicating the presence of both micropores and mesopores in the material. Powder XRD confirms retention of the amorphous nature of carbon in N-AC and O-AC, with peaks at 2θ ≈ 23.4° (002), turbostratically disordered graphitic layers, and 2θ ≈ 43.9° (100), corresponds to honeycomb domains (Fig. 1c).26 FT-IR analysis (Fig. 1d) reveals the parent AC signatures: a broad O–H stretch at ∼3435 cm−1 with the corresponding bending at 1637 cm−1, aliphatic C–H stretches at 2919/2859 cm−1, overlapping C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O modes at 2300–2000 cm−1, a broad C–O stretch centred at ∼1068 cm−1, and an out-of-plane C–H bend near 874 cm−1.4 Upon functionalization, N-AC exhibits N–H stretching at 3466 and 3139 cm−1 (free/protonated), an aromatic C–N stretch at 1296 cm−1, and ring C–H modes at 1531 and 742 cm−1, corroborating aniline incorporation.27 The band at 1296 cm−1 is assigned to the C–N stretching vibration of aromatic amine groups. Furthermore, the absorption peaks at 1531 and 742 cm−1 are consistent with C–H stretching and bending modes of the aromatic ring, suggesting successful incorporation of aniline on the carbon surface. O-AC shows a free phenolic O–H stretch near 3761 cm−1, phenolic O–H bending at 1364 cm−1, and additional aromatic C–H bands at 1470 and 605 cm−1.28
O modes at 2300–2000 cm−1, a broad C–O stretch centred at ∼1068 cm−1, and an out-of-plane C–H bend near 874 cm−1.4 Upon functionalization, N-AC exhibits N–H stretching at 3466 and 3139 cm−1 (free/protonated), an aromatic C–N stretch at 1296 cm−1, and ring C–H modes at 1531 and 742 cm−1, corroborating aniline incorporation.27 The band at 1296 cm−1 is assigned to the C–N stretching vibration of aromatic amine groups. Furthermore, the absorption peaks at 1531 and 742 cm−1 are consistent with C–H stretching and bending modes of the aromatic ring, suggesting successful incorporation of aniline on the carbon surface. O-AC shows a free phenolic O–H stretch near 3761 cm−1, phenolic O–H bending at 1364 cm−1, and additional aromatic C–H bands at 1470 and 605 cm−1.28
Thermogravimetric analysis shows single-stage decomposition for pristine AC, whereas N-AC and O-AC undergo two-stage mass loss (Fig. S1a, SI): an initial event below 100 °C (desorption of moisture) followed by decomposition above ∼250 °C, attributable to the loss of grafted organic moieties.
In the C 1s XPS spectra of pristine AC, three distinct binding energy peaks are observed at 284.7 eV, 286.0 eV, and 289.2 eV, which can be attributed to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively (Fig. 2a). The binding energies of C–O (286.4 eV) and C
O bonds, respectively (Fig. 2a). The binding energies of C–O (286.4 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.9 eV) bonds are slightly shifted towards higher binding energy in the C 1s XPS spectra of O-AC, which is due to the attachment of phenols on the carbon support (Fig. 2b). An additional C 1s XPS peak at 285.6 eV is observed in the XPS spectra of N-AC, which corresponds to the binding energy of C–N bonds suggesting the anchoring of aniline on the carbon support (Fig. 2c).
O (289.9 eV) bonds are slightly shifted towards higher binding energy in the C 1s XPS spectra of O-AC, which is due to the attachment of phenols on the carbon support (Fig. 2b). An additional C 1s XPS peak at 285.6 eV is observed in the XPS spectra of N-AC, which corresponds to the binding energy of C–N bonds suggesting the anchoring of aniline on the carbon support (Fig. 2c).
The N 1s spectrum of N-AC exhibits two peaks at 399.7 eV and 401.6 eV, corresponding to two chemically distinct nitrogen species (Fig. 2d). The lower-binding-energy component (399.7 eV) and the higher-binding-energy component (401.6 eV) are designated as Species I and Species II, respectively (Scheme 2a and b). In Species I, which accounts for approximately 53% of the total surface-bound aniline, the localized electronic environment of the aniline nitrogen remains largely preserved.11 In contrast, Species II (47%) represents nitrogen atoms whose local electronic environment is perturbed due to stronger interaction with surface sites. This preservation of the electronic environment around the N-atom of Species I enhances surface polarity, consistent with the strongly negative zeta potential of N-AC (−14 mV).
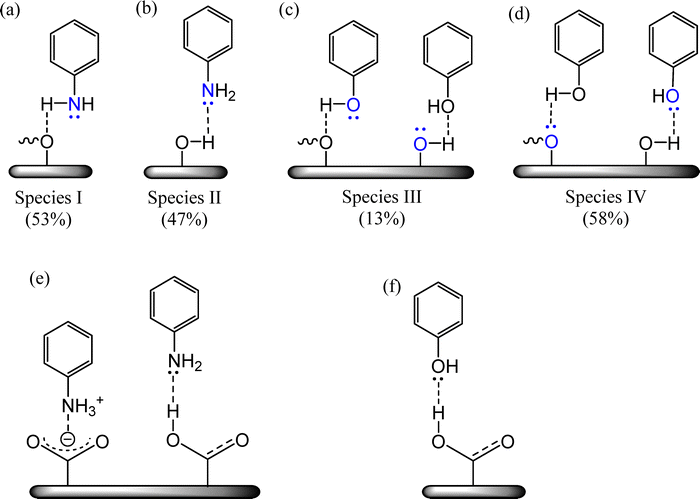 | ||
| Scheme 2 Proposed plausible surface interactions: (a) and (b) aniline-AC; (c) and (d) phenol-AC; (e) and (f) interaction of the surface –COOH group with aniline/phenol. | ||
The O 1s XPS spectra of AC exhibited three distinct peaks at 531.6 eV, 533.4 eV and 535.2 eV corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and –COOH bonds, respectively (Fig. 2e). Upon functionalization, the XPS peak associated with C–O bonds in the O 1s XPS spectra of N-AC undergoes splitting into two distinct components (Fig. 2f) at 533.3 eV and 532.0 eV that can be attributed to Species I and Species II (Scheme 2a and b). Likewise, the C–O feature in O-AC deconvolutes into two peaks at 531.6 and 533.1 eV (Fig. 2g), designated as Species III and Species IV, respectively (Scheme 2c and d). Species III, accounting for ∼13% of the total surface-bound phenol, corresponds to oxygen atoms that retain their native electronic environment, whereas Species IV (∼58%) represents oxygen centres that experience significant electronic perturbation due to strong surface interactions. This pronounced perturbation diminishes the overall surface polarity, consistent with the less negative zeta potential of O-AC (−1.89 mV). Additionally, the shifts observed in the C
O, C–O and –COOH bonds, respectively (Fig. 2e). Upon functionalization, the XPS peak associated with C–O bonds in the O 1s XPS spectra of N-AC undergoes splitting into two distinct components (Fig. 2f) at 533.3 eV and 532.0 eV that can be attributed to Species I and Species II (Scheme 2a and b). Likewise, the C–O feature in O-AC deconvolutes into two peaks at 531.6 and 533.1 eV (Fig. 2g), designated as Species III and Species IV, respectively (Scheme 2c and d). Species III, accounting for ∼13% of the total surface-bound phenol, corresponds to oxygen atoms that retain their native electronic environment, whereas Species IV (∼58%) represents oxygen centres that experience significant electronic perturbation due to strong surface interactions. This pronounced perturbation diminishes the overall surface polarity, consistent with the less negative zeta potential of O-AC (−1.89 mV). Additionally, the shifts observed in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH binding energies of N-AC and O-AC possibly result from the formation of hydrogen bonding or salt formation between aniline/phenol and the carbon support29 (Scheme 2e and f).
O and –COOH binding energies of N-AC and O-AC possibly result from the formation of hydrogen bonding or salt formation between aniline/phenol and the carbon support29 (Scheme 2e and f).
The 1H NMR spectrum of N-AC in DMSO-d6 (Fig. 3) exhibits signals for various protons (a′, b′, c′, and d′) of surface bound aniline,25 which are also confirmed by comparing with the 1H-NMR spectrum of free aniline. In the spectrum of N-AC, the signal a′ represents the surface-bound –NH2 protons, while the additional signal e′ corresponds to the protons of N–H present as [–(NH2)–H–O–CO–], consistent with the observed interaction between aniline and surface –COOH groups in the XPS analysis (Fig. 2f). The –NH2 proton signal (a′) is absent in the spectrum of aniline possibly due to rapid exchange of amino protons with the solvent.25 The signals m′, n′, o′, p′ in the 1H NMR spectrum of O-AC in DMSO-d6 represent the various protons of surface bound phenols, which are also confirmed by comparing with the 1H-NMR spectrum of free phenol (Fig. 3). Interestingly, the hydroxyl proton signal (m′) shows reduced intensity in the spectrum of O-AC, likely resulting from strong surface interactions that attenuate its NMR visibility.
 | ||
| Fig. 3 1H NMR spectra of aniline, N-AC, phenol, and O-AC [i: DMSO-d6 impurities; *: AC surface-functional-group protons]. | ||
3.2. Liquid phase adsorption and gas phase adsorption behaviour of N-AC and O-AC
The precise assessment of the adsorption efficiency of the as-synthesized N-AC and O-AC in the liquid phase has been carried out using aqueous MB as a model pollutant. N-AC and O-AC exhibited comparable MB adsorption efficiency (Fig. 4a) under identical conditions (100 ppm MB, 25 mL, 10 wt% adsorbent). The high MB adsorption capacity of O-AC, despite a ∼50% reduction in its surface area relative to N-AC, can be ascribed to the higher concentration of phenolic functionalities onto the activated carbon surface. The MB molecules are likely adsorbed through π–π stacking interactions and hydrogen bonding with surface functional groups on the as-synthesized N-AC and O-AC.28 Consequently, both materials achieve similar MB adsorption capacities. The comparable MB uptake observed for both O-AC and N-AC, despite their pronounced disparity in textural parameters, further indicates that the surface chemical characteristics play a dominant role in dictating the adsorption behaviour in the liquid phase. | ||
| Fig. 4 (a) Liquid-phase adsorption of MB (100 ppm, 25 mL, 10 wt% adsorbent) on AC, N-AC, and O-AC, and (b) CO2 and N2 adsorption isotherms of N-AC and O-AC at 298 K up to 1 bar. | ||
In contrast, N-AC exhibits a twofold enhancement in the CO2 adsorption capacity at 298 K and 1 bar compared to O-AC in the gas phase (Table 2). The superior adsorption performance of N-AC can be attributed to the electron-rich local environment generated by surface –NH2 groups, which enhances surface polarity and facilitates strong dipole–quadrupole interactions with CO2 molecules (quadrupole moment = 4.30 × 10−26 esu cm2).12 However, the electron-deficient local environment of surface –OH groups in O-AC significantly weakens adsorbate–adsorbent interactions and reduces CO2 affinity, resulting in diminished adsorption capacity. The adsorption performance of the as-synthesized materials in the gas phase thus appears to be governed primarily by the local electronic environment of the grafted aniline and/or phenolic moieties. Specifically, in N-AC, Species I dominate the surface electronic environment (Fig. 2d), where the –NH2 group retains its lone pair electrons that can delocalize into the aromatic framework, thereby increasing the surface electron density and strengthening quadrupolar coupling. This observation is consistent with the more negative zeta potential value of N-AC (–14.0 mV), indicating higher polarity and polarizability that favour physisorption. Conversely, in O-AC, the surface is enriched with Species IV (Fig. 2g), wherein the local electronic environment of –OH groups is perturbed. The low polarity of O-AC is reflected in its nearly neutral zeta potential (–1.89 mV), explaining the weakened quadrupole interactions. Therefore, this electronic disadvantage, low BET surface area and limited micropore volume of O-AC (Table 1) render it less suitable for gas-phase adsorption, whereas N-AC can be considered a promising candidate for dual-phase adsorption applications.
Overall, these results reveal a clear difference in the factors governing adsorption in the liquid and gas phases. In aqueous systems, adsorption mainly depends on the availability of π-domains and surface interaction sites such as π–π stacking and hydrogen bonding, which makes both N-AC and O-AC effective MB adsorbents. In contrast, CO2 adsorption in the gas phase is strongly influenced by surface polarity, electronic structure, and micropore accessibility. The nitrogen-enriched surface of N-AC therefore provides dual functionality: it supports π–π interactions for dye adsorption in the liquid phase and offers an electron-rich, polar surface that enhances quadrupolar interactions with CO2, leading to superior gas-phase performance.
3.3. Gas phase adsorption selectivity (CO2/N2: 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 85) and kinetics of N-AC and O-AC
85) and kinetics of N-AC and O-AC
CO2 and N2 adsorption isotherms (Fig. 4b) were employed to evaluate the CO2/N2 selectivity for N-AC and O-AC across 0–1 bar at 298 K, using Ideal Adsorbed Solution Theory (IAST) with a simulated flue-gas composition of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 CO2
85 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 (Fig. 5a). The single-component CO2 isotherms were independently fitted to the Freundlich model (Fig. 5b), from which saturation uptake and model parameters were extracted for subsequent selectivity calculations. The calculated values (Table 2) reveal significantly higher CO2/N2 selectivity in the low-pressure regime (0.01 bar), consistent with prior observations on heteroatom-doped carbons.4
N2 (Fig. 5a). The single-component CO2 isotherms were independently fitted to the Freundlich model (Fig. 5b), from which saturation uptake and model parameters were extracted for subsequent selectivity calculations. The calculated values (Table 2) reveal significantly higher CO2/N2 selectivity in the low-pressure regime (0.01 bar), consistent with prior observations on heteroatom-doped carbons.4
 | ||
Fig. 5 (a) CO2/N2 (0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85) selectivity of N-AC and O-AC, (b) Freundlich model fitted CO2 adsorption isotherm of N-AC and O-AC, (c) and (d) intraparticle diffusion kinetics of N-AC and O-AC. 0.85) selectivity of N-AC and O-AC, (b) Freundlich model fitted CO2 adsorption isotherm of N-AC and O-AC, (c) and (d) intraparticle diffusion kinetics of N-AC and O-AC. | ||
The Freundlich model fitting indicates adsorption on a heterogeneous surface comprising energetically distinct binding sites, consistent with the chemically diverse and electronically anisotropic nature of functionalized carbons. Such behaviour reflects non-ideal physisorption governed by multiple weak interactions, notably quadrupole–π and dipole–quadrupole coupling. The progressive uptake at higher pressures is also evident from the Type-I isotherm with its broad hysteresis loop in BET analysis (Fig. 1a), suggesting multilayer adsorption on defect-rich or high-energy domains. Collectively, these findings highlight the pivotal influence of surface heterogeneity, polarity, and pore architecture in dictating CO2 uptake, particularly under dilute gas conditions.
At low pressure (0.01 bar), N-AC demonstrates significantly higher CO2/N2 selectivity relative to O-AC, underscoring the decisive role of surface polarity and electronic structure in governing selective physisorption under dilute conditions. This enhancement arises from the predominant presence of Species I in N-AC, along with higher surface area and micropore volume of N-AC (Table 1), which provides a greater density of adsorption sites that are energetically favourable for CO2 but comparatively inaccessible to N2. However, at elevated pressures (approaching 1 bar), both N-AC and O-AC exhibit a measurable decline in CO2/N2 selectivity, due to saturation of high-affinity sites. Such pressure-dependent behaviour is characteristic of physisorption, wherein initial uptake is dominated by energetically favourable sites, followed by the engagement of less selective regions as pore-filling equilibrium is approached.4 To gain further insight into the adsorption dynamics, CO2 uptake kinetics were analysed using the pseudo first-order kinetic model (eqn (4)) and the Weber–Morris intraparticle diffusion model (eqn (5)).
The pseudo first-order kinetic model is given by:
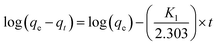 | (4) |
The Weber and Morris intraparticle diffusion model is given by:
| qt = KiDt0.5 + C | (5) |
The pseudo first-order model was evaluated by plotting ln(qe–qt) versus time (t) to obtain the rate constant kl (Fig. S2, SI), and the Weber–Morris model was assessed by plotting qt against t0.5. The experimental data showed that the Weber–Morris intraparticle diffusion model provides a better fit, and the fitting parameters are summarized in Table S1, SI. Under the studied conditions, intraparticle diffusion is the dominant rate-controlling step (Fig. 5c and d). In the Weber–Morris framework, a linear plot through the origin signifies exclusive intraparticle diffusion control; however, the nonzero intercepts observed here reveal that adsorption proceeds via multiple sequential mechanisms. The initial rapid linear region corresponds to surface adsorption at external active sites, followed by a second regime dominated by intraparticle diffusion into internal micropores. A final plateau marks the equilibrium stage, where uptake slows as saturation is approached. Importantly, N-AC displayed a 52.2% higher CO2 diffusion rate than O-AC, consistent with its larger micropore volume and improved pore accessibility (Table 1), which together facilitate more efficient gas transport through the framework.
4. Conclusions
Nitrogen- and oxygen-functionalized activated carbons (N-AC and O-AC) were synthesized through a mild aqueous anchoring strategy that preserved the textural properties of the parent carbon while inducing distinct electronic environments. Both the materials exhibited complete methylene blue adsorption (liquid phase) under identical conditions. However, N-AC demonstrated 2-fold enhanced CO2 uptake at 298 K and 1 bar, as compared to O-AC. In the liquid phase, dye adsorption was primarily governed by π–π stacking and hydrogen bonding rather than electronic factors, leading to comparable uptake for both materials. In N-AC, the –NH2 groups retained their native electronic configuration, producing a polar, electron-rich surface that favoured strong dipole–quadrupole interactions with CO2. Conversely, phenol anchoring in O-AC perturbed the –OH electronic environment, lowering surface polarity and weakening CO2 affinity. These results demonstrate that while liquid-phase adsorption is dominated by surface interactions, gas-phase performance critically depends on the retention or perturbation of the electronic environment of grafted guest molecules. The developed understanding on the functionalized adsorbent designing, could be useful for developing multifunctional carbon sorbents.Conflicts of interest
There are no conflicts of interest between the authors to declare.Data availability
The data analysis scripts of this article are available in the GitHub repository: https://github.com/sagnikm9-lab/Carbon_Paper_Sagnik_26082025.The data supporting this article have been included as part of the supplementary information (SI), which includes TGA profiles, kinetic fitting parameters. See DOI: https://doi.org/10.1039/d5nj03430b.
Acknowledgements
The authors acknowledge CSIR for financial support under project MMP-035201 and thank the Centralized Instrumentation Facility, CSMCRI Bhavnagar, for analytical support. Rahulbhai Parmar is thankful to UGC JRF for financial assistance. Appreciation is also extended to the Director, CSIR-CSMCRI, for continuous encouragement.References
- M. Roy and R. Saha, Intelligent Environmental Data Monitoring for Pollution Management, 2021, pp. 127–160 Search PubMed.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70–80 CrossRef CAS.
- J. Shao, Y. Wang, M. Che, Q. Xiao, M. Demir, M. K. Al Mesfer, L. Wang, X. Hu and Y. Liu, J. Energy Inst., 2025, 123, 102273 CrossRef CAS.
- G. Sethia and A. Sayari, Carbon, 2015, 93, 68–80 CrossRef CAS.
- J. Wang, Y. Yin, X. Liu, Y. Liu, Q. Xiao, L. Zhao, M. Demir, M. Ö. Alaş Çolak, L. Wang and X. Hu, Sep. Purif. Technol., 2025, 376, 134079 CrossRef CAS.
- J. Wang, Y. Wang, X. Liu, Q. Xiao, M. Demir, M. K. Almesfer, S. G. Colak, L. Wang, X. Hu and Y. Liu, Molecules, 2025, 30, 2564 CrossRef CAS.
- M. Pasha, H. Zhang, M. Shang, G. Li and Y. Su, Process Saf. Environ. Prot., 2022, 159, 106–119 CrossRef CAS.
- H. Kulkarni, C. Bari, S. Mukherjee, P. Gajera and G. Sethia, Carbon Trends, 2024, 17, 100398 CrossRef CAS.
- K. Y. Hor, J. M. C. Chee, M. N. Chong, B. Jin, C. Saint, P. E. Poh and R. Aryal, J. Cleaner Prod., 2016, 118, 197–209 CrossRef CAS.
- P. V. Messina and P. C. Schulz, J. Colloid Interface Sci., 2006, 299, 305–320 CrossRef CAS PubMed.
- S. Patel, S. Mukherjee, M. Neem, S. Neogi, K. L. Ameta, A. Mishra, M. P. Deshpande and M. K. Mishra, Colloids Surf., C, 2024, 2, 100047 Search PubMed.
- S. Mukherjee, S. Parmar, M. Neem, N. Chikhaliya, S. Neogi, K. L. Ameta and M. K. Mishra, ACS Appl. Nano Mater., 2025, 8, 15208–15222 CrossRef CAS.
- N. Mokhtari, M. Afshari and M. Dinari, Polymer, 2020, 195, 122430 CrossRef CAS.
- M. S. Shafeeyan, W. M. A. W. Daud, A. Houshmand and A. Shamiri, J. Anal. Appl. Pyrolysis, 2010, 89, 143–151 CrossRef CAS.
- M. Raninga, A. Mudgal, V. K. Patel, J. Patel and M. Kumar Sinha, Mater. Today Proc., 2023, 77, 286–294 CrossRef CAS.
- C. Y. Yin, M. K. Aroua and W. M. A. W. Daud, Sep. Purif. Technol., 2007, 52, 403–415 CrossRef CAS.
- R. T. Yang, Adsorbents: Fundamentals And Applications, John Wiley & Sons Inc., Hoboken, New Jersey, 2003 Search PubMed.
- A. Ali Abd, M. Roslee Othman and J. Kim, Environ. Sci. Pollut. Res., 2021, 28, 43329–43364 CrossRef.
- C. Y. Yin, M. K. Aroua and W. M. A. W. Daud, Sep. Purif. Technol., 2007, 52, 403–415 CrossRef CAS.
- M. S. Shafeeyan, W. M. A. W. Daud, A. Houshmand and A. Shamiri, J. Anal. Appl. Pyrolysis, 2010, 89, 143–151 CrossRef CAS.
- A. Ali Abd, M. Roslee Othman and J. Kim, Environ. Sci. Pollut. Res., 2021, 28, 43329–43364 CrossRef PubMed.
- H. Zentou, B. Hoque, M. A. Abdalla, A. F. Saber, O. Y. Abdelaziz, M. Aliyu, A. M. Alkhedhair, A. J. Alabduly and M. M. Abdelnaby, Carbon Capture Sci. Technol., 2025, 15, 100386 CAS.
- A. Abu-Nada, A. Abdala and G. McKay, J. Environ. Chem. Eng., 2021, 9, 105858 CrossRef CAS.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 RSC.
- S. Mukherjee, M. Singh, A. Ravani, A. Parekh, A. Shukla, S. Chaki, S. Neogi and M. K. Mishra, Microporous Mesoporous Mater., 2023, 359, 112636 CrossRef CAS.
- N. M. Keppetipola, M. Dissanayake, P. Dissanayake, B. Karunarathne, M. A. Dourges, D. Talaga, L. Servant, C. Olivier, T. Toupance, S. Uchida, K. Tennakone, G. R. A. Kumara and L. Cojocaru, RSC Adv., 2021, 11, 2854–2865 RSC.
- M. Raveendra, M. Chandrasekhar, K. Chandrasekhar Reddy, A. Venkatesulu, K. Sivakumar and K. Dayananda Reddy, Fluid Phase Equilib., 2018, 462, 85–99 CrossRef CAS.
- S. H. Paiman, M. A. Rahman, T. Uchikoshi, N. Abdullah, M. H. D. Othman, J. Jaafar, K. H. Abas and A. F. Ismail, J. Saudi Chem. Soc., 2020, 24, 896–905 CrossRef CAS.
- S. V. Fedorov, A. Y. Rulev, N. N. Chipanina, L. V. Sherstyannikova and V. K. Turchaninov, J. Mol. Struct., 2004, 691, 51–57 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |


