 Open Access Article
Open Access ArticleAntibacterial properties and underlying mechanisms of Mo2TiC2Tx and Mo2Ti2C3Tx MXenes targeting Escherichia coli (Gram-negative bacterium)
Mohsen
Pilevar
a,
Mostafa
Dadashi Firouzjaei
 *a,
Anupma
Thakur
*a,
Anupma
Thakur
 b,
B. S.
Nithin Chandran
b,
Sara
Wahib
c,
Delanie
Williams
a,
Hesam
Jafarian
a,
Carolina
Bryant
d,
Annabelle
Bedford
b,
B. S.
Nithin Chandran
b,
Sara
Wahib
c,
Delanie
Williams
a,
Hesam
Jafarian
a,
Carolina
Bryant
d,
Annabelle
Bedford
 b,
Adriana
Riveros
a,
Qiaoli
Liang
b,
Adriana
Riveros
a,
Qiaoli
Liang
 e,
Khaled A.
Mahmoud
c,
Mark
Elliott
e,
Khaled A.
Mahmoud
c,
Mark
Elliott
 *a and
Babak
Anasori
*bf
*a and
Babak
Anasori
*bf
aDepartment of Civil, Construction, and Environmental Engineering, University of Alabama, Tuscaloosa, AL 35487, USA. E-mail: mdfirouzjaei@ua.edu; melliott@eng.ua.edu
bSchool of Materials Engineering, Purdue University, West Lafayette, 47907 IN, USA. E-mail: banasori@purdue.edu
cQatar Environment and Energy Research Institute (QEERI), Hamad Bin Khalifa University, Qatar Foundation, P.O. Box 34110, Doha, Qatar
dDepartment of Chemical and Biological Engineering, University of Alabama, Tuscaloosa, AL 35487, USA
eDepartment of Chemistry and Biochemistry, University of Alabama, Tuscaloosa, AL 35487, USA
fSchool of Mechanical Engineering, Purdue University, West Lafayette, 47907 IN, USA
First published on 6th October 2025
Abstract
The widespread use of antibiotics has led to an increased number of antimicrobial-resistant (AMR) pathogens, highlighting the need for novel antibacterial nanomaterials with chemical and structural tunability. Here, we present the antibacterial properties/pathways of two molybdenum-based double transition metal (DTM) MXenes (Mo2TiC2Tx and Mo2Ti2C3Tx) and compare them with Ti3C2Tx MXene. We demonstrate that the antibacterial effectiveness of these MXenes is concentration- and time-dependent, with prolonged exposure time being more influential at lower concentration levels (<25 μg mL−1). Physical damage to E. coli cell walls by MXene nanoknives (sharp edges of MXene flakes), and disruption in metabolic functions through oxidative stress were key antibacterial pathways for Mo2TiC2Tx, Mo2Ti2C3Tx, and Ti3C2Tx MXenes. A 1 h sonication of MXene solutions reduced their flake sizes (average lateral size of 234 ± 163 nm) and led to substantial improvement of their antibacterial performance by bolstering the availability of nanoknives for physical damage to bacterial cells. However, prolonged sonication (2 h) resulted in reduced antibacterial effectiveness, potentially due to morphological defects of MXene flakes. We also studied the metal ion release and disc inhibition zone, which revealed no direct correlation between the MXenes’ antibacterial properties and the leaching of ions or fragments. This study demonstrates the potential for improving the antibacterial effectiveness of molybdenum-containing DTM MXenes by controlling their chemical and structural characteristics.
New conceptsThis study presents the first comprehensive investigation of molybdenum-containing double-transition metal (DTM) MXenes—Mo2TiC2Tx and Mo2Ti2C3Tx—as antibacterial agents. While previous reports primarily focused on mono-transition metal MXenes (e.g., Ti3C2Tx), this work reveals new structure–function relationships governing the antibacterial efficacy of Mo-based DTM MXenes. We demonstrate that nanoknife-enabled physical disruption of bacterial membranes is the dominant antibacterial mechanism, and that performance is significantly enhanced by flake size reduction through optimized sonication. Importantly, we decouple the contributions of oxidative stress and metal ion release, finding them to be negligible compared to physical interactions. These insights provide a critical step forward in understanding how compositional and morphological parameters influence the biocidal behavior of 2D materials, enabling the rational design of next-generation MXene-based antibacterial coatings and therapeutic platforms. |
Introduction
Antibiotics are widely used for treating microbial infectious diseases.1 However, pathogens are increasingly showing resistance to the most commonly used antibiotics due to the rise of drug-resistant microorganisms.2 It's projected that by 2050, antimicrobial resistance (AMR) will lead to 10 million fatalities annually, with associated costs of approximately $100 trillion.3 Hence, there is an urgent need for new approaches to combat AMR pathogens.Nanomaterial-based therapeutics have gained a lot of interest as they can be inherently biocidal, serve as carriers for antibacterial agents, or offer both functionalities.4 They can be utilized to overcome such infections by hindering the development of AMR bacteria,5,6 simultaneous delivery of multiple antimicrobial agents,7 and precise delivery of antimicrobial drugs to the infection site.8 The physicochemical properties (e.g., surface chemistry, particle/flake size, and morphology) of nanostructured materials can also influence their interactions with bacterial cells and consequently their antibacterial properties.9,10 Hence, utilizing nanostructured materials with tunable structure-composition properties could further bolster their effectiveness in therapeutic applications. In this regard, MXenes, an emerging class of two-dimensional (2D) materials, are an attractive choice for therapeutic and biomedical applications due to their structural tunability and functionality.11–13
MXenes can include one or more types of 3d–5d block transition metals of groups 3–6 of the periodic table interleaved with carbon and/or nitrogen.14 The antibacterial properties of mono-transition metal (mono-M) MXenes, 2D metal carbides/nitrides with only one type of transition metal (e.g., Ti3C2Tx), have been studied in recent years.15–18 Direct physical damage to bacterial cell walls by sharp edges of MXene flakes, termed “nanoknives”, and disruption in bacterial metabolic functions through oxidative stress have been reported as their primary antibacterial mode-of-action.15 The antibacterial effectiveness of mono-M MXenes (e.g., Ti3C2Tx, Nb2CTx) can also be affected by their structural and morphological characteristics, dispersibility, and surface charges.16,18,19 To the best of our knowledge, there has been only one study on the antibacterial properties of double-transition metal (DTM) MXenes (with two different transition metals in their structure), specifically those containing Ti and V in their structure (i.e., TiVCTx).20 However, there is no study on the antibacterial properties of DTM MXenes that contain molybdenum in their structures. Mo-containing DTM MXenes are of special interest because Mo ions, when integrated into nanostructured materials (e.g., MoS2, MoOx), can induce antibacterial properties by promoting oxidative stress and disrupting the metabolic functions of bacterial cells.21,22 Moreover, the integration of Mo ions in antibacterial pharmaceuticals can improve their stability while reducing the mammalian cytotoxicity levels associated with the antibacterial metal nanoparticles.23,24
In this study, the antibacterial properties of two Mo-containing DTM MXenes, namely Mo2TiC2Tx and Mo2Ti2C3Tx, were investigated and compared with Ti3C2Tx. Various antibacterial tests and characterizations were utilized to: (1) quantify and compare the antibacterial performance of Mo-containing DTM (Mo2TiC2Tx and Mo2Ti2C3Tx) with mono-M (Ti3C2Tx) MXenes, (2) investigate the impact of MXenes’ structural and morphological characteristics on their antibacterial effectiveness, and (3) elucidate the potential antibacterial modes-of-action for Mo2TiC2Tx and Mo2Ti2C3Tx MXenes. Escherichia coli (E. coli) was used as a representative Gram-negative bacterium due to its well-characterized physiology and extensive use as a model organism in microbiological and antibacterial studies.
Results and discussion
MXene synthesis and characterization
The unique crystal structure of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes (Fig. 1a) can influence their physicochemical properties, including their antibacterial effectiveness. The XRD patterns for Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes and their corresponding MAX phases are shown in Fig. 1b. The MAX phase peaks25,26 disappeared upon synthesis of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXene films. Since these MXene films were made by filtration of their delaminated single-flake nanosheets colloidal suspension (and not multilayer powders), only the 00L peaks are detected in the XRD patterns. We used delaminated single-flake nanosheets in our study to maximize the benefit from the large surface area of MXene flakes and their interactions with bacteria. The exact peak location and their corresponding peaks, along with the d-spacing calculated from the 00L peak position of the MAX and MXene XRD patterns, are provided in Table S1. Notably, the illustrated broadening and downshift in MAX phases’ peaks indicate an increase in their d-spacing, due to the removal of the A-layer and the presence of intercalated water or cations between the MXene sheets.25 Furthermore, the scanning electron microscopy (SEM) images demonstrate the bulk morphology of MAX phases (Fig. 1c), and the 2D structure of delaminated flakes of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes (Fig. 1d). The energy-dispersive X-ray (EDX) mapping analyses of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes further confirmed the elemental composition and uniform distribution of elements in the synthesized MXenes (Fig. S1 and Table S2). After confirming the successful synthesis of delaminated MXenes, we used them for our antibacterial experiments.Antibacterial performance assessment via growth-inhibition tests
A series of concentration- and time-dependent growth-inhibition tests were conducted to investigate the antibacterial efficacy of mono-M (i.e., Ti3C2Tx) and Mo-containing DTM MXenes (i.e., Mo2TiC2Tx and Mo2Ti2C3Tx) against E. coli as a Gram-negative bacterium (Materials and methods in SI). Growth-inhibition tests were initially carried out using phosphate-buffered saline (PBS) as the suspension medium due to its suitability for the preservation and growth of bacterial cells.27 The colloidal MXene suspensions exhibited minimal antibacterial efficacy (inhibition efficiency (IE) < 5%) even at the concentration of 100 μg mL−1 (Fig. S2). The visual appearance of colloidal suspensions hinted at potential MXene agglomeration inside the PBS solution, likely due to electrostatic and van der Waals interactions between MXenes’ functional groups and dissociated ions in the buffer solution, as reported in similar antibacterial studies of 2D materials.28,29 Therefore, modified growth-inhibition experiments were designed by replacing PBS with deionized (DI) water to promote uniform dispersion of MXene flakes. The hydrophilic nature of detected (Fig. S3) terminal groups (–F, Ti–O, and Mo–O) in Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes can promote the dispersibility of their flakes in DI water.30The modified growth-inhibition tests were carried out at different concentrations (5, 10, 25, 50, and 100 μg mL−1) of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes. All samples showed under 35% inhibition efficiency (IE) at the concentration of 5 μg mL−1 (Fig. S4). Increasing the MXene concentration to 10 μg mL−1 (Fig. 2a) resulted in <15% changes in IEs even after 2 h of contact time. However, extending the exposure time to 3 h led to a major improvement of IEs, at 75.3%, 65.4%, and 61.5% for Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes, respectively. Additionally, increasing the concentration of MXenes (≥25 μg mL−1) greatly enhanced their antibacterial performance while requiring shorter exposure times (Fig. 2b–d). For instance, at 25 μg mL−1 (Fig. 2b) and only after 1 h of exposure time, Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes obtained IEs of 92.2%, 89%, and 86.2%, respectively. By increasing the concentration to 100 μg mL−1 of MXene, Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx obtained IEs of 76.7%, 83.9%, and 80.9%, respectively, immediately upon exposure to the E. coli cells (Fig. 2d). The growth-inhibition results indicated that both concentration and exposure time of MXenes substantially affect the antibacterial effectiveness of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes. Notably, prolonged exposure time was more effective at lower MXene concentrations (<25 μg mL−1). The low IEs observed for the tested MXenes at low concentrations (<25 μg mL−1) may be attributed to electrostatic repulsion between the negatively charged surfaces of both E. coli cells and MXene flakes.18 The zeta potential measurements of Ti3C2Tx (−43.3 mV), Mo2TiC2Tx (−30.0 mV), and Mo2Ti2C3Tx (−31.0 mV) (Fig. S5) confirmed the negatively charged surface of these MXene nanosheets, further supporting the proposed mechanism. Table S3 presents our statistical analysis of the modified growth-inhibition data of MXene concentration, exposure time, and their interaction term on their overall antibacterial efficacy. The analyses also showed that the overall antibacterial performance of the three studied MXenes was comparable and not significantly different. We next investigated various antibacterial pathways to elucidate the findings from the modified growth-inhibition results.
Effects of sonication on antibacterial performance of MXenes
To investigate the nanoknives’ effects on MXenes’ antibacterial properties, we probe sonicated the MXene solutions for 1 h as a pretreatment step, to reduce their flake sizes and increase the exposed MXene edges. It is known that subjecting MXenes colloidal suspension to sonication reduces the lateral sizes while inducing minimal chemical alterations.31 After the 1-h sonication step, we conducted a time-dependent growth-inhibition test at fixed MXene concentrations of 10 μg mL−1 (Materials and methods in SI). The antibacterial performance of sonicated MXenes (Fig. 3a) was substantially improved compared to untreated samples (Fig. 2a). Notably, sonicated Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes achieved IEs above 98% (Fig. 3a) after 2 h of exposure time, whereas the untreated samples (Fig. 2a) only achieved IEs of 31.8%, 16.9%, and 13.9%, respectively. We measured the average MXene flake lateral sizes before and after 1 h sonication (Fig. 3b and Fig. S6 and S7). Before sonication, the average lateral flake size of untreated Ti3C2Tx was measured to be 2823 ± 287 nm, while 631 ± 280 and 895 ± 400 nm were obtained for Mo2TiC2Tx and Mo2Ti2C3Tx, respectively. After 1 h of sonication, flake sizes were reduced across all samples with Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes, obtaining an average flake size of 231 ± 142, 229 ± 183, and 242 ± 165 nm, respectively. The substantial size reduction in Ti3C2Tx flakes can be attributed to their initial large lateral sizes compared to Mo2TiC2Tx and Mo2Ti2C3Tx MXenes. It has been shown that MXenes with lateral sizes of a few μm are more susceptible to mechanical fragmentation under sonication31 compared to submicron flake sizes. The results suggest that the enhanced antibacterial performance of sonicated MXenes can be attributed to the reduced lateral sizes of their flakes, as more contact sites (edges) were available for direct physical damage to the bacterial cell walls.16The mass density of the studied MXenes is another critical factor influencing the availability and effectiveness of their flakes. To better assess this, the theoretical densities of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes were calculated alongside the estimated number of available flakes for each MXene at the same concentration of 10 μg mL−1 (after 1 h of sonication). Because of the lower theoretical density of Ti3C2Tx (ranging from 4.2 to 4.5 g cm−3), there are more Ti3C2Tx MXene flakes compared to the heavier M-containing MXenes, Mo2TiC2Tx, and Mo2Ti2C3Tx with densities in the range of 6.1 to 6.8 g cm−3 (Table S4). For example, in 1 mL of a MXene solution at 10 μg mL−1, we estimated 1.5 to 2 times more Ti3C2Tx MXene flakes (∼4.79 ± 0.14) × 1010 flakes per mL compared to Mo2TiC2Tx and Mo2Ti2C3Tx with (3.19 ± 0.06) × 1010 and (2.3 ± 0.04) × 1010 flakes per mL, respectively. This agrees with the higher IE (60.8%) of the sonicated Ti3C2Tx MXene after 1 h of exposure time compared to Mo2TiC2Tx and Mo2Ti2C3Tx MXenes. It also agrees with the slightly lower IEs for Mo2Ti2C3Tx compared to Mo2TiC2Tx. The relatively thicker Mo2Ti2C3Tx MXene (1.2 nm for Mo2Ti2C3Txvs. 0.9 nm for Ti3C2Tx and Mo2TiC2Tx) with the additional atomic layer in its flake structure can limit the availability of Mo2Ti2C3Tx nanoknives for physical contact with bacterial cells. Therefore, these findings suggest physical cell damage by “nanoknives” as one of the primary antibacterial mode-of-action for Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes, causing structural compromise and cytoplasmic leakage, which eventually leads to cell death.
The size-dependent antibacterial efficacy of Ti3C2Tx MXenes was further explored by extending the sonication duration to 2 h, followed by a time-dependent growth-inhibition test. After 2 h of exposure to E. coli cells (Fig. S7a), Ti3C2Tx MXenes showed a lower IE (17.4%) compared to 1-h sonicated samples (with an IE of 98.6%). The reduced antibacterial effectiveness of Ti3C2Tx MXenes by extending the sonication treatment (from 1 h to 2 h) suggests that there may be optimal morphological characteristics necessary for effective penetration of the cell membrane by MXene flakes. Longer sonication (2 h of probe sonication) could cause damage to MXene flakes and could even lead to localized oxidation,31–33 although our scanning electron microscopy (SEM) images (Fig. S7b) and transmission electron microscopy (see next section) did not show any evidence of oxidation or detectable damage. Additionally, prolonged probe sonication of MXenes has also been reported to turn MXene flakes into quantum dots (<10 nm) with rounded edges. These potential damages and sharp edge reduction could diminish the availability and effectiveness of nanoknives.34–36 Overall, the lower bactericidal effectiveness of MXenes suggests the flake nanoknives may be compromised by 2 h probe sonication.
The morphologies of both viable and damaged E. coli cells were also investigated via SEM imaging after collecting samples from growth-inhibition tests (Materials and methods in SI). Fig. 3c demonstrates E. coli cells after 3 h of incubation in the absence of colloidal Mo2TiC2Tx MXenes. The intact cellular cytoplasmic structure of the E. coli cells is indicative of healthy and viable conditions. Fig. 3d and e illustrate damaged E. coli cells alongside the sharp edges of Mo2TiC2Tx MXene flakes. The distinct morphological alteration in the cells can infer partial rupture of the cell wall, leading to the outflow of cell contents and the ultimate lysis of bacterial cells.16 Additional morphological characterizations of damaged E. coli cells, after exposure to Ti3C2Tx and Mo2Ti2C3Tx MXenes, are provided in Fig. S8.
Effects of oxidative stress induced by MXenes
To further investigate the antibacterial effects of untreated and sonicated Mo2TiC2Tx and Mo2Ti2C3Tx MXenes beyond membrane disruption, it is crucial to consider additional mechanisms like oxidative stress. Glutathione (GSH), a tripeptide containing a thiol group, acts as a key cellular antioxidant in bacteria, helping to maintain intracellular oxidative balance and protect cells from external electrophilic compounds.17 The oxidation of GSH, commonly used to indicate oxidative stress from nanomaterials, including MXenes, was measured here using Ellman's assay with untreated and sonicated Mo2TiC2Tx and Mo2Ti2C3Tx across concentrations of 10–100 μg mL−1 with incubation times of 2 and 4 hours (Materials and methods in SI). Oxidative stress mechanisms generally include ROS-mediated pathways, where reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and alkyl hydroperoxides induce cellular damage, and ROS-independent pathways, where cellular structures are directly oxidized, as seen in graphene toxicity studies.17After 2 h of incubation with Mo2TiC2Tx MXene, the GSH oxidation levels were relatively close (glutathione loss of ∼50 ± 3%) across different concentrations with or without the sonication treatment (Fig. 4a). However, after 4 h of incubation, a notable increase in GSH oxidation level was recorded for the 1 h sonicated Mo2TiC2Tx samples with the highest GSH loss (74.8%) achieved by 100 μg mL−1 MXenes (Fig. 4b). Additionally, extended sonication treatments (i.e., 2 h sonication time) resulted in minimal changes in the GSH oxidation levels (glutathione loss of ∼50 ± 4%) compared to untreated Mo2TiC2Tx MXenes across all conditions (Fig. 4b). This suggests that while sonication initially enhances GSH oxidation by Mo2TiC2Tx MXene, prolonged sonication may lead to stabilization or reduction in oxidation rates over time.
After 2 h of incubation with Mo2Ti2C3Tx MXene, the GSH oxidation levels showed minimal improvement across all concentrations and sonication conditions, with the highest GSH loss (37.8%) obtained by the 1 h sonicated samples at a concentration of 100 μg mL−1 (Fig. 4c). However, after 4 h of incubation, an increase in GSH oxidation (glutathione loss of 44.5%) was observed by 100 μg mL−1 of the 2 h sonicated Mo2Ti2C3Tx MXenes (Fig. 4d). This suggests that prolonged sonication, when coupled with extended incubation time, enhances the oxidative stress induced by Mo2Ti2C3Tx MXenes.
The GSH oxidation results highlight the importance of concentration, incubation time, and sonication duration on the oxidative stress induced by Mo2TiC2Tx and Mo2Ti2C3Tx MXenes. While higher MXene concentrations and extended incubation times resulted in slight to moderate improvements in their GSH oxidation levels, the impact of the sonication duration was not as straightforward. Notably, the highest GSH losses (%) were obtained using different sonication durations for each MXene type, namely 1 h sonication for Mo2TiC2Tx and 2 h sonication for Mo2Ti2C3Tx MXenes. The statistical significance of the variables (i.e., MXene concentration, exposure time, and sonication duration) on the GSH oxidation results was further investigated via a general linear model (Tables S5 and S6). Additionally, sonication treatment has been reported to improve the oxidative stress of Nb-containing mono-M MXenes (Nb2CTx and Nb4C3Tx) by increasing the interlayer spacing between their flakes.16 However, TEM imaging of untreated (Fig. 4e), 1 h sonicated (Fig. 4f), and 2 h sonicated (Fig. 4g) Mo2TiC2Tx MXenes showed no difference between their interlayer spacing, suggesting that the distance between flakes is not directly related to the oxidative stress posed by Mo2TiC2Tx MXenes. Similar to Mo2TiC2Tx MXene, the sonication treatment did not affect the interlayer spacing between Mo2Ti2C3Tx flakes (Fig. 4h–j). Overall, it can be inferred from Fig. 2 and 4a–d that oxidative stress, induced by Mo2TiC2Tx and Mo2Ti2C3Tx MXenes, had minimal contribution to their bactericidal properties. Additionally, the minimal differences in GSH oxidation levels between untreated and sonicated samples suggest that potential surface chemistry alterations, such as nano-titania (TiO2) formation, had limited impacts on the antibacterial properties of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes under normal light conditions.37
Metal ion release and disc inhibition assessment
The cumulative concentrations of the released metal ions (Ti and/or Mo) from untreated and 1 h sonicated Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx were measured to investigate their potential relationship with the antibacterial properties of the mentioned MXenes (Materials and methods in SI). For untreated Mo2TiC2Tx and Mo2Ti2C3Tx MXenes, the majority of Mo ions were released within the first hour of suspension, reaching concentrations of 3.64 and 0.92 μg mL−1, respectively (Fig. 5a). Minimal variation in the cumulative concentrations was recorded afterward (suspension time >1 h), with measured Mo concentrations of 3.87 and 1.01 μg mL−1 after 3 h of suspension for Mo2TiC2Tx and Mo2Ti2C3Tx, respectively. The 1-h sonication treatment resulted in increased concentrations of the released Mo ions from both Mo2TiC2Tx and Mo2Ti2C3Tx MXenes, with the highest cumulative concentrations reaching 5.71 μg mL−1 and 3.58 μg mL−1, respectively (Fig. 5b). As previously shown in Fig. 2a, prolonging the exposure times from 1 h to 3 h resulted in 42.6% and 37.59% improvement in IEs obtained by untreated Mo2TiC2Tx and Mo2Ti2C3Tx MXenes, respectively. Similarly, we recorded 46.8% and 71.7% improvement in IEs for the sonicated samples when the exposure time was extended from 1 h to 2 h (Fig. 3a). The notable improvement in their antibacterial performance did not correlate with the observed trends of the cumulative concentrations of Mo ions, released from Mo2TiC2Tx and Mo2Ti2C3Tx suspensions (Fig. 5a and b). Hence, it can be inferred that the released Mo ions were unlikely to play a crucial role in the overall antibacterial effectiveness of Mo2TiC2Tx and Mo2Ti2C3Tx MXenes.Similar to Mo ions, no meaningful correlation was observed between the cumulative concentration of released Ti ions and the antibacterial efficacy of untreated and sonicated Mo2TiC2Tx and Mo2Ti2C3Tx MXenes (Fig. S9). In addition, an analogous trend was observed in the concentration of released Ti ions from untreated Ti3C2Tx MXene, negating any potential correlation with previously reported IEs (Fig. 2a). The 1-h sonication treatment facilitated the release of Ti ions from Ti3C2Tx, resulting in a high concentration of 5.73 μg mL−1 measured after 1 h of suspension. This might suggest a potential relationship between released Ti ions and the high IE (61.0%) obtained by sonicated Ti3C2Tx MXene after 1 h of exposure time (Fig. 3a). However, minimal changes in released Ti ions were observed thereafter (suspension time >1 h), while the IE increased from 61.0% to 99.0% by extending the exposure time from 1 h to 2 h (Fig. 3a). Hence, it can be inferred that released Ti ions were not significantly influential in the overall antibacterial efficacy of Ti3C2Tx MXene. This was consistent with the reported bioinert properties of Ti ions and nanoparticles like nano-titania (TiO2).38,39 A comparative illustration of the cumulative concentrations of released metal ions and antibacterial performance of untreated and 1 h sonicated MXenes is further depicted in Fig. S10.
The antibacterial mode-of-action of mono-M (Ti3C2Tx) and Mo-containing DTM MXenes (Mo2TiC2Tx, and Mo2Ti2C3Tx) was further investigated via disc inhibition zone tests under a static mode (Materials and methods in SI). No apparent inhibitory zone was formed around the membrane coupons, coated with colloidal suspensions of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes (Fig. 5c). While the absence of an inhibition zone does not necessarily negate the antibacterial properties of tested MXenes, it highlights the structural stability of MXene sheets with minimal diffusion of potential antibacterial agents (e.g., leaching metal ions) under static conditions. In addition, the absence of inhibitory zones infers a substantial reduction in the antibacterial effectiveness of the tested MXenes, which can be due to the restricted effects of MXene nanoknives under static mode.
Conclusion
In summary, we showed that the antibacterial effectiveness of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes against E. coli depends on both concentration and duration of exposure, with longer exposure times being particularly influential at lower concentration levels (<25 μg mL−1). Notably, at 25 μg mL−1 and 1 h of exposure, all tested MXenes exhibited IE > 85%, identifying this as the lowest concentration that effectively inhibited bacterial growth across all samples. Further, ultrasonic-assisted experiments revealed that MXenes’ antibacterial mode-of-action is highly dependent on the size and morphology of the flakes (i.e., nanoknives), and by reducing the flake sizes (with an optimum sonication duration), their efficacy increases due to more exposed flake edges. Additional tests (GSH loss, ion release, and disc inhibition zone tests) elucidated that oxidative stress can minimally contribute to their antibacterial efficacy, while no conclusive relationship was observed between the release of antibacterial agents (e.g., leaching metal ions) from MXenes’ structure and their antibacterial properties.We summarized our proposed antibacterial pathways for Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx in Fig. 5d, with nanoknives and oxidative stress as their most likely antibacterial modes-of-action. The nanoknives played an important role in the overall antibacterial effectiveness of MXenes, with their impact highly affected by their size and morphology. Notably, a 1-h sonication treatment of all tested MXenes resulted in flakes with reduced sizes (234 ± 163 nm), leading to substantially improved antibacterial efficacy due to the enhanced availability of nanoknives. However, prolonged sonication treatments (2 h) substantially reduced their antibacterial effectiveness, further highlighting the importance of nanoknives' morphological characteristics for effective physical damage to bacterial cells. The exact relationship between the structural and morphological characteristics of nanoknives and their biocidal properties can be further studied for different classes of MXenes and other 2D materials. Also, given the high release concentration of Ti ions from MXenes and their bioinert properties, future studies can focus on the potential of MXenes for precise drug delivery to infection sites.
Experimental section
Materials
Trypticase soy broth (TSB), agar, phosphate buffer saline (PBS), and Escherichia coli (E. coli, ATCC 35695) were used in the antibacterial experiments. Glutaraldehyde (C5H8O2 >99%), osmium tetroxide (OsO4 >99%), Na2HPO4·7H2O, NaH2PO4·H2O, and ethanol (>99%) were purchased from Sigma-Aldrich and used for the preparation of cell-MXenes samples prior to scanning electron microscopy (SEM) imaging. 0.02-micron aluminum oxide membranes were purchased from Sterlitech and used as the substrate for the SEM samples. 0.2-micron PVDF filters were purchased from MilliporeSigma and used in the disc inhibition zone tests. In addition, 0.1 μm Supor filters were purchased from Pall Corporation and employed to separate metal ions from impurities before ion concentration measurements (via ICP-OES) in metal ion release tests. Tris Hydrochloride, 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), and Hydrogen peroxide solution were purchased from – and used in oxidative stress measurements by Ellman's assay.MXene synthesis and characterization
The synthesis and characterization of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes, along with their respective MAX phases, were carried out following established protocols (Materials and methods in SI).25,26,40,41 X-ray diffraction (XRD) measurements, field-emission scanning electron microscopy (FESEM), and high-resolution transmission electron microscopy (HR-TEM) were further utilized to characterize the synthesized MXenes (Materials and methods in SI).Antibacterial assessment experiments
The antibacterial performance of Ti3C2Tx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes was evaluated through a series of concentration- and time-dependent cell growth-inhibition tests. For this purpose, Escherichia coli (E. coli), as a model Gram-negative bacterium, was cultivated in the trypticase soy broth (TSB) at 37 °C overnight and sub-cultured to obtain the culture suspensions for the antibacterial experiments. 4 mL of the sub-cultured medium was then centrifuged at 6000 rpm (Multifuge X1R Centrifuge, Thermo Fisher Scientific, USA) for 5 minutes and repeatedly pelletized with PBS to remove any remaining macromolecules and growth medium components. The resultant pellet cells were then diluted with sterile deionized (DI) water to achieve the cell concentration of approximately 106 CFU mL−1, determined through optical density measurements at 600 nm wavelength (OD600). The concentration-dependent antibacterial performance of MXenes was assessed by using 5, 10, 25, 50, and 100 μg mL−1 of mono-M (Ti3C2Tx) and DTM (Mo2TiC2Tx and Mo2Ti2C3Tx) MXenes. For this purpose, previously prepared MXene stock solutions were diluted accordingly and added to E. coli suspension (106 CFU mL−1) to achieve the desired final concentrations. Then, the samples were incubated at 37 °C under 200 rpm shaking speed for 3 h. The bacterial concentration of samples was measured via the standard colony-forming unit (CFU) method at 1 h time intervals to evaluate the time-dependent antibacterial performance of the MXenes. To achieve this, 100 μL of serially diluted samples were plated on the solidified agar plates and put in the incubator (37 °C) for approximately 20 h. Colonies grown on each plate were then counted and used to calculate the bacterial concentration of each sample. Suspension of E. coli culture (106 CFU mL−1) in DI water (with no MXenes) was used as the control. Finally, the antibacterial performance of the samples was evaluated by calculating the inhibition efficiency (IE%) using the following equation: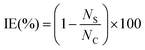 | (1) |
The antibacterial properties of MXenes were further assessed via various experiments such as probe sonication pretreatment, oxidative stress induced by MXenes, cumulative release of metal ions, and disc inhibition zone experiments (Materials and methods in SI).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Due to file size and institutional repository limitations, the datasets—including raw antibacterial assay data, characterization files (SEM, TEM, XRD), and image analyses—will be provided via direct email communication.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5nh00178a.
Acknowledgements
This research benefited greatly from funding provided by USDATAT-RWTS 00–69526, USEPA Cooperative Agreement MX-00D87019, The Richard Lounsbery Foundation, and the Transforming Wastewater Infrastructure in the United States project of Columbia World Projects. USDA or other agencies have not formally reviewed this paper, and the views expressed in this document are solely those of the authors and do not necessarily reflect those of the agencies. A. T., A. B., B. S. N. C. and B. A. acknowledge the support of the US National Science Foundation award number CMMI-2134607.References
- S. Marzouk, D. Nguyen and C. Sabet, Reviving antibiotic power, Nat. Rev. Chem., 2024, 1 Search PubMed.
- S. J. Lam, et al., Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers, Nat. Microbiol., 2016, 1(11), 1–11 CrossRef.
- J. O'Neill, Tackling drug-resistant infections globally: final report and recommendations. 2016 Search PubMed.
- P. Makvandi, et al., Metal-based nanomaterials in biomedical applications: antimicrobial activity and cytotoxicity aspects, Adv. Funct. Mater., 2020, 30(22), 1910021 CrossRef CAS.
- D. O. Schairer, et al., The potential of nitric oxide releasing therapies as antimicrobial agents, Virulence, 2012, 3(3), 271–279 CrossRef.
- K. Blecher, A. Nasir and A. Friedman, The growing role of nanotechnology in combating infectious disease, Virulence, 2011, 2(5), 395–401 CrossRef PubMed.
- L. Zhang, et al., Development of nanoparticles for antimicrobial drug delivery, Curr. Med. Chem., 2010, 17(6), 585–594 CrossRef CAS.
- J. G. Leid, et al., In vitro antimicrobial studies of silver carbene complexes: activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria, J. Antimicrob. Chemother., 2012, 67(1), 138–148 CrossRef CAS PubMed.
- F. Cao, et al., Defect-rich adhesive nanozymes as efficient antibiotics for enhanced bacterial inhibition, Angew. Chem., Int. Ed., 2019, 58(45), 16236–16242 CrossRef CAS.
- L. Zhang, et al., Nature-inspired construction of MOF@ COF nanozyme with active sites in tailored microenvironment and pseudopodia-like surface for enhanced bacterial inhibition, Angew. Chem., Int. Ed., 2021, 60(7), 3469–3474 CrossRef CAS.
- B. C. Wyatt, et al., Alkali cation stabilization of defects in 2D MXenes at ambient and elevated temperatures, Nat. Commun., 2024, 15(1), 6353 CrossRef CAS.
- K. Huang, et al., Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications, Chem. Soc. Rev., 2018, 47(14), 5109–5124 RSC.
- L. M. Dong, et al., Two-dimensional metal carbides and nitrides (MXenes): preparation, property, and applications in cancer therapy, Nanophotonics, 2020, 9(8), 2125–2145 CrossRef CAS.
- B. C. Wyatt, et al., Design of Atomic Ordering in Mo2Nb2C3T x MXenes for Hydrogen Evolution Electrocatalysis, Nano Lett., 2023, 23(3), 931–938 CrossRef CAS.
- A. Arabi Shamsabadi, et al., Antimicrobial mode-of-action of colloidal Ti3C2Tx MXene nanosheets, ACS Sustainable Chem. Eng., 2018, 6(12), 16586–16596 CrossRef CAS.
- R. P. Pandey, et al., Effect of sheet size and atomic structure on the antibacterial activity of Nb-MXene nanosheets, ACS Appl. Nano Mater., 2020, 3(11), 11372–11382 CrossRef CAS.
- K. Rasool, et al., Antibacterial activity of Ti3C2Tx MXene, ACS Nano, 2016, 10(3), 3674–3684 CrossRef CAS PubMed.
- F. Seidi, et al., MXenes Antibacterial Properties and Applications: A Review and Perspective, Small, 2023, 19(14), 2206716 CrossRef CAS PubMed.
- F. Perreault, et al., Antimicrobial properties of graphene oxide nanosheets: why size matters, ACS Nano, 2015, 9(7), 7226–7236 CrossRef CAS PubMed.
- Q. He, et al., Double transition-metal TiVCTX MXene with dual-functional antibacterial capability, Mater. Lett., 2022, 308, 131100 CrossRef CAS.
- S. Roy, et al., Mechanistic insight into the antibacterial activity of chitosan exfoliated MoS2 nanosheets: membrane damage, metabolic inactivation, and oxidative stress, ACS Appl. Bio Mater., 2019, 2(7), 2738–2755 CrossRef CAS PubMed.
- J. Liao, et al., Molybdenum-based antimicrobial nanomaterials: A comprehensive review, Nano Today, 2023, 50, 101875 CrossRef CAS.
- L. F. Deravi, J. D. Swartz and D. W. Wright, The biomimetic synthesis of metal oxide nanomaterials, Preface XV List of Contributors XIX, 2009, 16 Search PubMed.
- U. Kadiyala, N. A. Kotov and J. S. VanEpps, Antibacterial metal oxide nanoparticles: challenges in interpreting the literature, Curr. Pharm. Des., 2018, 24(8), 896–903 CrossRef CAS PubMed.
- B. Anasori, et al., Two-dimensional, ordered, double transition metals carbides (MXenes), ACS Nano, 2015, 9(10), 9507–9516 CrossRef CAS.
- A. Thakur, et al., Step-by-step guide for synthesis and delamination of Ti3C2Tx MXene, Small Methods, 2023, 7(8), 2300030 CrossRef CAS PubMed.
- C. H. Liao and L. Shollenberger, Survivability and long-term preservation of bacteria in water and in phosphate-buffered saline, Lett. Appl. Microbiol., 2003, 37(1), 45–50 CrossRef PubMed.
- S. F. Seyedpour, et al., Improved performance and antifouling properties of thin-film composite polyamide membranes modified with nano-sized bactericidal graphene quantum dots for forward osmosis, Chem. Eng. Res. Des., 2018, 139, 321–334 CrossRef CAS.
- X. Zou, et al., Mechanisms of the antimicrobial activities of graphene materials, J. Am. Chem. Soc., 2016, 138(7), 2064–2077 CrossRef CAS.
- S. Patra, et al., Hydrophobic MXene with enhanced electrical conductivity, Surf. Interfaces, 2023, 39, 102969 CrossRef CAS.
- K. Maleski, et al., Size-dependent physical and electrochemical properties of two-dimensional MXene flakes, ACS Appl. Mater. Interfaces, 2018, 10(29), 24491–24498 CrossRef CAS.
- M. Malaki, A. Maleki and R. S. Varma, MXenes and ultrasonication, J. Mater. Chem. A, 2019, 7(18), 10843–10857 RSC.
- A. Iqbal, et al., Improving oxidation stability of 2D MXenes: synthesis, storage media, and conditions, Nano Convergence, 2021, 8(1), 9 CrossRef CAS.
- G. Yang, et al., Biodegradable and photostable Nb2C MXene quantum dots as promising nanofluorophores for metal ions sensing and fluorescence imaging, Sens. Actuators, B, 2020, 309, 27735 CrossRef.
- Q. Zhang, et al., Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect, Nanoscale, 2020, 12(3), 1826–1832 RSC.
- Z. Wang, et al., Versatile cutting method for producing fluorescent ultrasmall MXene sheets, ACS Nano, 2017, 11(11), 11559–11565 CrossRef CAS PubMed.
- D. Tobaldi, et al., Silver-modified nano-titania as an antibacterial agent and photocatalyst, J. Phys. Chem. C, 2014, 118(9), 4751–4766 CrossRef CAS.
- S. Qin, et al., Approaches based on passive and active antibacterial coating on titanium to achieve antibacterial activity, J. Biomed. Mater. Res. Part A, 2018, 106(9), 2531–2539 CrossRef CAS PubMed.
- B. Li, et al., Preparation and antibacterial properties of plasma sprayed nano-titania/silver coatings, Mater. Chem. Phys., 2009, 118(1), 99–104 CrossRef CAS.
- B. Anasori, et al., Mo2TiAlC2: A new ordered layered ternary carbide, Scr. Mater., 2015, 101, 5–7 CrossRef CAS.
- T. S. Mathis, et al., Modified MAX phase synthesis for environmentally stable and highly conductive Ti3C2 MXene, ACS Nano, 2021, 15(4), 6420–6429 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |





