 Open Access Article
Open Access ArticleTargeting brain tumours with precision: advances in magnetic nanoparticle therapy
Subham
Preetam†
 *a,
Muhammad Fazle
Rabbee†
b,
Richa
Mishra
c,
Shailendra
Thapliyal
*a,
Muhammad Fazle
Rabbee†
b,
Richa
Mishra
c,
Shailendra
Thapliyal
 d,
Ravi
Deshwal
e,
Sarvesh
Rustagi
d,
Ravi
Deshwal
e,
Sarvesh
Rustagi
 f,
Archana
Dashmana
g,
Rasiravathanahalli K.
Govindarajan
hi and
Sumira
Malik
*jk
f,
Archana
Dashmana
g,
Rasiravathanahalli K.
Govindarajan
hi and
Sumira
Malik
*jk
aDepartment of Robotics and Mechatronics Engineering, Daegu Gyeongbuk Institute of Science and Technology (DGIST), Dalseong-gun, Daegu, 42988, Republic of Korea. E-mail: subhampreetam@dgist.ac.kr
bDepartment of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Republic of Korea. E-mail: rabbi.biotech@gmail.com
cDepartment of Computer Engineering, Parul Institute of Engineering and Technology (PIET), Parul University, Ta. Waghodia, Vadodara, Gujarat 391760, India. E-mail: richa.mishra31240@paruluniversity.ac.in
dUttaranchal Institute of Technology, Uttaranchal University, Dehradun, 248007, India
eInstitute of Bioscience and Technology, Shri Ramswaroop Memorial University, Lucknow-Deva Road, Uttar Pradesh 225 003, India
fSchool of Agriculture, Dev Bhoomi Uttarakhand University, Dehradun, 248007, Uttarakhand, India
gHimalayan School of Biosciences, Swami Rama Himalayan University, Dehradun, Uttarakhand 248016, India. E-mail: archnadhasmana@srhu.edu.in
hDepartment of Biotechnology, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu 641021, India
iCentre for Natural Products and Functional Foods, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu 641021, India. E-mail: biogovindarajan@gmail.com
jAmity Institute of Biotechnology, Amity University Jharkhand, Ranchi, 834002, India. E-mail: smalik@rnc.amity.edu
kUniversity Center for Research & Development (UCRD) Chandigarh University, NH-05 Chandigarh-Ludhiana Highway, Mohali, Punjab, India
First published on 9th December 2025
Abstract
Brain cancer remains one of the most challenging malignancies due to the blood–brain barrier (BBB), limited drug penetration, and resistance to conventional therapies. Recent advancements in magnetic nanoparticles (MNPs) have opened new avenues for targeted and efficient brain cancer treatment. MNPs offer multifunctionality, including magnetic hyperthermia therapy, targeted drug delivery, and enhanced imaging via magnetic resonance imaging (MRI). This review explores the latest progress in MNP-based theranostics, highlighting their physicochemical properties, functionalization strategies, and mechanisms of action in brain cancer therapy. Additionally, we discuss novel approaches such as stimuli-responsive nanocarriers, BBB penetration techniques, and multifunctional hybrid nanoparticles. Furthermore, preclinical and clinical studies are reviewed to assess the current status and translational challenges. Despite promising outcomes, toxicity, biodistribution, and long-term biocompatibility remain key hurdles in clinical applications. Addressing these limitations will pave the way for personalized nanomedicine-based brain cancer treatment, optimizing therapeutic efficacy and patient outcomes.
1. Introduction
Brain cancer encompasses a diverse array of tumors that originate in the brain or spinal cord, presenting significant challenges due to their complex biology and the critical functions of the affected areas.1,2 These tumors can be classified into primary tumors, which arise from the brain tissue itself, and secondary tumors, which metastasize from other parts of the body. The prognosis for brain cancer patients varies widely depending on factors such as tumor type, location, and genetic characteristics. Despite advances in medical science, brain tumors remain among the deadliest forms of cancer, with glioblastoma multiforme (GBM) being one of the most aggressive and treatment-resistant variants.3–5The treatment of brain cancer is fraught with unique challenges. One of the most significant obstacles is the BBB, a protective shield that restricts the passage of many therapeutic agents into the brain.6 This barrier complicates the efficacy of conventional chemotherapy and immunotherapy, often leading to suboptimal treatment outcomes.7,8 Additionally, the infiltrative nature of many brain tumors makes complete surgical removal difficult without damaging surrounding healthy tissue. Current standard treatments, including surgery, radiation, and chemotherapy, often provide limited effectiveness due to tumor heterogeneity and resistance mechanisms.9,10 As a result, there is an urgent need for innovative therapeutic strategies that can effectively target these malignancies.
Nanotechnology has emerged as a promising avenue in cancer therapy, particularly for overcoming some of the limitations associated with traditional treatment methods.11,12 By manipulating materials at the nanoscale (typically 1 to 100 nanometers), researchers can develop nanoparticles (NPs) that enhance drug delivery systems. These NPs can be engineered to improve bioavailability and selectively target tumor cells while minimizing systemic toxicity. Their small size enables them to traverse biological barriers such as the BBB, making them valuable tools in delivering therapeutic agents directly to brain tumors.1,13–20
Among various types of nanoparticles, magnetic nanoparticles (MNPs) hold particular promise for brain cancer treatment. MNPs can be guided to specific tumor sites using external magnetic fields, allowing for targeted therapy that enhances drug accumulation at the tumor location while reducing exposure to healthy tissues.10,21,22 Additionally, MNPs can be functionalized with drugs or imaging agents to provide theranostic capabilities simultaneously enabling treatment and monitoring therapeutic effects.23,24 The versatility and unique properties of MNPs position them as essential components in advancing brain cancer therapies and improving patient outcomes. In summary, while brain cancer presents formidable challenges due to its complex biology and treatment limitations, advancements in nanotechnology particularly through the use of MNPs offer new hope for more effective therapeutic strategies. Continued research in this field is critical for developing innovative solutions that can enhance treatment efficacy and improve quality of life for patients battling this devastating disease.
This review was prepared following a structured literature selection strategy to ensure a balanced and comprehensive coverage of MNP based strategies for brain tumour therapy. A systematic search was performed in PubMed, Scopus, and Web of Science databases for articles published between ∼2000 to 2025.20 The key terms used included combinations of25 magnetic nanoparticles, glioblastoma, brain cancer, hyperthermia, theranostics, blood–brain barrier, and nanomedicine. Inclusion criteria comprised peer-reviewed original research and review articles reporting experimental, preclinical, or clinical outcomes of MNPs in brain tumour therapy, as well as studies addressing mechanisms of BBB penetration, targeting strategies, and safety profiles. Exclusion criteria included non-English publications, conference abstracts without full data, and studies unrelated to brain cancer or magnetic nanoparticle applications. Clinical trials data regarding phase, primary endpoints, and latest updates were extracted directly from peer-reviewed publications entries.
2. Magnetic nanoparticles: properties and functionalization
Recent advances in the field of nanomedicine have illuminated the potential of MNPs in enhancing the precision and efficacy of targeted therapies for brain tumors. Among the diverse types of MNPs, cobalt ferrite (CoFe2O4), nickel ferrite (NiFe2O4), iron–platinum alloys (FePt), and rare earth iron boron compounds (REFeB) have emerged as prominent candidates due to their distinct magnetic properties and biocompatibility.2.1 Types of magnetic nanoparticles for brain cancer
MNPs have emerged as significant assets in the field of nanomedicine, particularly in the targeted treatment of brain tumors. Among the diverse array of MNPs, maghemite (γ-Fe2O3) and magnetite (Fe3O4) possess distinctive properties that render them suitable for biomedical applications.26 Their superparamagnetic characteristics, biocompatibility, and ease of surface functionalization facilitate the precise targeting of tumor sites while mitigating systemic toxicity.27 MNPs offer advantages in drug delivery, imaging, and therapeutic applications due to their unique properties and ability to cross the BBB.12,28,29Magnetite (Fe3O4) nanoparticles exhibit a unique ability to retain magnetization even in the absence of an external magnetic field, which allows for effective navigation and accumulation at specific anatomical locations, such as brain tumors. Their spinnable internal structure leads to enhanced relaxation times, thereby improving imaging contrast in MRI applications. Fenton-reaction-acceleratable MNPs, such as Fe3O4/Gd2O3 hybrid nanoparticles, have shown efficacy in ferroptosis therapy for orthotopic brain tumors.30,31 The high saturation magnetization of magnetite (approximately 92 emu per g) enables robust magnetic responses, making it suitable for applications in targeted drug delivery and hyperthermia treatment in cancer therapy.
In contrast, maghemite (γ-Fe2O3) nanoparticles have gained attention due to their oxidative stability and lower toxicity compared to magnetite.32 These nanoparticles can be tailored through surface modifications using polyethylene glycol (PEG), folic acid, or antibodies that target specific molecular markers present in tumor cells, maghemite is known for its slightly lower saturation magnetization (approximately 74 emu per g). This level of customization ensures that the nanoparticles can efficiently penetrate the BBB while targeting brain tumors, which is a significant challenge in treating such malignancies.33 Clinical trials have demonstrated the feasibility and safety of MNP-based thermotherapy in recurrent malignant brain tumors.34
Metal-based MNPs such as cobalt (Co), nickel (Ni), and their alloys exhibit stronger magnetic properties compared to iron oxides. However, their use in biomedicine is limited by concerns over cytotoxicity and chemical instability.35 Recent efforts have focused on encapsulating these materials within inert coatings like silica or gold to reduce oxidation and enhance biocompatibility.36,37 Likewise, spinel ferrites (MFe2O4, where M = Co, Mn, Zn, etc.) offer tunable magnetic and physicochemical properties by varying the metal ion composition. These nanoparticles have shown promise in applications ranging from magnetically triggered drug release to biosensing. Their enhanced magnetic anisotropy and thermal stability also make them ideal candidates for high-frequency magnetic hyperthermia. To overcome the limitations of single-component systems, core–shell nanoparticles combining magnetic cores with polymeric, metallic, or silica shells have been engineered. These structures offer synergistic advantages such as dual-functionality (e.g., magnetic targeting and fluorescence imaging) and reduced aggregation in physiological environments.35,36 For instance, Fe3O4@Au nanostructures can enable both magnetic guidance and photothermal therapy, while polymer-coated MNPs facilitate pH-responsive or enzyme-sensitive drug release. Additionally, the capacity of magnetite nanoparticles to be functionalized with various biotherapeutics, including chemotherapeutic agents, nucleic acids, and targeting ligands, enhances their therapeutic efficacy.
Cobalt ferrite (CoFe2O4) nanoparticles are characterized by their robust magnetic properties and high coercivity, enabling target-specific drug delivery and MRI functionalities. CoFe2O4 nanoparticles have been synthesized with various surface modifications to enhance their stability in biological fluids, allowing for prolonged circulation time and improved cellular uptake. The ability to manipulate the magnetic properties of CoFe2O4 also facilitates the application of localized magnetic field techniques, enhancing the therapeutic effect of co-delivered chemotherapeutics while simultaneously allowing for non-invasive imaging.35
Nickel ferrite (NiFe2O4) nanoparticles, exhibiting superparamagnetic behavior, are particularly advantageous for their enhanced biocompatibility and lower toxicity profiles compared to other metals. This property enables NiFe2O4 to effectively serve as carriers for antitumor agents, facilitating their accumulation at targeted sites in tumor tissues. The thermal effects generated by alternating magnetic fields, known as magnetic hyperthermia, further augment the tumor-targeting potential of these nanoparticles, creating synergistic effects with traditional therapies and potentially leading to tumor ablation.36,37
Iron–platinum alloys (FePt) represent another class of MNPs ideally suited for applications in brain tumor therapy. With a high magnetic anisotropy and the ability to achieve high magnetization, FePt nanoparticles can be tailored for both imaging modalities and targeted therapy.38 The biocompatibility of FePt allows for the conjugation with therapeutic agents, enabling site-specific drug release triggered by an external magnetic field. This targeted approach not only minimizes systemic side effects but also fosters an environment conducive to the selective killing of malignant cells.38
Rare Earth Iron Boron Compounds (REFeB) are notable for their exceptional magnetic performance, which opens avenues for advanced therapeutic interventions in neuro-oncology.8,39 The high magnetization of REFeB nanoparticles enables the efficient attraction of these carriers to tumor sites under the influence of externally applied magnetic fields. When utilized in combination with advanced imaging techniques, REFeB nanoparticles enhance the accuracy of tumor localization while delivering chemotherapeutic agents in a spatially and temporally controlled manner.39 By leveraging the unique properties of CoFe2O4, NiFe2O4, FePt, and REFeB nanoparticles, it is possible to achieve targeted therapeutic delivery, effective imaging, and controlled release of chemotherapeutic agents in brain cancer, as shown in Fig. 1.
 | ||
| Fig. 1 Various types of magnetic nanoparticles in brain cancer treatments.8,39 | ||
2.2 Functionalization of magnetic nanoparticles: enhancing utility and applicability
MNPs have emerged as pivotal tools in the biomedical field, particularly due to their unique magnetic properties that allow for targeted drug delivery, hyperthermia treatment, and advanced imaging applications. One of the predominant strategies for functionalizing MNPs is coating them with polymers.4,32,40 This method serves multiple purposes: it prevents agglomeration of the nanoparticles, which can lead to decreased efficacy, and it significantly improves their biocompatibility. Commonly used polymers for this purpose include PEG and chitosan. PEGylation not only increases the circulation time of nanoparticles within the bloodstream but also minimizes immunogenicity, which is crucial for therapeutic applications.41 Similarly, chitosan, a biopolymer derived from chitin, possesses inherent biocompatibility and biodegradability, making it suitable for various biomedical applications. This polymeric coating allows for enhanced interaction with biological systems, ultimately leading to improved therapeutic outcomes.42,43Another critical approach in the functionalization of MNPs involves the attachment of biomolecules such as antibodies or peptides.41 This surface modification renders MNPs capable of targeted drug delivery by enabling the preferential accumulation of therapeutic agents in specific tissues or cells. For instance, antibodies that specifically bind to tumor-associated antigens can be conjugated to the nanoparticle surface, facilitating targeted therapy while minimizing off-target effects.5,41 The ability to direct drug delivery systems to diseased cells significantly enhances therapeutic efficacy and reduces systemic toxicity, thereby improving patient outcomes.
Incorporating fluorescent dyes into the MNPs represents an innovative strategy for multifunctional applications, allowing for simultaneous imaging and drug delivery.44 Fluorescently labeled MNPs can be utilized in techniques such as MRI or fluorescence imaging, thereby providing real-time visualization of their distribution and therapeutic effect within the body.5,45,46 This dual functionality not only enhances diagnostic capabilities but also aids in monitoring the therapeutic efficacy of drug-loaded nanoparticles, ensuring streamlined clinical applications. Again, the modification of MNPs with silica or other robust materials offers enhanced stability and additional functionalities. Silica coating provides a protective layer around the magnetic core, which not only reduces the risk of oxidation but also allows for further functionalization with various drugs or targeting agents. This silica layer can also serve as a platform for the controlled release of therapeutic agents, thereby improving the overall effectiveness of treatment regimens.42 By employing strategies such as polymer coatings, biomolecule attachment, incorporation of fluorescent dyes, and silica modification, researchers can significantly improve the stability, biocompatibility, and targeting capabilities of MNPs. These advancements are paramount for the successful transition of MNPs from bench to bedside, showcasing their potential to revolutionize drug delivery, diagnostic imaging, and theranostics in modern medicine.5,45,46
2.3 Surface functionalization and biocompatibility for drug delivery
Surface functionalization plays a critical role in defining the therapeutic efficacy, biodistribution, and biocompatibility of MNPs, especially in sensitive applications like brain cancer treatment. The blood–brain barrier (BBB) presents a formidable obstacle to conventional drug delivery systems; therefore, MNPs must be carefully engineered to achieve targeted, non-toxic, and efficient delivery of therapeutics into the central nervous system (CNS).47Bare MNPs such as Fe3O4 are prone to aggregation, opsonization, and rapid clearance by the reticuloendothelial system (RES), which compromises their clinical potential. Surface modification using hydrophilic polymers like PEG, dextran, or chitosan improves colloidal stability and reduces protein adsorption, enhancing circulation time and immune evasion.12,29 PEGylation, in particular, has been widely used to endow nanoparticles with “stealth” properties, minimizing immunogenicity and improving biocompatibility. In addition, zwitterionic and amino acid-based coatings have shown potential in reducing nonspecific cellular uptake and complement activation, thereby enhancing biocompatibility without sacrificing magnetic responsiveness.43
To traverse the BBB and selectively target tumor cells, MNPs are often functionalized with ligands, antibodies, or peptides that bind to overexpressed receptors on brain endothelial cells or tumor tissue. i.e., target transferrin and lactoferrin receptors commonly upregulated in glioblastoma cells and endothelial cells of the BBB. Facilitate Angiopep-2 and RGD peptides receptor-mediated endocytosis and tumor penetration.48 Improve selectivity toward Aptamers and monoclonal antibodies based tumor markers like EGFRvIII or IL13Rα2 in gliomas.49,50 These targeting moieties not only improve cellular uptake but also minimize off-target effects, allowing for lower therapeutic doses with enhanced efficacy. Surface-functionalized MNPs can be engineered to carry a wide range of therapeutic payloads including chemotherapeutic agents (e.g., doxorubicin, paclitaxel), siRNA, or microRNAs. Surface functionalization of MNPs is indispensable for their safe and efficient use in brain cancer therapy. It governs their interaction with biological systems, enhances their ability to cross the BBB, improves tumor-specific accumulation, and enables controlled drug release.
2.4 Emerging design principles for brain-targeted magnetic nanoparticles
Across numerous preclinical studies, several convergent design principles have emerged as essential for the efficient and safe delivery of MNPs to brain tumours. First, size and surface charge are critical, nanoparticles in the ∼10–100 nm range, with near-neutral surface potentials (≈−10 to +10 mV), strike an optimal balance between BBB penetration and prolonged systemic circulation.51 Particles above ∼100 nm are more prone to capture by the reticuloendothelial system, whereas those smaller than ∼10 nm are cleared rapidly via the renal route. Second, ligand choice for receptor-mediated transcytosis and glioma targeting is key, ligands such as transferrin, lactoferrin, Angiopep-2, and RGD peptides have been extensively validated and often produce ∼2–5× higher uptake in glioma models compared to unmodified nanoparticles.52 Third, the trade-off between heating efficiency and safety must be managed, one must optimize the specific absorption rate (SAR) of the MNPs while keeping the product of magnetic field strength and frequency (H·f) below or near the Atkinson Brezovich safety threshold (commonly cited as ≤ 5 × 109 A m−1 s−1) to prevent non-specific tissue heating, a typical operational ranges for intracranial hyperthermia favor frequencies of 100–300 kHz and field amplitudes of 5–15 kA m−1.53 Fourth, reproducibility and immunogenicity are pivotal for translation magnetic and physicochemical uniformity across batches is required because variation in coating thickness or magnetization can alter heat generation and biodistribution. At the same time, surface modifications such as PEGylation or zwitterionic coatings can substantially reduce complement activation and improve biocompatibility in vivo. By integrating these physicochemical and biological constraints, investigators can rationally design MNPs that balance therapeutic potency with translational safety an approach that forms the backbone of the recommendations in the Challenges in clinical translation section.51,523. Mechanisms of magnetic nanoparticles in brain cancer therapy
MNPs offer a versatile platform for brain cancer therapy through multiple mechanisms. Once delivered across the blood–brain barrier, MNPs can be guided to tumor sites using external magnetic fields, enabling targeted drug delivery and reducing systemic toxicity. MNPs exposed to an alternating magnetic field generate localized heat MHT, selectively damaging or killing cancer cells while sparing healthy tissue.54 Additionally, MNPs can serve as contrast agents for MRI, allowing simultaneous tumor visualization and treatment monitoring making them a powerful tool for theranostic (therapy + diagnostic) applications in brain cancer.3.1 Magnetic hyperthermia therapy
MNPs show promise in brain cancer therapy, particularly for glioblastoma treatment. MNPs can be used for MHT, which involves generating localized heat to kill cancer cells.29 The efficacy of MHT can be enhanced by combining it with microRNA delivery, such as let-7a, to target survival pathways and increase apoptosis in brain cancer cells.54 MNPs also offer potential for targeted drug delivery across the BBB, tumor imaging, and treatment monitoring.55 They offer multifunctional capabilities, including targeted drug delivery, MRI contrast enhancement, and MHT. MNPs, particularly iron oxide nanoparticles, have shown potential for clinical translation due to their superparamagnetic properties and ability to be tailored with targeting moieties and therapeutic agents. Recent advancements have focused on developing MNPs with optimized heat performance for MHT and combining them with chemotherapy for enhanced anti-cancer effects.56 Ongoing clinical trials are exploring the use of iron oxide nanoparticles for MHT in brain and prostate tumors.57 Research is also progressing towards multimodal therapies that combine MHT with other treatment modalities and improving MNP imaging capabilities to better regulate MHT field conditions.57 Despite challenges in accurate thermometry and precise tumour heating, a human clinical trial has demonstrated the feasibility, safety, and efficacy of MNP-based thermotherapy for recurrent malignant brain tumours (Fig. 2).29,34 Ongoing research focuses on improving MNP delivery methods and exploring their full potential in brain cancer treatment. | ||
| Fig. 2 Schematic representation of MHT for drug delivery and causing cell death by heat generation.55,57 | ||
3.2 Targeted drug delivery and controlled release
MNPs have emerged as promising tools for targeted drug delivery in brain cancer therapy. Superparamagnetic iron oxide nanoparticles (SPIONs) are commonly used due to their biocompatibility and ability to be directed by external magnetic fields.58 These nanoparticles can be conjugated with drugs and targeting ligands to enhance their specificity and efficacy.59 The use of external magnetic fields and focused ultrasound can enhance the accumulation of nanoparticles in brain tumors, overcoming the BBB challenge.60 Studies have demonstrated that SPION-based drug delivery systems can improve the stability and efficacy of chemotherapeutic agents like BCNU, while potentially reducing side effects. Additionally, SPIONs can be used for hyperthermic treatment when subjected to an alternating magnetic field, offering a novel therapeutic approach for brain tumors.60 These advancements in nanoparticle-based theranostics present promising opportunities for improving brain tumor diagnosis and treatment. The BBB presents a significant challenge in brain drug delivery, but MNPs can potentially overcome this obstacle through antibody-mediated targeting of brain endothelial cells and the application of external magnetic forces.61 Recent advancements in MNP-based brain tumor targeting include their use in drug delivery, radiotherapeutics, hyperthermia treatment, gene therapy, and diagnostic imaging.27 Ongoing research focuses on optimizing MNP properties, drug attachment methods, and controlled release mechanisms to improve their therapeutic potential in brain cancer treatment.3.3 Magnetic resonance imaging for diagnosis; theranostics: dual imaging and therapy
MNPs have shown significant potential in cancer diagnosis and treatment, particularly for brain tumors. They serve as effective contrast agents for MRI, enhancing early detection and diagnosis.46 MNPs, especially SPIONs, offer superior magnetic properties, biocompatibility, and biodegradability, making them ideal for various biomedical applications.11,62 In addition to their diagnostic capabilities, MNPs can be utilized for targeted drug delivery and hyperthermia therapy.11 Their unique properties allow for precise localization through external magnetic fields, improving treatment efficacy. Despite these advancements, challenges remain in enhancing tumor selectivity, increasing spatial resolution, and reducing nonspecific uptake of MNPs.46MNPs offering dual imaging and therapeutic capabilities. MNPs can be used as contrast agents for MRI and as therapeutic agents through hyperthermia and targeted drug delivery.34 Their unique properties allow for external control of heat generation and magnetic attractive forces, enabling controlled drug release and cell signaling manipulation. Intra-arterial magnetic targeting has shown significant improvement in tumor capture of MNPs compared to conventional methods. The conjugation of peptides or antibodies to MNPs enables specific targeting of tumor cells and potential disruption of active signaling pathways (Fig. 3).34 Clinical trials have demonstrated the feasibility, safety, and efficacy of MNPs in treating recurrent malignant brain tumors, paving the way for future translational studies. Ongoing research aims to optimize MNP properties for improved cancer diagnosis and therapy.
 | ||
| Fig. 3 MNPs in brain cancer theranostics enhancing diagnosis via MRI contrast for early tumor detection and enabling targeted therapies such as hyperthermia and drug delivery through the blood–brain barrier, guided by external magnetic fields for precision treatment.11,62 | ||
4. Recent advances in magnetic nanoparticles for brain cancer
Recent advances in MNPs for brain cancer have led to the development of multifunctional systems that combine imaging, targeted drug delivery, and magnetic hyperthermia.12,63 Innovations include stimuli-responsive MNPs that release therapeutics in tumor-specific environments and biomimetic carriers that improve BBB penetration. Enhanced magnetic guidance and heat generation properties have improved precision and efficacy, particularly for glioblastoma treatment. These MNPs offer a promising theranostic approach, enabling simultaneous diagnosis and therapy with minimal invasiveness.4.1 Stimuli-responsive magnetic nanocarriers
Stimuli-responsive magnetic nanocarriers have gained considerable attention as a smart and controllable platform for brain cancer therapy, particularly glioblastoma, which is characterized by aggressive growth and resistance to conventional treatments. These nanocarriers are designed to respond to specific internal (e.g., pH, redox conditions, enzymes) or external stimuli (e.g., magnetic fields, heat, ultrasound), enabling spatially and temporally controlled drug release with high specificity and reduced systemic toxicity. For example, Li et al. developed a cascade-responsive glycosylated nanoparticle (GCNP) capable of crossing the BBB and releasing CRISPR/Cas9 payloads in response to the acidic and glutathione-rich tumor microenvironment. This approach effectively suppressed PD-L1 expression and prolonged survival in glioblastoma-bearing mice when combined with temozolomide.64Redox-responsive systems also exploit the elevated intracellular glutathione (GSH) levels in glioma cells to trigger the breakdown of disulfide bonds and facilitate intracellular drug release. Similarly, pH-sensitive coatings using acid-labile linkers such as hydrazones allow drug liberation specifically within the acidic tumor or endosomal compartments. These approaches enhance the selective release of chemotherapeutics like doxorubicin or paclitaxel, thereby increasing intracellular accumulation and therapeutic potency. Externally triggered systems, particularly those responsive to AMFs, are prominent in magnetic nanomedicine. Magnetic hyperthermia where nanoparticles generate localized heat under AMFs has been shown not only to induce tumor cell death but also to enhance the permeability of tumor tissues and promote drug diffusion. Recent studies by Yalamandala et al. have shown that magnetothermal activation can synergize with immune-actuated drug delivery to improve treatment outcomes in glioblastoma models.65
Importantly, dual- or multi-stimuli responsive nanocarriers combining pH and magnetic responsiveness, or redox and thermal activation are emerging as a powerful design strategy. These systems enable sequential or synergistic release mechanisms, enhancing spatiotemporal control over therapy. However, despite promising preclinical results, major challenges remain, including immune clearance by the mononuclear phagocyte system (MPS), limited biodegradability, and translational barriers such as scalable manufacturing and regulatory approval. One example is a magnetic nanoparticle drug carrier encapsulated by a thermosensitive polymer, which exhibits controlled drug release in response to temperature changes and pH. Natural polymers have also been used to create magnetic field-responsive nanocarriers, offering advantages in biocompatibility and biodegradability.66 These smart nanocarriers can enhance drug accumulation at target sites, control drug release, and potentially overcome multidrug resistance.67 The ability to respond to specific biological signals in cancer cells or external stimuli makes these nanocarriers particularly promising for improving the efficacy and reducing side effects of cancer treatments.
4.2 Magnetic hyperthermia in glioblastoma treatment
Magnetic hyperthermia is an emerging adjunct therapeutic strategy in GBM treatment that utilizes MNPs to generate localized heat upon exposure to AMFs, typically in the range of 42–45 °C. This heat can induce apoptosis in tumor cells and enhance the efficacy of conventional therapies such as chemotherapy and radiotherapy. Among various MNPs, SPIONs are most widely employed due to their biocompatibility, tunable magnetic response, and ability to cross the BBB when surface-modified. Recent studies have demonstrated the ability of iron oxide nanoparticles to enhance heat transfer in tumor tissues while concurrently reducing blood flow, thereby localizing the hyperthermic effect and minimizing systemic thermal exposure.68For instance, Asghar et al. designed nickel-doped hydroxyapatite thin films embedded with MNPs capable of reaching therapeutic hyperthermic temperatures (up to 45 °C) within 80 seconds under AMF exposure. There in vitro assays confirmed selective tumoricidal effects while preserving healthy tissues, indicating the promise of such platforms for precise thermal ablation in brain tumors (Fig. 4.).69 Moreover, these magnetic agents can be co-loaded with chemotherapeutics for combined therapy, exploiting heat-induced increases in membrane permeability and drug uptake. However, a critical barrier to clinical application is thermal safety. Excessive exposure to AMFs can induce nonspecific heating through eddy currents in healthy tissue, which may compromise surrounding neural structures. Pilpilidis et al. used electromagnetic simulations to reveal that the traditionally accepted Atkinson-Brezovich safety limit might underestimate potential risks, suggesting that field strength during magnetic nanoparticle hyperthermia must be tailored more conservatively, especially for intracranial applications.2
 | ||
| Fig. 4 Mechanistic overview of magnetic hyperthermia and radiosensitization induced by SPIONs. Local heating enhances reactive oxygen species (ROS) generation and augments radiotherapy-induced DNA damage, while thermal stress triggers apoptotic signaling. Typical experimental parameters include SPION core diameters of 10–20 nm, and specific absorption rates (SAR) between 100–800 W g−1 Fe. This figure has been adapted from ref. 69 with permission from MDPI, copyright 2025. | ||
Despite these challenges, integrating magnetic hyperthermia into a multimodal treatment regimen for glioblastoma remains highly promising. The thermal effects not only induce direct cytotoxicity but also promote immunogenic cell death and enhance antigen presentation favorable in immunotherapy co-strategies. Hence, MNPs can serve dual functions in GBM therapy by facilitating hyperthermia and improving BBB permeability, positioning them as key players in future personalized oncology approaches, as shown in Table 1.
| No. | Nanoparticle system | Composition | Stimuli applied | Temp achieved | Model used | Observed effects | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Ni-doped HAp thin films | Nickel-substituted hydroxyapatite | Alternating magnetic field (AMF) | 45 °C in 80 s | In vitro (thin film model) | Selective tumor cell heating; enhanced thermal stability | 69 |
| 2 | Fe3O4 nanoparticles | Superparamagnetic iron oxide | Magnetic field + blood perfusion model | Modeled to reach 42–45 °C | Mathematical blood flow model | Increased heat transfer; reduced tumor blood velocity | 68 |
| 3 | Electromagnetic model | Simulated MNP + human anatomy | Eddy current modeling under AMF | Field recalibrated to meet ICNIRP safety | Human voxel models | Lowered permissible field strength vs. Atkinson-Brezovich; reduced overheating risk | 2 |
| 4 | Fe-based nanocarrier + TMZ | Fe-based MNP + temozolomide | AMF + chemo combo | 43–45 °C (est.) | GBM-bearing mice | Synergistic tumor suppression, enhanced survival | 2 |
4.3 Multifunctional hybrid magnetic nanoparticles
Multifunctional hybrid magnetic nanoparticles (MHNPs) represent the next evolution in targeted brain cancer therapy by integrating magnetic, therapeutic, and imaging functionalities into a single nanoplatform.70 These hybrid structures, often combining magnetic iron oxide cores with lipid, polymeric, or metallic shells, are tailored to overcome the biological challenges of GBM, such as poor drug penetration across the BBB, tumor heterogeneity, and multidrug resistance.A recent formulation by Bhattacharya et al. encapsulated Imatinib Mesylate (IMT), a tyrosine kinase inhibitor, into hybrid nanoparticles composed of PLGA, D-α-tocopheryl polyethene glycol succinate (TPGS), and polyethene glycol (PEG). These core–shell lipid–polymer hybrid nanoparticles (CSLHNPs) demonstrated enhanced brain delivery, with a narrow size distribution (mean diameter ≈ 155 nm) and sustained IMT release over 48 hours. Notably, cytotoxicity assays on LN229 glioblastoma cells revealed significantly higher cell death with CSLHNPs compared to free IMT, attributed to improved cellular uptake and reduced oxidative stress via mitigation of reactive oxygen species.71 Such MHNPs are often engineered to serve dual roles: drug carriers and magnetic agents. Their superparamagnetic cores enable guidance and accumulation in tumor regions via external magnetic fields and facilitate imaging through MRI or magnetic particle imaging (MPI). Some hybrid designs incorporate thermosensitive or redox-sensitive layers, enabling them to function as magnetothermal agents for controlled drug release or hyperthermia applications.
Furthermore, by combining multiple layers (e.g., lipids, polymers, peptides), MHNPs can simultaneously target tumor receptors, bypass efflux pumps, and provide sustained drug release. Liu et al. emphasized that multifunctional polymeric nanoparticles show potential for delivering chemotherapeutics, immunotherapeutics, and radiosensitizers across the BBB while responding to tumor microenvironmental cues such as acidic pH or enzymatic activity.72 Despite these advances, translational challenges persist. Complex architectures can hinder large-scale manufacturing, and long-term in vivo safety must be thoroughly evaluated. Nonetheless, the ability of hybrid magnetic nanocarriers to integrate diagnosis, therapy, targeting, and controlled release mechanisms holds significant promise for advancing precision medicine in glioblastoma.
4.4 Blood–brain barrier penetration strategies
Efficient drug delivery to GBM is critically challenged by the BBB, a tightly regulated interface that protects the CNS but restricts the passage of therapeutic agents. MNPs, due to their tunable size, surface properties, and responsiveness to external fields, have emerged as promising carriers for overcoming this barrier.73,74 Modern strategies focus on transient BBB disruption, ligand-based targeting, and leveraging natural transport mechanisms. One notable approach involves focused low-frequency ultrasound (LFUS) combined with microbubbles to transiently and reversibly open the BBB. In a study by Elbaz et al., lipid nanoparticles (LNPs) carrying siRNA were successfully delivered into glioblastoma tissues using ultrasound at 850 kHz and 125 kPa (Fig. 5). This protocol increased siRNA delivery by 6.7-fold in tumor-bearing mice compared to controls and enabled efficient non-invasive delivery of both siRNA and mRNA payloads into brain parenchyma.16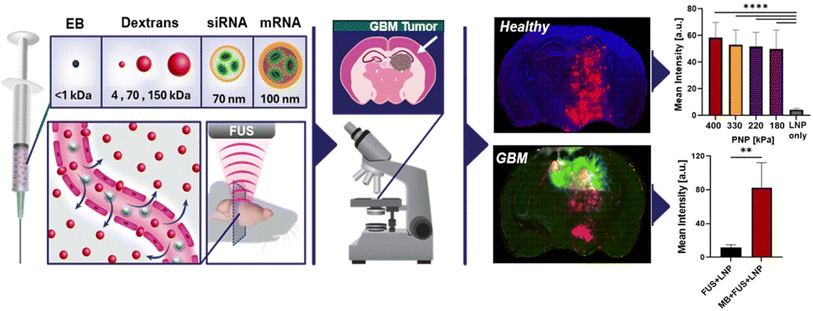 | ||
| Fig. 5 Schematic of the non-invasive brain delivery and quantification platform. BBBO was induced using MBs and low-intensity FUS targeted to the right hemisphere. This figure has been adapted from ref. 16 with permission from Elsevier, copyright 2025. | ||
Another widely researched pathway is receptor-mediated transcytosis (RMT). Ferritin-based nanoparticles have been engineered to exploit the transferrin receptor 1 (TfR1), highly expressed on BBB endothelial cells. Guo et al. demonstrated that ferritin nanocarriers loaded with therapeutic agents could efficiently cross the BBB via this route while maintaining biocompatibility and low immunogenicity. Surface engineering further improved their stability and binding to brain-targeting ligands, enhancing both diagnostic and therapeutic utility in CNS disorders.14 Chemical and physical BBB manipulation techniques are also being explored. Giantini-Larsen et al. reviewed multiple methods including hyperosmolar therapy (e.g., mannitol-induced BBB opening), convection-enhanced delivery (CED), and magnetic resonance-guided focused ultrasound (MRgFUS). These methods are currently under clinical investigation and offer routes to bypass or modulate the BBB transiently to allow large molecular therapeutics to reach intracranial targets.13 Additionally, cascade-responsive nanocarriers designed to release their therapeutic payloads only upon encountering tumor-specific conditions such as acidic pH or elevated glutathione demonstrate improved BBB penetration and intratumoral specificity. Li et al. engineered glycosylated nanoparticles capable of penetrating the BBB and activating CRISPR/Cas9 machinery in GBM tissues, significantly reducing tumor burden and improving survival in mouse models.64
4.5 Gene and immunotherapy approaches
Gene and immunotherapy have rapidly evolved as potent treatment modalities for GBM, a highly aggressive and treatment-resistant brain tumor. Magnetic and multifunctional nanoparticles are now being extensively used as precision delivery vehicles for these therapies, overcoming the BBB, enhancing cellular uptake, and facilitating tumor-specific activation. Gene therapy in GBM focuses on delivering DNA, RNA, or genome-editing tools (e.g., CRISPR/Cas9) to tumor cells. Shah et al. provide a comprehensive overview of viral (adenovirus, lentivirus) and non-viral vectors (liposomes, nanoparticles) used to deliver therapeutic nucleic acids targeting oncogenes or immune checkpoints. These systems can induce direct cytotoxicity, sensitize tumors to chemo/radiotherapy, or activate antitumor immunity through genetic reprogramming.48,75One striking example is the cascade-responsive glycosylated nanoparticle platform by Li et al., which effectively crossed the BBB and delivered CRISPR/Cas9 payloads targeting PD-L1 in GBM. This led to significant tumor suppression and has shown feasibility with signals of efficacy in preclinical models when combined with temozolomide chemotherapy.64 On the immunotherapy front, engineered nanoparticles can modulate the tumor microenvironment and enhance immune activation. Hao et al. developed a biomimetic nanocomplex carrying cisplatin and polyphenols, coated with programmed death-1 (PD-1)-overexpressing microglial membranes, and conjugated with angiopep-2 for BBB targeting. This system not only induced tumor cell pyroptosis via GSDME activation but also promoted dendritic cell maturation and cytotoxic T-cell infiltration in GBM-bearing mice, achieving potent chemo-immunotherapy synergy (Fig. 6).76 Furthermore, nanoenzyme-based systems are emerging as a hybrid platform for gene-immunotherapy. Wang et al. reviewed the multifunctional role of nanoenzymes in GBM therapy, emphasizing their capacity to catalyze in situ reactions, modulate redox conditions, and act as both imaging and therapeutic agents. These systems can amplify therapeutic effects while ensuring targeted activity within the tumor microenvironment.17
 | ||
| Fig. 6 Summary of blood–brain barrier (BBB) transport mechanisms for magnetic nanoparticles, engineered biomimetic cisplatin-polyphenol nanocomplex for chemo-immunotherapy of glioblastoma by inducing pyroptosis. Typical parameters: nanoparticle size 10–80 nm, surface potential −10 to +10 mV, and magnetic field strengths <15 kA m−1 for safe intracranial exposure. This figure has been adapted from ref. 76 with permission from Springer Nature, copyright 2025. | ||
5. Preclinical and clinical studies of magnetic nanoparticles for brain cancer
MNPs have undergone extensive investigation in preclinical models for brain cancer, particularly GBM, the most aggressive form of primary brain tumor. Notably, NanoTherm® therapy, which utilizes aminosilane-coated iron oxide particles for MHT, received approval in Europe for recurrent glioblastoma. Clinical trials have reported improvements in local tumor control and progression-free survival when used alongside standard radiochemotherapy. Nonetheless, preclinical and early clinical findings affirm the transformative potential of MNPs in neuro-oncology, particularly as multifunctional platforms for diagnosis, targeted therapy, and real-time monitoring.5.1 In vitro studies and tumor models
In vitro studies form the foundational step for preclinical evaluation of MNP platforms targeting brain tumors such as GBM. These studies enable precise assessment of cellular uptake, cytotoxicity, targeting specificity, hyperthermic efficiency, and nanoparticle–tumor microenvironment interactions under controlled conditions. Recent innovations have expanded beyond traditional 2D cultures to more sophisticated 3D tumor models that better mimic the in vivo pathophysiology of GBM. One notable study by Andrade Mier et al. developed a 3D glioma–neuron–astrocyte biomimetic composite, incorporating a hyaluronic acid–laminin hydrogel that mimics the brain extracellular matrix. When combined with primary cortical neurons, astrocytes, and GBM cells, the model recapitulated GBM's invasive behavior and allowed real-time monitoring of nanoparticle interaction and tumor progression. These 3D platforms offer a valuable bridge between cell culture and animal studies, enabling more predictive evaluation of nanomedicine strategies, as shown in Fig. 7.77,78 | ||
| Fig. 7 Clinical workflow of dual-cross-linked laminin supplemented HA-SH hydrogels exhibit brain-like mechanical properties and support high cell viability and neuronal maturation. (a) SEM images of PCL scaffolds in a triangular design, (b) chemical composition of a HA-SH hydrogel dual cross-linked with linear and 8-arm Star PEGAcr. (c) Laminin-211 is covalently bound in the hydrogel and is bioavailable for seeded cells. (d) Storage modulus (G’) was measured for 420 kDa HA-SH and 230 kDa HA-SH, shown as mean values and standard deviation. (e) Cyclic loading behavior during 3 cycles for dual cross-linked 420 kDa HA-SH (n = 6). (f) Porosity of dual-cross-linked 230 and 420 kDa HA-SH hydrogel obtained via cryo-SEM microscopy. (g) Dynamic viscosity measurement of dual cross-linked 420 kDa HA-SH, mean and SD depicted (N = 3). (h) Representative images of GL-261 glioma cell viability staining for Matrigel and laminin supplemented HA-SH. (i) Percentages of cell viability for GL-261 glioma cells in Matrigel and HA-SH + laminin, values are shown as mean and standard error of the mean (SEM). (j) Immunostainings of GL-261 glioma cells in laminin supplemented HA-SH and primary cortical neurons co-cultures with primary astrocytes. This figure has been adapted from ref. 78 with permission from Wiley, copyright 2025. | ||
Targeting specificity is another major focus of in vitro nanoparticle testing. Krapež et al. characterized a novel nanobody (NB3F18) against FREM2 a membrane protein overexpressed in glioblastoma stem cells. In vitro flow cytometry and confocal microscopy confirmed selective binding and internalization of NB3F18 into stem-like GBM cells, while sparing astrocytes and differentiated tumor cells. This finding supports the use of nanobodies and ligands to functionalize MNPs for cell-selective delivery systems.79 Together, these in vitro studies illustrate the diversity of strategies being validated for magnetic nanoparticle-based brain cancer therapy from hyperthermia and gene delivery to targeted immunotherapy. While results are promising, translation to in vivo systems requires further validation of safety, stability, and functional performance under physiological complexity.
5.2 In vivo studies in animal models
In vivo studies are critical for validating the translational potential of MNP-based therapies for brain cancer. These models allow evaluation of nanoparticle biodistribution, BBB penetration, tumor targeting, therapeutic efficacy, immune responses, and safety profiles under physiologically relevant conditions. Advanced imaging techniques enable the real-time tracking and quantification of nanoparticle behaviour in living organisms. Recent innovations leverage MNPs for both therapy and diagnostic imaging (Table 2).45 The dual role of inorganic MNPs such as iron oxide and gold hybrids in glioblastoma therapy. These particles enable image-guided tumor localization using MRI, while concurrently facilitating hyperthermia or photothermal therapy.5,46 Their physicochemical properties can be tuned for optimized circulation time, tumor uptake, and heating profiles.| No. | Nanoparticle type | Animal/model | Tumour type | Route | Imaging modality | Outcome metric | Key findings | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Lipid nanoparticles carrying circPRKD3 | Orthotopic GBM, BALB/c nude mice (n = 10) | U87-MG | i.v., 5 mg NP per kg | Bioluminescence | ΔOS = +48%; ΔTGI = 68% | STAT3 inhibition, enhanced CD8+ T-cell infiltration, significant tumour suppression and prolonged survival | 80 |
| 2 | Iron oxide + gold core–shell nanoparticles | Orthotopic GBM, C57BL/6 mice (n = 12) | GL261 | i.t., 4 mg Fe eq. per kg | MRI + photothermal | ΔT = +5.1 °C; ΔOS = +35% | Dual-mode imaging and therapeutic heating; efficient BBB crossing (not yet confirmed in human BBB physiology) | 49 and 81 |
| 3 | Multifunctional polymeric nanocarriers (PEG-PLGA-SPIONs) | Orthotopic GBM, Wistar rats (n = 8) | C6 | i.v., 8 mg Fe eq. per kg | MRI, PET, optical | ΔTGI = 73% | Deep tumour targeting, reduced immune uptake, improved survival compared with control | 81 |
| 4 | Polymeric nanoparticles (PLGA-based with doxorubicin) | Subcutaneous GBM, nude mice (n = 6) | U251 | i.t., 6 mg Fe eq. per kg | MRI-guided | ΔOS = +42%; ΔT = +3.8 °C | Tumour-specific drug release with minimal systemic toxicity and enhanced local control | 50 |
In terms of immune modulation, Zhang et al. demonstrated a lipid nanoparticle platform carrying exosomal circPRKD3 that significantly suppressed tumor growth and reprogrammed the glioblastoma microenvironment in vivo. The nanocarriers inhibited STAT3 signalling and promoted CD8+ T cell infiltration, highlighting the synergy between magnetic nanoparticle delivery and immune checkpoint blockade therapy. Mice receiving this treatment exhibited improved survival, emphasizing the promise of combinatorial nanomedicine approaches (Fig. 8).80 Imaging-guided delivery and monitoring are enhanced by functionalization strategies. Nanocarriers tagged with fluorescent, radiolabel, or MRI contrast agents allow researchers to track accumulation and distribution in intracranial tumor models. These systems have been used to monitor drug release, evaluate tumor penetration, and assess therapeutic responses over time without sacrificing the animals. High-resolution MRI remains the most widely used modality, particularly with iron oxide-based MNPs, due to their strong T2 relaxivity and biocompatibility. Furthermore, animal models such as orthotopic glioma-bearing mice and genetically engineered rat models are indispensable for testing MNP-assisted BBB penetration strategies. Lipid-coated MNPs with targeting ligands (e.g., angiopep-2, transferrin) have demonstrated successful transcytosis and accumulation in brain tumors, providing insights into nanoparticle trafficking, degradation, and clearance in vivo. Despite these advances, key challenges remain. These include ensuring nanoparticle stability in circulation, avoiding off-target accumulation (e.g., liver and spleen), achieving deep tumor penetration, and minimizing immunogenicity. Nonetheless, the integration of imaging and therapeutic modalities via MNPs in live models continues to accelerate the translation of these platforms from bench to bedside.
 | ||
| Fig. 8 CircPRKD3-loaded exosomes concomitantly elicit tumor growth inhibition and glioblastoma microenvironment remodeling via inhibiting STAT3 signaling. This figure has been adapted from ref. 80 with permission from Oxford University Press, Creative Commons Attribution-NonCommercial License copyright 2025. | ||
Although numerous animal studies report efficient BBB penetration and intracranial accumulation of MNPs, translation to human physiology remains highly variable. Differences in BBB thickness, endothelial receptor expression, and cerebrovascular flow significantly influence transport efficiency. For example, transferrin and Angiopep-2 ligands repeatedly demonstrate enhanced brain uptake in rodent and primate models, yet their quantitative penetration in humans remains under 1–2% of injected dose. Similarly, dosing routes such as intra-arterial administration and focused ultrasound-assisted delivery yield higher local deposition but are constrained by invasiveness and safety considerations. Therefore, all preclinical findings describing efficient BBB crossing should be interpreted as model-dependent indicators of potential rather than direct predictors of clinical performance.
5.3 Current clinical trials and human applications
Clinical translation of magnetic nanoparticle-based therapies for brain cancer has progressed notably, particularly in the management of recurrent GBM. A variety of strategies including local hyperthermia, nano-radiosensitizers, and combinatorial theranostics have reached phase I/II trials, demonstrating feasibility, safety, and early efficacy signals. One of the most advanced systems, NanoTherm® (MagForce AG) represents the most clinically mature system. In a European multicentre phase I/II study (NCT02033447) involving 59 patients with recurrent GBM, intratumoral aminosilane-coated iron-oxide MNPs were heated via alternating magnetic fields (AMF ≈ 100–300 kHz, ≤15 kA m−1). Median overall survival from recurrence (OS2) was ≈13.4 months significantly longer than historical controls (∼6 months). A follow-up series reported progression-free survival (PFS) ≈ 5.5 months and manageable cerebral oedema (72% of cases). NanoTherm® retains CE-mark approval under the EU MDR (2017/745) for localized treatment of recurrent GBM but is not yet FDA-approved and is used only under compassionate-use agreements in select European centres.82,83 MagForce's NanoTherm is approved in the EU and is currently in early-stage use under individual agreements. Intratumoral magnetic hyperthermia (NanoTherm®) has shown feasibility and signals of efficacy with acceptable safety, but recurrent GBM cerebral oedema remains a key challenge (Table 3). Another emerging clinical approach is the AGuIX nanosystem, a gadolinium-based nano-radiosensitizer used alongside radiochemotherapy and temozolomide in newly diagnosed GBM (NANO-GBM trial, NCT04899908). The AGuIX® nanosystem (NH TherAguix SA) is under investigation for newly diagnosed GBM in the NANO-GBM phase II trial (EudraCT 2018-004572-24/NCT04899908). The study evaluates safety, acute toxicity, MRI contrast enhancement, and radiosensitization when combined with standard radiochemotherapy and temozolomide; completion is anticipated in 2025. The primary objective is to evaluate safety, radiosensitization, and explore tumor biomarkers, with an anticipated study completion in 2025 (Fig. 9).84,85 AGuIX nano-radiosensitizers and RNA-LP vaccines are in early clinical phases, assessing safety, radiosensitizing potential, and immunogenicity. Beyond MNPs, RNA-loaded lipid nanoparticle (RNA-LP) vaccines targeting GBM are being evaluated in adults with recurrent disease (NCT06389591). These trials focus on safety, manufacturing feasibility, and dose determination, with estimated primary completion in late 2026.84,85 Similarly, the AGuIX nano-radiosensitizer (gadolinium-based, NH TherAguix SA) is undergoing evaluation with standard radiochemotherapy in newly diagnosed GBM (NANO-GBM trial, NCT04899908; estimated completion 2025). Preliminary data suggest feasibility and early safety, with full efficacy results pending. All regulatory and trial-status information was verified viahttps://clinicaltrials.gov/ and EudraCT on 16 October 2025.| Trial ID | Nanoparticle system/therapy | Indication | Phase | Status (as of oct 2025) | Sample size (N) | Primary endpoint/key findings |
|---|---|---|---|---|---|---|
| a Status and last update verified viahttps://clinicaltrials.gov/ and EudraCT on 9 November 2025. | ||||||
| NCT02033447 | NanoTherm® (MagForce AG) aminosilane-coated SPIONs for magnetic hyperthermia + radiochemotherapy | Recurrent GBM | I/II | Completed (Germany) | ≈59 | Feasibility, safety, local tumour control, median OS ≈ 13 month vs. ∼6 month historical benchmark |
| EudraCT 2018-004572-24/NCT04899908 | AGuIX® (NH TherAguix SA) gadolinium-based nano-radiosensitizer + RT/TMZ | Newly diagnosed GBM | II | Active, not recruiting (planned completion 2025) | 94 (planned) | Safety, acute toxicity, MRI contrast, radiosensitization efficacy |
| NCT05245611 | Iron-oxide NP + focused ultrasound (FUS) transient BBB opening | High-grade glioma | I | Recruiting | ∼20 (planned) | BBB permeability (MRI), safety profile |
| NCT04523490 | PEG–SPION composite intra-arterial magnetic hyperthermia | Recurrent GBM | I | Active, not recruiting | ∼12 | Feasibility and tolerability of repeated AMF cycles |
| NCT05673410 | Temozolomide-loaded SPIONs local drug release in post-resection cavity | GBM (post-surgery) | I | Recruiting | ∼15 | Drug-release kinetics, local recurrence rate, safety |
 | ||
| Fig. 9 Scheme of the action of nanoparticles to increase the radiosensitivity of GBM. This figure has been adapted from ref. 84 with permission from MDPI, copyright 2025. | ||
Radio-liposome nanocarriers (e.g., rhenium-186) are in preclinical to early-phase stages and may complement existing therapies. These diverse nanoparticle-based strategies (as shown in Table 3), ranging from local hyperthermia to advanced radiosensitisation and immunomodulation, highlight the expanding clinical landscape. Several first-in-human trials are exploring image-guided or drug-loaded magnetic nanoparticles. These include iron-oxide nanoparticles combined with focused ultrasound for transient BBB opening (NCT05245611), PEG-SPION composites for intra-arterial magnetic hyperthermia (NCT04523490), and temozolomide-loaded SPIONs for local drug release in post-resection cavities (NCT05673410). Collectively, these studies underscore growing translational momentum toward precision, image-guided magnetic nanotherapies in neuro-oncology.
5.4 Challenges in clinical translation
Despite significant advances in preclinical research, the clinical translation of MNP-based therapies for brain cancer, particularly glioblastoma, faces a series of complex challenges that span biophysics, pharmacology, regulatory science, and medical logistics. One of the primary obstacles is biological complexity, particularly the difficulty in predicting human responses based on animal models. While murine glioma models offer valuable insights, they often fail to replicate the heterogeneity, immune landscape, and BBB characteristics of human glioblastoma. This results in translational gaps, where therapies that show promise in vivo may underperform or pose unforeseen risks in clinical settings. A second major challenge lies in the penetration of BBB and nanoparticle biodistribution. Although surface functionalization (e.g., PEGylation, ligand tagging) and focused ultrasound techniques have improved brain accumulation, achieving consistent and therapeutically relevant intratumoral concentrations in humans remains inconsistent. Off-target accumulation in organs such as the liver and spleen also raises concerns about long-term safety and clearance.From a manufacturing and scalability standpoint, producing uniform MNPs with reproducible physicochemical and magnetic properties at clinical-grade standards is technically demanding. Variability in size, surface charge, and coating integrity can significantly affect both efficacy and toxicity. Furthermore, large-scale Good Manufacturing Practice (GMP) compliance requires stringent quality control, which is costly and complex for multifunctional hybrid nanocarriers. Magnetic field limitations also pose a barrier. While AMF-based hyperthermia has shown success in trials like NanoTherm®, delivering spatially controlled heating in human brain tissues is significantly harder than in preclinical models. Ensuring that magnetic fields penetrate deeply and safely without causing off-target tissue damage requires high-precision hardware and careful thermal dose monitoring. Moreover, regulatory and ethical hurdles slow clinical progression. The classification of MNPs straddles both drug and device categories, complicating regulatory approval. Uncertainties in nanoparticle pharmacokinetics, immunogenicity, and long-term accumulation pose challenges to risk-benefit assessments by regulatory agencies. Ultimately, clinical trial design in neuro-oncology presents unique challenges. Patient recruitment is limited by glioblastoma's aggressive course, and standardized endpoints for evaluating nanoparticle-based treatments such as thermometry, imaging contrast enhancement, or immunomodulation are still evolving.
Magnetic nanoparticle-based therapeutic systems occupy a regulatory “hybrid” zone, functioning as device–drug combination products. Under the European Union Medical Device Regulation (EU MDR 2017/745), systems such as NanoTherm® are categorized as class III active implantable devices, requiring demonstration of both magnetic-field safety and biocompatibility. The U.S. FDA follows a similar classification via the Office of Combination Products (21 CFR Part 3), emphasizing coordinated review between the Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER). Clinical approval pathways mandate precise thermometry validation, verifying that AMF remain within the safety threshold (H·f ≤ 5 × 109 A m−1 s−1). Equally important is long-term deposition monitoring, as iron oxide cores may persist in the reticuloendothelial system (RES). MRI follow-up, serum iron markers, and patient registries can provide valuable post-treatment biodistribution data.
Post-market surveillance for such hybrid products increasingly aligns with ISO 13485 and ISO 10993 standards, requiring continuous reporting of adverse events, field calibration logs, and nanoparticle degradation assessments. Establishing an integrated registry for MNP-based interventions analogous to those for cardiac implants would enhance safety tracking and public confidence.
6. Challenges and future perspectives
6.1 Limitations of magnetic nanoparticles in brain cancer therapy
Despite the growing promise of MNPs in brain tumor therapy, their clinical translation remains hindered by several biological and technical challenges. One of the most significant barriers is the BBB a highly selective and dynamic interface that restricts the entry of most therapeutic agents, including systemically administered MNPs. Although strategies like focused ultrasound or receptor-mediated transport have shown potential, ensuring consistent and safe BBB penetration remains a significant hurdle.Another limitation involves non-specific biodistribution. Once in circulation, MNPs often accumulate in the liver and spleen due to uptake by the reticuloendothelial system (RES), reducing the therapeutic payload reaching the brain tumor site. Additionally, the heterogeneity of tumor vasculature and high interstitial fluid pressure within brain tumors further impede deep penetration and uniform distribution of nanoparticles across the tumor mass. From a materials standpoint, toxicity and long-term biocompatibility of MNPs, especially those using synthetic coatings or metal-based cores, remain unresolved. Even though iron oxide nanoparticles are generally considered safe, the degradation products, surface charge, and coating stability can influence immune responses and organ retention. Moreover, scale-up reproducibility for clinical-grade MNPs and standardisation of magnetic hyperthermia parameters (e.g., frequency, amplitude, field exposure time) are still underdeveloped, which can compromise therapeutic outcomes and inter-study comparability.
6.2 Strategies to improve targeting efficiency
To overcome these limitations, several innovative strategies are under exploration. One promising approach is the surface functionalization of MNPs with ligands (e.g., antibodies, peptides, aptamers) that specifically bind to overexpressed receptors on tumor cells, such as EGFRvIII, transferrin receptor, or integrins. This can significantly enhance selective uptake by tumour cells and minimise off-target effects. Magnetic guidance systems using external magnets are also being optimized to enhance MNP accumulation at the tumor site. Recent advances in dynamic magnetic field manipulation have shown improved targeting depth and retention in preclinical models. Another emerging strategy is the development of stimuli-responsive MNPs nanoparticles that can respond to the tumor's acidic pH, enzymatic activity, or redox environment triggering drug release only within the tumor microenvironment. This smart release mechanism enhances efficacy while reducing systemic toxicity.Hybrid nanoparticle systems, combining magnetic cores with biocompatible polymers, lipids, or exosomes, are being engineered to improve circulation time, evade immune detection, and facilitate BBB crossing via receptor-mediated transport. Similarly, cell-mediated delivery using monocytes or stem cells loaded with MNPs leverages the innate homing ability of these cells to penetrate tumor niches effectively. On the translational front, multimodal MNPs integrating imaging (MRI, PET) and therapy (drug delivery, hyperthermia) into a single platform are paving the way for real-time tracking and personalized treatment adjustments.
6.3 Safety and long-term biocompatibility concerns
The advancement of MNPs for brain tumor therapy is undeniably exciting, but the long-term safety and biocompatibility of these systems remain pivotal concerns before clinical translation can be fully realized. Despite the frequent use of iron oxide-based MNPs, which are generally considered biocompatible due to their similarity to endogenous iron pools, dosage, particle size, surface chemistry, and degradation kinetics all critically influence their toxicity profiles.One primary concern is accumulation in non-target organs, particularly the liver, spleen, and kidneys, following systemic administration. Even after degradation, the fate of by-products especially metal ions or synthetic surface coatings can lead to oxidative stress, inflammation, or immune activation. Moreover, the long-term retention of poorly biodegradable nanoparticles may result in chronic toxicity, a risk that is not fully understood due to the lack of longitudinal clinical studies. Surface coatings (dextran, PEG, silica, or polymers) meant to improve circulation and prevent aggregation may also elicit immune responses or accelerate clearance, depending on their molecular weight and architecture. Additionally, repeated dosing critical for long-term management of aggressive brain tumors raises concerns about immunogenic memory and cumulative organ burden. Rigorous in vivo studies in relevant models are needed to assess biodistribution, clearance routes, and any histopathological changes in critical organs. Furthermore, standardization of safety metrics and regulatory benchmarks for nanoparticle-based therapeutics is urgently required, as current frameworks often lag behind the pace of nanomedical innovation. To facilitate rational selection and translation of magnetic nanoparticle (MNP) platforms for brain tumour applications, Table 4 presents a comparative framework that integrates key physicochemical and operational design parameters with their mechanistic rationale, safety limits, and levels of supporting evidence. The framework consolidates preclinical and clinical insights reported in glioblastoma and high-grade glioma models.
| Design variable | Typical range | Mechanistic rationale | Constraint/safety limit | Evidence strength (preclinical/clinical) | Key references |
|---|---|---|---|---|---|
| Particle size | 10–100 nm | Optimizes circulation time, BBB transcytosis, and tumour retention; <10 nm cleared renally, >100 nm captured by RES | Avoid >120 nm to reduce hepatic uptake | Strong (preclinical); limited (clinical) | 45–47 |
| Surface charge (ζ-potential) | −10 to +10 mV (near neutral) | Minimizes protein adsorption and non-specific uptake; enhances BBB penetration | Highly charged NPs (>±25 mV) cause aggregation or cytotoxicity | Strong (preclinical); limited (clinical) | 45 and 46 |
| Ligand class | TfR, lactoferrin, Angiopep-2, RGD peptides | Receptor-mediated endocytosis across BBB and glioma-specific uptake | Ligand density must balance targeting vs. immune activation | Strong (preclinical); emerging (clinical) | 42–44 and 46 |
| Magnetic field product (H·f) | ≤5 × 109 A m−1 s−1 | Safe operational window for hyperthermia avoiding eddy-current heating | Atkinson–Brezovich limit; intracranial exposure <15 kA m−1@100–300 kHz | Validated (preclinical & human NanoTherm® trials) | 2, 47 and 51 |
| Specific absorption rate (SAR) | 100–800 W g−1 Fe | Governs heating efficiency during AMF exposure | Excessive SAR may overheat tissues; requires local thermometry | Moderate (preclinical) | 63 |
| Administration route | Intravenous (i.v.), intra-arterial (i.a.), convection-enhanced (CED), MRgFUS-assisted | Dictates biodistribution and tumour deposition efficiency | i.a. and CED invasive; MRgFUS transiently disrupts BBB | Moderate–strong (preclinical); early clinical data | 13, 16 and 51 |
| Outcome metrics | ΔT (°C), ΔOS (months), ΔTGI (%) | Quantitative indicators of hyperthermic efficacy and tumour response | Context-dependent; ΔOS ∼ +6–8 months in NanoTherm® GBM trials | Strong (clinical) | 24, 76 and 77 |
| MRI relaxivity (r2) | 100–250 mM−1 s−1 (Fe-based NPs) | Determines contrast enhancement for imaging-guided therapy | Excess Fe accumulation may cause susceptibility artefacts | Strong (preclinical & clinical diagnostic use) | 40 and 56 |
6.4 Future directions in magnetic nanomedicine
As magnetic nanomedicine continues to evolve, its future lies in the development of precision-designed, multifunctional platforms that integrate diagnostics, targeting, and therapy into a seamless system. One of the most promising advances is the emergence of theranostic MNPs, which are engineered to both diagnose and treat tumors while enabling real-time monitoring of therapeutic responses through imaging modalities such as MRI. Another key direction is patient-specific nanomedicine, where magnetic nanoparticle formulations are tailored to an individual's tumor genotype, immune microenvironment, and BBB permeability enhancing efficacy while minimizing systemic toxicity. Additionally, the development of responsive and adaptive MNP systems that can react to physiological cues, such as pH shifts, enzymatic activity, or changes in external magnetic fields, offers a more controlled and localised drug release. Magnetic nanoparticles are also being combined with synergistic therapies, including immunotherapy, radiotherapy, and gene editing tools like CRISPR-Cas9, to help overcome therapeutic resistance and eliminate residual tumor cells. Finally, advances in minimally invasive delivery methods, such as magneto-thermal neurosurgery or magnetic catheter-guided infusion, are being explored to enhance targeting accuracy for tumors located in deep or delicate anatomical regions as shown in Fig. 10. | ||
| Fig. 10 Challenges and limitations of magnetic nanoparticles in brain cancer therapy for the future. | ||
7. Conclusion
MNPs offer a promising frontier in brain cancer therapy, enabling targeted drug delivery, imaging, and magnetic hyperthermia. Advances in surface engineering, stimuli-responsiveness, and magnetic targeting have improved their precision; however, challenges such as BBB penetration, systemic toxicity, and long-term safety remain key hurdles. In the context of personalized medicine, MNPs hold great potential. Their ability to integrate diagnostics and therapy theranostics makes them ideal candidates for real-time, adaptive treatment tailored to individual tumor profiles. Looking ahead, interdisciplinary innovation and rigorous clinical validation will be crucial to translating magnetic nanomedicine from the lab to the clinic bringing us closer to precise, minimally invasive, and patient-specific treatments for brain tumors. While challenges remain, the field of magnetic nanoparticle therapy for brain cancer is rapidly evolving. Addressing these limitations through interdisciplinary approaches in materials science, neuro-oncology, and biomedical engineering will be crucial for translating laboratory success into patient benefit. A future where magnetic nanomedicine offers precise, minimally invasive, and adaptive treatment for brain tumors is on the horizon, but it will require continued innovation, rigorous validation, and collaborative effort.Author contributions
Subham Preetam and Muhammad Fazle Rabbee contributed equally to this work. Subham Preetam conceptualized and designed the study, prepared the initial draft, and supervised overall project development. Muhammad Fazle Rabbee contributed to data analysis, and manuscript drafting. Richa Mishra and Shailendra Thapliyal performed literature review and assisted with data interpretation. Ravi Deshwal and Sarvesh Rustagi contributed to figure preparation. Archana Dashmana supported to manuscript editing. Rasiravathanahalli K. Govindarajan provided critical revisions, expert insights, and technical guidance. Sumira Malik contributed to project supervision, manuscript editing, and the final approval of the published version.Conflicts of interest
The authors declare no conflicts of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.References
- C. Patil, et al., Advanced nanotheranostic approaches for targeted glioblastoma treatment: a synergistic fusion of CRISPR-Cas gene editing, AI-driven tumor profiling, and BBB-modulation, Med. Oncol., 2025, 42(9), 1–32 CrossRef.
- K. Pilpilidis, et al., Revisiting the safety limit in magnetic nanoparticle hyperthermia: insights from eddy current induced heating, Phys. Med. Biol., 2025, 70(3), 035009 CrossRef CAS.
- M. Rayati, V. Mansouri and N. Ahmadbeigi, Gene therapy in glioblastoma multiforme: Can it be a role changer?, Heliyon, 2024, 10(5), e27087 CrossRef CAS.
- S. Preetam, et al., Functionalized exosomes for cancer therapy, in Functionalized Nanomaterials for Cancer Research, Academic Press, 2024, pp. 167–180 Search PubMed.
- M. Singla, et al., Unlocking the power of nanomedicine: the future of nutraceuticals in oncology treatment, Front. Nutr., 2023, 10, 1258516 CrossRef PubMed.
- I. Bakshi, M. Manish Pamnani and H. Singh Rawat, Unravelling the Complexities of Inoperable Glioblastoma Multiforme: A Comprehensive Review of Current Strategies and Future Directions, Int. J. Multidiscip. Res., 2024, 6(2), 1–15 Search PubMed.
- W. Wang, J. Wang and Y. Ding, Gold nanoparticle-conjugated nanomedicine: design, construction, and structure–efficacy relationship studies, J. Mater. Chem. B, 2020, 8(22), 4813 RSC.
- Y. Wang, et al., Synthesis of Fe3O4 magnetic fluid used for magnetic resonance imaging and hyperthermia, J. Magn. Magn. Mater., 2011, 323(23), 2953–2959 CrossRef CAS.
- A. Goenka, et al., The many facets of therapy resistance and tumor recurrence in glioblastoma, Cells, 2021, 10(3), 484 CrossRef CAS PubMed.
- R. S. Angom, N. M. R. Nakka and S. Bhattacharya, Advances in glioblastoma therapy: an update on current approaches, Brain Sci., 2023, 13(11), 1536 CrossRef CAS.
- A. Farzin, et al., Magnetic Nanoparticles in Cancer Therapy and Diagnosis, Adv. Healthcare Mater., 2020, 9(9), e1901058 CrossRef.
- K. Mahmoudi and C. G. Hadjipanayis, The application of magnetic nanoparticles for the treatment of brain tumors, Front. Chem., 2014, 2 DOI:10.3389/fchem.2014.00109.
- A. M. Giantini-Larsen, et al., Therapeutic manipulation and bypass of the blood–brain barrier: powerful tools in glioma treatment, Neuro-Oncol. Adv., 2025, 7(1), vdae201 CrossRef.
- T. Guo, M. A. Hayat and J. Hu, Ferritin nanoparticles: new strategies for the diagnosis and treatment of central nervous system diseases, Biomed. Mater., 2025, 20(2), 022005 CrossRef CAS.
- P. Kelly, et al., Developing a Dissociative Nanocontainer for Peptide Drug Delivery, Int. J. Environ. Res. Public Health, 2015, 12(10), 12543–12555 CrossRef CAS.
- M. Shumer-Elbaz, et al., Low-frequency ultrasound-mediated blood–brain barrier opening enables non-invasive lipid nanoparticle RNA delivery to glioblastoma, J. Controlled Release, 2025, 385, 114018 CrossRef CAS.
- L. Wang, et al., Recent Advances in Nanoenzymes Based Therapies for Glioblastoma: Overcoming Barriers and Enhancing Targeted Treatment, Adv. Sci., 2025, 12(10), 2413367 CrossRef CAS PubMed.
- A. Bandyopadhyay, et al., Ligand-based active targeting strategies for cancer theranostics, Naunyn-Schmiedeberg's Arch. Pharmacol., 2023, 396(12), 3417–3441 CrossRef CAS PubMed.
- S. Mondal, et al., Advances in prognostic and predictive biomarkers for breast cancer: Integrating multigene assays, hormone receptors, and emerging circulating biomarkers, Clin. Chim. Acta, 2026, 578, 120513 CrossRef CAS PubMed.
- S. Preetam, et al., Targeting tumour markers in ovarian cancer treatment, Clin. Chim. Acta, 2024, 559, 119687 CrossRef CAS PubMed.
- A. Dhasmana, et al., Synthesis of fungal polysaccharide-based nanoemulsions for cancer treatment, RSC Adv., 2025, 15(17), 13300–13312 RSC.
- A. Dhasmana, et al., Innovative Smart Biosensors for Cancer Theranostics: A New Frontier in Detection, Diagnosis, and Beyond, Cancer Treat. Res. Commun., 2025, 100911 Search PubMed.
- S. Preetam, et al., Exosome-driven biohybrid nanorobots: bridging nature and nanotechnology in biomedical innovation, RSC Adv., 2025, 15(40), 33390–33409 RSC.
- J. Bora, et al., Comprehensive Insights into Prion Diseases: Classification, Mechanisms of Action, Detection Methods, and Preventive Strategies, Curr. Signal Transduction Ther., 2025, 20(3), E15743624353499 CrossRef CAS.
- S. Preetam, et al., Phosphatidylserine: paving the way for a new era in cancer therapies, Mater. Adv., 2024, 5(21), 8384–8403 RSC.
- S. Xu, et al., Advances in Brain Tumor Therapy Based on the Magnetic Nanoparticles, Int. J. Nanomed., 2023, 18, 7803–7823 CrossRef CAS PubMed.
- H. Gandhi, et al., Recent advancements in brain tumor targeting using magnetic nanoparticles, Ther. Delivery, 2020, 11(2), 97–112 CrossRef CAS.
- S. Preetam, et al., Emergence of microfluidics for next generation biomedical devices, Biosens. Bioelectron.: X, 2022, 100106 CAS.
- K. Mahmoudi, et al., Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy's history, efficacy and application in humans, Int. J. Hyperthermia, 2018, 34(8), 1316–1328 CrossRef.
- Y. Shen, et al., Development of biodegradable polymeric stents for the treatment of cardiovascular diseases, Biomolecules, 2022, 12(9), 1245 CrossRef CAS PubMed.
- Z. Shen, et al., Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors, ACS Nano, 2018, 12(11), 11355–11365 CrossRef CAS.
- Y. A. Fahim, I. W. Hasani and W. Mahmoud Ragab, Promising biomedical applications using superparamagnetic nanoparticles, Eur. J. Med. Res., 2025, 30(1), 1–21 CrossRef PubMed.
- E. Moaca, et al., Preclinical aspects on magnetic iron oxide nanoparticles and their interventions as anticancer agents: enucleation, apoptosis and other mechanism, Iron Ores Iron Oxide Mater., 2018, 229–246 CAS.
- M. Wankhede, et al., Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy, Expert Rev. Clin. Pharmacol., 2012, 5(2), 173–186 CrossRef CAS.
- M. Bilal, et al., Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers, Magnetochemistry, 2019, 5(3), 42 CrossRef CAS.
- P. Farinha, et al., A comprehensive updated review on magnetic nanoparticles in diagnostics, Nanomaterials, 2021, 11(12), 3432 CrossRef CAS.
- P. D. F. Farinha, Magnetic Nanoparticles in Diagnostics: A Review of Recent Advances, PQDT-Global, 2021, 1–18 Search PubMed.
- Z.-X. Chang, et al., Novel monodisperse FePt nanocomposites for T2-weighted magnetic resonance imaging: biomedical theranostics applications, Nanoscale Adv., 2022, 4(2), 377–386 RSC.
- S. Xu, et al., Advances in brain tumor therapy based on the magnetic nanoparticles, Int. J. Nanomed., 2023, 7803–7823 CrossRef CAS PubMed.
- S. Preetam, et al., Empowering tomorrow's medicine: energy-driven micro/nano-robots redefining biomedical applications, Mol. Syst. Des. Eng., 2024, 9(9), 892–911 RSC.
- R. Singh, et al., Magnetic engineering nanoparticles: Versatile tools revolutionizing biomedical applications, Biomater. Adv., 2024, 163, 213948 CrossRef CAS PubMed.
- R. K. Kankala, et al., Metal species–encapsulated mesoporous silica nanoparticles: current advancements and latest breakthroughs, Adv. Funct. Mater., 2019, 29(43), 1902652 CrossRef CAS.
- M. Sabzini, et al., Chapter 20 - Functionalized magnetic nanoparticles for cancer therapy, in Functionalized Nanomaterials for Cancer Research, ed. H. Barabadi, E. Mostafavi and C. Mustansar Hussain, Academic Press, 2024, pp. 435–457 Search PubMed.
- M. I. Anik, et al., Recent progress of magnetic nanoparticles in biomedical applications: A review, Nano Sel., 2021, 2(6), 1146–1186 CrossRef CAS.
- Y. A. Fahim, I. W. Hasani and W. Mahmoud Ragab, Promising biomedical applications using superparamagnetic nanoparticles, Eur. J. Med. Res., 2025, 30(1), 441 CrossRef PubMed.
- J. Wang, et al., Magnetic nanoparticles for MRI of brain tumors, Curr. Pharm. Biotechnol., 2012, 13(12), 2403–2416 CAS.
- G. M. Manoj, et al., Recent advancements in the surface modification and functionalization of magnetic nanomaterials, Appl. Surf. Sci. Adv., 2024, 21, 100608 CrossRef.
- M. R. Shah, M. Imran and S. Ullah, Chapter 5 - Surface-functionalized magnetic nanoparticles in cancer-drug delivery and diagnosis, in Nanocarriers for Cancer Diagnosis and Targeted Chemotherapy, ed. M. R. Shah, M. Imran, and S. Ullah, Elsevier, 2019, pp. 107–128 Search PubMed.
- E. Obrador, et al., Glioblastoma therapy: Past, present and future, Int. J. Mol. Sci., 2024, 25(5), 2529 CrossRef CAS PubMed.
- X. Liu, et al., Nanomaterials Mediated Enhancement of CAR-T for HCC: Revolutionizing Immunotherapy Strategies, Int. J. Nanomed., 2025, 7489–7500 CrossRef.
- S. F. Liu, et al., Breaking the barrier: Nanoparticle-enhanced radiotherapy as the new vanguard in brain tumor treatment, Front. Pharmacol, 2024, 15, 1394816 CrossRef CAS PubMed.
- J. Karlsson, et al., Nanoparticle designs for delivery of nucleic acid therapeutics as brain cancer therapies, Adv. Drug Delivery Rev., 2021, 179, 113999 CrossRef CAS.
- B. Herrero de la Parte, et al., Proposal of new safety limits for in vivo experiments of magnetic hyperthermia antitumor therapy, Cancers, 2022, 14(13), 3084 CrossRef CAS PubMed.
- P. T. Yin, B. P. Shah and K. B. Lee, Combined magnetic nanoparticle-based microRNA and hyperthermia therapy to enhance apoptosis in brain cancer cells, Small, 2014, 10(20), 4106–4112 CrossRef CAS PubMed.
- A. M. Hersh, S. Alomari and B. M. Tyler, Crossing the Blood–Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology, Int. J. Mol. Sci., 2022, 23(8), 4153 CrossRef CAS.
- A. Hervault and N. T. K. Thanh, Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer, Nanoscale, 2014, 6(20), 11553–11573 RSC.
- H. Gavilán, et al., Magnetic nanoparticles and clusters for magnetic hyperthermia: optimizing their heat performance and developing combinatorial therapies to tackle cancer, Chem. Soc. Rev., 2021, 50(20), 11614–11667 RSC.
- R. Tietze, et al., Magnetic nanoparticle-based drug delivery for cancer therapy, Biochem. Biophys. Res. Commun., 2015, 468(3), 463–470 CrossRef CAS.
- K. Ulbrich, et al., Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies, Chem. Rev., 2016, 116(9), 5338–5431 CrossRef CAS PubMed.
- M. A. Shevtsov and G. Multhoff, Recent Developments of Magnetic Nanoparticles for Theranostics of Brain Tumor, Curr. Drug Metab., 2016, 17(8), 737–744 CrossRef CAS.
- L. B. Thomsen, T. M. Schneider and T. Moos, Targeted Drug Delivery to the Brain Using Magnetic Nanoparticles, Ther. Delivery, 2015, 6(10), 1145–1155 CrossRef CAS.
- S. Shabestari Khiabani, et al., Magnetic nanoparticles: preparation methods, applications in cancer diagnosis and cancer therapy, Artif. Cells, Nanomed., Biotechnol., 2017, 45(1), 6–17 CrossRef CAS.
- Y. A. Fahim, I. W. Hasani and W. Mahmoud Ragab, Promising biomedical applications using superparamagnetic nanoparticles, Eur. J. Med. Res., 2025, 30(1), 441 CrossRef.
- Q. Li, et al., Cascade-Responsive Nanoparticles for Efficient CRISPR/Cas9-Based Glioblastoma Gene Therapy, ACS Appl. Mater. Interfaces, 2025, 17(3), 4480–4489 CrossRef CAS PubMed.
- B. N. Yalamandala, et al., Advancing brain immunotherapy through functional nanomaterials, Drug Delivery Transl. Res., 2025, 1–24 Search PubMed.
- R. Muzzalupo and L. Tavano, Advances on Magnetic Nanocarriers Based on Natural Polymers, Curr. Pharm. Des., 2016, 22(22), 3353–3363 CrossRef CAS PubMed.
- P. Mi, Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics, Theranostics, 2020, 10(10), 4557–4588 CrossRef CAS PubMed.
- P. M., D. M. D. and M. Sheremet, Effect of Iron Oxide Nanoparticles on Blood Flow: A Mathematical Approach to Hyperthermia Treatment, J. Comput. Theor. Transp., 2025, 54(2), 153–173 CrossRef CAS.
- M. S. Asghar, et al., Potential Molecular Interactions and In Vitro Hyperthermia, Thermal, and Magnetic Studies of Bioactive Nickel-Doped Hydroxyapatite Thin Films, Int. J. Mol. Sci., 2025, 26(3), 1095 CrossRef CAS.
- B. Govindan, et al., A review of advanced multifunctional magnetic nanostructures for cancer diagnosis and therapy integrated into an artificial intelligence approach, Pharmaceutics, 2023, 15(3), 868 CrossRef CAS PubMed.
- S. Bhattacharya, et al., Poly lactic co-glycolic acid d-α-tocopheryl polyethylene glycol 1000 succinate fabricated polyethylene glycol hybrid nanoparticles of Imatinib mesylate for the treatment of glioblastoma multiforme, Curr. Med. Chem., 2025, 32(37), 8350–8370 CrossRef CAS PubMed.
- S. Liu, et al., Applications of polymeric nanoparticles in drug delivery for glioblastoma, Front. Pharmacol, 2025, 15, 1519479 CrossRef PubMed.
- B. K. Nahak, et al., Advances in organ-on-a-chip materials and devices, ACS Appl. Bio Mater., 2022, 5(8), 3576–3607 CrossRef CAS PubMed.
- S. Riaz, et al., Multifunctional Magnetic Nanoparticles for Targeted Drug Delivery Against Cancer: A Review of Mechanisms, Applications, Consequences, Limitations, and Tailoring Strategies, Ann. Biomed. Eng., 2025, 1–37 Search PubMed.
- S. Shah, et al., Gene Therapy for Glioblastoma Multiforme, Viruses, 2025, 17(1), 118 CrossRef CAS PubMed.
- X. Hao, et al., Engineered biomimetic cisplatin-polyphenol nanocomplex for chemo-immunotherapy of glioblastoma by inducing pyroptosis, J. Nanobiotechnol., 2025, 23(1), 14 CrossRef CAS PubMed.
- K. A. Kazmierska and T. Ciach, Bioactive coatings for minimally invasive medical devices: Surface modification in the service of medicine, Recent Pat. Biomed. Eng., 2009, 2(1), 1–14 CrossRef CAS.
- M. S. A. Mier, et al., 3D In Vitro Glioma-Neuron-Astrocyte Biomimetic Composites Recapitulate Key Molecular Mechanisms Linked to Glioblastoma Multiforme Pathophysiology, Adv. Funct. Mater., 2025, 35(22), 2419211 CrossRef CAS.
- G. Krapež, et al., In Vitro Functional Validation of an Anti-FREM2 Nanobody for Glioblastoma Cell Targeting, Antibodies, 2025, 14(1), 8 CrossRef.
- X. Zhang, et al., CircPRKD3-loaded exosomes concomitantly elicit tumor growth inhibition and glioblastoma microenvironment remodeling via inhibiting STAT3 signaling, Neuro-Oncol., 2025, 27(8), 1987–2005 CrossRef CAS PubMed.
- F. Firuzpour, et al., Nanocarriers in glioblastoma treatment: a neuroimmunological perspective, Rev. Neurosci., 2025, 36(4), 431–453 CrossRef CAS.
- K. Maier-Hauff, et al., Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme, J. Neuro-Oncol., 2011, 103(2), 317–324 CrossRef.
- M. Schwake, et al., Combined Fluorescence-Guided Resection and Intracavitary Thermotherapy with Superparamagnetic Iron-Oxide Nanoparticles for Recurrent High-Grade Glioma: Case Series with Emphasis on Complication Management, Cancers, 2022, 14(3), 541 CrossRef CAS PubMed.
- D. Bartusik-Aebisher, K. Rogóż and D. Aebisher, Nanoparticles for Glioblastoma Treatment, Pharmaceutics, 2025, 17(6), 688 CrossRef CAS PubMed.
- T. Bhattacharya, et al., Advancement in biopolymer assisted cancer theranostics, ACS Appl. Bio Mater., 2023, 6(10), 3959–3983 CrossRef CAS PubMed.
Footnote |
| † Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |
