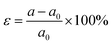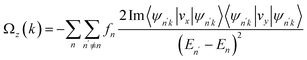Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valley splitting and anomalous valley Hall effect in MoTe2/CrSCl heterostructure
Jaehong
Park
,
Dongchul
Sung
,
Junho
Yun
and
Suklyun
Hong
 *
*
Department of Physics, Graphene Research Institute, Quantum Information Science and Technology Center, and KUU Quantum Materials·Devices International Research Center, Sejong University, Seoul 05006, Korea. E-mail: hong@sejong.ac.kr
First published on 5th December 2025
Abstract
Two-dimensional valleytronics offers a promising platform for novel information processing and quantum technologies by harnessing the valley degree of freedom. A key challenge lies in lifting valley degeneracy, for which magnetic proximity effects provide a promising route. Here, we demonstrate substantial valley splitting in a MoTe2/CrSCl heterostructure via first-principles calculations. We show that interlayer charge transfer and interfacial orbital hybridization critically govern the valley physics at the interface. Under a moderate in-plane tensile strain (3%) and an applied out-of-plane electric field (0.2 V Å−1), a sizable valley splitting of 63 meV emerges at the valence band maximum. These conditions induce hole doping and a pronounced Berry curvature of −23 Å2 at the K valley, realizing an electrically tunable anomalous valley Hall effect. Our findings establish the MoTe2/CrSCl interface as a versatile platform for valley-selective charge transport, opening pathways for valleytronic device applications.
1 Introduction
Valleys, which are local extrema in the electronic band structures of two-dimensional materials, provide a new degree of freedom that has led to the field of valleytronics.1–4 The valley index holds great potential for applications in information processing and quantum computing.5–7 In this context, monolayer transition metal dichalcogenides (TMDs; MX2, where M = Mo or W and X = S or Se) have attracted considerable attention. Due to their lack of inversion symmetry, TMDs exhibit two degenerate but inequivalent valleys located at the K and K′ points of the Brillouin zone.8 Furthermore, the strong spin–orbit coupling in TMDs gives rise to spin splitting at these valleys, and due to time-reversal symmetry the spin orientations at the K and K′ valleys point in opposite directions.9 This intrinsic spin–valley coupling in monolayer TMDs makes them highly promising candidates for valleytronic device applications.10–13A major challenge in utilizing the valley degree of freedom lies in lifting the valley degeneracy between the K and K′ points in the band structure of TMD materials. Since valley degeneracy is protected by time-reversal symmetry, breaking this symmetry is essential for valley manipulation. This can be achieved through two main approaches: optical and magnetic methods. First, the optical approach involves the use of circularly polarized light, which can selectively excite carriers in either the K or K′ valley depending on the light's polarization.14–17 However, this method faces practical limitations due to the need for precise control and the short carrier lifetime resulting from rapid recombination. Second, the magnetic approach provides a more robust means to break time-reversal symmetry and induce valley splitting. This category can be further divided into four strategies. (i) The application of an external magnetic field is a straightforward method,18–21 but the resulting valley splitting is relatively small (∼0.1–0.2 meV T−1), requiring extremely strong fields for practical use. (ii) Magnetic doping, where magnetic atoms are introduced into the TMD lattice, can generate local magnetic moments that interact with valley states. However, this often leads to the formation of impurity states, which significantly degrade carrier mobility and transport properties.22–24 (iii) A promising alternative is the construction of van der Waals (vdW) heterostructures with magnetic materials, which leverages the magnetic proximity effect to induce sizable valley splitting without introducing impurities.25,26 (iv) Ferrovalley materials, which possess intrinsic ferromagnetism and valley properties. In such systems, spontaneous valley polarization occurs even without external magnetic fields or doping, as in monolayer 2H-VSe2.27 Extensive theoretical and experimental efforts have therefore focused on the magnetic approach, particularly the realization of magnetic proximity effects in TMD-based heterostructures. Such systems combine nonmagnetic TMD layers with magnetic substrates such as EuO,28,29 EuS,30,31 MnO,32 CoO,33 and CrI3.34–37 For example, studies on WS2/EuS heterostructures have shown that the induced valley splitting decreases with increasing temperature and vanishes above the Curie temperature (Tc) of EuS. This underscores the need for high-Tc magnetic materials that can maintain valley splitting at elevated temperatures for practical valleytronic applications.
Monolayer CrSCl has recently been predicted to be a ferromagnetic semiconductor with a magnetic moment of 3 µB and a Curie temperature of 273 K.38,39 These properties make monolayer CrSCl a promising candidate as a magnetic layer in vdW heterostructures with TMDs, enabling the use of magnetic proximity effects to achieve robust and controllable valley splitting. Notably, monolayer CrSCl exhibits a Janus-type structure with asymmetric S and Cl atomic planes, leading to an intrinsic out-of-plane dipole and distinct top and bottom chemical environments. Such structural asymmetry can produce different valley and spin polarizations depending on the contact side, as demonstrated in other Janus and ferroelectric systems.40–42
In this work, we explore the magnetic proximity effect as a means to lift valley degeneracy in two-dimensional materials. To this end, we construct a MoTe2/CrSCl heterostructure and carry out density functional theory (DFT) and Berry curvature calculations to uncover the underlying mechanisms of valley splitting and valley-polarized transport. The intrinsic magnetic and structural properties of monolayer CrSCl make it a compelling substrate for proximity-induced valley manipulation. Owing to its structural asymmetry, the interface offers two different stacking configurations, enabling detailed investigation of interfacial interactions. Our study reveals that interlayer charge transfer and orbital hybridization play key roles in determining the extent of valley splitting, and that the symmetry breaking at the interface gives rise to nontrivial Berry curvature distributions leading to valley-polarized transport, relevant to valleytronic applications.
2 Computational details
We performed first-principles calculations using the Vienna Ab Initio Simulation Package (VASP),43,44 incorporating the generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE) functional.45 Ion-electron interactions were described using the projector augmented wave (PAW) method,46,47 and the van der Waals interaction was taken into consideration using the Grimme's DFT-D3 method.48 A plane-wave basis set with a cut-off energy of 500 eV was used to expand the electronic wave functions. For Brillouin-zone sampling, a Γ-centered 18 × 18 × 1 k-mesh was used. To consider the correlation effects in the Cr-3d orbital, the DFT + U method was applied with U − J = 2.1 eV.38,39 The convergence criteria were set to 0.001 eV Å−1 for forces and 10−8 eV for energy. Spin–orbit coupling (SOC) was included in all electronic structure calculations, and a vacuum space of 20 Å was introduced along the z direction. For the calculation of the Berry curvature, VASPBERRY49 code was used.3 Result and discussion
3.1 Optimized MoTe2/CrSCl heterostructures and their stability
First, we optimize the geometries of the vdW MoTe2/CrSCl heterostructures. The lattice constants of monolayer CrSCl and MoTe2 are 3.453 Å and 3.518 Å, respectively, resulting in a small lattice mismatch of 1.80%, which allows the construction of a commensurate 1 × 1 heterostructured supercell. To explore the interfacial properties, we consider eight possible stacking configurations of MoTe2/CrSCl heterostructure, labeled S-1 to S-4 and Cl-1 to Cl-4, depending on whether the S or Cl layer of CrSCl faces the Te atoms of MoTe2 at the interface (see Fig. 1).In S-1 and Cl-1, Te1 atoms are located above Cr atoms, while Mo atoms are above S (in S-1) or Cl (in Cl-1) atoms. In S-2 and Cl-2, Te1 atoms are above Cr atoms, and Mo atoms are above Cl (in S-2) or S (in Cl-2) atoms. In S-3 and Cl-3, Mo atoms are placed above Cr atoms, while Te1 atoms are above Cl (in S-3) or S (in Cl-3) atoms. In S-4 and Cl-4, Mo atoms are above S (in S-4) or Cl (in Cl-4) atoms, and Te1 atoms are above Cl (in S-4) or S (in Cl-4) atoms. In configurations S-2, S-4, Cl-2, and Cl-4, the MoTe2 layer is rotated by 180°, introducing structural asymmetry. Detailed atomic structures are presented in Fig. 1, and further information on each configuration is summarized in Table 1.
| Configuration | S-1 | S-2 | S-3 | S-4 | Cl-1 | Cl-2 | Cl-3 | Cl-4 |
|---|---|---|---|---|---|---|---|---|
| a 0 (Å) | 3.492 | 3.494 | 3.496 | 3.493 | 3.491 | 3.491 | 3.492 | 3.491 |
| E b (eV) | −0.28 | −0.27 | −0.28 | −0.29 | −0.22 | −0.22 | −0.22 | −0.22 |
| d (Å) | 3.21 | 3.26 | 3.17 | 3.12 | 3.33 | 3.35 | 3.37 | 3.33 |
| ΔKK′ (meV) | 27.9 | 35.2 | 33.3 | 6.9 | 4.1 | 5.3 | 6.7 | 0.5 |
To evaluate the relative stability of the different configurations, their binding energies are calculated. The binding energy Eb of each heterostructure is defined by Eb = EMoTe2/CrSCl − EMoTe2 − ECrSCl, where EMoTe2/CrSCl, EMoTe2, ECrSCl are the total energies of the heterostructure, monolayer MoTe2, and monolayer CrSCl, respectively. As shown in Table 1, all stacking configurations exhibit negative binding energies, indicating that they are thermodynamically stable. Notably, the S-side configurations consistently show lower binding energies than the Cl-side ones, suggesting that the S-interface structures are energetically more favorable.
3.2 Origin of the difference in valley splitting magnitude
The valley splitting of valence band maximum (VBM), denoted as ΔKK′, is defined as the energy difference between the VBMs at the K and K′ points. Overall, the S-side configurations exhibit larger valley splitting than their Cl-side counterparts. For detailed analysis, we select the configurations with the largest ΔKK′ values from each group: S-2 (35.2 meV) and Cl-3 (6.7 meV). Although S-2 is not the most stable among the S-side configurations, it is chosen for further investigation due to its prominent valley splitting. The calculated band structures for S-2 and Cl-3, obtained with spin–orbit coupling calculations, are shown in Fig. 2(c) and (e), respectively.To investigate the origin of the difference in valley splitting magnitude, we compare the work functions of monolayer MoTe2 and monolayer CrSCl, as well as the charge density differences in the S-2 and Cl-3 heterostructures, as illustrated in Fig. 2.
The work function of monolayer CrSCl depends on the surface termination, with values of 6.00 eV for the Cl-terminated side and 6.76 eV for the S-terminated side. In contrast, monolayer MoTe2 has a work function of 4.49 eV, as shown in Fig. 2(a). Since the work function of CrSCl is higher than that of MoTe2 on both surfaces, electrons are transferred from MoTe2 to CrSCl at the interface. Fig. 2(b) and (d) show the calculated charge density differences for the S-2 and Cl-3 configurations, respectively. A more pronounced charge redistribution is observed at the interface in the S-2 configuration compared to Cl-3. To quantify the interlayer charge transfer, we employ Bader charge analysis,50 revealing that 0.037 electrons are transferred from MoTe2 to CrSCl in S-2, whereas only 0.018 electrons are transferred in Cl-3. This difference can be attributed to the high electronegativity of Cl atoms, which tend to attract more charge than S atoms. As a result, stronger electron repulsion occurs on the Cl-terminated side, inhibiting interlayer charge transfer in Cl-3 compared to S-2.51 These results indicate that interlayer charge transfer plays a crucial role in determining the magnitude of valley splitting. This also explains why the Cl-side stacking is energetically less favorable and exhibits a larger interlayer distance: all Cl-side configurations already have more accumulated charge on the interfacial Cl atoms, which suppresses further charge transfer and weakens the interlayer coupling.
To further understand why S-2 exhibits the largest valley splitting among the S-side configurations, we first analyze the interlayer charge transfer in each case. The calculated charge transfer amounts for S-1 through S-4 are 0.039 e−, 0.037 e−, 0.042 e−, and 0.042 e−, respectively. Interestingly, S-2 shows the smallest interlayer charge transfer, despite exhibiting the largest valley splitting. To clarify this counterintuitive result, we examine the projected density of states (PDOS) for each configuration, as shown in Fig. 3. In S-2, strong hybridization occurs between Mo, Te1, and S atoms at the interface (Fig. 3(b)), resulting in the largest valley splitting. By contrast, S-1, S-3, and S-4 exhibit weaker hybridization (Fig. 3(a), (c), and (d)) and smaller valley splitting compared to S-2. In addition, in all cases, the VBM originates from the MoTe2 layer, while the conduction band minimum (CBM) is mainly contributed by the CrSCl layer. This confirms that all heterostructures exhibit staggered (type-II) band alignment,52 resulting in spatial separation of electrons and holes.
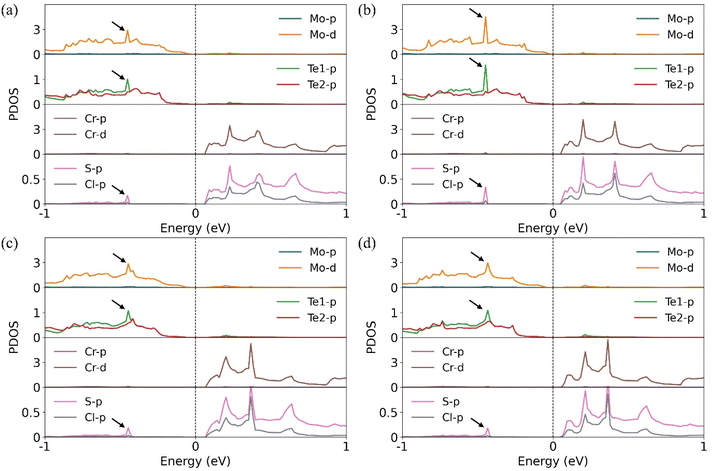 | ||
| Fig. 3 Projected density of states (PDOS) for each configuration: (a) S-1 (b) S-2 (c) S-3 and (d) S-4. The Fermi level is set to zero. | ||
These results suggest that both interlayer charge transfer and interfacial orbital hybridization contribute to the magnitude of valley splitting, with the latter playing a particularly important role in the case of S-2. In addition, S-4 and Cl-4 exhibit the weakest splitting, as their Mo atomic positions are not in good spatial superposition, that is, not vertically aligned with the magnetic Cr atoms.35
3.3 Tunable valley splitting via electric fields and strains
Next, we explore strategies for tuning the magnitude of valley splitting. Although the binding energies of all stacking configurations are quite similar, a mixture of different configurations may exist in experiments. Nevertheless, we focus on the S-2 configuration because it shows the largest valley splitting, making it the most representative case for exploring the tunability of valley splitting. As mentioned above, the valley splitting is closely related to the extent of interlayer charge transfer. External electric fields and biaxial strain can effectively tune the band alignment of the heterostructure, thereby modulating the interlayer charge transfer.53–55 To examine this effect, we first apply an out-of-plane (z-direction) electric field since the charge transfer from MoTe2 to CrSCl occurs predominantly along this direction. The resulting band structures under various electric field strengths are shown in Fig. 4. To enhance the charge transfer from MoTe2 to CrSCl, a positive electric field is applied. This leads to increased charge transfer, causing the CBM and VBM to move closer together, thereby enhancing the valley splitting, as shown in Fig. 4(a)–(c). In contrast, applying a negative electric field reduces the charge transfer from MoTe2 to CrSCl. This results in greater separation between the CBM and VBM and a corresponding decrease in valley splitting, as shown in Fig. 4(d)–(f). Table 2 summarizes the calculated charge transfer amounts and corresponding valley splitting values under various electric field conditions. | ||
| Fig. 4 Calculated band structures of the S-2 configuration under external electric fields: (a) 0.2 V Å−1, (b) 0.3 V Å−1, (c) 0.4 V Å−1, (d) −0.2 V Å−1, (e) −0.3 V Å−1, and (f) −0.4 V Å−1. | ||
| Electric field | −0.4 V Å−1 | −0.3 V Å−1 | −0.2 V Å−1 | 0 V Å−1 | 0.2 V Å−1 | 0.3 V Å−1 | 0.4 V Å−1 |
|---|---|---|---|---|---|---|---|
| Charge transfer (e−) | 0.02 | 0.024 | 0.028 | 0.037 | 0.048 | 0.053 | 0.059 |
| ΔKK′ (meV) | 14.5 | 18.5 | 23.4 | 35.2 | 50.4 | 58.9 | 65.6 |
Likewise, in-plane biaxial strain can also modulate the interlayer coupling and hence the valley splitting.32 The biaxial strain is defined as
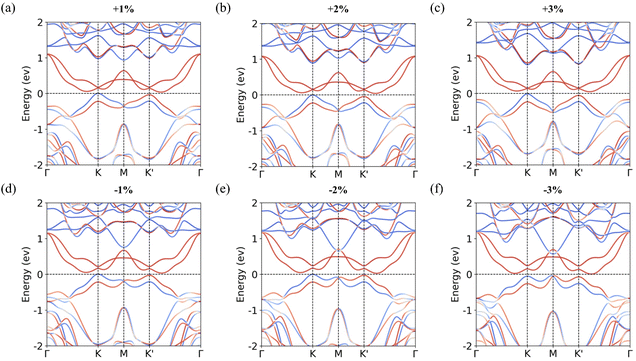 | ||
| Fig. 5 Calculated band structures for the S-2 configuration with in-plane biaxial strain. (a) ε = 1%, (b) ε = 2%, (c) ε = 3%, (d) ε = −1%, (e) ε = −2%, and (f) ε = −3%. | ||
To understand these variations, we analyze the interlayer distance and the amount of interlayer charge transfer, as summarized in Table 3. When tensile strain is applied, the interlayer distance decreases, thereby strengthening interlayer coupling.56 This stronger coupling promotes greater interlayer charge transfer, resulting in an increase in valley splitting. Conversely, the interlayer distance increases under compressive strain, the interlayer coupling strength becomes weaker, leading to reduced charge transfer and a corresponding decrease in valley splitting. Notably, under 3% compressive strain [Fig. 5(f)], the VBM at the M point surpasses that at the K and K′ points, causing the valley characteristics of the MoTe2/CrSCl heterostructure to disappear. Furthermore, a previous study has reported that in-plane tensile strain can enhance the Curie temperature (Tc) of monolayer CrSCl.38 Therefore, applying in-plane tensile strain serves as an effective approach to simultaneously enhance both the valley splitting and the Curie temperature in the MoTe2/CrSCl heterostructure.
| Strain | −3% | −2% | −1% | 0% | +1% | +2% | +3% |
|---|---|---|---|---|---|---|---|
| d (Å) | 3.33 | 3.3 | 3.28 | 3.26 | 3.23 | 3.2 | 3.18 |
| Charge transfer (e−) | 0.033 | 0.034 | 0.036 | 0.037 | 0.039 | 0.041 | 0.043 |
| ΔKK′ (meV) | 18.6 | 25.7 | 30.2 | 35.2 | 39.5 | 43.2 | 43.2 |
The overall trends of the valley splitting and interlayer charge transfer at the VBMs at the K and K′ point, as functions of the applied electric field and biaxial strain, are illustrated in Supplementary Fig. S1.
3.4 Anomalous valley Hall effect
In the MoTe2/CrSCl heterostructure, the breaking of both inversion symmetry and time-reversal symmetry gives rise to a nonzero Berry curvature at the K and K′ points, with opposite signs and differing magnitudes. The Berry curvature for all occupied states, derived from the Kubo formula, is defined by57,58where fn is the Fermi-Dirac distribution function, vx and vy are the velocity operators, and ψnk is the Bloch wavefunction associated with eigenvalue En. This Berry curvature induces an anomalous velocity of carriers at the K and K′ valleys, expressed as v ∼ E × Ωz(k),59 where E and Ωz(k) are the applied in-plane electric field and Berry curvature, respectively. When the Fermi level lies between the VBM of the K and K′ valleys, hole doping occurs only in the valley with the higher VBM energy. These carriers acquire an anomalous transverse velocity, resulting in valley-polarized transport, a phenomenon known as the anomalous valley Hall effect.27
To achieve a pronounced anomalous valley Hall effect and further enhance valley splitting, we apply a combination of a 0.2 V Å−1 out-of-plane electric field and 3% in-plane biaxial tensile strain—two modulation strategies previously shown to be effective. The resulting band structure, shown in Fig. 6(b), reveals a substantial valley splitting of 63.3 meV, along with hole doping in the K valley. Fig. 6(a) illustrates the Berry curvature under these conditions as a contour map in the 2D Brillouin zone and along a high-symmetry path, respectively. The Berry curvature at the K valley reaches −23.08 Å2. When an in-plane electric field is applied, spin-down hole carriers at the K valley acquire an upward anomalous velocity and accumulate along one side of the sample, as schematically depicted in Fig. 6(c). This results in a spin- and valley-dependent Hall voltage.
In realistic conditions, however, the Fermi level may not be precisely located between the two valleys. Even in such cases, a measurable transverse valley Hall voltage can still arise because the Berry curvature magnitudes at the K (23.08 Å2) and K′ (20.85 Å2) valleys are not identical. This asymmetry produces unequal anomalous velocities for carriers in the two valleys, leading to a net valley Hall voltage and maintaining spin-polarized transport due to the intrinsic spin–valley locking of the heterostructure.60
Under these combined conditions, the MoTe2/CrSCl heterostructure exhibits both a pronounced anomalous valley Hall effect and a high Curie temperature, making it a promising candidate for next-generation, high-performance valleytronic applications.
4 Conclusion
In summary, we conducted a comparative study of MoTe2/CrSCl heterostructures using DFT calculations to investigate the key factors affecting valley splitting. Our results reveal that interlayer charge transfer and interfacial orbital hybridization play critical roles in determining the magnitude of valley splitting. To further control the magnitude of the valley splitting, we applied both an external electric field and in-plane biaxial strain, each of which effectively influences the interlayer charge transfer. Under a positive electric field, the valley splitting increased significantly, while a slight increase was observed under biaxial tensile strain. When a 0.2 V Å−1 electric field and 3% biaxial tensile strain were applied simultaneously, the valley splitting reached 63.3 meV, accompanied by hole doping at the K point. This hole occupation at the K valley facilitates valley-polarized transport. These findings suggest that the MoTe2/CrSCl heterostructure is a promising candidate for future valleytronic device applications.Author contributions
Jaehong Park: conceptualization, investigation, formal analysis, visualization, data curation, writing – original draft. Dongchul Sung: formal analysis, writing – review and editing. Junho Yun: formal analysis. Suklyun Hong: conceptualization, investigation, formal analysis, supervision, writing – review and editing, resources, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The structure data for each stacking configuration can be found at https://doi.org/10.5281/zenodo.16760791.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5na00834d.
Acknowledgements
This work was supported by the NRF (RS-2024-00355708, RS-2024-00436306, 2022R1I1A1A01070953, and 2022M3H4A1A04096396) and the IITP (RS-2024-00437191) funded by the Ministry of Science and ICT (MSIT), Korea.References
- O. Gunawan, Y. Shkolnikov, K. Vakili, T. Gokmen, E. De Poortere and M. Shayegan, Phys. Rev. Lett., 2006, 97, 186404 CrossRef CAS PubMed.
- A. Rycerz, J. Tworzydło and C. Beenakker, Nat. Phys., 2007, 3, 172–175 Search PubMed.
- D. Xiao, W. Yao and Q. Niu, Phys. Rev. Lett., 2007, 99, 236809 CrossRef PubMed.
- J. R. Schaibley, H. Yu, G. Clark, P. Rivera, J. S. Ross, K. L. Seyler, W. Yao and X. Xu, Nat. Rev. Mater., 2016, 1, 16055 CrossRef CAS.
- Y. S. Ang, S. A. Yang, C. Zhang, Z. Ma and L. K. Ang, Phys. Rev. B, 2017, 96, 245410 CrossRef.
- S. A. Vitale, D. Nezich, J. O. Varghese, P. Kim, N. Gedik, P. Jarillo-Herrero, D. Xiao and M. Rothschild, Small, 2018, 14, 1801483 CrossRef PubMed.
- L. Li, L. Shao, X. Liu, A. Gao, H. Wang, B. Zheng, G. Hou, K. Shehzad, L. Yu and F. Miao, Nat. Nanotechnol., 2020, 15, 743–749 CrossRef CAS PubMed.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- Z. Y. Zhu, Y. C. Cheng and U. Schwingenschlögl, Phys. Rev. B, 2011, 84, 153402 CrossRef.
- D. Xiao, G.-B. Liu, W. Feng, X. Xu and W. Yao, Phys. Rev. Lett., 2012, 108, 196802 CrossRef PubMed.
- F. Bussolotti, H. Kawai, Z. E. Ooi, V. Chellappan, D. Thian, A. L. C. Pang and K. E. J. Goh, Nano Futures, 2018, 2, 032001 CrossRef.
- Y. Liu, Y. Gao, S. Zhang, J. He, J. Yu and Z. Liu, Nano Res., 2019, 12, 2695–2711 CrossRef CAS.
- S. Zhao, X. Li, B. Dong, H. Wang, H. Wang, Y. Zhang, Z. Han and H. Zhang, Rep. Prog. Phys., 2021, 84, 026401 CrossRef PubMed.
- H. Zeng, J. Dai, W. Yao, D. Xiao and X. Cui, Nat. Nanotechnol., 2012, 7, 490–493 CrossRef CAS PubMed.
- K. F. Mak, K. He, J. Shan and T. F. Heinz, Nat. Nanotechnol., 2012, 7, 494–498 CrossRef CAS PubMed.
- K. F. Mak, K. L. McGill, J. Park and P. L. McEuen, Science, 2014, 344, 1489–1492 CrossRef CAS PubMed.
- K. F. Mak, D. Xiao and J. Shan, Nat. Photonics, 2018, 12, 451–460 CrossRef CAS.
- Y. Li, J. Ludwig, T. Low, A. Chernikov, X. Cui, G. Arefe, Y. D. Kim, A. M. Van Der Zande, A. Rigosi and H. M. Hill, Phys. Rev. Lett., 2014, 113, 266804 CrossRef PubMed.
- D. MacNeill, C. Heikes, K. F. Mak, Z. Anderson, A. Kormányos, V. Zólyomi, J. Park and D. C. Ralph, Phys. Rev. Lett., 2015, 114, 037401 CrossRef CAS PubMed.
- A. Srivastava, M. Sidler, A. V. Allain, D. S. Lembke, A. Kis and A. Imamoğlu, Nat. Phys., 2015, 11, 141–147 Search PubMed.
- G. Aivazian, Z. Gong, A. M. Jones, R.-L. Chu, J. Yan, D. G. Mandrus, C. Zhang, D. Cobden, W. Yao and X. Xu, Nat. Phys., 2015, 11, 148–152 Search PubMed.
- Y. Cheng, Q. Zhang and U. Schwingenschlögl, Phys. Rev. B, 2014, 89, 155429 CrossRef.
- R. Peng, Y. Ma, S. Zhang, B. Huang and Y. Dai, J. Phys. Chem. Lett., 2018, 9, 3612–3617 CrossRef CAS PubMed.
- Q. Li, X. Zhao, L. Deng, Z. Shi, S. Liu, Q. Wei, L. Zhang, Y. Cheng, L. Zhang and H. Lu, ACS Nano, 2020, 14, 4636–4645 CrossRef CAS PubMed.
- M. Bora and P. Deb, J. Phys-Mater., 2021, 4, 034014 CrossRef CAS.
- E. M. Choi, K. I. Sim, K. S. Burch and Y. H. Lee, Adv. Sci., 2022, 9, 2200186 CrossRef CAS PubMed.
- W. Y. Tong, S. J. Gong, X. Wan and C. G. Duan, Nat. Commun., 2016, 7, 13612 CrossRef CAS PubMed.
- J. Qi, X. Li, Q. Niu and J. Feng, Phys. Rev. B, 2015, 92, 121403 CrossRef.
- Q. Zhang, S. A. Yang, W. Mi, Y. Cheng and U. Schwingenschlögl, Adv. Mater., 2016, 28, 959–966 CrossRef CAS PubMed.
- X. Liang, L. Deng, F. Huang, T. Tang, C. Wang, Y. Zhu, J. Qin, Y. Zhang, B. Peng and L. Bi, Nanoscale, 2017, 9, 9502–9509 RSC.
- C. Zhao, T. Norden, P. Zhang, P. Zhao, Y. Cheng, F. Sun, J. P. Parry, P. Taheri, J. Wang and Y. Yang, Nat. Nanotechnol., 2017, 12, 757–762 CrossRef CAS PubMed.
- L. Xu, M. Yang, L. Shen, J. Zhou, T. Zhu and Y. P. Feng, Phys. Rev. B, 2018, 97, 041405 CrossRef CAS.
- G. Yang, J. Li, H. Ma, Y. Yang, C. Li, X. Mao and F. Yin, Phys. Rev. B, 2018, 98, 235419 CrossRef CAS.
- K. L. Seyler, D. Zhong, B. Huang, X. Linpeng, N. P. Wilson, T. Taniguchi, K. Watanabe, W. Yao, D. Xiao and M. A. McGuire, Nano Lett., 2018, 18, 3823–3828 CrossRef CAS PubMed.
- Z. Zhang, X. Ni, H. Huang, L. Hu and F. Liu, Phys. Rev. B, 2019, 99, 115441 CrossRef CAS.
- T. Hu, G. Zhao, H. Gao, Y. Wu, J. Hong, A. Stroppa and W. Ren, Phys. Rev. B, 2020, 101, 125401 CrossRef CAS.
- Y. Qi, C. Yao, J. Zhao and H. Zeng, Phys. Rev. B, 2023, 108, 125304 CrossRef CAS.
- S. D. Guo, X. S. Guo, Y. T. Zhu and Y. S. Ang, Appl. Phys. Lett., 2022, 121, 062403 CrossRef CAS.
- Y. Hou, F. Xue, L. Qiu, Z. Wang and R. Wu, npj Comput. Mater., 2022, 8, 120 CrossRef CAS.
- H. Zeng, W. Zhang, C. Qiu, J. Zhao and D. Ding, Appl. Phys. Lett., 2025, 126, 061603 CrossRef CAS.
- Y. Qi, J. Zhao and H. Zeng, Appl. Phys. Lett., 2024, 125, 081603 CrossRef CAS.
- J. Zhao, X. Jin, H. Zeng, C. Yao and G. Yan, Appl. Phys. Lett., 2021, 119, 213101 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B, 1996, 54, 11169 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B, 1994, 50, 17953 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B, 1999, 59, 1758 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- H.-J. Kim, C. Li, J. Feng, J.-H. Cho and Z. Zhang, Phys. Rev. B, 2016, 93, 041404 CrossRef.
- G. Henkelman, A. Arnaldsson and H. Jónsson, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- H. Chen, J. Han, X. Deng, Z. Fan, L. Sun and Z. Zhang, Appl. Surf. Sci., 2022, 598, 153756 CrossRef CAS.
- S. Wang, H. Tian, C. Ren, J. Yu and M. Sun, Sci. Rep., 2018, 8, 12009 CrossRef PubMed.
- B. Zhou, Z. Li, J. Wang, X. Niu and C. Luan, Nanoscale, 2019, 11, 13567–13575 RSC.
- S. Wang and J. Yu, Thin Solid Films, 2018, 654, 107–115 CrossRef CAS.
- S. Wang and J. Yu, Appl. Phys. A, 2018, 124, 487 CrossRef.
- S. Pak, J. Lee, Y.-W. Lee, A.-R. Jang, S. Ahn, K. Y. Ma, Y. Cho, J. Hong, S. Lee and H. Y. Jeong, Nano Lett., 2017, 17, 5634–5640 CrossRef CAS PubMed.
- D. J. Thouless, M. Kohmoto, M. P. Nightingale and M. den Nijs, Phys. Rev. Lett., 1982, 49, 405 CrossRef CAS.
- Y. Yao, L. Kleinman, A. MacDonald, J. Sinova, T. Jungwirth, D.-s. Wang, E. Wang and Q. Niu, Phys. Rev. Lett., 2004, 92, 037204 CrossRef PubMed.
- D. Xiao, Y. Yao, Z. Fang and Q. Niu, Phys. Rev. Lett., 2006, 97, 026603 CrossRef PubMed.
- S. Wang, Mod. Phys. Lett. B, 2025, 39, 2530002 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |