Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Helical-structure transition and color changes in aromatic polyacetylenes under mechanochemical conditions: effect of the additive-alcohol chain length
Haruki
Ikushima
 a and
Yasuteru
Mawatari
a and
Yasuteru
Mawatari
 *ab
*ab
aGraduate School of Engineering, Muroran Institute of Technology, 27-1 Mizumoto-cho, Muroran, Hokkaido 050-8585, Japan. E-mail: mawatari@muroran-it.ac.jp
bThe Center for Rare Earths Research, Muroran Institute of Technology, 27-1 Mizumoto-cho, Muroran, Hokkaido 050-8585, Japan
First published on 9th October 2025
Abstract
This study investigates the control of higher-order polymer structures through mechanochemical (MC) synthesis, focusing on poly(2-ethynylnaphthalene) (P2EN) as a model helical poly(arylacetylene). The products of MC synthesis using linear alcohols (C1–C22) as additives were compared with those of conventional solution synthesis using these alcohols as solvents. Interestingly, the polymer color, which depends on helical conformation, is directly influenced by the carbon-chain length of the alcohol additive in MC synthesis. Short-chain alcohols produce yellow P2EN with extended helices (the cis-transoid structure), whereas long-chain alcohols yield red P2EN with contracted helices (the cis-cisoid structure). This structure-dependent color variation is exclusive to MC synthesis; solution polymerization consistently produces yellow P2EN with extended helices, regardless of the alcohol used. The results of this study suggest that under MC conditions, localized heat and pressure facilitate transitions from metastable cis-transoid to stable cis-cisoid conformations through specific high-affinity polymer/additive interactions. Thus, MC synthesis with appropriate additives can direct the synthesis of polymers with thermodynamically stable higher-order structures. The proposed approach offers an environmentally friendly method for controlling the conformation (and material properties) of polymers, potentially enabling the green industrial production of functional polymer materials.
Introduction
In mechanochemical (MC) synthesis, chemical reactions are induced by mechanical energy. Recently, owing to its solvent-free nature, MC synthesis has attracted significant attention in green chemistry.1–4 To establish this method as a major synthesis technique alongside solution synthesis, investigating the advantages specific to MC synthesis, such as improved reactivity and the ability to form unique structures, is vital. The neat grinding (NG) method, which uses no additives, involves mild reaction conditions5 and improved reactivities of poorly soluble compounds.6 Various additive methods are being investigated for reactivity improvements that cannot be ensured by using NG alone. Liquid-assisted grinding (LAG), which involves grinding with small amounts of liquid, is a commonly used method involving additives that can be used to control reaction pathways, improve reactivity,7 promote post-polymerization polymer modification, and suppress polymer-chain cleavage caused by mechanical stimulation.8 Ion- and liquid-assisted grinding (ILAG), which involves grinding with catalytic amounts of ionic salts and liquid additives, enables the quantitative and selective conversion of bismuth salicylate into various forms.9 Ionic liquid-assisted grinding (IL-AG) improves the rate of fluorination for aromatic and 1,3-dicarbonyl compounds.10 Polymer-assisted grinding (POLAG), which involves grinding with organic polymers, improves the efficiency of the solid-state cross-coupling of insoluble aryl halides11 and various other reactions, such as regioselective oxidative Heck coupling.12 Moreover, these additives can control the higher-order structures of products, such as the structures of metal–organic frameworks (MOFs) and the polymorphs of cocrystals. Friščić et al. reported on the structural control of products in the ILAG-based synthesis of MOFs.13 Hasa et al. showed that polymorphs can be structurally controlled by tuning the number of monomer units of polyethylene glycol added during the cocrystallization reaction of caffeine and glutaric acid.14 Although numerous studies investigate improvements in polymerization,15,16 the effect of additives on the higher-order structures of polymers remains largely unexplored to date. Controlling the higher-order structure of polymers, which significantly affects their functionality as materials, is extremely important.Previous studies have focused on controlling the higher-order structures of aromatic-substituted polyacetylenes (PAAs), a type of synthetic helical polymer.17–22 On polymerizing monomers in solution with the catalyst [Rh(norbornadiene)Cl]2-NEt3, the polymerization proceeds via a coordination-insertion mechanism23 (Fig. 1A), producing polymers with cis-configured geometric structures in the main chain.24–26 The secondary structure of these polymers comprises a helix, and their color varies with the solvent used, likely owing to different degrees of helix elongation and contraction in different solvents.27–30 Specifically, poly(2-ethynylnaphtalene) (P2EN) is obtained as a yellow powder consisting of an extended helical structure called cis-transoid (ct) when synthesized in ethanol, and as a red powder consisting of a contracted helical structure called cis-cisoid (cc) when synthesized in toluene (TOL) (Fig. 1B).31 Furthermore, upon immersion in TOL, P2EN powder with a metastable extended helical structure undergoes solid-state helical contraction, transitioning to a relatively stable contracted helical structure (Fig. 1B). As changes in the secondary structure of this polymer are accompanied with a visual color change, P2EN was selected for examining the effects of additives on secondary-structure changes in MC reactions.
A recent study demonstrated the MC polymerization of phenylacetylene-based monomers using the [Rh(norbornadiene)Cl]2-NEt3 catalyst system.32 The MC polymerization of 4-methoxycarbonylphenylacetylene, with the solid linear alcohol 1-hexadecanol as an additive, yields polymers without significantly decreasing both the cis content of the main chain and molecular weight of the polymer, indicating that the presence of additives is essential for the MC synthesis of aromatic polyacetylenes with high cis content in the main chains that can easily isomerize under pressure. Based on this research, to expand the range of applicable monomers for MC polymerization using the [Rh(norbornadiene)Cl]2-NEt3 catalyst system, the MC polymerization of 2EN, a naphthylacetylene-based monomer, was attempted. Using 1-hexadecanol as an additive produces a red powder, whereas LAG with EtOH, a liquid alcohol used in conventional solution polymerization, yields a yellow powder. Thus, in the MC synthesis of P2EN, the choice of additive influences the polymer color control, i.e., the formation of higher-order structures.
This study demonstrated the MC synthesis of P2EN using linear-alcohol additives with carbon numbers within 1–22 and examined the relationship between the carbon chains of the additives and the color difference depending on the helical structure of the synthesized P2ENs. Interestingly, when linear alcohols with different carbon numbers were used as additives in the MC synthesis of P2EN, the color of the synthesized polymers varied from yellow to red depending upon the carbon number (Fig. 1C). Furthermore, to investigate whether this phenomenon is specific to MC synthesis, the MC synthesis of P2EN was compared with solution synthesis using heat-melted alcohols as solvents. The color-control of polymers produced by the MC synthesis of P2EN through the choice of additives comprises a new strategy for higher-order polymer-structure modulation through additive selection in MC synthesis.
Results and discussion
Synthesis method-dependent color of polymers
MC polymerization was conducted using linear alcohols with different carbon numbers as additives. A high cis content is required for helical structure formation, which is the origin of the color variation observed for P2EN. The MC polymerization of 4-methoxycarbonylphenylacetylene using only monomers, Rh catalysts, and Et3N results in the formation of polymers with a low molecular weight and cis content.32 Interestingly, the addition of 1-hexadecanol (C16OH), a solid alcohol that does not participate in the reaction, to the MC polymerization system suppresses the decrease in molecular weight and cis–trans isomerization. In this study, MC polymerization using C16OH and EtOH, which is commonly used as a solvent in conventional solution synthesis, as additives resulted in the formation of red (Table 1, entry 6) and yellow (Table 1, entry 2) polymers, respectively. The Raman spectra of these polymers, as described in subsequent section, shows a high cis content. Therefore, similar to solid additives, liquid additives function as cushions that suppress cis-to-trans isomerization. The colors of polymers synthesized using linear alcohols with different carbon numbers as additives differed. Therefore, the MC polymerization of 2EN was attempted using various linear alcohols with carbon numbers within 1–22 as additives to clarify the relationship between the carbon number of the linear alcohol used as an additive and the color of the resulting polymer (Table 1, entry 1, 3, 4, 5, and 7). Regarding polymer abbreviations, the numbers represent the carbon number of the linear alcohol used as an additive or solvent, and “N” signifies cases where no additive was used. Additionally, for synthesis methods, MC synthesis is represented as “(M)” and solution synthesis as “(S)”. P1(M) is yellow; P4(M), P8(M), and P12(M) are orange; and P22(M) is almost the same red as P16(M). When hydrocarbons with carbon chains similar to those of the added alcohols were used as additives, the colors of the resulting polymers were almost identical. According to the literature, polymers derived from 2EN show variations in color depending on the type of polymerization solvent used in solution polymerization.31 However, in this study, the use of various alcohols as additives in solution synthesis resulted in yellow polymers in all cases (Table 1, entry 8–14). Interestingly, only in MC polymerization did the difference in the carbon number of the alcohol additive manifest as a difference in the color of P2EN. In contrast, PN(M), generated by NG without additives, was brown, which is distinct from the other polymers (entry 15 in Table 1 and Fig. S1). PN(M) is the only compound soluble in several organic solvents such as CHCl3, THF, and toluene because of its extremely low Mn and cis content (Table S1), i.e., pressure-induced chain scission and isomerization to the trans structure, as indicated by the GPC trace in Fig. S1 and the Raman spectra shown in Fig. 3.| Entry | P2EN | Polymerization temp. (°C) | H(CH2)nOH | Yield (%) | Color |
|---|---|---|---|---|---|
| a Reaction time of 30 min. b Conditions of the MC method: molar ratio of (2EN/Rh cat./Et3N) = 100/1/50. The weight of the 2EN and additive are 300 and 600 mg, respectively. c Conditions of the solution method: [2EN] = 0.2 mol L−1, molar ratio of [2EN]/[Rh cat.]/[Et3N] = 100/1/50. | |||||
| 1 | P1(M) | — | 1 | 88 |

|
| 2 | P2(M) | — | 2 | 96 |

|
| 3 | P4(M) | — | 4 | 94 |

|
| 4 | P8(M) | — | 8 | 93 |

|
| 5 | P12(M) | — | 12 | 91 |

|
| 6 | P16(M) | — | 16 | 97 |

|
| 7 | P22(M) | — | 22 | 75 |

|
| 8 | P1(S) | 60 | 1 | 98 |

|
| 9 | P2(S) | 75 | 2 | 99 |

|
| 10 | P4(S) | 75 | 4 | 97 |

|
| 11 | P8(S) | 75 | 8 | 98 |

|
| 12 | P12(S) | 75 | 12 | 99 |

|
| 13 | P16(S) | 75 | 16 | 96 |

|
| 14 | P22(S) | 75 | 22 | 99 |

|
| 15 | PN(M) | — | — | 77 |

|
The absorption maxima (λmax) in diffuse reflective UV–vis (DR UV–vis) spectra of P1(M), P2(M), P4(M), P8(M), and P12(M) were observed at ∼450 nm, while those of P16(M) and P22(M) were observed at ∼500 nm (Fig. 2). In the case of previous P2EN, the λmax of the ct and cc forms are observed at 445 and 510 nm, respectively.31 Therefore, the observed data suggest the formation of the ct structure for P1(M), P2(M), P4(M), P8(M), and P12(M), and cc structure for P16(M) and P22(M). Notably, the λmax of all solution-synthesized polymers was ∼450 nm, indicating the formation of ct structures. Interestingly, the λmax for P8(S)–P22(S), obtained through solution synthesis using alcohols with relatively long alkyl-chain lengths as solvents, remained unchanged at 445 nm; however, the absorption intensity near 530 nm increased slightly. These spectral features indicate the slight formation of the cc structure, which likely imparts a slight redness to the polymer. Therefore, in solution synthesis, as the alkyl-chain length of the solvent increases, the hydrophobic interactions between the solvent and polymer molecules become stronger, resulting in the partial formation of the relatively stable cc structure. This tendency is remarkably pronounced in MC synthesis, suggesting that P16(M) and P22(M) produce large amounts of the cc structure.
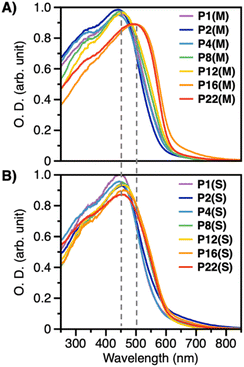 | ||
| Fig. 2 DRUV–vis spectra of P2ENs synthesized by (A) the MC method and (B) the solution synthesis observed on alumina powders. | ||
Raman study
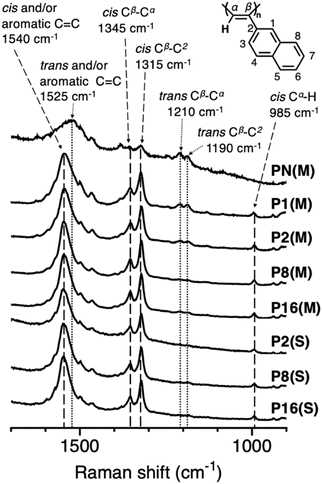
The cis content in the geometric structure of the main chain of the substituted polyacetylenes was estimated using solution-state 1H-NMR measurements. Because all P2ENs other than PN(M) are insoluble in common organic solvents, resonance Raman spectra were measured to qualitatively evaluate the geometric structure of the main chain, and each peak was assigned based on the literature (Fig. 3).31,33 In the spectrum of PN(M) without additives, intense peaks assigned to the trans structure were observed, while the peak assigned to cis Cα–H (at 985 cm−1) was not observed. Additionally, because only PN(M) is soluble in organic solvents, its 1H-NMR spectrum was measured (Fig. S2), and its cis content was calculated to be 10%, indicating that the majority of the main chain is in the trans structure. According to the literature, P2EN synthesized in EtOH shows a cis content of 22% when heat-treated at 200 °C for 1 min. The results of this study indicate that the energy generated by the ball-milling process used in this study likely exceeds the energy generated by heat treatment at 200 °C for 1 min.31 Notably, the Raman spectra of P2ENs obtained by MC synthesis and solution synthesis with additives contained intense peaks assigned to the cis structure, indicating that the majority of the main chain is in the cis structure. For these P2ENs, differences are observed in the peak-intensity ratio of the peak at 1210 cm−1 (attributed to trans Cβ–Cα) to that at 1345 cm−1 (due to cis Cβ–Cα); PN(M), P1(M), P2(M), and the other samples show approximate peak-intensity ratios of 1, 0.4, 0.2, and 0.1, respectively. This result suggests that using additive alcohols with a high carbon number in MC synthesis can effectively suppress the cis-to-trans isomerization of the polymer main chain. In resonance Raman spectroscopy, peaks from components with absorption bands near the irradiation wavelength, in this case 532 nm (corresponding to the trans form), were enhanced.34,35DSC study
The DSC traces of P2ENs were measured at a heating rate of 10 °C min−1 under Ar (Fig. 4). The trace shows two exothermic peaks at around 210 °C and 235 °C in all polymers. According to previous reports, these exothermic peaks can be attributed to the thermal cis-to-trans isomerization of the ct and cc structures, which comprise extended and contracted helical structures, respectively.31 However, the exothermic peak position observed in this study (near 235 °C) is at a lower temperature than that reported previously (247 °C). The cis-to-trans isomerization energy required for units located inside the cc sequence is expected to be greater than that required for units near the boundary with the ct structure (Fig. 5) because the highly regular π-stacking between the naphthyl groups of side chains formed by units inside the cc sequence hinders the rotational motion of the naphthyl groups, triggering cis-to-trans isomerization. Therefore, the shift of this peak to a lower temperature indicates a higher proportion of low-regularity chains between the naphthyl groups, i.e., in the boundary regions with ct. Thus, the appearance of the exothermic peak at around 235 °C indicates that the cc shows a relatively short persistence length. This hypothesis is validated by the XRD pattern of P2ENs; the peak intensity at 3.4 Å (corresponding to the distance between naphthyl groups in the side chains of cc) is extremely weak (Fig. S3). To represent the ratio of cc to ct in the polymer, the cc ratio was defined (Rcc = peak intensity near 235 °C/peak intensity at 210 °C), which is ∼1.0 for P2(M), P2(S), P8(S), and P16(S), 1.4 for P8(M), and 2.2 for P16(M). While the Rcc of all solution-synthesized P2ENs is almost the same, the Rcc of MC-synthesized P2ENs increases with increasing carbon number of the alcohol additive. Interestingly, even the spectrum of P16(M), which is a red sample, contains an exothermic peak attributed to the cis-to-trans isomerization of ct at approximately 210 °C. Notably, the spectrum of red ccP2EN synthesized in TOL does not contain an exothermic peak at 210 °C. Since the majority of P16(M) was confirmed to be cc with some ct, the ct formed through polymerization likely transitioned to cc under continuous MC conditions. This phenomenon is likely because the method used in this study differs from previously reported methods of cc formation, which involve polymerization using solvents with high affinity for the polymer, immersion to the solvents, or the heat-treatment of ct-formed polymers.31 | ||
| Fig. 5 Relationship between the persistence length of cc and the energy of cis-to-trans isomerization. | ||
Transition from ct to cc in the MC synthesis environment
To investigate the effect of the MC synthesis duration on the Rcc of the produced polymer, polymers were synthesized at reaction times of 5, 1, and 1/6 min (Table 2). Surprisingly, a yellow sample is synthesized within 10 s (P16(M)-1/6). On increasing the reaction time, the redness of the polymers gradually increases, with orange and red samples synthesized within 5 and 30 min, respectively (P16(M)-5 and P16(M), respectively) (entry 1 in Table 2 and entry 6 in Table 1, respectively). This time-dependent change suggests an increase in the Rcc of the polymer during MC synthesis.| Entry | P2EN | H(CH2)nOH | Ball-milling time (min) | Yield (%) | Color |
|---|---|---|---|---|---|
| a Mechanochemical method: molar ratio of (2EN/Rh cat./Et3N) = 100/1/50. The weight of the 2EN and additive are 300 and 600 mg, respectively. | |||||
| 1 | P16(M)-5 | 16 | 5 | 69 |

|
| 2 | P16(M)-1 | 16 | 1 | 39 |

|
| 3 | P16(M)-1/6 | 16 | 1/6 | 20 |

|
The Rcc of P16(M)-1/6, P16(M)-1, and P16(M)-5 determined from DSC are 0.9, 1.3, and 1.6, respectively (Fig. 6). Thus, the Rcc increases with increasing reaction time, indicating that the ct formed though polymerization transitions to cc under continuous MC conditions. Additionally, the exothermic peak attributed to the thermal cis-to-trans isomerization of cc was observed at 240 °C in all cases, indicating that the persistence length of cc remained constant, regardless of the reaction time.
Interestingly, in solution synthesis, alcohols with different carbon numbers do not produce polymers with different Rcc values (entry 8–14 in Table 1), indicating that contact with long-chain alcohols is not the only reason for the ct to cc transition of P16(M). Compared with ambient temperature and pressure conditions, the unique environment generated during MC synthesis, including localized heat and pressure, likely enhances the affinity between long-chain alcohol and polymer molecules. Consequently, long-chain alcohols can interact strongly with polymers frozen in the metastable ct structure, thereby undergoing facile transition to the stable cc structure. Moreover, on ball-milling P2(S), previously synthesized in EtOH, with 1-hexadecanol for 30 min, the sample becomes slightly reddish and the Rcc hardly increases. Thus, for facile ct to cc transition, polymer chains should be placed in an MC-synthesis environment with numerous long-chain alcohol molecules in close proximity.
Conclusions
In this study, P2EN was synthesized via MC synthesis using linear alcohols with carbon numbers ranging from 1 to 22 as additives and solution synthesis using the same linear alcohols as polymerization solvents. MC synthesis using linear-alcohol additives with a small carbon number resulted in the formation of yellow-powder P2EN comprising the ct structure with an extended helix, whereas using additives with a high carbon number resulted in red-powder P2EN comprising the cc structure with a contracted helix. This result is specific to MC synthesis; in conventional solution synthesis, yellow-powder P2EN containing extended helices is formed in all cases with no selectivity. Under MC conditions where localized heat and pressure occur, polymers frozen in metastable structures likely transition to stable structures owing to interactions with additives that show a high affinity toward the polymer. This study indicates that the use of additives with a high affinity for polymers in MC synthesis leads to the formation of stable higher-order structures. The additive-based control of higher-order structure formation through MC synthesis is also expected to be possible for other polymers. Controlling the higher-order structure of polymers enables the performance control of polymer materials. Therefore, this study confirms the high potential applicability of MC synthesis, which is more environmentally friendly than conventional solution methods, in industrial polymer-material manufacturing processes.Author contributions
H. I.: conceptualization, funding acquisition, investigation, methodology, project administration, validation, visualization, and writing – original draft. Y. M.: conceptualization, resources, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5mr00102a.Acknowledgements
This work was supported by JST SPRING, Grant Number JPMJSP2153.Notes and references
- D. Ceriotti, P. Marziani, F. M. Scesa, A. Collorà, C. L. Bianchi, L. Magagnin and M. Sansotera, Mechanochemical synthesis of fluorinated perovskites KCuF3 and KNiF3, RSC Mechanochem., 2024, 1, 520 RSC.
- L. Zhang, Q. Song, Y. Wang, R. Chen, Y. Xia, B. Wang, W. Jin, S. Wu, Z. Chen, A. Iqbal, C. Liu and Y. Zhang, Solvent-free mechanochemical synthesis of azo dyes, RSC Mechanochem., 2024, 1, 447 RSC.
- C. C. Sahu, S. Biswas, R. Hommelsheim and C. Bolm, Synthesis of α-ketothioamides with elemental sulfur under solvent-free conditions in a mixer mill, RSC Mechanochem., 2024, 1, 38 RSC.
- S. Elli, M. Caruso, A. Sacchetti and J. Martí-Rujas, Synthesis of a thermally stable 2D MOF via neat grinding and annealing of a panel-like triangular ligand with Zn(II), RSC Mechanochem., 2024, 2, 188 RSC.
- K. Kubota, R. Hisazumi, T. Seo and H. Ito, Mechanochemistry enabled highly efficient solvent-free deoxygenation of phosphine oxides in air, RSC Mechanochem., 2024, 1, 250 RSC.
- T. Seo, N. Toyoshima, K. Kubota and H. Ito, Tackling Solubility Issues in Organic Synthesis: Solid-State Cross-Coupling of Insoluble Aryl Halides, J. Am. Chem. Soc., 2021, 143, 6165 CrossRef CAS PubMed.
- P. Ying, J. Yu and W. Su, Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals, Adv. Synth. Catal., 2021, 363, 1246 CrossRef CAS.
- M. E. Skala, S. M. Zeitler and M. R. Golder, Liquid-assisted grinding enables a direct mechanochemical functionalization of polystyrene waste, Chem. Sci., 2024, 15, 10900 RSC.
- V. André, A. Hardeman, I. Halasz, R. S. Stein, G. J. Jackson, D. G. Reid, M. J. Duer, C. Curfs, M. T. Duarte and T. Friščić, Mechanosynthesis of the Metallodrug Bismuth Subsalicylate from Bi2O3 and Structure of Bismuth Salicylate without Auxiliary Organic Ligands, Angew. Chem., Int. Ed., 2011, 50, 7858 CrossRef PubMed.
- P. A. Zaikin, O. T. Dyan, I. R. Elanov and G. I. Borodkin, Ionic Liquid-Assisted Grinding: An Electrophilic Fluorination Benchmark, Molecules, 2021, 26, 5756 CrossRef CAS PubMed.
- K. Kubota, T. Seo and H. Ito, Solid-state cross-coupling reactions of insoluble aryl halides under polymer-assisted grinding conditions, Faraday Discuss., 2023, 241, 104 RSC.
- K. Xiang, H. Shou, C. Hu, W. Su and J. Yu, Mechanochemical aerobic oxidative Heck coupling by polymer-assisted grinding: cyclodextrin additive facilitating regioselectivity control, Green Chem., 2024, 26, 5890 RSC.
- I. Brekalo, K. Lisac, J. R. Ramirez, P. Pongrac, A. Puškarić, S. Valić, Y. Xu, M. Ferguson, J. M. Marrett, M. Arhangelskis, T. Friščić and K. T. Holman, Mechanochemical Solid Form Screening of Zeolitic Imidazolate Frameworks using Structure-Directing Liquid Additives, J. Am. Chem. Soc., 2025, 147, 27413 CrossRef CAS.
- D. Hasa, E. Carlino and W. Jones, Polymer-Assisted Grinding, a Versatile Method for Polymorph Control of Cocrystallization, Cryst. Growth Des., 2016, 16, 1772 CrossRef CAS.
- P. M. Rincon, M. Renner, L. Borchardt and P. Biessey, Process development and prospective life-cycle assessment of the mechanochemical depolymerization of polyethylene terephthalate, Chem. Eng. J., 2025, 509, 161411 CrossRef CAS.
- G. S. Lee, H. W. Lee, H. S. Lee, T. Do, J.-L. Do, J. Lim, G. I. Peterson, T. Friščić and J. G. Kim, Mechanochemical ring-opening metathesis polymerization: development, scope, and mechano-exclusive polymer synthesis, Chem. Sci., 2022, 13, 11496 RSC.
- X. Lu, L. Wang, L. Ren, W. Li and A. Zhang, Thermoresponsive helical dendronized poly(arylacetylene)s: modulating the dynamic chirality, Sci. China:Chem., 2025, 68, 1486 CrossRef CAS.
- S. Wang, X. Sun, H. Zeng, S. Xie, J. Zhang and X. Wan, Postpolymerization Modification-Induced Chiral Self-Assembly of Substituted Poly(phenylacetylene)s Based on Cis-Transoid to Cis-Cisoid Helical Transition, Macromolecules, 2025, 58, 1235 CrossRef CAS.
- M. Lago-Silva, M. M. Cid, E. Quiñoá and F. Freire, P/M Macromolecular Switch Based on Conformational Control Exerted by an Achiral Side Chain within an Axially Chiral Locked Pendant, J. Am. Chem. Soc., 2024, 146, 752 CrossRef CAS PubMed.
- Y. Nishikawa, D. Hirose, S. Sona and K. Maeda, Helicity induction and memory of a lipophilic Brønsted acid-type poly(phenylacetylene) in non-polar solvents, Chem. Commun., 2023, 59, 8226 RSC.
- S. Okuda, T. Ikai, H. Okutsu, M. Ando, M. Hattori, R. Ishidate and E. Yashima, Helix-Sense-Selective Memory Polymerization of Biphenylylacetylenes Bearing Carboxy and Amino Groups in Water, Angew. Chem., Int. Ed., 2024, 63, e202412752 CrossRef CAS PubMed.
- S. Huang, M. Teraguchi, T. Kaneko and T. Aoki, Absolute helix-sense-selective polymerization using circularly polarized light in one-handed helical channels, Macromol. Rapid Commun., 2025, 46, e2500185 CrossRef.
- Z. Ke, S. Abe, T. Ueno and K. Morokuma, Rh-Catalyzed Polymerization of Phenylacetylene: Theoretical Studies of the Reaction Mechanism, Regioselectivity, and Stereoregularity, J. Am. Chem. Soc., 2011, 133, 7926 CrossRef CAS PubMed.
- M. Tabata, T. Sone and Y. Sadahiro, Synthesis of monosubstituted polyacetylenes using Rh complex catalysts. Control of solid structure and π-conjugation length, Macromol. Chem. Phys., 1999, 200, 265 CrossRef CAS.
- M. Shiotsuki, F. Sanda and T. Masuda, Polymerization of substituted acetylenes and features of the formed polymers, Polym. Chem., 2011, 2, 1044 RSC.
- F. Sanda, M. Shiotsuki and T. Masuda, Controlled Polymerization of Phenylacetylenes Using Well-Defined Rhodium Catalysts, Macromol. Symp., 2015, 350, 67 CrossRef CAS.
- K. Huang, M. Tabata, Y. Mawatari, A. Miyasaka, E. Sato, Y. Sadahiro, Y. Kashiwaya and K. Ishii, Origin of the Color of p-Conjugated Columnar Polymers: Poly(p-n-Octylthiophenyl)acetylene Prepared Using a [Rh(norbornadiene)Cl]2 Catalyst, J. Polym. Sci., Part A:Polym. Chem., 2005, 43, 2836 CrossRef CAS.
- A. Miyasaka, M. Nakamura, Y. Mawatari, K. Orito and M. Tabata, A Novel Transparent Polyacetylene Bearing a Triphenylamine Moiety Prepared with a [Rh(norbornadiene)Cl]2 Catalyst, Macromol. Symp., 2006, 239, 13 CrossRef CAS.
- A. Motoshige, Y. Mawatari, Y. Yoshida, C. Seki, H. Matsuyama and M. Tabatam, Irreversible Helix Rearrangement from Cis-Transoid to Cis-Cisoid in Poly(p-n-hexyloxyphenylacetylene) Induced by Heat-Treatment in Solid Phase, J. Polym. Sci., Part A:Polym. Chem., 2012, 50, 3008 CrossRef CAS.
- Y. Mawatari, Y. Yoshida, K. Huang and M. Tabata, Methoxy-Group Control of Helical Pitch in Stereoregular Poly(2-ethynylmethoxynaphthalene) Prepared by Rhodium Complex Catalyst, Polymers, 2019, 11, 94 CrossRef PubMed.
- Y. Mawatari, A. Motoshige, Y. Yoshida, R. Motoshige, T. Sasaki and M. Tabata, Structural determination of stretched helix and contracted helix having yellow and red colors of poly(2-ethynylnaphthalene) prepared with a [Rh(norbornadiene)Cl]2-triethylamine catalyst, Polymer, 2014, 55, 2356 CrossRef CAS.
- H. Ikushima and Y. Mawatari, Mechanochemical Synthesis of Fragile Polymer: Synthesis of a Poly(phenylacetylene) Using a Ball-milling Approach, Chem. Lett., 2023, 52, 303 CrossRef CAS.
- Y. Mawatari, M. Tabata, T. Sone, K. Ito and Y. Sadahiro, Origin of color of π-conjugated columnar polymers. 1. Poly(p-3-methylbutoxy)phenylacetylene prepared using a [Rh(norbornadiene)Cl]2 catalyst, Macromolecules, 2001, 34, 3776 CrossRef CAS.
- D. B. Fitchen, Resonance Raman results in polyacetylene, Mol. Cryst. Liq. Cryst., 1982, 83, 95 CrossRef.
- S. Saito, M. Tasumi and C. Eugster, Resonance Raman spectra (5800–40 cm−1) of All-trans and 15-cis isomers of β-carotene in the solid state and in solution. Measurements with various laser lines from ultraviolet to red, J. Raman Spectrosc., 1983, 14, 299 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |