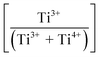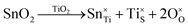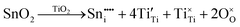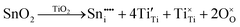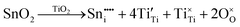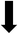Open Access Article
Open Access ArticleEffects of Sn4+ solid solubility mechanisms on the electromechanical and energy storage performance of (Ba0.85Ca0.15)(Ti0.92Zr0.08)O3
Tasmia
Zaman
 *ab,
Saroj Kumar
Bhattacharyya
*ab,
Saroj Kumar
Bhattacharyya
 b,
Sajjad Seifi
Mofarah
b,
Sajjad Seifi
Mofarah
 a,
Pramod
Koshy
a,
Pramod
Koshy
 a and
Charles Christopher
Sorrell
a and
Charles Christopher
Sorrell
 a
a
aSchool of Materials Science and Engineering, UNSW Sydney, NSW 2052, Australia. E-mail: t.zaman@unsw.edu.au
bSolid State and Elemental Analysis Unit, Mark Wainwright Analytical Centre, UNSW Sydney, NSW 2052, Australia
First published on 17th October 2025
Abstract
The electromechanical properties of piezoelectric materials are influenced significantly by the defect chemistry, which is determined by the solid-solubility and charge-compensation mechanisms. In the present work, the effects of Sn4+ doping of lead-free (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) ceramics on these parameters and the resultant electromechanical properties and energy-storage efficiencies are reported. The complex nature of the solid solubility mechanisms as a function of dopant content is elucidated through comprehensive analyses of the structures, microstructures, and surface chemistry. The corresponding charge compensation mechanisms are determined by correlating these characterization data with corresponding defect equilibria, which then provide the basis for the interpretation of the mechanisms governing the electromechanical properties and energy-storage efficiencies. The combined data for the surface Ti oxidation state (XPS) and bulk unit cell volumes (XRD) for the three observed polymorphs (orthorhombic Pmm2, tetragonal P4mm, and cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) reveal interstitial solid solubility at low (0.00 ≤ x ≤ 0.04) and high (0.08 ≤ x ≤ 0.10) Sn4+ doping levels, with intermediate (0.04 < x < 0.08) Sn4+ doping levels exhibiting mixed interstitial-substitutional solid solubility. The trends in the electromechanical properties correlate directly with the solid solubility mechanisms, with two resultant inflections at x = 0.04 (maximal defect concentration) and x = 0.08 (minimal defect concentration). These mechanisms significantly influence the electromechanical properties, where maxima occur for polarization at x = 0.04, bipolar strain at x = 0.08, and energy storage efficiency at x = 0.10. The latter is notable because this parameter reaches >95% across the wide temperature range of 25°–130 °C.
m) reveal interstitial solid solubility at low (0.00 ≤ x ≤ 0.04) and high (0.08 ≤ x ≤ 0.10) Sn4+ doping levels, with intermediate (0.04 < x < 0.08) Sn4+ doping levels exhibiting mixed interstitial-substitutional solid solubility. The trends in the electromechanical properties correlate directly with the solid solubility mechanisms, with two resultant inflections at x = 0.04 (maximal defect concentration) and x = 0.08 (minimal defect concentration). These mechanisms significantly influence the electromechanical properties, where maxima occur for polarization at x = 0.04, bipolar strain at x = 0.08, and energy storage efficiency at x = 0.10. The latter is notable because this parameter reaches >95% across the wide temperature range of 25°–130 °C.
New conceptsIt is universal to assume that BaTiO3 doping is substitutional. The present work documents data for five different and consistent analytical methods that show unambiguously that the solid solubility mechanism initially is interstitial. With increasing doping, the solubility limit is reached, initiating substitutional solid solubility. With yet more doping and the achievement of saturation solubility, the associated lattice distortion allows interstitial solid solubility to be reactivated. Differences: this assumption is a convenience that has become a universally accepted simplification. This work demonstrates across multiple platforms that this can be incorrect. This work also demonstrates that a single solid solubility mechanism is another potentially incorrect assumption. These observations build on a growing body of work that confirms that mixed solid solubility and changing solid solubility mechanisms occur commonly in other materials. This work is the first to show that this applies to BaTiO3-based materials. Insights: this work has ramifications for the functional materials industries because doping of these invariably is done using stoichiometric compensation, where dopant levels are decreased according to the dopant addition amount. When this assumption is incorrect, the result involves unrecognised metal vacancies, which inevitably have an effect on the functional properties. |
1. Introduction
Defects play a significant role in the response mechanisms of electroceramics, influencing the motion of domain walls,1–3 creating defect dipoles that interact with the spontaneous polarization,4–6 and lead to conductivity mechanisms.7–9 Understanding and controlling defects is essential for tailoring the properties and optimizing their performance in various applications, including sensors, actuators, and data- and energy-storage devices. The solid solubility mechanisms can often be elucidated through consideration of the defect equilibria in conjunction with supporting experimental data.10–15 Defect equilibria in electroceramics are expressed in terms of Kröger–Vink notation,10–16 which defines the defect chemistry. However, when Kröger–Vink notation16 was developed, there were limited means of examining variable cation valences, so this formalism does not provide a discrete notation to illustrate redox effects.10–15 Furthermore, while many oxides contain intrinsic vacancies and interstitials, doping and impurities lead to the formation of extrinsic vacancies; substitutionals; interstitials; and Frenkel, Schottky, and antisite pairs. The expression of the associated defect equilibria is contingent upon knowledge of the solid solubility mechanism, which is rarely the case. This differentiation is then critical to the assessment of the different charge compensation mechanisms such that both mass and charge balances are maintained.Lead-based Pb(Zr1−xTix)O3 (PZT) ceramics are used extensively for energy harvesting and storage applications as they exhibit higher power densities, faster charging/discharging, and superior performance compared to those of Li-ion batteries, electrochemical capacitors, and fuel cells. Moreover, the outstanding piezoelectric and ferroelectric performances extend these ceramics’ significance to electrocaloric and electromechanical applications.17–28 Further enhancement of these properties was found in other related materials, including Pb(Mg1/3Nb2/3)O3–PbTiO3 (PMN-PT) and Pb(Zn1/3Nb2/3)O3–PbTiO3 (PZN-PT) solid solutions.26–29 However, concerns about the toxicity of Pb and its impact on human health and the environment have restricted their applicability,37–40 thus directing research toward alternative lead-free materials.
Pure BaTiO3 is one of the potential lead-free candidates and it is well known to exhibit a high dielectric constant.41 However, owing to its low dielectric breakdown strength (∼1.2 kV42), low discharge energy density (370 mJ cm−3 (ref. 43)), and minimal energy storage efficiency (40.3%43), its applicability for high-energy-density materials is limited.44–46 A typical strategy to improve these performance parameters is doping the A-, B-, or both A- and B-sites of cubic BaTiO3;47–56 these sites are structured around an AO12 cuboctahedron and a BO6 octahedron. In this context, simultaneous A- and B-site doped lead-free (Ba,Ca)(Ti,Zr,Sn)O3 (BCTZS) is one of the most promising candidates and consequently has generated considerable interest based on demonstrated technical progress in the electromechanical and energy storage sectors over the last decade57–81 (Table S1). To date, there have been just over twenty studies on BCTZS.57–70,72,75–81 There are also several other works on BCTZS that report the use of dopants Pb2+,72 Sr2+,71,73 and Bi3+.74 Of these, ten are limited to reports of the structural evolution and electrical properties (2008–202359,60,64,65,70,75,77–80), five focus on the electrocaloric effect (2015,69 2016,58 2019,62,76 and 202073), three focus on the energy-harvesting performance (2020,81 2022,61 and 202366), and three focus on the energy storage efficiency (202263,67 and 202382). These also include six studies that examine the effects of varying the Ti4+/Sn4+ ratio.58,61,64,65,80,81 These studies reveal some ambiguity about the maximal Sn4+ solubility, where two of the studies reported it to be <6.0 mol%61,64 while the other four58,65,80,81 reported solid solubility up to 6.0–8.0 mol%.
Unfortunately, all twenty-one sets of publications assumed that substitutional solid solubility would occur. Furthermore, none of these studies investigated the solid solubilities, charge compensation mechanisms, or the associated defect chemistry and the resultant effects on the material properties, energy storage capacity, or electromechanical performance. Such an approach is critical because, if the interpretation of the solid solubility and/or charge compensation mechanism is incorrect, then this impacts on the accuracy of the resultant defect equilibria, thereby affecting appropriate assessment of the bases for the energy storage and electromechanical properties.
In contrast, the approach for halide perovskites is different. These perovskites are less stable than oxide perovskites owing to the larger anions, greater polarizability, less rigid BX6 octahedra, and greater extent of ionic bonding.83–90 Furthermore, the smaller and more covalent-bonding oxygen ion results in converse characteristics, thereby resulting in smaller interstices and more constrained structures.91
Consequently, both substitutional92–102 and interstitial solid solubilities99,103–112 have been reported for halide perovskites. There does not appear to be any example of experimental validation for the substitutional solid solubility. However, lattice parameter measurements from X-ray diffraction (XRD) were used to support the conclusion of interstitial solid solubility in at least eleven publications.99,103–112 This one experimental technique was used to support their claim. The possibility of interstitial solid solubility in oxide perovskites is rarely considered; when such consideration is given, it appears to have been limited to only two comparative computational studies of substitutional vs. interstitial solid solubility.113,114 In both of these studies, the simulations indicated that the former is favoured over the latter for oxide perovskites.
More generally, there are numerous experimental studies in which the solid solubility mechanism has been considered:
• Pb-based: Pb(Zr1−xTix)O3 (PZT),17,19,20,26–28 Pb(Mg1/3Nb2/3)O3–PbTiO3 (PMN-PT),29–32 and Pb(Zn1/3Nb2/3)O3–PbTiO3 (PZN-PT)33–36
• Pb-free: BaTiO3-based (BTO),115–120 (Ba1−xCax)(Ti1−yZry)O3-based (BCTZ),121–156 (Ba1−xCax)(Ti1−ySny)O3-based (BCTS),52,54,56,157–162 (Ba1−xCax)(Ti1−y−zZrySnz)O3-based (BCTZS),57,58,66,69,71,75–77,79,80,163 K0.5Na0.5NbO3-based (KNN),164–177 Bi0.5Na0.5TiO3-based (BNT),178–182 KNbO3-based (KN),183–187 and BiFeO3-based (BFO).188–190
In all cases, substitutional solid solubility was assumed but without any experimental evidence to support this conclusion. Although a few computational modelling studies suggest that what appears to be a universal assumption is justified, there remains an apparent absence of experimental validation. Consequently, the present work aims to probe this issue using a case study involving a specific system that is processed under specific conditions. The present work appears to be the first experimental investigation to provide evidence for the solid solubility mechanism, whether substitutional, interstitial, or mixed. The implications of this uncertainty highlight the potential to generate defect equilibria considerably different from those that result from the assumption of substitutional solid solubility.
The present work demonstrates the solid solubility mechanisms, charge compensation mechanisms, and corresponding defect chemistries involved in Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10). This phase was selected as a representative example of similar lead-free phases. Although preliminary stoichiometry compensation for the assumed substitution of Sn4+ for Ti4+ invariably is done on the basis of the assumption of substitutional solid solubility,57,58,66,69,71,75–77,79,80,163 the present work reveals that, for the specific experimental conditions used, this may not be a correct assumption. This contradiction has significant ramifications for the resultant defect equilibria.
The resultant solid solubility mechanisms in the present work are supported by five distinctly different sets of data, which are unambiguous in demonstrating both interstitial and mixed solid solubilities, where the former is dominant. These outcomes required the implementation of new concepts for Kröger–Vink redox charge compensation formalism,10–15 which have been made possible by the advent of new room-temperature characterization techniques. The capacity to examine resultant valences through X-ray photoelectron spectroscopy (XPS) allows assessment of the types of defects while photoluminescence (PL) spectroscopy allows assessment of their relative amounts. These data can be confirmed by complementary lattice parameter measurements by XRD and even true density. Combining these experimental data with defect equilibria offers the potential to elucidate the solid solubility mechanism without resorting to analytical techniques such as neutron diffraction (ND), X-ray absorption spectroscopy (XAS), small-angle X-ray scattering (SAXS), and solid-state nuclear magnetic resonance (NMR). Hence, the present work elucidates the roles of the solid solubility mechanisms, resultant charge compensation mechanisms, and underpinning defect equilibria in the modulation of the energy storage efficiency and electromechanical properties.
2. Experimental procedure
2.1. Materials synthesis
The conventional solid-state sintering technique was adopted for the synthesis of Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10). BaCO3, CaCO3, TiO2, ZrO2, and SnO2 (Sigma Aldrich, ACS reagent, purity ≥99.00 wt%) were used as initial raw materials. Stoichiometric compensation for the Sn4+ content was used on the basis of the preliminary assumption of substitutional solid solubility and neutral charge compensation. The powder was initially oven-dried at 100 °C for 24 h (heating rate: 300 °C h−1) to remove moisture. The appropriate proportions of the starting materials were measured using a digital balance (0.0001 g precision) and transferred to a high-density polyethylene (HDPE) ball mill (75 mm H × 65 mm Ø). The mill was filled to 2/3 volume with yttria-stabilized zirconia balls of 10 mm and 5 mm Ø, 60/40 wt% ratio, ∼60 g of powder was added, and ethanol was added until it reached the height of the balls (∼220 mL). Rotary ball milling was carried out at 265 rpm for 24 h. After milling, the slurry was transferred to a Pyrex beaker and dried in an oven at 100 °C for 24 h (heating rate: 300 °C h−1). The dried powders were deagglomerated by gentle hand-crushing using an agate mortar and pestle. The free-flowing powders were transferred into a Pt crucible for calcination at 1300 °C for 2 h (100 °C h−1 heating rate; natural cooling) in a muffle furnace (LABEC, Model CEMMS, Australia; 200 mm W × 400 mm L × 160 mm H, heating element MoSi2).After calcination, the reagglomerated powders again were deagglomerated by gentle hand-crushing using an agate mortar and pestle, after which they were ball milled for 96 h, dried, and deagglomerated as described above. The samples (pellets) were formed by uniaxial vacuum pressing (rotary vacuum pump) the powders in a hardened tool steel die (∼110 MPa pressure, ∼1 min holding time, ∼13 mm Ø, ∼2 mm thickness).
Five samples of identical composition were sandwiched in an alumina crucible (∼20 mm ID, 25 H), in which powder identical to that of the samples was used for embedding; each pellet was isolated by a layer of powder of ∼3 mm thickness (the diametral clearance to the crucible wall was ∼4 mm). These procedures were followed in order to reduce/eliminate volatilization and contamination during the sintering conditions. The pellets were sintered at 1500 °C for 12 h (100 °C h−1 heating rate; natural cooling) using the muffle furnace as described above. Any powder adhered to the pellets (∼10 mm diameter) could be removed easily by hand rubbing.
2.2. Characterization and testing
Before characterization, both planar surfaces of the pellets were wet-polished sequentially with SiC papers of varying grit sizes (800 (22.5 μm), 1200 (14.0 μm), and 2500 (7.8 μm)) at 200 rpm. The final thickness of the pellets was held at ∼1.5 mm, which was the thickness after reduction from the original ∼2.0 mm; thus, the analyses were for the bulk. After polishing, the pellets were cleaned in an ultrasonic bath using laboratory detergent and water, dried using heated flowing air for ∼1 min, and then air dried for 48 h. The pellets then were annealed at 200 °C for 24 h (heating rate of 300 °C h−1) in order to remove residual stress from polishing.Room temperature X-ray diffraction (XRD; PANalytical Empyrean II, Netherlands; 40 kV, 40 mA, CoKα radiation (0.1789 nm), 20–120° 2θ, 0.026° 2θ step size, 200 steps per s, 0.033° s−1 scan speed) was conducted on the pellets to determine the mineralogies. Si powder was used as an in situ standard (BDH Laboratory Chemicals, −325 mesh, 99% trace metals basis). Each pellet was inserted in a recessed sample holder and levelled to the plane of the sample holder surface. A small amount (∼0.01 g) of Si then was used to decorate the surface, leaving only a sparsely covered surface deposit of Si. The patterns were analyzed by Data Viewer PANalytical software and the peak positions then were normalized. Pawley fitting191 (HighScore Plus PANalytical software) was carried out in order to obtain qualitative and quantitative data for the phases present.
Although Rietveld refinement192 typically is applied for profile fitting for X-ray refinement, it requires a well-defined crystal structure, often including the assumption of specific lattice occupancy involving typically substitutional solid solubility. However, this assumption is commonly problematic in cases in which the solid solubility mechanism is unknown or there are mixed solid solubilities. This assumption may also be incorrect when multiple polymorphs are present, which introduces the potential for varying interstitial site filling. Since the present work reveals mixed Sn4+ solid solubilities and up to three simultaneously present polymorphs, Pawley fitting191,193 was used because it does not require detailed crystal structure parameters while providing approximate lattice parameters and peak profiles. Pawley fitting191 is an iterative technique that requires knowledge of the space group and the initial selection of unit cell parameters. The latter are refined progressively until the selected unit cell parameters match those following the fitting. This method effectively mitigates the potential for error when the preceding assumptions are relevant. However, it has the disadvantage that the deconvolutions of multiple peaks are less reliable than those of Rietveld refinement. Hence, while less precise than Rietveld refinement, it does not require the assumptions of crystal structure or lattice occupancy. In light of the comparative strengths and weaknesses of both approaches, the validity of the fitting can be assessed in light of non-XRD complementary data. Consequently, it has been utilized in recent work owing to this advantage.194–202
Surface chemical analyses were carried out using X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi spectrometer, Thermo Fisher Scientific, UK; monochromatic AlKα (1486.68 eV) soft X-ray source; 13.80 kV, 8.7 mA, 500 μm spot size). Although XPS is a surface analysis technique, the data are for the bulk since the surface examined was one quarter into the depth of the pellet.
The true density was determined by He pycnometry (AccuPyc II 1340; Micromeritics, Germany; line pressure >1.3 MPa, triple purge fill pressure 0.134 MPa, triple fill pressure 0.070 MPa, equilibration rate 0.035 kPa min−1).
Microstructural analyses were carried out using field emission scanning electron microscopy (FESEM; FEI Nova NanoSEM 450, USA; 30 kV, secondary electron (SE) mode, 3.5 spot size (30 μm aperture diameter, 140 pA)) on the unpolished and unetched surfaces. The electrical conductivity of each sample was increased using a sputter coater to deposit a ∼10 nm coating of Pt (Leica EM ACE600, Leica Mikrosysteme GmbH, Austria; Ar gas). The average grain sizes were determined by ImageJ software using twenty-five measurements over an area of ∼400 μm × ∼300 μm; corresponding histograms and frequency curves were also generated.
Field emission scanning electron microscopy (FESEM; FEI Nova NanoSEM 230, USA; 30 kV, secondary electron (SE) mode, 3.5 spot size (30 μm aperture diameter, 140 pA)) was used for regular elemental mapping, while a silicon drift detector (SDD) energy dispersive spectroscopy (EDS) detector (SDD-EDS; Bruker Xflash 6|30, Germany; 30 keV) was used to confirm the elemental distributions of the unpolished surfaces. A thin carbon coating (∼15 nm) was sputtered using a turbo-pumped carbon coater (DCT; NSC DCT, Iran, 13 kV) prior to EDS.
For electrical measurement, wet-polished samples were deposited with a ∼60 nm coating of Pt using a sputter coater (Leica EM ACE600, Leica Mikrosysteme GmbH, Austria; Ar gas), after which the samples were subjected to piezoelectric measurements (TF Analyzer 2000 System; aixACCT Systems, Germany; 1 Hz, 1 mA, 0.5–4.0 kV mm−1, 25°–140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, 5
°C, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C intervals) in a silicone-oil bath. Temperature-dependent dielectric and ferroelectric measurements were also obtained using the same TF Analyzer 2000 system (2.5 kV mm−1).
°C intervals) in a silicone-oil bath. Temperature-dependent dielectric and ferroelectric measurements were also obtained using the same TF Analyzer 2000 system (2.5 kV mm−1).
3. Results and discussion
3.1. Context
In the present work, the presence of multiple polymorphs and solid solubility mechanisms as well as the uncertainties of lattice occupancy of the dominant interstitial solid solubility mechanism requires the application of Pawley fitting rather than Rietveld refinement, the latter of which requires accurate structural data. The previous discussion concerning the pros and cons of Rietveld refinement and Pawley fitting indicate that the uncertainties associated with the latter are such that the XRD refinement should be confirmed by non-XRD complementary data. To this end, the XRD data are interpreted in terms of four non-XRD parameters. That is, XPS data are correlated with the effects of the three polymorphs detected by XRD. PL data then are correlated with the XPS data, following correlations between true density and bipolar strain data obtained with XPS and PL data. These correlations support the conclusion of the validity of the Pawley fitting for XRD refinement in the present work.3.2. XRD analysis
The structural features of Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) were determined using XRD (Fig. 1(a)). No additional peaks associated with secondary phases were detected, indicating the complete dissolution of the solutes (Ca2+, Zr4+, Sn4+). Semiquantitative analyses of the XRD data by Pawley fitting191 (Fig. 1(b)) confirm the presence of mixed polymorphs (orthorhombic Pmm2 and tetragonal P4mm) for all of the compositions. The corresponding XRD refinement parameters (R factors, goodness of fit (GoF)) are provided in Table S2. Furthermore, the changes in the trend of the lattice parameters (Fig. S1(a) and (b)), c/a ratio (Fig. S1(c)), and unit cell volumes (Fig. S1(d)) reveal a significant transition from a dominant Pmm2 (O) to a dominant P4mm (T) polymorph at x = 0.04 (first inflection). The pressure–temperature unary phase diagrams203–205 (Fig. S2) show that this orthorhombic → tetragonal phase transformation is stress-induced. At the highest Sn4+ doping level (x = 0.10), the formation of cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (C) along with the other two polymorphs (Pmm2 and P4mm) suggests the presence of a triple point (TP) at room-temperature (RT). The data for the c/a ratio at x = 0.04 and x = 0.08 are attributed to the achievement of saturation solid solubility, which will be discussed subsequently.
m (C) along with the other two polymorphs (Pmm2 and P4mm) suggests the presence of a triple point (TP) at room-temperature (RT). The data for the c/a ratio at x = 0.04 and x = 0.08 are attributed to the achievement of saturation solid solubility, which will be discussed subsequently.
3.3. Raman analysis
Fig. 1(c) shows the Raman data for Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) obtained at RT. The deconvoluted spectra (Fig. 1(d)) identify the characteristic vibrational modes,206–214 which again confirm the presence of mixed polymorphs (Pmm2 and P4mm) for all samples (Table S3). However, cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m at the highest Sn4+ doping level was not detected by this method due to peak overlap from the Pmm2 and P4mm polymorphs. Furthermore, the discrete Raman modes reveal the presence of several defects, viz., v-LVM (caused by interstitial solutes and or vacancies), s-LVM (caused by B-site substitution), and DARS (disorder-activated Raman scattering caused by structural distortion) at all Sn4+ doping levels.211,215–217 These data indicate the presence of mixed substitutional and interstitial solid solubility. Mixed polymorphs are known to enhance the ferroelectric and electromechanical properties,45,46,218–226 accelerate the charge and discharge rate,57,61,62,72,74,77,80 and improve thermal stability, resulting in materials with exceptional energy storage performance.
m at the highest Sn4+ doping level was not detected by this method due to peak overlap from the Pmm2 and P4mm polymorphs. Furthermore, the discrete Raman modes reveal the presence of several defects, viz., v-LVM (caused by interstitial solutes and or vacancies), s-LVM (caused by B-site substitution), and DARS (disorder-activated Raman scattering caused by structural distortion) at all Sn4+ doping levels.211,215–217 These data indicate the presence of mixed substitutional and interstitial solid solubility. Mixed polymorphs are known to enhance the ferroelectric and electromechanical properties,45,46,218–226 accelerate the charge and discharge rate,57,61,62,72,74,77,80 and improve thermal stability, resulting in materials with exceptional energy storage performance.
3.4. SEM and EDS analyses
Fig. 2 shows the field emission scanning electron microscopy (FESEM) images of the samples, which reveal highly dense microstructures for all compositions. Although the planar nature of many of the grain boundaries suggests microstructural stability, concentric growth artifacts remain visible. The corresponding energy dispersive spectrometry (EDS) elemental mappings (shown in insets) confirm the chemical homogeneity. Furthermore, the grain size distribution curves obtained using ImageJ software (Fig. S3) reveal that the average grain sizes vary in the range of ∼27–40 μm. | ||
| Fig. 2 FESEM images of surfaces of unpolished and unetched (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3: (a) x = 0.00, (b) x = 0.02, (c) x = 0.04, (d) x = 0.06, (e) x = 0.08, and (f) x = 0.10. | ||
3.5. XPS analysis
Fig. 3(a) shows the X-ray photoelectron spectroscopy (XPS) survey spectra for the samples. Since the XPS O 1s peak spectra reflect the Ti–O terminating lattice bonding,227 the subsurface cationic states can be identified. This approach is important because the surface metal species can be present in the form of undissolved, precipitated, and/or segregated solutes. The deconvoluted O 1s peaks shown in Fig. 3(b) correspond to lattice oxygen (Ti4+–O bond), the oxygen associated with an adjacent oxygen vacancy (Ti3+–O bond), and the oxygen in chemisorbed water (H2O/–OH bond). The effects of the oxygen bonding with Ba2+, Ca2+, Sn4+, and Zr4+ would be minimal because Ba2+, Ca2+, and Zr4+ have stable valences and Ca2+, Zr4+, and Sn4+ are present in low concentrations.The multiple Ti valence states evident in the O 1s XPS data are reflected in the normalized plot for 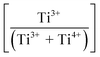 as a function of the Sn4+ doping level (Fig. 3(c)). The corresponding variations in unit cell volumes for Pmm2, and P4mm also are given. The correlation in trends between the subsurface (XPS) and bulk (XRD) data confirms the existence of three, sequential, solubility-driven, redox mechanisms. Furthermore, these data trends in redox confirm the occupancy of Sn4+, which is isovalent with Ti4+, in both substitutional and interstitial sites, thus reflecting mixed solid solubility. More broadly, initial dopant incorporation (Mechanism I: 0.00 < x ≤ 0.02) favors Ti4+ → Ti3+ reduction and associated interstitial solid solubility (ISS) in both Pmm2 and P4mm, as shown by the identical trends in unit cell volumes. Subsequent dopant incorporation (mechanism I: 0.02 < x ≤ 0.04) reveals a continued
as a function of the Sn4+ doping level (Fig. 3(c)). The corresponding variations in unit cell volumes for Pmm2, and P4mm also are given. The correlation in trends between the subsurface (XPS) and bulk (XRD) data confirms the existence of three, sequential, solubility-driven, redox mechanisms. Furthermore, these data trends in redox confirm the occupancy of Sn4+, which is isovalent with Ti4+, in both substitutional and interstitial sites, thus reflecting mixed solid solubility. More broadly, initial dopant incorporation (Mechanism I: 0.00 < x ≤ 0.02) favors Ti4+ → Ti3+ reduction and associated interstitial solid solubility (ISS) in both Pmm2 and P4mm, as shown by the identical trends in unit cell volumes. Subsequent dopant incorporation (mechanism I: 0.02 < x ≤ 0.04) reveals a continued 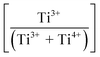 increase while the unit cell volumes follow opposite trends. The converse trend is interpreted in terms of the expansion of Pmm2 (from Ti4+ → Ti3+ reduction and continued interstitial solid solubility) and the forced contraction of adjacent P4mm (despite the continued interstitial solid solubility). At higher Sn4+ concentrations (mechanism II: 0.04 < x ≤ 0.08), the reverse Ti3+ → Ti4+ oxidation occurs. The trends in unit cell volumes for both polymorphs further suggest that the solid solubility mechanism for Pmm2 is consistently interstitial (from the linear baseline trend except at x = 0.04) while that for P4mm (from the staggered baseline) is subject to alteration. That is, these trends are interpreted in terms of the achievement of saturation interstitial solid solubility in P4mm and the onset of substitutional solid solubility (SSS). Hence, the absence of any charge compensation effect from the substitution of Sn4+ and Ti4+ effectively reduces the
increase while the unit cell volumes follow opposite trends. The converse trend is interpreted in terms of the expansion of Pmm2 (from Ti4+ → Ti3+ reduction and continued interstitial solid solubility) and the forced contraction of adjacent P4mm (despite the continued interstitial solid solubility). At higher Sn4+ concentrations (mechanism II: 0.04 < x ≤ 0.08), the reverse Ti3+ → Ti4+ oxidation occurs. The trends in unit cell volumes for both polymorphs further suggest that the solid solubility mechanism for Pmm2 is consistently interstitial (from the linear baseline trend except at x = 0.04) while that for P4mm (from the staggered baseline) is subject to alteration. That is, these trends are interpreted in terms of the achievement of saturation interstitial solid solubility in P4mm and the onset of substitutional solid solubility (SSS). Hence, the absence of any charge compensation effect from the substitution of Sn4+ and Ti4+ effectively reduces the 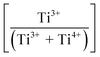 , which is equivalent to oxidation. Finally, at the highest Sn4+ doping levels (mechanism III: 0.08 < x ≤ 0.10), the
, which is equivalent to oxidation. Finally, at the highest Sn4+ doping levels (mechanism III: 0.08 < x ≤ 0.10), the 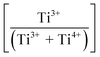 trend is reversed again, resulting in the onset of reduction (Ti4+ → Ti3+) owing to reintroduction of interstitial solid solubility in P4mm. This is interpreted in terms of the achievement of saturation substitutional solid solubility at x = 0.08, which distorted the lattice sufficiently to allow additional interstitial solid solubility. Since the linearity of the baseline in the XRD data for Pmm2 do not suggest the achievement of saturation solid solubility, then this polymorph plays no role in the generation of Pm
trend is reversed again, resulting in the onset of reduction (Ti4+ → Ti3+) owing to reintroduction of interstitial solid solubility in P4mm. This is interpreted in terms of the achievement of saturation substitutional solid solubility at x = 0.08, which distorted the lattice sufficiently to allow additional interstitial solid solubility. Since the linearity of the baseline in the XRD data for Pmm2 do not suggest the achievement of saturation solid solubility, then this polymorph plays no role in the generation of Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m at x = 0.10. Furthermore, the pressure–temperature unary phase diagrams203–205 and experimental observations228–231 show that the polymorphic phase transformation to Pm
m at x = 0.10. Furthermore, the pressure–temperature unary phase diagrams203–205 and experimental observations228–231 show that the polymorphic phase transformation to Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m is stress-induced. Hence, it is concluded that the second inflection in the redox data (x = 0.08) reflects the generation of Pm
m is stress-induced. Hence, it is concluded that the second inflection in the redox data (x = 0.08) reflects the generation of Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, which is formed directly by stress-induced polymorphic phase transformation from P4mm. The possible defect equilibria related to (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) are given in Table S4. Table 1 summarizes the relevant defect equilibria according to the XPS data and the preceding considerations. In all three cases, the observation of (1) dissolution of Sn4+ and (2) Ti4+ → Ti3+ reduction forces the charge-compensation mechanism to be redox-ionic10–15 except for the single case of substitutional solid solubility and neutral charge compensation.
m, which is formed directly by stress-induced polymorphic phase transformation from P4mm. The possible defect equilibria related to (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) are given in Table S4. Table 1 summarizes the relevant defect equilibria according to the XPS data and the preceding considerations. In all three cases, the observation of (1) dissolution of Sn4+ and (2) Ti4+ → Ti3+ reduction forces the charge-compensation mechanism to be redox-ionic10–15 except for the single case of substitutional solid solubility and neutral charge compensation.
3.6. PL analysis
In order to elucidate the effects of the solid solubility mechanisms on the defect chemistry, reflectance-induced photoluminescence (PL) analyses were carried out. The PL spectra peak intensities are inversely proportional to the rates of recombination, thus revealing electron–hole annihilation.232 More importantly, the PL spectra identify the presence of point defects by generating energy transitions between the ground and excited states.233 These transitions are associated with luminescence centers in the form of color centers (also known as F centers). Fig. 4(a) shows the PL spectra for Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10), which are correlated with ionic and electronic effects. The published four assignments (1–4)232,234 as defined in Table 2(a) are associated solely with intrinsic effects. The energies generating the relative intensities are ranked on the basis of thermodynamic reasoning. That is, the two electronic defect transitions (assignments 1 and 2) require greater energies to occur as they involve the destabilization of intrinsic oxygen vacancies . Conversely, although the other two intrinsic defect transitions (assignments 3 and 4) are ionic and hence suggest the energies from bond breaking, the charge-compensating Ti4+ → Ti3+ reduction potential is approximately nil.235 This suggests readily occurring transitions that initiate F+ (assignment 3) and F0 (assignment 4) color-center formation. The transition of lowest energy is assignment 5, which is proposed in the present work on the basis of the filling of an intrinsic oxygen vacancy
. Conversely, although the other two intrinsic defect transitions (assignments 3 and 4) are ionic and hence suggest the energies from bond breaking, the charge-compensating Ti4+ → Ti3+ reduction potential is approximately nil.235 This suggests readily occurring transitions that initiate F+ (assignment 3) and F0 (assignment 4) color-center formation. The transition of lowest energy is assignment 5, which is proposed in the present work on the basis of the filling of an intrinsic oxygen vacancy  , which generally is considered to occur very readily.236,237
, which generally is considered to occur very readily.236,237
| (a) | Assignment | Charge compensation | Defects and transitions | Decreasing enthalpy | Ref. |
|---|---|---|---|---|---|
| Intrinsic | 1 | Electronic |
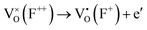
|
|
232 and 234 |
| 2 |

|
232 and 234 | |||
| 3 | Ionic |

|
234 | ||
| 4 |
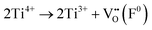
|
234 | |||
| 5 |

|
Present work |
Table 2(b) shows the combined effects of the extrinsic dopant (Sn4+) and its solid solubility mechanisms in relation to assignments 4 and 5. The column ‘Combined effects’ incorporates the nature of the Sn4+ solid solubility (substitutional or interstitial) with the single (assignment 5) or double (assignments 4 and 5) defect outcomes ( and/or
and/or 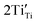 ). Fig. 4(b) shows that the inverse PL density at 570 nm for assignment 4 (Ti4+ → Ti3+ reduction,
). Fig. 4(b) shows that the inverse PL density at 570 nm for assignment 4 (Ti4+ → Ti3+ reduction, 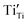 ) as a function of Sn4+ doping reaches a maximum at x = 0.04 (interstitial solid solubility) and a minimum at x = 0.08 (substitutional solid solubility). Accordingly, Table 2(b) suggests that the maximum at x = 0.04 is associated with interstitial solid solubility and hence dominated by Ti4+ → Ti3+ reduction
) as a function of Sn4+ doping reaches a maximum at x = 0.04 (interstitial solid solubility) and a minimum at x = 0.08 (substitutional solid solubility). Accordingly, Table 2(b) suggests that the maximum at x = 0.04 is associated with interstitial solid solubility and hence dominated by Ti4+ → Ti3+ reduction  over oxygen vacancy annihilation (assignment 5,
over oxygen vacancy annihilation (assignment 5,  ). In contrast, the minimum at x = 0.08 is associated with substitutional solid solubility and hence dominated by oxygen vacancy annihilation.
). In contrast, the minimum at x = 0.08 is associated with substitutional solid solubility and hence dominated by oxygen vacancy annihilation.
Since lower peak intensities and defect concentrations are inversely proportional, the inverse PL intensity at 570 nm contrasted with the 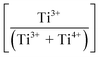 and true density as a function of Sn4+-doping level are shown in Fig. 4(b). These data reveal the maximal defect concentration (i.e., lowest stability) for x = 0.04, which results from the saturated interstitial solid solubility in P4mm. As discussed earlier, at this stage, substitutional solid solubility commences in P4mm, while interstitial solid solubility continues in Pmm2. At this point, the defect concentrations shift toward minima. These data reveal a critical observation for the present work that is at x = 0.04, the maximum defect concentration is dominated by the redox mechanism rather than oxygen vacancy annihilation. While at x = 0.08, oxygen vacancy annihilation dominates, thus stabilizing the lattice to a maximum at x = 0.08 (i.e., highest stability). Since interstitial solid solubility continues in Pmm2, then this continued increase in defect concentration of
and true density as a function of Sn4+-doping level are shown in Fig. 4(b). These data reveal the maximal defect concentration (i.e., lowest stability) for x = 0.04, which results from the saturated interstitial solid solubility in P4mm. As discussed earlier, at this stage, substitutional solid solubility commences in P4mm, while interstitial solid solubility continues in Pmm2. At this point, the defect concentrations shift toward minima. These data reveal a critical observation for the present work that is at x = 0.04, the maximum defect concentration is dominated by the redox mechanism rather than oxygen vacancy annihilation. While at x = 0.08, oxygen vacancy annihilation dominates, thus stabilizing the lattice to a maximum at x = 0.08 (i.e., highest stability). Since interstitial solid solubility continues in Pmm2, then this continued increase in defect concentration of 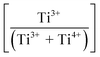 must be dominated by the intrinsic
must be dominated by the intrinsic  annihilation. The reverse trend at x = 0.10 is caused by the recurrence of interstitial solid solubility and increasing
annihilation. The reverse trend at x = 0.10 is caused by the recurrence of interstitial solid solubility and increasing 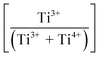 but also possibly the structural stress caused by the P4mm → Pm
but also possibly the structural stress caused by the P4mm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase transformation.
m phase transformation.
Fig. 4(b) integrates PL, XPS, and true density data, all of which provide quantitative estimates of defect concentrations. These data also highlight the risks of assuming substitutional solid solubility because this incorrect assumption results in the introduction of metal vacancies, which are integrated indirectly in the PL data. These particular defects thus are not considered part of the relevant defect equilibria because they do not require charge compensation as they are created by Ti deficiency. In this sense, these defects can be considered to be intrinsic defects, as in intrinsic oxygen vacancies in many oxides. This leads to a second oversight shown by the XPS data, which reveal the importance of redox charge compensation and the formation of Ti3+ defects through Ti4+ → Ti3+ reduction. The presence of point defects in the form of substitutional extrinsic dopants (weight gain or loss), interstitial extrinsic dopants (weight gain), and vacancies (weight loss) can be reflected through true density measurements, a technique that has been used previously.238,239 The similarities in trends between these three disparate techniques demonstrate that interstitial Sn4+ initiates redox charge compensation, where increasing x and the resultant irregular solid-solution mechanisms are reflected in corresponding changes in the 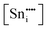 ,
, 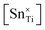 ,
,  , and
, and  . However, while the trends in the XRD data (Fig. S1) generally support these data, the correspondence is not perfect owing to the competing trends in lattice constants, c/a ratios, and unit cell volumes of the two polymorphs.
. However, while the trends in the XRD data (Fig. S1) generally support these data, the correspondence is not perfect owing to the competing trends in lattice constants, c/a ratios, and unit cell volumes of the two polymorphs.
3.7. Ferroelectric property analysis
In addition to consideration of the effects of the chemical, physical, and structural characteristics, the electromechanical properties of Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) were also examined. The RT unipolar polarization curves with no prepoling history (Fig. 4(c)) were used to calculate the coercive field (EC), which is shown as a function of [Sn4+] in Fig. 4(d). Relative to undoped (Ba0.85Ca0.15)(Ti0.92Zr0.08)O3, the addition of Sn4+ lowers the EC, making the materials ferroelectrically soft. The change is greatest for x = 0.02, indicating that only a small amount of Sn4+ is capable of reducing the energy barrier for domain switching. The negative slopes for interstitial Sn4+ compared to the nil slope of the substitutional Sn4+ indicates that the mechanism of solid solubility plays a key role in facilitating domain switching. Furthermore, the gradual approach of the EC toward 0 suggests the potential for complete elimination of the energy barrier to domain switching.The EC data for the doped samples are interpreted in terms of the PL data (Fig. 4(a) and (b)), which reveal the pervasive effect of intrinsic oxygen vacancies and associated color centers. Since oxygen vacancy annihilation (assignment 5) has the lowest energy requirement and so would be expected to occur readily, then this provides the driving force for the trend observed in Fig. 4(d). This is consistent with the effect of oxygen vacancies, which hinder domain wall motion through pinning effects.240–242 Thus, the gradual annihilation of intrinsic oxygen vacancies would cause a similar trend in increasing the soft ferroelectricity with increasing [Sn4+]. However, the monotonic trend in Fig. 4(d) in the EC does not mirror the multiple trends in Fig. 4(b). This is because Fig. 4(b) shows the effect of assignment 4 only (Ti reduction) and Fig. 4(d) shows the effect of assignment 5 only (oxygen vacancy annihilation). This differentiation thus allows decoupling of the data for the two solid solubility mechanisms. Such flexibility offers advantages over the more conventional approach for (Ba,Ca)(Ti,Sn,Zr)O360,62,63,67,75 that assumed B-site substitutional solid solubility (and hence neutral charge compensation) and unexplained charge compensation by oxygen vacancy formation. The alternative of A-site substitutional solid solubility would result in oxygen vacancy annihilation.
Fig. 5(a) shows the RT bipolar ferroelectric hysteresis (P–E) loops measured at 4.0 kV mm−1. All of the compositions exhibit well-shaped symmetric hysteresis loops with clear saturation, again confirming their conventional ferroelectric nature. However, with increasing Sn4+ doping level, the P–E loops become narrower, suggesting that Sn4+ reduces the maximal polarization (Pmax) as well as the remanent polarization (Pr), ultimately eliminating the Pr at x = 0.10. These trends clearly demonstrate that the addition of Sn4+ lowers the energy of the domain wall movement, thus facilitating the domain switching.243–245
The temperature-dependent bipolar hysteresis curves measured at 2.5 kV mm−1 in the range 25°–140 °C also are provided in Fig. S4. These data were used to determine Pmax (Fig. 5(b)) and Pr (Fig. S5). These data are consistent with the observation concerning Fig. 4(c), thus indicating that [Sn4+] effectively disrupts the long-range order, creating short-range polar regions that lower the energy required for domain wall movement, which is a requirement for high-performance energy storage materials. Furthermore, the variations in Pr with increasing temperature (Fig. S5) are consistent with a shift in polymorphic phase transformation temperatures at different Sn4+ doping levels. These data also reveal that the addition of Sn4+ lowers the tetragonal → cubic transformation temperature (Curie temperature, TC), ultimately reaching RT at x = 0.10.
3.8. Electromechanical property analysis
The unary phase diagrams for BaTiO3 (Fig. S2) show the influence of stress (and resultant strain) on the phase transformations of this material. The bipolar strain curves as a function of electric field are shown in Fig. 5(c), which reveals the change in shape of the strain curves with increasing Sn4+ content. These data confirm the gradual reduction in spontaneous strain. Fig. 5(d) shows the variations in the values of positive (Spos) and negative (Sneg) strains as a function of Sn4+ doping level, where the two inflections at x = 0.04 and x = 0.08 reflect relatively high respective net strain (ΔS) values of 0.125% and 0.144%.These data are also interpreted in terms of the PL data (Fig. 4(a) and (b)), which suggests that the gradual reduction in Sneg with increasing Sn4+ content is associated with the process of oxygen vacancy annihilation. In contrast, the Spos data show a decreasing trend but accompanied by small increases in strain at x = 0.04 and x = 0.08. Since the PL data for defect concentration (Fig. 4(b)) show a maximum at x = 0.04, then the correlation between the first inflection and the associated stress-induced orthorhombic → tetragonal phase transformation is clear. However, the second inflection at x = 0.08, which reveals minimal defect concentration, exhibits the highest strain in the doped samples. This is interpreted in terms of the transmission of stress and resultant strain, where strain is facilitated by stress transmission through a “perfect” lattice, in contrast with decreased strain from a more compliant lattice distorted by intrinsic 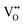 . As discussed, at x = 0.08, minimal defect concentration through maximal oxygen vacancy annihilation is expected, which decreases the compliance and so stiffens the lattice, thereby increasing the strain. The significant reduction in strain at x = 0.10 is attributed to the introduction of large-scale microstructural defects in the cubic (Pm
. As discussed, at x = 0.08, minimal defect concentration through maximal oxygen vacancy annihilation is expected, which decreases the compliance and so stiffens the lattice, thereby increasing the strain. The significant reduction in strain at x = 0.10 is attributed to the introduction of large-scale microstructural defects in the cubic (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) grains, thus resulting in considerable microstructural disturbance and resultant compliance.
m) grains, thus resulting in considerable microstructural disturbance and resultant compliance.
3.9. Dielectric property analysis
Fig. 6(a) shows the variation in maximal dielectric constant (εmax) as a function of electric field. These data reveal that the optimal samples exhibit εmax, values in the range 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15
000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (RT, 4.0 kV mm−1, 0.5 kHz) for x = 0.06–0.10. These data show an apparent inconsistency for x = 0.06 and 0.08. The transition from the higher value at x = 0.06 to the lower value at x = 0.08 would result from the transition from minimal to maximal substitutional solid solubility, this component of which effectively would not contribute to the dielectric polarization. This range in maximal dielectric constant may be contrasted with those for (Ba0.90Ca0.10)(Ti0.75Zr0.25)O3 (bulk material) of 1992 (RT, 2.0 kV mm−1, 10.0 kHz)246 and for (Ba0.85Ca0.15)(Ti0.90Zr0.10)O3 (thin film) of 1600 (RT 100.0 kV mm−1, 10.0 kHz).247 Furthermore, the low slopes of the plots in Fig. 6(a) indicate relatively stable dielectric constants for the range of fields examined (≤4.0 kV mm−1). Thus, these materials are potential candidates for energy storage since they exhibit very high dielectric constants.
000 (RT, 4.0 kV mm−1, 0.5 kHz) for x = 0.06–0.10. These data show an apparent inconsistency for x = 0.06 and 0.08. The transition from the higher value at x = 0.06 to the lower value at x = 0.08 would result from the transition from minimal to maximal substitutional solid solubility, this component of which effectively would not contribute to the dielectric polarization. This range in maximal dielectric constant may be contrasted with those for (Ba0.90Ca0.10)(Ti0.75Zr0.25)O3 (bulk material) of 1992 (RT, 2.0 kV mm−1, 10.0 kHz)246 and for (Ba0.85Ca0.15)(Ti0.90Zr0.10)O3 (thin film) of 1600 (RT 100.0 kV mm−1, 10.0 kHz).247 Furthermore, the low slopes of the plots in Fig. 6(a) indicate relatively stable dielectric constants for the range of fields examined (≤4.0 kV mm−1). Thus, these materials are potential candidates for energy storage since they exhibit very high dielectric constants.
3.10. Energy storage efficiency analysis
Fig. 6(b) shows the variation in charge (Wtot) and discharge energy densities (Wrec + Wloss) measured at room temperature as a function of Sn4+ doping level. These data reveal that the incorporation of Sn4+ is beneficial in reducing the loss energy densities (Wloss). The recoverable energy densities (Wrec) increase with increasing Sn4+ doping level up to x = 0.10, at which point the Wrec approaches Wtot, thus showing that the loss associated with the discharge energy density is negligible at x = 0.10.The data in Fig. 5(a) and Fig. S4 were used to calculate the energy storage efficiencies, which are shown in Fig. 6(c) and (d). The electric-field-induced energy storage efficiency (η) data in Fig. 6(c) suggest four performance regions (x = 0.00, 0.02–0.04, 0.06–0.08, and 0.10). These data are consistent with the bipolar strain data, which reflect the solid solubility mechanisms.
In the parent BaTiO3 structure, the two types of interstices are in twofold (unit cell edge) and threefold (tetrahedral void base) coordinations. With interstitial solid solubility, the introduction of the electronegative effect of the irregularly localized Sn4+ solutes would disrupt the long-range dipole order and generate short-range order. Such disruption is associated solely with relaxor ferroelectrics, which display a rapid response to applied electric fields and hence increased η. Since Sn4+-doped Ba(Ti1−xSnx)O3 (0.10 ≤ x ≤ 0.27) exhibits relaxor characteristics,248 then it is probable that, Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10) would exhibit relaxor behavior at x > 0.10.
The electric-field-induced temperature-dependent permittivity curves are given in Fig. S6, which suggests shifts in the polymorphic phase transformation temperatures toward RT with increasing Sn4+ doping level. These data explain the presence of Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m at x = 0.10, as detected by room-temperature XRD (Fig. 1(b)).
m at x = 0.10, as detected by room-temperature XRD (Fig. 1(b)).
Since thermal stability is an important aspect for practical applications of energy storage materials, then the energy storage efficiencies are shown in Fig. 6(d). These data were generated from the corresponding (20°–140 °C) bipolar hysteresis curves (Fig. S4). The trends in the data, which generally approach constancy, indicate the potential for both high and constant energy-storage efficiencies. This is particularly the case for x = 0.10, where η is >95% for RT to 130 °C.
Fig. 7(a) and (b) show plots of the energy-storage efficiencies (η) vs. recoverable energy densities (Wrec) reported during the period 2013–2024 for BaTiO3-based materials,50,63,67,68,122,249–273 including the relatively few studies of Sn4+-doped (Ba0.85Ca0.15)(Ti,Sn,Zr)O3 (202263,67 and 202382). The data shown in Fig. 7(a) and (b) are tabulated in Table S5. These data reveal that the majority (∼61%) of these studies involved the application of low electric fields (≤2.5 kV mm−1) and low temperatures (≤100 °C). Of these thirty publications, only two provided data for η for the simultaneous conditions of high electric field (>2.5 kV mm−1) and high temperature (>100 °C).68,273 This is a critical distinction since both of these parameters influence the η significantly.
Fig. 7(a) also reveals that nearly all of the previously reported BaTiO3-based studies exhibit comparatively low Wrec (grey-shaded region, <100 mJ cm−3). Conversely, the present study reports a linear trend with significantly higher values of η at RT and low electric fields. At higher temperatures and low electric field, the materials exhibit a linear trend in η for the materials that outperforms all reported equivalent data. Fig. 7(b) summarizes similar recent reports of energy storage performance in terms of η but at high electric fields. These data reveal that the highest Sn4+ doping level (x = 0.10), which is from the present work, exhibits an η value (>95%) that is the highest recorded for BaTiO3-based materials.
4. Conclusions
The present work highlights the significance of the basic science of piezoelectric ceramics using a lead-free analogue of BaTiO3-based materials, specifically Sn4+-doped (Ba0.85Ca0.15)([Ti0.92−xSnx]Zr0.08)O3 (x = 0.00–0.10). The effects of the solid solubility mechanism, charge-compensation process, and defect equilibria on the structural, chemical, electromechanical, and energy-storage properties were examined using a comprehensive suite of analytical techniques. Although such systems are considered universally to exhibit substitutional solid solubility, the present work demonstrates that, for this specific set of compositions and processing procedures, identification of the actual solid solubility mechanism, resultant charge compensation, and associated defect chemistry are essential in order to interpret materials properties and performance. The XRD and Raman data reveal the existence of mixed polymorphs (x = 0.00–0.08: Pmm2 + P4mm; x = 0.10: Pmm2 + P4mm + Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The XPS data confirm three, sequential, solubility-driven, redox mechanisms. The two inflections observed at x = 0.04 and x = 0.08 are stress-induced and attributed to the achievement of saturation solid solubility in P4mm. More broadly, x = 0.04 reveals saturation for interstitial solid solubility and x = 0.08 indicates saturation for substitutional solid solubility. The observation from the inverse PL data of the maximal defect concentration (i.e., lowest stability) at x = 0.04 reflects the dominance of redox-charge compensation. In contrast, the minimal defect concentration (i.e., highest stability) at x = 0.08 reflects the role of interstitial Sn4+ in annihilating intrinsic oxygen vacancies, thus stabilizing the structure. The expected higher stability at x = 0.10 was not observed owing to the introduction of a third phase Pm
m). The XPS data confirm three, sequential, solubility-driven, redox mechanisms. The two inflections observed at x = 0.04 and x = 0.08 are stress-induced and attributed to the achievement of saturation solid solubility in P4mm. More broadly, x = 0.04 reveals saturation for interstitial solid solubility and x = 0.08 indicates saturation for substitutional solid solubility. The observation from the inverse PL data of the maximal defect concentration (i.e., lowest stability) at x = 0.04 reflects the dominance of redox-charge compensation. In contrast, the minimal defect concentration (i.e., highest stability) at x = 0.08 reflects the role of interstitial Sn4+ in annihilating intrinsic oxygen vacancies, thus stabilizing the structure. The expected higher stability at x = 0.10 was not observed owing to the introduction of a third phase Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.
m.
The polarization data indicate a decrease in the oxygen-vacancy-induced pinning effect with increasing Sn4+ doping level. The bipolar strain data reveal high net strain (ΔS) values at x = 0.04 and x = 0.08 owing to changes in the solid solubility mechanism. These data reveal the great potential for x = 0.10 in energy storage, where an efficiency of >95% was achieved over the temperature range 25°–130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. This is the highest energy storage efficiency reported to date for the system (Ba,Ca)(Ti,Zr,Sn)O3. The other Sn4+-doped compositions from the present work also exhibit notably high energy-storage efficiencies up to 130 °C, thus making these materials suitable for high-temperature energy-storage applications.
°C. This is the highest energy storage efficiency reported to date for the system (Ba,Ca)(Ti,Zr,Sn)O3. The other Sn4+-doped compositions from the present work also exhibit notably high energy-storage efficiencies up to 130 °C, thus making these materials suitable for high-temperature energy-storage applications.
This synthesis of experimentation and mechanistic interpretation in the present work provides a basis for the engineering of piezoelectric materials with improved electromechanical properties and energy-storage performance. This approach can inform the strategic selection of dopants of specific characteristics that result in the establishment of different solid solubility mechanisms, corresponding charge-compensation processes, and associated defect equilibria, all of which play a pervasive role in the resultant properties.
More broadly, the present work applies a case study to test the apparent paradigm of the general assumption of substitutional solid solubility in the case of doping studies. This common practice for oxide perovskites and for many other materials, if not justified, has ramifications for the defect equilibria and associated performance of functional materials owing to the practice of stoichiometry compensation during fabrication. That is, the outcome can be unsuspected cation vacancies (ionic charge compensation), electron or hole generation (electronic charge compensation), or cation reduction (redox charge compensation). The present work shows that this assumption may not be justified. Thus, if there is one case of interstitial and/or mixed solid solubilities, there may be others.
The present work also introduces the possibility of determining the solid-solubility mechanism without resorting to the application of analytical techniques such as neutron diffraction (ND), X-ray absorption spectroscopy (XAS), small-angle X-ray scattering (SAXS), and/or solid-state nuclear magnetic resonance (NMR). The present work reveals that assessment of the defect equilibria through the use of common experimental techniques is a powerful yet simple method that can lead to the determination of the solid solubility mechanism. These methods, used singly or combined, include X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), photoluminescence (PL) by laser Raman microspectroscopy, and/or true density by He pycnometry.
Conflicts of interest
There are no conflicts to declare.Data availability
Any further data that support the findings of this study are available on request from the corresponding author.The supporting data are provided in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5mh01632k.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors acknowledge financial support from the Australian Government Research Training Program (RTP) and the subsidized analytical facilities provided by the Mark Wainwright Analytical Centre, UNSW. The authors also wish to acknowledge the significant assistance with the acquisition of the electromechanical measurements by Mr Bill Joe, Senior Technical Officer, School of Materials Science and Engineering, UNSW Sydney.References
- R. Bulanadi, K. Cordero-Edwards, P. Tückmantel, S. Saremi, G. Morpurgo, Q. Zhang, L. W. Martin, V. Nagarajan and P. Paruch, Phys. Rev. Lett., 2024, 133, 106801 CrossRef CAS PubMed.
- Y. X. Liu, H. C. Thong, Y. Y. S. Cheng, J. W. Li and K. Wang, J. Appl. Phys., 2021, 129, 024102 CrossRef CAS.
- D. Schrade, R. Mueller, D. Gross, T. Utschig, V. Y. Shur and D. C. Lupascu, Mech. Mater., 2007, 39, 161–174 CrossRef.
- K. Klyukin and V. Alexandrov, Phys. Rev. B, 2017, 95, 035301 CrossRef.
- P. Gao, C. T. Nelson, J. R. Jokisaari, S. H. Baek, C. W. Bark, Y. Zhang, E. Wang, D. G. Schlom, C. B. Eom and X. Pan, Nat. Commun., 2011, 2, 591 CrossRef PubMed.
- W. Wang, D. Zheng, M. Hu, S. Saeed, H. Liu, Y. Kong, L. Zhang and J. Xu, Materials, 2019, 12, 100 CrossRef CAS PubMed.
- Z. Xu, Y. X. Liu, M. Azadeh, H. C. Thong, Y. Jiang, F. Z. Yao, Z. X. Yue, Z. T. Zhang, Z. L. Tang, J. F. Li, H. Wang, T. Frömling and K. Wang, Angew. Chem., Int. Ed., 2023, 62, e202216776 CrossRef CAS PubMed.
- W. Liu, F. Li, M. Long, J. Zhai, C. Wang and L. Shan, J. Eur. Ceram. Soc., 2022, 42, 6295–6300 CrossRef CAS.
- X. Wu, L. Liu, X. Li, X. Zhao, D. Lin, H. Luo and Y. Huang, Mater. Chem. Phys., 2012, 132, 87–90 CrossRef CAS.
- H. Arandiyan, S. S. Mofarah, C. C. Sorrell, E. Doustkhah, B. Sajjadi, D. Hao, Y. Wang, H. Sun, B. J. Ni, M. Rezaei, Z. Shao and T. Maschmeyer, Chem. Soc. Rev., 2021, 50, 10116–10211 RSC.
- A. Chen, W. F. Chen, T. Majidi, B. Pudadera, A. Atanacio, M. Manohar, L. R. Sheppard, R. Liu, C. C. Sorrell and P. Koshy, Int. J. Hydrogen Energy, 2021, 46, 12961–12980 CrossRef CAS.
- W. F. Chen, H. Chen, P. Koshy, A. Nakaruk and C. C. Sorrell, Mater. Chem. Phys., 2018, 205, 334–346 CrossRef CAS.
- W. F. Chen, P. Koshy, Y. Huang, E. Adabifiroozjaei, Y. Yao and C. C. Sorrell, Int. J. Hydrogen Energy, 2016, 41, 19025–19056 CrossRef CAS.
- W. F. Chen, H. Chen, G. Bahmanrokh, P. Koshy, A. Nakaruk and C. C. Sorrell, Int. J. Hydrogen Energy, 2020, 45, 24558–24566 CrossRef CAS.
- X. Zhang, W. F. Chen, G. Bahmanrokh, V. Kumar, N. Ho, P. Koshy and C. C. Sorrell, Nano-Struct. Nano-Objects, 2020, 24, 100557 CrossRef CAS.
- F. A. Kröger and H. J. Vink, Solid State Phys., 1956, 3, 307–435 Search PubMed.
- A. Alasmari, P. Thakur, R. Sharma, T. B. Drye and S. R. Saha, Jpn. J. Appl. Phys., 1995, 34, 5683–5688 CrossRef.
- D.-C. Kong, Q.-Y. Zhu, D.-H. Guan, X.-X. Wang, Y.-L. Liu, H.-F. Wang and J.-J. Xu, Angew. Chem., Int. Ed., 2024, 64, e202418174 Search PubMed.
- M. Arora, S. Kaur, S. Kumar, K. M. Batoo, S. Hussain, A. Sharma, V. Arora, I. Sharma, M. Singh and A. Singh, Ceram. Int., 2024, 50, 10117–10130 CrossRef CAS.
- F. Craciun, F. Cordero, M. Cernea, V. Fruth, I. Atkinson, N. Stanica, B. S. Vasile, R. Trusca, A. Iuga, P. Galizia and C. Galassi, Ceram. Int., 2019, 45, 9390–9396 CrossRef CAS.
- M. K. Bhattarai, S. Shweta, S. Choudhary, H. M. Meyer, B. P. Thapaliya, B. R. Weiner, R. S. Katiyar and G. Morell, ACS Omega, 2024, 9, 19219–19226 CrossRef CAS PubMed.
- A. Joshi, S. Bandyopadhyay, A. Gupta, R. K. Srivastava and B. Nandan, J. Alloys Compd., 2023, 948, 169769 CrossRef CAS.
- M. T. Demirkan and M. Dogrusoz, Discov. Electrochem., 2025, 2, 1–12 CrossRef.
- Y. Zhang, M. Xie, J. Roscow, Y. Bao, K. Zhou, D. Zhang and C. R. Bowen, J. Mater. Chem. A, 2017, 5, 6569–6580 RSC.
- D. Fu, S. Sogen and H. Suzuki, ACS Appl. Electron. Mater., 2024, 6, 6384–6390 CrossRef CAS.
- P. Liu, M. Y. Li, Q. Zhang, W. Li, Y. Zhang, M. Shen, S. Qiu, G. Zhang and S. Jiang, J. Eur. Ceram. Soc., 2018, 38, 5396–5401 CrossRef CAS.
- S. H. Park, S. Ural, C. W. Ahn, S. Nahm and K. Uchino, Jpn. J. Appl. Phys., Part 1 Regul. Pap. Short Notes Rev. Pap., 2006, 45, 2667–2673 CrossRef CAS.
- S. K. Pradhan, S. N. Das, S. Bhuyan and R. N. P. Choudhary, Mater. Today Proc., 2019, 41, 312–315 CrossRef.
- K. Ran Han, J. Wung Jeong, C. S. Kim and Y. S. Kwon, Mater. Lett., 2006, 60, 3596–3600 CrossRef CAS.
- S. Kwon, E. M. Sabolsky and G. L. Messing, J. Am. Ceram. Soc., 2001, 84, 648–650 CrossRef CAS.
- M. Algueró, C. Alemany, L. Pardo and M. Pham-Thi, J. Am. Ceram. Soc., 2005, 88, 2780–2787 Search PubMed.
- J.-H. Chen and Y.-C. Liou, Ceram. Int., 2004, 30, 157–162 CrossRef CAS.
- J.-S. Park, J.-K. Lee, H.-M. Park and K. S. Hong, J. Am. Ceram. Soc., 2007, 90, 3512–3516 CrossRef CAS.
- H. Li, W. Liao, B. Sun, Y. Lu, X. He, Z. Xu, C. Huang, Z. Sun and T. Li, Ceram. Int., 2023, 49, 8325–8330 CrossRef CAS.
- D. Kobor, L. Lebrun, G. Sébald and D. Guyomar, J. Cryst. Growth, 2005, 275, 580–588 CrossRef CAS.
- B. Fang, R. Sun, Y. Shan, K. Tezuka and H. Imoto, J. Phys. Chem. Solids, 2009, 70, 893–899 CrossRef CAS.
- T. R. Shrout and S. J. Zhang, J. Electroceram., 2007, 19, 113–126 CrossRef.
- C. C. Tsai, W. H. Chao, S. Y. Chu, C. S. Hong, C. M. Weng and H. H. Su, AIP Adv., 2016, 6, 125024 CrossRef.
- G. Shirane and A. T. Tokyo, J. Phys. Soc. Jpn., 1952, 7, 5–11 CrossRef CAS.
- E. Sawaguchi, J. Phys. Soc. Jpn., 1953, 8, 615–629 CrossRef CAS.
- N. H. Yusoff, R. A. M. Osman, M. S. Idris, K. N. D. K. Muhsen and N. I. M. Nor, AIP Conf. Proc., 2020, 2203, 020038 CrossRef CAS.
- P. Gwizd, D. Sitko, I. Jankowska-sumara, D. Sitko, I. Jankowska-Sumara and M. Krupska-Klimczak, Phase Trans., 2021, 94, 192–198 CrossRef CAS.
- Y. Zhang, M. Cao, Z. Yao, Z. Wang, Z. Song and A. Ullah, Mater. Res. Bull., 2015, 67, 70–76 CrossRef CAS.
- T. Zhang, W. Li, W. Cao, Y. Hou, Y. Yu and W. Fei, Appl. Phys. Lett., 2016, 108, 162902 CrossRef.
- G. Sebald, L. Seveyrat, D. Guyomar, L. Lebrun, B. Guiffard and S. Pruvost, J. Appl. Phys., 2006, 100, 124112 CrossRef.
- J. Hagberg, A. Uusimäki and H. Jantunen, Appl. Phys. Lett., 2008, 92, 132909 CrossRef.
- Y. Zhang, J. Glaum, C. Groh, M. C. Ehmke, J. E. Blendell, K. J. Bowman and M. J. Hoffman, J. Am. Ceram. Soc., 2014, 97, 2885–2891 CrossRef CAS.
- V. Bijalwan, P. Tofel, J. Erhart and K. Maca, Ceram. Int., 2019, 45, 317–326 CrossRef CAS.
- S. Mahajan, O. P. Thakur, D. K. Bhattacharya and K. Sreenivas, Mater. Chem. Phys., 2008, 112, 858–862 CrossRef CAS.
- D. Zhan, Q. Xu, D. P. Huang, H. X. Liu, W. Chen and F. Zhang, J. Phys. Chem. Solids, 2018, 114, 220–227 CrossRef CAS.
- A. D. Loreto, A. Frattini and M. G. Stachiotti, Mater. Lett., 2017, 191, 69–72 CrossRef.
- W. Li, Z. Xu, R. Chu, P. Fu and G. Zang, J. Eur. Ceram. Soc., 2012, 32, 517–520 CrossRef CAS.
- Chitra, R. Laishram, S. Rajput and K. C. Singh, J. Alloys Compd., 2020, 812, 152128 CrossRef CAS.
- M. Chen, Z. Xu, R. Chu, Y. Liu, L. Shao, W. Li, S. Gong and G. Li, Mater. Lett., 2013, 97, 86–89 CrossRef CAS.
- L. Qifeng, M. Jingjun, M. Sharma and R. Vaish, J. Am. Ceram. Soc., 2019, 102, 5807–5817 CrossRef CAS.
- L. F. Zhu, B. P. Zhang, X. K. Zhao, L. Zhao, P. F. Zhou and J. F. Li, J. Am. Ceram. Soc., 2013, 96, 241–245 CrossRef CAS.
- Z. Liu, R. Yuan, D. Xue, W. Cao and T. Lookman, Acta Mater., 2018, 157, 155–164 CrossRef CAS.
- S. Patel, P. Sharma and R. Vaish, Phase Transitions, 2016, 89, 1062–1073 CrossRef CAS.
- S. Venkateshwarlu, L. K. Venkataraman, V. Segouin, F. P. Marlton, H. C. Hin, D. Chernyshov, Y. Ren, M. R. V. Jørgensen, S. Nayak, J. Rödel, L. Daniel and A. Pramanick, Commun. Phys., 2020, 3, 1–14 CrossRef.
- X. Wang and J. Liu, J. Ceram. Soc. Jpn., 2020, 128, 1013–1017 CrossRef CAS.
- S. Merselmiz, Z. Hanani, U. Prah, D. Mezzane, L. Hajji, Z. Abkhar, M. Spreitzer, D. Vengust, H. Uršič, D. Fabijan, A. G. Razumnaya, O. Shapovalova, I. A. Luk’yanchuk and Z. Kutnjak, Phys. Chem. Chem. Phys., 2022, 24, 6026–6036 RSC.
- S. Belkhadir, A. Neqali, M. Amjoud, D. Mezzane, A. Alimoussa, E. Choukri, Y. Gagou, I. Raevski, M. El Marssi, I. A. Luk’yanchuk, B. Rožič and Z. Kutnjak, J. Mater. Sci.: Mater. Electron., 2019, 30, 14099–14111 CrossRef CAS.
- S. Belkhadir, S. Khardazi, D. Mezzane, M. Amjoud, O. Shapovalova, V. Laguta, I. Raevski, K. Pushkarova, I. Lukyanchuk and M. El Marssi, Mater. Today Proc., 2022, 51, 2005–2014 CrossRef CAS.
- S. Khobragade, J. K. Devanuri and S. Patel, Phase Transitions, 2019, 92, 960–973 CrossRef CAS.
- B. G. Baraskar, P. S. Kadhane, T. C. Darvade, A. R. James and R. C. Kambale, Ferroelectr. Their Appl., IntechOpen, London, 2018, pp. 113–132 Search PubMed.
- K. N. D. K. Muhsen, R. A. M. Osman, M. S. Idris, N. I. M. Nadzri and M. H. H. Jumali, Ceram. Int., 2021, 47, 13107–13117 CrossRef CAS.
- Y. You and X. Guo, J. Alloys Compd., 2022, 918, 165557 CrossRef CAS.
- S. Khardazi, H. Zaitouni, A. Neqali, S. Lyubchyk, D. Mezzane, M. Amjoud, E. Choukri and Z. Kutnjak, J. Phys. Chem. Solids, 2023, 177, 111302 CrossRef CAS.
- H. Kaddoussi, A. Lahmar, Y. Gagou, J. L. Dellis, H. Khemakhem and M. El Marssi, Ceram. Int., 2015, 41, 15103–15110 CrossRef CAS.
- A. Pramanick, L. Daniel, S. Venkateshwarlu, V. Segouin and Y. Ren, J. Eur. Ceram. Soc., 2023, 43, 3236–3249 CrossRef CAS.
- X. Liu, Z. Chen, B. Fang, J. Ding, X. Zhao, H. Xu and H. Luo, J. Alloys Compd., 2015, 640, 128–133 CrossRef CAS.
- X. Wang, J. Liu, P. Liang and Z. Yang, J. Electron. Mater., 2018, 47, 6121–6127 CrossRef CAS.
- S. Patel and M. Kumar, EPJ Appl. Phys., 2020, 91, 20905 CrossRef CAS.
- S. Zhou, Q. Liu, P. Xu, L. Yao and E. Cai, Ferroelectrics, 2017, 510, 27–33 CrossRef CAS.
- K. N. D. K. Muhsen, R. A. M. Osman, M. S. Idris, M. H. H. Jumali and N. H. B. Jamil, J. Mater. Sci.: Mater. Electron., 2019, 30, 20654–20664 CrossRef CAS.
- P. Suresh, P. Mathiyalagan and K. S. Srikanth, Trans. Nonferrous Met. Soc. China, 2019, 29, 791–798 CrossRef CAS.
- H. Wang and J. Wu, J. Alloys Compd., 2014, 615, 969–974 CrossRef CAS.
- M. S. Yoon and S. C. Ur, Ceram. Int., 2008, 34, 1941–1948 CrossRef CAS.
- E. Cai, Q. Liu, F. Zeng, Y. Wang and A. Xue, Ceram. Int., 2018, 44, 788–798 CrossRef CAS.
- J. Wu, A. Habibul, X. Cheng, X. Wang and B. Zhang, Mater. Res. Bull., 2013, 48, 4411–4414 CrossRef CAS.
- S. Khobragade and S. Patel, J. Electron. Mater., 2020, 49, 1194–1203 CrossRef CAS.
- S. Khardazi, H. Zaitouni, A. Neqali, S. Lyubchyk, D. Mezzane, M. Amjoud, E. Choukri and Z. Kutnjak, J. Phys. Chem. Solids, 2023, 177, 111302 CrossRef CAS.
- R. Li, Y. Luo, L. Yao, L. Tian, Z. Sun, R. Wang and J. Xue, Appl. Phys. Lett., 2025, 126, 140501 CrossRef CAS.
- M. P. Hautzinger, W. Mihalyi-Koch and S. Jin, Chem. Mater., 2024, 36, 10408–10420 CrossRef CAS PubMed.
- S. Jin, ACS Energy Lett., 2021, 6, 3386–3389 CrossRef CAS.
- Z. Guo, J. Wang and W. J. Yin, Energy Environ. Sci., 2022, 15, 660–671 RSC.
- A. C. Ferreira, A. Létoublon, S. Paofai, S. Raymond, C. Ecolivet, B. Rufflé, S. Cordier, C. Katan, M. I. Saidaminov, A. A. Zhumekenov, O. M. Bakr, J. Even and P. Bourges, Phys. Rev. Lett., 2018, 121, 085502 CrossRef CAS PubMed.
- M. Gao, Y. Zhang, Z. Lin, J. Jin, M. C. Folgueras and P. Yang, Matter, 2021, 4, 3874–3896 CrossRef.
- M. Wuttig, C. F. Schön, M. Schumacher, J. Robertson, P. Golub, E. Bousquet, C. Gatti and J. Y. Raty, Adv. Funct. Mater., 2021, 32, 2110166 CrossRef.
- X. Liang, J. Klarbring, W. J. Baldwin, Z. Li, G. Csányi and A. Walsh, J. Phys. Chem. C, 2023, 127, 19141–19151 CrossRef CAS PubMed.
- M. A. Peña and J. L. G. Fierro, Chem. Rev., 2001, 101, 1981–2018 CrossRef PubMed.
- M. I. Khan, S. Hussain, M. Atif, J. E. Leal-Perez, B. A. García-Grajeda and J. M. Mendivil-Escalante, Results Chem., 2025, 13, 101974 CrossRef CAS.
- F. Zheng, W. Chen, T. Bu, K. P. Ghiggino, F. Huang, Y. Cheng, P. Tapping, T. W. Kee, B. Jia and X. Wen, Adv. Energy Mater., 2019, 9, 1901016 CrossRef.
- T. Cai, J. Wang, W. Li, K. Hills-Kimball, H. Yang, Y. Nagaoka, Y. Yuan, R. Zia and O. Chen, Adv. Sci., 2020, 7, 2001317 CrossRef CAS PubMed.
- X. Wang, Q. Shen, Y. Chen, N. Ali, Z. Ren, G. Bi and H. Wu, J. Alloys Compd., 2022, 895, 162610 CrossRef CAS.
- S. Tian, X. Zhou, C. Bi, X. Sun, M. Zhang, S. Yang and J. Tian, Adv. Opt. Mater., 2022, 10, 2200751 CrossRef CAS.
- Z. Zhang, J. Shang, H. Ge, Y. Zhang, Q. Chen, L. Zhou, W. Zhu, D. Chen, H. Xi, J. Zhang, C. Zhang and Y. Hao, Mater. Today Phys., 2023, 37, 101187 CrossRef CAS.
- A. P. Litvin, I. V. Margaryan, W. Yin, X. Zhang, W. Zheng and A. L. Rogach, Adv. Opt. Mater., 2024, 12, 2301001 CrossRef CAS.
- J. Gong, M. Yang, D. Rebollar, J. Rucinski, Z. Liveris, K. Zhu and T. Xu, Adv. Mater., 2018, 30, 1800973 CrossRef PubMed.
- G. C. Adhikari, S. Thapa, H. Zhu and P. Zhu, Adv. Opt. Mater., 2019, 7, 1900916 CrossRef CAS.
- S. Li, Z. Shi, F. Zhang, L. Wang, Z. Ma, D. Yang, Z. Yao, D. Wu, T. T. Xu, Y. Tian, Y. Zhang, C. Shan and X. J. Li, Chem. Mater., 2019, 31, 3917–3928 CrossRef CAS.
- A. Shapiro, M. W. Heindl, F. Horani, M. H. Dahan, J. Tang, Y. Amouyal and E. Lifshitz, J. Phys. Chem. C, 2019, 123, 24979–24987 CrossRef CAS.
- N. Phung, R. Félix, D. Meggiolaro, A. Al-Ashouri, G. Sousa, E. Silva, C. Hartmann, J. Hidalgo, H. Köbler, E. Mosconi, B. Lai, R. Gunder, M. Li, K. L. Wang, Z. K. Wang, K. Nie, E. Handick, R. G. Wilks, J. A. Marquez, B. Rech, T. Unold, J. P. Correa-Baena, S. Albrecht, F. De Angelis, M. Bär and A. Abate, J. Am. Chem. Soc., 2020, 142, 2364–2374 CrossRef CAS PubMed.
- W. Wei, J. Gebhardt, D. F. Urban and C. Elsässer, Phys. Rev. B, 2024, 109, 144104 CrossRef CAS.
- D. Bai, J. Zhang, Z. Jin, H. Bian, K. Wang, H. Wang, L. Liang, Q. Wang and S. F. Liu, ACS Energy Lett., 2018, 3, 970–978 CrossRef CAS.
- J. Cao, S. X. Tao, P. A. Bobbert, C. P. Wong and N. Zhao, Adv. Mater., 2018, 30, 1707350 CrossRef PubMed.
- F. Zhang, X. Sun, H. Xie, X. Cai, B. Zheng, H. Yu, E. Liu, X. Hao and M. Zhang, Chem. Mater., 2022, 34, 1010–1019 CrossRef CAS.
- P. Zhao, W. Yin, M. Kim, M. Han, Y. J. Song, T. K. Ahn and H. S. Jung, J. Mater. Chem. A, 2017, 5, 7905–7911 RSC.
- C. Zhou, T. Zhang, C. Zhang, X. Liu, J. Wang, J. Lin and X. Chen, Adv. Sci., 2022, 9, 2103491 CrossRef CAS PubMed.
- Z. Fang, H. He, L. Gan, J. Li and Z. Ye, Adv. Sci., 2018, 5, 1800736 CrossRef PubMed.
- Z. Guo, S. Zhao, A. Liu, Y. Kamata, S. Teo, S. Yang, Z. Xu, S. Hayase and T. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 19994–20003 CrossRef CAS PubMed.
- H. Sun, J. Zhang, X. Gan, L. Yu, H. Yuan, M. Shang, C. Lu, D. Hou, Z. Hu, Y. Zhu and L. Han, Adv. Energy Mater., 2019, 9, 1900896 CrossRef.
- A. Boonchun, M. F. Smith, B. Cherdhirunkorn and S. Limpijumnong, J. Appl. Phys., 2007, 101, 043521 CrossRef.
- J. E. Jaffe, R. M. Van Ginhoven and W. Jiang, Comput. Mater. Sci., 2012, 53, 153–157 CrossRef CAS.
- A. Moutaouaffiq, M. Belhajji, A. Didi Seddik, A. Tahiri, E. Ali, L. Hajji, A. Rjeb, P. Paramasivam, S. S. M. Ghoneim and A. B. A. Sharaf, Results Eng., 2025, 25, 104466 CrossRef CAS.
- D. He, Z. Wang, S. Ma, L. Yang, L. Tong, X. Zhou and T. Fan, J. Eur. Ceram. Soc., 2024, 44, 3824–3832 CrossRef CAS.
- P. J. Jumana, T. Karthik and V. Kumar, J. Alloys Compd., 2025, 1014, 178642 CrossRef CAS.
- V. S. Vinita, S. S. J. Dhas, S. Suresh, S. C. Jeyakumar, S. Srinivasan and C. S. Biju, J. Magn. Magn. Mater., 2023, 565, 170251 CrossRef.
- G. Y. Lee, G.-G. Lee, Y. S. Lim and S.-H. Yoon, Ceram. Int., 2025, 51, 11627–11633 CrossRef CAS.
- N. Tanwar, S. Upadhyay, R. Priya, S. Pundir, P. Sharma and O. P. Pandey, J. Solid State Chem., 2023, 317, 123674 CrossRef CAS.
- X. Chen, X. Ruan, K. Zhao, X. He, J. Zeng, Y. Li, L. Zheng, C. H. Park and G. Li, J. Alloys Compd., 2015, 632, 103–109 CrossRef CAS.
- K. Xu, P. Yang, W. Peng and L. Li, J. Alloys Compd., 2020, 829, 154516 CrossRef CAS.
- S. Saparjya, T. Badapanda, S. Behera, B. Behera and P. R. Das, Phase Transitions, 2020, 93, 245–262 CrossRef CAS.
- J. Gao, Q. Li, H. Liu, J. Shim, Q. Yan, Y. Zhang and X. Chu, Ceram. Int., 2015, 41, 2497–2501 CrossRef CAS.
- W. Li, Z. Xu, R. Chu, P. Fu and G. Zang, J. Am. Ceram. Soc., 2011, 94, 3181–3183 CrossRef CAS.
- W. Li, Z. Xu, R. Chu, P. Fu and P. An, Ceram. Int., 2012, 38, 4353–4355 CrossRef CAS.
- W. Li, Z. Xu, R. Chu, P. Fu and G. Zang, J. Alloys Compd., 2014, 583, 305–308 CrossRef CAS.
- Q. Li, Q. Zhang, W. Cai, C. Zhou, R. Gao, G. Chen, X. Deng, Z. Wang and C. Fu, Mater. Chem. Phys., 2020, 252, 123242 CrossRef CAS.
- F. Guo, W. Cai, R. Gao, C. Fu, G. Chen, X. Deng, Z. Wang and Q. Zhang, J. Electron. Mater., 2019, 48, 3239–3247 CrossRef CAS.
- Y. Yang, J. Guo, W. Ma, H. Zhao, M. Ma, J. Wu and M. Chi, J. Mater. Sci.: Mater. Electron., 2019, 30, 2854–2863 CrossRef CAS.
- P. Parjansri, U. Intatha and S. Eitssayeam, Mater. Res. Bull., 2015, 65, 61–67 CrossRef CAS.
- D. Zhang, Y. Zhang and S. Yang, J. Mater. Sci.: Mater. Electron., 2015, 26, 909–915 CrossRef CAS.
- R. Verma, A. Chauhan, K. M. Batoo, R. Kumar, M. Hadi and E. H. Raslan, Ceram. Int., 2021, 47, 15442–15457 CrossRef CAS.
- X. Xia, X. Jiang, C. Chen, X. Jiang, N. Tu and Y. Chen, Front. Mater. Sci., 2016, 10, 203–210 CrossRef.
- P. Parjansri and S. Eitssayeam, Ferroelectrics, 2018, 534, 63–70 CrossRef CAS.
- M. Gao, W. Ge, X. Li, H. Yuan, C. Liu, H. Zhao, Y. Ma and Y. Chang, Phys. Status Solidi A, 2020, 217, 12000253 CrossRef.
- Y. Tian, S. Li, Y. Gong, Y. Yu, Y. Tang, P. Liu and Q. Jing, Mater. Chem. Phys., 2018, 204, 163–170 CrossRef CAS.
- Y. Zhang, H. Deng, S. Si, T. Wang, D. Zheng, P. Yang and J. Chu, J. Am. Ceram. Soc., 2020, 103, 2491–2498 CrossRef CAS.
- Y. Zhang, H. Sun and W. Chen, J. Electron. Mater., 2016, 45, 5006–5016 CrossRef CAS.
- Z. Zhao, X. Li, H. Ji, Y. Dai and T. Li, J. Alloys Compd., 2015, 637, 291–296 CrossRef CAS.
- S. H. Shin, J. D. Han and J. Yoo, Mater. Lett., 2015, 154, 120–123 CrossRef CAS.
- U. Intatha, P. Parjansri, K. Pengpat, G. Rujijanagul, T. Tunkasiri and S. Eitssayeam, Integr. Ferroelectr., 2012, 139, 83–91 CrossRef CAS.
- J. Ma, X. Liu and W. Li, J. Alloys Compd., 2013, 581, 642–645 CrossRef CAS.
- W. Li, J. Hao, W. Bai, Z. Xu, R. Chu and J. Zhai, J. Alloys Compd., 2012, 531, 46–49 CrossRef CAS.
- W. Liu and S. Li, IEEE Trans. Dielectr. Electr. Insul., 2015, 22, 734–738 CAS.
- F. Zeng, Q. Liu, S. Peng, Y. Wang, E. Cai, A. Xue and S. Zhou, Ceram. Int., 2019, 45, 1416–1419 CrossRef CAS.
- J. Ma, X. Liu, M. Jiang, H. Yang, G. Chen, X. Liu, L. Qin and C. Luo, J. Mater. Sci.: Mater. Electron., 2014, 25, 992–996 CrossRef CAS.
- R. Hayati, M. A. Bahrevar, T. Ebadzadeh, V. Rojas, N. Novak and J. Koruza, J. Eur. Ceram. Soc., 2016, 36, 3391–3400 CrossRef CAS.
- Y. Tian, L. Wei, X. Chao, Z. Liu and Z. Yang, J. Am. Ceram. Soc., 2013, 96, 496–502 CrossRef CAS.
- S. Patel, A. Chauhan and R. Vaish, Appl. Mater. Today, 2015, 1, 37–44 CrossRef.
- J. Shi, X. Lu, J. Shao, B. Fang, S. Zhang, Q. Du, J. Ding, X. Zhao and H. Luo, Ferroelectrics, 2017, 507, 186–197 CrossRef CAS.
- R. Hayati, M. A. Bahrevar, Y. Ganjkhanlou, V. Rojas and J. Koruza, J. Adv. Ceram., 2019, 8, 186–195 CrossRef CAS.
- C. Han, J. Wu, C. Pu, S. Qiao, B. Wu, J. Zhu and D. Xiao, Ceram. Int., 2012, 38, 6359–6363 CrossRef CAS.
- P. Yong, D. Shihua, S. Tianxiu, Z. Qian and Z. Zuowei, Ferroelectrics, 2014, 463, 83–89 CrossRef CAS.
- Q. Li, W. Ma, J. Ma, X. Meng and B. Niu, Mater. Technol., 2016, 31, 18–23 CrossRef CAS.
- Y. Tian, S. Li, S. Sun, Y. Gong, T. Li, Y. Yu and Q. Jing, J. Electron. Mater., 2018, 47, 684–691 CrossRef CAS.
- D. Xue, Y. Zhou, H. Bao, J. Gao, C. Zhou and X. Ren, Appl. Phys. Lett., 2011, 99, 122901 CrossRef.
- M. Chen, Z. Xu, R. Chu, H. Qiu, M. Li, Y. Liu, L. Shao, S. Ma, W. Ji, W. Li, S. Gong and G. Li, Phys. B: Condens. Matter, 2014, 433, 43–47 CrossRef CAS.
- W. Li, Z. Xu, R. Chu, P. Fu and G. Zang, J. Eur. Ceram. Soc., 2012, 32, 517–520 CrossRef CAS.
- D. Lin, K. W. Kwok and H. L. W. Chan, Ceram. Int., 2014, 40, 6841–6846 CrossRef CAS.
- L. Zhao, B.-P. Zhang, P.-F. Zhou, L.-F. Zhu and N. Wang, Ceram. Int., 2016, 42, 1086–1093 CrossRef CAS.
- Z.-H. Chen, Z.-W. Li, J.-N. Ding, T.-X. Zhao, J.-H. Qiu, K.-Q. Zhu, J.-J. Xu and B. Zhang, J. Electron. Mater., 2018, 47, 3409–3413 CrossRef CAS.
- J. Gao, D. Xue, W. Liu, C. Zhou and X. Ren, Actuators, 2017, 6, 1–20 CrossRef.
- W. Shin, S. Kim, E. Kim, D. Kim, S. U. Oh, J. A. Lee, Y. W. Heo, J. H. Lee and S. Nahm, J. Korean Ceram. Soc., 2024, 61, 316–326 CrossRef CAS.
- X. Zhang, Y. Zhang, Q. Yin, H. Zhang, F. Lin, K. Hu, F. Si, Z. Du, C. Chen, Y. Peng and F. Shi, J. Electron. Mater., 2025, 54, 3260–3268 CrossRef CAS.
- T. Wang, M. Jiang, L. Li, S. Cheng, H. Lu, P. Ren, Y. Zhao and G. Rao, J. Alloys Compd., 2023, 935, 168126 CrossRef CAS.
- H. Zubairi, F. Hussain, S. Sheikh, A. A. Shaikh, D. Wang and I. M. Reaney, Mater. Sci. Eng., B, 2023, 296, 116632 CrossRef CAS.
- C. Liu, Z. Dai, Y. Zheng, X. Li, M. N. Rafiq, S. Tsukada, Y. Cong, T. Karaki and S. Zhou, J. Power Sources, 2025, 640, 236759 CrossRef CAS.
- Y. Xu, M. Jiang, Y. Zeng, Y. Ouyang, S. Cao, J. Song and G. Rao, Mater. Today Commun., 2025, 42, 111472 CrossRef CAS.
- X. Gao, H. Sun, H. Hao, C. Wang, Q. Shen and L. Zhang, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2023, 38, 261–266 CrossRef CAS.
- C. Kaushiga, S. Sakhuja, R. Devasaia, J. Kaarthik, G. Sradha, S. G. Reddy and V. Annapureddy, Appl. Phys. A: Mater. Sci. Process., 2025, 131, 1–13 CrossRef.
- W. Wu, D. Xiao, J. Wu, W. Liang, J. Li and J. Zhu, J. Alloys Compd., 2011, 509, L 284–L 288 CrossRef CAS.
- X. Jiang, Y. Chen, J. Wang, N. Tu and C. Chen, Ferroelectrics, 2010, 401, 72–78 CrossRef CAS.
- W. Liang, Z. Wang, D. Xiao, J. Wu, W. Wu, T. Huang and J. Zhu, Integr. Ferroelectr., 2012, 139, 63–74 CrossRef CAS.
- A. Kan, R. Onishi, S. Ohashi and H. Ogawa, J. Mater. Chem. C, 2022, 10, 6444–6455 RSC.
- S. Sahoo, D. K. Pradhan, S. Kumari, A. Sahu, K. S. Samantaray, C. Singh, A. Mishra, M. M. Rahaman, A. Kumar, R. Thomas, P. D. Rack and D. K. Pradhan, J. Appl. Phys., 2024, 135, 244101 CrossRef CAS.
- M. Dubernet, M. J. Pitcher, M. Zaghrioui, M. Bah, J. Bustillo, F. Giovannelli and I. Monot-Laffez, J. Eur. Ceram. Soc., 2022, 42, 2188–2194 CrossRef CAS.
- J. Suchanicz, M. Wąs, M. Nowakowska-Malczyk, P. Jelen, K. Konieczny, P. Czaja, K. Kluczewska-Chmielarz, D. Sitko, T. V. Kruzina and M. Sokolowski, Phase Transitions, 2020, 93, 877–882 CrossRef CAS.
- F. M. Volpi, T. J. Castro, A. Franco, S. O. Lira, P. C. Morais and S. W. da Silva, J. Alloys Compd., 2024, 1005, 175963 CrossRef CAS.
- M. Aggarwal, R. Goel, G. Sharma, A. K. Singh, S. Dhiman and S. Kumar, Mater. Chem. Phys., 2023, 310, 128503 CrossRef CAS.
- N. Rhimi, N. Dhahri, J. Laifi, F. Bourguiba, N. Abdelmoula, J. Dhahri, C. Castro and J. Juraszek, J. Mol. Struct., 2023, 1294, 136319 CrossRef CAS.
- S. Lenka, T. Badapanda, P. Nayak, S. Sarangi, S. Anwar and S. N. Tripathy, J. Mol. Struct., 2023, 1274, 134413 CrossRef CAS.
- L. Dong, D. S. Stone and R. S. Lakes, J. Appl. Phys., 2011, 109, 063531 CrossRef.
- J. A. Astudillo, S. A. Dionizio, J. L. Izquierdo, O. Morán, J. Heiras and G. Bolaños, AIP Adv., 2018, 8, 055817 CrossRef.
- P. Naik, S. K. Patri and J. Nanda, J. Alloys Compd., 2025, 1010, 177601 CrossRef CAS.
- Y. Wang, L. Wang, Y. Zhao and D. Yan, Ceram. Int., 2016, 42, 17911–17915 CrossRef CAS.
- S. Raja, R. Ramesh Babu, K. Ramamurthi and K. Sethuraman, Mater. Chem. Phys., 2018, 213, 130–139 CrossRef CAS.
- X. Wang, C. Wang, X. Yao, Y. Zhou, N. Liang, Q. Jin, K. Chen, M. He, E. Guo, C. Ge, G. Yang and K. Jin, ACS Appl. Electron. Mater., 2023, 5, 1234–1242 CrossRef CAS.
- L. Liao, Q. Yang, C. Cai, Y. Zhou, H. Sun, X. Huang, P. Chen, J. Wang, X. Li, X. Tian, S. Meng, X. Bai and L. Wang, Acta Mater., 2024, 273, 119986 CrossRef CAS.
- S. S. Brahma, J. Nanda, B. Munisha, S. Parida and S. R. Mohapatra, J. Magn. Magn. Mater., 2023, 587, 171268 CrossRef CAS.
- G. S. Pawley, J. Appl. Crystallogr., 1981, 14, 357–361 CrossRef CAS.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS.
- N. V. Y. Scarlett and I. C. Madsen, Powder Diffr., 2006, 21, 278–284 CrossRef CAS.
- E. Vega, M. Mollar and B. Marí, Energy Technol., 2020, 8, 1900743 CrossRef CAS.
- L. Atourki, E. Vega, M. Mollar, B. Marí, H. Kirou, K. Bouabid and A. Ihlal, J. Alloys Compd., 2017, 702, 404–409 CrossRef CAS.
- T. Baikie, Y. Fang, J. M. Kadro, M. Schreyer, F. Wei, S. G. Mhaisalkar, M. Graetzel and T. J. White, J. Mater. Chem. A, 2013, 1, 5628–5641 RSC.
- S. Waitschat, M. T. Wharmby and N. Stock, Dalton Trans., 2015, 44, 11235–11240 RSC.
- B. K. Heep, K. S. Weldert, Y. Krysiak, T. W. Day, W. G. Zeier, U. Kolb, G. J. Snyder and W. Tremel, Chem. Mater., 2017, 29, 4833–4839 CrossRef CAS.
- J. Euvrard, X. Wang, T. Li, Y. Yan and D. B. Mitzi, J. Mater. Chem. A, 2020, 8, 4049–4054 RSC.
- S. Pang, Y. Zhou, Z. Wang, M. Yang, A. R. Krause, Z. Zhou, K. Zhu, N. P. Padture and G. Cui, J. Am. Chem. Soc., 2016, 138, 750–753 CrossRef CAS PubMed.
- S. A. Dalrymple and G. K. H. Shimizu, J. Am. Chem. Soc., 2007, 129, 12114–12116 CrossRef CAS PubMed.
- Y. X. Ma, Z. J. Li, L. Wei, S. Y. Ding, Y. B. Zhang and W. Wang, J. Am. Chem. Soc., 2017, 139, 4995–4998 CrossRef CAS PubMed.
- S. A. Hayward and E. K. H. Salje, J. Phys.: Condens. Matter, 2002, 14, L599–L604 CrossRef CAS.
- J. Íñiguez and D. Vanderbilt, Phys. Rev. Lett., 2002, 89, 115503 CrossRef PubMed.
- T. Ishidate, S. Abe, H. Takahashi and N. Môri, Phys. Rev. Lett., 1997, 78, 2397–2400 CrossRef CAS.
- V. Bijalwan, P. Tofel and V. Holcman, J. Asian Ceram. Soc., 2018, 6, 384–393 CrossRef.
- S. M. Mane, P. M. Tirmali, S. L. Kadam, D. J. Salunkhe, C. B. Kolekar and S. B. Kulkarni, Adv. Appl. Ceram., 2017, 116, 325–332 CrossRef CAS.
- M. B. Abdessalem, A. Aydi and N. Abdelmoula, J. Alloys Compd., 2019, 774, 685–693 CrossRef CAS.
- V. S. Puli, D. K. Pradhan, W. Pérez and R. S. Katiyar, J. Phys. Chem. Solids, 2013, 74, 466–475 CrossRef.
- V. Buscaglia, S. Tripathi, V. Petkov, M. Dapiaggi, M. Deluca, A. Gajović and Y. Ren, J. Phys.: Condens. Matter, 2014, 26, 065901 CrossRef CAS PubMed.
- V. K. Veerapandiyan, S. Khosravi H, G. Canu, A. Feteira, V. Buscaglia, K. Reichmann and M. Deluca, J. Eur. Ceram. Soc., 2020, 40, 4684–4688 CrossRef CAS.
- S. Adireddy, C. Lin, B. Cao, W. Zhou and G. Caruntu, Chem. Mater., 2010, 22, 1946–1948 CrossRef CAS.
- J. Pokorńy, U. M. Pasha, L. Ben, O. P. Thakur, D. C. Sinclair and I. M. Reaney, J. Appl. Phys., 2011, 109, 114110 CrossRef.
- S. Fuentes, F. Céspedes, P. Muñoz, E. Chávez and L. Padilla-Campos, J. Chil. Chem. Soc., 2013, 58, 2077–2081 CrossRef CAS.
- A. Slodczyk and P. Colomban, Materials, 2010, 3, 5007–5028 CrossRef CAS PubMed.
- D. Schütz, M. Deluca, W. Krauss, A. Feteira, T. Jackson and K. Reichmann, Adv. Funct. Mater., 2012, 22, 2285–2294 CrossRef.
- H. Harima, J. Phys.: Condens. Matter, 2002, 14, R967–R993 CrossRef CAS.
- L. Mañosa, A. Planes and M. Acet, J. Mater. Chem. A, 2013, 1, 4925–4936 RSC.
- J. Peräntie, J. Hagberg, A. Uusimäki and H. Jantunen, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 134119 CrossRef.
- E. H. Birks, Phys. Status Solidi A, 1986, 94, 523–527 CrossRef CAS.
- B. Rožič, B. Malič, H. Uršič, J. Holc, M. Kosec, Z. Kutnjak, B. Malič, H. Uršič, J. Holc, M. Kosec and Z. Kutnjak, Ferroelectrics, 2011, 421, 103–107 CrossRef.
- D. Q. Xiao, Y. C. Wang, R. L. Zhang, S. Q. Peng, J. G. Zhu and B. Yang, Mater. Chem. Phys., 1998, 57, 182–185 CrossRef CAS.
- J. Peräntie, H. N. Tailor, J. Hagberg, H. Jantunen and Z.-G. Ye, J. Appl. Phys., 2013, 114, 174105 CrossRef.
- A. S. Mischenko, Q. Zhang, R. W. Whatmore, J. F. Scott and N. D. Mathur, Appl. Phys. Lett., 2006, 89, 242912 CrossRef.
- M. Valant, Prog. Mater. Sci., 2012, 57, 980–1009 CrossRef CAS.
- S. G. Lu and Q. Zhang, Adv. Mater., 2009, 21, 1983–1987 CrossRef CAS.
- Y. Jiang, C. Y. Toe, S. S. Mofarah, C. Cazorla, S. L. Y. Chang, Y. Yin, Q. Zhang, S. Lim, Y. Yao, R. Tian, Y. Wang, T. Zaman, H. Arandiyan, G. G. Andersson, J. Scott, P. Koshy, D. Wang and C. C. Sorrell, ACS Sustainable Chem. Eng., 2023, 11, 3370–3389 CrossRef CAS.
- G. Schmidt, H. Arndt, G. Borchhardt, J. von Cieminski, T. Petzsche, K. Borman, A. Sternberg, A. Zirnite and V. A. Isupov, Phys. Status Solidi A, 1981, 63, 501–510 CrossRef CAS.
- B. A. Tuttle, P. Yang, J. H. Gieske, J. A. Voigt, T. W. Scofield, D. H. Zeuch and W. R. Olson, J. Am. Ceram. Soc., 2001, 84, 1260–1264 CrossRef CAS.
- F. Chen, Y. H. Li, G. Y. Gao, F. Z. Yao, K. Wang, J. F. Li, X. L. Li, X. Y. Gao and W. Wu, J. Am. Ceram. Soc., 2015, 98, 1372–1376 CrossRef CAS.
- L. M. Riemer, K. V. Lalitha, X. Jiang, N. Liu, C. Dietz, R. W. Stark, P. B. Groszewicz, G. Buntkowsky, J. Chen, S. T. Zhang, J. Rödel and J. Koruza, Acta Mater., 2017, 136, 271–280 CrossRef CAS.
- X. Zheng, S. S. Mofarah, C. Cazorla, R. Daiyan, A. A. Esmailpour, J. Scott, Y. Yao, S. Lim, V. Wong, E. Y. Chen, H. Arandiyan, P. Koshy and C. C. Sorrell, Adv. Funct. Mater., 2021, 31, 2103171 CrossRef CAS.
- J. Wang, E. Gürdal, A. Horneber, S. Dickreuter, S. Kostcheev, A. J. Meixner, M. Fleischer, P. M. Adam and D. Zhang, Nanoscale, 2018, 10, 8240–8245 RSC.
- Y. Jiang, C. Y. Toe, S. S. Mofarah, C. Cazorla, S. L. Y. Chang, Y. Yin, Q. Zhang, S. Lim, Y. Yao, R. Tian, Y. Wang, T. Zaman, H. Arandiyan, G. G. Andersson, J. Scott, P. Koshy, D. Wang and C. C. Sorrell, ACS Sustainable Chem. Eng., 2023, 11, 3370–3389 CrossRef CAS.
- W. M. Haynes, CRC Handbook of Chemistry and Physics, Taylor & Francis Inc, Boca Raton, 2014, p. 2704 Search PubMed.
- J. He, B. Baldassarri and C. Wolverton, Phys. Rev. B, 2023, 108, 104103 CrossRef CAS.
- Y. Kumagai, N. Tsunoda, A. Takahashi and F. Oba, Phys. Rev. Mater., 2021, 5, 123803 CrossRef CAS.
- M. E. Straumanis and S. Zyszczynski, J. Appl. Crystallogr., 1970, 3, 1–6 CrossRef CAS.
- M. E. Straumanis, T. Ejima and W. J. James, Acta Crystallogr., 1961, 14, 493–497 CrossRef CAS.
- J. E. Garcia, V. Gomis, R. Perez, A. Albareda and J. A. Eiras, Appl. Phys. Lett., 2007, 91, 042902 CrossRef.
- W. L. Warren, G. E. Pike, K. Vanheusden, D. Dimos, B. A. Tuttle and J. Robertson, J. Appl. Phys., 1996, 79, 9250–9257 CrossRef CAS.
- S. Takahash, Ferroelectrics, 1982, 41, 143–156 CrossRef.
- R. Yimnirun, R. Wongmaneerung, S. Wongsaenmai, A. Ngamjarurojana, S. Ananta and Y. Laosiritaworn, Appl. Phys. Lett., 2007, 90, 112906 CrossRef.
- R. Yimnirun, S. Wongsaenmai, R. Wongmaneerung, N. Wongdamnern, A. Ngamjarurojana, S. Ananta and Y. Laosiritaworn, Phys. Scr., 2007, 184, 184–189 CrossRef.
- B. Wen, Y. Zhang, X. Liu, L. Ma and X. Wang, J. Mater. Sci., 2012, 47, 4299–4304 CrossRef CAS.
- X.-G. Tang and H. L. Chan, J. Appl. Phys., 2020, 97, 034109 CrossRef.
- S. R. Reddy, V. Venkata, B. Prasad, S. Bysakh, V. Shanker, N. Hebalkar and S. K. Roy, J. Mater. Chem. C, 2019, 7, 7073–7082 RSC.
- M. A. Ansari and K. Sreenivas, Ceram. Int., 2019, 45, 20738–20749 CrossRef CAS.
- Y. Lin, D. Li, M. Zhang, S. Zhan, Y. Yang, H. Yang and Q. Yuan, ACS Appl. Mater. Interfaces, 2019, 11, 36824–36830 CrossRef CAS PubMed.
- V. S. Puli, D. K. Pradhan, D. B. Chrisey, M. Tomozawa, G. L. Sharma, J. F. Scott and R. S. Katiyar, J. Mater. Sci., 2013, 48, 2151–2157 CrossRef CAS.
- X. Chen, X. Chao and Z. Yang, Mater. Res. Bull., 2019, 111, 259–266 CrossRef CAS.
- Z. Hanani, S. Merselmiz, M. Amjoud, D. Mezzane, M. Lahcini, J. Ghanbaja, M. Spreitzer, D. Vengust, M. E. Marssi, I. A. Luk’yanchuk, Z. Kutnjak, B. Rožič and M. Gouné, J. Mater., 2022, 8, 873–881 Search PubMed.
- A. B. Swain, V. Subramanian and P. Murugavel, Ceram. Int., 2018, 44, 6861–6865 CrossRef CAS.
- R. L. Nayak, Y. Zhang, S. S. Dash and M. P. K. Sahoo, Ceram. Int., 2022, 48, 10803–10816 CrossRef CAS.
- A. Lakouader, H. Mezzourh, D. Mezzane, M. Amjoud, L. Hajji, E. H. Choukri, I. A. Luk’yanchuk, Z. Kutnjak and M. E. Marssi, J. Mater. Sci.: Mater. Electron., 2022, 33, 14381–14396 CrossRef CAS.
- P. Vineetha, R. Jose, A. Vijay, P. S. Charan and K. V. Saravanan, Mater. Res. Express, 2022, 9, 066303 CrossRef.
- S. T. Dang, L. L. Xue, L. F. He, Y. C. Shi, H. N. Li, Y. C. Hu, J. Shang, S. Q. Yin and X. W. Wang, J. Mater. Sci.: Mater. Electron., 2022, 33, 26100–26112 CrossRef CAS.
- A. R. Jayakrishnan, K. V. Alex, A. Thomas, J. P. B. Silva, K. Kamakshi, N. Dabra, K. C. Sekhar, J. Agostinho Moreira and M. J. M. Gomes, Ceram. Int., 2019, 45, 5808–5818 CrossRef CAS.
- F. C. Su, X. B. Guo, X. L. Lu, Z. Su, W. H. Qiu, X. G. Tang, S. F. Li and W. H. Li, Phys. B: Condens. Matter, 2023, 651, 414597 CrossRef CAS.
- T. Badapanda, S. Chaterjee, A. Mishra, R. Ranjan and S. Anwar, Phys. B: Condens. Matter, 2017, 521, 264–269 CrossRef CAS.
- W. Cai, Q. Zhang, C. Zhou, R. Gao, S. Zhang, Z. Li, R. Xu, G. Chen, X. Deng, Z. Wang and C. Fu, J. Mater. Sci.: Mater. Electron., 2020, 31, 9167–9175 CrossRef CAS.
- V. S. Puli, D. K. Pradhan, I. Coondoo, N. Panwar, S. Adireddy, S. Luo, R. S. Katiyar and D. B. Chrisey, J. Phys. D: Appl. Phys., 2019, 52, 255304 CrossRef CAS.
- D. Zhan, Q. Xu, D. P. Huang, H. X. Liu, W. Chen and F. Zhang, J. Alloys Compd., 2016, 682, 594–600 CrossRef CAS.
- A. Dahri, Y. Gagou, N. Abdelmoula, H. Khemakhem and M. E. Marssi, Ceram. Int., 2022, 48, 3157–3171 CrossRef CAS.
- X. Wang, S. Chen, Y. Liang, B. Zhang, X. Shi, R. Zhang, Y. Shi, Z. Ren, R. Liu, Y. Hu, J. Shang and S. Yin, Eur. Phys. J.: Appl. Phys., 2022, 97, 1–12 CrossRef.
- E. V. Ramana, N. M. Ferreira, A. Mahajan, D. M. Tobaldi, I. Bdikin, B. Rožič, Z. Kutnjak and M. A. Valente, J. Eur. Ceram. Soc., 2021, 41, 6424–6440 CrossRef CAS.
- Q. Zhang, B. Chen, D. Wu, Z. Peng, X. Qiao, X. Chao and Z. Yang, Ceram. Int., 2020, 46, 28285–28291 CrossRef CAS.
- X. W. Wang, B. H. Zhang, Y. Y. Li, Y. C. Shi, L. Y. Sun, G. Feng, C. L. Li, Y. F. Liang, Y. P. Zheng, S. Y. Shang, J. Shang, Y. C. Hu and S. Q. Yin, J. Mater. Sci.: Mater. Electron., 2020, 31, 4732–4742 CrossRef CAS.
- S. Merselmiz, Z. Hanani, D. Mezzane, M. Spreitzer, A. Bradeško, D. Fabijan, D. Vengust, M. B. Amjoud, L. Hajji, Z. Abkhar, A. G. Razumnaya, B. Rožič, I. A. Luk’yanchuk and Z. Kutnjak, Ceram. Int., 2020, 46, 23867–23876 CrossRef CAS.
- H. Mezzourh, S. Belkhadir, D. Mezzane, M. Amjoud, E. Choukri, A. Lahmar, Y. Gagou, Z. Kutnjak and M. E. Marssi, Phys. B: Condens. Matter, 2021, 603, 412760 CrossRef CAS.
- S. Merselmiz, Z. Hanani, D. Mezzane, A. G. Razumnaya, M. Amjoud, L. Hajji, S. Terenchuk, B. Rožič, I. A. Luk’yanchuk and Z. Kutnjak, RSC Adv., 2021, 11, 9459–9468 RSC.
- Z. Hanani, D. Mezzane, M. Amjoud, A. G. Razumnaya, S. Fourcade, Y. Gagou, K. Hoummada, M. E. Marssi and M. Gouné, J. Mater. Sci.: Mater. Electron., 2019, 30, 6430–6438 CrossRef CAS.
- Z. Hanani, S. Merselmiz, D. Mezzane, M. Amjoud, A. Bradeško, B. Rožič, M. Lahcini, M. E. Marssi, A. V. Ragulya, I. A. Luk’Yanchuk, Z. Kutnjak and M. Gouné, RSC Adv., 2020, 10, 30746–30755 RSC.
| This journal is © The Royal Society of Chemistry 2026 |