 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The dark matter in water, air and land: from microplastic to invisible nanoplastics
Martina H. Stenzel
School of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: M.Stenzel@unsw.edu.au
First published on 4th December 2025
Abstract
Microplastic is well known and has been the subject of many review articles. In recent years, an increasing number of reports have documented the presence of nanoplastics—plastic particles smaller than 1 µm—in various environments, from the ocean to the human brain. In this article, I focus on nanoparticles and what we do and do not understand about their effects on our health. After an introduction to nanoplastics and their size relative to a single polymer chain, the degradation process that produces nanoplastics, similar to microplastics, is briefly summarized. Due to their high surface area, nanoplastics can behave differently in solution because they tend to aggregate. After reviewing the presence of microplastics and nanoplastics in humans, insights from the established field of nanomedicine are used to explore how nanoplastics may enter the bloodstream and reach the brain. This also includes the topic of protein corona formation, which influences the fate of nanoplastics in the body. Finally, a brief summary on the impact of plastic particles on health is provided, focusing on reports comparing nano- and microplastics. This article concludes with how materials scientists and chemists can contribute to addressing the rising plastic pollution problem.
New conceptsEveryone has heard of microplastics, but the term nanoplastic is only now entering the public consciousness. Recent articles have identified trigger points, showing that we have large amounts of nanoplastics in our oceans—an amount that far exceeds microplastics. Furthermore, plastic has been found in the brain, and closer inspection revealed it to be nanoplastic. We do not yet know how dangerous plastic truly is to our health, but initial reports, often using engineered micro- or nanoplastic beads, suggest it could be harmful. This feature article explores the pathways from plastic waste to nanoplastics, concluding with an examination of our current understanding of the differences between micro- and nanoplastics and their potential health implications. Although research on nanoplastic and its health effects is still limited, the mature field of nanomedicine can help us understand how nanoplastics in the environment may absorb small compounds like pollutants, form a biocorona with, for example, proteins, and transfer from the stomach, after ingestion with food and water, to the brain. |
Introduction
There is an abundance of microplastics in the ocean and in soil, mainly based on the polymers shown in Fig. 1. Pictures of coloured plastic pieces that are around the size of a grain of sand circulate widely on the internet. A recent UNESCO report paints a very dire picture of the state of our oceans. Around 8 to 10 million plastic ends up in the ocean and, alarmingly, it is estimated that by 2050, more plastic will be found in the ocean than fish.1 Numerous local studies worldwide focused on quantifying the issue. The reality is that it is not as simple as collecting and counting the plastic bottles floating on water. Exposure to the elements results in the degradation of plastic into small pieces. Some of them are visible with the naked eye, others require a light microscope. In 2004, Thompson and co-workers were the first to report on the occurrence of microplastics.2 Since then, the problem is omnipresent with man-made plastics being discovered in land, air, animals, drinking water and more.3 The term microplastic refers to a vast and diverse group of particles formed from the breakdown of plastics, classified by their size, which ranges from 1 µm to 5 mm. The variety of chemical structures is huge. The most commonly produced plastics are summarised in Fig. 1, but there are many more. Just behind the term of Nylon are hidden several structural varieties that have all different properties. Even if we pick up two microplastic pieces of nylon from two different origins, they might vary in their molecular weight and in other aspects. The reality is that the plastic we use is a mixture of different ingredients. Polymers, which are the underpinning macromolecules, are processed in plastics by blending them with other polymers and with additives. These additives, which are small molecules, can serve different purposes, including enhancing the longevity of the plastic, providing colour, enhancing flexibility or rendering them with other properties.4 These additives are not covalently attached to the polymer and can leach out during degradation, causing additional environmental damage.4 | ||
| Fig. 1 Main commercial plastics. Source: Wikipedia, https://upload.wikimedia.org/wikipedia/commons/5/5f/Plastics_Summary.svg, CC0 1.0 DEED, CC0 1.0 Universal, Author: Orion Lawlor. | ||
While there is a lot of focus on microplastic pollution, with many active community groups collecting samples and assessing the problems worldwide, recent reports have shown that nanoplastics are just as abundant. A 2023 study, published in Nature in 2025, highlighted that the main plastic pollution is nanoplastics, not microplastics, with around 1.5–32.0 mg m−3 of PET, PS and PVC being found in various ocean layers. The authors estimated that the amount of nanoplastic found in the temperate to subtropical North Atlantic is around 27 million tonnes, which is exceeding or similar to the amount of large-scale plastic found in all oceans.5 The authors concluded that “nanoplastics comprise the dominant fraction of marine plastic pollution”.5
Nanoplastics are defined by a size between 1–1000 nm, but I would advise against searching for nanoplastics of 1 nm. A monomer, a standard building block, typically measures about 500 pm to 1 nm. In commercial applications, many polymers consist of 500 to 1000 repeating units, but some can be significantly larger, such as Ultra-high-molecular-weight polyethylene (UHMWPE), which can have up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 repeating units. The contour length of a PS with 1000 units is around 24 nm, but polymers do not exist as extended as extended chains but as coils. An expression of “size” can be the random-flight end-to-end distance that increases in size with
000 repeating units. The contour length of a PS with 1000 units is around 24 nm, but polymers do not exist as extended as extended chains but as coils. An expression of “size” can be the random-flight end-to-end distance that increases in size with  and equates for a polymer with 1000 repeating units to 15.4 nm.6 This is only a mathematical model; in reality, environmental factors play a role. However, it should serve as an indication of how large a single polymer chain can be. Once we enter the realm of nanoplastics, other physical properties come into play. A plastic particle with a diameter of 1 mm has the same mass as 1012 nanoparticles, each with a diameter of 100 nm, assuming they are made from the same material. At the same time, we created a 10
and equates for a polymer with 1000 repeating units to 15.4 nm.6 This is only a mathematical model; in reality, environmental factors play a role. However, it should serve as an indication of how large a single polymer chain can be. Once we enter the realm of nanoplastics, other physical properties come into play. A plastic particle with a diameter of 1 mm has the same mass as 1012 nanoparticles, each with a diameter of 100 nm, assuming they are made from the same material. At the same time, we created a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times increase in surface area, which means that now surface properties play an important role.
000 times increase in surface area, which means that now surface properties play an important role.
If we were to engineer 100 nm nanoparticles in the lab using any hydrophobic polymers such as PS, PP, PE, or others, we would observe that these nanoparticles quickly aggregate to form large visible particles in water as a means to minimise surface energy.7 However, alterations to the surface properties through chemical reactions or adsorption of compounds found in the environment, facilitated by various interactions such as H-bonding, van der Waals forces or others, can create nanoparticles with colloidal stability.8 Now, we have nanoparticles dissolved in aqueous solution that are invisible to the naked eye as long as the size remains below a critical scattering threshold. These nanoplastic particles sit alongside other nanoparticles found in nature.9 These naturally occurring nanoparticles can be living, such as viruses, but also include non-biological ones like nano-sized soot particles, calcium ion particles in drinking water, or inorganic nanoparticles produced by bacteria.9,10 Naturally occurring nanoparticles as well as man-made nanoplastic particles would behave very differently in a biological system compared to microplastics, as they can invade living organisms to an extent that microplastics cannot.9
In this focus article, the challenges related to nanoplastics will be examined. While the formation of microplastics is widely addressed, awareness of nanoplastics is only now beginning to grow. Clarivate Web of Science lists 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 766 articles mentioning microplastics, whereas only 1409 documents focus on nanoplastics while writing this article. Until 2020, there were just a few reports on nanoplastics, but the field has since expanded rapidly. An alarming report from 2025 showed the presence of plastic in the brain. The particles found were not microplastics, but the authors found particles below 200 nm in size.11 The question is how nanoplastics are formed and how they can pass through the relatively impermeable blood–brain barrier (BBB). Researchers apply knowledge from nanomedicine, a field that started in the 1990s, to better understand the effects of nanoplastics on human health. However, several challenges still need to be addressed. While the impact of nanoplastics on our health is particularly interesting for medical researchers, materials scientists also play a role in helping detect nanoplastics or in developing polymer materials that are less likely to contribute to future pollution.
766 articles mentioning microplastics, whereas only 1409 documents focus on nanoplastics while writing this article. Until 2020, there were just a few reports on nanoplastics, but the field has since expanded rapidly. An alarming report from 2025 showed the presence of plastic in the brain. The particles found were not microplastics, but the authors found particles below 200 nm in size.11 The question is how nanoplastics are formed and how they can pass through the relatively impermeable blood–brain barrier (BBB). Researchers apply knowledge from nanomedicine, a field that started in the 1990s, to better understand the effects of nanoplastics on human health. However, several challenges still need to be addressed. While the impact of nanoplastics on our health is particularly interesting for medical researchers, materials scientists also play a role in helping detect nanoplastics or in developing polymer materials that are less likely to contribute to future pollution.
How do nanoplastics end up in the environment?
Humans do not litter microplastics or nanoplastics; they litter large plastic items. In some countries, more than 2 kg of plastic is emitted into the ocean per person.12 This amounts to around 100 plastic bottles, assuming a weight of about 20 g per bottle. We are all familiar with pictures of rivers that have floating layers of plastic waste. At this point, the plastic waste can be scooped up and processed safely. However, once nature exerts its full force, plastics start breaking down. We have all seen the degradation process looking at the plastic products around the house: the ones kept inside the house can last many years, but a plastic garden chair starts to yellow quickly, crumbles, and then breaks,13 often without much prior warning that the end of structural integrity has been reached. Degradation of plastics can be accelerated by light (photodegradation and photooxidation), heat (thermal degradation), chemicals (oxidation, hydrolysis), microorganisms (biodegradation), mechanical force (physical degradation) and even electrical fields (Fig. 2). Several review articles describe plastic degradation in detail; only the main aspects are highlighted here.13–17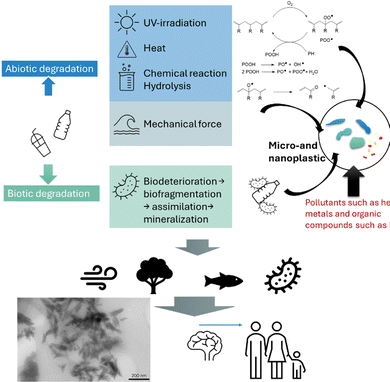 | ||
| Fig. 2 Pathways of plastic degradation. The inserted TEM shows the nanoplastic pieces found in the brain by Nihart et al.,11 reprinted from Springer Nature Medicine https://creativecommons.org/licenses/by-nc-nd/4.0/. | ||
Degradation by light and oxidation
Plastics in the environment are subject to light exposure that can trigger the degradation or ageing of polymers,18 either in the presence or absence of oxygen.19,20 UV irradiation at approximately 300 nm can directly break C–C and C–H bonds. However, the presence of chromophores, like carbonyl groups in the polymer, can accelerate this process. Certain compounds, such as metal compounds, pollutants, or oxygen, can have catalytic effects and serve as photosensitizers.21 The process catalyzed by oxygen is called photooxidation and can be divided into two mechanisms: the formation of singlet oxygen, which can lead to the formation of peroxides along the polymer chain, or the formation of a radical on the polymer that can react with oxygen, serving as an initiator for subsequent propagation across different parts of the polymer chains.19 The oxygen-centred radical that is formed in the process allows polymer chain scission (Fig. 2). The rate of degradation is dependent on the intensity of light, the type of polymer, exposure time, and even the inclination angle.22–24 UV-light can result in the formation of microplastics, but also nanoplastics, albeit this process is more pronounced when in combination with some mechanical agitation. UV light alone can enable the degradation into nanoparticles,25 but simulated beach conditions using a standardized UV aging experiment revealed that after the initial formation of microplastics, nanoplastics less than 50 nm in size start to emerge.26Degradation by heat
Polymers are generally stable at moderate temperatures. They can transition from glassy to rubbery states if the glass transition temperature is reached at higher temperatures, but they do not visibly degrade until temperatures approach 200 °C or higher.27 At this point, polymers degrade in a radical process encompassing initiation, propagation, and termination similar to that of UV degradation.28 However, decomposition can already occur at lower temperatures, such as 30 °C, resulting in the slow release of oligomeric compounds.29 It is therefore not surprising that microplastics are present when plastic is heated, such as with plastic teabags in hot water.30 The Nylon and PET components of the tea bag started breaking down after only 5 min in hot water, releasing micron and nano-sized particles that affected the swimming behaviour of Daphnia magna, a planktonic crustacean.30 Similar results were observed when hot solutions were filled into PP infant feeding bottles,31 or when silicone-rubber baby teats are disinfected with steam,32 both processes led to the formation of nanoplastics. Plastic containers together with their content are often placed in microwave ovens, which can locally generate very high heat. PP containers were found to release 4.22 million microplastic and 2.11 billion nanoplastic particles from only one square centimetre of plastic area within 3 min of microwave heating.33Degradation by mechanical abrasion
Light and heat can compromise the chemical structure through chain scission. However, it is often the movement of plastic, which includes abrasion from objects, sheer stress by tearing, or tensile stress by stretching, that causes the breakage of plastic into smaller pieces.34 Under mechanical stress, polymers might initially respond with realignment of the polymer chains, but with increasing force, they can break, especially along entanglements or knots.35 Most plastic is exposed to a variety of such external forces during usage, depending on the application. An example for mechanical force is washing clothes. Each household produces microplastics simply by washing clothes.36 A study showed that 124 to 308 mg microfibres per kg of washed polyester were produced, resulting in a set of recommendations on how to reduce microplastic pollution, such as the use of cold water or the installation of filters.37,38 There is little discussion about the formation of nanoplastics, but when it comes to clothing, it is important to note that many clothing items are treated with engineered nanoparticles that might detach during mechanical stress. This can be mistaken for the formation of nanofibers generated during stress on the fabric. Other sources of microplastic in the household, that are subject to mechanical stress, include plastic chopping boards,39 and a variety of food packaging, as reviewed earlier.40 Microplastics have recently been found in unexpected places, like used chewing gum, which is polyvinyl acetate that breaks down through repeated mechanical stress.41 A significant contributor to the microplastic pollution are tires, including bike tires, with a study finding that a mountain bike loses 3.62 g of plastic on a 100 km ride.42 Even indoor climbing venues are not safe, as the constant mechanical stress on climbing shoes leads to the emission of large amounts of microplastic nanoparticles.43While most studies have focused on microplastics, it is possible that nanoplastics might also be present. A study that accelerated the mechanical stress effect by blending polystyrene coffee cup lids immersed in water found that plastic can break down into pieces ranging from 100 to 200 nm within just 5 minutes. Microscopy analysis revealed the presence of a range of different shapes, including spindle-like structures.44 This was also observed during simulations of water shear forces, where nanoplastics of approximately 50 nm in size were rapidly produced from microplastics.45
Chemical reaction
Plastics can degrade by non-radical reactions.18 This includes the reaction with ozone on double bonds, such as SEBS plastics, which is widely used in the automotive industry, for example.46 Hydrolysable polymers with esters or amides in the backbone can theoretically be cleaved in an aqueous environment, but higher temperatures or pH values that are not neutral are required to accelerate the reaction.46 At neutral conditions in a marine environment, it takes around 4.5 years for 50% of PET bonds to cleave when the ocean temperature is 35 °C, but 162 years if it is only 30 °C.47 Earlier studies only focused on the formation of microplastics in the process of degradation,48 but there is now an increased awareness that nanoplastic will also be generated.49Biodegradation
While polymers exhibit stability in pure aqueous environments, their degradation within biological systems is often accelerated by the synergistic effects of mechanical forces, ultraviolet (UV) radiation-induced photodegradation, enzymatic activity, and microbial colonization. The weathering of polymeric materials initially results in fragmentation into smaller particles, thereby increasing the surface area available for subsequent chemical degradation reactions catalyzed by various environmental factors.50 Many polyester polymers, such as PLA or PET, can be readily hydrolysed with the help of lipases, esterases, and others, but also polymers such as PVC and PP show signs of degradation when exposed to microorganisms such as fungi.51 There is growing interest in developing genetically modified bacteria to help break down commodity polymers produced by radical polymerization, which are regarded as more difficult to degrade.52 Biodegradation can be unpredictable, and labels in plastic items such as biodegradable or compostable do not mean that the plastic bag is completely depleted, as this will depend on subtle environmental differences. Placing in soil, hot compost, sea water, or simply in open air will lead to widely different degradation rates, to the point of showing little biodegradation.17,53,54Biodegradation can also occur when plastics are ingested. Microplastics are internalised via drinking water, seafood, salt, honey, and many other food items.55 But not all plastic can be broken down in the stomach. Large pieces of plastic often pass through the gastrointestinal (GI) system, which includes the stomach, colon, and intestines, unchanged. This is evidenced by the many LEGO pieces swallowed by children that only reappear after a few days, as shown by a study in which six paediatricians each swallowed a piece of LEGO.56 Of course, large amounts of plastic can block the stomach or physically damage it and therefore result in the death of many smaller animals.57 For smaller plastic pieces, the residence time in the stomach is often not long enough, and despite the high acidity in the stomach and the presence of bacteria and enzymes, medium-sized plastic pieces do not provide enough surface area to trigger substantial changes. However, there is evidence that smaller plastic pieces with higher surface area might indeed degrade in the intestines, subject to the nature of the polymer used. Understandably, esters such as PLA or PET might show quick signs of aging, but even polymers such as PS that are considered hard to degrade start breaking down, as they were observed to undergo oxidation on the surface when exposed to conditions found in the stomach.58 While the low pH value in the stomach can facilitate hydrolysis, gut bacteria can assist in the degradation. Plastic-degrading bacteria were recently found in human faeces that could oxidize the surface of LDPE and PP and partly depolymerize the polymer chain.59
Degradation to nano-sized particles and their analysis
Effect of the plastic particle size on degradation
The occurrence of degradation reactions will also depend on the size of the plastic piece. Mechanical forces play more an effect in larger plastic pieces, where shear forces significantly contribute to the process and accelerate the breakdown into microplastic and even nanoplastic.45,60 With declining size, the surface area is becoming more dominant compared to the bulk of the material. It is therefore expected that reactions that target the functionality of the surface and the underlying layer would speed up the degradation process. Hydrolytic reactions, for example, are accelerated when the plastic particles become smaller, as these reactions rely on direct access to functional groups on the surface.61 This, however, does not mean that plastic pieces quickly dissolve into monomeric building blocks once they are nano-sized. At this stage, the morphology of the plastic particle will influence the process. Since most polymers are semi-crystalline and chain scission mainly occurs in the amorphous regions, crystalline areas tend to resist further degradation.49 In this study, the authors emphasized that the fact that commercial polymers are often semi-crystalline is an important aspect of understanding the degradation of micro- and nanoplastics.Effect of the type of environment
Plastic degrades through a combination of mechanisms, resulting in a wide variation in degradation rates depending on the location. Factors like soil or water, hot sunny locations or dry cold climates, mechanical stress or undisturbed conditions, all create unique chemical reactions that can lead to plastic breakage to microplastics and then nanoplastics.Degradation in the environment is complex and often based on a feedback system. For example, the presence of microplastics was found to affect earthworms, who responded by increasing the number of burrows and thus may increase earth porosity.73 These changes in soil structure will subsequently influence plastic degradation. The literature is filled with complex relationships between plastic particles and living organisms, emphasizing the challenge of fully understanding the effects of plastics.
Identifying nanoplastics
One of the issues, however, is the difficulty in detecting nanoplastics. Current analytical techniques for analyzing plastic pollution are more tailored towards identifying the type of plastic than the particle size. Spectroscopic techniques such as Raman, Fourier Transform Infrared (FTIR) spectroscopy, or even nuclear magnetic resonance (NMR), paired with thermo-analytical techniques such as gas chromatography–mass spectrometry (GC–MS) (often coupled with other techniques) or differential scanning calorimetry (DSC), help to identify the type of plastic and they are used to analyse micro- and nanoplastics. However, the size and shape identification of nanoplastics is more challenging.Examples include a membrane based on nanowires that can capture nanoplastic, which are then detected with surface-enhanced Raman spectroscopy (SERS).83 The cavities in MOFs can be used to extract nanoplastics from solutions as shown with a chromium-based MOF.84 Researchers have developed an optical sieve that can detect nanoplastic and their size using Mie void resonances. According to the authors, this technique could be combined with μ-FTIR or µm-Raman spectroscopy to elucidate the structure of the nanoplastic particles.85
The reader is referred to comprehensive review articles that discuss and compare available techniques to detect micro-and nanoplastics, including the advancements in microscopy, spectroscopy, and AI-supported techniques.75,76,86
Despite the difficulty of identifying nanoplastic, they have now been widely observed in nature. In 2017, researchers found nanoplastics in the North Atlantic,87 but since then, nanoplastics have been discovered everywhere, including in 2021 in soil,88 in 2022 in polar ice,89 in 2021 in the snow,90 and in tissue in humans, as it will be discussed below. The recently published study outlined the widespread occurrence of nanoplastic in the ocean.5
Nanoparticles may not be nanoparticles in their biological environment
However, just because nano-sized plastic pieces were detected under the microscope, it does not mean they exist as isolated pieces in the environment.91 As discussed in the beginning, a reduction in size will lead to a larger surface area. As a result, the type of functional groups on the surface will influence what happens to these nanoparticles. Hydrophobic groups or hydrophilic and potentially charged groups will determine if these nanoparticles are colloidally stable or if they aggregate. In solution, this is described by the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory. This theory combines attractive van-der Waals forces and electrostatic repulsion, which is caused by the double layer of ions, also discussed in terms of the zetapotential of the nanoparticle. The total potential energy describes the balance between both forces and thus determines stability. While the colloidal stability of engineered nanoparticles is relatively well understood, the solution behaviour of micro- and nanoplastics is complex. The surface of these plastic particles will undergo a range of chemical changes during the weathering process.92 Photooxidation usually leads to an increase in oxygen-containing functional groups, such as hydroxyl or carboxyl groups, that will help with solubility in aqueous solutions. This often coincides with changes in surface structure, such as increased roughness. This alone will already influence whether the plastic particles aggregate. However, the particles also strongly interact with other compounds present in their environment. In nature, this can include the formation of biofilm and the absorption of environmental contaminants such as metal ions and organic compounds that change surface properties.92 Depending on the chemistry of these coated plastic pieces, they can either be stable in the aqueous system or start aggregating randomly.93 The rate of aggregation will be determined not only by the composition of the plastic surface but also by the nature of the surroundings.93 The size here plays an important role as nanoparticles aggregate faster than micron-sized particles due to their high surface energy.94 Once the plastic pieces are ingested, they might be subject to further degradation as the low pH value of the stomach or the different enzymes might alter the surface properties again. Particles that enter the bloodstream will most likely be covered by a protein corona, which will determine the ultimate fate of the plastic pieces (Fig. 3). The types of proteins on the surface and their denaturation state will influence biodistribution, as well as whether the plastic pieces remain stable in the bloodstream.95 | ||
| Fig. 3 Transport of micro- and nanoplastics from the stomach or intestine to the blood ort lymphatic circulation (parts of this picture were created with https://BioRender (https://BioRender)). | ||
How much plastic is in our body?
Humans are exposed to plastics in numerous ways.96 As mentioned above, a significant amount of plastic is ingested through contaminated food or water, but plastic can also enter the body when previously uncontaminated food or water comes into contact with plastic containers that release plastic particles upon heating or other degenerative forces. Depending on the type and size of the plastic pieces, plastic is excreted through the stool. In a study with healthy volunteers, around 20 microplastic pieces between 50–500 µm in size per 10 g of stool were detected.97 This amount of particles found in faeces can be directly linked to the exposure to plastic food packaging.98 This will mean that plastic will go back into the ecosystem.99 Other options include the inhalation of plastic particles from the air.100 Moreover, the skin is frequently in contact with plastic, whether it is clothing or other plastic items, such as phones or personal care products. Healthy skin usually serves as a barrier,101 although it was proposed that nanoplastics might display some toxicological effects on the skin.102The main question on everyone's mind is whether micro- and nanoplastics are dangerous to humans. First of all, we need to explore where plastic can be found in the body. This is not an easy undertaking, as many organs can only be analysed after the person is deceased. It is therefore not surprising that we know the most about micro- and nanoplastic by analysing the placenta (Table 1). In the first study, published in 2021, microplastics were detected in 4 out of the 12 placenta samples, with particles measuring approximately 10 µm in size, primarily composed of PP.103 Another study found microplastic pieces larger than 50 µm in the placenta,104 but also in the baby's first stool, the meconium, which is clear evidence that microplastic is transferred from the mother to the baby. Microscopy techniques, such as FT-IR or Raman, were typically the method of choice for counting micron-sized microplastic particles. Occasionally, fluorescence microscopy was also used after visualising the microplastic with dyes such as Nile Red (Table 1). While seeing these large plastic pieces in organs can be confronting, a real game changer was the introduction of pyrolysis-gas chromatography and mass spectrometry (Py-GC–MS), which is capable of quantifying plastic in tissue, independent of the particle size.105 However, a very recent study raised concerns about its suitability in complex media like blood, indicating that the concentration values, particularly for PVS and PE, are not reliable.106 In a 2024 study by Garcia et al., between 6.5 and 685 µg of plastic was found in one gram of placental tissue (mean 126.8 ± 147.5 µg g−1).107 The amount varied widely, with a specific measurement of 685 µg found in the placenta of one mother, equating to more than 0.06 wt%. Let's compare microscopy samples with these results: microplastic pieces vary widely in size and shape, but let's assume one piece with a prolate shape with a length of 10 µm and a thickness of 2 µm, which equates to a volume of V = 20.9 µm3. A piece like that would weigh approximately 2 × 10−5 µg, which is only a fraction of the 126.8 µg of polymer per g tissue found in the study by Gacia et al. (Table 1). This calculation is only a rough example, but it highlights that microscopy might reveal less than 1% of the plastics present in the tissue. The invisible part, nanoplastics, makes up more than 99%, and can only be visualized by techniques that are suitable for nanoscopic analysis. Table 1 lists how microscopy studies uncovered a few microplastic pieces in a gram of tissue in various organs, while Py-GC–MS exposed large plastic quantities that can only be assigned to nanoplastic pieces. We are only at the beginning of understanding how much plastic in the form of nanoplastic has accumulated in the body, but the initial studies provide an insight that is concerning.
| System | Organ | Analytical technique | Concentration: wt plastic per wt biological sample | Amount of pieces | Size of plastic particles | Dominant type of plastic | Ref. |
|---|---|---|---|---|---|---|---|
| Nervous system | Brain (2024) | 4917 µg g−1 | Below 200 nm, spherical and anisotropic | PE | Nihart et al.11 | ||
| Digestive system and urinary system | Liver (2024) | 433 µg g−1 | Rod-shape 1–5 µm (potentially aggregates) and smaller shard-like below 400 nm | PE, PP | Nihart et al.11 | ||
| Liver (2022) | Fluorescence microscopy | 0 particles per g (kidney) | 3.3 to 20.1 µm | PS, PVC, PET | Horvatits et al.108 | ||
| µRaman spectroscopy | 1.1 particles per g (spleen) | ||||||
| 0.7 particles per g (healthy liver) | |||||||
| 8.4 particles per g (liver with cirrosis) | |||||||
| Kidney (2024) | 404 µg g−1 | Rod-shape 1–5 µm (potentially aggregates) and smaller shard-like below 400 nm | PE, PP | Nihart et al.11 | |||
| Circulatory system | Blood | Py-GC/MS | 1.6 µg ml−1 | Size not reported | PET, PS, PP | Lesli et al.109 | |
| Carotid plaque (2024) | Py-GC/MS | 21.7 ± 24.5 µg g−1 (PE) | Jagged-edged nanoparticles below 1 µm | PE, PVC | Marfella et al.110 | ||
| TEM | 5.2 ± 2.4 µg g−1 (PVC) | ||||||
| SEM | |||||||
| IRMS | |||||||
| Corona | Py-GC/MS | 118.66 ± 53.87 µg g−1 | Size not reported | PET | Liu et al.111 | ||
| Coronary artery | |||||||
| Carotid artery | |||||||
| Heart, different parts | LDIR | 60–75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 504 particles per g 504 particles per g |
20 to 469 µm | PET, PE | Yang et al.112 | ||
| SEM | Fibre-like | ||||||
| Reproductive system | Placenta (2021) | Visible light microscopy | 12 pieces in the placentas of four women of samples sizes of 23.3 ± 5.7 g | 5–10 µm | PP | Ragusa et al.103 | |
| Raman microscopy | All the analyzed MPs were pigmented. | ||||||
| Peripheral and central placenta, meconium | FT-IR microscopy | A particle found in 1 × 1 × 1 cm Placenta sample and in some Meconium samples | >50 µm | PS, PP | Braun et al.104 | ||
| Placenta | LDIR | 2.70 ± 2.65 particles per g | 20.34 to 307.29 µm | PVC, PP, PBS, PET | Zhu et al.113 | ||
| Placenta | Fluorescence microscopy | 126.8 ± 147.5 µg g−1 | 153.07 ± 84.45 per 0.4 g | Size not reported | PE dominant | Garcia et al.107 | |
| Py-GC–MS | |||||||
| Preterm placenta | Py-GC–MS | 224.7 ± 180.7 µg g−1 | Size not reported | PE dominant | Jochum et al.114 | ||
| Placenta | 175.5 ± 137.9 µg g−1 | ||||||
| Testes | 328.44 µg g−1 | Size not reported | PE dominant | Hu et al.115 | |||
| Testes and Semen | LDIR | 15.34 ± 23.31 µg g−1 (semen) | 0.23 ± 0.45 particles per mL (Semen) | 96.19 ± 74.17 µm (fibres, films, flakes and others) | PVC and PE (semen) | Zhao et al.116 | |
| 11.60 ± 15.52 particles per g (testis) | 83.15 ± 56.25 µm | PS (testis) | |||||
| Respiratory system | Lung | Raman spectroscopy | 0.56 particles per g | 5.5 µm particles, and fibres of 8.12 to 16.8 µm | PP and PE | Amato-Lourenco et al.117 | |
| Light microscopy | |||||||
| Lung | µFTIR | upper (0.80 ± 0.96 MP g−1), middle/lingular (0.41 ± 0.37 MP g−1), and the lower (3.12 ± 1.30 MP g−1) | 223.10 ± 436.16 µm (length) and 22.21 ± 20.32 µm fibre, fragment, films | PP and PET | Jenner et al.118 | ||
| Lung | µFTIR | 24 microplastics (>20 µm) in 100 human lung tissues | 1000–5000 µm length fibres | Polyester and natural fibres | Chen et al.119 | ||
| SEM |
How does plastic get into organs?
The fate of these plastic particles in the lungs (after inhalation) or the stomach (after ingestion) depends on the properties of the particles. Size will play a major role, as will shape and surface chemistry. Plastic that will find its way into the stomach will be exposed to very acidic environments and a gastrointestinal epithelium that does not allow the transport of larger molecules from the stomach to the blood. In general, the gastrointestinal (GI) tract, encompassing the oesophagus, stomach, and intestine, is relatively impermeable to many compounds.120 The transport across these barriers has been the focus of attention in the field of nanomedicine. Researchers have developed particles of different sizes, shapes, and properties and investigated how they can cross these impermeable barriers. Nanoparticles can cross through the transcellular route through epithelial cells, paracellular pathways between cells along the tight junctions, using the M-cells of the Peyer's patches or via receptor and transcytosis-mediated endocytosis (Fig. 3). While many of these pathways are irrelevant for larger micron-sized particles, the M cells in Peyer's patches can internalise microplastics of several micrometers. Similar to all cell internalization pathways, the uptake by M cells depends on the types of proteins that can be found on the surface.121 It appears, though, that there is an upper size limit as plastic particles that are larger than 150 µm are predominantly excreted.122 Translocation into the bloodstream increases as the size of the plastic particle decreases because additional transport pathways open up (Fig. 3). Particles in the low micron size can now use phagocytosis or endocytosis to move into the bloodstream, albeit the paracellular route can be ruled out when the particles are still in the microrange.123 Uptake is further facilitated when these plastic pieces are below 1 µm. A study on engineered nanoparticles of various sizes showed that nanoparticles below 100 nm have significantly higher permeation across intestinal cells than spheres of 1000 nm.124 The authors also prepared disk- and rod-shaped nanoparticles and observed significant uptake. Understanding the unique behavior of anisotropic nanoparticles is important because TEM analysis of plastics in organs revealed that most pieces are non-spherical (Table 1 and TEM insert in Fig. 2).The field of nanomedicine also provides us with plenty of studies on the effect of size and surface properties on biodistribution.125 Particles with sizes between 50 and around 100 nm usually have a long circulation time in the bloodstream as they are only slowly detected by the mononuclear phagocytic system (MPS).125 A landmark paper also showed the significantly extended circulation time when non-spherical, worm-like nanoparticles were used.126 The differences in circulation time between spherical nanoparticles and other shapes are crucial when dealing with nanoplastics. TEM analysis revealed a myriad of shapes, which are the result of various erosion processes, but the shapes are rarely spherical. Moreover, the surface chemistry will play a role. In nanomedicine, blood clearance is often evaded by coating nanoparticles with polymers such as poly(ethylene glycol) PEG and zwitterionic structures that can introduce stealth properties to the nanoparticles.127 The surface chemistry of nanoplastic is given by the degradation mechanism. While commodity plastics are hydrophobic, introducing functional groups will not only enhance the colloidal stability, but it will also influence the interaction with biological entities such as proteins, as discussed earlier. A protein corona that contains predominantly opsonin proteins will have a shorter circulation time than one with dysopsonins.128 After circulating in the bloodstream for a few hours, or in some cases several days, the nanoparticles begin accumulating in various organs, primarily in the liver and spleen, unless they have been excreted by the kidneys. It is therefore not surprising that large amounts of micro- and nanoplastics have been found in these two organs (Table 1). The final destination of nanoparticles is, however, hard to predict, although attempts have been made to link the properties of nanoparticles with in vivo outcomes.129 Even very small changes to the nanoparticle properties can result in different protein coronas and biodistributions, which is explored in nanomedicine to target a range of organs.129
While the reader probably quietly accepts that plastic was found in the liver, an organ whose role is to collect and excrete unwanted products, discovering that plastic was found in the brain is concerning.11 Nanoparticles are widely developed as a way to treat brain diseases such as brain cancer, neurodegenerative diseases, or Alzheimer's disease. However, the primary challenge that still exists is effectively delivering nanoparticles across the BBB.130 That engineered plastic nanoparticles, which are spherical in shape and made from commodity polymers, can penetrate this layer was indeed shown in animal studies.131 Key to brain entry was not only the size of the particle,132 as only nanoplastics, but not microplastics, could cross the BBB, but also the composition of the protein corona.132 Indeed, from the nanomedicine field, we know that the smaller nanoplastic pieces are, the more dangerous they will be in crossing the BBB.133
Does more plastic exposure lead to higher accumulation in the organs?
The studies listed in Table 1 only had a handful of participants in each study, and it is difficult at this point to draw clear conclusions. In general, there seems to be some correlation between what we eat and drink and the amount of plastic found in our bodies.98,134 However, the authors listed in Table 1 all exercise caution about potential relationships, as the number of samples is often limited. There seems to be, for example, a moderate correlation between the amount of bottled water a volunteer drank and the microplastic pieces in faeces.135 However, in this study, the authors only counted microplastics, while the amount of nanoplastics may be huge but invisible. In another study, this time involving infants, a PET content of 36 µg per gram of stool was measured using solid-phase extraction (SPE). In contrast, only 2.6 µg was found in adults. The excessive use of plastic products like bottles during infant feeding could explain the difference.136,137 In a study that tried to compare the consumption of bottled water with the occurrence of microplastics in semen, no such correlation was found.116 A direct correlation between plastic consumption and enhanced accumulation can, therefore, not confidently be drawn at this stage, as there are probably too many factors that play a role.One would also expect plastic accumulation to increase with age, but the absence of a direct correlation highlights the complexity of accumulation and clearance. For example, the concentration of microplastics in human testes was found to be independent of age and more likely related to the process of sperm production.115 Similar observations were made when linking the amount of plastic in the brain with age, where there was a lack of correlation.11 Yet, when comparing samples collected eight years ago with recent samples, there was a noticeable increase in the amount of plastic deposited in the organs, which is in line with the increase in plastic pollution in the environment.11 It appears that plastic accumulation in the organ plateaus as incoming plastic is also slowly cleared again, as observed in a zebrafish study.138 In contrast, when analysing the presence of microfibres in lung tissue, the authors found a clear increase with age.119
The concentration in various organs seems to be a function of the unique physiology of each living being. An enhanced concentration of microplastics was found in patients with liver cirrhosis. It was proposed that the “leaky gut” in these patients allows additional migration of microplastics into the liver.108 A huge amount of nanoplastic was measured in the brains of dementia patients. It was highlighted that it is likely the inability of these brains to purge nanoplastic, although it might be tempting to link nanoplastics with dementia.11 However, the authors state clearly that this is not an indication that nanoplastic will cause dementia, but they also warn that more needs to be done to understand the effect on human health. At this point we do not have enough knowledge to separate cause and effect. Does the enhanced accumulation trigger a disease, or does the disease lead to enhanced accumulation? An example of the former might be the higher amount of microplastics found in lung cancer,119 where it appears that plastic fibres in ground-glass nodules (GGNs) in the lung might have contributed to the progression of the disease.
Is nanoplastic dangerous?
The complex relationship between the plastic accumulation of microplastics in various organs and potential health effects has been subject to a range of reviews,96,123,139–143 therefore, only a brief overview is given here.Ultimately, the question will be whether micro- or nanoplastic will make me sick? Unfortunately, we do not have sufficient human data yet to answer this, but there are enough in vitro and in vivo data using animal studies to show that we should be concerned. There is a large body of work on the toxicity of micro-and nanoplastics that proposes that plastic in our body might cause adverse health effects. However, setting up suitable studies is complex. In many cases, the researchers used engineered microplastics, which are often spherical particles made from commodity polymers, with a well-defined surface chemistry. Apart from micro- and nanoplastics being irregular in shape, we also know that plastic composition from various sources can vary substantially, as each plastic item produced has a different mixture of additives. However, these engineered nanoparticles allow us to gain some insight into potential toxic effects.
The initial step in toxicity testing is to study micro- and nanoplastics on cell lines similar to what researchers in the nanomedicine sector would do. From there, we know that nanoplastics can be readily taken up by a large variety of different cells while microplastics struggle to gain entry.96 Macrophages, which play an important role in the immune system, are known to phagocytose even micron-sized particles.80 The more particles enter the cells, the more likely they are to cause cell stress or even cell death; therefore, size-dependent effects are observed in some reports.144,145 Plastic particles often do not directly display cytotoxicity, only at relatively high concentrations.146,147 Again, this is often a function of size as nanoplastics in the low nanometer range were found to be toxic already at low concentrations, while larger particles require higher concentrations to be cytotoxic towards various phagocytes.145 Cytotoxicity is often not directly observed but manifests as a range of more subtle interferences with cells since nanoplastics can interfere with the mitochondria,148 inhibit membrane transporters,148 trigger the production of reactive oxygen species (ROS),144,146 or cytokines144 or lead to an increase in a range of other cell markers that might indicate cell stress.145,149 The effects of micro- and nanoplastics on cytotoxicity, immune response, oxidative stress, barrier properties, and genotoxicity were compared in a meta-analysis, and the authors found that the shape of the plastic particle is a much stronger predictor of toxicity than its size or polymer type.150 Nanoplastic pieces with rough edges and irregular shapes showed noticeably more toxicity than spherical ones, which can be concerning, considering that most naturally occurring nanoplastic pieces are non-spherical.151 It also highlights that engineered spherical nanoparticles may not be the best models to test plastic toxicity.
Organoids and spheroids have recently emerged as an alternative tool to study the effects of plastic particles.152–156 Next to toxicology information, organoids and spheroids can provide information on the ability of plastic pieces to penetrate into tissue.157 Cortical or cerebral, kidney, cardiac, lung or liver organoids could reveal significant damage after exposure to predominantly nanoplastic particles as small as 100 nm.156 Organoids were able to uncover various toxic effects, but according to the author of a recent review paper, organoids need to become more complex and include features such as vasculature and immune cells. Additionally, the authors recommended that the experiments should better simulate long-term exposure to nanoplastics.156
What is, however, on people's minds is how nanoplastic might affect their health or their ability to have healthy children. As highlighted earlier, we don’t have enough data on human studies, but in vivo work using animals can shed some light on this.
Digestive system and urinary system
The first line of contact of micro and nano plastics is with the digestive system and urinary system. Particles that are ingested will potentially damage the stomach or intestine, and after entering the bloodstream, will be transported to the kidneys, liver, and bladder. It is evident that damages already occur in the stomach and intestine, with the various effects being summarized in review articles.158,159 Feeding mice with 5 µm PS particles over 28 days led to oxidative damage, inflammation, and disturbances of the intestinal microbiota.160 This led further to an increase in undesirable gut bacteria and increased levels of interleukin-1α in blood serum, a hallmark of inflammation as observed after feeding mice with micron-sized PE.161 Similar results were observed after feeding micron-sized PE to zebrafish, including intestinal inflammation and increased infection probability.162 While a pro-inflammatory response has been observed with nanoplastics in vitro,163 there is only a limited number of studies that look at the effects of nanoplastics in animal models. A direct comparison of micro- and nanoplastic on zebrafish showed that while all particles affected the microbiome and the intestinal immune cells, only nanoplastic led to genetic changes that led to increased ROS production and mucus secretion.164The observed disturbance of the microbiome will affect the gut-liver axis and can potentially result in liver diseases such as liver fibrosis and cirrhosis, as reviewed here.165 Accumulation of microplastics in the liver is widely observed, which is not surprising considering that the liver and spleen filter the blood to remove harmful substances. Once lodged there, microplastics can cause oxidative stress, alter metabolism, increase cell death, and inflammation.165 Most studies used microplastics, but there are a few studies using fish that show that nanoplastics are just as dangerous.166 Zebrafish that were exposed to 50 nm PS particles for 28 days showed not only signs of liver damage, but the authors found evidence for the depolymerization of PS in the liver by the cytochrome P450 enzymes, which may lead to the formation of cytotoxic styrene and styrene oxide.167 A review that directly compared studies on microplastics and nanoplastics and their effect on the liver found that microplastics are more likely to accumulate in the liver and cause hepatotoxicity.158 According to these initial reports, it appears that microplastics could be more damaging than nanoplastics to the liver.158
Nervous system
Microplastics can cause significant neurotoxicity in mammals and fish,168,169 and an increasing number of studies now include nanoplastics. Micro- and nanoplastices were directly compared when mice were given drinking water containing PS particles with diameters of 500 nm, 4, and 10 µm, respectively, over 180 days. The smallest nanoparticles were able to cross the BBB to a significant extent, but nevertheless, the authors found no size effect on subsequently studied cognitive dysfunction, only a concentration effect.170 This does not mean that nanoplastic is not potentially more dangerous, as shown in studies on fish using 70 nm and 5 µm PS particles. While both particles led to significant toxic effects, nanoplastic reduced the activity of acetylcholinesterase (AChE), an enzyme that controls nerve transmission.171 Several studies showed that nanoplastics, when fed to mice and rats, result in significant behaviour changes, cellular dysfunction, as reviewed in detail here.172 For example, PS particles measuring 30–50 nm in size, and administered orally to mice, were capable of reaching the brain, where they induced cognitive impairment, most likely by modulating gene expression in microglia.173Blood, circulatory and lymphatic system
Once micro-and nanoplastics cross into the bloodstream, they will immediately be exposed to the innate immune system, most likely to neutrophils.174 Plastic particles have been widely found in the blood of animals and humans.175 Several studies on fish exposed the changes in neutrophils and the subsequent effect on the fish population, which includes reduced disease resistance,174 but also chromosomal harm to fish erythrocytes.166 In mice, an increase in eosinophils and basophilic leucocytes176 and altered lipid metabolism, as evidenced by elevated serum markers, were found.177 While the effects of plastic particles on blood cells and cardiovascular parameters are evident as reviewed here,175 there are few studies comparing micro- and nanoplastics in vivo. One study that investigated the effect of plastic particles of 500 nm and 5 µm in size reported an increase in body weight after plastic ingestion. The mice fed with the 500 nm nanoplastic particles had significantly higher glucose levels than the control group.176Once in the blood, the particle can be shuttled to the lymph nodes and various organs178 with nano-sized plastic pieces reaching the lymph nodes more rapidly than microplastics.179 Plastic was detected in humans in the aorta, coronary arteries, and carotid arteries, and the concentration was correlated to the disease history of the individual humans.110,111 The authors suggested a potential connection between the presence of large amounts of nanoplastics in carotid plaques and the occurrence of stroke, myocardial infarction, and other deaths in patients.110 Moreover, it is also believed that plastic particles might be linked to atherosclerosis.103 However, the authors measured the content using Py-GC/MS and did not distinguish between micro- and nanoplastic, which might have shed light on the effect of size differences. Finally, the plastic pieces might reach the spleen, where they can cause adverse effects.159
Reproductive system
The effects of micro- and nanoplastics on the reproductive system are widely studied and the subject of many review articles,180–183 and the main effects are summarise in Fig. 4. In this field, there is a substantially greater volume of research focusing on human subjects relative to studies examining health effects on other organs. This disparity can be primarily attributed to the relative ease of obtaining biological samples such as placentas or semen, whereas obtaining other organs is generally limited to specimens from diseased individuals or those who have undergone partial organ resection. Consequently, studies involving the uterus, cervix, or testis tend to be scarce and often characterized by limited sample sizes. For example, uterine fibroids, which are benign tumors of the uterus, are usually removed together with the neighboring myometrial tissue during surgery.134 It was observed that there was more microplastic in tissue that contained fibroids than in healthy tissue. What was particularly interesting about this study was the link between these findings and the lifestyle choices of the tissue donors. It was discovered that a higher consumption of takeaway meals and bottled water led to increased plastic accumulation, highlighting that most plastic found in the body is ingested directly through contaminated food.134 Microplastics are also found in testis (on average 328.44 µg per gram tissue)115 and uterus (amount not determined).184 Analysis of tissue samples from cervical cancer patients showed that when microplastics were found, metabolic changes in cervical invasive cancer tissues were measured, but the researchers did not draw any conclusions about what this means for our health.185 However, some concerning correlations have been reported in recent years. For example, reduced foetal growth is associated with an abundance of plastic in the placenta of women,186 while plastic in the women's villous tissues is thought to lead to an increased occurrence of miscarriages.187 | ||
| Fig. 4 Visual representation highlighting the impacts of micro- and nanoplastics (MNP) on the male and female reproductive system. Inspired and adapted from Ye et al.190 | ||
As the number of studies on humans is limited, researchers have used animal models to learn more about this topic. The extent to which microplastics may influence fertility rates or adversely affect offspring remains to be fully explored.180 In mice, administration of plastic particles led to reduced fertility, an abnormal sex ratio in their offspring,184 increased embryo resorption rate,188 and embryonic growth retardation.189 Several animal studies suggest that disruptions in the neuroendocrine system via the hypothalamic-pituitary-gonadal (HPG) axis may affect the synthesis and secretion of sex hormones, in addition to endometrial dysfunction, atrophy, fibrosis, and other adverse effects, as reviewed here.180 Comparison of micro-and nanoplastics in zebrafish led to the conclusion that nanoplastics might be able to reach areas such as the yolk sac, which microplastics cannot reach,180 but in general, the number of studies comparing the effects of different sizes of plastic particles on reproductive health is limited.190 A study that uses PS beads in the size of 100 nm, 3 µm, and 10 µm found a larger accumulation of nanoplastic in the testis compared to the microplastic particles, but not in the epididymis.191 Nanoplastics are also more likely to cross the placental barrier to reach the foetal brain in mice.192 Depending on the surface charge of the nanoparticles, damages to the foetal brain by ROS were observed.192 Next to damages to the brain of the unborn mouse during gestation, nanoplastic can also be more easily delivered from the female to the offspring through breast milk.193 Both particles, micron- and nano-sized, reduced fetal growth, but this study on mice did not find significant differences between 50 nm and 5 µm particles.194 A study that followed the health of mice from the gestational stage to puberty after their mothers were fed plastic particles of 500 nm and 5 µm, respectively.195 The offspring showed metabolic changes although they were never exposed to plastic directly, which could either mean that the maternal metabolism was affected or that plastic particles passed through the placenta. Interestingly, this effect was more pronounced with microplastics.
Respiratory system
It is not unexpected that plastic particles should have a profound impact on the respiratory system, considering that inhalation is a common pathway of entry. As reviewed elsewhere,100,196 the deposition of plastic particles on the lungs can potentially cause respiratory diseases, as shown in animal models. However, Vasse and Melgert also concluded that more work is needed to understand the effect of actual environmental plastic pollution on human health.196 However, it is reasonable to expect that nanoplastics might cause more damage than microplastics, as they are more likely to reach the respiratory bronchioles, alveolar ducts, and alveoli, whereas larger particles tend to remain in the upper respiratory system.197In summary, there is evidence indicating that plastic particles may have detrimental health effects. However, it remains unclear at this point whether nanoplastics pose a greater or lesser risk compared to larger plastic particles, as studies that compare sizes directly are limited. Moreover, many studies employ engineered spherical PS nanoparticles while actual micro-and nanoplastic particles are often fibrous, rough with many edges, or film-like in appearance. Furthermore, certain studies employ exceedingly high concentrations of plastic particles in animal experiments (several milligrams of plastic per kilogram per day), which may not be representative of typical human consumption. While it was proposed that we eat the equivalent of one credit card per week (5 grams), Pletz revisited these numbers and proposed that 4.1 µg per week is a more likely number.198 Even with a 5 g intake by a 70 kg adult, the daily dose is 10 mg per kg, which is lower than the concentrations used in some studies.
Is nanoplastic more dangerous than microplastic?
If we reduce the size of a plastic particle from 5 µm to 50 nm, we increase the surface area relative to the volume by a factor of 100. Therefore, the types of functional groups on the surface will determine the fate of the plastic pieces. It is the degradation pathways, the degradation environment such as soil, air or water, the season, the type of polymer, and even subtle differences in the constitution or stereochemistry of a polymer, such as molecular weight, branching and tacticity, that will influence the rate of degradation, but also the type of functional groups that will dominate the surface.199–201 This interface will control if the nanoplastic pieces are stable in water or if and how they interact with other compounds.202 Both processes are interrelated. Surface functionalities such as charged groups will ensure water-solubility, colloidal stability, but they will also decide with what organic molecule or biomaterial they interact. This corona of absorbed molecules will then decide the stability in water, which is either long-term colloidal stability or aggregation into larger micron-sized aggregates. Nano-sized particles are more likely to circulate around the blood stream for an extended period of time, they are more likely to enter cells in the body or even cross the BBB. In contrast, the surface of microplastics is relatively small. There is less room to absorb any molecules, such as pollutants, and they are likely to be cleared via the liver. Although more work needs to done, nanoplastic is likely more dangerous.Responsible plastic use
It is evident that there are many unknowns about the effects of plastic particles on our health, but we need to err on the side of caution and reconsider how we use plastics. Plastic is a unique material that is irreplaceable in our daily lives for certain applications, but in other areas, we need to consider how to reduce plastic consumption, reuse items, or recycle them. An informed population is a prerequisite for this step. For example, it is not widely known that washing of clothes made from synthetic fibres such as Nylon is a huge contributor to microplastic pollution.203 A recent citizen science project in the UK showed that households grossly underestimate how many plastic items they dispose of (plastic blindness),204 but awareness of it can help with behaviour change. The “One Planet Network”, a global community of practitioners, policymakers and experts, governments, businesses, civil societies, academia and international organisations, has assembled strategies that help with behavior change.205 While there is a focus on personal responsibility, the responsibility also lies with big corporations and lawmakers.Plastic pollution can be reduced by better remediation techniques, such as the removal of plastic particles from storm water, better waste water sludge management, the development of a circular economy, better waste management and many other approaches203,206 More importantly, policies need to be in place that aid the process. Tackling plastic pollution is a major aim of the UN environmental program,207 and law makers around the world already act upon. Examples are the “National Plastic Plan” in Australia,208 the “Plastic strategy” by the European Commission,209 but also international agreements are being developed that seek to tackle the plastic problem (UNEA resolution (5/14)).210 Policies include the phasing out of single-use plastic, improving waste management, reducing plastic leakage, and appropriately financing the circular economy, with some responsibility given to the private sector.211
Until then, it is recommended to limit personal exposure to plastics. This includes the protection from airborne plastic particles, which is particularly crucial for workers in recycling plants. This aspect is often overlooked: recycling plants, where plastic is shredded, can be a source of micro- and nanoplastics formation.203 On a personal level, households can reduce the amount of plastic particles in the air by using air purifiers equipped with HEPA filters, which can help alleviate exposure,212 consider the use of plastic items in the kitchen and household,72 and other plastic items, such as water in cheap plastic bottles.40
The role of chemists and materials scientists
Understanding the health effects of micro- and nanoplastics will need to be the subject of many studies for biomedical researchers in the coming years, as we still do not understand the magnitude of the problem. This problem can only be tackled by combining the effects of interdisciplinary teams comprising analytical chemists, environmental chemists, food, soil, water scientists, toxicologists, and medical researchers. Materials scientists can play a central role in the discussion around plastic pollution as they can contribute to our understanding of the behaviour of plastic particles, but they can also be instrumental in helping to reduce plastic waste. The following outlines areas where polymer scientists can potentially contribute to the discussion:Making plastic more durable and repairable
This may be a strange demand, but it needs to be considered that plastic materials have properties that cannot be found with any other materials. Plastic materials are known to save lives and can help reduce energy consumption, among other favourable properties. There are plastic items we want to be durable and to last for a long time, yet many have been found to break down during usage.213 Some plastic pollution is the product of abrasion, such as tires, or premature breakage. As a result, plastic is released in the environment or contributes to landfills. To extend the lifetime of a plastic consumer item, it is desirable to be able to repair it or reshape it into an alternative product. The development of self-healing plastic214 or dynamic materials that can be upcycled215,216 Even broken plastic might be a valuable raw material in such a case.Creating better micro-and nanoplastic reference materials
Many animal studies have used spherical PS particles that are commercially available as models to test the effects on health. However, we know from the plastic pieces found in various tissues that plastic particles are neither spherical nor made from PS, as the dominant plastic material found in tissue was often PE (Table 1). The impact of the shape on biodistribution and cell uptake is significant. Additionally, PS cannot be directly compared to PE because one is an amorphous polymer and the other is highly crystalline, which influences degradation. Micro- and nanoplastics have diverse shapes and a broad size range,217 making it challenging to predict their health effects. While it is feasible to use actual micro-and nanoplastic samples from nature, the issue would be that the size, composition and surface chemistry represent a very unique fingerprint of the geographic area and time point that created these specific plastic pieces. Materials scientists could potentially help to create models using accelerated aging conditions while providing in-depth structural characterization, such as size, shape, and surface functionalities. A variety of approaches have been summarised here,75 but it is evident that some reliable techniques to generate nano-sized materials are still missing. A notable challenge involves generating nanoplastics with surface chemistries that closely emulate the characteristics of natural nanoplastics. While accelerated procedures are never on par with conditions found in nature,218 they can still serve as better models than the ones currently used. Materials scientists can, moreover, think about introducing labels in the process that facilitate subsequent biological analysis. Introducing stable or radioisotopes is one option, but it is not readily available to all labs. Alternatives could be metal-doped nanoparticles.219,220 However, whatever functionality is introduced, care should be taken not to significantly alter the surface chemistry, as this may lead to the adsorption of different proteins, thus different biodistributions.Developing techniques to identify nanoplastics
As Ivleva emphasized,75 the analysis of nanoplastics within environmental matrices remains a significant analytical challenge. Conventional methods for microplastic detection, primarily based on diffraction techniques, lack the requisite resolution to identify nanoparticles at the nanometer scale. Moreover, visualizing low-abundance nanoplastics against complex backgrounds containing naturally occurring nanoparticles—including viruses, organic matter, and inorganic particles—poses additional difficulties. Preconcentration, enrichment, and fractionation procedures can enhance detection sensitivity; however, the advancement and integration of hyphenated analytical techniques, wherein two complementary methods are combined, are arguably the most promising strategies for the effective visualization and characterization of nanoplastics amidst diverse environmental constituents. Because nanoplastics are usually found in low concentrations in most environments, developing reliable analytical techniques that prevent false positives poses extra challenges. Once such samples are transferred to the lab for analysis, the samples are introduced to an environment that is filled with plastic products such as gloves, stoppers and syringes. There is an increasing awareness that analysis of samples that contain plastic nanoparticles requires additional care to avoid further contamination221 but also more thought needs to be devoted to the development of control experiments that help verify detection thresholds and the absence of false positives.75Contributing to the discussion on standards
While materials scientists are not necessarily policymakers, their involvement in the discussion on standardized protocols is crucial. This would include the discussion on the best way of separating micro-and nanoplastics from the surrounding media, such as natural inorganic nanoparticles and organic matter, etc., and the establishment of protocols and procedures that have limited false readings and can be universally applied. The International Standardization Organisation has now established the first internationally recognized microplastic testing standards, ISO 24187, “Principles for the analysis of microplastics present in the environment.” This is a first step forward, but this standard focuses only on microplastics. Therefore, informed discussion towards clear guidelines on nanoplastics is needed.Analysing the interaction between nanoplastic with pollutants
While current research primarily focuses on assessing the toxicity of micro- and nanoplastics, predominantly utilizing engineered polystyrene (PS) beads, the potential risks may be more alarming. Traditionally, polymer scientists exploit the ability of plastics to interact with small molecules, such as additives, to enhance desired material properties. In natural environments, this mechanism inadvertently occurs as plastic particles either absorb pollutants into their bulk or adsorb contaminants onto their surfaces, potentially facilitating the transfer of harmful substances. Depending on the polymer and the pollutant, accumulation is not limited to the surface but these small molecules will penetrate deeper into the nanoplastic material. This can lead to unexpected toxicity. While some pollutants may not be toxic because they lack a pathway to enter cells in the body, nanoplastics can now transport them directly into cells, where they are released. It was discovered that a mixture of microplastics and perfluoroalkyl substances (PFAS) exhibits synergistic toxicity compared to PFAS and plastic particles alone.222 Similar effects were observed with heavy metals.223 This interaction depends not only on the compatibility between the polymer and the compound but also on the presence of functional groups, which typically result from the degradation process. Additionally, amorphous polymers can absorb significant amounts of low molecular weight compounds if the Flory–Huggings interaction parameter χ is favorable. In contrast, crystalline polymers generally have limited space to accommodate potential pollutants. It is important to remember that loading pollutants in bulk or on the surface will alter the properties of nanoplastic particles, thus affecting colloidal stability, protein adsorption, and ultimately determining the fate of these nanoplastics in the body. This relationship between the size of the plastic particles, the degradation mechanism that determines functionality of the surface, the type of pollutants, and the resulting potentially toxic warheads is complex, and further work is required to fully understand how plastic will increase the toxicity of environmental pollutants.Developing strategies to remove plastic from complex media
There are several proposed strategies for detoxifying the body from plastic, including the use of melatonin, probiotics, and other compounds that appear to mitigate the effects of plastic in animals.212 Very recently, extracorporeal apheresis, which is the filtration of the blood, was tested as a way to remove microplastics from the blood,224 although approaches like this need to be followed up with more studies, including detailed quantification and size analysis. Most of these studies are preliminary and require more attention from medical scientists.Materials scientists can, however, help to create better techniques to remove micro-and nanoplastics from the environment as part of a remediation process. Currently, a range of techniques are already applied to remove micro-and nanoplastics from wastewater, such as filtration, centrifugation, and flocculation, but not all of them are suitable for nanoplastics.225 New ideas are currently being explored to remove nanoplastics from the environment, such as the electrophoretic removal of nanoplastics from industrial wastewater,226,227 the photolytic decomposition of plastic,225 the use of bioreactors,225 or the adsorption of nanoplastics onto other materials.225 Most of these techniques appear to be successful in removing nanoplastics from water, but it is evident that other matrices, such as soil, are more challenging. Isolating nanoplastics from complex media, however, is an important step in enabling robust quantitative analysis.
Developing an understanding that polyethylene is not polyethylene
Just because two plastic items are made from polyethylene, it does not mean they are the same; this statement applies to any plastic items. There are differences in additive, molecular weight, crystallinity, branching, and so on that result in different behaviours in the environment, such as different degrees of degradation.228 This can lead to a large amount of data as each polymer has its unique degradation rate in a specific environment. Machine Learning could be applied more widely in the future to help identify the link between the type of polymer and its degradation.229 The subtle differences between polymer structure and outcome are not only evident when analysing the behaviour of the nanoplastic in the environment. Very recently, it was shown that even Py-GC–MS, a standard technique to identify plastics, could not provide reliable results when PS of different tacticities (syndiotactic, isotactic and atactic) were measured.230 This means that we need to adjust some analytical techniques and rethink its robustness.Participation in networks
This complex topic can only be understood through collaboration among researchers from different fields. Chemists and materials scientists contribute their expertise on analyzing micro- and nanoplastics and designing reference materials, but their role extends further. Large-scale comparison studies across multiple labs help validate methods and ensure their accuracy. These interlaboratory studies are already underway or have been completed, such as a study involving 84 labs that compared microplastic detection methods.231 Other networks explored better reporting options and open-access tools.232 A network can help establish key principles for managing plastics, which can become government-supported standards such as the Australian Standard AS ISO 24187 “Principles for the analysis of microplastics present in the environment”.233 This entails academics dedicating some of their time and resources, but it is a crucial step toward addressing this issue.Creating alternative polymers for single-use plastic
While the mantra should be to avoid single-use plastic at all costs and for everyone to take on the responsibility to limit daily plastic usage, there are still fields where plastic is necessary. An example would be the need for sterile catheters, blister packs for medicine, packaging that can enhance the shelf-life of food, or personal protective equipment. Biodegradable plastics could be an alternative as they are designed to degrade fully. However, this approach has caveats. Comparison of biodegradable microplastics with that of commodity polymers shows that degradable materials pose similar challenges such as the adsorption of contaminants, but they can also display negative effects towards marine life234 or modulate the carbon and nitrogen cycle in marine sediment.235 A recent review warned that we should not replace one problem with another, as there is evidence that incompletely degraded plastics might affect the ecosystem.236 While polymer scientists have many innovative ideas regarding the design of new degradable polymers, their concepts need to be discussed with ecotoxicologists in a timely manner.Conclusions
There is no denying that excessive plastic consumption, especially single-use plastics, has created a significant environmental problem. Now, increasing evidence shows that tiny plastic particles enter humans and are detected in blood and organs, including the brain. The plastic particles found in the brain measure 200 nm in size; therefore, we need to expand our discussion from microplastics to nanoplastics. Nanoplastics are produced in similar ways to microplastics. However, when discussing nanoplastics, new aspects need to be considered, such as the crystallinity of the polymer, which affects degradation. The results of degradation are often fiber-like pieces, irregular with rugged edges. Sometimes, they resemble film pieces, but they are rarely spherical particles. When transitioning from microplastic to nanoplastic, other effects come into play, such as the large surface area. The type of functional groups on the surface, usually resulting from the degradation pathway, will influence colloidal stability and the adsorption of biomolecules. This determines whether the nanoplastic particles are nano-sized entities or large micron-sized aggregates in an aqueous environment. How these nanoplastic particles reach the brain can be understood through the field of nanomedicine. From there, it becomes clear that size, shape, and surface functionality— which influence the protein corona composition— are highly important parameters that shape the behavior of nanoparticles. This also highlights that published toxicity studies with round engineered nanoparticles may not indicate actual toxicity effects, as we know that non-spherical structures can behave quite differently. Materials scientists can help create better reference materials, but they can also develop reference materials that can be easily detected in tissue and complex biological solutions, as these small nanoparticles are harder to identify than microplastics. While we often discuss microplastics, it appears that we need to shine a spotlight on nanoplastics. This complex problem can only be tackled when materials scientists, analytical chemists, environmental scientists, medical researchers, and researchers from related fields work together.Author contributions
The article has been written by MS with no further support.Conflicts of interest
There are no conflicts to declare.Data availability
This is a review article and does not contain any original data.Acknowledgements
MS would like to thank the Australian Research Council (ARC) for funding (FL200100124).Notes and references
- https://oceanliteracy.unesco.org/plastic-pollution-ocean/.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle and A. E. Russell, Science, 2004, 304, 838 CrossRef CAS PubMed.
- A. A. Koelmans, P. E. Redondo-Hasselerharm, N. H. M. Nor, V. N. de Ruijter, S. M. Mintenig and M. Kooi, Nat. Rev. Mater., 2022, 7, 138–152 CrossRef.
- N. R. Maddela, D. Kakarla, K. Venkateswarlu and M. Megharaj, J. Environ. Manage., 2023, 348, 119364 CrossRef CAS PubMed.
- S. ten Hietbrink, D. Materić, R. Holzinger, S. Groeskamp and H. Niemann, Nature, 2025, 643, 412–416 CrossRef CAS PubMed.
- H. Aoki, K. Mori, T. Takahashi and S. Ito, Chem. Phys., 2013, 419, 54–58 CrossRef CAS.
- D. Vollath, F. D. Fischer and D. Holec, Beilstein J. Nanotechnol., 2018, 9, 2265–2276 CrossRef CAS PubMed.
- A. M. N. A. A. Rahman, A. Rusli, M. K. Abdullah, R. K. Shuib, Z. A. A. Hamid, K. M. Ku Ishak, M. Mohd Zaini Makhtar, M. Jaafar and M. D. Shafiq, Water Sci. Eng., 2024, 17, 361–370 CrossRef.
- S. S. Raut, R. Singh and U. M. Lekhak, Next Sustain., 2024, 3, 100037 CrossRef.
- S. Griffin, M. I. Masood, M. J. Nasim, M. Sarfraz, A. P. Ebokaiwe, K. H. Schäfer, C. M. Keck and C. Jacob, Antioxidants, 2018, 7(1), 3 CrossRef PubMed.
- A. J. Nihart, M. A. Garcia, E. El Hayek, R. Liu, M. Olewine, J. D. Kingston, E. F. Castillo, R. R. Gullapalli, T. Howard, B. Bleske, J. Scott, J. Gonzalez-Estrella, J. M. Gross, M. Spilde, N. L. Adolphi, D. F. Gallego, H. S. Jarrell, G. Dvorscak, M. E. Zuluaga-Ruiz, A. B. West and M. J. Campen, Nat. Med., 2025, 31, 1114–1119 CrossRef CAS PubMed.
- https://ourworldindata.org/plastic-pollution.
- S. S. Ali, T. Elsamahy, E. Koutra, M. Kornaros, M. El-Sheekh, E. A. Abdelkarim, D. Zhu and J. Sun, Sci. Total Environ., 2021, 771, 144719 CrossRef CAS PubMed.
- Y. An, A. Padermshoke, T. Van Nguyen and A. Takahara, Langmuir, 2024, 40, 9336–9344 CrossRef CAS PubMed.
- S. Kliem, M. Kreutzbruck and C. Bonten, Materials, 2020, 13, 4586 CrossRef CAS PubMed.
- J. Zhou, T.-G. Hsu and J. Wang, Angew. Chem., Int. Ed., 2023, 62, e202300768 CrossRef CAS PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustainable Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- B. Singh and N. Sharma, Polym. Degrad. Stab., 2008, 93, 561–584 CrossRef CAS.
- E. Yousif and R. Haddad, SpringerPlus, 2013, 2, 398 CrossRef PubMed.
- B. Gewert, M. M. Plassmann and M. MacLeod, Environ. Sci. Process. Impacts, 2015, 17, 1513–1521 RSC.
- K. Zhang, A. H. Hamidian, A. Tubić, Y. Zhang, J. K. H. Fang, C. Wu and P. K. S. Lam, Environ. Pollut., 2021, 274, 116554 CrossRef CAS PubMed.
- T. Lu, E. Solis-Ramos, Y. Yi and M. Kumosa, Polym. Degrad. Stab., 2018, 154, 203–210 CrossRef CAS.
- F. Eisenreich, Angew. Chem., Int. Ed., 2023, 62, e202301303 CrossRef CAS PubMed.
- A. Delre, M. Goudriaan, V. H. Morales, A. Vaksmaa, R. T. Ndhlovu, M. Baas, E. Keijzer, T. de Groot, E. Zeghal, M. Egger, T. Röckmann and H. Niemann, Mar. Pollut. Bull., 2023, 187, 114544 CrossRef CAS PubMed.
- J. Gigault, B. Pedrono, B. Maxit and A. Ter Halle, Environ. Sci.: Nano, 2016, 3, 346–350 RSC.
- P. Pfohl, M. Wagner, L. Meyer, P. Domercq, A. Praetorius, T. Hüffer, T. Hofmann and W. Wohlleben, Environ. Sci. Technol., 2022, 56, 11323–11334 CrossRef CAS PubMed.
- V. Kholodovych and W. J. Welsh, in Physical Properties of Polymers Handbook, ed. J. E. Mark, Springer, New York, New York, NY, 2007, pp. 927–938 DOI:10.1007/978-0-387-69002-5_54.
- T. Faravelli, M. Pinciroli, F. Pisano, G. Bozzano, M. Dente and E. Ranzi, J. Anal. Appl. Pyrolysis, 2001, 60, 103–121 CrossRef CAS.
- H. Kimukai, Y. Kodera, K. Koizumi, M. Okada, K. Yamada, T. Hiaki and K. Saido, Appl. Sci., 2020, 10, 5100 CrossRef CAS.
- L. M. Hernandez, E. G. Xu, H. C. E. Larsson, R. Tahara, V. B. Maisuria and N. Tufenkji, Environ. Sci. Technol., 2019, 53, 12300–12310 CrossRef CAS PubMed.
- D. Li, Y. Shi, L. Yang, L. Xiao, D. K. Kehoe, Y. K. Gun’ko, J. J. Boland and J. J. Wang, Nat. Food, 2020, 1, 746–754 CrossRef CAS PubMed.
- Y. Su, X. Hu, H. Tang, K. Lu, H. Li, S. Liu, B. Xing and R. Ji, Nat. Nanotechnol., 2022, 17, 76–85 CrossRef CAS PubMed.
- K. A. Hussain, S. Romanova, I. Okur, D. Zhang, J. Kuebler, X. Huang, B. Wang, L. Fernandez-Ballester, Y. Lu, M. Schubert and Y. Li, Environ. Sci. Technol., 2023, 57, 9782–9792 CrossRef CAS PubMed.
- C. B. Crawford and B. Quinn, in Microplastic Pollutants, ed. C. B. Crawford and B. Quinn, Elsevier, 2017, pp. 57–100 DOI:10.1016/B978-0-12-809406-8.00004-9.
- N. Chongvimansin and T. C. O’Connor, Macromolecules, 2025, 58, 1787–1794 CrossRef CAS PubMed.
- I. E. Napper and R. C. Thompson, Mar. Pollut. Bull., 2016, 112, 39–45 CrossRef CAS PubMed.
- F. De Falco, E. Di Pace, M. Cocca and M. Avella, Sci. Rep., 2019, 9, 6633 CrossRef PubMed.
- A. P. Periyasamy, Toxics, 2023, 11, 575 CrossRef CAS PubMed.
- H. Yadav, M. R. H. Khan, M. Quadir, K. A. Rusch, P. P. Mondal, M. Orr, E. G. Xu and S. M. Iskander, Environ. Sci. Technol., 2023, 57, 8225–8235 CrossRef CAS PubMed.
- E. B. Jadhav, M. S. Sankhla, R. A. Bhat and D. S. Bhagat, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100608 CAS.
- https://www.acs.org/pressroom/presspacs/2025/march/chewing-gum-can-shed-microplastics-into-saliva-pilot-study-finds.html.
- F. Sommer, L. Brockmann, M. J. Steinbauer and V. Audorff, Sci. Total Environ., 2025, 969, 178971 CrossRef CAS PubMed.
- A. Sherman, T. Masset, L. Wimmer, L. K. Maruschka, L. A. Dailey, T. Hüffer, F. Breider and T. Hofmann, ACS ES&T Air, 2025, 2, 930–942 Search PubMed.
- M. T. Ekvall, M. Lundqvist, E. Kelpsiene, E. Šileikis, S. B. Gunnarsson and T. Cedervall, Nanoscale Adv., 2019, 1, 1055–1061 RSC.
- S. Monira, R. Roychand, F. I. Hai, M. Bhuiyan and B. K. Pramanik, Environ. Pollut., 2025, 364, 125310 CrossRef CAS PubMed.
- N. S. Allen, M. Edge, D. Mourelatou, A. Wilkinson, C. M. Liauw, M. Dolores Parellada, J. A. Barrio and V. Ruiz Santa Quiteria, Polym. Degrad. Stab., 2003, 79, 297–307 CrossRef CAS.
- D. Stanica-Ezeanu and D. Matei, Sci. Rep., 2021, 11, 4431 CrossRef CAS PubMed.
- M. Arhant, M. Le Gall, P.-Y. Le Gac and P. Davies, Polym. Degrad. Stab., 2019, 161, 175–182 CrossRef CAS.
- N. F. Mendez, V. Sharma, M. Valsecchi, V. Pai, J. K. Lee, L. S. Schadler, A. J. Müller, S. Watson-Sanders, M. Dadmun, G. Kumaraswamy and S. K. Kumar, Nat. Commun., 2025, 16, 3051 CrossRef CAS PubMed.
- G.-X. Wang, D. Huang, J.-H. Ji, C. Völker and F. R. Wurm, Adv. Sci., 2021, 8, 2001121 CrossRef CAS PubMed.
- M. Srikanth, T. S. R. S. Sandeep, K. Sucharitha and S. Godi, Bioresour. Bioprocess., 2022, 9, 42 CrossRef PubMed.
- D. Martín-González, C. de la Fuente Tagarro, A. De Lucas, S. Bordel and F. Santos-Beneit, Int. J. Mol. Sci., 2024, 25, 5536 CrossRef PubMed.
- I. E. Napper and R. C. Thompson, Environ. Sci. Technol., 2019, 53, 4775–4783 CrossRef CAS PubMed.
- S.-J. Royer, F. Greco, M. Kogler and D. D. Deheyn, PLoS One, 2023, 18, e0284681 CrossRef CAS PubMed.
- K. D. Cox, G. A. Covernton, H. L. Davies, J. F. Dower, F. Juanes and S. E. Dudas, Environ. Sci. Technol., 2019, 53, 7068–7074 CrossRef CAS PubMed.
- A. Tagg, D. Roland, G. S. Leo, K. Knight, H. Goldstein, T. Davis and Don’t Forget The Bubbles, J. Paediatr. Child Health, 2019, 55, 921–923 CrossRef PubMed.
- C. R. Multisanti, S. Ferrara, G. Piccione and C. Faggio, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2025, 291, 110149 CAS.
- P. Krasucka, A. Bogusz, E. Baranowska-Wójcik, B. Czech, D. Szwajgier, M. Rek, Y. S. Ok and P. Oleszczuk, Sci. Total Environ., 2022, 843, 157108 CrossRef CAS PubMed.
- Y. Jang, I. Nyamjav, H. R. Kim, D.-E. Suh, N. Park, Y. E. Lee and S. Lee, Sci. Total Environ., 2024, 929, 172775 CrossRef CAS PubMed.
- K. Waldschläger and H. Schüttrumpf, Environ. Sci. Technol., 2019, 53, 13219–13227 CrossRef PubMed.
- S. Hino, N. Kawasaki, N. Yamano, T. Nakamura and A. Nakayama, Mater. Chem. Phys., 2023, 303, 127813 CrossRef CAS.
- Z. Chen, X. Shi, J. Zhang, L. Wu, W. Wei and B.-J. Ni, Water Res.: X, 2023, 19, 100169 CAS.
- S. Shrestha, B. Wang and P. Dutta, Adv. Colloid Interface Sci., 2020, 279, 102162 CrossRef CAS PubMed.
- A. Choudhury, F. Z. Simnani, D. Singh, P. Patel, A. Sinha, A. Nandi, A. Ghosh, U. Saha, K. Kumari, S. K. Jaganathan, N. K. Kaushik, P. K. Panda, M. Suar and S. K. Verma, Ecotoxicol. Environ. Saf., 2023, 259, 115018 CrossRef CAS PubMed.
- P. Pfohl, K. Santizo, J. Sipe, M. Wiesner, S. Harrison, C. Svendsen and W. Wohlleben, Microplast. Nanoplast., 2025, 5, 7 CrossRef CAS.
- S. Xiao, Y. Cui, J. Brahney, N. M. Mahowald and Q. Li, Nat. Geosci., 2023, 16, 863–870 CrossRef CAS.
- E.-L. Ng, E. Huerta Lwanga, S. M. Eldridge, P. Johnston, H.-W. Hu, V. Geissen and D. Chen, Sci. Total Environ., 2018, 627, 1377–1388 CrossRef CAS PubMed.
- E. Karalija, M. Carbó, A. Coppi, I. Colzi, M. Dainelli, M. Gašparović, T. Grebenc, C. Gonnelli, V. Papadakis, S. Pilić, N. Šibanc, L. Valledor, A. Poma and F. Martinelli, J. Hazard. Mater., 2022, 438, 129450 CrossRef CAS PubMed.
- L. Jia, L. Liu, Y. Zhang, W. Fu, X. Liu, Q. Wang, M. Tanveer and L. Huang, Front. Plant Sci., 2023, 14, 1226484 CrossRef PubMed.
- J. Boctor, F. C. Hoyle, M. A. Farag, M. Ebaid, T. Walsh, A. S. Whiteley and D. V. Murphy, Environ. Sci. Eur., 2025, 37, 68 CrossRef.
- V. Suresh, R. Shams, K. K. Dash, A. M. Shaikh and K. Béla, J. Agric. Food Res., 2025, 20, 101788 CAS.
- V. K. Snekkevik, M. Cole, A. Gomiero, M. Haave, F. R. Khan and A. L. Lusher, Heliyon, 2024, 10, e35022 CrossRef PubMed.
- E. Huerta Lwanga, H. Gertsen, H. Gooren, P. Peters, T. Salánki, M. van der Ploeg, E. Besseling, A. A. Koelmans and V. Geissen, Environ. Pollut., 2017, 220, 523–531 CrossRef CAS PubMed.
- D. Debraj and L. Mulky, Environ. Sci. Eur., 2025, 14, 259–272 CAS.
- N. P. Ivleva, Chem. Rev., 2021, 121, 11886–11936 CrossRef CAS PubMed.
- A. Nene, S. Sadeghzade, S. Viaroli, W. Yang, U. P. Uchenna, A. Kandwal, X. Liu, P. Somani and M. Galluzzi, Environ. Sci. Eur., 2025, 37, 7 CrossRef.
- N. Qian, X. Gao, X. Lang, H. Deng, T. M. Bratu, Q. Chen, P. Stapleton, B. Yan and W. Min, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2300582121 CrossRef CAS PubMed.
- E.-S. Yu, E. T. Jeong, S. Lee, I. S. Kim, S. Chung, S. Han, I. Choi and Y.-S. Ryu, ACS Nano, 2023, 17, 2114–2123 CrossRef CAS PubMed.
- R. Gillibert, G. Balakrishnan, Q. Deshoules, M. Tardivel, A. Magazzù, M. G. Donato, O. M. Maragò, M. Lamy de La Chapelle, F. Colas, F. Lagarde and P. G. Gucciardi, Environ. Sci. Technol., 2019, 53, 9003–9013 CrossRef CAS PubMed.
- S. Ganda, C. K. Wong, J. Biazik, R. Raveendran, L. Zhang, F. Chen, N. Ariotti and M. H. Stenzel, ACS Appl. Mater. Interfaces, 2022, 14, 35333–35343 CrossRef CAS PubMed.
- D. Ho and J. Masura, Colorants, 2025, 4, 20 CrossRef CAS.
- B. Nguyen, D. Claveau-Mallet, L. M. Hernandez, E. G. Xu, J. M. Farner and N. Tufenkji, Acc. Chem. Res., 2019, 52, 858–866 CrossRef CAS PubMed.
- Q. Yang, S. Zhang, J. Su, S. Li, X. Lv, J. Chen, Y. Lai and J. Zhan, Environ. Sci. Technol., 2022, 56, 10818–10828 CrossRef CAS PubMed.
- S. Modak, M. Kasula and M. R. Esfahani, ACS Appl. Eng. Mater., 2023, 1, 744–755 CrossRef CAS.
- D. Ludescher, L. Wesemann, J. Schwab, J. Karst, S. B. Sulejman, M. Ubl, B. O. Clarke, A. Roberts, H. Giessen and M. Hentschel, Nat. Photonics, 2025, 19, 1138–1145 CrossRef CAS.
- S. Choi, S. Lee, M.-K. Kim, E.-S. Yu and Y.-S. Ryu, Anal. Chem., 2024, 96, 8846–8854 CrossRef CAS PubMed.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono, L. Brach and J. Gigault, Environ. Sci. Technol., 2017, 51, 13689–13697 CrossRef CAS PubMed.
- A. Wahl, C. Le Juge, M. Davranche, H. El Hadri, B. Grassl, S. Reynaud and J. Gigault, Chemosphere, 2021, 262, 127784 CrossRef CAS PubMed.
- D. Materić, H. A. Kjær, P. Vallelonga, J.-L. Tison, T. Röckmann and R. Holzinger, Environ. Res., 2022, 208, 112741 CrossRef PubMed.
- D. Materić, E. Ludewig, D. Brunner, T. Röckmann and R. Holzinger, Environ. Pollut., 2021, 288, 117697 CrossRef PubMed.
- A. Pradel, C. Catrouillet and J. Gigault, NanoImpact, 2023, 29, 100453 CrossRef CAS PubMed.
- Y. Xu, Q. Ou, J. P. van der Hoek, G. Liu and K. M. Lompe, Environ. Sci. Technol., 2024, 58, 991–1009 CrossRef CAS PubMed.
- X. Wang, N. Bolan, D. C. W. Tsang, B. Sarkar, L. Bradney and Y. Li, J. Hazard. Mater., 2021, 402, 123496 CrossRef CAS PubMed.
- H. Sun, R. Jiao, G. An, H. Xu and D. Wang, J. Environ. Sci., 2021, 103, 33–42 CrossRef CAS PubMed.
- M. Mahmoudi, M. P. Landry, A. Moore and R. Coreas, Nat. Rev. Mater., 2023, 8, 422–438 CrossRef PubMed.
- Y. Li, L. Tao, Q. Wang, F. Wang, G. Li and M. Song, Environ. Health, 2023, 1, 249–257 CAS.
- P. Schwabl, S. Köppel, P. Königshofer, T. Bucsics, M. Trauner, T. Reiberger and B. Liebmann, Ann. Intern. Med., 2019, 171, 453–457 CrossRef PubMed.
- C. Hartmann, I. Lomako, C. Schachner, E. El Said, J. Abert, V. Satrapa, A.-M. Kaiser, H. Walch and S. Köppel, Sci. Total Environ., 2024, 951, 175825 CrossRef CAS PubMed.
- M. C. Rillig and A. Lehmann, Science, 2020, 368, 1430–1431 CrossRef CAS PubMed.
- S. C. Saha and G. Saha, Heliyon, 2024, 10, e24355 CrossRef CAS PubMed.
- E. Proksch, J. M. Brandner and J.-M. Jensen, Exp. Dermatol., 2008, 17, 1063–1072 CrossRef PubMed.
- W. Han, J. Cui, G. Sun, X. Miao, Z. Pufang and L. Nannan, Environ. Pollut., 2024, 349, 123874 CrossRef PubMed.
- A. Ragusa, A. Svelato, C. Santacroce, P. Catalano, V. Notarstefano, O. Carnevali, F. Papa, M. C. A. Rongioletti, F. Baiocco, S. Draghi, E. D'Amore, D. Rinaldo, M. Matta and E. Giorgini, Environ. Int., 2021, 146, 106274 CrossRef CAS PubMed.
- T. Braun, L. Ehrlich, W. Henrich, S. Koeppel, I. Lomako, P. Schwabl and B. Liebmann, Pharmaceutics, 2021, 13, 921 CrossRef CAS PubMed.
- Y. Picó and D. Barceló, TrAC, Trends Anal. Chem., 2020, 130, 115964 CrossRef.
- C. Rauert, N. Charlton, A. Bagley, S. A. Dunlop, C. Symeonides and K. V. Thomas, Environ. Sci. Technol., 2025, 59, 1984–1994 CrossRef CAS PubMed.
- M. A. Garcia, R. Liu, A. Nihart, E. El Hayek, E. Castillo, E. R. Barrozo, M. A. Suter, B. Bleske, J. Scott, K. Forsythe, J. Gonzalez-Estrella, K. M. Aagaard and M. J. Campen, Toxicol. Sci, 2024, 199, 81–88 CrossRef CAS PubMed.
- T. Horvatits, M. Tamminga, B. Liu, M. Sebode, A. Carambia, L. Fischer, K. Püschel, S. Huber and E. K. Fischer, EBioMedicine, 2022, 82, 104147 CrossRef CAS PubMed.
- H. A. Leslie, M. J. M. van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Environ. Int., 2022, 163, 107199 CrossRef CAS PubMed.
- R. Marfella, F. Prattichizzo, C. Sardu, G. Fulgenzi, L. Graciotti, T. Spadoni, N. D'Onofrio, L. Scisciola, R. La Grotta, C. Frigé, V. Pellegrini, M. Municinò, M. Siniscalchi, F. Spinetti, G. Vigliotti, C. Vecchione, A. Carrizzo, G. Accarino, A. Squillante, G. Spaziano, D. Mirra, R. Esposito, S. Altieri, G. Falco, A. Fenti, S. Galoppo, S. Canzano, F. C. Sasso, G. Matacchione, F. Olivieri, F. Ferraraccio, I. Panarese, P. Paolisso, E. Barbato, C. Lubritto, M. L. Balestrieri, C. Mauro, A. E. Caballero, S. Rajagopalan, A. Ceriello, B. D'Agostino, P. Iovino and G. Paolisso, N. Engl. J. Med., 2024, 390, 900–910 CrossRef CAS PubMed.
- S. Liu, C. Wang, Y. Yang, Z. Du, L. Li, M. Zhang, S. Ni, Z. Yue, K. Yang, Y. Wang, X. Li, Y. Yang, Y. Qin, J. Li, Y. Yang and M. Zhang, J. Hazard. Mater., 2024, 469, 133855 CrossRef CAS PubMed.
- Y. Yang, E. Xie, Z. Du, Z. Peng, Z. Han, L. Li, R. Zhao, Y. Qin, M. Xue, F. Li, K. Hua and X. Yang, Environ. Sci. Technol., 2023, 57, 10911–10918 CrossRef CAS PubMed.
- L. Zhu, J. Zhu, R. Zuo, Q. Xu, Y. Qian and L. An, Sci. Total Environ., 2023, 856, 159060 CrossRef CAS PubMed.
- M. Jochum, M. Garcia, A. Hammerquist, J. Howell, M. Stanford, R. Liu, M. Olewine, E. E. Hayek, E. Phan, L. Showalter, C. Shope, M. Suter, M. Campen, K. Aagaard and E. Barrozo, Res. Sq., 2025 DOI:10.21203/rs.3.rs-5903715/v1.
- C. J. Hu, M. A. Garcia, A. Nihart, R. Liu, L. Yin, N. Adolphi, D. F. Gallego, H. Kang, M. J. Campen and X. Yu, Toxicol. Sci, 2024, 200, 235–240 CrossRef CAS PubMed.
- Q. Zhao, L. Zhu, J. Weng, Z. Jin, Y. Cao, H. Jiang and Z. Zhang, Sci. Total Environ., 2023, 877, 162713 CrossRef CAS PubMed.
- L. F. Amato-Lourenço, R. Carvalho-Oliveira, G. R. Júnior, L. dos Santos Galvão, R. A. Ando and T. Mauad, J. Hazard. Mater., 2021, 416, 126124 CrossRef PubMed.
- L. C. Jenner, J. M. Rotchell, R. T. Bennett, M. Cowen, V. Tentzeris and L. R. Sadofsky, Sci. Total Environ., 2022, 831, 154907 CrossRef CAS PubMed.
- Q. Chen, J. Gao, H. Yu, H. Su, Y. Yang, Y. Cao, Q. Zhang, Y. Ren, H. Hollert, H. Shi, C. Chen and H. Liu, Environ. Sci. Eur., 2022, 34, 25 CrossRef CAS.
- S. Ahadian, J. A. Finbloom, M. Mofidfar, S. E. Diltemiz, F. Nasrollahi, E. Davoodi, V. Hosseini, I. Mylonaki, S. Sangabathuni, H. Montazerian, K. Fetah, R. Nasiri, M. R. Dokmeci, M. M. Stevens, T. A. Desai and A. Khademhosseini, Adv. Drug Delivery Rev., 2020, 157, 37–62 CrossRef CAS PubMed.
- M. Smith, N. Thomas, P. Jenkins, N. Miller, D. Cremaschi and C. Porta, Exp. Physiol., 1995, 80, 735–743 CrossRef CAS PubMed.
- J. C. Prata, Crit. Rev. Environ. Sci. Technol., 2023, 53, 1489–1511 CrossRef CAS.
- H. Bouwmeester, P. C. H. Hollman and R. J. B. Peters, Environ. Sci. Technol., 2015, 49, 8932–8947 CrossRef CAS PubMed.
- A. Banerjee, J. Qi, R. Gogoi, J. Wong and S. Mitragotri, J. Controlled Release, 2016, 238, 176–185 CrossRef CAS PubMed.
- M. J. Ernsting, M. Murakami, A. Roy and S. D. Li, J. Controlled Release, 2013, 172, 782–794 CrossRef CAS PubMed.
- H. B. Haroon, A. C. Hunter, Z. S. Farhangrazi and S. M. Moghimi, Adv. Drug Delivery Rev., 2022, 188, 114396 CrossRef CAS PubMed.
- J. Lu, X. Gao, S. Wang, Y. He, X. Ma, T. Zhang and X. Liu, Exploration, 2023, 3, 20220045 CrossRef PubMed.
- E. Papini, R. Tavano and F. Mancin, Front. Immunol., 2020, 11, 567365 CrossRef CAS PubMed.
- Q. Cheng, T. Wei, L. Farbiak, L. T. Johnson, S. A. Dilliard and D. J. Siegwart, Nat. Nanotechnol., 2020, 15, 313–320 CrossRef CAS PubMed.
- D. Wu, Q. Chen, X. Chen, F. Han, Z. Chen and Y. Wang, Signal Transduction Targeted Ther., 2023, 8, 217 CrossRef PubMed.
- A. Kaushik, A. Singh, V. Kumar Gupta and Y. K. Mishra, Chemosphere, 2024, 361, 142380 CrossRef CAS PubMed.
- V. Kopatz, K. Wen, T. Kovács, A. S. Keimowitz, V. Pichler, J. Widder, A. D. Vethaak, O. Hollóczki and L. Kenner, Nanomaterials, 2023, 13, 1404 CrossRef CAS PubMed.
- O. Betzer, M. Shilo, R. Opochinsky, E. Barnoy, M. Motiei, E. Okun, G. Yadid and R. Popovtzer, Nanomedicine, 2017, 12, 1533–1546 CrossRef CAS PubMed.
- H. Xu, C. Dong, Z. Yu, Z. Hu, J. Yu, D. Ma, W. Yao, X. Qi, Y. Ozaki and Y. Xie, Environ. Pollut., 2024, 360, 124632 CrossRef CAS PubMed.
- N. Zhang, Y. B. Li, H. R. He, J. F. Zhang and G. S. Ma, Sci. Total Environ., 2021, 767, 144345 CrossRef CAS PubMed.
- J. Zhang, L. Wang, L. Trasande and K. Kannan, Environ. Sci. Technol. Lett., 2021, 8, 989–994 CrossRef CAS.
- C. Mišľanová, M. Valachovičová and Z. Slezáková, Life, 2024, 14, 371 CrossRef PubMed.
- T. Habumugisha, Z. Zhang, C. Fang, C. Yan and X. Zhang, Sci. Total Environ., 2023, 893, 164840 CrossRef CAS PubMed.
- M. S. Yee, L. W. Hii, C. K. Looi, W. M. Lim, S. F. Wong, Y. Y. Kok, B. K. Tan, C. Y. Wong and C. O. Leong, Nanomaterials, 2021, 11(2), 496 CrossRef CAS PubMed.
- L. Zhou, L. Ran, Y. He and Y. Huang, Mol. Med. Rep., 2025, 31, 98 CAS.
- P. Li and J. Liu, Environ. Sci. Technol., 2024, 58, 3065–3078 CAS.
- E. Winiarska, M. Jutel and M. Zemelka-Wiacek, Environ. Res., 2024, 251, 118535 CrossRef CAS PubMed.
- Q. Shi, J. Tang, R. Liu and L. Wang, Crit. Rev. Environ. Sci. Technol., 2022, 52, 3863–3895 CrossRef CAS.
- J. Hwang, D. Choi, S. Han, J. Choi and J. Hong, Sci. Total Environ., 2019, 684, 657–669 CrossRef CAS PubMed.
- B. Prietl, C. Meindl, E. Roblegg, T. R. Pieber, G. Lanzer and E. Fröhlich, Cell Biol. Toxicol., 2014, 30, 1–16 CrossRef CAS PubMed.
- A. Poma, G. Vecchiotti, S. Colafarina, O. Zarivi, M. Aloisi, L. Arrizza, G. Chichiriccò and P. Di Carlo, Nanomaterials, 2019, 9, 1299 CrossRef CAS PubMed.
- K. A. Hussain, S. Romanova, I. Okur, D. Zhang, J. Kuebler, X. Huang, B. Wang, L. Fernandez-Ballester, Y. Lu, M. Schubert and Y. Li, Environ. Sci. Technol., 2023, 57, 9782–9792 CrossRef CAS PubMed.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Chemosphere, 2019, 221, 333–341 CrossRef CAS PubMed.
- K. E. Goodman, T. Hua and Q.-X. A. Sang, ACS Omega, 2022, 7, 34136–34153 CrossRef CAS PubMed.
- E. Danopoulos, M. Twiddy, R. West and J. M. Rotchell, J. Hazard. Mater., 2022, 427, 127861 CrossRef CAS PubMed.
- D. Choi, J. Hwang, J. Bang, S. Han, T. Kim, Y. Oh, Y. Hwang, J. Choi and J. Hong, Sci. Total Environ., 2021, 752, 142242 CrossRef CAS PubMed.
- A. S. Winkler, A. Cherubini, F. Rusconi, N. Santo, L. Madaschi, C. Pistoni, G. Moschetti, M. L. Sarnicola, M. Crosti, L. Rosso, P. Tremolada, L. Lazzari and R. Bacchetta, Environ. Int., 2022, 163, 107200 CrossRef CAS PubMed.
- T. Hua, S. Kiran, Y. Li and Q.-X. A. Sang, J. Hazard. Mater., 2022, 435, 128884 CrossRef CAS PubMed.
- Z. Hou, R. Meng, G. Chen, T. Lai, R. Qing, S. Hao, J. Deng and B. Wang, Sci. Total Environ., 2022, 838, 155811 CrossRef CAS PubMed.
- J. Cong, J. Wu, Y. Fang, J. Wang, X. Kong, L. Wang and Z. Duan, Environ. Int., 2024, 188, 108744 CrossRef CAS PubMed.
- Y. Cho and H. N. Kim, Organoid, 2025, 5, e5 CrossRef.
- H. Lu and M. H. Stenzel, Small, 2018, 14, 1702858 CrossRef PubMed.
- K. Yin, Y. Wang, H. Zhao, D. Wang, M. Guo, M. Mu, Y. Liu, X. Nie, B. Li, J. Li and M. Xing, Sci. Total Environ., 2021, 774, 145758 CrossRef CAS.
- G. L. Lopez and A. Lamarre, Reprod. Toxicol., 2025, 135, 108951 CrossRef CAS PubMed.
- S. Liu, H. Li, J. Wang, B. Wu and X. Guo, Sci. Total Environ., 2022, 833, 155198 CrossRef CAS PubMed.
- B. Li, Y. Ding, X. Cheng, D. Sheng, Z. Xu, Q. Rong, Y. Wu, H. Zhao, X. Ji and Y. Zhang, Chemosphere, 2020, 244, 125492 CrossRef CAS PubMed.
- Y. Yuan, M. S. Sepúlveda, B. Bi, Y. Huang, L. Kong, H. Yan and Y. Gao, Chemosphere, 2023, 311, 137048 CrossRef CAS PubMed.
- A. Weber, A. Schwiebs, H. Solhaug, J. Stenvik, A. M. Nilsen, M. Wagner, B. Relja and H. H. Radeke, Environ. Int., 2022, 163, 107173 CrossRef CAS PubMed.
- W. Gu, S. Liu, L. Chen, Y. Liu, C. Gu, H.-Q. Ren and B. Wu, Environ. Sci. Technol., 2020, 54, 3417–3427 CrossRef CAS PubMed.
- C. C. Chiang, H. Yeh, R. F. Shiu, W. C. Chin and T. H. Yen, World J. Gastroenterol., 2024, 30, 1011–1017 Search PubMed.
- I. Brandts, M. Cánovas, A. Tvarijonaviciute, M. Llorca, A. Vega, M. Farré, J. Pastor, N. Roher and M. Teles, Environ. Res., 2022, 212, 113433 CrossRef CAS PubMed.
- A. Rehman, F. Huang, Z. Zhang, T. Habumugisha, C. Yan, U. Shaheen and X. Zhang, Environ. Int., 2024, 187, 108713 Search PubMed.
- M. Prüst, J. Meijer and R. H. S. Westerink, Part. Fibre Toxicol., 2020, 17, 24 CrossRef PubMed.
- L. G. A. Barboza, L. R. Vieira, V. Branco, N. Figueiredo, F. Carvalho, C. Carvalho and L. Guilhermino, Aquat. Toxicol., 2018, 195, 49–57 CrossRef CAS PubMed.
- H. Jin, C. Yang, C. Jiang, L. Li, M. Pan, D. Li, X. Han and J. Ding, Environ. Health Perspect., 2022, 130, 107002 CrossRef CAS PubMed.
- H. Yang, H. Xiong, K. Mi, W. Xue, W. Wei and Y. Zhang, J. Hazard. Mater., 2020, 388, 122058 CrossRef CAS PubMed.
- A. M. Araújo, C. Mota, H. Ramos, M. A. Faria, M. Carvalho and I. M. P. L. V. O. Ferreira, Arch. Toxicol., 2025, 99, 3505–3525, DOI:10.1007/s00204-025-04091-3.
- Y. M. M. Paing, Y. Eom, G. B. Song, B. Kim, M. G. Choi, S. Hong and S. H. Lee, Sci. Total Environ., 2024, 924, 171681 CrossRef CAS PubMed.
- W. Yang, N. Jannatun, Y. Zeng, T. Liu, G. Zhang, C. Chen and Y. Li, Front. Toxicol., 2022, 4, 956885 CrossRef PubMed.
- L. Geppner, J. Hellner and M. Henjakovic, Environ. Res., 2025, 273, 121254 CrossRef CAS PubMed.
- J. Zhao, D. Gomes, L. Jin, S. P. Mathis, X. Li, E. C. Rouchka, H. Bodduluri, D. J. Conklin and T. E. O’Toole, Ecotoxicol. Environ. Saf., 2022, 232, 113239 Search PubMed.
- M. Zhang, J. Shi, Y. Zhu, H. Pan, L. Song and H. Deng, Environ. Pollut., 2024, 345, 123441 Search PubMed.
- A. Banerjee and W. L. Shelver, J. Food Prot., 2021, 84, 1480–1495 CrossRef CAS PubMed.
- P. U. Jani, D. E. McCarthy and A. T. Florence, Int. J. Pharm., 1992, 86, 239–246 CrossRef CAS.
- M. Wang, Y. Wu, G. Li, Y. Xiong, Y. Zhang and M. Zhang, Sci. Total Environ., 2024, 935, 173177 CrossRef CAS PubMed.
- K. Hunt, A. Davies, A. Fraser, C. Burden, A. Howell, K. Buckley, S. Harding and D. Bakhbakhi, BJOG, 2024, 131, 675–683 CrossRef CAS PubMed.
- N. Kumar and M. Mangla, Biol. Reprod., 2025, 112, 1028–1038 CrossRef CAS PubMed.
- M. Ali-Hassanzadeh, N. Arefinia, Z.-A.-S. Ghoreshi, H. Askarpour and H. Mashayekhi-Sardoo, Reprod. Toxicol., 2025, 135, 108932 CrossRef CAS PubMed.
- X. Qin, M. Cao, T. Peng, H. Shan, W. Lian, Y. Yu, G. Shui and R. Li, Environ. Sci. Technol., 2024, 58, 10482–10493 CrossRef CAS PubMed.
- H. Xu, C. Dong, T. Xiang, X. Shentu, Z. Yu, J. Xu, J. Yu, D. Ma and Y. Xie, J. Hazard. Mater., 2025, 483, 136656 Search PubMed.
- F. Amereh, N. Amjadi, A. Mohseni-Bandpei, S. Isazadeh, Y. Mehrabi, A. Eslami, Z. Naeiji and M. Rafiee, Environ. Pollut., 2022, 314, 120174 CrossRef CAS PubMed.
- S. Wan, X. Wang, W. Chen, M. Wang, J. Zhao, Z. Xu, R. Wang, C. Mi, Z. Zheng and H. Zhang, Part. Fibre Toxicol., 2024, 21, 13 Search PubMed.
- J. Hu, X. Qin, J. Zhang, Y. Zhu, W. Zeng, Y. Lin and X. Liu, Reprod. Toxicol., 2021, 106, 42–50 CrossRef CAS PubMed.
- J. Bai, Y. Wang, S. Deng, Y. Yang, S. Chen and Z. Wu, Part. Fibre Toxicol., 2024, 21, 36 CrossRef CAS PubMed.
- J. Ye, Y. Ren, Y. Dong and D. Fan, Toxicology, 2024, 504, 153792 CrossRef CAS PubMed.
- Z. S. Yang, Y. L. Bai, C. H. Jin, J. Na, R. Zhang, Y. Gao, G. W. Pan, L. J. Yan and W. Sun, Biomed. Environ. Sci., 2022, 35, 1025–1037 Search PubMed.
- D. Yang, J. Zhu, X. Zhou, D. Pan, S. Nan, R. Yin, Q. Lei, N. Ma, H. Zhu, J. Chen, L. Han, M. Ding and Y. Ding, Environ. Int., 2022, 166, 107362 CrossRef CAS PubMed.
- B. Jeong, J. Y. Baek, J. Koo, S. Park, Y.-K. Ryu, K.-S. Kim, S. Zhang, C. Chung, R. Dogan, H.-S. Choi, D. Um, T.-K. Kim, W. S. Lee, J. Jeong, W.-H. Shin, J.-R. Lee, N.-S. Kim and D. Y. Lee, J. Hazard. Mater., 2022, 426, 127815 CrossRef CAS PubMed.
- Z. Aghaei, J. G. Sled, J. C. Kingdom, A. A. Baschat, P. A. Helm, K. J. Jobst and L. S. Cahill, Environ. Sci. Technol. Lett., 2022, 9, 426–430 CrossRef CAS.
- T. Luo, Y. Zhang, C. Wang, X. Wang, J. Zhou, M. Shen, Y. Zhao, Z. Fu and Y. Jin, Environ. Pollut., 2019, 255, 113122 CrossRef CAS PubMed.
- G. F. Vasse and B. N. Melgert, Eur. Respir. Rev., 2024, 33, 230226 CrossRef PubMed.
- X. Huang, S. C. Saha, G. Saha, I. Francis and Z. Luo, Environmen. Adv., 2024, 16, 100525 CAS.
- M. Pletz, J. Hazard. Mater. Lett., 2022, 3, 100071 CrossRef CAS.
- C. K. H. Cheung and C. Not, Mar. Pollut. Bull., 2024, 207, 116809 Search PubMed.
- A. Foetisch, M. Filella, B. Watts, M. Bragoni and M. Bigalke, Microplast. Nanoplast., 2023, 3, 18 CrossRef CAS PubMed.
- T. Cao, M. Zhao, T. Zhang and W. Chen, Environ. Sci.: Nano, 2025, 12, 232–240 Search PubMed.
- K. Tallec, O. Blard, C. González-Fernández, G. Brotons, M. Berchel, P. Soudant, A. Huvet and I. Paul-Pont, Chemosphere, 2019, 225, 639–646 Search PubMed.
- K. Syberg, M. B. Nielsen, N. B. Oturai, L. P. W. Clausen, T. M. Ramos and S. F. Hansen, J. Hazard. Mater. Adv., 2022, 5, 100044 Search PubMed.
- K. Whitman, C. Bowyer, M. Nieto-Garcia, G. Georgiou, T. Evans and S. Fletcher, Environ. Sci. Policy, 2025, 173, 104218 CrossRef.
- https://www.campaignsthatwork.org/.
- J. N. Meegoda and M. C. Hettiarachchi, Int. J. Environ. Res. Public Health, 2023, 20, 5555 Search PubMed.
- https://www.unep.org/plastic-pollution.
- https://www.dcceew.gov.au/environment/protection/waste/plastics-and-packaging.
- https://environment.ec.europa.eu/strategy/plastics-strategy_en.
- T. R. Walker, Mar. Pollut. Bull., 2022, 176, 113447 CrossRef CAS PubMed.
- https://www.oecd.org/en/publications/policy-scenarios-for-eliminating-plastic-pollution-by-2040_76400890-en.html.
- F. Jahedi and N. Jaafarzadeh Haghighi Fard, Toxicol. Rep., 2025, 14, 102043 CrossRef CAS PubMed.
- R. C. Hale, A. E. King, J. M. Ramirez, M. La Guardia and C. Nidel, Environ. Sci. Techno. Lett., 2022, 9, 798–807 CrossRef CAS.
- S. Utrera-Barrios, R. Verdejo, M. A. López-Manchado and M. Hernández Santana, Mater. Horiz., 2020, 7, 2882–2902 RSC.
- J. Liu and M. W. Urban, Mater. Horiz., 2025, 12, 7060–7065, 10.1039/D5MH00710K.
- B. Wang, Y. Wang, S. Du, J. Zhu and S. Ma, Mater. Horiz., 2023, 10, 41–51 RSC.
- J. Gigault, H. El Hadri, B. Nguyen, B. Grassl, L. Rowenczyk, N. Tufenkji, S. Feng and M. Wiesner, Nat. Nanotechnol., 2021, 16, 501–507 CrossRef CAS PubMed.
- M. Frigione and A. Rodríguez-Prieto, Polymers, 2021, 13(16), 2688 CrossRef CAS PubMed.
- D. M. Mitrano, A. Beltzung, S. Frehland, M. Schmiedgruber, A. Cingolani and F. Schmidt, Nat. Nanotechnol., 2019, 14, 362–368 CrossRef CAS PubMed.
- Y. Luo, L. Li, Y. Feng, R. Li, J. Yang, W. J. G. M. Peijnenburg and C. Tu, Nat. Nanotechnol., 2022, 17, 424–431 CrossRef CAS PubMed.
- G. Kutralam-Muniasamy, V. C. Shruti, F. Pérez-Guevara and P. D. Roy, Sci. Total Environ., 2023, 856, 159164 CrossRef CAS PubMed.
- T. Soltanighias, A. Umar, M. Abdullahi, M. A.-E. Abdallah and L. Orsini, Environ. Pollut., 2024, 363, 125133 CrossRef CAS PubMed.
- Y. Cao, M. Zhao, X. Ma, Y. Song, S. Zuo, H. Li and W. Deng, Sci. Total Environ., 2021, 788, 147620 CrossRef CAS PubMed.
- S. R. Bornstein, T. Gruber, D. Katsere, A. El Attaoui, L. Wohlsperger, M. Yaman, W. Kanczkowski, G. Piwernetz, R. Straube, K. Voit-Bak, J. Licinio and C. Steenblock, Brain Med., 2025, 1, 52–53 Search PubMed.
- M. Keerthana Devi, N. Karmegam, S. Manikandan, R. Subbaiya, H. Song, E. E. Kwon, B. Sarkar, N. Bolan, W. Kim, J. Rinklebe and M. Govarthanan, Sci. Total Environ., 2022, 845, 157168 CrossRef CAS PubMed.
- A. Abdeljaoued, B. L. Ruiz, Y.-E. Tecle, M. Langner, N. Bonakdar, G. Bleyer, P. Stenner and N. Vogel, Nat. Commun., 2024, 15, 5437 CrossRef CAS PubMed.
- A. Abdeljaoued, B. L. Ruiz, Y.-E. Tecle, P. Stenner and N. Vogel, Water Res., 2026, 288, 124627 CrossRef CAS PubMed.
- L. He, J. Ding, S. S. Yang, Y. N. Zang, J. W. Pang, D. Xing, L. Y. Zhang, N. Ren and W. M. Wu, Environ. Sci. Technol., 2024, 58, 6647–6658 CrossRef CAS PubMed.
- K. Min, J. D. Cuiffi and R. T. Mathers, Nat. Commun., 2020, 11, 727 CrossRef CAS PubMed.
- H. Chen, K. V. Thomas and C. Rauert, Microplast. Nanoplast., 2025, 5, 29 CrossRef CAS.
- D. Ciornii, V.-D. Hodoroaba, N. Benismail, A. Maltseva, J. F. Ferrer, J. Wang, R. Parra, R. Jézéquel, J. Receveur, D. Gabriel, A. Scheitler, C. van Oversteeg, J. Roosma, A. van Renesse van Duivenbode, T. Bulters, M. Zanella, A. Perini, F. Benetti, D. Mehn, G. Dierkes, M. Soll, T. Ishimura, M. Bednarz, G. Peng, L. Hildebrandt, M. Peters, S.-K. Kim, J. Türk, F. Steinfeld, J. Jung, S. Hong, E.-J. Kim, H.-W. Yu, S. Klockmann, C. Krafft, J. Süssmann, S. Zou, A. ter Halle, A. M. Giovannozzi, A. Sacco, M. Fadda, M. Putzu, D.-H. Im, N. Nhlapo, P. Carrillo-Barragán, N. Schmidt, D. Herzke, A. Gomiero, A. Jaén-Gil, D. J. E. Cabanes, M. Doedt, V. Cardoso, A. Schmitz, M. Hawly, H. Mo, J. Jacquin, A. Mechlinski, G. A. Adediran, J. Andrade, S. Muniategui-Lorenzo, A. Ramsperger, M. G. J. Löder, C. Laforsch, T. Cirkovic Velickovic, D. Fabbri, I. Coralli, S. Federici, B. M. Scholz-Böttcher, J. la Nasa, G. Biale, C. Rauert, E. D. Okoffo, A. Undas, L. An, V. Wachtendorf, P. Fengler and K. Altmann, Analyt. Chem., 2025, 97, 8719–8728 CrossRef CAS PubMed.
- A. Bakir, A. R. McGoran, B. Silburn, J. Russell, H. Nel, A. L. Lusher, R. Amos, R. S. Shadrack, S. J. Arnold, C. Castillo, J. F. Urbina, E. Barrientos, H. Sanchez, K. Pillay, L. Human, T. Swartbooi, M. R. Cordova, S. Y. Sani, T. W. A. W. Wijesinghe, A. A. D. Amarathunga, J. Gunasekara, S. Somasiri, K. Mahatantila, S. Liyanage, M. Müller, Y. Y. Hee, D. F. Onda, K. M. Jansar, Z. Shiraz, H. Amir and A. G. Mayes, Sci. Rep., 2024, 14, 12714 CrossRef CAS PubMed.
- https://www.iso.org/standard/78033.html accessed 11/08/2025.
- M. Chen, F. Chen, Z. Li, M. R. Haider, J. Wei, G. Chen, W. Wang and J. Wang, TrAC, Trends Anal. Chem., 2023, 169, 117381 CrossRef CAS.
- C. Sanz-Lázaro, N. Casado-Coy and A. Beltrán-Sanahuja, Sci. Total Environ., 2021, 756, 143978 CrossRef PubMed.
- G. O. Lara-Topete, J. D. Castanier-Rivas, M. F. Bahena-Osorio, S. Krause, J. R. Larsen, F. J. Loge, J. Mahlknecht, M. S. Gradilla-Hernández and M. E. González-López, Sci. Total Environ., 2024, 944, 173735 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |
