Illuminating liver fibrosis: recent progress in the design and applications of highly sensitive fluorescent probes
Yutong
Lv†
ab,
Zhe
Ma†
b,
Yue
Chong†
a,
Zhenlong
Wang
*a,
Li
Xue
*a and
Fu
Wang
 *ab
*ab
aDepartment of Urology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi 710004, China. E-mail: zhenlongw2001@xjtu.edu.cn; xueli1979@xjtu.edu.cn; wangfu@xjtu.edu.cn
bInstitute of Medical Engineering, School of Basic Medical Sciences, Xi'an Jiaotong University, Xi'an 710061, China
First published on 5th September 2025
Abstract
Liver fibrosis involves excessive, disorganized extracellular matrix deposition in the liver, critically driving progression from chronic liver disease to cirrhosis and determining patient prognosis. Although histological examination of liver tissue biopsies continues to serve as the most reliable diagnostic approach, the development of precise detection methods remains crucial for enabling timely therapeutic interventions and improving patient management. Recent advances in fluorescent probes have transformed the detection of liver fibrosis, enabling real-time, non-invasive visualization of biomarkers and microenvironmental changes. Based on its design strategy, targeted objects and functional characteristics, this review systematically classifies state-of-the-art fluorescent probes into five categories: probes directly targeting liver fibrosis markers, enzyme-activated probes, microenvironment-responsive probes, intracellular targeting probes, and multifunctional theranostic probes. Among them, near-infrared II (NIR-II, 1000–1700 nm) imaging and genetically encoded probes boost molecular precision and resolution, yet clinical application faces challenges from limited tissue penetration and poor biocompatibility. Consequently, future research in this field will concentrate on the development of NIR II probes, the discovery of biomarkers in biofluids, and the design of new therapeutic interventions. By elucidating design principles and applications, this review aims to bridge the gap between molecular innovation and clinical practice, ultimately advancing precision medicine for liver fibrosis.
Wider impactThe development and application of fluorescent probes for monitoring liver fibrosis hold significant potential to transform the diagnosis and management of chronic liver diseases. By enabling non-invasive, real-time, and high-resolution detection of fibrotic progression, these probes could reduce the reliance on invasive biopsies, improving patient comfort and accessibility to early diagnosis. This technology may also facilitate personalized treatment strategies by allowing dynamic assessment of therapeutic responses, ultimately improving clinical outcomes. Beyond hepatology, advances in molecular imaging could inspire similar approaches for other fibrotic diseases (e.g., pulmonary or renal fibrosis), fostering interdisciplinary innovation. Widespread adoption could lower healthcare costs through early intervention and reduce the global burden of end-stage liver complications, aligning with global health priorities like the WHO's goal to combat non-communicable diseases. |
1. Introduction
Liver fibrosis represents a progressive pathological condition marked by abnormal accumulation of extracellular matrix (ECM) proteins, serving as a key transitional stage in various chronic liver disorders, including viral hepatitis, alcohol-associated liver disease, and non-alcoholic steatohepatitis (NASH).1 Globally, chronic liver diseases impact over 1.5 billion individuals, and without early therapeutic measures, fibrotic progression can lead to irreversible cirrhosis or hepatocellular carcinoma.2–4Specifically, the pathophysiological core of hepatic fibrosis lies in the abnormal repair response induced by chronic liver injury, involving dynamic interactions among multiple cellular and molecular mechanisms. Chronic liver injury (e.g., viral hepatitis, alcohol, or metabolic factors) first triggers an inflammatory response, prompting Kupffer cells to release pro-inflammatory cytokines (e.g., TNF-α and IL-6) and chemokines that recruit neutrophils and monocytes, establishing a chronic inflammatory microenvironment.5 During this process, the central role of hepatic stellate cells (HSCs) in fibrosis is evidenced by the observation that their activation is pivotal in driving ECM overproduction through complex signaling pathways, such as transforming growth factor-βeta/Sma- and Mad-related proteins and platelet-derived growth factor BB/platelet-derived growth factor receptor βeta (TGF-β/Smad and PDGF-BB/PDGFR-β).6–11 Under injury signaling, quiescent hepatic stellate cells (HSCs) transition into a myofibroblast-like phenotype, massively secreting extracellular matrix (ECM) components (e.g., type I collagen and fibronectin) while maintaining activation through autocrine loops.12 Concurrently, ECM metabolic imbalance manifests as suppressed matrix metalloproteinase (MMP) activity and upregulated tissue inhibitors of metalloproteinases (TIMPs), leading to insufficient collagen degradation and abnormal deposition, ultimately forming fibrous septa.13 Additionally, oxidative stress and endoplasmic reticulum stress exacerbate HSC activation via the reactive oxygen species-nuclear dactor kappa-B (ROS-NF-κB) pathway, while vascular remodeling (e.g., sinusoidal capillarization) further promotes hypoxia and fibrotic progression.14 Despite advances in understanding the mechanisms of fibrosis, early diagnosis and accurate staging remain formidable challenges due to the silent nature of early fibrosis and the limitations of conventional diagnostic tools.
The clinical diagnosis and monitoring of liver fibrosis continue to evolve with technological advancements, yet current methods face multiple limitations, driving the exploration of novel imaging techniques. Liver biopsy, as the traditional gold standard, is difficult to repeat due to invasive risks (e.g., hemorrhage incidence ∼0.4%, mortality 0.11%15) and sampling errors16 (assessing only ∼1/50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of the liver parenchyma), particularly limiting its use in pediatric patients or those with coagulation disorders. Transient elastography (e.g., FibroScan®), while non-invasive and convenient, suffers from insufficient sensitivity for early-stage fibrosis (F1–F2) and is significantly influenced by obesity, hepatic steatosis, and operator experience.17 Serum biomarkers (e.g., FIB-4 and APRI) can indicate fibrosis risk but lack spatial resolution to localize lesions and are prone to interference from age, platelet count, and extrahepatic diseases.18 These limitations underscore the pressing need for non-invasive real-time diagnostic techniques that can capture dynamic molecular and microenvironmental changes in fibrotic livers.2 In clinical settings, the application of fluorescent probes is progressively transitioning from laboratory research to practical diagnostic and therapeutic tools, demonstrating core value across multiple domains including early disease detection, intraoperative navigation, therapeutic monitoring, and pathological analysis.
000 of the liver parenchyma), particularly limiting its use in pediatric patients or those with coagulation disorders. Transient elastography (e.g., FibroScan®), while non-invasive and convenient, suffers from insufficient sensitivity for early-stage fibrosis (F1–F2) and is significantly influenced by obesity, hepatic steatosis, and operator experience.17 Serum biomarkers (e.g., FIB-4 and APRI) can indicate fibrosis risk but lack spatial resolution to localize lesions and are prone to interference from age, platelet count, and extrahepatic diseases.18 These limitations underscore the pressing need for non-invasive real-time diagnostic techniques that can capture dynamic molecular and microenvironmental changes in fibrotic livers.2 In clinical settings, the application of fluorescent probes is progressively transitioning from laboratory research to practical diagnostic and therapeutic tools, demonstrating core value across multiple domains including early disease detection, intraoperative navigation, therapeutic monitoring, and pathological analysis.
In this context, near-infrared (NIR) imaging has emerged as a research hotspot due to its unique ability to visualize molecular and functional activities. Compared to ultrasound elastography, NIR imaging employs targeted probes (e.g., MMP-13-activated fluorescent probes) to specifically label collagen synthesis or degradation, providing dynamic metabolic insights beyond static stiffness measurements. While NIR imaging is inferior to magnetic resonance elastography (MRE) in penetration depth (<2 cm) and whole-organ imaging, its micrometer-level resolution enables precise localization of early fibrotic lesions and differentiation between reversible fibrosis and mature scars (e.g., using LOXL2-targeted probes to mark cross-linked collagen). Additionally, NIR's radiation-free nature and low cost make it promising for long-term follow-up or intraoperative navigation (e.g., guiding precision liver biopsy). However, clinical translation of NIR imaging remains constrained by unvalidated probe targeting efficiency, signal attenuation in deep tissues, and the lack of standardized quantification methods.
Future breakthroughs should focus on multimodal technology integration, such as combining NIR with ultrasound elastography to complement molecular specificity with anatomical resolution, or developing NIR-II window probes (1000–1700 nm) to enhance penetration depth. Concurrently, advancing probes targeting key fibrotic markers (e.g., integrin αvβ3 and PDGFRβ) into clinical trials will accelerate the transition of NIR from the lab to the bedside, ultimately enabling precise, dynamic, and personalized management of liver fibrosis (Table 1).
| Technology | Spatial resolution | Molecular specificity | Penetration depth | Dynamic monitoring | Clinical applicability |
|---|---|---|---|---|---|
| Ultrasound elastography | ∼1–5 mm | None | ∼5–8 cm | No | High (widely used) |
| Magnetic resonance elastography (MRE) | ∼0.5–1 mm | Low | Whole-body | No | Medium (requires MRI equipment) |
| CT/MRI | ∼0.5–1 mm | Low | Whole-body | No | High |
| Near-infrared (NIR) imaging | ∼10–100 μm | High | <2 cm | Yes | Low (preclinical stage) |
This review systematically classifies advances in fluorescent probes for liver fibrosis over the past five years, highlighting their design principles, biomedical applications and translational potential. The probes are classified according to detection targets. Following this, we scrutinize key gaps: biocompatibility optimization and challenges in clinical validation. To conclude, we propose future research directions advocating for collaboration across disciplines to bridge the gap between laboratory findings and clinical practice faster.
2. Liver fibrosis fluorescence targeting different detection targets
Liver fibrosis is driven by dynamic changes in molecular and cellular components, so detecting dynamic changes in these components can be a key target for probe design.1 Depending on the detection goal, this review will describe five classes of fluorescent probes: probes directly targeting fibrosis biomarkers, enzyme-activated probes responding to fibrosis signals, microenvironmental-responsive probes, intracellular targeting probes, and multifunctional theranostic probes. This section will systematically review the design principles and applications of these probes (Table 2).2.1. Direct targeting of fibrosis biomarkers
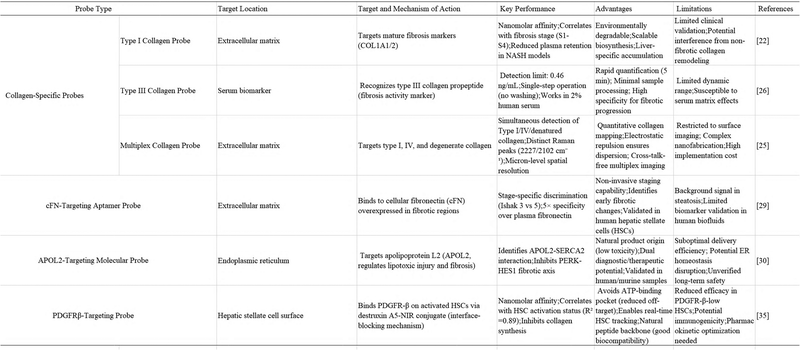
Liver fibrosis is characterized by two critical pathological processes: the activation of hepatic stellate cells (HSCs) and the dynamic reorganization of extracellular matrix (ECM) components.19 The direct targeting of fibrosis biomarkers, including collagen isoforms, cell fibronectin (cFN) and hematopoietic stem cell surface markers, can facilitate the accurate location of fibrotic lesions. These probes typically rely on ligand–receptor interaction or antibody–antigen binding, exhibiting high specificity. However, it is crucial to optimize the affinity and biodistribution for optimal performance. | ||
| Fig. 1 Targeted imaging of type I collagen in liver fibrosis using the Col1-TRAP fluorescent probe. (A) Schematic of the Col1-TRAP fluorescent probe. (B) Representative imaging of mouse liver. The figures were adapted from Wang, A.L., et al., 202422 with permission from Elsevier, copyright 2024. | ||
The accumulation of type I collagen, occurring from the early (S1) to the late (S4) stages of fibrosis, drives the formation of fibrous septa, thereby exacerbating the fibrotic state.23,24 Concurrently, the rise in type IV collagen results in the disruption of liver structure throughout the fibrotic stage. The presence of denatured collagen (a marker of collagen degradation and turnover) provides further evidence of the continued progression of fibrosis. Linge Nian et al.25 engineered peptide-based SF-I, SF-IV, and SF-D probes, which are tailored for targeted imaging of type I, type IV and degenerate collagen. These probes are capable of high spatial resolution fingerprinting of collagen in liver fibrosis. The probes comprise silver nanoparticles (Ag NPs), Raman reporter genes, and FAM-labelled collagen-targeting peptides. They exhibit distinct Raman peaks at 2227, 2154, and 2102 cm, enabling multiplexed imaging without cross-talk. The results of fluorescence-guided SERS imaging demonstrate the capacity of the method to identify, localize and quantify collagen, thereby offering new insights into the role of collagen in the development of liver fibrosis. The incorporation of Asp residues is intended to maintain the negative charge state of the CTP, which induces electrostatic repulsion and thus ensures significant dispersion and stability of the SERS probe.
Pre-type III collagen N-terminal peptide (P-III-NP), a biomarker linked to fibrotic progression in the liver and heart, can be detected using a novel single-step fluorescent immunosensor developed by Yi et al.26 Their approach utilized a quenchbody (Q-body) system, where an anti-P-III-NP single-chain variable fragment (scFv) was engineered with a Cys-tag for site-specific fluorophore conjugation. To generate the scFv, total mRNA was isolated from hybridoma cells, followed by cDNA synthesis, PCR amplification of the variable region, and subsequent sequencing. Recombinant expression in E. coli Shuffle T7 yielded 9.8 mg L−1 of the purified scFv. Fluorescence labeling was achieved via maleimide–thiol coupling, with different spacer arm lengths tested to optimize signal response. Among the tested configurations, the TAMRA-C6-conjugated Q-body exhibited antigen-dependent fluorescence quenching, enabling sensitive detection of P-III-NP—with a limit of detection (LOD) of 0.46 ng mL−1 in buffer and 1.0 ng mL−1 in 2% human serum. This one-step immunoassay eliminates the need for washing steps, offering rapid and efficient quantification of the procollagen type III N-terminal peptide, a key indicator of fibrotic disease.
Peptide-based probes SF-I, SF-IV, and SF-D are engineered for specific targeting of type I collagen, type IV collagen, and denatured collagen, respectively. Utilizing distinct Raman signatures, these probes enable high spatial resolution multiplexed imaging of collagen subtypes without signal crosstalk. Crucially, fluorescence-guided SERS imaging with these probes allows identification, localization, and quantification of collagen alterations in fibrotic livers, revealing characteristic changes compared to healthy tissue architecture.
Recent advances in molecular imaging have led to the development of novel fluorescence probes utilizing a nucleic acid aptamer (ZY-1) specific to intracellular fibronectin (cFN), a critical ECM component whose expression markedly increases during hepatic fibrogenesis.28In vitro studies using human hepatic stellate cells (HSCs) confirmed ZY-1's high binding specificity for cFN. Building upon this finding, investigators engineered multiple ZY-1-conjugated fluorescent markers and evaluated their dynamic imaging capabilities in murine models with carbon tetrachloride-induced hepatic fibrosis at various progression stages (Fig. 2). As shown in Fig. 2A, upregulation of cellular fibronectin (cFN) enables specific binding of the DNA aptamer ZY-1 to activated LX-2 hepatic stellate cells in vitro. Through real-time imaging and area-under-the-curve (AUC) analyses, ZY-1 fluorescent probes achieve early detection and staging of liver fibrosis by distinguishing CCl4-induced murine models from healthy controls. Notably, these ZY-1-derived probes achieved unprecedented discrimination between initial fibrotic changes (stage 6 of Ishak 3) and severe fibrosis (stage 6 of Ishak 5), representing a significant breakthrough in fibrosis staging technology. This foundational research establishes ZY-1-based imaging agents as potential clinical tools for timely detection and progression monitoring of liver fibrosis, offering new possibilities for diagnostic approaches in hepatic and other fibrotic disorders.29
 | ||
| Fig. 2 Fluorescent probes conjugated to the ZY-1 aptamer allow specific visualization of intracellular fibronectin (cFN) for assessment of liver fibrosis progression. (A) Real-time imaging and AUC analysis of ZY-1-based fluorescent probes in normal mice and CCl4-induced liver fibrosis mice allow early detection and staging of liver fibrosis. (B) and (C) In vivo molecular imaging of liver fibrosis with fluorescent probes and ex vivo imaging of the corresponding major organs. The figures were adapted from Ge, M., et al. 202429 with permission from Elsevier, copyright 2024. | ||
 | ||
| Fig. 3 Targeted imaging of APOL2 in liver fibrosis using DP fluorescent probes. (A) Schematic design of DP fluorescent probes. (B) and (C) Immunofluorescence images showing co-localization of APOL2 (green) and α-SMA (red) in hepatic tissues from both murine and human samples. The figures were adapted from Gan, L., et al. 202530 with permission from Springer Nature, copyright 2025. | ||
Researchers have explored the potential of destruxin A5 as a fluorescent probe for HSCs, due to its unique targeting properties.35 Coupling destruxin A5 with near-infrared fluorescent moieties allows for the construction of molecular probes capable of real-time tracing of activated HSCs. These probes have three advantages: (1) high specificity: based on the interfacial blocking mechanism, the probes can accurately identify activated HSCs with high PDGFR-β expression, avoiding the non-specific labelling of hepatic parenchymal cells; (2) dynamic monitoring: combining with the in vivo imaging technology, the spatial distribution of HSCs and the changes in activation status can be monitored and can be visualized in real time in fibrotic livers; (3) evaluation of the therapeutic efficacy: through the probes, the signal intensity and the degree of fibrosis can be quantified. Quantitative correlation between signal intensity and the fibrosis degree, and the therapeutic response of antifibrotic drugs (e.g., destruxin A5 derivatives) can be objectively assessed. In addition, the natural cyclic peptide backbone of destruxin A5 gives the probe good biocompatibility, which provides the basis for its clinical translation.
Despite the encouraging indications, further development of the optimization of destruxin A5 probes is required to address the challenges associated with pharmacokinetic characterization and multimodal imaging integration. The identification of destruxin A5 represents a significant advancement, offering a highly selective instrument for the study of PDGF-BB/PDGFR-β signaling. Furthermore, it provides a novel avenue for translating molecular mechanisms into clinical applications, facilitating targeted diagnosis and treatment of liver fibrosis.
2.2. Fluorescent probes based on enzyme activity
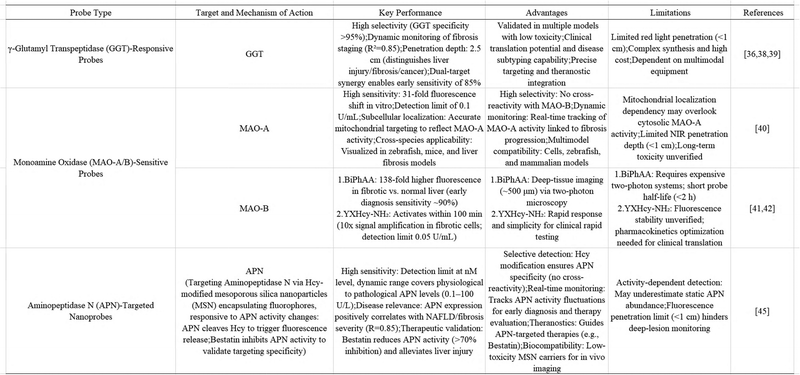
The progression of liver fibrosis is closely related to the abnormal regulation of specific enzyme activities. These enzymes play a key role in extracellular matrix remodeling, oxidative stress and inflammatory signaling pathways. Fluorescent probes based on enzyme activity can detect enzyme activity changes in the pathological microenvironment in real time and in situ by designing enzyme-responsive molecular switches, providing dynamic molecular information for disease staging and treatment evaluation. This section systematically reviews the fluorescent probes developed in recent years for liver fibrosis-related enzymes, focusing on their design principles and application breakthroughs in preclinical models. By combining quantitative imaging data with histopathological verification, these probes not only reveal the role of temporal and spatial heterogeneity of enzyme activity in fibrosis, but also lay a molecular foundation for the development of enzyme-targeted therapy (Table 3).The Rho-GGT probe demonstrated effective detection of endogenous GGT activity in both LX-2 cell cultures and zebrafish models. Importantly, this imaging agent successfully monitored dynamic GGT changes in murine models of both acute hepatic damage and fibrotic liver disease. When applied to pharmacologically treated fibrotic mice, Rho-GGT provided quantitative assessment of GGT activity that correlated with established clinical parameters for fibrosis staging and therapeutic monitoring. Notably, the probe's imaging performance in hepatic tissue shows significant translational potential, as it can be integrated with conventional serum biomarkers and diagnostic modalities. Histopathological analysis confirmed consistent fluorescence patterns and morphological features between liver specimens from experimental mice and human patients with fibrosis (Fig. 4). This successful validation suggests that Rho-GGT enables accurate spatial mapping of GGT activity in hepatic injury, offering promising applications for both preclinical research and clinical diagnostics.
 | ||
| Fig. 4 Fluorescent probe Rho-GGT for the detection of GGT. (A) Schematic design of Rho-GGT. (B) In the fibrosis intervention study, silybin was administered orally to model mice, while glutamlhydrine and Rho-GGT were delivered via injection. (C) Representative hepatic tissue sections from normal, fibrotic, and treated groups were subjected to histological examination and fluorescence imaging. The figures were adapted from Wang, K., et al. 202336 with permission from Elsevier, copyright 2023. | ||
For further accurate detection of GGT, Wang et al.38 developed the probe ETYZE-GGT, which achieved bimodal imaging in far-infrared fluorescence (FL) and photoacoustic (PA) modes in several hepatocellular carcinoma (HCC)-related models. The selection of typical γ-glutamyl recognition groups and benzo-indole-xanthene signalling reporter genes ensured stability and adaptability to a range of conditions. The pattern of GGT mutation differs in cases of hepatocellular injury, liver fibrosis and hepatocellular carcinoma (HCC). In general, GGT levels in the context of liver injury exhibit a rapid increase to a certain value, while in liver fibrosis, they display relatively high fluctuations. In hepatocellular carcinoma, however, they tend to remain at relatively high levels. By conceptualizing the detection of GGT as a dynamic indicator rather than a simple value, it is possible to distinguish between these three processes. Furthermore, the researchers extended the probe's conjugate system to increase the emission wavelength, thereby enhancing the depth of penetration and potentially rendering it suitable for human tissue imaging.
While optical imaging probes show promise for hepatic fibrosis detection, their clinical application is often limited by poor tissue penetration depth, restricting in vivo imaging performance. To overcome this limitation, Miao and colleagues39 engineered a dual-modality probe combining activatable fluorescence and photoacoustic imaging capabilities for specific fibrosis visualization. The molecular architecture incorporates a near-infrared thioxanthene-semicarbocyanine fluorophore conjugated with: a γ-glutamyl transpeptidase (GGT)-cleavable moiety and a cyclic RGD peptide for integrin targeting. This innovative design confers two critical functions: selective accumulation in fibrotic tissue through integrin binding, and subsequent activation of both fluorescent and photoacoustic signals upon GGT-mediated uncaging. Such target-responsive signal amplification enables accurate spatial mapping of fibrotic lesions, demonstrating the probe's potential for non-invasive diagnosis of early-stage hepatic fibrosis. This work establishes a novel design paradigm for developing dual-targeted molecular imaging agents for fibrotic diseases.
Subsequently, Tang et al.41 developed a TP fluorescence imaging probe to characterize monoamine oxidase-B (MAO-B) BiPhAA in vivo. The use of TP fluorescence imaging revealed that liver tissue from fibrotic mice exhibited fluorescence at a level 138 times greater than that observed in normal tissue, thereby enabling the differentiation between the two types of mice. The developed BiPhAA probe demonstrates superior sensitivity and precision as a fluorescent imaging agent for early-stage fibrosis detection. Additionally, this molecular tool shows potential for elucidating pathogenic mechanisms involved in hepatic fibrogenesis.
Furthermore, Mingzhao Sun et al.42 developed an enzyme-regulated activated fluorescent probe, YXHcy-NH2, for the detection of monoamine oxidase-B activity in living cells and in vivo (Fig. 5). The developed probe demonstrates excellent biocompatibility, target selectivity, and detection sensitivity. Upon activation by MAO-B within 100 minutes, it generates strong fluorescence emission at its characteristic wavelength. Experimental results revealed a 10-fold increase in the fluorescence signal in fibrotic LX-2 cells compared to healthy controls, along with a 5-fold enhancement in liver tissues from fibrotic mice relative to normal specimens. This work establishes a straightforward, rapid, and sensitive approach for in situ MAO-B imaging in living systems, offering promising potential for early-stage liver fibrosis diagnosis.
 | ||
| Fig. 5 The YXHcy-NH2 optical probe enables targeted MAO-B detection in fibrotic liver tissue. (A) Structural composition and operational mechanism of the molecular probe. (B) Comparative in vivo fluorescence imaging of MAO-B expression in mice at varying fibrosis severity levels. The figures were adapted from Sun, M., et al. 202442 with permission from Elsevier, copyright 2024. | ||
 | ||
| Fig. 6 Hcy-APN probe for liver fibrosis detection. (A) Detection mechanism of Hcy-APN@MSN against APN. (B) Imaging of APN levels in mouse models of liver fibrosis. The figures were adapted from Sun, X., et al. 202445 with permission from American Chemical Society, copyright 2024. | ||
2.3. Microenvironment-responsive probes
The liver fibrotic microenvironment is a dynamic interplay of physical and chemical alterations that drive disease progression.31,46 Physical changes (e.g., increased tissue stiffness and organelle structural remodeling) and chemical imbalances (e.g., oxidative/reductive stress and enzyme dysregulation) collectively serve as hallmarks of fibrosis.47,48 Probes responsive to these microenvironmental cues not only enhance diagnostic accuracy but also provide mechanistic insights into fibrogenesis (Table 4).Physical properties of the fibrotic liver, such as elevated viscosity and organelle deformation, are critical indicators of ECM deposition and cellular dysfunction.49,50 Probes targeting these biomechanical parameters enable non-invasive mapping of fibrosis severity and spatial heterogeneity. Despite their promise, challenges remain in achieving tissue-specific targeting and minimizing interference from non-fibrotic pathologies (e.g., inflammation-induced edema). Below, we discuss three key subcategories of physical microenvironment sensors.
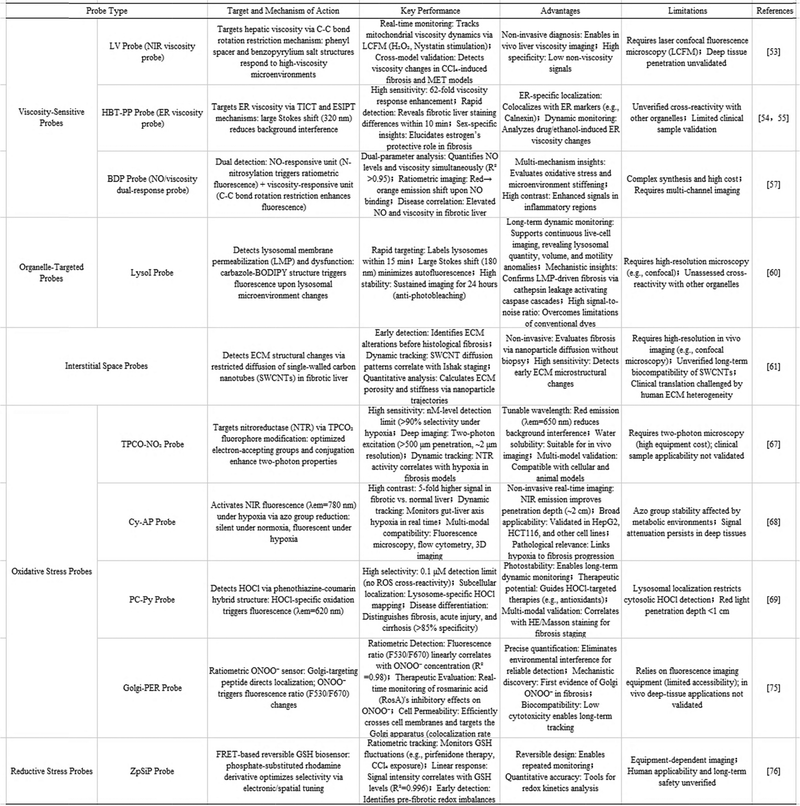
Elevated endoplasmic reticulum viscosity shows significant correlation with liver fibrosis development, suggesting its potential as a clinical biomarker.54 The research team has developed HBT-PP, the first viscosity-responsive near-infrared (NIR) fluorescent probe targeting the ER, for use in studying the pathogenesis of liver fibrosis. The probe combines two distinct mechanisms, namely twisted intramolecular charge transfer (TICT) and excited-state intramolecular proton transfer (ESIPT), with a selective response to viscosity (∼62-fold) and an exceptionally large Stokes shift (∼320 nm). The HBT-PP probe demonstrates selective localization to the endoplasmic reticulum, facilitating real-time monitoring of viscosity changes induced by pharmaceutical compounds or ethanol exposure in cellular systems. Notably, compared to healthy liver, this imaging tool has revealed sex-dependent variations in hepatic fibrogenesis and provided visual evidence for estrogen's protective effects against disease progression. Remarkably, fibrotic liver tissue showed differential staining patterns within just 10 minutes of HBT-PP application, suggesting its utility for rapid clinical assessment of fibrosis severity. These findings collectively highlight HBT-PP's significant value as a research tool for studying ER-related pathophysiological mechanisms.55
Emerging research suggests that abnormal nitric oxide (NO) concentrations and elevated hepatic viscosity could serve as diagnostic indicators for liver fibrosis.56 In response to this finding, Song et al. (2023) designed a fluorescence ratio-based sensing probe, BDP, to simultaneously detect upregulated NO and microenvironmental viscosity for fibrosis assessment. As depicted in the figure, the probe incorporates an aromatic secondary amine with high electron density as the NO-responsive unit, where N-nitrosylation modulates the intramolecular charge transfer (ICT) process for ratiometric imaging (Fig. 7A). At low viscosity, fluorescence quenching occurs due to unrestricted C–C bond rotation between the N-methylaniline moiety and the BODIPY fluorophore. However, in the highly viscous fibrotic liver microenvironment, restricted molecular rotation enhances fluorescence emission. Upon NO binding, the probe undergoes rapid conversion from a secondary amine to an N-nitrosamine, accompanied by a spectral shift from red to orange emission. Comparative imaging analysis revealed that fibrotic liver tissues from murine models exhibited pronounced inflammatory infiltration, along with significantly intensified NO fluorescence signals and higher viscosity than healthy controls (Fig. 7B).57
 | ||
| Fig. 7 Dual-parameter detection using the ratiometric probe BDP for nitric oxide and viscosity monitoring in hepatic fibrosis. (A) Working principle of the BDP probe, demonstrating its simultaneous response to NO concentration and microenvironmental viscosity changes. (B) Comparative fluorescence imaging analysis of NO levels in hepatic tissues from healthy control mice and fibrotic model mice, with the corresponding ex vivo organ distribution profiles. The figures were adapted from Han, S., et al. 202357 with permission from Elsevier, copyright 2023. | ||
Key findings include: (1) SWCNT trajectory analysis revealed decreasing exploration distances correlating with disease progression. (2) The restricted nanotube mobility resulted from spatial confinement rather than changes in diffusion kinetics. (3) This methodology demonstrated superior sensitivity for early fibrosis detection compared to conventional histopathology. This nanotechnology-based approach provides new insights into the biophysical microenvironmental changes accompanying fibrogenesis, offering potential for both early diagnosis and fundamental studies of disease mechanisms.58
2.4. Intracellular targeting probes
 | ||
| Fig. 8 BODIPY carbazole derivative probe for lysosomal imaging. (A) Schematic illustration of BODIPY. (B) Confocal images of LysoI-labeled lysosomes and a commercial nuclear labeling dye (Syto63) co-staining in normal LX2 cells and LX2 cells with liver fibrosis induced by TGF-β. The figures were adapted from Su, L., et al. 202561 with permission from American Chemical Society, copyright 2025. | ||
2.4.2.1. Oxidative stress probes. The pathogenesis and progression of hepatic fibrosis are closely linked to the propagation of reactive oxygen species (ROS) cascades. The superoxide anion (O2−), the primary ROS, is generated at mitochondrial and plasma membranes by NADPH oxidase (NOX). This species drives NF-κB pathway activation, inducing the release of inflammatory mediators.9 Its downstream metabolite hydrogen peroxide (H2O2) acts as a messenger in TGF-β signaling, directly stimulating collagen synthesis in hepatic stellate cells (HSCs).65 Within iron-overloaded microenvironments, H2O2 undergoes Fenton reactions yielding highly reactive hydroxyl radicals (˙OH), which provoke DNA strand breaks and lipid peroxidation in hepatocytes.66 Furthermore, neutrophil-derived hypochlorous acid (HOCl) and inflammation-site peroxynitrite (ONOO−) contribute to protein halogenation and cellular necrosis, establishing a self-perpetuating cycle. Recent advances in molecular probe technology enable precise mapping of ROS dynamics, marked by key developments: the genetically encoded probe SOx-FRP67 leverages fluorescence resonance energy transfer (FRET) to achieve macrophage-specific O2− imaging. Its 0.5 μm spatial resolution demonstrated that metformin suppresses O2− generation by 60% through NOX4 inhibition.
However, clinical translation faces three persistent challenges: (a) Limited specificity: certain chemical probes (e.g., boronate-based) fail to discriminate between H2O2, ONOO−, and HOCl.68 (b) Inadequate dynamic range: pathological ROS concentrations can reach millimolar levels, exceeding the typical probe saturation threshold of 500 μM. (c) Metabolic instability: reduced CYP450 activity in fibrotic patients accelerates degradation of cyanine-benzopyran-based probes.69
The research team led by Zhang developed a pair of wavelength-tunable fluorescent dyes by modifying TPQL structures with electron-accepting groups and expanded conjugation systems.70,71 Among these, TPCO2 shows particular promise for biological imaging applications due to its bright red emission, favorable two-photon characteristics, and good water solubility - features that collectively enable deep-tissue visualization with superior signal discrimination. Based on the excellent photophysical characteristics of TPCO2, two distinct fluorescent probes were developed. Among them, TPCO–NO2 demonstrates remarkable selectivity and sensitivity toward nitroreductase (NTR), allowing for precise monitoring of NTR expression levels in cellular models under varying hypoxic conditions. Notably, this probe has been effectively utilized for high-contrast imaging of NTR activity in liver fibrosis models, providing a valuable tool for assessing hypoxia severity during fibrotic progression.72
The research team led by Li et al. developed a novel hypoxia-responsive fluorescent probe named Cy-AP, which integrates a semi-cyanine fluorophore with an azo-based recognition moiety. Under normoxic conditions, this probe remains non-fluorescent. However, in hypoxic environments, the azo group undergoes reductive cleavage to form an amino group, triggering strong fluorescence emission. In vitro experiments revealed that Cy-AP displays excellent sensitivity and specificity toward sodium dithionite (Na2S2O4). This probe was successfully applied for hypoxia detection in four distinct cell lines (HepG2, HCT 116, HeLa, and MCF-7) using fluorescence microscopy, flow cytometry, and three-dimensional imaging techniques. Additionally, owing to its near-infrared (NIR) emission properties, Cy-AP enabled real-time monitoring of hypoxic conditions in mouse models of liver fibrosis. Notably, this probe provided the first experimental evidence supporting the functional existence of the “gut-liver axis” in vivo through hypoxia tracking. As the first NIR fluorescent probe specifically designed for hypoxia detection in enterohepatic studies, Cy-AP represents a powerful tool for investigating the pathological mechanisms underlying liver fibrosis and may contribute to the development of improved therapeutic strategies for liver-related diseases.73
In 2023, Yuan and colleagues introduced an innovative strategy for tracking liver fibrosis development through a fluorescent probe specifically targeting hypochlorous acid (HOCl).74 The designed probe, based on a phenothiazine-coumarin hybrid structure (PC-Py), displayed outstanding detection capabilities, including high sensitivity and selectivity toward HOCl, robust photostability, and low cellular toxicity. Notably, PC-Py could accurately measure trace amounts of HOCl and monitor minute fluctuations at intracellular HOCl concentrations. These results highlight HOCl's role as a potential diagnostic marker for distinguishing between progressive liver fibrosis and acute drug-induced hepatic damage, offering valuable insights for early-stage fibrosis identification and therapeutic intervention. The probes were predominantly localized within lysosomes, rather than mitochondria. Concurrently, the findings were corroborated by trichrome staining in conjunction with hematoxylin–eosin and Masson, which elucidated the structural alterations and progression of liver fibrosis in tissue sections. The observations indicate that HOCl has the potential to serve as a biomarker for differentiating between liver fibrosis, acute liver injury, and cirrhosis. This makes the probe an appropriate tool for monitoring HOCl-induced cellular alterations and a promising avenue for the diagnosis and treatment of liver-related disorders.
Accumulation of peroxynitrite (ONOO−), a potent oxidizing agent, has been demonstrated to contribute to the pathogenesis of hepatic fibrosis, as supported by multiple studies.75–79 To enable precise detection and evaluation of treatment efficacy in fibrotic liver disease, Zhang Tianao and colleagues80 designed a selective ratiometric fluorescence sensor named Golgi-PER, which exhibits remarkable sensitivity in monitoring ONOO− levels (Fig. 9). The probe exhibits favorable biocompatibility, optimal cell membrane permeability and effective Golgi-targeting capability, thereby enabling its utilization for ratiometric monitoring of ONOO− fluctuations within the Golgi apparatus and the identification of polyphenol inhibitors in living cells. Concurrently, the therapeutic response of liver fibrosis to rosmarinic acid (RosA) was meticulously visualized for the first time by overexpression of Golgi ONOO− in liver fibrosis using Golgi-PER as a probe. Consequently, this probe can be an effective and useful tool for investigating the occurrence, development and treatment of liver fibrosis.
 | ||
| Fig. 9 Golgi-PER probe for ONOO− imaging. (A) Detection principle of Golgi-PER, a ratiometric fluorescent probe targeting ONOO− in the Golgi apparatus. (B) Tracking peroxynitrite (ONOO−) dynamics in liver fibrosis models using Golgi-PER. The figures were adapted from Zhang, T., et al. 202480 with permission from Elsevier, copyright 2024. | ||
Oxidative stress serves as a core pathological mechanism driving disease progression in hepatic fibrosis, involving an imbalance of multiple reactive oxygen species (ROS). Beyond the previously detailed hypochlorous acid (HOCl) and peroxynitrite (ONOO−), other critical ROS—including hydrogen peroxide (H2O2), hydroxyl radical (˙OH), superoxide anion (O2˙−), and singlet oxygen (1O2)—play significant roles in hepatic stellate cell (HSC) activation, extracellular matrix deposition, and inflammatory cascades.81 The aberrant accumulation of these ROS exacerbates fibrosis by promoting lipid peroxidation, DNA damage, and collagen cross-linking. Recent advances in highly specific fluorescent probes have significantly enhanced monitoring capabilities for these molecular dynamics, providing new tools for early diagnosis and mechanistic studies of hepatic fibrosis. The following sections systematically elaborate on the design principles, application performance, and limitations of various ROS probes.
H2O2, a key signaling molecule in hepatic fibrosis, exhibits elevated concentrations directly correlated with TGF-β pathway activation and HSC stimulation. For instance, the IR-990 probe developed by Tian et al.82 achieved triple optimization through molecular engineering: emission of intense NIR-II fluorescence at 990 nm; generation of a large Stokes shift (200 nm) upon reaction with H2O2, effectively circumventing tissue autofluorescence; quantitative detection range spanning 2–250 μM with a low detection limit (0.59 μM), enabling precise capture of H2O2 fluctuations in pathological microenvironments. In validation studies, this probe not only successfully visualized endogenous H2O2 bursts in HepG2 cells but also achieved a high signal-to-noise ratio (11.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in an acetaminophen (APAP)-induced liver injury mouse model, representing the first demonstration of noninvasive DILI progression monitoring. Addressing the critical challenge of microenvironment specificity, the Z-1065 probe developed by Chen's team83 employs a mitochondrial targeting strategy. This probe exhibits excellent selectivity (<5% interference from other ROS) and biocompatibility (negligible cytotoxicity). Its innovative value lies in: confirmed response specificity through >80% fluorescence reduction upon treatment with the ROS scavenger N-acetylcysteine (NAC), successful spatiotemporal imaging of mitochondrial H2O2 bursts at the subcellular scale in an isoniazid (INH)-induced liver injury model, revealing spatial co-localization patterns between oxidative stress and hepatic damage.
1) in an acetaminophen (APAP)-induced liver injury mouse model, representing the first demonstration of noninvasive DILI progression monitoring. Addressing the critical challenge of microenvironment specificity, the Z-1065 probe developed by Chen's team83 employs a mitochondrial targeting strategy. This probe exhibits excellent selectivity (<5% interference from other ROS) and biocompatibility (negligible cytotoxicity). Its innovative value lies in: confirmed response specificity through >80% fluorescence reduction upon treatment with the ROS scavenger N-acetylcysteine (NAC), successful spatiotemporal imaging of mitochondrial H2O2 bursts at the subcellular scale in an isoniazid (INH)-induced liver injury model, revealing spatial co-localization patterns between oxidative stress and hepatic damage.
Hydroxyl radicals (˙OH) and superoxide anions (O2˙−) act as central mediators of hepatocyte lipid peroxidation and collagen cross-linking due to their potent oxidizing capacity and ultrashort half-lives (nanosecond scale).84,85 For ˙OH detection, Yu et al.86 reported the novel, highly selective turn-on fluorescent probe BIJ-H. Specific for ˙OH, its oxidative dehydrogenation generates the fluorescent product BIJ, emitting significantly enhanced near-infrared (NIR) fluorescence at 625 nm. BIJ-H exhibits stability under physiological conditions, rapid response kinetics, high selectivity for ˙OH, and a low detection limit (0.1379 μM). Studies demonstrate that BIJ-H enables sensitive, spatiotemporally resolved imaging of both exogenous and endogenous ˙OH in HepG2 cells. It successfully monitored ˙OH level fluctuations in acetaminophen (APAP)-induced liver injury mouse models, demonstrating significant potential for assessing oxidative stress in ˙OH-related pathologies. Targeting O2˙−, Wang et al.87 developed the activatable NIR fluorescent probe QL-3F, specifically designed for this key ROS. Its core advantage—a large Stokes shift—enables specific localization and response to site-specific O2˙− generation. At the cellular level, QL-3F precisely monitored endogenous O2˙− dynamics within hepatocytes (HepG2 cells), triggered by stimuli such as lipopolysaccharide (LPS), phorbol ester (PMA), and APAP-induced hepatotoxicity.
Singlet oxygen (1O2), generated in periductal fibrosis regions via bilirubin photosensitization, promotes myofibroblast differentiation and collagen deposition. The BAD probe,88 a BODIPY-anthracene derivative, activates NIR fluorescence (λem = 780 nm) upon 1O2-triggered [4+2] cycloaddition. It achieved the first spatially resolved imaging of periductal fibrosis, offering a new tool for studying primary sclerosing cholangitis mechanisms. However, future clinical translation requires integration with bile duct-targeted delivery systems to enhance specificity.
2.4.2.2. Reductive stress probes. Reductive stress in hepatic fibrosis manifests as a systemic imbalance of antioxidant molecular networks, with core mechanisms involving three key molecular entities: (a) diminished hydrogen sulfide (H2S) synthesis: mediated through downregulation of cystathionine γ-lyase (CSE) expression in hepatocytes,89 attenuating H2S-mediated suppression of the TGF-β/Smad3 pathway and accelerating collagen deposition. (b) Cysteine (Cys) depletion driving glutathione (GSH) cycle collapse: reduced Cys concentrations in portal hypertension models disrupt the γ-glutamyl cycle, inducing mitochondrial oxidative phosphorylation uncoupling and hepatocyte apoptosis.90 Concomitantly, a positive shift ≥20 mV in the Cys/cystine (CySS) redox potential (Eh) emerges as a biomarker for hepatic stellate cell (HSC) activation. (c) Loss of reduced thioredoxin (Trx) status: decreased Trx-(SH)2 in NASH models sustains apoptosis signal-regulating kinase 1 (ASK1) activation, promoting fibrosis via the p38-MAPK pathway.91
Advances in probe technology targeting these pathways focus on precise localization and dynamic monitoring. Li et al.92 developed the liver-targeted fluorescent probe series H2S-YL for in situ specific detection of hepatic hydrogen sulfide (H2S). Its core innovation integrates a cholic acid (CA) moiety as an active liver-targeting unit. Binding to hepatobiliary-specific transporters drives precise probe accumulation within liver tissue. This hepatobiliary-specific localization enables in situ, high-efficiency monitoring of dynamic H2S fluctuations in both physiological conditions and drug-induced liver injury (DILI) models, facilitating assessment of its correlation with DILI severity. Validation confirms that H2S-YL combines rapid response kinetics (<3 min), high sensitivity (LOD = 0.83 μM), and excellent H2S selectivity, enabling successful site-specific H2S imaging in both in vitro (hepatocytes) and in vivo (murine liver) settings.
Cui et al. developed ZpSiP, a novel reversible FRET-based biosensor capable of precise glutathione (GSH) quantification in living systems. The research team engineered this probe through strategic modification of phosphate-substituted rhodamine derivatives, optimizing both electronic configuration and steric hindrance to achieve selective reactivity. As shown in Fig. 10, the probe enabled ratiometric fluorescence monitoring of hepatic GSH fluctuations in fibrotic liver models under different experimental conditions, including pirfenidone treatment and graded CCl4 exposure. Key findings include: (1) an excellent linear response (R2 = 0.996) between probe signal intensity and tissue GSH concentrations, (2) successful demonstration of real-time GSH tracking in hepatic tissue, (3) validation of the probe's sensitivity for early detection of oxidative stress-related lesions. This work represents a significant advancement in molecular imaging, providing researchers with a robust tool for quantitative assessment of redox dynamics in biological systems.93
 | ||
| Fig. 10 ZpSiP probes for GSH imaging. (A) Synthetic routes and reaction principles of four ratiometric GSH-responsive probes. (B) Ratiometric fluorescence visualization of hepatic GSH dynamics in murine models. The figures were adapted from Li, N., et al. 202393 with permission from John Wiley and Sons, copyright 2023. | ||
Jiao et al.94 established the thioredoxin/thioredoxin reductase (Trx/TrxR) system as a pivotal molecular target in hepatic fibrosis progression, demonstrating its critical role for the first time.1 Their study revealed that the compound BS, a specific TrxR inhibitor, exhibited significant therapeutic efficacy in a carbon tetrachloride (CCl4)-induced mouse model of hepatic fibrosis. The primary cellular target of BS is hepatic stellate cells (HSCs), the central effector cells in fibrosis. BS effectively suppressed HSC activation. At the molecular level, BS acts on the transforming growth factor-β1/Smads (TGF-β1/Smads) signaling pathway. By precisely inhibiting signal transduction through this pathway, BS disrupts downstream profibrotic events, thereby ameliorating fibrotic severity. This study not only validated the Trx/TrxR system as a viable therapeutic target for antifibrotic intervention but also highlighted BS as a multifunctional compound. Its mechanism—targeted TrxR inhibition coupled with blockade of the TGF-β1/Smads pathway—provides a solid theoretical and experimental foundation for developing BS into a novel targeted therapeutic agent for clinical hepatic fibrosis management.
Clinical translation faces three principal challenges: (a) detection interference: hypoalbuminemia (<30 g L−1) increases free cysteine (Cys) concentration, inducing false positives in Cy-OFF-type fluorescence-quenching probes. (b) Insufficient dynamic range: pathological glutathione (GSH) fluctuations (0.1–10 mM) exceed the linear detection range (upper limit: 5 mM) of probes like ZpSiP. (c) Low delivery efficiency: liver-targeting efficiency of H2S prodrugs (e.g., NaHS) remains below 10%.
2.5. Multifunctional probes and emerging technologies
 | ||
| Fig. 11 pLVX-COL1A1-EGFP reporting system. (A) Schematic illustrating the pLVX-COL1A1-EGFP reporter construct and its functional mechanism. (B) Dose-dependent effects of dihydrotanshinone I and artesunate on COL1A1 and EGFP transcript levels in LX-2-CE cells. The figures were adapted from Wang, L., et al. 202396 with permission from Journal of Peking University (Health Sciences), copyright 2023. | ||
 | ||
| Fig. 12 Dual-functional NIR-II nanoprobe for liver fibrosis theranostics. (A) Design strategy of the NIR-responsive nanoprobe integrating NO gas therapy with real-time fibrosis monitoring through ratiometric fluorescence imaging. (B) In vivo NIR-II fluorescence visualization of therapeutic NO delivery in murine liver fibrosis models. The figures were adapted from Jiang, M., et al. 202397 with permission from Elsevier, copyright 2023. | ||
Facing the persistent clinical challenge of liver fibrosis lacking effective treatments, Ribera et al.98 developed an innovative targeted photothermal therapy (PTT) strategy using gold nanorods (GNRs). Their approach capitalizes on the critical role of activated hepatic stellate cells (HSCs) and the overexpression of PDGFRβ on their surface during fibrosis progression. The researchers engineered anti-PDGFRβ antibody-coated GNRs designed to specifically bind activated HSCs. Utilizing the natural liver tropism of nanoparticles combined with this active targeting, the constructs selectively accumulated on target cells. Upon exposure to near-infrared (NIR) light, the plasmonic properties of the GNRs generated localized heat, inducing photothermal ablation specifically within targeted HSCs. In a CCl4-induced mouse model of liver fibrosis, this nanoplatform-mediated PTT demonstrated significant therapeutic efficacy: it successfully reduced established fibrosis, diminished liver inflammation, and alleviated hepatocyte damage. The authors highlight this strategy's strength in targeting the core pathological process of fibrosis (activated HSC proliferation) irrespective of the underlying disease etiology, presenting it as a promising baseline for developing novel, pathology-focused antifibrotic therapies applicable potentially to fibrosis in other organs. This work exemplifies the potential of nanotechnology to fundamentally alter therapeutic strategies for complex chronic diseases.
Gold nanorods (Au NRs) enable combined photoacoustic imaging and photothermal therapy.99 Xiang et al.100 developed a gold nanostar (GNS)-based probe (GLTTs) engineered for specific targeting of receptors highly expressed on hepatocyte membranes. This active targeting was achieved by modifying the nanostars with glycyrrhetinic acid (GA), a ligand known to bind specific receptors abundant on the surface of liver parenchymal cells. Leveraging this mechanism, GLTTs demonstrated significantly enhanced accumulation in liver tissue (12.85-fold higher signal intensity) compared to unmodified GNS. This efficient hepatic parenchymal cell targeting proved foundational for the successful application of surface-enhanced Raman scattering (SERS) technology, enabling in situ detection of early molecular changes associated with fibrosis and the identification of 10 diagnostic biomarker peaks. This strategy establishes a crucial tool for non-invasive early diagnosis.
However, both materials face challenges in clinical translation. The heavy metal toxicity of cadmium-based QDs has spurred the development of cadmium-free alternatives (e.g., InP/ZnS QDs),103 while the long-term retention risks of carbon nanoparticles require size optimization and surface modifications to improve renal clearance.104 Furthermore, the heterogeneity of fibrotic liver tissues (e.g., necrotic areas or steatosis) may interfere with nanoparticle optical signals, necessitating the use of targeting ligands (e.g., collagen-binding peptides) or dual-modal imaging strategies to enhance detection specificity.
2.6. Technical hurdles in probe synthesis
The preparation of fibrosis-targeted fluorescent probes involves multi-step processes with critical technical bottlenecks: (1) ligand conjugation efficiency: covalent coupling of targeting moieties (e.g., aptamers and antibodies) to fluorophores often yields <30% conjugation efficiency due to steric hindrance, necessitating costly HPLC purification (e.g., ZY-1 aptamer probes require 5-step purification, doubling production costs). (2) Nanoparticle batch consistency: colloidal probes (e.g., Ag@MSN) exhibit ±15% size variability across batches when synthesized via traditional sol–gel methods, impairing biodistribution reproducibility. Microfluidic synthesis could reduce this to <5% but requires substantial capital investment. (3) Long-term stability: lyophilized probes (e.g., protein-based Col1-TRAP) lose 40% activity after 6-month storage at −80 °C, highlighting the need for cryoprotectant optimization.3. Clinical challenges
At present, the translation of these promising laboratory findings into validated clinical tools remains in its nascent stages. Despite the wealth of preclinical data, the number of fluorescent probes that have progressed to formal human clinical trials for liver fibrosis diagnosis is remarkably limited. A pivotal study conducted by de Lédinghen et al.105 exemplifies clinical utility: among 286 patients, a sequential protocol (M probe first, XL probe if M fails) achieved reliable liver stiffness measurements (LSM) in 91.2% of cases with comparable diagnostic accuracy, proving particularly critical for obese/BMI > 30 kg m−2 cohorts where conventional methods fail. To date, the vast majority of these innovative probes exist primarily as research tools, with only a handful venturing into early-phase human studies.This stark gap underscores the critical hurdles impeding clinical integration. Successfully deploying these probes requires overcoming key challenges: long-term biocompatibility/toxicity concerns, limited imaging depth, suboptimal pharmacokinetics/targeting efficiency, and the urgent need for standardized validation across diverse patient populations and liver disease etiologies.
3.1. Biocompatibility and long-term toxicity
The biocompatibility of fluorescent probes, along with their long-term toxicity, is one of the central barriers to their movement from the laboratory to clinical applications. Although many probes have demonstrated good biosafety in short-term experiments, e.g., no acute inflammation or organ damage was induced after intravenous injection, the potential risks of these probes in long-term, repeated use remain a major blind spot. At present, we cannot know whether the probes interfere with the normal function of immune cells or induce chronic inflammation, and whether the various degradation products of the probes have insidious effects on long-term liver and kidney functions. These issues still need to be systematically evaluated in future studies.Beyond acute safety assessments, clinical limitations persist in probe biocompatibility. For instance, antibody-conjugated probes (e.g., anti-APOL2 probes) may trigger immune reactions (e.g., ADA formation) upon repeated administration, compromising both imaging accuracy and patient safety. Critically, fluorophore degradation products (e.g., cyanine dye-derived indoles) exhibit unpredictable hepatic accumulation in cirrhotic patients with impaired detoxification pathways, posing risks of secondary toxicity.
3.2. Imaging depth
The optical performance of existing probes is still limited by the maximum emission wavelength, resulting in suboptimal imaging depth and resolution. The tissue penetration ability of many of the current probes is insufficient, and the fluorescent signals are not able to reach the deep tissues of the liver and clearly outline the subtle changes in the fibrotic areas of the liver, thus failing to present a comprehensive, three-dimensional picture of the temporal and spatial progression of the disease.Clinical applicability of optical probes is constrained by inherent physical limitations. While NIR-II probes (e.g., Ag2S quantum dots) achieve deeper tissue penetration (∼5 mm), their signals remain insufficient for visualizing posterior liver segments in obese patients (BMI >30), where adipose layers exceed 4 cm. This necessitates complementary modalities like intraoperative laparoscopic imaging, increasing procedural invasiveness.
3.3. Pharmacokinetics and targeting efficiency
The in vivo distribution, clearance rate and targeting efficiency of probes directly affect the imaging signal-to-noise ratio. Currently, there is a common problem of non-specific enrichment or too fast renal clearance of probes. These problems greatly affect the accuracy and sensitivity of the diagnosis.3.4. Standardization and clinical validation
There is a lack of standardized criteria for the evaluation of probe performance, including sensitivity thresholds, specificity validation methods, and stability indicators. In addition, most of the existing studies are limited to mouse models, and large-scale multicenter clinical trials are urgently needed to verify their reliability in human liver fibrosis.Clinical translation further requires addressing cost-effectiveness and scalability. Current probe synthesis often relies on multi-step organic reactions or recombinant protein expression, which are resource-intensive and hinder mass production. For example, collagen-targeting protein probes (e.g., Col1-TRAP) necessitate mammalian cell culture systems, raising manufacturing costs by ∼30% compared to small-molecule probes. Additionally, regulatory hurdles, such as good manufacturing practice (GMP) compliance for clinical-grade probes, demand standardized protocols for purification, sterilization, and stability testing—areas still underdeveloped for most experimental probes.
4. Future directions
To overcome the above challenges, future research can focus on the following directions:4.1. Important directions for technological innovation
In the future, fluorescent probes for liver fibrosis can focus on three directions: improving probe sensitivity and precision, probe function integration, and clinical utility.4.2. Interdisciplinary integration
The design of novel probes can be based on multidisciplinary integration, and AI can accelerate the virtual screening of probe molecular libraries to predict the best combinations of targeting moieties and fluorophores, dramatically shortening the development cycle. For example, generative AI models can design novel probe backbones that combine high affinity and low toxicity. Secondly, materials science can be integrated into probe development, such as biocompatible materials to improve the in vivo distribution and metabolic efficiency of probes, and microfluidic organ-chip technology to simulate the microenvironment of liver fibrosis in a high-throughput manner, accelerating the validation of probe performance.Recent advances in interdisciplinary research have significantly enhanced the diagnostic and therapeutic potential of fluorescent probes for liver fibrosis. Compared to traditional imaging modalities, fluorescence-based strategies now synergize with emerging technologies to address critical limitations. For instance, photoacoustic imaging (PAI) combines the molecular specificity of fluorescent probes with deep-tissue penetration (up to 5 cm), enabling quantitative mapping of collagen deposition in cirrhotic livers with submillimeter resolution. Meanwhile, CRISPR-based biosensors have been engineered to detect fibrosis-associated microRNAs in serum, achieving attomolar sensitivity through fluorophore-tagged sgRNA systems.
Compared with existing technologies, fluorescent probes achieve 10× higher molecular specificity for early-stage fibrosis, while MRI provides whole-organ stiffness mapping but lacks biomarker resolution. Also, surface-enhanced Raman scattering (SERS) probes enable multiplexed collagen typing but require complex instrumentation, whereas fluorescence imaging offers real-time bedside applicability. This interdisciplinary convergence not only refines diagnostic accuracy but also paves the way for personalized antifibrotic therapies.
4.3. Establishment of standards related to probe design and applications
To robustly facilitate the clinical translation of molecular imaging probes, establishing a comprehensive standardization framework is essential. This begins with defining unified performance metrics: internationally recognized consensus standards must be developed to evaluate core indicators such as sensitivity, specificity, limit of detection (LOD), dynamic range, photostability, batch-to-batch consistency, shelf-life stability, and biocompatibility/toxicity profiles. Building on these metrics, standardized validation protocols are critical, requiring rigorous procedures to validate probe performance across diverse model systems—from cell lines and primary cells to animal models—and ultimately in human samples, including standardized methods for correlating probe signals with gold-standard diagnostics like histopathology. Furthermore, the creation of well-characterized reference sample banks containing human liver tissues (normal and across various fibrosis stages and etiologies) and biofluids (serum, plasma) is vital, providing essential materials for probe calibration, validation, and reliable inter-laboratory comparisons. Open science practices, notably sharing probe characterization data, imaging protocols, and validation results, are fundamental for reproducibility and trust-building. In this context, clinically relevant platforms (e.g., humanized organoids and advanced in vitro models) play a pivotal role: they permit standardized, controlled testing and personalized probe development, thereby ensuring consistent clinical translation.5. Conclusion
In the biomedical domain, fluorescence imaging has emerged as a powerful tool for investigating hepatic fibrosis, offering novel insights into its pathological mechanisms. Notably, the advancement of organic small-molecule fluorescent probes has become a key breakthrough in this research area, enabling more precise visualization and analysis of disease progression. This technological progress has substantially enhanced our comprehension of fibrotic liver disorders at the molecular level. Due to their precise localization and sensitive feedback, these probes are capable of closely tracking a wide range of biologically active substances and cells that are closely related to the pathological evolution of liver fibrosis. These include collagen, hepatic stellate cells and other core markers, which have greatly advanced the diagnostic research of liver fibrosis. Despite the impressive results, the road ahead is still challenging, and numerous challenges remain to be addressed. The transition from preclinical success to clinical utility demands urgent resolution of two bottlenecks: (1) clinical limitations rooted in human biological complexity (e.g., immune reactivity and anatomical variability) and (2) scalable probe synthesis with batch-to-batch consistency. Industrial partnerships must prioritize GMP-compliant production lines for ligand-functionalized nanoparticles, reducing costs by >50% through microfluidic synthesis platforms.In conclusion, in order to transform the cutting-edge laboratory findings into efficacious clinical tools and illuminate the path to recovery for the majority of liver disease patients, researchers must cultivate the field of molecular design, dismantle the disciplinary silos, and harness the synergies of multiple fields to collectively overcome the existing challenges and accelerate the development of liver fibrosis diagnosis and treatment.
Author contributions
Conceptualization: Fu Wang; methodology: Yutong Lv, Zhe Ma, Yue Chong; formal analysis and investigation: Yutong Lv, Zhe Ma, Yue Chong; software: Yutong Lv, Zhe Ma, Yue Chong; writing – original draft preparation: Yutong Lv, Zhe Ma; writing – review and editing: Fu Wang; funding acquisition: Fu Wang; supervision: Fu Wang, Li Xue, Zhenlong Wang. All authors reviewed the manuscript.Conflicts of interest
No potential conflicts of interest were disclosed.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 32271512 and 82572281) and Natural Science Basic Research Program of Shaanxi (no. 2022JC-56 and 2023-JC-ZD-43).References
- R. Bataller and D. A. Brenner, Liver fibrosis, J. Clin. Invest., 2005, 115(2), 209–218 CrossRef CAS.
- J. Y. Chen, D. Thakar and T. T. Chang, Liver Fibrosis: Current Approaches and Future Directions for Diagnosis and Treatment, in Fibrosis in Disease: An Organ-Based Guide to Disease Pathophysiology and Therapeutic Considerations, ed M. S. Willis, C. C. Yates, and J. C. Schisler, Springer International Publishing, Cham, 2019, pp. 387–417 Search PubMed.
- S. K. Asrani, et al., Burden of liver diseases in the world, J. Hepatol., 2019, 70(1), 151–171 CrossRef PubMed.
- P. Byass, The global burden of liver disease: a challenge for methods and for public health, Med. Glob. Health, 2014, 12, 159 Search PubMed.
- K. Liu, F.-S. Wang and R. Xu, Neutrophils in liver diseases: pathogenesis and therapeutic targets, Cell. Mol. Immunol., 2021, 18(1), 38–44 CrossRef CAS.
- M. M. Aydın and K. C. Akçalı, Liver fibrosis, Turk J Gastroenterol, 2018, 29(1), 14–21 CrossRef PubMed.
- T. Higashi, S. L. Friedman and Y. Hoshida, Hepatic stellate cells as key target in liver fibrosis, Adv. Drug Delivery Rev., 2017, 121, 27–42 CrossRef CAS.
- D. Schuppan, et al., Liver fibrosis: Direct antifibrotic agents and targeted therapies, Matrix Biol., 2018, 68–69, 435–451 CrossRef CAS.
- M. Parola and M. Pinzani, Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues, Mol. Aspects Med., 2019, 65, 37–55 CrossRef CAS.
- T. Kisseleva and D. Brenner, Molecular and cellular mechanisms of liver fibrosis and its regression, Nat. Rev. Gastroenterol. Hepatol., 2021, 18(3), 151–166 CrossRef PubMed.
- M. Zhao, et al., Targeting fibrosis: mechanisms and clinical trials, Signal Transduction Targeted Ther., 2022, 7(1), 206 CrossRef.
- V. Taru, et al., Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation, J. Hepatol., 2024, 81(5), 895–910 CrossRef CAS.
- L. Shan, et al., Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis, Biomed. Pharmacother., 2023, 161, 114472 CrossRef CAS PubMed.
- T. Chen, et al., Liver sinusoidal endothelial cells in hepatic fibrosis: opportunities for future strategies, Biochem. Biophys. Res. Commun., 2025, 766, 151881 CrossRef CAS.
- D. C. Howlett, et al., Findings of the UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor and major complications and procedure-related mortality, Radiology, 2013, 266(1), 226–235 CrossRef PubMed.
- A. B. Chowdhury and K. J. Mehta, Liver biopsy for assessment of chronic liver diseases: a synopsis, Clin. Exp. Med., 2023, 23(2), 273–285 CrossRef PubMed.
- L. Castera, X. Forns and A. Alberti, Non-invasive evaluation of liver fibrosis using transient elastography, J. Hepatol., 2008, 48(5), 835–847 CrossRef.
- R. Masuzaki, et al., Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives, Int. J. Mol. Sci., 2020, 21(14), 4906 CrossRef CAS PubMed.
- E. E. Powell, V. W.-S. Wong and M. Rinella, Non-alcoholic fatty liver disease, Lancet, 2021, 397(10290), 2212–2224 CrossRef CAS.
- B. Gao and R. Bataller, Alcoholic liver disease: pathogenesis and new therapeutic targets, Gastroenterology, 2011, 141(5), 1572–1585 CrossRef CAS.
- A. Campos-Murguía, et al., Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease, World J. Gastroenterol., 2020, 26(39), 5919–5943 CrossRef PubMed.
- A. L. Wang, et al., Collagen-targeted protein nanomicelles for the imaging of non-alcoholic steatohepatitis, Acta Biomater., 2024, 187, 291–303 CrossRef CAS PubMed.
- W.-C. Zhou, Q.-B. Zhang and L. Qiao, Pathogenesis of liver cirrhosis, World J. Gastroenterol., 2014, 20(23), 7312–7324 CrossRef.
- C. Trautwein, et al., Hepatic fibrosis: Concept to treatment, J. Hepatol., 2015, 62(1), S15–S24 CrossRef CAS PubMed.
- L. Nian, et al., Multiplex Collagen Fingerprinting for the Staging of Hepatic Fibrosis Using High-Precision Fluorescence-Guided SERS Imaging, Anal. Chem., 2024, 96(42), 16649–16657 CrossRef CAS.
- J. Y. Yi, et al., One-step detection of procollagen type III N-terminal peptide as a fibrosis biomarker using fluorescent immunosensor (quenchbody), Anal. Chim. Acta, 2024, 1317, 342887 CrossRef CAS PubMed.
- V. Hernandez-Gea and S. L. Friedman, Pathogenesis of liver fibrosis, Annu. Rev. Pathol., 2011, 6, 425–456 CrossRef CAS PubMed.
- Y. Zou, et al., A DNA Aptamer Targeting Cellular Fibronectin Rather Than Plasma Fibronectin for Bioimaging and Targeted Chemotherapy of Tumors, Adv. Funct. Mater., 2022, 32(41), 2205002 CrossRef CAS.
- M. Ge, et al., Cellular fibronectin-targeted fluorescent aptamer probes for early detection and staging of liver fibrosis, Acta Biomater., 2024, 190, 579–592 CrossRef CAS PubMed.
- L. Gan, et al., A natural small molecule alleviates liver fibrosis by targeting apolipoprotein L2, Nat. Chem. Biol., 2025, 21(1), 80–90 CrossRef CAS.
- T. Kisseleva and D. Brenner, Molecular and cellular mechanisms of liver fibrosis and its regression. Nature Reviews, Gastroenterol. Hepatol., 2021, 18(3), 151–166 Search PubMed.
- H. L. R. A. S. L. Friedman, Activation of hepatic stellate cells- A key issue in liver fibrosis., Front. Biosci., 2002, 7, 808–826 CrossRef.
- Z. Chen, et al., Targeted Drug Delivery to Hepatic Stellate Cells for the Treatment of Liver Fibrosis, J. Pharmacol. Exp. Ther., 2019, 370(3), 695–702 CrossRef CAS.
- A. A. Elnfarawy, et al., Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats, Hum. Exp. Toxicol., 2021, 40(2), 355–368 CrossRef CAS.
- X. Wang, et al., Targeting the PDGF-B/PDGFR-β Interface with Destruxin A5 to Selectively Block PDGF-BB/PDGFR-ββ Signaling and Attenuate Liver Fibrosis, EBioMedicine, 2016, 7, 146–156 CrossRef PubMed.
- K. Wang, et al., Constructing a novel fluorescence detection method for γ-glutamyltranspeptidase and application on visualizing liver injury, Biosens. Bioelectron., 2023, 219, 114767 CrossRef CAS PubMed.
- D. Gosalia, et al., Accuracy of Noninvasive Diagnostic Tests for the Detection of Significant and Advanced Fibrosis Stages in Nonalcoholic Fatty Liver Disease: A Systematic Literature Review of the US Studies, Diagnostics, 2022, 12, 11 CrossRef PubMed.
- K. Wang, et al., Multifunctional fluorescence/photoacoustic bimodal imaging of γ-glutamyltranspeptidase in liver disorders under different triggering conditions, Biomaterials, 2024, 310, 122635 CrossRef CAS.
- M. Miao, et al., An activatable near-infrared molecular reporter for fluoro-photoacoustic imaging of liver fibrosis, Biosens. Bioelectron., 2023, 235, 115399 CrossRef CAS PubMed.
- Z.-M. Yang, et al., Mitochondrial-Targeted and Near-Infrared Fluorescence Probe for Bioimaging and Evaluating Monoamine Oxidase A Activity in Hepatic Fibrosis, ACS Sens., 2020, 5(4), 943–951 CrossRef CAS.
- N. Fan, et al., Rapid Two-Photon Fluorescence Imaging of Monoamine Oxidase B for Diagnosis of Early-Stage Liver Fibrosis in Mice, Anal. Chem., 2021, 93(18), 7110–7117 CrossRef CAS PubMed.
- M. Sun, et al., Evaluation of monoamine oxidase B fluctuation in liver fibrosis cell and mice models via a specificity fluorescent probe, Sens. Actuators, B, 2024, 417, 136111 CrossRef CAS.
- U. Lendeckel, et al., The Role of the Ectopeptidase APN/CD13 in Cancer, Biomedicines, 2023, 11, 3 CrossRef.
- S. Nohara, et al., Aminopeptidase N (APN/CD13) as a target molecule for scirrhous gastric cancer, Clin. Res. Hepatol. Gastroenterol., 2016, 40(4), 494–503 CrossRef CAS.
- X. Sun, et al., A Nanofluorescent Probe for Evaluating the Fluctuation of Aminopeptidase N in Nonalcoholic Fatty Liver Disease and Hepatic Fibrosis, Anal. Chem., 2024, 96(36), 14639–14649 CrossRef CAS PubMed.
- J. Berumen, et al., Liver fibrosis: Pathophysiology and clinical implications, Wiley Interdiscip. Rev.: Mech. Dis., 2020, 13(1), e1499 Search PubMed.
- L. Hammerich and F. Tacke, Hepatic inflammatory responses in liver fibrosis, Nat. Rev. Gastroenterol. Hepatol., 2023, 20(10), 633–646 CrossRef CAS PubMed.
- Y. Meng, et al., The role of hepatic microenvironment in hepatic fibrosis development, Ann. Med., 2022, 54(1), 2829–2843 CrossRef PubMed.
- Y. Koyama and D. A. Brenner, Liver inflammation and fibrosis, J. Clin. Invest., 2017, 127(1), 55–64 CrossRef PubMed.
- F. Kai, A. M. Leidal and V. M. Weaver, Tension-induced organelle stress: an emerging target in fibrosis, Trends Pharmacol. Sci., 2025, 46(2), 117–131 CrossRef CAS.
- Y. Zhang, et al., A Mitochondrial-Targeting Near-Infrared Fluorescent Probe for Visualizing and Monitoring Viscosity in Live Cells and Tissues, Anal. Chem., 2019, 91(15), 10302–10309 CrossRef CAS PubMed.
- Z. Zou, et al., Real-Time Visualizing Mitophagy-Specific Viscosity Dynamic by Mitochondria-Anchored Molecular Rotor, Anal. Chem., 2019, 91(13), 8574–8581 CrossRef CAS PubMed.
- W. Zhang, et al., Viscosity-sensitive NIR probe for in vivo imaging of Early-Stage Hepatic Fibrosis, J. Mater. Chem. B, 2022, 10 CAS.
- J. Maiers and H. Malhi, Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis, Semin. Liver Dis., 2019, 39(02), 235–248 CrossRef CAS PubMed.
- Z. Liu, et al., Visualization of the protective role of estrogen against female liver fibrosis via an ER viscosity NIR fluorescent probe, Sci. China: Chem., 2025, 68(1), 360–368 CrossRef CAS.
- Y. Iwakiri, Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase, Clin. Mol. Hepatol., 2015, 21, 4 Search PubMed.
- S. Han, et al., Accurate diagnosis of hepatic fibrosis with dual detection of nitric oxide and viscosity by a ratiometric fluorescent probe, Chem. Eng. J., 2023, 463, 142383 CrossRef CAS.
- A. Lee, et al., Identification of Early Stage Liver Fibrosis by Modifications in the Interstitial Space Diffusive Microenvironment Using Fluorescent Single-Walled Carbon Nanotubes, Nano Lett., 2024, 24(18), 5603–5609 CrossRef CAS PubMed.
- J. H. Lee, et al., CCCP induces hepatic stellate cell activation and liver fibrogenesis via mitochondrial and lysosomal dysfunction, Free Radicals Biol. Med., 2024, 225, 181–192 CrossRef CAS PubMed.
- M. Itoh, et al., Lysosomal cholesterol overload in macrophages promotes liver fibrosis in a mouse model of NASH, J. Exp. Med., 2023, 220, 11 CrossRef.
- L. Su, et al., Development and Application of a BODIPY Carbazole Derivative Probe for Lysosomal Imaging: Insights into Lysosomal Dynamics and Dysfunction in Inflammation-Related Diseases, ACS Appl. Mater. Interfaces, 2025, 17(1), 607–616 CrossRef CAS.
- J. Cai, et al., The roles and mechanisms of hypoxia in liver fibrosis, J. Transl. Med., 2021, 19(1), 186 CrossRef PubMed.
- M. Chen, et al., Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling, Autophagy, 2017, 13(11), 1813–1827 CrossRef PubMed.
- P. Pelegrin, F. Alegre and A. Feldstein, Inflammasomes in Liver Fibrosis, Semin. Liver Dis., 2017, 37(02), 119–127 CrossRef.
- M. C. Iglesias-De La Cruz, et al., Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells, Kidney Int., 2001, 59(1), 87–95 CrossRef CAS PubMed.
- T. Tsuchida, et al., A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer, J. Hepatol., 2018, 69(2), 385–395 CrossRef PubMed.
- B. Hochreiter, A. P. Garcia and J. A. Schmid, Fluorescent proteins as genetically encoded FRET biosensors in life sciences, Sensors, 2015, 15(10), 26281–26314 CrossRef CAS.
- J. Gong, et al., Diaminonaphthalene Boronic Acid (DANBA): New Approach for Peroxynitrite Sensing Site, Angew. Chem., Int. Ed., 2024, 63(49), e202409295 CrossRef CAS.
- T. C. Peterson, et al., Hepatic fibrosis and cytochrome P450: experimental models of fibrosis compared to AHR knockout mice, Hepatol. Res., 2000, 17(2), 112–125 CrossRef PubMed.
- S. Schuster, et al., Triggering and resolution of inflammation in NASH, Nat. Rev. Gastroenterol. Hepatol., 2018, 15(6), 349–364 CrossRef CAS.
- M. Parola and G. Robino, Oxidative stress-related molecules and liver fbrosis, J. Hepatol., 2001, 35(2), 297–306 CrossRef CAS PubMed.
- X. Zhang, et al., Evolving a novel red-emitting two-photon dye with optically tunable amino group for monitoring the degree of hypoxia during liver fibrosis, Chin. Chem. Lett., 2023, 34(5), 107835 CrossRef CAS.
- Y. Tian, et al., Novel Strategy for Validating the Existence and Mechanism of the “Gut–Liver Axis” in Vivo by a Hypoxia-Sensitive NIR Fluorescent Probe, Anal. Chem., 2020, 92(6), 4244–4250 CrossRef CAS PubMed.
- F. Yuan, et al., Demonstrating HOCl as a potential biomarker for liver fibrosis using a highly sensitive fluorescent probe, Sens. Actuators, B, 2023, 378, 133219 CrossRef CAS.
- E. Yang, J. Lee and J.-W. Park, Ethanol induces peroxynitrite-mediated toxicity through inactivation of NADP + -dependent isocitrate dehydrogenase and superoxide dismutase, Biochimie, 2008, 90, 1316–1324 CrossRef CAS.
- Y. Zhang and D. Ma, Selective detection of peroxynitrite in living cells by a near-infrared diphenyl phosphinate-based dicyanoisophorone probe, Spectrochim. Acta, Part A, 2021, 244, 118890 CrossRef CAS PubMed.
- K. Wang, et al., Multifunctional lysosome-targetable fluorescent probe for imaging peroxynitrite in acute liver injury model, Chem. Eng. J., 2023, 455, 140491 CrossRef CAS.
- Y. Zhong, et al., Biomarker-Responsive Fluorescent Probes for In-Vivo Imaging of Liver Injury, Chem. – Asian J., 2022, 17(9), e202200038 CrossRef CAS PubMed.
- D. Sun, et al., Recent advance of fluorescent probes for detection of drug-induced liver injury markers, Chin. Chem. Lett., 2022, 33(10), 4478–4494 CrossRef CAS.
- T. Zhang, et al., Visulization of peroxynitrite variation for accurate diagnosis and assessing treatment response of hepatic fibrosis using a Golgi-targetable ratiometric fluorescent probe, J. Photochem. Photobiol., B, 2024, 257, 112950 CrossRef CAS.
- T. Luangmonkong, et al., Targeting Oxidative Stress for the Treatment of Liver Fibrosis, Rev. Physiol. Biochem. Pharmacol., 2018, 175, 71–102 CAS.
- Y. Tian, et al., H2O2-Activated NIR-II Fluorescent Probe with a Large Stokes Shift for High-Contrast Imaging in Drug-Induced Liver Injury Mice, Anal. Chem., 2022, 94(32), 11321–11328 CrossRef CAS.
- H. Chen, et al., A novel NIR-II fluorescent probe for hydrogen peroxide detection in drug-induced liver injury, Chem. Commun., 2024, 60(71), 9618–9621 RSC.
- C. Dong, et al., A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs), Chemosphere, 2022, 308, 136205 CrossRef CAS.
- C. A. Juan, et al., The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies, Int. J. Mol. Sci., 2021, 22, 9 Search PubMed.
- M. Yu, et al., A turn-on fluorescent probe for imaging of hydroxyl radicals in drug-induced liver injury, Spectrochim. Acta, Part A, 2025, 329, 125569 CrossRef CAS PubMed.
- Q.-Q. Wang, et al., A superoxide anion activatable near-infrared fluorescent probe with a large Stokes shift for imaging of drug-induced liver injury, Microchem. J., 2024, 200, 110288 CrossRef CAS.
- Z. Wang and J. Zhao, Bodipy–Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet–Triplet Annihilation Upconversion, Org. Lett., 2017, 19(17), 4492–4495 CrossRef CAS PubMed.
- W. Xu, et al., Hepatocellular cystathionine γ lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor, Hepatology, 2022, 76, 6 CrossRef.
- Y. Iwakiri and J. Trebicka, Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy, JHEP Rep., 2021, 3(4), 100316 CrossRef PubMed.
- T. Wang, et al., Small-molecule inhibitors targeting apoptosis signal-regulated kinase 1, Eur. J. Med. Chem., 2023, 262, 115889 CrossRef CAS PubMed.
- Y. Li, et al., A novel liver-targeted and highly selective fluorescent probe for hepatic hydrogen sulfide detection in the diagnosis of drug-induced liver injury, Eur. J. Med. Chem., 2025, 291, 117640 CrossRef CAS PubMed.
- N. Li, et al., A Substituted-Rhodamine-Based Reversible Fluorescent Probe for In Vivo Quantification of Glutathione, Angew. Chem., Int. Ed., 2023, 62(12), e202217326 CrossRef CAS PubMed.
- W. Jiao, et al., Therapeutic Effects of an Inhibitor of Thioredoxin Reductase on Liver Fibrosis by Inhibiting the Transforming Growth Factor-β1/Smads Pathway, Front. Mol. Biosci., 2021, 8, 690170 CrossRef CAS PubMed.
- W. J. Peveler, et al., A Rapid and Robust Diagnostic for Liver Fibrosis Using a Multichannel Polymer Sensor Array, Adv. Mater., 2018, 30(28), e1800634 CrossRef.
- L. Wang, et al., [Establishment of a reporter system for estimating activation of human hepatic stellate cells based on COL1A1 promoter and enhanced green fluorescent protein], J. Peking Univ., Health Sci., 2023, 55(5), 876–885 CAS.
- M. Jiang, et al., NIR-II emissive nanoprobe for non-invasive monitoring of liver fibrosis and in-situ ratiometric visualization of NO gas-based therapy process, Chem. Eng. J., 2023, 475, 145977 CrossRef CAS.
- J. Ribera, et al., Treatment of Hepatic Fibrosis in Mice Based on Targeted Plasmonic Hyperthermia, ACS Nano, 2021, 15(4), 7547–7562 CrossRef CAS PubMed.
- J. Zhong, et al., Imaging-guided high-efficient photoacoustic tumor therapy with targeting gold nanorods, Nanomedicine, 2015, 11(6), 1499–1509 CrossRef CAS PubMed.
- S. Xiang, et al., SERS diagnosis of liver fibrosis in the early stage based on gold nanostar liver targeting tags, Biomater. Sci., 2021, 9(14), 5035–5044 RSC.
- A. Moreno-Lanceta, et al., RNF41 orchestrates macrophage-driven fibrosis resolution and hepatic regeneration, Sci. Transl. Med., 2023, 15(704), eabq6225 CrossRef CAS PubMed.
- X. Zhang, et al., Plasmonic-Fluorescent Janus Ag/Ag2S Nanoparticles for In Situ H2O2-Activated NIR-II Fluorescence Imaging, Nano Lett., 2021, 21(6), 2625–2633 CrossRef CAS PubMed.
- C. Zhu, et al., Recent advances in non-toxic quantum dots and their biomedical applications, Prog. Nat. Sci.: Mater. Int., 2019, 29(6), 628–640 CrossRef CAS.
- S. Mosleh-Shirazi, et al., Renal clearable nanoparticles: An expanding horizon for improving biomedical imaging and cancer therapy, Mater. Today Commun., 2021, 26, 102064 CrossRef CAS.
- V. de Lédinghen, et al., Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: Comparison between M and XL probe of FibroScan®, J. Hepatol., 2012, 56(4), 833–839 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |