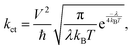Non-radiative recombination energy losses in Y-series asymmetric acceptor-based organic solar cells
Yongjie
Cui
ab,
Zhaohan
Chai
a,
Shenbo
Zhu
b,
Zihua
Wu
 a,
Huaqing
Xie
a and
Huawei
Hu
a,
Huaqing
Xie
a and
Huawei
Hu
 *b
*b
aSchool of Energy and Materials, Shanghai Polytechnic University, Shanghai 201209, China
bState Key Laboratory of Advanced Fiber Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China. E-mail: huaweihu@dhu.edu.cn
First published on 24th September 2025
Abstract
The molecular engineering of Y6 has marked a paradigm shift in the development of non-fullerene acceptors (NFAs) for organic solar cells (OSCs), enabling remarkable enhancements in power conversion efficiencies (PCEs) through its distinct A–DA′D–A architecture and optimized intermolecular packing. However, further advancement is hindered by persistent non-radiative recombination energy losses (ΔE3), which predominantly originate from molecular relaxation and sub-gap charge recombination pathways. Among various structural optimization strategies, the implementation of molecular asymmetry has recently emerged as a promising approach to suppress ΔE3 without compromising light absorption or charge transport. This review systematically summarizes recent progress in asymmetric Y-series NFAs, including modifications involving central cores, terminal groups, side chains, and multi-site asymmetrization. Emphasis is placed on the mechanistic understanding of how specific asymmetries influence molecular energetics, exciton dynamics, and non-radiative decay processes. Theoretical models and empirical correlations are discussed to elucidate the structure–ΔE3 relationship. Finally, key challenges and prospective design principles for the rational development of next-generation asymmetric NFAs are outlined.
Wider impactThis review provides a comprehensive analysis of asymmetric molecular design strategies for Y-series non-fullerene acceptors (NFAs) in organic solar cells (OSCs), with a particular emphasis on suppressing non-radiative recombination energy losses (ΔE3)—a major bottleneck limiting further efficiency improvements. By systematically categorizing recent advances in asymmetric modifications to central cores, terminal groups, and side chains, this work demonstrates how molecular asymmetry can precisely modulate molecular interactions, optimize active layer morphology, and reduce voltage losses. The mechanistic insights derived from both theoretical and experimental studies provide a clear structure–property–performance relationship, offering guidance for the rational design of high-efficiency OSC materials. |
1. Introduction
Organic solar cells (OSCs) have demonstrated broad prospects in application fields such as distributed photovoltaics and building-integrated photovoltaics due to their advantages of flexibility, semi-transparency, low cost, lightweight, and environmental friendliness. They are expected to contribute to achieving national energy-saving, emission-reduction, and “dual-carbon” strategic goals.1–6 The active layer, composed of donor and acceptor materials as a critical component of OSC devices, is primarily where exciton generation, diffusion, dissociation, as well as charge transport and collection occur, playing a decisive role in determining photovoltaic performance.7–10 In recent years, thanks to the innovative design of non-fullerene acceptors (NFAs) and continuous optimization of device processing, the power conversion efficiency (PCE) of OSCs has exceeded 20%.11–16 However, compared to silicon-based and perovskite solar cells, the efficiency of OSCs remains constrained by moderate open-circuit voltage (VOC) caused by relatively large energy losses. In solar cells, energy loss is defined as the difference between the optical bandgap of the active layer and the energy corresponding to the VOC (Eloss = Eg − qVOC, q is the elementary charge). Inorganic solar cells exhibit an energy loss of 0.3 eV, whereas state-of-the-art OSCs still suffer from a significant energy loss of 0.6 eV, far exceeding the theoretical Shockley–Queisser (SQ) limit.17–19 In recent years, reducing energy loss to achieve a higher VOC without sacrificing short-circuit current (JSC) and fill factor (FF) has become the key to breakthrough performances in OSCs. Consequently, numerous researchers have begun exploring material systems with low voltage loss through molecular structure design.The development of representative NFAs has evolved through the ITIC series,20–22 M series,23–28 and Y series,29 with a gradual reduction in molecular symmetry—specifically transitioning from centrosymmetric linear conjugated backbones to axially symmetric banana-shaped frameworks. Among them, the Y6-series symmetric acceptors feature an A–DA′D–A-type structural framework, where the DA'D structure effectively broadens the material's absorption spectrum while reducing voltage loss in devices, thereby achieving a balance between VOC and JSC. Additionally, the introduction of pyrrole nitrogen into the conjugated backbone can effectively suppress excessive aggregation and enhance intermolecular π–π interactions, improving charge transport properties.30–34 However, Y6-series acceptors still exhibit significant non-radiative recombination energy losses (ΔE3) in devices. To address this, researchers worldwide have explored asymmetric strategies, including modifications to terminal groups, central cores, and side chains. Y-series asymmetric acceptors have larger dipole moments and stronger intermolecular binding energies, leading to enhanced intermolecular interactions.35 Consequently, asymmetric strategies can effectively optimize the aggregation behavior of organic photovoltaic materials, improve molecular assembly, and facilitate the formation of an optimal active layer morphology, thereby enhancing charge generation and transport. Moreover, the reduced uniformity of charge distribution on the surface of asymmetric acceptor molecules helps fine-tune intramolecular charge transfer and improve optical absorption. Asymmetric design can also relax the selection rules for electronic transitions, enabling higher photoluminescence quantum yields and thereby suppressing non-radiative voltage losses.36,37 Although several review articles have summarized Y-series asymmetric acceptor molecules, few have focused on their mechanistic role in modulating non-radiative voltage losses.
Based on this, our review first outlines the origin and classification of energy loss in OSCs, followed by a summary of the molecular design strategies aimed at reducing ΔE3. Specifically, Y6-derived asymmetric acceptors containing core engineering, terminal modulation, side-chain optimization and multiple asymmetric strategies, as well as their effects on the corresponding device performance, are discussed, as shown in Fig. 1. Core engineering can be categorized into core expansion/shortening, heteroatom substitution and their isomerization on the Y6 central core. These strategies influence molecular configuration, optical absorption, dielectric properties and voltage loss, thereby modulating device photovoltaic performance. Terminal modification involves asymmetric halogenation of terminals (2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile, IC) and introducing new terminal units, primarily regulating intermolecular packing and π–π interactions. Side-chain optimization includes two asymmetric design approaches: inner-side and outer-side alkyl chain engineering, which tune intermolecular interactions and active-layer morphology. The combination of these strategies is a current research focus for Y6-based asymmetric acceptors, leveraging synergistic effects to optimize molecular orientation, packing, morphology, and voltage loss for enhanced photovoltaic performance. Finally, we summarize theoretical insights into how asymmetric acceptors regulate ΔE3, establishing a structure–property relationship to guide the rational design of high-performance asymmetric acceptors.
2. The origin and classification of energy loss in OSCs
In the device architecture of OSCs, the active layer absorbs sunlight to generate excitons, which then diffuse to the donor–acceptor interface. Driven by the energy level offset at this interface, the excitons (mostly in the first singlet excited state, S1, i.e., the localized exciton state, LE) first dissociate to form a charge transfer (CT) state. This is followed by vibrational relaxation to the lowest-energy CT state, after which the CT-state excitons further dissociate into free charges that are eventually transported to the electrodes and collected by the respective anode and cathode (Fig. 2).38–40 For efficient exciton dissociation and CT-state formation, an appropriate energy level offset between the donor and acceptor is required. Additionally, extra energy is needed at the donor–acceptor interface to dissociate the CT-state excitons. Consequently, while the CT process enhances charge extraction efficiency, it also leads to increased energy loss.41 Furthermore, the disordered molecular packing in bulk heterojunction (BHJ) structures introduces significant ΔE3. The total Eloss in BHJ devices can be decomposed into three components (Fig. 2):33,42–44Eloss = (Eg − qVSQOC) + (qVSQOC − qVradOC) + (qVradOC − qVOC) = (Eg − qVSQOC) + qΔVrad,below![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gapOC + qΔVnon-radOC = ΔE1 + ΔE2 + ΔE3, gapOC + qΔVnon-radOC = ΔE1 + ΔE2 + ΔE3, |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gapOC is the voltage loss caused by below-bandgap radiative recombination, and ΔVnon-radOC is the voltage loss caused by non-radiative recombination. In the equation, ΔE1 is the radiative recombination above the optical bandgap and an inevitable fundamental loss in all solar cells. ΔE2 is the radiative recombination below the optical bandgap. ΔE3 is quantifiable through the relationship: ΔE3 = −kT
gapOC is the voltage loss caused by below-bandgap radiative recombination, and ΔVnon-radOC is the voltage loss caused by non-radiative recombination. In the equation, ΔE1 is the radiative recombination above the optical bandgap and an inevitable fundamental loss in all solar cells. ΔE2 is the radiative recombination below the optical bandgap. ΔE3 is quantifiable through the relationship: ΔE3 = −kT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(EQEEL), where k is Boltzmann's constant and T is the temperature in Kelvin. Notably, higher device electroluminescence efficiency directly reduces ΔE3. The EQEEL depends on the ratio of radiative (kr) to total recombination coefficients (kr + knr), scaling with kr/(kr + knr).45,46 While ΔE1 and ΔE2 have been minimized to levels comparable with those of perovskite and inorganic solar cells, ΔE3-related non-radiative voltage losses now constitute the primary limitation for further VOC enhancement.47 Asymmetric acceptor-based devices can achieve lower ΔE3, and a series of theoretical studies have revealed their mechanism based on experimental results.
ln(EQEEL), where k is Boltzmann's constant and T is the temperature in Kelvin. Notably, higher device electroluminescence efficiency directly reduces ΔE3. The EQEEL depends on the ratio of radiative (kr) to total recombination coefficients (kr + knr), scaling with kr/(kr + knr).45,46 While ΔE1 and ΔE2 have been minimized to levels comparable with those of perovskite and inorganic solar cells, ΔE3-related non-radiative voltage losses now constitute the primary limitation for further VOC enhancement.47 Asymmetric acceptor-based devices can achieve lower ΔE3, and a series of theoretical studies have revealed their mechanism based on experimental results.
 | ||
| Fig. 2 (a) Energy level diagram depicting the energy of ground-state (S0), S1 and triplet (T1) excitons, CT state, and free carriers. The green arrow indicates optical absorption transitions within the neat narrow-gap material phase, and the yellow arrow indicates optical absorption by interfacial CT states. The red arrow indicates radiative excited-state decay. (b) The absorptance (blue curves) and emission (yellow curves) of SQ type devices (top) and real-word OSC devices (bottom). Reproduced with permission.48 Copyright 2020, Wiley-VCH. | ||
3. Material design of Y-series asymmetric NFAs
Since Zou et al. first reported the novel molecule Y6 in 2019,31 a series of Y6-derivatives have been developed and designed, significantly enhancing the efficiency of OSCs. Although these molecules differ in structural unit composition, they share some key design principles: the central core exhibits good planarity and rigidity, ensuring stronger electron delocalization and near-infrared absorption; the strongly electron-withdrawing terminal groups facilitate intramolecular charge transfer while forming robust π–π stacking to provide charge transport channels; and the side chains guarantee good solubility and suppress excessive molecular aggregation. From the perspective of molecular structure, the asymmetric strategy for NFAs has also been implemented based on these three aspects, achieving optimized active layer morphology and reduced ΔE3.3.1. Asymmetric central core-based NFAs
The asymmetric central core strategy includes two approaches: either altering the conjugation length (extending or shortening it) or keeping it unchanged through heteroatom substitution. As for the former strategy, the assembly behaviors of these materials both in solution and thin films exhibit significant differences, thereby leading to distinct nanoscale phase separation and molecular packing in the active layer. These morphological differences directly affect exciton dissociation, charge transport, and recombination processes, ultimately leading to considerable variations in photovoltaic performance. For the latter strategy, these materials can effectively preserve the configuration of Y6 and exhibit favorable packing behavior. Furthermore, the heteroatoms can enhance the electron-donating ability of the central core, thereby promoting intramolecular charge transfer, which ultimately leads to broadened absorption and improved device performance.49–51By adjusting the conjugation length of the Y6 central core, NFAs with different molecular configurations were constructed. Yang et al. synthesized an asymmetric acceptor AY6 (Fig. 3) using a cross conceptual strategy integrating the structural features of IT6-4F and Y6. AY6 exhibits an X-shaped configuration with poor crystallization but forms a favorable intermixed phase with polymer donor PM6, enhancing face-on orientation and π–π stacking in blend films (Fig. 4). This morphology improves exciton dissociation and charge transport, achieving an efficiency of 15.6% with a higher VOC of 0.87 V compared to Y6.52 Alex et al. constructed Z-shaped and W-shaped asymmetric acceptors BP5T-4F and ABP4T-4F (Fig. 3), respectively, by introducing thiophene to extend the conjugation length of the central core and employing a central core isomerization strategy. Compared to C-shaped symmetric acceptors, the asymmetric acceptors exhibit increased dielectric constants, thereby reducing the binding energy of the charge transfer state and enhancing charge generation. Furthermore, the asymmetric design suppresses charge recombination and reduces ΔE3.53 Subsequently, Alex et al. designed and synthesized two benzo[c][1,2,5]thiadiazole (BT)-based asymmetric acceptors, BP6T-4F and ABP6T-4F (Fig. 3). Compared to the M-shaped BP6T-4F, the C-shaped ABP6T-4F exhibited a higher dielectric constant and lower exciton binding energy, thereby promoting more efficient exciton dissociation (Fig. 4). Furthermore, its blend film with PM6 demonstrated optimized micromorphology and appropriate phase-separation domains, ultimately achieving a device efficiency of 15.8%.54 Yang et al. designed and synthesized two asymmetric acceptors, BDTP-4F and BTDTP-4F (Fig. 3), featuring C-shaped and S-shaped conformations, respectively. Compared with BTDTP-4F, BDTP-4F exhibited a higher-lying lowest unoccupied molecular orbital (LUMO) energy level and a blue-shifted absorption spectrum. Moreover, its blend film demonstrated a favorable nanofibril network structure, appropriate phase separation, preferential face-on molecular orientation, and enhanced intermolecular interactions, ultimately achieving a device efficiency of 15.42%.55 Further advancing the design, Yang et al. introduced pyrrole and selenophene units into the Y6 core to construct two asymmetric acceptors, BTN-4F and BTSe-4F (Fig. 3). Among them, BTN-4F exhibited a slightly upshifted energy level and a red-shifted absorption spectrum. When paired with PM6, the corresponding device achieved an efficiency of 15.82%.56 Zhou et al. discovered that when the conjugated core of the central unit contains seven or nine rings with [2+2], [2+4] or [1+3] models, the device efficiency is superior to that based on [2+1] or [2+3] models. This enhancement is attributed to the stronger dipole moment, which promotes favorable face-on molecular orientation and improved π–π stacking.57
 | ||
| Fig. 3 Chemical structures of asymmetric central cores with different conjugation length-based NFAs. | ||
 | ||
| Fig. 4 (a) 2D-GIWAXS patterns for IT-4F, AY6 and Y6 blend films. Reproduced with permission.52 Copyright 2020, The Royal Society of Chemistry. (b) Optimal capacitance (Cp) and relative dielectric constant (εr) of pure and blend films as a function of frequency. Reproduced with permission.54 Copyright 2021, Wiley-VCH. | ||
Heteroatom substitution primarily involves replacing the sulfur atoms on the outer thiophene units of the Y6 central core with selenium or nitrogen atoms, while maintaining the same conjugation length as that of Y6. Zhu et al. replaced the S on the inner thiophene side of the Y6 core with N to construct the asymmetric acceptor SN (Fig. 5). Compared with Y6, SN exhibited a 40 nm redshift in its absorption peak and enhanced the intramolecular charge transfer effect. Notably, the devices based on SN achieved a non-radiative voltage loss of 0.15 eV.58 Wang et al. introduced a selenium atom at the outermost position of the Y6 core to design the asymmetric acceptor A-WSSe-Cl (Fig. 5), which exhibited stronger and more compact intermolecular π–π stacking interactions. The corresponding device achieved an efficiency of 17.51%.59 Based on this, Wang et al. constructed three isomeric acceptors (S-CSeF, A-ISeF and A-OSeF, Fig. 5) by introducing selenium atoms into the central, inner, and outer positions of the Y6 core, respectively. Among them, asymmetric acceptor A-OSeF exhibited tighter π–π stacking, a more ordered three-dimensional network packing and more efficient charge hopping pathways (Fig. 6). Moreover, the blend film based on A-OSeF demonstrated favorable fibril-like phase separation morphology and achieved a device efficiency of 18.5%.60 Therefore, by combining an asymmetric central core with precise heteroatom modulation, it is possible to simultaneously enhance JSC and FF values without sacrificing the VOC, thereby achieving higher device efficiency.
 | ||
| Fig. 5 Chemical structures of asymmetric central cores with equal conjugation length and heteroatom-based NFAs. | ||
 | ||
| Fig. 6 The single crystallographic structures, the detailed π–π stackings and 3D network packing of S-CSeF, A-ISeF and A-OSeF. Reproduced with permission.60 Copyright 2023, Wiley-VCH. | ||
In summary, for the design of an asymmetric central core structure, maintaining an odd number of rings and incorporating selenium atom substitution should be prioritized. The odd-numbered ring configuration promotes favorable molecular packing and enhanced charge delocalization, while selenium substitution improves the quinoidal characteristic and reduces energy loss. These synergistic effects contribute to suppressed charge recombination and improved charge transport, ultimately enabling devices to achieve superior performance with a high VOC (Table 1).
| Acceptors | Donors | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | ΔE3 (eV) | Ref. |
|---|---|---|---|---|---|---|---|
| AY6 | PM6 | 0.87 | 23.54 | 76.3 | 10.8 | — | 52 |
| BP5T-4F | PM6 | 0.888 | 24.60 | 76.3 | 16.70 | 0.242 | 53 |
| ABP4T-4F | PM6 | 0.922 | 22.00 | 75.1 | 15.20 | 0.206 | 53 |
| BP6T-4F | PM6 | 0.910 | 11.00 | 64.2 | 6.43 | — | 54 |
| ABP6T-4F | PM6 | 0.880 | 24.67 | 73.01 | 15.81 | — | 54 |
| BDTP-4F | PM6 | 0.895 | 22.54 | 75.5 | 15.24 | — | 55 |
| BTDTP-4F | PM6 | 0.866 | 21.25 | 71.3 | 13.12 | — | 55 |
| BTN-4F | PM6 | 0.816 | 25.05 | 77.3 | 15.82 | — | 56 |
| BTSe-4F | PM6 | 0.811 | 22.52 | 75.4 | 13.79 | — | 56 |
| SN | PM6 | 0.820 | 25.14 | 68.9 | 14.30 | 0.15 | 58 |
| A-WSSe-Cl | PM6 | 0.850 | 26.58 | 77.50 | 17.51 | — | 59 |
| A-ISeF | PM1 | 0.885 | 26.60 | 74.6 | 17.60 | 0.219 | 60 |
| A-OSeF | PM1 | 0.880 | 27.20 | 77.3 | 18.50 | 0.220 | 60 |
3.2. Asymmetry terminal-based NFAs
The charge transport in NFAs primarily occurs through terminal-to-terminal interactions.61,62 The electronegativity of the terminal groups plays a critical role in the overall electron affinity and optical bandgap of the material.63,64 Asymmetric terminal-based NFAs can be constructed through a facile one-pot stepwise Knoevenagel condensation, allowing the fine-tuning of energy levels and molecular packing. By rationally selecting terminal groups, such as combining strong and weak electron-withdrawing groups, these materials can significantly suppress ΔE3 by reducing charge recombination and enhancing electroluminescence efficiency. This strategy promotes a better balance between VOC and JSC, leading to improved overall device performance.Chen et al. constructed three asymmetric acceptors (SY1, SY2 and SY3) by replacing the fluorine atoms on Y6's terminals with chlorine. Among them, SY1 exhibited a shallower LUMO energy level and enhanced molecular packing, ultimately achieving a device efficiency of 16.83% with an VOC of 0.871 V.65 Chen et al. developed asymmetric acceptors BTP-S1 and BTP-S2 (Fig. 7) by substituting Y6 terminal groups with halogenated indandione. The BTP-S2 variant with six chlorine atoms exhibited an enhanced electroluminescence quantum yield (EQEEL), leading to significantly reduced ΔE3 and energy loss. The asymmetric molecular design simultaneously facilitated more efficient hole transfer to PM6. These synergistic effects ultimately achieved a champion efficiency of 16.37%.66 Later, Chen et al. extended the conjugation of the IC terminal groups in BO-4Cl to construct two asymmetric acceptors, BTP-S8 and BTP-S9 (Fig. 7), which exhibited improved charge separation and reduced non-radiative losses. The alkyl-shortened BTP-S9 demonstrated enhanced terminal-group stacking and a reduced π–π stacking distance, ultimately achieving a device efficiency of 17.56% with a high FF of 78.44%.67 Yan et al. designed an asymmetric acceptor, namely BTP-2F-ThCl (Fig. 7), by replacing the IC-2F terminal group of Y6 with a chlorinated thiophene-fused ring. This modification effectively balanced the VOC and JSC in the corresponding devices, ultimately achieving a high efficiency of 17.06%.68 Subsequently, Chen et al. further designed an asymmetric acceptor AC9 (Fig. 7) by replacing the terminal groups of BTP-eC9 with chlorinated thiophene-fused rings. The corresponding device achieved an optimal trade-off between charge generation and non-radiative charge recombination, ultimately yielding a champion efficiency of 18.43%.69 Subsequently, Chen et al. employed halogenated indandione, chlorinated thiophene-fused rings and IC-2Cl to synthesize asymmetric acceptors BO-5Cl and BO-6Cl (Fig. 7). Both acceptors exhibited reduced ΔE3 and higher VOC.70 Min et al. utilized IC-2F and IC-2Cl to design and synthesize an asymmetric acceptor, namely BTP-2F-2Cl (Fig. 7). When incorporated into the PM6:L8-BO system, it broadened light absorption, improved molecular packing and suppressed non-radiative charge recombination, ultimately achieving a remarkable ternary device efficiency of 19.17%.71 Chen et al. developed a novel terminal unit, TPC-Cl, by conjugatively extending the IC group with a chlorinated thiophene moiety. This unit was employed to construct an asymmetric acceptor, BTP-T-3Cl. The corresponding device exhibited optimized film morphology and efficient charge generation, ultimately achieving an efficiency of 17.61% with an energy loss of 0.51 eV.72 Fei et al. developed a novel terminal group, imide-containing 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (IIC), featuring strong electron-withdrawing capability and modifiable N-substitution sites. This group was utilized to design asymmetric acceptors, including BTP-IIC-2Cl and BTP-IIC-BO-2Cl (Fig. 7). Compared to BTP-IIC-BO-2Cl, devices based on BTP-IIC-2Cl exhibited broader spectral absorption, more balanced charge transport and superior molecular packing, ultimately achieving a champion efficiency of 17.12%.73 Kim et al. introduced a novel terminal unit, 9H-indeno[1,2-b]pyrazine-2,3,8-tricarbonitrile (IPC1CN), to construct asymmetric acceptors IPC1CN-BBO-IC2F and IPC1CN-BBO-IC2Cl. This molecular design effectively modulated light absorption and energy levels while enhancing charge carrier mobility and enabling favorable film morphology, which is critical for facilitating large-area blade-coating fabrication.74 Peng et al. designed a brominated terminal group IC-FBr, and applied it to modify the terminals of L8-BO, constructing the asymmetric acceptor BTP-3FBr. When paired with D18, the system achieved optimized crystalline orientation and thin-film morphology, ultimately yielding a high efficiency of 18.34%.75 Yan et al. incorporated a difluoro-methoxylated terminal group to develop the asymmetric acceptor BTP-BO-4FO (Fig. 7), which exhibited upshifted energy levels, a larger dipole moment, and continuous crystallinity. Devices based on BTP-BO-4FO achieved an impressive efficiency of 18.62% with a VOC of 0.933 V.76 Subsequently, Yan et al. further employed brominated and chlorinated-methoxylated terminals to develop asymmetric acceptors BTP-2FBrO and BTP-2FClO based on BTP-eC9. Both exhibited optimized aggregation behavior, modest molecular crystallinity, and a high VOC of 0.96 V (Fig. 8). As a result, the champion device based on BTP-2FClO achieved a remarkable efficiency of 19.34%.77 He et al. proposed a chlorine-mediated asymmetric design strategy and synthesized four isomeric acceptors including BO3Cl-α, BO3Cl-γ, BO3Cl-β, and BOEH3Cl-β (Fig. 7). The study revealed that enhanced chlorine dispersion facilitated exciton dissociation and suppressed charge recombination. Ultimately, the BOEH3Cl-β-based device achieved an efficiency exceeding 19%.78 Zhu et al. designed the asymmetric acceptor Z8 by incorporating tethered phenyl groups on the inner side of its terminal asymmetric acceptor. The delocalized excitons induced by this strategy can minimize non-radiative energy loss and charge recombination, while simultaneously tuning intermolecular interactions to enhance exciton dissociation. As a result, the Z8-based ternary device achieved an outstanding efficiency of 20.2%, demonstrating the efficacy of this molecular engineering approach.79
 | ||
| Fig. 8 AFM height images, 2D GIWAXS images and corresponding 1D GIWAXS profiles for all blend films. Reproduced with permission.77 Copyright 2025, Wiley-VCH. | ||
In summary, the rational design of asymmetric terminals presents a highly promising structural modification strategy, which enables the reduction of ΔE3 below 0.2 eV. Therefore, during the design of asymmetric terminals, emphasis should be placed on selecting complementary electron-withdrawing groups with tailored electronegativity and steric hindrance to optimize molecular packing, suppress non-radiative recombination, and enhance charge transport. Simultaneously, energy level alignment with the donor material must be carefully modulated to minimize voltage losses while maintaining efficient charge generation and dissociation, ultimately leading to improved VOC and device performance (Table 2).
| Acceptors | Donors | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | ΔE3 (eV) | Ref. |
|---|---|---|---|---|---|---|---|
| SY1 | PM6 | 0.871 | 25.41 | 76.0 | 16.83 | — | 65 |
| SY2 | PM6 | 0.852 | 25.29 | 74.3 | 16.01 | — | 65 |
| SY3 | PM6 | 0.858 | 25.54 | 74.1 | 16.23 | — | 65 |
| BTP-S1 | PM6 | 0.934 | 22.39 | 72.69 | 15.21 | 0.22 | 66 |
| BTP-S2 | PM6 | 0.945 | 24.07 | 72.02 | 16.37 | 0.20 | 66 |
| BTP-S8 | PM6 | 0.861 | 25.92 | 75.09 | 16.76 | 0.241 | 67 |
| BTP-S9 | PM6 | 0.852 | 26.96 | 75.45 | 17.33 | 0.234 | 67 |
| BTP-2F-ThCl | PM6 | 0.869 | 25.38 | 77.40 | 17.06 | 0.22 | 68 |
| AC9 | PM6 | 0.871 | 26.75 | 79.00 | 18.43 | 0.238 | 69 |
| BO-5Cl | PM6 | 0.958 | 22.57 | 70.10 | 15.02 | 0.184 | 70 |
| BO-6Cl | PM6 | 0.944 | 23.22 | 72.90 | 15.94 | 0.194 | 70 |
| BTP-2F-2Cl | PM1 | 0.861 | 27.35 | 78.16 | 18.40 | 0.208 | 71 |
| BTP-T-3Cl | PM6 | 0.893 | 26.02 | 75.79 | 17.61 | 0.22 | 72 |
| BTP-IIC-2Cl | PM6 | 0.820 | 27.22 | 76.73 | 17.12 | 0.232 | 73 |
| BTP-IIC-BO-2Cl | PM6 | 0.870 | 24.43 | 77.61 | 16.50 | 0.225 | 73 |
| IPC1CN-BBO-IC2F | PM6-PBDBT(55) | 0.833 | 24.38 | 64.10 | 13.02 | — | 74 |
| IPC1CN-BBO-IC2Cl | PM6-PBDBT(55) | 0.836 | 25.94 | 65.09 | 14.12 | — | 74 |
| BTP-3FBr | D18 | 0.921 | 25.60 | 77.78 | 18.34 | — | 75 |
| BTP-BO-4FO | PM6 | 0.933 | 25.79 | 77.40 | 18.62 | — | 76 |
| BTP-2FBrO | PM6 | 0.968 | 22.63 | 72.90 | 15.97 | — | 77 |
| BTP-2FClO | PM6 | 0.961 | 23.30 | 73.22 | 16.40 | — | 77 |
| BO3Cl-α | PM6 | 0.890 | 21.25 | 74.56 | 14.11 | 0.228 | 78 |
| BO3Cl-γ | PM6 | 0.856 | 25.98 | 75.74 | 16.85 | 0.227 | 78 |
| BO3Cl-β | PM6 | 0.863 | 27.25 | 77.64 | 18.25 | 0.217 | 78 |
| BOEH3Cl-β | PM6 | 0.906 | 25.79 | 81.36 | 19.02 | 0.213 | 78 |
| Z8 | D18 | 0.890 | 26.70 | 78.40 | 18.60 | 0.19 | 79 |
3.3. Asymmetric side chain-based NFAs
The side chains of NFAs critically influence their crystallinity, packing behavior, and solubility.80,81 Therefore, rational asymmetric modification of the inner and outer side chains in Y6-based acceptors can effectively optimize photovoltaic performance. By fine-tuning the length, branching position, and polarity of alkyl chains on different molecular sites, one can achieve enhanced molecular ordering and appropriate phase separation in the bulk heterojunction. This optimization improves charge transport, reduces recombination losses, and increases VOC, thereby contributing to higher efficiency and stability in OSCs.Huang et al. engineered the asymmetric acceptor EH-HD-4F (Fig. 9) by modifying the inner alkyl chains of Y6, effectively enhancing its optical absorption and molecular orientation. When blended with PM6, the resulting device achieved an efficiency of 18.3% with a high JSC density of 27.48 mA cm−2.82 Later, Huang et al. further developed asymmetric acceptors with halogenated inner-side chains, DT-C8Cl and DT-C8BTz (Fig. 9), where halogen atoms could form non-covalent interactions with O, S and Se, leading to finely tuned film morphology (Fig. 10(a)). Ultimately, devices based on DT-C8Cl achieved an efficiency of 19.4%, attributed to enhanced π–π stacking, improved charge transport and reduced charge recombination. Moreover, the non-covalent interactions induced by the halogenated alkyl chains effectively suppressed unfavorable morphological evolution, thereby significantly improving the thermal and storage stability of the devices.83 Hu et al. synthesized asymmetric acceptors BTP-9F and BTP-17F (Fig. 9) by modulating the semi-fluorinated side chains on the pyrrole nitrogen. Among them, the BTP-9F-based device demonstrated more efficient polaron generation kinetics, reduced trap-state density, lower charge recombination, and superior vertical morphology, ultimately achieving an efficiency of 17.2%.84 Based on this, Hu et al. further constructed asymmetric acceptors EH-17F and HD-17F (Fig. 9) by modulating the size and length of the inner alkyl chains, incorporating optimally fluorinated side chains. As a result, the corresponding devices achieved improved molecular packing and morphology, ultimately delivering PCEs of 10.9% and 14.5%, respectively.85 Yan et al. designed an asymmetric acceptor, Y6-1O (Fig. 9), by replacing the outer alkyl chains of Y6 with alkoxy chains. Compared to Y6, Y6-1O exhibited better solubility and a higher VOC in the devices, ultimately achieving an efficiency of 16.1%.86 Based on this work, Yan et al. further constructed an asymmetric acceptor, BTP1O-4Cl-C12 (Fig. 9), through terminal-chlorination and inner alkyl chain modification. This strategy effectively balanced VOC and JSC while optimizing film morphology, ultimately achieving a device efficiency of 17.1%.87 Chen et al. developed asymmetric acceptors BTP-F0 and BTP-F5 (Fig. 9) through a side-chain fluorination strategy. Theoretical calculations revealed that fluorination increases the total average electrostatic potential (ESP) and charge balance factor of the acceptors, thereby modulating intermolecular interactions driven by ESP and precisely tuning molecular packing and active-layer morphology. As a result, BTP-F5-based binary and ternary devices achieved efficiencies of 17.3% and 19.2%, respectively.88 Sun et al. engineered the asymmetric acceptor L8-BO-C4 (Fig. 9) by strategically modulating the branching position of the outer alkyl chains on L8-BO. This design yielded similar void sizes, enhanced crystallinity (Fig. 10(b)) and a high photoluminescence quantum yield compared to L8-BO. Remarkably, the resulting ternary device achieved a record-breaking efficiency of 20.42%, with a VOC of 0.894 V and an FF of 81.6%.89 Li et al. developed asymmetric acceptors namely BTP-C11-TBO and BTP-BO-TBO (Fig. 9) featuring alkyl/thienyl hybrid side chains. This design effectively modulates intermolecular interactions to achieve higher luminescence efficiency and reduced energy loss. The corresponding devices demonstrated balanced charge transport and prolonged carrier lifetime. Ultimately, the BTP-BO-TBO-based device achieved an efficiency of 19.76% with a VOC of 0.913 V and an FF of 81.17%.90
 | ||
| Fig. 10 (a) The schematic presentation of noncovalent interactions induced by flexible haloalkyl chains and heterojunction structure with “rivet”-like halogen bonds. Reproduced with permission.83 Copyright 2024, Wiley-VCH. (b) 3D interpenetrating packing structures and 2D-GIWAXS images of L8-BO and L8-BO-C4. Reproduced with permission.89 Copyright 2025, Springer Nature. | ||
These results indicate that through the asymmetric side chain strategy, an optimal active layer morphology can be achieved while minimizing energy loss, leading to superior photovoltaic performance (Table 3). Therefore, when implementing this strategy, it is essential to select halogenated alkane chains for the inner side to enhance dipole interactions and energy level alignment, and incorporate branched alkyl chains combined with heterocyclic units for the outer side to improve solubility and fine-tune molecular packing and crystallinity.
| Acceptors | Donors | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | ΔE3 (eV) | Ref. |
|---|---|---|---|---|---|---|---|
| EH-HD-4F | PM6 | 0.840 | 27.50 | 79.30 | 18.30 | — | 82 |
| DT-C8Cl | D18 | 0.851 | 28.17 | 80.90 | 19.12 | 0.238 | 83 |
| DT-C8BTz | D18 | 0.849 | 26.75 | 79.00 | 17.67 | 0.245 | 83 |
| BTP-9F | PM6 | 0.848 | 27.00 | 75.10 | 17.20 | 0.264 | 84 |
| BTP-17F | PM6 | 0.832 | 25.20 | 66.80 | 14.2 | 0.285 | 84 |
| EH-17F | PM6 | 0.830 | 24.10 | 54.50 | 10.90 | 0.286 | 85 |
| HD-17F | PM6 | 0.860 | 25.20 | 67.00 | 14.50 | 0.268 | 85 |
| Y6-1O | PM6 | 0.890 | 23.20 | 78.30 | 16.10 | — | 86 |
| BTP1O-4Cl-C12 | PM6 | 0.910 | 23.85 | 78.80 | 17.10 | — | 87 |
| BTP-F0 | PM1 | 0.863 | 26.20 | 71.00 | 16.10 | — | 88 |
| BTP-F5 | PM1 | 0.855 | 26.90 | 75.10 | 17.30 | — | 88 |
| L8-BO-C4 | PM6 | 0.878 | 27.64 | 81.50 | 19.78 | 0.210 | 89 |
| BTP-C11-TBO | PM6 | 0.856 | 27.35 | 79.06 | 18.51 | 0.215 | 90 |
| BTP-BO-TBO | PM6 | 0.913 | 26.67 | 81.17 | 19.76 | 0.198 | 90 |
3.4. Multiple asymmetric NFAs
The asymmetric strategies targeting the central core, terminal groups, and side chains have successfully suppressed ΔE3 and enhanced device performance. Recently, chemists have begun employing multiple asymmetric approaches to synergistically optimize energy level alignment, molecular packing, and film morphology, thereby further improving charge generation and transport, as well as advancing the efficiency of asymmetric NFAs.Ma et al. developed dual-asymmetric acceptors, asy-YC11 and bi-asy-YC12 (Fig. 11), by modifying the alkyl chains of a selenophene-based asymmetric acceptor. Among them, bi-asy-YC12 exhibited appropriate phase separation and higher molecular ordering, leading to improved VOC and JSC, as well as an efficiency of 17.16% in the corresponding devices.91 Wang et al. engineered dual-asymmetric acceptors (AYT11Se9-Cl and AYT9Se11-Cl, Fig. 11) of the A–D1A′D2–A type by modifying an asymmetric selenophene-based core with tailored side chains. Compared to AYT11Se9-Cl, AYT9Se11-Cl exhibited denser and more ordered molecular packing. Additionally, its blend film demonstrated optimal phase separation and a pronounced face-on molecular orientation. Ultimately, the AYT9Se11-Cl-based device achieved an efficiency of 18.12%.92 Chen et al. combined core modulation and terminal-group engineering to construct dual-asymmetric acceptors, A-SSe-TCF and A-SSe-LSF (Fig. 11), both of which exhibited broadened light absorption and a significant dipole moment difference between their half-molecular structures. Among them, the A-SSe-TCF-based blend system demonstrated optimized phase separation, a vertically graded compositional distribution, and more ordered π–π stacking. The corresponding device achieved an efficiency of 18.53% alongside high VOC and JSC density. Furthermore, when incorporated into the PM6:L8-BO ternary system, the resulting device achieved a remarkable efficiency of 19.73%, highlighting the synergistic benefits of this molecular design.93 Wang et al. employed a multi-asymmetric strategy involving core, terminal, and side-chain modifications to construct four acceptors (DASe-4F, DASe-4Cl, TASe-2Cl2F and TASe-2F2Cl, Fig. 11). Among them, the trip-asymmetric TASe-2F2Cl exhibited a densely packed 3D network structure (Fig. 12), excellent crystallinity and improved electron mobility. Its blend film demonstrated optimal phase separation and highly ordered molecular stacking. As a result, the TASe-2F2Cl-based device achieved an impressive efficiency of 19.32% alongside an ultralow ΔE3 of 0.179 eV.94
 | ||
| Fig. 12 3D stacking viewed from the conjugated planes for A-OSeF, DASe-4Cl, and TASe-2F2Cl. Reproduced with permission.94 Copyright 2025, Wiley-VCH. | ||
In summary, the integration of a selenium-substituted central core with an odd number of rings, halogenated terminal units, and outer alkyl chains has enabled device efficiency of above 19% with ΔE3 below 0.2 eV (Table 4). Current dual-asymmetric strategies mainly encompass combinations of the core and side chains, as well as the core and terminal groups. The latter approach, however, often results in isomer formation. Effective separation of these isomers is critical to unraveling the structure–property relationships in multi-asymmetric acceptors and ultimately improving device efficiency. This challenge must be addressed when implementing a core-terminal dual-asymmetric design. Furthermore, outer alkyl chains incorporating heterocyclic units and inner chlorinated alkyl chains can also be explored for application in multi-asymmetric strategies.
| Acceptors | Donors | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] | ΔE3 (eV) | Ref. |
|---|---|---|---|---|---|---|---|
| asy-YC11 | PM6 | 0.849 | 26.74 | 72.70 | 16.52 | 0.219 | 91 |
| bi-asy-YC12 | PM6 | 0.871 | 26.39 | 0.743 | 17.16 | 0.225 | 91 |
| AYT11Se9-Cl | PM6 | 0.842 | 26.86 | 77.47 | 17.52 | — | 92 |
| AYT9Se11-Cl | PM6 | 0.843 | 27.40 | 78.43 | 18.12 | — | 92 |
| A-SSe-TCF | PM6 | 0.851 | 27.86 | 78.14 | 18.53 | 0.244 | 93 |
| A-SSe-LSF | PM6 | 0.853 | 26.43 | 73.95 | 16.68 | 0.240 | 93 |
| DASe-4F | PM1 | 0.858 | 27.58 | 77.22 | 18.27 | 0.203 | 94 |
| DASe-4Cl | PM1 | 0.852 | 26.71 | 75.80 | 17.25 | 0.210 | 94 |
| TASe-2Cl2F | PM1 | 0.853 | 26.10 | 73.20 | 16.30 | 0.196 | 94 |
| TASe-2F2Cl | PM1 | 0.879 | 27.52 | 79.88 | 19.32 | 0.179 | 94 |
4. Theoretical study of ΔE3 in Y-series asymmetric NFAs
To understand and reduce ΔE3 in non-fullerene OSCs, researchers have conducted extensive mechanistic and theoretical studies. Drawing on the intensity borrowing mechanism, Nelson,95 Gao,96 Brédas97 and others proposed a three-state model involving the CT state, the LE state, and a hybrid CT/LE state. Electronic coupling between the LE and CT states plays a critical role in non-radiative voltage loss. If the electronic coupling is zero, the non-radiative voltage loss depends linearly on energy offset; if coupling is present and increases, the non-radiative voltage loss is reduced. Gao et al. selected a series of non-fullerene acceptors and donors with varying energy offsets and found that a smaller energy difference between the CT and LE states enhances charge transfer rates. The radiative capacity of the CT state can be increased via the LE state through intensity borrowing. Moreover, a highly emissive LE state provides an additional radiative relaxation pathway for the CT state, leading to higher EQEEL and reduced ΔE3.96 Using time-dependent density functional theory (TD-DFT) calculations on the DRTB-T/IT-4F system, Yi et al. found that the CT state can borrow considerable oscillator strength from the LE state, and may even undergo complete hybridization with the LE state. This LE/CT hybridization facilitates efficient charge generation.98 Hou et al. introduced the concept of molecular surface ESP to explain efficient charge separation even under small driving forces in non-fullerene systems. A larger difference in ESP between donor and acceptor facilitates a stronger interfacial electric field, promoting charge generation.99 In subsequent research, the team studied the donor PM6 paired with acceptors BTP-0F, BTP-2F, BTP-4F, and BTP-6F. Both theoretical and experimental results showed that stronger electrostatic interaction enhances hybridization between CT and LE states, which improves charge generation but may also lead to higher ΔE3.100 Thus, by incorporating CT–LE hybridization into non-radiative recombination models, a new mechanism has been proposed to rationalize how NFA systems achieve high PCE with low non-radiative voltage losses, even under small energy offsets.As for theoretical study in Y-series asymmetric NFAs, Chen et al. employed molecular dynamics (MD) simulations to obtain blend models of different systems and utilized TD-DFT calculations to investigate the nature of interfacial CT states. The results demonstrate that asymmetric acceptors can form more diverse D:A interfacial conformations compared to symmetrical acceptors, while also inducing more favorable blend interfacial energetics (ΔELE-CT) (Fig. 13(a)). This optimized interface facilitates better balance between charge generation (larger ΔELE-CT) and recombination processes (smaller ΔELE-CT) while achieving a lower ΔE3.70 Based on this, Zuo et al. employed all-atom MD simulations and found that asymmetric acceptors also form diverse D:A interfaces with the higher interfacial CT state energy (ECT) which is directly relevant to ΔE3 (Fig. 13(b)). Experiments revealed that asymmetric acceptors exhibit lower ΔECT (ΔECT = Eoptg − ECT) and suppressed triplet (T1) formation (Fig. 2). Furthermore, according to the equation  , where kCT-T is the energy transfer rate from 3CT to T1, the lower kCT-T of asymmetric acceptors leads to a reduced voltage loss.101 Lu et al. combined investigations into the crystalline packing behavior of asymmetric acceptors with DFT calculations to quantify intermolecular interaction energies. They found that asymmetric acceptors exhibit stronger intermolecular interactions, with van der Waals attraction dominating between different terminal groups (Fig. 13(c)). Furthermore, compared to symmetric acceptors, devices based on asymmetric acceptors showed reduced ΔE3, which aligns with the calculation findings. The decreased ΔE3 may be attributed to the enhanced electronic coupling95,102 induced by the larger dipole moment of asymmetric acceptors, thereby promoting efficient exciton diffusion and reducing non-radiative decay rates.103 Through DFT calculations, Chen et al. discovered that asymmetric acceptors exhibit reduced disorder in surface electrostatic potential distribution and lower average electrostatic potential values, thereby weakening electrostatic interactions with PM6. Furthermore, the increased semi-molecular dipole moment difference and enhanced terminal electrostatic potential difference in asymmetric acceptors promote exciton dissociation and strengthen π–π interactions between terminal groups. The weakened D:A interactions caused by modified electrostatic potentials lead to decreased miscibility, consequently affecting both the vertical composition distribution of the active layer and the built-in potential. Additionally, this reduces the hybridization ratio of CT states at the D:A interface, resulting in improved EQEEL and suppression of ΔE3.104,105 Given the close relationship between the energy loss and charge transport properties, Li et al. employed DFT calculations to investigate the effects of asymmetric outer alkyl chain strategy on the electronic structure properties. In organic semiconductors, charge transport occurs via a hopping mechanism, and its rate can be determined using the Marcus rate equation:
, where kCT-T is the energy transfer rate from 3CT to T1, the lower kCT-T of asymmetric acceptors leads to a reduced voltage loss.101 Lu et al. combined investigations into the crystalline packing behavior of asymmetric acceptors with DFT calculations to quantify intermolecular interaction energies. They found that asymmetric acceptors exhibit stronger intermolecular interactions, with van der Waals attraction dominating between different terminal groups (Fig. 13(c)). Furthermore, compared to symmetric acceptors, devices based on asymmetric acceptors showed reduced ΔE3, which aligns with the calculation findings. The decreased ΔE3 may be attributed to the enhanced electronic coupling95,102 induced by the larger dipole moment of asymmetric acceptors, thereby promoting efficient exciton diffusion and reducing non-radiative decay rates.103 Through DFT calculations, Chen et al. discovered that asymmetric acceptors exhibit reduced disorder in surface electrostatic potential distribution and lower average electrostatic potential values, thereby weakening electrostatic interactions with PM6. Furthermore, the increased semi-molecular dipole moment difference and enhanced terminal electrostatic potential difference in asymmetric acceptors promote exciton dissociation and strengthen π–π interactions between terminal groups. The weakened D:A interactions caused by modified electrostatic potentials lead to decreased miscibility, consequently affecting both the vertical composition distribution of the active layer and the built-in potential. Additionally, this reduces the hybridization ratio of CT states at the D:A interface, resulting in improved EQEEL and suppression of ΔE3.104,105 Given the close relationship between the energy loss and charge transport properties, Li et al. employed DFT calculations to investigate the effects of asymmetric outer alkyl chain strategy on the electronic structure properties. In organic semiconductors, charge transport occurs via a hopping mechanism, and its rate can be determined using the Marcus rate equation:
 | ||
| Fig. 13 (a) PM6:BO-4Cl and (b) PM6:BO-5Cl complexes, as well as the relevant energies of the LE and CT states and their differences. Reproduced with permission.70 Copyright 2022, Springer Nature. (b) The distribution of CT energy of donor PM6 with end groups (A1, A2) of different acceptors. Reproduced with permission.101 Copyright 2024, Springer Nature. (c) Decomposed interaction energies between respective end groups of BTP-S1 and BTP-S2. Reproduced with permission.103 Copyright 2024, Wiley-VCH. (d) The reorganization energies of acceptors in the related transitions among the S0, S1, and the anionic state during the photoelectric conversion processes: λEET, λED and λCR are the reorganization energies for exciton energy transfer, exciton dissociation and nonradiative charge recombination, respectively. Reproduced with permission.90 Copyright 2025, Springer Nature. | ||
In summary, based on theoretical studies, the potential mechanisms of asymmetric acceptors at the terminal and side chain in inhibiting ΔE3 have been revealed: (1) asymmetric acceptors at the terminal and side chains form more diverse blended conformations with the donor while exhibiting more favorable ΔELE-CT; (2) the larger dipole moment of terminal asymmetric acceptors induces stronger electronic coupling between CT and LE states; (3) the proportion of CT states in its hybridization with the LE state decreases at the D:A blending interface for terminal asymmetric acceptors; (4) side chain asymmetric acceptors exhibit suppressed electron-vibration coupling (Fig. 14). The current mechanism is primarily derived from terminal- and side chain asymmetric acceptors, whereas the role of the central core and multiply asymmetric acceptors in regulating ΔE3 requires systematic investigation. Additionally, the existing conclusions are based on specifically designed asymmetric molecules, lacking exploration of universal principles. Therefore, with the continuous development of asymmetric acceptors and further in-depth theoretical studies, the intrinsic relationship between the molecular structure of asymmetric acceptors and ΔE3 in devices will become clearer.
5. Summary and outlook
The development of Y-series asymmetric acceptors represents a transformative advancement in OSCs, addressing critical challenges such as ΔE3 while pushing device efficiencies beyond 19%. This review systematically categorizes asymmetric design strategies into four key approaches: core engineering, terminal modulation, side-chain optimization, and multiple asymmetric integration. Core engineering, involving conjugation extension, heteroatom substitution, and isomerization, enhances dielectric properties and molecular packing. Terminal modifications, such as halogenation and conjugated extensions, optimize intermolecular π–π interactions and reduce ΔE3, enabling devices with efficiencies exceeding 19%. Side-chain engineering, including halogenated alkyl chains and fluorinated designs, fine-tunes morphology and crystallinity, contributing to a record efficiency of 20.42% in ternary systems. Multi-asymmetric strategies synergistically combine these approaches to minimize ΔE3 while maximizing charge transport. Crucially, theoretical studies reveal that asymmetric acceptors reduce ΔE3 by diversifying donor–acceptor interfaces, exhibiting more favorable ΔELE-CT, enhancing dipole moments, decreasing the CT state ratio and suppressing electron-vibration coupling. These innovations collectively demonstrate that asymmetric molecular design is pivotal for balancing VOC, JSC, and FF, thereby overcoming the efficiency bottlenecks of OSCs.Despite remarkable progress, several challenges and opportunities lie ahead for Y-series asymmetric acceptors. First, preliminary studies have been conducted on the regulation mechanism of ΔE3 based on asymmetric terminal-group and side-chain-based acceptors, but there is still a lack of refined structural characteristic parameters for asymmetric acceptors to establish the relationship between asymmetric molecular structures and non-radiative voltage loss. Building on this, machine learning methods could be integrated to accelerate the discovery of optimal asymmetric configurations. Next, acceptor materials constructed through multiple asymmetric strategies have demonstrated significant potential in realizing efficiency breakthroughs. However, such materials often contain isomers in their products, which hinders the study of structure–performance relationships and the improvement of device performance. Therefore, selecting appropriate synthetic methods to prepare highly pure and high-yield multiple asymmetric acceptors is an urgent challenge in this field. Then, current devices based on terminal and side chain asymmetric acceptors have better efficiency than that of central core asymmetric acceptors, and thus the design strategy based on dual asymmetric modifications of terminal-groups and side chains should be applied to develop high-efficiency NFAs with a low voltage loss. Ultimately, stability under operational conditions demands further improvement, leveraging halogenated side chains or cross-linkable moieties to enhance robustness. Additionally, integrating asymmetric acceptors with emerging technologies, such as tandem cells, semi-transparent devices, or indoor photovoltaics, could unlock new applications. Finally, unifying design principles across diverse material systems may yield universal strategies for minimizing energy losses. By addressing these challenges, asymmetric acceptors could not only bridge the efficiency gap with silicon and perovskite solar cells but also enable OSCs to meet the demands of next-generation renewable energy systems. The future of this field lies in interdisciplinary collaboration, combining molecular design, device engineering, and industrial partnerships to translate laboratory breakthroughs into real-world solutions.
Author contributions
Writing – original draft: Y. C., Z. C. and H. H.; writing – reviewing & editing: Y. C., S. Z., Z. W., H. X. and H. H.; funding acquisition: Y. C. and H. H.; supervision: Y. C. and H. H.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included, and no new data were generated or analyzed as part of this review.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22475037 and 22405166), the “Chenguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 24CGA71), the Fundamental Research Funds for the Central Universities (2232023A-01) and the Key Lab of Fluorine and Silicon for Energy Materials and Chemistry of the Ministry of Education, Jiangxi Normal University (No. KFSEMC-202202).References
- Y. Li, X. Huang, H. K. M. Sheriff and S. R. Forrest, Nat. Rev. Mater., 2022, 8, 186–201 CrossRef
.
- J. Luke, E. J. Yang, C. Labanti, S. Y. Park and J.-S. Kim, Nat. Rev. Mater., 2023, 8, 839–852 CrossRef CAS
.
- Z. Wang, Y. Bo, P. Bai, S. Zhang, G. Li, X. Wan, Y. Liu, R. Ma and Y. Chen, Science, 2023, 382, 1291–1296 CrossRef CAS
.
- W. Fan, Nat. Mater., 2025, 24, 324–325 CrossRef CAS
.
- W. Xiong, Y. Cui, Z. Zhang, S. Zhu, Z. Wang, Z. Chai, H. Hu and Y. Chen, Angew. Chem., Int. Ed., 2025, 64, e202500085 CrossRef CAS
.
- W. Liang, S. Zhu, K. Sun, J. Hai, Y. Cui, C. Gao, W. Li, Z. Wu, G. Zhang and H. Hu, Adv. Funct. Mater., 2024, 35, 2415499 CrossRef
.
- Z. Wang, S. Zhu, X. Peng, S. Luo, W. Liang, Z. Zhang, Y. Dou, G. Zhang, S. Chen, H. Hu and Y. Chen, Angew. Chem., Int. Ed., 2024, 64, e202412903 CrossRef
.
- L. Lyu, Z. Zhang, R. Lyu, S. Zhu, Y. Cui and H. Hu, ChemPhotoChem, 2024, 8, e202300256 CrossRef
.
- S. Zhu, L. Lyu, Y. Li, W. Li, Y. Cui and H. Hu, ACS Appl. Mater. Interfaces, 2024, 16, 33928–33934 CrossRef CAS
.
- W. Xiong, C. Zhang, S. Zhu, W. Liang, Z. Wang, G. Zhang, Z. Ma and H. Hu, ACS Appl. Polym. Mater., 2024, 6, 13735–13743 CrossRef CAS
.
- Y. Jiang, K. Liu, F. Liu, G. Ran, M. Wang, T. Zhang, R. Xu, H. Liu, W. Zhang, Z. Wei, Y. Cui, X. Lu, J. Hou and X. Zhu, Adv. Mater., 2025, 37, e2500282 CrossRef
.
- S. Zhu, Y. Cui, W. Xiong, C. Zhang, Y. Jiang, F. Liu, X. Shi, Z. Ma, X. Zhu, Y. Liao and H. Hu, Adv. Energy Mater., 2025, 15, 2502643 CrossRef CAS
.
- Z. Xu, S. Ding, W. Shi, W. Zhao, X. Cao, Z. Yao, Y. Guo, G. Long, C. Li, X. Wan and Y. Chen, Angew. Chem., Int. Ed., 2025, 64, e202504616 CrossRef CAS PubMed
.
- H. Lu, D. Li, W. Liu, G. Ran, H. Wu, N. Wei, Z. Tang, Y. Liu, W. Zhang and Z. Bo, Angew. Chem., Int. Ed., 2024, 63, e202407007 CrossRef CAS PubMed
.
- Z. Chen, J. Ge, W. Song, X. Tong, H. Liu, X. Yu, J. Li, J. Shi, L. Xie, C. Han, Q. Liu and Z. Ge, Adv. Mater., 2024, 36, 2406690 CrossRef CAS
.
- L. Chen, W. Liang, A. A. Sergeev, J. Y. L. Lai, X. Zeng, K. S. Wong, J. Zhang, S. H. Pun, H. Yan and H. Hu, Energy Environ. Sci., 2025, 18, 6608–6617 RSC
.
- M. Azzouzi, J. Yan, T. Kirchartz, K. Liu, J. Wang, H. Wu and J. Nelson, Phys. Rev. X, 2018, 8, 031055 CAS
.
- N. Wei, Y. Guo, H. Song, Y. Liu, H. Lu and Z. Bo, ChemSusChem, 2025, 18, e202402169 CrossRef CAS PubMed
.
- W. Liang, L. Chen, Z. Wang, Z. Peng, L. Zhu, C. H. Kwok, H. Yu, W. Xiong, T. Li, Z. Zhang, Y. Wang, Y. Liao, G. Zhang, H. Hu and Y. Chen, Adv. Energy Mater., 2024, 14, 2303661 CrossRef CAS
.
- X. Wan, C. Li, M. Zhang and Y. Chen, Chem. Soc. Rev., 2020, 49, 2828–2842 RSC
.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS
.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148–7151 CrossRef CAS
.
- H. Shi, H. Guo, D. Cai, J.-Y. Wang, Y. Ma and Q. Zheng, Energy Environ. Sci., 2025, 18, 2895–2904 RSC
.
- X. Xiong, S. Wan, B. Hu, Y. Li, Y. Ma, G. Lu, H. Fu and Q. Zheng, Adv. Energy Mater., 2024, 14, 2401816 CrossRef CAS
.
- Y. Zhu, H. Guo, X. Xiong, D. Cai, Y. Ma and Q. Zheng, Adv. Mater., 2024, 36, 2314169 CrossRef CAS
.
- D. Cai, J. Zhang, J.-Y. Wang, Y. Ma, S. Wan, P. Wang, Z. Wei and Q. Zheng, J. Mater. Chem. A, 2020, 8, 24543–24552 RSC
.
- Y. Ma, R. Sun, Z. Chen, S. Zhang, D. Cai, S. Wan, W. Lin, S.-Q. Zhang, Q. Tu, W. Ma, J. Min, X. Hao and Q. Zheng, Nano Energy, 2023, 107, 108116 CrossRef CAS
.
- D. Cai, Y. Ma, K. Xing, J.-Y. Wang, S. Luan, C. Tang, Y. Zhu, S.-C. Chen and Q. Zheng, Chem, 2024, 10, 3131–3147 CAS
.
- Q. Yue, W. Liu and X. Zhu, J. Am. Chem. Soc., 2020, 142, 11613–11628 CrossRef CAS PubMed
.
- K. Jiang, Q. Wei, J. Y. L. Lai, Z. Peng, H. K. Kim, J. Yuan, L. Ye, H. Ade, Y. Zou and H. Yan, Joule, 2019, 3, 3020–3033 CrossRef CAS
.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS
.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nat. Energy, 2021, 6, 605–613 CrossRef CAS
.
- S. Liu, J. Yuan, W. Deng, M. Luo, Y. Xie, Q. Liang, Y. Zou, Z. He, H. Wu and Y. Cao, Nat. Photonics, 2020, 14, 300–305 CrossRef CAS
.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 CrossRef CAS
.
- D. Li, C. Sun, T. Yan, J. Yuan and Y. Zou, ACS Cent. Sci., 2021, 7, 1787–1797 CrossRef CAS PubMed
.
- C. Li, H. Fu, T. Xia and Y. Sun, Adv. Energy Mater., 2019, 9, 1900999 CrossRef
.
- J. Song and Z. Bo, Chin. Chem. Lett., 2023, 34, 108163 CrossRef CAS
.
- T. Fritsch, J. Kurpiers, S. Roland, N. Tokmoldin, S. Shoaee, T. Ferron, B. A. Collins, S. Janietz, K. Vandewal and D. Neher, Adv. Energy Mater., 2022, 12, 2200641 CrossRef CAS
.
- A. A. Bakulin, A. Rao, V. G. Pavelyev, P. H. M. v Loosdrecht, M. S. Pshenichnikov, D. Niedzialek, J. Cornil, D. Beljonne and R. H. Friend, Science, 2012, 335, 1340–1344 CrossRef CAS PubMed
.
- J. Liu, S. Chen, D. Qian, B. Gautam, G. Yang, J. Zhao, J. Bergqvist, F. Zhang, W. Ma, H. Ade, O. Inganäs, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 16089 CrossRef CAS
.
- G. Han and Y. Yi, Adv. Theory Simul., 2019, 2, 1900067 CrossRef
.
- Q. Liu and K. Vandewal, Adv. Mater., 2023, 35, 2302452 CrossRef CAS PubMed
.
- N. Banerji, Nat. Energy, 2021, 6, 775–776 CrossRef
.
- B. Fan, X. Du, F. Liu, W. Zhong, L. Ying, R. Xie, X. Tang, K. An, J. Xin, N. Li, W. Ma, C. J. Brabec, F. Huang and Y. Cao, Nat. Energy, 2018, 3, 1051–1058 CrossRef CAS
.
- Y. Wang, J. Yu, R. Zhang, J. Yuan, S. Hultmark, C. E. Johnson, N. P. Gallop, B. Siegmund, D. Qian, H. Zhang, Y. Zou, M. Kemerink, A. A. Bakulin, C. Müller, K. Vandewal, X.-K. Chen and F. Gao, Nat. Energy, 2023, 8, 978–988 CrossRef
.
- G. Cai, Z. Chen, M. Li, Y. Li, P. Xue, Q. Cao, W. Chi, H. Liu, X. Xia, Q. An, Z. Tang, H. Zhu, X. Zhan and X. Lu, Adv. Sci., 2022, 9, 2103428 CrossRef CAS
.
- D. He, F. Zhao, C. Wang and Y. Lin, Adv. Funct. Mater., 2022, 32, 2111855 CrossRef CAS
.
- F. Zhao, H. Zhang, R. Zhang, J. Yuan, D. He, Y. Zou and F. Gao, Adv. Energy Mater., 2020, 10, 2002746 CrossRef CAS
.
- B. Fan, F. Lin, X. Wu, Z. Zhu and A. K. Jen, Acc. Chem. Res., 2021, 54, 3906–3916 CrossRef CAS PubMed
.
- F. Qi, L. Jones, K. Jiang, S.-H. Jang, W. Kaminsky, J. Oh, H. Zhang, Z. Cai, C. Yang, K. L. Kohlstedt, G. C. Schatz, F. Lin, T. Marks and A. K. Y. Jen, Mater. Horiz., 2021, 9, 403 RSC
.
- F. Qi, B. Fan, Q. Fan and A. K. Y. Jen, InfoMat, 2024, 6, e12595 CrossRef CAS
.
- W. Gao, X. Ma, Q. An, J. Gao, C. Zhong, F. Zhang and C. Yang, J. Mater. Chem. A, 2020, 8, 14583–14591 RSC
.
- W. Gao, H. Fu, Y. Li, F. Lin, R. Sun, Z. Wu, X. Wu, C. Zhong, J. Min, J. Luo, H. Y. Woo, Z. Zhu and A. K. Y. Jen, Adv. Energy Mater., 2020, 11, 2003177 CrossRef
.
- W. Gao, B. Fan, F. Qi, F. Lin, R. Sun, X. Xia, J. Gao, C. Zhong, X. Lu, J. Min, F. Zhang, Z. Zhu, J. Luo and A. K. Y. Jen, Adv. Funct. Mater., 2021, 31, 2104369 CrossRef CAS
.
- Z. Luo, R. Ma, Y. Xiao, T. Liu, H. Sun, M. Su, Q. Guo, G. Li, W. Gao, Y. Chen, Y. Zou, X. Guo, M. Zhang, X. Lu, H. Yan and C. Yang, Small, 2020, 16, e2001942 CrossRef PubMed
.
- Z. Luo, R. Ma, Y. Xiao, F. Ni, T. Liu, Q. Zhan, H. Sun, Y. Chen, Y. Zou, X. Lu, H. Yan and C. Yang, Small Struct., 2020, 2, 2000052 CrossRef
.
- Y. Zhang, Y. Ji, Y. Zhang, W. Zhang, H. Bai, M. Du, H. Wu, Q. Guo and E. Zhou, Adv. Funct. Mater., 2022, 32, 2205115 CrossRef CAS
.
- W. Liu, S. Sun, L. Zhou, Y. Cui, W. Zhang, J. Hou, F. Liu, S. Xu and X. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202116111 CrossRef CAS PubMed
.
- C. Yang, Q. An, H. R. Bai, H. F. Zhi, H. S. Ryu, A. Mahmood, X. Zhao, S. Zhang, H. Y. Woo and J. L. Wang, Angew. Chem., Int. Ed., 2021, 60, 19241–19252 CrossRef CAS PubMed
.
- C. Yang, Q. An, M. Jiang, X. Ma, A. Mahmood, H. Zhang, X. Zhao, H. F. Zhi, M. H. Jee, H. Y. Woo, X. Liao, D. Deng, Z. Wei and J. L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202313016 CrossRef CAS PubMed
.
- G. Han, T. Hu and Y. Yi, Adv. Mater., 2020, 32, 2000975 CrossRef CAS PubMed
.
- W. Zheng, J. Liu, Y. Guo, G. Han and Y. Yi, Adv. Funct. Mater., 2021, 32, 2108551 CrossRef
.
- T. Li, G. Hu, L. Tao, J. Jiang, J. Xin, Y. Li, W. Ma, L. Shen, Y. Fang and Y. Lin, Sci. Adv., 2023, 9, eadf6152 CrossRef CAS PubMed
.
- M. V. Putz, N. Russo and E. Sicilia, Theor. Chem. Acc., 2005, 114, 38–45 Search PubMed
.
- T. Liu, Y. Zhang, Y. Shao, R. Ma, Z. Luo, Y. Xiao, T. Yang, X. Lu, Z. Yuan, H. Yan, Y. Chen and Y. Li, Adv. Funct. Mater., 2020, 30, 2000456 CrossRef CAS
.
- S. Li, L. Zhan, Y. Jin, G. Zhou, T. K. Lau, R. Qin, M. Shi, C. Z. Li, H. Zhu, X. Lu, F. Zhang and H. Chen, Adv. Mater., 2020, 32, 2001160 CrossRef CAS PubMed
.
- S. Li, L. Zhan, N. Yao, X. Xia, Z. Chen, W. Yang, C. He, L. Zuo, M. Shi, H. Zhu, X. Lu, F. Zhang and H. Chen, Nat. Commun., 2021, 12, 4627 CrossRef CAS PubMed
.
- Z. Luo, R. Ma, T. Liu, J. Yu, Y. Xiao, R. Sun, G. Xie, J. Yuan, Y. Chen, K. Chen, G. Chai, H. Sun, J. Min, J. Zhang, Y. Zou, C. Yang, X. Lu, F. Gao and H. Yan, Joule, 2020, 4, 1236–1247 CrossRef CAS
.
- C. He, Z. Bi, Z. Chen, J. Guo, X. Xia, X. Lu, J. Min, H. Zhu, W. Ma, L. Zuo and H. Chen, Adv. Funct. Mater., 2022, 32, 2112511 CrossRef CAS
.
- C. He, Z. Chen, T. Wang, Z. Shen, Y. Li, J. Zhou, J. Yu, H. Fang, Y. Li, S. Li, X. Lu, W. Ma, F. Gao, Z. Xie, V. Coropceanu, H. Zhu, J. L. Bredas, L. Zuo and H. Chen, Nat. Commun., 2022, 13, 2598 CrossRef CAS PubMed
.
- R. Sun, Y. Wu, X. Yang, Y. Gao, Z. Chen, K. Li, J. Qiao, T. Wang, J. Guo, C. Liu, X. Hao, H. Zhu and J. Min, Adv. Mater., 2022, 34, e2110147 CrossRef PubMed
.
- Y. Pan, X. Zheng, J. Guo, Z. Chen, S. Li, C. He, S. Ye, X. Xia, S. Wang, X. Lu, H. Zhu, J. Min, L. Zuo, M. Shi and H. Chen, Adv. Funct. Mater., 2021, 32, 2108614 CrossRef
.
- S. Shi, S. Zhang, X. Yao, K. Xian, D. Han, Y. Zhu, Y. Li, X. Tu, Z. Tang, L. Ye, H. Zhong and Z. Fei, J. Mater. Chem. C, 2023, 11, 11943 RSC
.
- P. Gopikrishna, J. Rhee, S. Yoon, D. Um, H. Jin, Y. Jun, H. Choi, H. J. Son and B. Kim, Adv. Funct. Mater., 2023, 33, 2305541 CrossRef CAS
.
- M. Deng, X. Xu, Y. Duan, W. Qiu, L. Yu, R. Li and Q. Peng, Adv. Mater., 2023, 36, 2308216 CrossRef
.
- W. Wu, B. Zou, R. Ma, J. Yao, C. Li, Z. Luo, B. Xie, M. Qammar, T. A. Dela Pena, M. Li, J. Wu, C. Yang, Q. Fan, W. Ma, G. Li and H. Yan, Small, 2024, 20, e2402793 CrossRef PubMed
.
- B. Zou, A. Liang, P. Ding, J. Yao, X. Zeng, H. Li, R. Ma, C. Li, W. Wu, D. Chen, M. Qammar, H. Yu, J. Yi, L. Guo, S. H. Pun, J. E. Halpert, G. Li, Z. Kan and H. Yan, Angew. Chem., Int. Ed., 2025, 65, e202415332 Search PubMed
.
- H. Lai, Y. Lang, Y. Luo, Z. Deng, Y. Wang, D. Qiu, R. Sun, G. Zhang, J. Wu, G. Li and F. He, Adv. Energy Mater., 2025, 2406097 CrossRef
.
- Y. Jiang, S. Sun, R. Xu, F. Liu, X. Miao, G. Ran, K. Liu, Y. Yi, W. Zhang and X. Zhu, Nat. Energy, 2024, 9, 975–986 CrossRef CAS
.
- L. Tian, C. Liu and F. Huang, Sci. China: Chem., 2023, 66, 788–805 Search PubMed
.
- Z. Luo, T. Xu, C. Zhang and C. Yang, Energy Environ. Sci., 2023, 16, 2732–2758 RSC
.
- S. Chen, L. Feng, T. Jia, J. Jing, Z. Hu, K. Zhang and F. Huang, Sci. China: Chem., 2021, 64, 1192–1199 CrossRef CAS
.
- S. Chen, S. Zhu, L. Hong, W. Deng, Y. Zhang, Y. Fu, Z. Zhong, M. Dong, C. Liu, X. Lu, K. Zhang and F. Huang, Angew. Chem., Int. Ed., 2024, 63, e202318756 CrossRef CAS
.
- X. Zhou, W. Liang, R. Ma, C. Zhang, Z. Peng, T. A. P. Dela Peña, J. Wu, Z. Ma, Y. Liao, G. Li and H. Hu, Energy Environ. Sci., 2024, 17, 7762–7771 RSC
.
- S. Liu, T. Li, X. Zhou, W. Liang, S. Zhu, W. Xiong, Y. Cui, H. Hu and Y. Chen, Chem. Commun., 2024, 60, 12589 RSC
.
- Y. Chen, F. Bai, Z. Peng, L. Zhu, J. Zhang, X. Zou, Y. Qin, H. K. Kim, J. Yuan, L. K. Ma, J. Zhang, H. Yu, P. C. Y. Chow, F. Huang, Y. Zou, H. Ade, F. Liu and H. Yan, Adv. Energy Mater., 2020, 11, 2003141 CrossRef
.
- Y. Chen, R. Ma, T. Liu, Y. Xiao, H. K. Kim, J. Zhang, C. Ma, H. Sun, F. Bai, X. Guo, K. S. Wong, X. Lu and H. Yan, Adv. Energy Mater., 2021, 11, 2003777 CrossRef CAS
.
- H. Hu, S. Liu, J. Xu, R. Ma, Z. Peng, T. A. Dela Pena, Y. Cui, W. Liang, X. Zhou, S. Luo, H. Yu, M. Li, J. Wu, S. Chen, G. Li and Y. Chen, Angew. Chem., Int. Ed., 2024, 63, e202400086 CrossRef CAS PubMed
.
- C. Li, J. Song, H. Lai, H. Zhang, R. Zhou, J. Xu, H. Huang, L. Liu, J. Gao, Y. Li, M. H. Jee, Z. Zheng, S. Liu, J. Yan, X. K. Chen, Z. Tang, C. Zhang, H. Y. Woo, F. He, F. Gao, H. Yan and Y. Sun, Nat. Mater., 2025, 24, 433–443 CrossRef CAS PubMed
.
- J. Guo, S. Qin, J. Zhang, C. Zhu, X. Xia, Y. Gong, T. Liang, Y. Zeng, G. Han, H. Zhuo, Y. Li, L. Meng, Y. Yi, J. Chen, X. Li, B. Qiu and Y. Li, Nat. Commun., 2025, 16, 1503 CrossRef CAS
.
- Q. Fan, R. Ma, J. Yang, J. Gao, H. Bai, W. Su, Z. Liang, Y. Wu, L. Tang, Y. Li, Q. Wu, K. Wang, L. Yan, R. Zhang, F. Gao, G. Li and W. Ma, Angew. Chem., Int. Ed., 2023, 62, e202308307 CrossRef CAS PubMed
.
- X. Zhao, Q. An, H. Zhang, C. Yang, A. Mahmood, M. Jiang, M. H. Jee, B. Fu, S. Tian, H. Y. Woo, Y. Wang and J. L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202216340 CrossRef CAS PubMed
.
- H. Yin, Y. Cui, D. Chen, S. Liu, T. Wu, M. Yu, L. Ye, A. Liang and Y. Chen, J. Am. Chem. Soc., 2025, 147, 8796–8808 CrossRef CAS PubMed
.
- C. Yang, Y. Gao, H. Zhang, Z. F. Yao, E. L. Li, H. H. Guan, H. F. Zhi, Q. Yuan, M. H. Jee, H. Y. Woo, J. Min and J. L. Wang, Angew. Chem., Int. Ed., 2025, 137, e202506795 CrossRef
.
- F. D. Eisner, M. Azzouzi, Z. Fei, X. Hou, T. D. Anthopoulos, T. J. S. Dennis, M. Heeney and J. Nelson, J. Am. Chem. Soc., 2019, 141, 6362–6374 CrossRef CAS
.
- D. Qian, Z. Zheng, H. Yao, W. Tress, T. R. Hopper, S. Chen, S. Li, J. Liu, S. Chen, J. Zhang, X. K. Liu, B. Gao, L. Ouyang, Y. Jin, G. Pozina, I. A. Buyanova, W. M. Chen, O. Inganäs, V. Coropceanu, J. L. Bredas, H. Yan, J. Hou, F. Zhang, A. A. Bakulin and F. Gao, Nat. Mater., 2018, 17, 703–709 CrossRef CAS PubMed
.
- X. K. Chen, V. Coropceanu and J. L. Bredas, Nat. Commun., 2018, 9, 5295 CrossRef CAS PubMed
.
- G. Han and Y. Yi, J. Phys. Chem. Lett., 2019, 10, 2911–2918 CrossRef CAS PubMed
.
- H. Yao, Y. Cui, D. Qian, C. S. Ponseca, A. Honarfar, Y. Xu, J. Xin, Z. Chen, L. Hong, B. Gao, R. Yu, Y. Zu, W. Ma, P. Chabera, T. Pullerits, A. Yartsev, F. Gao and J. Hou, J. Am. Chem. Soc., 2019, 141, 7743–7750 CrossRef CAS PubMed
.
- Y. Xu, H. Yao, L. Ma, L. Hong, J. Li, Q. Liao, Y. Zu, J. Wang, M. Gao, L. Ye and J. Hou, Angew. Chem., Int. Ed., 2020, 132, 9089–9095 CrossRef
.
- J. Huang, T. Chen, L. Mei, M. Wang, Y. Zhu, J. Cui, Y. Ouyang, Y. Pan, Z. Bi, W. Ma, Z. Ma, H. Zhu, C. Zhang, X.-K. Chen, H. Chen and L. Zuo, Nat. Commun., 2024, 15, 3287 CrossRef CAS PubMed
.
- A. J. Gillett, A. Privitera, R. Dilmurat, A. Karki, D. Qian, A. Pershin, G. Londi, W. K. Myers, J. Lee, J. Yuan, S. J. Ko, M. K. Riede, F. Gao, G. C. Bazan, A. Rao, T. Q. Nguyen, D. Beljonne and R. H. Friend, Nature, 2021, 597, 666–671 CrossRef CAS PubMed
.
- X. Xia, L. Mei, R. Sun, S. Li, C. Y. Chen, J. M. Lin, J. Min, H. Chen, X. K. Chen and X. Lu, Adv. Energy Mater., 2024, 14, 2303785 CrossRef CAS
.
- Y. Cui, P. Zhu, H. Hu, X. Xia, X. Lu, S. Yu, H. Tempeld, R.-A. Eichel, X. Liao and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202304931 CrossRef CAS
.
- Y. Cui, P. Zhu, X. Shi, X. Liao and Y. Chen, J. Phys. Chem. C, 2021, 125, 10250–10259 CrossRef CAS
.
- Y. Guo, L. Zhu, R. Duan, G. Han and Y. Yi, Chem. – Eur. J., 2023, 29, e202203356 CrossRef CAS
.
- Y. Shi, Y. Chang, K. Lu, Z. Chen, J. Zhang, Y. Yan, D. Qiu, Y. Liu, M. A. Adil, W. Ma, X. Hao, L. Zhu and Z. Wei, Nat. Commun., 2022, 13, 3256 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2026 |