 Open Access Article
Open Access ArticleExploiting intracellular oncogenic proteins to release cytotoxins
Matthias
Schild
 and
Dennis
Gillingham
and
Dennis
Gillingham
 *
*
Department of Chemistry, University of Basel, 4056 Basel, Switzerland. E-mail: dennis.gillingham@unibas.ch
First published on 15th January 2026
Abstract
The success of antibody-drug conjugates has demonstrated the value of targeted delivery strategies for cytotoxic molecules. However, many oncogenic drivers remain inaccessible to antibodies due to their intracellular location, and these drivers are currently mainly addressed using small molecule inhibitors. This work explores repurposing such inhibitors for the intracellular delivery and controlled release of cytotoxic payloads. Using click-to-release chemistry, a pre-targeting strategy was developed where inhibitor-tetrazine conjugates enable selective activation of systemically administered trans-cyclooctene (TCO) caged prodrugs. This concept was demonstrated using the epidermal growth factor receptor (EGFR), a key therapeutic target in non-small cell lung cancer. An afatinib-tetrazine conjugate achieved sufficient intracellular retention in EGFR-overexpressing cells to enable toxicity recovery from a TCO-protected monomethyl auristatin E (MMAE) derivative. Successful intracellular targeting and controlled payload release establish a foundation for expanding the scope of targeted drug delivery to previously inaccessible oncogenic drivers.
Introduction
Systemically administered chemotherapy and targeted oncology agents have proven highly effective in many types of cancer. Especially with chemotherapeutic agents, however, dose-limiting acute and long-term side effects limit their potential.1–4 In recent decades, active or ligand-targeted delivery methods have emerged to overcome toxicity by delivering the payload specifically to the desired site, thereby reducing systemic exposure.5,6 A typical approach is to couple an unspecific drug to a ligand that binds tightly with a cancer-associated biomarker. The targeting ligands can vary in composition (e.g. there are many cases of small molecules7,8 or peptides),9–11 but antibody-drug conjugates (ADCs), where antibodies are linked to cytotoxic payloads, are currently the most successful embodiment of the approach.12,13Upon reaching the intended site, payloads generally require release from their delivery carriers to become therapeutically active, except in certain cases such as with radioligands. This release is commonly facilitated by differences in stimuli between malignant and healthy tissue or through lysosomal cleavage after internalization. While enzymatic cleavage is the most widely used approach, differences in pH or redox environment have also been exploited.14 Unfortunately, these stimuli are not perfectly binary; hence an exogenous trigger could be helpful in minimizing premature and off-target release. Light-activation15 and click-to-release16 chemistry are the most common options. The click-to-release approach is particularly compelling; here (most commonly) alcohols in allylic positions on trans-cyclooctene (TCOs) can be eliminated following an inverse electron demand Diels–Alder (IEDDA)17 reaction with a tetrazine. By eliminating the need for endogenous release control, click-to-release has opened up a new field of research, with controlled release having been demonstrated using ADCs,18,19 peptide-drug conjugates,20,21 carbon nanotubes,22 nanoparticles,23 micelles,24–26 supramolecular assembly-based strategies/enzyme-instructed supramolecular self-assembly (EISA),27–29 metabolic glycoengineering,30 hydrogels,31–33 and small molecules.34,35
Targeted delivery for oncology requires distinguishing characteristics between healthy and cancerous tissue. This differentiation is typically achieved by targeting cancer specific cell surface markers (i.e. antibody or peptide targeting), or by exploiting drug activation driven by chemical or physiological differences in the tumor microenvironment.36 Many oncoproteins, however, remain inaccessible to these targeting methods because they are intracellular.37 This work proposes exploiting such intracellular oncogenic proteins for pre-targeting a click-to-release agent (here a tetrazine-bound inhibitor). Subsequent treatment with TCO-caged prodrugs would then trigger an IEDDA/allylic elimination cascade to release the drug. This strategy would have the dual benefit of inhibiting the original oncoprotein, while also releasing a toxin in response to its presence, which may hinder the emergence of resistance.38 PET tracers based on clinically approved small molecule inhibitors have successfully imaged tumors with specific oncoprotein expression, as demonstrated in human trials for the G12C mutation of Kirsten rat sarcoma gene (KRASG12C)39 and epidermal growth factor receptor (EGFR).40 Although cases of using intracellular targets to aid in drug release are rare, recently developed covalent addition/elimination systems are exciting innovations.41–44 Here we establish a viable working system for click-to-release activation based on covalent targeting of EGFR (Fig. 1). EGFR ticked all the boxes as a targeting handle since it is frequently mutated and/or overexpressed in malignant tumors,45 and has been a major focus of drug development (10 of the 80 FDA-approved kinase inhibitors target EGFR or the ERBB subfamily), meaning many good inhibitors are available.46
Results
Molecular design and target engagement
We settled on the covalent EGFR inhibitor afatanib (Afa, see bottom right of Scheme 1, first disclosed in 200847 and approved by the FDA in 2013)48 as the targeting ligand49 because of its covalent mechanism, and its established track record for integration in bifunctional molecules. For example, Afa derivatives have been used to visualize EGFR-expressing tumors in mice50 and humans,40 and also been used to create bifunctional protein degraders.51 Based on this prior art, we designed and synthesized a tetrazine bearing version of Afa (Afa-Tz, Scheme 1) where the linker connecting the tetrazine is installed at the position of the tetrahydrofuran ring in Afa. For the tetrazine, we selected the methyl/aryl substituted derivative (synthesis described in SI) because it is a workhorse structure in IEDDA bioconjugation52 and has even been used in phase I clinical trials for click-to-release activation.53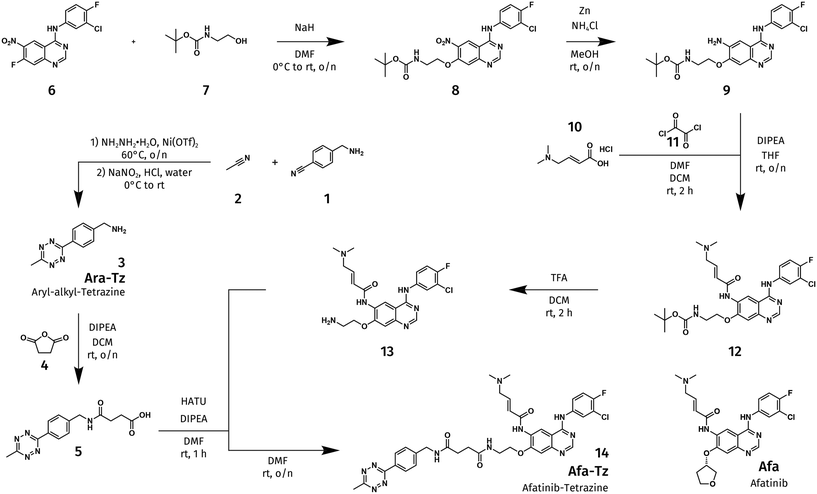 | ||
| Scheme 1 Synthesis of a version of afatinib bearing a tetrazine (Afa-Tz). Experimental details can be found in the SI. | ||
Before performing toxin release (Fig. 2A), we first needed to validate EGFR engagement and target selectivity with the Afa-Tz probe, as well as its proficiency at IEDDA. We therefore prepared a non-releasing TCO derivative bearing a Cy5.5 dye (Cy5.5-nrTCO, Fig. 2B), and used it to examine covalent targets of Afa-Tz in in-gel fluorescence assays (Fig. 2C–E). Specifically, the epidermoid carcinoma cells A431 (which are high in EGFR)47,54 were first treated with Afa-Tz for the appropriate time and then lysed, Cy5.5-nrTCO was then added before loading the samples on a gel (Fig. 2C). The data indicate (Fig. 2D) that Afa-Tz labels EGFR at both 1 μM and 500 nM, with optimal labelling at 12 h, although a robust signal is already seen at 1 h. Importantly, if we pre-treat the cells with the parent inhibitor Afa, the labelling with Afa-Tz is blocked and no fluorescent band is observed (Fig. 2E). EGFR western blotting (Fig. 5A) confirms the identity of the labelled band, and the lack of any other fluorescent bands in the complete gels (see Fig. S5 and S6) speaks to highly selective labelling. An interesting side-note is that when we block EGFR with Afa, there is a noticeable increase in off-target labelling (see last column of Fig. 2E). This can be rationalized by considering that when EGFR is not available as a rapid sink for Afa-Tz, its increased effective concentration promotes off-target, lower affinity binding events.
Click-to-release of the topoisomerase poison doxorubicin (Dox) and tubulin poison monomethylauristatin E (MMAE)
As our initial TCO-caged drug candidates we selected two well-studied cytotoxins: Dox and MMAE. Dox (Fig. 3A) is a topoisomerase poison that is widely used as chemotherapeutic agent,55 and whose known toxicity profile continues to drive efforts to develop safer delivery methods.56 It was among the first drugs demonstrated in click-to-release systems16 and now frequently serves as a benchmark for evaluating novel drug activation strategies57,58 and other papers citing.16MMAE (Fig. 3A) is a synthetic derivative of the tubulin poison Dolastatin,59 and can only be used as a therapeutic when conjugated with an antibody due to its high systemic toxicity.60 The synthesis of caged versions of these agents, Dox-TCO and MMAE-TCO followed established protocols57,61 and proceeded without event (detailed synthesis in the SI). We first evaluated the impact of TCO caging on the cytotoxic potency of both drugs independent of EGFR-targeting effects. We did this by measuring cell viability changes in A431 cells upon click-to-release reactions with the activating tetrazine, Afa-Tz.Dox-TCO displayed a 10-fold increase in IC50 compared to free Dox (Fig. 3A(iii); tabulated data for cell viability experiments can be found in the SI), a relatively modest attenuation. This raised concerns that the inherent background toxicity of Dox-TCO might overshadow potential benefits from selective activation – a concern highlighted by Morese et al.41 in their study of covalent ligand-directed release of 5-fluorouracil upon EGFR binding. In contrast, MMAE-TCO exhibited a substantial 111-fold increase in IC50 over free MMAE (8.1 ± 0.8 nM vs. 73 ± 10 pM), indicating a far greater attenuation of toxicity and thus a larger therapeutic window for activation. In parallel with these efforts we also examined the change in IC50 caused by the chemical changes we introduced on Afa to make Afa-Tz. Importantly, the Afa-Tz IC50 is 25 ± 9 μM (see Fig. S2), which is significantly higher than the Dox-TCO and MMAE-TCO IC50s, meaning there is low risk that effects below an IC50 of 10 μM have any connection to EGFR inhibition. Although Afa-Tz toxicity arising from EGFR inhibition is therapeutically desirable, for quantitatively studying the pre-targeting effect it is ideal to have a window where the pre-targeting driven toxicity is clearly differentiated from inhibition.
We next performed co-incubation experiments to evaluate re-activation efficiency of caged drugs by treating cells simultaneously with Afa-Tz and the respective TCO-caged drug (see Fig. 3B(i)). For Dox-TCO, using 5 μM Afa-Tz restored cytotoxicity to levels approaching free Dox (IC50 420 ± 100 nM). Even at lower Afa-Tz concentrations (1 μM), substantial cytotoxic enhancement was observed, clearly confirming the click-to-release activation. MMAE-TCO activation proved more effective: co-incubation with 5 μM Afa-Tz enhanced toxicity nearly 30-fold compared to MMAE-TCO alone (see Fig. 3B(ii)). At 1 μM Afa-Tz, activation remained strong and consistent down to 100 nM MMAE-TCO, underscoring MMAE's sensitivity to the click-to-release mechanism.
In vivo pre-targeting would rely on a subject's circulatory system to distribute the pre-targeting agent, where it would be transiently exposed to all tissues and cells, but gradually accumulate in the target tissue because that event is irreversible.62 To simulate these conditions in cell culture we used washing steps after a specified incubation time. Specifically, after seeding, cells were treated (i.e. pre-targeted) with Afa-Tz and incubated for a defined period (data in Fig. 2D led us to select 1–6 h), followed by multiple medium changes to remove unbound Afa-Tz. Subsequently, TCO-caged drug dilutions were added and the standard viability assay was run. Initial pre-targeting experiments with Dox-TCO (1 μM Afa-Tz for 1 h, followed by extensive washing) failed to demonstrate enhanced cytotoxicity compared to Dox-TCO alone. Increasing incubation times (up to 6 h) or Afa-Tz concentrations (5 μM) also yielded no improvement, suggesting that pre-targeting efficacy with Dox-TCO is limited by insufficient tetrazine retention or inadequate potency or both (see Fig. 4A(i)). Given these limitations, we shifted focus to MMAE-TCO, hoping that its higher potency would improve outcomes. Indeed, pre-targeting experiments with MMAE-TCO (5 μM Afa-Tz, 1 h) successfully demonstrated toxicity recovery, reducing the IC50 from 8.1 ± 0.8 nM (MMAE-TCO alone) to 1.0 ± 0.3 nM. Optimization of pre-targeting conditions further enhanced recovery, reaching an IC50 as low as 280 ± 50 pM (5 μM, 6 h; see Fig. 4A(ii)).
Our pre-targeting strategy relies on tetrazine retention through covalent binding to EGFR. Hence as a control for nonspecific retention, we performed pre-targeting assays with a minimal aryl-alkyl-tetrazine (Ara-Tz, see Scheme 1) under identical conditions to those used for Afa-Tz. These experiments confirmed that toxicity recovery depended primarily on specific EGFR engagement by Afa-Tz, although there does seem to be some background retention as Ara-Tz treatment leads to a slight increase in MMAE-TCO toxicity (see Fig. 4B). Co-incubation of MMAE-TCO with Afa-Tz or Ara-Tz (see Fig. 3B(ii) and S3), showed similar toxicity recovery (approximately 20-fold), confirming that the Tz incorporated in Ara-Tz is as efficient as the Afa-Tz in the IEDDA reaction. Under our initial conditions (5 μM and 1 h, see Fig. 4B), Ara-Tz pre-targeting showed only a two-fold toxicity increase relative to MMAE-TCO, indicating good (but incomplete) washout. In contrast, Afa-Tz demonstrated superior retention with an eight-fold toxicity increase (see Fig. 4A(ii)). At lower concentrations (1 μM and 1 h), both compounds showed similar but low toxicity recovery, suggesting that any observed activation by Afa-Tz at these lower concentrations likely resulted from unspecific retention rather than selective EGFR binding. Unselective cellular retention is a well-documented challenge in fluorescent imaging63,64 where background fluorescence can persist after multiple washes over extended periods.65–67 Nevertheless, the correlation between reactivation and EGFR expression supports EGFR-dependent click-to-release.
EGFR dependence
To examine the extent of EGFR dependency, we performed pre-targeting experiments on a panel of cell lines differing in EGFR expression: EGFR-positive A431 and MDA-MB-468, and EGFR-negative MCF-7 and SW620. Western blotting confirmed strong EGFR expression in A431 and MDA-MB-468, and minimal expression in MCF-7 and SW620 (Fig. 5A). Consistent with these expression patterns, target engagement assays showed robust Afa-Tz binding exclusively in EGFR-positive cells (Fig. 5B). Since Afa is a pan-ERBB inhibitor, the negative control cell lines should also express low levels of HER2, HER3, and HER4, and since we see no off-target bands in the target engagement assay, we can conclude that covalent targeting of other related ERBB proteins is not a confounding factor. | ||
| Fig. 5 Pre-targeting efficacy correlates with EGFR expression across cell lines. A. Western blot analysis reveals high EGFR expression in A431 and MDA-MB-468 and low expression in MCF-7 and SW620. Values from band integration normalized to total protein stain. B. In-gel fluorescence experiment (as in Fig. 2D) across the cell line panel. Strong bands at ∼175 kDa from Afa-Tz treatment are observed only in EGFR-high cell lines and can be blocked by pretreatment with Afa indicating selective EGFR targeting. C. Cell viability comparison across cell lines showing toxicities for parent drug MMAE, pro-drug MMAE-TCO, and pre-targeting with Afa-Tz (5 μM, 1 h) followed by MMAE-TCO treatment. EGFR-high cell lines demonstrate stronger toxicity recovery (8-fold and 7-fold enhancement) compared to EGFR-low cell lines (3-fold and 4-fold enhancement), though background activation is observed in all cases. Data points represent independent biological experiments, bars and error bars indicate mean and SEM, respectively. | ||
Having established the extent of covalent binding of Afa-Tz in the cell line panel, experiments were conducted to assess the various toxicities induced by this system. Initial measurements focused on baseline toxicities of MMAE and the protected drug MMAE-TCO. As shown in Fig. 5C, distinct toxicity profiles emerged across the cell lines. In A431, MMAE displayed an IC50 of 73 ± 10 pM, while MMAE-TCO showed reduced toxicity with an IC50 of 8.1 ± 0.8 nM, representing a 111-fold reduction. Similar fold-changes in toxicity were observed in MDA-MB-468 (115-fold) and SW620 (98-fold), though at higher absolute IC50 values. MDA-MB-468 exhibited IC50 values of 143 ± 11 pM for MMAE and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 800 pM for MMAE-TCO, while SW620 showed IC50 values of 105 ± 7 pM for MMAE and 10
500 ± 800 pM for MMAE-TCO, while SW620 showed IC50 values of 105 ± 7 pM for MMAE and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ± 700 pM for MMAE-TCO. MCF-7 cells showed a notably different pattern, with MMAE-TCO maintaining similar toxicity (IC50 = 7900 ± 800 pM) to other cell lines, but MMAE showing reduced potency (IC50 = 160 ± 30 pM), resulting in only a 49-fold reduction. In all cases, MMAE-TCO co-incubated with 5 μM Afa-Tz was approximately 5 times less toxic than MMAE itself, with the relative shift between cell lines remaining consistent.
300 ± 700 pM for MMAE-TCO. MCF-7 cells showed a notably different pattern, with MMAE-TCO maintaining similar toxicity (IC50 = 7900 ± 800 pM) to other cell lines, but MMAE showing reduced potency (IC50 = 160 ± 30 pM), resulting in only a 49-fold reduction. In all cases, MMAE-TCO co-incubated with 5 μM Afa-Tz was approximately 5 times less toxic than MMAE itself, with the relative shift between cell lines remaining consistent.
As toxicity recovery could be shown in all cell lines from co-incubating Afa-Tz and MMAE-TCO, we next pre-targeted the Afa-Tz to EGFR before treating with MMAE-TCO. As previously shown, A431 exhibited an 8-fold increase in toxicity under our standard pre-targeting conditions (5 μM for 1 h) with Afa-Tz (Fig. 4A(ii)). Applying these conditions in MDA-MB-468 led to a very similar 7-fold increase in toxicity. Although some toxicity recovery was observed in the negative control cell lines (see EGFR low cells in Fig. 5C), we could always clearly distinguish the EGFR effect.
Discussion
Most current click-to-release drug activation systems rely primarily on specific cell surface receptors or specific biomarkers in the tumor stroma to achieve targeting. In contrast, our approach uniquely exploits the stoichiometric binding of a tetrazine-conjugated ligand (Afa-Tz) to individual EGFR molecules inside the cell. While offering new opportunities for selectivity, our approach limits the maximum achievable drug release to the number of available EGFR binding sites per cell. Consequently, achieving therapeutically effective drug concentrations via this mechanism depends on the potency of the selected cytotoxic payload and the overexpression of the targeting receptor. Calculations indicate68 that even under ideal conditions, EGFR-targeted release of a drug like Dox would generate intracellular concentrations approaching only 1 μM. Given the moderate potency of Dox (IC50 around 0.42 μM in A431 cells), our system was insufficient to achieve observable therapeutic effects (top pathway in Fig. 6). This highlights the necessity of using more potent cytotoxins to fully exploit the potential of stoichiometric click-to-release strategies (represented graphically in Fig. 6). To address this, we turned to MMAE, a highly potent tubulin inhibitor widely utilized as an ADC payload. MMAE is several orders of magnitude more toxic than Dox, making it an ideal candidate to overcome stoichiometric limitations inherent in EGFR-targeted click-to-release. Indeed, our results confirmed that TCO-caged MMAE exhibited significantly greater potency and therapeutic window upon activation compared to Dox-TCO.Our caged constructs showed only moderate toxicity attenuation, which would result in substantial systemic toxicity – a key limitation to practical applications. However, this is a known problem in the field and others are actively investigating linker chemistry,69 release chemistry, and attachment position to improve this point.70,71 A more systematic investigation into drug “cage-ability” – with the goal of developing caged payloads that are substantially less toxic in their inactivated state – would benefit the field as a whole, as improved caging strategies can be directly integrated into any click-to-release platform.
Importantly, click-to-release activation of MMAE-TCO with Afa-Tz was successful even at low tetrazine concentrations, underscoring the sensitivity of MMAE to this activation mechanism. Pre-targeting assays further validated that effective drug activation was dependent on EGFR expression, although nonspecific cellular retention of the activating tetrazine remains a challenge requiring optimization. Importantly, our careful comparative analysis between two separate payloads across several cell lines gives a roadmap for future designs. Specifically, we now understand that complete deactivation of the caged pro-drug is important and that the bulk cellular retention of the pre-targeting agent should be quantified. The availability of clinically validated covalent ligands for important oncology and immunology targets is expanding rapidly, hence an exciting future prospect would be to repurpose some of these ligand/oncoprotein conjugates to drive pre-targeting. Moving beyond EGFR is also an important future direction, as is the possibility of achieving catalysis by using reversible covalent ligands72 (catalysis in receptor) or delivering molecular glue or PROTAC degraders (catalytic loss of target) or both.73,74 Related concepts are already being explored in the ADC world.75,76
Overall, our study demonstrates the critical importance of cytotoxic potency, target expression, and selective retention in designing effective pre-targeted drug delivery systems. MMAE's superior potency and robust activation profile position it as particularly suited to EGFR-targeted click-to-release approaches, highlighting a promising pathway for developing more effective and selective cancer therapies. Although targeting intracellular cancer-specific targets will likely not have the same target scope as extracellular ones, there are clear potential use cases, such as in G12C mutations of KRAS,77,78 in cysteine mutants of p53,79 or highly overexpressed kinases (like EGFR selected here) that contain ligandable cysteines.
Author contributions
M. S. conceptualized the study, developed and performed experiments, analyzed and visualized data and wrote the original draft and edited and revised the manuscript. D. G. initiated and conceptualized the study, supervised the project, and edited and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information: the SI includes detailed synthesis protocols and associated characterization data, uncropped gels related to images in the figures, detailed biological protocols including IC50 determinations. See DOI: https://doi.org/10.1039/d5md00764j.
Acknowledgements
We would like to thank Dr. Seemon Coomar and Dr. Angel Cores for fruitful discussions as well as Dr. Basilius Sauter for input on data analysis and visualization. We are grateful to Minqi Pan for assistance with NMR spectroscopy and HRMS measurements and Hortense Donteville for contributions to the synthesis. The University of Basel and the European Research Council (Horizon 2020, ExploDProteins, 866345) are gratefully acknowledged for supporting this work.References
- I. Raber and A. Asnani, Cardioprotection in cancer therapy: novel insights with anthracyclines, Cardiovasc. Res., 2019, 115, 915–921 Search PubMed.
- N. M. Kuderer, A. Desai, M. B. Lustberg and G. H. Lyman, Mitigating acute chemotherapy-associated adverse events in patients with cancer, Nat. Rev. Clin. Oncol., 2022, 19, 681–697 CrossRef PubMed.
- M. B. Lustberg, N. M. Kuderer, A. Desai, C. Bergerot and G. H. Lyman, Mitigating long-term and delayed adverse events associated with cancer treatment: implications for survivorship, Nat. Rev. Clin. Oncol., 2023, 20, 527–542 Search PubMed.
- R. Juthani, S. Punatar and I. Mittra, New light on chemotherapy toxicity and its prevention, BJC Rep., 2024, 2, 41 CrossRef PubMed.
- Z. Zhao, A. Ukidve, J. Kim and S. Mitragotri, Targeting Strategies for Tissue-Specific Drug Delivery, Cell, 2020, 181, 151–167 CrossRef CAS PubMed.
- M. Srinivasarao and P. S. Low, Ligand-Targeted Drug Delivery, Chem. Rev., 2017, 117, 12133–12164 CrossRef CAS PubMed.
- L. Wang, M. Tran, E. D'Este, J. Roberti, B. Koch, L. Xue and K. Johnsson, A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy, Nat. Chem., 2020, 12, 165–172 CrossRef CAS PubMed.
- T. K. Patel, N. Adhikari, S. A. Amin, S. Biswas, T. Jha and B. Ghosh, Small molecule drug conjugates (SMDCs): an emerging strategy for anticancer drug design and discovery, New J. Chem., 2021, 45, 5291–5321 RSC.
- V. P. Chavda, H. K. Solanki, M. Davidson, V. Apostolopoulos and J. Bojarska, Peptide-Drug Conjugates: A New Hope for Cancer Management, Molecules, 2022, 27, 7232 CrossRef CAS PubMed.
- T. T. Dean, J. Jelú-Reyes, A. L. C. Allen and T. W. Moore, Peptide–Drug Conjugates: An Emerging Direction for the Next Generation of Peptide Therapeutics, J. Med. Chem., 2024, 67, 1641–1661 CrossRef CAS PubMed.
- B. Zhang, M. Wang, L. Sun, J. Liu, L. Yin, M. Xia, L. Zhang, X. Liu and Y. Cheng, Recent Advances in Targeted Cancer Therapy: Are PDCs the Next Generation of ADCs?, J. Med. Chem., 2024, 67, 11469–11487 CrossRef CAS PubMed.
- C. Dumontet, J. M. Reichert, P. D. Senter, J. M. Lambert and A. Beck, Antibody–drug conjugates come of age in oncology, Nat. Rev. Drug Discovery, 2023, 22, 641–661 CrossRef CAS PubMed.
- Z. Fu, S. Li, S. Han, C. Shi and Y. Zhang, Antibody drug conjugate: the “biological missile” for targeted cancer therapy, Signal Transduction Targeted Ther., 2022, 7, 93 CrossRef CAS PubMed.
- J. D. Bargh, A. Isidro-Llobet, J. S. Parker and D. R. Spring, Cleavable linkers in antibody–drug conjugates, Chem. Soc. Rev., 2019, 48, 4361–4374 Search PubMed.
- E. Maurits, M. J. v. d. Graaff, S. Maiorana, D. P. A. Wander, P. M. Dekker, S. Y. v. d. Zanden, B. I. Florea, J. J. C. Neefjes, H. S. Overkleeft and S. I. v. Kasteren, Immunoproteasome Inhibitor–Doxorubicin Conjugates Target Multiple Myeloma Cells and Release Doxorubicin upon Low-Dose Photon Irradiation, J. Am. Chem. Soc., 2020, 142, 7250–7253 Search PubMed.
- R. M. Versteegen, R. Rossin, W. t. Hoeve, H. M. Janssen and M. S. Robillard, Click to Release: Instantaneous Doxorubicin Elimination upon Tetrazine Ligation, Angew. Chem., Int. Ed., 2013, 52, 14112–14116 CrossRef CAS PubMed.
- M. L. Blackman, M. Royzen and J. M. Fox, Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity, J. Am. Chem. Soc., 2008, 130, 13518–13519 Search PubMed.
- R. Rossin, R. M. Versteegen, J. Wu, A. Khasanov, H. J. Wessels, E. J. Steenbergen, W. ten Hoeve, H. M. Janssen, A. H. A. M. van Onzen, P. J. Hudson and M. S. Robillard, Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice, Nat. Commun., 2018, 9, 1484 CrossRef PubMed.
- R. Rossin, S. M. J. v. Duijnhoven, W. t. Hoeve, H. M. Janssen, L. H. J. Kleijn, F. J. M. Hoeben, R. M. Versteegen and M. S. Robillard, Triggered Drug Release from an Antibody–Drug Conjugate Using Fast “Click-to-Release” Chemistry in Mice, Bioconjugate Chem., 2016, 27, 1697–1706 CrossRef CAS PubMed.
- X. He, J. Li, X. Liang, W. Mao, X. Deng, M. Qin, H. Su and H. Wu, An all-in-one tetrazine reagent for cysteine-selective labeling and bioorthogonal activable prodrug construction, Nat. Commun., 2024, 15, 2831 Search PubMed.
- M. Chang, F. Gao, D. Pontigon, G. Gnawali, H. Xu and W. Wang, Bioorthogonal PROTAC Prodrugs Enabled by On-Target Activation, J. Am. Chem. Soc., 2023, 145, 14155–14163 Search PubMed.
- H. Li, J. Conde, A. Guerreiro and G. J. L. Bernardes, Tetrazine Carbon Nanotubes for Pretargeted In Vivo “Click-to-Release” Bioorthogonal Tumour Imaging, Angew. Chem., Int. Ed., 2020, 59, 16023–16032 Search PubMed.
- I. Khan, P. F. Agris, M. V. Yigit and M. Royzen, In situ activation of a doxorubicin prodrug using imaging-capable nanoparticles, Chem. Commun., 2016, 52, 6174–6177 Search PubMed.
- F. Suehiro, S. Fujii and T. Nishimura, Bioorthogonal micellar nanoreactors for prodrug cancer therapy using an inverse-electron-demand Diels–Alder reaction, Chem. Commun., 2022, 58, 7026–7029 RSC.
- L. Zuo, J. Ding, C. Li, F. Lin, P. R. Chen, P. Wang, G. Lu, J. Zhang, L.-L. Huang and H.-Y. Xie, Coordinating bioorthogonal reactions with two tumor-microenvironment-responsive nanovehicles for spatiotemporally controlled prodrug activation, Chem. Sci., 2020, 11, 2155–2160 Search PubMed.
- X. Xie, B. Li, J. Wang, C. Zhan, Y. Huang, F. Zeng and S. Wu, Bioorthogonal Nanosystem for Near-Infrared Fluorescence Imaging and Prodrug Activation in Mouse Model, ACS Mater. Lett., 2019, 1, 549–557 Search PubMed.
- Q. Yao, F. Lin, X. Fan, Y. Wang, Y. Liu, Z. Liu, X. Jiang, P. R. Chen and Y. Gao, Synergistic enzymatic and bioorthogonal reactions for selective prodrug activation in living systems, Nat. Commun., 2018, 9, 5032 CrossRef PubMed.
- C. Wu, J. Xie, Q. Yao, Y. Song, G. Yang, J. Zhao, R. Zhang, T. Wang, X. Jiang, X. Cai and Y. Gao, Intrahippocampal Supramolecular Assemblies Directed Bioorthogonal Liberation of Neurotransmitters to Suppress Seizures in Freely Moving Mice, Adv. Mater., 2024, 36, e2314310 Search PubMed.
- Q. Yao, S. Gao, C. Wu, T. Lin and Y. Gao, Enzymatic non-covalent synthesis of supramolecular assemblies as a general platform for bioorthogonal prodrugs activation to combat drug resistance, Biomaterials, 2021, 277, 121119 Search PubMed.
- M. M. A. Mitry, M. L. Dallas, S. Y. Boateng, F. Greco and H. M. I. Osborn, Selective activation of prodrugs in breast cancer using metabolic glycoengineering and the tetrazine ligation bioorthogonal reaction, Bioorg. Chem., 2024, 147, 107304 Search PubMed.
- J. M. M. Oneto, I. Khan, L. Seebald and M. Royzen, In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma, ACS Cent. Sci., 2016, 2, 476–482 Search PubMed.
- M. Czuban, S. Srinivasan, N. A. Yee, E. Agustin, A. Koliszak, E. Miller, I. Khan, I. Quinones, H. Noory, C. Motola, R. Volkmer, M. D. Luca, A. Trampuz, M. Royzen and J. M. M. Oneto, Bio-Orthogonal Chemistry and Reloadable Biomaterial Enable Local Activation of Antibiotic Prodrugs and Enhance Treatments against Staphylococcus aureus Infections, ACS Cent. Sci., 2018, 4, 1624–1632 Search PubMed.
- K. Wu, N. A. Yee, S. Srinivasan, A. Mahmoodi, M. Zakharian, J. M. M. Oneto and M. Royzen, Click activated protodrugs against cancer increase the therapeutic potential of chemotherapy through local capture and activation, Chem. Sci., 2021, 12, 1259–1271 Search PubMed.
- M. Chang, Y. Dong, H. Xu, A. B. Cruickshank-Taylor, J. S. Kozora, B. Behpour and W. Wang, Senolysis Enabled by Senescent Cell-Sensitive Bioorthogonal Tetrazine Ligation, Angew. Chem., Int. Ed., 2024, 63, e202315425 Search PubMed.
- N. A. M. Ligthart, M. A. R. de Geus, M. A. T. van de Plassche, D. Torres García, M. M. E. Isendoorn, L. Reinalda, D. Ofman, T. van Leeuwen and S. I. van Kasteren, A Lysosome-Targeted Tetrazine for Organelle-Specific Click-to-Release Chemistry in Antigen Presenting Cells, J. Am. Chem. Soc., 2023, 145, 12630–12640 CrossRef CAS PubMed.
- W. A. Denny, Tumor-activated Prodrugs—A New Approach to Cancer Therapy, Cancer Invest., 2004, 22, 604–619 Search PubMed.
- F. Martínez-Jiménez, F. Muiños, I. Sentís, J. Deu-Pons, I. Reyes-Salazar, C. Arnedo-Pac, L. Mularoni, O. Pich, J. Bonet, H. Kranas, A. Gonzalez-Perez and N. Lopez-Bigas, A compendium of mutational cancer driver genes, Nat. Rev. Cancer, 2020, 20, 555–572 Search PubMed.
- R. Yaeger, R. Mezzadra, J. Sinopoli, Y. Bian, M. Marasco, E. Kaplun, Y. Gao, H. Zhao, A. D. C. Paula, Y. Zhu, A. C. Perez, K. Chadalavada, E. Tse, S. Chowdhry, S. Bowker, Q. Chang, B. Qeriqi, B. Weigelt, G. J. Nanjangud, M. F. Berger, H. Der-Torossian, K. Anderes, N. D. Socci, J. Shia, G. J. Riely, Y. R. Murciano-Goroff, B. T. Li, J. G. Christensen, J. S. Reis-Filho, D. B. Solit, E. d. Stanchina, S. W. Lowe, N. Rosen and S. Misale, Molecular characterization of acquired resistance to KRAS G12C-EGFR inhibition in colorectal cancer, Cancer Discovery, 2022, 13, 41–55 CrossRef PubMed.
- X. Li, J. Ye, J. Wang, Z. Quan, G. Li, W. Ma, M. Zhang, W. Yang, J. Wang, T. Ma, F. Kang and J. Wang, First-in-Humans PET Imaging of KRASG12C Mutation Status in Non–Small Cell Lung and Colorectal Cancer Patients Using 18F PFPMD, J. Nucl. Med., 2023, 64, 1880–1888 CrossRef CAS PubMed.
- X. Sun, Z. Xiao, G. Chen, Z. Han, Y. Liu, C. Zhang, Y. Sun, Y. Song, K. Wang, F. Fang, X. Wang, Y. Lin, L. Xu, L. Shao, J. Li, Z. Cheng, S. S. Gambhir and B. Shen, A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management, Sci. Transl. Med., 2018, 10, eaan8840 CrossRef PubMed.
- P. A. Morese, N. Anthony, M. Bodnarchuk, C. Jennings, M. P. Martin, R. A. Noble, N. Phillips, H. D. Thomas, L. Z. Wang, A. Lister, M. E. M. Noble, R. A. Ward, S. R. Wedge, H. L. Stewart and M. J. Waring, Targeting Cytotoxic Agents through EGFR-Mediated Covalent Binding and Release, J. Med. Chem., 2023, 66(17), 12324–12341 CrossRef CAS PubMed.
- R. N. Reddi, A. Rogel, R. Gabizon, D. G. Rawale, B. Harish, S. Marom, B. Tivon, Y. S. Arbel, N. Gurwicz, R. Oren, K. David, J. Liu, S. Duberstein, M. Itkin, S. Malitsky, H. Barr, B.-Z. Katz, Y. Herishanu, I. Shachar, Z. Shulman and N. London, Sulfamate Acetamides as Self-Immolative Electrophiles for Covalent Ligand-Directed Release Chemistry, J. Am. Chem. Soc., 2023, 145, 3346–3360 CrossRef CAS PubMed.
- R. N. Reddi, A. Rogel, E. Resnick, R. Gabizon, P. K. Prasad, N. Gurwicz, H. Barr, Z. Shulman and N. London, Site-Specific Labeling of Endogenous Proteins Using CoLDR Chemistry, J. Am. Chem. Soc., 2021, 143, 20095–20108 CrossRef CAS PubMed.
- R. N. Reddi, E. Resnick, A. Rogel, B. V. Rao, R. Gabizon, K. Goldenberg, N. Gurwicz, D. Zaidman, A. Plotnikov, H. Barr, Z. Shulman and N. London, Tunable Methacrylamides for Covalent Ligand Directed Release Chemistry, J. Am. Chem. Soc., 2021, 143, 4979–4992 CrossRef CAS PubMed.
- C. L. Arteaga and J. A. Engelman, ERBB Receptors: From Oncogene Discovery to Basic Science to Mechanism-Based Cancer Therapeutics, Cancer Cell, 2014, 25, 282–303 Search PubMed.
- R. Roskoski, Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update, Pharmacol. Res., 2024, 200, 107059 Search PubMed.
- D. Li, L. Ambrogio, T. Shimamura, S. Kubo, M. Takahashi, L. R. Chirieac, R. F. Padera, G. I. Shapiro, A. Baum, F. Himmelsbach, W. J. Rettig, M. Meyerson, F. Solca, H. Greulich and K. K. Wong, BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models, Oncogene, 2008, 27, 4702–4711 CrossRef CAS PubMed.
- R. T. Dungo and G. M. Keating, Afatinib: First Global Approval, Drugs, 2013, 73, 1503–1515 CrossRef CAS PubMed.
- F. Solca, G. Dahl, A. Zoephel, G. Bader, M. Sanderson, C. Klein, O. Kraemer, F. Himmelsbach, E. Haaksma and G. R. Adolf, Target Binding Properties and Cellular Activity of Afatinib (BIBW 2992), an Irreversible ErbB Family Blocker, J. Pharmacol. Exp. Ther., 2012, 343, 342–350 Search PubMed.
- S. Liu, W. Song, X. Gao, Y. Su, E. Gao and Q. Gao, Discovery of Nonpeptide, Reversible HER1/HER2 Dual-Targeting Small-Molecule Inhibitors as Near-Infrared Fluorescent Probes for Efficient Tumor Detection, Diagnostic Imaging, and Drug Screening, Anal. Chem., 2019, 91, 1507–1515 CrossRef CAS PubMed.
- G. M. Burslem, B. E. Smith, A. C. Lai, S. Jaime-Figueroa, D. C. McQuaid, D. P. Bondeson, M. Toure, H. Dong, Y. Qian, J. Wang, A. P. Crew, J. Hines and C. M. Crews, The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study, Cell Chem. Biol., 2018, 25, 67–77 e63 Search PubMed.
- B. L. Oliveira, Z. Guo and G. J. L. Bernardes, Inverse electron demand Diels–Alder reactions in chemical biology, Chem. Soc. Rev., 2017, 46, 4895–4950 RSC.
- J. M. McFarland, M. a. Alečković, G. Coricor, S. Srinivasan, M. Tso, J. Lee, T.-H. Nguyen and J. M. M. a. Oneto, Click Chemistry Selectively Activates an Auristatin Protodrug with either Intratumoral or Systemic Tumor-Targeting Agents, ACS Cent. Sci., 2023, 9, 1400–1408 CrossRef CAS PubMed.
- G. T. Merlino, Y.-H. Xu, S. Ishii, A. J. L. Clark, K. Semba, K. Toyoshima, T. Yamamoto and I. Pastan, Amplification and Enhanced Expression of the Epidermal Growth Factor Receptor Gene in A431 Human Carcinoma Cells, Science, 1984, 224, 417–419 CrossRef CAS PubMed.
- S. Sritharan and N. Sivalingam, A comprehensive review on time-tested anticancer drug doxorubicin, Life Sci., 2021, 278, 119527 Search PubMed.
- P. Schöffski, J.-P. Delord, E. Brain, J. Robert, H. Dumez, J. Gasmi and A. Trouet, First-in-man phase I study assessing the safety and pharmacokinetics of a 1-hour intravenous infusion of the doxorubicin prodrug DTS-201 every 3 weeks in patients with advanced or metastatic solid tumours, Eur. J. Cancer, 2017, 86, 240–247 CrossRef PubMed.
- R. Rossin, S. M. J. van Duijnhoven, W. ten Hoeve, H. M. Janssen, L. H. J. Kleijn, F. J. M. Hoeben, R. M. Versteegen and M. S. Robillard, Triggered Drug Release from an Antibody–Drug Conjugate Using Fast “Click-to-Release” Chemistry in Mice, Bioconjugate Chem., 2016, 27, 1697–1706 CrossRef CAS PubMed.
- I. Khan, P. F. Agris, M. V. Yigit and M. Royzen, In situ activation of a doxorubicin prodrug using imaging-capable nanoparticles, Chem. Commun., 2016, 52, 6174–6177 Search PubMed.
- R. L. Bai, G. R. Pettit and E. Hamel, Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites, J. Biol. Chem., 1990, 265, 17141–17149 CrossRef CAS PubMed.
- A. Younes, U. Yasothan and P. Kirkpatrick, Brentuximab vedotin, Nat. Rev. Drug Discovery, 2012, 11, 19–20 Search PubMed.
- S. Gnaim, A. Scomparin, S. Das, R. Blau, R. Satchi-Fainaro and D. Shabat, Direct Real-Time Monitoring of Prodrug Activation by Chemiluminescence, Angew. Chem., Int. Ed., 2018, 57, 9033–9037 CrossRef CAS PubMed.
- M. Handula, K.-T. Chen and Y. Seimbille, IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications, Molecules, 2021, 26, 4640 CrossRef CAS PubMed.
- J. J. Gruskos, G. Zhang and D. Buccella, Visualizing Compartmentalized Cellular Mg2+ on Demand with Small-Molecule Fluorescent Sensors, J. Am. Chem. Soc., 2016, 138, 14639–14649 CrossRef CAS PubMed.
- L. Wang, M. Tran, E. D'Este, J. Roberti, B. Koch, L. Xue and K. Johnsson, A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy, Nat. Chem., 2020, 12, 165–172 Search PubMed.
- E. Kozma, I. Nikić, B. R. Varga, I. V. Aramburu, J. H. Kang, O. T. Fackler, E. A. Lemke and P. Kele, Hydrophilic trans-Cyclooctenylated Noncanonical Amino Acids for Fast Intracellular Protein Labeling, ChemBioChem, 2016, 17, 1518–1524 CrossRef CAS PubMed.
- H.-J. Chen, C. Y. Chew, E.-H. Chang, Y.-W. Tu, L.-Y. Wei, B.-H. Wu, C.-H. Chen, Y.-T. Yang, S.-C. Huang, J.-K. Chen, I. C. Chen and K.-T. Tan, S-Cis Diene Conformation: A New Bathochromic Shift Strategy for Near-Infrared Fluorescence Switchable Dye and the Imaging Applications, J. Am. Chem. Soc., 2018, 140, 5224–5234 Search PubMed.
- J. E. Pigga, J. E. Rosenberger, A. Jemas, S. J. Boyd, O. Dmitrenko, Y. Xie and J. M. Fox, General, Divergent Platform for Diastereoselective Synthesis of trans-Cyclooctenes with High Reactivity and Favorable Physiochemical Properties, Angew. Chem., Int. Ed., 2021, 60, 14975–14980 CrossRef CAS PubMed.
- M. Srinivasarao, C. V. Galliford and P. S. Low, Principles in the design of ligand-targeted cancer therapeutics and imaging agents, Nat. Rev. Drug Discovery, 2015, 14, 203–219 Search PubMed.
- W. Kuba, B. Sohr, P. Keppel, D. Svatunek, V. Humhal, B. Stöger, M. Goldeck, J. C. T. Carlson and H. Mikula, Oxidative Desymmetrization Enables the Concise Synthesis of a trans-Cyclooctene Linker for Bioorthogonal Bond Cleavage, Chem. – Eur. J., 2023, 29, e202203069 CrossRef CAS PubMed.
- J. M. McFarland, M. Alečković, G. Coricor, S. Srinivasan, M. Tso, J. Lee, T.-H. Nguyen and J. M. Mejía Oneto, Click Chemistry Selectively Activates an Auristatin Protodrug with either Intratumoral or Systemic Tumor-Targeting Agents, ACS Cent. Sci., 2023, 9, 1400–1408 Search PubMed.
- X. Xu, Y. Wang, Y. Shi, X. Wang, X. Tong, Y. Chen, Y. Zhao, J. Chen, W. Guo and Y. Zheng, Development of Cyclooctyne-Nitrone Based Click Release Chemistry for Bioorthogonal Prodrug Activation both In Vitro and In Vivo, J. Am. Chem. Soc., 2025, 147, 34425–34437 CrossRef CAS PubMed.
- I. M. Serafimova, M. A. Pufall, S. Krishnan, K. Duda, M. S. Cohen, R. L. Maglathlin, J. M. McFarland, R. M. Miller, M. Frödin and J. Taunton, Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles, Nat. Chem. Biol., 2012, 8, 471 CrossRef CAS PubMed.
- D. Lu, X. Yu, H. Lin, R. Cheng, E. Y. Monroy, X. Qi, M. C. Wang and J. Wang, Applications of covalent chemistry in targeted protein degradation, Chem. Soc. Rev., 2022, 51, 9243–9261 RSC.
- H. Kiely-Collins, G. E. Winter and G. J. L. Bernardes, The role of reversible and irreversible covalent chemistry in targeted protein degradation, Cell Chem. Biol., 2021, 28, 952–968 CrossRef CAS PubMed.
- M. a. Maneiro, N. Forte, M. M. Shchepinova, C. S. Kounde, V. Chudasama, J. R. Baker and E. W. Tate, Antibody–PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4, ACS Chem. Biol., 2020, 15, 1306–1312 CrossRef CAS PubMed.
- K. Tsuchikama, Y. Anami, S. Y. Y. Ha and C. M. Yamazaki, Exploring the next generation of antibody–drug conjugates, Nat. Rev. Clin. Oncol., 2024, 21, 203–223 CrossRef CAS PubMed.
- J. Hallin, L. D. Engstrom, L. Hargis, A. Calinisan, R. Aranda, D. M. Briere, N. Sudhakar, V. Bowcut, B. R. Baer, J. A. Ballard, M. R. Burkard, J. B. Fell, J. P. Fischer, G. P. Vigers, Y. Xue, S. Gatto, J. Fernandez-Banet, A. Pavlicek, K. Velastagui, R. C. Chao, J. Barton, M. Pierobon, E. Baldelli, E. F. Patricoin, D. P. Cassidy, M. A. Marx, I. I. Rybkin, M. L. Johnson, S.-H. I. Ou, P. Lito, K. P. Papadopoulos, P. A. Jänne, P. Olson and J. G. Christensen, The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients, Cancer Discovery, 2020, 10(1), 54–71 CrossRef CAS PubMed.
- M. R. Janes, J. Zhang, L.-S. Li, R. Hansen, U. Peters, X. Guo, Y. Chen, A. Babbar, S. J. Firdaus, L. Darjania, J. Feng, J. H. Chen, S. Li, S. Li, Y. O. Long, C. Thach, Y. Liu, A. Zarieh, T. Ely, J. M. Kucharski, L. V. Kessler, T. Wu, K. Yu, Y. Wang, Y. Yao, X. Deng, P. P. Zarrinkar, D. Brehmer, D. Dhanak, M. V. Lorenzi, D. Hu-Lowe, M. P. Patricelli, P. Ren and Y. Liu, Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor, Cell, 2018, 172, 578–589.e517 CrossRef CAS PubMed.
- A. Sadagopan, M. Carson, E. J. Zamurs, N. Garaffo, H.-J. Chang, S. L. Schreiber, M. Meyerson and W. J. Gibson, Mutant p53 protein accumulation is selectively targetable by proximity-inducing drugs, Nat. Chem. Biol., 2025 DOI:10.1038/s41589-025-02051-7.
| This journal is © The Royal Society of Chemistry 2026 |





