Open Access Article
Open Access ArticleShortcut to highly π-extended optoelectronic systems based on the dibenzothiophene core
Clara
Fabregat
 ab,
Roger
Bujaldón
ab,
Roger
Bujaldón
 *ab,
Silvia
Oliva
a,
Jaume
Garcia-Amorós
*ab,
Silvia
Oliva
a,
Jaume
Garcia-Amorós
 ab,
Dmytro
Volyniuk
c,
Melika
Ghasemi
c,
Juozas V.
Grazulevicius
ab,
Dmytro
Volyniuk
c,
Melika
Ghasemi
c,
Juozas V.
Grazulevicius
 c,
Joaquim
Puigdollers
c,
Joaquim
Puigdollers
 d and
Dolores
Velasco
d and
Dolores
Velasco
 *ab
*ab
aGrup de Materials Orgànics, Departament de Química Inorgànica i Orgànica, Secció de Química Orgànica, Universitat de Barcelona, Martí i Franquès 1, Barcelona, E-08028, Spain. E-mail: rr.bujaldon@ub.edu; dvelasco@ub.edu
bInstitut de Nanociència i Nanotecnologia (IN2UB), Barcelona, E-08028, Spain
cDepartment of Polymer Chemistry and Technology, Kaunas University of Technology, Barsausko 59, Kaunas, Lithuania
dDept. Enginyeria Electrònica, Universitat Politècnica de Catalunya, Jordi Girona 1–3, Barcelona, E-08034, Spain
First published on 11th November 2025
Abstract
The Scholl reaction stands as a versatile tool to synthetize multiple π-extended systems via intramolecular C–C oxidative couplings. A prime example is the expansion of dibenzothiophene to polycyclic butterfly-shaped heterocycles, which claim key characteristics in diverse optoelectronic applications. Herein we describe a protocol from commercial tetrabromothiophene based on sequential one-pot Suzuki–Miyaura reactions followed by the Scholl reaction. This strategy permits rapid access to complex constructions fusing up to 11 rings in just two steps and improved yields. The proposed π-extensions successfully reduced the HOMO energy levels in the solid state to align with the gold work function (5.1 eV), while offering tunable photophysical properties. Remarkably, phenanthrene as scaffold endowed the core with a hole mobility value of 7 × 10−5 cm2 V−1 s−1 in OTFTs and excellent air-stability, with a shelf lifetime exceeding one year. Moreover, the inclusion of sulfurated units unlocked room temperature phosphorescence under oxygen-free conditions, a highly sought-after characteristic in metal- and halogen-free constructions. Their RTP quantum yields when introduced into a Zeonex matrix are as high as 14%, with oxygen-sensitive photoluminiscence that goes from deep-blue to yellow-orange. Altogether, this strategy holds great potential and versatility in developing adaptable materials for multiple functionalities.
Introduction
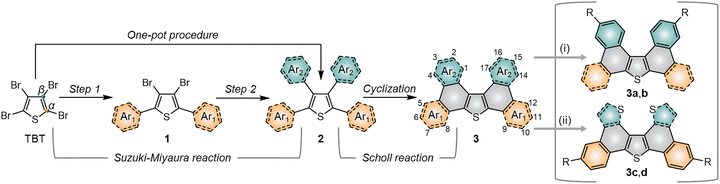
Organic materials provide unique characteristics that set them apart from their inorganic competitors, making them pivotal in next-generation electronics. Their inherent assets encompass from low-cost production processes to the fabrication of large-area and flexible devices.1–3 Moreover, their optoelectronic features can be easily adapted from a structural point of view to match the envisioned application.For organic semiconductors, π-conjugated systems with adequate energy levels generally grant a steady π–π stacking, translating into an efficient and fast charge transport.4,5 Thiophene is a recognized building block in the field of organic electronics.6–9 Indeed, nuclei such as benzothieno[3,2-b][1]benzothiophene (BTBT)10–14 or dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene (DNTT)15–18 are clear examples of thiophene-containing heterocycles currently acknowledged as top-performing organic semiconductors. Alternative π-expanded systems comprising branched and twisted architectures based on diphenanthro[9,10-b:9′,10′-d]thiophene (DPT) are equally engaging. Synthetically, the production of this latter core involves a 4-fold coupling of aromatic moieties to tetrabromothiophene (TBT), generally through the Suzuki–Miyaura reaction, and a subsequent intramolecular cyclization via the Scholl reaction19 (Scheme 1). Considering the easy availability of TBT as starting material and the immense library of aromatic commercially-available boronic acids, this strategy opens the way to diverse and tailored materials. So far, this approach has been exploited with assorted polycyclic structures.20–32
The DPT nucleus 3 features a butterfly-shaped π-system that deviates from planarity due to the steric hindrance between the C–H bonds in positions 1 and 17 (Ar2 in Scheme 1), a characteristic that is generally avoided in organic electronics. Regardless, the successful integration of some derivatives as hole transporting materials in devices encompassing single crystal FETs22 and perovskite solar cells33 certainly proves their potential. Non-planar π-conjugated systems, apart from offering alternative packing arrangements,34 can also circumvent the insolubility issues that hamper some top-performing materials for their use in more economical solution-processed devices.35–37 Moreover, this core has been recently confirmed as a metal- and halogen-free platform towards room temperature phosphorescence (RTP),38 a highly sought-after feature in applications like phosphorescent OLEDs, data encryption and oxygen sensing.39–43 Given the multifunctionality of this core, the access to diverse and more complex structures was appealing, especially via a versatile and straightforward synthetic route encompassing a broader scope of substituents.
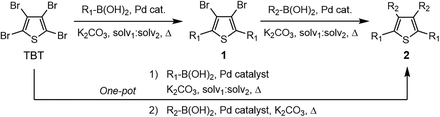
With this in mind, we designed two sets of compounds: (i) derivatives 3a,b, based on the expansion of the π-system through the aromatics in positions α of thiophene, especially those fusing numerous rings; (ii) derivatives 3c,d, based on the incorporation of sulfurated heterocycles in β to target more planar structures. Indeed, the substitution of C–H of phenyls by sulfur lessens the steric hindrance in that position, as demonstrated by some reported crystal structures.44 Nevertheless, either the incorporation of bulky aromatic extensions or the construction of more planar π-extended heterocycles could easily derive into major solubility problems. Thus, we introduced long alkyl chains to ensure reasonable solubility of the final products. Moreover, alkyl chains greatly modulate intermolecular interactions, which are key in solid-state properties.45–50 Overall, these designs imply a heterogeneous substitution to α and β positions of thiophene, requiring two consecutive two-fold cross-couplings instead of a homogeneous tetrasubstitution. In this step, we evaluated the viability of a one-pot procedure towards a more direct synthetic route.51 Fusing the central thiophene ring with additional thiophene derivatives at the periphery, however, represented a tricky point. Indeed, the precedents on thiophene-derived scaffolds coupled to tetrabromothiophene via the Suzuki–Miyaura reaction are scarce and often infructuous, especially to positions β.52 Alternatively, the few successful examples substitute the corresponding boronic acids for their respective pinacol esters53 or trihydroxyboronate salts.44 The Stille54 and Kumada55,56 cross-coupling reactions are also valid to achieve so. Since boronic acids are generally more accessible, though, we surveyed different synthetic conditions to prioritize their use.
The potential of this strategy is confirmed with the synthesis of the target π-extended compounds via sequential one-pot Suzuki–Miyaura and Scholl reactions, and the assessment of their photophysical, electrochemical and thermal properties. All derivatives were also investigated in two high-impact areas: as semiconductor materials in organic thin-film transistors (OTFTs) and as pure halogen-free organic emitters displaying RTP.
Experimental section
The information regarding chemicals, synthetic procedures and characterization is detailed in the SI.Structural characterization
1H NMR spectra were collected using either a Varian Mercury or a Bruker Avance III spectrometer operating at 400 MHz. 13C NMR measurements were carried out on a Bruker Avance III system at 100 MHz. All spectra were analysed with MestReNova software (version 14.0.0-23239), using the solvent signal as reference. Mass spectrometry was conducted on an Applied Biosystems MDS SCIEX 4800 instrument in reflector mode via MALDI-TOF. Single-crystal X-ray diffraction data were collected using a Bruker D8 Venture system equipped with a multilayer monochromator and a Mo Kα microfocus source (λ = 0.71073 Å). Data integration was performed using the Bruker SAINT software package with a narrow-frame algorithm. Structure solution and refinement were carried out using the SHELXTL software suite.Electrochemical and thermal characterization
Cyclic voltammetry measurements were conducted for the electrochemical characterization under argon atmosphere and quiescent conditions at 100 mV s−1 using a cylindrical three-electrode cell connected to a potentiostat/galvanostat (Autolab PGSTAT30) with NOVA software. An Ag/Ag+ electrode (10−3 M AgNO3 in acetonitrile) was used as the reference, a glassy carbon electrode was employed as the working one, while a platinum wire served as the counter electrode. The analysed compounds were dissolved in distilled dichloromethane (1 mM) with tetrabutylammonium hexafluorophosphate (TBAP) as the supporting electrolyte (0.1 M). All voltammetries were referred to the Fc+/Fc redox couple. Ionization potentials (IP) were estimated from the onset of the first oxidation peak (Eoxonset) as: IP = Eoxonset + 5.39 eV, with 5.39 eV being the energy level of the Fc+/Fc couple on the Fermi scale.57 Electron affinities (EA) were estimated as EA = IP – Eoptgap, in which the optical band gap (Eoptgap) was extracted from the onset wavelength of the UV-vis absorption spectrum. Ionization potentials in films (IPfilm) were acquired via electron photoemission spectroscopy in air. The samples were prepared by vacuum deposition (<10−6 mbar) over glass slides coated with fluorine-doped SnO2. The setup was composed of a 6517B Keithley electrometer, an ASBN-D130-CM deep UV deuterium light source and a CM110 1/8 m monochromator. The photoelectron emission spectra were acquired by irradiating the samples with increasing excitation energies, while monitoring the resulting photoemission current as a function of the excitation energy. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) thermograms were acquired under nitrogen atmosphere in a scan rate of 10 °C min−1, using a TA Instruments Q2000 calorimeter and a TA Instruments Q50, respectively.Charge transport characterization
Samples for time-of-flight (TOF) measurements were prepared by vacuum evaporation (<10−6 mbar) onto glass substrates coated with a 100 nm layer of indium-tin oxide (ITO). Subsequently, an aluminum layer (70 nm) was analogously deposited through a shadow mask to define the electrodes. Charge carriers were generated using a pulsed Nd:YAG laser (EKSPLA NL300) operating at an excitation wavelength of 355 nm and a pulse duration of 3–6 ns. The surface potential was controlled with a Keithley 6517B electrometer, and photocurrent transients were recorded using a Tektronix TDS 3032C oscilloscope. The carrier transit times (tt) were determined from the inflection point observed in the transient curve plotted on a log–log scale. Charge carrier drift mobility (μTOF) was calculated using the relation μTOF = d2/(U·tt), where d is the film thickness and U is the applied voltage at the moment of photoexcitation. The thickness of the films were measured using a Profilm3D profilometer. Zero-field mobilities (μ0,TOF) and the field-activation parameter (α) were extracted using the Poole–Frenkel-type relation μTOF = μ0,TOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(α·E1/2), where E is the applied electric field.
exp(α·E1/2), where E is the applied electric field.
OTFT devices were fabricated in a bottom-gate, top-contact configuration on thermally oxidized crystalline SiO2/Si wafers (SiO2 thickness of ca. 135 nm) coated with a polystyrene (PS) layer. The pre-treatment of the substrate and deposition of PS were carried out using a reported method.46 The organic semiconductors were deposited by thermal evaporation under vacuum (<10−6 mbar). Sublimation temperatures were monitored for each compound to stabilize the deposition rate at 0.3 Å s−1 until reaching a film thickness of 75 nm. Gold contacts were subsequently deposited in a separate vacuum chamber using a shadow mask to define the device architecture. Each substrate contained 16 analogous OTFT devices, with channel dimensions of 2 mm (W) and 80 µm (L). Electrical characterization was performed under ambient conditions in the dark with a Keithley 2636A source meter. Charge carrier mobility values were extracted in the saturation regime (μsat) as ID = (μsatCox(VG − Vth)2W)/2L, where Cox is the capacitance per unit area of the gate insulator. All devices were stored under ambient conditions in the dark.
Photophysical characterization
Absorption and emission spectra in dichloromethane were collected at room temperature in a Varian Cary UV-Vis-NIR 500E spectrophotometer and a PTI 810 fluorimeter, respectively. Fluorescence quantum yields in solution (Φf,sol) were quantified in toluene with an integrating sphere using an Edinburgh Instruments FLS980 fluorescence spectrophotometer. Films for the photophysical characterization were prepared through the drop-casting method, in which the components were dissolved in toluene and carefully deposited onto quartz substrates to ensure full surface coverage. The solvent was then allowed to evaporate slowly under ambient conditions to promote uniform film formation. The composition of the drop-casted solutions was as follows: fluorophore (1 mg mL−1) for the characterization in the solid state, and Zeonex (4 mg mL−1) and fluorophore (1 wt% with respect to Zeonex) for Zeonex-based films. Absorption and emission spectra in films were collected using a PerkinElmer Lambda 35 UV/Vis spectrophotometer and an Edinburgh Instruments FLS980 fluorescence spectrophotometer, respectively. Fluorescence quantum yields in the solid state were also quantified with an integrating sphere.Results and discussion
Synthesis
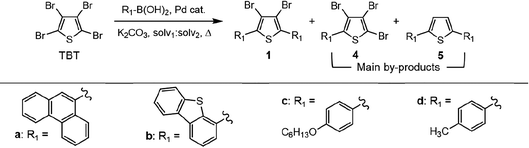
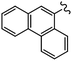
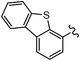
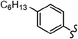
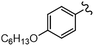
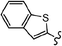
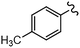
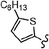
As stated, we designed two types of derivatives incorporating: (i) large aromatic scaffolds in α positions (9-phenanthrenyl and 4-dibenzothienyl for 3a and 3b, respectively) and 4-hexylphenyl moieties in β positions to preserve some solubility; (ii) 4-alkylphenyl scaffolds in α positions and sulfurated heterocycles in β positions to target more planar structures (4-phenyloxyphenyl and 2-benzothienyl for 3c and 4-methylphen-2-yl and 5-hexylthien-2-yl for 3d). For this, we first surveyed different established methods in the synthesis of intermediates 1a–d to identify the most suitable conditions. Table 1 summarizes the main synthetic results and conditions towards the disubstituted intermediates 1a–d.| Conditions | Solv1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Solv2 Solv2 |
Ratio (v/v) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1a (%) | By-prod. (%) | 1b (%) | By-prod. (%) | 1c (%) | By-prod. (%) | 1d (%) | By-prod. (%) | |||
| a Pd cat. refers to Pd(PPh3)2Cl2 (0.05 eq.) and PPh3 (0.10 eq.), carried out at 110 °C overnight. b Pd cat. refers to Pd(PPh3)4 (0.05 eq.), carried out under reflux overnight. c Not detected. | ||||||||||
| Aa | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
56 | (—)c | (—)c | 71 (5b) | (—)c | 69 (5c) | 42 | (—)c |
| Bb | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
26 | 23 (4a) | 46 | (—)c | 45 | (—)c | 58 | (—)c |
| Ca | Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
12 | 6 (4a) | 41 | 26 (4b) | 19 | 71 (4c) | 3 | 71 (4d) |
| Db | Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
51 | (—)c | 70 | (—)c | 38 | 36 (5c′) | 83 | (—)c |
The use of Pd(PPh3)2Cl2 as catalyst, PPh3 as ligand and K2CO3 as base in a mixture of DMF/water as solvent (cond. A), being successful for tetrasubstitutions in 9H-carbazole58 and thiophene,37 was optimal for incorporating 9-phenanthrene in 1a, but induced debromination as a side-reaction when incorporating heteroatom-containing moieties, resulting in by-products 5b,c instead of the expected 1b,c. As reported in the literature, β positions with respect to the heteroatom are particularly vulnerable to dehalogenation in this type of systems.59–63 Thus, the use of DMF/water was not suitable for a 2-fold all-encompassing coupling to TBT. The substitution of DMF by THF with Pd(PPh3)4 as catalyst in milder conditions (cond. B) deactivated the dehalogenation pathway, successfully yielding compounds 1b,c in a ca. 45%. It was also beneficial in the case of 1d, increasing from 42 to 58%, while 1a dropped from 56 to 26%. The use of toluene/water with Pd(PPh3)2Cl2/PPh3 (cond. C), which was similarly used in the literature,51 underperformed in all cases, majorly leading to the monosubstituted counterpart 4. The combination of toluene with methanol and Pd(PPh3)4 as catalyst (cond. D)23,64 resulted in the choicest option, with yields surpassing those of the aforementioned conditions. Particularly, the yields of 1b and 1d raised from 46 and 58% to 70 and 83%, respectively, in relation to THF/water. Therefore, the subsequent coupling towards structure 2 was performed using these conditions and the second best-performing ones for comparison, i.e. DMF/water for 2a and THF/water for 2b–d. Table 2 compiles the main results. The use of toluene/methanol greatly ameliorated the yields of all tetrasubstituted derivatives. This is especially notable for 2b, increasing from 35 to 60%. The inclusion of sulfurated scaffolds in β positions was further improved with Pd(AcO)2 as catalyst, SPhos as ligand and potassium phosphate as base (cond. E).52 Remarkably, the one-pot process did not only shorten the synthetic route, but also granted higher yields of precursors 2a–d than the 2-step procedure. The choice of toluene/methanol as solvent grants a more universal method to introduce a diverse array of scaffolds in a one-pot way.
| Substituent | Methoda | Yield (%) | |||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | Solv1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Solv2 Solv2 |
1 | 2 | Globalc | One-potd | |
a Conditions: (A) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 8 H2O 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v with Pd(PPh3)2Cl2 (5 mol%) and PPh3 (10 mol%) at 110 °C; (B) THF 1 v/v with Pd(PPh3)2Cl2 (5 mol%) and PPh3 (10 mol%) at 110 °C; (B) THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 6 H2O 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v with Pd(PPh3)4 (5 mol%) under reflux; (D) toluene 1 v/v with Pd(PPh3)4 (5 mol%) under reflux; (D) toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 3 MeOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v with Pd(PPh3)4 (5 mol%) under reflux.
b Single-step yield of 2 from intermediate 1.
c Yield of 2 comprising steps 1 and 2.
d Yield of 2 on the one-pot process from TBT.
e Cond. E: the second coupling implied the addition of benzothien-2-ylboronic acid in 1c or 5-hexylthienyl as a pinacol ester65 in 1d (4 eq.), Pd(AcO)2 (5 mol%) as catalyst, SPhos (20 mol%) as ligand and K3PO4 (4 eq.) as base.
f These conditions were not suitable for the coupling of pinacol boronic esters. 1 v/v with Pd(PPh3)4 (5 mol%) under reflux.
b Single-step yield of 2 from intermediate 1.
c Yield of 2 comprising steps 1 and 2.
d Yield of 2 on the one-pot process from TBT.
e Cond. E: the second coupling implied the addition of benzothien-2-ylboronic acid in 1c or 5-hexylthienyl as a pinacol ester65 in 1d (4 eq.), Pd(AcO)2 (5 mol%) as catalyst, SPhos (20 mol%) as ligand and K3PO4 (4 eq.) as base.
f These conditions were not suitable for the coupling of pinacol boronic esters.
|
|||||||
| a |
|

|
DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
56 | 55 | 31 | 70 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
51 | 86 | 44 | 73 | |||
| b |
|
|
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
46 | 76 | 35 | 53 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
70 | 86 | 60 | 69 | |||
| c |
|
|
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
45 | 52 | 23 | 34 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
38 | 71e | 27e | 46e | |||
| d |
|
|
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
58 | (—)f | (—)f | (—)f |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
83 | 78e | 65e | 76e | |||
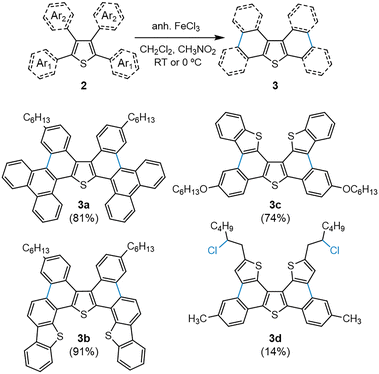
The Scholl reaction was carried out under standard conditions, as shown in Scheme 2. These conditions were successful in yielding compounds 3a and 3c in an 81 and 74%, respectively, but failed for the other two due to polymerization and other side reactions. To avoid these, we reduced the temperature to 0 °C, achieving an excellent 91% for 3b and a 14% for 3d. The unprecedented chlorination at aliphatic positions in 3d even at low temperature, which was confirmed via NMR spectroscopy (Fig. S1), could expand the applicability of the Scholl reaction. Overall, this synthetic strategy permits the construction of highly extended heterocycles up to 11-ring from TBT in just two steps.
It should be highlighted that, despite the bulkiness and π-extension of 3a, its non-planarity makes it highly soluble in all type of organic solvents, including non-polar examples like hexane (ca. 3 mg mL−1 at room temperature). This characteristic is prone to facilitating its implementation in solution-processed displays for a low-cost and greener manufacturing.
Single-crystal analysis
The single-crystal analysis of 3a permitted to get further insight into its structure and arrangement. It could be crystallized in space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) of the triclinic system in two alternative arrangements, depending on the solvent mixture: the first one was obtained from a mixture of hexane and dichloromethane (Fig. 1a), and the second one, from dichloromethane and ethanol (Fig. 1b). Both structures embedded solvent molecules. As observed, they corroborate the non-planarity of 3a, with torsion angles around 25°. The π–π stacking in this type of materials generally takes place between dimers comprising both atropoisomers, since this combination offers a better match. The first packing present π-stacking distances between atropoisomers that go from 3.3 to 3.9 Å, whereas the closest π–π interaction between dimers comes from slipped stacking distances of 3.87 Å, with additional stabilization from alkylic C–H⋯π and edge-to-edge interactions. The second crystal emphasizes even more the potential of 3a, with consistent π–π stacking interaction between atropoisomers as close as 3.3 Å. The dimers establish edge-to-face interactions between each other with distances up to 3.66 Å, with a resulting packing that could be assimilated to a γ type.66,67 This type of packing presents two different π–π stacking directions, which endows the crystal with alternative hopping routes in charge-transporting applications. The crystallographic data is detailed in the SI.
of the triclinic system in two alternative arrangements, depending on the solvent mixture: the first one was obtained from a mixture of hexane and dichloromethane (Fig. 1a), and the second one, from dichloromethane and ethanol (Fig. 1b). Both structures embedded solvent molecules. As observed, they corroborate the non-planarity of 3a, with torsion angles around 25°. The π–π stacking in this type of materials generally takes place between dimers comprising both atropoisomers, since this combination offers a better match. The first packing present π-stacking distances between atropoisomers that go from 3.3 to 3.9 Å, whereas the closest π–π interaction between dimers comes from slipped stacking distances of 3.87 Å, with additional stabilization from alkylic C–H⋯π and edge-to-edge interactions. The second crystal emphasizes even more the potential of 3a, with consistent π–π stacking interaction between atropoisomers as close as 3.3 Å. The dimers establish edge-to-face interactions between each other with distances up to 3.66 Å, with a resulting packing that could be assimilated to a γ type.66,67 This type of packing presents two different π–π stacking directions, which endows the crystal with alternative hopping routes in charge-transporting applications. The crystallographic data is detailed in the SI.
Electrochemical and thermal properties
Table 3 compiles the electrochemical and thermal properties of 3a–d and those of DPT for comparison. Their optical band gaps, extracted from the onset of their absorption spectra in dichloromethane solutions, are quite divergent. Derivative 3a presents the smallest one of 2.74 eV, suggesting a higher conjugation and extension of the π-system despite its non-planarity. The values for those including sulfurated extensions (3b–d) go from 3.03 to 3.32 eV, which are still lower than the 3.33 eV observed for DPT. As shown in the cyclic voltammograms (Fig. S3), all compounds underwent an oxidation process that was used to estimate their IP values, being around 5.9 eV. The IP values were also determined in the solid state via photoelectron emission spectrometry (Fig. S4), exhibiting a considerable decrease with respect to the values obtained for solutions presumably due to an effective π-stacking and delocalization. In fact, they cover the range from 4.80 to 5.21 eV, thus lowering the value relative to that of the DPT nucleus (5.96 eV). Hence, the proposed π-extensions place this core closer to the gold work function (ca. 5.1 eV). A neutral p-type semiconductor should feature an IP higher than 4.9 eV.4 Otherwise, a shallow HOMO energy level would make it prone to reducing ambient oxygen in the presence of humidity. Consequently, compounds 3a and 3d are the fittest to the gold work function while granting long-term electrochemical stability. Apart, all derivatives excel in terms of thermal stability, with 3a–c exhibiting Td values beyond 400 °C based on a 5% weight loss in thermogravimetric analysis (TGA). Considering that the value for DPT is 257 °C, the proposed π-extensions effectively increase the thermal stability of the main nucleus, and therefore are appropriate for a vacuum-deposition process. Their melting points (Tm), determined via differential scanning calorimetry (DSC), were significantly higher than room temperature, i.e. 271, 246 and 254 °C for 3b–d, respectively. In the case of 3a, if any, it was not detected below 300 °C. The DSC and TGA curves are represented in Fig. S5 and S6, respectively. The characteristics of compounds 3a–d anticipated great potential in the optoelectronic field. Thus, we evaluated the performance of these compounds as organic materials for two different functionalities: for semiconductor layers in OTFT devices and as RTP emitters for sensing or lighting displays.| E optgap (eV) | E oxonset (V) | IPc (eV) | EAd (eV) | IPfilme (eV) | T m (°C) | T d (°C) | |
|---|---|---|---|---|---|---|---|
| a Optical energy gaps, estimated from the absorption spectrum (Fig. S10c). b Onset oxidation potentials vs. Fc+/Fc, determined via CV in 1 mM solutions in CH2Cl2. c Ionization potentials in CH2Cl2, estimated as IP = Eoxonsetvs. Fc+/Fc + 5.39. d Electron affinities in CH2Cl2, estimated as EA = IP − Eoptgap. e Ionization potentials in the solid state, extracted from the photoelectron emission spectra of vacuum-deposited films. f Melting temperatures obtained from DSC at a scan rate of 10 °C min−1. g Decomposition temperatures obtained from the 5% weight loss in TGA at a heating rate of 10 °C min−1. | |||||||
| DPT37 | 3.33 | 0.83 | 6.22 | 2.89 | 5.96 | 265 | 257 |
| 3a | 2.74 | 0.50 | 5.89 | 3.15 | 5.01 | — | 425 |
| 3b | 3.03 | 0.50 | 5.89 | 2.86 | 4.87 | 271 | 468 |
| 3c | 3.32 | 0.54 | 5.93 | 2.61 | 4.80 | 246 | 415 |
| 3d | 3.27 | 0.57 | 5.96 | 2.76 | 5.21 | 254 | 260 |
Semiconductor properties
The semiconductor properties of compounds 3a–d were dually surveyed using the time-of-flight (TOF) technique and integrated in OTFT devices. Table 4 collects the charge mobility values extracted from both techniques as well as the OTFT characteristics. The transient curves acquired via TOF in the linear scale presented dispersive patterns, so the transient times were picked from the photocurrent transients in the log–log scale (Fig. S7 and S8). All the materials behaved as p-type semiconductors, with 3a showing the best μh,TOF with an inherent value of 8.3 × 10−5 cm2 V−1 s−1 at an applied electric field of 6 × 105 V cm−1. Compound 3d exhibited slightly lower values, while 3b,c underperformed with a μh,TOF in the order of 5 × 10−6 cm2 V−1 s−1. These preliminary results suggest the potential of this type of constructions as semiconductors regardless of the planarity of the π-system.| μ h,TOF (cm2 V−1 s−1) | μ 0,TOF (cm2 V−1 s−1) | α (cm−1/2 V−1/2) | μ h,max (cm2 V−1 s−1) | μ h,avg (cm2 V−1 s−1) | V th (V) | I on/Ioffd (A A−1) | |
|---|---|---|---|---|---|---|---|
| a Hole mobility extracted from vacuum-evaporated layers (the specific thicknesses are indicated in Fig. S7) via the TOF technique at an electric field of 6.0 × 105 V cm−1. b Zero-field mobility. c Field dependence parameter. d Maximum hole mobility value and Ion/Ioff ratio from an individual OTFT device. e Average hole mobility and threshold voltage of a set of six analogous devices measured on the same day. f Not detected. | |||||||
| 3a | 8.3 × 10−5 | 5.5 × 10−6 | 0.0045 | 6.9 × 10−5 | (6.3 ± 0.3) × 10−5 | –11 ± 2 | 103 |
| 3b | 5.0 × 10−6 | 1.8 × 10−8 | 0.0094 | 5.1 × 10−6 | (4.6 ± 0.4) × 10−6 | –9 ± 3 | 102 |
| 3c | 4.2 × 10−6 | 4.0 × 10−9 | 0.0080 | 8.0 × 10−5 | (6.8 ± 0.9) × 10−5 | –12 ± 2 | 103 |
| 3d | 2.3 × 10−5 | 5.3 × 10−7 | 0.0047 | (—)f | (—)f | (—)f | (—)f |
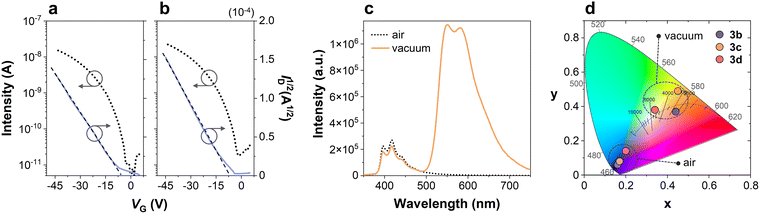
In OTFTs, the organic materials were deposited under vacuum over polystyrene (PS)-coated SiO2/Si substrates in a standard bottom gate-top contact geometry. The incorporation of a PS layer at the dielectric/semiconductor interface is a well-known strategy to reduce the wettability of the dielectric and promote improved molecular order and morphology.68,69 The mobility values extracted for 3a,b were consistent with those estimated from TOF measurements. Specifically, compound 3a showed an analogous μh,avg of 6.3 × 10−5 cm2 V−1 s−1, whereas 3b underperformed with a μh,avg of 4.6 × 10−6 cm2 V−1 s−1. Fig. 2a and b displays the OTFT characteristics of 3a both at the initial measurement and after one year of storage. As observed, 3a maintains nearly ideal behavior with highly linear characteristics. It also demonstrates remarkable air stability, retaining comparable parameters after one year of shelf storage under ambient conditions. The evolution of its μh,avg over time is represented in Fig. S9a. Indeed, 3a possesses the most appropriate IP in the solid state, being the closest to the gold work function while remaining low-lying enough to ensure air stability. Besides, its single-crystal structures reveal favorable π-stacking regardless of its non-planarity. On the other hand, compound 3c increased the inherent TOF mobility by an order of magnitude when incorporated in an OTFT prototype, whereas 3d could not be measured due to the poor quality of the deposited layers. The OTFT characteristics of compounds 3b,c are shown in Fig. S9b and c. Although the extracted hole mobility values remain lower than those of top-performing materials, these are still noteworthy due to their unique, non-planar structural features. Furthermore, this approach offers promising opportunities for the development of stable and easily processable materials, thanks to its straightforward engineering potential.
Photophysical properties
An equally sought-after feature of DPT-based structures resides on their photoluminescence, especially RTP.37 For this, we surveyed the luminescent properties of compounds 3a–d in different media, as compiled in Table 5. Analogously, the characteristics of precursors 2a–d were also surveyed for comparison (Table S3). All compounds emit within the UV-blue region when dissolved in toluene except for 3a, whose emission red-shifts to the blue-green due to the effective π-conjugation with the phenanthrene scaffolds. Compound 3a also exhibits the highest fluorescence quantum yield (Φf,DCM = 14%), surpassing that of DPT. In the solid state, the emission of all compounds undergo a bathochromic shift into the green region, accompanied by the decrease in Φf,film due to the intermolecular interaction. The exception is the Φf,film of 3b, which slightly increases from 4.2 to 5.9%. On the other hand, Φf of the non-cyclized analogs 2a–d are below 2% regardless of the medium. All absorption and emission spectra are compiled in the SI.| λ em,sol (nm) | Φ f,sol (%) | λ em,film (nm) | Φ f,film (%) | λ em,ZEONEX (nm) | I vac/Iaird | I Phos/IFLe | Φ air (%) | Φ vac (%) | Φ RTP (%) | CIE,airh | CIE,vach | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Maximum emission wavelengths (λem,sol) at λex = 330 nm and fluorescence quantum yields (Φf,sol) in toluene, determined using an integrating sphere. b Maximum emission wavelengths (λem,film) at λex = 330 nm and fluorescence quantum yields (Φf,film) in spin-coated films, determined using an integrating sphere. c Maximum emission wavelengths in a Zeonex film (λem,ZEONEX) at λex = 330 nm and fluorescence quantum yields under air (Φair) in Zeonex, measured using an integrating sphere. The values in parenthesis correspond to the maximum wavelength of the RTP band meaured under vacuum. d Photoluminescence intensity ratio between vacuum and aerated conditions in Zeonex, calculated as: Ivac/Iair = Areavac/Areaair. e Photoluminescence intensity ratio between the phosphorescence and fluorescence contributions in Zeonex under vacuum, calculated as: IPhos/IFL = AreaPhos/AreaFL. f Photoluminescence quantum yields in Zeonex under vacuum (Φvac), estimated as Φvac = (Ivac/Iair)Φair. g RTP quantum yields in Zeonex under vacuum (ΦRTP), estimated as ΦRTP = (IPhos/(IPhos + IFL,vac))Φvac. h CIE 1931 coordinates, calculated from the corresponding photoluminescence spectra under aerated (CIE,air) and vacuum (CIE,vac) conditions. | ||||||||||||
| DPT37 | 394 | 3.2 | 449, 481 | 4.7 | 393 (585) | 4.5 | 4.1 | 2.7 | 12.2 | 9.8 | (0.17, 0.04) | (0.47, 0.47) |
| 3a | 454, 475 | 14.0 | 469, 491 | 1.1 | 456, 476 (—) | 1.0 | — | 11 | 11 | — | (0.16, 0.06) | (0.16, 0.06) |
| 3b | 402, 424 | 4.2 | 473 | 5.9 | 409, 428 (578) | 3.8 | 3.7 | 4.4 | 16.7 | 13.1 | (0.16, 0.06) | (0.44, 0.37) |
| 3c | 396, 414 | 2.1 | 449, 478 | 0.8 | 396, 417 (564) | 9.0 | 10.7 | 1.7 | 15.3 | 13.8 | (0.17, 0.08) | (0.45, 0.49) |
| 3d | 389, 408 | 1.6 | 462, 521 | 0.3 | 395 (551) | 1.7 | 1.2 | 1.5 | 2.6 | 1.1 | (0.20, 0.14) | (0.34, 0.38) |
For RTP analysis, we evaluated the molecular dispersions of all compounds in a polymeric matrix of Zeonex with a concentration of 1 wt% under air and vacuum conditions. Zeonex performs as a rigid and inert host that quenches vibrational relaxation processes, promoting an emissive decay from the triplet to the ground state in the absence of oxygen. Emission of compound 3a was equivalent under both conditions, thus discarding the presence of RTP for this particular structure (Fig. S12a). Contrarily, compound 3c displayed the largest Ivac/Iair ratio, which quantifies the difference of emission intensity between vacuum and air equilibrated conditions, with a value as high as 9. The photoluminescence spectra of a film of Zeonex doped with 3c (1 wt%) under air and vacuum are shown in Fig. 2c. This underscores the potential of 3c as an RTP sensor. Its emission goes from deep blue CIE coordinates (0.17, 0.08) to yellow ones (0.45, 0.49) as the concentration of oxygen decreases (Fig. 2d). Temperature-dependent studies, the results of which are shown in Fig. S13, also corroborate that the yellow emission band derives from RTP instead of thermally activated delayed fluorescence (TADF). The films containing compounds 3b,d also revealed RTP (Fig. S12), with Ivac/Iair ratios of 3.8 and 1.7, respectively. Under vacuum, the CIE coordinates of the emission of 3b are placed within the orange region, while the film containing 3d exhibits white emission due to its balanced Ivac/Iair ratio. Therefore, the photoluminescence intensity and color under vacuum can be easily fine-tuned through the molecular design. The introduction of sulfurated π-extended moieties in 3b and 3c afforded the highest RTP quantum yields (ΦRTP) of their molecular mixtures with Zeonex of 13.1 and 13.8%, respectively, surpassing that of the molecular mixture with parent DPT. The photoluminescence spectra of the molecular dispersions of 3b and 3c in Zeonex also revealed a clear reduction of the singlet–triplet splitting energy (ΔEST) with respect to the system containing DPT, as detailed in Fig. S14. Specifically, DPT exhibits a ΔEST of 1.01 eV,38 whereas this value decreases to 0.80 eV for the films with both 3b and 3c. A narrower singlet–triplet gap is prone to promoting intersystem crossing, which further supports the enhanced RTP in these two cases. Contrastingly, ΔEST slightly increases to 1.16 eV for the films with 3d, which is consistent with its weaker RTP contribution. The films containing precursors 2a–d do not exhibit RTP, underlining the pivotal role of rigidity conferred through the Scholl reaction. These molecular structures could therefore inspire novel designs towards accessible and efficient halogen- and metal-free RTP emitters.
Conclusions
We synthetized a set of dibenzothiophene-derived heterocycles through a concise and efficient protocol that combines sequential Suzuki–Miyaura and Scholl reactions. The Suzuki–Miyaura couplings were optimized using a mixture of toluene/methanol as solvent, which broadens the substrate scope while affording higher yields. Moreover, it admits a one-pot procedure, further enhancing the efficiency while reducing the number of synthetic steps. This two-step approach, starting from commercially available reagents, permits the construction of complex polycyclic architectures containing up to 11 fused rings with significantly improved overall yields. The resulting π-systems display adjustable photophysical, electrochemical and thermal properties that upgrade those of the parent DPT core. The phenanthrene-containing derivative, with an appropriate IP value of 5.01 eV in the solid state, outperforms as a stable p-type semiconductor in OTFTs exhibiting a μh of 7 × 10−5 cm2 V−1 s−1 and an excellent shelf-lifetime exceeding one year under ambient conditions. Moreover, the inclusion of sulfur-rich π-extensions enabled RTP under oxygen-free conditions, achieving quantum yields as high as 14% when dispersed in a Zeonex film. The presence of RTP highly modulates the colour of the photoluminescence from deep blue to yellow-orange as the oxygen content decreases, which entails potential in fields such as lighting and sensing. Overall, this synthetic strategy gives access to multifunctional π-extended materials with potential for optoelectronic applications ranging from p-type semiconductors to molecular sensors.Author contributions
Clara Fabregat: methodology, validation, formal analysis, investigation, data curation, writing – original draft. Roger Bujaldón: conceptualization, methodology, validation, investigation, writing – original draft, writing – review & editing, visualization. Sílvia Oliva: validation, investigation. Jaume Garcia-Amorós: validation, writing – review & editing, supervision, project administration. Dmytro Volyniuk: methodology, formal analysis, investigation, data curation. Melika Ghasemi: investigation, data curation. Juozas V. Grazulevicius: resources, writing – review & editing, supervision, funding acquisition. Joaquim Puigdollers: resources, writing – review & editing, supervision. Dolores Velasco: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma01124h.CCDC 2489351 and 2489352 contain the supplementary crystallographic data for this paper.70a,b
Acknowledgements
Authors gratefully acknowledge financial support by Ministerio de Ciencia, Innovación y Universidades (grant number PID2023-151915NB-I00) and Horizon Europe, the European Union's framework programme for research and innovation (R&I) for 2021–2027, project HELIOS (grant agreement no. 101155017). C. F. is grateful for the predoctoral grant FI AGAUR from Generalitat de Catalunya. The authors also want to thank the CCiTUB for the use of their equipment, and especially acknowledge Cristina Puigjaner (X-Ray Diffraction Unit) for the elucidation of the single-crystal structures and M![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) Antònia Molins and Vicky Muñoz (NMR unit) for their assistance with 2D NMR spectroscopy.
Antònia Molins and Vicky Muñoz (NMR unit) for their assistance with 2D NMR spectroscopy.
References
- M. Sawatzki-Park, S.-J. Wang, H. Kleemann and K. Leo, Chem. Rev., 2023, 123, 8232–8250 CrossRef CAS PubMed.
- F. Wu, Y. Liu, J. Zhang, S. Duan, D. Ji and H. Yang, Small Methods, 2021, 5, 2100676 CrossRef CAS PubMed.
- A. D. Scaccabarozzi, A. Basu, F. Aniés, J. Liu, O. Zapata-Arteaga, R. Warren, Y. Firdaus, M. I. Nugraha, Y. Lin, M. Campoy-Quiles, N. Koch, C. Müller, L. Tsetseris, M. Heeney and T. D. Anthopoulos, Chem. Rev., 2021, 122, 4420–4492 CrossRef PubMed.
- H. Bronstein, C. B. Nielsen, B. C. Schroeder and I. McCulloch, Nat. Rev. Chem., 2020, 4, 66 CrossRef CAS.
- S. M. Elbert, E. Bolgert, O. T. A. Paine, F. Ghalami, W.-S. Zhang, U. Zschieschang, F. Rominger, D. Popp, H. Klauk, M. Elstner and M. Mastalerz, Org. Chem. Front., 2024, 11, 5340 RSC.
- H. Shudo, P. Wiesener, E. Kolodzeiski, K. Mizukami, D. Imoto, H. Mönig, S. Amirjalayer, H. Sakamoto, H. Klaasen, B. J. Ravoo, N. Kimizuka, A. Yagi and K. Itami, Nat. Commun., 2025, 16, 1074 CrossRef CAS PubMed.
- H. M. F. Elnagdy, Dyes Pigm., 2024, 229, 112251 CrossRef CAS.
- A. Cuadrado, R. Bujaldón, C. Fabregat, J. Puigdollers and D. Velasco, Org. Electron., 2024, 107, 107020 CrossRef.
- A. Nitti, M. Scagliotti, L. Beverina, L. Mariucci, M. Rapisarda and D. Pasini, Mater. Adv., 2023, 4, 4590–4597 RSC.
- A. Babuji, I. Temiño, A. Pérez-Rodríguez, O. Solomeshch, N. Tessler, M. Vila, J. Li, M. Mas-Torrent, C. Ocal and E. Barrena, ACS Appl. Mater. Interfaces, 2020, 12, 28416 CrossRef CAS.
- B. Lu, S. Zhang, D. Liu, W. Jin, D. Li, Z. Liu and T. He, Org. Chem. Front., 2025, 12, 422 RSC.
- Y. Yuan, G. Giri, A. L. Ayzner, A. P. Zoombelt, S. C. B. Mannsfeld, J. Chen, D. Nordlund, M. F. Toney, J. Huang and Z. Bao, Nat. Commun., 2014, 5, 3005 CrossRef.
- H. Iino, T. Usui and J. Hanna, Nat. Commun., 2015, 6, 6828 CrossRef CAS PubMed.
- A. Y. Amin, A. Khassanov, K. Reuter, T. Meyer-Friedrichsen and M. Halik, J. Am. Chem. Soc., 2012, 134, 16548 CrossRef CAS PubMed.
- T. Yamamoto and K. Takimiya, J. Am. Chem. Soc., 2007, 129, 2224 CrossRef CAS.
- J. W. Borchert, B. Peng, F. Letzkus, J. N. Burghartz, P. K. L. Chan, K. Zojer, S. Ludwigs and H. Klauk, Nat. Commun., 2019, 10, 1119 CrossRef PubMed.
- B. Peng, K. Cao, A. H. Y. Lau, M. Chen, Y. Lu and P. K. L. Chan, Adv. Mater., 2020, 32, 2002281 CrossRef CAS.
- K. Nakayama, Y. Hirose, J. Soeda, M. Yoshizumi, T. Uemura, M. Uno, W. Li, M. J. Kang, M. Yamagishi, Y. Okada, E. Miyazaki, Y. Nakazawa, A. Nakao, K. Takimiya and J. Takeya, Adv. Mater., 2011, 23, 1626 CrossRef CAS PubMed.
- G. M. Badger, B. J. Christie, J. M. Pryke and W. H. F. Sasse, J. Chem. Soc., 1957, 4417–4419 RSC.
- S. Venkateswarlu, S. P. Prakoso, S. Kumar and Y.-T. Tao, J. Chin. Chem. Soc., 2020, 67, 437–445 CrossRef CAS.
- S. Venkateswarlu, S. Kumar and Y.-T. Tao, Asian J. Org. Chem., 2021, 10, 2251–2261 CrossRef CAS.
- S. Venkateswarlu, S. P. Prakoso, S. Kumar, M.-Y. Kuo and Y.-T. Tao, J. Org. Chem., 2019, 84, 10990 CrossRef CAS.
- S. Maddala, K. Kollimalaian, A. Samal and V. Parthasarathy, Tetrahedron, 2024, 151, 133807 CrossRef CAS.
- M. Sankarrao, S. Maddala and V. Parthasarathy, Adv. Synth. Catal., 2024, 366, 4114–4121 CrossRef CAS.
- S. Maddala, S. Mahanthi and V. Parthasarathy, Chem. – Eur. J., 2025, 31, e01724 CrossRef CAS PubMed.
- C.-X. Liu, H. Wang, J.-Q. Du, K.-Q. Zhao, P. Hu, B.-Q. Wang, H. Monobe, B. Heinrich and B. Donnio, J. Mater. Chem. C, 2018, 6, 4471–4478 RSC.
- J.-F. Hang, H. Lin, K.-Q. Zhao, P. Hu, B.-Q. Wang, H. Monobe, C. Zhu and B. Donnio, Eur. J. Org. Chem., 2021, 1989–2002 CrossRef CAS.
- T. Ma, H.-F. Wang, K.-Q. Zhao, B.-Q. Wang, P. Hu, H. Monobe, B. Heinrich and B. Donnio, ChemPlusChem, 2019, 84, 1439–1448 CrossRef CAS.
- C.-Y. Zeng, W.-J. Deng, K.-Q. Zhao, C. Redshaw and B. Donnio, Chem. – Eur. J., 2024, 30, e202400296 CrossRef CAS PubMed.
- T. Ma, Y.-J. Zhong, H.-F. Wang, K.-Q. Zhao, B.-Q. Wang, P. Hu, H. Monobe and B. Donnio, Chem. – Asian J., 2021, 16, 1106–1117 CrossRef CAS PubMed.
- W.-J. Deng, S. Liu, H. Lin, K.-X. Zhao, X.-Y. Bai, K.-Q. Zhao, P. Hu, B.-Q. Wang, H. Monobe and B. Donnio, New J. Chem., 2022, 46, 7936–7949 RSC.
- K.-C. Zhao, J.-Q. Du, H.-F. Wang, K.-Q. Zhao, P. Hu, B.-Q. Wang, H. Monobe, B. Heinrich and B. Donnio, Chem. – Asian J., 2019, 14, 462–470 CrossRef CAS PubMed.
- S. Venkateswarlu, Y.-D. Lin, K.-M. Lee, K.-L. Liau and Y.-T. Tao, ACS Appl. Mater. Interfaces, 2020, 12, 50495 CrossRef CAS.
- M. Nagase, R. Yoshida, S. Nakano, T. Hirose and Y. Segawa, Chem. Commun., 2025, 61, 11187–11190 RSC.
- L. Ueberricke and M. Mastalerz, Chem. Rec., 2021, 21, 558 CrossRef CAS PubMed.
- L. Fang, Y. Zhang, Y. Cai, J. Zhang, Y. Wei, Y. Yuan and P. Wang, Energy Environ. Sci., 2023, 16, 5231 RSC.
- L. Knight, J. C. Jimenez, Q. Tran, M. Zhao, M. H. Pugh, C. D. Brancel, H. Zhang, R. Li, Y. Yuan, Y. Li, L. Zhu and G. Sauvé, Mater. Adv., 2024, 5, 6145–6153 RSC.
- C. Fabregat, R. Bujaldón, J. Garcia-Amorós, D. Volyniuk, M. Ghasemi, J. V. Grazulevicius and D. Velasco, Dyes Pigm., 2025, 243, 113053 CrossRef CAS.
- Z. Xu, D. Hean, J. Yuan and M. O. Wolf, Recent Progress in Pure Organic Room Temperature Phosphorescence of Small Molecular Host–Guest Systems, ACS Mater. Lett., 2021, 3, 652–657 CrossRef.
- P. Pander, A. Swist, J. Soloducho and F. B. Dias, Dyes Pigm., 2017, 142, 315–322 CrossRef CAS.
- S. Han, Y. Li, Z. Wang and X. Li, Dyes Pigm., 2024, 228, 112243 CrossRef CAS.
- Y. Wang, X. Qi, Z. Ye, Y. Yan, Q. Chen and Y. Yang, Dyes Pigm., 2024, 224, 112486 Search PubMed.
- H. Bhatia and D. Ray, Mater. Adv., 2020, 1, 1858–1865 RSC.
- J. Wang, H. Xu, B. Li, X.-P. Cao and H.-L. Zhang, Tetrahedron, 2012, 68, 1192 CrossRef CAS.
- W. Cai, J. Lee, Y. Zhao, B. Kang and G. Zhang, Mater. Adv., 2023, 4, 3344–3350 RSC.
- R. Bujaldón, A. Vilche, J. Puigdollers, C. Puigjaner, X. Alcobé and D. Velasco, ACS Appl. Electron. Mater., 2023, 5, 3675–3684 CrossRef.
- R. Bujaldón, A. Cuadrado, D. Volyniuk, J. V. Grazulevicius, J. Puigdollers and D. Velasco, Coatings, 2023, 13, 896 CrossRef.
- M. Reig, G. Bagdziunas, A. Ramanavicius, J. Puigdollers and D. Velasco, Phys. Chem. Chem. Phys., 2018, 20, 17889–17898 RSC.
- T. Lei, J.-Y. Wang and J. Pei, Chem. Mater., 2014, 26, 594–603 CrossRef CAS.
- J. C. Jimenez, Q. Tran, M. H. Pugh, C. D. Brancel, A. L. Rheingold and G. Sauvé, Dyes Pigm., 2023, 208, 110858 CrossRef.
- K. M. Saini, R. K. Saunthwal and A. K. Verma, Org. Biomol. Chem., 2017, 15, 10289–10298 RSC.
- T. T. Dang, T. T. Dang, N. Rasool, A. Villinger, H. Reinke, C. Fischer and P. Langer, Adv. Synth. Catal., 2009, 351, 1595–1609 CrossRef.
- X. Liu, Y. Wang, J. Gao, L. Jiang, X. Qi, W. Hao, S. Zou, H. Zhang, H. Li and W. Hu, Chem. Commun., 2014, 50, 442 RSC.
- J. Urieta-Mora, I. Zimmermann, J. Aragó, A. Molina-Ontoria, E. Ortí, N. Martín and M. K. Nazeeruddin, Chem. Mater., 2019, 31, 6435 CrossRef CAS.
- Y. Xing, X. Xu, F. Wang and P. Lu, Opt. Mater., 2006, 29, 407 CrossRef CAS.
- H. Muraoka, T. Tanifuji and S. Ogawa, Chem. Lett., 2011, 40, 964 CrossRef CAS.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367–2371 CrossRef CAS.
- S. Kumar and Y.-T. Tao, J. Org. Chem., 2015, 80, 5066 CrossRef CAS PubMed.
- J. Sherwood, J. H. Clark, I. J. S. Fairlamb and J. M. Slattery, Green Chem., 2019, 21, 2164 RSC.
- Z. Ahmadi and J. S. McIndoe, Chem. Commun., 2013, 49, 11488 RSC.
- S. T. Handy, H. Bregman, J. Lewis, X. Zhang and Y. Zhang, Tetrahedron Lett., 2003, 44, 427 CrossRef CAS.
- L. Jedinák, R. Zátopková, H. Zemánková, A. Šustková and P. Cankař, J. Org. Chem., 2017, 82, 157 CrossRef.
- S. T. Handy and D. Mayi, Tetrahedron Lett., 2007, 48, 8108 CrossRef CAS PubMed.
- S. Maddala, A. Panua and P. Venkatakrishnan, Chem. – Eur. J., 2021, 27, 16013–16020 CrossRef CAS PubMed.
- C.-H. Lee and K. N. Plunkett, Org. Lett., 2013, 15, 1202–1205 CrossRef CAS PubMed.
- J. E. Campbell, J. Yang and G. M. Day, J. Mater. Chem. C, 2017, 5, 7574 RSC.
- G. R. Desiraju and A. Gavezzotti, Acta Crystallogr., 1989, B45, 473 CAS.
- S. Fritz, T. M. Kraft, M. Sommer and J. C. Brendel, Appl. Phys. A: Mater. Sci. Process., 2015, 118, 809–815 CrossRef.
- M. M. Hasan, M. M. Islam, X. Li, M. He, R. Manley, J. Chang, N. Zhelev, K. Mehrotra and J. Jang, IEEE Trans. Electron Devices, 2020, 67, 1751–1756 CAS.
- (a) CCDC 2489351: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2pkcn; (b) CCDC 2489352: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2pkcpb.
| This journal is © The Royal Society of Chemistry 2026 |