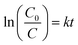Open Access Article
Open Access ArticleEnhanced photocatalytic degradation of pollutants via MoS2-integrated DyCrO3 nanostructures
Md. Mahbubar
Rahman
,
Md. Sobuj
Hossain
 ,
Tasnim
Jahan
,
Tasnim
Jahan
 and
M. A.
Basith
*
and
M. A.
Basith
*
Nanotechnology Research Laboratory, Department of Physics, Bangladesh University of Engineering and Technology, Dhaka-1000, Bangladesh. E-mail: mabasith@phy.buet.ac.bd
First published on 6th January 2026
Abstract
Water contamination by persistent dyes and antibiotics is a major environmental concern. Here, a DyCrO3/MoS2 S-scheme heterojunction photocatalyst was synthesized via a simple hydrothermal method to enhance solar-light-driven degradation efficiency. Structural and electronic analyses (XRD, FESEM, TEM, XPS, UV-vis, PL, Mott–Schottky) confirm well-dispersed MoS2 nanosheets, oxygen vacancies, improved visible-light absorption, and favorable band alignment. MoS2 incorporation reduced the band gap from 2.14 to 1.72 eV and prevented DyCrO3 aggregation, yielding particles of 28 ± 7 to 32 ± 12 nm. The optimized DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composite (10 mg) degraded 84.95% of levofloxacin and 78.97% of methylene blue within 240 min, with apparent quantum yields of 37.88% and 39.59%, respectively, and strong cycle stability. Active-species trapping identified photogenerated holes as the dominant oxidants, supporting an S-scheme mechanism. These results demonstrate that MoS2-engineered DyCrO3 nanostructures provide an efficient and durable platform for solar-driven wastewater purification.
15%) composite (10 mg) degraded 84.95% of levofloxacin and 78.97% of methylene blue within 240 min, with apparent quantum yields of 37.88% and 39.59%, respectively, and strong cycle stability. Active-species trapping identified photogenerated holes as the dominant oxidants, supporting an S-scheme mechanism. These results demonstrate that MoS2-engineered DyCrO3 nanostructures provide an efficient and durable platform for solar-driven wastewater purification.
1 Introduction
Rapid industrialization has exacerbated environmental pollution, with chemical industries-including textiles, paper, paint, and cosmetics-discharging substantial amounts of non-biodegradable chemicals, antibiotics, and hazardous dyes into aquatic systems.1 Studies indicate that 30–90% of antibiotics enter wastewater as parent compounds or metabolites.2 Synthetic dyes (e.g., methylene blue, rhodamine B), pharmaceutical antibiotics (e.g., levofloxacin, ciprofloxacin), and pesticides are now prevalent in water bodies, posing severe ecological and health risks.3 In Bangladesh, levofloxacin concentrations have been reported up to <LOD-710 ng L−1.4 The complex aromatic and heterocyclic structures of these contaminants make them resistant to conventional treatments such as adsorption, reverse osmosis, chlorination, chemical oxidation, coagulation, biological degradation, and ozonolysis,5–7 which may also generate secondary pollutants. Microbial treatments are often ineffective against bio-persistent compounds, underscoring the urgent need for advanced and efficient remediation strategies.8Advanced oxidation processes (AOPs) have emerged as effective approaches for degrading persistent organic pollutants into CO2, H2O, and biodegradable intermediates. Among various AOPs, semiconductor-based photocatalysis has attracted considerable attention due to its ability to directly harness solar energy for the generation of highly reactive species, including superoxide (˙O2−), hydroxyl radicals (˙OH), and photogenerated holes.9,10 In particular, visible-light-responsive semiconductors with engineered band structures and heterojunction interfaces are actively pursued to enhance solar utilization, suppress charge-carrier recombination, and improve photocatalytic stability for the efficient removal of dyes and emerging pharmaceutical contaminants.9–12 Since visible light accounts for 43% of solar radiation, compared to 4% for UV light, visible-light-driven photocatalysts are particularly desirable.13 While TiO2, ZnO, and Fe2O3 have been extensively explored,14–16 TiO2's wide bandgap and rapid electron–hole recombination limit its visible-light efficiency.17
Rare-earth orthochromites, especially DyCrO3, have emerged as promising visible-light-responsive photocatalysts.18 DyCrO3 exhibits favorable charge carrier dynamics, nanoscale particle size, tunable surface properties, oxygen vacancies, and a bandgap of 2.72 eV, which collectively enhance adsorption, charge separation, and redox activity.19–23 However, challenges such as particle agglomeration, limited surface area, and incomplete charge separation restrict its photocatalytic potential.24 In this study, DyCrO3 nanoparticles were synthesized via a sol–gel method at 750 °C to investigate their photocatalytic performance.
Nanostructured transition-metal oxides (TMOs) and two-dimensional materials, particularly MoS2, have attracted attention for photocatalytic applications due to their high surface area, layered structure, excellent electron mobility, and tunable electronic properties.25–28 Integrating DyCrO3 with MoS2 nanosheets forms an S-scheme n–n heterojunction, generating a built-in electric field that drives photogenerated electrons and holes in opposite directions, suppressing recombination and enhancing charge carrier lifetimes.29–31 MoS2 also prevents DyCrO3 aggregation, provides abundant catalytic sites, and improves structural stability, while DyCrO3 mitigates MoS2 restacking, facilitating light penetration and charge transport. This synergy enhances reactive oxygen species formation.
Despite extensive studies on rare-earth orthochromites and two-dimensional sulfide photocatalysts, the integration of MoS2 with DyCrO3 for simultaneous dye and antibiotic degradation under solar irradiation remains underexplored. Herein, we construct a MoS2-integrated DyCrO3 S-scheme heterojunction that enhances interfacial charge separation and reactive oxygen species generation, leading to efficient visible-light-driven degradation of both methylene blue and levofloxacin. This work elucidates the structure-function correlation induced by MoS2 incorporation and highlights MoS2-engineered DyCrO3 as an effective photocatalyst for solar wastewater remediation. DyCrO3–MoS2 nanocomposites with optimized MoS2 loadings (5–20 wt%) were systematically developed, where both components function as n-type semiconductors with well-matched band structures that facilitate directional charge migration.18,32,33 The S-scheme architecture exploits the layered nature of MoS2 to increase accessible active sites, inhibit particle aggregation, and enhance recyclability. Detailed TRPL, PEC, XPS, Raman, and AQY analyses collectively establish a comprehensive structure–property–performance relationship, validating the DyCrO3–MoS2 composite as an efficient and durable photocatalyst.
2 Materials preparation and experimental techniques
2.1 Materials
The chemicals used in this study included dysprosium(III) nitrate pentahydrate (Dy(NO3)3·5H2O, 99.8%), chromium(III) nitrate nonahydrate (Cr(NO3)3·9H2O, 98.0%), citric acid (C6H8O7, 99.5%), ammonium hydroxide (NH4OH, 35.05 g mol−1), molybdenum disulfide (MoS2, 99% metal basis), and ethylene glycol (CH2OHCH2OH, 99%). Additional reagents included levofloxacin (LFX, 98%), methylene blue (MB, 98%), isopropanol (IPA, 60.1 g mol−1), ethanol (CH3CH2OH), acrylamide (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCONH2, 99%), potassium dichromate (K2Cr2O7, 99.8%), disodium EDTA (99.8%), N-methyl-2-pyrrolidone (NMP, C5H9NO, 99.8%), polyvinylidene fluoride (PVDF, [CH2CF2]n, 99.8%), and sodium sulfate (Na2SO4, 99%). All chemicals were purchased from Sigma-Aldrich (Germany) and used without further purification.
CHCONH2, 99%), potassium dichromate (K2Cr2O7, 99.8%), disodium EDTA (99.8%), N-methyl-2-pyrrolidone (NMP, C5H9NO, 99.8%), polyvinylidene fluoride (PVDF, [CH2CF2]n, 99.8%), and sodium sulfate (Na2SO4, 99%). All chemicals were purchased from Sigma-Aldrich (Germany) and used without further purification.
2.2 Synthesis methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) sample, 198 mg of pre-synthesized DyCrO3 nanoparticles (sol–gel method) and 22 mg of MoS2 nanosheets were dispersed in 50 mL of a 1
5%) sample, 198 mg of pre-synthesized DyCrO3 nanoparticles (sol–gel method) and 22 mg of MoS2 nanosheets were dispersed in 50 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of deionized water and ethanol. The suspension was magnetically stirred for 3 h to ensure homogeneity, followed by hydrothermal treatment in a Teflon-lined stainless-steel autoclave at 180 °C for 12 h. After naturally cooling to room temperature, the product was collected by centrifugation, washed repeatedly with deionized water and ethanol, and dried at 120 °C for 12 h. Nanocomposites with higher MoS2 contents (10, 15, and 20 wt%) were synthesized using the same procedure by adjusting the MoS2 amount accordingly.
1 (v/v) mixture of deionized water and ethanol. The suspension was magnetically stirred for 3 h to ensure homogeneity, followed by hydrothermal treatment in a Teflon-lined stainless-steel autoclave at 180 °C for 12 h. After naturally cooling to room temperature, the product was collected by centrifugation, washed repeatedly with deionized water and ethanol, and dried at 120 °C for 12 h. Nanocomposites with higher MoS2 contents (10, 15, and 20 wt%) were synthesized using the same procedure by adjusting the MoS2 amount accordingly.
2.3 Characterization
The crystalline structure of the synthesized materials was analyzed using X-ray diffraction (XRD) on a Rigaku SmartLab diffractometer with Cu Kα radiation (λ = 1.5406 Å). The resulting diffraction patterns were refined via Rietveld analysis using the FullProf Suite to extract structural parameters. Surface morphology and microstructural features were examined using field emission scanning electron microscopy (FESEM, GeminiSEM 360, Zeiss, Germany) and transmission electron microscopy (TEM, Talos F200X, Thermo Fisher Scientific, USA). Elemental composition was determined through energy-dispersive X-ray (EDX) spectroscopy. Surface chemical states and binding energy profiles were probed using X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, USA). Optical properties were evaluated by UV-vis absorption spectroscopy (UV-2600, Shimadzu, Japan), while photoluminescence (PL) spectra were recorded on an RF-6000 spectrofluorophotometer (Shimadzu, Japan). Mott–Schottky (MS) measurements were performed at room temperature using a standard three-electrode setup on an electrochemical workstation (Autolab PGSTAT302N, Metrohm, Germany) to assess the semiconductor properties of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite. The photocatalytic performance of the DyCrO3–MoS2 (85%
15%) nanocomposite. The photocatalytic performance of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite was evaluated through the degradation of methylene blue (MB) and levofloxacin (LFX) under simulated solar irradiation. A 500 W Hg–Xe arc lamp, providing a power density of 100 mW cm−2, was used as the solar simulator for these studies.
15%) nanocomposite was evaluated through the degradation of methylene blue (MB) and levofloxacin (LFX) under simulated solar irradiation. A 500 W Hg–Xe arc lamp, providing a power density of 100 mW cm−2, was used as the solar simulator for these studies.
2.4 Electrochemical characterization for Mott–Schottky analysis
A schematic representation of the electrode fabrication process,35,36 and the electrochemical cell configuration employed for the Mott–Schottky measurements is provided in Fig. S2, with further procedural details outlined in the SI.2.5 Photocatalytic characterization
A detailed discussion of the photocatalytic characterization, accompanied by the relevant schematic illustration in Fig. S3, is presented in the article in the SI.3 Results and discussion
3.1 Structural, morphological and chemical state analysis
Rietveld-refined XRD patterns of DyCrO3–MoS2 nanocomposites with varying MoS2 loadings are shown in Fig. 1(a)–(d), confirming the coexistence of orthorhombic DyCrO3 (space group Pnma)18,24,34 and hexagonal MoS2 (space group P63/mmc).29,30 The Rietveld-refined XRD profile of pure MoS2 (Fig. S4, SI) exhibits sharp diffraction peaks, with the (0 0 2) reflection at 14.436° showing the highest intensity. The orthorhombic DyCrO3 framework provides a stable backbone for uniform MoS2 dispersion, while the hexagonal MoS2 lattice facilitates ion intercalation, enhancing both structural integrity and photocatalytic performance.Crystallite sizes of the DyCrO3–MoS2 composites with 5, 10, 15, and 20 wt% MoS2 were calculated as 17.06, 19.28, 23.15, and 21.23 nm, respectively, using the Scherrer equation24 (Table S1, SI). These sizes are significantly smaller than that of pure DyCrO3 nanoparticles (26 nm),18 indicating the formation of nanoscale crystallites and suggesting that MoS2 incorporation increases the surface area.
Notably, the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) sample shows a shift in the (0 0 2) peak near 2θ ≈14°, with an associated increase in interlayer spacing (inset of Fig. 1(b)), implying modifications in the MoS2 interplanar distance. This peak shift likely enhances photocatalytic performance by strengthening interfacial interactions between DyCrO3 and MoS2, facilitating more efficient charge separation.
15%) sample shows a shift in the (0 0 2) peak near 2θ ≈14°, with an associated increase in interlayer spacing (inset of Fig. 1(b)), implying modifications in the MoS2 interplanar distance. This peak shift likely enhances photocatalytic performance by strengthening interfacial interactions between DyCrO3 and MoS2, facilitating more efficient charge separation.
In contrast, a reduction in interlayer spacing at higher MoS2 loadings suggests possible MoS2 aggregation, which may hinder photocatalytic efficiency by reducing available surface area. The pronounced peak intensity at 2θ ≈ 14.19° further verifies the successful formation of DyCrO3–MoS2 nanocomposites across all compositions. Detailed structural parameters, including refined lattice constants and goodness-of-fit χ2 values for both MoS2 and DyCrO3–MoS2 systems, are presented in Table S1 in the SI.
FESEM images of DyCrO3–MoS2 nanocomposites with varying MoS2 concentrations (Fig. 2(a)–(d)) highlight distinct morphological features. EDX spectra and particle size distributions of DyCrO3–MoS2 nanocomposites with compositions (wt%) of DyCrO3 and MoS2 are 95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05%, 90%
05%, 90%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%, 85%
10%, 85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%, and 80%
15%, and 80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%, and along with the EDX profile and sheet thickness of MoS2 nanosheets, are illustrated in Fig. S5 in the SI. The estimated MoS2 nanosheets thickness exhibits around 10.99 nm (Fig. S5(j), in the SI). Moreover, the particle size of DyCrO3–MoS2 (95%
20%, and along with the EDX profile and sheet thickness of MoS2 nanosheets, are illustrated in Fig. S5 in the SI. The estimated MoS2 nanosheets thickness exhibits around 10.99 nm (Fig. S5(j), in the SI). Moreover, the particle size of DyCrO3–MoS2 (95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05%), DyCrO3–MoS2 (90%
05%), DyCrO3–MoS2 (90%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%), DyCrO3–MoS2 (85%
10%), DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), and DyCrO3–MoS2 (80%
15%), and DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%) nanocomposites were found to be in the range of 28 ± 7 to 32 ± 12 nm, In comparison, DyCrO3 nanoparticles range from 28 to 45 nm in size,18 both of which play critical roles in influencing the composites’ functionality. Moreover, mass and atomic percentages of elements in MoS2 nanosheets, and at varying MoS2 contents in DyCrO3–MoS2 nanocomposites were determined by EDX and theoretical analysis, which are presented in Table S2 in the SI. In the DyCrO3–MoS2 (95%
20%) nanocomposites were found to be in the range of 28 ± 7 to 32 ± 12 nm, In comparison, DyCrO3 nanoparticles range from 28 to 45 nm in size,18 both of which play critical roles in influencing the composites’ functionality. Moreover, mass and atomic percentages of elements in MoS2 nanosheets, and at varying MoS2 contents in DyCrO3–MoS2 nanocomposites were determined by EDX and theoretical analysis, which are presented in Table S2 in the SI. In the DyCrO3–MoS2 (95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05%) sample (Fig. 2(a)), prominent thick sheet-like morphologies indicate strong interfacial interactions between DyCrO3 and MoS2, resulting in a compact and well-integrated structure. With an increased MoS2 content of 10% (Fig. 2(b)), more intricate sheet architectures emerge, characterized by dispersed MoS2 clusters and reduced nanoparticle agglomeration. These features are favorable for photocatalysis, as they contribute to increased surface area, enhanced pollutant adsorption, and more efficient generation of reactive species. At the DyCrO3–MoS2 (85%
05%) sample (Fig. 2(a)), prominent thick sheet-like morphologies indicate strong interfacial interactions between DyCrO3 and MoS2, resulting in a compact and well-integrated structure. With an increased MoS2 content of 10% (Fig. 2(b)), more intricate sheet architectures emerge, characterized by dispersed MoS2 clusters and reduced nanoparticle agglomeration. These features are favorable for photocatalysis, as they contribute to increased surface area, enhanced pollutant adsorption, and more efficient generation of reactive species. At the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composition (Fig. 2(c)), the nanocomposite displays well-defined, larger MoS2 sheets interlaced with DyCrO3 nanoparticles, indicating strong heterointerfacial contact. In the DyCrO3–MoS2 (80%
15%) composition (Fig. 2(c)), the nanocomposite displays well-defined, larger MoS2 sheets interlaced with DyCrO3 nanoparticles, indicating strong heterointerfacial contact. In the DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%) sample (Fig. 2(d)), MoS2 sheets appear more extensively integrated within the DyCrO3 matrix, accompanied by increased sheet thickness. This may impede efficient charge transfer during photocatalytic reactions. While enhanced interfacial interactions contribute to structural integrity, the higher MoS2 content also promotes phase separation, which can compromise compositional uniformity. These observations highlight the importance of optimizing MoS2 loading to achieve a balance between structural coherence and photocatalytic efficiency.
20%) sample (Fig. 2(d)), MoS2 sheets appear more extensively integrated within the DyCrO3 matrix, accompanied by increased sheet thickness. This may impede efficient charge transfer during photocatalytic reactions. While enhanced interfacial interactions contribute to structural integrity, the higher MoS2 content also promotes phase separation, which can compromise compositional uniformity. These observations highlight the importance of optimizing MoS2 loading to achieve a balance between structural coherence and photocatalytic efficiency.
Energy-dispersive X-ray spectroscopy (EDS) mapping offers further insight into the spatial distribution of elements within the DyCrO3–MoS2 nanocomposites. In the DyCrO3–MoS2 (95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05%) sample (Fig. 2(e)), a uniform distribution of dysprosium (Dy), chromium (Cr), and oxygen (O) confirms the integrity of the DyCrO3 matrix, while the homogeneous presence of molybdenum (Mo), and sulfur (S) indicates successful MoS2 incorporation. At 10% MoS2 loading (Fig. 2(f)), more pronounced regions rich in Mo and S are evident, suggesting localized MoS2 clustering. The DyCrO3–MoS2 (85%
05%) sample (Fig. 2(e)), a uniform distribution of dysprosium (Dy), chromium (Cr), and oxygen (O) confirms the integrity of the DyCrO3 matrix, while the homogeneous presence of molybdenum (Mo), and sulfur (S) indicates successful MoS2 incorporation. At 10% MoS2 loading (Fig. 2(f)), more pronounced regions rich in Mo and S are evident, suggesting localized MoS2 clustering. The DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) sample (Fig. 2(g)) exhibits well-defined Mo and S domains, consistent with the presence of larger MoS2 sheets seen in FESEM analysis. A further increase to 20% MoS2 (Fig. 2(h)) results in intensified signals from Mo and S, confirming a higher degree of MoS2 integration. Elemental mapping conducted for both MoS2 nanosheets and DyCrO3–MoS2 composites (Fig. S6–S10, in the SI) validates the compositional uniformity critical for photocatalytic activity.
15%) sample (Fig. 2(g)) exhibits well-defined Mo and S domains, consistent with the presence of larger MoS2 sheets seen in FESEM analysis. A further increase to 20% MoS2 (Fig. 2(h)) results in intensified signals from Mo and S, confirming a higher degree of MoS2 integration. Elemental mapping conducted for both MoS2 nanosheets and DyCrO3–MoS2 composites (Fig. S6–S10, in the SI) validates the compositional uniformity critical for photocatalytic activity.
TEM analysis (Fig. 2(i) and (j)) further corroborates the morphology of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite, confirming the interconnected nanocrystalline structure observed in FESEM images. While FESEM estimated particle sizes for 5, 10, 15, and 20 wt% loading of MoS2 composites as 28.68 ± 7, 30.91 ± 12, 32.78 ± 13, and 32.40 ± 12 nm, respectively (Fig. S5 in the SI) TEM imaging (Fig. 2(i)) reveals more uniform, irregular spherical particles averaging 18 ± 10 nm in size for DyCrO3–MoS2 (85%
15%) nanocomposite, confirming the interconnected nanocrystalline structure observed in FESEM images. While FESEM estimated particle sizes for 5, 10, 15, and 20 wt% loading of MoS2 composites as 28.68 ± 7, 30.91 ± 12, 32.78 ± 13, and 32.40 ± 12 nm, respectively (Fig. S5 in the SI) TEM imaging (Fig. 2(i)) reveals more uniform, irregular spherical particles averaging 18 ± 10 nm in size for DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite, as shown in Fig. S11. The HRTEM image (Fig. 2(j)) displays clear lattice fringes with d-spacing values of 0.347 nm for the (1 1 1) plane of orthorhombic DyCrO3 nanoparticles and 0.216 nm for the (0 0 4) plane of hexagonal MoS2 nanosheets, consistent with XRD results. The SAED pattern (Fig. 2(k)) exhibits prominent concentric rings, confirming the polycrystalline nature of the DyCrO3–MoS2 (85%
15%) nanocomposite, as shown in Fig. S11. The HRTEM image (Fig. 2(j)) displays clear lattice fringes with d-spacing values of 0.347 nm for the (1 1 1) plane of orthorhombic DyCrO3 nanoparticles and 0.216 nm for the (0 0 4) plane of hexagonal MoS2 nanosheets, consistent with XRD results. The SAED pattern (Fig. 2(k)) exhibits prominent concentric rings, confirming the polycrystalline nature of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composite. Collectively, these morphological, elemental, and structural characterizations highlight the DyCrO3–MoS2 (85%
15%) composite. Collectively, these morphological, elemental, and structural characterizations highlight the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composition as the most structurally stable and catalytically favorable configuration, owing to its high crystallinity, uniform distribution of active sites, and enhanced interfacial integration.
15%) composition as the most structurally stable and catalytically favorable configuration, owing to its high crystallinity, uniform distribution of active sites, and enhanced interfacial integration.
X-ray photoelectron spectroscopy (XPS) analysis of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite provides crucial insights into its surface chemistry and electronic structure. The full survey spectrum (Fig. S12(a)) confirms the presence of Dy, Cr, O, Mo, and S elements. The chemical state analysis of DyCrO3–MoS2 (85%
15%) nanocomposite provides crucial insights into its surface chemistry and electronic structure. The full survey spectrum (Fig. S12(a)) confirms the presence of Dy, Cr, O, Mo, and S elements. The chemical state analysis of DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite, as depicted in Fig. 3, was carried out to determine its surface composition. The formation of oxygen vacancies,24 arising from missing oxygen atoms in the lattice, is governed by the coexistence of mixed oxidation states of transition metal ions (Cr2+/Cr3+) and rare-earth ions (Dy3+/Dy4+). In Fig. 3(a) and (b), the high-resolution Dy 4d and Dy 3d spectra reveal peaks characteristic of Dy4+, and Dy3+, respectively, while the Cr 2p region displays a mixture of Cr2+ and Cr3+ oxidation states (Fig. 3(c)). The Mo 3d spectrum (Fig. 3(d)) indicates the coexistence of Mo4+ and Mo6+ species, confirming the formation of Mo–S bonds, which is further supported by the S 2p (Fig. 3(e)) spectrum indicating successful sulfur incorporation from MoS2.29,30,37 Furthermore, the O 1s spectrum depicted in Fig. 3(f) shows a dominant contribution from oxygen vacancies, in addition to signals corresponding to metal–oxygen bonds and hydroxyl groups.24,38–41
15%) nanocomposite, as depicted in Fig. 3, was carried out to determine its surface composition. The formation of oxygen vacancies,24 arising from missing oxygen atoms in the lattice, is governed by the coexistence of mixed oxidation states of transition metal ions (Cr2+/Cr3+) and rare-earth ions (Dy3+/Dy4+). In Fig. 3(a) and (b), the high-resolution Dy 4d and Dy 3d spectra reveal peaks characteristic of Dy4+, and Dy3+, respectively, while the Cr 2p region displays a mixture of Cr2+ and Cr3+ oxidation states (Fig. 3(c)). The Mo 3d spectrum (Fig. 3(d)) indicates the coexistence of Mo4+ and Mo6+ species, confirming the formation of Mo–S bonds, which is further supported by the S 2p (Fig. 3(e)) spectrum indicating successful sulfur incorporation from MoS2.29,30,37 Furthermore, the O 1s spectrum depicted in Fig. 3(f) shows a dominant contribution from oxygen vacancies, in addition to signals corresponding to metal–oxygen bonds and hydroxyl groups.24,38–41
Notably, the oxygen vacancy concentration in the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composite (66.06%) is substantially higher than that in pristine DyCrO3 nanoparticles (40%),18 suggesting enhanced electron trapping, as depicted in Fig. 3(f). This increase in oxygen vacancies is likely to suppress charge carrier recombination, contributing to improved photocatalytic efficiency. Additionally, the presence of mixed valence states of Cr and Mo, combined with abundant oxygen vacancies, facilitates effective charge separation and promotes the formation of reactive oxygen species (ROS). These oxygen vacancies also serve as active sites for pollutant adsorption and ROS generation, further enhancing the overall photocatalytic performance of the composite.
15%) composite (66.06%) is substantially higher than that in pristine DyCrO3 nanoparticles (40%),18 suggesting enhanced electron trapping, as depicted in Fig. 3(f). This increase in oxygen vacancies is likely to suppress charge carrier recombination, contributing to improved photocatalytic efficiency. Additionally, the presence of mixed valence states of Cr and Mo, combined with abundant oxygen vacancies, facilitates effective charge separation and promotes the formation of reactive oxygen species (ROS). These oxygen vacancies also serve as active sites for pollutant adsorption and ROS generation, further enhancing the overall photocatalytic performance of the composite.
3.2 Optical characterization
UV-vis absorbance spectra and Tauc plots were used to estimate the optical band gaps of the DyCrO3–MoS2 nanocomposites.42 According to the Tauc plots in Fig. 4(a)–(d), the optical band gaps for DyCrO3–MoS2 (95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05%), DyCrO3–MoS2 (90%
05%), DyCrO3–MoS2 (90%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%), DyCrO3–MoS2 (85%
10%), DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), and DyCrO3–MoS2 (80%
15%), and DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%) are 2.14 eV, 2.07 eV, 1.91 eV, and 1.73 eV, respectively. With increasing MoS2 incorporation, the band gap narrows, indicating improved visible-light absorption. Moreover, by using the Tauc model, 1.724 eV is estimated as the band gap of MoS2 (Fig. S13). Nanocomposites' decreased band gap indicates that MoS2 insertion successfully alters the electrical band structure, promoting better charge separation and transfer.
20%) are 2.14 eV, 2.07 eV, 1.91 eV, and 1.73 eV, respectively. With increasing MoS2 incorporation, the band gap narrows, indicating improved visible-light absorption. Moreover, by using the Tauc model, 1.724 eV is estimated as the band gap of MoS2 (Fig. S13). Nanocomposites' decreased band gap indicates that MoS2 insertion successfully alters the electrical band structure, promoting better charge separation and transfer.
Steady-state photoluminescence (PL) spectra of DyCrO3–MoS2 nanocomposites with varying MoS2 content are shown in Fig. 4, providing insights into charge carrier dynamics and optical properties. Emission peaks corresponding to optical band gaps of 2.14 eV, 1.94 eV, 1.85 eV, and 1.69 eV are observed in the PL spectra presented in Fig. 4(e)–(h), recorded at an excitation wavelength of 220 nm for DyCrO3–MoS2 (95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05%), DyCrO3–MoS2 (90%
05%), DyCrO3–MoS2 (90%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%), DyCrO3–MoS2 (85%
10%), DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), and DyCrO3–MoS2 (80%
15%), and DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%), respectively. The redshift in PL emission peaks and the reduced band gap with increasing MoS2 content indicate enhanced solar light absorption. These results suggest that a 15 wt% MoS2 content is optimal for improving the photocatalytic activity of DyCrO3–MoS2 nanocomposites.
20%), respectively. The redshift in PL emission peaks and the reduced band gap with increasing MoS2 content indicate enhanced solar light absorption. These results suggest that a 15 wt% MoS2 content is optimal for improving the photocatalytic activity of DyCrO3–MoS2 nanocomposites.
The Mott–Schottky plots in Fig. 4(i)–(k) verify the n-type semiconducting behavior of DyCrO3–MoS2 nanocomposites,24,43,44 with flat band potentials (Vfb) of −0.49 V, −0.61 V, and −0.53 V (vs. Ag/AgCl) for DyCrO3, MoS2, and DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), respectively. XPS analysis revealed that the valence band of the 15 wt% MoS2-loaded composite i.e., DyCrO3–MoS2 (85%
15%), respectively. XPS analysis revealed that the valence band of the 15 wt% MoS2-loaded composite i.e., DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) is positioned at 1.54 V (Fig. 10(g)). Using the optical band gap values in combination with the Vfb measurements, the calculated band edge positions as discussed in details in the SI, were illustrated in Fig. 4(p), giving VBM/CBM values of 2.327 V/−0.393 V, 1.411 V/−0.313 V, and 1.477 V/−0.433 V (vs. NHE) for DyCrO3, MoS2, and DyCrO3–MoS2 (85%
15%) is positioned at 1.54 V (Fig. 10(g)). Using the optical band gap values in combination with the Vfb measurements, the calculated band edge positions as discussed in details in the SI, were illustrated in Fig. 4(p), giving VBM/CBM values of 2.327 V/−0.393 V, 1.411 V/−0.313 V, and 1.477 V/−0.433 V (vs. NHE) for DyCrO3, MoS2, and DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), respectively.
15%), respectively.
To further verify the charge-carrier recombination dynamics, time-resolved photoluminescence (TRPL) measurements were performed for the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite under 375 nm pulsed excitation. The decay curve (Fig. 4(l)) was fitted using a bi-exponential function, yielding an average lifetime of approximately 3.05 ns. The prolonged lifetime compared to typical oxide-based photocatalysts indicates efficient separation and slower recombination of photogenerated charge carriers across the DyCrO3/MoS2 interface.24 This result aligns well with the steady-state PL quenching and the reduced charge-transfer resistance observed from EIS, confirming the superior photoinduced charge-separation efficiency of the DyCrO3–MoS2.
15%) nanocomposite under 375 nm pulsed excitation. The decay curve (Fig. 4(l)) was fitted using a bi-exponential function, yielding an average lifetime of approximately 3.05 ns. The prolonged lifetime compared to typical oxide-based photocatalysts indicates efficient separation and slower recombination of photogenerated charge carriers across the DyCrO3/MoS2 interface.24 This result aligns well with the steady-state PL quenching and the reduced charge-transfer resistance observed from EIS, confirming the superior photoinduced charge-separation efficiency of the DyCrO3–MoS2.
The more negative CBM values enhance the thermodynamic driving force for the reduction of oxygen to ˙O2−, a key ROS in organic pollutant degradation. Similarly, the positive VBM values facilitate water oxidation to ˙OH, another potent ROS, thereby supporting efficient photocatalysis. In addition, the electrochemical impedance spectroscopy (EIS) results, illustrated in Fig. 4(m)–(o) for DyCrO3 nanoparticles, MoS2 nanosheets, and DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite, reveal the absence of arc radii in the insets of Fig. 4(m)–(o), indicating minimal charge transfer resistance. This reflects efficient charge carrier separation and suppressed electron–hole recombination45,46 contributing to the improved photocatalytic performance.
15%) nanocomposite, reveal the absence of arc radii in the insets of Fig. 4(m)–(o), indicating minimal charge transfer resistance. This reflects efficient charge carrier separation and suppressed electron–hole recombination45,46 contributing to the improved photocatalytic performance.
3.3 Photoelectrochemical analysis
Photoelectrochemical (PEC) investigations were performed to elucidate the interfacial charge-transfer behaviour of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite under dark and illuminated conditions (Fig. 5). The linear sweep voltammetry (LSV) curves (Fig. 5a) reveal a pronounced enhancement in current density upon illumination, increasing from 3.6 A g−1 in the dark to 9.9 A g−1 at 2.8 V under simulated solar light. This more than twofold increase in photocurrent directly confirms the efficient generation and separation of photoinduced charge carriers within the DyCrO3/MoS2 heterojunction. The superior photocurrent response under illumination originates from the S-scheme charge-transfer pathway that drives spatially separated redox reactions across the interface. The cyclic voltammetry (CV) profiles (Fig. 5b) recorded at a scan rate of 100 mV s−1 further substantiate this behaviour, displaying a substantially larger enclosed area under light irradiation compared with the dark condition. The enlarged CV loop implies higher capacitive response, improved interfacial redox kinetics, and enhanced reversibility of charge storage processes triggered by photoexcitation. Electrochemical impedance spectroscopy (EIS) measurements (Fig. 5c) complement these findings. The Nyquist plots exhibit a markedly smaller semicircle radius under illumination, indicating a lower charge-transfer resistance (Rct) and faster interfacial electron migration. The inset highlights the high-frequency region, where the slope increase signifies improved ionic/electronic conductivity and reduced recombination probability. Collectively, the LSV, CV, and EIS analyses confirm that DyCrO3–MoS2 (85%
15%) nanocomposite under dark and illuminated conditions (Fig. 5). The linear sweep voltammetry (LSV) curves (Fig. 5a) reveal a pronounced enhancement in current density upon illumination, increasing from 3.6 A g−1 in the dark to 9.9 A g−1 at 2.8 V under simulated solar light. This more than twofold increase in photocurrent directly confirms the efficient generation and separation of photoinduced charge carriers within the DyCrO3/MoS2 heterojunction. The superior photocurrent response under illumination originates from the S-scheme charge-transfer pathway that drives spatially separated redox reactions across the interface. The cyclic voltammetry (CV) profiles (Fig. 5b) recorded at a scan rate of 100 mV s−1 further substantiate this behaviour, displaying a substantially larger enclosed area under light irradiation compared with the dark condition. The enlarged CV loop implies higher capacitive response, improved interfacial redox kinetics, and enhanced reversibility of charge storage processes triggered by photoexcitation. Electrochemical impedance spectroscopy (EIS) measurements (Fig. 5c) complement these findings. The Nyquist plots exhibit a markedly smaller semicircle radius under illumination, indicating a lower charge-transfer resistance (Rct) and faster interfacial electron migration. The inset highlights the high-frequency region, where the slope increase signifies improved ionic/electronic conductivity and reduced recombination probability. Collectively, the LSV, CV, and EIS analyses confirm that DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) demonstrates superior photoelectrochemical activity under visible-light excitation due to efficient interfacial charge separation and accelerated surface redox dynamics, thereby substantiating its outstanding photocatalytic performance.
15%) demonstrates superior photoelectrochemical activity under visible-light excitation due to efficient interfacial charge separation and accelerated surface redox dynamics, thereby substantiating its outstanding photocatalytic performance.
3.4 Evaluation of photocatalytic performance
The solar-driven photocatalytic performance of DyCrO3–MoS2 nanocomposites was evaluated using two representative water pollutants: LFX, a colorless antibiotic, and MB, a colored dye. While LFX exhibited substantial degradation under sunlight, MB showed an even more pronounced response, highlighting the influence of pollutant optical properties on photocatalytic activity. This comparative approach allowed for a comprehensive investigation of photocatalytic behavior under different optical conditions.
Fig. 6(a) presents the photocatalytic degradation efficiency of LFX as a function of DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%) nanocomposite dosage under solar irradiation. The corresponding absorbance spectra for various dosages (5, 10, 20, and 40 mg) are shown in Fig. S14, in the SI. Initially, increasing the catalyst dosage enhanced the degradation rate due to the greater availability of active sites, which improved light absorption and facilitated the generation of reactive oxygen species (ROS), including OH˙ and ˙O2−. The degradation efficiency peaked at 10 mg, while further increases in dosage led to a plateau at 20 mg and a notable decline at 40 mg. This decrease is attributed to excessive particle loading, causing agglomeration and reduced light penetration, thereby lowering photocatalytic activity. Consequently, 10 mg was identified as the optimal dosage, balancing active surface area with effective light utilization.
20%) nanocomposite dosage under solar irradiation. The corresponding absorbance spectra for various dosages (5, 10, 20, and 40 mg) are shown in Fig. S14, in the SI. Initially, increasing the catalyst dosage enhanced the degradation rate due to the greater availability of active sites, which improved light absorption and facilitated the generation of reactive oxygen species (ROS), including OH˙ and ˙O2−. The degradation efficiency peaked at 10 mg, while further increases in dosage led to a plateau at 20 mg and a notable decline at 40 mg. This decrease is attributed to excessive particle loading, causing agglomeration and reduced light penetration, thereby lowering photocatalytic activity. Consequently, 10 mg was identified as the optimal dosage, balancing active surface area with effective light utilization.
Similar trends were observed for both LFX (Fig. 6(b)) and MB (Fig. 6(c)) across different MoS2 loadings. Lower MoS2 contents (5, and 10 wt%) resulted in reduced degradation efficiency, likely due to insufficient active sites and less effective ROS generation. Conversely, excessive MoS2 incorporation (20 wt%) negatively impacted activity, presumably due to increased nanosheet thickness and agglomeration, which limited light penetration and decreased accessible surface area, hindering ROS formation. The degradation efficiency of LFX and MB under solar irradiation at the optimized 10 mg dosage for different MoS2 loadings is shown in Fig. S15 and S16, respectively. Pseudo-first-order kinetic analysis for LFX degradation with varying DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%) dosages (Fig. 6(d)) revealed the highest rate constant of 0.01154 min−1 at 10 mg.
20%) dosages (Fig. 6(d)) revealed the highest rate constant of 0.01154 min−1 at 10 mg.
Using this optimized 10 mg dosage, DyCrO3–MoS2 nanocomposites with varying MoS2 concentrations were tested for LFX and MB degradation. Both pollutants exhibited increased reaction rate constants with higher MoS2 content. Notably, the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composition demonstrated the highest pseudo-first-order rate constants: 0.01416 min−1 for LFX (Fig. 6(e)) and 0.00829 min−1 for MB (Fig. 6(f)), reflecting the optimal balance of active surface area and light absorption. Based on these results, the 10 mg dosage of the DyCrO3–MoS2 (85%
15%) composition demonstrated the highest pseudo-first-order rate constants: 0.01416 min−1 for LFX (Fig. 6(e)) and 0.00829 min−1 for MB (Fig. 6(f)), reflecting the optimal balance of active surface area and light absorption. Based on these results, the 10 mg dosage of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite was selected for all subsequent photocatalytic studies to ensure consistent and efficient performance.
15%) nanocomposite was selected for all subsequent photocatalytic studies to ensure consistent and efficient performance.
The corresponding UV-vis absorption spectra (Fig. 7(a) and (b)) show a progressive decline in peak intensity accompanied by a hypochromic shift over time, confirming the effective photocatalytic degradation of LFX and MB by the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite.43,47 These results underscore the photocatalytic competence of the nanocomposite under simulated solar irradiation. The degradation efficiency was quantitatively determined using the following expression.18,24
15%) nanocomposite.43,47 These results underscore the photocatalytic competence of the nanocomposite under simulated solar irradiation. The degradation efficiency was quantitatively determined using the following expression.18,24
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite exhibited superior photocatalytic activity. After 240 minutes of irradiation with a 500 W Hg–Xe lamp, the DyCrO3–MoS2 (85%
15%) nanocomposite exhibited superior photocatalytic activity. After 240 minutes of irradiation with a 500 W Hg–Xe lamp, the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite exhibited degradation efficiencies of 84.95% for LFX and 79.87% for MB, highlighting its superior photocatalytic performance (Fig. 7(c)). The catalyst also showed an efficient degradation of CIP (68.51%) under identical conditions (Fig. S17). Pseudo-first-order kinetic analyses for LFX (0.00794 min−1) and MB (0.00606 min−1) degradation revealed markedly higher reaction rates with the DyCrO3–MoS2 (85%
15%) nanocomposite exhibited degradation efficiencies of 84.95% for LFX and 79.87% for MB, highlighting its superior photocatalytic performance (Fig. 7(c)). The catalyst also showed an efficient degradation of CIP (68.51%) under identical conditions (Fig. S17). Pseudo-first-order kinetic analyses for LFX (0.00794 min−1) and MB (0.00606 min−1) degradation revealed markedly higher reaction rates with the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite than the control samples, confirming its efficacy for removing pharmaceutical and dye pollutants from wastewater (Fig. 7(d)). These results highlight the synergistic enhancement in photocatalytic performance attributed to the incorporation of MoS2.
15%) nanocomposite than the control samples, confirming its efficacy for removing pharmaceutical and dye pollutants from wastewater (Fig. 7(d)). These results highlight the synergistic enhancement in photocatalytic performance attributed to the incorporation of MoS2.
Notably, the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposites demonstrated significantly higher photocatalytic activity than pristine DyCrO3 nanoparticles, which achieved only 70% MB degradation under identical conditions.18 This enhancement emphasizes the critical role of MoS2 in promoting charge separation and extending light absorption, thereby improving overall photocatalytic performance. Interestingly, during the first 120 minutes, LFX degradation (70%) was approximately 25% higher than that of MB (45%), highlighting the influence of pollutant optical properties and reaction kinetics. These results collectively underscore the DyCrO3–MoS2 (85%
15%) nanocomposites demonstrated significantly higher photocatalytic activity than pristine DyCrO3 nanoparticles, which achieved only 70% MB degradation under identical conditions.18 This enhancement emphasizes the critical role of MoS2 in promoting charge separation and extending light absorption, thereby improving overall photocatalytic performance. Interestingly, during the first 120 minutes, LFX degradation (70%) was approximately 25% higher than that of MB (45%), highlighting the influence of pollutant optical properties and reaction kinetics. These results collectively underscore the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite as the most structurally and catalytically favorable composition for solar-driven pollutant degradation.
15%) nanocomposite as the most structurally and catalytically favorable composition for solar-driven pollutant degradation.
To further elucidate the degradation mechanism, kinetic studies were performed using a pseudo-first-order model. The experimental data were fitted according to the following expression:18,24,48
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%), the highest rate constant of 0.01154 min−1 was obtained (Fig. 6(d)). Notably, the same optimized dosage of DyCrO3–MoS2 (85%
20%), the highest rate constant of 0.01154 min−1 was obtained (Fig. 6(d)). Notably, the same optimized dosage of DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) exhibited even higher rate constants of 0.01416 min−1 for LFX (Fig. 6(e)) and 0.00829 min−1 for MB (Fig. 6(f)). In contrast, pristine DyCrO3 nanoparticles showed a significantly lower rate constant of 0.00472 min−1 for MB degradation,18 indicating that MoS2 incorporation more than doubled the photocatalytic efficiency.
15%) exhibited even higher rate constants of 0.01416 min−1 for LFX (Fig. 6(e)) and 0.00829 min−1 for MB (Fig. 6(f)). In contrast, pristine DyCrO3 nanoparticles showed a significantly lower rate constant of 0.00472 min−1 for MB degradation,18 indicating that MoS2 incorporation more than doubled the photocatalytic efficiency.
Our composite shows an apparent rate constant of 0.00606 min−1 for methylene blue (MB, 12 mg L−1) and 0.00794 min−1 for levofloxacin (LFX, 10 mg L−1) using only 10 mg catalyst (0.1 g L−1) under a 500 W Hg–Xe lamp for 240 min, achieving ∼80–85% degradation efficiency. In comparison, bulk MoS2 (20 mg, sunlight, 240 min) shows 63% degradation with k = 3.5 × 10−3 min−1,49 layered MoS2 nanosheets (20 mg, 150 W visible lamp, 120 min) exhibit k = 7.7 × 10−3 min−1 (oxidative) and 81.7 × 10−3 min−1 (reductive),50 while a ZnS:CdS mixture (50 mg, 500 W halogen lamp, 360 min) records k = 3.61 × 10−3 min−1.51 Despite employing 2–5 times less catalyst, the DyCrO3–MoS2 composite demonstrates comparable or better kinetics and significantly broader pollutant applicability. Importantly, most MoS2- and ZnS-based systems focus solely on dye degradation, whereas our study extends to LFX, a non-chromophoric antibiotic resistant to photosensitized oxidation. Achieving high degradation of both dye and antibiotic pollutants under identical visible-light conditions highlights the robustness and universality of the DyCrO3–MoS2 photocatalyst.
The superior performance of the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite can be attributed to a synergistic combination of factors. The 15 wt% MoS2 loading provides an optimal balance between active catalytic sites and effective light penetration, while simultaneously enhancing charge carrier separation through efficient electron transfer pathways. This minimizes electron–hole recombination, promoting the generation of reactive oxygen species (ROS) that drive pollutant degradation. Although the DyCrO3–MoS2 (80%
15%) nanocomposite can be attributed to a synergistic combination of factors. The 15 wt% MoS2 loading provides an optimal balance between active catalytic sites and effective light penetration, while simultaneously enhancing charge carrier separation through efficient electron transfer pathways. This minimizes electron–hole recombination, promoting the generation of reactive oxygen species (ROS) that drive pollutant degradation. Although the DyCrO3–MoS2 (80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%) sample exhibits a slightly narrower band gap, this advantage is offset by reduced light accessibility and potential recombination effects, making the DyCrO3–MoS2 (85%
20%) sample exhibits a slightly narrower band gap, this advantage is offset by reduced light accessibility and potential recombination effects, making the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) composition the most efficient overall.
15%) composition the most efficient overall.
To further evaluate the catalytic potential, the activation energy (Ea) for LFX degradation was determined using the Arrhenius equation:24,54
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T curve. Fig. 8 illustrates the temperature-dependent degradation of LFX. In the absence of a photocatalyst (Fig. 8(a)), the rate constants at 10, 30, and 50 °C are 0.0022, 0.00622, and 0.01188 min−1, respectively, reflecting a thermally activated process. With the DyCrO3–MoS2 (85%
k vs. 1/T curve. Fig. 8 illustrates the temperature-dependent degradation of LFX. In the absence of a photocatalyst (Fig. 8(a)), the rate constants at 10, 30, and 50 °C are 0.0022, 0.00622, and 0.01188 min−1, respectively, reflecting a thermally activated process. With the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite (Fig. 8(b)), the corresponding rate constants increase to 0.0039, 0.0076, and 0.0123 min−1, indicating a substantial acceleration of the degradation process.
15%) nanocomposite (Fig. 8(b)), the corresponding rate constants increase to 0.0039, 0.0076, and 0.0123 min−1, indicating a substantial acceleration of the degradation process.
The Arrhenius plots (Fig. 8(c)) reveal that Ea decreases from 30.03 ± 3.87 kJ mol−1 (without photocatalyst) to 21.49 ± 1.05 kJ mol−1 in the presence of DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), confirming the enhanced photocatalytic performance of the nanocomposite. For comparison, pristine DyCrO3 exhibits an Ea of 20.85 kJ mol−1.18 The slightly higher activation energy for the composite is still indicative of efficient catalysis and arises from the synergistic interplay between DyCrO3 and MoS2. This synergy improves solar light absorption, promotes charge separation, and enhances ROS generation, collectively contributing to superior photocatalytic degradation.
15%), confirming the enhanced photocatalytic performance of the nanocomposite. For comparison, pristine DyCrO3 exhibits an Ea of 20.85 kJ mol−1.18 The slightly higher activation energy for the composite is still indicative of efficient catalysis and arises from the synergistic interplay between DyCrO3 and MoS2. This synergy improves solar light absorption, promotes charge separation, and enhances ROS generation, collectively contributing to superior photocatalytic degradation.
Overall, the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite demonstrates strong potential as a highly efficient, solar-driven photocatalyst, suitable for sustainable wastewater treatment and environmental remediation.
15%) nanocomposite demonstrates strong potential as a highly efficient, solar-driven photocatalyst, suitable for sustainable wastewater treatment and environmental remediation.
Fig. 9(a) illustrates that the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite exhibited the highest apparent quantum yield (AQY) values after the first photocatalytic cycle, achieving 37.88% for LFX, 39.59% for MB, and 36.21% for CIP. In contrast, after seven consecutive degradation cycles, the AQY values slightly decreased to 35.37%, 36.68%, and 34.76% for LFX, MB, and CIP, respectively, indicating excellent photocatalytic stability and reusability. The minor decline in AQY may be attributed to partial catalyst surface fouling or slight loss during recovery, rather than structural degradation. These results suggest that this composition offers an optimal synergy between charge separation efficiency, visible-light absorption, and active surface reactivity. Furthermore, this composition demonstrated inferior AQY performance, underscoring the pivotal role of MoS2 content in enhancing photon-to-chemical energy conversion. Overall, these findings confirm that the DyCrO3–MoS2 (85%
15%) nanocomposite exhibited the highest apparent quantum yield (AQY) values after the first photocatalytic cycle, achieving 37.88% for LFX, 39.59% for MB, and 36.21% for CIP. In contrast, after seven consecutive degradation cycles, the AQY values slightly decreased to 35.37%, 36.68%, and 34.76% for LFX, MB, and CIP, respectively, indicating excellent photocatalytic stability and reusability. The minor decline in AQY may be attributed to partial catalyst surface fouling or slight loss during recovery, rather than structural degradation. These results suggest that this composition offers an optimal synergy between charge separation efficiency, visible-light absorption, and active surface reactivity. Furthermore, this composition demonstrated inferior AQY performance, underscoring the pivotal role of MoS2 content in enhancing photon-to-chemical energy conversion. Overall, these findings confirm that the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite functions as a highly efficient, durable, and recyclable solar-driven photocatalyst, particularly effective for the degradation of organic pollutants such as MB, LFX, and CIP, making it a promising candidate for sustainable wastewater purification.
15%) nanocomposite functions as a highly efficient, durable, and recyclable solar-driven photocatalyst, particularly effective for the degradation of organic pollutants such as MB, LFX, and CIP, making it a promising candidate for sustainable wastewater purification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite possesses favorable band edge positions that promote efficient charge separation and facilitate the formation of OH˙ and ˙O2− radicals, thereby enhancing overall photocatalytic performance.
15%) nanocomposite possesses favorable band edge positions that promote efficient charge separation and facilitate the formation of OH˙ and ˙O2− radicals, thereby enhancing overall photocatalytic performance.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite demonstrated excellent reusability, retaining approximately 79.27% and 72.79% of its initial degradation efficiency for both LFX and MB after seven cycles, respectively. This minimal reduction in activity highlights the catalyst's robust structural and chemical stability under repeated solar irradiation, confirming its durability and reinforcing its potential for real-world wastewater treatment applications. The negligible decline in activity emphasizes the resilience of the photocatalyst, making it a strong candidate for scalable and sustainable environmental remediation strategies.
15%) nanocomposite demonstrated excellent reusability, retaining approximately 79.27% and 72.79% of its initial degradation efficiency for both LFX and MB after seven cycles, respectively. This minimal reduction in activity highlights the catalyst's robust structural and chemical stability under repeated solar irradiation, confirming its durability and reinforcing its potential for real-world wastewater treatment applications. The negligible decline in activity emphasizes the resilience of the photocatalyst, making it a strong candidate for scalable and sustainable environmental remediation strategies.
To further elucidate the structural integrity and durability of the nanocomposite, post-cycle characterization was conducted. Rietveld refinement of XRD patterns for the DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite before and after seven photocatalytic cycles is shown in Fig. 10(a) and (b). The crystal structure remains largely unaltered, with crystallite sizes of 22.88 nm before cycling and 21.98 nm after seven cycles (SI Table S1). Preservation of crystallinity is crucial for maintaining steady photocatalytic performance, as structural changes could disrupt active sites and hinder intercomponent interactions.
15%) nanocomposite before and after seven photocatalytic cycles is shown in Fig. 10(a) and (b). The crystal structure remains largely unaltered, with crystallite sizes of 22.88 nm before cycling and 21.98 nm after seven cycles (SI Table S1). Preservation of crystallinity is crucial for maintaining steady photocatalytic performance, as structural changes could disrupt active sites and hinder intercomponent interactions.
FESEM images (Fig. 10(c) and (d)) further confirm the morphological stability of the nanocomposite. The MoS2 nanosheets retain their sheet-like morphology, while DyCrO3 nanoparticles exhibit only minor agglomeration and a slight increase in sheet thickness after four cycles. This morphological preservation ensures that active sites remain accessible, sustaining high photocatalytic activity over repeated use.
Surface chemical states were evaluated using XPS. Survey spectra (Fig. S12(a)) confirm the presence of Dy, Cr, Mo, S, and O before and after seven cycles, demonstrating no elemental loss. Dy 4d and Dy 3d spectra (Fig. S12(b) and (c)) display unchanged binding energies and peak symmetry, verifying the stability of Dy3+ and Dy–O coordination. The O 1s spectra (Fig. 10(e) and (f)) show nearly identical lattice-oxygen, oxygen-vacancy, and hydroxyl components, with the vacancy fraction decreasing only slightly from 69.06% to 66.36%, indicating preservation of defect sites essential for charge separation and ROS generation. In the Cr 2p region (Fig. S12(d)), Cr3+ and minor Cr2+ species remain present, with a small increase in Cr2+ after cycling, suggesting mild surface reduction that may support interfacial charge compensation. The Mo 3d spectrum (Fig. S12(e) and (f)) shows significant evolution in the Mo 3d and S 2p regions. The Mo 3d spectrum (Fig. S12(e)) of the fresh catalyst exhibits a well-defined Mo 3d doublet associated with Mo6+ (Mo 3d5/2 at 235.75 eV) and Mo4+ (232.44 and 229.13 eV) contributions. However, after seven photocatalytic cycles, the characteristic Mo 3d doublet almost completely disappears, and the region collapses. This pronounced loss of the doublet may be attributed to the extensive Mo6+ → Mo4+ reduction and the generation of defect-associated Mo–S active sites through redox-driven surface reconstruction.57 A comparable transformation is observed in the S 2p spectrum (Fig. S12(f)), where the well-resolved S2− doublet present in the pristine sample is significantly suppressed after cycling; notably, the S 2p1/2 peak effectively disappears, and the remaining S 2p signal broadens with an enhanced S–O contribution. This behaviour signifies the formation of sulfur vacancies and mild surface oxidation during sustained photocatalysis, while the persistence of the S 2p3/2 component confirms that the MoS2 lattice framework remains largely intact. Such defect generation is widely recognized to facilitate interfacial charge transport and contributes to the excellent catalytic durability of the composite. Electronic and vibrational stability were further examined using VB-XPS and Raman spectroscopy. The VB-XPS spectra (Fig. 10(g)) show an unchanged valence-band maximum at ∼1.54 eV after seven cycles, confirming preservation of electronic structure and band alignment. Raman spectra (Fig. 10(h)) maintain all characteristic DyCrO3 modes (Dy–O bending, Cr–O stretching, octahedral vibrations58,59) and MoS2 bands (E12g at ∼383 cm−1, A1g at ∼408 cm−1 (ref. 60 and 61)) with negligible intensity variation, indicating no structural degradation or phase change. FTIR spectra (Fig. 10(i)) show minimal differences before and after photocatalysis, with characteristic S–S stretching at 959 cm−1,62 Mo–S vibrations near 600 cm−1,63,64 Cr–O stretching at 524 cm−1, and Dy3+-related phonon modes near 445 cm−1 (ref. 18) preserved. The peak at ∼571.83 cm−1 remains consistent with MoS2 signatures.65
Collectively, the XRD, FESEM, XPS, VB-XPS, Raman, and FTIR results confirm that DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) maintains its crystal structure, morphology, surface chemistry, electronic configuration, and vibrational framework during repeated cycling, demonstrating outstanding durability and suitability for long-term photocatalytic applications.
15%) maintains its crystal structure, morphology, surface chemistry, electronic configuration, and vibrational framework during repeated cycling, demonstrating outstanding durability and suitability for long-term photocatalytic applications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite is attributed to an S-scheme heterojunction mechanism under visible-light irradiation (Fig. 11 and 12). Both DyCrO3 and MoS2 are n-type semiconductors, with the conduction band (CB) of DyCrO3 (−0.393 V, Fig. 4(p)) more negative than that of MoS2 (−0.313 V, Fig. 4(p)), as determined via Tauc plots (Fig. 8(b) of our previous report,18 S13) and Mott–Schottky analyses (Fig. 4(i) and (j)). When the two semiconductors form a heterojunction, electrons transfer from DyCrO3 (reduction photocatalyst, RP) to MoS2 (oxidation photocatalyst, OP), creating an internal electric field directed from DyCrO3 to MoS2 (Fig. 11(c)).
15%) nanocomposite is attributed to an S-scheme heterojunction mechanism under visible-light irradiation (Fig. 11 and 12). Both DyCrO3 and MoS2 are n-type semiconductors, with the conduction band (CB) of DyCrO3 (−0.393 V, Fig. 4(p)) more negative than that of MoS2 (−0.313 V, Fig. 4(p)), as determined via Tauc plots (Fig. 8(b) of our previous report,18 S13) and Mott–Schottky analyses (Fig. 4(i) and (j)). When the two semiconductors form a heterojunction, electrons transfer from DyCrO3 (reduction photocatalyst, RP) to MoS2 (oxidation photocatalyst, OP), creating an internal electric field directed from DyCrO3 to MoS2 (Fig. 11(c)).
This interfacial electric field, together with Fermi-level alignment and Coulombic interactions (Fig. 11(a) and (b)), induces band bending and selective recombination of low-energy carriers. Specifically, CB electrons of MoS2 recombine with VB holes of DyCrO3 at the interface (Fig. 11(c)), while high-energy electrons in DyCrO3 CB and holes in MoS2 VB are preserved. It should be noted that charge separation in the DyCrO3–MoS2 S-scheme heterojunction is governed by interfacial band bending and the built-in electric field rather than direct long-range valence-band-to-valence-band hole transfer. These charge carriers drive the generation of reactive oxygen species (ROS), such as ˙O2− and OH˙, responsible for the photocatalytic degradation of LFX and MB (Fig. 7 and 11).
In addition to heterojunction formation, oxygen vacancies in DyCrO3 (Fig. 11) play a critical role in enhancing photocatalytic performance. These vacancies act as electron-capturing centers, improving charge separation and facilitating ROS generation. Adsorbed oxygen reacts with electrons at these vacancies to form ˙O2− radicals, while holes oxidize water or hydroxyl ions to produce OH˙ radicals. MoS2 nanosheets contribute further by providing abundant active sites for pollutant adsorption and promoting interfacial electron transfer, which suppresses electron–hole recombination. The photocatalytic degradation process can be summarized as:
| h+ + pollutants → byproducts |
| OH˙ + pollutants → byproducts |
Consequently, the combined effects of oxygen vacancies in DyCrO3 and the structural and electronic features of MoS2 result in efficient charge separation, enhanced ROS generation, and improved adsorption, collectively driving the high photocatalytic activity of the nanocomposite. The S-scheme architecture, optimized DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%), and 10 mg dosage ensure maximal light absorption, interfacial charge transfer, and active-site availability, making the DyCrO3–MoS2 nanocomposite highly effective for solar-driven environmental remediation (Fig. 10 and 11). Overall, the integration of MoS2 with DyCrO3 offers several advantages for visible-light photocatalysis. The formation of an S-scheme heterojunction significantly enhances charge separation and suppresses electron–hole recombination, leading to higher degradation efficiency compared to pristine DyCrO3. The optimized DyCrO3–MoS2 (85%
15%), and 10 mg dosage ensure maximal light absorption, interfacial charge transfer, and active-site availability, making the DyCrO3–MoS2 nanocomposite highly effective for solar-driven environmental remediation (Fig. 10 and 11). Overall, the integration of MoS2 with DyCrO3 offers several advantages for visible-light photocatalysis. The formation of an S-scheme heterojunction significantly enhances charge separation and suppresses electron–hole recombination, leading to higher degradation efficiency compared to pristine DyCrO3. The optimized DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite also benefits from improved surface activity and stability. However, the photocatalytic performance depends on the MoS2 loading ratio, as excessive MoS2 may induce light-shielding effects, highlighting the importance of compositional optimization.
15%) nanocomposite also benefits from improved surface activity and stability. However, the photocatalytic performance depends on the MoS2 loading ratio, as excessive MoS2 may induce light-shielding effects, highlighting the importance of compositional optimization.
4 Conclusions
A DyCrO3/MoS2 S-scheme heterojunction photocatalyst was synthesized via a simple hydrothermal approach and thoroughly characterized. The S-scheme interface established a strong internal electric field that enhanced charge separation and reactive oxygen species generation. The optimized DyCrO3–MoS2 (85%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15%) nanocomposite achieved 84.95% degradation of levofloxacin and 78.97% degradation of methylene blue within 240 min under visible light. TRPL, PEC, band-edge analysis, and radical-trapping experiments confirmed efficient carrier utilization and improved light harvesting, resulting in superior kinetics and quantum yield. Post-cycling XPS and Raman analyses verified excellent structural stability and reusability. Overall, the DyCrO3–MoS2 heterostructure demonstrates strong potential as a durable and efficient solar-driven photocatalyst for wastewater purification. Future work may expand its applicability to a broader range of emerging pollutants.
15%) nanocomposite achieved 84.95% degradation of levofloxacin and 78.97% degradation of methylene blue within 240 min under visible light. TRPL, PEC, band-edge analysis, and radical-trapping experiments confirmed efficient carrier utilization and improved light harvesting, resulting in superior kinetics and quantum yield. Post-cycling XPS and Raman analyses verified excellent structural stability and reusability. Overall, the DyCrO3–MoS2 heterostructure demonstrates strong potential as a durable and efficient solar-driven photocatalyst for wastewater purification. Future work may expand its applicability to a broader range of emerging pollutants.
Author contributions
Md. Mahbubar Rahman: investigation; methodology; visualization; writing – original draft. Md. Sobuj Hossain: investigation; writing – review & editing. Tasnim Jahan: investigation; writing – review & editing. M. A. Basith: conceptualization; resources; funding acquisition; validation; supervision; writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental method; UV absorption spectra of LFX and MB; apparent quantum yield (AQY) calculation; comparative photocatalytic performance of DyCrO3 with other perovskite oxide; activation energy experiment; Rietveld refinement XRD patterns of DyCrO3 nanoparticle pre and post cycle photocatalysis. In this study, DyCrO3/MoS2 S-scheme nanostructures were synthesized by integrating DyCrO3 nanoparticles (an n-type semiconductor) with MoS2 nanosheets (an n-type semiconductor). See DOI: https://doi.org/10.1039/d5ma01025j.Acknowledgements
We sincerely acknowledge the Committee for Advanced Studies and Research (CASR) at the Bangladesh University of Engineering and Technology (BUET), which has been instrumental in the successful execution of this research.Notes and references
- M. Sakamoto, T. Ahmed, S. Begum and H. Huq, Sustainability, 2019, 11, 1951 CrossRef.
- H. Gao, J. Angadi, S. Wang, X. Zhou, H. Yang, A. M. Al-Enizi, M. Ubaidullah, B. Pandit, D. Li and M. Gupta, Adv. Sustainable Syst., 2024, 8, 2300533 CrossRef CAS.
- R. Shanmuganathan, M. S. Kadri, T. Mathimani, Q. H. Le and A. Pugazhendhi, Chemosphere, 2023, 332, 138812 CrossRef CAS PubMed.
- U. Salma, Y. Nishimura, M. Tokumura, A. Hossain, K. Watanabe, K. Noro, M. Raknuzzaman, T. Amagai and M. Makino, Chemosphere, 2025, 370, 143956 CrossRef CAS PubMed.
- S. Gupta, Y. Mittal, R. Panja, K. B. Prajapati and A. K. Yadav, in Current Developments in Biotechnology and Bioengineering, ed. S. Kumar, R. Kumar and A. Pandey, Elsevier, 2021, pp. 47–75 Search PubMed.
- P. V. Messina and P. C. Schulz, J. Colloid Interface Sci., 2006, 299, 305–320 CrossRef CAS PubMed.
- S. Muthupoongodi, T. Linda, X. S. Shajan, L. Mitu and S. Balakumar, Polym. Bull., 2018, 75, 1867–1893 CrossRef CAS.
- P. N. Egbuikwem, G. C. Obiechefu, F. I. Hai, M. C. E. Devanadera and D. P. Saroj, Environ. Technol. Rev., 2021, 10, 77–110 CrossRef.
- J. S. Chang, Y. W. Phuan, M. N. Chong and J. D. Ocon, J. Ind. Eng. Chem., 2020, 83, 303–314 CrossRef CAS.
- R. Gade, J. Ahemed, K. L. Yanapu, S. Y. Abate, Y.-T. Tao and S. Pola, J. Environ. Chem. Eng., 2018, 6, 4504–4513 CrossRef CAS.
- M. Subburu, R. Gade, V. Guguloth, P. Chetti, K. R. Ravulapelly and S. Pola, J. Photochem. Photobiol., A, 2021, 406, 112996 CrossRef CAS.
- K. Masula, Y. Bhongiri, G. R. Rao, P. V. Kumar, S. Pola and M. Basude, Opt. Mater., 2022, 126, 112201 CrossRef.
- M. Pirhashemi and A. Habibi-Yangjeh, Ceram. Int., 2015, 41, 14383–14393 CrossRef CAS.
- Q. Guo, C. Zhou, Z. Ma and X. Yang, J. Adv. Mater., 2019, 31, 1901997 CrossRef CAS PubMed.
- A. Mclaren, T. Valdes-Solis, G. Li and S. C. Tsang, J. Am. Chem. Soc., 2009, 131, 12540–12541 CrossRef CAS PubMed.
- C. Hitam and A. Jalil, J. Environ. Manage., 2020, 258, 110050 CrossRef CAS PubMed.
- J. A. Rengifo-Herrera and C. Pulgarin, J. Chem. Eng., 2023, 146875 CrossRef CAS.
- M. M. Rahman, F. Yasmeen, M. Tarek and M. Basith, J. Alloys Compd., 2025, 1010, 177295 CrossRef CAS.
- Y. F. Abed, S. Das, M. S. Ali, Z. Rana and M. A. Basith, Mater. Lett., 2022, 318, 132159 CrossRef CAS.
- M. Rani, S. Dahiya and N. Panwar, Ceram. Int., 2022, 48, 19925–19936 CrossRef CAS.
- Q. Ji, L. Bi, J. Zhang, H. Cao and X. S. Zhao, Energy Environ. Sci., 2020, 13, 1408–1428 RSC.
- M. Periyasamy, S. Sain, U. Sengupta, M. Mandal, S. Mukhopadhyay and A. Kar, Mater. Adv., 2021, 2, 4843–4858 RSC.
- H. Xiao, P. Liu, W. Wang, R. Ran, W. Zhou and Z. Shao, Energy Fuels, 2020, 34, 9208–9221 CrossRef CAS.
- M. Tarek, F. Yasmeen and M. A. Basith, J. Mater. Chem. A, 2024, 12, 25475–25490 RSC.
- U. Chinnathambi, R. Chandrapal, R. Aiswarya, B. Palanivel and T. Kalaivani, J. Cluster Sci., 2025, 36, 43 CrossRef CAS.
- X. Yang, L. Wen, H. Huang, Y. Wang, L. Wei and J. Yang, Solid State Sci., 2022, 133, 107014 CrossRef CAS.
- X. Qiu, T. Zhang, Z. Dai, R. Cao and T. Wei, Ionics, 2022, 28, 939–949 CrossRef CAS.
- R. Mohammed, M. E. M. Ali, E. Gomaa and M. Mohsen, Environ. Nanotechnol. Monit. Manage., 2023, 19, 100772 CAS.
- A. M. Tama, S. Das, S. Dutta, M. Bhuyan, M. Islam and M. A. Basith, RSC Adv., 2019, 9, 40357–40367 RSC.
- M. Tarek and M. A. Basith, J. Mater. Chem. C, 2023, 11, 16605–16622 RSC.
- Q. Chen, J.-L. Li, Q.-L. Mo and F.-X. Xiao, Chem. Eng. J., 2024, 497, 154584 CrossRef CAS.
- R. Kumari and R. Kumar, ECS J. Solid State Sci. Technol., 2023, 12, 097004 CrossRef CAS.
- H. Khan, A. Mehtab, J. Ahmed, S. E. Lofland, K. V. Ramanujachary and T. Ahmad, ChemNanoMat, 2023, 9, e202300091 CrossRef CAS.
- M. Tarek, F. Yasmeen and M. A. Basith, Nanoscale, 2025, 17, 6620–6636 RSC.
- M. Islam, M. Tarek, M. A. Adib and M. A. Basith, J. Phys. D: Appl. Phys., 2024, 57, 215302 CrossRef CAS.
- F. Yasmeen, M. Tarek and M. A. Basith, ACS Appl. Mater. Interfaces, 2024, 16, 47535–47550 CrossRef CAS PubMed.
- S. Hasan, A. H. Reaz, S. Das, C. K. Roy and M. A. Basith, J. Mater. Chem. C, 2022, 10, 7980–7996 RSC.
- M. Islam, M. Tarek, R. Rashid, M. Bally, F. Ara and M. A. Basith, Mater. Adv., 2025, 6, 1379–1391 RSC.
- D.-Q. Yang and E. Sacher, J. Phys. Chem. C, 2009, 113, 6418–6425 CrossRef CAS.
- R. Liu, Z. Wang, S. Peng, J. Bi, J. Wu and Z.-G. Ye, J. Am. Ceram. Soc., 2020, 103, 1097–1104 CrossRef CAS.
- S. Jain, J. Shah, N. S. Negi, C. Sharma and R. K. Kotnala, Int. J. Energy Res., 2019, 43, 4743–4755 CrossRef CAS.
- P. Makuła, M. Pacia and W. Macyk, How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra, 2018 Search PubMed.
- M. A. Adib, F. Sharmin and M. A. Basith, Nanoscale Adv., 2023, 5, 6194–6209 RSC.
- M. Hosen, M. Tarek, M. Bhuyan, M. A. Basith and I. Syed, Nanoscale Adv., 2025, 7, 1742–1753 RSC.
- T. Xiong, H. Wang, Y. Zhou, Y. Sun, W. Cen, H. Huang, Y. Zhang and F. Dong, Nanoscale, 2018, 10, 8066–8074 RSC.
- Y. Li, L. Ding, Y. Guo, Z. Liang, H. Cui and J. Tian, ACS Appl. Mater. Interfaces, 2019, 11, 41440–41447 CrossRef CAS PubMed.
- M. S. Ali, S. Das, Y. F. Abed and M. A. Basith, Phys. Chem. Chem. Phys., 2021, 23, 22184–22198 RSC.
- V. Ramar and K. Balasubramanian, ACS Appl. Nano Mater., 2021, 4, 5512–5521 CrossRef CAS.
- N. Chaudhary, P. Yadav and V. Bisht, AIP Conf. Proc., 2020, 2276, 020030 CrossRef.
- J. Kisała, R. Wojnarowska-Nowak and Y. Bobitski, Sci. Rep., 2023, 13, 14148 CrossRef PubMed.
- N. Soltani, E. Saion, M. Z. Abdul Hamid and A. Rezaee Khadangi, Int. J. Mol. Sci., 2012, 13, 12242–12258 CrossRef CAS PubMed.
- E. Muetterties, Science, 1977, 196, 839–848 CrossRef CAS PubMed.
- O. Christensen, A. Bagger and J. Rossmeisl, ACS Catal., 2024, 14, 2151–2161 CrossRef CAS.
- J. Velázquez, R. Fernández-González, L. Daz, E. P. Melián, V. Rodrguez and P. Núñez, J. Alloys Compd., 2017, 721, 405–410 CrossRef.
- M. J. Muñoz-Batista, A. Kubacka and M. Fernández-Garca, Catal. Sci. Technol., 2014, 4, 2006–2015 RSC.
- J. Barrio, D. Mateo, J. Albero, H. Garca and M. Shalom, Adv. Energy Mater., 2019, 9, 1902738 CrossRef CAS.
- X. Zhang, X. Zhang, Y. Liu, X. Li, Y. Wang, Y. Zhao and H. Huang, Appl. Catal., B, 2020, 263, 118358 CrossRef.
- M. C. Weber, J. Kreisel, P. A. Thomas, M. Newton, K. Sardar and R. I. Walton, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 054303 CrossRef.
- L. Li, F. Chen, J.-Q. Lu and M.-F. Luo, J. Phys. Chem. A, 2011, 115, 7972–7977 CrossRef CAS PubMed.
- S. Cortijo-Campos, P. Kung, C. Prieto and A. de Andres, J. Phys. Chem. C, 2021, 125, 23904–23910 CrossRef CAS.
- T. Livneh and J. E. Spanier, 2D Mater., 2015, 2, 035003 CrossRef.
- K. R. Mamaghani and N. Parvin, J. Mater. Sci., 2023, 58, 12182–12201 CrossRef CAS.
- R. Leelavathi, K. Vivekanandan and V. Hariharan, Acta Phys. Pol., A, 2024, 145, 361–369 CrossRef CAS.
- A. Kaur, S. Rana, A. Bharti, G. R. Chaudhary and N. Prabhakar, Microchim. Acta, 2021, 188, 222 CrossRef CAS PubMed.
- S. Ahmad, I. Khan, A. Husain, A. Khan and A. M. Asiri, Polymers, 2020, 12, 3047 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |