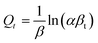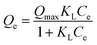Open Access Article
Open Access ArticleTailored magnetic hybrid composites with recoverable properties for efficient Cr(VI) adsorption and reduction: a synergistic experimental and theoretical study
Abdelaziz
Imgharn
 *ab,
Mohammed
Elhoudi
a,
Samira
El omari
a,
Kamal
Ait El Bacha
a,
Mohamed
Laabd
*ab,
Mohammed
Elhoudi
a,
Samira
El omari
a,
Kamal
Ait El Bacha
a,
Mohamed
Laabd
 a,
Lahcen
Bazzi
c and
Abdallah
Albourine
ac
a,
Lahcen
Bazzi
c and
Abdallah
Albourine
ac
aLaboratory of Materials and Environment, Faculty of Sciences, Ibn Zohr University, Agadir, Morocco. E-mail: abdelaziz.imgharn@edu.uiz.ac.ma
bInternational Water Research Institute (IWRI), Mohammed VI Polytechnic University, Ben Guerir 43150, Morocco
cLaboratory of Industrial Engineering, Energy and Environment (LI3E), SupMTI Rabat, Morocco
First published on 27th October 2025
Abstract
Despite polyaniline's simple synthesis methods and proven capabilities pollutant removal applications, its recovery from solutions remains a major challenge that hampers its application. Therefore, developing approaches for synthesizing an efficient and easily recoverable polyaniline (PANI) adsorbent is crucial. In this study, we designed a magnetic Fe3O4–cysteine-functionalized PANI (Fe3O4–Cys–PANI) adsorbent via in situ polymerization. The resulting magnetic adsorbent was characterized by several analytical techniques (e.g., FTIR spectroscopy, XRD, porosity measurements and SEM-EDS), and the results indicated that it exhibited tunable features for Cr(VI) detoxification. A systematic experimental study revealed that the maximum Cr(VI) detoxification yield (98.12%) was achieved with an adsorbent dose of 0.25 g L−1 and at pH 2.0. The Freundlich isotherm models and the pseudo-second-order kinetics were appropriate for predicting the Cr(VI) removal process. The Monte Carlo simulations further elucidated the Cr(VI) adsorption process, revealing an adsorption energy of Eads = −55.613 kcal mol−1 on Fe3O4–Cys–PANI. The significantly negative adsorption energy further supported the experimental findings, confirming the spontaneity of the process and high energy efficiency. Additionally, the Fe3O4–Cys–PANI magnetic composite showed excellent decontamination, outstanding regeneration capacity and prominent reusability, making it a promising candidate for industrial wastewater treatment.
1. Introduction
Chromium (Cr) toxins are found in almost every component of the environment, including soil, air, and water.1–3 Cr(VI) oxyanions, common forms of the heavy metal chromium, raise a significant concern due to their toxic properties and tendency to accumulate in organisms. Acute exposure to Cr(VI) can lead to diarrhea, kidney failure, nausea, liver diseases, ulcer formation, lung cancer and respiratory issues.4,5 Reducing Cr(VI) to Cr(III) is indeed an ideal option, given the much higher toxicity of Cr(VI) compared to Cr(III).6 Hence, it is crucial to explore and develop effective methods that can concurrently reduce Cr(VI) to Cr(III) and eliminate it from the environment. The conventional approaches for wastewater treatment involve membrane filtration, electrochemical processes, coagulation and flocculation, bioremediation, and advanced oxidation processes.4,6 Nevertheless, these approaches require high-energy systems, which can increase their operating costs. It is particularly advantageous to adsorb and reduce Cr(VI) ions from solutions using the adsorption–reduction process. Thus, it is essential to develop low-cost, dual-purpose, and highly efficient materials for adsorption–reduction.A variety of N-containing functional group organic polymers have been used in hybrid composites in recent years to adsorb and reduce Cr(VI).7–9 The unique conjugation system, doping/dedoping properties, and excellent stability of these composites have led to their excessive use in hybrids. Besides their interrelationship with Cr(VI)'s negative charge, nitrogen atoms in amines and imines can donate electrons to reduce Cr(VI) ions into less-toxic Cr(III) ions.10 Therefore, polyaniline (PANI) possesses all the aforementioned features, making it a promising adsorber/reducer for Cr(VI) detoxification.11 Recent investigations have reaffirmed its outstanding performance in Cr(VI) removal and revealed its potential for the adsorption of a wide range of organic pollutants from aqueous media. Nevertheless, as for other adsorbent materials, the main cornerstone of PANI for further application in the field of adsorption is its difficult recovery from solutions, which can lead to secondary pollution. Due to the absence of polarity on PANI, it cannot be controlled by magnetic fields.12 Typically, PANI powders are separated from liquid solutions by centrifugation or filtration, both of which are expensive.13,14
To address this issue and prevent secondary pollution after adsorption processes, incorporating magnetic materials enables easy recovery. It is therefore proposed to combine PANI with magnetic particles to adsorb heavy metals in a simple, low-cost and fast way for practical procedures.15–17 The Fe3O4 magnetic particles were chosen not only to tackle the PANI's drawback – its recovery – but also to contribute toward the reduction of toxic Cr(VI) to the less harmful Cr(III) form, making it an efficient water purification material.10,18 Furthermore, functionalizing PANI with cysteine significantly enhances its adsorption capacity and selectivity for Cr(VI) by introducing additional functional groups that facilitate strong and selective binding of Cr(VI) oxyanions. Magnetic cysteine–PANI composites have not yet been reported, representing an efficient approach that combines enhanced adsorption performance with the magnetic functionality that ensures easy recovery and reusability of the adsorbent. This integration ensures efficient pollutant removal and prevents secondary contamination of water by facilitating its separation from treated water.
This research endeavor holds immense significance in the field of water environmental remediation, aiming to mitigate the detrimental effects of Cr(VI) in water systems. Herein, a tailored hybrid composite was developed and characterized, in which Fe3O4, cysteine and polyaniline were combined to provide functional groups, magnetic recoverability, and improved Cr(VI) adsorption capacity. A systematic study was conducted to evaluate the influences of solution pH, temperature, adsorbent dose, contact time and selectivity on the Cr(VI) detoxification. Finally, the regeneration of Fe3O4–Cys–PANI was performed to assess its reusability.
2. Experimental
2.1. Chemicals
Aniline (C6H7N) (≥99%), sodium hydroxide (NaOH) (≥99%), ammonium persulfate ((NH4)2S2O8) (≥98.5%), ammonia (NH4OH) (≥99%), iron(III) chloride (FeCl3·6H2O) (≥99%), iron(II) chloride (FeCl2·4H2O) (≥98.8%), ethanol (C2H6O) (≥99%), acetone (≥99.8%), hydrochloric acid (HCl) (37%), potassium dichromate (K2Cr2O7) (≥99.5%), phosphoric acid (H3PO4) (86%), 1,5-diphenylcarbazide (95%) and sulfuric acid (H2SO4) (≥98%) were purchased from Sigma-Aldrich.2.2. Preparation of magnetic Fe3O4–Cys–PANI with recoverability
The Fe3O4 nanoparticles were synthesized by a co-precipitation method. Briefly, 1 M FeCl2·4H2O and 2 M FeCl3·6H2O were introduced into deionized water at a molar ratio of 1/2. NH4OH (2 M) was added dropwise to the solution under continuous stirring at 60 °C for 4 h. The resulting nanoparticles were magnetically separated, washed with distilled water, and dried at 70 °C.The magnetic Fe3O4–Cys–PANI composite was synthesized via in situ oxidative polymerization in an ice bath. In this process, 1 g of Fe3O4 nanoparticles and 1 g of cysteine were first dispersed in 100 mL of 0.01 M HCl and sonicated for 1 h to ensure uniform distribution and surface functionalization. Subsequently, 0.25 mL of purified aniline monomer was introduced into the Fe3O4–cysteine suspension, allowing cysteine to act as a co-functional agent during the polymerization step. The mixture was then stirred for 2 h. Then, ammonium persulfate (APS), dissolved in 50 mL of 0.01 M HCl with a 1/2 monomer (aniline)-to-oxidant (APS) molar ratio, was added dropwise to the suspension while maintaining the ice bath. Polymerization proceeded under continuous stirring for 12 h. The resulting Fe3O4–Cys–PANI composite was washed with ethanol and distilled water and dried at 70 °C. This tailored design strategy was selected to combine the high affinity of cysteine functional groups, the conductive framework of PANI, and the magnetic separability of Fe3O4, thereby enhancing the overall adsorption efficiency.
2.3. Characterization of the adsorbent
The porosity of Fe3O4–Cys–PANI was evaluated by N2 adsorption at −196 °C in a volumetric analyzer. Prior to the measurements, the sample was outgassed at 120 °C for 12 h under vacuum. The total pore volume (Vtotal), specific surface area (SBET), and the micropore volume (VMICRO) were acquired from the gas adsorption data. The Fe3O4–Cys–PANI surface features were visualized by scanning electron microscopy (SEM, JEOL JSM-IT200, operating at an accelerating voltage of 10–20 kV) coupled with energy-dispersive X-ray spectrometry (EDS) analysis. The zero-charge point (PZC) of the Fe3O4–Cys–PANI sample was investigated by a potentiometric titration route. The crystal structures of the hybrid composites were investigated by X-ray powder diffraction (XRD) analysis (EMPYREAN PANALYTICAL diffractometer). Fourier-transform infrared (FTIR) spectra of the prepared materials were visualized using an FT-IR spectrometer (ALPHA-Bruker Optics, Germany) with KBr pellets in the range of 400 to 4000 cm−1.2.4. Batch experiments and regeneration
The adsorption assays were conducted to evaluate Cr(VI) decontamination on the Fe3O4–Cys–PANI surface. The tests were carried out in Erlenmeyer flasks containing 20 mg L−1 Cr(VI) solutions (100 mL) with the composite studied (0.025 g) under continuous stirring at constant temperature. After each adsorption experiment, Fe3O4–Cys–PANI was separated easily from the Cr(VI) solution by a magnet. The equilibrium concentration of Cr(VI) was complexed with 1,5-diphenylcarbazide and then analyzed using a UV-2300 spectrophotometer at 540 nm. The removed efficiency R(%) and the adsorption uptake Qe (mg g−1) were determined using the following equations:19 | (1) |
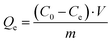 | (2) |
Water treatment involves regenerating adsorbents to restore their original properties for reuse.20 In this process, after recovering the material using a magnet after its use, 50 mL of 0.4 M NaOH solution was added to an Erlenmeyer flask containing Fe3O4–Cys–PANI and soaked for 2 hours at 25 °C. After soaking in NaOH, the regenerated sample was rinsed with distilled water and then treated with 0.8 M HCl. Subsequently, a fresh Cr(VI) solution was used under the same conditions for five adsorption–desorption runs.
2.5. Computational details
The crystallographic structure of studied Fe3O4 was obtained from previous studies.33,34 To predict the more adapted Fe3O4 surfaces for the simulations process, DFT with a plane-wave basis set was utilized. As a result, the simulations were carried out on Fe3O4 periodic crystal surfaces in a simulation box of 30.16 Å × 30.16 Å × 23.29 Å, which consists of 15 Å of substrates and a vacuum region of 30 Å thickness. The adsorption (EAds) energy of Cr(VI) ions on each substrate type was calculated using eqn (3):35
| Eads = Esyst − (Esubs/surf+sol + Eiso/subs) | (3) |
All computations were performed using the BIOVIA Materials Studio 6.0 software package (Dassault Systèmes, San Diego).36
3. Results and discussion
3.1. Characterization
 | ||
| Fig. 1 (a) SEM images of Fe3O4 and (c) Fe3O4–Cys–PANI and their corresponding (b) and (d) EDS elemental spectra. (e) N2 adsorption isotherms curves of Fe3O4–Cys–PANI. | ||
The specific surface area of Fe3O4–Cys–PANI was determined using isothermal N2 adsorption–desorption measurements, as shown in Fig. 1(e). In conformity with the IUPAC classification, the N2 adsorption–desorption isotherm of our adsorbent shows a typical IV isotherm with a H3-type hysteresis loop, thus indicating the occurrence of mesoporous structures (with pore sizes between 2 and 50).37 Furthermore, its surface area composite is about 19 m2 g−1 and the total pore volume is 0.046 cm3 g−1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of the quinoid structure in PANI, confirming its successful polymerization.41,42 A weak peak at 2575 cm−1 corresponds to the S–H stretching vibrations.10 Furthermore, all other characteristic absorption bands of Fe3O4 are observed in the spectra of Fe3O4–Cys–PANI composite. It is noteworthy that there is a decrease in the intensity of the peak at 580 cm−1, attributed to Fe3O4, accompanied by a slight shift observed in the band at 550 cm−1, which moves to 558 cm−1. This shift indicates the interaction between Fe3O4 and Cys–PANI in the composite.
N stretching vibration of the quinoid structure in PANI, confirming its successful polymerization.41,42 A weak peak at 2575 cm−1 corresponds to the S–H stretching vibrations.10 Furthermore, all other characteristic absorption bands of Fe3O4 are observed in the spectra of Fe3O4–Cys–PANI composite. It is noteworthy that there is a decrease in the intensity of the peak at 580 cm−1, attributed to Fe3O4, accompanied by a slight shift observed in the band at 550 cm−1, which moves to 558 cm−1. This shift indicates the interaction between Fe3O4 and Cys–PANI in the composite.
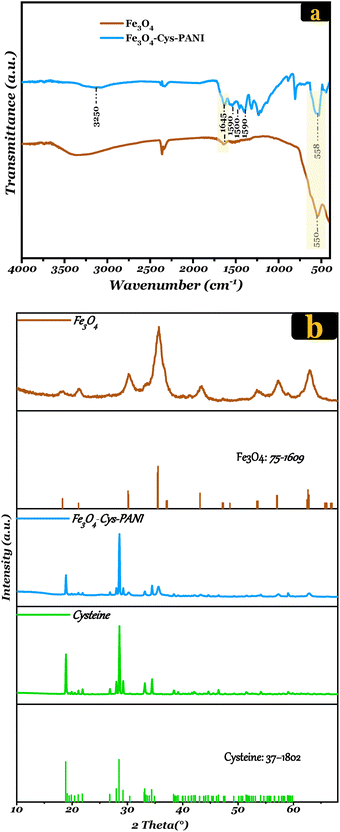 | ||
| Fig. 2 (a) XRD patterns of Fe3O4 and Fe3O4–Cys–PANI composite. (b) FTIR spectra of Fe3O4, Cysteine and Fe3O4–Cys–PANI. | ||
The XRD patterns of cysteine and Fe3O4–Cys–PANI are presented in Fig. 2(b). The XRD analyses were performed to examine the phase and crystal structure of the synthesized materials. The findings indicate that the XRD pattern of pure Fe3O4 corresponds to orthorhombic magnetite (JCPDS 75-1609, space group Imma and no. 74).43 Notably, the diffraction peaks observed at 2θ = 18.49, 30.34°, 35.65°, 43.31°, 53.82°, 57.32°, and 62.93° can be ascribed to the crystal planes of (0 1 1), (2 0 0), (1 0 3), (0 0 4), (2 0 4), (3 2 1), and (4 0 0), respectively. For cysteine, the pattern shows main peaks centered at 18.91° (1 0 0), 28.58° (0 0 1), 33.12° (1 1 2), 34.45° (1 1 6) and 38.44° (2 0 3), according to JCPDS 37-1802.44 The Fe3O4–Cys–PANI diffractogram depicts the coexistence of both Fe3O4 and cysteine phases with a reduction in the intensity of their main peaks. Additionally, the lack of impurity in the XRD patterns suggests the successful design of the Fe3O4–Cys–PANI composite. In addition, the crystallite size was calculated using the Scherrer equation (eqn (4)):
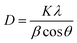 | (4) |
3.2. Adsorption experiments
Loading effect. The impact of loading on the detoxification of 20 ppm Cr(VI) was investigated in the 0.125–1.5 g L−1 loading range. It can be observed from Fig. 3(a) that the Cr(VI) decontamination efficiency and its adsorbed amount on Fe3O4–Cys–PANI vary at different loading levels. There are relatively few surface-active sites at lower doses, causing Cr(VI)-detoxification performance to be irrelevant. However, at an Fe3O4–Cys–PANI dose of 0.25 g L−1, the adsorption percentage notably increased to 98.12% due to more available binding sites for Cr(VI) detoxification. Beyond this dose, further increases in the Fe3O4–Cys–PANI concentration did not significantly affect the Cr(VI) removal efficiency, likely because the available Cr(VI) ions in the solution became limited relative to the adsorbent surface sites. Therefore, 0.25 g L−1 was used for further experiments.
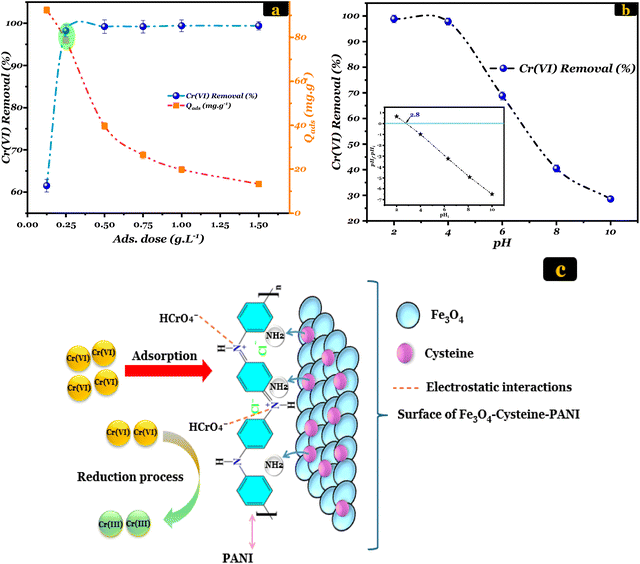 | ||
| Fig. 3 (a) Effect of adsorbent dosage and (b) effect of pH on Cr(VI) removal. (c) Probable mechanism of Cr(VI) removal. | ||
pH effect and mechanism. Both the Cr(VI) solution and the adsorbent's surface charge depend on the pH of the solution, which is paramount in the Cr(VI) adsorption process.48 The interactions occurring at the interface between these two entities are directly affected by the acidity or basicity of the solution. However, prior to investigating the influence of pH on Cr(VI) adsorption, it is essential to check the adsorbent's surface charge as a function of pH (inset of Fig. 3(b)). The point of zero charge (PZC) value of the Fe3O4–Cys–PANI composite was found to be 2.8. This reveals that the surface of the as-prepared Fe3O4–Cys–PANI material is positively charged at pH levels below 2.8, while the surface becomes negatively charged at pH levels above 2.8. Hence, a comprehensive study was conducted to evaluate the impact of solution pH on the efficiency of Cr(VI) detoxification using Fe3O4–Cys–PANI. The pH values were systematically varied from 2.0 to 10.0 while maintaining consistent experimental conditions: an adsorbent dose of 0.25 g L−1, an initial Cr(VI) concentration of 20 mg L−1, a temperature (T) of 298 K, and a contact time of 90 minutes. Under lower pH conditions, as depicted in Fig. 3(b), the magnetic Fe3O4–Cys–PANI material exhibited its maximum potential for detoxifying Cr(VI). It is crucial to consider the various forms of Cr(VI) species present in aqueous solutions within the studied pH range. In acidic media (pH < 6), the predominant species are HCrO4− and Cr2O72−, which possess strong oxidizing ability and high mobility. As the solution becomes more acidic, the amino groups of the Fe3O4–Cys–PANI composite become protonated, resulting in positively charged active sites that enhance the electrostatic attraction of these negatively charged Cr(VI) species (eqn (5) and (6)).10 This initial adsorption process facilitates the close interaction between the Cr(VI) ions and the redox-active surface of the composite.
Subsequently, an in situ reduction of Cr(VI) to Cr(III) occurs through electron transfer from the Fe2+/Fe3+ redox couple in Fe3O4 and from the nitrogen-containing functional groups introduced by cysteine and polyaniline (eqn (7) and (8)).41,49,50 The presence of cysteine enhances the availability of electron-donating nitrogen sites, while the conjugated structure of PANI promotes charge transfer and electron mobility, both of which accelerate the reduction process. The reduced Cr(III) species, being less toxic and less soluble, can then chelate with amine and imine nitrogen groups on the composite surface, forming stable coordination bonds that prevent their release back into the solution.
| –NH3+ + HCrO4− → –NH3+–HCrO4− | (5) |
| –NH3+ + Cr2O72− → –NH3+–Cr2O72− | (6) |
| HCrO4− + 7H++ 3e− → Cr3+ + 4H2O | (7) |
| Cr2O72− + 14H++ 6e− → 2Cr3+ + 7H2O | (8) |
Conversely, at pH = 10, the Cr(VI) removal decreased as the pH values increased. Only 28.57% of Cr(VI) ions were decontaminated using our magnetic composite. Therefore, a pH value of 2 was selected for the subsequent experiments. The schematic representation is provided in Fig. 3(c) for the probable mechanism by which Cr(VI) is adsorbed to the Fe3O4–Cys–PANI material.
Adsorption kinetics. The contact time of the absorbent and the ability to adsorb quickly are the key features for its successful use in practical applications.51 The result of adsorbent/adsorbate contact time (from 2 to 180 min) on the decontamination of Cr(VI) oxyanions by the Fe3O4–Cys–PANI composite surface was investigated under the following operational conditions: an adsorbent dosage of 0.25 g L−1 was used in 100 mL of Cr(VI) solution with initial 20 mg L−1 concentration at pH 2 and 25 °C (Fig. 4). A better rate of Cr(VI) oxyanion uptake was noticed when the contact time was increased in the first 30 min, but then it became slower, and equilibrium was attained after 60 min. Accordingly, the removal ability of the Fe3O4–Cys–PANI composite was referred to the availability of Cr(VI) oxyanion-binding sites on the Fe3O4–Cys–PANI surface. We fit the kinetic data using pseudo-first-order, pseudo-second-order, Elovitch, and intra-particle diffusion kinetic models, and the findings are presented in Fig. 4 and Table 1. According to the table, R2 of the pseudo-second-order kinetic model was found to be higher than that obtained for others. As the pseudo-second-order kinetic model had a higher correlation with laboratory data, its Qe value was also similar to the actual experiments’ Qe value.
 | ||
| Fig. 4 Pseudo-first-order, pseudo-second-order, Elovitch and Weber–Morris intraparticle diffusion linear plots for Cr(VI) adsorption by Fe3O4–Cys–PANI. | ||
| Weber–Morris model Qt = kintt1/2 + C | |||
|---|---|---|---|
| Initial linear portion | Second linear portion | ||
| k int.1 | C 1 | k int.2 | C 2 |
| 6.731 | 33.98 | 0.065 | 76.14 |
The experimental results were further fitted by the intra-particle diffusion kinetic model (inset of Fig. 4). According to the multilinear pattern of the intra-particle diffusion plot, Cr(VI) transfer to solid surfaces is governed by two distinct phases. Initial Cr(VI) ion migration from the aqueous medium to the Fe3O4–Cys–PANI composite interface indicates a higher mass flow rate for Cr(VI) ion transfer from the liquid to the absorbent. Adsorption equilibrium is reached after Fe3O4–Cys–PANI surface sites are saturated, internal diffusion resistance increases, and the surface sites of the adsorbent are eventually saturated.
Isotherm models. It is a mandatory tool to consider the distribution of Cr(VI) oxyanions from the liquid phase to Fe3O4–Cys–PANI (solid phase) up to the equilibrium stage under the controlled (fixed) conditions.13,51 A pair of conventional adsorption isotherms (Langmuir and Freundlich) have been examined to explain the type of isotherm, the adsorption mechanism, the adsorbent affinity, the reaction nature, whether multilayer or monolayer adsorption, and the maximum capacity of adsorption. Fig. 5(a) and Table 2 show that the Freundlich model anticipates the adsorption of Cr(VI) ions over the Fe3O4–Cys–PANI composite better than the Langmuir model, based on the comparison of regression coefficients.
 | ||
| Fig. 5 (a) Non-linear isotherm plots and (b) comparison of our composite with other materials reported in the literature. | ||
Comparison. Compared with other reported adsorbents, Fe3O4–Cys–PANI shows excellent adsorption performance (Fig. 5(b) and Table 3). The literature reports numerous studies on the adsorption of Cr(VI) from water using a variety of materials. The composite exhibits an outstanding maximum uptake capacity of 933.15 mg g−1. Thus, it has the potential to be an effective material for removing Cr(VI). Fe3O4–Cys–PANI can, therefore, be recommended as a promising and cost-effective wastewater decontamination adsorbent.
| Adsorbent | Q max (mg g−1) | Operating conditions | Ref. | ||
|---|---|---|---|---|---|
| Conc (mg L−1) | Ads. dose (g L−1) | pH | |||
| Polyaniline@almond shells | 324.48 | 100–400 | 2 | 4.5 | 52 |
| DBSA-PANI/MWCNTs | 55.55 | 20–140 | 1.33 | 2 | 53 |
| Fe3O4@polypyrrol | 322.58 | 50–450 | 1 | 2 | 54 |
| PANI@graphene oxide-CNT | 142.86 | 25–200 | 1 | 2 | 55 |
| polyaniline-NF/Ox-g-C3N4 | 178.57 | 25–300 | 0.6 | 2 | 56 |
| ZnCl2 modified vinegar residue biochar | 236.8 | 10–1500 | 2 | 2 | 57 |
| MSEP/PPy | 108.85 | 10–100 | 0.5 | 2 | 58 |
| Polypyrrole/Fe3O4 magnetic nanocomposite | 169.4–243.9 | 200–600 | 2 | 2 | 59 |
| Hydrous zirconium oxide | 61 | 100–200 | 2 | 2 | 60 |
| Fe 3 O 4 –Cys–PANI | 933.15 | 20–500 | 0.25 | 2 | Current study |
Thermodynamics. A study of the temperature effect on the adsorption of Cr(VI) onto Fe3O4–Cys–PANI was conducted in order to gain a deeper understanding of the thermodynamic conduct of the adsorption procedure.61,62Eqn (9) and (10) were used to evaluate the results:
 | (9) |
K d is the constant distribution equilibrium specified by the following equation:
 | (10) |
As part of this research, the Cr(VI) adsorption by Fe3O4–Cys–PANI was examined at three different temperatures: 298 K, 308 K and 318 K (Fig. 6(a) and Table 4). From the experimental data, standard thermodynamic parameters were estimated for understanding the thermodynamic behavior. It can be demonstrated that the ΔG° values are negative, provided that the removal of Cr(VI) occurred spontaneously onto the Fe3O4–Cys–PANI surface sites. Furthermore, the gradual decrease in the absolute values of ΔG° as the temperature increases suggests that higher temperatures are more favorable to global adsorption. The positive ΔH° value suggests that the adsorption of Cr(VI) onto Fe3O4–Cys–PANI is an endothermic process. This is supported by the positive ΔS° value, indicating the strong affinity of Fe3O4–Cys–PANI surfaces for Cr(VI) ions at the adsorbent–adsorbate interface.
 | ||
| Fig. 6 (a) van’t Hoff law plot. (b) Regeneration cycles. (c) Effect of co-interfering ions. (d) Selectivity towards orange G (OG), methylene blue (MB) dyes and 4-nitrophenol (4-NP). | ||
| ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | ΔG° (kJ mol−1) | ||
|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||
| 30.973 | 17.361 | −14.37 | −15.56 | −16.77 |
Regeneration. The critical factor in assessing the practical applications of the designed adsorbent lies in its regeneration potential.10,63 After the adsorption test, the recovered Fe3O4–Cys–PANI was reutilized for five runs, as shown in Fig. 6(b). The efficiency of Cr(VI) decontamination using Fe3O4–Cys–PANI provides excellent regenerability and outstanding reusability. This suggests that Fe3O4–Cys–PANI maintains its adsorption performance even after multiple regeneration runs, making it a promising candidate for practical applications in water treatment.
Selectivity. There is no doubt that wastewater is polluted by heavy metals and other organic and inorganic pollutants as well.55,64 Therefore, assessing the influence of co-interfering ions is necessary. Herein, a series of adsorption tests were performed, maintaining the Cr(VI) concentration at 20 mg L−1 and the concentrations of co-interfering ions (CO32−, SO42−, and NO3−) at 100 mg L−1. As shown in Fig. 6(c), the presence of CO32− and NO3− ions did not impact the decontamination of Cr(VI). However, SO42− ions caused a slight decline of 2.5%. This indicates the selectivity of the Fe3O4–Cys–PANI composite in detoxifying Cr(VI) oxyanions. Furthermore, the adsorption behavior of Fe3O4–Cys–PANI was tested with three pollutants: orange G dye (OG), methylene blue dye (MB), and 4-nitrophenol (4-NP) (Fig. 6(d)). The results showed that Fe3O4–Cys–PANI achieved an impressive decontamination rate of over 94% for Cr(VI) oxyanions, with an adsorption capacity around 933.15 mg g−1, whereas the adsorption rates for the other pollutants were below 60%. Compared to the other pollutants, Fe3O4–Cys–PANI has a higher selectivity for Cr(VI).
3.3. Theoretical results
The optimized molecular structure, 3D representation of HOMO and LUMO and ESP map are shown in Fig. 7. The HOMO and LUMO (EHOMO and ELUMO) energies belong to the popular quantum chemical descriptors.69–72 As stated previously, the HOMO corresponds to the area in a molecule that can donate electrons (electron-rich region) to the adsorbent surface, while the LUMO specifies the region within the molecule (electron-poor region) that tends to accept electrons from the adsorbent during the adsorption process.73 The figure reveals that the carboxylic group, linked to the thiol group, forms an angle of approximately 5° with the remaining part of the molecule, indicating that the native configuration of cysteine is nearly planar. This molecule's planarity may be crucial when considering how cysteine interacts with PANI and the Fe3O4–Cys–PANI composite. According to a report, the planar structure can offer a greater surface area for interaction with the Fe3O4 combination.74,75
The adsorption of cysteine on the Fe3O4 surface was quantitatively inspected by calculating the adsorption energy (EAds, eqn (10)), which is the energy necessary during the adsorption of cysteine on the Fe3O4 Surface. According to the equilibrium configurations shown in Fig. 8, the adsorption energy (Eads = −91.681 kcal mol−1) was achieved by carrying out MC calculations. Furthermore, cysteine was situated about parallel to the surface of Fe3O4. The large negative value of the adsorption energy for Fe3O4–Cys inferred that the adsorption was exothermic and stable, and this composite was formed through the sp3 nitrogen atom of cysteine and Fe3O4 in a flat mode as well as hydrogen bonds.
 | ||
| Fig. 8 Equilibrated adsorption configuration of cysteine on Fe3O4 surfaces in the aqueous phase, on side and top views. | ||
According to the obtained results, the values of adsorption energy of PANI are largely negative, suggesting spontaneous adsorption of PANI on Fe3O4–Cys (Eads = −165.6 kcal mol−1), inferring that the physisorption could lead to the formation of a new composite that was reactive and exothermic. PANI molecules were located in the parallel position on Fe3O4–Cys (Fig. 9). If we consider the Cys–PANI interaction, the unprotonated oxygen and oxygen sp2 atoms of cysteine were directed toward the PANI molecules, involving its favorable interaction with it, as displayed in Fig. 9. The remarkable adsorption capacity of PANI on Fe3O4–Cys was attributed to strong interactions of PANI molecules on the Fe3O4–Cys surface to produce a Fe3O4–Cys–PANI hybrid composite.
 | ||
| Fig. 9 Side and top views for the lowest energy geometries derived from MC for the PANI molecules adsorbed onto the interface of Fe3O4–Cys. | ||
To further understand the adsorption behavior observed at different pH levels, it is essential to investigate the interaction energy between Cr(VI) ions and the Fe3O4–Cys–PANI interface. In this regard, experimental findings were supplemented with theoretical simulations to gain a deeper understanding of adsorption mechanisms. The interaction between the adsorbate ion and the interface of Fe3O4–Cys and polyaniline (PANI) is a significant source of valuable information. By analyzing this interaction, adsorption energies associated with this method may be calculated, providing crucial insights into the system's energy and durability. MC/SA simulation was used to elucidate the adsorption of Cr(VI) ions in the aqueous phase, and the results are exhibited in Fig. 10 by considering the aqueous system during this simulation, the adsorption energy of Cr(VI) ions on Fe3O4–Cys–PANI was calculated to be Eads = −55.613 kcal mol−1. Because the Monte Carlo simulation produced such a negative result, it validated experimental findings, and the process was stable and spontaneous, and Cr(VI) can be effectively adsorbed on Fe3O4–Cys–PANI.80,81 According to this figure, the adsorption orientation of Cr(VI) ions is strongly influenced by the chemical nature of the Fe3O4–Cys–PANI substrate. The calculated distance between the nitrogen atom of the cysteine moiety and the chromate ion is approximately 2.96 Å, while the distance between the nitrogen atom of the polyaniline heterocycle and the chromate ion is around 3.07 Å. In contrast, the distance between the ferric ion of Fe3O4 and the chromium atom is noticeably shorter, indicating stronger interactions and distinct reactivities among the active sites. These results suggest that electrostatic interactions initially guide and pre-orient the chromate ions near the surface, facilitating subsequent strong bonding and contributing to the overall stability of the adsorbed complex. Furthermore, the Fe3O4–Cys–PANI system exhibited the lowest adsorption energy, confirming its enhanced affinity and stability toward Cr(VI) species.82,83 This suggested that the Cr(VI) ion's adsorption process was more robust, steady, and spontaneous on the Fe3O4–Cys–PANI system. Therefore, this provided a good clarification for why the elimination efficiency of Cr ion was improved after the use of the Fe3O4–Cys–PANI hybrid composite.72,84
4. Conclusion
To sum up, the magnetic Fe3O4–Cys–PANI composite was successfully synthesized and characterized, exhibiting tunable properties for efficient Cr(VI) detoxification. The functionalization with cysteine introduced N-containing groups that enhanced the surface reactivity and facilitated the interaction with Cr(VI) species. The magnetic adsorbent achieved a maximum Cr(VI) removal efficiency of 98.12% at an adsorbent dose of 0.25 g·L−1 and pH 2, where acidic conditions favored both the reduction of Cr(VI) to Cr(III) and the adsorption of Cr2O72− and HCrO4− ions through electrostatic attraction. Monte Carlo simulations revealed a high adsorption energy of −55.613 kcal·mol−1, confirming the strong binding affinity between Cr(VI) species and the active sites of Fe3O4–Cys–PANI, in agreement with experimental observations. Freundlich isotherm and pseudo-second-order kinetic models accurately described the Cr(VI) decontamination behavior. Moreover, the Fe3O4–Cys–PANI magnetic composite demonstrated exceptional selectivity towards Cr(VI) oxyanions and significant regeneration capacities and reusability as well. Although this study presents promising results in depolluting Cr(VI) solutions under controlled conditions, further work is needed to evaluate the economic feasibility of the tailored Fe3O4–Cys–PANI composite in real wastewater treatment applications.Author contributions
A. Imgharn: formal analysis, data curation, writing – original draft; M. El Houdi: formal analysis, writing – original draft; S. El Omari: methodology, data curation; K. Ait El Bacha: investigation; M. Laabd: validation, review & editing; L. Bazzi: validation, review & editing; A. Albourine: conceptualization, validation, revision, editing, supervision, project administration.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data for this article are available from the corresponding author.Acknowledgements
The authors received no specific funding for this work.References
- Z. Rahman and L. Thomas, Chemical-Assisted Microbially Mediated Chromium (Cr)(VI) Reduction Under the Influence of Various Electron Donors, Redox Mediators, and Other Additives: An Outlook on Enhanced Cr(VI) Removal, Front. Microbiol., 2021, 11, 619766, DOI:10.3389/fmicb.2020.619766.
- L. Wang, Y. Wang, F. Ma, V. Tankpa, S. Bai, X. Guo and X. Wang, Mechanisms and Reutilization of Modified Biochar Used for Removal of Heavy Metals from Wastewater: A Review, Sci. Total Environ., 2019, 668, 1298–1309, DOI:10.1016/j.scitotenv.2019.03.011.
- J. Liang, X. Huang, J. Yan, Y. Li, Z. Zhao, Y. Liu, J. Ye and Y. Wei, A Review of the Formation of Cr(VI) via Cr(III) Oxidation in Soils and Groundwater, Sci. Total Environ., 2021, 774, 145762, DOI:10.1016/j.scitotenv.2021.145762.
- R. Zheng, J. Li, R. Zhu, R. Wang, X. Feng, Z. Chen, W. Wei, D. Yang and H. Chen, Enhanced Cr(VI) Reduction on Natural Chalcopyrite Mineral Modulated by Degradation Intermediates of RhB, J. Hazard. Mater., 2022, 423, 127206, DOI:10.1016/j.jhazmat.2021.127206.
- P. Sharma, S. P. Singh, S. K. Parakh and Y. W. Tong, Health Hazards of Hexavalent Chromium (Cr(VI)) and Its Microbial Reduction, Bioengineered, 2022, 13(3), 4923–4938, DOI:10.1080/21655979.2022.2037273.
- A. Imgharn, M. Laabd, Y. Naciri, A. Hsini, F.-Z. Mahir, H. Zouggari and A. Albourine, Insights into the Performance and Mechanism of PANI@Hydroxapatite-Montmorillonite for Hexavalent Chromium Cr(VI) Detoxification, Surf. Interfaces, 2023, 36, 102568, DOI:10.1016/j.surfin.2022.102568.
- B. Kumari, R. K. Tiwary and M. Yadav, Non Linear Regression Analysis and RSM Modeling for Removal of Cr(VI) from Aqueous Solution Using PANI@WH Composites, Mater. Chem. Phys., 2022, 290, 126457, DOI:10.1016/j.matchemphys.2022.126457.
- L. Xiang, C.-G. Niu, N. Tang, X.-X. Lv, H. Guo, Z.-W. Li, H.-Y. Liu, L.-S. Lin, Y.-Y. Yang and C. Liang, Polypyrrole Coated Molybdenum Disulfide Composites as Adsorbent for Enhanced Removal of Cr(VI) in Aqueous Solutions by Adsorption Combined with Reduction, Chem. Eng. J., 2021, 408, 127281, DOI:10.1016/j.cej.2020.127281.
- R. Ansari and A. Fallah Delavar, Removal of Cr(VI) Ions from Aqueous Solutions Using Poly 3-Methyl Thiophene Conducting Electroactive Polymers, J. Polym. Environ., 2010, 18(3), 202–207, DOI:10.1007/s10924-010-0199-7.
- M. Laabd, A. Imgharn, A. Hsini, Y. Naciri, M. Mobarak, S. Szunerits, R. Boukherroub and A. Albourine, Efficient Detoxification of Cr(VI)-Containing Effluents by Sequential Adsorption and Reduction Using a Novel Cysteine-Doped PANi@faujasite Composite: Experimental Study Supported by Advanced Statistical Physics Prediction, J. Hazard. Mater., 2022, 422, 126857, DOI:10.1016/j.jhazmat.2021.126857.
- J. Qiu, F. Liu, S. Cheng, L. Zong, C. Zhu, C. Ling and A. Li, Recyclable Nanocomposite of Flowerlike MoS2@Hybrid Acid-Doped PANI Immobilized on Porous PAN Nanofibers for the Efficient Removal of Cr(VI), ACS Sustainable Chem. Eng., 2018, 6(1), 447–456, DOI:10.1021/acssuschemeng.7b02738.
- M. H. Mahmoudian, A. Azari, A. Jahantigh, M. Sarkhosh, M. Yousefi, S. A. Razavinasab, M. Afsharizadeh, F. Mohammadi Shahraji, A. Pour Pasandi, A. Zeidabadi, T. Ilaghinezhad Bardsiri and M. Ghasemian, Statistical Modeling and Optimization of Dexamethasone Adsorption from Aqueous Solution by Fe3O4@NH2-MIL88B Nanorods: Isotherm, Kinetics, and Thermodynamic, Environ. Res., 2023, 236, 116773, DOI:10.1016/j.envres.2023.116773.
- R.-S. Juang, Y.-C. Yei, C.-S. Liao, K.-S. Lin, H.-C. Lu, S.-F. Wang and A.-C. Sun, Synthesis of Magnetic Fe3O4/Activated Carbon Nanocomposites with High Surface Area as Recoverable Adsorbents, J. Taiwan Inst. Chem. Eng., 2018, 90, 51–60, DOI:10.1016/j.jtice.2017.12.005.
- Y.-F. Lin and C.-Y. Chang, Magnetic Mesoporous Iron Oxide/Carbon Aerogel Photocatalysts with Adsorption Ability for Organic Dye Removal, RSC Adv., 2014, 4(54), 28628, 10.1039/c4ra03436h.
- A. Nasiri, M. A. Gharaghani, G. Yazdanpanah, H. Mahdizadeh and N. Amirmahani, Facile, Fast, and Green Synthesis of CoFe2O4@CMC/Cysteine as a Novel Magnetic Nanobiocomposite Adsorbent for Tetracycline Removal from Aqueous Media, J. Polym. Environ., 2025, 33(9), 4212–4236, DOI:10.1007/s10924-025-03629-x.
- S. Abolghasemi, A. Nasiri, M. Hashemi, S. Rajabi and F. Rahimi, Magnetic Nanocomposites: Innovative Adsorbents for Antibiotics Removal from Aqueous Environments–a Narrative Review, Appl. Water Sci., 2025, 15(2), 30, DOI:10.1007/s13201-025-02360-1.
- S. Rajabi, H. Hashemi, M. R. Samaei, A. Nasiri, A. Azhdarpoor and S. Yousefinejad, Enhanced Sonophoto-Catalytic and Adsorption Capabilities of Fe3O4@MC/MWCNT-CuO/Ag for Petrochemical Organic Pollutants Degradation from Industrial Process Streams, Arabian J. Chem., 2024, 17(11), 105994, DOI:10.1016/j.arabjc.2024.105994.
- T. T. H. Chu and M. V. Nguyen, Improved Cr(VI) Adsorption Performance in Wastewater and Groundwater by Synthesized Magnetic Adsorbent Derived from Fe3O4 Loaded Corn Straw Biochar, Environ. Res., 2023, 216, 114764, DOI:10.1016/j.envres.2022.114764.
- A. Pasgar, A. Nasiri and N. Javid, Single and Competitive Adsorption of Cu2+ and Pb2+ by Tea Pulp from Aqueous Solutions, Environ. Health Eng. Manage. J., 2021, 9(1), 65–74, DOI:10.34172/EHEM.2022.08.
- A. Imgharn, L. Anchoum, A. Hsini, Y. Naciri, M. Laabd, M. Mobarak, N. Aarab, A. Bouziani, S. Szunerits, R. Boukherroub, R. Lakhmiri and A. Albourine, Effectiveness of a Novel polyaniline@Fe-ZSM-5 Hybrid Composite for Orange G Dye Removal from Aqueous Media: Experimental Study and Advanced Statistical Physics Insights, Chemosphere, 2022, 295, 133786, DOI:10.1016/j.chemosphere.2022.133786.
- R. Dennington; T. A. Keith and J. M. Millam, GaussView, Version 6.0. 16., Semichem Inc, Shawnee Mission KS, 2016 Search PubMed.
- E. Caldeweyher, C. Bannwarth and S. Grimme, Extension of the D3 Dispersion Coefficient Model, J. Chem. Phys., 2017, 147(3), 034112, DOI:10.1063/1.4993215.
- P. J. Hay and W. R. Wadt, Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg, J. Chem. Phys., 1985, 82(1), 270–283, DOI:10.1063/1.448799.
- M. N. Rasul, A. Anam, M. A. Sattar, A. Manzoor and A. Hussain, DFT Based Structural, Electronic and Optical Properties of B1−xInxP (x = 0.0, 0.25, 0.5, 0.75, 1.0) Compounds: PBE-GGA vs. mBJ-Approaches, Chin. J. Phys., 2018, 56(6), 2659–2672 CrossRef CAS.
- M. Arshad Javid, Z. U. Khan, Z. Mehmood, A. Nabi, F. Hussain, M. Imran, M. Nadeem and N. Anjum, Structural, Electronic and Optical Properties of LiNbO3 Using GGA-PBE and TB-mBJ Functionals: A DFT Study, Int. J. Mod. Phys. B, 2018, 32(14), 1850168 CrossRef CAS.
- M. Elhoudi, N. Aarab and M. Laabd, Quantum Chemical Approach (DFT) of the Binary Complexation of Hg(II), Moroccan J. Chem., 2021, 3, 406–415 Search PubMed.
- M. J. Frisch; G. W. Trucks; H. B. Schlegel; G. E. Scuseria; M. A. Robb; J. R. Cheeseman; G. Scalmani; V. Barone; G. A. Petersson; H. Nakatsuji; X. Li; M. Caricato; A. Marenich; J. Bloino; B. G. Janesko; R. Gomperts; B. Mennucci; H. P. Hratchian; J. V. Ortiz; A. F. Izmaylov; J. L. Sonnenberg; D. Williams-Young; F. Ding; F. Lipparini; F. Egidi; J. Goings; B. Peng; A. Petrone; T. Henderson; D. Ranasinghe; V. G. Zakrzewski; J. Gao; N. Rega; G. Zheng; W. Liang; M. Hada; M. Ehara; K. Toyota; R. Fukuda; J. Hasegawa; M. Ishida; T. Nakajima; Y. Honda; O. Kitao; H. Nakai; T. Vreven; K. Throssell; J. A. Montgomery Jr.; J. E. Peralta; F. Ogliaro; M. Bearpark; J. J. Heyd; E. Brothers; K. N. Kudin; V. N. Staroverov; T. Keith; R. Kobayashi; J. Normand; K. Raghavachari; A. Rendell; J. C. Burant; S. S. Iyengar; J. Tomasi; M. Cossi; J. M. Millam; M. Klene; C. Adamo; R. Cammi; J. W. Ochterski; R. L. Martin; K. Morokuma; O. Farkas; J. B. Foresman and D. J. Fox, Gaussian 09, Rev. D.01, Gaussian Inc., Wallingford, CT, 2016 Search PubMed.
- M. Cossi; N. Rega; G. Scalmani; V. Barone; D. Chimica; F. Ii and C. M. S. Angelo Molecules in Solution with the C-PCM Solvation Model. 2003.
- A. P. Scott and L. Radom, Harmonic Vibrational Frequencies: An Evaluation of Hartree-Fock, Møller-Plesset, Quadratic Configuration Interaction, Density Functional Theory, and Semiempirical Scale Factors, J. Phys. Chem., 1996, 100(41), 16502–16513, DOI:10.1021/jp960976r.
- S. Baroni, P. Giannozzi and E. Isaev, Density-Functional Perturbation Theory for Quasi-Harmonic Calculations, Rev. Mineral. Geochem., 2010, 71(1), 39–57 CrossRef CAS.
- S. Kirkpatrick, C. D. Gelatt Jr and M. P. Vecchi, Optimization by Simulated Annealing, Science, 1983, 220(4598), 671–680 CrossRef CAS.
- P. J. M. Van Laarhoven; E. H. L. Aarts; P. J. M. van Laarhoven and E. H. L. Aarts, Simulated Annealing, Springer, 1987 Search PubMed.
- National Center for Biotechnology Information (2025). PubChem Substance Record for SID 85240249, 25233-30-1, Source: ChEBI. Retrieved February 19, 2025 from https://pubchem.ncbi.nlm.nih.gov/substance/85240249. No Title.
- I. A. M. Ahmed and B. A. Maher, Identification and Paleoclimatic Significance of Magnetite Nanoparticles in Soils, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(8), 1736–1741, DOI:10.1073/pnas.1719186115.
- I. B. Obot, K. Haruna and T. A. Saleh, Atomistic Simulation: A Unique and Powerful Computational Tool for Corrosion Inhibition Research, Arabian J. Sci. Eng., 2019, 44, 1–32 CrossRef.
- Materials Studio. Accelrys Software Inc., S. Diego. N. that; 2, some of these codes were also offered as part of the C.; From, application that preceded M. S. 2013. No Title.
- B. Vellaichamy and P. Periakaruppan, Synergistic Combination of a Novel Metal-Free Mesoporous Band-Gap-Modified Carbon Nitride Grafted Polyaniline Nanocomposite for Decontamination of Refractory Pollutant, Ind. Eng. Chem. Res., 2018, 57(19), 6684–6695, DOI:10.1021/acs.iecr.8b01098.
- X. Song, C. Ren, Q. Zhao and B. Su, Simultaneous Removal of Cr(VI) and Triclosan from Aqueous Solutions through Fe3O4 Magnetic Nanoscale-Activated Persulfate Oxidation, Chem. Eng. J., 2020, 381, 122586, DOI:10.1016/j.cej.2019.122586.
- I. M. Minisy, N. A. Salahuddin and M. M. Ayad, Adsorption of Methylene Blue onto Chitosan–Montmorillonite/Polyaniline Nanocomposite, Appl. Clay Sci., 2021, 203, 105993, DOI:10.1016/j.clay.2021.105993.
- A. Nasiri, M. R. Heidari, N. Javid and G. Yazdanpanah, New Efficient and Recyclable Magnetic Nanohybrid Adsorbent for the Metronidazole Removal from Simulated Wastewater, J. Mater. Sci.: Mater. Electron., 2022, 33(33), 25103–25126, DOI:10.1007/s10854-022-09216-3.
- A. Chaoui, A. Imgharn, A. C. Estrada, A. B. Hamou, S. Farsad, N. Nouj, M. Ez-zahery, T. Trindade, A. Albourine and N. E. Alem, Chromium (VI) Remediation via Biochar@polyaniline Composite: Advancing Water Treatment Using Biogas Residue Digestate, Adv. Compos. Hybrid Mater., 2025, 8(4), 312, DOI:10.1007/s42114-025-01345-7.
- S. El Omari, A. Imgharn, Y. Abdellaoui, O. May Tzuc, A. Albourine, L. Bazzi, M. Laabd and K. Benhabib, A Hybrid Polyaniline/Dolomite–Palygorskite Framework for Environmental Remediation: Experimental Design and Molecular-Level Adsorption Interpretation, Mater. Adv., 2025, 6(20), 7409–7426, 10.1039/D5MA00660K.
- Astuti, S. Arief, M. Muldarisnur, Zulhadjri and S. R. A. Usna, Enhancement in Photoluminescence Performance of Carbon-Based Fe3O4@ZnO–C Nanocomposites, Vacuum, 2023, 211, 111935, DOI:10.1016/j.vacuum.2023.111935.
- S. Song, X. Qiu, S. Huang, H. Tian, R. Pu, J. Huang, J. Su, Y.-L. Tang, L. Huang, X. Luo, W. He, Q. Ni and W. Zhang, Cystine-Modified Lignin–Copper Coordination Nanocarriers Improve the Therapeutic Efficacy of Tyrosine Kinase Inhibition via Cuproptosis, ACS Appl. Mater. Interfaces, 2025, 17(6), 9074–9086, DOI:10.1021/acsami.4c20305.
- A. Cervellino; R. Frison; N. Masciocchi and A. Guagliardi, X-Ray Powder Diffraction Characterization of Nanomaterials, in X-ray and Neutron Techniques for Nanomaterials Characterization, ed. C. S. S. R. Kumar, Springer Berlin Heidelberg, Berlin, Heidelberg, 2016, pp 545–608 DOI:10.1007/978-3-662-48606-1_10.
- A. Imgharn, T. Sun, J. Nicolle, Y. Naciri, A. Hsini, A. Albourine and C. Ania, A Simple Approach to Prepare a C3N4/MoO3 Heterojunction with Improved Photocatalytic Performance for the Degradation of Methylparaben, Catalysts, 2024, 14(3), 170, DOI:10.3390/catal14030170.
- A. L. Patterson, The Scherrer Formula for X-Ray Particle Size Determination, Phys. Rev., 1939, 56(10), 978–982, DOI:10.1103/PhysRev.56.978.
- Q. Wang, L. Li, Y. Tian, L. Kong, G. Cai, H. Zhang, J. Zhang, W. Zuo and B. Wen, Shapeable Amino-Functionalized Sodium Alginate Aerogel for High-Performance Adsorption of Cr(VI) and Cd(II): Experimental and Theoretical Investigations, Chem. Eng. J., 2022, 446, 137430, DOI:10.1016/j.cej.2022.137430.
- W. Wang, C. Chen, X. Huang, S. Jiang, J. Xiong, J. Li, M. Hong, J. Zhang, Y. Guan, X. Feng, W. Tan, F. Liu, L.-J. Ding and H. Yin, Chromium(VI) Adsorption and Reduction in Soils under Anoxic Conditions: The Relative Roles of Iron (Oxyhr)Oxides, Iron(II), Organic Matters, and Microbes, Environ. Sci. Technol., 2024, 58(41), 18391–18403, DOI:10.1021/acs.est.4c08677.
- A. Hsini, R. Haounati, A. Imgharn, Y. Naciri, R. E. Malekshah, A. Shaim, S. Szunerits, R. Boukherroub and A. Albourine, 1,2,4,5-Benzene Tetracarboxylic Acid-Doped Polyaniline/Protonated Carbon Nitride Nanostructures for Cr(VI) Adsorption in Water, ACS Appl. Nano Mater., 2024, 7(11), 13050–13061, DOI:10.1021/acsanm.4c01503.
- F.-X. Dong, L. Yan, X.-H. Zhou, S.-T. Huang, J.-Y. Liang, W.-X. Zhang, Z.-W. Guo, P.-R. Guo, W. Qian, L.-J. Kong, W. Chu and Z.-H. Diao, Simultaneous Adsorption of Cr(VI) and Phenol by Biochar-Based Iron Oxide Composites in Water: Performance, Kinetics and Mechanism, J. Hazard. Mater., 2021, 416, 125930, DOI:10.1016/j.jhazmat.2021.125930.
- A. Hsini, A. Essekri, N. Aarab, M. Laabd, A. Ait Addi, R. Lakhmiri and A. Albourine, Elaboration of Novel polyaniline@Almond Shell Biocomposite for Effective Removal of Hexavalent Chromium Ions and Orange G Dye from Aqueous Solutions, Environ. Sci. Pollut. Res., 2020, 27(13), 15245–15258, DOI:10.1007/s11356-020-08039-1.
- R. Kumar, M. O. Ansari and M. A. Barakat, DBSA Doped Polyaniline/Multi-Walled Carbon Nanotubes Composite for High Efficiency Removal of Cr(VI) from Aqueous Solution, Chem. Eng. J., 2013, 228, 748–755, DOI:10.1016/j.cej.2013.05.024.
- M. Chigondo, H. K. Paumo, M. Bhaumik, K. Pillay and A. Maity, Magnetic Arginine-Functionalized Polypyrrole with Improved and Selective Chromium(VI) Ions Removal from Water, J. Mol. Liq., 2019, 275, 778–791, DOI:10.1016/j.molliq.2018.11.032.
- M. O. Ansari, R. Kumar, S. A. Ansari, S. P. Ansari, M. A. Barakat, A. Alshahrie and M. H. Cho, Anion Selective pTSA Doped Polyaniline@graphene Oxide-Multiwalled Carbon Nanotube Composite for Cr(VI) and Congo Red Adsorption, J. Colloid Interface Sci., 2017, 496, 407–415, DOI:10.1016/j.jcis.2017.02.034.
- R. Kumar, M. A. Barakat and F. A. Alseroury, Oxidized G-C3N4/Polyaniline Nanofiber Composite for the Selective Removal of Hexavalent Chromium, Sci. Rep., 2017, 7(1), 12850, DOI:10.1038/s41598-017-12850-1.
- K. Ding, X. Zhou, H. Hadiatullah, Y. Lu, G. Zhao, S. Jia, R. Zhang and Y. Yao, Removal Performance and Mechanisms of Toxic Hexavalent Chromium (Cr(VI)) with ZnCl2 Enhanced Acidic Vinegar Residue Biochar, J. Hazard. Mater., 2021, 420, 126551, DOI:10.1016/j.jhazmat.2021.126551.
- Q. Zhou, J. Huang, X. Zhang and Y. Gao, Assembling Polypyrrole Coated Sepiolite Fiber as Efficient Particle Adsorbent for Chromium(VI) Removal with the Feature of Convenient Recycling, Appl. Clay Sci., 2018, 166, 307–317, DOI:10.1016/j.clay.2018.09.031.
- G. Chen, C. Qiao, Y. Wang and J. Yao, Synthesis of Magnetic Gelatin and Its Adsorption Property for Cr(VI), Ind. Eng. Chem. Res., 2014, 53(40), 15576–15581, DOI:10.1021/ie502709u.
- L. A. Rodrigues, L. J. Maschio, R. E. Da Silva and M. L. C. P. Da Silva, Adsorption of Cr(VI) from Aqueous Solution by Hydrous Zirconium Oxide, J. Hazard. Mater., 2010, 173(1–3), 630–636, DOI:10.1016/j.jhazmat.2009.08.131.
- A. Al Shra’ah, A. T. Al-Fawwaz, M. M. Ibrahim and E. Alsbou, Remediation of Methyl Orange Dye in Aqueous Solutions by Green Microalgae (Bracteacoccus Sp.): Optimization, Isotherm, Kinetic, and Thermodynamic Studies, Separations, 2024, 11(6), 170, DOI:10.3390/separations11060170.
- H. Zeng, H. Zeng, H. Zhang, A. Shahab, K. Zhang, Y. Lu, I. Nabi, F. Naseem and H. Ullah, Efficient Adsorption of Cr (VI) from Aqueous Environments by Phosphoric Acid Activated Eucalyptus Biochar, J. Cleaner Prod., 2021, 286, 124964, DOI:10.1016/j.jclepro.2020.124964.
- A. Mohamed, L. Yu, Y. Fang, N. Ashry, Y. Riahi, I. Uddin, K. Dai and Q. Huang, Iron Mineral-Humic Acid Complex Enhanced Cr(VI) Reduction by Shewanella Oneidensis MR-1, Chemosphere, 2020, 247, 125902, DOI:10.1016/j.chemosphere.2020.125902.
- S. Banerjee, S. Dubey, R. K. Gautam, M. C. Chattopadhyaya and Y. C. Sharma, Adsorption Characteristics of Alumina Nanoparticles for the Removal of Hazardous Dye, Orange G from Aqueous Solutions, Arab. J. Chem., 2019, 12(8), 5339–5354, DOI:10.1016/j.arabjc.2016.12.016.
- Y. Gu, X. Chen, L. Liu, S. Wang, X. Yu, Z. Jia and X. Zhou, Cr (VI)-Bioremediation Mechanism of a Novel Strain Bacillus Paramycoides Cr6 with the Powerful Ability to Remove Cr(VI) from Contaminated Water, J. Hazard. Mater., 2023, 455, 131519 CrossRef CAS PubMed.
- B. Eyvazi, A. Jamshidi-Zanjani and A. K. Darban, Synthesis of Nano-Magnetic MnFe2O4 to Remove Cr(III) and Cr(VI) from Aqueous Solution: A Comprehensive Study, Environ. Pollut., 2020, 265, 113685 CrossRef CAS.
- M. Elhoudi, A. Hsini, M. El Houdi, R. Lakhmiri and A. Albourine, A Theoretical Investigation of Complexation for Pyrimidine Bases with Hg2+ and Cd2+ by DFT Method, Nanotechnol. Environ. Eng., 2021, 6(2), 1–13, DOI:10.1007/s41204-021-00128-x.
- T. Laktif, A. Imgharn, A. Hsini, M. Elhoudi, N. Aarab, M. Laabd, R. Lakhmiri and A. Albourine, Sunflower Seed Shells@ Polyaniline: A Novel Composite for the Removal of Pharmaceutical Pollutants from Wastewater, Int. J. Environ. Anal. Chem., 2022, 1–18 Search PubMed.
- V. Choudhary, A. Bhatt, D. Dash and N. Sharma, DFT Calculations on Molecular Structures, HOMO–LUMO Study, Reactivity Descriptors and Spectral Analyses of Newly Synthesized Diorganotin(IV) 2-chloridophenylacetohydroxamate Complexes, J. Comput. Chem., 2019, 40(27), 2354–2363 CrossRef CAS PubMed.
- D. B. Boyd, The Power of Computational Chemistry to Leverage Stress Testing of Pharmaceuticals, In Pharmaceutical stress testing, CRC Press, 2005, pp 377–440 Search PubMed.
- J. Aires-de-sousa, Machine Learning Methods to Predict Density Functional Theory B3LYP Energies of HOMO and LUMO Orbitals, J. Chem. Inf. Model., 2017, 57(1), 11–21, DOI:10.1021/acs.jcim.6b00340.
- M. Elhoudi, R. Oukhrib, C. A Celaya, D. G. Araiza, Y. Abdellaoui, I. Barra, Y. Brahmi, H. Bourzi, M. Reina, A. Albourine and H. Abou Oualid, Comparison of Green Bio-Based Cerium/Alginate vs. Copper/Alginate Beads: A Study of Vibrational and Thermal Properties Using Experimental and Theoretical Methods, J. Mol. Model., 2022, 28(2), 1–15 CrossRef PubMed.
- M. Miar, A. Shiroudi, K. Pourshamsian, A. R. Oliaey and F. Hatamjafari, Theoretical Investigations on the HOMO–LUMO Gap and Global Reactivity Descriptor Studies, Natural Bond Orbital, and Nucleus-Independent Chemical Shifts Analyses of 3-Phenylbenzo[d]Thiazole-2(3H)-Imine and Its Para-Substituted Derivatives: Solvent and Substituent Effects, J. Chem. Res., 2021, 45(1–2), 147–158 CrossRef CAS.
- P. Hobza, H. L. Selzle and E. W. Schlag, Potential Energy Surface of the Benzene Dimer: Ab Initio Theoretical Study, J. Am. Chem. Soc., 1994, 116(8), 3500–3506 CrossRef CAS.
- N. Malikova, I. Pastoriza-Santos, M. Schierhorn, N. A. Kotov and L. M. Liz-Marzán, Layer-by-Layer Assembled Mixed Spherical and Planar Gold Nanoparticles: Control of Interparticle Interactions, Langmuir, 2002, 18(9), 3694–3697 CrossRef CAS.
- R. Haounati, H. Ighnih, R. E. Malekshah, S. Alahiane, F. Alakhras, E. Alabbad, H. Alghamdi, H. Ouachtak, A. A. Addi and A. Jada, Exploring ZnO/Montmorillonite Photocatalysts for the Removal of Hazardous RhB Dye: A Combined Study Using Molecular Dynamics Simulations and Experiments, Mater. Today Commun., 2023, 35, 105915 CrossRef CAS.
- I. Salahshoori, A. Mohseni, M. N. Jorabchi, S. Ghasemi, M. Afshar and S. Wohlrab, Study of Modified PVDF Membranes with High-Capacity Adsorption Features Using Quantum Mechanics, Monte Carlo, and Molecular Dynamics Simulations, J. Mol. Liq., 2023, 375, 121286 CrossRef CAS.
- I. Lebkiri, B. Abbou, R. Hsissou, Z. Safi, M. Sadiku, A. Berisha, A. El Amri, Y. Essaadaoui, L. Kadiri and A. Lebkiri, Investigation of the Anionic Polyacrylamide as a Potential Adsorbent of Crystal Violet Dye from Aqueous Solution: Equilibrium, Kinetic, Thermodynamic, DFT, MC and MD Approaches, J. Mol. Liq., 2023, 372, 121220 CrossRef CAS.
- P. Taylor; R. L. C. Akkermans; N. A. Spenley and S. H. Robertson, Monte Carlo Methods in Materials Studio, Molecular Simulation, Taylor & Francis, 2013, pp. 1153–1164 DOI:10.1080/08927022.2013.843775.
- X. Xing, X. Ren, N. S. Alharbi and C. Chen, Efficient Adsorption and Reduction of Cr(VI) from Aqueous Solution by Santa Barbara Amorphous-15 (SBA-15) Supported Fe/Ni Bimetallic Nanoparticles, J. Colloid Interface Sci., 2023, 629, 744–754 CrossRef CAS PubMed.
- Y. Abdellaoui, C. A. Celaya, M. Elhoudi, R. Boualou, H. Agalit, M. Reina, P. Gamero-Melo and H. A. Oualid, Understanding of Vibrational and Thermal Behavior of Bio-Based Doped Alginate@nickel Cross-Linked Beads: A Combined Experimental and Theoretical Study, J. Mol. Struct., 2022, 1249, 131524, DOI:10.1016/j.molstruc.2021.131524.
- K. Ait, E. Bacha, A. Imgharn, A. Hsini, H. Zouggari, F. Mahir, B. Selhami, R. Lakhmiri, M. Laabd, H. Ejazouli and A. Albourine, Arginine-Polyaniline Embedded Jujube Shells Composite for Outstanding Cr(VI) Detoxification from Aqueous Solution Arginine – Polyaniline Embedded Jujube Shells Composite for Outstanding Cr(VI) Detoxification from Aqueous Solution, Water, Air, Soil Pollut., 2024, 235, 380, DOI:10.1007/s11270-024-07194-2.
- A. Hsini; R. Haounati; A. Imgharn; Y. Naciri; R. E. Malekshah; A. Shaim; S. Szunerits; R. Boukherroub and A. Albourine Carbon Nitride Nanostructures for Cr(VI) Adsorption in Water 2024, VI DOI:10.1021/acsanm.4c01503.
- Y. Abdellaoui, H. Abou, A. Hsini and B. El, Synthesis of Zirconium-Modified Merlinoite from Fly Ash for Enhanced Removal of Phosphate in Aqueous Medium: Experimental Studies Supported by Monte Carlo/SA Simulations, Chem. Eng. J., 2021, 404, 126600, DOI:10.1016/j.cej.2020.126600.
| This journal is © The Royal Society of Chemistry 2026 |