 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of chewed gum for treating oil sands tailings and adsorbing organic dyes and heavy metal ions
Wei Zhanga,
Amirhossein Farshchi-Andisib,
Maryam Salami*ab and
Michael J. Serpe *a
*a
aFaculty of Science, Department of Chemistry, University of Alberta, Edmonton, Alberta T6G 2G2, Canada. E-mail: serpe@ualberta.ca; msalami2@ualberta.ca
bDepartment of Food Science, Engineering, and Technology, College of Agriculture and Natural Resources, Karaj Campus, University of Tehran, Karaj, Iran
First published on 16th January 2026
Abstract
Here, we investigated the use of waste chewing gum as an environmentally problematic waste product for use as a sorbent for treating tailings pond water (TPW) from the Alberta oil sands. TPW has been found to contain toxic substances, including heavy metals, certain naphthenic acids, and polycyclic aromatic hydrocarbons. These pollutants are known to pose significant risks to the environment and living organisms. In this study, we demonstrated that the gum sorbent exhibited good sorption affinity for model compounds, e.g., crystal violet (CV), rhodamine B (RB), and methylene blue (MB). Furthermore, a slight decrease in adsorption efficiency was observed over four reuse cycles. Good removal efficiency of heavy metal ions (Pb2+, Fe3+, Cu2+, Zn2+, Hg2+, Ag+, and Cd2+) was also observed, especially for lead (100%), iron (99%), and copper (98%), and even in heavy metal ion mixtures. The greatest reduction in TPW cytotoxicity was shown by filtration columns prepared with gum and silica, based on cell viability measured using HeLa cells, even when compared to columns containing charcoal and silica. Interestingly, higher levels of untreated organic carbon were found in the filtrate from these gum-based columns, suggesting that the gum might have reacted with the harmful compounds rather than simply trapping them. These results point to chewing gum as a recyclable waste material that is promising for the development of effective waste-based systems for cleaning industrial wastewater.
1. Introduction
Alberta has the world's fourth-largest oil reserves, consisting of 165.4 billion barrels of oil sands comprising bitumen, water, and mineral solids.1 The oil sands deposit in northeastern Alberta produces nearly two-thirds of the total bitumen in Alberta.2 Surface mining and bitumen extraction from these sands is the largest soil excavation/relocation operation in the world.3,4 This operation involves the removal of muskeg (composed of decomposed plant material), together with the sand, silt, clay, and shale that lie above the bitumen-rich deposits.5 After that, bitumen is extracted using the Clark caustic hot water process, which requires between 0.2 and 2.6 barrels of water for each barrel of bitumen produced.1,6Oil sands tailings are liquid wastes generated from the process of removing bitumen from the oil sands. These wastes hold water, sand, fine clay, leftover bitumen, and natural compounds like naphthenic acids.7 A significant share of the organic compounds in this water comes from naphthenic acids (NAs) and polycyclic aromatic hydrocarbons (PAHs), which together account for more than half of the total organics and are widely known for their toxic effects on both plants and animals.5,8 However, slurry or tailings are continuously produced and stored in tailings ponds.2 Problems with this method include the release of carbon emissions into the atmosphere and the risk of groundwater contamination from the ponds.4
Previously, oil sands process water (OSPW) and tailings must be stored in on-site tailings ponds under Alberta's zero-discharge policy.1,2 As much as 83% of these tailings are water, most of which stays trapped and unused, forming tailings ponds water (TPW).7 Potential seepage from tailings ponds into surface water or groundwater is a known risk. For example, oil sands process water has been detected in two tributaries of the Athabasca River, McLean Creek and Lower Beaver River, near tailings ponds in Alberta, Canada.5 Because of this, effective methods must be found to recycle the water and reclaim the land from the water in tailings ponds.
Water pollution has grown rapidly alongside industry rise and cities’ spread. This pollution is mainly caused by the inadequate waste management practices, which include heavy metals; other charged ions, molecules, and particles; organics (including pharmaceuticals and synthetic dyes).9,10 Among various pollutants, organic dyes have become widespread contaminants in natural waters. Due to their resistance to environmental degradation, organic dyes can harm aquatic life and pose risks to human health.9,11 These toxic dyes are known to be potentially teratogenic, mutagenic, and carcinogenic. As a result, increasing attention has been directed toward treating dye-contaminated wastewater.10,12
Crystal violet, rhodamine B, and methylene blue are synthetic organic dyes derived from carbon-based structures that are produced from chemical synthesis. Crystal violet (CV) is a triphenylmethane dye with a long-lasting deep violet color used frequently in applications related to textile dyeing, printing inks, and as a microbiological stain.13,14 Rhodamine B (RB) is a highly soluble cationic xanthene based dye noted for its bright pink to red color that is used for textiles, as a printing pigment, within animal medicine, food products, and as a fluorescent tracer in environmental monitoring.15,16 Methylene Blue (MB), a thiazine dye exhibiting intense blue coloration, is extensively utilized in textile dyeing, biological staining, medical diagnostics, and a range of medical and cosmetic applications.13,17 In addition, heavy metal ions, often found above safe levels in water sources, are highly toxic and resistant to breakdown, remaining in the environment and accumulating through the food chain over time in a process known as bioaccumulation and biomagnification.11,18
Previous research has reported various methods for removing dyes from water, nanofibrous membrane filtration,19 biocoagulation,13 photocatalytic degradation,10,20,21 and adsorption.22 Recently, nanofiber membranes,19 precipitation method,18 adsorption,11 and other techniques have been employed to remove heavy metals from water. Among these methods, adsorption has been identified as a promising option for the removal of both dyes and heavy metal ions, owing to its simplicity, low cost, adaptability, and efficiency.9,23 In this context, preparing adsorbents from solid waste is a practical option.18
Chewing gum is made up of a polymer that cannot be digested, and because it is not biodegradable, it has caused increasing social and environmental worries.24,25 The most commonly used polymers for chewing gum bases are styrene–butadiene and isobutylene–isoprene (butyl rubber) copolymers, poly(isobutylene), polyethylene, polyvinyl acetate, and many other vinyl ester polymers.26,27 Most methods of removing chewed gum are costly, carbon-emitting, and cause air pollution.28 However, while recycling and reusing discarded gum for products such as iontronic devices,29 superhydrophobic material,30 and bioethanol production31 has been explored as an innovative approach to address this environmental challenge, these efforts alone are insufficient.
Here, we showed that recovered waste chewing gum could be transformed into a sorbent, and associated filter, capable of adsorbing organic dyes and heavy metal ions while reducing water toxicity in oil sand tailings ponds. This approach is believed to address the gum waste problem and offer a novel solution for treating the water in these tailings ponds.
2. Materials and methods
2.1 Materials
Three types of chewing gum were purchased from the market: Excel Peppermint (gum 1), Trident Peppermint (gum 2), and Dubble Bubble Crybaby Sour Gumballs (gum 3).Three different commercial chewing gum samples were purchased from the local market and coded as gum 1, gum 2, and gum 3. Rhodamine B (C28H31ClN2O3), methylene blue (C16H18ClN3S), crystal violet (C25H30N3Cl), activated charcoal powder, flash silica, and sand were obtained from Sigma Aldrich (Oakville, Ontario) and used without further purification. 0.2 µm syringe filters (Basix™ Nylon, Fisher Scientific, USA) and 25 mm syringe filters with 0.2 µm nylon membranes (VWR International, USA) were obtained. Metal salts used for ion preparation comprised ferric chloride (FeCl3; Sigma-Aldrich, Germany), copper(II) sulfate pentahydrate (CuSO4·5H2O; Sigma-Aldrich, Japan), silver nitrate (AgNO3; Sigma-Aldrich, Ontario), mercuric nitrate monohydrate (Hg(NO3)2·H2O; J.T. Baker Chemical Co., Phillipsburg, NJ, USA), as well as cadmium nitrate, lead nitrate, and zinc nitrate (Cd(NO3)2, Pb(NO3)2, Zn(NO3)2; Anachemia Canada Inc., Montreal, Quebec, Canada). Hydrogen chloride and sodium hydroxide were also obtained from Fisher Scientific (Ottawa, Ontario). 95% ethanol and 99.9% toluene were purchased from Sigma Aldrich (St Louis, Missouri) and used as received. Deionized water was filtered to a resistivity of 18.2 MΩ-cm using a Milli-Q Plus system (Millipore Co.).
2.2 Isolation of polymers from chewing gum
All chewing gum samples were chewed for a minimum of 30 min, as obtained from commercial sources. The chewed gum was sequentially washed three times with toluene, ethanol, and water to eliminate soluble components. The solid residues were then separated from the gum-solvent mixture by centrifugation. Following washing, the solids were dried overnight under vacuum conditions. Gums 1, 2, and 3 were finally obtained.2.3 Organic dye removal
| A = εbc | (1) |
 | (2) |
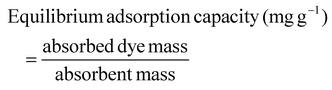 | (3) |
Absorbance (A) is related to concentration (c), ε is the molar extinction coefficient, and b is the path length in centimeters, C0 is the initial concentration of the dye before adsorption and C1 is the concentration after the adsorption.
2.4 Heavy metal ion removal
A solution of each heavy metal was prepared at a 50 mg L−1 concentration. A 10 mL sample of this solution was placed in a 15 mL centrifuge tube. Then, 0.1 g of gum powder was added to the tube. The mixture was shaken for 1 h. After shaking, the sample was centrifuged (15 min, 25 °C, 4000 rpm). The clear liquid (supernatant) was poured into a separate test tube. Inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer, USA) was used to evaluate heavy metals in the water. The concentration of metals was determined using a standard calibration curve made from solutions with known metal concentrations.2.5 Oil sand TPW treatment
| Column | Charcoal (g) | Silica (g) | Gum (g) |
|---|---|---|---|
| Values are expressed as mean ± standard deviation (n = 3). | |||
| 1 | 5.14 g ± 0.01 | — | — |
| 2 | 3.00 g ± 0.01 | 3.01 g ± 0.01 | — |
| 3 | — | 3.01 g ± 0.01 | 1.51 g ± 0.01 |
| 4 | — | 7.47 g ± 0.01 | — |
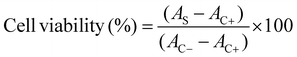 | (4) |
3. Results and discussion
3.1 Adsorptive removal of organic dyes
CV has been widely used in printing, cleaning products, and Gram staining to aid in the classification of bacteria.13 Organic dyes are known for their persistence in the environment and resistance to natural degradation. For this reason, they are considered suitable model dyes for evaluating the effectiveness of dye removal methods. Fig. 3 examines the adsorption experiment using gum 1. For measurement, the solution was diluted tenfold before being analyzed by UV-Vis spectroscopy. The adsorbent was added from 0 to 300 mg to 8 mL of CV solution at a concentration of 50 mg L−1 and pH levels of 3, 7, and 11. A decrease in absorbance intensity at the 590 nm peak was observed with increasing adsorbent dosage across all three pH values.Furthermore, greater adsorption of CV was achieved at pH 7 compared to pH 3, as shown in Fig. 2a and b. It is worth noting that the initial pH value of the TPW is reported to be ∼8.33 At low pH levels, the surface of the adsorbent becomes positively charged due to protonation.34–36 Since CV dye also carries a positive charge, electrostatic repulsion occurs between the dye and the surface. Excess hydrogen ions (H+) also compete with the dye molecules for active adsorption sites. Consequently, the dye uptake is significantly reduced under acidic conditions.34
However, dye adsorption decreased without adding adsorbent at pH 11 (Fig. 2c). Tahar et al. (2025) reported that the stability of CV is strongly influenced by pH.14 When the pH exceeds 10, the dye degrades gradually over time, while at pH levels above 11.5, the degradation becomes much faster, and a solution containing 20 mg L−1 of CV can become decolorized entirely in less than 30 min. The pronounced decrease in the absorbance of CV at high pH is due to a chemical transformation, in which the violet cationic species (CV+) is converted into a colorless neutral triphenylmethanol form (CVOH0) through the nucleophilic attack of hydroxide ions on the central carbon.14,37 However, although CV is not stable in highly alkaline conditions, the absorbance is still observed to drop as the amount of gum increases. Research conducted by Thurakkal & Porel, (2023) showed that a covalent organic polymer based on dithiocarbamate, despite its electron-rich structure, was found to exhibit reduced ability to remove the cationic dye CV when the pH was changed.9 Therefore, changes in pH can cause the charge of chemical species to be altered, which may interfere with the efficiency of dye removal. These findings suggest that dye levels in solutions can be reduced by gum across a range of pH values. In the end, removing CV was less effective under acidic and alkaline conditions than at neutral pH. This result was in agreement with findings reported in previous studies.9,13
The rate at which the dyes adsorb/absorb is considered important for practical use; faster adsorption results in reduced removal time. The presence of more available sites leads to an increase in the rate of dye absorption. Therefore, 100 mg of the gum was reacted with 50 mg L−1 CV at pH 7 for 2, 4, 6, 8, and 10 min. Fig. 2d shows that the absorbance peaks (2 to 10 min) were closely aligned and remained relatively constant. This means that nearly all of the 50 mg L−1 CV was adsorbed by the material within just 2 min. Finally, the adsorption was found to remain almost constant after 2 min, leading to overlapping peaks.
To visually assess dye removal using gum as a sorbent, a 0.2-micron membrane was modified by injecting a gum suspension into it. Through this process, gum particles were caused to adhere to the inner surface of the filter. CV solutions were then passed through the modified membrane. In contrast to the unmodified filter, the liquid collected from the modified membrane was observed to be colourless (Fig. 3). These results indicate that the gum rapidly and effectively adsorbs the dye. This finding, therefore, suggests a promising approach for designing simple and efficient dye removal devices.
 | ||
| Fig. 3 Visual comparison of (a) unmodified and (b) modified filters used for CV solution removal, showing their appearance from the beginning to the end of the separation process. | ||
Three different brands of gum, each 100 mg, were used to treat solutions of CV, RB, and MB. In each case, the dye was adsorbed from the solution, although the extent of adsorption was influenced by both the type of gum and the chemical structure of the dye. As shown in Fig. 4a, the best performance with CV was achieved by gum 3. This is due to stronger bonding of the dye with that gum. Accordingly, the removal efficiency and adsorption capacity of CV by 100 mg of gum (mean ± SD, n = 3) followed the order: gum 3 (93.88 ± 3.72%, 0.5633 ± 0.0310 mg g−1) > gum 2 (87.68 ± 4.27%, 0.5261 ± 0.0256 mg g−1) > gum 1 (84.75 ± 3.72%, 0.5085 ± 0.0223 mg g−1). For RB, the order was: gum 1 (58.72 ± 1.71%, 0.5872 ± 0.0171 mg g−1) > gum 3 (40.17 ± 1.94%, 0.4017 ± 0.0194 mg g−1) > gum 2 (22.25 ± 1.68%, 0.2225 ± 0.0168 mg g−1). For MB, the order was: gum 2 (43.63 ± 2.53%, 0.1745 ± 0.0101 mg g−1) > gum 3 (28.13 ± 0.81%, 0.1125 ± 0.0032 mg g−1) > gum 1 (22.73 ± 1.18%, 0.0909 ± 0.0047 mg g−1). Overall, the adsorption of RB and MB was not as strong as that of CV. However, it should be noted that the adsorbent was prepared from waste material derived from chewing gum. Despite this, removal efficiencies as high as 99% for CV, 60% for RB, and 46% for MB were recorded in the best-performing samples.
Reusability is considered a key parameter in assessing the performance of an adsorbent for practical applications.9 Materials that can be regenerated and used again are known to reduce waste management issues related to spent adsorbents, and they may also help reduce some of the overall treatment costs.38 To prepare the adsorbent for subsequent adsorption cycles, it must be thoroughly washed to ensure all remaining color is completely removed. In this work, the reusability of gum 1 for dye removal was examined by measuring its removal efficiency, which is defined as the amount of dye adsorbed relative to the original dye concentration. As shown in Fig. 4c, the gum suspension was observed to be white before use. After reaction with CV, visible coloration occurred due to dye adsorption. The adsorbed dye was removed by washing the gum several times with ethanol. Following washing, a return to a white colour was observed. No absorbance at 590 nm was detected in the UV-Vis spectrum of the washing liquid, indicating that dye removal had been achieved. The regenerated gum was reused for dye removal in aqueous solution. Based on Fig. 4b, effective removal of both CV and RB was maintained by gum 1 over four repeated cycles. Based on these results, an 11% decrease in the adsorption of CV and an 8% decrease in the adsorption of RB were recorded between the first and fourth cycles. In comparison, a more pronounced decline in performance has been reported by other studies, with reductions ranging from approximately 25% to 47% between the initial and final cycles.9,13,34 However, the number of reuses for each material and the corresponding reduction in performance compared to the initial cycle are presented in Table 2.
| Dye | Absorbent (source) | RE (%) | qe (mg g−1) | C (mg L−1) | AD (g L−1) | Reusability (reduction%) | Ref. |
|---|---|---|---|---|---|---|---|
| RE: removal efficiency, qe: equilibrium adsorption capacity, C: concentration, AD: adsorbent dosage, CS: chemical synthesis, WM: waste materials, E; experimental, M: modelled. | |||||||
| CV | Dithiocarbamat (CS) | 100 | 250 (E) | 250 | 5 | 5 (25) | 9 |
| Avocado seed (WM) | 94 | — | 50 | 1 | 5 (47) | 13 | |
| Water treatment sludge (WM) | 89 | 10 (M) | 50–250 | 2 | 5 (25) | 34 | |
| Chewed gum (WM) | 99 | 0.6 (E) | 12 | 20 | 4 (11) | This work | |
| RB | Sulfonated tetraphenylethylene (CS) | 100 | 2232 (M) | 180–270 | 0.2 | 5 (0) | 12 |
| ZnSnO3/Zn2SnO4 (CS) | 93 | — | 50 | 0.3 | 6 (5) | 39 | |
| Chewed gum (WM) | 60 | 0.6 (E) | 17 | 17 | 4 (8) | This work | |
| MB | Activated carbon obtained from mangosteen peel (WM) | 100 | 871 (M) | 300–500 | 0.5 | 4 (80) | 40 |
| Industrial carpet waste (WM) | 100 | 2 (E) | 10–20 | 10 | — | 41 | |
| Avocado seed (WM) | 92 | — | 50 | 1 | 5 (56) | 13 | |
| Carbon nano-onions (WM) | 99 | 43 (M) | 10–100 | 0.25–2 | 4 (−9) | 38 | |
| Printer powder nanocomposite (WM) | 95 | 489 (M) | 10–100 | 0.2–1.2 | 6 (10) | 42 | |
| Chewed gum (WM) | 46 | 0.2 (E) | 6 | 14 | 4 (66) | This work | |
Nonetheless, a more noticeable drop in removal efficiency was observed for MB. This decline is likely caused by partial blocking of some active sites on the adsorbent surface, due to the stronger binding of MB to the gum. As a result, the removal performance was reduced in the following cycles.10,22 Even after washing with a saturated sodium chloride solution, complete regeneration of the gum was not achieved. These findings suggest that MB is not mainly adsorbed through electrostatic interactions. Finally, the ability to reuse the gum improves the economic feasibility of this method for real wastewater treatment applications.
In order to compare previous studies, Table 2 summarizes the adsorption of CV, RB, and MB based on the type of adsorbent and its source, dye concentration, adsorbent dosage, removal efficiency, equilibrium adsorption capacity, reusability, and the approximate percentage decrease in performance from the first to the final cycle. In many previous studies, adsorbents were produced using chemical synthesis. In contrast, this research has successfully used gum waste, achieving effective removal of these three dyes and promising results in removing heavy metals.
3.2 Adsorptive removal of heavy metal ions
Metals with a density greater than 5 g cm−3 are classified as heavy metals. Toxic metals such as mercury, copper, cadmium, lead, zinc, and valuable metals like silver are important members of this group.11 Lead, mercury, and cadmium are recognized as the most significant heavy metal ions due to their harmful effects on the environment.11,43 Gum 1 powder was tested to evaluate its ability to remove heavy metal ions. Different heavy metal ions were selected, as wastewater usually contains several kinds. Each metal ion solution was prepared at a concentration of 50 mg L−1. An equal amount of gum powder (100 mg) was added to each solution. After the reaction, different final concentrations of the metal ions were recorded (Fig. 5a). This indicated that the gum powder binds various ions with different strengths. The strongest binding was observed with Pb2+, followed by Fe3+, Cu2+, Zn2+, Hg2+, Ag+, and Cd2+. To better understand the adsorption range of heavy metals that were removed during the preliminary test (Pb2+, Fe3+), different amounts of gum powder were examined based on ICP-OES analysis. Amounts of 10, 20, 30, 40, 50, and 100 mg of gum powder were mixed with 10 mL of Pb(NO3)2 and Fe(NO3)3 metal ion solutions at 50 mg L−1. As shown in Fig. 5c and d, 20 mg of gum powder was sufficient for completely removing Pb2+ ions, while almost all Fe3+ ions were removed using only 10 mg of gum powder.A solution was prepared with various proportions of Pb(NO3)2, Fe(NO3)3, CuSO4·5H2O, and Hg(NO3)2·H2O to simulate an industrial wastewater scenario. The solution was tested before and after being treated with 10 mg of gum powder. The net intensity of each metal ion was measured. As shown in Fig. 5b, relatively strong competition was observed among the metal ions for adsorption in the mixed-metal system. A reduction in adsorption capacity was observed, suggesting that competition among metal ions for active surface sites persists, even when some ions like Pb2+ and Fe3+ exhibit strong binding affinity. The adsorption of heavy metals is influenced by both the physical and chemical properties of the metal ions. For example, the high electronegativity of mercury or the large ionic radius of lead may interfere with the adsorption of coexisting metal ions such as copper. On the other hand, the relatively small size and low electronegativity of iron allowed it to be more effectively adsorbed when in competition with other metal ions.43 It is worth noting that the adsorbent showed higher selectivity toward iron, mercury, and lead. Among them, Fe3+ showed the highest adsorption, likely due to its smaller ionic radius, which allowed it to compete more effectively for active sites. This behavior may also be closely related to the hydration abilities of the involved metal ions.18 In general, the goal was to confirm that gum powder remains effective even when multiple ions are present together, and the exact net intensities were not the primary focus here. The reported values are semi-quantitative and should be understood as relative rather than absolute measurements.
In addition, a filter was modified with gum powder, following the method used in section 3.1. A FeCl3 solution was passed through the modified filter. The solution became colourless after passing through. A commercial filter was tested in the same way. It did not remove any colour, as seen by the naked eye (Fig. 6).
 | ||
| Fig. 6 (a) shows the result after 1000 mg L−1 of Fe3+ was passed through an unmodified filter. Figure (b) shows the result after the same solution was passed through a gum-modified filter. | ||
A comparison of heavy metal removal reported in previous studies is presented in Table 3. The data are categorized based on the type and source of the adsorbent, removal efficiency, equilibrium adsorption capacity, heavy metal concentration, adsorbent dosage, and reaction time. In the present work, the removal efficiency for Pb2+ was below the method detection limit, while those for Fe3+, Cu2+, Zn2+, Hg2+, Ag+, and Cd2+ were 99%, 98%, 90%, 64%, 56%, and 48%, respectively. Under the tested conditions, over 90% of Pb2+, Fe3+, and Cu2+ were eliminated. This adsorbent performed better than similar studies that evaluated several heavy metals.
| Absorbent (source) | Heavy metals | RE (%) | qe (mg g−1) | C (mg L−1) | AD (g L−1) | RT (min) | Ref. |
|---|---|---|---|---|---|---|---|
| RE: removal efficiency, qe: equilibrium adsorption capacity, C: concentration, AD: adsorbent dosage, RT: reaction time, CS: chemical synthesis, WM: waste materials, E; experimental, M: modelled. | |||||||
| Sludge-based biochar/chitosan composite (CS) | Pb, Cu, Se, Cr | 94, 97, 88, 84 | 0.010, 0.121, 0.010, 0.049 (E) | <0.07 | 0.5 | 15–90 | 44 |
| A bio-composite of cellulose@CaCO3 (CS) | Pb2+ | 100 | 602.3 (M) | 50 | 10 | 30 | 45 |
| Cotton stalks & date palm stones (WM) | Cd2+, Pb2+, Zn2+ | 98, 92, 79 | 2.67, 2.88, 2.19 (M) | 30 | 10 | 30 | 43 |
| Flowerlike calcium silicate hydrate from coal fly ash (WM) | Cd2+ | 100 | 320 (E) | 300 | 1 | 120 | 18 |
| Chewed gum (WM) | Pb2+, Fe3+, Cu2+, Zn2+, Hg2+, Ag+, Cd2 | 100, 99, 98, 90, 64, 56, 48 | 5, 4.95, 4.9, 4.5, 3.2, 2.8, 2.4 (E) | 50 | 10 | 60 | This work |
3.3 Treatment of oil sands TPW
Produced water from oilfields is considered a major global challenge for the oil and gas industry, as it contains salts, suspended solids, heavy metals, and various ions. It is also known to include harmful organic compounds such as naphthenic acids, phenols, polycyclic aromatic hydrocarbons, and BTEX compounds (benzene, toluene, ethylbenzene, and xylenes).5 Among these, naphthenic acids have been identified as key contributors to the overall toxicity of TPW and are known to pose serious risks to the health of living organisms.8 Therefore, we wanted to show a real world application of gum as a sorbent. Fig. 7 shows that the filtrate from charcoal-treated tailings pond water (TPW) was very clear. This suggests that most organic molecules were adsorbed onto the surface of the activated charcoal. The porous structure of the charcoal allowed toxins to be bound to exposed sites. In contrast, the untreated TPW shown in the same figure appeared cloudy and yellowish. This indicates that most dissolved chemicals and solids remained in the solution before treatment. Charcoal and silica columns were prepared by placing a layer of sand first, followed by 3 grams of charcoal suspended in deionized water. Another layer of sand was added, then 3 grams of flash silica suspended in deionized water, and finally, a top layer of sand was placed. TPW was added slowly, drop by drop. The filtrate collected from this column was as clear as that from the charcoal column. This clarity was mainly attributed to the strong adsorptive ability of charcoal, which effectively removed organic molecules. A column with silica and gum was prepared using layers of sand, 3 grams of silica suspended in deionised water, another sand layer, 1.5 grams of gum, and a final sand layer. The gum was placed above the silica to prevent it from seeping into the lower sand and cotton layers. Only 1.5 grams of gum was used, as larger amounts caused the column to become too tight for water to pass through. Tailings pond water (TPW) was added slowly, drop by drop, and the filtrate collected was slightly darker than that from the charcoal column. A second column containing 1.5 grams of gum and 3 grams of charcoal was also prepared; however, it was impermeable to TPW.Accordingly, an in vitro cytotoxicity test was carried out using various filtration columns. Each filter was tested in five replicates. In this assay, the unfiltered TPW was used as the positive control and the sample without TPW as the negative control. The average cell viability (mean ± standard deviation) of all treated samples was standardized with the control groups to evaluate cell viability. As expected, the lowest cell viability was recorded for the unfiltered TPW. When TPW was passed through a charcoal filter, cell viability increased to 40.31 ± 11.24%. A similar result, 40.16 ± 9.69%, was observed after filtration through a charcoal and silica column. This result was anticipated because activated charcoal is known to be most effective at removing organic acids, which are the primary contributors to TPW toxicity.7,11 Although when TPW was filtered through silica, cell viability reached only 26.99 ± 11.66%. This confirmed that silica was less effective than charcoal, even though a larger amount was used. Nonetheless, the column containing only silica filtered the water the fastest, as silica is more permeable than charcoal and gum. In contrast, TPW filtered through a column containing silica and gum showed the highest cell viability, recorded at 60.75 ± 8.77%. In this case, gum was more effective than activated charcoal.
Non-purgeable organic carbon (NPOC) data were collected to determine whether the gum adsorbed or reacted with material from TPW (Fig. 7). Values are expressed as mean ± standard deviation (n = 3). Initially, the untreated TPW showed an NPOC level of 67.40 ± 0.86 mg L−1, which was expected due to its high organic carbon content. After treatment, this level was reduced to 3.70 ± 0.35 mg L−1 by the charcoal column. Furthermore, this value decreased even further to 1.77 ± 0.17 mg L−1 when silica was added to the charcoal, which is consistent with the known adsorption capabilities of activated charcoal. This result aligns with what is known about charcoal's ability to remove organic matter. However, contrary to expectations, the silica and gum column exhibited a higher NPOC value of 71.20 ± 0.78 mg L−1 than pure TPW. This suggests that the gum likely reacted with compounds in the TPW, probably the naphthenic acids. Such a reaction might explain why less cytotoxicity was observed earlier. Nevertheless, more work is needed to understand which parts of the gum remain after washing, what reactions are occurring, and what new substances are forming.
4. Conclusions
This study has shown that waste chewing gum holds substantial potential, renewable material for treating tailings pond water (TPW) from oil sands operations. A variety of pollutants were effectively removed by the gum-based materials, including organic dyes such as CV, RB, and MB, as well as toxic heavy metals like Pb2+, Fe3+, Cu2+, Zn2+, Hg2+, Ag+, and Cd2+. Removal efficiency reached 100 percent for lead, and over 98 percent for iron, copper, and CV. In addition, more than 60 percent removal was achieved for zinc, mercury, and RB. Reusability tests confirmed that gum maintained high adsorption efficiency over multiple cycles, with only a slight decline in performance, indicating its durability as an adsorbent. Among the filtration systems tested, the column containing silica and gum brought about the most significant reduction in TPW cytotoxicity, as shown by cell viability results using HeLa cells. Although an unexpected increase in non-purgeable organic carbon (NPOC) was observed in this setup, the elevated levels are likely the result of chemical interactions between the gum and specific components of TPW, such as naphthenic acids, rather than simple surface adsorption. These findings suggest that chewing gum may do more than trap harmful substances. It may also take part in chemical reactions that reduce the biological toxicity of these compounds.While there are many benefits to the materials presented here, there are limitations. Specifically, the work is limited in how well it can explain the adsorption process. The mechanism cannot be confirmed at this stage because detailed structural and chemical characterization is not complete, e.g. FTIR, XPS, SEM, and BET. As a result, the findings were based observed adsorption properties. Identifying surface groups, chemical structure, morphology and porosity of a sample will improve our understanding of the adsorption mechanism and provide insight into how the material's structure and properties relate to each other, which will be the subject of future studies.
Conflicts of interest
There are no conflicts to declare.Data availability
Data availability statement available upon request.Acknowledgements
M. J. S. acknowledges funding from the University of Alberta (the Department of Chemistry and the Faculty of Science), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), the Alberta Advanced Education & Technology Small Equipment Grants Program (AET/SEGP).References
- D. C. C. da S. Medeiros, P. Chelme-Ayala and M. Gamal El-Din, Chem. Eng. J., 2023, 463, 142329 CrossRef CAS.
- N. Eltoukhy Allam, M. Nabeel Anwar, P. V. Kuznetsov, A. C. Ulrich and B. R. Dhar, Chem. Eng. J., 2022, 437, 135162 CrossRef CAS.
- F. Nwaishi, R. M. Petrone, J. S. Price, S. J. Ketcheson, R. Slawson and R. Andersen, Ecol. Eng., 2015, 81, 471–480 CrossRef.
- D. Wang, H. Tao, K. Wang, X. Tan and Q. Liu, Fuel, 2022, 316, 123395 CrossRef CAS.
- M. Arslan, M. Usman and M. G. El-Din, Water Res., 2024, 255, 121502 CrossRef CAS PubMed.
- K. A. Clark and D. S. Pasternack, Ind. Eng. Chem., 1932, 24, 1410–1416 CrossRef CAS.
- J. Li, Z. T. How and M. G. El-Din, Sci. Total Environ., 2023, 856, 159079 CrossRef CAS PubMed.
- L. Yang, A. Bekele and M. G. El-Din, Environ. Res., 2024, 252, 118972 CrossRef CAS PubMed.
- L. Thurakkal and M. Porel, Eur. Polym. J., 2023, 194, 112169 Search PubMed.
- K. Nagaraja, M. Arunpandian and O. Tae, Int. J. Biol. Macromol., 2024, 273, 133123 CrossRef CAS PubMed.
- A. Singh, S. S. Shah, C. Sharma, V. Gupta, A. K. Sundramoorthy, P. Kumar and S. Arya, J. Environ. Chem. Eng., 2024, 12, 113032 CrossRef CAS.
- M. Shi, K. Zhang, Q. Zhuang, C. Zhang, X. Lin, A. Xie and W. Dong, Colloids Surf., A, 2022, 647, 128948 CrossRef CAS.
- A. Zourif, A. Benbiyi, S. Kouniba and M. El Guendouzi, Sustainable Chem. Pharm., 2024, 39, 101621 Search PubMed.
- L. B. Tahar, R. Mogharbel, Y. Hameed, A. Noubigh, M. J. A. A. E. Abualreish, A. H. Alanazi and M. R. Hatshan, Results Chem., 2025, 14, 102152 Search PubMed.
- T. A. Saleh, M. Tuzen and A. Sarı, Environ. Sci. Pollut. Res., 2021, 28, 55655–55666 Search PubMed.
- G. Sharifzade, A. Asghari and M. Rajabi, RSC Adv., 2017, 7, 5362–5371 RSC.
- A. Mills, D. Hazafy, J. Parkinson, T. Tuttle and M. G. Hutchings, Dyes Pigm., 2011, 88, 149–155 CrossRef CAS.
- L. Xing, J. Luo, H. Jiang, X. Zhang, M. Rao and G. Li, Sep. Purif. Technol., 2024, 348, 127690 CrossRef CAS.
- S. Wu, W. Shi, K. Li, J. Cai, C. Xu, L. Gao, J. Lu and F. Ding, Int. J. Biol. Macromol., 2023, 239, 124264 CrossRef CAS PubMed.
- K. Nagaraja, B. Mallika, M. Arunpandian and T. H. Oh, Int. J. Biol. Macromol., 2025, 305, 140962 CrossRef CAS PubMed.
- J. Liu, T. Shu, L. Su, X. Zhang and M. J. Serpe, RSC Adv., 2018, 8, 16850–16857 RSC.
- R. A. S. Alatawi, Int. J. Biol. Macromol., 2025, 299, 140086 CrossRef CAS PubMed.
- J. Song, Z. Huang and M. G. El-Din, Chem. Eng. J., 2021, 421, 129937 CrossRef CAS.
- A. A. Oladipo, E. O. Ahaka and M. Gazi, Chem. Eng. Commun., 2021, 208, 220–232 Search PubMed.
- X. Yin, K. Sun, J. Cui, H. Zhang and X. Li, ACS Appl. Electron. Mater., 2025, 7, 5195–5204 CrossRef CAS.
- E. Tisdale and C. Wilkins, Anal. Chim. Acta, 2014, 820, 92–103 CrossRef CAS PubMed.
- N. Konar, I. Palabiyik, O. S. Toker and O. Sagdic, Trends Food Sci. Technol., 2016, 55, 29–38 CrossRef CAS.
- H. Yu, L. Yu, X. Qi, J. Cui, K. Wu, Y. Liu, L. Chen and X. Li, J. Mater. Chem. C, 2023, 11, 10455–10463 RSC.
- B. Cheng and P. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 6731–6738 CrossRef CAS PubMed.
- J. Zhu, L. Li, M. Dong, Z. Zeng and L. Sun, Colloids Surf., A, 2023, 675, 132128 CrossRef CAS.
- D. Yüceşen Serbest, N. Baylan and S. Çehreli, Indian Chem. Eng., 2024, 66, 152–163 CrossRef.
- Z. M. Markovic, L. M. Harhaji-Trajkovic, B. M. Todorovic-Markovic, D. P. Kepić, K. M. Arsikin, S. P. Jovanović, A. C. Pantovic, M. D. Dramićanin and V. S. Trajkovic, Biomaterials, 2011, 32, 1121–1129 CrossRef CAS PubMed.
- J. Wan, T. Chakraborty, C. (Charles) Xu and M. B. Ray, Sep. Purif. Technol., 2019, 211, 448–455 CrossRef CAS.
- D. A. El-Emam, A. H. Elezaby, M. A. Zeyadah and M. A. El-Sonbati, Sci. Rep., 2025, 15, 17426 CrossRef CAS PubMed.
- F. Ahmadijokani, H. Molavi, M. Amini, A. Bahi, S. Wuttke, T. M. Aminabhavi, M. Kamkar, O. J. Rojas, F. Ko and M. Arjmand, Chem. Eng. J., 2023, 466, 143119 CrossRef CAS.
- D. Li, Y. Sun, Y.-L. Yang, X.-L. Shi, D.-A. Xie, L. Nie, J.-G. Chen, Z. Luo, H.-J. Chen, C.-A. Yang and Z.-G. Chen, Sustainable Mater. Technol., 2024, 39, e00834 CrossRef CAS.
- E. Q. Adams and L. Rosenstein, J. Am. Chem. Soc., 1914, 36, 1452–1473 CrossRef CAS.
- D. Patel, K. M. Tripathi and R. K. Sonwani, ACS Omega, 2024, 9, 30834–30845 CrossRef CAS PubMed.
- Z. Li, Q. Li, X. Liu, C. Yang and Y. Zhou, Mater. Res. Bull., 2022, 156, 111980 CrossRef.
- Z. Zhang, L. Xu, Y. Liu, R. Feng, T. Zou, Y. Zhang, Y. Kang and P. Zhou, Microporous Mesoporous Mater., 2021, 315, 110904 CrossRef CAS.
- K. Janbooranapinij, A. Yimponpipatpol, N. Ngamthanacom and G. Panomsuwan, Clean. Eng. Technol., 2021, 4, 100150 CrossRef.
- M. A. Bhakare, P. S. Dhumal, M. P. Bondarde, K. D. Lokhande and S. Some, Colloids Surf., A, 2024, 685, 133196 CrossRef CAS.
- H. Nagy, M. Fawzy, E. Hafez and A. E. D. Mahmoud, Environ. Sci. Pollut. Res., 2024, 31, 39849–39865 CrossRef PubMed.
- J. Song, S. A. Messele, L. Meng, Z. Huang and M. G. El-Din, Water Res., 2021, 194, 116930 CrossRef CAS PubMed.
- H.-X. Zhao, Y. Wang, J.-J. Yang, J.-G. Zhang, Y.-R. Guo, S. Li and Q.-J. Pan, Chem. Eng. J., 2023, 454, 140297 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





