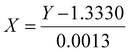Open Access Article
Open Access ArticleEffects of polyether polyol hydroxyl equivalent weight on controlled release polyurethane coatings of urea (46-0-0)
Alex J.
Kosanovich
 *,
Omar
Jalife
,
Yi
Fan
,
Abhishek
Shete
and
Yasmin
Srivastava
*,
Omar
Jalife
,
Yi
Fan
,
Abhishek
Shete
and
Yasmin
Srivastava
The Dow Chemical Company, 230 Abner Jackson Pkwy, Lake Jackson, TX 77566, USA. E-mail: AJKosanovich@dow.com
First published on 23rd October 2025
Abstract
Layer-by-layer polyurethane coatings incorporating wax additives were applied to urea granules (46-0-0) to investigate the influence of poly(propylene oxide) triol molecular weight on controlled-release fertilizer (CRF) performance. Triols ranging from 260–1000 g mol−1 were evaluated at constant coating weight, triethanolamine active catalyst inclusion, temperature, and isocyanate index to modulate hydroxyl equivalent weight (HEW), calculated crosslink density, and measured glass transition temperature (Tg) for the coating formulations. Castor oil-derived polyurethane served as a benchmark to validate the chosen coating process variables. Lower-HEW triols required increased polyisocyanate content to maintain stoichiometry, resulting in higher crosslink densities and enhanced nutrient release control. In contrast, the glycerol-based coating, despite a high calculated crosslink density and measured Tg failed to form uniform films, leading to negligible release. These results establish a structure–property relationship linking poly(propylene oxide) triol molecular weight to CRF performance, enabling tunable release profiles through precise polyurethane formulation.
Introduction
The United Nations Sustainable Development Goal (SDG) #2 is described as ending hunger globally.1 With increasing population growth has come the intensifying need for improved crop yields to meet corresponding food demands. In sustaining these demands, farmers utilize fertilizers, either in natural form (animal/human waste-based products)2 or synthetic3 to provide the plants with necessary nutrients. Urea (46-0-0)4 is 46 wt% nitrogen and serves as a primary source of nitrogen for growing staple commodity crops, such as corn and wheat.5 Urea is highly water soluble which requires it to be dispersed either in multiple or blended allocations and in a controlled fashion to mitigate potential for overdosage and root burn.6 Additionally, its usage must be closely monitored to reduce risks of environmental leaching which can lead to deleterious effects on plants, water, and wildlife.7Controlled release fertilizers (CRFs) are granular nutrients where a thin coating of polymer or other barrier material has been utilized to manage the overall diffusion of water and rate of nutrient availability. By effectively leveraging this technology, the nutrient use efficiency (NUE) of the fertilizer is increased by designed release profiles which correspond to the real-time nutritional needs of the crop.8 While tested in a laboratory in a controlled environment, coatings must be designed to be robust as in an applied agricultural setting, controlled release fertilizers must contend with variables such as soil conditions and pH, microbial activity, and fluctuating temperature while still providing smooth release performance. While various other polymeric materials9 have been used, polyurethanes consistently present one of the most robust and tunable polymer coatings to enable controlled release coatings of urea (46-0-0) with reproducible kinetics.10 Polyurethanes are generated by the addition reaction of a polyhydroxylated compound, or polyol, with a polyisocyanate containing two or more isocyanate (R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups to form carbamate linkages. The composition, functionality, and molecular weight of the polyol and isocyanate components determine the material properties of the resultant polyurethane polymeric material. Various polyisocyanates can be and are often utilized to provide crosslinked polyurethane coatings including isophorone diisocyanate11 (IPDI), 1,6-hexamethylene diisocyanate12 (HDI), isomeric mixtures of toluene diisocyanate13 (TDI), and monomeric (MDI) or polymeric methylene diphenyl diisocyanate (pMDI).14 The addition polymerization of polyol to isocyanate is often catalyzed by tertiary amines such as triethylene diamine or organometallic tin, bismuth, or zinc catalysts. This work utilizes spherical urea (46-0-0) granules and successive layer-by-layer application of polymeric MDI and polyol components in the presence of a wax additive to provide a set of controlled release fertilizer coatings which allow for a range of release performances. By generating polyurethane coatings using poly(propylene oxide) triols of various molecular weights, a structure–property relationship was established, revealing key factors that drive release performance.
O) groups to form carbamate linkages. The composition, functionality, and molecular weight of the polyol and isocyanate components determine the material properties of the resultant polyurethane polymeric material. Various polyisocyanates can be and are often utilized to provide crosslinked polyurethane coatings including isophorone diisocyanate11 (IPDI), 1,6-hexamethylene diisocyanate12 (HDI), isomeric mixtures of toluene diisocyanate13 (TDI), and monomeric (MDI) or polymeric methylene diphenyl diisocyanate (pMDI).14 The addition polymerization of polyol to isocyanate is often catalyzed by tertiary amines such as triethylene diamine or organometallic tin, bismuth, or zinc catalysts. This work utilizes spherical urea (46-0-0) granules and successive layer-by-layer application of polymeric MDI and polyol components in the presence of a wax additive to provide a set of controlled release fertilizer coatings which allow for a range of release performances. By generating polyurethane coatings using poly(propylene oxide) triols of various molecular weights, a structure–property relationship was established, revealing key factors that drive release performance.
Experimental
Materials
All chemical components were used as received. Castor oil (CAS #8001-79-4) and glycerol (99.5%) were purchased from Sigma Aldrich. All poly(propylene oxide) triols (P1000, P700, P450, P260) were obtained from the Dow Chemical Company and are available as VORANOL™ 8150, VORANOL™ 2070, VORANOL™ CP 450, and VORANOL™ 230-660 polyols, respectively. Polymeric methylenediphenyldiisocyanate (pMDI) was obtained from the Dow Chemical Company and is available as PAPI™ 27 polymeric MDI. Triethanolamine (TEOA, 99%) was obtained from the Dow Chemical Company. C30+HA wax granules were purchased from the Chevron Phillips Chemical Company. Urea (46-0-0) granules (SGN 250) were purchased from American Plant Food Corp. and kept in an oven at 50 °C for 2 h prior to coating.Thermal analysis
To produce polyurethane plaques suitable for testing, each coating formulation was added to a FlackTek™ cup (30 g of total mass) and mixed at 2000 rpm for 30s before being poured into a circular metal paint can lid treated with urethane mold release and allowed to cure for 7 days after which the solid plaque was removed and tested for thermal properties. Full thermal analysis methods can be found in the SI.Coating procedure
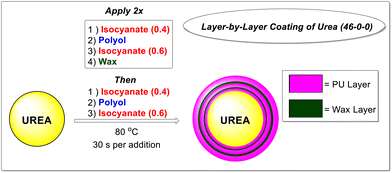
Urea prills (1.0 kg) were kept in an oven set to 80 °C for up to 24 h prior to coating experiments. Using gloves for hot material handling, the prills were transferred from the oven to the coater (see SI for coater geometry and details) and rotation was initiated at 40 rpm. An externally placed heat gun was used to heat and maintain the temperature of the rotating prills at 80 °C, and an IR temperature probe was utilized to ensure temperature stability prior to addition of coating materials. Once the temperature was stabilized, the coating was applied as three layers in layer-by-layer fashion where the first two polyurethane layers were followed by addition of a wax layer, with a third, final polyurethane layer (see SI for full procedure details). Following application of the coating, the granules were allowed to cool to below 50 °C, where free flowing granule behavior was observed, before being discharged from the coater and allowed to sit for 7 days to complete cure, prior to release profile testing.Refractive index (RI) calibration
As light travels through a transparent material, or solution, its velocity is altered and can be measured. Solvated materials influence that refractive index (RI) value. Using these principles, a calibration curve was generated by measuring the RI, using a Reichert AR 200 refractometer, of aqueous urea (46-0-0) solutions with varied concentrations (see SI). The equation can be rearranged to solve for dissolved urea in wt% as such:where, X = wt% released urea; Y = refractive index (RI).
Analysis of urea release performance
After a minimum of 7 days post-coating, the urea release profiles were measured by taking 10 g of coated material and placing it in a jar with 100 mL of DI H2O and kept at room temperature. The refractive index of a small aliquot was collected after 14, 28, 56, and 84 days, or until >95% urea release was measured.Results and discussion
The properties and performance of coated urea substrates are highly dependent upon process conditions such as temperature, rotation rate, timing, and coating weight. These affect coating uniformity, thickness, and batch cycle times.15 Once process conditions have been optimized, the chemistry of the polyurethane itself can be modified through judicious choice of isocyanate and polyol components. The ultimate cure rate, hydrophobicity, and crosslink density can be effectively tuned and connected to controlled release performance. Generally, release of a conformally coated, water-soluble granule occurs in stages where release of the highly soluble urea is enacted by an initial saturation of the coating shell with water, after which solvation of the granule and osmosis, or transfer of the nutrient solute occurs via transport through the shell structure.16 In a properly applied polyurethane coating without defect, water slowly diffuses into the crosslinked polymer network, dissolves the urea, and causes its release at a controlled rate.Composition of polyurethane coatings
Coatings were applied in layer-by-layer fashion at 80 °C. To promote the kinetics of polymer formation and maintain relevant molar ratios, each polyol selected was blended with a 10 wt% inclusion of triethanolamine (TEOA).17 The polyisocyanate was applied to the coated material in two portions (40% and 60% of the total addition) with the full portion of polyol blend applied between them, constituting the polyurethane layer (Scheme 1).To enable a crosslinked, thermosetting polyurethane coating, PAPI™ 27 polymeric methylenediphenyldiisocyanate (pMDI) of 2.7 average isocyanate functionality, was utilized. Further, it was qualitatively determined that an isocyanate index (the molar ratio of NCO to OH) of 1.4 would provide optimal coating performance and component weight ratios (see SI) for this study. Three total polyurethane layers were applied, with two total wax layers placed between each. The inclusion of a wax additive both aids hydrophobic performance, and fosters coating uniformity.18 For this purpose, a conveniently granulated C30 wax with a melting range of ca. 50–60 °C was used. Thus, a total coating weight of 3.2 wt% was applied to each granule, with 2.7 wt% polyurethane and 0.5 wt% wax total. This lower coating weight still protects the prill and improves crush strength (see SI) while preserving nitrogen availability of the urea granule.19 The various chemical structures of the chemically reactive components used are shown in Scheme 2.
 | ||
| Scheme 2 Chemical structures of components which are combined to generate polyurethane fertilizer coatings. | ||
Castor oil is commonly utilized as a hydrophobic, isocyanate-reactive component in the formation of polyurethanes and was chosen to provide baseline formulation performance for a controlled release urea coating.20 Poly(propylene oxide) triols are ubiquitous in the polyurethanes industry for their applicability in the creation of flexible foams, coatings, adhesives, sealants, and elastomers.21 These species provide greater hydrophobicity22 than poly(ethylene oxide) polyols and are commercially available in a range of molecular weights. For this study, polyurethane coatings were generated from poly(propylene oxide) triols of molecular weights from 260–1000 g mol−1 and tested for their controlled release. Glycerol was also chosen for comparison as the lowest molecular weight, isocyanate-reactive triol from which castor oil and poly(propylene oxide) triols are derived.
Calculated crosslink density
Crosslink density was normalized to elastically equivalent tetra-functional crosslinks (i.e.: 2 trifunctional crosslinks that become linked are elastically equivalent to 1 tetra-functional cross-link). The following equation derived from the work of Macosko and Miller23 was used to calculate the expected crosslink density for each thermoset polyurethane formulation (equation used to calculate theoretical crosslink density of thermoset polyurethane coating formulations (eqn (1)): | (1) |
Castor oil has a reported averaged hydroxyl functionality of 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and is primarily constituted of trifunctional ricinolein, among other glyceride species. Glycerol and all poly(propylene oxide) triol components were presumed to be trifunctional. The polymeric MDI is sold as having an average functionality of 2.7 isocyanate groups. Table 1 reveals that as lower hydroxyl equivalent weight polyols are utilized, the calculated crosslink density of the resultant polyurethane increases significantly.
24 and is primarily constituted of trifunctional ricinolein, among other glyceride species. Glycerol and all poly(propylene oxide) triol components were presumed to be trifunctional. The polymeric MDI is sold as having an average functionality of 2.7 isocyanate groups. Table 1 reveals that as lower hydroxyl equivalent weight polyols are utilized, the calculated crosslink density of the resultant polyurethane increases significantly.
| Coating | Polyol | Blend OH# (mg KOH per g) | Blend HEW (g mol−1) | T g (°C) | Calculated crosslink density (mmol g−1) |
|---|---|---|---|---|---|
| A | Castor | 261 | 215 | 15.2 | 2.24 |
| B | P1000 | 263 | 213 | 28.4 | 2.45 |
| C | P700 | 329 | 171 | 51.1 | 2.75 |
| D | P450 | 449 | 125 | 49.1 | 3.17 |
| E | P260 | 692 | 81 | 128.7 | 5.31 |
| F | Glycerol | 1758 | 32 | 127.2 | 4.47 |
Thermal analysis of coating formulations
Polyurethane plaques of coatings A–F were made using 30 g total material cured at room temperature over 7 days, to mirror the cure profile of the coated granules. Following this, each polyurethane formulation was subjected to thermal analysis to determine their glass transition temperature (Tg) and onset of any notable polymer degradation (Table 1, vide supra). Coatings A–E produced plaques which exhibited sufficient curing within 24 h. However, coating F was observed to remain without sufficient green strength (i.e.: not fully cured) for >24 h after its generation. The plaque made using coating A based on castor oil remained physically flexible even at room temperature and provided a Tg of 15.2 °C. The formulations based on poly(propylene oxide) triols produced plaques with increased Tg correlated to an increase in calculated crosslink density. For example, the P1000 based coating B formulation with a calculated crosslink density of 2.45 mmol g−1 resulted in a measured Tg of 28.4 °C, whereas a 12% increased calculated crosslink density of 2.75 mmol in coating C results in a 80% higher Tg of 51.1 °C. However, the P450-based formulation, coating D, with a calculated crosslink density of 3.17 mmol g−1 resulted in a similar measured Tg of 49.1 °C, indicating that a potential thermal property plateau is met within this crosslink density regime. Further evidencing this observation, P260-based formulation, coating E with a calculated crosslink density of 5.31 mmol g−1 resulted in a greatly increased Tg of 128.7 °C, with the glycerol-based coating F providing a nearly equivalent Tg (127.2 °C) despite a lower calculated crosslink density of 4.47 mmol g−1, potentially indicating a separate thermal plateau at a higher crosslink density regime.Effects of hydroxyl equivalent weight on formulation ratios
All coatings were applied at 2.7 wt% polyurethane and formulated at an isocyanate index of 1.4. Polyol components were applied as blends containing 10 wt% of triethanolamine (TEOA) as a reactive catalyst and crosslinker to promote coating cure kinetics. The differences in hydroxyl number (OH#) and hydroxyl equivalent weight (HEW) are shown in Table 2 (vide infra). As TEOA is added, the OH# of the blend increases significantly, and the hydroxyl equivalent weight of the isocyanate-reactive portion decreases. This in turn increases the weight percentage of PAPI™ 27 polyisocyanate which comprises the coating itself. For castor oil, the blend with TEOA has a calculated hydroxyl equivalent weight of 215 g mol−1, and the polyol constitutes 54 wt% of the polyurethane coating formulation. Comparatively, the TEOA blend with glycerol has a calculated hydroxyl equivalent weight of 32 g mol−1, where the polyol is 15 wt% of the polyurethane coating when applied. This has the effect of lessening the physical amount of material able to be applied to the granular urea (46-0-0) while maintaining the prescribed molar ratios. Aside from the molecular composition of the polyurethane, the critical need for a conformal coating to entirely encapsulate the highly soluble urea granule requires that a requisite minimum mass of both the polyol and isocyanate are added to physically coat the entire granule prior to the urethanization reaction.| Coating | Reagent | OH# | HEW | 10% TEOA blend OH# | Blend HEW | wt% iso | wt% poly |
|---|---|---|---|---|---|---|---|
| A | Castor | 165 | 340 | 261 | 215 | 46% | 54% |
| B | P1000 | 168 | 333 | 263 | 213 | 47% | 53% |
| C | P700 | 238 | 236 | 329 | 171 | 52% | 48% |
| D | P450 | 374 | 150 | 449 | 125 | 60% | 40% |
| E | P260 | 660 | 85 | 692 | 81 | 70% | 30% |
| F | Glycerol | 1829 | 31 | 1758 | 32 | 85% | 15% |
Controlled release performance
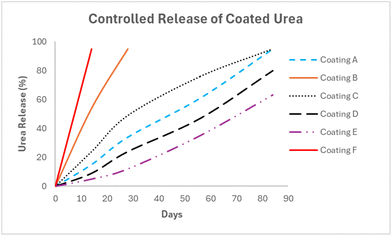
Once applied to the granular urea, the polymer is allowed to cure for 7 days prior to controlled release testing. Release is measured via refractive index (see Experimental) at predetermined times which are shown in Table 3 and represented graphically in Fig. 1.| Coating | Polyol | PU wt% | Wax wt% | Iso (index) | Urea release (%) | |||
|---|---|---|---|---|---|---|---|---|
| 14 d | 28 d | 56 d | 84 d | |||||
| A | Castor | 2.7 | 0.5 | 1.4 | 15 | 34 | 61 | >95 |
| B | P1000 | 2.7 | 0.5 | 1.4 | 54 | >95 | — | — |
| C | P700 | 2.7 | 0.5 | 1.4 | 24 | 49 | 76 | >95 |
| D | P450 | 2.7 | 0.5 | 1.4 | 9 | 24 | 47 | 80 |
| E | P260 | 2.7 | 0.5 | 1.4 | 5 | 12 | 35 | 63 |
| F | Glycerol | 2.7 | 0.5 | 1.4 | >95 | — | — | — |
Given its high usage25 in PU thermoset coating applications, a castor oil-based polyurethane formulation (coating A) was chosen to provide benchmark release performance. Coating A has a blend hydroxyl equivalent weight (HEW) of 215 g mol−1 with a calculated crosslink density of 2.24 mmol g−1. Coating A provided slow release over the measured period, notably only releasing 34% after 28 days and 61% after 56 days. Coating B was based on P1000, the 1000 g mol−1 poly(propylene oxide) triol, however, despite its similar blend HEW of 213 g mol−1 and higher calculated crosslink density of 2.45 mmol g−1, it released quickly, with 54% after 14 days, and >95% within 28 days. Coating C is generated using P700 and the blend has a HEW of 171 g mol−1 with calculated crosslink density of 2.75 mmol g−1. A suitable release profile was obtained with only 49% release after 28 days, and 76% release observed after 56 days. Seeking to increase the crosslink density of the polyurethane, coating D used P450 with a blend HEW of 125 g mol−1 and calculated crosslink density of 3.17 mmol g−1 and achieved excellent slowed release behavior with only 24% release after 28 days, 47% after 56 days, and only 80% release after 84 days. Similarly, increasing the crosslink density in coating E by using P260 provided a blend HEW of 81 g mol−1 and calculated crosslink density of 5.31 mmol g−1. Coating E provided remarkable slow release of 12% after 28 days, 35% after 56 days, and still only 63% urea release after 84 days. A further attempt to examine a lower blend HEW was performed in coating F where glycerol was used with 10 wt% TEOA to generate an isocyanate-reactive component with HEW of 32 g mol−1, and while the calculated crosslink density was 4.47 g mol−1, the coating showed no slow release performance with full (>95%) urea release prior to the first 14 days.
Analysis of release performance
There are notable differences in coating performance which require further analysis. Despite coating A and coating B using similar isocyanate-reactive blends (CastorversusP1000) with coating A having a 46 wt% proportion of pMDI and coating B having a 47 wt% proportion and Coating A having a lower calculated crosslink density, the large difference in observed release performance likely originates from structural differentiation. Ricinolein, as the primary component of castor oil has significant aliphatic content with a small relative number of hydrophilic functionalities, or hydroxyl endgroups, whereas P1000 contains a propylene oxide repeat unit which constitutes the backbone of the polyol architecture and therefore includes more hydrophilic groups. Thus, the highly hydrophobic structure of castor oil as the isocyanate-reactive component versus the P1000 may lead to the significantly slowed release behavior. However, potential differences in reactivity or cure profile during coating cannot be ruled out at this stage. Analysis of coatings B–E compare poly(propylene oxide) triol performance to crosslink density (Fig. 2).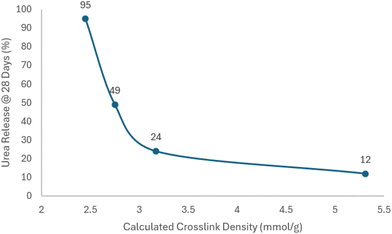 | ||
| Fig. 2 Graphical representation for poly(propylene oxide) triol based coatings B–E showing relationship between calculated crosslink density and urea release at 28 days. | ||
In this respect, there is a clear step change in performance going from coating B to coating C (>95% versus 49% urea release in 28 days). The blend HEW is decreased significantly and the calculated crosslink density was increased >10% from 2.45 to 2.75 mmol g−1. Also of note, as the isocyanate index of 1.4 was kept constant throughout the study, the proportion of pMDI was increased from 47 wt% in coating B to 52 wt% in coating C. These effects are seen as well in moving from coating C to coating D where the HEW is decreased and the calculated crosslink density is additionally increased by 15%. Additionally, coating D uses 60 wt% pMDI as part of its formulation, and its release profile only shows 24% release after 28 days, now with the feature of providing slowed release of 80% after 84 days. Coating E shows an exacerbated effect of decreased HEW and a 67% increased calculated crosslink density versus coating D going from 3.17 to 5.31 mmol g−1, with a 70 wt% portion of pMDI in the coating formulation. Surprisingly, coating E provided additional step-change slow-release performance with only 12% release after 28 days and 63% release after 84 days, indicating that a separate crosslink density regime provided access to differentiated release performance. Despite a clear relationship between crosslink density and release performance of the poly(propylene oxide) triols, no trendline equation was able to be applied such as to be suitably predictive. Coating F which relied upon a glycerol-based blend was comprised of 85 wt% pMDI but showed no controlled release performance. This lack of continued improvement in release, despite a heightened calculated crosslink density is likely due to the ratios in coating F where too little of the isocyanate-reactive blend was physically able to be applied to properly provide a uniform coating at 2.7 wt% polyurethane. Therefore, it is not clear for coating F whether the performance limitations lie in the chemistry or the set coating process itself. Further process modifications to optimize this glycerol-based formulation to achieve greater prill coverage were not explored. However, for the directly compared blends made with poly(propylene oxide) triols it may be that the hydrophobic effects brought by the increased pMDI inclusion needed at decreased HEW of the blend may foster greater hydrophobicity, assuming a conformal coating of the granule can be physically achieved.
Conclusions
In conclusion, polyurethane coatings were applied in layer-by-layer fashion with a wax additive to urea (46-0-0) granules to affect controlled release fertilizer (CRF) performance. Process and formulation conditions such as total coating wt%, TEOA inclusion, temperature, and isocyanate index were held constant to determine the structure–property relationship between crosslink density and controlled release of polyurethane films made from various poly(propylene oxide) triols of different molecular weights (260–1000 g mol−1). Castor oil was chosen in this study as a common isocyanate-reactive component to benchmark CRF performance and ensure that the poly(propylene oxide) triols were achieving suitable release under the given conditions. It was revealed that as hydroxyl equivalent weight (HEW) is decreased among the poly(propylene oxide) triols, that the commensurate proportion of polyisocyanate in the coating was increased as well as the calculated crosslink density, leading to slower controlled release, and greater performance of the coating. While thermal analysis evidenced a direct relationship between crosslink density regimes and glass transition temperature (Tg) of the polyurethane formulation, there was little relationship revealed between measured Tg of the formulation and controlled release performance of the coating. The glycerol-based blend, coating F, while bearing a theoretically high crosslink density and measured Tg similar to the high-performance coating E based on P260, likely did not have a high enough inclusion under the chosen coating process parameters to fully coat the granules prior to cure and therefore provided no release performance in the coating.Author contributions
Alex Kosanovich: conceptualization, data Curation, formal analysis, investigation, writing – original draft; supervision; Omar Jalife: investigation, methodology; Yi Fan: methodology, validation, writing – review and editing; Abhishek Shete: data curation, formal analysis; Yasmin Srivastava: resources, supervision, methodology, writing–review and editing.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5lp00257e.Acknowledgements
The authors would like to acknowledge The Dow Chemical Company, Polyurethanes Business for support of this study.References
- For a list of the UN Sustainability Development Goals see: https://www.ungeneva.org/en/about/topics/sustainable-development-goals.
- M. Kacprzak, Sewage slude as a source of organic to be used as soil improvement, in Water Management and Circular Economy, ed. M. G. Zamparas and G. L. Kyriakopoulos, Elsevier, 2023, ch. 13, pp. 303–316, ISBN 9780323952804 Search PubMed.
- L. Babcock-Jackson, T. Konovalova, J. P. Krogman, R. Bird and L. Lotti Díaz, J. Agric. Food Chem., 2023, 71, 8265–8296 CrossRef CAS PubMed.
- Fertilizers are often described by their NPK notation denoting the wt% composition of nitrogen, phosphorous, and potassium, respectively.
- A. M. Motasim, A. W. Samsuri, A. S. A. Sukor, A. Akter and A. M. Amin, Discov. Agric., 2024, 2, 56 CrossRef.
- For references to fertilizer overdosage and its effects see: https://pubs.extension.oregonstate.edu./pnw659/fertilizer-burn.
- N. S. Hina, Water, 2024, 16, 457 CrossRef CAS.
- D. Lawrencia, S. K. Wong, D. Y. S. Low, B. H. Goh, J. K. Goh, U. R. Ruktanonchai, A. Soottitantawat, L. H. Lee and S. Y. Tang, Plants, 2021, 10, 238 CrossRef CAS.
- (a) Y.-C. Yang, M. Zhang, Y. Li, X.-H. Fan and Y.-Q. Geng, J. Agric. Food. Chem., 2012, 60, 11229–11237 CrossRef CAS PubMed; (b) Y. Li, C. Jia, X. Zhang, Y. Jiang, M. Zhang, P. Lu and H. Chen, Prog. Org. Coat., 2018, 119, 50–56 CrossRef CAS; (c) K. Lubkowski, A. Smorowska, B. Grzmil and A. Kozlowska, J. Agric. Food Chem., 2015, 63, 2597–2605 CrossRef CAS PubMed.
- R. Bortoletto-Santos, C. Ribeiro and W. L. Polito, J. Appl. Polym. Sci., 2016, 133, 43790 CrossRef.
- L. Yuan, J. Zhi, C. Wang, J. Wang, L. Cao, Y. Yang, G. Zhao, C. Lu and X. Lu, Sci. Rep., 2024, 14, 20965 CrossRef CAS PubMed.
- E. Yousefi, M. Reza Ghadimi, S. Amirpoor and A. Dolati, Appl. Surf. Sci., 2018, 454, 201–209 CrossRef CAS.
- Z. Huang, R. S. Gurney, Y. Wang, W. Han, T. Wang and D. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 7488–7497 CrossRef CAS.
- Y. Yakabe, K. M. Henderson, W. C. Thompson, D. Pemberton, B. Tury and R. E. Bailey, Environ. Sci. Technol., 1999, 33, 2579–2583 CrossRef CAS.
- Y. N. Srivastava, A. J. Kosanovich, Y. Fan, D. Abebe, P. Boopalachandran, K. Pal and J. C. Medina, 2023, WO2023183236.
- N. Lakshani, H. S. Wijerathne, C. Sandaruwan, N. Kottegoda and V. Karunarathne, ACS Agric. Sci. Technol., 2023, 3, 939–956 CrossRef CAS.
- O. Jalife, A. J. Kosanovich and Y. N. Srivastava, 2024, WO2024263478.
- A. J. Kosanovich, P. Agarwal, J. C. Medina, Y. N. Srivastava, M. J. Cotanda Santapau and Y. Fan, 2025, US20250019321A1.
- H. Mansouri, H. A. Said, H. Noukrati, A. Oukarroum, H. B. Youcef and F. Perreault, Adv. Sustain. Syst., 2023, 7, 2300149 CrossRef CAS.
- H. Lu, Y. Chen, C. Dun, X. Hu, R. Wang, P. Cui, H. Zhang and H. Zhang, BMC Chem., 2025, 19, 154 CrossRef CAS.
- S. A. Umoren, M. M. Solomon and V. S. Saji, Polyethers, in Polymeric Materials in Corrosion Inhibition Fundamentals and Applications, Elsevier, 2022, ch. 17, pp. 399–417, ISBN 9780128238547 Search PubMed.
- U. Dorn and S. Enders, Fluid Phase Equilib., 2016, 424, 58–67 CrossRef CAS.
- (a) C. W. Macosko and D. R. Miller, Macromolecules, 1976, 9, 199 CrossRef CAS; (b) D. R. Miller and C. W. Macosko, Macromolecules, 1976, 9, 206 CrossRef CAS.
- M. Ionescu, D. Radojčić, X. Wan, M. L. Shrestha, Z. S. Petrović and T. A. Upshaw, Eur. Polym. J., 2016, 84, 736–749 CrossRef CAS.
- (a) S. S. Panda, B. P. Panda, S. K. Nayak and S. Mohanty, Polym.-Plast. Technol. Eng., 2018, 57, 500–522 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |