Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Batteries to the rescue: the formation of Pt bioelectrocatalysts with Shewanella oneidensis MR-1 and commercial batteries
Matei Tom
Iacob
 *a,
Adrian
Ghinea
b,
Ana-Maria
Moroşanu
b,
Ioan
Ardelean†
b,
Şerban N.
Stamatin
*a and
Cristina
Moisescu
b
*a,
Adrian
Ghinea
b,
Ana-Maria
Moroşanu
b,
Ioan
Ardelean†
b,
Şerban N.
Stamatin
*a and
Cristina
Moisescu
b
aFaculty of Physics, University of Bucharest, Atomistilor 405, 077125 Magurele, Ilfov, Romania. E-mail: tom.iacob@3nanosae.org
bInstitute of Biology Bucharest of the Romanian Academy, Spl. Independentei no. 296, sect. 6, 060031, Bucharest, Romania
First published on 8th October 2025
Abstract
Platinum nanoparticles are active towards many reactions relevant to energy and environmental applications. The biosynthesis of platinum nanoparticles from ionic platinum solutions needs an electron donor to yield nanoparticles. Molecular hydrogen and formate are the electron donors of choice for generating platinum nanoparticles and can be used as reducing agents without microorganisms. We hypothesized that the metal ions adsorbed on a microorganism surface can be transformed to nanoparticles in the presence of an electron source, even in the absence of an organic electron donor. To test the hypothesis, a bioelectrochemical setup was designed that allowed the connection of a commercial 1.5 V battery to a bioelectrode in a platinum solution. The platinum reduction efficiency of the battery-bioelectrode system was compared to experiments that involved (1) a battery and an abiotic electrode, and (2) a bioelectrode that was not connected to the battery. The reduction efficiency was determined by a combined study of optical emission spectroscopy and atomic emission spectroscopy. Platinum nanoparticles were revealed by transmission electron microscopy. The electrochemical performance was evaluated by cyclic voltammetry. Platinum nanoparticles were formed only when the bioelectrode was connected to the battery, evidenced by the well-known platinum cyclic voltammetry.
Introduction
Platinum (Pt) is a member of the platinum group of metals—a set of highly catalytically active metals that are crucial for many energy and environmental applications, such as fuel cells.1 The growing interest in platinum nanoparticles (NPs) for applications across various fields has highlighted concerns about the pollution associated with their chemical and physical syntheses, which often involve harmful, hazardous, energy-intensive, or costly chemicals.2–4 Therefore, there is currently a growing interest in nanoparticle biosynthesis with the help of microorganisms, a synthesis that requires easier and more ecological conditions.Reports on the biological synthesis of Pt NPs are scarce. The first emphasis on the reduction of Pt(IV) was aided by the use of sulfate-reducing bacteria from aqueous solution as well as from industrial wastewater.5–7 There are several reports on the biosynthesis of Pt NPs by diverse groups of microorganisms, such as Plectonema boryanum UTEX 485,8Calothrix sp.,9Desulfovibrio sp.,10Shewanella algae,11Shewanella putrefaciens,12Escherichia coli MC4100 (ref. 13) and halophilic bacteria.14,15
It is known that the genus Shewanella includes the largest number of species capable of interacting and synthesizing a large range of metallic nanoparticles. Shewanella oneidensis MR-1 is one of the most widely used and represents a model bacterium for the biosynthesis of nanoparticles. This group of dissimilatory metal-reducing bacteria has an extracellular electron transfer mechanism through which they can transfer electrons into the surrounding environment. This capability enables the extracellular reduction of metals, which provides a great advantage in the bio-fabrication of abiotic nanomaterials, including metal nanoparticles. To date, only three accounts report the biosynthesis of Pt NPs by Shewanella oneidensis MR-1.16–18 The genus Shewanella includes a large number of bacterial species with great respiratory versatility. The best known and most commonly used strain is Shewanella oneidensis MR-1, the representative strain of the genus due to its ability to use the widest variety of electron acceptors, such as oxygen, nitrates, sulfur compounds, organic matter, and most importantly for the present study, metals.19 In addition, S. oneidensis MR-1 is one of the most electroactive bacteria known, and can discharge and receive electrons from electrodes in bioelectrochemical systems, recommending it as the first choice for our attempt to biosynthesize Pt nanoparticles.
The type of electron donor can significantly affect the formation of NPs by bacterial cells. The most common source of electron donors for the bioformation of Pt NPs with S. oneidensis MR-1 is molecular hydrogen (H2), formate and lactate.16,20,21 Formate and H2 are the most efficient and most commonly utilized electron donors, but they are known to abiotically reduce palladium ions in the absence of bacteria.22,23 A similar formate or H2 pathway can reduce Pt as well. Lactate is relatively inert and can be used to reduce Pt(IV) to Pt NPs, although the yield is significantly lower.16 The challenging conclusion is that Pt NPs can be biosynthesized while H2 and formate are known as Pt(IV) reducing agents. To emphasize the ability of S. oneidensis MR-1 to biosynthesize Pt NPs, one needs an experimental setup in which Pt reducing agents are absent. Using an abiotic source of electrons, such as batteries, could be a promising alternative.
Herein, we propose a bioelectrochemical solution to form bioelectrodes that are composed of microorganisms decorated with Pt NPs (Fig. S1). To demonstrate the benefit of our method, we chose the simple, yet insufficiently studied system: Shewanella oneidensis MR-1 and Pt NPs. Fig. S1 shows the experimental setup and illustrates the (bio)electrode formed in the current study. To the best of our knowledge, this is the first work that couples Shewanella oneidensis MR-1 to a battery in order to form a bioelectrode.
Materials and methods
Materials
Chemicals used in this work without further purification were: sodium chloride (98%, VWR Chemicals), potassium chloride (97%, VWR Chemicals), disodium hydrogen phosphate (98%, VWR Chemicals), potassium dihydrogen phosphate (98%, VWR Chemicals), sodium bicarbonate (98%, Chimreactiv S.R.L, Romania), dihydrogen hexachloroplatinate(IV) hydrate (99.9%, Alfa Aesar, Germany), sodium pyrophosphate (98%, Alfa Aesar, Germany), Tween 20 (Millipore Sigma; formerly Sigma-Aldrich, Switzerland), ethanol (96%, VWR Chemicals), glutaraldehyde (25%, VWR Chemicals), and 2,3,5-triphenyltetrazolium chloride (VWR Chemicals, Belgium). Deionized (DI) water with a resistivity of 18.2 MΩ cm was obtained from a LaboStar PRO DI 4 water purification system (Evoqua Water Technologies, USA) and used in all the experiments.Formation of bioelectrodes
Shewanella oneidensis MR-1 (LMG 19005) was purchased from the BCCM/LMG Bacterium Collection (Gent, Belgium). Biofilms of S. oneidensis MR-1 on Ti were obtained according to a previously described procedure.24 A culture vessel with 100 mL of Luria–Bertani (LB) broth25 was inoculated with S. oneidensis MR-1 seed culture (1% v/v) and incubated under aerobic conditions (at 30 °C). After 24 h, a sterile titanium electrode (area = 1 × 4 cm2) was placed inside the culture vessel and incubated for another 72 h (at 30 °C, without stirring). The electrode with the attached biofilm was removed from the broth, washed thoroughly with sterile sodium bicarbonate buffer (30 mM), and then fixed into the cap of a 15 mL tube together with a sterile abiotic titanium electrode that worked as a counter electrode.Three experimental setups were tested in this work (Fig. S1). B-Pt was an abiotic setup consisting of a titanium electrode connected to the battery (1.5 V AA-type battery, Varta, Germany) at constant voltage (the current was measured between 10 and 40 μA). S-Pt was a biotic setup composed of a titanium electrode with an S. oneidensis MR-1 biofilm that was not connected to a battery. SB-Pt was a titanium electrode with an S. oneidensis MR-1 biofilm that was connected to a battery. Each sample was analysed in triplicate. A blank for the SB-Pt sample contained a titanium electrode with a heat-degraded S. oneidensis MR-1 biofilm (121 °C, 20 min) and connected to the battery. All setups were immersed in sodium bicarbonate buffer with 1 mM H2PtCl6 and incubated at 30 °C for at least 3 days. The biofilm and abiotic electrodes were connected to the negative and positive terminals of the battery, respectively. No other electron donors were used in this study.
The entire setup was placed inside an airtight anaerobic GENbag (bioMérieux, France) to ensure anaerobic conditions for NP synthesis. The GENbag had been previously flushed with N2. To ensure the removal of any residual oxygen left inside the plastic bag, an anaerobic generator sachet was added to the GENbag.
Characterization
A UV-vis spectrometer (Jasco V550, Tokyo, Japan) was used to identify the platinum complex in the final solution. Elemental analysis was used to determine the concentration of platinum according to a modified protocol.26 ICP-OES was performed on a Perkin Elmer, Avio 200 (Waltham, MA, USA). A 2 mL aliquot was removed from each sample and digested in 12 mL of aqua regia in a microwave reactor (Milestone Microwave Labstation, Bergamo, Italy). The program consisted of a heating step to 180 °C (16 °C min−1) and maintenance at 180 °C for 30 minutes. After mineralization, the volume was brought up to 27 mL. The final solution was diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and analysed by ICP-OES. Every sample was measured 3 times, and values were reported as averages with error bars (= 95% confidence interval).
10) and analysed by ICP-OES. Every sample was measured 3 times, and values were reported as averages with error bars (= 95% confidence interval).
Titanium electrodes with bacterial biofilms (S-Pt and SB-Pt) were washed with sterile phosphate-buffered saline (PBS)27 and fixed overnight with 2.5% glutaraldehyde in 0.1 M phosphate buffer (4 °C). The electrodes were immersed in a detachment solution to separate bacteria from the electrode. A modified protocol was used for biofilm detachment.28 The electrodes were treated for 10 minutes in an ultrasound bath (SONOREX™ Digital 10 P Ultrasonic bath, Bandelin Electronics, Germany) in a solution of sodium pyrophosphate (3 mM) and Tween 20 (10 μg mL−1). The detached bacteria were recovered by centrifugation (7000 rpm, 10 min, Thermo Fisher, Germany) and dehydrated through an ascending alcohol series (10%, 50%, 70%, 80%, 90%, 2 × 100%), with each change lasting 20 minutes. After each change, the cells were recovered by centrifugation (7000 rpm, 10 min). Shortly before TEM observations, one drop of cell suspension was placed onto a Formvar-coated Cu TEM grid (MilliporeSigma; formerly Sigma-Aldrich, USA) and left to air dry at room temperature. No additional staining (i.e., contrast enhancement) was used. The samples were imaged using a JEOL JEM-1400 (Jeol, Japan) transmission electron microscope operated at 80 kV accelerating voltage and visualized with a Quemesa CCD camera (Olympus Soft Imaging Solutions).
Cell viability
Biofilm viability was determined using the 2,3,5-triphenyltetrazolium chloride (TTC) staining method and acetone as extractant.28,29 A sterile 1% (w/v) TTC stock solution was prepared by dissolving the powder in sterile distilled water and filtered using a 0.22 μm PES sterile syringe filter (Labbox Labware S.L., Spain). To rinse the attached biofilm and remove unbound cells, S-Pt and SB-Pt were washed with sterile PBS (pH 7.4). Samples were incubated at 30 °C under aerobic conditions in a solution of LB broth and TTC (0.02% w/v). The bioelectrodes were removed after 24 h and washed in 15 mL of sterile PBS of pH 7.4. Samples were placed for 10 min in 8.5 mL of acetone to dissolve the formazan crystals bound to the bioelectrode. The absorption at 485 nm (A485) was measured with a Specord 210 Plus spectrophotometer (Analytik Jena, Germany).Electrochemical characterization
Electrochemical characterization was carried out in a 2-compartment electrochemical cell in a 3-electrode configuration. A saturated calomel electrode (SCE) was used as a reference electrode with a measured standard potential of 0.24 V vs. a dynamic hydrogen electrode (Hydroflex, Gaskatel, Germany). All potentials are referenced against SCE unless otherwise stated. A piece of carbon felt (approx. 10 cm2) was used as a counter electrode. The working electrodes were the as-obtained samples without further treatment. 0.5 M NaHCO3 bicarbonate solution was used as the supporting electrolyte throughout the experiment. Cyclic voltammograms were recorded from −0.8 to 1 V vs. SCE at 0.02 V s−1 on a Voltalab PGZ-301.Results
Bioreduction efficiency
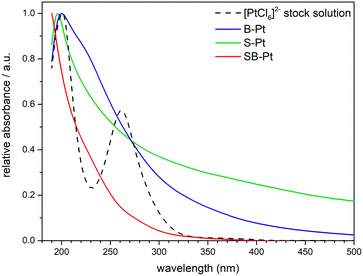
UV-vis spectra were collected from the solution at the end of the experiment and compared to the initial stock solution containing 1 mM [PtCl6]2− (prepared from H2PtCl6) (Fig. 1). The Pt4+ in [PtCl6]2− needs 4 electrons to generate a Pt nanoparticle with a subsequent 2-electron reduction to a Pt2+ intermediate. [PtCl6]2− shows the well-known peaks at approx. 210 and 270 nm.30 A reduction in the absorbance at 270 nm or a shift towards smaller wavelengths is the usual method to confirm the formation of a Pt nanoparticle via 4-electron or partial 2-electron reduction, respectively. The abiotic sample (i.e., B-Pt) showed at least 3 features: at 210, 230 and 270 nm (Fig. S2). The peak at 230 nm was previously assigned to Pt2+ species in nanoparticle formation from [PtCl6]2−. Pt2+ can be ascribed to the partial (2-electron reduction) of [PtCl6]2−. S-Pt showed the presence of the peak at 210 nm, while the other features were hard to identify due to a complex background. The peak at 270 nm was absent for SB-Pt. There is a clear difference in the background between S-Pt and SB-Ptm, which makes the UV-vis analysis challenging.To understand the reduction mechanism of [PtCl6]2− by S-Pt and SB-Pt, the remaining liquid sample after each experiment was further characterized by ICP-OES (Fig. 2). The dotted and solid lines show 0% and 100% reduction efficiency, respectively (Fig. 2). Reduction efficiency was calculated using eqn (1):
 | (1) |
 | ||
| Fig. 3 Bright-field TEM images of S-Pt (A and B) and SB-Pt (C and D) samples with 2000 nm (A and C) and 500 nm (B and D) scale bar. | ||
Transmission electron microscopy (TEM) investigations
Fig. 3 shows the TEM investigation of S-Pt and SB-Pt. S. oneidensis MR-1 had the well-known appearance of round-shaped rods (blue arrows) with a length of 2–3 μm (Fig. 3A and B). Pt NPs should appear as black dots on the microorganism surface (red circles). Fig. 3A and B show that S-Pt does not generate NPs, which is in line with the UV-vis analysis (Fig. 1). Black dots appear on the surface of the microorganisms in SB-Pt (red circles in Fig. 3C and D). The black dots tend to agglomerate in larger structures (yellow circle, Fig. 3C). In the absence of any other metallic species, it can be concluded that the black dots were Pt NPs. It is challenging to estimate the dimensions of the metal NPs, although analysis of the TEM images indicates diameters below 10 nm (Fig. 3D). Further nanoparticle characterization falls beyond the general scope of the work at hand. Pt NPs were present mainly on the microorganism surface. One may suggest that the nanoparticles were produced by S. oneidensis MR-1 only when connected to the battery (SB-Pt).Cell viability
Exposure of a thin bacterial biofilm to high concentrations of Pt for long periods of time has the potential to adversely affect the biofilm. Additional physiological stress, such as platinum toxicity, influences the efficiency of the Pt NP biosynthesis. It is thus important that the viability of the bacterial biofilm be preserved. The metabolic status of S. oneidensis MR-1 biofilms during incubation in bicarbonate buffer with 1 mM [PtCl6]2− was investigated through the 2,3,5-triphenyltetrazolium chloride (TTC) staining method.28 Unlike other staining methods, the tetrazolium salts assay does not stain different biological molecules within the biofilm (i.e., polysaccharides or DNA, proteins) but measures the metabolic activity of each bacterial cell, making it possible to distinguish between metabolically active cells, referred to as viable cells, and inactive cells.28,30–33 In the presence of bacteria, TTC is reduced to 1,3,5-triphenylformazan (TFP)34,35 an intracellularly formed insoluble red crystal. This enables the staining and counting of adhered, metabolically active cells. The formation of red formazan represents a qualitative indication of bacterial activity, whereas measurement of colour intensity for the resuspended red formazan, using the UV-vis absorbance at 485 nm, could provide quantitative evidence of the impact of toxic metals on a bacterial population.The aim of this investigation was to measure the ability of S. oneidensis MR-1 cells grown as biofilms on titanium electrodes to maintain an endogenous metabolism in the absence of any organic source of electrons added into the suspension buffer, measured as the ability to reduce artificial electron acceptors such as TTC.36 These measurements were made before and after biofilm incubation in the bicarbonate buffer with [PtCl6]2−.
Biofilm viability was determined by measuring the absorbance at 485 nm at the beginning of the experiment (BOE) and end of the experiment (EOE) of a formazan solution. The reader should bear in mind that this is a surface-sensitive reaction (i.e., only the microorganisms in direct contact with formazan can be evaluated). The viability of the S. oneidensis MR-1 biofilm was considered to be 100% at BOE. After exposing the cells for 72 h to 1 mM Pt, with no additional carbon source, the viability of the biofilm decreased to (33 ± 6)% at EOE. Even though the biofilm was clearly affected, S. oneidensis MR-1 cells were able to cope with the difficult conditions, maintaining a viable population until the end of the experiment. The viability assessment is directly correlated with the formation of Pt NPs, as no Pt reduction was detected in the experiments with heat-degraded biofilm, demonstrating the involvement of active bacterial cells in the synthesis of biologically mediated Pt NPs.
Electrochemical characterization
Fig. 4 shows the electrochemical characterization of the samples towards water splitting. It is well known that the typical Pt CV has a butterfly appearance, which can be divided into hydrogen (at potentials smaller than −0.2 V vs. SCE) and oxygen (at potentials larger than 0 V vs. SCE) regions.37 Positive currents are specific for oxidation reactions, such as hydrogen oxidation (at potentials smaller than −0.2 V vs. SCE) and water oxidation (at potentials larger than 0 V vs. SCE). Negative currents are specific for reduction reactions, such as proton reduction to hydrogen (at potentials smaller than −0.6 V vs. SCE) and the oxygen reduction reaction (at potentials larger than −0.6 V vs. SCE). | ||
| Fig. 4 Cyclic voltammograms recorded at room temperature in 0.5 M NaHCO3 (pH = 6.8) at 0.02 V s−1 from −0.8 to 1 V vs. SCE. | ||
Pt electrochemical features were absent from the S-Pt CV (Fig. 4). The current in Fig. 4 for S-Pt is multiplied by a factor of 20 to allow comparison among all samples. The double-charge layer in S-Pt is considerably smaller than that in SB-Pt. A similar biofilm was expected to grow on both samples, S-Pt and SB-Pt, in stark contrast to the electrochemical results. The proton reduction to hydrogen and minor oxygen evolution at potentials larger than 0.6 V vs. SCE suggests that the underlying Ti plate may be exposed. The CV for the abiotic electrode, B-Pt, is presented in Fig. S4.
The smallest double-layer charge was observed for B-Pt, which can be explained by the thin film structure usually resulting from metal electrodeposition.38 The anodic and cathodic sweeps are widely separated, indicating a large double-layer charge. Usually, a large double-layer charge is specific to electrodes with high roughness. Microorganisms forming the biofilm can lead to a large double-layer charge. SB-Pt showed the sum of the contributions of the Pt nanoparticles and microorganisms.
Discussion
There has been a surge of interest in the biosynthesis of noble metal nanoparticles, although the actual details of NP bioformation remain elusive.39 There are two components at play: (1) biosorption of the metal ion of choice on the surface of the microorganism and (2) the formation of the metallic electronic band by the reduction of ionic species. The Pt ion in the current study is Pt4+ (i.e., [PtCl4]2−), which is known to be more challenging to reduce than [PtCl6]2–.39 [PtCl6]2− has been shown to have a lower sorption efficiency on S. oneidensis than [PtCl6]2–.16 Indeed, our results suggest that S. oneidensis alone (i.e., without the addition of electron donors) does not produce Pt NPs from [PtCl6]2− (S-Pt in Fig. 1–3). In our setup (Fig. S1), microorganisms were restricted only to the available area of the Ti electrode, which is a key difference from bioreactors under continuous stirring. Therefore, it was challenging to measure the sorption of [PtCl6]2− and compare it to previously reported values.16However, SB-Pt produces nanoparticles at (72 ± 5)% (Fig. 2) in the absence of in situ electron donors, such as H2, formate, or lactate. Indeed, it is challenging to confirm the exact mechanism for Pt electroreduction. However, it can be concluded that Pt nanoparticles were formed only when an external electron source was connected.
TEM investigations (Fig. 3) showed that only SB-Pt generates NPs. It was believed that the biosynthesis of metallic nanoparticles would exhibit increased selectivity, which has been disproved.13 Usually, the biosynthesis of metal nanoparticles is conducted in bioreactors where the microorganisms are stirred in a broth in the presence of an in situ electron donor, such as H2, formate, or lactate.16,40 Other studies have focused on Escherichia coli, dissolving the microorganism in NaOH to obtain electrical conductivity.13 Our battery-bioelectrode system, demonstrated here with S. oneidensis and Pt, is expected to be adaptable to other metal precursors, such as Pd(II), based on established S. oneidensis-mediated syntheses of Pd and Pd–Pt alloys.17–19,38 Additionally, other Shewanella species are known to reduce metals, including Au(III), Ag(I), Cr(VI), and Cu(II),40,41 suggesting that future studies could explore these alternatives to enhance the system's sustainability and expand its applications in bioelectrochemical technologies.
Conclusions
A new bioelectrochemical setup was designed for the formation of bioelectrocatalysts. A battery was connected to a preformed bioelectrode in a Pt solution. Nanoparticles were biosynthesized on the microorganism surface in the absence of in situ electron donors only when it was connected to the battery. The Pt bioelectrocatalyst was electrochemically compared to an abiotic electrode (without microorganisms) and a biotic electrode (without a battery). Electrochemical results showed that the Pt bioelectrocatalyst has the potential to be used as a biocathode where electrons can be exchanged between the microorganism and the abiotic electrocatalyst. We have put forward a simple and affordable solution to construct an active biocathode formed from Pt-decorated microorganisms.Author contributions
M. T. I and A. G contributed equally; investigation – M. T. I, A. G. and A.-M. M; formal analysis – I. A., S. N. S and C. M; data curation – S. N. S; writing (original draft) – M. T. I., A. G., I. A., S. N. S and C. M.; writing review and editing – S. N. S. and C. M.; funding acquisition – I. A. and S. N. S; supervision – I. A., S. N. S. and C. M.Conflicts of interest
There are no conflicts to declare.Data availability
Data can be obtained via request from E-mail: tom.iacob@3nanosae.org; . The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5lf00223k.Acknowledgements
This work was funded by the contract 76PCCDI/2018, Project “Eco-innovative technologies for recovering platinum group metals from used catalytic converters” (ECOTECH-GMP) and project no. RO1567-IBB05/2025 from the Institute of Biology Bucharest of Romanian Academy.Notes and references
- H. A. Gasteiger, S. S. Kocha, B. Sompalli and F. T. Wagner, Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs, Appl. Catal., B, 2005, 56(1–2), 9–35, DOI:10.1016/j.apcatb.2004.06.021.
- Y. Govender, T. Riddin, M. Gericke and C. G. Whiteley, Bioreduction of platinum salts into nanoparticles: A mechanistic perspective, Biotechnol. Lett., 2009, 31(1), 95–100, DOI:10.1007/s10529-008-9825-z.
- T. Herricks, J. Chen and Y. Xia, Polyol Synthesis of Platinum Nanoparticles: Control of Morphology with Sodium Nitrate, Nano Lett., 2004, 4(12), 2367–2371, DOI:10.1021/nl048570a.
- A. Syed and A. Ahmad, Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum, Colloids Surf., B, 2012, 97, 27–31, DOI:10.1016/j.colsurfb.2012.03.026.
- K. J. Rashamuse, C. C. Z. Mutambanengwe and C. G. Whiteley, Enzymatic recovery of platinum (IV) from industrial wastewater using a biosulphidogenic hydrogenase, Afr. J. Biotechnol., 2008, 7(8), 1087–1095, DOI:10.5897/AJB08.085.
- K. J. Rashamuse and C. G. Whiteley, Bioreduction of Pt (IV) from aqueous solution using sulphate-reducing bacteria, Appl. Microbiol. Biotechnol., 2007, 75(6), 1429–1435, DOI:10.1007/s00253-007-0963-3.
- T. Riddin, M. Gericke and C. G. Whiteley, Biological synthesis of platinum nanoparticles: Effect of initial metal concentration, Enzyme Microb. Technol., 2010, 46(6), 501–505, DOI:10.1016/j.enzmictec.2010.02.006.
- M. F. Lengke, M. E. Fleet and G. Southam, Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial Biomass with a palladium(II) chloride complex, Langmuir, 2007, 23(17), 8982–8987, DOI:10.1021/la7012446.
- R. Brayner, H. Barberousse, M. Hemadi, C. Djedjat, C. Yéprémian, T. Coradin, J. Livage, F. Fiévet and A. Couté, Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route, J. Nanosci. Nanotechnol., 2007, 7(8), 2696–2708, DOI:10.1166/jnn.2007.600.
- M. Martins, C. Mourato, S. Sanches, J. P. Noronha, M. T. B. Crespo and I. A. C. Pereira, Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds, Water Res., 2017, 108, 160–168, DOI:10.1016/j.watres.2016.10.071.
- Y. Konishi, K. Ohno, N. Saitoh, T. Nomura, S. Nagamine, H. Hishida, Y. Takahashi and T. Uruga, Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae, J. Biotechnol., 2007, 128(3), 648–653, DOI:10.1016/j.jbiotec.2006.11.014.
- K. Tanaka and N. Watanabe, Study on the coordination structure of Pt sorbed on bacterial cells using X-ray absorption fine structure spectroscopy, PLoS One, 2015, 10(5), 1–12, DOI:10.1371/journal.pone.0127417.
- G. Attard, M. Casadesús, L. E. MacAskie and K. Deplanche, Biosynthesis of platinum nanoparticles by Escherichia coli MC4100: Can such nanoparticles exhibit intrinsic surface enantioselectivity?, Langmuir, 2012, 28(11), 5267–5274, DOI:10.1021/la204495z.
- S. Maes, M. Claus, K. Verbeken, E. Wallaert, R. de Smet, F. Vanhaecke, N. Boon and T. Hennebel, Platinum recovery from industrial process streams by halophilic bacteria: Influence of salt species and platinum speciation, Water Res., 2016, 105, 436–443, DOI:10.1016/j.watres.2016.09.023.
- S. Maes, R. Props, J. P. Fitts, R. de Smet, R. Vilchez-Vargas, M. Vital, D. H. Pieper, F. Vanhaecke, N. Boon and T. Hennebel, Platinum Recovery from Synthetic Extreme Environments by Halophilic Bacteria, Environ. Sci. Technol., 2016, 50(5), 2619–2626, DOI:10.1021/acs.est.5b05355.
- S. Maes, R. Props, J. P. Fitts, R. de Smet, F. Vanhaecke, N. Boon and T. Hennebel, Biological recovery of platinum complexes from diluted aqueous streams by axenic cultures, PLoS One, 2017, 12(1), 1–17, DOI:10.1371/journal.pone.0169093.
- Y. Tuo, G. Liu, B. Dong, Y. Yu, C. Liu, Y. Zhao and J. Zhang, Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol, Environ. Sci. Pollut. Res., 2017, 24, 5249–5258, DOI:10.1007/s11356-016-8276-7.
- (a) H. Xu, Y. Xiao, M. Xu, H. Cui, L. Tan, N. Feng, X. Liu, G. Qiu, H. Dong and J. Xie, Microbial synthesis of Pd–Pt alloy nanoparticles using Shewanella oneidensis MR-1 with enhanced catalytic activity for nitrophenol and azo dyes reduction, Nanotechnology, 2019, 30(6), 065607, DOI:10.1088/1361-6528/aaf2a6Ikeda; (b) S. Iacob, Y. Takamatsu, M. Tsuchiya, K. Suga, Y. Tanaka, A. Kouzuma and K. Watanabe, Shewanella oneidensis MR-1 as a bacterial platform for electro-biotechnology, Essays Biochem., 2021, 65(2), 355–364, DOI:10.1042/EBC20200178.
- W. de Windt, P. Aelterman and W. Verstraete, Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls, Environ. Microbiol., 2005, 7(3), 314–325, DOI:10.1111/j.1462-2920.2004.00696.x.
- V. D. Rajput, T. Minkina, R. L. Kimber, V. K. Singh, S. Shende, A. Behal, S. Sushkova, S. Mandzhieva and J. R. Lloyd, Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella, Appl. Environ. Microbiol., 2021, 87(22), e0139021, DOI:10.1128/AEM.01390-21.
- O. Yishai, S. N. Lindner, J. Gonzalez de la Cruz, H. Tenenboim and A. Bar-Even, The formate bio-economy, Curr. Opin. Chem. Biol., 2016, 35, 1–9, DOI:10.1016/j.cbpa.2016.07.005.
- C. Zhou, A. Ontiveros-Valencia, Z. Wang, J. Maldonado, H. P. Zhao, R. Krajmalnik-Brown and B. E. Rittmann, Palladium Recovery in a H2-Based Membrane Biofilm Reactor: Formation of Pd(0) Nanoparticles through Enzymatic and Autocatalytic Reductions, Environ. Sci. Technol., 2016, 50(5), 2546–2555, DOI:10.1021/acs.est.5b05318.
- G. Gallegos Ortega, V. E. Reyes Cruz, G. Urbano Reyes, D. Manzano Arredonda, M. A. Veloz Rodríguez, A. Trujillo Estrada, M. Pérez Labra and J. A. Cobos Murcia, Biofilm formation on Titanium and Titanium Oxide and its Characterization and Electrochemical Properties, Int. J. Electrochem. Sci., 2019, 14, 10162–10175, DOI:10.20964/2019.11.05.
- J. Sambrook, E. F. Fritsch and T. Maniatis, Molecular Cloning: a Laboratory Manual, Cold Spring Harbor Laboratory, 2nd edn, 1989 Search PubMed.
- E. Vasile, A. Ciocanea, V. Ionescu, I. Lepadatu, C. Diac and S. N. Stamatin, Making precious metals cheap: A sonoelectrochemical – Hydrodynamic cavitation method to recycle platinum group metals from spent automotive catalysts, Ultrason. Sonochem., 2021, 72, 105404, DOI:10.1016/j.ultsonch.2020.105404.
- B. Chazotte, Labeling Golgi with Fluorescent Ceramides, Cold Spring Harb. Protoc., 2012, 2012(8), pdb.prot070599, DOI:10.1101/pdb.prot070599.
- C. M. Araya, A. Cazorla and I. Reche, Detachment Procedure of Bacteria from Atmospheric Particles for Flow-cytometry Counting, Bio-Protoc., 2019, 9(12), e3273, DOI:10.21769/BioProtoc.3273.
- H. L. Brown, A. H. M. van Vliet, R. P. Betts and M. Reuter, Tetrazolium reduction allows assessment of biofilm formation by Campylobacter jejuni in a food matrix model, J. Appl. Microbiol., 2013, 115(5), 1212–1221, DOI:10.1111/jam.12316.
- C. F. Nørgaard, S. N. Stamatin and E. M. Skou, Redeposition of electrochemically dissolved platinum as nanoparticles on carbon, Int. J. Hydrogen Energy, 2014, 39(30), 17322–17326, DOI:10.1016/j.ijhydene.2014.08.054.
- R. P. Tengerdy, J. G. Nagy and B. Martin, Quantitative measurement of bacterial growth by the reduction of tetrazolium salts, Appl. Microbiol., 1967, 15(4), 954–955, DOI:10.1128/am.15.4.954-955.1967.
- G. Schaule, H. C. Flemming and H. F. Ridgway, Use of 5-cyano-2,3-ditolyl tetrazolium chloride for quantifying planktonic and sessile respiring bacteria in drinking water, Appl. Environ. Microbiol., 1993, 59(11), 3850–3857, DOI:10.1128/aem.59.11.3850-3857.1993.
- E. A. Trafny, R. Lewandowski, I. Zawistowska-Marciniak and M. Stepińska, Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metalworking fluids, World J. Microbiol. Biotechnol., 2013, 29(9), 1635–1643, DOI:10.1007/s11274-013-1326-0.
- M. Bačkor and D. Fahselt, Tetrazolium reduction as an indicator of environmental stress in lichens and isolated bionts, Environ. Exp. Bot., 2005, 53(2), 125–133, DOI:10.1016/j.envexpbot.2004.03.007.
- E. T. M. Berends, A. R. Horswill, N. M. Haste, M. Monestier, V. Nizet and M. von Köckritz-Blickwede, Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps, J. Innate Immun., 2010, 2(6), 576–586, DOI:10.1159/000319909.
- Y. Xiao, E. Zhang, J. Zhang, Y. Dai, Z. Yang, H. E. M. Christensen, J. Ulstrup and F. Zhao, Extracellular polymeric substances are transient media for microbial extracellular electron transfer, Sci. Adv., 2017, 3(7), 1–9, DOI:10.1126/sciadv.1700623.
- C. Diac, F. I. Maxim, R. Tirca, A. Ciocanea, V. Filip, E. Vasile and S. N. Stamatin, Electrochemical recycling of platinum group metals from spent catalytic converters, Metals, 2020, 10(6), 1–11, DOI:10.3390/met10060822.
- P. N. Bartlett, R. P. Birkin and M. A. Ghanem, Electrochemical deposition of macroporous platinum, palladium and cobalt films using polystyrene latex sphere templates, Chem. Commun., 2000, 1671–1672, 10.1039/B004398M.
- K. B. Tan, D. Sun, J. Huang, T. Odoom-Wubah and Q. Li, State of arts on the bio-synthesis of noble metal nanoparticles and their biological application, Chin. J. Chem. Eng., 2021, 30, 272–290, DOI:10.1016/j.cjche.2020.11.010.
- A. J. Stephen, N. v. Rees, I. Mikheenko and L. E. Macaskie, Platinum and palladium bio-synthesized nanoparticles as sustainable fuel cell catalysts, Front. Energy Res., 2019, 7, 1–13, DOI:10.3389/fenrg.2019.00066.
- R. Wu, L. Cui, L. Chen, C. Wang, C. Cao, G. Sheng, H. Yu and F. Zhao, Effects of Bio-Au Nanoparticles on Electrochemical Activity of Shewanella oneidensis Wild Type and ΔomcA/mtrC Mutant, Sci. Rep., 2013, 3307, DOI:10.1038/srep03307.
- V. D. Rajput, T. Minkina, R. L. Kimber, V. K. Singh, S. Shende, A. Behal, S. Sushkova, S. Mandzhieva and J. R. Lloyd, Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella, Appl. Environ. Microbiol., 2021, 87, e01390-21, DOI:10.1128/AEM.01390-21.
Footnote |
| † We dedicate this work to the memory of Ioan I. Ardelean, who passed away before the completion of this manuscript. |
| This journal is © The Royal Society of Chemistry 2026 |