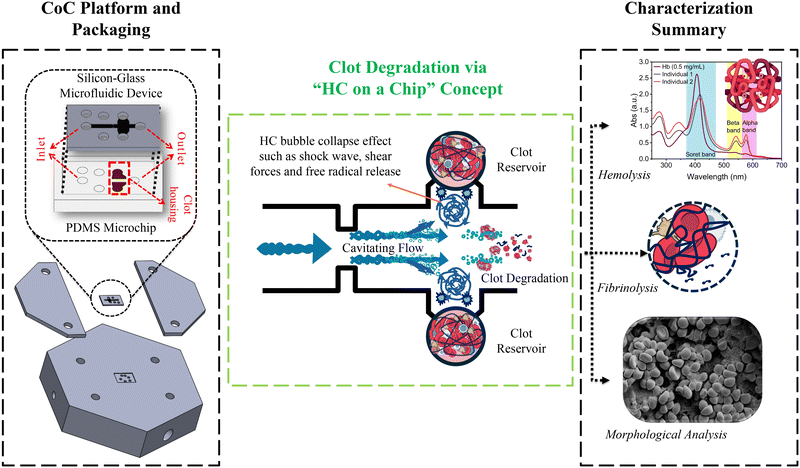
Thrombolytic potential of the “hydrodynamic cavitation on a chip” concept: insights into clot degradation
Abuzer Alp
Yetisgin†
 ab,
Beyzanur
Ozogul†
ab,
Beyzanur
Ozogul†
 ab,
Unal
Akar
ab,
Rabia
Mercimek
ab,
Unal
Akar
ab,
Rabia
Mercimek
 ab,
Seyedali Seyedmirzaei
Sarraf
d,
Tugrul
Elverdi
e,
Ehsan
Amani
f,
Dmitry
Grishenkov
g,
Ali
Koşar
ab,
Seyedali Seyedmirzaei
Sarraf
d,
Tugrul
Elverdi
e,
Ehsan
Amani
f,
Dmitry
Grishenkov
g,
Ali
Koşar
 *a and
Morteza
Ghorbani
*a and
Morteza
Ghorbani
 *abc
*abc
aFaculty of Engineering and Natural Sciences, Sabanci University, 34956 Tuzla, Istanbul, Turkey. E-mail: kosara@sabanciuniv.edu; mghorbani@sabanciuniv.edu
bSabanci University Nanotechnology Research and Application Center, 34956 Tuzla, Istanbul, Turkey
cCenter of Excellence for Functional Surfaces and Interfaces for Nano-Diagnostics (EFSUN), Sabanci University, Orhanli, 34956, Tuzla, Istanbul, Turkey
dDepartment of Mechanical Engineering and Mechanics, Lehigh University, Bethlehem, PA 18015, USA
eDivision of Hematology, Department of Internal Medicine, Cerrahpasa Faculty of Medicine, Istanbul University-Cerrahpasa, Istanbul, Turkey
fDepartment of Mechanical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Iran
gDepartment of Biomedical Engineering and Health Systems, KTH Royal Institute of Technology, SE-141 57 Stockholm, Sweden
First published on 2nd October 2025
Abstract
Thrombolysis is essential for treating vascular conditions such as pulmonary embolism and deep vein thrombosis, yet current thrombolytic drug-based approaches have notable limitations in efficacy and safety. Hydrodynamic cavitation (HC) offers drug-free clot degradation through mechanical disruption. In this study, the effects of HC exposure on thrombolysis were investigated using a clot-on-a-chip (CoC) platform. In this regard, the thrombolytic potential of HC exposure was evaluated by analyses involving hemolysis and fibrinolysis. Furthermore, the results were compared with acoustic cavitation (AC), a widely studied alternative. According to the obtained results, HC exposure (482 kPa, 120 s) resulted in 12.1% released hemoglobin and a 53.4% reduction in clot mass. In contrast, AC exposure (24 kHz, 50% nominal output power, 30 s) led to a 1.3-fold greater mass reduction with 26.8% released hemoglobin, likely due to additional thermal effects. Morphological analyses revealed that HC treatment significantly reduced red blood cell density in a pressure- and time-dependent manner. Notably, HC treatment effectively eroded blood clots by hemolysis with slight fibrinolysis, whereas clot erosion in AC was primarily due to hemolysis. HC achieved thrombolysis comparable to or better than AC, offering a safer, more targeted strategy, especially for disease due to RBC-rich clots such as non-cardioembolic stroke. The findings will advance mechanistic understanding of cavitation-induced clot degradation and support HC's clinical potential for thrombosis treatment.
1 Introduction
Thrombosis is a pathological condition of significant health risks characterized by the formation of a blood clot within a blood vessel. It can develop in both the venous and arterial systems, leading to serious complications such as deep vein thrombosis (DVT), pulmonary embolism (PE), and stroke. According to the World Health Organization (WHO), stroke globally ranks as the third most common cause of death, accounting for approximately 10% of total deaths in 2021.1 Any disruption in blood circulation caused by a clot might result in ischemia, cell death, and an inflammatory response. To counteract these effects, thrombolytic treatments, known as thrombolysis, have been developed to rapidly restore blood flow by dissolving abnormal intravascular clots, thereby preventing ischemic damage.2 In the treatment of acute ischemic stroke, systemic thrombolysis is commonly performed using an intravenous recombinant tissue plasminogen activator (rtPA). The thrombolytic agent rtPA facilitates the enzymatic breakdown of fibrin—the extracellular framework of an acute thrombus—into D-dimer protein fragments and other fibrin degradation products.3,4 However, this approach is limited by a 4.5 hour therapeutic window and an increased risk of bleeding complications.5,6 Another approach, catheter-directed thrombolysis (CDT), delivers thrombolytic agents directly to the clot, increasing local drug concentration while potentially reducing systemic side effects and enhancing clot dissolution. Despite these advantages, CDT has challenges, such as an increased risk of bleeding and hemorrhagic stroke.7 In more severe cases of DVT, thrombolytic drugs such as streptokinase, urokinase, and rtPA have been used; nevertheless, they pose a significant risk of bleeding.8 Additionally, mechanical thrombectomy and catheter-directed thrombolytics are viable options; however, improper filtration might increase the risk of PE and hemorrhage.9Adjuvant therapies aim to overcome the limitations of current thrombolysis techniques, prompting the development of technically feasible and potentially effective treatments such as sonothrombolysis.10 This approach is based on the cavitation phenomenon and is a form of cavitation-assisted thrombolysis. Cavitation occurs when the local pressure of a liquid drops below its vapor saturation pressure, triggering phase change.11 This can be achieved, for instance, by applying a tension (as in hydrodynamic cavitation (HC) or acoustic cavitation (AC)) or depositing energy (as with electrical discharge or a laser).12 AC is a well-established field and has been employed in various engineering applications, including chemical processing, wastewater treatment13 and cleaning.14 In AC, ultrasound waves generate pressure gradients, leading to alternating zones of compression and rarefaction (stretching) in fluid. Cavitation bubbles form during the rarefaction cycle if the tensile forces are strong enough to reduce the static pressure of the liquid below its vapor saturation pressure. The AC bubbles can oscillate in the applied ultrasonic field (stable cavitation) or undergo a violent collapse following a quick increase in size (inertial cavitation).
While the intense collapse of cavitation bubbles might cause damage (e.g., to ship propellers or heart valves), the released energy can also be harnessed for beneficial purposes in various biomedical applications such as cell lysis,15 dermatologic treatments,16 cancer treatment17 and thrombolysis.18 Recent studies have shown that inertial cavitation is generated inside a blood clot during focused ultrasonography.19,20 Accordingly, blood flow can be restored if the clot is broken up by the mechanical stress caused by collapsing bubbles. In the context of cavitation in microfluidic devices, Gao et al. demonstrated that AC at 28 kHz is optimal for dissociating blood clots.21 Additionally, AC can temporarily increase the cell membrane permeability, enhancing the localized delivery of medications or thrombolytic agents.22
Unlike AC, HC generates pressure variations by rapidly forcing liquid through a narrow flow constriction, leading to a phase change in the resulting low-pressure zones. The fluid properties, flow rate, and constriction geometry are important parameters that induce HC by affecting its critical features such as shear forces and shockwaves.23 These features lead to the physical and mechanical effects of the cavity dynamics and collapse, which offer a number of benefits in biotechnology.24 Cell membranes can be gently broken down by shear forces for applications such as extracting DNA or releasing intracellular contents.25 Conversely, shockwaves can be employed for more forceful disruption, such as breaking down hard tissues or rendering microbes inactive.26 The combination of HC with microfluidics offers advantages over traditional methods, including reduced liquid volume requirements, improved monitoring and real-time analysis, and precise control over bubble formation and behavior.27 Microfluidic devices enable even finer control over this process, a feature particularly valuable in biotechnological applications such as targeted cell lysis, controlled drug delivery, and biomolecule manipulation.27,28
Recently, the promising results obtained from HC in medical applications27,28 have motivated researchers to further explore its potential as a thrombolytic technique. Motivated by this potential, this study aims to investigate the effectiveness of the “HC on a chip” concept (microscale HC) for clot degradation using a clot-on-a-chip (CoC) platform (Fig. 1). The CoC platform consists of two integrated microfluidic device components: a silicon-glass microfluidic device designed to facilitate microscale HC at low upstream pressures (≤482 kPa) and a polydimethylsiloxane (PDMS) microchip that houses the blood clot. Hemolysis and fibrinolysis analyses were performed to evaluate the thrombolytic potential following microscale HC treatment. For this, UV-vis spectroscopy was used to quantify hemoglobin (Hb) levels, oxygenated hemoglobin (Oxy-Hb), and total protein levels, while D-dimer analysis was employed to assess fibrin degradation. Our previous work29 demonstrated that HC effectively degrades blood clots, as evidenced by mass and diameter reduction, along with structural damage observed via field emission-scanning electron microscopy (FE-SEM) analysis. In this study, we further extend the physical characterization, including SEM analysis, conducted in that prior work.29 Moreover, we present a comprehensive comparison of the effects of microscale HC and AC on clot breakdown by evaluating the energy density, as well as by conducting the same biological and physical characterization. Given that AC is a widely studied adjuvant thrombolysis approach, this comparison will provide a valuable perspective on the effectiveness of microscale HC. Thus, we further substantiate the potential of microscale HC as a novel thrombolysis technique by incorporating D-dimer and Hb analyses, which reinforce our findings. These results support the translation of microscale HC into a viable thrombolytic method for future clinical applications.
2 Results
2.1 Evaluation of thrombolytic potential
Comprehensive biological evaluations were conducted to assess the thrombolytic potential of microscale HC using the CoC platform, and AC via a low frequency sonotrode (24 kHz), as standalone approaches for addressing health issues such as PE and DVT. Before the utilization of the CoC platform for thrombolysis via microscale HC, the flow regimes and associated cavitation patterns were determined by using a high-speed camera. The onset and development of HC were characterized at three upstream pressures: 69 kPa (no cavitation), 172 kPa (cavitation inception), and 482 kPa (fully developed cavitation), corresponding to Reynolds numbers of 1600, 2933, and 4973, and cavitation numbers of 0.475, 0.364, and 0.357, respectively (Fig. S1).29 The associated average bulk flow velocities under these conditions were calculated to be 16.67, 30.55, and 51.80 m s−1, confirming the progressive transition from laminar to cavitating flow regimes.29 The clot was positioned 920 μm downstream from the cavitation interfaces, where the collapse of HC bubbles and the resulting shockwaves and microjets generate localized mechanical forces sufficient to cause thrombolysis within the microfluidic device (Fig. S2 and S3).29 The formation of cavitation vortices and atomized spray zones was consistently observed under both thrombus-present and thrombus-absent conditions. High-speed imaging and flow field characterization under controlled upstream pressure (Fig. S4) confirm that the thrombus, positioned near the outlet chamber, does not interfere with cavitation dynamics or alter vortex symmetry. These results indicate that the engineered shear force zones and surface roughness modifications effectively stabilize cavitation behaviour, independent of thrombus-induced local flow resistance.Thrombolytic potential was determined by assessing hemolysis—characterized by the degradation of red blood cells (RBCs) and the release of Hb—and fibrinolysis, indicated by the release of a D-dimer due to fibrin degradation.30 To elucidate the thrombolytic potential of microscale HC in the CoC platform both qualitatively and quantitatively, Hb, Oxy-Hb, and total protein were measured using peaks at 414 (Soret band), 540 (beta band), and 280 nm of the obtained UV-vis spectra, respectively (Fig. S5).31 D-dimer levels were determined via ELISA, while SEM micrographs of eroded blood clots and RBC density analyses from these micrographs were also collected. Additionally, changes in Oxy-Hb and total protein levels were examined for various microscale HC and AC treatment parameters. The microscale HC and AC parameters for each case are summarized in Table 1.
| Experimental test rig | Case | Upstream pressure [kPa] or NOPa [%] | Time [s] | HC flow pattern conditions | Medium |
|---|---|---|---|---|---|
| a For cases in microscale HC, upstream pressure values and for cases in AC, nominal output power (NOP) were investigated. | |||||
| Microscale HC | Case H1 | 69 | 120 | Non-cavitation (control) | Phosphate-buffered saline (PBS) (pH 7.4 at RT) |
| Case H2 | 172 | 90 | Cavitation inception | ||
| Case H3 | 172 | 120 | |||
| Case H4 | 482 | 30 | Fully developed cavitation | ||
| Case H5 | 482 | 60 | |||
| Case H6 | 482 | 90 | |||
| Case H7 | 482 | 120 | |||
| AC | Case-Ctrl | — | 120 | PBS (pH 7.4 at 37 ± 2 °C) | |
| Case A1 | 20 | 10 | |||
| Case A2 | 20 | 30 | |||
| Case A3 | 50 | 10 | |||
| Case A4 | 50 | 30 | |||
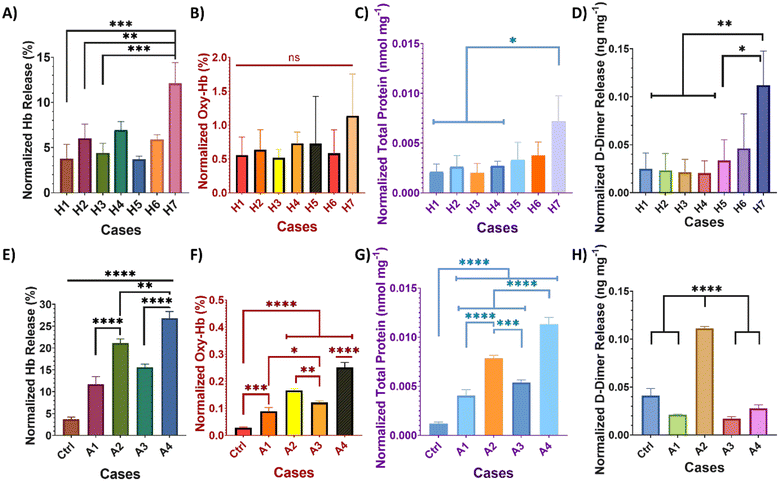
The amounts of released Hb increase with the pressure, as shown in Fig. 2-A. The highest Hb release of 12.1 ± 2.3% is observed in case H7, indicative of fully developed cavitating flow conditions. In comparison, the control sample for microscale HC (no cavitation conditions), corresponding to case H1 with an upstream pressure of 69 kPa, exhibits an Hb release of 3.8 ± 1.6% (p = 0.0006). Meanwhile, samples exposed to HC inception conditions at an upstream pressure of 172 kPa in cases H2 and H3 have Hb releases of 6.0 ± 1.6% (p = 0.0035) and 4.4 ± 1.1% (p = 0.0005), respectively (Tukey-adjusted p-values; full pairwise results given in Table S2). Thus, microscale HC treatment at an upstream pressure of 482 kPa in case H7 significantly degrades clot samples through enhanced hemolysis. Similarly, Hb release from clots degraded by AC treatment increases with either higher nominal output power (NOP) or longer treatment duration. For AC treatments lasting 30 s, Hb releases are obtained to be 26.8 ± 1.6% and 21.2 ± 0.9% in cases A4 and A2, respectively (Fig. 2-E). In comparison, 10 s of AC treatments result in Hb releases of 15.6 ± 0.7% and 11.7 ± 1.8% in cases A3 and A1, respectively. When compared to the maximum Hb release observed in microscale HC treatment, AC treatment leads to approximately two-fold higher hemolysis efficiency. Moreover, all AC treatments lead to significantly higher Hb release (p < 0.0001) compared to the control group (case-Ctrl), where clots were immersed in PBS (pH 7.4, 37 ± 2 °C) for 120 s. Comparisons of different treatment durations at a constant NOP, or vice versa, clearly indicate that Hb release significantly increases with the treatment time or NOP.
Simultaneously, Oxy-Hb amounts were obtained from the absorbance peaks at 540 nm in the UV-vis spectra. Previous studies suggested that the absorbance peaks at 540 nm and 570 nm increase with the Oxy-Hb concentration in the blood, as observed by comparing diluted blood samples from individuals with a Hb standard (Fig. S5).31,32 Furthermore, a higher Oxy-Hb content in the samples is indicated by a peak shift from 407 nm (in the Hb standard) to 414 nm (in clot erosion samples and diluted blood from individuals). The results prove that microscale HC-treated clot samples do not exhibit significant differences in Oxy-Hb levels among themselves (p = 0.5986); however, the Oxy-Hb release slightly increases with the treatment time and pressure, reaching a maximum of 1.14 ± 0.62% for samples of case H7 (Fig. 2-B). In contrast, AC-treated samples display a similar trend in Hb release, with Oxy-Hb amounts increasing in response to the higher energy input (Fig. 2-F). Nevertheless, compared to microscale HC-treated samples, AC-treated samples release significantly less Oxy-Hb. Even the highest Oxy-Hb level in AC-treated samples is only 0.25 ± 0.02% (observed in case A4). This value is half the lowest Oxy-Hb level observed in microscale HC-treated samples in case H1 (at an upstream pressure of 69 kPa for 120 s) and nearly five times lower than that observed in case H7 (an upstream pressure of 482 kPa for 120 s). Consequently, microscale HC generates more radical oxygen species, leading to more induction of Oxy-Hb compared to AC treatment.33,34
The total protein amounts in treated blood clots were determined using the absorbance peak at 280 nm (Abs280) from UV-vis spectra, which arises from the absorption of aromatic amino acids in proteins (including tryptophan, tyrosine, and phenylalanine), and were obtained using the Beer–Lambert law.35 Since blood is a complex medium containing a wide variety of cells and compounds—including albumins and globulins—the normalized total protein is reported in nanomoles per milligram (nmol mg−1).35 The AC-treated samples demonstrate a significant increase in total protein release compared to the case-Ctrl (Fig. 2-G). For AC treatments lasting 30 seconds, the samples treated at 50% NOP (case A4) release total protein of 0.0113 ± 6.9 × 10−4 nmol mg−1, while those treated at 20% NOP (case A2) release 0.0079 ± 3.1 × 10−4 nmol mg−1 (p < 0.0001). For 10 second treatments, the samples treated at 50% NOP (case A3) release 0.0054 ± 3.0 × 10−4 nmol mg−1, and those at 20% NOP (case A1) release 0.0040 ± 6.2 × 10−4 nmol mg−1 total protein. An increase in treatment time results in a two-fold increase in the total protein release, a change more pronounced than the corresponding increase in Hb release under the same conditions. Most microscale HC-treated samples, except for case H7, have no significant total protein release compared to the non-cavitation sample (case H1). Case H7 resulted in a significant total protein release of 0.0071 ± 26.0 × 10−4 nmol mg−1 (p < 0.05) compared to treatments in cases H1, H2, H3, and H4 (Fig. 2-C). Additionally, the microscale HC treatment in case H7 leads to a higher total protein release than the 10 second AC treatments (cases A1 and A3).
The fibrinolysis observed after microscale HC treatment follows a similar trend in hemolysis, as shown in Fig. 2-D. D-dimer release increases significantly with both pressure and treatment duration in microscale HC treatment. Notably, the case H7 treatment causes a D-dimer release of 0.112 ± 35.6 × 10−3 ng mg−1, which is significantly higher than that observed for cases H1 (p = 0.0071), H2 (p = 0.006), H3 (p = 0.0052), H4 (p = 0.0048), and H5 (p = 0.0161). In contrast, the D-dimer release in AC-treated samples shows a different fibrinolysis trend compared to hemolysis (Fig. 2-H); here, the highest D-dimer release is observed for case A2, with 0.111 ± 18.7 × 10−3 ng mg−1 (p < 0.0001), which is significantly higher than in the other AC-treated samples. Moreover, the highest D-dimer levels of microscale HC (case H7) and AC treatment (case A2) are comparable.
2.2 Morphological analysis of blood clots
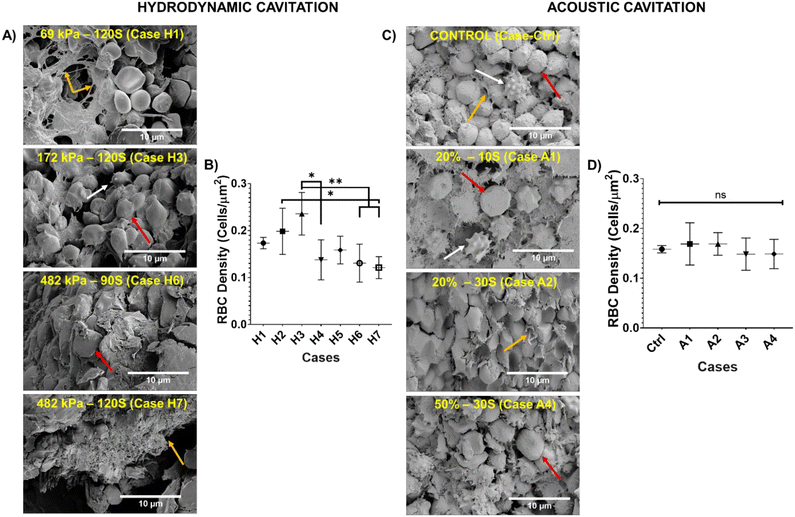
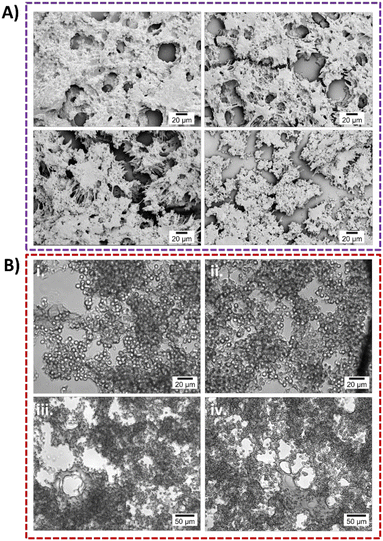
Fig. 3 presents SEM micrographs of blood clots subjected to microscale HC at varying upstream pressures and AC at different NOP levels across a range of treatment durations. SEM analysis indicates that the clots are composed of RBCs, fibrin, and white blood cells, mirroring the structure of in vivo clots.36 Blood clots treated with microscale HC at different upstream pressures and durations, and the resulting morphological changes were examined and are shown in Fig. 3-A. In this figure, red, white, and yellow arrows indicate RBCs, white blood cells, and fibrin fibers, respectively. In the first row, undisturbed fibrin fibers and RBCs, which are solely affected by the fluid flow at an upstream pressure of 69 kPa for 120 s (case H1), are observed in the absence of microscale HC exposure. In the second row, minor damage—particularly to fibrin fibers—can be observed at an upstream pressure of 172 kPa where the samples were exposed to cavitation inception conditions for 120 s (case H3). In the third row, structural integrity is further damaged since fully developed cavitating flow was applied to the blood clot at an upstream pressure of 482 kPa for 90 seconds (case H6). RBCs and fibrin fibers show progressive disruption, supporting the occurrence of hemolysis and fibrinolysis as evidenced by Hb and D-dimer analysis results. Further loss of structure in blood clots can be seen in the fourth row due to increased duration of microscale HC exposure (120 s) at an upstream pressure of 482 kPa (case H7). In addition, gradual changes of clot structure upon microscale HC treatment for each parameter are given in Fig. S6.The density of RBCs was calculated for each microscale HC treatment case from SEM micrographs using the ImageJ software as shown in Fig. 3-B. RBC density is the highest in case H3 with 0.23 ± 0.04 cells per μm2, which is significantly higher than that observed in cases H4 (p = 0.0129), H5 (p = 0.0286), H6 (p = 0.0014) and H7 (p = 0.0010). The second highest RBC density is observed in case H2, with an upstream pressure of 172 kPa for 90 seconds, with 0.19 ± 0.01 cells per μm2. When the upstream pressure is increased to 482 kPa (microscale HC exposure for 30 s), the RBC density dramatically decreases to 0.13 ± 0.04 cells per μm2. Even though the RBC density under 60 s of microscale HC exposure at the upstream pressure of 482 kPa is higher (0.15 ± 0.02 cells per μm2) compared to 30 s, the lowest RBC density (0.12 ± 0.02 cells per μm2) is seen at the upstream pressure of 482 kPa after 120 s of microscale HC exposure, which represents the most intense HC conditions in this study. This aligns with the qualitative results of SEM analysis shown in Fig. 3-A. In addition, Hb release also correlates with the results of RBC density calculation upon microscale HC exposure. Accordingly, the highest release of Hb (12.1%) is obtained in case H7. Additionally, the Hb release is higher in case H4 with 6.9% compared to Hb release in case H5 with 3.7%. This suggests that the hemolysis must occur in case H7 at an upstream pressure of 482 kPa for 120 seconds.
The SEM micrographs of blood clots subjected to AC at varying NOPs and durations are presented in Fig. 3-C. Similar to Fig. 3-A, the magnification is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×, with red arrows indicating RBCs, white arrows indicating white blood cells, and yellow arrows marking fibrin fibers. The first row represents the control group, where no treatment exists, showing an undisturbed blood clot structure. The second row displays the effects of AC exposure in case A1 (20% NOP for 10 s), revealing minor fibrin fiber degradation while preserving the overall clot structure. The third row corresponds to case A2 (20% NOP for 30 s), where more pronounced damage is observed in both RBCs and fibrin fibers. The fourth row illustrates case A4 (50% NOP for 30 s). In this case, fibrin fiber destruction and significant morphological degradation of RBCs are evident. In addition, gradual changes of the clot structure following AC treatment for each case are shown in Fig. S7. RBC density was quantified from SEM micrographs using the ImageJ software as shown in Fig. 3-D, with the control group exhibiting an RBC density of 0.15 ± 0.007 cells per μm2. The highest RBC densities, 0.16 ± 0.04 cells per μm2 and 0.16 ± 0.02 cells per μm2, are observed in cases A1 and A2, respectively. In contrast, the lowest RBC densities, 0.14 ± 0.03 cells per μm2 and 0.14 ± 0.02 cells per μm2, are recorded in cases A3 and A4, respectively, indicating that an increase in AC NOP leads to a reduction in RBC density. The changes between groups are non-significant.
000×, with red arrows indicating RBCs, white arrows indicating white blood cells, and yellow arrows marking fibrin fibers. The first row represents the control group, where no treatment exists, showing an undisturbed blood clot structure. The second row displays the effects of AC exposure in case A1 (20% NOP for 10 s), revealing minor fibrin fiber degradation while preserving the overall clot structure. The third row corresponds to case A2 (20% NOP for 30 s), where more pronounced damage is observed in both RBCs and fibrin fibers. The fourth row illustrates case A4 (50% NOP for 30 s). In this case, fibrin fiber destruction and significant morphological degradation of RBCs are evident. In addition, gradual changes of the clot structure following AC treatment for each case are shown in Fig. S7. RBC density was quantified from SEM micrographs using the ImageJ software as shown in Fig. 3-D, with the control group exhibiting an RBC density of 0.15 ± 0.007 cells per μm2. The highest RBC densities, 0.16 ± 0.04 cells per μm2 and 0.16 ± 0.02 cells per μm2, are observed in cases A1 and A2, respectively. In contrast, the lowest RBC densities, 0.14 ± 0.03 cells per μm2 and 0.14 ± 0.02 cells per μm2, are recorded in cases A3 and A4, respectively, indicating that an increase in AC NOP leads to a reduction in RBC density. The changes between groups are non-significant.
3 Discussion
Previous studies primarily focused on the combination of sonothrombolysis with thrombolytic drugs—such as rtPA (alteplase), urokinase, or tenecteplase—to achieve synergistic thrombolytic efficacy.30,37–39 Additionally, some studies utilized microbubbles or nanodroplets to deliver thrombolytic drugs to clots, demonstrating improved thrombolytic efficacy and reduced therapeutic dosages.20,40–42 Notably, most of these studies suggest that cavitation alone offers insufficient thrombolytic efficacy. However, our results clearly show that microscale HC treatment of blood clots in the CoC platform induces significant thrombolysis without the need for thrombolytic drugs.Although differences in thrombolytic rates—measured as the mass change of clots—are not statistically significant among the various microscale HC treatment cases, more than 35% of the clot mass was degraded starting from HC inception conditions (172 kPa; Fig. S8). In comparison, the highest mass reduction is observed at an upstream pressure of 482 kPa after 120 s of microscale HC treatment, yielding a 53.4 ± 4.9% reduction. Meanwhile, 30 s of AC treatment (24 kHz) at 50% NOP results in a greater reduction of 67.9 ± 6.8% (Fig. S8). The thrombolytic efficiency of this AC frequency range was discussed by Gao et al., where they suggested that between 25 and 30 kHz frequencies, 28 kHz with 2 s actuation showed the highest thrombolytic efficiency.21 However, their experiments were performed using a microfluidic platform, which may not fully reflect the conditions of mesoscale clots treated with a sonotrode.
As previously demonstrated via SEM micrographs, the human blood clots analyzed in this study are composed of RBCs, immune cells, platelets, and fibrin fibers. Consequently, three primary mechanisms contribute to the reduction in clot volume: (i) the breakdown of RBCs (hemolysis), (ii) degradation of the fibrin network (fibrinolysis), and (iii) clot deformation without actual lysis.20 This study demonstrates that all three mechanisms contribute to clot degradation upon cavitation exposure, as confirmed by Hb and D-dimer measurements and SEM analysis. The degradation of the fibrin network is a key factor in clot destruction and the risk of re-occlusion.20 However, until now, the impact of HC on fibrin network integrity has not been systematically evaluated. To specifically assess the fibrinolytic contribution to clot degradation, the D-dimer level was measured in this study, as it is a well-established biomarker of fibrin degradation.43 Because the D-dimer is a terminal, soluble product of cross-linked fibrin proteolysis, it cannot re-polymerize.44 Thus, any re-occlusion risk in vivo stems from residual macro-fragments or de novo coagulation on injured surfaces, not from ‘chemical re-assembly’ of the degraded network.45 In our experiments, we observed no re-occlusion at the 1.8 mm outlet, consistent with surface-erosive lysis. On the other hand, evaluation of distal embolization and re-thrombosis under plasma flow will be addressed in catheter-relevant models beyond the scope of this study.
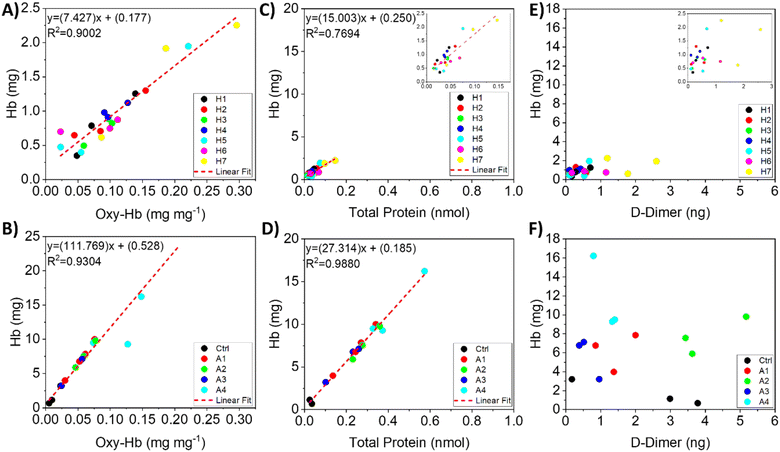
Previous studies suggested that hemolysis is the primary contributor to clot erosion in the absence of fibrinolysis, as reflected by D-dimer release; however, cavitation also mechanically disrupts fibrin networks, thereby enhancing thrombolysis.30,46 The comparison in the hemolysis and fibrinolysis outcomes of microscale HC- and AC-treated blood clots reveals that cavitation-induced thrombolysis primarily occurs via hemolysis (Fig. 4 and S9). The Hb results indicate releases of 12.1 ± 2.3% after 120 s of microscale HC treatment at an upstream pressure of 482 kPa (case H7) and 26.8 ± 1.6% for 30 s of AC treatment at 50% NOP (case A4). Since these Hb values were normalized to the initial clot masses, they suggest that a significant fraction of the observed mass change is attributable to hemolysis. Moreover, the relationship between Hb and Oxy-Hb, as shown in Fig. 4-A, reveals a linear increase in Oxy-Hb with the Hb levels. This linearity is also confirmed for AC treatment; however, AC has a steeper slope, implying that Hb release is proportionally higher than Oxy-Hb release (Fig. 4-B). Furthermore, a significant fraction of the Hb in the samples is seen in the form of Oxy-Hb—calculated to be 1.14 ± 0.62% (approximately 9.4% of the released Hb) after 120 s of microscale HC treatment at an upstream pressure of 482 kPa (case H7)—compared to 0.25 ± 0.02% (approximately 0.9% of the released Hb) after 30 s of AC treatment at 50% NOP (case A4). This can be explained by the findings from a recent study, which confirmed the dominant role of chemical effects during microscale HC bubble collapse, leading to hydroxyl radical production from H2O dissociation under supercritical conditions.33 Moreover, compared to AC treatment at 35 kHz, HC treatment yields higher levels of free hydroxyl radical production.33 Therefore, the higher Oxy-Hb release observed in microscale HC-treated samples proves that Hb undergoes further oxidation due to the chemical effects of bubble collapse.34,47
In terms of total protein release, both AC and microscale HC treatments have linear correlations with their respective Hb releases (Fig. 4-C and D). AC treatment results in a higher Hb release relative to total protein release, whereas the release rates of Hb and total protein are similar in microscale HC treatment. This observation suggests that microscale HC treatment could lead to lysis of not only RBCs but also other blood components such as thrombocytes and leukocytes.48 This might indicate that the destructive effects of HC bubble collapse can penetrate deeper into the blood clots, while AC predominantly disrupts RBCs near the surface, leading to more Hb release.38,49 The deeper penetration of HC bubbles and their subsequent collapse effects are also caused by the closer positioning of clots to the cavitation vortex.29
Another important aspect of thrombolysis is fibrinolysis, which involves the degradation of fibrinogen and fibrin fibers within the thrombus, ultimately resulting in the release of the D-dimer.50 As mentioned previously, hemolysis is considered as the primary mechanism underlying cavitation-induced thrombolysis.20,30,38,46 According to Petit et al., in the absence of lytic drugs (e.g., rtPA), neither ultrasound alone nor the combination of ultrasound (1 MHz, 1.3 MPa, 0.8 duty cycle) and microbubbles produced any D-dimer, whereas the addition of rtPA resulted in a release of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ng mL−1 D-dimer after 30 min.20 Similarly, a recent study by Li et al. showed that an ultrasound-alone group (1 MHz) did not release any D-dimer in a microfluidic blood-on-a-chip system; however, the combination of ultrasound and urokinase led to the release of approximately 10
100 ng mL−1 D-dimer after 30 min.20 Similarly, a recent study by Li et al. showed that an ultrasound-alone group (1 MHz) did not release any D-dimer in a microfluidic blood-on-a-chip system; however, the combination of ultrasound and urokinase led to the release of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 D-dimer after 10 min.38 In contrast, our study reports a small amount of D-dimer release with the cavitation effect alone. Blood clots increase D-dimer levels—ranging in the low nanogram scale—after 120 s of microscale HC treatment at an upstream pressure of 482 kPa showing a particularly significant D-dimer release compared to other cases (Fig. 4-E and F). These results clearly demonstrate that D-dimer release in microscale HC treatment is proportional to both the applied pressure and treatment duration. It is possible to achieve fibrinolysis comparable to that of lytic drugs if the system safety can be adequately managed. Moreover, previous studies confirmed that cavitation inception could reduce the diameter of fibrin fibers while increasing their surface area.51 Interestingly, AC treatment displays a different trend in D-dimer release relative to Hb release; specifically, 30 s of AC treatment at 20% NOP releases significantly more D-dimer than other AC treatment cases and control groups.
000 ng mL−1 D-dimer after 10 min.38 In contrast, our study reports a small amount of D-dimer release with the cavitation effect alone. Blood clots increase D-dimer levels—ranging in the low nanogram scale—after 120 s of microscale HC treatment at an upstream pressure of 482 kPa showing a particularly significant D-dimer release compared to other cases (Fig. 4-E and F). These results clearly demonstrate that D-dimer release in microscale HC treatment is proportional to both the applied pressure and treatment duration. It is possible to achieve fibrinolysis comparable to that of lytic drugs if the system safety can be adequately managed. Moreover, previous studies confirmed that cavitation inception could reduce the diameter of fibrin fibers while increasing their surface area.51 Interestingly, AC treatment displays a different trend in D-dimer release relative to Hb release; specifically, 30 s of AC treatment at 20% NOP releases significantly more D-dimer than other AC treatment cases and control groups.
Additionally, the thrombolysis rate after 30 s of AC treatment at 50% NOP (case A4) is higher, showing almost a 1.3-fold greater reduction in blood clot mass compared to 120 s of microscale HC treatment at an upstream pressure of 482 kPa (case H7). This enhanced thrombolytic rate may be attributable to temperature increases of 4.3 ± 0.6 °C and 3.3 ± 0.6 °C observed after 30 s of AC treatment at 50% and 20% NOPs, respectively (Fig. S10), which could also limit the clinical applicability of these AC cases.52,53 According to Sakharov et al., an AC-induced temperature rise from 37 °C to 43 °C (using 1 MHz, 2 W cm−2) resulted in a 1.3-fold increase in lysis, a finding that is similarly observed in our study.53 In contrast, Nahirnyak et al. suggested that AC operating at frequencies below 3.5 MHz and intensities below 0.5 W cm−2 does not contribute to thrombolysis via a thermal mechanism.54 Although some studies controversially debated the thermal effects in sonothrombolysis, safety concerns have not been fully addressed.52,54,55 In this context, microscale HC treatment offers a safer alternative to sonothrombolysis while providing comparable thrombolytic efficacy.29,56 Overall, microscale HC treatment has high thrombolytic potential by combining both hemolysis and modest fibrinolysis and disruption of fibrin fibers, whereas AC treatment primarily erodes clots via hemolysis. These findings are corroborated by morphological observations of the clots after treatment.
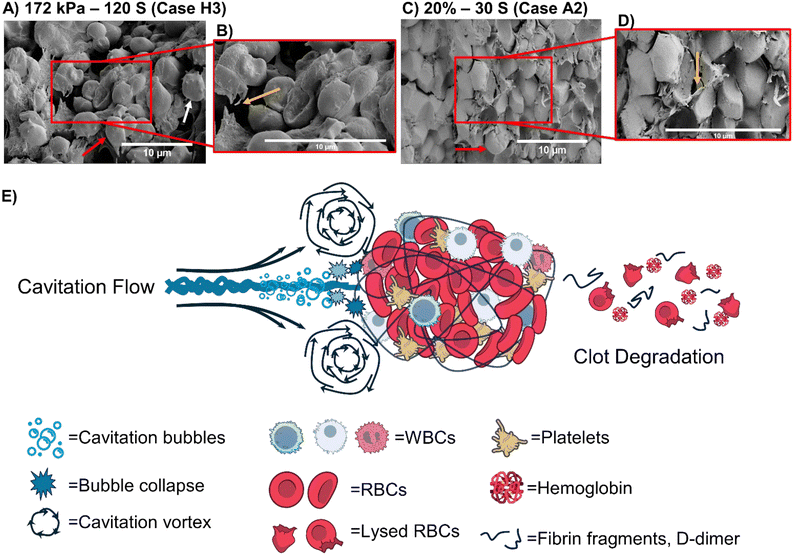
Further analysis of pre-treatment clot composition was performed by imaging cryosectioned blood clots using both SEM and brightfield microscopy. These images revealed that the clots were densely packed with red blood cells, indicating that an RBC-rich composition typically formed under low-shear conditions, as commonly observed in pathologies such as venous thrombosis and non-cardioembolic stroke (Fig. 5).57 In addition, the clots exhibited a highly porous structure with sparsely crosslinked fibrin fibers, suggesting lower stiffness compared to fibrin-rich thrombi.58,59 Such structural characteristics are known to confer increased susceptibility to mechanical disruption, aligning with the hemolysis-driven lysis observed in our HC experiments. HC generates vapor bubbles that expand and collapse, producing localized high-pressure transients, shear forces, and high-speed microjets. Asymmetric bubble collapse near the clot surface results in microjets that mechanically rupture fibrin fibers,58 while bubble oscillations align fibers circumferentially, making them more prone to breakage under stress.60,61 These effects are particularly pronounced in loosely crosslinked, RBC-rich thrombi as also observed in our study.62 Cavitation also induces vortices and microstreaming, contributing to shear-induced erosion of fibrin structures.58 In parallel, cavitation-induced hemolysis releases intracellular components such as Hb, which may enhance fibrinolysis through biochemical mechanisms such as pH shifts or enzymatic activation.53,63 Stable bubbles may further aid in clearing fibrin degradation products via micro-pumping and stirring effects.53,64 Fibrinolysis, assessed by D-dimer release, showed that the HC conditions of case H7 achieved levels comparable to the AC conditions of case A2, despite the absence of thrombolytic drugs. Importantly, the hemolysis-intensive nature of microscale HC may represent a safer therapeutic option for thrombosis treatment, particularly under conditions like non-cardioembolic stroke, where RBC-rich clots are the primary cause.59
The SEM results shown in Fig. 6 indicate that hemolysis is more pronounced than fibrinolysis in clot degradation for both microscale HC and AC. This conclusion is supported by the released levels of Hb and D-dimers, confirming that hemolysis serves as the primary mechanism of clot breakdown upon cavitation exposure, with fibrinolysis playing a secondary role.
The results presented in Fig. 6 provide a detailed examination of the structural alterations in blood clots following exposure to microscale HC and AC, elucidating the underlying mechanisms driving thrombolysis. The high-magnification SEM images confirm that cavitation-induced forces significantly compromise the clot integrity through fibrin disruption and RBC hemolysis. HC exposure at an upstream pressure of 172 kPa for 120 s (case H3) leads to pronounced fibrin fiber fragmentation and RBC lysis, as evident in Fig. 6-A and B. The cavitation phenomenon generates vapor bubbles that rapidly expand and collapse, producing localized high-pressure spikes and microjets. These forces mechanically disrupt the fibrin network, weakening the structural integrity of the clot.65–68 The schematic representation in Fig. 6-E further illustrates this mechanism, highlighting how HC bubble collapse generates vortices and shear forces that erode the fibrin scaffold. Additionally, the collapse of cavitation bubbles generates microjets that exert localized, high-impact forces on RBCs, leading to their rupture and the subsequent release of Hb and intracellular components.68 This hemolytic effect further accelerates clot degradation by disrupting the clot stability and promoting fibrinolysis.63 Similarly, AC exposure at 20% NOP for 30 s (case A2) induces significant clot disintegration, as shown in Fig. 6-C and D. The SEM images reveal that AC primarily causes RBC deformation and fibrin disruption, reinforcing the role of cavitation in clot breakdown. Notably, hemolysis appears to be the predominant mechanism of thrombolysis, with RBC deformation being more pronounced than fibrin degradation. The comparison of the clot morphology before and after cavitation exposure (Fig. 3-A, first and fourth rows) suggests that mechanical stress from cavitation preferentially targets RBCs, further supporting the notion that hemolysis is the primary driver of cavitation-induced clot degradation. These findings align with previous studies indicating that cavitation enhances streaming, generates vortices, and produces localized mechanical stresses capable of breaking down clot structures.13 The rapid energy transfer from bubble collapse fragments fibrin fibers while concurrently inducing RBC lysis, establishing an efficient thrombolytic process. The combined effects of fibrin fiber disruption and RBC hemolysis create optimal conditions for clot erosion.
Hendley et al. investigated clot breakdown via histotripsy and a lytic agent, assessing fibrinolysis through D-dimer and Hb measurements as well as clot mass loss.69 They reported that in the absence of rtPA, D-dimer production was comparable to that of clots exposed to plasma alone, suggesting that the mechanical effects of AC alone do not induce fibrinolysis. In contrast, our study demonstrates fibrin degradation occurring under both AC and microscale HC exposures, even in the absence of thrombolytic agents. Importantly, this drug-free approach has the potential to reduce the risk of hemorrhagic complications, a major concern associated with conventional thrombolytic therapies.70
HC is at least ten-fold more energy-efficient than AC in microbiological disruption and industrial processing, according to studies by Save et al.71,72 and Lévêque et al.73 A notable difference in energy generation and efficiency is seen when comparing the energy inputs of microscale HC and AC. According to the experimental data, microscale HC energy values are significantly lower, ranging from 2.76 W s (in case H1) to a maximum of 46.92 W s (in case H7), while AC requires a significantly greater energy input, reaching 1250.1 W s in case A4 (Fig. 7 and SI section S1). This significant difference implies that AC offers far longer localized energy concentration, making it more suitable for applications requiring intense energy transmission. The energy-efficient thrombolysis of microscale HC is evident from the hemolysis, fibrinolysis, and morphological changes observed in this study, while transmitting over 58 times less energy than the highest AC treatment parameter.
4 Experimental
4.1 Materials
Calcium chloride, PBS, ethyl alcohol, and glutaraldehyde were obtained from MERCK KgaA (Darmstadt, Germany) for the blood clot studies. A PDMS polymer and SYLGARD 184 silicone elastomer kit were acquired from Dow Corning (Midland, MI) for fabricating PDMS microchips, while Hb (H7379) and D-dimer ELISA kits (RAB0648) were purchased from Sigma-Aldrich (Burlington, MA).4.2 Design of the CoC platform
A CoC platform comprising a dual-microfluidic chip system was developed to investigate the effect of microscale HC on clot degradation. The system, which integrates a silicon-glass microfluidic device with a PDMS microchip, was successfully assembled and prepared for HC exposure. Cavitating flows occur inside the microchannel of the silicon-glass microfluidic device. The PDMS microchip serves as a housing to securely hold the clot. The clot housings in the PDMS microchip were designed in such a way that they were aligned with the reservoir locations on the silicon-glass microfluidic device. The CoC platform is thus formed by bringing the silicon-glass microfluidic device and the PDMS microchip into intimate contact using a special sandwich holder (Fig. 1 and S11 and S12) which can withstand high pressures and be integrated into the experimental setup. All dimensions of the CoC platform are provided in Table S6 of SI section S2.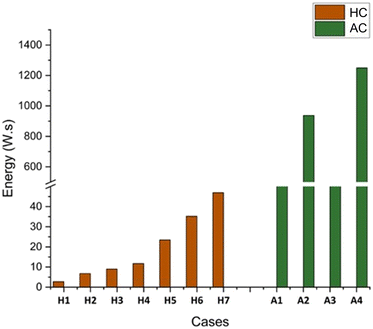 | ||
| Fig. 7 Comparison of the energy inputs (W s) of microscale HC and AC treatments according to working conditions. | ||
4.3 Fabrication of the CoC platform
4.4 The experimental setups and procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio of buffer to blood) within a fume hood. The mixture was gently stirred for 10 seconds to ensure a uniform distribution of calcium ions. The samples were then incubated at 37 °C in a water bath for 4 hours to simulate body temperature. After incubation, the resulting blood clot was immediately placed into a PDMS microchip for subsequent hydrodynamic cavitation (HC) treatment. This prompt handling was intended to preserve the clot's structural integrity and to minimize the risk of artifacts during the HC procedure.
10 ratio of buffer to blood) within a fume hood. The mixture was gently stirred for 10 seconds to ensure a uniform distribution of calcium ions. The samples were then incubated at 37 °C in a water bath for 4 hours to simulate body temperature. After incubation, the resulting blood clot was immediately placed into a PDMS microchip for subsequent hydrodynamic cavitation (HC) treatment. This prompt handling was intended to preserve the clot's structural integrity and to minimize the risk of artifacts during the HC procedure.
The pre-treatment blood clots were further characterized by using brightfield and low magnification SEM imaging. Briefly, clots were washed 3 times with PBS and fixated in 4% paraformaldehyde (PFA) for 24 h. Afterward, clots were embedded in resin (Cryomatrix, Epredia) and cryosectioned into 20 μm-thick sections by using a cryotome (Cryostar NX50, Epredia). The clot sections were imaged under a brightfield microscope (Carl-Zeiss Axio Observer Z.1) for 20× and 40× magnifications. Then, the clot sections were coated with Au/Pd for 160 s with 40 mA current and imaged under SEM (Zeiss LEO Supra 35 VP) at 1000× magnification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 fps, and resolution of 1280 × 800 pixels) and a macrocamera lens (model K2 DistaMax, focal length of 50 mm, f-number of 1.2). To ensure sufficient lighting and enhance the visibility in high-speed recordings, a point halogen light source was utilized.
200 fps, and resolution of 1280 × 800 pixels) and a macrocamera lens (model K2 DistaMax, focal length of 50 mm, f-number of 1.2). To ensure sufficient lighting and enhance the visibility in high-speed recordings, a point halogen light source was utilized.
4.5 Evaluation of thrombolytic potential
Solutions collected from clots degraded by cavitation were analyzed using UV-vis spectrophotometry and an ELISA assay kit to elucidate the effects of cavitation on thrombolysis. For microscale HC, the volumes of the collected solutions were carefully measured using a volumetric flask to ensure accurate calculations of Hb, Oxy-Hb, total protein, and D-dimer amounts. In the case of AC, a constant volume of 75 mL PBS was used throughout the experiments.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 824 M−1 cm−1.76 For comparative analysis of microscale HC and AC samples, all results were normalized to the initial masses of the blood clots.
824 M−1 cm−1.76 For comparative analysis of microscale HC and AC samples, all results were normalized to the initial masses of the blood clots.
4.6 Morphological analysis of clots after thrombolysis
The effects of cavitation on blood clot thrombolysis were also evaluated using SEM (Zeiss LEO Supra 35 VP) imaging. Following microscale HC and AC treatment, blood clots were prepared in a fume hood according to a previously established method.29 The clots were washed three times in PBS solution and fixed in 2.5% glutaraldehyde at room temperature for 18 hours. After fixation, they were rinsed twice in PBS for 5 minutes each to remove any residual glutaraldehyde. The specimens were then dehydrated using graded ethanol concentrations (1.8%, 10%, 20%, 40%, 50%, 70%, 80%, and 90%), followed by two immersions in 100% ethanol for 2 minutes each. The dehydrated samples were allowed to rest at room temperature for 24 hours in a fume hood. Prior to SEM analysis, the samples were sputter coated with an Au–Pd layer for 120 seconds at a current of 40 mA (Cressington Sputter Coater 108). Field-emission SEM micrographs were captured at magnifications of 5000×, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×, and 20
000×, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× with an accelerating voltage of 2 kV to assess the sample morphology. The micrographs were obtained by combining signals from the secondary electron and in-lens detectors with a signal mixing ratio of 0.6. Additionally, red blood cell densities (cells per μm2) were determined using the ImageJ software from 5000× magnified micrographs of at least three separate areas per sample (see SI section S4 for the ImageJ macro code and a representative demonstration of RBC counting from SEM micrographs in Fig. S16).
000× with an accelerating voltage of 2 kV to assess the sample morphology. The micrographs were obtained by combining signals from the secondary electron and in-lens detectors with a signal mixing ratio of 0.6. Additionally, red blood cell densities (cells per μm2) were determined using the ImageJ software from 5000× magnified micrographs of at least three separate areas per sample (see SI section S4 for the ImageJ macro code and a representative demonstration of RBC counting from SEM micrographs in Fig. S16).
4.7 Data reduction for cavitation input energy:
PHc = ![[Q with combining dot above]](https://www.rsc.org/images/entities/i_char_0051_0307.gif) ·ΔP ·ΔP | (1) |
![[Q with combining dot above]](https://www.rsc.org/images/entities/i_char_0051_0307.gif) is the flow rate (m3 s−1), and ΔP is the pressure difference (Pa), which is the pressure drop across the silicon-glass microfluidic device (in Pa). The applied pressure was measured using a pressure gauge, referencing atmospheric pressure. Since the outlet of the system was directly open to the ambient atmosphere, the pressure difference (ΔP) across the device was calculated as the gauge pressure at the inlet.77
is the flow rate (m3 s−1), and ΔP is the pressure difference (Pa), which is the pressure drop across the silicon-glass microfluidic device (in Pa). The applied pressure was measured using a pressure gauge, referencing atmospheric pressure. Since the outlet of the system was directly open to the ambient atmosphere, the pressure difference (ΔP) across the device was calculated as the gauge pressure at the inlet.77
The total microscale HC input energy is defined as:
| EHC = PHC·t | (2) |
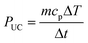 | (3) |
| Euc = PucΔt | (4) |
4.8 Statistical analysis
All experiments were conducted in triplicate, and the data were represented as the mean ± standard deviation (SD). The groups were compared using one-way ANOVA (GraphPad Prism 8.0.1), followed by Tukey's multiple comparison test (ns for p ≥ 0.05, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001). A p-value of less than 0.05 was considered as statistically significant.Conclusions
This study proves that thrombolysis can be effectively induced using the microscale HC approach via the CoC platform, primarily through hemolysis, with additional support from fibrinolysis. Comprehensive biological and physical evaluations revealed that microscale HC treatment degraded blood clots through RBC lysis, evidenced by increased Hb and Oxy-Hb release, as well as fibrin disruption, indicated by elevated D-dimer levels. Interestingly, the highest fibrinolysis for AC was observed in case A2 (20% NOP for 30 s) which is comparable to the highest value obtained in microscale HC for case H7. This finding suggests that mechanical disruption alone may facilitate fibrinolysis, challenging previous assumptions that enzymatic activity is required for clot degradation under AC exposure. Although a higher overall mass reduction was obtained for AC for case A4—primarily via hemolysis and associated thermal effects—a more balanced thrombolytic effect was achieved with microscale HC for case H7 without reliance on adjunct thrombolytic drugs. Moreover, further oxidation of Hb was demonstrated by more induction of Oxy-Hb in microscale HC compared to AC treatment, which was induced by free radicals generated during microscale HC, suggesting an additional chemical contribution to clot erosion. Finally, morphological changes observed after thrombolysis treatments further confirmed the effect of microscale HC treatment on RBC lysis and fibrin breakage and degradation. As a result, the potential of microscale HC as a safer, scalable, energy-efficient, and more targeted therapeutic strategy for health problems related to RBC-rich clots such as non-cardioembolic stroke was revealed. In near future studies, the development of a medical device prototype with a flexible catheter-like design for HC-mediated thrombolysis will be made, and its clinical efficiency will be assessed, with the expectation that the “HC on a chip” concept will be successfully translated into a viable clinical methodology.Author contributions
Abuzer Alp Yetisgin: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); supervision (supporting). Beyzanur Ozogul: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal). Unal Akar: data curation (equal); formal analysis (equal); investigation (equal); software (equal); validation (equal); visualization (equal). Rabia Mercimek: data curation (equal); formal analysis (equal); methodology (equal); software (equal); validation (equal); visualization (equal). Seyedali Seyedmirzaei Sarraf: investigation (supporting); methodology (supporting). Dmitry Grishenkov: investigation (supporting); methodology (supporting); resources (equal); visualization (supporting). Ehsan Amani: formal analysis (equal); investigation (equal); methodology (equal). Tugrul Elverdi: formal analysis (equal); methodology (equal); resources (equal). Ali Koşar: conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review & editing (lead). Morteza Ghorbani: conceptualization (equal); funding acquisition (equal); methodology (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review & editing (lead).Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the SI. Supplementary information: Supplementary movies show clot degradation by hydrodynamic cavitation on a clot-on-a-chip during fully developed cavitation (482 kPa). See DOI: https://doi.org/10.1039/d5lc00482a.Acknowledgements
This study was supported by TUBITAK (The Scientific and Technological Research Council of Turkey) 1001 - The Scientific and Technological Research Projects Funding Program grant no. 221M421, the Royal Society via the Isaac Newton International Fellowship with grant number NIF\R1\221238, the TÜBA-GEBİP Award of Turkish Academy of Sciences (TÜBA) and the BAGEP Award of the Science Academy.References
- O. World Health, The Top 10 Causes of Death, https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death, (accessed 03/02/2025).
- T. Young, J. Perianesth. Nurs., 2022, 37, E8 CrossRef.
- M. Righini, A. Perrier, P. De Moerloose and H. Bounameaux, J. Thromb. Haemostasis, 2008, 6, 1059–1071 CrossRef CAS.
- M. Yepes, in Encyclopedia of Signaling Molecules, ed. S. Choi, Springer International Publishing, Cham, 2018, pp. 5465–5471, DOI:10.1007/978-3-319-67199-4_101941.
- W. Hacke, M. Kaste, E. Bluhmki, M. Brozman, A. Davalos, D. Guidetti, V. Larrue, K. R. Lees, Z. Medeghri, T. Machnig, D. Schneider, R. von Kummer, N. Wahlgren, D. Toni and E. Investigators, N. Engl. J. Med., 2008, 359, 1317–1329 CrossRef CAS.
- C. Wang, Z. Zhai, Y. Yang, Q. Wu, Z. Cheng, L. Liang, H. Dai, K. Huang, W. Lu, Z. Zhang, X. Cheng, Y. H. Shen and G. China Venous Thromboembolism Study, Chest, 2010, 137, 254–262 CrossRef CAS.
- D. Fleck, H. Albadawi, F. Shamoun, G. Knuttinen, S. Naidu and R. Oklu, Cardiovasc. Diagn. Ther., 2017, 7, S228–S237 CrossRef.
- H. D. White and D. P. Chew, Lancet, 2008, 372, 570–584 CrossRef CAS.
- M. J. Sharafuddin and M. E. Hicks, J. Vasc. Interv. Radiol., 1997, 8, 911–921 CrossRef CAS.
- A. J. Dixon, J. Li, J. M. R. Rickel, A. L. Klibanov, Z. Y. Zuo and J. A. Hossack, Ann. Biomed. Eng., 2019, 47, 1012–1022 CrossRef PubMed.
- C. E. Brennen, Cavitation and Bubble Dynamics, Cambridge University Press, Cambridge, 2013 Search PubMed.
- F. R. Young, Cavitation, Imperial College Press, World Scientific, 1999 Search PubMed.
- K. Fedorov, X. Sun and G. Boczkaj, Chem. Eng. J., 2021, 417, 128081 CrossRef CAS.
- B. Verhaagen and D. Fernandez Rivas, Ultrason. Sonochem., 2016, 29, 619–628 CrossRef CAS.
- R. M. Trujillo, G. Almanza, D. Sanchez-Saldana, O. Rosand, M. Hoydal, M. Fernandino and C. A. Dorao, Lab Chip, 2023, 23, 4773–4782 RSC.
- E. L. Tanzi, C. C. Capelli, D. W. Robertson, B. LaTowsky, C. Jacob, O. Ibrahim and M. S. Kaminer, Lasers Surg. Med., 2022, 54, 121–128 CrossRef PubMed.
- J. Cao, C. L. Hu, H. Zhou, F. Q. Qiu, J. F. Chen, J. Zhang and P. T. Huang, Ultrasound Med. Biol., 2021, 47, 323–333 CrossRef PubMed.
- Z. G. Li, A. Q. Liu, E. Klaseboer, J. B. Zhang and C. D. Ohl, Lab Chip, 2013, 13, 1144 RSC.
- L. Auboire, D. Fouan, J. M. Gregoire, F. Ossant, C. Plag, J. M. Escoffre and A. Bouakaz, Pharmaceutics, 2021, 13, 1566 CrossRef CAS PubMed.
- B. Petit, F. Yan, P. Bussat, Y. Bohren, E. Gaud, P. Fontana, F. Tranquart and E. Allémann, J. Drug Delivery Sci. Technol., 2015, 25, 29–35 CrossRef CAS.
- Y. Gao, M. Wu, B. I. Gaynes, R. S. Dieter and J. Xu, Lab Chip, 2021, 21, 3707–3714 RSC.
- S. Shin Low, C. Nong Lim, M. Yew, W. Siong Chai, L. E. Low, S. Manickam, B. Ti Tey and P. L. Show, Ultrason. Sonochem., 2021, 80, 105805 CrossRef PubMed.
- M. Ghorbani, A. K. Sadaghiani, M. Yidiz and A. Koşar, J. Mech. Sci. Technol., 2017, 31, 235–247 CrossRef.
- H. Zheng, Y. Zheng and J. Zhu, Engineering, 2022, 19, 180–198 CrossRef CAS.
- F. Zhao, Z. Wang and H. Huang, Processes, 2024, 12, 2059 CrossRef CAS.
- J. A. McAteer and A. P. Evan, Semin. Nephrol., 2008, 28, 200–213 CrossRef.
- M. Ghorbani, A. K. Sadaghiani, M. Yidiz and A. Kosar, J. Mech. Sci. Technol., 2017, 31, 235–247 CrossRef.
- A. P. Keller, Cavitation scale effects—empirically found relations and the correlation of cavitation number and hydrodynamic coefficients, in CAV 2001: Fourth International Symposium on Cavitation, Pasadena, CA, USA, 2001 Search PubMed.
- B. Ozogul, U. Akar, R. Mercimek, F. R. Talabazar, S. S. Sarraf, A. S. Aghdam, A. A. Hamedani, L. G. Villanueva, D. Grishenkov, E. Amani, T. Elverdi, M. Ghorbani and A. Koşar, Adv. NanoBiomed Res., 2024, 5, 2400112 CrossRef.
- S. A. Hendley, J. D. Paul, A. D. Maxwell, K. J. Haworth, C. K. Holland and K. B. Bader, IEEE Trans. Ultrason. Ferroelectr. Freq. Control, 2021, 68, 2942–2952 Search PubMed.
- C. Heckl, A. Lang, A. Ruhm, R. Sroka, T. Duffield, M. Vogeser and M. Paal, J. Biophotonics, 2021, 14, e202000461 CrossRef CAS PubMed.
- P. Liu, Z. Zhu, C. Zeng and G. Nie, J. Biomed. Opt., 2012, 17, 125002 CrossRef.
- F. Rokhsar Talabazar, A. Sheibani Aghdam, M. Jafarpour, D. Grishenkov, A. Koşar and M. Ghorbani, Chem. Eng. J., 2022, 445, 136734 CrossRef CAS.
- W. G. Zijlstra and A. Buursma, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1997, 118, 743–749 CrossRef.
- V. A. Buzanovskii, Rev. J. Chem., 2017, 7, 79–124 CrossRef CAS.
- C. Maegerlein, B. Friedrich, M. Berndt, K. E. Lucia, L. Schirmer, H. Poppert, C. Zimmer, J. Pelisek, T. Boeckh-Behrens and J. Kaesmacher, Interv. Neuroradiol., 2018, 24, 70–75 CrossRef PubMed.
- T. Fruhwald, U. Gartner, N. Stockmann, J. H. Marxsen, C. Gramsch and F. C. Roessler, BMC Neurol., 2019, 19, 181 CrossRef PubMed.
- Y. Li, Y. Li and H. Chen, Artif. Organs, 2024, 48, 734–742 CrossRef CAS PubMed.
- J. Tang, J. Tang, Y. Liao, L. Bai, T. Luo, Y. Xu and Z. Liu, Heliyon, 2024, 10, e26624 CrossRef CAS PubMed.
- Y. Pan, Y. Li, Y. Chen, J. Li and H. Chen, Adv. Healthcare Mater., 2024, 13, e2303358 CrossRef.
- Y. Pan, Y. Li, Y. Li, X. Zheng, C. Zou, J. Li and H. Chen, Adv. Healthcare Mater., 2023, 12, e2202281 CrossRef PubMed.
- X. Zheng, Y. Pan, Z. Wang and S. Zhang, J. Thromb. Thrombolysis, 2024, 57, 1056–1066 CrossRef PubMed.
- S. S. Adam, N. S. Key and C. S. Greenberg, Blood, 2009, 113, 2878–2887 CrossRef CAS PubMed.
- M. Franchini, D. Focosi, M. P. Pezzo and P. M. Mannucci, Haematologica, 2024, 109, 1035–1045 CrossRef CAS PubMed.
- N. Venketasubramanian, L. L. L. Yeo, B. Tan and B. P. L. Chan, J. Cardiovasc. Dev. Dis., 2024, 11, 75 Search PubMed.
- S. Yang, C. Zemzemi, D. S. Escudero, D. C. Vela, K. J. Haworth and C. K. Holland, Ultrasound Med. Biol., 2024, 50, 1167–1177 CrossRef PubMed.
- M. Maleki, F. R. Talabazar, S. H. Davoudian, M. Dular, A. Kosar, M. Petkovsek, A. Smid, M. Zupanc and M. Ghorbani, Chem. Eng. J., 2025, 511, 161976 CrossRef CAS.
- I. Namli, Z. Karavelioglu, S. S. Sarraf, A. S. Aghdam, R. Varol, A. Yilmaz, S. B. Sahin, B. Ozogul, D. N. Bozkaya, H. F. Acar, H. Uvet, S. Cetinel, O. Kutlu, M. Ghorbani and A. Kosar, Lab Chip, 2023, 23, 2640–2653 RSC.
- Z. Q. Tan, E. H. Ooi, Y. S. Chiew, J. J. Foo, Y. K. Ng and E. T. Ooi, Comput. Biol. Med., 2024, 181, 109061 CrossRef.
- A. Elnager, W. Z. Abdullah, R. Hassan, Z. Idris, N. Wan Arfah, S. A. Sulaiman and Z. Mustafa, Adv. Hematol., 2014, 2014, 1–8 CrossRef PubMed.
- I. N. Chernysh, C. E. Everbach, P. K. Purohit and J. W. Weisel, J. Thromb. Haemostasis, 2015, 13, 601–609 CrossRef CAS.
- R. Morihara, T. Yamashita, Y. Osakada, T. Feng, X. Hu, Y. Fukui, K. Tadokoro, M. Takemoto and K. Abe, J. Cereb. Blood Flow Metab., 2022, 42, 1322–1334 CrossRef CAS.
- D. V. Sakharov, R. T. Hekkenberg and D. C. Rijken, Thromb. Res., 2000, 100, 333–340 CrossRef CAS PubMed.
- V. Nahirnyak, T. D. Mast and C. K. Holland, Ultrasound Med. Biol., 2007, 33, 1285–1295 CrossRef PubMed.
- K. B. Bader, F. Padilla, K. J. Haworth, N. Ellens, D. Dalecki, D. L. Miller and K. A. Wear, J. Ultrasound Med., 2024, 44, 381–433 CrossRef PubMed.
- E. Kestek, U. Akar, S. Seyedmirzaei Sarraf, O. Kanbur, U. Gorkem Kirabali, H. Eda Sutova, M. Ghorbani, O. Kutlu, H. Uvet, A. Isin Dogan Ekici, S. Ekici, G. Kozalak and A. Kosar, Ultrason. Sonochem., 2025, 114, 107223 CrossRef CAS PubMed.
- Z. Zeng, T. Nallan Chakravarthula, A. Christodoulides, A. Hall and N. J. Alves, J. Mater. Sci.: Mater. Med., 2023, 34, 24 CrossRef CAS PubMed.
- H. L. Weiss, P. Selvaraj, K. Okita, Y. Matsumoto, A. Voie, T. Hoelscher and A. J. Szeri, J. Acoust. Soc. Am., 2013, 133, 3159–3175 CrossRef PubMed.
- P. Jolugbo and R. A. S. Ariens, Stroke, 2021, 52, 1131–1142 CrossRef CAS PubMed.
- K. C. Gersh, K. E. Edmondson and J. W. Weisel, J. Thromb. Haemostasis, 2010, 8, 2826–2828 CrossRef CAS PubMed.
- A. E. X. Brown, R. I. Litvinov, D. E. Discher, P. K. Purohit and J. W. Weisel, Science, 2009, 325, 741–744 CrossRef CAS PubMed.
- P. Riha, X. Wang, R. Liao and J. F. Stoltz, Clin. Hemorheol. Microcirc., 1999, 21, 45–49 CAS.
- R. Devanagondi, X. Zhang, Z. Xu, K. Ives, A. Levin, H. Gurm and G. E. Owens, J. Vasc. Interv. Radiol., 2015, 26, 1559–1565 CrossRef PubMed.
- S. Datta, C. C. Coussios, L. E. McAdory, J. Tan, T. Porter, G. De Courten-Myers and C. K. Holland, Ultrasound Med. Biol., 2006, 32, 1257–1267 CrossRef PubMed.
- Y. H. Chuang, P. W. Cheng and P. C. Li, IEEE Trans. Ultrason. Ferroelectr. Freq. Control, 2013, 60, 97–104 Search PubMed.
- A. D. Maxwell, S. Park, C. A. Cain and Z. Xu, Trapping of solid particles by cavitation-induced acoustic streaming, 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 2011, pp. 1506–1509 Search PubMed.
- A. Soltani, R. Singhal, M. Obtera, R. A. Roy, W. M. Clark and D. R. Hansmann, J. Thromb. Thrombolysis, 2011, 31, 71–84 CrossRef PubMed.
- H. L. Weiss, P. Selvaraj, K. Okita, Y. Matsumoto, A. Voie, T. Hoelscher and A. J. Szeri, J. Acoust. Soc. Am., 2013, 133, 3159–3175 CrossRef PubMed.
- S. A. Hendley, J. D. Paul, A. D. Maxwell, K. J. Haworth, C. K. Holland and K. B. Bader, IEEE Trans. Ultrason. Ferroelectr. Freq. Control, 2021, 68, 2942–2952 Search PubMed.
- Y. Kasahara, T. Nakagomi, T. Matsuyama, D. Stern and A. Taguchi, Stroke, 2012, 43, 499–506 CrossRef CAS PubMed.
- S. S. Save, A. B. Pandit and J. B. Joshi, Chemical Engineering Journal and the Biochemical Engineering Journal, 1994, 55, B67–B72 Search PubMed.
- S. S. Save, A. B. Pandit and J. B. Joshi, Food Bioprod. Process., 1997, 75, 41–49 CrossRef.
- J.-M. Lévêque, G. Cravotto, F. Delattre and P. Cintas, in Organic Sonochemistry, ed. J.-M. Lévêque, G. Cravotto, F. Delattre and P. Cintas, Springer International Publishing, Cham, 2018, ch. 8, pp. 113–123, DOI:10.1007/978-3-319-98554-1_8.
- N. T. Blum, C. M. Gyorkos, S. J. Narowetz, E. N. Mueller and A. P. Goodwin, ACS Appl. Mater. Interfaces, 2019, 11, 36324–36332 CrossRef CAS PubMed.
- M. T. Hopp, S. M. Vaidya, K. M. Grimmig, L. J. Strudthoff, J. C. Clauser, X. Yuan, S. Singh, J. Muller, J. Oldenburg, I. Hamza and D. Imhof, Anal. Chim. Acta, 2024, 1312, 342766 CrossRef CAS PubMed.
- G. J. Ma, A. R. Ferhan, J. A. Jackman and N. J. Cho, Commun. Mater., 2020, 1, 45 CrossRef.
- P. R. Gogate and A. B. Pandit, Rev. Chem. Eng., 2001, 17, 1–85 CAS.
- T. Mason, J. Lorimer, D. Bates and Y. Zhao, Ultrason. Sonochem., 1994, 1, S91–S95 CrossRef CAS.
Footnote |
| † Abuzer Alp Yetisgin and Beyzanur Ozogul contributed equally to this work as first authors. |
| This journal is © The Royal Society of Chemistry 2026 |