A new potential synthetic pyrrhotite reference material for Fe–S isotope microanalysis
Xiao-Yan
Liu
a,
Lei
Chen
 *abc,
Fu-De
Zhao
a,
Fei
Huang
d,
Qiu-Li
Li
*abc,
Fu-De
Zhao
a,
Fei
Huang
d,
Qiu-Li
Li
 e,
Hui-Min
Yu
e,
Hui-Min
Yu
 f and
Xian-Hua
Li
f and
Xian-Hua
Li
 e
e
aSchool of Earth Sciences and Resources, China University of Geosciences (Beijing), Beijing, China. E-mail: chenlei1211@gmail.com; chenlei1211@cugb.edu.cn
bMining Research Institute of Baotou Steel (Group) Corp., Baotou, China
cMNR Key Laboratory for Exploration Theory & Technology of Critical Mineral Resources, China University of Geosciences (Beijing), Beijing, China
dSchool of Resources and Civil Engineering, Northeastern University, Shenyang, China
eState Key Laboratory of Lithospheric and Environmental Coevolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China
fState Key Laboratory of Lithospheric and Environmental Coevolution, School of Earth and Space Sciences, University of Science and Technology of China, Hefei, China
First published on 31st October 2025
Abstract
Pyrrhotite is a common Fe sulfide mineral in meteorites and magmatic sulfide ore deposits. It can be used to trace the origin of metals, as well as the formation and evolution of ore deposits. The natural pyrrhotite reference materials have been extensively consumed with the development of in situ techniques. In this study, we synthesized pyrrhotite by the hydrothermal method and evaluated its suitability as a reference material for in situ Fe and S isotope analysis. The synthetic pyrrhotite obtained through a hydrothermal experiment exhibits uniform surfaces and dense structures. The electron probe microanalysis (EPMA) verified the homogeneity of the major elements. The results of laser ablation multiple-collector inductively coupled plasma mass spectrometry (LA-MC-ICP-MS) for the iron isotopes and secondary ion mass spectrometry (SIMS) for the sulfur isotopes demonstrate the homogeneity of Fe and S isotopes, respectively. For in situ microanalysis, the two standard deviations (2SD) of iron and sulfur isotope compositions of SY-Po are 0.47‰ (2SD, n = 30) and 0.53‰ (2SD, n = 133), respectively. The solution MC-ICP-MS analyses and isotope ratio mass spectrometry (IRMS) results for SY-Po determined that the best recommended δ56Fe value is 0.39 ± 0.03‰ (2SD, n = 3) and δ34S value is −1.16 ± 0.23‰ (2SD, n = 11). Synthetic pyrrhotite SY-Po is a new potential reference material for in situ Fe and S isotope microanalysis, intended for tracing the formation processes of Fe sulfide minerals in magmatic sulfide ore deposits and meteorites.
1. Introduction
The Fe–S isotope geochemistry of sulfides has now become a powerful tool for studying various geological processes, including magmatic, metamorphic, sedimentary, hydrothermal, and biological processes.1–8 Pyrrhotite (Fe1−xS with 0 ≤ x ≤ 0.125) is a common iron sulfide in magmatic and hydrothermal ore deposits and meteorites.9–11 The iron and sulfur isotope compositions of pyrrhotite have been widely applied in tracing the formation, evolution, and source of ore-forming materials of magmatic sulfide deposits (e.g., ref. 12–17). Besides, the sulfur isotope compositions of pyrrhotite and troilite (variant of pyrrhotite18) have become an essential tool for studying the evolution process of the lunar magma ocean, e.g., homogeneity of lunar mantle sulfur isotopes19,20 or degassing process.20The microanalysis of Fe and S isotope ratios can be conducted by laser ablation multiple-collector inductively coupled plasma mass spectrometry (LA-MC-ICP-MS21,22) and secondary ion mass spectrometry (SIMS23–25). These two in situ analytical methods offer significant advantages over conventional bulk analysis techniques, including efficient sample introduction,26 high spatial resolution,27–30 and the capability to detect isotopic variations at the micrometre scales.31–33 However, instrument fractionation and matrix effects from LA-MC-ICP-MS and SIMS still limit the accurate determination of the Fe–S isotope ratios.34–36 Previous research results have demonstrated that the use of matrix-matched reference materials (RMs) can effectively eliminate the matrix effects and instrument fractionation generated during the analysis process.37,38 Therefore, the development of a well-characterized reference material is significant for the accuracy of the Fe–S isotope compositions of pyrrhotite in microbeam analysis.
Some studies have developed natural pyrrhotite RMs for Fe isotope analysis (JC-Po38 and Ll-Po,39Table 1) or for S isotope analysis (Po-10,40 Alexo,12 YP136,41 Ll-Po![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 and JC-Po,38Table 1). The amount of natural pyrrhotite RMs is minimal and non-renewable. In recent years, several laboratories have conducted methodological studies on the synthesis of sulfide RMs for isotope analysis, including the natural sulfide powder pressed pellet method,27,42 rapid hot-pressing method,43 plasma-activated sintering (PAS),44,45 high-temperature melting (HTM),28,45 and hydrothermal method.46 The synthesis methods of pyrrhotite mainly include dry methods47,48 and wet-chemical routes (e.g., hydrothermal method,49,50 electro-synthesis method51 and heating-up method52). Among these, the hydrothermal method is much more suitable for synthesizing pyrrhotite that meets the requirements of in situ analysis morphology.53 Previous studies have demonstrated that the pyrrhotite synthesized through hydrothermal experiments exhibits a plate-like morphology, characterized by large grains, a clean surface, and no cracks.49,50,53 Although the hydrothermal method can be used to synthesize pyrrhotite, there are currently no studies on the synthetic pyrrhotite as a RM for Fe–S isotope microanalysis.
39 and JC-Po,38Table 1). The amount of natural pyrrhotite RMs is minimal and non-renewable. In recent years, several laboratories have conducted methodological studies on the synthesis of sulfide RMs for isotope analysis, including the natural sulfide powder pressed pellet method,27,42 rapid hot-pressing method,43 plasma-activated sintering (PAS),44,45 high-temperature melting (HTM),28,45 and hydrothermal method.46 The synthesis methods of pyrrhotite mainly include dry methods47,48 and wet-chemical routes (e.g., hydrothermal method,49,50 electro-synthesis method51 and heating-up method52). Among these, the hydrothermal method is much more suitable for synthesizing pyrrhotite that meets the requirements of in situ analysis morphology.53 Previous studies have demonstrated that the pyrrhotite synthesized through hydrothermal experiments exhibits a plate-like morphology, characterized by large grains, a clean surface, and no cracks.49,50,53 Although the hydrothermal method can be used to synthesize pyrrhotite, there are currently no studies on the synthetic pyrrhotite as a RM for Fe–S isotope microanalysis.
| Standard | Material | Solution δ56Fea (‰) | In situ δ 56Fe min.a (‰) | In situ δ 56Fe max.a (‰) | Reproducibility 2SDb | Spot size (µm) | Equipment | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Values for δ56Fe given in per mil relative to IRMM-014. b 2SD is defined as intra-grain micro-beam analytical uncertainty. c Values for δ34S given in per mil relative to Vienna-Cañon Diablo Troilite (V-CDT). | ||||||||
| JC-Po | Pyrrhotite | −0.34 | −0.72 | 0.05 | 0.33 | 35 | LA | 38 |
| Ll-Po | Pyrrhotite | −0.62 | −0.83 | −0.48 | 0.18 | 30–40 | (fs)-LA | 39 |
| Standard | Material | IRMS δ34Sc (‰) | In situ δ 34S min.c (‰) | In situ δ 34S max.c (‰) | Reproducibility 2SDb | Spot size (µm) | Equipment | Ref. |
|---|---|---|---|---|---|---|---|---|
| Po-10 | Pyrrhotite | 6.10 | — | — | 0.40 | 20 | Ion microprobe | 40 |
| Alexo | Pyrrhotite | 5.23 | 4.81 | 5.78 | 0.30 | 20–30 | SIMS | 12 |
| YP136 | Pyrrhotite | 1.50 | 1.50 | 2.00 | 0.30 | 10 | SIMS | 41 |
| Ll-Po | Pyrrhotite | 6.42 | 5.67 | 6.74 | 0.38 | 40 | (fs)-LA | 39 |
| JC-Po | Pyrrhotite | 0.06 | −0.33 | 0.34 | 0.27 | 20 | SIMS | 38 |
In this study, we first proposed a new pyrrhotite RM synthesized by hydrothermal experiments. The morphology, textures, element compositions, and Fe and S isotope ratios in the synthetic samples were studied. The homogeneity of Fe and S isotopes of the synthetic pyrrhotite was evaluated by LA-MC-ICP-MS and SIMS, respectively. Finally, our results suggest that synthetic pyrrhotite SY-Po is a new potential pyrrhotite RM for the microbeam determination of Fe–S isotope ratios. This RM is available in sufficient quantities and is renewable, allowing it to be shared with other microbeam analysis laboratories.
2. Sample description and preparation
The synthesis experiment was carried out in the School of Resources and Civil Engineering, Northeastern University. The hydrothermal experiment is a crucial method for synthesizing pyrrhotite. The hydrothermal experiments were conducted in a 100 mL polytetrafluoroethylene (PTFE) liner, sealed with a cylindrical lid, and subsequently placed in a stainless steel autoclave. Ferrous sulfate heptahydrate (FeSO4·7H2O, 0.5 mol L−1) and thioacetamide (CH3CSNH2, TAA, 0.5 mol L−1) were separately dissolved in volumetric flasks to prepare the initial reaction solution. Subsequently, the ferrous sulfate and TAA solutions were transferred into a beaker and thoroughly mixed to achieve a molar ratio of Fe to S of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The resulting homogeneous mixture was then transferred into the pre-cleaned PTFE liner, which was filled to 60% of its total volume. Finally, the liner was securely sealed and placed inside the stainless steel autoclave for further processing. The autoclaves should be placed in a drying oven at 200 °C for 24 hours. Ferrous sulfate (FeSO4·7H2O) was dissolved in the reaction solution to form Fe2+. TAA was heated to decompose and produce H2S. At the end of the experiment, the steel autoclaves were cooled naturally in air for nearly 7.5 hours, and then the PTFE liners were removed. After cooling and filtration, the final experimental product was a pile of dark grey solid material. Then, the solid was ultrasonically cleaned three times with high-concentration alcohol to remove small mineral particles suspended in the upper layer. Finally, the cleaned solid was allowed to dry naturally. The yield of pyrrhotite was highest under these experimental conditions.
3. The resulting homogeneous mixture was then transferred into the pre-cleaned PTFE liner, which was filled to 60% of its total volume. Finally, the liner was securely sealed and placed inside the stainless steel autoclave for further processing. The autoclaves should be placed in a drying oven at 200 °C for 24 hours. Ferrous sulfate (FeSO4·7H2O) was dissolved in the reaction solution to form Fe2+. TAA was heated to decompose and produce H2S. At the end of the experiment, the steel autoclaves were cooled naturally in air for nearly 7.5 hours, and then the PTFE liners were removed. After cooling and filtration, the final experimental product was a pile of dark grey solid material. Then, the solid was ultrasonically cleaned three times with high-concentration alcohol to remove small mineral particles suspended in the upper layer. Finally, the cleaned solid was allowed to dry naturally. The yield of pyrrhotite was highest under these experimental conditions.
To facilitate observation and study, a portion of over 100 synthetic pyrrhotite grains was handpicked from the solid part. Some of them were placed on epoxy resin bases with a diameter of 2.5 cm for taking BSE photographs, EPMA analysis, and in situ Fe–S isotope tests. Another part of the synthetic pyrrhotite powder was used for taking morphological photos of pyrrhotite and bulk Fe–S isotope analysis.
3. Analytical methods
To determine the chemical and isotope composition of potential pyrrhotite RMs, six analytical techniques were employed. Firstly, we performed wavelength dispersive spectroscopy (WDS) by electron probe microanalysis (EPMA) on multiple grains of synthetic pyrrhotite to ensure the chemical composition homogeneity of all sample grains. Secondly, the morphology, potential chemical zonation, mineralogical inclusions, and fractures of pyrrhotite were observed using a scanning electron microscope (SEM) and a field-emission scanning electron microscope (FESEM). Thirdly, the multiple grains were analyzed by bulk Fe–S analysis methods to determine the isotope compositions. Bulk Fe and S analyses were completed by solution MC-ICP-MS and isotope ratio mass spectrometry (IRMS), respectively. Finally, more than 100 µm of synthetic pyrrhotite grains were analyzed for in situ Fe–S isotope by LA-MC-ICP-MS and SIMS, respectively, to determine the reproducibility of the grains. Each analysis technique is described in detail below.3.1 Electron probe microanalysis (EPMA)
The major element chemical compositions were obtained by using a JEOL JXA-8100 microprobe equipped with a fully automated five X-ray WDS at the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Beijing, China. The operating conditions consisted of an acceleration voltage of 15 kV, a probe current of 20 nA, and a beam diameter of 5 µm. The following sulfides were used as standards in this study: FeS2 (Fe, S), FeAsS (As), PbS (Pb), CuFeS2 (Cu), ZnS (S), NiS (Ni), and CoS (Co). The detection limits for each element were Fe (126 ppm), S (126 ppm), As (478 ppm), Pb (616 ppm), Cu (111 ppm), Zn (189 ppm), Ni (96 ppm), and Co (88 ppm).3.2 Field emission scanning electron microscope (FESEM)
The morphology of SY-Po pyrrhotite grains was observed using a Thermo Fisher Apreo 2C FESEM at the Analytical and Testing Center, Northeastern University, China. Before analysis, the pyrrhotite grains were fixed to a copper column with conductive adhesive and coated with ∼10 nm thick carbon for electrical conduction. The FESEM images were obtained at an accelerating voltage of 20 kV and a beam current of 20 nA, with a working distance of 15 mm.3.3 LA-MC-ICP-MS iron isotope ratios measurement
The homogeneity test of iron isotopes from pyrrhotite was conducted at the Beijing Createch Testing Technology Co., Ltd. The Fe isotopes were measured using a ThermoFinnigan Neptune Plus multi-collection inductively coupled plasma mass spectrometry (high-resolution MC-ICP-MS) combined with a RESOlution-193 laser ablation system. The working parameters of MC-ICP-MS are detailed in ref. 38.The analytical conditions for Fe isotope measurements involved an ablation pit of 20 µm diameter, an ablation time of 10 s, a repetition rate of 6 Hz, and a laser beam energy density of 2 J cm−2. The ultrapure water was introduced into the ICP to form a “wet” plasma atmosphere, which can effectively suppress the matrix effect during the Fe isotope analysis.54,55 Since argon nitrides and argon oxides polyatomic ions are produced in the plasma sources during LA-MC-ICP-MS measurements, these polyatomic ions have the same isobaric mass as 54Fe, 56Fe, 57Fe, and 58Fe. Therefore, LA-MC-ICP-MS measurements of Fe isotopes were performed at high mass resolution (M/ΔM ≈ 9000, 5–95% peak side width definition) to exclude polyatomic ions interference with Fe isotopes during the experiment.56 A detector array of Faraday cups is set to acquire the ion beams of 53Cr, 54Fe, 56Fe, 57Fe, 58Fe, and 60Ni. 53Cr, and 60Ni are used to correct the potential isobaric interference of 54Fe and 58Fe, respectively.57 The SSB (Sample-Standard Bracketing) method was employed for mass fractionation correction. The JC-Po RM was used as the quality control sample for in situ Fe isotope analysis of pyrrhotite.38 The RMs were measured for drift monitoring before LA-MC-ICP-MS analysis of every 2–3 samples. The measurement values of δ56FeIRMM-014 in JC-Po were −0.39 ± 0.38‰ (2SD, n = 12), which were consistent with reference values within the uncertainty.38
The measured 56Fe/54Fe and 57Fe/54Fe ratios were converted to δ56Fe and δ57Fe values through normalizing by using the IRMM-014 compositions (56Fe/54Fe = 15.6979, 57Fe/54Fe = 0.3625 (ref. 58)) as follows,
| δ56Femeasured in per mil = (56Fe/54Femeasured ÷ 15.6929 − 1) × 1000 | (1) |
| δ57Femeasured in per mil = (57Fe/54Femeasured ÷ 0.3625 − 1) × 1000 | (2) |
The δiFemeasured values were normalised with the average value of solution MC-ICP-MS measurements following the equation below,
| δiFefinal = δiFemeasured + (mean of δiFesolution MC-ICP-MS − mean of δiFemeasured) | (3) |
The long-term external reproducibility in high-resolution mode was better than 0.50‰ for δ56Fe at 2SD.
3.4 Solution MC-ICP-MS iron isotope ratios measurement
The Fe isotope ratios were analysed at the State Key Laboratory of Lithospheric and Environmental Coevolution, School of Earth and Space Sciences, University of Science and Technology of China (USTC). The purification procedure and mass-spectrometer setups refer to the methods presented in previous studies.59 In the purification process, Fe is first purified by anion resin (Bio-Rad AG1-X8) under 6 N HCl conditions. Hydrochloric acid washes away the remaining matrix elements. Then, Fe is eluted with 0.4 N HCl and H2O and 6 N HCl. The total procedural blanks for Fe were less than 10 ng, which is insignificant relative to the amount of Fe (≥20 µg) in all chemical purification processes. The iron isotope compositions of synthetic pyrrhotite have been measured by the standard bracketing method using a Neptune-Plus at the University of Science and Technology of China. 53Cr, 54Fe, 56Fe, 57Fe, 58Fe, and 60Ni were measured in the static mode of L3, L1, H1, H2, and H4 Faraday cups, respectively. To avoid the Cr and Ni elements that produced interference. The Fe isotope results are expressed as deviations of an iron isotope ratio of a sample from that of the RM IRMM-014 in per mil (56Fe/54Fe = 15.6979, 57Fe/54Fe = 0.3625)58:| δiFe = [(iFe/54Fe)measured/(iFe/54Fe)standard − 1] × 1000 | (4) |
The long-term external reproducibility in high-resolution mode was better than 0.05‰ for δ56Fe at 2SD.15,59
3.5 IRMS sulfur isotope measurement
A total of 11 SY-Po pyrrhotite samples were selected randomly for sulfur isotope analysis. Eight samples were analyzed at the State Key Laboratory of Critical Mineral Research and Exploration, Institute of Geochemistry, CAS. Three samples were analyzed at the Stable Isotope Laboratory at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, CAS. Pure sulfide grains were manually selected under a binocular microscope. Sulfur isotope compositions for SY-Po pyrrhotite and Tianyu-Py pyrite RM were determined using the EA-IRMS system. Approximately 0.08 mg of sulfide sample was combined with V2O5 and combusted at 1000 °C in an elemental analyzer, and the product SO2 was analyzed mass spectrometrically on a MAT 253 mass spectrometer (Thermo Fisher Scientific Inc.). All laboratory standards used were calibrated by international standard samples IAEA-S-1, IAEA-S-2, and IAEA-S-3. The sulfur isotope is expressed in standard δ notation as per mil (‰) deviations from V-CDT.Sulfur isotope analysis by IRMS was performed to determine δ34S value of Tianyu-Py pyrite RM at three laboratories (Table 2). The IRMS results of Tianyu-Py pyrite RM determined that the best recommended δ34S value is 3.98 ± 0.23‰ (2SD, n = 19).
| Lab. | Replicates | Tianyu-Py RM |
|---|---|---|
| δ 34S (‰) | ||
| a Lab. 1: State Key Laboratory of Critical Mineral Research and Exploration, Institute of Geochemistry, CAS; Lab. 2: Beijing Research Institute of Uranium Geology; Lab. 3: Stable Isotope Laboratory at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, CAS. | ||
| 1 | 1 | 4.19 |
| 2 | 4.15 | |
| 3 | 4.07 | |
| 4 | 4.15 | |
| 5 | 4.05 | |
| 6 | 4.00 | |
| 7 | 3.98 | |
| Mean | 4.08 ± 0.16 (2SD) | |
| 2 | 1 | 3.97 |
| 2 | 3.97 | |
| 3 | 3.85 | |
| 4 | 3.97 | |
| 5 | 3.97 | |
| 6 | 3.90 | |
| 7 | 3.78 | |
| 8 | 3.82 | |
| 9 | 3.84 | |
| Mean | 3.90 ± 0.15 (2SD) | |
| 3 | 1 | 3.99 |
| 2 | 3.96 | |
| 3 | 4.08 | |
| Mean | 4.01 ± 0.12 (2SD) | |
| Grand mean | 3.98 ± 0.23 (2SD) | |
3.6 SIMS sulfur isotope measurement
The sulfur isotopes (32S, 33S, and 34S) of SY-Po pyrrhotites were measured using a Cameca IMS-1280 at the Institute of Geology and Geophysics (Beijing), Chinese Academy of Sciences. The analytical procedures and instrument parameters were detailed in Chen et al., 2015.60 A gold coat of ∼50 nm thickness was applied to the sample surface to ensure electrical conductivity, and the sample was then loaded into the sample chamber. The Cs+ primary ion beam with an intensity of ca. 2.5 nA (Gaussian mode with a primary beam aperture of 400 µm to reduce aberrations) is accelerated under the condition of 10 kV. The beam was scanned ∼20 µm × 20 µm during analysis to reduce the depth of the crater. Utilisation of a normal-incidence electron flood gun (NEG) to neutralise the possible positive charge buildup in the analysis area. The accelerating voltage for obtaining negative ions is −10 kV. The field aperture was set to 2000 µm, and the transfer-optics magnification was adjusted to give a field of view of 125 µm (FA = 8000). Before starting the session (5 eV gap with respect to the maximum), the mechanical position of the energy slit was calibrated and its width set to 25 eV. Sulfur isotopes were measured in multi-collector mode using three off-axis Faraday cups (L′2, L1, and H1). Each Faraday cup detector was positioned along the focal plane for the simultaneous measurements of 32S, 33S, and 34S. The entrance slit width of each detector is 60 µm, and the exit slit width is 500 µm. The mass resolution power (MRP) of 2400 was employed to have the top peaks on all the masses. Meanwhile, nuclear magnetic resonance (NMR) regulation is utilised to maintain the stability of the magnetic field. The intensity of 32S was typically 1 × 109 cps. Each analysis takes about 4.5 min, consisting of 30 s pre-sputtering, 60 s of automated centering of secondary ions, and 160 s integrating sulfur isotope signals (40 cycles × 4 s).Measurements of 34S/32S and 33S/32S ratios were normalized by using the Vienna-Cañon Diablo Troilite (V-CDT) compositions (34S/32S = 0.044163, 33S/32S = 0.007877)61 as follows,
| δ34Smeasured in per mil = (34S/32Smeasured ÷ 0.044163 − 1) × 1000 | (5) |
| δ33Smeasured in per mil = (33S/32Smeasured ÷ 0.007877 − 1) × 1000 | (6) |
Sonora pyrite RM was used as a running standard for sulfur isotope analysis sessions. Every 5 to 10 SY-Po pyrrhotite analyses were bracketed by 2 or 3 Sonora RM analyses in sessions 1 and 2. Sonora and Tianyu-Py pyrite RMs were used to monitor the instrumental drift.37,60 Every 5 Tianyu-Py analyses were bracketed by 2 or 3 Sonora RM analyses in session 3.
The δxSmeasured values were normalized with the average value of SY-Po measured by IRMS. The formula is as follows:
| δxSfinal = δxSmeasured + (mean of δxS IRMS − mean of δxSmeasured) | (7) |
In this study, the average value of δ34S corrected by Sonora for Tianyu-Py RM was 4.05 ± 0.47‰ (2SD, n = 25, Table S1). The calibrated δ34S values from SIMS were consistent with the δ34S value of Tianyu-Py pyrite RM obtained from the IRMS (3.98 ± 0.23‰, 2SD, n = 19), within the error range. The in situ sulfur isotope measurements of pyrite RMs prove that the sulfur isotope ratios of sulfide minerals obtained by SIMS are reliable at IGGCAS.
4. Results and discussion
4.1 Characteristics of the synthetic pyrrhotite
The products obtained from the hydrothermal experiments in this study include pyrrhotite and a few fine-grained pyrite (Fig. 1A). Firstly, the BSE images and photomicrograph show that the surface of the synthetic pyrrhotite is clean and uniform, with few cracks. The grain sizes range from 10 µm to 150 µm (Fig. 1B and C). These crack areas should be avoided as much as possible during microbeam analysis. Among them, pyrite is distributed in circular granular form around pyrrhotite, with grain sizes ranging from 3 µm to 10 µm (Fig. 1A). The SY-Po pyrrhotites have regular morphology with flat planes (Fig. 1D).The major element compositions of individual spots from SY-Po pyrrhotite are listed in Table S2. In this study, we randomly selected 20 spots on SY-Po pyrrhotite grains for major elements analysis by EPMA. The chemical compositions of SY-Po pyrrhotite are fairly homogeneous, with the concentration of Fe ranging from 58.98 wt% to 61.40 wt% and the concentration of S ranging from 38.86 wt% to 40.00 wt%. The average concentrations of other elements (n = 20) are As 0.05 wt%, Pb 0.04 wt%, Cu 0.01 wt%, Zn 0.01 wt%, Ni 0.00 wt%, and Co 0.05 wt%. The BSE image (Fig. 1B) of SY-Po pyrrhotite shows no internal growth or zonation. WDS analysis indicated that the sample is stoichiometric pyrrhotite.
4.2 Fe and S isotope compositions of the synthetic pyrrhotite
The measurement results of the solution MC-ICP-MS for SY-Po synthetic pyrrhotite are shown in Table 3 and Fig. 2A, with data presented as the means ± 2SD. Three samples of SY-Po synthetic pyrrhotite were selected as a single sample. The results show limited variation in δ56Fe values between 0.38‰ and 0.41‰, and the average δ56Fe value was 0.39 ± 0.03‰ (2SD, n = 3, Fig. 2A). The grand mean value is interpreted as the best recommended Fe isotope values for SY-Po synthetic pyrrhotite.| Sample | δ 56Fe (‰) | 2SD (‰) |
|---|---|---|
| SY-Po | 0.38 | 0.05 |
| 0.38 | 0.05 | |
| 0.41 | 0.02 | |
| Mean | 0.39 ± 0.03‰ (2SD) | |
Sulfur isotope compositions determined at the two different laboratories were consistent. All pyrrhotites were supplied as single samples. Eleven measurements of sulfur isotopes from each sample were conducted by IRMS. The results are shown in Table 4. The SY-Po values of all δ34S ranged from −1.31‰ to −0.98‰ and give an average of −1.16 ± 0.23‰ (2SD, n = 11, Fig. 2B). The grand mean value is interpreted as the best recommended S isotope values for SY-Po synthetic pyrrhotite.
| Lab. | Replicates | SY-Po |
|---|---|---|
| δ 34S (‰) | ||
| a Lab. 1: State Key Laboratory of Critical Mineral Research and Exploration, Institute of Geochemistry, CAS; Lab. 2: Stable Isotope Laboratory at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, CAS. | ||
| 1 | 1 | −1.23 |
| 2 | −1.13 | |
| 3 | −1.05 | |
| 4 | −1.31 | |
| 5 | −1.27 | |
| 6 | −1.30 | |
| 7 | −1.11 | |
| 8 | −1.22 | |
| Mean | −1.20 ± 0.19 (2SD) | |
| 2 | 1 | −0.98 |
| 2 | −1.02 | |
| 3 | −1.12 | |
| Mean | −1.04 ± 0.14 (2SD) | |
| Grand mean | −1.16 ± 0.23 (2SD) | |
4.3 In situ Fe and S isotope ratios of the synthetic pyrrhotite
The results of in situ Fe isotope analysis conducted by LA-MC-ICP-MS are shown in Table S3 and graphically presented in Fig. 2A. A total of 30 in situ Fe isotope ratio analysis spots for SY-Po synthetic pyrrhotite were conducted by LA-MC-ICP-MS. The range of δ56Fe values of SY-Po synthetic pyrrhotite is from −0.12‰ to 0.76‰ (δ56Femean = 0.39‰), with a distribution close to Gaussian with standard deviation of 0.47 (2SD, Fig. 2C). The cracks in the grains caused the wide range of Fe isotope ratios, so that the flat and smooth area on the surface of grains is needed to be chosen carefully during the analysis.SIMS analyses comprising a total of 133 sulfur isotope ratio measurements were undertaken in two sessions. The graphically presented in Fig. 2B. The mean δ34S values of SY-Po in session 1 and session 2 were −1.16 ± 0.51‰ (2SD, n = 54) and −1.16 ± 0.55‰ (2SD, n = 79), respectively. The values of all δ34S ranged from −1.81‰ to −0.55‰, yielding a mean of −1.16‰ with a distribution close to Gaussian with two standard deviations of 0.53 (2SD, n = 133, Fig. 2D). The results show limited variations with excellent repeatability (Fig. 2B and D), and the raw data are given in Table S1.
The homogeneity of samples is the most basic requirement for preparing reference materials. Thus, the homogeneity of SY-Po synthetic pyrrhotite was tested according to the national regulations for reference material preparation (ISO Guide 35 2017).62 The heterogeneity of the composition of synthetic pyrrhotite is uncertain. Therefore, RMs prepared from synthetic materials should be subjected to an experimental homogeneity study.62 Based on the results of previous studies, an acceptable estimate of the between-unit variance for uncertainty evaluation can be obtained with nine or more degrees of freedom. In this study, fifteen grains of synthetic pyrrhotite were randomly selected to test the homogeneity of the Fe and S isotope ratios from SY-Po pyrrhotite. Two subsamples were extracted from each grain, and each subsample is an independent sample. Results of isotope analyses of the replicates are expressed as Xi1 and Xi2 in Table S4, and the average of duplicate pairs is given by ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) . Homogeneity tests were conducted under the same strict conditions, all tests were conducted in the same laboratory, by the same analyst using the same analytical method, and all samples were tested in the same session.
. Homogeneity tests were conducted under the same strict conditions, all tests were conducted in the same laboratory, by the same analyst using the same analytical method, and all samples were tested in the same session.
Single-factor ANOVA statistical analysis method was used to examine the inhomogeneity between-grains. The experimental F ratio is the ratio of the among-grain variance (Samong2) and the within-grain variance (Swithin2), and the formula is as follows:
 | (8) |
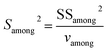 | (9) |
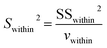 | (10) |
| vamong = m − 1 | (11) |
| vwithin = m(n − 1) | (12) |
The results are shown in Table 5. F-Testing demonstrates that the samples have very good homogeneity [F < Fcritical (vamong, vwithin); critical value of F for α = 5%]. Differences in isotope ratios were caused mainly by the repeatability of the method, rather than the heterogeneity of the reference materials.
| Sample | Isotope ratio |
![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif)
|
SSamong | SSwithin | F | F 0.05 (14, 15) |
|---|---|---|---|---|---|---|
| a n = 2; m = 15; vamong = 14; vwithin = 15. | ||||||
| SY-Po | 34S/32S | 0.0426 | 9.0639 × 10−4 | 9.6971 × 10−4 | 1.00 | 2.42 |
| SY-Po | 56Fe/54Fe | 16.2515 | 2.2204 × 10−4 | 1.7099 × 10−4 | 1.39 | 2.42 |
4.4 The analytical feasibility of SY-Po synthetic pyrrhotite RM
Numerous studies have shown that the iron and sulfur isotope compositions of pyrrhotite in magmatic sulfide deposits are highly variable, ranging from −1.48‰ to 0.05‰ (ref. 6, 7 and 15) and −6.90‰ to 3.18‰,14,17,32 respectively. Previous studies have shown that the δ34S values of troilites in lunar soil, measured by LA-MC-ICP-MS and NanoSIMS, range from −1.60‰ to 2.00‰.19,20 The ranges of Fe and S isotope variations in natural pyrrhotite samples are all greater than the error (2SD) of SY-Po synthetic pyrrhotite by microanalysis. Therefore, the two standard deviations (2SD, Fig. 2A and B) of the SY-Po synthetic pyrrhotite in this study are sufficient to distinguish the natural variation characteristics of pyrrhotite, which means that SY-Po synthetic pyrrhotite RM is suitable for Fe–S isotope analysis in lunar soil and geological samples.Besides, the δ34S values of the currently used pyrrhotite RMs range from 0.06‰ to 6.42‰, and the δ56Fe values range from −0.62‰ to −0.34‰ (Table 1). The newly introduced SY-Po synthetic pyrrhotite RM (δ56Fe = 0.39‰, δ34S = −1.16‰, Fig. 3) in this study effectively expands the coverage range of Fe and S isotopes of reported pyrrhotite RMs, filling the gap of reliable standards for wide-range in situ Fe–S isotope analysis.
 | ||
| Fig. 3 Plots of (A) δ57Fe vs. δ56Fe and (B) δ33S vs. δ34S for all pyrrhotite RMs reported in the literature and this study. Error crosses represent the reproducibility (at 2SD) by microanalysis. Note: SY-Po (this study), JC-Po (ref. 38), Ll-Po (ref. 39), YP-136 (ref. 41), and Po-10 (ref. 40). | ||
5. Conclusion
This study demonstrates that the Fe–S isotope compositions of the synthetic pyrrhotite (SY-Po) are homogeneous. The SY-Po synthetic pyrrhotite is suitable as a RM for Fe and S isotope ratios microanalysis using LA-MC-ICP-MS and SIMS. For the SY-Po synthetic pyrrhotite, the best recommended values of δ56Fe and δ34S are 0.39 ± 0.03‰ (2SD, n = 3) and −1.16 ± 0.23‰ (2SD, n = 11), respectively. This study contributes to the intersection of isotope geochemistry and materials science. Approximately 10 g of SY-Po synthesis pyrrhotite grains are currently available and can be obtained by contacting the corresponding author.Author contributions
Lei Chen and Xian-Hua Li conceived the presented idea. Xian-Hua Li encouraged Lei Chen to investigate the reference materials for microanalysis and supervised the project. Xiao-Yan Liu, Fu-De Zhao, Fei Huang, and Hui-Min Yu carried out the experiments. Qiu-Li Li contributed to the interpretation of the results. Xiao-Yan Liu and Lei Chen wrote the manuscript. All the authors provided critical feedback and helped shape the research and experiments.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ja00310e.Acknowledgements
We thank Jiao Li, Hong-Xia Ma, and Lin Meng for assistance in sample preparation. We also thank Dr Ning An, Jing Gu, Juan Han, Guo-Qiang Tang, and Ze-Xian Cui for sulfur isotope analysis and measurements. The manuscript benefited from detailed and constructive reviews from two anonymous reviewers, which are gratefully appreciated. We thank the editor Dr Elizabeth Bedwell for editorial handling and useful suggestions. This work was supported by the Major Research Plan of the National Natural Science Foundation of China (92262303), the National Key Research and Development Program of China (2023YFF0804200), the National Natural Science Foundation of China (42472128), the Central Guidance on Local Science and Technology Development Fund (24ZYQF001), the Key Research and Development Program of Gansu Province (24YFGA015), and the Program of Basic Geological Survey of Gansu Province.References
- J. Foden, P. A. Sossi and C. M. Wawryk, Lithos, 2015, 212–215, 32–44 CrossRef CAS.
- Y. W. Bao, M. J. Zhang, H. F. Zhang, Y. T. Feng, X. F. Li and J. He, Ore Geol. Rev., 2024, 173, 106224 CrossRef.
- S. J. Liu, B. Chen, J. H. Zheng, Y. F. Wu, C. Bao and G. C. Zhao, Gondwana Res., 2022, 106, 126–141 CrossRef CAS.
- C. Archer and D. Vance, Geology, 2006, 34, 153–156 CrossRef CAS.
- Y. W. Zhang, H. R. Fan, M. Santosh, L. W. Xie, F. F. Hu, X. Liu, H. L. Hu and X. H. Li, J. Asian Earth Sci., 2022, 230, 105217 CrossRef.
- L. D. Bilenker, D. Weis, J. S. Scoates and E. Perry, Econ. Geol., 2018, 113, 1181–1192 CrossRef.
- M. J. Brzozowski, D. J. Good, C. Z. Wu and W. Q. Li, Chem. Geol., 2021, 576, 120282 CrossRef CAS.
- A. J. Hall, A. J. Boyce and A. E. Fallick, Chem. Geol., 1987, 65, 305–310 CAS.
- N. Belzile, Y. W. Chen, M. F. Cai and Y. R. Li, J. Geochem. Explor., 2004, 84, 65–76 CrossRef CAS.
- D. Rickard and G. W. Luther, Chem. Rev., 2007, 107, 514–562 CrossRef CAS PubMed.
- S. J. Barnes, D. A. Holwell and M. Le Vaillant, Elements, 2017, 13, 89–95 CrossRef CAS.
- C. LaFlamme, L. Martin, H. Jeon, S. M. Reddy, V. Selvaraja, S. Caruso, T. H. Bui, M. P. Roberts, F. Voute, S. Hagemann, D. Wacey, S. Littman, B. Wing, M. Fiorentini and M. R. Kilburn, Chem. Geol., 2016, 444, 1–15 CrossRef CAS.
- E. M. Ripley, B. W. Wernette, A. Ayre, C. S. Li, J. M. Smith, B. S. Underwood and R. R. Keays, Geochim. Cosmochim. Acta, 2017, 214, 226–245 CrossRef CAS.
- S. X. Cao, H. Kaliasiha, Y. F. Deng, S. Wei, F. Yuan, W. D. Li, Z. X. Tan and J. J. Han, Chin. J. Geol., 2024, 59, 1588–1602 Search PubMed.
- X. Ding, E. M. Ripley, W. Z. Wang, C. H. Li and F. Huang, Geochim. Cosmochim. Acta, 2019, 261, 327–341 CrossRef CAS.
- B. Zhu, H. F. Zhang, X. M. Zhao and Y. S. He, Ore Geol. Rev., 2016, 74, 139–150 CrossRef.
- J. H. Zheng, B. Chen, S. J. Liu and C. Bao, Am. Mineral., 2021, 106, 430–442 CrossRef.
- J. G. Moreau, A. Jõeleht, J. Aruväli, M. J. Heikkilä, A. N. Stojic, T. Thomberg, J. Plado and S. Hietala, Meteorit. Planet. Sci., 2022, 57, 588–602 CrossRef CAS.
- Z. C. Wang, Y. H. Li, W. Zhang, Q. He, F. B. Pan, Z. C. Hu, K. Q. Zong, Y. T. Feng, H. Becker, J. M. D. Day, W. L. Song, H. J. Hui, F. Moynier, Y. Jiang, X. J. Zhang, Z. B. She, X. Wu, L. Xiao and L. Wang, Geochim. Cosmochim. Acta, 2024, 368, 168–184 CrossRef CAS.
- X. Y. Liu, J. L. Hao, R. Y. Li, Y. Y. He, H. C. Tian, S. Hu, J. Li, L. X. Gu, W. Yang and Y. T. Lin, Geophys. Res. Lett., 2022, 49, e2022GL099922 CrossRef CAS.
- I. Horn, F. von Blanckenburg, R. Schoenberg, G. Steinhoefel and G. Markl, Geochim. Cosmochim. Acta, 2006, 70, 3677–3688 CrossRef CAS.
- L. Chen, H. L. Yuan, K. Y. Chen, Z. A. Bao, L. M. Zhu and P. Liang, J. Asian Earth Sci., 2019, 176, 325–336 CrossRef.
- J. Marin Carbonne, C. Rollion-Bard and B. Luais, Chem. Geol., 2011, 285, 50–61 CrossRef CAS.
- V. Pasquier, E. S. Rego, J. Dupeyron, A. S. Bouvier, T. Bovay, M. Robyr and J. M. Carbonne, Geostand. Geoanal. Res., 2024, 48, 423–431 CrossRef CAS.
- J. M. Carbonne, V. Busigny, J. Miot, C. R. Bard, E. Muller, N. Drabon, D. Jacob, S. Pont, M. Robyr, T. R. R. Bontognali, C. François, S. Reynaud, M. Van Zuilen and P. Philippot, Geobiology, 2020, 18, 306–325 CrossRef PubMed.
- C. González de Vega, M. Costas Rodríguez, T. Van Acker, S. Goderis and F. Vanhaecke, Anal. Chem., 2020, 92, 3572–3580 CrossRef.
- J. L. Fu, Z. C. Hu, W. Zhang, L. Yang, Y. S. Liu, M. Li, K. Q. Zong, S. Gao and S. H. Hu, Anal. Chim. Acta, 2016, 911, 14–26 CrossRef CAS PubMed.
- L. Chen, K. Y. Chen, Z. Bao, P. Liang, T. T. Sun and H. L. Yuan, J. Anal. At. Spectrom., 2017, 32, 107–116 RSC.
- N. T. Kita, J. M. Huberty, R. Kozdon, B. L. Beard and J. W. Valley, Surf. Interface Anal., 2011, 43, 427–431 CrossRef CAS.
- M. Oeser, S. Weyer, I. Horn and S. Schuth, Geostand. Geoanal. Res., 2014, 38, 311–328 CrossRef CAS.
- J. Ma, X. B. Lü, A. Escolme, S. Li, N. G. Zhao, X. F. Cao, L. J. Zhang and F. Lu, Ore Geol. Rev., 2018, 98, 38–61 CrossRef.
- Y. J. Li, H. Ji, J. J. Xiong, Z. K. Chen, T. Ulrich and J. H. Wei, Ore Geol. Rev., 2024, 165, 105913 CrossRef.
- R. Kozdon, N. T. Kita, J. M. Huberty, J. H. Fournelle, C. A. Johnson and J. W. Valley, Chem. Geol., 2010, 275, 243–253 CrossRef CAS.
- B. Rusk, Econ. Geol., 2009, 104, 601–602 CrossRef CAS.
- W. Zhang and Z. C. Hu, Spectrochim. Acta, Part B, 2020, 171, 105929 CrossRef CAS.
- N. T. Kita, T. Ushikubo, B. Fu and J. W. Valley, Chem. Geol., 2009, 264, 43–57 CrossRef CAS.
- L. Chen, Y. Feng, H. M. Yu, W. Zhang, J. T. Kang, F. Huang, Z. Hu and X. H. Li, J. Anal. At. Spectrom., 2022, 37, 2300–2308 RSC.
- L. Chen, Y. Liu, Y. Li, Q. L. Li and X. H. Li, J. Anal. At. Spectrom., 2021, 36, 1431–1440 RSC.
- Y. T. Feng, W. Zhang, Z. C. Hu, T. Luo, Q. L. Li and J. Y. Liu, Geostand. Geoanal. Res., 2024, 48, 227–244 CrossRef CAS.
- S. E. Gilbert, L. V. Danyushevsky, T. Rodemann, N. Shimizu, A. Gurenko, S. Meffre, H. Thomas, R. R. Large and D. Death, J. Anal. At. Spectrom., 2014, 29, 1042–1051 RSC.
- R. C. Li, X. P. Xia, S. H. Yang, H. Y. Chen and Q. Yang, Geostand. Geoanal. Res., 2019, 43, 177–187 CrossRef CAS.
- Z. A. Bao, L. Chen, C. L. Zong, H. L. Yuan, K. Y. Chen and M. N. Dai, Int. J. Mass Spectrom., 2017, 421, 255–262 CrossRef CAS.
- J. Tian, Z. A. Bao, K. Y. Chen, N. Lv, W. Q Yang, X. J. Nie and H. L. Yuan, J. Anal. At. Spectrom., 2024, 39, 1503–1513 RSC.
- X. J. Nie, Z. A. Bao, C. L. Zong, N. Lv, K. Y. Chen and H. L. Yuan, J. Anal. At. Spectrom., 2023, 38, 1065–1075 RSC.
- Y. T. Feng, W. Zhang, Z. C. Hu, T. Luo, M. Li, Y. S. Liu, H. Liu and Q. L. Li, J. Anal. At. Spectrom., 2022, 37, 551–562 RSC.
- Y. T. Feng, W. Zhang, Z. C. Hu, Y. S. Liu, K. Chen, J. L. Fu, J. Y. Xie and Q. H. Shi, J. Anal. At. Spectrom., 2018, 33, 2172–2183 RSC.
- G. Kullerud, Bull. Geol. Soc. Finl., 1986, 58, 293–305 CAS.
- F. Xia, J. W. Zhou, A. Pring, Y. Ngothai, B. O’Neill, J. Brugger, G. Chen and C. Colby, React. Kinet. Catal. Lett., 2007, 92, 257–266 CrossRef CAS.
- L. Meng, F. Huang, X. Q. Wang, W. Y. Gao, B. M. Zhang, D. Song, G. L. Li and B. Y. Zhang, J. Geochem. Explor., 2020, 219, 106636 CrossRef CAS.
- L. A. Figueroa, N. Torres-Gómez, R. G. Contreras, A. R. Vilchis-Nestor, O. M. Alvarez, L. S. A. Torres and M. C. Arenas-Arrocena, Prog. Nat. Sci.:Mater. Int., 2018, 28, 447–455 CrossRef.
- S. F. Sadr and S. Mosivand, Ceram. Int., 2024, 50, 33123–33130 CrossRef.
- D. M. Roberts, A. R. Landin, T. G. Ritter, J. D. Eaves and C. R. Stoldt, Sci. Rep., 2018, 8, 6591 CrossRef PubMed.
- H. Y. Xian, J. X. Zhu, X. L. Liang and H. P. He, RSC Adv., 2016, 6, 31988–31999 RSC.
- X. Y. Zheng, B. L. Beard and C. M. Johnson, J. Anal. At. Spectrom., 2018, 33, 68–83 RSC.
- K. Y. Chen, H. L. Yuan, Z. A. Bao and N. Lv, At. Spectrosc., 2021, 42, 282–293 CAS.
- S. Weyer and J. B. Schwieters, Int. J. Mass Spectrom., 2003, 226, 355–368 CrossRef CAS.
- G. L. Arnold, S. Weyer and A. D. Anbar, Anal. Chem., 2004, 76, 322–327 CrossRef CAS PubMed.
- P. D. P. Taylor, R. Maeck and P. De Bièver, Int. J. Mass Spectrom. Ion Processes, 1992, 121, 111–125 CrossRef CAS.
- Y. J. An, J. X. Huang, W. L. Griffin, C. Z. Liu and F. Huang, Geochim. Cosmochim. Acta, 2017, 200, 167–185 CrossRef CAS.
- L. Chen, X. H. Li, J. W. Li, A. H. Hofstra, Y. Liu and A. E. Koenig, Miner. Deposita, 2015, 50, 643–656 CrossRef CAS.
- T. Ding, S. Valkiers, H. Kipphardt, P. De Bièvre, P. D. P. Taylor, R. Gonfiantini and R. Krouse, Geochim. Cosmochim. Acta, 2001, 65, 2433–2437 CrossRef CAS.
- ISO Guide 35 Reference Materials — Guidance for Characterization and Assessment of Homogeneity and Stability, International Organization for Standardization, Geneva, 2017. Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |


