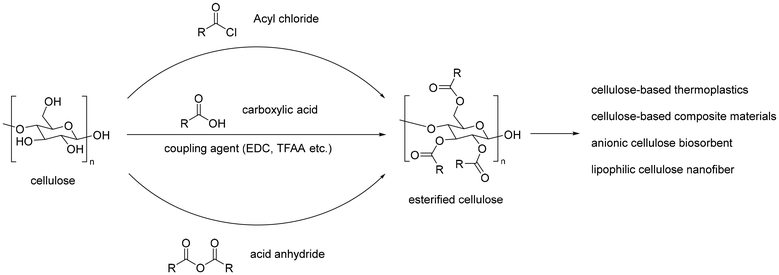
Open Access Article
Open Access ArticleMechanochemical transformations of polysaccharides to value added products: a review with Green Chemistry evaluation
Galen
Yang†
 a,
Yasmeen
Jaberi†
a,
Edmond
Lam†
a,
Yasmeen
Jaberi†
a,
Edmond
Lam†
 a and
Audrey
Moores†
a and
Audrey
Moores†
 *ab
*ab
aCentre in Green Chemistry and Catalysis, Department of Chemistry, McGill University, 801 Sherbrooke St. West, Montréal, QC H3A 0B8, Canada. E-mail: audrey.moores@mcgill.ca
bDepartment of Materials Engineering, McGill University, 3610 University Street, Montréal, Quebec H3A 0C5, Canada
First published on 13th January 2026
Abstract
Biopolymers, particularly polysaccharides, are a renewable feedstock with the potential to reduce reliance on petrochemicals and enable decarbonization and circularity efforts. Their sustainable chemical modification is essential to expand their use in the industry, yet this goal has proven hard to achieve because of their poor processability attributed to low solubility in most solvents. Mechanochemistry is a fast-emerging technique enabling the effective chemical transformation of materials in the solid state. It is effective for the chemical modification of biopolymers and composites with lower reagent, solvent and energy use compared to solution phase methods. Herein, we review recent progress in the development of mechanochemical methodologies for polysaccharide transformations, including depolymerization, nano-extraction, and chemical functionalization. We compare in detail the different techniques and their outcomes in terms of the functional properties of the final products, as well as the green metrics of each method based on reported parameters. Conclusions are then drawn to direct future research directions to expand the range of new functional biomaterials mechanochemistry that can be produced in a more sustainable manner.
Green foundation1. Recent advances in mechanochemistry have transformed polysaccharide valorization by enabling solvent-free or solvent-minimized reactions, significantly lowering reagent and energy use. Mechanochemical depolymerization, nano-extraction, and functionalization now achieve outcomes comparable or superior to traditional solution-phase methods. Innovations such as aging-assisted processes extend reactivity while maintaining polymer integrity, providing efficient access to new biopolymer architectures.2. This research sits at the intersection of sustainable chemistry, materials science, and circular bioeconomy. Mechanochemical polysaccharide modification addresses global challenges in decarbonization, waste valorization, and plastic replacement by converting abundant biomass into high-value materials without reliance on petrochemicals. Its solvent-free nature and compatibility with insoluble substrates make it a model for sustainable process design. The field also demonstrates how physical energy inputs can replace hazardous reagents, aligning with the 12 Principles of Green Chemistry and inspiring innovation beyond biomass processing. 3. The full realization of the potential of this research requires that mechanochemical processes scaling is developed. Integrating real-time monitoring, and green metrics such as PMI and E-factor systematically will also further improve the field. Insights from this review highlight the need for systematic benchmarking of sustainability parameters, mechanistic understanding to guide rational process design and well as toxicity considerations. As green chemistry evolves toward circular and low-carbon manufacturing, these strategies will shape a new generation of functional biopolymers derived through energy-efficient, solvent-free transformations—cementing mechanochemistry as a cornerstone of sustainable materials science. |
1. Introduction
Polymers are consistently ranked among the most important materials in modern civilization due to their wide range of applications, stemming from the diversity of their functional groups and structures that define their physicochemical properties.1–3 However, the mass production and widespread use of synthetic polymers have also introduced significant environmental challenges throughout their entire lifecycle. Many common synthetic polymers such as nylon,4,5 polyolefin,6 polystyrene,7 polyester,8 polyurethane,9 Teflon,10 and epoxy are all derived from petrochemicals,11 which require extensive refining and processing of crude oil, resulting in substantial environmental impacts such as pollution, biodiversity loss, and climate change.12,13 Synthetic polymers often lack biodegradability, leading to plastic waste persisting in the environment long after human consumption.14,15 Disposal strategies such as incineration, further contribute to the release of toxic compounds and carbon dioxide in the atmosphere,16 a primary driver of global warming and climate change,17,18 while land-filling and release directly in the environment lead to water, soil and air pollution in the form of leached molecules, nano-, micro- and macro-plastics. This situation calls for urgent collective efforts from society to preserve our planet for future generations.19Biopolymers have a crucial role in the process of decarbonization to reduce our reliance on petrochemical-derived synthetic polymers.20–22 Unlike synthetic polymers, every biopolymer can be traced back to photosynthesis which converts carbon dioxide and water (H2O) into organic matter. The biochemical processes of living organisms then transform this simple organic matter into a diverse array of compounds that support life. It is important to stress that such schemes must be associated with sustainable land management, forestry and agricultural practices to truly yield environmental benefits and avoid unintended consequences such as biodiversity loss. Among the major classes of biopolymers polysaccharides are the most abundant,23 with cellulose and chitin ranking first and second, respectively.21,24
Lignocellulosic and chitinous materials are widely available in biomass waste, such as sidestreams from the wood, agricultural, and food industries, making them ideal feedstocks for promoting a circular economy.21,25–27 However, pristine polysaccharides often lack sufficient functionalities and properties that limit their ability to serve as substitutes for a broader range of synthetic polymers. For instance, most polysaccharides are hydrophilic, and are incompatible with many synthetic polymers that are hydrophobic, as well as with applications requiring water resistance.28–31 On the other hand, plastics feature mechanical and durability properties allowing them to perform well in diverse conditions, which native lignocellulosic or chitinous materials cannot achieve.32
Several approaches have been developed to enable the use of polysaccharide and achieve desired properties. (1) Deconstruction typically proceeds through the depolymerization of polysaccharides into their monomers, followed by further treatments to access platform chemicals,33–35 and eventually end products, including de novo polymers.36 One prime example of the deconstructive strategy is the production of polylactic acid using lactic acid obtained from fermentation of starchy biomass,37,38 and the synthesis of polyethylene furanoate (PEF) from sugars through furan dicarboxylic acid as a platform molecule.36 Such strategies have been intensely researched and provide production pathways comparable to petrochemistry but suffer from the drawback of poor atom economy and the need for many reaction steps. (2) Nano-extraction involves the controlled and limited depolymerization of polysaccharides to produce nanoscale materials such as cellulose or chitin nanocrystals, nanofibers, or chitosan nanowhiskers,39,40 typically through acidic or oxidative pathways.41–43 These nanomaterials can be used directly or incorporated into composites44,45 to create synergies between the properties of multiple materials or to enhance performance.46,47(3) Transformation strategies rely on maintaining most or all the biopolymer backbone, focusing on its modification to achieve the desired properties. Chemical grafting of new functionalities onto these biopolymers gives access to robust materials, with modified solubility, chemical, optical, and mechanical properties.48,49 Examples of transformation strategies include mass produced materials such as nitrocellulose and starch acetate.50,51 Such transformations can also enable the combination of polysaccharide derivatives with other components to form biocomposites, creating synergies between the properties of multiple materials.52,53 Transformation strategies have the potential to be more sustainable as they minimize steps to product, although accessing precise, high-performance properties in the targeted materials may be challenging.
All these strategies are of interest and need to be harnessed for a more sustainable future. However, their implementation requires careful consideration, guided by the 2025 Stockholm Declaration on Chemistry for the Future,54 which emphasizes five pillars: (i) the sustainable and safe design of chemical products and processes, (ii) the reduction of risks, (iii) the integration of sustainability into education, (iv) ensuring data transparency, and (v) alignment with supportive policies. These strategies all face significant challenges: the most abundant lignocellulosic and chitinous polymers are insoluble in common solvents,55,56 and extensive inter- and intramolecular hydrogen bonding networks within these polymers57 reduce their chemical reactivity.58 These properties naturally evolved in polysaccharides to act as an integral protective strategy for living organisms, making homogeneous solution-based and heterogeneous dispersion-based methods of polysaccharide modification ineffective unless harsh reaction conditions including high temperatures and extreme acidic or basic environments, along with large excess of reagents which are applied individually or together to promote the chemical reactions.59–62
In recent years, mechanochemistry has emerged as a successful strategy to overcome some or all these limitations in processing and functionalizing polysaccharides. Mechanochemistry is defined as the science of chemical reactions that is induced by the direct absorption of mechanical energy,63–65 where the energy can be delivered either with grinding media typically in various types of ball mills through impact and shear,66,67 or without grinding media, where acoustic waves introduces compression and shear. Examples of the latter include the conventional mortar and pestle,68 extruders,69,70 roller mills,71,72 pan mills,73 and the more recently developed resonant acoustic mixing (RAM).74,75 Mechanochemistry is particularly well-suited for reactions involving insoluble substrates as reactivity is not reliant on the dissolution of reagents.76 Additionally, the limited use of solvents and reagents allows for mechanochemistry to enhance the overall green metrics of polysaccharide modification such as the process mass intensity (PMI).77 In the last 15 years, the use of mechanochemistry for the valorization of polysaccharides has exploded, with tremendous development in all three strategies: (1) deconstruction, (2) nano-extraction and (3) functionalization. In this context, accelerated aging, also simply referred to as aging has recently been established as an effective complement to mechanical activation. Rather than relying on high reagent loadings and prolonged milling, aging allows the reaction mixture, once activated, to rest under mild or ambient conditions. During this stationary period, reactions proceed through adsorption, penetration and diffusion, of substrates.78–83 This approach has been successfully employed in a series of polysaccharide modification studies by our group84–88 as well as by others.72,89–94 In many cases, aging has been found to extend transformations while simultaneously reducing the energy consumption associated with mechanical treatment. Importantly, it also helps prevent unintended structural damage to polysaccharide substrates, offering an appealing unified strategy that promotes higher reaction efficiency than conventional solution phase synthesis while operating under milder conditions than purely mechanochemical methods.
In this review, we critically examine the methods of mechanochemical and aging processing for deconstruction, nano-extracting and functionalization of different polysaccharides, namely cellulose, starch, chitin and chitosan, including with the use of mechanoenzymology. We highlight the conditions of these mechanochemical transformations, the efficacy of the processes, and the performance of the resulting products in their applications. Furthermore, we identify key areas for further exploration, to ultimately enhance the overall green chemistry metrics and improve the feasibility and practicality of mechanochemical polysaccharide modifications for producing functional biopolymers as substitutes for conventional synthetic polymers. Considering the importance of this field, several reviews were published, covering part of the topics gathered here.95–98 Kobayashi and coworkers described in 2024 reports on the mechanochemical depolymerization of polysaccharides with a focus on mechanistic considerations.99 Kerton and coworker described in 2021 the use mechanochemistry to valorize biomass, with examples from polysaccharides and biominerals.98 These two reviews do not cover functionalization and nano-extraction strategies. In 2022, our review focused only on functionalization and nano-extraction with an important focus on the applications.100 Since then, De Borggraeve and coworkers as well as Hernáiz and coworkers, they both highlighted the sustainability advantages of polysaccharide functionalization via mechanochemistry;101,102 However, these works did not integrate green chemistry principles or apply quantitative metrics for process evaluation. We identify this as a gap in the field, underscoring the need for an extended discussion that systematically addresses the green aspects of all polysaccharide mechanochemical functionalization strategies within a single review, which had yet to be done. Specifically, in this critical review, we discuss the advantages of mechanochemistry and aging based methods as compared to liquid state transformations, the generality of these methods across polysaccharide reactivities as well as the selectivity of such methods for deconstruction, nano-extracting and functionalization, and finally toxicity considerations in such schemes.
2. Mechanochemical tools and aging strategies used in polyssacharide functionalization
Tools used in mechanochemistry and aging have been reviewed before and we point the reader to existing references for more details (Fig. 2).66,103–107 In this section we will focus on highlighting the equipment most used in the research covered in this review. Mechanical pretreatment is common in biomass processing, and many of the same tools have been adapted for mechanochemistry, particularly ball mills. Vibrational and planetary ball mills are most frequently employed. In ball-milling, the balls transfer energy to the material, with vibrational mills typically using one or two balls.105 Key parameters including the number, size, mass, and material of the balls, as well as the size and material of the grinding jars determine ball movement, while milling frequency and duration define the total mechanical energy delivered. The addition of other grinding media or liquid additives for liquid-assisted grinding (LAG),108 further defines the milling conditions in a mechanochemical treatment. Planetary milling combines two rotational motions: rotation of the jars around the planetary axis and simultaneous rotation around their own Z-axes, typically in the opposite direction.103 This counter-rotation generates strong shear and impact forces between the balls and the vessel walls. Smaller balls in larger numbers are often employed to enhance mixing and activation. While similar to vibrational ball milling in relying on the ball and jar setup, the energy input in planetary milling is governed primarily by the rotational speed (rpm) and milling duration. Variations in the motion can tune milling intensity; for example, high-energy ball mills, a variant of planetary mills, generate stronger shear and impact forces by forcing multi-directional ball movement, making them particularly efficient for substrate comminution.109Mechanochemical transformations may also be performed without balls as grinding media. In several examples discussed in this review, researchers have employed pan mills, where the material placed in the grinding chamber is crushed by heavy rollers.110 Gear-like teeth on the roller surfaces provide both strong compression and shear, enabling mechanochemical transformations. Because pan-milling is not conducted in a sealed vessel, the total energy input is typically quantified by the number of cycles the material passes through the rollers rather than by continuous reaction time. Offering more process control, reactive extrusion has emerged as a versatile flow-based technique for mechanochemical synthesis.69 One or two rotating metal screws, configured with alternating conveying and kneading blocks, apply strong shear and compressive forces to the substrate, initiating chemical transformations. What distinguishes reactive extrusion from other methods is its modularity: the arrangement of functional blocks can be customized to meet the requirements of a given reaction. In addition, precise temperature control is possible, as each barrel section can be independently heated, making the technique especially attractive for syntheses requiring simultaneous mechanical and thermal activation.
In several examples, aging, also known as accelerated aging, is used in conjunction with ball-milling. Aging is a diffusion-based technique in which a substrate mixture is left stationary at room temperature or under mild heating, in a dry atmosphere, high humidity, or in the presence of reagent vapors.78–83 In many cases, this approach has proven highly effective in improving reaction efficiency in terms of reagent conversion rates, while reducing energy consumption compared to extended mechanical treatment.
3. Review of functionalization strategies, organized by substrates and reaction
3.1. Cellulose
Cellulose is the most abundant biopolymer in the biosphere accounting for about 1.5 trillion tonnes available annually.21 It is a linear biopolymer composed of D-glucose units linked by β-1,4 glycosidic bonds, which give cellulose its characteristic rigidity and strength. Found in the cell walls of plants and in the extracellular matrix of certain bacteria,42,111,112 cellulose forms microfibrils with high crystallinity.113 There is continuous growth of interest in the development of pathways to valorize cellulose by chemical modification.114,115| Active reagent | Equipment | Conditions | Product | Yield | Ref. |
|---|---|---|---|---|---|
| Layered delaminated kaolinite (Al2Si2O7·2H2O) | Mixer mill | 3 h | Water-soluble sugars | 84% | Blair and coworkers120 |
| Layered niobium molybdate (HNbMoO6) | Planetary ball mill | 800 rpm, 24 h | Water-soluble sugars | 72% | Takagaki and coworkers121 |
| Impregnated acid (H2SO4, HCl, or p-TSA) | Planetary ball mill | 800 rpm, 2–5 h | Water-soluble sugars | 80% | Schüth and coworkers122 |
| Impregnated H2SO4 | Simoloyer ball mill | 527 rpm, 35 min | Glucose | 65% | Rinaldi and coworkers123 |
| Impregnated H2SO4 | Vibrational ball mill | 30 Hz, 60 min | Water-soluble sugars | 99% | Yu and coworkers124 |
| Fatty alcohol, H2SO4 catalyst | Mechanochemical stirring | 700 rpm, 30 min, 110 °C | Fatty alcohol-grafted cellulose oligomers | >60% | Yan and coworkers125 |
| Fatty amine, H2SO4 (pre-impregnated) | Mechanochemical stirring | 700 rpm, 30–75 min, 120 °C | Cellulose oligomeric glycosylamine | >50% | Yan and coworkers126 |
| Activated carbon | Planetary ball mill | 60 rpm, 2 d | Glucose | 88% | Fukuoka and coworkers127 |
| Carbonaceous solid acid | Planetary ball mill | 500 rpm, 4 h | Glucose | 88% | Qi and coworkers128 |
| Sulfonated carbon catalyst | Planetary ball mill | 300 rpm, 18 h | Glucose | 10% | Vogel and coworkers129 |
| T. longibrachiatum cellulase | Vibrational ball mill | 30 Hz, 5 min, 55 min aging, 12 cycles | Glucose | 50% | Auclair and coworkers89 |
| Al2(SO4)3 | Planetary ball mill | 350 rpm, 4 h | HMF | 44.6% | Qi and coworkers130 |
Blair and Schüth laid foundation in the development of the method to hydrolyze cellulose by mechanochemistry through two distinct pathways. Blair and coworkers identified kaolinite, a phyllosilicate minerals, effective in solubilizing cellulose: milling microcrystalline cellulose (MCC) (1 g) with kaolinite (1 g) solubilized 84% cellulose, yielding glucose, fructose, and dehydration products including 5-hydroxymethylfurfural (HMF), levoglucosenone and levoglucosan.120 With a similar strategy, Takagaki and coworkers screened 13 oxides; layered niobium molybdate (HNbMoO6) gave 72% water-soluble sugars after 24 h milling (800 rpm).121 This Brønsted acid facilitated chain insertion and random glycosidic bond cleavage.
The methodologies Schüth and coworkers developed involves impregnating cellulose with acids assisted by diethyl ether. They first tested impregnated cellulose with sulfuric acid (H2SO4), p-toluenesulfonic acid (p-TSA), or HCl before milling.122 With 0.88 mmol g−1 H2SO4, complete conversion to soluble oligomers with degree of polymerization (DP) over 3 was achieved in 2 h (800 rpm). At kilogram scale, a Simoloyer mill processed α-cellulose (1 kg) impregnated with H2SO4 (0.8 mmol g−1). Milling at 527 rpm for 35 min produced 93% soluble products, mostly oligosaccharides. A saccharification step at 145 °C raised glucose yield to 65% with a total specific energy consumption for cellulose hydrolysis of 3450 kWh t−1.123 Eliminating organic solvent, Yu and coworkers impregnated MCC in diluted H2SO4 solution (0.5 mmol g−1) and removed excess water in the slurry prior to ball-milling (30 Hz, 60 min). The process yielded ∼99% soluble oligomers, 14% with DP < 5. Subsequent hydrolysis (0.25 wt% H2SO4, 150 °C, 5 min) gave 91% glucose.124
Yan and coworkers developed a top-down mechanochemical approach to cellulose oligosaccharide surfactants via thermally-enhanced alcoholysis of MCC.125 Milling MCC with concentrated H2SO4 (1 wt%) and fatty alcohols (C6–C12) at 110 °C afforded >60% biomass conversion to fatty alcohol-grafted cellulose oligomers (DP ≈ 12), with predominant C1 substitution. The method was applicable to raw lignocellulosic feedstocks including wheat bran and poplar sawdust, albeit at lower conversion. The resulting water-soluble oligosaccharides exhibited low critical micelle concentrations (0.1–1 mmol L−1), comparable to commercial methyl ester sulfonate surfactants, highlighting their potential as sustainable bio-based alternatives.
Using a related one-pot mechanochemical protocol, the same group synthesized cellulose oligomeric glycosylamine surfactants by reacting crude cellulooligosaccharides with fatty amines (C8–C14).126 Extended milling and heating at 120 °C afforded >50% conversion and degrees of functionalization up to 0.15. The glycosylamine derivatives showed surface activity comparable to the alcohol-grafted analogues with critical micelle concentrations slightly exceeding 1 mmol L–1, while the incorporation of amine functionalities imparted broad-spectrum antimicrobial activity, as demonstrated against Escherichia coli and Bacillus subtilis.
Fukuoka and coworkers pioneered the use of carbonaceous solid acid catalysts in mechanochemical pretreatment to selectively produce oligosaccharides and promote subsequent hydrothermal hydrolysis. In their study, MCC with alumina balls and activated carbon was milled (60 rpm, 2 d), then hydrothermally hydrolyzed in 0.012% hydrochloric acid (HCl) (20 min, 453 K), giving 98% conversion and 88% glucose yield.127 Qi and coworkers achieved comparable results (88.0% glucose) with reduced mechanical treatment, by milling MCC with a carbonaceous solid acid catalyst (500 rpm, 4 h), followed by hydrothermal hydrolysis (200 °C, 1 h); without milling, yield dropped to 11.2%. Addition of 0.02 wt% HCl boosted glucose yield from 52.8% to 88.0%.128 Vogel and coworkers milled MCC with sulfonated carbon catalyst (300 rpm, 18 h), then hydrolyzed at 165 °C under 20 bar with 1 mM ethylenediaminetetraacetic acid, reaching 27.2% conversion, with 17.4% oligosaccharides.129
Enzymatic routes under mechanochemical conditions have also been developed. Auclair and coworkers applied mechanoenzymatic reactive aging, or “RAging” (5 min milling, 55 min aging, repeated 12 cycles) with cellulase and trace water, converting 50% MCC to glucose in 12 h.89 This achieved > 20× throughput compared to conventional enzymatic digestion. Applied to raw biomass, 1.5 mmol glucose per g was obtained.
Other than glucose, the complete depolymerization of cellulose can yield aromatic by-products such as furfural and HMF, which are valuable lignocellulose-derived platform chemicals.120,123,129 Qi and coworkers targeted HMF production by milling cellulose with a Lewis and Brønsted acid, Al2(SO4)3 at 350 rpm, for 4 h, followed by hydrolysis in a water-γ-valerolactone biphasic solvent system (165 °C, 50 min), reaching 44.6% HMF yield.130
| Substrate | Active reagent | Equipment | Condition | Yield | Characteristics | Ref. |
|---|---|---|---|---|---|---|
| a Process does not include mechanical treatment. | ||||||
| Bamboo pulp | H3PO4 | Stirring ball mill | 400 rpm, 2.5 h | 77.4% | Rod-like | Lu and coworkers135 |
| Bamboo pulp | PTA | Planetary ball mill | — | 88.4% | CrI = 80% | Huang and coworkers136 |
| Fluffed cotton fiber | ChCl/OAD NADES | Vibrational ball mill | 30 Hz, 1.5 h | 65.0% | CrI = 92% | Bras and coworkers139 |
| CNFs | HCl gasa | N/A | 0.6 or 1.0 bar HCl gas for 30 min | N/A | CNC-like rods | Kontturi and coworkers140 |
| MCC | APS | Mortar & pestle, shaker incubator | 3 min mixing, 2 d HHSA | N/A | Carboxylated (DO = 20%) | Moores and coworkers88 |
| MCC or Wood pulp | CDEEA·HCl, NaOH, TBAH | Vibrational ball mill | 29.5 Hz, 10 min 3 h aging | 63.7% | ζ-potential = + 68 mV | Moores and coworkers143 |
| MCC | T. longibrachiatum cellulase | Roller mill | 100 rpm, 48 h | 49.3% | CrI = 77% | Su and coworkers146 |
Mechanochemical strategies for CNC production have recently been intensely researched. Lu and coworkers demonstrated ball-milling of bamboo pulp with phosphoric acid (H3PO4) followed by ultrasonication, yielding 77.4% CNCs with rod-like morphology (100–200 × 15–30 nm).135 Similarly, Huang and coworkers applied milling with phosphotungstic acid (PTA) and hydrothermal hydrolysis, reaching 88.4% yield and a CrI of 79.6%, with enhanced thermal stability compared to the starting pulp (CrI 63.7%).136,137
Integration with natural deep eutectic solvents (NADES)138 has also advanced the field. Bras and coworkers reported a one-step choline chloride (ChCl)/oxalic acid dihydrate (OAD) NADES process, achieving 65% yield versus 27% for citric acid monohydrate (CAM)-based NADES, with CNCs showing CrI of 92%, surface charge of 120 μmol g−1, and ∼143 nm length.139
Another approach to the mineral acid-assisted production of CNC was demonstrated by Kontturi and coworkers where they observed the hydrolysis of CNFs on silicon dioxide by HCl gas, leading to CNC-like rods with lengths of 68 or 284 nm, depending on pressure.140 In their work they demonstrated the power of aging to achieve CNC production with HCl gas, while completely avoiding mechanical damage the delicate nanocellulose.
Besides acidic routes, oxidative pathways have been important benchmarks for liquid state CNC synthesis. 2,2,6,6-Tetramethylpiperidine-1-oxylradical (TEMPO)-mediated oxidation42,141 and ammonium persulfate (APS) oxidation142 both promote CNF defibrillation by introducing anionic carboxyl groups to the cellulose substrate. Our group demonstrated mechanochemical high-humidity shaker aging (HHSA), producing CNCs ∼220 × 5 nm with degree of oxidation (DO) = 0.20.88 This work importantly combined a very short manual mixing (3 min) with aging to achieve high quality CNCs with limited energy input.
Our group recently proposed another strategy to help the defibrillation process with the introduction of cationic charges by mechanochemistry. Grafted cationic tertiary amine groups promoted defibrillation, producing CNFs and CNCs (Fig. 3).143 Interestingly, CNFs (1.2 μm × 2 nm) were directly obtained from the mechanochemical and aging-based treatment of softwood bleached kraft pulp (SBKP) with 2-chloro-N,N-diethylethylamine hydrochloride (CDEEA·HCl), followed by short ultrasonication, hence by-passing complex defibrillation techniques.
 | ||
| Fig. 3 Mechanochemical and aging based synthesis of DEEA-MCC, DEEA-SBKP, and DEEA-chitin through SN2-type aminoalkylation of polysaccharide substrate with in situ-generated CDEEA electrophile, followed by ultrasonication defibrillation to produce cationic nanopolysaccharides DEEA-substrateUT; Figure: TEM images of defibrillated samples (A) DEEA-MCCUT, (B) DEEA-SBKPUT, (C) DEEA-chitinUT. Reproduced with permission from ref. 143. Copyright © 2025 Royal Society of Chemistry. | ||
Mechanoenzymatic methods are emerging,144–146 as shown by Su and coworkers, who combined ball-milling with cellulase hydrolysis, affording 49.3% CNC yield, CrI 76.8%, and average length 457 nm.146
In the latter four examples,88,143 aging played a key role in minimizing the milling step and serves as the primary reaction stage, offering a strategy for producing nanocellulose while mitigating undesired degradation and amorphization of the cellulose crystallites.88,147,148
| Substrate | Active reagent | Equipment | Condition | Product | Highest DS | Characteristics | Ref. |
|---|---|---|---|---|---|---|---|
| MCC | Oleic acid, TsCl | Magnet mortar | 150 rpm, 13 h | Cellulose oleate | 0.21 | Thermoplastic | Andou and coworkers149 |
| Regenerated cellulose | Oleic acid, EDC·HCl | Planetary ball mill | 500 rpm, 2 h | Cellulose oleate | 2.55 | Thermoplastic | Yang and coworkers150 |
| MCC | Fatty acyl chlorides, TFAA | Planetary ball mill | 500 rpm, 4 h | FACEs | 2.32 | Thermoplastic | Yang and coworkers152 |
| MCC | Carboxylic acids, TFAA | Planetary ball mill | 500 rpm, 4 h | Cellulose esters | 2.9 | Thermoplastic | Yang and coworkers155 |
| SACP, NR | Acetic anhydride, stearic acid | Pan mill | 60 rpm, 2 cycles | NR vulcanizates | — | Cellulose-filler reinforcement | Lu and coworkers156 |
| Fibrous cellulose, MPE | Maleic anhydride | Planetary ball mill | 250 rpm, 24 h | Cellulose/PE | — | Improved toughness and ductility | Hirotsu and coworkers159 |
| Fibrous cellulose, MAPP | Maleic anhydride | Planetary ball mill | 250 rpm, 8 h | Cellulose/PP | — | Core–shell structure | Hirotsu and coworkers160 |
| Bleached hardwood pulp, PVA | SA | Pan mill | 30 rpm, 20 cycles | Crosslinked PVA/cellulose | — | Improved toughness and ductility | Lu and coworkers161 |
| SBKP, PP | Pimelic acid, acetic anhydride | Planetary ball mill | 120 min | Cellulose/PP | — | Improved toughness and ductility | Wu and coworkers167 |
| Cellulose fiber | SA | Pan mill | 60 rpm, 30 cycles | Anionic cellulose | 0.24 | Biosorbent | Lu and coworkers73 |
| MCC | Maleic anhydride | High energy ball mill | 700 rpm, 4 h | Anionic cellulose | 0.72 | Unsaturated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group C group |
Khaligh and coworkers173 |
| CNF | Fatty acids | Rolling mill | 350–400 rpm, 6 h | Lipophilic CNFs | 0.46 | Disperse in organic solvent | Wei and coworkers71 |
3.1.3.1 Cellulose thermoplastic. Andou and coworkers prepared cellulose oleate from MCC via mechanochemistry using a magnet mortar with oleic acid (OA) and 1-butyl-3-methylimidazolium acetate (BmimOAc).149 Sulfonate esters were introduced with p-toluenesulfonyl chloride (TsCl), giving a maximum degree of substitution (DS) of 0.210 at 100 °C after 12 h, slightly decreasing to 0.204 after 24 h due to hydrolysis. CrI dropped modestly from 86.3% (MCC) to 80.8%, indicating limited amorphization.
Yang and coworkers reported thermoplastic cellulose oleate via two-stage planetary ball-milling (Fig. 4).150 Regenerated cellulose, 6 equivalents (equiv) of OA, and 7.2 equiv. carbodiimide hydrochloride (EDC·HCl) were milled, followed by 4-dimethylaminopyridine (DMAP) addition to accelerate esterification. The process yielded pure cellulose oleate with DS 2.55, later moulded into transparent, flexible, hydrophobic films, offering an alternative to solvent-intensive acid-catalyzed acylation.151 Expanding this strategy, Yang converted MCC into melt-processable cellulose stearate thermoplastics using stearyl chloride and trifluoroacetic anhydride (TFAA) in a planetary ball mill.152 Direct mechanochemical esterification required ∼1/8th the pyridine or TFAA compared to conventional heterogeneous methods.153,154 Fatty acyl chlorides (C10–C18) produced fatty acid cellulose esters (FACEs) with DS 2.18–2.62, all processable by hot-pressing (160 °C, 10 MPa), though samples showed slight yellowing from pyridine.150,152 To mitigate this, Yang later developed a TFAA-assisted milling method using carboxylic acids, yielding similar thermoplastic esters.155
3.1.3.2 Cellulose composite. Cellulose esterification has also been used as a method to produce composite materials (Table 3). Lu and coworkers prepared surface-acetylated cellulose powder (SACP) by pan-milling cellulose with acetic anhydride (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, 60 rpm, 2 cycles).156 Milling disrupted H-bonding, exposed OH groups, and enabled acetylation.157,158 The synthesized SACP was then compounded with natural rubber in a laboratory two-roll mill in the presence of zinc oxide (ZnO), stearic acid, N-cyclohexyl benzthiazyl sulphenamide (CBS) accelerator, and sulfur for vulcanization. When 30 parts per hundred resin SACP was compounded with natural rubber, the vulcanizate showed tensile strength of 14.5 MPa vs. 8.9 MPa for pristine cellulose, and elongation at break of 930% vs. 875%.
4 ratio, 60 rpm, 2 cycles).156 Milling disrupted H-bonding, exposed OH groups, and enabled acetylation.157,158 The synthesized SACP was then compounded with natural rubber in a laboratory two-roll mill in the presence of zinc oxide (ZnO), stearic acid, N-cyclohexyl benzthiazyl sulphenamide (CBS) accelerator, and sulfur for vulcanization. When 30 parts per hundred resin SACP was compounded with natural rubber, the vulcanizate showed tensile strength of 14.5 MPa vs. 8.9 MPa for pristine cellulose, and elongation at break of 930% vs. 875%.
Hirotsu and coworkers demonstrated the role of mechanochemistry in cellulose-polyolefin ester composites. Ball-milling cellulose with maleated polyethylene (MPE) followed by melt-mixing gave composites with 19.3–22.3% bonded MPE, compared to only 3.1% for melt-mixing alone.159,160 This strong covalent ester linkage led to tensile elongation of 73% vs. 32% for untreated samples. Using maleic anhydride-grafted polypropylene (MAPP), mechanochemical treatment yielded 10.2 wt% bonded MAPP vs. 0.9 wt% for melt-mixing only, forming a core–shell structure with improved interfacial adhesion.160
Lu later applied pan-milling to produce polyvinyl alcohol (PVA)/cellulose composites using succinic anhydride (SA) as crosslinker.73,161 The process consumed 61.6% SA and gave a tensile strength of 18 MPa and modulus of 66 MPa, compared to 14 MPa and 45 MPa for neat PVA.
Wu and coworkers addressed PP/cellulose incompatibility162,163 by generating β-phase nucleating sites via mechanochemical acylation.164–167 SBKP was first milled to 71 μm activated powders, which was then co-milled 120 min with mixed anhydride/pimelic acid/calcium carbonate (CaCO3), producing esterified cellulose that nucleated β-PP crystals (>50%).167 The modified cellulose powders in PP (5% loading) yielded tensile strength of 43.2 MPa and elongation at break of 422%.
3.1.3.3 Anionic cellulose. Carboxylated cellulose is a staple in industrial use of cellulose.168 Its negative charges169,170 render it highly hydrophilic and has enabled applications such as high-performance films, biodegradable materials, and as a nanofiller in various composites, while its ability to bind toxic heavy metal ions is helpful in water decontamination.171,172 Lu and coworkers reported a solvent-free mechanochemical method to carboxylated cellulose for use in Pb2+ removal.73 Cellulose and SA (3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were milled at 60 rpm for 30 cycles, affording material with 2.70 mmol g−1 carboxylate groups, characterized by Fourier-transform infrared spectroscopy (FT-IR). In adsorption tests, it removed 421.8 mg g−1 Pb2+ (84.4%) from a 500 mg L−1 solution, significantly higher than unmodified cellulose (26.4%).
2) were milled at 60 rpm for 30 cycles, affording material with 2.70 mmol g−1 carboxylate groups, characterized by Fourier-transform infrared spectroscopy (FT-IR). In adsorption tests, it removed 421.8 mg g−1 Pb2+ (84.4%) from a 500 mg L−1 solution, significantly higher than unmodified cellulose (26.4%).
Khaligh and coworkers used high-energy ball-milling (700 rpm, 4 h, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 feed-to-ball ratio, 0.5 mm balls) for esterification of MCC with maleic anhydride.173 The process achieved DS = 0.72, corresponding to 3.10 mmol g−1 carboxylates, with over 86% ring opening and grafting efficiency, confirmed by conductometric titration, back titration, and thermogravimetry-differential scanning calorimetry (TG-DSC). This efficient, additive-free method required only water for quenching and purification.
50 feed-to-ball ratio, 0.5 mm balls) for esterification of MCC with maleic anhydride.173 The process achieved DS = 0.72, corresponding to 3.10 mmol g−1 carboxylates, with over 86% ring opening and grafting efficiency, confirmed by conductometric titration, back titration, and thermogravimetry-differential scanning calorimetry (TG-DSC). This efficient, additive-free method required only water for quenching and purification.
3.1.3.4 Lipophilic cellulose. Mechanochemical esterification is an effective method to functionalize already defibrillated CNFs. The efficacy of mechanochemical esterification of CNFs is dependent on multiple factors including, but not limited to, the surface area of dried CNFs, the carboxylic acid for the esterification reaction, and the energy input during ball-milling.71 Wei and coworkers discovered that the addition of 10 wt% tert-butanol (TBA) to a 3.3 wt% aqueous slurries of CNFs prevented the collapse of CNFs during lyophilization and afforded CNFs with a significantly increased specific surface area of 23.47 m2 g−1 compared to 2.44 m2 g−1 measured from the CNFs lyophilized in water only. The increased surface area significantly enhanced the DS in the mechanochemical esterification of CNF. This process involved milling one equiv. of granulated CNF with a low-speed rolling mill at 350–400 rpm for 6 h, along with two equiv. of acylimidazole intermediates. These intermediates were prepared by milling equal molar of fatty acids with carbonyldiimidazole. The addition of 10% TBA during lyophilization increased the DS in the optimized esterification reaction. Specifically, DS rose from 0.27 to 0.46 for hexanoic acid (C6), 0.11 to 0.25 for lauric acid (C12), 0.05 to 0.13 for OA, and 0.12 to 0.29 for stearic acid (C18). The esterified CNFs, with increased DS and higher surface area, demonstrated improved dispersion stability at 0.5 wt% in organic solvent as these CNFs remained well-dispersed after being resuspended in EtOH or chloroform by sonication.
Chen and coworkers functionalized cellulose in lignin-containing bamboo powder (49.25% cellulose, 12.95% lignin, 21.44% hemicellulose) using 3,4-dichlorophenyl isocyanate.180 One gram pretreated powder (0.49 g cellulose) was milled with 1.74 g isocyanate (3 equiv.) in 20 mL dimethyl sulfoxide (DMSO) at 300 rpm for 6 h, followed by 5 min ultrasonication. The process yielded thin CNFs with fiber width of 2 ± 1 nm characterized by atomic force microscopy (AFM), with urethane formation confirmed by FT-IR. X-ray Diffraction (XRD) showed no crystallinity loss, and CNF films blocked >90% UV light while maintaining >80% transmittance. Ball-milling thus promoted carbamation, hydrophilization, and partial defibrillation to lignin-containing CNFs.
Xiao and coworkers prepared carboxymethylated CNCs via mechanochemistry.188 MCC (5 g, 1 equiv.) was first activated with sodium hydroxide (NaOH) (0.2 g, 0.16 equiv.) under argon and trace water, then ball-milled with agate beads at 600 rpm for 2 h. Sodium chloroacetate (0.2 g, 0.06 equiv.) was added and the reaction micture was milled 7 h, yielding DS = 0.99. However, titration suggested DS 720% above the theoretical maximum, highlighting the need for reliable characterization. H2O addition (2 mL, η = 0.37 μL mg−1) enhanced hydration and reaction efficiency, giving nearly tripled DS compared to dry milling. Milling reduced particle size, promoted defibrillation into rods, and induced near-complete amorphization. Toxicological tests indicated low cytotoxicity, suggesting biocompatibility.
Kaabel and coworkers reported a two-step mechanochemical/aging approach to obtain C6-substituted aminated and carboxylic esterified CNCs.90 Acid-hydrolyzed CNCs (200 mg, 1 equiv.) were tosylated with TsCl (360 mg, 1.5 equiv.) by ball-milling (25 Hz, 60 min) under LAG conditions (pyridine, η = 0.36 μL mg−1), yielding low DS = 0.11 to preserve crystallinity (CrI = 75%). Extended milling increased DS to 0.29 with 11 pt% CrI loss. Tosylated CNCs were then reacted with nucleophiles (0.708 mmol, 7.7 equiv.) via 5 min milling and 3 d aging at 55 °C, achieving near-quantitative substitution (DSnuc ≈ 0.10). In the second milling stage the newly administrated nucleophiles react exclusively with the anhydrous glucose tosylate electrophile leaving the result repeating units untouched. In the second milling stage, the newly introduced nucleophiles react exclusively with the anhydroglucose tosylate electrophiles formed in the first step, leaving the remaining repeating units unaffected. This high mechanochemical selectivity not only minimizes side-products and undesired derivatives but also calls for careful control of reagent loading to avoid unnecessary excess, in accordance with green chemistry principles. Transmission electron microscopy (TEM) showed rod-shaped CNCs with slight aggregation, attributed to both alkyl chain addition and mechanical treatment.
Mechanochemical phosphorylation has also been applied to bulk cellulose. Laoutid and coworkers milled MCC (20 g) with P4O10 (26 g, 0.75 equiv.) at 200 rpm for 1 h without urea, affording enough grafting efficiency (33% P, 4.15 wt%) to increase thermal degradation temperature.195 Adding urea improves efficiency and lubrication. Su and coworkers milled MCC with P4O10 and urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) at 650 rpm for 90 min, followed by 30 min aging at 150 °C, producing anionic P-MCC defibrillated into P-CNCs (∼4 mmol g−1 charge, 65% P grafted, CrI = 73.3%).91 TEM showed uniform rod-shaped P-CNCs (254.9 ± 27.3 × 7.8 ± 1.2 nm), showcasing the power of this method. This work was the only one to our knowledge providing a life cycle assessment (LCA) comparing a mechanochemical process with a solution phase one (Fig. 5). It revealed a > 10% reduction in five evaluated environmental impacts (global warming potential, primary energy demand, resource depletion water, acidification potential, and ecotoxicity) and 24.8% average reduction versus heat-assisted pre-phosphorylation, mainly by eliminating energy-intensive drying (electricity demand: 0.579 → 0.362 kWh g−1).196
5) at 650 rpm for 90 min, followed by 30 min aging at 150 °C, producing anionic P-MCC defibrillated into P-CNCs (∼4 mmol g−1 charge, 65% P grafted, CrI = 73.3%).91 TEM showed uniform rod-shaped P-CNCs (254.9 ± 27.3 × 7.8 ± 1.2 nm), showcasing the power of this method. This work was the only one to our knowledge providing a life cycle assessment (LCA) comparing a mechanochemical process with a solution phase one (Fig. 5). It revealed a > 10% reduction in five evaluated environmental impacts (global warming potential, primary energy demand, resource depletion water, acidification potential, and ecotoxicity) and 24.8% average reduction versus heat-assisted pre-phosphorylation, mainly by eliminating energy-intensive drying (electricity demand: 0.579 → 0.362 kWh g−1).196
Even under mechanochemical conditions, the reaction with cellulose is usually performed in a H2O/EtOH mixture medium, due to the necessity for silane hydrolysis to occur to introduce active silanol species. Jing and coworkers and Lee and coworkers conducted similar wet ball-milling reactions.201,202
Jing and coworkers prepared amine-functionalized silylated microfibrillated (MFC) with (3-aminopropyl)triethoxysilane (APTES) (2 g MFC, 0.2 equiv. APTES in 3 mL H2O/100 mL EtOH) at 600 rpm for 24 h in a zirconia (ZrO2) planetary mill jar.201 Quantitative efficiency was not reported, but vacuum-dried films showed high optical transparency (∼80%) and enhanced hydrophobicity (contact angle 133.2 ± 3.4° vs. fully hydrophilic MFC). Lee and coworkers silylated MCC with trialkoxysilanes including methyltrimethoxysilane (MTMS), vinyltrimethoxysilane (VTMS), γ-glycidoxypropyltrimethoxysilane (GPTMS), and phenyltrimethoxysilane (PTMS) to introduce alkyl, vinyl, epoxy, or aromatic groups respectively.202 One gram MCC was milled with 33 equiv. silane in H2O/EtOH (17 g/13 g) in a ZrO2 planetary mill. Same as the other work, the reaction efficiency was not quantified with DS; Si–O–C bonds were confirmed qualitatively by FT-IR and scanning electron microscopy (SEM) coupled with energy dispersive X-ray spectroscopy (EDS) while XRD showed minor CrI loss. When used as fillers in silicone foams, silylated MCC greatly improved tensile strength (397 kPa), 44.1× and 5.4× higher than neat silicone foam and foam with pristine MCC, respectively.
Solala and coworkers studied this using styrene-modified cellulose: cotton linters were cryomilled with styrene and stainless steel balls, generating maximum radicals after 40 min.206 In styrene's presence, electron paramagnetic resonance (EPR) intensity decreased while molecular weight (Mw) was stabilized, attributed to low styrene binding. The process was judged inefficient for industry, though inert atmosphere might reduce oxygen side reactions.
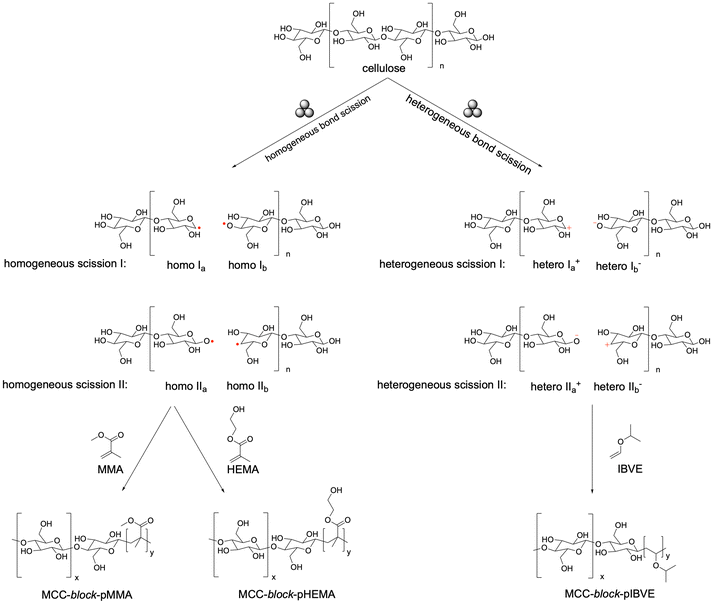
Mechanistically, radicals arise from homogeneous scission of β-1,4-glycosidic bonds, as shown by Sakaguchi (Scheme 2).207 Ohura and coworkers exploited this by synthesizing a diblock copolymer: MCC (50 mg) and 2-hydroxyethyl methacrylate (pHEMA) (50 mg) were milled with 10 g alumina balls in vacuum for 6 h, where cellulose radicals initiated polymerization, yielding MCC-block-pHEMA (37 mol% pHEMA, DP = 69).208 In contrast, heterogeneous scission produces mechano-ions. Sakaguchi and coworkers reported MCC milled with isobutyl vinyl ether (IBVE) in a vibrational glass mill (23 h, 77 K, vacuum), generating ionic yield of 0.692.209,210 The resulting mechano-cations (MCC+) initiated cationic polymerization, forming MCC-block-PIBVE, where IBVE bonded to MCC+ that produces new MCC mechano-cations and propagated PIBVE chains on the cellulose surface.211
3.2. Starch
Starch is an insoluble, semi-crystalline polysaccharide that serves as storage carbohydrate in some plants. Like cellulose, starch is also composed of D-glucose units. However, it simultaneously contains both linear amylose and highly branched amylopectin.212 The most common application of starch is in the food and beverage sector where it is used as an thickening agent,213 although it is also used as a feedstock in the chemical industry for bioplastics production or as a fermentation substrate for bioethanol.100,213–215Traditional starch sources, such as potato and corn starch, can raise ethical concerns as they compete with food supplies. Researchers have turned to the by-products of protein extraction from legumes and pulses as sources for starch.215,216 In our literature searches, the only types of mechanochemical modification of starch that involved the formation of new covalent bonds are esterification and etherification.
| Substrate | Active reagent | Equipment | Condition | Product | Highest DS | Characteristics | Ref. |
|---|---|---|---|---|---|---|---|
| a With overestimation, 8 times higher than stoichiometric maximum. | |||||||
| Cassava starch | Acetic anhydride | Rolling machine | 30 Hz, 18 h | Starch acetate | 0.023 | Improved solubility | Dai and coworkers72 |
| Mung bean starch | Acetic anhydride | Mortar grinding machine | 20 rpm, 48 h | Starch acetate | 0.026 | Improved solubility | Dai and coworkers92 |
| Cassava starch | Lauric acid | Stirring ball mill | 375 rpm, 1 h | Starch laurate | 0.040 | Improved hydrophobicity | Huang and coworkers217 |
| Cassava starch | OSA | Stirring ball mill | 375 rpm, 1.5 h | Starch octenyl succinate | 0.040 | High emulsion stability | Huang and coworkers218 |
| Waxy rice starch | OSA | Planetary ball mill | 450 rpm, 20 h | Starch octenyl succinate | 0.032 | High solubility, low viscosity | Zhao and coworkers219 |
| Corn starch | Lauroyl chloride, pyridine | Planetary ball mill | 500 rpm, 1 h 110 °C, 1 h | Starch laurate | 2.7 | Thermal processibility, water resistance | Hou and coworkers93 |
| Corn starch | Salicylic acid, tartaric acid, glycerol | Single-screw extruder | 90 to 120 °C, 15 g min−1, 100 rpm | Salicylated starch | 0.28a | Thermal processibility, antioxidant | Gutiérrez and coworkers223 |
Huang and coworkers developed ball-milling esterification using a stirring ball mill.217 Cassava starch, lauric acid, and potassium carbonate (K2CO3) catalyst yielded starch laurate (DS 0.0398) after 1 h milling at 60 °C, 375 rpm, with reduced crystallinity (measured by XRD) due to disrupted H-bonding (Scheme 3). Using octenyl succinic anhydride (OSA) and sodium carbonate (Na2CO3) under similar conditions (90 min) produced OSA-starch (DS 0.0397), which was soluble in cold water up to 5.3 wt% with ∼88% transparency and enhanced emulsion stability (k < 2.0 × 10−4).218
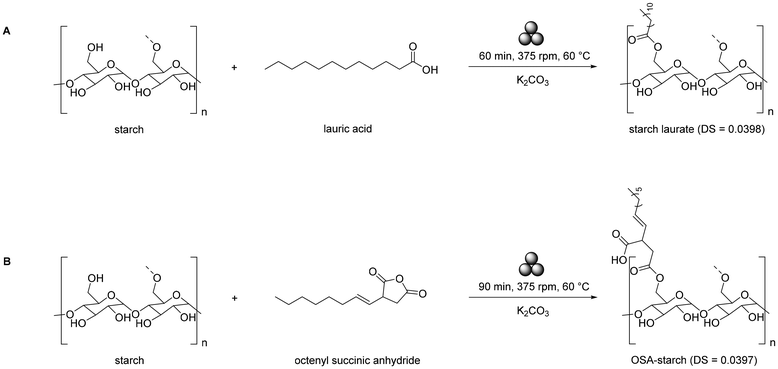 | ||
| Scheme 3 Mechanochemical starch esterification with (A) lauric acid and (B) octenyl succinic anhydride. | ||
Zhao and coworkers explored the mechanochemical octenyl succinylation of starch using a planetary mill (450 rpm, agate jar and balls), where waxy rice starch was reacted with OSA in the presence of NaOH as a catalyst.219,220 In their optimization, the highest reaction efficiency was obtained with 0.9 wt% NaOH relative to starch. Although increasing OSA loading led to a higher DS up to 0.032 at 9 wt% OSA, it simultaneously decreased the reaction efficiency from 75% at 3 wt% to 60% at 4 wt%. Under optimal conditions (0.9 wt% NaOH, 3 wt% OSA, 10 h milling), a DS of 0.017 and 60% reaction efficiency were achieved. Ball-milling reduced the CrI of OSA-starch from 18% to 4.12% after 10 h and rendered the samples fully amorphous after 30–50 h. The resulting OSA-starches exhibited high solubility (>20%) but relatively low viscosity and rigidity compared to conventionally prepared counterparts.
Hou, who previously contributed to several studies on mechanochemical cellulose esterification,150,152,155 reported pyridine-catalyzed esterification of corn starch with lauroyl chloride.93 Corn starch (0.324 g), lauroyl chloride (3.938 g, 9 equiv.), and pyridine (1.898 g, 12 equiv.) were milled at 500 rpm for 1 h, then aged 1 h at 110 °C, yielding DS 2.7. Claimed as more sustainable than N,N-dimethylacetamide (DMAc)/lithium chloride (LiCl) or pyridine solution (E-factors: 14.20, 5.21, vs. 2.05),221,222 this assessment ignored EtOH used in purification, and the method left 70% unreacted lauroyl chloride as waste.
Gutiérrez and coworkers combined organocatalysis, flow mechanochemistry, and extrusion for salicylated starch.223 Corn starch (140 g), glycerol (60 g), salicylic acid (4.0 g, 0.034 equiv.), and tartaric acid (4.35 g, 0.034 equiv.) were extruded (90–120 °C, 100 rpm, 15 g min−1). Titration reported DS 0.28 ± 0.06, exceeding the theoretical maximum (0.034), due to method overestimation. Solid-state cross-polarization/magic-angle spinning (CP-MAS) 13C nuclear magnetic resonance spectroscopy (NMR) confirmed ester bonds. Films of salicylated starch showed 3% swelling in water, 0.099 MPa Young's modulus, 10% elongation, and slight antioxidant activity (6%), suggesting potential for food packaging.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) a premixed blend of corn starch and NaOH (1.6 equiv.) with monochloroacetic acid (0.8 equiv.) in the presence of a catalytic amount of tetrabutylammonium bromide (0.1 mol%) under LAG condition with isopropanol (i-PrOH) at η = 0.1 μL mg−1.232 This method afforded carboxymethyl starch with a moderate DS = 0.631, where the DS increased steadily over the first 6 h of milling before plateauing. Although the process requires high energy input due to prolonged intense milling, it achieved notably high reaction efficiency, with 79% conversion of monochloroacetic acid to grafted carboxymethyl groups.
4) a premixed blend of corn starch and NaOH (1.6 equiv.) with monochloroacetic acid (0.8 equiv.) in the presence of a catalytic amount of tetrabutylammonium bromide (0.1 mol%) under LAG condition with isopropanol (i-PrOH) at η = 0.1 μL mg−1.232 This method afforded carboxymethyl starch with a moderate DS = 0.631, where the DS increased steadily over the first 6 h of milling before plateauing. Although the process requires high energy input due to prolonged intense milling, it achieved notably high reaction efficiency, with 79% conversion of monochloroacetic acid to grafted carboxymethyl groups.
3.3. Chitin
Chitin, or poly(N-acetyl-β-D-glucosamine) is one of the most abundant biopolymers second only to cellulose.24 It is also the most abundant nitrogen-containing biopolymer widely found in the exoskeleton of arthropods and the cell walls of fungi and yeast.29 Like cellulose, chitin also serves as a structural component in living organisms in the form of highly crystalline microfibrils.40 Chitin can be depolymerized to form D-acetylglucosamine (GlcNAc) monomers or oligomers for applications in nutraceutics, biomedical or fertilizers;233,234 it can also be converted to nanofibers,235 and nanocrystals for various uses.40,88,236,237 Importantly, chitin featuring an amide functionality which can be deacetylated to yield chitosan, or poly(β-D-glucosamine).238,239 The nitrogen-containing nature of chitin and its derivatives makes them viable precursors for nitrogen-doped carbon materials capable of metal immobilization. Such materials have been prepared using mechanochemical techniques and applied in a range of contexts, including organic dye degradation,240 bio-imaging,241,242 and, more recently, cross-coupling catalysis.243 While these developments represent an important advance in the valorization of chitinous waste, they do not involve intentional bond breaking or covalent bond formation within the polysaccharide backbone itself. In these systems, chitin and chitosan function primarily as structural supports or precursors rather than as chemically modified polysaccharides as defined by the three valorization strategies discussed in this review. Consequently, these applications are not covered in detail in the following sections. Readers are referred to a dedicated review from our group for a comprehensive discussion of mechanochemical transformations of biomass into functional material.100| Substrate | Active reagent | Equipment | Condition | Product | Yield | Ref. |
|---|---|---|---|---|---|---|
| α-Chitin | Kaolinite | Mixer ball mill | 1080 cycles | Chitin oligosaccharides | 76% | Kerton and coworkers244 |
| Chitin | Impregnated H2SO4 | Planetary ball mill | 500 rpm, 6 h | Chitin oligosaccharides | >99% | Fukuoka and coworkers245 |
| Chitin | Air-oxidized activated carbon catalyst | Planetary ball mill | 500 rpm, 48 h | Chitin oligosaccharides | 66% | Fukuoka and coworkers249 |
| Chitin, chitinous biomass | Aspergillus niger chitinase | Vibrational ball mill | 30 Hz, 5 min, 12 h aging, 20 cycles | GlcNAc monomer | 24% | Auclair and coworkers94 |
Kaolinite was applied by Kerton and coworkers for chitin amorphization/hydrolysis.244 Milling α-chitin (CrI = 91%) with kaolinite (1080 cpm, 120 min) reduced CrI to 35% and increased solubilization (11.0% vs. 6.3% initially). Higher ball packing and longer milling (240 min) maximized soluble product yield to 76%. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-ToF) confirmed GlcNAc monomers and oligomers (DP 2–5).
Fukuoka and coworkers systematically studied mechanochemical chitin hydrolysis. Milling H2SO4-impregnated chitin (500 rpm, 6 h) achieved full conversion to water-soluble compounds, with 41.8% GlcNAc monomers/oligomers (DP < 5).245 The small physisorbed water in chitin enabled acid-catalyzed hydrolysis without added solvent, while N-acetyl groups were fully retained, unlike in conventional heterogeneous hydrolysis.246 Subsequent hydrolysis/methanolysis gave up to 53% GlcNAc and 70% 1-O-methyl-N-acetylglucosamine. Fukuoka's group also produced oligosaccharides using solid acid catalysts to avoid mineral acid by-products.245,247,248
Kobayashi and coworkers introduced an oxidized carbon catalyst (AC-Air, 0.90 mmol g−1 carboxyl, 0.84 mmol g−1 phenolic).249 Milling with AC-Air (48 h) solubilized 72 wt% chitin with 91% selectivity for oligosaccharides. Fukuoka's density functional theory (DFT) studies revealed that planetary milling induced subnano Newton tensile/compressive forces that activated chitin glycosidic bonds.250
Mechanoenzymatic method has also been applied. Auclair and coworkers used RAging (repeated 5 min milling at 30 Hz + 12 h aging, 20 cycles) with chitinase to convert 200 mg chitin to 47 mg GlcNAc and minor dimers/trimers.89,94 When applied to acetic acid (AcOH)-washed shrimp (AcShrimp) and crab shells (AcCrab), yields improved (51 mg GlcNAc from AcShrimp, 49 mg from AcCrab, 10 cycles). This selective route offers a simple valorization pathway for chitinous biomass.
We also extended our cationic tertiary-aminated CNF/CNC synthesis strategy to chitin.143 Practical grade chitin was briefly ball-milled with CDEEA·HCl and NaOH under LAG conditions using TBAH solution for 10 min, followed by aging for 3 h. After purification, the resulting material was defibrillated by ultrasonication, yielding individualized ChNCs dispersed in water. In contrast to the anionic oxidized ChNCs obtained via the HHSA method, these ChNCs were highly cationic (ζ-potential = +68 mV) and exhibited a higher aspect ratio (average length 425 nm, average width 2 nm). This morphology is likely attributable to the strong electrostatic repulsion from the high DS of 0.79. Notably, without requiring excess reagent, the process was remarkably efficient, achieving a low PMIreaction of 4.0.
To construct flexible PVA/chitin films, solid-state shear milling (S3 M) was used both to comminute chitin and disperse it into PVA (Fig. 7).263 Ten cycles of neat S3 M reduced chitin particle size to 51.03 μm and decreased CrI from 95.01% to 61.54%, improving processability. A second S3 M stage produced PVA/chitin composites with flat, smooth morphology confirmed by SEM, indicating good PVA-chitin compatibility. Films obtained by hot pressing showed hydrophilicity suitable for wet biological surfaces, unlike hydrophobic PVA.264 Incorporation of 10 wt% chitin increased tensile strength to 23.25 MPa compared to 19.12 MPa for neat PVA, while maintaining flexibility and foldability.
Lignin, an abundant biopolymer and pulp industry sidestream,256,265 was mechanochemically combined with chitin by Bartczak and coworkers to prepare a chitin-lignin filler for polyurethane (PUR) composites.266 Chitin and hydrogen peroxide (H2O2)-activated alkali lignin were ball milled at 600 rpm for 2 h in a planetary mill, yielding filler particles ∼300 nm in size. During PUR foaming, the filler reacted with isocyanate groups via its hydroxyls, reinforcing the composite. Adding 10% filler extended rising time from 93 to 126 s, increased free rise density from 46.3 to 52.3 kg m−3, and raised tensile strength from 118 to 135 kPa due to chitin/lignin crosslinking with PUR chains.
3.4. Chitosan
Chitosan, obtained by deacetylating >50% of chitin's D-acetyl-glucosamine units,267 contains protonatable primary amines that enable solubility below pH 6.0.268 Dissolved chitosan functions as a flocculant for water remediation,269 and its amine-driven antimicrobial activity supports uses in nutraceutical,270 biomedical,271,272 coatings,273 and packaging.274 With ∼8% nitrogen by Mw, it also serves as a slow-release fertilizer.275 Unlike cellulose or chitin, even high-Mw chitosan is water-soluble, making it one of the most studied polysaccharides for chemical modification.238,239![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH ratio up to 4
NaOH ratio up to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaching 93.5%.279 Unlike ball-milling, the softer method increased CrI from 61.3% to 68.3–75.3%.
1, reaching 93.5%.279 Unlike ball-milling, the softer method increased CrI from 61.3% to 68.3–75.3%.
| Substrate | Active reagent | Equipment | Condition | Product | Highest DS or DDA | Characteristics | Ref. |
|---|---|---|---|---|---|---|---|
| Chitin, shrimp shell | NaOH | Planetary ball mill | 700 rpm, 80 min | Chitosan | DDA 76.4% | Low Mw | Yan and coworkers278 |
| Shrimp chitin | NaOH | Vibrational ball mill | 5 min, 3 d aging | Chitosan | DDA 95.0% | High Mw | Moores and coworkers84 |
| ChNCs | NaOH | Mortar and pestle | Manual grinding, 6 d aging | ChsNCs | DDA 65.5% | Moderate CrI Gels with alginate | Moores and coworkers85 |
| Crab chitin | NaOH | Mortar and pestle | Manual grinding, 30 min | Chitosan | DDA 93.5% | High CrI | Usman and coworkers279 |
| Chitosan | 1,3-Propane sultone | Vibrational ball mill | 30 Hz, 80 min | N-Sulfonated chitosan | DS 78.0% | water-soluble | Stevens and coworkers287 |
| Prawn chitosan | Benzaldehyde derivatives | Shaker ball mill | 700 cycles min−1, 10 min | Chitosan SBs | DS 79.5% | Aromatic | Hernawan and coworkers291 |
| Chitosan | Aldehydes, NaBH4 | Vibrational ball mill | 29.5 Hz, 30 min, 2 stages, 3 d aging | N-Alkylated chitosan | DS 99.1% | Aldehyde species dependent | Moores and coworkers87 |
| Low Mw chitosan | Aldehydes, NaBH4 | Vibrational ball mill | 29.5 Hz, 30 min 29.5 Hz, 120 min | N-Alkylated chitosan | DS 64.4% | Thiophene π–π stacking | Stevens and coworkers296 |
| Chitosan | CDMEA, NaOH | Vibrational ball mill, planetary ball mill | 29.5 Hz, 30 min, 2 d aging; 300 rpm, 30 min, 2 d aging | N-,O-Aminoalkylated chitosan | DS 1.67 | High amine loading, CO2-switchable | Moores and coworkers299 |
| Chitosan | H3PO3, paraformaldehyde | Planetary ball mill | 780 rpm, 7 h | N-Phosphonated chitosan | DS 95.6% | Gypsum scale inhibition | Mady and coworkers303 |
| Crab chitosan | Allyl bromide | Twin-screw extruder | −5 °C, several min | Allylchitosan | DS 50.0% | Photosensitive | Bardakova and coworkers304 |
| Chitosan | Potassium persulfate | Planetary ball mill | 225 rpm, 1 or 3 h | Oxidized chitosan | N/A | High adsorption capacity | Cagnetta and coworkers305 |
Hernawan and coworkers prepared air-stable chitosan Schiff bases (SBs) with benzaldehyde derivatives by solvent-free ball-milling.291 Prawn chitosan and aldehydes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were milled in polytetrafluoroethylene (PTFE) jars at 700 cycles min−1 for 10 min. DS depended on aldehyde: 34.3% (4-methoxybenzaldehyde), 79.5% (2-hydroxybenzaldehyde), and 5.5% (4-hydroxy-3-methoxybenzaldehyde). Like other SBs,291–293 they were hydrolytically unstable in acidic solution,294,295 limiting applications.
1) were milled in polytetrafluoroethylene (PTFE) jars at 700 cycles min−1 for 10 min. DS depended on aldehyde: 34.3% (4-methoxybenzaldehyde), 79.5% (2-hydroxybenzaldehyde), and 5.5% (4-hydroxy-3-methoxybenzaldehyde). Like other SBs,291–293 they were hydrolytically unstable in acidic solution,294,295 limiting applications.
To overcome this, our group developed a two-step reductive alkylation via ball-milling and aging (Scheme 4).87 Chitosan and aldehydes were first milled (30 min, 29.5 Hz, PTFE jar, with H2O) to form SBs, then reduced without purification by milling with sodium borohydride (NaBH4) under LAG condition with EtOH (30 min, 29.5 Hz), followed by 3 d aging. The method was found effective across 20 aldehydes with many afforded high DS, with products displaying altered solubility: bulky alkyl chains made chitosan hydrophobic, while reductive alkylation with 4-(dimethylamino)benzaldehyde introduced high-pKa amines, affording solubility up to pH 12. The approach was efficient with PMI = 36.
 | ||
| Scheme 4 Mechanochemical synthesis of N-alkylated chitosan through reductive amination via processes reported by (a) Moores and coworkers, (b) Stevens and coworkers. | ||
Independently, Stevens and coworkers developed similar protocols using low Mw chitosan.296 One-step milling (500 mg chitosan + 1.0 equiv. aldehyde, 30 Hz, 30 min, stainless steel jar, 2 balls) produced chitosan SBs. For N-alkylated chitosan, NaBH4 was added and the mixture milled for 2 h, giving 6 derivatives with DS up to 64.4% (1H NMR). Compared to our method, this was faster but with lower DS (e.g., N-(furan-2-yl)methyl chitosan: 56% vs. 75%). N-(Thiophen-2-yl)methyl chitosan showed π–π stacking, suggesting optoelectronic potential.297 The authors also reported green metrics in this work including PMIreaction = 2.77, PMIisolation = 209, and reaction mass efficiency ≈ 100%.
Subsequently our group reported a one-pot SN2-type aminoalkylation of chitosan with tertiary amines for CO2-switchable applications.298,299 Chitosan, 2-chloro-N,N-dimethylethylamine·hydrochloride (CDMEA·HCl), NaOH, and catalytic amount of TBAH solution were milled in a PTFE jar with a ZrO2 ball on a vibrational mill for 30 min, followed by in-jar aging at room temperature for 2 d. The reactive CDMEA electrophile was generated in situ and reacted with C2 amine and C6 alcohol groups, producing DMEA-chitosan with up to 14.9% amine nitrogen (DS = 3.00), comparable to synthetic CO2-switchable poly(N,N-dimethylallylamine).300 Unlike solvothermal synthesis, which only gave C6–O substitution,301 the mechanochemical route avoided aqueous acid (which protonates C2 amines) and enabled full nucleophilicity. Aging reduced energy input to 1/6th (598 kJ g−1) compared to extended milling that affords same high DS. Scaling on a planetary ball mill to 10 g afforded DS = 1.60, 91% yield, and PMIreaction = 2.4. The product showed CO2-switchable properties in water for multiple cycles.302
Mady and coworkers synthesized phosphonated chitosan (P-chitosan) as a green oilfield scale inhibitor via the Moedritzer–Irani reaction under LAG condition.303 Low Mw chitosan, phosphorous acid (H3PO3) (3 equiv.), and paraformaldehyde (3 equiv.) were milled in a ZrO2 jar at 780 rpm for 7 h with H2O (η = 0.26 μL mg−1). The water-soluble P-chitosan reached DS = 0.956. LAG improved mobility and diffusion of reactants, enhancing reaction efficiency. In antiscaling tests, P-chitosan matched or exceeded aminotrismethylenephosphonic acid and carboxymethyl inulin against gypsum, calcite, and heidrun calcite. It retained full activity after 1 week at 130 °C, giving 100% inhibition at 2–100 ppm (gypsum) and 10–100 ppm (heidrun calcite).
Bardakova and coworkers produced photosensitive allylchitosan by TSE mechanochemistry.304 Chitosan, allyl bromide, and NaOH were kneaded at −5 °C, affording allylchitosan with DS up to 50%. The material was structured into multilayer 3D scaffolds by vinyl photocrosslinking (325 nm, 15 mW). In vivo implantation in rats restored large tissue defects, demonstrating that mechanochemistry can produce biocompatible photosensitive chitosan derivatives with vinyl groups for mild, solvent-free scaffold fabrication.
3.5. Other polysaccharides
Beyond lignocellulose and chitinous biopolymers, other renewable polysaccharides such as pectin, alginate, and bacterial polysaccharides have been less explored in mechanochemical chemical modification, often serving as additives or coatings.309–311 Mechanochemistry has been applied to study metal–polymer interactions in composite synthesis. For instance, Naimi-Jamal and coworkers employed ball-milling for a one-pot in situ synthesis of alginate-coated imidazole zinc-based metal–organic framework nanoparticles (ZIF-8@alginate NPs). Sodium alginate was co-milled with zinc acetate dihydrate and 2-methylimidazole in a stainless steel jar with two balls using a vibrational ball mill at 28 Hz for 1 h.309 This produced spherical ZIF-8@alginate NPs (50–100 nm) with a surface area of 476.18 m2 g−1 and 9.9 nm pores characterized by Brunauer–Emmett–Teller (BET) analysis. FT-IR spectra indicated no new absorption bands, suggesting the coating arose from dynamic interactions, including hydrogen bonding and ionic interactions between zinc cations and alginate carboxylates.Coordination chemistry is another strategy for polysaccharide-based composites. Luque and coworkers investigated fungal polysaccharides in enhancing zinc oxide (ZnO) NP biocompatibility.312 Zinc nitrate hexahydrate and fungal polysaccharides (from Abortiporus biennis, Lentinus tigrinus, Rigidororus microporus, and Ginkgo polypore) were milled at 350 rpm for 30 min in a stainless steel jar with 18 balls using a Retsch-PM100 planetary mill. The resulting jelly-like nanohybrids were oven-dried and calcined to remove unbound organics. Nitrogen adsorption showed BET surface areas of 15–20 m2 g−1, pore diameters of 20–28 nm, and pore volumes of 0.06–0.13 cm3 g−1. Diffuse reflectance infrared Fourier transform spectroscopy (DRIFT) confirmed Zn coordination with polysaccharide –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, and XRD revealed high crystallinity with a wurtzite structure and slightly reduced unit cell. Toxicological evaluation showed EC50 values of 50 μg mL−1 (A549 cells) and 100 μg mL−1 (SH-SY5Y cells), higher than uncoated commercial ZnO powders, indicating improved biocompatibility.312–315
O groups, and XRD revealed high crystallinity with a wurtzite structure and slightly reduced unit cell. Toxicological evaluation showed EC50 values of 50 μg mL−1 (A549 cells) and 100 μg mL−1 (SH-SY5Y cells), higher than uncoated commercial ZnO powders, indicating improved biocompatibility.312–315
4. Critical discussion
4.1 Advantages of mechanochemistry and aging over liquid state transformations
To demonstrate the capability of mechanochemistry and aging in enhancing the green metrics of polysaccharide chemical modification, we highlight three representative examples chosen among the three pillars of this review: deconstruction, nano-extraction, and functionalization.Conventional acid-catalyzed hydrolysis of cellulose with dilute H2SO4 is typically performed at elevated temperatures above 170 °C,316 well beyond the boiling point of water. This not only results in high energy consumption but also poses significant operational risks, as it requires pressurized equipment to handle the highly corrosive liquid. For example, Thompson and Grethlein reported hydrolysis of a 5 wt% water slurry of newsprint as a cellulose source under extreme conditions: 240 °C in a flow reactor with 1 wt% H2SO4 (0.33 equiv.) present in the slurry.317 These aggressive conditions enabled rapid hydrolysis, yielding ∼50% glucose in only 0.22 min using a specially designed reactor. However, the authors identified the cellulose slurry concentration as the main bottleneck to process efficiency, since higher concentrations hindered flow and mixing.317 More recently, Fierro and coworkers hydrolyzed cotton cellulose at a reduced temperature of 160 °C but with significantly higher H2SO4 loading. Heating a 1.25 wt% cellulose suspension containing 5 wt% H2SO4 (6.5 equiv.) yielded 20% glucose in 2 h. They concluded that the high crystallinity of cellulose with tightly associated polymeric chains necessitates harsh conditions combining elevated temperature with high acid loading.318
Ball-milling addresses both mixing and amorphization effectively, making cellulose hydrolysis less wasteful. In the three examples discussed, H2SO4 loading was reduced to 0.14,122 0.08,319 and 0.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 by impregnating cellulose prior to milling. In all cases, hydrolysis was completed within 2 h, or as short as 35 min using a Simoloyer ball mill at kilogram scale without heating. Near-quantitative conversion of cellulose to water-soluble glucose or low-DP oligomers was achieved without any subsequent hydrothermal treatment. Mechanochemistry thus enabled a ∼50-fold reduction in acid consumption from Fierro's study for the same chemical transformation, meanwhile eliminating the hazardous hot corrosive acid solution under pressure, all within comparable timescales. The solid-state nature of mechanochemistry also allows more elegant process design, enabling the replacement of conventional mineral acids with recyclable solid acids in other examples.120,121,127–129 This not only simplifies the isolation and purification of hydrolyzed sugars but also opens opportunities to design more benign acid catalysts, better aligning the process with safety-related green chemistry principles.
123 by impregnating cellulose prior to milling. In all cases, hydrolysis was completed within 2 h, or as short as 35 min using a Simoloyer ball mill at kilogram scale without heating. Near-quantitative conversion of cellulose to water-soluble glucose or low-DP oligomers was achieved without any subsequent hydrothermal treatment. Mechanochemistry thus enabled a ∼50-fold reduction in acid consumption from Fierro's study for the same chemical transformation, meanwhile eliminating the hazardous hot corrosive acid solution under pressure, all within comparable timescales. The solid-state nature of mechanochemistry also allows more elegant process design, enabling the replacement of conventional mineral acids with recyclable solid acids in other examples.120,121,127–129 This not only simplifies the isolation and purification of hydrolyzed sugars but also opens opportunities to design more benign acid catalysts, better aligning the process with safety-related green chemistry principles.
Mechanochemical techniques have shown a similar impact in reducing acid consumption in the synthesis of acid-hydrolyzed nanopolysaccharides,135,136 selectively hydrolyzing the amorphous regions of native polysaccharides. Additionally, mechanochemistry has also proven influential in accessing oxidatively carboxylated nanopolysaccharides. Interestingly, TEMPO-mediated oxidation, the gold standard for the synthesis of CNF,320 CNC, and ChNC254 using TEMPO, sodium bromide, and sodium hypochlorite under maintained basic pH, has not been adapted to mechanochemical conditions. The HHSA technique developed by our group demonstrates that dynamic aging can enhance the green chemistry metrics from the solution phase APS pathway for producing carboxylated nanopolysaccharides, which conventionally requires refluxing native crystalline polysaccharides with 4 equiv. APS in aqueous solution at 60 °C for 16 h.88,321 Although the APS loading in HHSA was not drastically reduced (3.5 equiv.), the oxidation was more effective, as indicated by fivefold higher DO values for ChNCs produced mechanochemically compared to the solution method (0.25 vs. 0.05).88 In this example, the aging temperature (50 °C) was close to that of solvothermal condition, yet the solid-state mixing provided by mechanochemistry was pivotal in achieving ultra-high local APS concentrations during high-humidity aging with dynamic water adsorption, resulting in improved reaction efficiency. Equally important to this transformation, the aging process compensated for the mild mechanical treatment of manual grinding, avoiding the extensive amorphization typically caused by ball-milling. While amorphization can be beneficial for complete polysaccharide hydrolysis, it is undesirable in nano-extraction processes where preserving the native crystalline domains is essential. When evaluated with green chemistry metrics, by eliminating bulk solvent, the HHSA method generates less solvent waste, as reflected in its significantly improved PMIreaction of 12.5 compared to 203 for the solution phase process.
The advantages of mechanochemistry in functionalizing native polysaccharides can be divided into two main aspects. The first, though less relevant in hydrolysis and nano-extraction, lies in enabling new reactivity, selectivity, and compatibility by avoiding unwanted solvent effects that typically arise from dissolving the polysaccharide substrate to achieve mixability and processability. This benefit is particularly evident in the mechanochemical functionalization of chitosan, where the nucleophilicity of the C2 amine is fully expressed for a range of reactions discussed in this review, by avoiding the aqueous acidic environment required for its dissolution. We have observed such pattern in a few of our own works as well as other reports from other colleagues, such as the prevention of imine hydrolysis that facilitated chitosan reductive amination to introduce new functional groups, including those bearing acid-sensitive acetal protections;87,296 and in the SN2-type N-aminoalkylation, which is otherwise inhibited in acidic media.299
The second advantage of mechanochemical polysaccharide functionalization is the high reaction efficiency and low waste generation observed across all three classes of modifications reviewed. A direct comparison can be drawn between transformations achievable by both solution phase and mechanochemical methods. For instance, the conventional synthesis of carboxymethyl starch involves reacting a 4 wt% starch suspension in a H2O/i-PrOH mixture (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 w/w) with 3.2 wt% NaOH and 1.7 equiv. monochloroacetic acid at 60 °C, yielding carboxymethyl starch with a DS of 0.53, corresponding to 31% conversion of the Williamson etherification agent.322 Subsequent optimization by Tijsen and coworkers, adjusting the H2O/i-PrOH ratio to 10
80 w/w) with 3.2 wt% NaOH and 1.7 equiv. monochloroacetic acid at 60 °C, yielding carboxymethyl starch with a DS of 0.53, corresponding to 31% conversion of the Williamson etherification agent.322 Subsequent optimization by Tijsen and coworkers, adjusting the H2O/i-PrOH ratio to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 w/w, did not significantly improve efficiency.323,324 In contrast, the mechanochemical approach developed by Zhu and coworkers achieved a higher DS of 0.631 using less than half the amount of monochloroacetic acid (0.8 equiv.) and only a minimal amount of i-PrOH as a LAG additive rather than as a bulk solvent.232 The mechanochemical method thus afforded a markedly higher 79% conversion rate and a nearly ideal PMIreaction of 1.55 compared to 21.98 for the conventional process, reflecting its greatly reduced solvent demand.
90 w/w, did not significantly improve efficiency.323,324 In contrast, the mechanochemical approach developed by Zhu and coworkers achieved a higher DS of 0.631 using less than half the amount of monochloroacetic acid (0.8 equiv.) and only a minimal amount of i-PrOH as a LAG additive rather than as a bulk solvent.232 The mechanochemical method thus afforded a markedly higher 79% conversion rate and a nearly ideal PMIreaction of 1.55 compared to 21.98 for the conventional process, reflecting its greatly reduced solvent demand.
While the effect of aging in conjunction with mechanochemistry has not been systematically studied, it has been widely employed in a number of the studies featured in this review to reduce the extent of required mechanical treatment, as noted by several researchers.72,84–94,140,143,191,299,303 The energy demand associated with the aging step varies considerably among these reports: some require heating at elevated temperatures, whereas others demonstrate that thermal input is not essential for achieving an effective aging process.87,143,299 In such cases, the overall energy consumption can be viewed as proportionally reduced relative to the extent of mechanical treatment replaced by aging, in addition to preventing undesired degradation that mechanochemical techniques might otherwise induce.
We provide in Table 7 a qualitative comparison of mechanochemical/aging and solution-phase polysaccharide transformations. This comparison is based on key CHEM-21 sustainability parameters and offers a quick overview of the sustainability benefits of the topic discussed.
| Parameter (CHEM-21 dimension) | Mechanochemistry/Aging | Solution-phase techniques |
|---|---|---|
| Solvent | None or minimal (LAG; aging under solvent vapor) in reaction stage; often reduced in separation/purification | Bulk solvents typically required in reaction stage for dissolution/dispersion and heating |
| Reaction time | Minutes to hours of milling; aging allows conversion without continuous energy input, although over extended time (days) | Hours to days, often requiring prolonged heating or stirring |
| Energy | Mechanical energy activates substrates; reduced thermal input; aging decouples reaction progress from continuous energy supply | Thermal energy for heating, reflux, and solvent removal |
| Conversion | Often stoichiometric or near-stoichiometric; high local concentrations | Frequently large reagent excess to overcome solubility and diffusion limits |
| PMIreaction | Low to moderate; minimal solvent contribution | High, dominated by solvent mass |
| PMIworkup | Low to moderate; simple (washing, drying); often no solvent-intensive purification | High, multi-step extraction, precipitation, filtration, and drying |
| E-Factor | Do not display difference when transformations occur through the same reaction pathway | |
| Atom economy | ||
| CHEM-21 overall assessment | Favorable across solvent, waste, and energy metrics | Penalized by solvent intensity and waste generation |
4.2 Mechanochemistry and aging as a tunable approach to polysaccharide depolymerization, nano-extraction and functionalization
In this review, we have demonstrated that mechanochemistry and aging-based approached are now developing into a mature field. Interestingly, this review underscores the many similarities between the methods developed for cellulose, starch, chitin, and chitosan. For instance, the esterification reaction is often the model reaction developed in this space. Reaction conditions are remarkably similar among substrates such as common fatty acids71,149,150,217 and acetic anhydride,72,92,156 pointing to the generality of these methods. This applies as well to reactions such as the SN2 alkylation reaction, which functions in a fairly similar way with chitosan,299 chitin, and cellulose.143These methods can also be modulated to achieve the different targets of polysaccharide valorization, namely depolymerization, nano-extraction and functionalization, as illustrated in Fig. 1. Ball milling, especially if extended for one or several hours will lead to polysaccharide amorphization. This can assist with depolymerization and functionalization, as it can help expose and activate the polymer for reactivity. It may however interfere with nano-extraction, a method relying on the crystalline arrangement of the biopolymer to preserve its nanostructure. The use of aging can help mitigate this problem, as demonstrated by us and others.88,140 It is also possible that functionalization and depolymerization can interfere with one another. No group has discussed this aspect so far, as common Mw measurements methods, relying on standard-calibrated GPC, can not reliably measure the Mw of functionalized polysaccharides yet. Future works should be geared to solving this challenge so as to further discuss the selectivity of these methods.
4.3 Toxicity considerations
Toxicity is a central aspect of green chemistry, reflected in both Principle 4, which calls for the design of safer chemicals, and Principle 3, which emphasizes the use of less hazardous synthetic methods.325 Polysaccharides are inherently low in biotoxicity and have therefore found extensive use in biomedical and food applications for this reason.326,327 However, chemical modification of these otherwise benign materials still demands the highest caution to minimize toxicity-related environmental impacts throughout the lifecycle of polysaccharide-derived products.328Examples discussed in this review include reagents with recognized GHS health hazard classifications, such as persulfate oxidizers,142,305 the electrophilic 1,3-propane sultone,287 and the activating agents TFAA152 and CDI.71 Several other reagents are acutely toxic under GHS classification, including EDC·HCl and DMAP used in cellulose esterification;150 furfural and NaBH4 in chitosan reductive amination;87,296 sodium chloroacetate,188 CDMEA·HCl,300 and CDEEA·HCl143 as alkylating agents for cellulosic and chitinous polysaccharides; as well as allyl bromide employed in allylchitosan synthesis.304
Importantly, mechanochemical modification of polysaccharides, while sometimes still requiring these toxic reagents, can mitigate their overall toxicity-related environmental impacts by improving reaction efficiency and thereby reducing reagent quantities. Conversely, whenever mechanochemistry enables alternative synthetic pathways that avoid toxic substances, these routes should be prioritized. For instance, in many cases featured in this review, cellulose or starch esterification was accomplished efficiently using carboxylic acids, acid anhydrides, or acyl chlorides without employing activating agents.71,73,93,173 Similarly, the elimination or minimization of solvent use in mechanochemical systems greatly reduces the risk of inhalation or dermal exposure to toxic organic solvents such as DMAc, commonly used with LiCl to solubilize starch for esterification.221
On the other hand, assessing the toxicity of functionalized polysaccharides is far more challenging. Many of these products are newly accessible only through mechanochemical synthesis, and their biological effects remain largely unknown. In general, molecules with molecular weights exceeding 700 g mol−1 cannot readily penetrate cellular phospholipid bilayers and thus are unlikely to exhibit direct toxic effects.329 Nevertheless, the biodegradability of functionalized polysaccharides may lead to the formation of oligomers (DP < 3) or monomers of sufficiently low molecular weight to pose potential risks.330,331 Accordingly, evaluating the toxicity of such functionalized polysaccharides, as well as their metabolites, will require multidisciplinary collaborations, representing one of the most pressing current challenges in advancing green and sustainable polysaccharide chemistry.
4.4 Economic considerations and industrial relevance of mechanochemical polysaccharide transformations
Beyond environmental benefits, mechanochemistry has the potential to also deliver economic advantages for polysaccharide valorization. While the cost of waste biomass-derived polysaccharides is low and competes favorably with virgin source,332 other raw chemicals and auxiliaries used in the process have a large impact on the final price tag. Mechanochemistry reduces reliance on bulk solvents and minimizing reagent use and thus positively impacts marginal cost. This also decreases downstream separation and waste treatment expenses. Several examples in this review demonstrate low PMI values and high reaction efficiencies achieved without solvent-intensive processing,85–88,143,192,232,287,296,299 suggesting favourable operating costs compared to conventional solution-phase methods.Energy consumption is another crucial economic factor in the production of polysaccharide-based functional materials. While mechanical activation requires a significant initial energy investment, this can be offset by shortened treatment times with aging, eliminating the need for solvent heating, and reducing drying steps. Furthermore, aging-based strategies separate chemical conversion from continuous mechanical input, enabling reactions to occur under mild conditions with minimal additional energy. This is demonstrated by the life cycle assessment of mechanochemical cellulose phosphorylation,91 which shows reduced electricity consumption and overall environmental impact compared to heat-assisted solution-phase processes, highlighting potential cost savings.
From an industrial perspective, mechanochemical platforms such as reactive extrusion and RAM are inherently scalable and compatible with continuous processing. These technologies are already widely used in the food,333 polymer,334 and materials industries,335 lowering barriers to adoption for polysaccharide modification. Furthermore, the ability to process insoluble, heterogeneous biomass feedstocks without prior dissolution simplifies process design and reduces capital investment in tightly controlled solvent handling and corrosion-resistant equipment.
Nevertheless, economic challenges remain. Capital costs for specialized milling or extrusion equipment, wear on grinding media, and the need for careful process optimization to prevent over-milling are all important factors. The use of mechanical equipment by many industries, such as the mining,336 forestry,337 and food,338 for applications for kg to ton scales, are offering opportunities for the commercialization of mechanochemical processes. Future studies performed on the scale up for the mechanochemical transformation of biomass will provide invaluable insights on the feasibility of such processes on scale, while clarifying questions on marginal and capital costs associated with them. Such work should combine techno-economic analysis with life cycle assessment will be crucial for quantitatively comparing mechanochemical routes to established industrial processes beyond lab scale techniques. Ultimately, the convergence of improved green metrics, scalable processing technologies, and reduced process complexity positions mechanochemistry as a promising and economically viable foundation for functional polysaccharide-based materials production as a sustainable alternative to fossil-based polymers.
5. Conclusion and future outlooks
Polysaccharides have received major interest as a feedstock for materials traditionally derived from petrochemical sources. Among abundantly available polysaccharides, cellulose has been studied the most and has been demonstrated as a sustainable feedstock for hydrolysis to produce sugars and nanocellulose, chemical modification to make thermoplastic, and composite formulation with other polymers. Mechanochemistry facilitates these transformations with the benefit of reduced solvent use to potentially lower the cost of polysaccharide-based material production, as well as improve the sustainability of such modification processes in reducing energy consumption, increasing reaction efficiency, and decreasing waste generation (PMI). This trend also applies to other polysaccharides such as chitin and chitosan.However, the current studies also highlight some important knowledge gaps in this field of research: (1) many polysaccharides have not been extensively investigated as a substrate for modification compared to cellulose, starch, and chitin; (2) the scope of chemical transformations has largely been limited to esterification reactions; (3) the continued use of excess reagents, as opposed to catalytic amounts, for polysaccharide modification by mechanochemistry; (4) high energy input during mechanochemical process associated with high ball count-to-reactant (substrate) ratio and lengthy milling time; (5) insufficient study on the scalability of mechanochemical modifications beyond multigram scales; (6) insufficient use of green metrics to evaluate the processes under mechanochemical conditions; (7) the narrow focus on ball mills as the equipment; (8) lack of in-depth mechanistic study on the impact of mechanochemistry to polymer properties such as the Mw during chemical modification; (9) absence of toxicology investigations related to the degradation of chemically modified polysaccharides; and (10) inconsistency in quality of the biomass source and insufficient quantities to meet supply chain needs.
As further studies aim to address these gaps, it is anticipated that mechanochemical polysaccharide modification remains an emerging field of research with significant potential to access high performing, modified polysaccharides at scale to compete with petrochemical-derived materials.
Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.Abbreviations
| AC-Air | oxidized carbon catalyst |
| AcCrab | Acetic acid-washed crab shells |
| AcOH | Acetic acid |
| AcShrimp | Acetic acid-washed shrimp shells |
| AFM | Atomic force microscopy |
| APS | Ammonium persulfate |
| APTES | (3-Aminopropyl)triethoxysilane |
| BET | Brunauer–Emmett–Teller |
| BmimOAc | 1-Butyl-3-methylimidazolium acetate |
| CAM | Citric acid monohydrate |
| CaO2 | Calcium peroxide |
| CDEEA·HCl | 2-Chloro-N,N-diethylethylamine hydrochloride |
| CDMEA·HCl | 2-Chloro-N,N-dimethylethylamine·HCl |
| ChCl | Choline chloride |
| ChNC | Chitin nanocrystal |
| ChsNC | Chitosan nanocrystal |
| CNC | Cellulose nanocrystal |
| CNF | Cellulose nanofibre |
| CO2 | Carbon dioxide |
| CP-MAS | Cross-polarization/magic-angle spinning |
| CrI | Crystallinity index |
| DDA | Degree of deacetylation |
| DFT | Density functional theory |
| DMAc | N,N-Dimethylacetamide |
| DMAP | 4-Dimethylaminopyridine |
| DMSO | Dimethyl sulfoxide |
| DO | Degree of oxidation |
| DRIFT | Diffuse reflectance infrared Fourier transform spectroscopy |
| DS | Degree of substitution |
| EDC·HCl | Carbodiimide hydrochloride |
| EDS | Dispersive X-ray spectroscopy |
| EPR | Electron paramagnetic resonance |
| EPTMAC | (2,3-Epoxypropyl) trimethylammonium chloride |
| EtOH | Ethanol |
| FACEs | Fatty acid cellulose esters |
| FT-IR | Fourier-transform infrared spectroscopy |
| GlcNAc | D-Acetylglucosamine |
| GPC | Gel permeation chromatography |
| GPTMS | γ-Glycidoxypropyltrimethoxysilane |
| H2O | Water |
| H2O2 | Hydrogen peroxide |
| H3PO3 | Phosphorous acid |
| H3PO4 | Phosphoric acid |
| HHSA | High-humidity shaker aging |
| HMF | 5-Hydroxymethylfurfural |
| IBVE | Isobutyl vinyl ether |
| i-PrOH | Isopropanol |
| K2CO3 | Potassium carbonate |
| K2S2O8 | Potassium persulfate |
| LAG | Liquid-assisted grinding |
| LCA | Life cycle assessment |
| LiCl | Lithium chloride |
| MALDI-ToF | Matrix-assisted laser desorption ionization-time of flight mass spectrometry |
| MAPP | Maleic anhydride-grafted polypropylene |
| MCC | Microcrystalline cellulose |
| MCC+ | Microcrystalline cellulose mechano-cations |
| MFC | Microfibrillated cellulose |
| MPE | Maleated polyethylene |
| MTMS | Methyltrimethoxysilane |
| M w | Molecular weight |
| Na2CO3 | Sodium carbonate |
| Na2CO3·1.5H2O2 | Sodium percarbonate |
| NaBH4 | Sodium borohydride |
| NaCl | Sodium chloride |
| NADES | Natural deep eutectic solvents |
| NaOH | Sodium hydroxide |
| NMR | Nuclear magnetic resonance spectroscopy |
| NPs | Nanoparticles |
| OA | Oleic acid |
| OAD | Oxalic acid dihydrate |
| OSA | Octenyl succinic anhydride |
| P-chitosan | Phosphonated chitosan |
| P-CNC | Phosphorylated CNC |
| P4O10 | Phosphorus pentoxide |
| PDI | Polydispersity index |
| PEF | Polyethylene furanoate |
| pHEMA | 2-Hydroxyethyl methacrylate |
| PMI | Process mass intensity |
| PrS | 1,3-Propane sultone |
| PTA | Phosphotungstic acid |
| PTFE | Polytetrafluoroethylene |
| PTMS | Phenyltrimethoxysilane |
| PUR | Polyurethane |
| PVA | Polyvinyl alcohol |
| Raging | Reactive aging |
| RAM | Resonant acoustic mixing |
| RH | Relative humidity |
| S3 M | Solid-state shear milling |
| SA | Succinic anhydride |
| SACP | Surface-acetylated cellulose powder |
| SBs | Schiff bases |
| SEM | Scanning electron microscopy |
| TBA | tert-Butanol |
| TBAH | Tetrabutylammonium hydroxide |
| TEM | Transmission electron microscopy |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxylradical |
| TFAA | Trifluoroacetic anhydride |
| TG-DSC | Thermogravimetry-differential scanning calorimetry |
| TsCl | p-Toluenesulfonyl chloride |
| TSE | Twin screw extrusion |
| VTMS | Vinyltrimethoxysilane |
| XRD | X-ray diffraction |
| ZIF-8@alginate NPs | Zinc-based metal–organic framework nanoparticles |
| ZnO | Zinc oxide |
Conflicts of interest
There is no conflict to declare.Data availability
This article is a review and all its material are based on analysis of articles cited in the bibliography. There is no other data to report or share.Acknowledgements
The authors would like to thank the Natural Science and Engineering Research Council of Canada (NSERC)-Discovery Grant (RGPIN-2024-05617 (A. M.)), the Fonds de Recherche du Québec – Nature et Technologies (FRQNT) - the Centre for Green Chemistry and Catalysis (CGCC, https://doi.org/10.69777/265155), and McGill University including the Heather Munroe-Blum Fellowships (G. Y.) in Green Chemistry for their financial support.References
-
J. Brandrup, E. H. Immergut, E. A. Grulke, A. Abe and D. R. Bloch, Polymer handbook, Wiley New York, 1999, vol. 89 Search PubMed
.
-
J. E. Mark, Physical properties of polymers handbook, Springer, 2007, vol. 1076 Search PubMed
.
-
W. J. Roff and J. R. Scott, Fibres, films, plastics and rubbers: a handbook of common polymers, Elsevier, 2013 Search PubMed
.
- J. M. García, F. C. García, F. Serna and J. L. de la Peña, High-performance aromatic polyamides, Prog. Polym. Sci., 2010, 35(5), 623–686, DOI:10.1016/j.progpolymsci.2009.09.002
.
-
J. A. Brydson, Plastics materials, Elsevier, 1999 Search PubMed
.
-
W. Kaminsky, Polyolefins, in Handbook of Polymer Synthesis, CRC Press, 2004, pp. 13–84 Search PubMed
.
- I. M. Maafa, Pyrolysis of Polystyrene Waste: A Review, Polymers, 2021, 13(2), 225 CrossRef CAS PubMed
.
- B. H. A. Rehm, Polyester synthases: natural catalysts for plastics, Biochem. J., 2003, 376(1), 15–33, DOI:10.1042/bj20031254
.
- J. O. Akindoyo, M. D. H. Beg, S. Ghazali, M. R. Islam, N. Jeyaratnam and A. R. Yuvaraj, Polyurethane types, synthesis and applications – a review, RSC Adv., 2016, 6(115), 114453–114482, 10.1039/C6RA14525F
.
- G. J. Puts, P. Crouse and B. M. Ameduri, Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer, Chem. Rev., 2019, 119(3), 1763–1805, DOI:10.1021/acs.chemrev.8b00458
.
- F.-L. Jin, X. Li and S.-J. Park, Synthesis and application of epoxy resins: A review, J. Ind. Eng. Chem., 2015, 29, 1–11, DOI:10.1016/j.jiec.2015.03.026
.
- H. Wake, Oil refineries: a review of their ecological impacts on the aquatic environment, Estuarine, Coastal Shelf Sci., 2005, 62(1), 131–140, DOI:10.1016/j.ecss.2004.08.013
.
- D. Rourke and S. Connolly, Just oil? the distribution of environmental and social impacts of oil production and consumption, Ann. Rev. Environ. Resour., 2003, 28, 587–617, DOI:10.1146/annurev.energy.28.050302.105617
.
- C. J. Moore, Synthetic polymers in the marine environment: A rapidly increasing, long-term threat, Environ. Res., 2008, 108(2), 131–139, DOI:10.1016/j.envres.2008.07.025
.
- D. Hu, M. Shen, Y. Zhang, H. Li and G. Zeng, Microplastics and nanoplastics: would they affect global biodiversity change? Environ, Sci. Pollut. Res., 2019, 26(19), 19997–20002, DOI:10.1007/s11356-019-05414-5
.
- M. S. Qureshi, A. Oasmaa, H. Pihkola, I. Deviatkin, A. Tenhunen, J. Mannila, H. Minkkinen, M. Pohjakallio and J. Laine-Ylijoki, Pyrolysis of plastic waste: Opportunities and challenges, J. Anal. Appl. Pyrolysis, 2020, 152, 104804, DOI:10.1016/j.jaap.2020.104804
.
- B. R. T. Simoneit, P. M. Medeiros and B. M. Didyk, Combustion Products of Plastics as Indicators for Refuse Burning in the Atmosphere, Environ. Sci. Technol., 2005, 39(18), 6961–6970, DOI:10.1021/es050767x
.
- R. Verma, K. S. Vinoda, M. Papireddy and A. N. S. Gowda, Toxic Pollutants from Plastic Waste- A Review, Proc. Environ. Sci., 2016, 35, 701–708, DOI:10.1016/j.proenv.2016.07.069
.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782, DOI:10.1126/sciadv.1700782
.
- J. Baranwal, B. Barse, A. Fais, G. L. Delogu and A. Kumar, Biopolymer: A Sustainable Material for Food and Medical Applications, Polymers, 2022, 14(5), 983 CrossRef CAS PubMed
.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Cellulose: Fascinating Biopolymer and Sustainable Raw Material, Angew. Chem., Int. Ed., 2005, 44(22), 3358–3393, DOI:10.1002/anie.200460587
.
- M. Mujtaba, L. Fernandes Fraceto, M. Fazeli, S. Mukherjee, S. M. Savassa, G. Araujo de Medeiros, A. do Espírito Santo Pereira, S. D. Mancini, J. Lipponen and F. Vilaplana, Lignocellulosic biomass from agricultural waste to the circular economy: a review with focus on biofuels, biocomposites and bioplastics, J. Cleaner Prod., 2023, 402, 136815, DOI:10.1016/j.jclepro.2023.136815
.
- R. A. Gross and B. Kalra, Biodegradable Polymers for the Environment, Science, 2002, 297(5582), 803–807, DOI:10.1126/science.297.5582.803
.
- R. N. Tharanathan and F. S. Kittur, Chitin—The Undisputed Biomolecule of Great Potential, Crit. Rev. Food Sci. Nutr., 2003, 43(1), 61–87, DOI:10.1080/10408690390826455
.
- J. L. Vidal, T. Jin, E. Lam, F. Kerton and A. Moores, Blue is the new green: Valorization of crustacean waste, Curr. Res. Green Sustainable Chem., 2022, 5, 100330, DOI:10.1016/j.crgsc.2022.100330
.
- H. P. S. Abdul Khalil, A. H. Bhat and A. F. Ireana Yusra, Green composites from sustainable cellulose nanofibrils: A review, Carbohydr. Polym., 2012, 87(2), 963–979, DOI:10.1016/j.carbpol.2011.08.078
.
- X. Chen, H. Yang and N. Yan, Shell Biorefinery: Dream or Reality?, Chem. – Eur. J., 2016, 22(38), 13402–13421, DOI:10.1002/chem.201602389
.
- F. Rol, M. N. Belgacem, A. Gandini and J. Bras, Recent advances in surface-modified cellulose nanofibrils, Prog. Polym. Sci., 2019, 88, 241–264, DOI:10.1016/j.progpolymsci.2018.09.002
.
- M. Rinaudo, Chitin and chitosan: Properties and applications, Prog. Polym. Sci., 2006, 31(7), 603–632, DOI:10.1016/j.progpolymsci.2006.06.001
.
- S. N. Pawar and K. J. Edgar, Alginate derivatization: A review of chemistry, properties and applications, Biomaterials, 2012, 33(11), 3279–3305, DOI:10.1016/j.biomaterials.2012.01.007
.
- M. J. Lundahl, A. G. Cunha, E. Rojo, A. C. Papageorgiou, L. Rautkari, J. C. Arboleda and O. J. Rojas, Strength and Water Interactions of Cellulose I Filaments Wet-Spun from Cellulose Nanofibril Hydrogels, Sci. Rep., 2016, 6(1), 30695, DOI:10.1038/srep30695
.
- P. Cazón, G. Velazquez, J. A. Ramírez and M. Vázquez, Polysaccharide-based films and coatings for food packaging: A review, Food Hydrocolloids, 2017, 68, 136–148, DOI:10.1016/j.foodhyd.2016.09.009
.
- L. T. Mika, E. Cséfalvay and Á. Németh, Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability, Chem. Rev., 2018, 118(2), 505–613, DOI:10.1021/acs.chemrev.7b00395
.
- N. M. Delzenne, Oligosaccharides: state of the art, Proc. Nutr. Soc., 2003, 62(1), 177–182, DOI:10.1079/PNS2002225
.
- T. E. McAlindon, M. P. LaValley, J. P. Gulin and D. T. Felson, Glucosamine and Chondroitin for Treatment of OsteoarthritisA Systematic Quality Assessment and Meta-analysis, J. Am. Med. Assoc., 2000, 283(11), 1469–1475, DOI:10.1001/jama.283.11.1469
.
- E. de Jong, H. A. Visser, A. S. Dias, C. Harvey and G.-J. M. Gruter, The Road to Bring FDCA and PEF to the Market, Polymers, 2022, 14(5), 943 CrossRef CAS PubMed
.
- Y. Ohkouchi and Y. Inoue, Direct production of l(+)-lactic acid from starch and food wastes using Lactobacillus manihotivorans LMG18011, Bioresour. Technol., 2006, 97(13), 1554–1562, DOI:10.1016/j.biortech.2005.06.004
.
- Y. Cheng, S. Deng, P. Chen and R. Ruan, Polylactic acid (PLA) synthesis and modifications: a review, Front. Chem. China, 2009, 4(3), 259–264, DOI:10.1007/s11458-009-0092-x
.
- B. Thomas, M. C. Raj, A. K. B, R. M. H, J. Joy, A. Moores, G. L. Drisko and C. Sanchez, Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications, Chem. Rev., 2018, 118(24), 11575–11625, DOI:10.1021/acs.chemrev.7b00627
.
- T. Jin, T. Liu, E. Lam and A. Moores, Chitin and chitosan on the nanoscale, Nanoscale Horiz., 2021, 6(7), 505–542, 10.1039/d0nh00696c
.
- T. Jin, D. Kurdyla, S. Hrapovic, A. C. W. Leung, S. Régnier, Y. Liu, A. Moores and E. Lam, Carboxylated Chitosan Nanocrystals: A Synthetic Route and Application as Superior Support for Gold-Catalyzed Reactions, Biomacromolecules, 2020, 21(6), 2236–2245, DOI:10.1021/acs.biomac.0c00201
.
- T. Saito, Y. Nishiyama, J.-L. Putaux, M. Vignon and A. Isogai, Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose, Biomacromolecules, 2006, 7(6), 1687–1691, DOI:10.1021/bm060154s
.
- J. D. Goodrich and W. T. Winter, α-Chitin Nanocrystals Prepared from Shrimp Shells and Their Specific Surface Area Measurement, Biomacromolecules, 2007, 8(1), 252–257, DOI:10.1021/bm0603589
.
- Z. Yang, H. Zhao, Y. Huang and H. Liu, Preparation of porous TEMPO-oxidized cellulose nanofiber (TOCNF) composites via Pickering-emulsion template for adsorption of aromatic pollutants, Cellulose, 2025, 32(7), 4289–4311, DOI:10.1007/s10570-025-06539-2
.
- C. Lu, S. Gong, Y. Xia, N. Xu, J. Yu, C. Wang, J. Wang, Q. Yong and F. Chu, Muscle-like self-strengthening poly(vinyl alcohol)/cellulose/MXene composite hydrogel by mechanical training for wearable electronics, Int. J. Biol. Macromol., 2025, 322, 146706, DOI:10.1016/j.ijbiomac.2025.146706
.
- A. Isogai, Cellulose Nanofibers: Recent Progress and Future Prospects, J. Fiber Sci. Technol., 2020, 76(10), 310–326, DOI:10.2115/fiberst.2020-0039
.
- T. Li, C. Chen, A. H. Brozena, J. Y. Zhu, L. Xu, C. Driemeier, J. Dai, O. J. Rojas, A. Isogai, L. Wågberg and L. Hu, Developing fibrillated cellulose as a sustainable technological material, Nature, 2021, 590(7844), 47–56, DOI:10.1038/s41586-020-03167-7
.
- D. M. Suflet, G. C. Chitanu and V. I. Popa, Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose, React. Funct. Polym., 2006, 66(11), 1240–1249, DOI:10.1016/j.reactfunctpolym.2006.03.006
.
-
T. Heinze, T. Liebert and A. Koschella, Esterification of polysaccharides, Springer Science & Business Media, 2006 Search PubMed
.
- F. Sullivan, L. Simon, N. Ioannidis, S. Patel, Z. Ophir, C. Gogos, M. Jaffe, S. Tirmizi, P. Bonnett and P. Abbate, Chemical reaction modeling of industrial scale nitrocellulose production for military applications, AlChE J., 2020, 66(7), e16234, DOI:10.1002/aic.16234
.
-
N. R. Choudhury, Acetylated Starch Market Growth & Demand Forecast 2022 to 2032, Future Market Insights, United States, 2022 Search PubMed
.
- S. Afewerki, A. Sheikhi, S. Kannan, S. Ahadian and A. Khademhosseini, Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics, Bioeng. Transl. Med., 2019, 4(1), 96–115, DOI:10.1002/btm2.10124
.
- L. Avérous, C. Fringant and L. Moro, Plasticized starch–cellulose interactions in polysaccharide composites, Polymer, 2001, 42(15), 6565–6572, DOI:10.1016/S0032-3861(01)00125-2
.
- P. Anastas, P. Licence and J. B. Zimmerman, The 2025 Stockholm Declaration on Chemistry for the Future, Environ. Sci. Technol., 2025, 59(21), 10121–10121, DOI:10.1021/acs.est.5c06442
.
- B. Lindman, G. Karlström and L. Stigsson, On the mechanism of dissolution of cellulose, J. Mol. Liq., 2010, 156(1), 76–81, DOI:10.1016/j.molliq.2010.04.016
.
- V. Zargar, M. Asghari and A. Dashti, A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications, ChemBioEng Rev., 2015, 2(3), 204–226, DOI:10.1002/cben.201400025
.
- C. Y. Liang and R. H. Marchessault, Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses, J. Polym. Sci., 1959, 37(132), 385–395, DOI:10.1002/pol.1959.1203713209
.
- Y. Yu, T. Tyrikos-Ergas, Y. Zhu, G. Fittolani, V. Bordoni, A. Singhal, R. J. Fair, A. Grafmüller, P. H. Seeberger and M. Delbianco, Systematic Hydrogen–Bond Manipulations To Establish Polysaccharide Structure–Property Correlations, Angew. Chem., 2019, 131(37), 13261–13266, DOI:10.1002/ange.201906577
.
- Y. Hu, Y. Li and F.-J. Xu, Versatile Functionalization of Polysaccharides via Polymer Grafts: From Design to Biomedical Applications, Acc. Chem. Res., 2017, 50(2), 281–292, DOI:10.1021/acs.accounts.6b00477
.
- X. Meng and K. J. Edgar, “Click” reactions in polysaccharide modification, Prog. Polym. Sci., 2016, 53, 52–85, DOI:10.1016/j.progpolymsci.2015.07.006
.
- A. Kirschning, N. Dibbert and G. Dräger, Chemical Functionalization of Polysaccharides—Towards Biocompatible Hydrogels for Biomedical Applications, Chem. – Eur. J., 2018, 24(6), 1231–1240, DOI:10.1002/chem.201701906
.
- K. Kurita, Controlled functionalization of the polysaccharide chitin, Prog. Polym. Sci., 2001, 26(9), 1921–1971, DOI:10.1016/S0079-6700(01)00007-7
.
- J.-L. Do and T. Friščć, Mechanochemistry: a force of synthesis, ACS Cent. Sci., 2017, 3(1), 13–19, DOI:10.1021/acscentsci.6b00277
.
- T. Friščić, New opportunities for materials synthesis using mechanochemistry, J. Mater. Chem., 2010, 20(36), 7599–7605, 10.1039/C0JM00872A
.
- Mechano-chemical reaction. 5.0.0 ed., International Union of Pure and Applied Chemistry (IUPAC), 2025.
- G.-W. Wang, Mechanochemical organic synthesis, Chem. Soc. Rev., 2013, 42(18), 7668–7700, 10.1039/C3CS35526H
.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Ball milling in organic synthesis: solutions and challenges, Chem. Soc. Rev., 2011, 40(5), 2317–2329, 10.1039/C0CS00195C
.
- K. Tanaka and F. Toda, Solvent-Free Organic Synthesis, Chem. Rev., 2000, 100(3), 1025–1074, DOI:10.1021/cr940089p
.
- R. R. A. Bolt, J. A. Leitch, A. C. Jones, W. I. Nicholson and D. L. Browne, Continuous flow mechanochemistry: reactive extrusion as an enabling technology in organic synthesis, Chem. Soc. Rev., 2022, 51(11), 4243–4260, 10.1039/D1CS00657F
.
- F. Gomollón-Bel, Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable, Chem. Int., 2019, 41, 12–17 Search PubMed
.
- M. Mavlan, T. Chang, R. Feng, J. R. Wilkinson, R. J. Nicholas, N. B. Idahagbon, J. P. Youngblood and A. Wei, Mechanochemical esterification of cellulose nanofibers lyophilized from eutectic water–tert-butanol mixtures, Cellulose, 2023, 30(14), 8805–8817, DOI:10.1007/s10570-023-05435-x
.
- A. Pan, Y. Dai, H. Hou, W. Wang, X. Ding, H. Zhang, X. Li and H. Dong, Preparation of acetylated starch by rolling-assisted method and its influence mechanism, J. Food Meas. Charact., 2020, 14(2), 623–631, DOI:10.1007/s11694-019-00308-z
.
- W. Zhang, C. Li, M. Liang, Y. Geng and C. Lu, Preparation of carboxylate-functionalized cellulose via solvent-free mechanochemistry and its characterization as a biosorbent for removal of Pb2+ from aqueous solution, J. Hazard. Mater., 2010, 181(1), 468–473, DOI:10.1016/j.jhazmat.2010.05.036
.
- J. D. Thorpe, J. Marlyn, S. G. Koenig and M. J. Damha, Synthesis of short DNA and RNA fragments by resonant acoustic mixing (RAM), RSC Mechanochem., 2024, 1(3), 244–249, 10.1039/D4MR00009A
.
- D. Farajat, J.-L. Do, P. Forgione, T. Friščić, L. A. Cuccia and C.-J. Li, Shaking Up the Friedländer Reaction: Rapid, Scalable Mechanochemical Synthesis of Polyaryl-Substituted Quinolines, Adv. Synth. Catal., 2024, 366(24), 5135–5143, DOI:10.1002/adsc.202400862
.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. Harris, G. Hyett and W. Jones, Mechanochemistry: opportunities for new and cleaner synthesis, Chem. Soc. Rev., 2012, 41(1), 413–447 RSC
.
- K. J. Ardila-Fierro and J. G. Hernández, Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry, ChemSusChem, 2021, 14(10), 2145–2162, DOI:10.3390/life9020052
.
- F. Qi, R. S. Stein and T. Friščić, Mimicking mineral neogenesis for the clean synthesis of metal–organic materials from mineral feedstocks: coordination polymers, MOFs and metal oxide separation, Green Chem., 2014, 16(1), 121–132, 10.1039/C3GC41370E
.
- M. J. Cliffe, C. Mottillo, R. S. Stein, D.-K. Bučar and T. Friščić, Accelerated aging: a low energy, solvent-free alternative to solvothermal and mechanochemical synthesis of metal–organic materials, Chem. Sci., 2012, 3(8), 2495–2500, 10.1039/C2SC20344H
.
- P. Tang, C. Jia, Y. Jiang, W. Gong, X. Cao, J. Yang and W. Yuan, Reactivity Studies of Metal–Organic Frameworks under Vapor-Assisted Aging: Structural Interconversions and Transformations, Eur. J. Inorg. Chem., 2016, 36, 5617–5622, DOI:10.1002/ejic.201600907
.
- M. Đud, O. V. Magdysyuk, D. Margetić and V. Štrukil, Synthesis of monosubstituted thioureas by vapour digestion and mechanochemical amination of thiocarbamoyl benzotriazoles, Green Chem., 2016, 18(9), 2666–2674, 10.1039/c6gc00089d
.
- D. Braga, S. L. Giaffreda, F. Grepioni, M. R. Chierotti, R. Gobetto, G. Palladino and M. Polito, Solvent effect in a “solvent free” reaction, CrystEngComm, 2007, 9(10), 879–881, 10.1039/B711983F
.
- D. Cinčić, I. Brekalo and B. Kaitner, Effect of atmosphere on solid-state amine–aldehyde condensations: gas-phase catalysts for solid-state transformations, Chem. Commun., 2012, 48(95), 11683–11685, 10.1039/C2CC36357G
.
- T. Di Nardo, C. Hadad, A. Nguyen Van Nhien and A. Moores, Synthesis of high molecular weight chitosan from chitin by mechanochemistry and aging, Green Chem., 2019, 21(12), 3276–3285, 10.1039/C9GC00304E
.
- T. Jin, T. Liu, S. Jiang, D. Kurdyla, B. A. Klein, V. K. Michaelis, E. Lam, J. Li and A. Moores, Chitosan nanocrystals synthesis via aging and application towards alginate hydrogels for sustainable drug release, Green Chem., 2021, 23(17), 6527–6537, 10.1039/D1GC01611C
.
- G. Yang, E. Lam and A. Moores, Controlled Chitosan Molecular Weight Reduction by Mechanochemical and Aging-Based Phosphoric Acid Hydrolysis, ACS Sustainable Chem. Eng., 2023, 11(20), 7765–7774, DOI:10.1021/acssuschemeng.3c00367
.
- G. Yang, S. Régnier, N. Huin, T. Liu, E. Lam and A. Moores, Mechanochemical and aging-based reductive amination with chitosan and aldehydes affords high degree of substitution functional biopolymers, Green Chem., 2024, 26(9), 5386–5396, 10.1039/D4GC00127C
.
- T. Jin, T. Liu, F. Hajiali, M. Santos, Y. Liu, D. Kurdyla, S. Régnier, S. Hrapovic, E. Lam and A. Moores, High-Humidity Shaker Aging to Access Chitin and Cellulose Nanocrystals, Angew. Chem., Int. Ed., 2022, 61(42), e202207206, DOI:10.1002/anie.202207206
.
- F. Hammerer, L. Loots, J. L. Do, J. P. D. Therien, C. W. Nickels, T. Friščić and K. Auclair, Solvent–Free Enzyme Activity: Quick, High–Yielding Mechanoenzymatic Hydrolysis of Cellulose into Glucose, Angew. Chem., Int. Ed., 2018, 57(10), 2621–2624, DOI:10.1002/anie.201711643
.
- D. Langerreiter, N. L. Attallah, I. Schlapp-Hackl, M. A. Kostiainen and S. Kaabel, Mechanochemical modification of cellulose nanocrystals by tosylation and nucleophilic substitution, Green Chem., 2024, 26(18), 9823–9832, 10.1039/d4gc03378g
.
- X. Gao, L. Zhang, M. Cui, W. Qi, H. L. Lam, R. Huang and R. Su, Integrating solvent-free mechanochemistry and heat curing for the green production of highly charged and highly crystalline phosphorylated cellulose nanocrystals, Chem. Eng. J., 2025, 511, 162260, DOI:10.1016/j.cej.2025.162260
.
- K. Zhang, Y. Dai, H. Hou, X. Li, H. Dong, W. Wang and H. Zhang, Influences of grinding on structures and properties of mung bean starch and quality of acetylated starch, Food Chem., 2019, 294, 285–292, DOI:10.1016/j.foodchem.2019.05.055
.
- M. An, P. Yuan, Y. Chen, F. Yang, H. Sun, Y. Zheng, X. Lin, C. Liu, H. Lei and D. Hou, Combination of ball milling mechanochemistry with thermal treatment for sustainable preparation of starch laurates, Int. J. Biol. Macromol., 2025, 310, 143219, DOI:10.1016/j.ijbiomac.2025.143219
.
- J. P. D. Therien, F. Hammerer, T. Friščić and K. Auclair, Mechanoenzymatic Breakdown of Chitinous Material to N-Acetylglucosamine: The Benefits of a Solventless Environment, ChemSusChem, 2019, 12(15), 3481–3490, DOI:10.1002/cssc.201901310
.
- S. Kuga and M. Wu, Mechanochemistry of cellulose, Cellulose, 2019, 26(1), 215–225, DOI:10.1007/s10570-018-2197-1
.
- L. Jicsinszky, F. Bucciol, S. Chaji and G. Cravotto, Mechanochemical Degradation of Biopolymers, Molecules, 2023, 28(24), 8031 CrossRef CAS PubMed
.
- T. A. Akopova, T. N. Popyrina and T. S. Demina, Mechanochemical Transformations of Polysaccharides: A Systematic Review, Int. J. Mol. Sci., 2022, 23(18), 10458 CrossRef CAS PubMed
.
-
G. Margoutidis and F. M. Kerton, Biomass Processing via Mechanochemical Means, in Biomass Valorization, 2021, pp. 343–365 Search PubMed
.
- H. Kobayashi and A. Fukuoka, Mechanochemical Hydrolysis of Polysaccharide Biomass: Scope and Mechanistic Insights, ChemPlusChem, 2024, e202300554, DOI:10.1002/cplu.202300554
.
- F. Hajiali, T. Jin, G. Yang, M. Santos, E. Lam and A. Moores, Mechanochemical Transformations of Biomass into Functional Materials, ChemSusChem, 2022, 15(7), e202102535, DOI:10.1002/cssc.202102535
.
- A. Perona, P. Hoyos, Á. Farrán and M. J. Hernáiz, Current challenges and future perspectives in sustainable mechanochemical transformations of carbohydrates, Green Chem., 2020, 22(17), 5559–5583, 10.1039/D0GC00901F
.
- C. C. Piras, S. Fernández-Prieto and W. M. De Borggraeve, Ball, milling: a green technology for the preparation and functionalisation of nanocellulose derivatives, Nanoscale Adv., 2019, 1(3), 937–947, 10.1039/c8na00238j
.
- C. F. Burmeister and A. Kwade, Process engineering with planetary ball mills, Chem. Soc. Rev., 2013, 42(18), 7660–7667, 10.1039/C3CS35455E
.
- L. Takacs, The historical development of mechanochemistry, Chem. Soc. Rev., 2013, 42(18), 7649–7659, 10.1039/C2CS35442J
.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Hallmarks of mechanochemistry: from nanoparticles to technology, Chem. Soc. Rev., 2013, 42(18), 7571, 10.1039/c3cs35468g
.
- T. Friščić, C. Mottillo and H. M. Titi, Mechanochemistry for Synthesis, Angew. Chem., Int. Ed., 2020, 59(3), 1018–1029, DOI:10.1002/anie.201906755
.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Advancing mechanochemical synthesis by combining milling with different energy sources, Nat. Rev. Chem., 2022, 7(1), 51–65, DOI:10.1038/s41570-022-00442-1
.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome, CrystEngComm, 2009, 11(3), 418–426, 10.1039/B815174A
.
-
L. Yang, Nanotechnology-enhanced orthopedic materials: fabrications, applications and future trends, Woodhead Publishing, Cambridge, U.K., 1st edn, 2015 Search PubMed
.
- X. Liu, H. Wen, B. Guo, C. Lv, W. Shi, W. Kang, J. Zhang, R. Yuan and C. Zhang, Pan-Milling: Instituting an All-Solid-State Technique for Mechanical Metastable Oxides as High-Performance Lithium-Ion Battery Anodes, Adv. Energy Mater., 2021, 11(14), 2100310, DOI:10.1002/aenm.202100310
.
- H. P. S. Abdul Khalil, Y. Davoudpour, M. N. Islam, A. Mustapha, K. Sudesh, R. Dungani and M. Jawaid, Production and modification of nanofibrillated cellulose using various mechanical processes: A review, Carbohydr. Polym., 2014, 99, 649–665, DOI:10.1016/j.carbpol.2013.08.069
.
- A. C. Corrêa, E. de Morais Teixeira, L. A. Pessan and L. H. C. Mattoso, Cellulose nanofibers from curaua fibers, Cellulose, 2010, 17(6), 1183–1192, DOI:10.1007/s10570-010-9453-3
.
-
T. Heinze, Cellulose: Structure and Properties, in Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials, ed. O. J. Rojas, Springer International Publishing, Cham, 2016, pp. 1–52 Search PubMed
.
- H. Shaghaleh, X. Xu and S. Wang, Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives, RSC Adv., 2018, 8(2), 825–842, 10.1039/C7RA11157F
.
- F. Güleç, A. Parthiban, G. C. Umenweke, U. Musa, O. Williams, Y. Mortezaei, H. Suk-Oh, E. Lester, C. C. Ogbaga, B. Gunes and J. A. Okolie, Progress in lignocellulosic biomass valorization for biofuels and value-added chemical production in the EU: A focus on thermochemical conversion processes, Biofuels, Bioprod. Biorefin., 2024, 18(3), 755–781, DOI:10.1002/bbb.2544
.
-
L.-t. Fan, M. M. Gharpuray and Y.-H. Lee, Cellulose hydrolysis, Springer Science & Business Media, 2012, vol. 3 Search PubMed
.
- P. L. Dhepe and A. Fukuoka, Cellulose Conversion under Heterogeneous Catalysis, ChemSusChem, 2008, 1(12), 969–975, DOI:10.1002/cssc.200800129
.
- A. Zebda, S. Cosnier, J. P. Alcaraz, M. Holzinger, A. Le Goff, C. Gondran, F. Boucher, F. Giroud, K. Gorgy, H. Lamraoui and P. Cinquin, Single Glucose Biofuel Cells Implanted in Rats Power Electronic Devices, Sci. Rep., 2013, 3(1), 1516, DOI:10.1038/srep01516
.
- H.-S. Lee, M. B. Salerno and B. E. Rittmann, Thermodynamic Evaluation on H2 Production in Glucose Fermentation, Environ. Sci. Technol., 2008, 42(7), 2401–2407, DOI:10.1021/es702610v
.
- S. M. Hick, C. Griebel, D. T. Restrepo, J. H. Truitt, E. J. Buker, C. Bylda and R. G. Blair, Mechanocatalysis for biomass-derived chemicals and fuels, Green Chem., 2010, 12(3), 468–474, 10.1039/B923079C
.
- S. Furusato, A. Takagaki, S. Hayashi, A. Miyazato, R. Kikuchi and S. T. Oyama, Mechanochemical Decomposition of Crystalline Cellulose in the Presence of Protonated Layered Niobium Molybdate Solid Acid Catalyst, ChemSusChem, 2018, 11(5), 888–896, DOI:10.1002/cssc.201702305
.
- N. Meine, R. Rinaldi and F. Schüth, Solvent-Free Catalytic Depolymerization of Cellulose to Water-Soluble Oligosaccharides, ChemSusChem, 2012, 5(8), 1449–1454, DOI:10.1002/cssc.201100770
.
- M. D. Kaufman Rechulski, M. Käldström, U. Richter, F. Schüth and R. Rinaldi, Mechanocatalytic Depolymerization of Lignocellulose Performed on Hectogram and Kilogram Scales, Ind. Eng. Chem. Res., 2015, 54(16), 4581–4592, DOI:10.1021/acs.iecr.5b00224
.
- Y. Yu, Y. Long and H. Wu, Near-Complete Recovery of Sugar Monomers from Cellulose and Lignocellulosic Biomass via a Two-Step Process Combining Mechanochemical Hydrolysis and Dilute Acid Hydrolysis, Energy Fuels, 2016, 30(3), 1571–1578, DOI:10.1021/acs.energyfuels.5b02196
.
- J. Mi, S. Yu, C. Fang, S. Shen, K. H. Ng and N. Yan, Mechanochemical Top-Down Production of Oligosaccharide-Based Surfactants from Cellulosic Biomass, ACS Sustainable Chem. Eng., 2025, 13(12), 4790–4799, DOI:10.1021/acssuschemeng.4c10358
.
- J. Mi, K. L. Chan, K. H. Ng, Q. Wu, C. Fang, Y. Lim and N. Yan, Mechanochemical Upcycling of Biomass into Antimicrobial Oligomeric Glycosylamine Surfactants, ACS Sustainable Chem. Eng., 2025, 13(29), 11569–11578, DOI:10.1021/acssuschemeng.5c04262
.
- H. Kobayashi, M. Yabushita, T. Komanoya, K. Hara, I. Fujita and A. Fukuoka, High-yielding one-pot synthesis of glucose from cellulose using simple activated carbons and trace hydrochloric acid, ACS Catal., 2013, 3(4), 581–587, DOI:10.1021/cs300845f
.
- M. Qiu, C. Bai, L. Yan, F. Shen and X. Qi, Efficient Mechanochemical-Assisted Production of Glucose from Cellulose in Aqueous Solutions by Carbonaceous Solid Acid Catalysts, ACS Sustainable Chem. Eng., 2018, 6(11), 13826–13833, DOI:10.1021/acssuschemeng.8b01910
.
- D. Scholz, J. Xie, O. Kröcher and F. Vogel, Mechanochemistry-assisted hydrolysis of softwood over stable sulfonated carbon catalysts in a semi-batch process, RSC Adv., 2019, 9(57), 33525–33538, 10.1039/C9RA07668A
.
- F. Shen, S. Sun, X. Zhang, J. Yang, M. Qiu and X. Qi, Mechanochemical-assisted production of 5-hydroxymethylfurfural from high concentration of cellulose, Cellulose, 2020, 27(6), 3013–3023, DOI:10.1007/s10570-020-03008-w
.
- J. Wang, X. Liu, T. Jin, H. He and L. Liu, Preparation of nanocellulose and its potential in reinforced composites: A review, J. Biomater. Sci., Polym. Ed., 2019, 30(11), 919–946, DOI:10.1080/09205063.2019.1612726
.
- J. Ramasamy and M. Amanullah, Nanocellulose for oil and gas field drilling and cementing applications, J. Pet. Sci. Eng., 2020, 184, 106292, DOI:10.1016/j.petrol.2019.106292
.
- A. Meftahi, P. Samyn, S. A. Geravand, R. Khajavi, S. Alibkhshi, M. Bechelany and A. Barhoum, Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications, Carbohydr. Polym., 2022, 278, 118956, DOI:10.1016/j.carbpol.2021.118956
.
- S. Doobary, V. Apostolopoulou-Kalkavoura, A. P. Mathew and B. Olofsson, Nanocellulose: New horizons in organic chemistry and beyond, Chem, 2024, 10(11), 3279–3293, DOI:10.1016/j.chempr.2024.09.007
.
- Q. Lu, W. Lin, L. Tang, S. Wang, X. Chen and B. Huang, A mechanochemical approach to manufacturing bamboo cellulose nanocrystals, J. Mater. Sci., 2015, 50(2), 611–619, DOI:10.1007/s10853-014-8620-6
.
- Q. Lu, Z. Cai, F. Lin, L. Tang, S. Wang and B. Huang, Extraction of Cellulose Nanocrystals with a High Yield of 88% by Simultaneous Mechanochemical Activation and Phosphotungstic Acid Hydrolysis, ACS Sustainable Chem. Eng., 2016, 4(4), 2165–2172, DOI:10.1021/acssuschemeng.5b01620
.
- J. E. Sealey, G. Samaranayake, J. G. Todd and W. G. Glasser, Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose, J. Polym. Sci., Part B: Polym. Phys., 1996, 34(9), 1613–1620, DOI:10.1002/(SICI)1099-0488(19960715)34:9<1613::AID-POLB10>3.0.CO;2-A
.
- L. Benvenutti, A. A. F. Zielinski and S. R. S. Ferreira, Which is the best food emerging solvent: IL, DES or NADES?, Trends Food Sci. Technol., 2019, 90, 133–146, DOI:10.1016/j.tifs.2019.06.003
.
- L. Douard, M. N. Belgacem and J. Bras, Extraction of Carboxylated Nanocellulose by Combining Mechanochemistry and NADES, ACS Sustainable Chem. Eng., 2022, 10(39), 13017–13025, DOI:10.1021/acssuschemeng.2c02783
.
- P. Spiliopoulos, S. Spirk, T. Pääkkönen, M. Viljanen, K. Svedström, L. Pitkänen, M. Awais and E. Kontturi, Visualizing Degradation of Cellulose Nanofibers by Acid Hydrolysis, Biomacromolecules, 2021, 22(4), 1399–1405, DOI:10.1021/acs.biomac.0c01625
.
- T. Saito, S. Kimura, Y. Nishiyama and A. Isogai, Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose, Biomacromolecules, 2007, 8(8), 2485–2491, DOI:10.1021/bm0703970
.
- A. C. W. Leung, S. Hrapovic, E. Lam, Y. Liu, K. B. Male, K. A. Mahmoud and J. H. T. Luong, Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure, Small, 2011, 7(3), 302–305, DOI:10.1002/smll.201001715
.
- G. Yang, Y. Tomita, A. J. Richard, S. Fujisawa, E. Lam, T. Saito and A. Moores, Low-energy synthesis of individualized pH-responsive cationic cellulose nanofibers and chitin nanocrystals by mechanochemistry and aging, Nanoscale Horiz., 2025, 11(1), 170–184, 10.1039/d5nh00597c
.
- J. Arciszewski and K. Auclair, Mechanoenzymatic Reactions Involving Polymeric Substrates or Products, ChemSusChem, 2022, 15(7), e202102084, DOI:10.1002/cssc.202102084
.
- Q. Zhang, Y. Chen and W. Su, Highly efficient preparation of cellulose nanocrystals by mechano-enzymatic hydrolysis: a mechanism study, Catal. Sci. Technol., 2023, 13(3), 618–623, 10.1039/D2CY01904C
.
- Q. Zhang, Z. Lu, C. Su, Z. Feng, H. Wang, J. Yu and W. Su, High yielding, one-step mechano-enzymatic hydrolysis of cellulose to cellulose nanocrystals without bulk solvent, Bioresour. Technol., 2021, 331, 125015, DOI:10.1016/j.biortech.2021.125015
.
- T. Di Nardo and A. Moores, Mechanochemical amorphization of chitin: impact of apparatus material on performance and contamination, Beilstein J. Org. Chem., 2019, 15, 1217–1225, DOI:10.3762/bjoc.15.119
.
- R. Avolio, I. Bonadies, D. Capitani, M. E. Errico, G. Gentile and M. Avella, A multitechnique approach to assess the effect of ball milling on cellulose, Carbohydr. Polym., 2012, 87(1), 265–273, DOI:10.1016/j.carbpol.2011.07.047
.
- J. Lease, T. Kawano and Y. Andou, Esterification of Cellulose with Long Fatty Acid Chain through Mechanochemical Method, Polymers, 2021, 13(24), 4397 CrossRef CAS PubMed
.
- D.-F. Hou, M.-L. Li, C. Yan, L. Zhou, Z.-Y. Liu, W. Yang and M.-B. Yang, Mechanochemical preparation of thermoplastic cellulose oleate by ball milling, Green Chem., 2021, 23(5), 2069–2078, 10.1039/d0gc03853a
.
- J. A. Ávila Ramírez, E. Fortunati, J. M. Kenny, L. Torre and M. L. Foresti, Simple citric acid-catalyzed surface esterification of cellulose nanocrystals, Carbohydr. Polym., 2017, 157, 1358–1364, DOI:10.1016/j.carbpol.2016.11.008
.
- D.-F. Hou, M.-L. Li, P.-Y. Li, L. Zhou, K. Zhang, Z.-Y. Liu, W. Yang and M.-B. Yang, Efficient Conversion of Cellulose to Thermoplastics by Mechanochemical Esterification, ACS Sustainable Chem. Eng., 2023, 11(20), 7655–7663, DOI:10.1021/acssuschemeng.2c07364
.
- C. J. Maim, J. W. Mench, D. L. Kendall and G. D. Hiatt, Aliphatic Acid Esters of Cellulose. Preparation by Acid-Chloride-Pyridine Procedure, Ind. Eng. Chem., 1951, 43(3), 684–688, DOI:10.1021/ie50495a033
.
- T. Nakano, Mechanism of Thermoplasticity for Chemically-Modified Wood, Holzforschung, 1994, 48(4), 318–324, DOI:10.1515/hfsg.1994.48.4.318
.
- M.-L. Li, D.-F. Hou, P.-Y. Li, Z.-W. Feng, Y.-H. Huang, F. Wang, Y.-M. Zhai, X.-R. Sun, K. Zhang, B. Yin, W. Yang and M.-B. Yang, One-Step Solvent-Free Strategy to Efficiently Synthesize High-Substitution Cellulose Esters, ACS Sustainable Chem. Eng., 2024, 12(26), 9669–9681, DOI:10.1021/acssuschemeng.4c00953
.
- W. Zhang, X. Zhang, M. Liang and C. Lu, Mechanochemical preparation of surface-acetylated cellulose powder to enhance mechanical properties of cellulose-filler-reinforced NR vulcanizates, Compos. Sci. Technol., 2008, 68(12), 2479–2484, DOI:10.1016/j.compscitech.2008.05.005
.
- A. Biswas, R. L. Shogren and J. L. Willett, Solvent-Free Process to Esterify Polysaccharides, Biomacromolecules, 2005, 6(4), 1843–1845, DOI:10.1021/bm0501757
.
- W. Qiu, F. Zhang, T. Endo and T. Hirotsu, Milling-induced esterification between cellulose and maleated polypropylene, J. Appl. Polym. Sci., 2004, 91(3), 1703–1709, DOI:10.1002/app.13368
.
- F. Zhang, W. Qiu, L. Yang, T. Endo and T. Hirotsu, Mechanochemical preparation and properties of a cellulose–polyethylene composite, J. Mater. Chem., 2002, 12(1), 24–26, 10.1039/b108255h
.
- W. Qiu, T. Endo and T. Hirotsu, Interfacial interactions of a novel mechanochemical composite of cellulose with maleated polypropylene, J. Appl. Polym. Sci., 2004, 94(3), 1326–1335, DOI:10.1002/app.21123
.
- Y. Niu, X. Zhang, X. He, J. Zhao, W. Zhang and C. Lu, Effective dispersion and crosslinking in PVA/cellulose fiber biocomposites via solid-state mechanochemistry, Int. J. Biol. Macromol., 2015, 72, 855–861, DOI:10.1016/j.ijbiomac.2014.09.042
.
- D. T. Quillin, D. F. Caulfield and J. A. Koutsky, Crystallinity in the polypropylene/cellulose system. I. Nucleation and crystalline morphology, J. Appl. Polym. Sci., 1993, 50(7), 1187–1194, DOI:10.1002/app.1993.070500709
.
- A. S. Nielsen and R. Pyrz, A Raman study into the effect of transcrystallisation
on thermal stresses in embedded single fibres, J. Mater. Sci., 2003, 38(3), 597–601, DOI:10.1023/A:1021866429394
.
- T. Foresta, S. Piccarolo and G. Goldbeck-Wood, Competition between α and γ phases in isotactic polypropylene: effects of ethylene content and nucleating agents at different cooling rates, Polymer, 2001, 42(3), 1167–1176, DOI:10.1016/S0032-3861(00)00404-3
.
- S. C. Tjong, J. S. Shen and R. K. Y. Li, Impact fracture toughness of β-form polypropylene, Scr. Metall. Mater., 1995, 33(3), 503–508, DOI:10.1016/0956-716X(95)00225-K
.
- S. C. Tjong, R. K. Y. Li and T. Cheung, Mechanical behavior of CaCO3 particulate-filled β-crystalline phase polypropylene composites, Polym. Eng. Sci., 1997, 37(1), 166–172, DOI:10.1002/pen.11657
.
- L. Huang, Q. Wu, Q. Wang and M. Wolcott, Interfacial crystals morphology modification in cellulose fiber/polypropylene composite by mechanochemical method, Composites, Part A, 2020, 130, 105765, DOI:10.1016/j.compositesa.2020.105765
.
- M. S. Rahman, M. S. Hasan, A. S. Nitai, S. Nam, A. K. Karmakar, M. S. Ahsan, M. J. A. Shiddiky and M. B. Ahmed, Recent Developments of Carboxymethyl Cellulose, Polymers, 2021, 13(8), 1345, DOI:10.3390/polym13081345
.
- V. Marchetti, A. Clément, P. Gérardin and B. Loubinoux, Synthesis and use of esterified sawdusts bearing carboxyl group for removal of cadmium(II) from water, Wood Sci. Technol., 2000, 34(2), 167–173, DOI:10.1007/s002260000040
.
- A.-A. M. A. Nada and M. L. Hassan, Ion exchange properties of carboxylated bagasse, J. Appl. Polym. Sci., 2006, 102(2), 1399–1404, DOI:10.1002/app.24255
.
- M. A. Momodu and C. Anyakora, Heavy Metal Contamination of Ground Water: The Surulere Case Study, Res. J. Environ. Earth Sci., 2010, 2, 39–43 CAS
.
- L. A. Malik, A. Bashir, A. Qureashi and A. H. Pandith, Detection and removal of heavy metal ions: a review, Environ. Chem. Lett., 2019, 17(4), 1495–1521, DOI:10.1007/s10311-019-00891-z
.
- L. Zaharani, M. K. Vequizo, Z. R. Amiri, M. R. Johan, W. H. A. Majid and N. G. Khaligh, Development of a new sustainable greener strategy for cellulose functionalization: A mechanochemical catalyst-free and solvent-free process in ambient conditions, Int. J. Biol. Macromol., 2025, 304, 140907, DOI:10.1016/j.ijbiomac.2025.140907
.
- G. Siqueira, J. Bras and A. Dufresne, New Process of Chemical Grafting of Cellulose Nanoparticles with a Long Chain Isocyanate, Langmuir, 2010, 26(1), 402–411, DOI:10.1021/la9028595
.
- K. Joseph, S. Thomas and C. Pavithran, Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites, Polymer, 1996, 37(23), 5139–5149, DOI:10.1016/0032-3861(96)00144-9
.
- L. Ladouce, E. Fleury, C. Gousse, R. Cantiani, H. Chanzy and G. Excoffier, Cellulose microfibrils with modified surface, preparation method and use thereof. US 6703497 B1, Mar. 9, 2004, 2004.
- P. Stenstad, M. Andresen, B. S. Tanem and P. Stenius, Chemical surface modifications of microfibrillated cellulose, Cellulose, 2008, 15(1), 35–45, DOI:10.1007/s10570-007-9143-y
.
- G. Siqueira, J. Bras and A. Dufresne, Cellulose Whiskers versus Microfibrils: Influence of the Nature of the Nanoparticle and its Surface Functionalization on the Thermal and Mechanical Properties of Nanocomposites, Biomacromolecules, 2009, 10(2), 425–432, DOI:10.1021/bm801193d
.
- J. Pescheux-Sergienko, C. Sillard, M. N. Belgacem and J. Bras, Simultaneous Comminution and Hydrophobization of Cellulose Fibers by Mechanochemistry, ACS Sustainable Chem. Eng., 2024, 12(45), 16540–16552, DOI:10.1021/acssuschemeng.4c04209
.
- X. Chen, J. Liu, D. Gao, M. Wu and Y. Huang, Preparation of ultrafine hydrophobic lignin-contained cellulose nanofiber via mechanochemical method and its films, Ind. Crops Prod., 2024, 222, 120064, DOI:10.1016/j.indcrop.2024.120064
.
- S. Ahola, M. Österberg and J. Laine, Cellulose nanofibrils—adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive, Cellulose, 2008, 15(2), 303–314, DOI:10.1007/s10570-007-9167-3
.
- D. Li, C.-S. Lee, Y. Zhang, R. Das, F. Akter, A. K. Venkatesan and B. S. Hsiao, Efficient removal of short-chain and long-chain PFAS by cationic nanocellulose, J. Mater. Chem. A, 2023, 11(18), 9868–9883, 10.1039/d3ta01851b
.
- Z. Lu, X. An, H. Zhang, L. Liu, H. Dai, H. Cao, B. Lu and H. Liu, Cationic cellulose nano-fibers (CCNF) as versatile flocculants of wood pulp for high wet web performance, Carbohydr. Polym., 2020, 229, 115434, DOI:10.1016/j.carbpol.2019.115434
.
- Y. Li, L. Zhu, N. Grishkewich, K. C. Tam, J. Yuan, Z. Mao and X. Sui, CO2-Responsive Cellulose Nanofibers Aerogels for Switchable Oil–Water Separation, ACS Appl. Mater. Interfaces, 2019, 11(9), 9367–9373, DOI:10.1021/acsami.8b22159
.
- P. Eskandari, H. Roghani-Mamaqani, M. Salami-Kalajahi and Z. Abousalman-Rezvani, Modification of cellulose nanocrystal with dual temperature- and CO2-responsive block copolymers for ion adsorption applications, J. Mol. Liq., 2020, 310, 113234, DOI:10.1016/j.molliq.2020.113234
.
- J. Glasing, P. G. Jessop, P. Champagne and M. F. Cunningham, Graft-modified cellulose nanocrystals as CO2-switchable Pickering emulsifiers, Polym. Chem., 2018, 9(28), 3864–3872, 10.1039/C8PY00417J
.
- J. Glasing, J. Bouchard, P. G. Jessop, P. Champagne and M. F. Cunningham, Grafting well-defined CO2-responsive polymers to cellulose nanocrystals via nitroxide-mediated polymerisation: effect of graft density and molecular weight on dispersion behaviour, Polym. Chem., 2017, 8(38), 6000–6012, 10.1039/C7PY01258F
.
- Z. Xiao, Q. Zhao, Q. Li, Y. Wang, H. Zheng, H. Wang, J. Huang and J. Mao, Mechanochemistry-Assisted Fabrication of (Carboxymethyl)cellulose Mediated by Minute Surface-Confined Water, ACS Sustainable Chem. Eng., 2024, 12(41), 14999–15011, DOI:10.1021/acssuschemeng.4c03456
.
- I. A. Udoetok, R. M. Dimmick, L. D. Wilson and J. V. Headley, Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution, Carbohydr. Polym., 2016, 136, 329–340, DOI:10.1016/j.carbpol.2015.09.032
.
- A. Pei, N. Butchosa, L. A. Berglund and Q. Zhou, Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes, Soft Matter, 2013, 9(6), 2047, 10.1039/c2sm27344f
.
- T. Nikonovich, Y. Yu, M. Korkiakoski, C. Yang, I. Seitz, D. Langerreiter, M. A. Kostiainen, E. Anaya-Plaza and S. Kaabel, Solid-State Synthesis of Cationic Cellulose Fibers from Low-Processed Cotton for Efficient Virus Capture, ACS Sustainable Chem. Eng., 2025, 13(42), 18223–18231, DOI:10.1021/acssuschemeng.5c07884
.
- J. A. Sirviö, Cationization of lignocellulosic fibers with betaine in deep eutectic solvent: Facile route to charge stabilized cellulose and wood nanofibers, Carbohydr. Polym., 2018, 198, 34–40, DOI:10.1016/j.carbpol.2018.06.051
.
- V. Kokol, M. Božič, R. Vogrinčič and A. P. Mathew, Characterisation and properties of homo- and heterogenously phosphorylated nanocellulose, Carbohydr. Polym., 2015, 125, 301–313, DOI:10.1016/j.carbpol.2015.02.056
.
- B. G. Fiss, L. Hatherly, R. S. Stein, T. Friščić and A. Moores, Mechanochemical Phosphorylation of Polymers and Synthesis of Flame-Retardant Cellulose Nanocrystals, ACS Sustainable Chem. Eng., 2019, 7(8), 7951–7959, DOI:10.1021/acssuschemeng.9b00764
.
- M. Aaddouz, F. Laoutid, J. Mariage, J. Lazko, B. Yada, E. M. Mejdoubi, A. Toncheva and P. Dubois, Facile Preparation Route of Cellulose-Based Flame Retardant by Ball-Milling Mechanochemistry, Molecules, 2024, 29(24), 6065, DOI:10.3390/molecules29246065
.
- X. Gao, L. Zhang, M. Cui, R. Huang, W. Qi and R. Su, Pre-phosphorylation for facile production of phosphorylated cellulose nanocrystals with high charge content: an optimised design and life cycle assessment, Green Chem., 2023, 25(13), 5041–5050, 10.1039/D3GC00478C
.
- P. Mischnick, M. Lange, M. Gohdes, A. Stein and K. Petzold, Trialkylsilyl derivatives of cyclomaltoheptaose, cellulose, and amylose: rearrangement during methylation analysis, Carbohydr. Res., 1995, 277(1), 179–187, DOI:10.1016/0008-6215(95)00202-5
.
- M. Abdelmouleh, S. Boufi, M. N. Belgacem, A. P. Duarte, A. Ben Salah and A. Gandini, Modification of cellulosic fibres with functionalised silanes: development of surface properties, Int. J. Adhes. Adhes., 2004, 24(1), 43–54, DOI:10.1016/S0143-7496(03)00099-X
.
- R. M. Neves, H. L. Ornaghi, A. J. Zattera and S. C. Amico, The influence of silane surface modification on microcrystalline cellulose characteristics, Carbohydr. Polym., 2020, 230, 115595, DOI:10.1016/j.carbpol.2019.115595
.
- Y. Ding, K. Tu, I. Burgert and T. Keplinger, Janus wood membranes for autonomous water transport and fog collection, J. Mater. Chem. A, 2020, 8(42), 22001–22008, 10.1039/d0ta07544b
.
- M. Jing, L. Zhang, Z. Fan, X. Liu, Y. Wang, C. Liu and C. Shen, Markedly improved hydrophobicity of cellulose film via a simple one-step aminosilane-assisted ball milling, Carbohydr. Polym., 2022, 275, 118701, DOI:10.1016/j.carbpol.2021.118701
.
- K. Lee, Y. L. Sim, H. Jeong, A. Kim, Y. Lee, S. E. Shim and Y. Qian, Mechanochemically functionalized and fibrillated microcrystalline cellulose as a filler in silicone foam: An integrated experimental and simulation investigation, Carbohydr. Polym., 2024, 327, 121660, DOI:10.1016/j.carbpol.2023.121660
.
- W. Zhang, X. Yang, C. Li, M. Liang, C. Lu and Y. Deng, Mechanochemical activation of cellulose and its thermoplastic polyvinyl alcohol ecocomposites with enhanced physicochemical properties, Carbohydr. Polym., 2011, 83(1), 257–263, DOI:10.1016/j.carbpol.2010.07.062
.
- D. N.-S. Hon, Formation and behavior of mechanoradicals in pulp cellulose, J. Appl. Polym. Sci., 1979, 23(5), 1487–1499, DOI:10.1002/app.1979.070230519
.
- M. Sakaguchi and J. Sohma, ESR evidence for main-chain scission produced by mechanical fracture of polymers at low temperature, J. Polym. Sci., Polym. Phys. Ed., 1975, 13(6), 1233–1245, DOI:10.1002/pol.1975.180130614
.
- I. Solala, U. Henniges, K. F. Pirker, T. Rosenau, A. Potthast and T. Vuorinen, Mechanochemical reactions of cellulose and styrene, Cellulose, 2015, 22(5), 3217–3224, DOI:10.1007/s10570-015-0724-x
.
- M. Sakaguchi, T. Ohura, T. Iwata and Y. Enomoto-Rogers, Nano cellulose particles covered with block copolymer of cellulose and methyl methacrylate produced by solid mechano chemical polymerization, Polym. Degrad. Stab., 2012, 97(3), 257–263, DOI:10.1016/j.polymdegradstab.2011.12.022
.
- T. Ohura, Y. Tsutaki and M. Sakaguchi, Novel Synthesis of Cellulose-Based Diblock Copolymer of Poly(hydroxyethyl methacrylate) by Mechanochemical Reaction, Sci. World J., 2014, 2014(1), 127506, DOI:10.1155/2014/127506
.
- M. Sakaguchi, M. Makino, T. Ohura and T. Iwata, The correlation between the ionic degree of covalent bond comprising polymer main chain and the ionic yield due to mechanical fracture, Polymer, 2014, 55(8), 1917–1919, DOI:10.1016/j.polymer.2014.02.056
.
- M. Sakaguchi, M. Makino, T. Ohura and T. Iwata, Mechanoanions Produced by Mechanical Fracture of Bacterial Cellulose: Ionic Nature of Glycosidic Linkage and Electrostatic Charging, J. Phys. Chem. A, 2012, 116(40), 9872–9877, DOI:10.1021/jp306261k
.
- T. Motokawa, M. Makino, Y. Enomoto-Rogers, T. Kawaguchi, T. Ohura, T. Iwata and M. Sakaguchi, Novel method of the surface modification of the microcrystalline cellulose powder with poly(isobutyl vinyl ether) using mechanochemical polymerization, Adv. Powder Technol., 2015, 26(5), 1383–1390, DOI:10.1016/j.apt.2015.07.012
.
- L. Copeland, J. Blazek, H. Salman and M. C. Tang, Form and functionality of starch, Food Hydrocolloids, 2009, 23(6), 1527–1534, DOI:10.1016/j.foodhyd.2008.09.016
.
-
A.-C. Eliasson, Starch in food: Structure, function and applications, CRC press, 2004 Search PubMed
.
- J. L. d. Maia, J. S. Cardoso, D. J. d. S. Mastrantonio, C. K. Bierhals, J. B. Moreira, J. A. V. Costa and M. G. d. Morais, Microalgae starch: A promising raw material for the bioethanol production, Int. J. Biol. Macromol., 2020, 165, 2739–2749, DOI:10.1016/j.ijbiomac.2020.10.159
.
- P. Adewale, M. S. Yancheshmeh and E. Lam, Starch modification for non-food, industrial applications: Market intelligence and critical review, Carbohydr. Polym., 2022, 291, 119590, DOI:10.1016/j.carbpol.2022.119590
.
- S. K. Bardhan, S. Gupta, M. E. Gorman and M. A. Haider, Biorenewable chemicals: Feedstocks, technologies and the conflict with food production, Renewable Sustainable Energy Rev., 2015, 51, 506–520, DOI:10.1016/j.rser.2015.06.013
.
- Y. Zhang, T. Gan, H. Hu, Z. Huang, A. Huang, Y. Zhu, Z. Feng and M. Yang, A Green Technology for the Preparation of High Fatty Acid Starch Esters: Solid-Phase Synthesis of Starch Laurate Assisted by Mechanical Activation with Stirring Ball Mill as Reactor, Ind. Eng. Chem. Res., 2014, 53(6), 2114–2120, DOI:10.1021/ie403186h
.
- H. Hu, W. Liu, J. Shi, Z. Huang, Y. Zhang, A. Huang, M. Yang, X. Qin and F. Shen, Structure and functional properties of octenyl succinic anhydride modified starch prepared by a non–conventional technology, Starch/Staerke, 2016, 68(1–2), 151–159, DOI:10.1002/star.201500195
.
- M. Chen, T. Yin, Y. Chen, S. Xiong and S. Zhao, Preparation and characterization of octenyl succinic anhydride modified waxy rice starch by dry media milling, Starch/Staerke, 2014, 66(11–12), 985–991, DOI:10.1002/star.201400015
.
- N. Li, M. Niu, B. Zhang, S. Zhao, S. Xiong and F. Xie, Effects of concurrent ball milling and octenyl succinylation on structure and physicochemical properties of starch, Carbohydr. Polym., 2017, 155, 109–116, DOI:10.1016/j.carbpol.2016.08.063
.
- A. Vanmarcke, L. Leroy, G. Stoclet, L. Duchatel-Crépy, J.-M. Lefebvre, N. Joly and V. Gaucher, Influence of fatty chain length and starch composition on structure and properties of fully substituted fatty acid starch esters, Carbohydr. Polym., 2017, 164, 249–257, DOI:10.1016/j.carbpol.2017.02.013
.
- A. David, G. Stoclet, N. Joly, C. Ribeiro, N. Descamps, D. Lourdin and V. Gaucher, Structure and Mechanical Behavior of Fully Substituted Acid Starch Esters, Macromol. Chem. Phys., 2023, 224(19), 2300177, DOI:10.1002/macp.202300177
.
- V. T. Troncoso, O. Hernandez-Hernandez, M. V. Alvarez, A. G. Ponce, J. R. Mendieta and T. J. Gutiérrez, Organocatalytically salicylated starch-based food packaging obtained via reactive extrusion/thermo-molding, Int. J. Biol. Macromol., 2025, 320, 146116, DOI:10.1016/j.ijbiomac.2025.146116
.
- S. F. Li, J. M. V. Mujyambere and M. Liu, Synthesis of Carboxymethyl Starch with High Degree of Substitution by a Modified Dry Process, Adv. Mater. Res., 2011, 233–235, 306–310, DOI:10.4028/www.scientific.net/AMR.233-235.306
.
- Y. Bi, M. Liu, L. Wu and D. Cui, Synthesis of carboxymethyl potato starch and comparison of optimal reaction conditions from different sources, Polym. Adv. Technol., 2008, 19(9), 1185–1192, DOI:10.1002/pat.1102
.
- O. S. Lawal, M. D. Lechner, B. Hartmann and W.-M. Kulicke, Carboxymethyl Cocoyam Starch: Synthesis, Characterisation and Influence of Reaction Parameters, Starch/Staerke, 2007, 59(5), 224–233, DOI:10.1002/star.200600594
.
- A. A. Ragheb, H. S. El-Sayiad and A. Hebeish, Preparation and Characterization of Carboxymethyl Starch (CMS) Products and Their Utilization in Textile Printing, Starch/Staerke, 1997, 49(6), 238–245, DOI:10.1002/star.19970490605
.
- K. Sangseethong, S. Ketsilp and K. Sriroth, The Role of Reaction Parameters on the Preparation and Properties of Carboxymethyl Cassava Starch, Starch/Staerke, 2005, 57(2), 84–93, DOI:10.1002/star.200400302
.
- T. Spychaj, K. Wilpiszewska and M. Zdanowicz, Medium and high substituted carboxymethyl starch: Synthesis, characterization and application, Starch/Staerke, 2013, 65(1–2), 22–33, DOI:10.1002/star.201200159
.
- P. N. Bhandari and M. A. Hanna, Continuous Solventless Extrusion Process for Producing Sodium Carboxymethyl Starch Suitable for Disintegrant Applications in Solid Dosage Forms, Ind. Eng. Chem. Res., 2011, 50(22), 12784–12789, DOI:10.1021/ie200311w
.
- P. N. Bhandari and M. A. Hanna, Preparation of highly substituted carboxymethyl starch using a twin–screw extruder, Starch/Staerke, 2011, 63(12), 771–779, DOI:10.1002/star.201100035
.
- M. Zhou, L. Shi, F. Cheng, Y. Lin and P.-X. Zhu, High-Efficient Preparation of Carboxymethyl Starch via Ball Milling With Limited Solvent Content, Starch/Staerke, 2018, 70(5–6), 1700250, DOI:10.1002/star.201700250
.
-
S. K. Halder and K. C. Mondal, Microbial Valorization of Chitinous Bioresources for Chitin Extraction and Production of Chito-Oligomers and N-Acetylglucosamine: Trends, Perspectives and Prospects, in Microbial Biotechnology: Volume 2. Application in Food and Pharmacology, ed. J. K. Patra, G. Das and H.-S. Shin, Springer Singapore, Singapore, 2018, pp. 69–107 Search PubMed
.
-
A. Shrotri, H. Kobayashi and A. Fukuoka, Chapter Two - Catalytic Conversion of Structural Carbohydrates and Lignin to Chemicals, in Advances in Catalysis, ed. C. Song, Academic Press, 2017, vol. 60, pp. 59–123 Search PubMed
.
- S. Ifuku and H. Saimoto, Chitin nanofibers: preparations, modifications, and applications, Nanoscale, 2012, 4(11), 3308–3318, 10.1039/c2nr30383c
.
- L. Bai, L. Liu, M. Esquivel, B. L. Tardy, S. Huan, X. Niu, S. Liu, G. Yang, Y. Fan and O. J. Rojas, Nanochitin: Chemistry, Structure, Assembly, and Applications, Chem. Rev., 2022, 122(13), 11604–11674, DOI:10.1021/acs.chemrev.2c00125
.
- Y. Fan, T. Saito and A. Isogai, Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization, Carbohydr. Polym., 2010, 79(4), 1046–1051, DOI:10.1016/j.carbpol.2009.10.044
.
- H. El Knidri, R. Belaabed, A. Addaou, A. Laajeb and A. Lahsini, Extraction, chemical modification and characterization of chitin and chitosan, Int. J. Biol. Macromol., 2018, 120, 1181–1189, DOI:10.1016/j.ijbiomac.2018.08.139
.
- H. Sashiwa and S.-i. Aiba, Chemically modified chitin and chitosan as biomaterials, Prog. Polym. Sci., 2004, 29(9), 887–908, DOI:10.1016/j.progpolymsci.2004.04.001
.
- R. Mulongo-Masamba, M. El Hazzat, A. El Hamidi, M. Halim and S. Arsalane, New functional β-chitin/calcium phosphate as promising support of copper nanocatalyst for the reductive degradation of methylene blue, Int. J. Environ. Sci. Technol., 2019, 16(12), 8117–8128, DOI:10.1007/s13762-019-02353-z
.
- Z. Bujňáková, E. Dutková, M. Kello, J. Mojžiš, M. Baláž, P. Baláž and O. Shpotyuk, Mechanochemistry of Chitosan-Coated Zinc Sulfide (ZnS) Nanocrystals for Bio-imaging Applications, Nanoscale Res. Lett., 2017, 12(1), 328, DOI:10.1186/s11671-017-2103-z
.
- Z. Bujňáková, E. Dutková, A. Zorkovská, M. Baláž, J. Kováč, M. Kello, J. Mojžiš, J. Briančin and P. Baláž, Mechanochemical synthesis and in vitro studies of chitosan-coated InAs/ZnS mixed nanocrystals, J. Mater. Sci., 2017, 52(2), 721–735 CrossRef
.
- O. Trentin, D. Ballesteros-Plata, E. Rodríguez-Castellón, L. Puppulin, M. Selva, A. Perosa and D. Rodríguez-Padrón, Upcycling of Chitin to Cross-Coupling Catalysts: Tailored Supports and Opportunities in Mechanochemistry, ChemSusChem, 2025, 18(1), e202401255, DOI:10.1002/cssc.202401255
.
- G. Margoutidis, V. H. Parsons, C. S. Bottaro, N. Yan and F. M. Kerton, Mechanochemical Amorphization of α-Chitin and Conversion into Oligomers of N-Acetyl-d-glucosamine, ACS Sustainable Chem. Eng., 2018, 6(2), 1662–1669, DOI:10.1021/acssuschemeng.7b02870
.
- M. Yabushita, H. Kobayashi, K. Kuroki, S. Ito and A. Fukuoka, Catalytic Depolymerization of Chitin with Retention of N-Acetyl Group, ChemSusChem, 2015, 8(22), 3760–3763, DOI:10.1002/cssc.201501224
.
- A. Einbu and K. M. Vårum, Depolymerization and de-N-acetylation of chitin oligomers in hydrochloric acid, Biomacromolecules, 2007, 8(1), 309–314, DOI:10.1021/bm0608535
.
- P. Dornath, H. J. Cho, A. Paulsen, P. Dauenhauer and W. Fan, Efficient mechano-catalytic depolymerization of crystalline cellulose by formation of branched glucan chains, Green Chem., 2015, 17(2), 769–775, 10.1039/c4gc02187h
.
- A. Shrotri, L. K. Lambert, A. Tanksale and J. Beltramini, Mechanical depolymerisation of acidulated cellulose: understanding the solubility of high molecular weight oligomers, Green Chem., 2013, 15(10), 2761–2768, 10.1039/C3GC40945G
.
- H. Kobayashi, Y. Suzuki, T. Sagawa, M. Saito and A. Fukuoka, Selective Synthesis of Oligosaccharides by Mechanochemical Hydrolysis of Chitin over a Carbon-Based Catalyst, Angew. Chem., Int. Ed., 2023, 62(3), e202214229, DOI:10.1002/anie.202214229
.
- H. Kobayashi, Y. Suzuki, T. Sagawa, K. Kuroki, J.-Y. Hasegawa and A. Fukuoka, Impact of tensile and compressive forces on the hydrolysis of cellulose and chitin, Phys. Chem. Chem. Phys., 2021, 23(30), 15908–15916, 10.1039/d1cp01650d
.
- M. J. Hülsey, Shell biorefinery: A comprehensive introduction, Green Energy Environ., 2018, 3(4), 318–327, DOI:10.1016/j.gee.2018.07.007
.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green chemistry and the ocean-based biorefinery, Green Chem., 2013, 15(4), 860–871, 10.1039/C3GC36994C
.
- C. Chen, Q. Wu, Z. Wan, Q. Yang, Z. Xu, D. Li, Y. Jin and O. J. Rojas, Mildly processed chitin used in one-component drinking straws and single use materials: Strength, biodegradability and recyclability, Chem. Eng. J., 2022, 442, 136173, DOI:10.1016/j.cej.2022.136173
.
- Y. Fan, T. Saito and A. Isogai, Chitin Nanocrystals Prepared by TEMPO-Mediated Oxidation of α-Chitin, Biomacromolecules, 2008, 9(1), 192–198, DOI:10.1021/bm700966g
.
- R. H. Marchessault, F. F. Morehead and N. M. Walter, Liquid Crystal Systems from Fibrillar Polysaccharides, Nature, 1959, 184(4686), 632–633, DOI:10.1038/184632a0
.
- E. Lam and J. H. T. Luong, Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals, ACS Catal., 2014, 4(10), 3393–3410, DOI:10.1021/cs5008393
.
- S. Deepthi, J. Venkatesan, S.-K. Kim, J. D. Bumgardner and R. Jayakumar, An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering, Int. J. Biol. Macromol., 2016, 93, 1338–1353, DOI:10.1016/j.ijbiomac.2016.03.041
.
- S. Peter, N. Lyczko, D. Gopakumar, H. J. Maria, A. Nzihou and S. Thomas, Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review, Waste Biomass Valorization, 2021, 12(9), 4777–4804, DOI:10.1007/s12649-020-01244-6
.
- M. J. Ahmed, B. H. Hameed and E. H. Hummadi, Review on recent progress in chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants, Carbohydr. Polym., 2020, 247, 116690, DOI:10.1016/j.carbpol.2020.116690
.
- R. Nasrin, S. Biswas, T. U. Rashid, S. Afrin, R. A. Jahan, P. Haque and M. M. Rahman, Preparation of Chitin-PLA laminated composite for implantable application, Bioact. Mater., 2017, 2(4), 199–207, DOI:10.1016/j.bioactmat.2017.09.003
.
- D. Meng, J. Xie, G. I. N. Waterhouse, K. Zhang, Q. Zhao, S. Wang, S. Qiu, K. Chen, J. Li, C. Ma, Y. Pan and J. Xu, Biodegradable Poly(butylene adipate-co-terephthalate) composites reinforced with bio-based nanochitin: Preparation, enhanced mechanical and thermal properties, J. Appl. Polym. Sci., 2020, 137(12), 48485, DOI:10.1002/app.48485
.
- T. M. Aida, K. Oshima, C. Abe, R. Maruta, M. Iguchi, M. Watanabe and R. L. Smith, Dissolution of mechanically milled chitin in high temperature water, Carbohydr. Polym., 2014, 106, 172–178, DOI:10.1016/j.carbpol.2014.02.009
.
- X. Ye, Z. Wang, Q. Wang, L. Zhu, L. Yang and D. Xu, Solid-State Utilization of Chitin to Construct Flexible Films with Enhanced Biocompatibility and Mechanical Performance, ACS Sustainable Chem. Eng., 2024, 12(11), 4497–4505, DOI:10.1021/acssuschemeng.3c07491
.
- L. Wang, T. Gong, W. Ming, X. Qiao, W. Ye, L. Zhang and C. Pan, One step preparation of multifunctional poly (ether sulfone) thin films with potential for wound dressing, Biomater. Adv., 2022, 136, 212758, DOI:10.1016/j.bioadv.2022.212758
.
- C. Chio, M. Sain and W. Qin, Lignin utilization: A review of lignin depolymerization from various aspects, Renewable Sustainable Energy Rev., 2019, 107, 232–249, DOI:10.1016/j.rser.2019.03.008
.
- P. Bartczak, M. Wysokowski, K. Szylińczuk, M. Odalanowska, T. Jesionowski and S. Borysiak, Green synthesis of chitin/lignin based-polyurethane composites, Ind. Crops Prod., 2023, 204, 117237, DOI:10.1016/j.indcrop.2023.117237
.
- S. M. Taghizadeh and G. Davari, Preparation, characterization, and swelling behavior of N-acetylated and deacetylated chitosans, Carbohydr. Polym., 2006, 64(1), 9–15, DOI:10.1016/j.carbpol.2005.10.037
.
- I. A. Sogias, V. V. Khutoryanskiy and A. C. Williams, Exploring the Factors Affecting the Solubility of Chitosan in Water, Macromol. Chem. Phys., 2010, 211(4), 426–433, DOI:10.1002/macp.200900385
.
- R. Yang, H. Li, M. Huang, H. Yang and A. Li, A review on chitosan-based flocculants and their applications in water treatment, Water Res., 2016, 95, 59–89, DOI:10.1016/j.watres.2016.02.068
.
-
J.-Y. Je and S.-K. Kim, Chapter 7 - Chitosan as Potential Marine Nutraceutical, in Advances in Food and Nutrition Research, ed. S.-K.
Kim, Academic Press, 2012, vol. 65, pp. 121–135 Search PubMed
.
- R. C. F. Cheung, T. B. Ng, J. H. Wong and W. Y. Chan, Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications, Mar. Drugs, 2015, 13(8), 5156–5186 CrossRef CAS PubMed
.
- H. Hamedi, S. Moradi, S. M. Hudson, A. E. Tonelli and M. W. King, Chitosan based bioadhesives for biomedical applications: A review, Carbohydr. Polym., 2022, 282, 119100, DOI:10.1016/j.carbpol.2022.119100
.
- P. Homayonpour, H. Jalali, N. Shariatifar and M. Amanlou, Effects of nano-chitosan coatings incorporating with free /nano-encapsulated cumin (Cuminum cyminum L.) essential oil on quality characteristics of sardine fillet, Int. J. Food Microbiol., 2021, 341, 109047, DOI:10.1016/j.ijfoodmicro.2021.109047
.
- R. Priyadarshi and J.-W. Rhim, Chitosan-based biodegradable functional films for food packaging applications, Innovative Food Sci. Emerging Technol., 2020, 62, 102346, DOI:10.1016/j.ifset.2020.102346
.
- D. Prajapati, A. Pal, C. Dimkpa, Harish, U. Singh, K. A. Devi, J. L. Choudhary and V. Saharan, Chitosan nanomaterials: A prelim of next-generation fertilizers; existing and future prospects, Carbohydr. Polym., 2022, 288, 119356, DOI:10.1016/j.carbpol.2022.119356
.
- K. L. B. Chang, G. Tsai, J. Lee and W.-R. Fu, Heterogeneous N-deacetylation of chitin in alkaline solution, Carbohydr. Res., 1997, 303(3), 327–332, DOI:10.1016/S0008-6215(97)00179-1
.
- A. Domard and M. Rinaudo, Preparation and characterization of fully deacetylated chitosan, Int. J. Biol. Macromol., 1983, 5(1), 49–52, DOI:10.1016/0141-8130(83)90078-8
.
- X. Chen, H. Yang, Z. Zhong and N. Yan, Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan, Green Chem., 2017, 19(12), 2783–2792, 10.1039/C7GC00089H
.
- M. A. Asrahwi, N. A. Rosman, N. N. M. Shahri, J. H. Santos, E. Kusrini, S. Thongratkaew, K. Faungnawakij, S. Hassan, A. H. Mahadi and A. Usman, Solid-state mechanochemical synthesis of chitosan from mud crab (Scylla serrata) chitin, Carbohydr. Res., 2023, 534, 108971, DOI:10.1016/j.carres.2023.108971
.
- C. Chatelet, O. Damour and A. Domard, Influence of the degree of acetylation on some biological properties of chitosan films, Biomaterials, 2001, 22(3), 261–268, DOI:10.1016/S0142-9612(00)00183-6
.
- H. Zhang and S. H. Neau, In vitro degradation of chitosan by a commercial enzyme preparation: effect of molecular weight and degree of deacetylation, Biomaterials, 2001, 22(12), 1653–1658, DOI:10.1016/S0142-9612(00)00326-4
.
- C. R. Huei and H.-D. Hwa, Effect of molecular weight of chitosan with the same degree of deacetylation on the thermal, mechanical, and permeability properties of the prepared membrane, Carbohydr. Polym., 1996, 29(4), 353–358, DOI:10.1016/S0144-8617(96)00007-0
.
- W. Wang, Y. Du, Y. Qiu, X. Wang, Y. Hu, J. Yang, J. Cai and J. F. Kennedy, A new green technology for direct production of low molecular weight chitosan, Carbohydr. Polym., 2008, 74(1), 127–132, DOI:10.1016/j.carbpol.2008.01.025
.
- Z. Jia and D. Shen, Effect of reaction temperature and reaction time on the preparation of low-molecular-weight chitosan using phosphoric acid, Carbohydr. Polym., 2002, 49(4), 393–396, DOI:10.1016/S0144-8617(02)00026-7
.
- C. K. S. Pillai, W. Paul and C. P. Sharma, Chitin and chitosan polymers: Chemistry, solubility and fiber formation, Prog. Polym. Sci., 2009, 34(7), 641–678, DOI:10.1016/j.progpolymsci.2009.04.001
.
- M. W. Anthonsen and O. Smidsrød, Hydrogen ion titration of chitosans with varying degrees of N-acetylation by monitoring induced 1H-NMR chemical shifts, Carbohydr. Polym., 1995, 26(4), 303–305, DOI:10.1016/0144-8617(95)00010-5
.
- C. Van Poucke, A. Vandeputte, S. Mangelinckx and C. V. Stevens, Green mechanochemical synthesis of water-soluble N-sulfonated chitosan, Green Chem., 2023, 25(11), 4271–4281, 10.1039/D3GC00549F
.
- G. S. Georgiev, E. B. Kamenska, E. D. Vassileva, I. P. Kamenova, V. T. Georgieva, S. B. Iliev and I. A. Ivanov, Self-Assembly, Antipolyelectrolyte Effect, and, Nonbiofouling Properties of Polyzwitterions, Biomacromolecules, 2006, 7(4), 1329–1334, DOI:10.1021/bm050938q
.
- B.-O. Jung, J. Na and C. H. Kim, Synthesis of chitosan derivatives with anionic groups and its biocompatibility in vitro, J. Ind. Eng. Chem., 2007, 13(5), 772–776 CAS
.
- A. Heydari, M. Darroudi and I. Lacík, Efficient N-sulfopropylation of chitosan with 1,3-propane sultone in aqueous solutions: neutral pH as the key condition, React. Chem. Eng., 2021, 6(11), 2146–2158, 10.1039/D1RE00089F
.
- F. A. W. Fatika, M. Anwar, D. J. Prasetyo, W. A. Rizal, R. Suryani, P. Yuliyanto, S. Hariyadi, A. Suwanto, N. A. Bahmid, S. K. Wahono, F. H. Sriherfyna, C. D. Poeloengasih, B. Purwono, E. Agustian, R. Maryana and H. Hernawan, Facile fabrication of chitosan Schiff bases from giant tiger prawn shells (Penaeus monodon) via solvent-free mechanochemical grafting, Int. J. Biol. Macromol., 2023, 247, 125759, DOI:10.1016/j.ijbiomac.2023.125759
.
- R. Antony, T. Arun and S. T. D. Manickam, A review on applications of chitosan-based Schiff bases, Int. J. Biol. Macromol., 2019, 129, 615–633, DOI:10.1016/j.ijbiomac.2019.02.047
.
- V. Pawariya, S. De and J. Dutta, Chitosan-based Schiff bases: Promising materials for biomedical and industrial applications, Carbohydr. Polym., 2024, 323, 121395, DOI:10.1016/j.carbpol.2023.121395
.
- E. H. Cordes and W. P. Jencks, On the Mechanism of Schiff Base Formation and Hydrolysis, J. Am. Chem. Soc., 1962, 84(5), 832–837, DOI:10.1021/ja00864a031
.
- E. H. Cordes and W. P. Jencks, The Mechanism of Hydrolysis of Schiff Bases Derived from Aliphatic Amines, J. Am. Chem. Soc., 1963, 85(18), 2843–2848, DOI:10.1021/ja00901a037
.
- C. Van Poucke, S. Mangelinckx and C. V. Stevens, Shaking up conjugates between chitosan and aldehydes via mechanochemistry, RSC Mechanochem., 2025, 2(1), 142–151, 10.1039/d4mr00110a
.
- F. Parenti, F. Tassinari, E. Libertini, M. Lanzi and A. Mucci, Π-Stacking Signature in NMR Solution Spectra of Thiophene-Based Conjugated Polymers, ACS Omega, 2017, 2(9), 5775–5784, DOI:10.1021/acsomega.7b00943
.
- M. F. Cunningham and P. G. Jessop, An introduction to the principles and fundamentals of CO2-switchable polymers and polymer colloids, Eur. Polym. J., 2016, 76, 208–215, DOI:10.1016/j.eurpolymj.2016.01.036
.
- G. Yang, R. S. Korchinsky, J. Sauvé-St-Pierre, P. G. Jessop, E. Lam and A. Moores, Mechanochemical and Aging–Based SN2 Method to Access CO2–Responsive, High–Amine–Loading Chitosan, ChemSusChem, 2025, 18(19), e202501187, DOI:10.1002/cssc.202501187
.
- M. Ramezani, S. N. Ellis, A. Riabtseva, M. F. Cunningham and P. G. Jessop, CO2-Responsive Low Molecular Weight Polymer with High Osmotic Pressure as a Draw Solute for Forward Osmosis, ACS Omega, 2023, 8(51), 49259–49269, DOI:10.1021/acsomega.3c07644
.
- J.-Y. Je and S.-K. Kim, Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation, Bioorg. Med. Chem., 2006, 14(17), 5989–5994, DOI:10.1016/j.bmc.2006.05.016
.
-
P. G. Jessop and M. F. Cunningham, CO2-switchable Materials: Solvents, Surfactants, Solutes and Solids, The Royal Society of Chemistry, London, UK, 2020 Search PubMed
.
- M. F. Mady, E. Haukereid, S. Abdel-Azeim, I. A. Hussein and M. A. Kelland, A sustainable approach to synthesize phosphonated chitosan using ball milling and its application for oilfield scale management, Green Chem., 2022, 24(18), 7171–7183, 10.1039/d2gc02102a
.
- K. N. Bardakova, T. A. Akopova, A. V. Kurkov, G. P. Goncharuk, D. V. Butnaru, V. F. Burdukovskii, A. A. Antoshin, I. A. Farion, T. M. Zharikova, A. B. Shekhter, V. I. Yusupov, P. S. Timashev and Y. A. Rochev, From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives, Mar. Drugs, 2019, 17(1), 48, DOI:10.3390/md17010048
.
- M. Vakili, W. Qiu, G. Cagnetta, J. Huang and G. Yu, Solvent-free mechanochemical mild oxidation method to enhance adsorption properties of chitosan, Front. Environ. Sci. Eng., 2021, 15(6), 128, DOI:10.1007/s11783-021-1416-4
.
- X. Yan, X. Liu, C. Qi, C. Lin, P. Li and H. Wang, Disposal of hexabromocyclododecane (HBCD) by grinding assisted with sodium persulfate, RSC Adv., 2017, 7(38), 23313–23318, 10.1039/C7RA02689G
.
- M. Bengisu and E. Yilmaz, Oxidation and pyrolysis of chitosan as a route for carbon fiber derivation, Carbohydr. Polym., 2002, 50(2), 165–175, DOI:10.1016/S0144-8617(02)00018-8
.
- M. Vakili, W. Qiu, G. Cagnetta, J. Huang and G. Yu, Mechanochemically oxidized chitosan-based adsorbents with outstanding Penicillin G adsorption capacity, J. Environ. Chem. Eng., 2021, 9(4), 105454, DOI:10.1016/j.jece.2021.105454
.
- T. Azizi Vahed, M. R. Naimi-Jamal and L. Panahi, Alginate-coated ZIF-8 metal-organic framework as a green and bioactive platform for controlled drug release, J. Drug Delivery Sci. Technol., 2019, 49, 570–576, DOI:10.1016/j.jddst.2018.12.022
.
- W. Xu, S. Chen, L. Song, H. Jin, F. Pu, W. Su, Z. Lou and X. Xu, Mechanochemical synthesis of cysteine-gum acacia intermolecular complex for multiple metal(loid) sequestration from herbal extracts, Chemosphere, 2023, 338, 139612, DOI:10.1016/j.chemosphere.2023.139612
.
- L. Cai, J. Lin, E. Fan, F. Wu, R. Chen and L. Li, Eco-Friendly Organic Acid-Assisted Mechanochemical Process for Metal Extraction from Spent Lithium-Ion Batteries, ACS Sustainable Chem. Eng., 2022, 10(32), 10649–10657, DOI:10.1021/acssuschemeng.2c02553
.
- C. Xu, M. Ojeda, R. A. D. Arancon, A. A. Romero, J. L. Domingo, M. Gómez, J. Blanco and R. Luque, Bioinspired Porous ZnO Nanomaterials from Fungal Polysaccharides: Advanced Materials with Unprecedented Low Toxicity in Vitro for Human Cells, ACS Sustainable Chem. Eng., 2015, 3(11), 2716–2725, DOI:10.1021/acssuschemeng.5b00568
.
- X. Yang, X. Liu, H. Lu, X. Zhang, L. Ma, R. Gao and Y. Zhang, Real-Time Investigation of Acute Toxicity of ZnO Nanoparticles on Human Lung Epithelia with Hopping Probe Ion Conductance Microscopy, Chem. Res. Toxicol., 2012, 25(2), 297–304, DOI:10.1021/tx2004823
.
- T. Kang, R. Guan, X. Chen, Y. Song, H. Jiang and J. Zhao, In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells, Nanoscale Res. Lett., 2013, 8(1), 496, DOI:10.1186/1556-276X-8-496
.
- I. L. Hsiao and Y.-J. Huang, Effects of serum on cytotoxicity of nano- and micro-sized ZnO particles, J. Nanopart. Res., 2013, 15(9), 1829, DOI:10.1007/s11051-013-1829-5
.
- R. Rinaldi and F. Schüth, Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes, ChemSusChem, 2009, 2(12), 1096–1107, DOI:10.1002/cssc.200900188
.
- D. R. Thompson and H. E. Grethlein, Design and Evaluation of a Plug Flow Reactor for Acid Hydrolysis of Cellulose, Ind. Eng. Chem. Prod. Res. Dev., 1979, 18(3), 166–169, DOI:10.1021/i360071a003
.
- S. Morales-Delarosa, J. M. Campos-Martin and J. L. G. Fierro, Optimization of the process of chemical hydrolysis of cellulose to glucose, Cellulose, 2014, 21(4), 2397–2407, DOI:10.1007/s10570-014-0280-9
.
- O. Bobleter, W. Schwald, R. Concin and H. Binder, Hydrolysis of Cellobiose in Dilute Sulpuric Acid and Under Hydrothermal Conditions, J. Carbohydr. Chem., 1986, 5(3), 387–399, DOI:10.1080/07328308608058843
.
- A. Isogai, T. Saito and H. Fukuzumi, TEMPO-oxidized cellulose nanofibers, Nanoscale, 2011, 3(1), 71–85, 10.1039/C0NR00583E
.
-
J. H. Luong, E. Lam, C. W. Leung, S. Hrapovic and K. B. Male, Chitin nanocrystals and process for preparation thereof, US10214596B2, 2016
.
- M. I. Khalil, A. Hashem and A. Hebeish, Carboxymethylation of Maize Starch, Starch/Staerke, 1990, 42(2), 60–63, DOI:10.1002/star.19900420209
.
- C. J. Tijsen, H. J. Scherpenkate, E. J. Stamhuis and A. A. C. M. Beenackers, Optimisation of the process conditions for the modification of starch, Chem. Eng. Sci., 1999, 54(13), 2765–2772, DOI:10.1016/S0009-2509(98)00321-2
.
- C. J. Tijsen, H. J. Kolk, E. J. Stamhuis and A. A. C. M. Beenackers, An experimental study on the carboxymethylation of granular potato starch in non-aqueous media, Carbohydr. Polym., 2001, 45(3), 219–226, DOI:10.1016/S0144-8617(00)00243-5
.
-
P. T. Anastas and J. C. Warner, Green chemistry: Theory and practice, 1998, vol. 29 Search PubMed
.
- H. Seddiqi, E. Oliaei, H. Honarkar, J. Jin, L. C. Geonzon, R. G. Bacabac and J. Klein-Nulend, Cellulose and its derivatives: towards biomedical applications, Cellulose, 2021, 28(4), 1893–1931, DOI:10.1007/s10570-020-03674-w
.
- S. Jobling, Improving starch for food and industrial applications, Curr. Opin. Plant Biol., 2004, 7(2), 210–218, DOI:10.1016/j.pbi.2003.12.001
.
- S. Sala, F. Biganzoli, E. S. Mengual and E. Saouter, Toxicity impacts in the environmental footprint method: calculation principles, Int. J. Life Cycle Assess., 2022, 27(4), 587–602, DOI:10.1007/s11367-022-02033-0
.
- H. Nikaido and M. Vaara, Molecular basis of bacterial outer membrane permeability, Microbiol. Rev., 1985, 49(1), 1–32 Search PubMed
. 0146-0749/85/010001-32$02.00/0.
- C. M. Buchanan, R. M. Gardner and R. J. Komarek, Aerobic biodegradation of cellulose acetate, J. Appl. Polym. Sci., 1993, 47(10), 1709–1719, DOI:10.1002/app.1993.070471001
.
- C. Fringant, M. Rinaudo, N. Gontard, S. Guilbert and H. Derradji, A Biogradable Starch Based Coating to Waterproof Hydrophilic Materials, Starch/Staerke, 1998, 50(7), 292–296, DOI:10.1002/(SICI)1521-379X(199807)50:7<292::AID-STAR292>3.0.CO;2-#
.
- N. Yan and X. Chen, Sustainability: Don't waste seafood waste, Nature, 2015, 524, 155–157, DOI:10.1038/524155a
.
- D. Le Roux, B. Vergnes, M. Chaurand and J. Abécassis, A thermomechanical approach to pasta extrusion, J. Food Eng., 1995, 26(3), 351–368, DOI:10.1016/0260-8774(94)00060-M
.
- E. Cook, M. Derks and C. A. Velis, Plastic waste reprocessing for circular economy: A systematic scoping review of risks to occupational and public health from legacy substances and extrusion, Sci. Total Environ., 2023, 859, 160385, DOI:10.1016/j.scitotenv.2022.160385
.
- D. E. Crawford and J. Casaban, Recent Developments in Mechanochemical Materials Synthesis by Extrusion, Adv. Mater., 2016, 28(27), 5747–5754, DOI:10.1002/adma.201505352
.
-
P. Baláž, High-Energy Milling, in Mechanochemistry in Nanoscience and Minerals Engineering, Springer Berlin Heidelberg, Berlin, Heidelberg, 2008, pp 103–132 Search PubMed
.
- A. Tohry, S. Chehreh Chelgani, S. S. Matin and M. Noormohammadi, Power-draw prediction by random forest based on operating parameters for an industrial ball mill, Adv. Powder Technol., 2020, 31(3), 967–972, DOI:10.1016/j.apt.2019.12.012
.
-
G. Saravacos and A. E. Kostaropoulos, Mechanical Processing Equipment, in Handbook of Food Processing Equipment, Springer International Publishing, Cham, 2016, pp. 149–232 Search PubMed
.
Footnote |
| † Present Addresses: Centre in Green Chemistry and Catalysis, Department of Chemistry, McGill University, Montréal, Québec H3A 0B8, Canada. |
| This journal is © The Royal Society of Chemistry 2026 |