Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development and systematic evaluation of aqueous triazole chloride-based deep eutectic solvents for efficient CO2 capture
Qiangbing
Shi
 a,
Kaige
Jia
a,
Xiangping
Zhang
a,
Kaige
Jia
a,
Xiangping
Zhang
 bc,
Chuan
Wang
d,
Paul
Cobden
bc,
Chuan
Wang
d,
Paul
Cobden
 d,
Anna-Maria
Beregi Amnéus
e,
David
Muren
f and
Xiaoyan
Ji
d,
Anna-Maria
Beregi Amnéus
e,
David
Muren
f and
Xiaoyan
Ji
 *a
*a
aEnergy Engineering, Department of Engineering Science and Mathematics, Luleå University of Technology, Luleå 97187, Sweden. E-mail: xiaoyan.ji@ltu.se
bState Key Laboratory of Heavy Oil Processing, College of Chemical Engineering and Environment, China University of Petroleum, Beijing, 102249, China
cCenter of Ionic Liquids and Green Energy, Beijing Key Laboratory of Solid State Battery and Energy Storage Process, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China
dSwerim AB, Luleå 97125, Sweden
eSMA Mineral AB, Filipstad 68227, Sweden
fLinde Gas AB, Luleå 97188, Sweden
First published on 12th December 2025
Abstract
Deep eutectic solvents (DESs) have attracted considerable attention as promising alternatives to conventional solvents for mitigating CO2 emissions due to their tunable structures, low volatility, and promising physicochemical properties. In this work, a series of [Triz]Cl/amine DESs were designed and synthesized and then formulated as 30 wt% aqueous solutions (30 wt% DES + 70 wt% H2O) to systematically investigate how the type of hydrogen bond donor (HBD) affects their physicochemical properties, thermal stability, and CO2 capture performance, and to identify the most effective solvent; their CO2 absorption capacity, absorption rate, thermal stability, and desorption efficiency were determined experimentally, and a novel stepwise evaluation strategy was employed for identification. [Triz]Cl/DETA was identified, exhibiting significantly enhanced performance, with CO2 absorption capacity, absorption rate, thermal stability, and cyclic loading increased by 34%, 12%, 114%, and 39%, respectively, when compared with the conventional monoethanolamine (MEA). Its viscosity (both before and after CO2 absorption), oxidative stability, and corrosion resistance were further studied, confirming the superior performance, and the reaction mechanism was also elucidated. This work provides valuable insights into the structure–property relationships of DESs and establishes [Triz]Cl/DETA-based solvents as promising candidates for efficient and sustainable CO2 capture applications.
Green foundation1. We performed structure–function analysis for HBD in aqueous [TrizCl]-based DESs and identified [Triz]Cl/DETA that outperforms MEA while lowering volatility and corrosion, yielding design rules (optimization of amine density, flexibility, and H-bonding).2. 30 wt% [Triz]Cl/DETA delivers 0.17 g-CO2 per g-solvent, Ka = 0.183 min−1, a desorption efficiency of 57% at 110 °C, and a cyclic loading of 0.09 g-CO2 per g-solvent; the post-loading viscosity is ∼7.5 mPa s. The corrosion rates are markedly lower than those of 30 wt% MEA (unloaded: 1.2 × 10−4vs. 2.3 × 10−4 mm a−1; loaded: 7.5 × 10−4vs. 1.8 × 10−3 mm a−1). No FTIR-detectable oxidation was observed after 5 h of O2. 3. Regeneration temperature/energy (target ≤100 °C) via composition tuning is reduced; durability (extension of O2 exposure beyond 5 h) is verified; and corrosion is optimized by optimizing the DES–water ratio and materials selection, while quantifying solvent loss relative to that of MEA. |
1. Introduction
The combustion of fossil fuels has driven industrial modernization but has also produced unprecedented anthropogenic CO2 emissions.1 The concentration of CO2 in the atmosphere has continued to rise and reached 422.5 ppm in 2024,2 the first year on record with a global average temperature about 1.5 °C above pre-industrial levels.3 It has been observed that the global per capita CO2 emissions and the global mean temperature have risen substantially since the 19th century, demonstrating a clear positive correlation. Therefore, CO2 mitigation and removal have become critical scientific and technological concerns for achieving global climate goals and maintaining climate stability.4To mitigate these challenges, carbon capture, utilization, and storage (CCUS)5 has been considered indispensable, owing to its application in large-scale industrial operation. Within the CCUS chain, CO2 capture serves as a critical component, making the development of efficient CO2 capture paramount. Among the available technologies for CO2 capture, chemical-based absorption is widely regarded as the most promising one, due to its high selectivity, large absorption capacity, technological maturity, and flexibility for integration with existing industrial systems. For the chemical absorption routes, amine-based solvents, such as monoethanolamine (MEA), diethanolamine (DEA), methyl diethanolamine (MDEA), and their blends, are the most widely employed in research and industrial practice.5 Among the studied amines, primary and secondary amines (e.g., MEA and DEA) provide high absorption capacities and fast CO2 capture, but their use is often constrained by volatility, corrosivity, and high regeneration energy demand; tertiary amines (e.g., MDEA) exhibit lower volatility and reduced corrosivity, but their inherently low CO2 absorption rates remain a significant drawback.6 Hence, the development of alternative absorbents remains imperative to overcome the limitations of conventional systems.
Ionic liquids (ILs) exhibit exceptional properties, including high thermal stability, negligible volatility, and tunable structures, making them highly adaptable for diverse applications.7 Nevertheless, the high production cost of conventional ILs remains a critical barrier to their large-scale deployment. Deep eutectic solvents (DESs) are a distinct class of IL analogues composed of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA).8 DESs not only retain many of the advantageous properties of ILs but also offer additional benefits, such as low cost and biocompatibility. Development of DESs has become more prominent than that of conventional ILs in recent years.
To develop DESs as absorbents for CO2 capture, recent studies have highlighted that the choice of HBD is decisive.9 For instance, Jiang et al.10 showed that employing acylamido compounds as HBDs led to notably high capacities, and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN)/2-imidazolidone (EU) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) reached 0.23 g-CO2 per g-solvent at 45 °C and 1 bar, attributed to multiple N-site interactions that enhance chemisorption. Likewise, within a chloride–amine family using a fixed HBA ([MEA]Cl), replacing the HBD from MEA to ethylenediamine (EDA) markedly increased the capacity, where [MEA]Cl/EDA achieved ∼0.30 g-CO2 per g-solvent under ambient pressure.11 With choline chloride as the HBA, switching the HBD from urea to ethylene glycol reduces viscosity but also weakens chemisorption strength and decreases equilibrium loading, revealing a rate–capacity trade-off controlled by the HBD identity.12 Similarly, the superbase 1,1,3,3-tetramethylguanidine (TMG) was used as the HBA and paired with polyol HBDs (e.g., glycerol and ethylene glycol) to tune both the absorption kinetics and the energy required for desorption via shifting the dominant binding pathway (from carbamate to bicarbonate under humid conditions).13
1) reached 0.23 g-CO2 per g-solvent at 45 °C and 1 bar, attributed to multiple N-site interactions that enhance chemisorption. Likewise, within a chloride–amine family using a fixed HBA ([MEA]Cl), replacing the HBD from MEA to ethylenediamine (EDA) markedly increased the capacity, where [MEA]Cl/EDA achieved ∼0.30 g-CO2 per g-solvent under ambient pressure.11 With choline chloride as the HBA, switching the HBD from urea to ethylene glycol reduces viscosity but also weakens chemisorption strength and decreases equilibrium loading, revealing a rate–capacity trade-off controlled by the HBD identity.12 Similarly, the superbase 1,1,3,3-tetramethylguanidine (TMG) was used as the HBA and paired with polyol HBDs (e.g., glycerol and ethylene glycol) to tune both the absorption kinetics and the energy required for desorption via shifting the dominant binding pathway (from carbamate to bicarbonate under humid conditions).13
Despite growing evidence highlighting the importance of the HBD component for the performance of DESs, previous research14 has encountered three primary challenges. First, the formulation space was only partially investigated by focusing on EDA as the HBD and varying the types of HBAs, thus leaving the vast spectrum of potential HBDs largely unexplored. Second, the EDA-based DESs demonstrated only moderate thermal stability, surpassing that of MEA, but still inadequate at relatively high temperatures and with extended cycling. Third, crucial durability indicators relevant to practical applications, such as oxidative stability, corrosion resistance, and cyclic stability, have not been systematically evaluated or sufficiently considered.
Additionally, for the DESs with outstanding CO2 capture capacities, it is common for a sharp viscosity rise to occur following the CO2 absorption, which has frequently been overlooked.15,16 For example, neat [MEA]Cl/MDEA shows a multi-fold viscosity increase (from 275 to 1500 mPa s) upon CO2 loading, which severely suppresses mass transfer and lowers the absorption rate.17 To address this issue, water, an inexpensive and environmentally benign cosolvent, is highly effective at adjusting viscosity. For example, a 40 wt% [MEA]Cl/EDA + 60 wt% H2O solution maintained a low viscosity both before and after CO2 absorption (4.4 and 13.3 mPa s at 25 °C, respectively).18 Similarly, a [Ch]Cl/MEA solution containing 75 vol% H2O exhibited a high CO2 absorption capacity of 0.19 g-CO2 per g-solvent and low viscosity (2.2 mPa s) at 20 °C and 1.5 MPa.19
To address the research gaps and given prior evidence that 1,2,4-triazolium chloride ([Triz]Cl) demonstrates favorable CO2 absorption/desorption efficiency and thermal stability, in this work, a series of [Triz]Cl-based DESs was designed and synthesized by pairing [Triz]Cl with structurally varied amine HBDs and then mixing with water as a cosolvent to form aqueous solutions. A stepwise method was used to identify promising DESs based on the CO2 absorption capacity, absorption rate, thermal stability, and cyclic loading under identical conditions. The most effective DES was subsequently subjected to application-relevant evaluations, including viscosity measurements before and after CO2 absorption, oxidative stability testing, corrosion compatibility assessment, and mechanistic analysis of the CO2 capture. Through this approach, a practical design and screening strategy was developed to identify DESs as absorbents for CO2 capture, and guidance directly relevant to industrial CO2 capture deployment was provided.
2. Experimental
2.1. Materials
CO2 (99.99%), 1,2,4-triazole (Triz, Macklin, 99%, CAS no. 288-88-0), hydrochloric acid (HCl, 37%, Macklin, CAS no. 7647-01-0), ethylenediamine (EDA, Lingfeng, 99.5%, CAS no. 107-15-3), diethylenetriamine (DETA, Macklin, 99%, CAS no. 111-40-0), tetraethylenepentamine (TEPA, Macklin, 95%, CAS no. 112-57-2), N-methyldiethanolamine (MDEA, Macklin, 98%, CAS no. 105-59-5), diethanolamine (DEA, Macklin, 99%, CAS no. 111-42-2), monoethanolamine (MEA, Macklin, 99%, CAS no. 141-43-5), 2-amino-2-methyl-propanol (AMP, Sigma-Aldrich, >95%, CAS no. 124-68-5) and aminoethylethanolamine (AEEA, Macklin, 99%, CAS no. 111-41-1) were used in this study. The structures of all studied HBDs are shown in Table 1.2.2. Preparation of DESs
The HBA, [Triz]Cl, was synthesized via a one-step anion-exchange method. During the synthesis, equimolar HCl was added dropwise into a round-bottom flask containing an aqueous 1,2,4-triazole solution, under stirring in an ice bath (1 °C) to minimize HCl volatilization. Afterward, the mixture was stirred at room temperature for 3 hours, followed by rotary evaporation at 60 °C and vacuum drying at the same temperature for 3 hours to remove excess water. To eliminate the remaining impurities, the sample was then connected to an ultra-high vacuum pump at 70 °C, yielding white crystalline [Triz]Cl. The DES, for example, [Triz]Cl/DETA, was prepared by mixing [Triz]Cl with DETA at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, followed by stirring at 60 °C for 3–5 hours to obtain a clear and homogeneous solvent. Subsequently, [Triz]Cl/DETA was diluted with H2O at a mass ratio of 3
5, followed by stirring at 60 °C for 3–5 hours to obtain a clear and homogeneous solvent. Subsequently, [Triz]Cl/DETA was diluted with H2O at a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to produce the aqueous [Triz]Cl/DETA solution for CO2 capture. Other aqueous DES solutions were prepared following the same procedure.
7 to produce the aqueous [Triz]Cl/DETA solution for CO2 capture. Other aqueous DES solutions were prepared following the same procedure.
2.3. Characterization
13C NMR spectra were measured using a 60 MHz NMR spectrometer (Spinsolve 60). Fourier Transform Infrared (FTIR, Nicolet 8700) spectroscopy was employed to identify functional groups of samples in the range of 400–4000 cm−1 with a resolution of 4 cm−1. Thermal stability was evaluated via thermogravimetric analysis (TGA) using a TA SDT650 instrument, with DES samples heated from room temperature to 400 °C at a rate of 10 °C min−1 under an N2 flow of 60 mL min−1. Viscosity measurements of the solvents before and after CO2 absorption were performed at 25 °C using an Anton Paar 2000 ME viscometer (accuracy ±0.35%). Oxidative stability was evaluated by comparing FTIR spectra recorded before and after 5 h of exposure to O2 and N2.2.4. CO2 absorption capacity and rate
The CO2 absorption capacity was measured using a gravimetric method,18 following the procedure described in our previous work, as shown in Fig. S1.14 Briefly, about 7 g of solvent was placed in a 15 mL gas absorption tube, which was immersed in a water bath maintained at a constant temperature. CO2 gas was then bubbled into the solvent under atmospheric pressure at a flow rate of 60 mL min−1, controlled by a mass flow controller (YJ-700C). The entire gas absorption tube was weighed at regular intervals using an electronic analytical balance with an accuracy of ±0.1 mg. Absorption equilibrium was reached when there was no further increase in mass. Each experiment was repeated at least twice, and the average value was reported with an uncertainty of ±0.01 g-CO2 per g-solvent. Before conducting experiments on the systems studied in this work, a 30 wt% MEA aqueous solution was investigated to ensure experimental reliability and accuracy, and a 30 wt% [Triz]Cl/EDA aqueous solution was also tested as a representative DES. Details of the uncertainty analysis and the corresponding raw data are provided in Fig. S2 of the SI.The kinetics of the CO2 capture process were analyzed, where the instantaneous CO2 absorption rate (rt, g-CO2 per (g-solvent min)) and the apparent absorption rate constant (Ka, min−1) were calculated based on formulas derived from previous studies,20 as shown below:
 | (1) |
| nt = ne(1 − e−Kat) | (2) |
2.5. Solvent regeneration
The solvent regeneration process was conducted in a three-neck flask containing CO2-saturated DES, which was heated to 110 °C in an oil bath and continuously stirred at 150 rpm, as shown in Fig. S1. To recover water vapor released alongside CO2, the gas stream was passed through a serpentine condenser maintained at 1 °C, followed by concentrated sulfuric acid to remove residual moisture.21 The desorption rate (rd, g-CO2 per (g-solvent min)) was obtained from eqn (3), and then the CO2 cyclic loading (nd) was obtained from the integral of rd over time as shown in eqn (4). The CO2 desorption efficiency (η) was calculated from the cyclic loading and the saturated CO2 capacity ne as expressed in eqn (5): | (3) |
 | (4) |
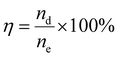 | (5) |
2.6. Corrosion analysis
To study the corrosive effects of the absorbents on steel, a three-electrode electrochemical system was used.22 The setup consists of a 20# carbon steel working electrode (W.E.), an Ag/AgCl (saturated KCl) reference electrode (R.E.), and a platinum counter electrode (C.E.). Before testing, the steel samples were cleaned with acetone and ethanol. Electrochemical measurements were performed using a CHI760E Electrochemical Workstation. Each specimen was stabilized at the open-circuit potential (OCP). Tafel polarization was recorded by scanning from −100 to +100 mV versus OCP at a rate of 10 mV s−1. The corrosion current density (icorr) was obtained by Tafel extrapolation, and the corrosion rate (CR, mm a−1) was calculated as | (6) |
3. Results and discussion
3.1. Structural and functional features of HBDs
HBDs play a critical role in the CO2 capture performance of DESs, with representative examples and their corresponding absorption capacities summarized in Table 2. Depending on their functional groups, HBDs can participate in different types of hydrogen-bonding and CO2 interaction mechanisms. The most studied classes include acylamides, polyols, carboxylic acids, alkanolamines, and polyamines. Acylamido-based HBDs, such as urea, contain both carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and amino (–NH2) groups, which form strong hydrogen bonds with HBAs, significantly lowering the eutectic temperature.23 Moreover, as shown in Fig. 1(a), their amine groups can chemically interact with CO2 to form carbamate-like intermediates, enhancing CO2 capture efficiency.10 However, the relatively high volatility and limited chemical stability of some acylamides under regeneration conditions constrain their large-scale applicability.
O) and amino (–NH2) groups, which form strong hydrogen bonds with HBAs, significantly lowering the eutectic temperature.23 Moreover, as shown in Fig. 1(a), their amine groups can chemically interact with CO2 to form carbamate-like intermediates, enhancing CO2 capture efficiency.10 However, the relatively high volatility and limited chemical stability of some acylamides under regeneration conditions constrain their large-scale applicability.
 | ||
| Fig. 1 Representative CO2 capture pathways involving different HBD types in DESs: (a) acylamide, (b) polyol, and (c) amine. | ||
| HBD type | DESs (molar ratio) | HBD | T (K)/P (bar) | Capacity (g-CO2 per g-solvent) | Ref. |
|---|---|---|---|---|---|
| Acylamido compounds | [Ch]Cl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Urea | 313.15/123.4 | 0.04 | 23 |
DBN/DMU (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Dimethylolurea (DMU) | 318.15/1.0 | 0.04 | 10 | |
DBN/DMLU (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1,3-Dimethylurea (DMLU) | 318.15/1.0 | 0.17 | ||
DBN/EU (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2-Imidazolidone (EU) | 318.15/1.0 | 0.23 | ||
DBN/EU (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2-Imidazolidone (EU) | 318.15/1.0 | 0.19 | ||
| Polyols | [TETA]Cl/DEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Diethylene glycol (DEG) | 313.15/1.0 | 0.16 | 25 |
[TETA]Cl/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Ethylene glycol (EG) | 313.15/1.0 | 0.18 | ||
[N2222]Triz/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Ethylene glycol (EG) | 298.15/1.0 | 0.13 | 26 | |
[N2222]Im/EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Ethylene glycol (EG) | 298.15/1.0 | 0.13 | ||
[Ch]Cl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Glycerol (Gly) | 298.2/1.0 | 0.02 | 31 | |
| Carboxylic acids | [BHDE]Cl/L (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Lactic acid (L) | 298.15/2.8 | 0.001 | 27 |
[BHDE]Cl/Ac (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Acetic acid (Ac) | 298.15/2.1 | 0.003 | ||
[N4444]Cl/DECA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Decanoic acid (DECA) | 308.15/0.9 | 0.002 | 28 | |
[N8881]Cl/DECA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Decanoic acid (DECA) | 323.15/0.9 | 0.002 | ||
[N8888]Cl/DECA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Decanoic acid (DECA) | 323.15/0.9 | 0.002 | ||
| Alcohol amine | [TEPA]Cl/MEA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Monoethanolamine (MEA) | 298.15/1.0 | 0.41 | 9 |
[DEA]Cl/MDEA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Methyldiethanolamine (MDEA) | 298.15/1.0 | 0.11 | 17 | |
[MDEA]Cl/MDEA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Methyldiethanolamine (MDEA) | 298.15/1.0 | 0.06 | ||
| Polyamine | [MEA]Cl/EDA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Ethylenediamine (EDA) | 328.15/1.0 | 0.36 | 29 |
[MEA]Cl/DETA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Diethyltriamine (DETA) | 313.15/1.0 | 0.18 | 30 | |
[MEA]Cl/TETA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Triethylenetetramine (TETA) | 313.15/1.0 | 0.17 | ||
[MEA]Cl/TEPA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Tetraethylenepentamine (TETA) | 313.15/1.0 | 0.10 |
Polyol-based HBDs, such as ethylene glycol (EG) and glycerol, contain multiple hydroxyl (–OH) groups, which facilitate extensive hydrogen-bonding networks with HBAs, thereby promoting CO2 absorption physically and chemically.24 For example, when using EG and diethylene glycol (DG) as HBDs, and triethylenetetramine chloride ([TETA]Cl) as the HBA to prepare various DESs, it is found that EG or DG can activate the –NH– and –NH2 groups in [TETA]Cl, thereby improving the basicity of the DESs and enhancing CO2 capacity.25 FTIR and NMR analyses have demonstrated that EG can chemically react with CO2 to produce carbonate species, facilitated by activation of its hydroxyl oxygen through interaction with the cationic HBA (Fig. 1b).26 In addition, polyol-based DESs exhibit high thermal stability and low volatility, which is beneficial for solvent regeneration and long-term stability.
Carboxylic acid-based HBDs can be categorized into hydrophilic and hydrophobic types, both primarily capturing CO2 through physical absorption.27 Hydrophilic acids (e.g., lactic acid and formic acid) form dense hydrogen bonding networks via their –COOH groups, depressing melting points and increasing polarity to enhance CO2 solubility. In contrast, hydrophobic acids (e.g., dodecanoic acid) yield less polar DESs that demonstrate superior thermal stability and require lower energy for solvent regeneration.28
Amino alcohols and polyamines are among the most promising HBD families owing to their low cost, high reactivity toward CO2, and favorable physicochemical characteristics.17 Amino alcohols possess both amine (–NH2) and hydroxyl (–OH) groups, offering dual sites for hydrogen bonding and CO2 activation,9 while polyamines are rich in multiple nucleophilic amine sites, capable of forming strong hydrogen bonding networks with HBAs.29 These features enable the formation of stable and dense hydrogen bonding networks with HBAs, thereby facilitating the self-assembly of low-melting-point DESs and providing abundant reactive sites for CO2 interaction.30 As depicted in Fig. 1(c), primary and secondary amines react directly with CO2 to form carbamate species, while tertiary amines facilitate CO2 hydration to produce bicarbonate ions in aqueous media. This chemical versatility enables amine-based DESs to achieve high absorption capacities and fast kinetics compared with other HBD types.
As summarized in Table 2, DESs incorporating amine-based HBDs consistently exhibit superior CO2 absorption capacities. Therefore, in this work, amine-based HBDs were selected to systematically investigate the structure–property relationships. More specifically, EDA, DETA, TEPA, MEA, DEA, MDEA, AMP, and AEEA were chosen, which encompass a broad range of molecular architectures, from polyamines to alkanolamines and tertiary amines, enabling a systematic comparison of how the number of amine groups, steric hindrance, and hydroxyl substitution influence CO2 absorption capacity, kinetics, and thermal stability.
3.2. Absorption performance of aqueous DESs
Based on the findings in section 3.1, eight amines with potentially superior CO2 absorption capacities were selected as the HBDs for this study. For a DES solution, the ratio of HBA to HBD and the content of water will also influence the properties and performance. Previously, Shukla et al.32 systematically investigated the properties of [Im]Cl/EDA at various HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratios (1
HBD molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 1
5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). The results showed that although [Im]Cl/EDA (1
6). The results showed that although [Im]Cl/EDA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) exhibited a slightly lower CO2 absorption capacity than [Im]Cl/EDA (1
5) exhibited a slightly lower CO2 absorption capacity than [Im]Cl/EDA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), its thermal degradation was significantly lower than that of the 1
6), its thermal degradation was significantly lower than that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 counterparts. Additionally, the viscosity of the 30 wt% [Im]Cl/EDA (1
6 counterparts. Additionally, the viscosity of the 30 wt% [Im]Cl/EDA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) aqueous solution was found to be below 5 mPa s. Therefore, a 30 wt% DES aqueous solution with an HBA
5) aqueous solution was found to be below 5 mPa s. Therefore, a 30 wt% DES aqueous solution with an HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio of 1
HBD molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was prepared for all the studied DESs in this work to reflect practical application scenarios.
5 was prepared for all the studied DESs in this work to reflect practical application scenarios.
| DESs | CO2 absorption capacity (g-CO2 per g-solvent) | Instantaneous rate (g-CO2 per (g-solvent min)) | Apparent rate constant Ka (min−1) |
|---|---|---|---|
| [Triz]Cl/EDA | 0.18 | 0.025 | 0.20 |
| [Triz]Cl/DETA | 0.17 | 0.024 | 0.18 |
| [Triz]Cl/AMP | 0.10 | 0.013 | 0.12 |
| [Triz]Cl/AEEA | 0.09 | 0.011 | 0.15 |
| [Triz]Cl/MDEA | 0.04 | 0.001 | 0.03 |
| [Triz]Cl/MEA | 0.08 | 0.011 | 0.14 |
| [Triz]Cl/TEPA | 0.13 | 0.010 | 0.09 |
| [Triz]Cl/DEA | 0.05 | 0.007 | 0.15 |
To comprehensively compare the CO2 absorption capacity and apparent rate constant of aqueous DESs with different HBDs, as well as to compare their performance relative to conventional MEA, the absorption performance data of all tested DESs are summarized in Fig. 2(d). The data demonstrate that [Triz]Cl/EDA and [Triz]Cl/DETA exhibit significantly superior performance in both CO2 absorption capacity and apparent absorption rate constant compared to the other DESs and MEA. This may be attributed to their higher amine density and the lower steric hindrance. These findings highlight the critical role of HBD selection in optimizing CO2 capture performance.
3.3. Thermal stability of DESs
As illustrated in Fig. 3, the thermal stability of [Triz]Cl-based DESs (neat, water-free samples) was evaluated using thermogravimetric analysis (TGA) and differential thermogravimetry (DTG). The onset temperature (Tonset) and the peak temperature (Tpeak) were used to quantitatively evaluate the thermal stability of the DESs. Tonset is defined as the temperature at which the weight baseline intersects the tangent of the TGA curve, corresponding to the beginning of thermal decomposition, as shown in Fig. S4. In contrast, Tpeak represents the temperature at which the sample exhibits the maximum decomposition rate and corresponds to the peak in the DTG curve. | ||
| Fig. 3 (a) TGA and (b) DTG curves of pure DESs with different HBDs, compared with MEA, DETA, and [Triz]Cl. | ||
The results show that all DESs exhibit higher thermal stability than pure MEA (Table 4), confirming that DES formation enhances solvent stability. Specifically, MEA exhibited the lowest stability, with a Tonset of 83 °C and a Tpeak of only 110 °C. Among the samples, [Triz]Cl/TEPA demonstrated the highest Tonset (261 °C) and Tpeak (295 °C), alongside minimal weight loss (0.7% at 110 °C). This superior stability is attributed to the high molecular complexity of TEPA, which facilitates the formation of extensive hydrogen-bonding networks with the HBA, thereby enhancing the structural integrity of the DES. In contrast, [Triz]Cl/EDA displayed the lowest thermal stability among the polyamine-based DESs, with a Tonset of only 90 °C and a substantial weight loss of 23%. This stark difference is primarily due to the low molecular weight and boiling point of EDA, resulting in high volatility and increased susceptibility to thermal degradation. [Triz]Cl/DETA, on the other hand, exhibited intermediate stability, with a Tonset of 178 °C, a Tpeak of 207 °C, and negligible weight loss (0.7%), reflecting a balance between molecular complexity and volatility. [Triz]Cl/MDEA and [Triz]Cl/AEEA exhibited relatively high Tonset values of 199 °C and 224 °C, respectively, along with low weight losses (0.7% and 0.2%), indicating favorable thermal resilience. In contrast, [Triz]Cl/MEA and [Triz]Cl/AMP showed relatively low Tonset temperatures (155 °C and 160 °C, respectively) and higher weight losses (1.6% and 5.7%).
| DES | T onset (°C) | T peak (°C) | Weight loss at 110 °C (%) |
|---|---|---|---|
| [Triz]Cl/EDA | 90 | 109 | 23.0 |
| [Triz]Cl/DETA | 178 | 207 | 0.7 |
| [Triz]Cl/TEPA | 261 | 295 | 0.7 |
| [Triz]Cl/MEA | 155 | 186 | 1.6 |
| [Triz]Cl/DEA | 172 | 259 | 1.1 |
| [Triz]Cl/MDEA | 199 | 247 | 0.7 |
| [Triz]Cl/AEEA | 224 | 247 | 0.2 |
| [Triz]Cl/AMP | 160 | 171 | 5.7 |
| MEA | 83 | 110 | 40.0 |
| DETA | 101 | 141 | 13.5 |
| [Triz]Cl | 255 | 304 | 0.1 |
Overall, the thermal stability of DESs followed the trend: [Triz]Cl/TEPA > [Triz]Cl/AEEA > [Triz]Cl/MDEA > [Triz]Cl/DETA > [Triz]Cl/DEA > [Triz]Cl/AMP > [Triz]Cl/MEA > [Triz]Cl/EDA > MEA. These findings highlight that the thermal stability of DESs is predominantly governed by the physical properties and structural characteristics of the HBD, as well as the strength of hydrogen bonding between the HBD and HBA. For further analysis, Fig. S3(b) shows that the Tonset and Tpeak of the DESs increase with the boiling point of the HBD. DESs based on higher-boiling, higher-molecular-weight HBDs (e.g., TEPA, AEEA, and MDEA) are more thermally stable than those using the lower-boiling HBDs (e.g., MEA and EDA). This suggests that stronger hydrogen-bonding networks and less volatile HBDs enhance the intrinsic thermal stability of the DESs.
3.4. First-step screening and desorption performance of aqueous DESs
As illustrated in Fig. 2(d), 30 wt% [Triz]Cl/EDA and [Triz]Cl/DETA demonstrate significantly superior CO2 capture performance, exhibiting higher absorption capacities (0.18–0.17 g-CO2 per g-solvent) and faster absorption rate constants (0.20–0.18 min−1) compared to other 30 wt% DES aqueous solutions (0.05–0.13 g-CO2 per g-solvent; 0.03–0.15 min−1) and 30 wt% MEA (0.12 g-CO2 per g-solvent; 0.16 min−1). These distinctive advantages have qualified 30 wt% [Triz]Cl/EDA and [Triz]Cl/DETA as primary candidates for subsequent investigations. Meanwhile, thermal stability analysis revealed that pure [Triz]Cl/TEPA and [Triz]Cl/AEEA possess exceptional thermal resistance with high Tonset values of 261 °C and 224 °C, respectively, ranking highest among all the evaluated DESs. However, [Triz]Cl/TEPA exhibited suboptimal absorption kinetics (0.09 min−1), rendering it industrially impractical. In contrast, although [Triz]Cl/AEEA exhibited a lower CO2 absorption capacity and rate constant (0.09 g-CO2 per g-solvent and 0.15 min−1) than MEA, its outstanding thermal stability (Tonset = 224 °C, 0.2 wt% weight loss at 110 °C) made it a more promising candidate. Therefore, [Triz]Cl/AEEA was selected as the third potential absorbent, alongside [Triz]Cl/EDA and [Triz]Cl/DETA, for further study (Fig. 4).As a key step in the absorption–desorption cycle, CO2 desorption is equally vital as absorption, as it governs the overall energy efficiency and long-term sustainability of the process. In this work, desorption at 110 °C was identified as the optimal condition, providing a favorable balance between the amount of CO2 desorbed and the desorption rate, as shown in Fig. S5. To this end, the desorption performance of the CO2-saturated aqueous DES solutions was evaluated by measuring the time-dependent CO2 desorption rate, desorption efficiency, and cyclic loading. As shown in Fig. 5(a), the peak desorption rates of [Triz]Cl/DETA, [Triz]Cl/EDA, [Triz]Cl/AEEA, and MEA are 0.011, 0.013, 0.009, and 0.009 g-CO2 per (g-solvent min), respectively. In comparison, [Triz]Cl/EDA and [Triz]Cl/DETA exhibited desorption rates 44% and 22% higher than that of MEA, respectively, whereas [Triz]Cl/AEEA showed a similar desorption rate to MEA. Furthermore, as shown in Fig. 5(b), the desorption efficiencies of [Triz]Cl/DETA, [Triz]Cl/EDA, [Triz]Cl/AEEA, and MEA are 57%, 46%, 56%, and 57%, respectively. Correspondingly, their CO2 cyclic loadings, presented in Fig. 5(c), are 0.09, 0.08, 0.05, and 0.07 g-CO2 per g-solvent, respectively. As a result, [Triz]Cl/DETA demonstrated both the highest cyclic loading and the highest desorption efficiency, highlighting its potential as a highly regenerable absorbent. Notably, although [Triz]Cl/EDA exhibited a higher cyclic loading than MEA, it showed the lowest desorption efficiency. This may be attributed to the stronger CO2 binding affinity of EDA, which impedes complete desorption under moderate thermal conditions.
 | ||
| Fig. 5 (a) CO2 desorption curves, (b) desorption efficiencies, and (c) CO2 cyclic loading of aqueous DESs and MEA. | ||
3.5. Global comparison and second-step screening
Based on a comprehensive evaluation of key performance metrics, including CO2 absorption capacity, apparent rate constant, thermal stability, desorption efficiency, and cyclic loading, as shown in Table 5, the 30 wt% [Triz]Cl/DETA aqueous solution was identified as the most promising absorbent. It exhibits high absorption performance (nt = 0.17 g-CO2 per g-solvent, Ka = 0.18 min−1), outstanding thermal stability (Tonset = 178 °C with only 0.7% weight loss at 110 °C), and the highest cyclic CO2 loading (0.09 g-CO2 per g-solvent), while maintaining a desorption efficiency comparable to that of MEA.| Absorbent | Absorption capacity nt (g-CO2 per g-solvent) | Apparent rate constant Ka (min−1) | T onset (°C) | Weight loss at 110 °C (%) | Desorption efficiency η (%) | Cyclic loading nc (g-CO2 per g-solvent) |
|---|---|---|---|---|---|---|
| [Triz]Cl/DETA | 0.17 | 0.18 | 178 | 0.7 | 57% | 0.09 |
| [Triz]Cl/EDA | 0.18 | 0.20 | 90 | 23 | 46% | 0.08 |
| [Triz]Cl/AEEA | 0.09 | 0.15 | 224 | 0.2 | 56% | 0.05 |
| MEA | 0.12 | 0.16 | 83 | 40 | 57% | 0.07 |
To further integrate and compare the multiple performance indicators of DESs, a radar chart was constructed using normalized data, as shown in Fig. 6. All performance metrics were normalized to a 0–1 range using Min–Max scaling methods (eqn (S1) in the SI), with weight loss converted to residual mass. This comprehensive visualization enables a direct comparison of overall performance across different systems. Based on this analysis, the 30 wt% [Triz]Cl/DETA aqueous solution was also identified as the most promising absorbent (Fig. 4).
3.6. Further research on [TrizCl][DETA]
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching vibrations, respectively, which are indicative of carbamate formation. Peaks at 1090 and 1162 cm−1 were assigned to C–N and symmetric C–O stretching,36 while a shoulder near 1327 cm−1 reflected the characteristic vibration of carbamate C
O and C–O stretching vibrations, respectively, which are indicative of carbamate formation. Peaks at 1090 and 1162 cm−1 were assigned to C–N and symmetric C–O stretching,36 while a shoulder near 1327 cm−1 reflected the characteristic vibration of carbamate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.11
O groups.11
In parallel, Fig. 10(b) shows the 13C NMR spectra of [Triz]Cl/DETA before and after CO2 absorption. A new peak at ∼165 ppm appeared in the 13C NMR spectrum of CO2-loaded [Triz]Cl/DETA, which was attributed to the carbonyl carbon of the carbamate species (–NHCOO–), confirming that chemical absorption occurred via the formation of zwitterionic intermediates, followed by stabilization through proton transfer. New signals were observed near 48 ppm, corresponding to the –CH2–N– groups formed after the CO2 absorption. Moreover, a slight upfield shift near 45 ppm indicates changes in the local electronic environment of the amine carbon atoms, reflecting hydrogen-bond rearrangement and proton exchange during carbamate formation.37 These observations collectively support the reaction of amine sites with CO2.38
To further clarify which component interacts with CO2 and how the DES structure is formed, the FTIR spectra of 30 wt% DETA, 30 wt% [Triz]Cl, and 30 wt% [Triz]Cl/DETA were compared (Fig. S6). New bands at 2361 and 1255 cm−1 appear only for 30 wt% [Triz]Cl/DETA, indicating specific hydrogen-bond interactions between the HBA ([Triz]Cl) and HBD (DETA). After CO2 absorption, 30 wt% [Triz]Cl shows only minor spectral changes, whereas 30 wt% DETA clearly displays new carbamate bands, and the CO2-loaded spectrum of 30 wt% [Triz]Cl/DETA closely matches that of CO2-loaded DETA. These results confirm that DETA provides the chemically active –NH– sites for CO2 binding, while [Triz]Cl mainly acts as the hydrogen-bond acceptor matrix rather than reacting directly with CO2.
The respective roles of [Triz]Cl and DETA are also reflected in the macroscopic CO2 absorption behaviour and thermal stability. As shown in Fig. S7 (SI), 30 wt% DETA shows the highest CO2 capacity (0.20 g-CO2 per g-solvent) but a slower absorption rate than 30 wt% [Triz]Cl/DETA, whereas 30 wt% [Triz]Cl exhibits only a very low CO2 capacity (0.02 g-CO2 per g-solvent). In 30 wt% [Triz]Cl/DETA, the DETA content is 24.9 wt%. For comparison, the CO2 absorption capacity of 24.9 wt% DETA was estimated, proving a value of 0.16 g-CO2 per g-solvent, which is almost the same as that of 30 wt% [Triz]Cl/DETA (0.17 g-CO2 per g-solvent). Therefore, DETA provides the main chemically active –NH– sites for CO2 binding. The thermal stabilities of pure DETA and [Triz]Cl were also measured under the same conditions for comparison (Fig. 3). Both the Tonset and Tpeak values of DETA are lower than those of [Triz]Cl/DETA, whereas [Triz]Cl shows higher Tonset and Tpeak values than both DETA and [Triz]Cl/DETA. These observations confirm that [Triz]Cl does not significantly absorb CO2 and primarily acts as a hydrogen-bond acceptor framework, thereby improving the physicochemical properties of the DES (e.g., thermal stability).
It should be noted that this simplified metric reflects only the sensible-heat contribution. Indeed, the energy demand for solvent regeneration is a joint contribution of sensible heat to increase temperature, CO2 absorption enthalpy, and energy used for vaporization. This work focused on the screening of effective solvents. Once the effective solvents are identified, in our future work, equilibrium and calorimetric measurements together with process-level simulations will be carried out to quantify the overall regeneration energy of the promising DES systems.
4. Conclusions
In this study, a series of DESs were synthesized using [Triz]Cl as the HBA and eight different amine-based compounds as HBDs to evaluate the relationship between HBD structure and CO2 capture performance. The results demonstrated that the molecular structure and functionality of the HBD significantly influenced the CO2 absorption capacity, absorption rate, thermal stability, and desorption performance. Specifically, short-chain polyamines such as EDA and DETA exhibited high CO2 absorption capacities (0.18 and 0.17 g-CO2 per g-solvent, respectively) owing to their dense –NH– active sites that facilitate carbamate formation; the thermal stability of the DESs follows the trend [Triz]Cl/TEPA > [Triz]Cl/AEEA > [Triz]Cl/MDEA > [Triz]Cl/DETA > [Triz]Cl/DEA > [Triz]Cl/AMP > [Triz]Cl/MEA > [Triz]Cl/EDA, emphasizing that stronger hydrogen-bond networks and higher molecular weights of the HBD enhance resistance to decomposition. Among them, 30 wt% [Triz]Cl/DETA exhibited the highest cyclic CO2 loading (0.09 g-CO2 per g-solvent) and desorption efficiency (57%), coupled with superior oxidative and corrosion stability, confirming its industrial applicability. FTIR and 13C NMR analyses confirmed the formation of the carbamate species, providing clear evidence of chemical absorption of CO2 in the [Triz]Cl/DETA systems. Overall, this work establishes clear structure–property correlations for DES-based CO2 absorbents, demonstrating that optimizing amine density, molecular flexibility, and hydrogen-bonding interactions is key to balancing absorption performance, regeneration efficiency, and stability. These features suggest that the 30 wt% [Triz]Cl/DETA aqueous solution is a more energy-efficient and equipment-friendly CO2 absorbent than the 30 wt% MEA aqueous solution, offering a clear green chemistry advantage.Author contributions
Qiangbing Shi: data curation and writing – original draft. Kaige Jia: writing – original draft. Xiangping Zhang: methodology and validation. Chuan Wang: validation. Paul Cobden: validation. Anna-Maria Beregi Amnéus: validation. David Muren: validation. Xiaoyan Ji: validation, conceptualization, supervision, and funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. Supplementary information: a schematic diagram of the CO2 absorption and desorption apparatus, TG and DTG curves, CO2 desorption behavior at different temperatures, and details of the min-max scaling method. See DOI: https://doi.org/10.1039/d5gc05611j.Acknowledgements
This work was financially supported by the Swedish Energy Agency (Energimyndigheten) (P2021-00004), the European Union, STINT (CH2019-8287), and the National Key Research and Development Program of China (2024YFE0206200).References
- H. C. Lau and S. C. Tsai, Energies, 2023, 16, 7800 CrossRef CAS.
- I. E. Agency, Global Energy Review 2025, Paris, 2025 Search PubMed.
- H. Wu, Q. Li, M. Sheng, Z. Wang, S. Zhao, J. Wang, S. Mao, D. Wang, B. Guo, N. Ye, G. Kang, M. Li and Y. Cao, J. Membr. Sci., 2021, 624, 119137 CrossRef CAS.
- A. G. Olabi and M. A. Abdelkareem, Renewable Sustainable Energy Rev., 2022, 158, 112111 CrossRef CAS.
- D. Garza, P. Dargusch and D. Wadley, Energies, 2023, 16, 4090 CrossRef CAS.
- I. M. Bernhardsen and H. K. Knuutila, Int. J. Greenhouse Gas Control, 2017, 61, 27–48 CrossRef CAS.
- G. Cui, J. Wang and S. Zhang, Chem. Soc. Rev., 2016, 45, 4307–4339 RSC.
- D. O. Abranches and J. A. P. Coutinho, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 141–163 CrossRef CAS PubMed.
- J. Ju, D. Choi, S. Cho, Y. Yoo and D. Kang, Chem. Eng. J., 2024, 496, 153922 CrossRef CAS.
- B. Jiang, J. Ma, N. Yang, Z. Huang, N. Zhang, X. Tantai, Y. Sun and L. Zhang, Energy Fuels, 2019, 33, 7569–7577 CrossRef CAS.
- T. J. Trivedi, J. H. Lee, H. J. Lee, Y. K. Jeong and J. W. Choi, Green Chem., 2016, 18, 2834–2842 RSC.
- S. Imteyaz and P. P. Ingole, J. Mol. Liq., 2023, 376, 121436 CrossRef CAS.
- B. Lu, Y. Zeng, M. Chen, S. Zhang and D. Yang, Atmosphere, 2024, 15, 229 CrossRef CAS.
- Q. Shi, K. Jia, X. Zhang, C. Wang, P. Cobden, A.-M. B. Amnéus, D. Muren and X. Ji, Chem. Eng. J., 2025, 522, 167866 CrossRef CAS.
- F. Gabriele, M. Chiarini, R. Germani, M. Tiecco and N. Spreti, J. Mol. Liq., 2019, 291, 111301 CrossRef CAS.
- K. G. Jia, Q. B. Shi and X. Y. Ji, Green Chem. Eng., 2025, 6, 562–571 CrossRef CAS.
- N. Ahmad, X. Lin, X. Wang, J. Xu and X. Xu, Fuel, 2021, 293, 120466 CrossRef CAS.
- S. Foorginezhad and X. Ji, Sep. Purif. Technol., 2024, 347, 127593 CrossRef CAS.
- M. Yan, Q. Huan, Y. Zhang, W. Fang, F. Chen, A. Pariatamby, E. Kanchanatip and H. Wibowo, Biomass Convers. Biorefin., 2024, 14, 283–297 CrossRef CAS.
- S. Foorginezhad and X. Ji, Carbon Capture Sci. Technol., 2025, 15, 100385 CAS.
- L. Xiao, Z. Qiu, S. Feng, X. Duan, Z. Zhao, Y. Liu and L. Ma, Chem. Eng. Process., 2024, 204, 109931 CrossRef CAS.
- E. I. Ahmed, K. S. Ryder and A. P. Abbott, Electrochim. Acta, 2021, 397, 139284 CrossRef CAS.
- X. Li, M. Hou, B. Han, X. Wang and L. Zou, J. Chem. Eng. Data, 2008, 53, 548–550 CrossRef CAS.
- S. Sarmad, J. P. Mikkola and X. Ji, ChemSusChem, 2017, 10, 324–352 CrossRef CAS PubMed.
- K. Zhang, Y. Hou, Y. Wang, K. Wang, S. Ren and W. Wu, Energy Fuels, 2018, 32, 7727–7733 CrossRef CAS.
- G. Cui, M. Lv and D. Yang, Chem. Commun., 2019, 55, 1426–1429 RSC.
- S. Sarmad, Y. Xie, J.-P. Mikkola and X. Ji, New J. Chem., 2017, 41, 290–301 RSC.
- L. F. Zubeir, D. J. G. P. van Osch, M. A. A. Rocha, F. Banat and M. C. Kroon, J. Chem. Eng. Data, 2018, 63, 913–919 CrossRef CAS PubMed.
- S. K. Shukla and J.-P. Mikkola, Chem. Commun., 2019, 55, 3939–3942 RSC.
- X. Song, J. Yuan, C. Yang, G. Deng, Z. Wang and J. Gao, Renewable Sustainable Energy Rev., 2023, 184, 113499 CrossRef CAS.
- S. Sarmad, J.-P. Mikkola and X. Ji, ChemSusChem, 2017, 10, 324–352 CrossRef CAS PubMed.
- S. K. Shukla, Y. L. Wang, A. Laaksonen and X. Ji, Chem. Commun., 2023, 59, 10516–10519 RSC.
- F. Meng, T. Ju, S. Han, L. Lin, J. Li, K. Chen and J. Jiang, Sep. Purif. Technol., 2023, 310, 123195 CrossRef CAS.
- Y. G. Ko, S. S. Shin and U. S. Choi, J. Colloid Interface Sci., 2011, 361, 594–602 CrossRef CAS PubMed.
- J. T. Cullinane and G. T. Rochelle, Ind. Eng. Chem. Res., 2006, 45, 2531–2545 CrossRef CAS.
- S. F. R. Taylor, M. McClung, C. McReynolds, H. Daly, A. J. Greer, J. Jacquemin and C. Hardacre, Ind. Eng. Chem. Res., 2018, 57, 17033–17042 CrossRef CAS.
- X. Sun, S. Zeng, G. Li, Y. Bai, M. Shang, J. Zhang and X. Zhang, AIChE J., 2024, 70, e18376 CrossRef CAS.
- X. Zhan, B. Lv, K. Yang, G. Jing and Z. Zhou, Environ. Sci. Technol., 2020, 54, 6281–6288 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |