Electrochemical reduction of sulfoxides to thioethers with hydrosilanes catalyzed by a recyclable ionic liquid
Zhaoxin
Wei
b,
Ziren
Chen
 b,
Fei
Xue
b,
Bin
Wang
b,
Fei
Xue
b,
Bin
Wang
 b,
Yu
Xia
b,
Yu
Xia
 b,
Shaofeng
Wu
b,
Yonghong
Zhang
b,
Shaofeng
Wu
b,
Yonghong
Zhang
 b,
Weiwei
Jin
b,
Weiwei
Jin
 d,
XueFeng
Jiang
d,
XueFeng
Jiang
 *bc and
Chenjiang
Liu
*bc and
Chenjiang
Liu
 *ab
*ab
aCollege of Chemistry and Chemical Engineering, Xinjiang Normal University, Urumqi 830054, P. R. China. E-mail: pxylcj@126.com
bUrumqi Key Laboratory of Green Catalysis and Synthesis Technology, Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education & Xinjiang Uygur Autonomous Region, State Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources, College of Chemistry, Xinjiang University, Urumqi 830017, P. R. China. E-mail: pxylcj@126.com
cHainan Institute of East China Normal University, State Key Laboratory of Petroleum Molecular & Process Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Process, School of Chemistry and Molecular Engineering, East China Normal University, 3663 North Zhongshan Road, Shanghai 200062, P. R. China. E-mail: xfjiang@chem.ecnu.edu.cn
dKey Laboratory of Specialty Agri-Product Quality and Hazard controlling Technology of Zhejiang Province, College of Life Sciences, China Jiliang University, Hangzhou 310018, P. R. China. E-mail: wwjin0722@cjlu.edu.cn
First published on 27th November 2025
Abstract
The first electrochemical reduction of sulfoxides to thioethers has been achieved using a recyclable ionic liquid as both catalyst and electrolyte, without metal catalysts, stoichiometric redox reagents, or external electrolytes. The ionic liquid showed no significant loss of catalytic activity over six cycles. Various sulfoxides (32 examples) were efficiently reduced to the corresponding thioethers in up to 98% yield using hydrosilanes as the activating agent. Notably, the protocol exhibits robust scalability under optimized conditions.
Green foundation1. We present a paired electrolysis strategy that efficiently reduces sulfoxides to thioethers at room temperature. This approach employs a recyclable ionic liquid as both catalyst and electrolyte, in combination with hydrosilanes as the activating agents.2. This protocol requires no additional metal catalysts, stoichiometric redox reagents, or external supporting electrolytes, fundamentally minimizing waste generation and the use of hazardous substances. Moreover, the method operates without an inert atmosphere, is simple to perform, and employs an ionic liquid catalyst that can be reused for six cycles without significant loss of activity, thereby reducing material consumption. 3. For a greener future, this electrochemical process could be adapted to a simpler and more sustainable continuous-flow regime to enhance reaction efficiency and pave the way for industrial production. |
Introduction
The thioether moiety is not only widely distributed in pharmaceuticals1 and natural products,2 but also plays vital roles in medicinal chemistry3 and materials science.4 29% of FDA-approved sulfur-bearing small-molecule drugs contain thioether functionalities5 (Fig. 1). Given the broad applications of thioethers, their synthesis has become a central topic in synthetic chemistry.6The catalytic reduction of sulfoxides to sulfides constitutes a cornerstone transformation in modern synthetic organic chemistry. However, the existing methods7 face significant limitations, including (1) energy-intensive conditions (e.g., high temperature or pressure), (2) residual metal contamination from transition-metal catalysts, which precludes pharmaceutical applications, (3) cumbersome workup procedurs, and (4) inefficient reaction outcomes (e.g., low yields or selectivity). Thus, developing a sustainable, metal-free, simple operation and efficient protocol for sulfide synthesis remains a critical challenge.
As a green chemistry paradigm, electrochemical synthesis has gained widespread application in organic transformations owing to its mild conditions and superior atom economy.8 Nevertheless, electrochemical approaches for sulfoxide reduction remain underdeveloped. To date, only two effective electrochemical deoxygenation strategies have been documented (Scheme 1a). In 2014, Waldvogel's group9 first achieved efficient electrochemical sulfoxide reduction under ambient conditions. However, this method suffered from non-green characteristics, notably requiring strongly acidic electrolytes and toxic lead electrodes. In 2021, Guo's group10 tackled the toxicity issue by employing an aluminum anode system in place of lead, achieving broad substrate compatibility. Nevertheless, this system introduced new challenges: (1) gradual degradation of the sacrificial anode, (2) the necessity for strictly anaerobic conditions, and (3) a reliance on Lewis acids and supporting electrolytes—all of which impede practical scalability.
Hydrosilanes have gained considerable attention as environmentally benign reducing agents in green organic synthesis.11 Nonetheless, their application in sulfoxide reduction still faces significant challenges: current catalytic systems typically require transition metals (e.g., Pt, Ru) or strong Lewis acids (e.g., boron compounds) as catalysts12 (Scheme 1b). These methodologies not only increase the synthetic expense but also introduce issues of metal residue.
To address these limitations, building upon our group's expertise in ionic liquid catalysis,13 we herein present an efficient and sustainable electrochemical protocol for the metal-free reduction of sulfoxides to thioethers under ambient conditions. This strategy employs a recyclable ionic liquid that serves dual functions as both electrolyte and catalyst, coupled with cost-effective graphite electrodes, and proceeds efficiently at room temperature in air. As a result of these features, our approach represents an environmentally benign and practically attractive alternative for thioether synthesis.
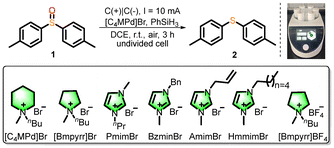
With the assistance of a six-position parallel current/voltage/electrode-regulated electrochemical reactor, optimization experiments were conducted with 4,4′-dimethylphenyl sulfoxide (1) as the model substrate. Optimal conditions were identified as follows: graphite as anode and cathode, [C4MPd]Br as both a catalyst and an electrolyte, phenylsilane as the activating agent, and DCE as solvent, under constant current (10 mA, 3 h) with a yield of 98% (Table 1, entry 1). Bromide-based ionic liquids with piperidinium or pyrrolidinium cations demonstrated higher activity than those with imidazolium cations (entries 2–6). No reaction occurred when [Bmpyrr]BF4 was used instead of [C4MPd]Br (entry 7). Silanes screening revealed an inverse relationship between phenyl substitution degree and yield (entries 8–9). Suboptimal results were obtained with alternative cathodes (Ni, stainless steel, etc., entries 10 and 11, Table S2-1, entries 15–21) or solvents (DCM, MeCN, entries 12 and 13). Neither current intensity nor reaction time variations improved yields (Table S2-1, entries 28–37). Control experiments confirmed the necessity of both constant current and ionic liquid (entries 14 and 15).
| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: graphite plate as anode and cathode, constant current = 10 mA, 1 (0.3 mmol), [C4MPd]Br (0.6 eq.), PhSiH3 (2.0 eq.), DCE (5.0 mL), room temperature, 3 h, undivided cell, under air. b Yield of isolated product. n.d. = not detected. SS = stainless steel. | ||
| 1 | None | 98 |
| 2 | [Bmpyrr]Br instead of [C4MPd]Br | 90 |
| 3 | PmimBr instead of [C4MPd]Br | 30 |
| 4 | BzmimBr instead of [C4MPd]Br | 35 |
| 5 | AmimBr instead of [C4MPd]Br | 50 |
| 6 | HmmimBr instead of [C4MPd]Br | 47 |
| 7 | [Bmpyrr]BF4 instead of [C4MPd]Br | n.d. |
| 8 | Ph2SiH2 instead of PhSiH3 | 42 |
| 9 | Ph3SiH instead of PhSiH3 | Trace |
| 10 | C/Ni instead of C/C | 70 |
| 11 | C/SS instead of C/C | 62 |
| 12 | DCM instead of DCE | 70 |
| 13 | MeCN instead of DCE | 52 |
| 14 | without constant current | n.d. |
| 15 | without [C4MPd]Br | n.d. |
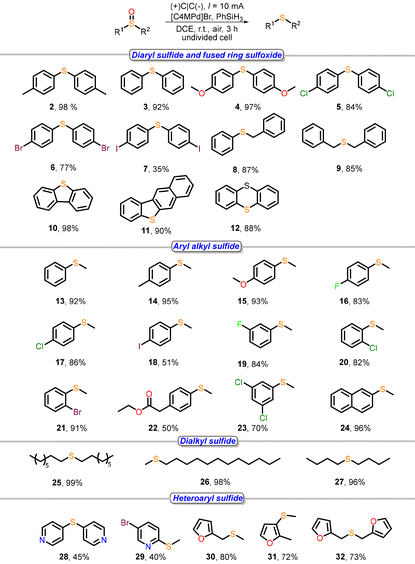
With the optimized conditions in hand, we evaluated the reaction scope using various sulfoxide substrates (Table 2). Unsubstituted diphenyl sulfoxide exhibited excellent reactivity, affording the desired product 3 in 92% yield. The electronic effects of substituents on the phenyl rings were then investigated. Both electron-donating groups (methyl, methoxy) and electron-withdrawing groups (Cl, Br) were well tolerated, delivering products 2–6 in high yields (77–98%). However, 4,4′-diiodophenyl sulfoxide showed low reactivity, yielding 7 with only 35%. Notably, the system demonstrated excellent compatibility with benzyl-substituted sulfoxides (benzyl phenyl sulfoxide and dibenzyl sulfoxide), providing products 8 and 9 in 87% and 85% yields, respectively. Fused-ring sulfoxides exhibited exceptional reactivity, furnishing products 10–12 with 88–98% yields.
| a Reaction conditions: graphite plate as anode and cathode, constant current = 10 mA, sulfoxides (0.3 mmol), [C4MPd]Br (0.6 eq.), PhSiH3 (2.0 eq.), DCE (5.0 mL), room temperature, 3 h, undivided cell, under air, yield of isolated product. |
|---|
|
We next explored the applicability of methyl aryl sulfoxides. The electronic nature of the para-substituents significantly influenced reactivity: substrates bearing I or (ethoxycarbonyl)methyl group gave lower yields (18, 51%; 22, 50%), while those with electron-donating (methyl, methoxy) or moderately electron-withdrawing halides (F, Cl, Br) performed well (50–95% yields, 13–23). Remarkably, 2-(methylsulfinyl)naphthalene was exceptionally reactive, yielding 24 in 96%.
Finally, we examined aliphatic and heterocyclic sulfoxides. Dialkyl sulfoxides demonstrated outstanding reactivity, with di-n-butyl, di-n-hexyl, and dodecyl methyl sulfoxide being reduced to the corresponding thioethers (25–27) with 93–96% yields. Heterocycle-containing sulfoxides (pyridyl, furyl) also proved viable, affording products 28–32 in moderate to good yields.
To evaluate the scalability of this method, a gram-scale constant-current electrolysis (30 mA, 9 h, rt) of 4,4′-dimethylphenyl sulfoxide 1 (8 mmol) was conducted, affording the desired product 2 in 96% yield (1.65 g), which demonstrates its potential for practical synthesis (Scheme 2a). It is noteworthy that the ionic liquid [C4MPy]Br could be conveniently recovered and reused. Upon completion of the reaction, the mixture was partitioned between water and ethyl acetate, allowing [C4MPy]Br to be isolated from the aqueous phase. The ionic liquid was subsequently dried under vacuum and successfully employed in six consecutive reaction cycles with no significant decrease in activity (Scheme 2b).
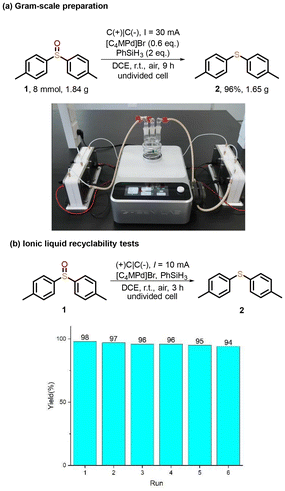 | ||
| Scheme 2 (a) Gram-scale electrochemistry synthesis of di-p-tolylsulfane (b) lonic liquid recyclability tests. | ||
To verify the potential involvement of radical intermediates during the reaction process, radical trapping experiments were conducted (Table 3). The results demonstrated a significant decrease in reaction yields with increasing amounts of the radical scavenger TEMPO. Furthermore, silicon-centered radical intermediate was successfully detected by high-resolution mass spectrometry (HRMS).
To elucidate the reaction mechanism, cyclic voltammetry (CV) experiments were first conducted (Fig. 2). The black curve represents the system containing only compound 1. Under standard conditions, the green curve exhibits an oxidation peak at 0.64 V with a current of 76.7 μA. When [C4MPd]Br was replaced by TBAClO4, the cyclic voltammogram (red curve) exhibited no discernible oxidation peak, implying that [C4MPd]Br played a catalytic role in the reaction. The blue curve, recorded in the absence of phenylsilane, exhibited no oxidation peak, further supporting the essential roles of both [C4MPd]Br and phenylsilane in the reaction. Moreover, the green curve exhibited a well-defined reduction peak at −0.39 V (current: −31.8 μA), which is indicative of electron transfer at the cathode.
High-resolution mass spectrometry (HRMS) analysis unambiguously confirmed the formation of silanol compounds under standard reaction conditions (Scheme 3a). To gain deeper mechanistic insights, in situ FTIR spectroscopy was employed to monitor the reaction in real time (Scheme 3b). The IR spectral data revealed two key observations: (1) the characteristic S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching absorption at 920 cm−1 exhibited a progressive decrease in intensity with reaction time; (2) a new absorption band emerged at 1130 cm−1, which was unequivocally assigned to the Si–O stretching vibration and showed gradual intensification as the reaction proceeded. These experimental results provide definitive evidence that the oxygen atom from sulfoxides is eliminated in the form of silanol, offering crucial support for the proposed reaction mechanism.
O stretching absorption at 920 cm−1 exhibited a progressive decrease in intensity with reaction time; (2) a new absorption band emerged at 1130 cm−1, which was unequivocally assigned to the Si–O stretching vibration and showed gradual intensification as the reaction proceeded. These experimental results provide definitive evidence that the oxygen atom from sulfoxides is eliminated in the form of silanol, offering crucial support for the proposed reaction mechanism.
Based on mechanistic studies and literature precedent, a plausible pathway for the electrocatalytic deoxygenative reduction of sulfoxides to sulfides is proposed (Scheme 4). The mechanism initiates with single-electron oxidation of bromide anions at the anode to generate bromine radical.14 The radical subsequently abstract a hydrogen atom from the hydrosilane via hydrogen atom transfer (HAT), affording silyl radical15 along with regeneration of bromide ion and proton. The silyl radical is then oxidized at the anode to form silyl cation,16 which engage in nucleophilic attack with the sulfoxide to form intermediate A. Cathodic reduction of A yields the sulfide product and releases a siloxide anion. This anion is subsequently protonated to form silanol.
Conclusions
In summary, we developed a green and efficient protocol for S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond cleavage, achieving the first electrochemical transformation of sulfoxides to thioethers using recyclable ionic liquids as electrolyte and catalyst, phenylsilane as the activating reagent. This metal-free, redox-reagent-free strategy requires no additional supporting electrolyte and demonstrates remarkable advantages: (1) operational simplicity (no inert atmosphere protection required), (2) excellent substrate scope (32 examples, up to 98% yield) and (3) outstanding scalability (successful gram-scale synthesis).
O bond cleavage, achieving the first electrochemical transformation of sulfoxides to thioethers using recyclable ionic liquids as electrolyte and catalyst, phenylsilane as the activating reagent. This metal-free, redox-reagent-free strategy requires no additional supporting electrolyte and demonstrates remarkable advantages: (1) operational simplicity (no inert atmosphere protection required), (2) excellent substrate scope (32 examples, up to 98% yield) and (3) outstanding scalability (successful gram-scale synthesis).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc05274b.Acknowledgements
The authors acknowledge the financial support from the Tianshan Talents Program for Leading Talents in Science and Technology Innovation (no. 2022TSYCLJ0016), the National Natural Science Foundation of China (no. 22361044 and 22201241), the Key Program of the Natural Science Foundation of Xinjiang Uygur Autonomous Region (no. 2022D01D06), and the Tianchi Talents Introduction Program (no. 5105240151a).References
- (a) M. Feng, B. Tang, H. S. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed; (b) C. T. B. Ferro, B. F. D. Santos, C. D. G. D. Silva, G. Brand and N. L. d. C. Domingues, Curr. Org. Synth., 2020, 17, 192–210 CrossRef CAS PubMed; (c) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2014, 57, 2832–2842 CrossRef CAS PubMed.
- (a) C. S. Kim, J. Oh, L. Subedi, S. Y. Kim, S. U. Choi and K. R. Lee, J. Nat. Prod., 2018, 81, 2129–2133 CrossRef CAS PubMed; (b) O. Goethe, A. Heuer, X. Ma, Z. Wang and S. B. Herzon, Nat. Prod. Rep., 2019, 36, 220–247 RSC; (c) N. Wang, P. Saidhareddy and X. Jiang, Nat. Prod. Rep., 2020, 37, 246–275 RSC.
- A. S. Surur, L. Schulig and A. Link, Arch. Pharm., 2019, 352, 1800248 Search PubMed.
- X. Li, W. Ma, H. Li, Q. Zhang and H. Liu, Coord. Chem. Rev., 2020, 408, 213191 CrossRef CAS.
- K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed.
- (a) T. B. Nguyen, Adv. Synth. Catal., 2017, 359, 1066–1130 CrossRef CAS; (b) H.-T. Gao, P.-L. Ou, H. Ma, H. Zhang, L.-M. Zhang, Y.-L. Xu, Y.-Z. Guo, D.-B. Hao, C.-L. Chen, D.-S. Li, S.-F. Yuan and T. Wu, Green Chem., 2025, 27, 9768–9776 RSC; (c) X.-R. Li, R.-J. Zhang, Y. Xiao, Q.-X. Tong and J.-J. Zhong, Org. Chem. Front., 2024, 11, 646–653 RSC; (d) W. Zhang, M. Huang, Z. Zou, Z. Wu, S. Ni, L. Kong, Y. Zheng, Y. Wang and Y. Pan, Chem. Sci., 2021, 12, 2509–2514 RSC; (e) Y.-F. Wei, W.-C. Gao, H.-H. Chang and X. Jiang, Org. Chem. Front., 2022, 9, 6684–6707 RSC.
- (a) H. Ishikawa, S. Yamaguchi, A. Nakata, K. Nakajima, S. Yamazoe, J. Yamasaki, T. Mizugaki and T. Mitsudome, JACS Au, 2022, 2, 419–427 CrossRef CAS PubMed; (b) Y. Kuwahara, Y. Yoshimura, K. Haematsu and H. Yamashita, J. Am. Chem. Soc., 2018, 140, 9203–9210 CrossRef CAS PubMed; (c) T. Mitsudome, Y. Takahashi, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2014, 53, 8348–8351 CrossRef CAS PubMed; (d) A. S. Touchy, S. M. A. Hakim Siddiki, W. Onodera, K. Kon and K.-i. Shimizu, Green Chem., 2016, 18, 2554–2560 RSC; (e) K. Yao, Z. Yuan, S. Jin, Q. Chi, B. Liu, R. Huang and Z. Zhang, Green Chem., 2020, 22, 39–43 RSC; (f) F.-F. Xu, Z.-L. Ruan and P.-Q. Huang, Org. Chem. Front., 2024, 11, 2448–2456 RSC; (g) X. Gao, F. Chen, M. Y. Jin and C. Xu, Org. Biomol. Chem., 2024, 22, 3215–3219 RSC.
- (a) Y. Zhang, Z.-L. Zhou, J.-H. Li and Y.-T. Li, Chem. Rec., 2025, 25, e202400263 CrossRef CAS PubMed; (b) R. Zhang, M. Liu, Z. Zhao and Y. Qiu, Green Chem., 2025, 27, 1658–1666 RSC; (c) W. Zeng, Y. Wang, C. Peng and Y. Qiu, Chem. Soc. Rev., 2025, 54, 4468–4501 RSC; (d) C. Liu, R. Zhang, Y. Liu and Y. Zhang, Chin. J. Chem., 2025, 43, 3302–3328 CrossRef CAS; (e) Z. Wang, R. Shi, J. Zhang, L.-B. Zhang, L. Wen and W. Guo, Chin. J. Chem., 2025, 43, 2213–2218 CrossRef CAS; (f) H. Long, T.-S. Chen, J. Song, S. Zhu and H.-C. Xu, Nat. Commun., 2022, 13, 3945 CrossRef CAS PubMed; (g) J. C. Siu, N. Fu and S. Lin, Acc. Chem. Res., 2020, 53, 547–560 CrossRef CAS PubMed; (h) P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed; (i) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed; (j) D. Yang, Z. Guan, Y. Peng, S. Zhu, P. Wang, Z. Huang, H. Alhumade, D. Gu, H. Yi and A. Lei, Nat. Commun., 2023, 14, 1476 CrossRef CAS PubMed; (k) Y. Yuan and A. Lei, Acc. Chem. Res., 2019, 52, 3309–3324 CrossRef CAS PubMed; (l) Z.-Y. Fan, Y. Zhu, L. Tang, J.-Y. He and C. Zhu, Green Synth. Catal., 2025 DOI:10.1016/j.gresc.2025.06.014.
- C. Edinger and S. R. Waldvogel, Eur. J. Org. Chem., 2014, 5144–5148 CrossRef CAS.
- Z. Kong, C. Pan, M. Li, L. Wen and W. Guo, Green Chem., 2021, 23, 2773–2777 RSC.
- H. Yang, Z. Zhou, C. Tang and F. Chen, Chin. Chem. Lett., 2024, 35, 109257 CrossRef CAS.
- (a) F. Takahashi, K. Nogi and H. Yorimitsu, Eur. J. Org. Chem., 2020, 3009–3012 CrossRef CAS; (b) S. C. A. Sousa, C. J. Carrasco, M. F. Pinto and B. Royo, ChemCatChem, 2019, 11, 3839–3843 CrossRef CAS; (c) S. Enthaler and M. Weidauer, Catal. Lett., 2011, 141, 833–838 CrossRef CAS; (d) J. M. S. Cardoso and B. Royo, Chem. Commun., 2012, 48, 4944–4946 RSC; (e) N. Sakai, R. Shimada and Y. Ogiwara, Asian J. Org. Chem., 2021, 10, 845–850 CrossRef CAS.
- (a) H. Li, C. Liu, Y. Zhang, Y. Sun, B. Wang and W. Liu, Org. Lett., 2015, 17, 932–935 CrossRef CAS PubMed; (b) D. Cao, Y. Zhang, C. Liu, B. Wang, Y. Sun, A. Abdukadera, H. Hu and Q. Liu, Org. Lett., 2016, 18, 2000–2003 CrossRef CAS PubMed; (c) X. Dong, R. Wang, W. Jin and C. Liu, Org. Lett., 2020, 22, 3062–3066 CrossRef CAS PubMed; (d) R. Wang, N. Zhang, Y. Zhang, B. Wang, Y. Xia, K. Sun, W. Jin, X. Li and C. Liu, Green Chem., 2023, 25, 3925–3930 RSC; (e) Z. Wei, Z. Chen, F. Xue, Y. Yue, S. Wu, Y. Zhang, B. Wang, Y. Xia, W. Jin and C. Liu, Green Chem., 2024, 26, 10189–10195 RSC.
- H.-T. Tang, J.-S. Jia and Y.-M. Pan, Org. Biomol. Chem., 2020, 18, 5315–5333 RSC.
- Z. Fu, F. Xiao, J. Yin, F. Tong, S. Guo and H. Cai, Green Chem., 2024, 26, 5838–5844 RSC.
- H. Liang, L.-J. Wang, Y.-X. Ji, H. Wang and B. Zhang, Angew. Chem., Int. Ed., 2021, 60, 1839–1844 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |