An electrochemically promoted modular synthesis of diverse glycomimetics via acyloxyphosphonium ions
Yi-Min
Jiang†
,
Cheng-Lin
Ding†
,
Guizhen
Zhang
,
Hongbao
Sun
* and
Jie
Liu
 *
*
State Key Laboratory of Biotherapy and Cancer Center, Department of Radiology, Huaxi MR Research Center (HMRRC), Institution of Radiology and Medical Imaging, Psychoradiology Key Laboratory of Sichuan Province, West China Hospital, Sichuan University, Chengdu 610041, China. E-mail: hbsun7@sina.com; liujie2011@scu.edu.cn
First published on 14th November 2025
Abstract
The installation of sugar residues can significantly enhance the hydrophilicity, pharmacological activity, and bioavailability of natural products and bioactive compounds. In this study, we developed an electrochemical strategy for the efficient conjugation of sugar units to target molecules, enabling the synthesis of glycomimetics (C-acyl and C-heteroaryl glycosides). This approach leverages the anodic oxidation of iodide anions to mediate the oxidation of triphenylphosphine (Ph3P), generating electrophilic acyloxyphosphonium ions in situ. This mechanism facilitates direct coupling between nucleophilic molecules (N-, S-, O-, C-) and glycosyl carboxylic acids without the need for additional oxidants, metal catalysts, or condensing agents. Under optimized conditions, the reaction demonstrates broad applicability across various glycosyl carboxylic acids and structurally complex target molecules, exhibiting excellent functional group compatibility with yields of up to 99%. Furthermore, this methodology has been successfully applied to incorporate sugar motifs into diverse bioactive scaffolds, including drug molecules and natural products, as well as to construct sugar-containing linkers for bioconjugation, polysaccharide amide fragments, and pharmacologically relevant glycosyl oxazoles. These results highlight the method's considerable potential in the development of bioactive molecules.
Green foundation1. This study introduces an electrochemical glycosylation method applicable to a broad range of molecules. Conducted under mild conditions at room temperature using recyclable acetonitrile, this approach overcomes the compatibility limitations between glycosyl donors and target molecules typical of conventional methods, facilitating greener and more sustainable glycosylation processes.2. This eco-friendly method operates at room temperature without chemical oxidants or metal catalysts, achieving yields up to 99% with broad complex molecular substrate compatibility. Its successful application with respect to bioactive molecules and gram-scale synthesis highlights its potential as a versatile and sustainable glycosylation strategy. 3. Future work could focus on using aqueous or biobased solvents, recycling the Ph3P/nBu4NI catalyst, and extending the method to biological applications such as the introduction of sugar moieties into bioactive macromolecules like proteins. Reducing electrolyte waste would also improve sustainability. |
Introduction
The installation of sugar residues is considered an effective structural modification method for various natural products and bioactive compounds, enhancing their hydrophilicity and thereby improving their pharmacological activity and bioavailability.1–3 Over recent decades, significant efforts have been made to develop glycosylation methodologies, driven by the potential applications of sugar-containing structures in medicinal chemistry and chemical biology.4–8 The key reaction involves the formation of glycosidic bonds, which connect sugar moieties to aglycones to produce C-,9,10N-,11,12O-,13,14 and S-glycosides.15,16 Each type exhibits a distinct linkage pattern, imparting unique chemical and biological properties to these compounds.However, these methodologies still cannot fully meet the demand for conjugation of sugar units to a broader range of target molecules. As part of a diversity-oriented synthesis project, there is a growing demand for versatile and efficient methods for the conjugation of sugar units to target molecules. Developing new glycosyl donors and corresponding methodologies represents an effective approach to achieve this goal. In 2023, the Niu research group reported on the glycosidation of pyridine structures using glycosyl sulfinates as a precursor and the glycosidation of carboxyl structures using glycosyl allyl sulfones as a precursor.17,18 The Lei group and Zhu group respectively reported glycal boronates as glycosyl donors for glycosylation of C–H bonds and Suzuki–Miyaura cross-couplings, synthesizing C1-glycals.19,20 These methods enable the introduction of sugar motifs into bioactive and pharmaceutical molecules under mild conditions (Scheme 1A). Inspired by these advances, we envisioned that sugars containing carboxyl groups may be good sugar donors, and their carboxyl groups can serve as functionalized handles for synthesizing functional compounds containing sugar structures, which is beneficial for the development of methodologies for installing sugar structures.
Glycosyl carboxylic acids, particularly uronic acids, are naturally abundant metabolites of sugars.21–24 Currently, decarboxylative radical processes for non-classical glycosylation to construct sugar-containing compounds are widely reported,16,25–28 while methods utilizing carboxylic acid groups to form non-glycosidic linkages remain limited. Employing the carboxylic acid group of glycosyl carboxylic acids to form ester and amide linkages with alcohols and amines serves as the most direct and effective glycoconjugation strategy. Additionally, glycosyl carboxylic acid derivatives (such as uronamides) exhibit various biological activities and can be used as antibiotics, anticancer agents, and cholinesterase (ChE) inhibitors, among others.29–36 Glucopyranosyl nucleic amides (GNAs) are oligomers composed of aminouronic acids (also termed sugar amino acids, SAAs), which demonstrate sequence-specific binding affinity and selectivity that may exceed that of natural DNA and RNA.37 Moreover, a prodrug activator SQL70 targeting tumors also involves glycosamide fragments in its structure, introducing key tetrazine structures through amide bonds.38 The above research results demonstrate the cruciality of the structure of glycosyl carboxylic acid derivatives. If this type of reaction is developed into a modular reaction, it will be possible to quickly expand the space of sugar-containing structures and construct a diverse molecular compound library of sugar-containing structures for bioactivity screening (Scheme 1B).39,40 However, current methods for forming amides and esters face significant challenges: expensive condensation reagents, harsh reaction conditions, uncontrollable side reactions, and narrow substrate scope, especially poor compatibility with sugar-containing substrates.36 Consequently, developing greener and more efficient methodologies with enhanced compatibility for carbohydrate substrates is critically important.
Trivalent phosphines are widely employed in deoxygenative substitution of hydroxyl and carboxyl groups. The reaction proceeds via formation of the reactive acyloxyphosphonium or alkoxyphosphonium intermediates. Subsequently, the nucleophilic substitution proceeds with displacement of phosphine oxide as the driving force.41–45 However, these reactions typically require stoichiometric oxidants or reductants to achieve phosphorus valence changes and pose significant purification challenges, resulting in reagent waste and diminished atom economy. Moreover, the use of oxidants causes compatibility issues with sugar structures, limiting this strategy's application in glycoconjugation (Scheme 1C).46–50 Therefore, a set of conditions that can avoid the use of stoichiometric amounts of activation reagents while maintaining broad substrate scope is highly desired. Electrochemical synthesis technology, which facilitates redox reactions through electron transfer at electrode surfaces, is recognized as an innovative solution to replace traditional stoichiometric redox reagents.51–56 The electrosynthesis utilizing anodic oxidation of trivalent phosphine to generate phosphonium ions may effectively circumvent the use of oxidants. Furthermore, by precisely regulating the electrode potential within a broader redox potential window, the electrochemical approach enables flexible modulation of reaction parameters, making it advantageous for application to a wider range of substrates compared to oxidant-dependent reactions. Given the great potential of synthetic electrochemistry in the exploration of novel redox reactivity, we envisage the preparation of multiple glycomimetics57via in situ generation of acyloxyphosphonium ions from triphenylphosphine by electrolysis and further explore the compatibility of the reaction with different types of nucleophiles, as well as drugs and natural products with complex structures (Scheme 1D). Remarkably, this method efficiently generates glycomimetic (C-acyl and C-heteroaryl glycoside) compounds while attaching sugar motifs to target molecules, serving as a valuable complement to existing C-glycoside synthesis. Furthermore, glycosyl carboxylic acid substrates are readily available, and the reaction avoids stereoselectivity control issues, which also provides the advantage of producing stereoretentive glycomimetics (C-acyl and C-heteroaryl glycosides).
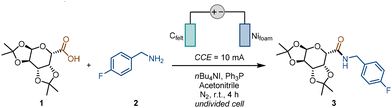
The feasibility of the new electrosynthesis method for C-acyl glycoside compounds was investigated. With 1,2:3,4-di-O-isopropylidene-α-D-galacturonic acid (1) and 4-fluorbenzylamine (2) as model substrates in acetonitrile, using a carbon felt anode, and a nickel foam cathode in an undivided cell under constant-current electrolysis (CCE = 10 mA, t = 4 h) at room temperature, the target uronamide (3) was successfully isolated in 91% yield (Table 1, entry 1). It was found that the choice of anode was crucial because the replacement of carbon felt by either a carbon rod electrode or a carbon cloth electrode led to significant decreases in yield (Table 1, entry 2). Different cathodes had little influence on reaction yield. Whether platinum or nickel was used as the cathode, the reaction was achieved with an excellent yield (for both, yields >99% were detected) (Table 1, entry 3). In contrast, nickel foam was chosen due to its low cost and ease of trimming. Both dichloromethane (DCM) and N,N-dimethylformamide (DMF) were explored, and the yields were similar to those obtained with acetonitrile, which was prioritized as a greener solvent due to its lower environmental impact and ease of recycling (Table 1, entry 4). The presence of trivalent phosphines was essential, as no reaction occurred in the absence of Ph3P (Table 1, entry 5), and the significance of an acyloxyphosphonium ion originating from trivalent phosphine was demonstrated. When P(OCy)3 or P(OMe)3 was applied, the yield decreased slightly (Table 1, entry 6). Nevertheless, triphenylphosphine (Ph3P) would be more suitable because Ph3P, as a stable solid, is inexpensive and easier to handle, and the reduction of triphenylphosphine oxide (Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) to Ph3P proceeds more efficiently than that of alkyl phosphines.58–66 The following experiment shows that, tetra-n-butylammonium iodide (nBu4NI) serves as both an electrolyte and a mediator in this reaction. When nBu4NCl and nBu4NBr were employed, the desired product was obtained in similar yields. However, the in situ generated iodine species exhibited milder oxidation ability than the high-valence Cl and Br species (Table 1, entry 7). In contrast, when nBu4NClO4 was used as the electrolyte, a lower yield was observed, attributed to the influence of overpotentials under direct electrolysis (Table 1, entry 8).56,67–69 Therefore, nBu4NI proved to be the superior choice, offering greater compatibility with multiple functional groups and complex structures. Additionally, experiments were also conducted under standard conditions without drying. It was found that the reaction still proceeded in the presence of water. However, water affected the efficiency of the nucleophilic reaction, resulting in a decrease in conversion rate and consequently a reduction in yield (Table 1, entry 9). The control experiment confirmed that no reaction occurred without passing electricity (Table 1, entry 10). Furthermore, we also attempted to compare electrochemical reaction conditions with the typical TBTU/DCC/Mitsunobu condensation reaction conditions (see the SI for details).
O) to Ph3P proceeds more efficiently than that of alkyl phosphines.58–66 The following experiment shows that, tetra-n-butylammonium iodide (nBu4NI) serves as both an electrolyte and a mediator in this reaction. When nBu4NCl and nBu4NBr were employed, the desired product was obtained in similar yields. However, the in situ generated iodine species exhibited milder oxidation ability than the high-valence Cl and Br species (Table 1, entry 7). In contrast, when nBu4NClO4 was used as the electrolyte, a lower yield was observed, attributed to the influence of overpotentials under direct electrolysis (Table 1, entry 8).56,67–69 Therefore, nBu4NI proved to be the superior choice, offering greater compatibility with multiple functional groups and complex structures. Additionally, experiments were also conducted under standard conditions without drying. It was found that the reaction still proceeded in the presence of water. However, water affected the efficiency of the nucleophilic reaction, resulting in a decrease in conversion rate and consequently a reduction in yield (Table 1, entry 9). The control experiment confirmed that no reaction occurred without passing electricity (Table 1, entry 10). Furthermore, we also attempted to compare electrochemical reaction conditions with the typical TBTU/DCC/Mitsunobu condensation reaction conditions (see the SI for details).
| Entry | Deviation from standard reaction conditions | Yield (%) |
|---|---|---|
| a Undivided cell, graphite felt anode, Ni foam cathode, 1 (0.4 mmol), 2 (0.8 mmol), nBu4NI (0.4 mmol), and triphenylphosphine (0.8 mmol) in MeCN (8.0 mL) at room temperature under nitrogen, constant current = 10 mA, 4 h, Q = 3.7 F mol−1, faradaic efficiency of 3 is 49%, current efficiency of the electrolysis reaction is 85.8%. Yields were determined by 1H NMR with 1,3,5-trimethoxylbenzene as the internal standard. b Isolated yields. n.d. = not detected. | ||
| 1 | None | >99 (91b) |
| 2 | C rod, C cloth as the anode | 78, 82 |
| 3 | Pt, Ni as the cathode | >99, >99 |
| 4 | DCM, DMF as the solvent | 97, 93 |
| 5 | Without Ph3P | n.d. |
| 6 | P(Cy)3, P(OMe)3 instead of Ph3P | 96, 95 |
| 7 | nBu4NBr, nBu4NCl as the electrolyte | 98, 95 |
| 8 | nBu4NClO4 as the electrolyte | 88 |
| 9 | Without drying | 68 |
| 10 | No current | n.d. |
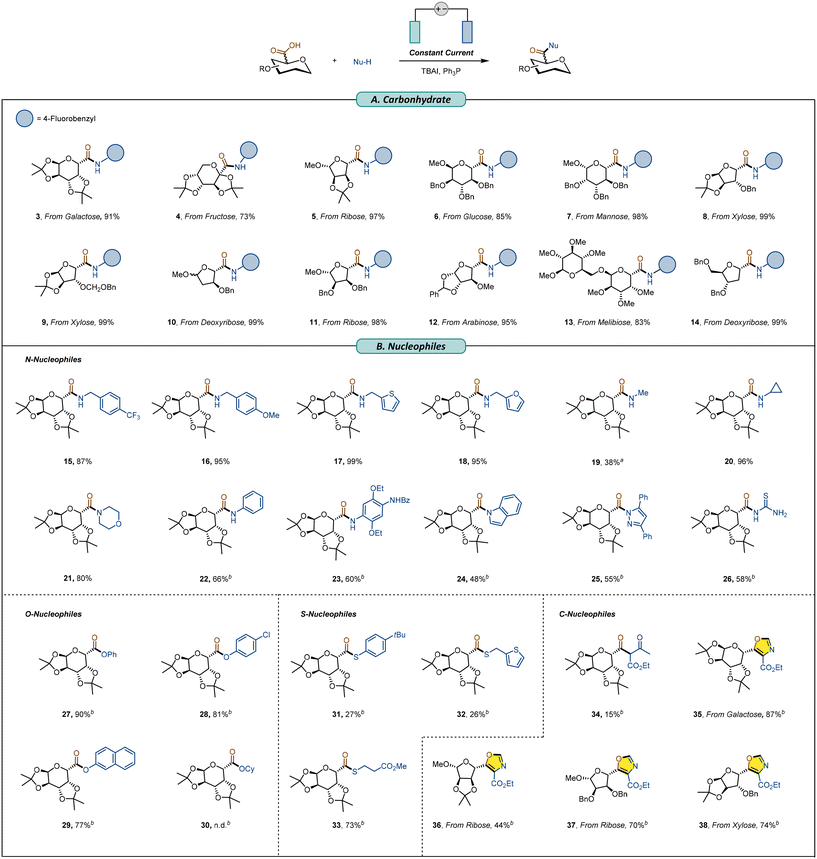
With the optimal reaction conditions determined, the substrate scope of this electrochemical synthesis of glycomimetic compounds was explored (Scheme 2). Various glycosyl carboxylic acids derived from various monosaccharides, including fructose (4, 73% yield), ribose (5 and 11, 97% and 98% yields, respectively), glucose (6, 85% yield), mannose (7, 98% yield), xylose (8 and9, 99% yield each), deoxyribose (10, 99% yield), and arabinose (12, 95% yield) were coupled to afford the corresponding amides in excellent yields. Glycosyl carboxylic acids prepared from disaccharides such as melibiose (13, 99% yield) were compatible with the reaction system. Additionally, C1-carboxy deoxyriboside was also highly stoichiometrically transformed into the corresponding amide (14). Attempts were also made with unprotected uronic acid, but no product formation was detected. The scope of amines was examined as well. Diverse amines were all competently employed under these reaction conditions (15–27). Aliphatic amines, including primary and secondary amines, were successfully converted to uronamides (15–21) in moderate to excellent yields, and amines containing the structure of thiophene (17) and furan (18) were compatible with the reaction system. Aromatic amines can also furnish the target products in good yields (22, 23), but because of their weak nucleophilicity,11 it is necessary to add a base to assist in the deprotonation of acids and nucleophiles, and thus promote the progression of nucleophilic reactions, thereby improving the reaction efficiency (see Table S2 for details of the investigation on the necessity of the base). Moreover, under these reaction conditions, nitrogen-containing heterocyclic compounds such as indole (24) and pyrazole (25), which are difficult to directly condense with carboxylic acids owing to their low nucleophilicity,70 successfully gave the desired products in moderate yields. Furthermore, thiourea (26) was employed, and the target product was isolated in 58% yield.
To further demonstrate the generality of this reaction, we utilized various non-amine nucleophiles, which successfully underwent the process to give the corresponding glycol-mimetics (Scheme 2B). Phenols gave the corresponding glycosyl carboxylic acid esters (27–29) in good to excellent yields. In contrast, aliphatic alcohols were incompatible and failed to transform into corresponding esters, due to possible competition between the hydroxyl group in the aliphatic alcohol and the carboxyl substrate during activation. When thiols and thiophenols were used as nucleophiles, the reaction proceeded smoothly, yielding the target glycosyl carboxylic acid thioester products (31–33) in 26%–73% yields. In addition to heteroatom nucleophiles, β-dicarbonyl compounds were employed as nucleophiles and the desired C-acyl glycoside (34) was obtained in a yield of 15%. Interestingly, isocyanoacetate esters proved compatible with C-nucleophilic reaction systems. Subsequent cycloaddition enabled the synthesis of glycosyl oxazoles, which are C-heteroaryl glycosides, successfully introducing the sugar units to the oxazole ring structure (for optimization of conditions for the synthesis of glycosyl oxazoles, see SI Table S1 for details). Using this method, various glycosyl oxazole derivatives were successfully obtained with isolated yields of 44%–87%, including galacturonic acid (35), riburonic acid (36, 37), and xyluronic acid (38).
We next directed our investigation to applications in glycoconjugation (Scheme 3). In drug research and development, synthesizing drugs with carbohydrate fragments is essential. As hydrophilic molecules, saccharides can form hydrogen bonds with water, endowing the modified drugs with better solubility, thus, enhancing their bioavailability. Additionally, adding sugar fragments can improve drug targeting and mitigate issues like widespread distribution and severe adverse effects.1–3 To bring about glycoconjugation of bioactive functional molecules under our conditions, different kinds of complex compounds were investigated. First, various drugs and natural products with complex structures were allowed to be transformed to corresponding carbohydrate-modified products (Scheme 3A). Salicylic acid (39) with anti-inflammatory activity71 and carvacrol (40) as the natural bioactive substance extracted from Lamiaceae plants72 were all modified by sugar fragments in 58% and 75% yields, respectively. Dehydroabietylamine is a synthetic derivative of rosin, which presents a wide range of bioactivities, including anti-bacterial, anti-fungal, anti-herpetic, anti-dengue, and anti-cancer activities.73–77 Under electrolysis conditions, mannose (41) and N-dehydroabietyluronamides (42) were successfully isolated in 65% and 80% yields, respectively. Polysaccharides linked by amide bonds are considered fragments of glucopyranosyl nucleic amides (GNAs) with multiple biological activities.37 Under galvanostatic electrolysis systems, the synthesis of GNA analogues was investigated. The glucosamine (α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 1
β = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) reacted with galacturonic acid under electrolysis, and N-glycosyluronamides (43) are obtained in an equivalent conversion, with more β-configuration products being obtained (α
1) reacted with galacturonic acid under electrolysis, and N-glycosyluronamides (43) are obtained in an equivalent conversion, with more β-configuration products being obtained (α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 1
β = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7) due to the β-configuration of the glucosamine having reduced steric hindrance. Sertraline (44),78 maprotiline (45),79 and levomilnacipran (46),80 which have been used to treat depression, were all compatible under the conditions and furnished in good yields. In these products, the isomers were detected due to the restricted rotation of the tertiary amide groups. For sulfamethoxazole, an antibiotic containing an aniline structure,81 glycoconjugation can also be successfully achieved under these reaction conditions (47, 72% yield). The N-glycosyl structures of both donepezil (treatment for Alzheimer's disease)82 and linezolid (providing therapeutic effects on multiple drug-resistant bacteria)83 were synthesized in yields of 77% (48) and 86% (49), respectively.
1.7) due to the β-configuration of the glucosamine having reduced steric hindrance. Sertraline (44),78 maprotiline (45),79 and levomilnacipran (46),80 which have been used to treat depression, were all compatible under the conditions and furnished in good yields. In these products, the isomers were detected due to the restricted rotation of the tertiary amide groups. For sulfamethoxazole, an antibiotic containing an aniline structure,81 glycoconjugation can also be successfully achieved under these reaction conditions (47, 72% yield). The N-glycosyl structures of both donepezil (treatment for Alzheimer's disease)82 and linezolid (providing therapeutic effects on multiple drug-resistant bacteria)83 were synthesized in yields of 77% (48) and 86% (49), respectively.
Furthermore, since sugar-modified amino acids or peptides are regarded as post-translational modified products that play fundamental roles in diverse biological processes,84 various amino acids were employed under the electrolytic conditions (Scheme 3B). The α-amino of glycine and phenylalanine derivatives has been successfully transformed into target sugar-containing amino acids (50, 51) in 79% and 64% yields, respectively. The dipeptide was also applied in this reaction, and the desired product 52 was obtained in 23% yield. The abovementioned amino acid esters are commercially available as their hydrochloride salts and without the pre-preparation of the free amine.
Except for the α-amino group, the side chains of amino acids are invariably the sites for modification. The amino of lysine (53), the indole of tryptophan (54), the thiol of cysteine (55), and the phenol of tyrosine (56) could all be modified by sugar, and target compounds were obtained in 50%–94% yields. In addition to drug and natural product modifications, this electrosynthesis approach was applied to prepare oxazol-5-yl glycosides. Since oxazole derivatives exhibit many significant bioactivities, oxazoles are ubiquitous and have wide application potential in the chemical engineering and pharmaceutical fields.85–88C1-Heteroaryl galactosides are recognized as a class of structures used as lectin inhibitors. Their selectivity is influenced by heteroaryl and aryl substituents to give compounds selective for either galectin-1 or galectin-3. The selectivity induced by C1-heteroaryl groups is superior to lactose, and the compounds may exhibit enhanced hydrolytic stability and drug-like properties compared to natural sugars.89 Under standard conditions, our method can also rapidly synthesize different C1-heteroaryl galactoside analogues, such as galacturonic acid reacting with methyl (57), tert-butyl (58), benzyl (59), and cyclohexyl (60) iso-cyanurate, to furnish high-yield products for structure–activity relationship studies (Scheme 3C).
Based on the recognition of specific sugar structures by cell surface receptors,90 the design of sugar-targeted multi-functional molecules has broad application prospects in protein labelling and tracking,91 targeted degradation,92 and drug delivery.90,93 Therefore, we attempted to prepare different types of sugar-containing linkers, which can be applied to research in the above fields (Scheme 3D). The flexible alkyl or PEG linkers containing the amino (61), alkynyl (62), and carboxyl (63) groups were employed and successfully altered to the corresponding products in equivalent conversion. On the other hand, the tetrazine-containing compound for bioorthogonal chemistry94 was employed in the reaction system and successfully converted into corresponding glycosyl-tetrazine derivative (64) in a yield of 66%. Due to the potential applications of the sugar-based linker, the gram-scale preparation of 61 was investigated (Scheme 3E). Starting from 9.6 mmol of the glycosyl carboxylic acid (1), the desired uronamide (61) was obtained in 91% yield (3.9 g) by electrolysis with a constant current of 80 mA for 11 h, which validated the potential for large-scale production. Upon treatment of the product with hydrochloric acid, the Boc-deprotected product (61-a) was obtained in 95% yield.
To gain more insight into the mechanism, we conducted several mechanistic experiments. First, cyclic voltammetry (CV) studies were performed. Two oxidation peaks of nBu4NI were found to be 0.62 V (versus Ag/AgCl) and 0.97 V (versus Ag/AgCl). This might be attributed to the oxidation of I− to I˙, and I˙ to I+, respectively, and the reductive peak of I+ to I˙ was found to be 0.63 V (versus Ag/AgCl) (Fig. 1a, red curve). The single electron oxidation potential of PPh3 was 1.42 V (versus Ag/AgCl) (Fig. 1a, blue curve). The oxidation peak of glycosyl carboxylic acid was not found, which was considered to be difficult to oxidize. The results demonstrated a preferential electrochemical oxidation of I− over other components, implying that the anodic oxidation process initiates primarily through I− oxidation rather than PPh3 (Fig. 1a). Additionally, when the mixture of nBu4NI and Ph3P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) was employed, the complete disappearance of the reduction peak of the iodine cation (I+ to I˙) was detected, and an obvious catalytic current of I˙ to I+ was induced (Fig. 1b, purple curve).56,95 The results of CV studies indicated that the I+ generated from double oxidation of I− was captured by Ph3P rapidly to form [I–PPh3]+, the detection of which was attempted by 31P NMR (see the SI for details).
2 molar ratio) was employed, the complete disappearance of the reduction peak of the iodine cation (I+ to I˙) was detected, and an obvious catalytic current of I˙ to I+ was induced (Fig. 1b, purple curve).56,95 The results of CV studies indicated that the I+ generated from double oxidation of I− was captured by Ph3P rapidly to form [I–PPh3]+, the detection of which was attempted by 31P NMR (see the SI for details).
 | ||
| Fig. 1 Cyclic voltammetry studies. (a) Studies on substrates. (b) Studies on the mixture of Ph3P and nBu4NI. See Supplementary Information, section 5.1 for experimental details. | ||
Furthermore, radical-trapping experiments with 1,1-diphenylethylene and (1-(2-phenylcyclopropyl)vinyl) benzene (Scheme 4) were conducted under standard conditions. The radical-trapping products were not detected, and uronamide (3) was detected by QNMR, which was defined in 99% and 97% yield, respectively. The experimental results indicated that the addition of radical scavengers had no effect on the reaction. Therefore, the above experimental results suggested that a radical mechanism could be excluded from the reaction system.
The crossover experiment using two different glycosyl carboxylic acids also confirmed that the reaction mainly proceeds via a nucleophilic mechanism, and no radical pathway was observed (see the SI for details). On the other hand, we compared the conversion rates and yields of reactions involving aniline and phenol under the same electrolytic conditions, both in the presence and absence of a base. It was found that in the absence of a base, the yields of both aniline and phenol decreased, and their conversion rates also dropped significantly under the same conditions. This may be because the basicity of the amino group in aniline compensates for part of the base's function, resulting in a smaller degree of decrease compared to phenol (Table S2). From these comparisons, it can be concluded that for substrates with weaker nucleophilicity, the presence of a base can help nucleophiles deprotonate to enhance their nucleophilicity, and also assist acids in deprotonation, promoting the formation of acyloxyphosphonium ions and thus improving reaction efficiency.
Based on the aforementioned studies and related literature surveys,96–98 a plausible mechanism for the electrochemical synthesis of glycomimetics was proposed (Scheme 5). Initially, the iodine cation generated from the iodine anion via anodic oxidation on the surface of the anode97,98 was combined with PPh3 to form the [I-PPh3]+ intermediate A. Subsequently, glycosyl carboxylic acid B is attacked by A to yield active acyloxyphosphonium ion C. Finally, C is attacked by a nucleophile to produce the desired glycomimetic D.
Conclusions
In conclusion, our innovative electrochemical condensation strategy provides a highly efficient approach for synthesizing diverse sugar-based bioactive compounds. This study employs electrochemically generated iodine cations (I+) to indirectly oxidize Ph3P, achieving in situ generation of acyloxyphosphonium ions, directly driving the coupling of glycosyl carboxylic acids with nucleophiles such as amines, phenols, thiols, and β-dicarbonyl compounds without the need for oxidizing agents, metal catalysts, or condensation agents. The strategy demonstrated exceptional substrate compatibility (e.g., pentoses, hexoses, disaccharides), achieving yields generally exceeding 90%. Its scope was successfully expanded to weakly nucleophilic heterocycles and complex drug molecules, and applications in constructing sugar-based linkers, glycosyl oxazoles, and GNA amide fragments. The high yield of the gram-scale reaction proved the potential of the scalable synthesis of complex glycoconjugates. We considered that this novel approach with green chemistry features and functional compatibility emerges with broad application potential in drug solubility enhancement, targeted delivery system design, etc. Additionally, related bioactive research into our sugar-modified drugs and novel heterocyclic glycosides will also be systematically pursued in the future.Author contributions
J. Liu conceived the research idea. H. Sun and J. Liu directed the project. Y.-M. Jiang optimized the reaction conditions, and developed the reaction and extended the scope of these transformations. C.-L. Ding synthesized all substrates. Y.-M. Jiang, C.-L. Ding, and G. Zhang analyzed the data. Y.-M. Jiang wrote the manuscript with input from all the authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: materials, methods, experimental details, additional data, and NMR spectra. See DOI: https://doi.org/10.1039/d5gc04739k.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22271200), and the Sichuan Science and Technology Program (2025ZNSFSC0692, 2023NSFSC1716). We thank X. Wang from the Analytical and Testing Center, Sichuan University for assistance with NMR analysis.References
- A. Das, A. K. Nayak, B. Mohanty and S. Panda, Int. Scholarly Res. Not., 2011, 2011, 819765 Search PubMed
.
- J. Morimoto, K. Miyamoto, Y. Ichikawa, M. Uchiyama, M. Makishima, Y. Hashimoto and M. Ishikawa, Sci. Rep., 2021, 11, 12697 CrossRef CAS PubMed
.
- H. Gao and G. Huang, Bioorg. Med. Chem., 2018, 26, 5578–5581 CrossRef CAS
.
- W. Shang and D. Niu, Acc. Chem. Res., 2023, 56, 2473–2488 CrossRef CAS
.
- M. J. McKay and H. M. Nguyen, ACS Catal., 2012, 2, 1563–1595 CrossRef CAS PubMed
.
- M. Wenninger, M. L. Poulsen, M. E. Larsen and N. M. Hauser, Synthesis, 2024, 1265–1279 Search PubMed
.
- H. Wang, P. Wu, X. Zhao, J. Zeng and Q. Wan, Acta Chim. Sin., 2019, 77, 231–241 CrossRef CAS
.
- C. C. Santiago, N. B. Rengel, P. S. Fernandez and A. Ponzinibbio, Mini-Rev. Org. Chem., 2025, 22, 199–213 CrossRef CAS
.
- W. Shang, R. Shi and D. Niu, Chin. J. Chem., 2023, 41, 2217–2236 CrossRef CAS
.
- S. P. Parida, T. Das, M. A. Ahemad, T. Pati, S. Mohapatra and S. Nayak, Carbohydr. Res., 2023, 530, 108856 CrossRef CAS
.
- Q. Sun, Q. Wang, W. Qin, K. Jiang, G. He, M. J. Koh and G. Chen, Nat. Synth., 2024, 3, 623–632 CrossRef CAS
.
- C. Xu, Q. Zhang and Y. Yusupu, ChemBioChem, 2025, 26, e202400864 CrossRef CAS
.
- X. Zhao, Z. Zhang, J. Xu, N. Wang, N. Huang and H. Yao, J. Org. Chem., 2023, 88, 11735–11747 CrossRef CAS PubMed
.
- C. Zhang, H. Zuo, G. Y. Lee, Y. Zou, Q.-D. Dang, K. N. Houk and D. Niu, Nat. Chem., 2022, 14, 686–694 CrossRef CAS PubMed
.
- Y. Liu, Y. Wang, J. Chen, N. Wang, N. Huang and H. Yao, J. Org. Chem., 2024, 89, 8815–8827 CrossRef CAS
.
- Z. Le, S. He, J. Hou, M. Ye, J. Chen, G. Lv, T. Huang, Z. Yang and Y. Wu, Chem. Commun., 2023, 59, 13759–13762 RSC
.
- H. Zuo, C. Zhang, Y. Zhang and D. Niu, Angew. Chem., Int. Ed., 2023, 62, e202309887 CrossRef CAS PubMed
.
- S. Xu, W. Zhang, C. Li, Y. Li, H. Zeng, Y. Wang, Y. Zhang and D. Niu, Angew. Chem., Int. Ed., 2023, 62, e202218303 CrossRef CAS
.
- A. Chen, Y. Han, R. Wu, B. Yang, L. Zhu and F. Zhu, Nat. Commun., 2024, 15, 5228 CrossRef CAS PubMed
.
- S. Wang, K. Chen, F. Guo, W. Zhu, C. Liu, H. Dong, J.-Q. Yu and X. Lei, ACS Cent. Sci., 2023, 9, 1129–1139 CrossRef CAS PubMed
.
- T. Mehtiö, M. Toivari, M. G. Wiebe, A. Harlin, M. Penttilä and A. Koivula, Crit. Rev. Biotechnol., 2016, 36, 904–916 CrossRef
.
- A. Farrán, C. Cai, M. Sandoval, Y. Xu, J. Liu, M. J. Hernáiz and R. J. Linhardt, Chem. Rev., 2015, 115, 6811–6853 CrossRef
.
- H. Pińkowska, M. Krzywonos, P. Wolak and A. Złocińska, Green Process. Synth., 2019, 8, 683–690 Search PubMed
.
- P. N. Amaniampong, A. Karam, Q. T. Trinh, K. Xu, H. Hirao, F. Jérôme and G. Chatel, Sci. Rep., 2017, 7, 40650 CrossRef
.
- C. Zhang, D. He, Z. Ma, M. Wang, Y. Zhu, Y. Liu, J. Chen, L. Guo, G. Lv and Y. Wu, J. Org. Chem., 2024, 89, 10112–10126 CrossRef CAS
.
- C. Zhang, D. He, Z. Ma, M. Wang, Y. Zhu, Y. Liu, J. Chen, G. Lv and Y. Wu, Chem. Commun., 2024, 60, 5860–5863 RSC
.
- J. R. Romeo, J. D. Lucera, D. Jensen, L. M. Davis and C. S. Bennett, Org. Lett., 2023, 25, 3760–3765 CrossRef CAS PubMed
.
- R. Qi, C. Wang, Z. Ma, H. Wang, Q. Chen, L. Liu, D. Pan, X. Ren, R. Wang and Z. Xu, Angew. Chem., Int. Ed., 2022, 61, e202200822 CrossRef CAS
.
- N. M. Xavier, A. Porcheron, D. Batista, R. Jorda, E. Řezníčková, V. Kryštof and M. C. Oliveira, Org. Biomol. Chem., 2017, 15, 4667–4680 RSC
.
- N. M. Xavier, S. Schwarz, P. D. Vaz, R. Csuk and A. P. Rauter, Eur. J. Org. Chem., 2014, 2770–2779 CrossRef CAS
.
- N. M. Xavier, R. Goncalves-Pereira, R. Jorda, D. Hendrychová and M. C. Oliveira, Pure Appl. Chem., 2019, 91, 1085–1105 CrossRef CAS
.
- Z. Jiang, J. Sung, X. Wang, Y. Zhang, Y. Wang, H. Zhou and L. Wen, RSC Adv., 2021, 11, 31235–31259 RSC
.
- C. M. Timmers, J. J. Turner, C. M. Ward, G. A. van der Marel, M. L. C. E. Kouwijzer, P. D. J. Grootenhuis and J. H. van Boom, Chem. – Eur. J., 1997, 3, 920–929 CrossRef CAS
.
- X. Bian, X. Fan, C. Ke, Y. Luan, G. Zhao and A. Zeng, Bioorg. Med. Chem., 2013, 21, 5442–5450 CrossRef CAS PubMed
.
- S. Knapp and V. K. Gore, Org. Lett., 2000, 2, 1391–1393 CrossRef CAS PubMed
.
- Z. Hricovíniová, M. Hricovíni, V. Brezová and P. Magdolen, Tetrahedron: Asymmetry, 2016, 27, 361–368 CrossRef
.
- S. A. W. Gruner, E. Locardi, E. Lohof and H. Kessler, Chem. Rev., 2002, 102, 491–514 CrossRef CAS PubMed
.
- J. M. McFarland, M. Alečković, G. Coricor, S. Srinivasan, M. Tso, J. Lee, T.-H. Nguyen and J. M. Mejía Oneto, ACS Cent. Sci., 2023, 9, 1400–1408 CrossRef CAS
.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS
.
- J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249–1262 RSC
.
- O. Mitsunobu and M. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380–2382 CrossRef CAS
.
- R. H. Beddoe, K. G. Andrews, V. Magné, J. D. Cuthbertson, J. Saska, A. L. Shannon-Little, S. E. Shanahan, H. F. Sneddon and R. M. Denton, Science, 2019, 365, 910–914 CrossRef CAS PubMed
.
- R. Appel, Angew. Chem., Int. Ed. Engl., 1975, 14, 801–811 CrossRef
.
- J. M. Lipshultz and A. T. Radosevich, J. Am. Chem. Soc., 2021, 143, 14487–14494 CrossRef CAS
.
- K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551–2651 CrossRef CAS
.
- J. Su, J.-N. Mo, X. Chen, A. Umanzor, Z. Zhang, K. N. Houk and J. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202112668 CrossRef CAS PubMed
.
- D. Hirose, T. Taniguchi and H. Ishibashi, Angew. Chem., Int. Ed., 2013, 52, 4613–4617 CrossRef CAS
.
- D. Hirose, M. Gazvoda, J. Košmrlj and T. Taniguchi, Chem. Sci., 2016, 7, 5148–5159 RSC
.
- D. Hirose, M. Gazvoda, J. Košmrlj and T. Taniguchi, Org. Lett., 2016, 18, 4036–4039 CrossRef CAS
.
- J. A. Buonomo and C. C. Aldrich, Angew. Chem., Int. Ed., 2015, 54, 13041–13044 CrossRef CAS
.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230 CrossRef CAS PubMed
.
- K. D. Moeller, Chem. Rev., 2018, 118, 4817–4833 CrossRef CAS
.
- S. R. Waldvogel, S. Lips, M. Selt, B. Riehl and C. J. Kampf, Chem. Rev., 2018, 118, 6706 CrossRef CAS
.
- Y. Yuan and A. Lei, Nat. Commun., 2020, 11, 802 CrossRef CAS PubMed
.
- T. H. Meyer, I. Choi, C. Tian and L. Ackermann, Chem, 2020, 6, 2484–2496 CAS
.
- R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC
.
- Z. Zhou, Y. Ai, S. Xu, X. Lin, Y. Ping and W. Kong, Angew. Chem., Int. Ed., 2025, 64, e202510594 CrossRef CAS
.
- S. Nagahara, Y. Okada, Y. Kitano and K. Chiba, Chem. Sci., 2021, 12, 12911–12917 RSC
.
- D. Hérault, D. H. Nguyen, D. Nuel and G. Buono, Chem. Soc. Rev., 2015, 44, 2508–2528 RSC
.
- H. Fritzsche, U. Hasserodt and F. Korte, Chem. Ber., 1965, 98, 171–174 CrossRef CAS
.
- K. Naumann, G. Zon and K. Mislow, J. Am. Chem. Soc., 1969, 91, 7012–7023 CrossRef CAS
.
- M.-L. Schirmer, S. Jopp, J. Holz, A. Spannenberg and T. Werner, Adv. Synth. Catal., 2016, 358, 26–29 CrossRef CAS
.
- Y. Li, S. Das, S. Zhou, K. Junge and M. Beller, J. Am. Chem. Soc., 2012, 134, 9727–9732 CrossRef CAS PubMed
.
- C. Petit, E. Poli, A. Favre-Réguillon, L. Khrouz, S. Denis-Quanquin, L. Bonneviot, G. Mignani and M. Lemaire, ACS Catal., 2013, 3, 1431–1438 CrossRef CAS
.
- J. S. Elias, C. Costentin and D. G. Nocera, J. Am. Chem. Soc., 2018, 140, 13711–13718 CrossRef CAS
.
- S. Manabe, C. M. Wong and C. S. Sevov, J. Am. Chem. Soc., 2020, 142, 3024–3031 CrossRef CAS PubMed
.
- E. Steckhan, Angew. Chem., Int. Ed. Engl., 1986, 25, 683–701 CrossRef
.
-
E. Steckhan, Organic syntheses with electrochemically regenerable redox systems, 1987 Search PubMed
.
- N. O. Yurii, N. E. Michail and I. N. Gennady, Russ. Chem. Rev., 2009, 78, 89 CrossRef
.
- A. Umehara, S. Shimizu and M. Sasaki, ChemCatChem, 2023, 15, e202201596 CrossRef CAS
.
- E. M. E. Sykes, D. White, S. McLaughlin and A. Kumar, Can. J. Microbiol., 2023, 70, 1–14 CrossRef
.
- M. Imran, M. Aslam, S. A. Alsagaby, F. Saeed, I. Ahmad, M. Afzaal, M. U. Arshad, M. A. Abdelgawad, A. H. El-Ghorab, A. Khames, M. A. Shariati, A. Ahmad, M. Hussain, A. Imran and S. Islam, Food Sci. Nutr., 2022, 10, 3544–3561 CrossRef CAS PubMed
.
- S.-G. Zhang, Y.-Q. Wan and W.-H. Zhang, J. Nat. Prod., 2024, 87, 924–934 CrossRef CAS PubMed
.
- B. Goodson, A. Ehrhardt, S. Ng, J. Nuss, K. Johnson, M. Giedlin, R. Yamamoto, W. H. Moos, A. Krebber, M. Ladner, M. B. Giacona, C. Vitt and J. Winter, Antimicrob. Agents Chemother., 1999, 43, 1429–1434 CrossRef CAS PubMed
.
- V. C. Roa-Linares, Y. M. Brand, L. S. Agudelo-Gomez, V. Tangarife-Castaño, L. A. Betancur-Galvis, J. C. Gallego-Gomez and M. A. González, Eur. J. Med. Chem., 2016, 108, 79–88 CrossRef CAS PubMed
.
- Y. Chen, Z.-X. Lin and A.-M. Zhou, Nat. Prod. Res., 2012, 26, 2188–2195 CrossRef CAS PubMed
.
- K. Kovaleva, O. Oleshko, E. Mamontova, O. Yarovaya, O. Zakharova, A. Zakharenko, A. Kononova, N. Dyrkheeva, S. Cheresiz, A. Pokrovsky, O. Lavrik and N. Salakhutdinov, J. Nat. Prod., 2019, 82, 2443–2450 CrossRef CAS PubMed
.
- B. K. Koe, A. Weissman, W. M. Welch and R. G. Browne, J. Pharmacol. Exp. Ther., 1983, 226, 686–700 CrossRef CAS
.
- Z. Zhou and S. Liu, Exp. Clin. Endocrinol. Diabetes, 2022, 130, 596–603 CrossRef CAS PubMed
.
- R.-M. Ragguett, S. J. Yim, P. T. Ho and R. S. Mclntyre, Expert Opin. Pharmacother., 2017, 18, 2017–2024 CrossRef CAS PubMed
.
- H. Hoffmann, S. Baldofski, K. Hoffmann, S. Flemig, C. P. Silva, V. I. Esteves, F. Emmerling, U. Panne and R. J. Schneider, Talanta, 2016, 158, 198–207 CrossRef CAS PubMed
.
- J. T. Brewster II, S. Dell'Acqua, D. Q. Thach and J. L. Sessler, ACS Chem. Neurosci., 2019, 10, 155–167 CrossRef
.
- R. T. Girase, I. Ahmad, J. M. Oh, B. Mathew, S. K. Vagolu, T. Tønjum, D. Sriram, J. Kumari, N. C. Desai, Y. Agrawal, H. Kim and H. M. Patel, ACS Med. Chem. Lett., 2024, 15, 924–937 CrossRef CAS PubMed
.
- I. Paolini, F. Nuti, M. de la Cruz Pozo-Carrero, F. Barbetti, B. Kolesinska, Z. J. Kaminski, M. Chelli and A. M. Papini, Tetrahedron Lett., 2007, 48, 2901–2904 CrossRef CAS
.
- J. Li, A. W. G. Burgett, L. Esser, C. Amezcua and P. G. Harran, Angew. Chem., Int. Ed., 2001, 40, 4770–4773 CrossRef CAS PubMed
.
- H. Garrido-Hernandez, M. Nakadai, M. Vimolratana, Q. Li, T. Doundoulakis and P. G. Harran, Angew. Chem., Int. Ed., 2005, 44, 765–769 CrossRef CAS PubMed
.
- Á. Pintér and G. Haberhauer, Eur. J. Org. Chem., 2008, 2375–2387 CrossRef
.
- Y. Luo, K. Ji, Y. Li and L. Zhang, J. Am. Chem. Soc., 2012, 134, 17412–17415 CrossRef CAS
.
- A. Dahlqvist, H. Leffler and U. J. Nilsson, ACS Omega, 2019, 4, 7047–7053 CrossRef CAS
.
- L. Su, Y. Feng, K. Wei, X. Xu, R. Liu and G. Chen, Chem. Rev., 2021, 121, 10950–11029 CrossRef CAS PubMed
.
- W. Lin, Y. Du, Y. Zhu and X. Chen, J. Am. Chem. Soc., 2014, 136, 679–687 CrossRef CAS
.
- F. Jia, Y. Xiao, Y. Feng, J. Yan, M. Fan, Y. Sun, S. Huang, W. Li, T. Zhao, Z. Han, S. Hou and J. Chai, Nat. Plants, 2024, 10, 2014–2026 CrossRef CAS PubMed
.
- C.-H. Lee, S. Park, S. Kim, J. Y. Hyun, H. S. Lee and I. Shin, Chem. Sci., 2024, 15, 555–565 RSC
.
- G. Zhao, Z. Li, R. Zhang, L. Zhou, H. Zhao and H. Jiang, Front. Mol. Biosci., 2022, 9, 1055823 CrossRef CAS PubMed
.
- K. Liu, C. Song and A. Lei, Org. Biomol. Chem., 2018, 16, 2375–2387 RSC
.
- X. Guo, N. G. Price and Q. Zhu, Org. Lett., 2024, 26, 7347–7351 CrossRef CAS PubMed
.
- N. Chen and H.-C. Xu, Green Synth. Catal., 2021, 2, 165–178 Search PubMed
.
- Y. Wu, J.-Y. Chen, H.-R. Liao, X.-R. Shu, L.-L. Duan, X.-F. Yang and W.-M. He, Green Synth. Catal., 2021, 2, 233–236 Search PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |