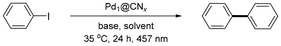
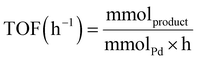Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-driven and green Ullmann homocoupling with a Pd single-atom catalyst
Areti Moutsioua,
Theodore A. Gazisa,
Luis A. Ciprianoa,
Mert Can Incea,
Ik Seon Kwonb,
Nicolò Allasia a,
Sadaf Fatima Jafricd,
Elisa Borfecchia
a,
Sadaf Fatima Jafricd,
Elisa Borfecchia cd,
Lorenzo Mino
cd,
Lorenzo Mino cd,
Martin Sterrer
cd,
Martin Sterrer e,
Giovanni Di Liberto
e,
Giovanni Di Liberto f and
Gianvito Vilé
f and
Gianvito Vilé *a
*a
aDepartment of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, Piazza Leonardo da Vinci 32, 20133 Milano, Italy. E-mail: gianvito.vile@polimi.it
bDepartment of Energy Science and Engineering, Kunsan National University, 558 Daehak-ro, Gunsan-si, Republic of Korea
cDepartment of Chemistry, University of Torino, Via Pietro Giuria 7, Torino, 10125 Italy
dNanostructured Interfaces and Surfaces (NIS) Interdepartmental Centre, University of Torino, Via Pietro Giuria 7, Torino, 10125 Italy
eInstitute of Physics, University of Graz, Universitätsplatz 5, 8010 Graz, Austria
fDepartment of Materials Science, University of Milan Bicocca, Via Roberto Cozzi 55, 20125 Milano, Italy
First published on 18th December 2025
Abstract
The sustainable synthesis of biaryl skeletons via Ullmann-type C–C coupling remains a challenge in organic synthesis. Herein, we report a series of Pd single-atom catalysts supported on mesoporous graphitic carbon nitride (CNx) that promote the visible-light-driven homocoupling of aryl halides under ambient reaction conditions and with high efficiency and recyclability. Spectroscopic and microscopic analyses confirmed the atomic dispersion of Pd within CNx and elucidated its local coordination environment, while demonstrating that the structural framework of the support remained intact upon metal incorporation. Mechanistic studies combining operando X-ray absorption spectroscopy and density functional theory revealed a reversible, light-induced change in Pd coordination, that is linked to the catalytic turnover. Finally, techno-economic analysis and life cycle assessment validated the sustainability of the protocol, highlighting its reduced environmental footprint compared to conventional approaches. Collectively, these findings demonstrate that photoactive single-atom catalysts are a promising platform for efficient, stable, and sustainable biaryl synthesis, paving the way for more sustainable and efficient C–C coupling methodologies.
Green foundation1. We demonstrate that Ullmann homocoupling, a benchmark C–C bond-forming reaction, proceeds under green conditions using a palladium single-atom catalyst on carbon nitride (Pd1@CNx). This catalyst combines high activity and stability with minimal metal use, lowering costs and dependence on scarce palladium.2. Our Pd1@CNx cuts CO2 emissions by ∼70%, water use by ∼75%, energy by 62%, and Pd precursor needs vs. nanoparticle systems by 93%. These results demonstrate how atomic-level catalyst design combined with light-driven activation can enable greener chemical transformations. 3. Greener advances could include solar-to-chemical integration, replacing LEDs with sunlight, bioderived supports and solvent-free or aqueous media, and circular strategies for Pd recovery and catalyst recycling. Such optimizations would move our approach toward a truly circular, carbon-neutral platform for sustainable C–C coupling. |
Introduction
The Ullmann homocoupling reaction, long regarded as a cornerstone of synthetic chemistry, is indispensable for constructing biaryl scaffolds that constitute key motifs in pharmaceuticals, agrochemicals, and functional materials.1 Initially performed with stoichiometric reagents, this transformation has progressively evolved into a catalytic protocol employing homogeneous transition metals, such as phosphine-ligated Pd(0)/Pd(II) complexes,1,2 which enable lower coupling temperatures and offer broader substrate tolerance. Despite these advances, homogeneous systems remain prone to rapid deactivation, residual metal contamination in the final product, and challenges in purification and recycling.Heterogeneous catalysts employing Pd nanoparticles3 or bimetallic alloys4,5 offer improved stability and reusability, but introduce new limitations, such as harsh operating conditions,6 reliance on strong bases,5,7 and the need for reductants.4,6,7 Moreover, these catalysts suffer from low atom economy due to the low utilisation of bulk metal atoms.8 Furthermore, their catalytic activity is governed by unevenly distributed active sites, often located at edges or corners, and structural defects. This complicates active site identification and hinders efforts to correlate structure with performance. As a result, the rational design of heterogeneous Ullmann catalysts remains elusive.
Single-atom catalysts (SACs) have emerged as a transformative class of materials that bridge the gap between homogeneous and heterogeneous systems. By isolating individual metal atoms on solid supports, SACs combine the atomic efficiency and site uniformity of homogeneous catalysts with the robustness and reusability of heterogeneous systems.8–11 In addition, their coordinatively unsaturated environment confers distinctive electronic properties to the metal atom that enhance reactant adsorption and catalytic activity.12,13 However, their application to complex organic reactions has remained underexplored. For the Ullmann C–C homocoupling, only one example involving a bimetallic gold–palladium single-atom alloy catalyst7 has been reported to date. While effective, the protocol required strong bases (NaOH) and additional reductants (ascorbic acid), which limit compatibility with sensitive substrates and add complexity to the operation.
Photocatalysis offers a sustainable route to overcome these challenges by enabling reactions under ambient conditions, exploiting light as a renewable energy source, and minimising the use of hazardous reagents.14–17 Among the available catalysts,18,19 carbon nitride (CNx) stands out for its tuneable band structure, high thermal and chemical stability, and facile synthesis.20 Moreover, its triazine cavities provide anchoring sites that can stabilise isolated metal atoms, making it an ideal support for SACs.21–23 However, visible-light-driven Ullmann-type C–C coupling using SACs has not yet been demonstrated.
Herein, we report a family of Pd SACs supported on mesoporous graphitic carbon nitride (Pd1@CNx) that mediate the Ullmann homocoupling of aryl halides under ambient conditions and visible light irradiation. This system achieves efficient coupling under conditions significantly milder than those of conventional protocols. The results establish the untapped potential of photoactive SACs in C–C bond formation and highlight their broader promise for sustainable strategies in organic synthesis.
Results and discussion
Preparation and characterisation of the SACs
The introduction of Pd single atoms on mesoporous graphitic carbon nitride (Pd1@CNx) was achieved by co-condensation of K2PdCl4 with cyanamide, in the presence of SiO2 nanoparticles as a hard template. This templating step assists in achieving a mesoporous structure while also stabilising the metal by lowering the surface energy of the metal atoms, which are otherwise prone to migration, particularly during pyrolysis.24 A heating treatment at 550 °C, followed by template removal with (NH4)HF2, afforded the desired catalysts. The final composition and textural properties of the obtained catalysts are shown in Table 1.| Catalyst | Ca (wt%) | Na (wt%) | Ha (wt%) | Pdb (wt%) | SBETc (m2 g−1) |
|---|---|---|---|---|---|
| a CHN elemental analysis.b ICP-OES data.c BET method applied on the adsorption branch of the N2 isotherm in the 0.05 < p/p0 < 0.3 range. | |||||
| CNx | 30.4 | 52.6 | 1.80 | — | 185 |
| 0.43-Pd1@CNx | 31.4 | 53.1 | 1.97 | 0.43 | 279 |
| 0.67-Pd1@CNx | 30.9 | 52.2 | 2.42 | 0.67 | 254 |
| 1.32-Pd1@CNx | 32.4 | 54.7 | 2.10 | 1.32 | 246 |
Elemental CHN analysis revealed that all materials display a stoichiometric C/N ratio of ca. 0.60. The marginal variability in the ratios in both Pd-loaded and pristine CNx samples can be ascribed to the incomplete polymerisation during the thermal process which gives rise to structural defects and residual impurities in the carbon nitride materials, in line with previous reports.25 In addition, inductively coupled plasma-optical emission spectroscopy (ICP-OES) data demonstrated that varying the amount of metal precursor during synthesis allowed for controlled tuning of the Pd loading on the support, ranging from 0.43 to 1.32 wt%.
The crystallinity and phase purity of the Pd-based SACs was verified by X-ray diffraction (XRD) analysis (Fig. 1a). The XRD patterns displayed two distinctive diffraction peaks at 2θ angles of approximately 13° and 27°, corresponding to the trigonal nitrogen linkages of the triazine moieties and the interplanar stacking of aromatic rings, respectively.26 Notably, no other diffraction peaks were present, implying the absence of metallic nanoparticles or other crystalline impurities in the samples. Moreover, across the Pd-loaded samples, the diffraction profiles remain essentially identical to pristine CNx, indicating that the Pd loading does not significantly disturb the long-range order of the CNx framework, even at the highest loading. To evaluate the surface area and porosity of the catalysts, N2 physisorption studies at −196 °C were conducted. These textural measurements revealed high specific surface areas for all materials (Table 1). The high surface area and mesoporous nature of the catalysts allow for enhanced exposure of the active sites to the reaction mixture and improve light penetration, promoting more uniform photoexcitation of active sites.27 Specifically, the obtained isotherms displayed type IV behaviour with H3 hysteresis loops (Fig. 1b) and average pore diameters of ca. 10 nm (Fig. 1c), confirming the mesoporous structure of the materials. Overall, the surface area and pore diameter of the Pd-loaded materials were greater than bare carbon nitride, revealing that the mesoporosity was preserved after Pd incorporation, and importantly, no pore collapse or decrease in surface area was observed.
Transmission electron microscopy (TEM) analysis shows a lack of discernible metal clusters, indicating well-dispersed Pd species within the CNx framework (Fig. 1d). Notably, using high-resolution TEM (HR-TEM), we could observe individual metal atoms uniformly dispersed on the support (Fig. 1e). The micrograph revealed isolated bright spots corresponding to single Pd atoms, confirming their dispersion at the atomic level and proving that agglomeration of Pd atoms into larger clusters and nanoparticles did not occur during catalyst synthesis. To evaluate the role of SiO2 in Pd dispersion, a reference material was prepared under identical conditions but without the addition of SiO2. TEM analysis of the synthesised material revealed the presence of Pd aggregates (Fig. S1), demonstrating that single-atom dispersion is achieved better when a highly-porous material is used as support.
Further insights into the chemical state of carbon nitride and active Pd species on its surface were obtained by X-ray photoelectron spectroscopy (XPS). The analysis was performed on the highest-loading 1.32-Pd1@CNx, as a representative catalyst, since the stronger Pd signal enhanced sensitivity and ensured reliable analysis of the active sites. Two peaks were observed in the Pd 3d spectrum (Fig. 1i), corresponding to Pd 3d5/2 and Pd 3d3/2 signals, with a Pd 3d5/2 binding energy of 338.7 eV, assigned to positively charged Pd species.28,29 The C 1s signal (Fig. 1g) consists of three contributions, with binding energies of 287.9 eV, 285.7 eV, and 284.7 eV that are ascribed to sp2-hybridised N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds in the tri-s-triazine ring of carbon nitride, C–NHx bonds, and adventitious carbon (C–C/C–H). The signal present in the N 1s spectrum (Fig. 1h) could be deconvoluted into three peaks at 400.8 eV, 399.4 eV, and 398.4 eV, corresponding to pyrrolic nitrogen (C–NH2 and C–NH–C), graphitic nitrogen (N–C3), and pyridinic nitrogen (C
N bonds in the tri-s-triazine ring of carbon nitride, C–NHx bonds, and adventitious carbon (C–C/C–H). The signal present in the N 1s spectrum (Fig. 1h) could be deconvoluted into three peaks at 400.8 eV, 399.4 eV, and 398.4 eV, corresponding to pyrrolic nitrogen (C–NH2 and C–NH–C), graphitic nitrogen (N–C3), and pyridinic nitrogen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C).30
N–C).30
To comprehend the electronic and local structure of Pd1@CNx, X-ray absorption spectroscopy (XAS) was utilised. Analysis was performed on 1.32-Pd1@CNx to understand the electronic and local structure from its Pd and N K edges, using PdO powder and Pd foil as references in the Pd K edge XAS data. Fig. 2a illustrates the X-ray absorption near edge structure (XANES) for the Pd K edge, where 1.32-Pd1@CNx displays a distinct spectral shape compared to Pd and PdO reference samples, indicating a different local atomic environment. This confirms that the local structure of 1.32-Pd1@CNx is neither metallic Pd nor PdO.
Fig. 2b displays the extended X-ray absorption fine structure (EXAFS) data for the Pd K edge through the Fourier transformation of the X-ray absorption spectrum from rising edges to 1200 eV. Specifically, 1.32-Pd1@CNx displays non-phase-corrected peaks near 1.53 Å, corresponding to the Pd–N interatomic distance. This suggests primary bonding of Pd atoms with N atoms in the CNx support with no apparent Pd–Pd bonding observed. In the case of PdO powder, the Pd–O peak was observed near 1.57 Å, at a slightly longer distance than the Pd–N bond in 1.32-Pd1@CNx. Finally, intense Fourier transform features peaks are observed above 2.5 Å, indicating the presence of Pd–Pd interatomic distances for both PdO and Pd foil, which are not detected in 1.32-Pd1@CNx.
The structural characteristics of surrounding nitrogen were examined by measuring the N K edge for 1.32-Pd1@CNx (Fig. 2c). Strong peaks near 399 eV were observed in 1.32-Pd1@CNx, indicating the electronic transition from N 1s orbital to the π* transition of pyrrolic and pyridinic nitrogen originating from the CNx support. Notably, the peak intensity of graphitic nitrogen is weaker than pyrrolic/pyridinic nitrogen due to the preliminary nitrogen form in CNx being pyridinic with unpaired electrons.
Quantitative values from measured EXAFS data, such as interatomic distance, Debye–Waller factor, and coordination number, obtained through EXAFS fitting analysis, are summarised in Table S1. In 1.32-Pd1@CNx, the coordination number for the Pd–N bond is 4, consistent with the nature of SACs with an M–N4 structure. In the wavelet-transformed EXAFS (WT-EXAFS) data in Fig. 2d, the prominent maximum is observed around 4.2 Å−1 in k space, indicating Pd–N bond. The analysis demonstrated that the Pd atoms in 1.32-Pd1@CNx are predominantly surrounded by N atoms, aligning with the EXAFS data. In the case of PdO powder and Pd foil, the maxima region in k space are present at 8–10 Å−1, implying the existence of Pd–Pd bonds in these samples. However, it must be noted that XAS provides averaged information over all Pd sites, and the high synthesis temperature combined with the intrinsic defects of the CNx substrate may introduce some heterogeneity in Pd coordination. Nonetheless, XANES and EXAFS analyses indicate predominantly atomically dispersed Pd–N species, though complete homogeneity cannot be ensured.
Catalytic performance of the Pd-based catalysts
To optimise the reaction parameters for the photocatalytic Ullmann homocoupling, iodobenzene was employed as a model substrate (Table 2). The choice of base and solvent significantly impacted reaction efficiency, with trends explained by base strength and solvation effects. Specifically, an array of bases was initially examined (entries 1–9). Under benchmark conditions employing 0.67-Pd1@CNx, methanol (MeOH), and 1 M aqueous NaOH, the reaction yielded a TOF of 1.80 h−1 (entry 1). While NaOH, as a strong base, efficiently deprotonates intermediate species, its inherent nucleophilicity induces side reactions, reducing the overall efficiency. At the other end of the spectrum, Na2CO3 (entry 2, TOF = 1.21 h−1), Cs2CO3 (entry 3, TOF = 1.29 h−1), and K2CO3 (entry 4, TOF = 0.59 h−1), despite their low nucleophilicity, produced suboptimal TOFs, likely due to their weak basicity. K3PO4 (entry 5) emerged as the most effective base, achieving a TOF of 1.85 h−1, minimising side reactions while ensuring efficient deprotonation. Finally, organic bases proved unsuitable, consistently yielding low TOFs (entries 6–9). Considering solvent selection, the polar protic nature of methanol (entry 5) allowed it to solvate the base and stabilise charged intermediate species through hydrogen bonding.31 In contrast, isopropanol (i-PrOH), acetonitrile (MeCN), and dimethylformamide (DMF) all resulted in lower TOFs (entries 10–12). In particular, i-PrOH promoted the competitive dehalogenation reaction, decreasing the coupling reaction efficiency.| Entry | Base | Base equiv. | Solvent | Conversion (%) | TOF (h−1) |
|---|---|---|---|---|---|
| Unless indicated otherwise, the reaction conditions were 0.67-Pd1@CNx (5 mg), iodobenzene (0.1 mmol), base (0.1–0.2 mmol), and solvent (2 mL).a Catalyst amount increased to 7.5 mg. TOF: mmolproduct mmolPd−1 h−1. | |||||
| 1 | NaOHaq (1 M) | 1 | MeOH | 76 | 1.80 |
| 2 | Na2CO3 | 1 | MeOH | 44 | 1.21 |
| 3 | Cs2CO3 | 1 | MeOH | 49 | 1.29 |
| 4 | K2CO3 | 1 | MeOH | 26 | 0.59 |
| 5 | K3PO4 | 1 | MeOH | 59 | 1.85 |
| 6 | i-Pr2NH | 1 | MeOH | 9 | 0.46 |
| 7 | Bu2NH | 1 | MeOH | 21 | 0.24 |
| 8 | Et3N | 1 | MeOH | 26 | 0.25 |
| 9 | DIPEA | 1 | MeOH | 21 | 0.38 |
| 10 | K3PO4 | 1 | i-PrOH | 33 | 0.22 |
| 11 | K3PO4 | 1 | MeCN | 0 | 0 |
| 12 | K3PO4 | 1 | DMF | 0 | 0 |
| 13 | K3PO4 | 2 | MeOH | 99 | 2.65 |
| 14a | K3PO4 | 2 | MeOH | 99 | 1.56 |
Doubling the equivalents of K3PO4 in MeOH (entry 13) resulted in a TOF of 2.65 h−1. This increase in K3PO4 concentration likely enhanced deprotonation efficiency, without introducing significant nucleophilic side reactions. Further investigations revealed that increasing the catalyst amount (entry 14) led to a reduction in TOF. Control experiments (Table S2) confirmed the importance of light (no reaction in its absence), metal catalyst (no biphenyl formation using bare CNx), and base (traces in its absence).
To assess the effect of Pd loading on catalytic performance, Pd1@CNx catalysts with varying Pd contents were tested under the optimised reaction conditions (Fig. 3a). Switching from the lower-loading 0.43-Pd1@CNx (TOF of 2.25 h−1) to 0.67-Pd1@CNx improved the TOF (2.65 h−1), likely due to a greater number of active Pd single-atom sites facilitating higher catalytic turnover. However, when 1.32-Pd1@CNx was employed, the TOF dropped significantly to 1.44 h−1, suggesting that beyond an optimal loading, additional Pd does not enhance catalytic activity proportionally.
Ensuring stability in heterogeneous catalysis is key to minimising performance fluctuations across multiple reaction runs. To assess this within our catalytic protocol, the reaction kinetics were monitored over 24 h (Fig. 3b). Increasing product formation was observed for the first 14 h to a TOF value of 3.79 h−1, whereupon a plateau was reached, and only marginal improvements were observed for the remaining 10 h. Notably, this TOF, to the best of our knowledge, outperformed previous photocatalytic Ullmann homocouplings (Table S3). The durability of the catalyst was further investigated over three successive runs by filtering, washing, drying, and reusing it. Recycling was performed at low conversion (<40%) to avoid operating in a reactant-limited regime (Fig. 3c), followed by a hot filtration test under identical conditions (Fig. S2). After rapid removal of the catalyst, metal-free CNx photocatalyst was added to the filtrate and maintained under the same conditions. No further biphenyl formation was observed, confirming the absence of catalytically active Pd species in solution. The recovered catalyst was reused in consecutive reactions with comparable activity, confirming its recyclability and the heterogeneous nature of the catalysis. Additionally, recycling at full conversion was performed to assess stability and structural integrity under prolonged operating conditions (Fig. 3d). The catalyst retained high activity, exhibiting only a slight decrease to 96% of its original performance. Further testament to the catalyst's stability was provided by a battery of characterisation techniques performed after the third reaction cycle (Fig. S3). XRD and BET measurements revealed no significant alterations in the catalyst structure, surface area, or porosity. TEM analysis confirmed the absence of metal clusters, demonstrating that metal aggregation did not occur during catalysis. Finally, ICP-OES analysis of the powdered catalyst showed no leaching of Pd during the reactions.
With a reaction profile and the optimal reaction conditions established (Table 2, entry 13), we next sought to expand the substrate scope of our 0.67-Pd1@CNx catalyst to aryl halides possessing a variety of functional groups (Scheme S1). Initially, we examined the coupling efficiency of different halobenzenes. The poor leaving group nature of chlorobenzene rendered it inactive for this transformation. However, both iodobenzene and bromobenzene successfully furnished biphenyl 1 with TOFs of 2.65 and 2.25 h−1, respectively. Steric hindrance was also detrimental to coupling efficiency. In comparison to p-biaryl 1a, sterically demanding aryl halides led to decreased TOFs for m-biaryl 1b and o-biaryl 1c.
Further investigations into the substrate scope revealed distinct trends based on the electronic nature of the aryl halides. A marked preference for aryl iodides bearing electron-donating groups was observed, as demonstrated by the 4.96 and 5.10 h−1 TOFs achieved with compounds 1a and 1d, respectively. Conversely, aryl halides with electron-withdrawing substituents delivered more modest TOFs, reaching up to 2.98 h−1 for 1e. The TOFs declined further for the more strongly deactivated dihalobiphenyls 1f (2.45 h−1) and 1g (2.71 h−1), consistent with the diminished electron density of these substrates. This trend is attributed to the substrate-dependent susceptibility of the C–I bond to reductive cleavage: aryl iodides bearing strongly electron-withdrawing groups undergo more facile bond dissociation, due to electron transfer from the carbon nitride support, thereby promoting dehalogenation. In contrast, inactive substrates bearing electron-donating groups exhibit less efficient C–I bond activation, favouring the desired coupling pathway.
Next, the catalyst compatibility with heteroaromatic halides was explored. These substrates generally exhibit lower reactivity in coupling reactions. In line with this trend, our 0.67-Pd1@CNx catalyst delivered bipyridine 1h in a modest 1.59 h−1 TOF. Despite the limited TOF, this result highlights the catalyst's versatility in accommodating even challenging, less reactive aryl halides.
Finally, we successfully utilised the catalyst for the synthesis of hetero-coupled aryl halides (1i–1m), achieving TOFs of up to 1.79 h−1 for 1l. Notably, the cross-coupled products showed similar selectivity to the homocoupled products of the unactivated aryl iodides. In contrast, activated aryl halides exhibited significantly lower selectivity for the corresponding homocoupled products, consistent with their limited reactivity during the homocoupling studies.
Mechanistic investigations
To study possible changes in the electronic properties of the metal active site and its stability, XAS measurements were performed under reaction conditions. As can be seen in Fig. 4, upon irradiating the sample at 457 nm under reaction conditions, a progressive reduction in the white line intensity and a shift of the edge position to lower energies (left) are observed (grey curves). The transient phase lasts about 30 minutes, after which a photo-stationary state is reached. This suggests a decrease in the Pd site oxidation state and a change in its coordination. When visible-light irradiation is suspended, as evident from comparing the black and red curves, the system reverts to its initial state with 4-fold coordination and higher oxidation state, which further corroborate the stability of the catalytic system. The light-induced changes in Pd coordination observed under photocatalytic conditions are consistent with previous studies on SACs, where photoexcitation has been shown to alter the metal's oxidation state and coordination environment,32 demonstrating how light can uniquely modulate the structural dynamics, activity, and stability of SACs. In our system, which operates without strong bases or homogeneous ligands, illumination is the primary factor driving modifications in the Pd site structure. This behaviour aligns with C–C coupling studies on SACs performed under thermal conditions, where bases and solvents were found to exert only minor effects on the electronic properties of the metal centres.33,34Based on the experimental insights, we considered a Pd atom with a +2 oxidation state and a 4-fold coordination in the triazine cavity of CNx (Fig. 5a), in accordance with previous studies.35 More specifically, we recently demonstrated, using X-ray spectroscopy, infrared spectroscopy of probe molecules, and quantum chemical simulations, that during the synthesis of CNx-based SACs, a Ni atom assumes a four-fold coordination to the matrix.35 Similarly, in a Pd-based covalent organic framework, we observed that the metal atom is stabilised when occupying a similar cavity.36 Based on these observations, the choice of the simulated Pd-SAC model is considered reasonable. We modelled the Ullmann-type C–C homocoupling of iodobenzene (RI) to biphenyl (BP) (Fig. 5b). The Pd atom is coordinated to four nitrogen atoms with an average bond length of 2.11 Å (Fig. S4a), where the Pd atom donates +0.9|e| to the CNx support, as quantified by atomic charge analysis according to the Quantum Theory of Atoms In Molecules (QTAIM), by using Henkelman's algorithm.37 The 4-fold coordination renders the Pd atom weakly reactive, and a perturbation of its local geometry is needed to make it able to promote the reaction. We hypothesised that Pd1@CNx undergoes a low-energy photoexcitation of +1.79 eV, which corresponds to a sub-gap transition enabled by Pd-induced states within the CNx (ca. 2.1 eV).38 This excitation redistributes electron density around the Pd atom, leading to a calculated contraction of the average Pd–N bond length from 2.11 Å to 1.98 Å. This contraction is well within the range observed for metal centres undergoing changes in oxidation/coordination (ca. 0.1–0.15 Å) or for different adsorption geometries in Pd1@CNx systems.34,39
The overall reaction for the Ullmann homocoupling process is exergonic (ΔG = −1.98 eV) and it consumes two iodobenzene (RI) molecules to release biphenyl (BP) and two iodine ions (2RI → BP + 2I−). The proposed mechanism involves the following elementary steps:
| RI + * → RI* | (1) |
| RI* → R* + I− | (2) |
| R* + RI → R*R + I− | (3) |
| R*R → BP* | (4) |
| BP* → BP + * | (5) |
The first step involves the adsorption of the reactant RI to the single metal site (*), to obtain an adsorbed species of the type RI*, with calculated Pd–C and Pd–I distances equal to 2.09 Å and 2.58 Å respectively, compatible with activated intermediates. This process is endergonic and has an energetic cost of ΔG = +0.89 eV (Fig. 5c, I). Indeed, this activation step facilitates the formation of the reaction intermediate (R*), and an iodine ion is released in a favourable process (ΔG = −0.89 eV) (Fig. 5c, II). The formation of R* implies a reduction of the Pd–C bond length from 2.09 Å to 1.95 Å, in line with the qualitative description of the bond order conservation principle. To prove that the R* intermediate is the most stable isomer that can be formed when iodobenzene reacts with the catalyst, we simulated other possible isomers (Fig. S5), and we found that indeed this isomer is energetically the most favourable. In the third step, the R* intermediate reacts with a second iodobenzene molecule to form the R*R intermediate. This transformation is endergonic, requiring an energy input of approximately +0.58 eV, and represents a selectivity-controlling stage in the reaction pathway. In the R*R intermediate, the two phenyl groups remain bonded to the Pd with a bond length of 2.05 Å, while the Pd–N bond length increases from 1.98 Å in the R* intermediate to 2.04 Å, and the second iodine ion is released (Fig. 5c, III). The subsequent step entails the rearrangement of the two phenyl groups on the Pd@CNx, leading to the formation of the fourth intermediate BP*, which resembles a biphenyl molecule in the gas phase (Fig. 5c, IV). The process is highly exergonic, ΔG = −1.98 eV, and only one carbon from the likely-biphenyl molecule is bonded to the Pd atom with a bond length of 2.50 Å. Within the fourth step, the Pd–N bond length decreases from 2.04 Å in the R*R intermediate to 1.99 Å. The last step is also exergonic, ΔG = −0.58 eV, and involves the release of the biphenyl molecule and the free catalyst (*). Notably, during the reaction, the metal atom changes its coordination to bind reaction intermediates before recovering its initial 4-fold coordination in the triazine cavity of CNx, in line with the operando XAS investigations. Fig. 5c shows the Gibbs free energy profile calculated at T = 308.15 K. Specific bond distances for each intermediate and the catalysts are reported in Fig. S4, whereas the calculated zero-point energy and entropy of the gas-phase molecules and each intermediate are provided in Table S4.
Techno-economic and sustainability assessment
To assess their techno-economic and environmental performance, our SAC (Pd1@CNx, as employed in Table 2, entry 13) and a reported nanoparticle-based system (PdNP@CNx) were benchmarked against a representative homogeneous protocol through techno-economic analysis (TEA) and life-cycle assessment (LCA) (Fig. 6, Fig. S6–S13, and Tables S5–S7). The homogeneous and heterogeneous systems were comparatively evaluated across two key economic indicators and five major sustainability metrics, including raw material cost, energy consumption, CO2 emissions, water consumption, carcinogenic toxicity, fine particulate matter formation, and ocean acidification. The Pd1@CNx catalyst exhibited substantial improvements across all sustainability parameters relative to the homogeneous counterpart, achieving reductions of 64% in water consumption, 61% in carcinogenic toxicity, 65% in particulate matter formation, and 64% in ocean acidification. Notably, raw material expenses were reduced by 87% owing to the high cost of the [Au2(dppm)2]Cl2 catalyst in the homogeneous system. Despite these clear sustainability advantages, the homogeneous protocol displayed higher energy efficiency, primarily due to the multi-step and energy-intensive synthesis of heterogeneous catalysts. Following this comparison, the study focuses exclusively on the heterogeneous systems, with particular emphasis on the comparative techno-economic and environmental evaluation of Pd1@CNx and the literature-reported PdNP@CNx. | ||
| Fig. 6 Comparative evaluation of homogeneous (red) and heterogeneous (blue) systems across key economic and sustainability metrics. | ||
Initially, a TEA was conducted, as illustrated in Fig. 7a and detailed in Tables S8 and S9. A substantial reduction in raw material costs was observed for Pd1@CNx compared to PdNP@CNx, resulting in a decrease from 193.0 to 37.1 € gproduct−1 year−1. The catalyst production cost and solvent expenses were identified as the major contributors to this cost cut. Annual operational costs (OpEx), evaluated across seven cost categories including raw materials, labor, maintenance, utilities, operating charges, lab overhead, and other expenses (Fig. 7b and Tables S10 and S11), also decreased by nearly 70%, while the total energy consumption (Fig. 7c and detailed in Table S12) was reduced by 62% when Pd1@CNx was used instead of PdNP@CNx.
 | ||
| Fig. 7 Techno-economic evaluation of Pd1@CNx and PdNP@CNx reaction conditions in terms of (a) raw material cost, (b) OpEx, and (c) energy consumption. | ||
The environmental impacts of the catalytic processes employing Pd1@CNx and PdNP@CNx were evaluated through an LCA. In the initial phase of the analysis, the two reactions were compared across key environmental indicators, including carbon emissions (kg CO2 equiv. gproduct−1) and water consumption (m3 gproduct−1). By exploiting Pd1@CNx in the reaction, CO2 emissions were mitigated from 58.6 to 19.2 kg CO2 equiv. gproduct−1, representing a 67% reduction compared to the nanoparticle-based protocol (Fig. 8a and Tables S13 and S14). This improvement was primarily driven by the more sustainable synthesis of the catalyst (Pd1@CNx catalyst), which alone contributed to a cut of 38.9 kg CO2 equiv. gproduct−1. In addition, the replacement of the dioxane–H2O mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) with methanol had a positive effect on the emissions. In the PdNP@CNx system, solvent-related emissions accounted for 1.1 kg CO2 equiv. gproduct−1, whereas methanol in the Pd1@CNx protocol reduced the emissions to 0.4 kg CO2 equiv. gproduct−1. Water consumption was also evaluated, showing a 74% reduction under Pd1@CNx conditions, decreasing from 4.7 to 1.2 m3 gproduct−1 (Fig. 8a and Tables S15 and S16). A key factor in this improvement was the lower Pd loading in the Pd1@CNx catalyst, which reduced the water required during metal precursor processing from 4.6 to 1.1 m3 gproduct−1 compared to the nanoparticle-based counterpart.
5 v/v) with methanol had a positive effect on the emissions. In the PdNP@CNx system, solvent-related emissions accounted for 1.1 kg CO2 equiv. gproduct−1, whereas methanol in the Pd1@CNx protocol reduced the emissions to 0.4 kg CO2 equiv. gproduct−1. Water consumption was also evaluated, showing a 74% reduction under Pd1@CNx conditions, decreasing from 4.7 to 1.2 m3 gproduct−1 (Fig. 8a and Tables S15 and S16). A key factor in this improvement was the lower Pd loading in the Pd1@CNx catalyst, which reduced the water required during metal precursor processing from 4.6 to 1.1 m3 gproduct−1 compared to the nanoparticle-based counterpart.
A broader evaluation was performed to capture aggregated environmental impacts on three major damage categories: human health, ecosystem quality, and critical resources (Fig. 8b and Tables S17 and S18). A reduced overall environmental impact was associated with Pd1@CNx conditions relative to the nanoparticle-based system, with damage reductions of 72% in human health, 73% in ecosystem quality, and 68% in resources. Building upon the endpoint analysis, the assessment was further extended to include midpoint level indicators and implemented into a planetary boundary framework (Fig. 8c and detailed in Tables S19 and S20) to characterise individual environmental stressors with greater precision. The impact on ocean acidification was notably reduced under reaction conditions exploiting Pd1@CNx, from 2.5 × 10−1 to 5.7 × 10−2 mol H+ equiv. gproduct−1. This outcome was primarily linked to the reduced Pd content and the solvent system employed. Specifically, the use of Pd1@CNx catalyst led to significantly lower acidification impact, showing a 78% reduction relative to the PdNP@CNx system, decreasing from 2.4 × 10−1 to 5.2 × 10−2 mol H+ equiv. gproduct−1. Furthermore, the use of methanol in the Pd1@CNx protocol resulted in a reduction in the solvent-derived acidification impact from 4.5 × 10−3 to 1.4 × 10−3 mol H+ equiv. gproduct−1, reflecting its lower acidification potential compared to the dioxane–H2O system. The genetic and functional biosphere integrities also followed a similar trend and decreased by 79% and 68%, respectively. Moreover, under Pd1@CNx conditions, the impact on climate change was reduced by 67% and 65%, considering the effects of radiative forcing and carbon emissions, respectively. Atmospheric aerosol loading is a critical parameter influencing Earth's radiative balance and air quality, thereby playing a pivotal role in human health. The use of Pd1@CNx in the catalytic method enhanced process sustainability, reducing atmospheric aerosol impacts by 78%. Land system change, closely associated with deforestation as a primary environmental pressure, was notably mitigated under the Pd1@CNx protocol. The impact in this category was reduced by 93%, corresponding to a land use saving of 5.5 × 10−1 m2 crop equiv. gproduct−1 compared to the PdNP@CNx conditions. Further reductions were observed in the biogeochemical flows, where the use of Pd1@CNx lowered the impacts on phosphorus and nitrogen cycles by 73% and 64%, respectively. Lastly, the impacts on green and blue waters were investigated in the context of freshwater change. The conditions with Pd1@CNx exhibited a much greener performance for both freshwater impact categories, and the impacts were minimised by 87% and 86%, respectively.
We next examined catalyst synthesis routes, quantifying carbon emissions to assess the environmental burden associated with the preparation of the catalytic material (Fig. S14 and Tables S21–S26). Also in this case, the synthesis of the nanoparticle-based catalyst led to much greater carbon emissions. In comparison, the preparation of the Pd1@CNx catalyst resulted in a 69% reduction in carbon emissions, highlighting the environmental advantages of the protocol. One of the major reasons for this reduction was the energy-intensive synthetic protocol of the reported PdNP@CNx catalyst, wherein 84% of the total carbon emissions originated from energy consumption. This high energy demand was further amplified by the low catalyst mass yield (ca. 50%), which increased the energy input required per gram of catalyst synthesised. Another notable contributor to the elevated carbon emissions was the selection of the Pd precursor (K2PdCl6), which accounted for 13% of the total emissions owing to the carbon-intensive production of Pd metal for precursor synthesis. In contrast, the Pd1@CNx synthesis protocol significantly reduced carbon emissions by addressing the energy-intensive characteristics of the nanoparticle-based route, achieving a 65% decrease in energy consumption. Finally, PdNP@CNx catalyst required a higher Pd precursor consumption (ca. 0.7 gprecursor gcatalyst−1), which increased the resource intensity of the process. In contrast, the Pd1@CNx catalyst led to a 93% reduction in precursor-related consumption. The results corroborated the advantage of SACs in minimising the use of critical raw materials, in contrast to nanoparticle systems where a larger fraction of the metal is required and remains inactive in the catalytic process.
Conclusion
We developed a family of Pd1@CNx catalysts with tuneable Pd loadings between 0.43–1.32 wt%. Advanced spectroscopy (XPS and XAS) unambiguously confirmed atomically dispersed Pd in a Pd–N4 coordination environment without Pd–Pd contributions assigned to metal clusters and nanoparticles. In the photocatalytic Ullmann homocoupling of aryl halides, the optimal catalyst (0.67-Pd1@CNx) achieved a TOF of 2.65 h−1 under visible light, with reaction kinetics reaching 3.79 h−1 after 14 h, which surpass previously reported photocatalytic systems. The catalyst also exhibited good stability, retaining 96% activity after three cycles, without evidence of aggregation or Pd leaching. DFT calculations and operando XAS revealed a light-induced Pd2+ coordination dynamics that enables substrate activation, consistent with a low-energy barrier (ΔG‡ ≈ +0.58 eV) for the selectivity-controlling step. From a techno-economic perspective, the Pd1@CNx protocol reduced raw material costs from 193.0 to 37.1 € gproduct−1 year−1 and operating expenses by ca. 70% relative to Pd nanoparticle benchmarks. Energy consumption was also lowered by 62%, while life cycle assessment showed ca. 70% less CO2 emissions. Collectively, these results demonstrate that Pd SACs not only unlock superior photocatalytic Ullmann couplings but also establish a scalable and environmentally sustainable route to C–C bond formation.Materials and methods
Catalyst preparation
The Pd single atoms anchored on CNx catalysts were prepared according to a modified literature procedure.40 Cyanamide (3 g) and the appropriate amount of K2PdCl4 were added to a 40% aqueous dispersion of 12 nm SiO2 particles (7.5 g). Specifically, 30 mg (0.09 mmol), 52 mg (0.16 mmol), and 85 mg (0.26 mmol) of K2PdCl4 were used to prepare the 0.43 wt%, 0.67 wt%, and 1.32 wt% Pd1@CNx catalysts, respectively. The suspension was stirred at 80 °C for 16 h, leading to complete water evaporation. The resultant solid mixture was heated to 550 °C at a rate of 2.2 °C min−1 and maintained at that temperature for 4 h. The obtained orange powder was briefly ground and washed with an (NH4)HF2 solution (12 g in 50 mL of water) for 24 h to remove the silica template. The solid was collected by filtration, washed with water (3 × 100 mL) and ethanol (100 mL), and dried in an oven at 65 °C overnight to yield 1.5 g of the desired Pd1@CNx. Reference CNx was prepared similarly but without the addition of metal precursor (K2PdCl4).Catalyst characterisation
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was conducted on a PerkinElmer Optima 8300 ICP-OES spectrometer to determine the Pd content of Pd1@CNx. The elemental composition of samples was determined using a Vario MICRO Elemental Analyzer through combustion analysis at temperatures exceeding 1000 °C in an oxygen-rich environment. Powder X-ray diffraction (XRD) was performed on a Bruker D2 Phaser diffractometer equipped with a Cu Kα radiation source (λ = 1.54 Å), using a 2θ step size of 0.016° and a counting time of 0.4 s per step. Nitrogen physisorption measurements were performed on a Micromeritics ASAP 2020 instrument at −196 °C after degassing the samples at 150 °C for 24 h. The specific surface areas were calculated by applying the Brunauer–Emmett–Teller (BET) model to the adsorption branch in the p/p0 range from 0.05 to 0.30. Transmission electron microscopy (TEM) studies were conducted using a Philips CM200 FEG instrument operated at 200 kV equipped with a cold field emission gun. High-resolution TEM (HR-TEM) studies were performed using the Grand-ARM300F (JEOL, Japan) with a cold field emission gun operated at 300 kV. Aberration correction of the image-forming as well as of the probe-forming lenses enabled sub-Å resolution. For X-ray photoelectron spectroscopy (XPS) measurements, the powdered catalyst was distributed and pressed onto a double-sided carbon adhesive tape, which was fixed on a flag-style sample holder and then introduced into the ultrahigh-vacuum analysis chamber via a load-lock. The carbon tape was fully covered to ensure that no additional signal contributions were detected. The analysis chamber was equipped with a dual anode X-ray source (XR50, Specs) and a hemispherical electron energy analyser (Argus CU, Scienta Omicron). The XP spectra of the catalyst samples were acquired with Al Kα radiation (1486.7 eV) at normal emission. For the Pd 3d, C 1s, and N 1s detail spectra, the analyser pass energy was set to 50 eV. Charging effects were considered by relating the BE scale to the C 1s signal of the aromatic carbon at 284.7 eV. X-ray absorption spectroscopy (XAS) measurements for the Pd K edge were carried out at the Pohang Light Source-II (PLS-II) 8C Nanoprobe XAFS beamline (BL8C). A double crystal monochromator with Si (111) crystal was used to deliver a monochromatic X-ray beam. The slits used in all measurements had an aperture of 0.5 mm (vertical) × 1 mm (horizontal). The measurements were performed at the Pd K edge in transmission mode using gas ionisation chambers as the detectors.Photocatalytic experiments
A 4 mL glass vial was charged with catalyst (5–7.5 mg), the appropriate aryl halide (0.1 mmol), base (0.1–0.2 mmol), and solvent (2 mL), and sealed with a silicone septum. The experiments were conducted in a PhotoCube™ reactor (ThalesNano Inc.) under visible light irradiation (λ = 457 nm) for 24 h at 35 °C. For the reaction optimisation, the crude was filtered through a Nylon syringe filter (0.45 μm) after reaction completion and analysed by high-performance liquid chromatography (HPLC). Aliquots of 100 μL from the reaction mixtures were diluted with 1.5 mL of MeCN and injected into an Agilent 1200 chromatograph (G1315D UV detector at λ = 210 nm). The stationary phase used was a C18 HypersilGOLD 5 μm 175 Å column (Thermo Scientific) and the eluent was a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mixture of MeCN/H2O (flow rate: 0.7 mL min−1 at 40 °C). Conversion and TOF were determined after HPLC calibration with commercial standards. Specifically, the amount of product (mmol), quantified by HPLC, was used to calculate the TOF according to the following equation, where mmolPd is the Pd content in the catalyst (mmol) and h the reaction time:
40 mixture of MeCN/H2O (flow rate: 0.7 mL min−1 at 40 °C). Conversion and TOF were determined after HPLC calibration with commercial standards. Specifically, the amount of product (mmol), quantified by HPLC, was used to calculate the TOF according to the following equation, where mmolPd is the Pd content in the catalyst (mmol) and h the reaction time:For recycling studies, after reaction completion, the catalyst was separated by filtration, washed with water (15 mL) and methanol (15 mL), dried in an oven at 60 °C, and reused in the next cycle. After the recycling tests, the Pd loading of the recovered catalyst was detected by ICP-OES to be 0.67 wt%. For recycling at low conversion, the reaction was stopped after 4 h. To investigate Pd leaching, a hot filtration test was performed under the same conditions. After rapid catalyst removal, metal-free CNx (5 mg) and K3PO4 (0.2 mmol) were added to the filtrate, and the mixture was maintained under identical conditions. To investigate the generality of the reaction, a substrate scope was conducted using a diverse array of substrates under the optimised conditions. After each reaction, the catalyst was separated by filtration and washed with methanol (15 mL). The solvent was then removed under reduced pressure. The resultant crude mixture was treated with H2O (10 mL) and extracted with ethyl acetate (3 × 10 mL). The organic phase was dried with MgSO4, filtered, concentrated under reduced pressure, and re-dissolved in CDCl3. Dibromomethane or 2,2,2-trifluoroethanol (0.1 mmol) was added as an external standard and the mixture was analysed by means of NMR spectroscopy. Yields determined by 1H or 19F NMR spectroscopy.
DFT calculations
Spin-polarised DFT calculations were performed with the VASP code,41–43 using the generalised gradient approximation, as implemented in the Perdew–Burke–Ernzerhof (PBE) functional.44 Dispersion forces have been included according to Grimme's D3 parametrisation.45 The valence electrons have been expanded on a set of plane waves with a kinetic energy cutoff of 450 eV, whereas the core electrons were treated with the projector augmented wave approach (PAW).46,47 The threshold criteria for electronic and ionic loops were set to 1 × 10−6 eV and 1 × 10−2 eV Å−1, respectively. The sampling of the reciprocal space was reduced to the gamma point because of the cell size. Single-point PBE048,49 calculations were performed on top of PBE-optimised structures to improve the description of the electronic structure. This strategy allows us to avoid intensive geometry optimisations with hybrid functionals with an acceptable error bar of about 0.1 eV.50 As a catalyst, we considered a newly elucidated carbon nitride structure, which some of us recently validated through experimental results and DFT simulations.35 The optimised lattice parameters for the CNx are: a = 15.593 Å, b = 20.698 Å, γ = 90.2°. Each intermediate's binding energies (ΔE) were calculated with respect to the free molecular species and the catalyst. The Gibbs energies (ΔG) were evaluated by adopting the thermochemistry approach of Nørskov and co-workers, including zero-point energy correction and entropy terms.51,52 The entropy of adsorbed species and gas-phase molecules was calculated through the harmonic approximation, following the methodology previously reported by some of the authors,53 and accounting for the reaction temperature (35 °C); the entropy of solid-state species was considered equal to zero.52 To account for the Pd +2 oxidation state, we simulated all systems with a +2 charge, e.g., [Pd@CNx]2+, following a previous study.35Operando XAS
Pd K edge XAS measurements under reaction conditions were performed at the ID24-DCM beamline of the European Synchrotron Radiation Facility (ESRF) in Grenoble, France. The spectra were collected in fluorescence mode, employing a capillary to host the sample in powder form. The capillary was connected to a liquid-flow setup to circulate the reaction solution containing iodobenzene, solvent, and base. The operando experiment was conducted under visible light irradiation (λ = 457 nm). Background subtraction, energy alignment, and edge jump normalisation were carried out with the ATHENA software as part of the Demeter suite.54Techno-economic analysis
Techno-economic assessments were conducted using Aspen Plus® V11.55 The simulations incorporated a stoichiometric reactor model with fractional conversion parameters to accurately reflect the experimentally observed performance of the processes under steady-state conditions. The thermophysical and chemical properties of all components were sourced from integrated databases (APV110, APESV110, and NISTV110)56 within the software environment. Simulation inputs, including temperature, pressure, and feed streams, were applied identically with experimental operating conditions. In order to compute the total energy consumption of the reactions, electricity was accounted for as a utility input. Cost estimations were determined from real reactor volumes using the Aspen Process Economic Analyzer (APEA). To evaluate raw material costs, the unit prices of the chemical components were obtained from Merck's commercial database.57Life-cycle assessment
Author contributions
A. M. was responsible for catalyst preparation and characterisation, collected catalytic data, and carried out operando XAS experiments. T. A. G. generalised the findings to more reactants. L. A. C. and G. D. L. performed DFT calculations. I. S. K. conducted XAS studies, while M. S. performed XPS studies. M. C. I. conducted techno-economic analysis and life-cycle assessment. N. A. and S. F. J. carried out operando XAS experiments. L. M. and E. B. supervised the operando XAS studies and L. M. did data analysis. G. V. coordinated and supervised the project. All authors contributed to writing the manuscript and approved its final version.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Supplementary information (SI): additional characterisation and catalytic results of the materials; EXAFS fitting results; additional DFT calculations; TEA and LCA details; and NMR characterisations of the reaction products. See DOI: https://doi.org/10.1039/d5gc04693a.
Acknowledgements
A. M. and N. A. thank financial support from the European Commission under the Horizon Europe's Global Challenges and European Industrial Competitiveness programme (“SusPharma”, grant agreement 101057430). T. A. G. gratefully acknowledge financial support from the European Commission through a Marie Skłodowska-Curie Postdoctoral Fellowship (“SOLCAT”, grant agreement 101152890). G. V. and L. A. C. thank the European Research Council (“SAC_2.0”, grant agreement 101075832) for an ERC Starting Grant. M. C. I. thanks the European Commission's Horizon Europe research and innovation programme for the Marie Skłodowska-Curie doctoral fellowship (“GreenDigiPharma”, grant agreement 101073089). G. D. L. and L. M. have received funding from the Italian Ministry of University and Research (MUR) and the European Union – Next Generation EU, Mission 4, Component 1 through the PRIN project “UNDERSAC” (CUP: 2022LRPSTS). The authors thank the European Synchrotron Radiation Facility (ESRF) for allocation of beamtime at the beamline ID24-DCM and Dr Davide Salusso (ESRF) for the support during experiment CH-7183. The authors also kindly acknowledge Xiufang He for support during operando XAS experiments, Cinzia Ferrario for TEM analyses, and Grazia Isa Carla Righetti for support with catalytic data not shown in this paper regarding Pd-based catalysts.References
- F. Khan, M. Dlugosch, X. Liu and M. G. Banwell, Acc. Chem. Res., 2018, 51, 1784–1795 CrossRef PubMed.
- X. Gong, J. Wu, Y. Meng, Y. Zhang, L.-W. Ye and C. Zhu, Green Chem., 2019, 21, 995–999 RSC.
- A. Kamal, V. Srinivasulu, B. N. Seshadri, N. Markandeya, A. Alarifi and N. Shankaraiah, Green Chem., 2012, 14, 2513 RSC.
- H. Jiang, J. Xu, S. Zhang, H. Cheng, C. Zang and F. Bian, Catal. Sci. Technol., 2021, 11, 219–229 RSC.
- R. N. Dhital, C. Kamonsatikul, E. Somsook, K. Bobuatong, M. Ehara, S. Karanjit and H. Sakurai, J. Am. Chem. Soc., 2012, 134, 20250–20253 CrossRef PubMed.
- X. Tian, Y. Guo, W. An, Y.-L. Ren, Y. Qin, C. Niu and X. Zheng, Nat. Commun., 2022, 13, 6186 CrossRef PubMed.
- L. Zhang, A. Wang, J. T. Miller, X. Liu, X. Yang, W. Wang, L. Li, Y. Huang, C.-Y. Mou and T. Zhang, ACS Catal., 2014, 4, 1546–1553 CrossRef.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef.
- V. B. Saptal, V. Ruta, M. A. Bajada and G. Vilé, Angew. Chem., Int. Ed., 2023, 62, e202219306 CrossRef PubMed.
- H. B. Kale, A. D. Kute, R. P. Gaikwad, P. Fornasiero, R. Zbořil and M. B. Gawande, Coord. Chem. Rev., 2024, 502, 215602 Search PubMed.
- X. Cui, W. Li, P. Ryabchuk, K. Junge and M. Beller, Nat. Catal., 2018, 1, 385–397 CrossRef.
- L.-H. Xu, W. Liu and K. Liu, Adv. Funct. Mater., 2023, 33, 2304468 Search PubMed.
- V. Giulimondi, S. Mitchell and J. Pérez-Ramírez, ACS Catal., 2023, 13, 2981–2997 Search PubMed.
- C. Gao, J. Low, R. Long, T. Kong, J. Zhu and Y. Xiong, Chem. Rev., 2020, 120, 12175–12216 CrossRef PubMed.
- S. Wu and P. Schmuki, Adv. Mater., 2024, 37, 2414889 CrossRef PubMed.
- A. Moutsiou, A. Olivati, L. A. Cipriano, A. Sivo, S. M. Collins, Q. M. Ramasse, I. S. Kwon, G. Di Liberto, M. Kanso, R. Wojcieszak, G. Pacchioni, A. Petrozza and G. Vilé, ACS Catal., 2025, 15, 5601–5613 CrossRef PubMed.
- W. Li, X. Zheng, B. Xu, Y. Yang, Y. Zhang, L. Cai, Z. Wang, Y. Yao, B. Nan, L. Li, X. Wang, X. Feng, M. Antonietti and Z. Chen, Angew. Chem., Int. Ed., 2024, 63, e202320014 CrossRef PubMed.
- S. Wang, H. Wang and B. König, J. Am. Chem. Soc., 2021, 143, 15530–15537 Search PubMed.
- A. Savateev, I. Ghosh, B. König and M. Antonietti, Angew. Chem., Int. Ed., 2018, 57, 15936–15947 Search PubMed.
- G. F. S. R. Rocha, M. A. R. Da Silva, A. Rogolino, G. A. A. Diab, L. F. G. Noleto, M. Antonietti and I. F. Teixeira, Chem. Soc. Rev., 2023, 52, 4878–4932 RSC.
- M. Gawande, P. Fornasiero and R. Zbořil, ACS Catal., 2020, 10, 2231–2259 CrossRef.
- G. Vilé, D. Albani, M. Nachtegaal, Z. Chen, D. Dontsova, M. Antonietti, N. López and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2015, 54, 11265–11269 CrossRef PubMed.
- S. Büchele, A. Yakimov, S. M. Collins, A. Ruiz-Ferrando, Z. Chen, E. Willinger, D. M. Kepaptsoglou, Q. M. Ramasse, C. R. Müller, O. V. Safonova, N. López, C. Copéret, J. Pérez-Ramírez and S. Mitchell, Small, 2022, 18, 2202080 CrossRef PubMed.
- L. Jiao, R. Zhang, G. Wan, W. Yang, X. Wan, H. Zhou, J. Shui, S.-H. Yu and H.-L. Jiang, Nat. Commun., 2020, 11, 2831 CrossRef PubMed.
- V. Ruta, A. Sivo, L. Bonetti, M. A. Bajada and G. Vilé, ACS Appl. Nano Mater., 2022, 5, 14520–14528 Search PubMed.
- A. Sivo, V. Ruta, V. Granata, O. Savateev, M. A. Bajada and G. Vilé, ACS Sustainable Chem. Eng., 2023, 11, 5284–5292 CrossRef PubMed.
- K. S. Lakhi, D.-H. Park, K. Al-Bahily, W. Cha, B. Viswanathan, J.-H. Choy and A. Vinu, Chem. Soc. Rev., 2017, 46, 72–101 Search PubMed.
- R. Arrigo, M. E. Schuster, Z. Xie, Y. Yi, G. Wowsnick, L. L. Sun, K. E. Hermann, M. Friedrich, P. Kast, M. Hävecker, A. Knop-Gericke and R. Schlögl, ACS Catal., 2015, 5, 2740–2753 Search PubMed.
- Z. Chen, E. Vorobyeva, S. Mitchell, E. Fako, M. A. Ortuño, N. López, S. M. Collins, P. A. Midgley, S. Richard, G. Vilé and J. Pérez-Ramírez, Nat. Nanotechnol., 2018, 13, 702–707 CrossRef PubMed.
- M. A. Bajada, G. Di Liberto, S. Tosoni, V. Ruta, L. Mino, N. Allasia, A. Sivo, G. Pacchioni and G. Vilé, Nat. Synth., 2023, 2, 1092–1103 CrossRef.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH, Weinheim, 2011 Search PubMed.
- L. Piccolo, P. Afanasiev, F. Morfin, T. Len, C. Dessal, J.-L. Rousset, M. Aouine, F. Bourgain, A. Aguilar-Tapia, O. Proux, Y. Chen, L. Soler and J. Llorca, ACS Catal., 2020, 10, 12696–12705 Search PubMed.
- M. E. Usteri, G. Giannakakis, A. Bugaev, J. Pérez-Ramírez and S. Mitchell, ACS Catal., 2024, 14, 12635–12646 Search PubMed.
- G. Giannakakis, M. E. Usteri, A. Bugaev, A. Ruiz-Ferrando, D. Faust Akl, N. López, S. Fantasia, K. Püntener, J. Pérez-Ramírez and S. Mitchell, ACS Catal., 2025, 15, 284–295 CrossRef PubMed.
- N. Allasia, S. Xu, S. F. Jafri, E. Borfecchia, L. A. Cipriano, G. Terraneo, S. Tosoni, L. Mino, G. Di Liberto, G. Pacchioni and G. Vilé, Small, 2025, 21, e2408286 Search PubMed.
- V. B. Saptal, C. Saetta, A. Laufenböck, M. Sterrer, I. S. Kwon, A. Lucotti, M. Tommasini, O. Tomanec, A. Bakandritsos, G. Di Liberto, G. Pacchioni and G. Vilé, J. Am. Chem. Soc., 2025, 147, 18524–18540 CrossRef PubMed.
- G. Henkelman, A. Arnaldsson and H. Jónsson, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- G. Gao, Y. Jiao, E. R. Wacławik and A. Du, J. Am. Chem. Soc., 2016, 138, 6292–6297 CrossRef PubMed.
- E. Biasin, T. B. van Driel, K. S. Kjær, A. O. Dohn, M. Christensen, T. Harlang, P. Chabera, Y. Liu, J. Uhlig, M. Pápai, Z. Németh, R. Hartsock, W. Liang, J. Zhang, R. Alonso-Mori, M. Chollet, J. M. Glownia, S. Nelson, D. Sokaras, T. A. Assefa, A. Britz, A. Galler, W. Gawelda, C. Bressler, K. J. Gaffney, H. T. Lemke, K. B. Møller, M. M. Nielsen, V. Sundström, G. Vankó, K. Wärnmark and S. E. Canton, Phys. Rev. Lett., 2016, 117, 013002 CrossRef PubMed.
- G. Vilé, G. Di Liberto, S. Tosoni, A. Sivo, V. Ruta, M. Nachtegaal, A. H. Clark, S. Agnoli, Y. Zou, A. Savateev, M. Antonietti and G. Pacchioni, ACS Catal., 2022, 12, 2947–2958 Search PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 Search PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 Search PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 Search PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982–9985 Search PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 Search PubMed.
- G. Di Liberto, L. A. Cipriano and G. Pacchioni, ACS Catal., 2022, 12, 5846–5856 CrossRef CAS.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. R. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef.
- J. K. Nørskov, T. Bligaard, J. Rossmeisl and C. H. Christensen, Nat. Chem., 2009, 1, 37–46 CrossRef PubMed.
- E. Di Simone, G. Vilé, G. Di Liberto and G. Pacchioni, ACS Catal., 2025, 15, 447–456 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 Search PubMed.
- Aspen Plus 11, Aspen Technology Inc., Bedford, MA, USA, 2019 Search PubMed.
- Aspen Plus 11 User Guide, Aspen Technology Inc., Bedford, MA, USA, 2019 Search PubMed.
- Sigma-Aldrich, Merck KGaA, Darmstadt, Germany, https://www.sigmaaldrich.com/IT/it (accessed July 6, 2025).
- ISO, ISO 14040: Environmental management, life cycle asssessment, principles and framework, 2006; https://www.iso.org/standard/37456.html (accessed July 6, 2025).
- L. Benini, S. Sala, S. Manfredi and M. Góralczyk, Quantitative Methods for Life Cycle Assessment, Publications Office of the European Union, Luxembourg, 2014, p. JRC92824 Search PubMed.
- K. Richardson, W. Steffen, W. Lucht, J. Bendtsen, S. E. Cornell, J. F. Donges, M. Drüke, I. Fetzer, G. Bala, W. von Bloh, G. Feulner, S. Fiedler, D. Gerten, T. Gleeson, M. Hofmann, W. N. Huiskamp, M. Kummu, C. Mohan, D. Nogués-Bravo, S. Petri, M. Porkka, S. Rahmstorf, S. Schaphoff, K. Thonicke, A. Tobian, V. Virkki, L. Wang-Erlandsson, L. Weber and J. Rockström, Sci. Adv., 2023, 9, eadh2458 Search PubMed.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- S. Andreasi Bassi, F. Biganzoli, N. Ferrara, A. Amadei, A. Valente, S. Sala and F. Ardente, Updated characterisation and normalisation factors for the Environmental Footprint 3.1 method, Publications Office of the European Union, Luxembourg, 2023, p. JRC130796 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |