Selective hydrodeoxygenation of lignin phenolics to cyclohexanols over low-Ru catalysts
Rumin
Ma
,
Xueying
Gao
,
Xiancheng
Li
,
Shuizhong
Wang
 * and
Guoyong
Song
* and
Guoyong
Song
 *
*
State Key Laboratory of Efficient Production of Forest Resources, Beijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing 100083, People's Republic of China. E-mail: szwang@bjfu.edu.cn; songg@bjfu.edu.cn
First published on 18th November 2025
Abstract
The selective conversion of lignin-derived phenolics into cyclohexanols through demethoxylation and ring hydrogenation, while preserving hydroxyl groups, remains a significant catalytic challenge but offers a green alternative to fossil resources. Here, we report a low-loaded Ru catalyst supported on Al2O3 (Ru0.36CN/γ-Al2O3) that enables efficient production of cyclohexanols from lignin-derived phenolics in water. Under optimal conditions, 4-propylguaiacol is converted to 4-propylcyclohexanol in 77% yield. The catalyst also exhibits excellent recyclability and broad substrate scope. A plausible reaction pathway is proposed based on detailed studies of intermediate compounds. Moreover, lignin-derived bio-oil obtained from the reductive catalytic fractionation (RCF) of Chinese fir is upgraded to alkyl cyclohexanols with a yield of 23.2 wt% and a selectivity of 69.5%. This work highlights the potential of low-loaded Ru catalysts for the sustainable production of cyclohexanols from lignin-derived feedstocks.
Green foundation1. We report a sustainable catalytic strategy to produce cyclohexanols from renewable lignin in water using a low-loading Ru catalyst. By providing a biomass-based alternative to petroleum-derived precursors and operating in a benign solvent, this approach advances greener production of cyclohexanols used in nylons, plasticizers, pharmaceuticals, and fine chemicals.2. Our green chemistry achievements include the use of a realistic wood-derived feedstock, a low-loading yet efficient Ru catalyst, and a benign aqueous medium to produce cyclohexanols. The process delivers an E-factor of 28.7, which falls within the typical range for commodity chemical production. 3. This process could be further improved by replacing the precious Ru catalyst with Earth-abundant metals. While our catalyst functions successfully for four cycles, slight hydrolysis of the γ-Al2O3 support in the fifth cycle highlights the need for enhanced long-term stability. |
Introduction
Cyclohexanols are crucial intermediates for manufacturing nylon, plasticizers, pharmaceuticals, and fine chemicals.1–3 Their annual production exceeds 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons,4 with the global manufacturing market projected to grow from $11.5 billion in 2024 to approximately $16.2 billion by 2031.5 Currently, cyclohexanol is commercially produced either by the direct oxidation of petroleum-derived cyclohexane or by the hydrogenation of fossil-based phenol.1 However, these routes usually require harsh reaction conditions, exhibit low conversion and selectivity, and involve energy-intensive separation processes.6,7 Therefore, developing efficient and sustainable pathways for producing cyclohexanols from renewable biomass is of considerable interest and importance.
000 tons,4 with the global manufacturing market projected to grow from $11.5 billion in 2024 to approximately $16.2 billion by 2031.5 Currently, cyclohexanol is commercially produced either by the direct oxidation of petroleum-derived cyclohexane or by the hydrogenation of fossil-based phenol.1 However, these routes usually require harsh reaction conditions, exhibit low conversion and selectivity, and involve energy-intensive separation processes.6,7 Therefore, developing efficient and sustainable pathways for producing cyclohexanols from renewable biomass is of considerable interest and importance.
Lignin, a major component of lignocellulosic biomass, represents the largest renewable source of aromatic compounds on Earth.8–10 It is a natural polymer composed of methoxylated phenylpropane units, p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) moieties, primarily connected by C–O and C–C linkages.8,11,12 Lignin can be converted into monophenolic compounds bearing diverse side chains through strategies such as pyrolysis, hydrogenolysis, and oxidation.13–15 Typically, lignin-depolymerized monophenols contain a phenyl ring substituted with a phenolic group, a para-alkyl substituent, and one or two ortho-methoxy groups, rendering them ideal feedstocks for the production of cyclohexanol derivatives. However, this transformation remains challenging because demethoxylation, ring hydrogenation, and the undesired dehydroxylation share similar energy barriers under reductive conditions.7 Heterogeneous catalysts with high loadings of noble metals (such as Ru,16–20 Pd,21,22 and Pt23) or non-noble metals (such as Ni,24–29 Co,30–32 and Mo33) have been shown to efficiently convert lignin-derived monophenols into cyclohexanols (Fig. 1). For instance, the Pt1/NiAl-LDH catalyst (2.1 wt% Pt) reported by Lan et al. can catalyse the hydrodeoxygenation (HDO) of 4-propylguaiacol (4-PrG) to 4-propylcyclohexanol (4-PrCHOL) with 100% conversion and 90% selectivity.23 Hu et al. designed a bimetallic RuCoNx/NC catalyst (9.2 wt% Ru, 5.3 wt% Co), which exhibited 92% selectivity for 4-propylcyclohexanol at 210 °C.34 Zhang and coworkers reported a magnetic 0.2Co1-NPs@NC catalyst (16.7 wt% Co) that achieved selective HDO of 4-propylguaiacol to cyclohexanols with 92.8% yield.31 While noble metal-based catalysts generally exhibit superior performance compared to their non-precious counterparts, their scarcity, high cost, and low atom utilisation limit their practical application on a large scale.
 | ||
| Fig. 1 Schematic overview of the catalytic HDO of lignin-derived aromatics into cyclohexanols with high- and low-loaded metal catalysts. | ||
Reducing metal loading while increasing the dispersion of active sites can enhance catalytic activity and improve metal atom utilisation.35,36 Nevertheless, reports on low-loaded (<1 wt%) catalysts for efficient hydrodeoxygenation (HDO) of lignin-derived monomers to cyclohexanols remain scarce. Han et al. showed that Ru single atoms on CeO2 (0.1 wt% Ru) efficiently catalyse benzene ring hydrogenation and demethoxylation in guaiacol, yielding 99.9% cyclohexanol.7 Lu and co-workers reported that Ru supported on tungstated zirconia (0.67 wt% Ru) enabled the HDO of guaiacol to cyclohexanol with a 92% yield.37 Wan et al. found that Ru single atoms on a titania/carbon composite (0.2 wt% Ru) facilitated selective HDO of guaiacol to cyclohexanol with 99% yield.38 Notably, these studies focused on simple phenolics rather than complex lignin-derived oils with multiple phenolic compounds.
Herein, we report a low-loaded Ru catalyst supported on γ-Al2O3, denoted as Ru0.36CN/γ-Al2O3 (0.36 wt% Ru), for the selective HDO of lignin-derived phenolics to cyclohexanols. This catalyst exhibited high activity, excellent selectivity, and remarkable stability for converting 4-PrG to 4-PrCHOL via selective demethoxylation and aromatic ring hydrogenation. Experiments with model compounds enabled the proposal of a plausible reaction pathway under the current conditions. This catalyst showed broad feedstock compatibility, efficiently converting a range of lignin-derived phenolic monomers and dimers. Moreover, when depolymerised softwood lignin oil is used, the catalyst delivers high yields with excellent selectivity toward cyclohexanol derivatives.
Results and discussion
Synthesis and characterisation of the catalyst
The Ru0.36CN/γ-Al2O3 catalyst was prepared via a wet impregnation of γ-Al2O3 with RuIII(tpy)Cl3 (tpy = 2,2′,2″-terpyridine) as the precursor, followed by pyrolysis at 600 °C under a N2 atmosphere (see the SI). For comparison, a series of RuxCN/γ-Al2O3 catalysts with Ru loadings of 0.10, 0.25, and 0.67 wt% were also prepared using the same method.The Ru content in the Ru0.36CN/γ-Al2O3 catalyst was determined to be 0.36 wt% by inductively coupled plasma optical emission spectrometry (ICP-OES) analysis (Table S1). The N2 adsorption–desorption isotherms of Ru0.36CN/γ-Al2O3 displayed a typical type IV pattern,39,40 indicative of a mesoporous structure, with a specific surface area of 137.2 m2 g−1 and a pore volume of 0.24 cm3 g−1 (Fig. S1 and Table S2). Powder X-ray diffraction (XRD) analysis revealed distinct diffraction peaks at 2θ = 28.0°, 35.1°, and 54.2°, which were indexed to the (110), (101), and (211) planes of RuO2 (JCPDS No. 40-1290), confirming the presence of crystalline RuO2 species (Fig. 2a).41,42 Notably, no diffraction peaks corresponding to metallic Ru were detected. In addition, the characteristic peaks corresponding to the γ-Al2O3 support were also observed at 2θ = 37.6°, 45.9°, and 67.0°, being consistent with the standard pattern of γ-Al2O3 (JCPDS No. 10-0425).43 X-ray photoelectron spectroscopy (XPS) analysis confirmed the presence of Ru, Al, O, C, and a trace amount of N in the Ru0.36CN/γ-Al2O3 catalyst (Fig. S2). In the high-resolution Ru 3d spectrum (Fig. 2b), only the Ru 3d5/2 signal was clearly detected due to the inherent overlap between the Ru 3d3/2 and C 1s regions. A distinct peak at 280.9 eV was assigned to Ru4+, suggesting the presence of oxidised Ru species.41 To further clarify the oxidation state of Ru, the Ru 3p spectrum was also recorded (Fig. 2c). The characteristic Ru 3p spectrum displayed a spin–orbit doublet at 462.4 eV (Ru 3p3/2) and 483.8 eV (Ru 3p1/2), corresponding to Ru4+, along with two satellite peaks at 465.9 eV and 486.7 eV (denoted as Sat.).41,42,44 Hydrogen temperature-programmed reduction (H2-TPR) of Ru0.36CN/γ-Al2O3 was conducted to investigate the reducibility of the catalysts. The sample exhibited two distinct reduction peaks: a low-temperature peak at around 200 °C, corresponding to the reduction of weakly bound Ru species, and a broad high-temperature peak centered at approximately 450 °C, attributable to the reduction of Ru species strongly interacting with Al2O3 (Fig. S3).45
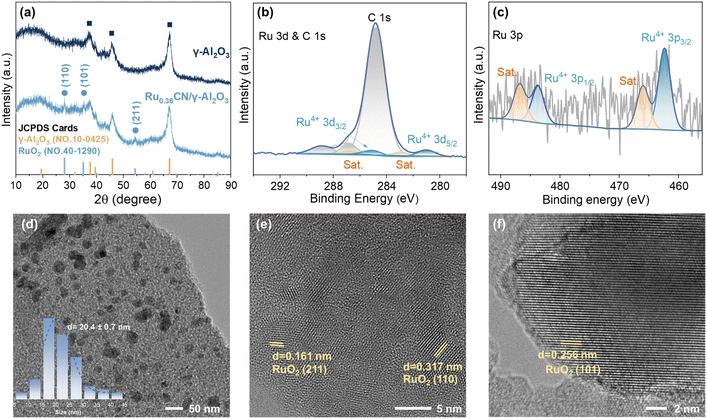 | ||
| Fig. 2 Morphological and structural characterisation of Ru0.36CN/γ-Al2O3: (a) XRD patterns; (b and c) XPS spectra of Ru 3d and Ru 3p, respectively; and (d–f) HR-TEM images. | ||
High-resolution TEM (HR-TEM) images of Ru0.36CN/γ-Al2O3 revealed that RuO2 nano-crystallites were dispersed on the support, with an average particle diameter of approximately 20.4 nm (Fig. 2d–f). Distinct lattice fringes with interplanar spacings of 0.161, 0.317, and 0.256 nm were observed, which, according to Bragg's equation, correspond to the (211), (110), and (101) crystal planes of RuO2, respectively.41,42 These observations were in line with the results from XRD and XPS analyses, further confirming that the Ru species in the catalyst predominantly existed in the form of RuO2.
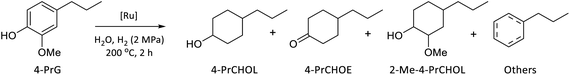
HDO of 4-propylguaiacol into 4-propylcyclohexanol
To evaluate the catalytic performance of the as-prepared catalysts, 4-propylguaiacol, a typical lignin-depolymerised product,46,47 was selected as the model substrate. A preliminary screening of supports (α-Al2O3 and γ-Al2O3) and solvents (H2O, n-hexane, and MeOH) was performed, and γ-Al2O3 and water were identified as the optimal support and solvent, respectively (Tables S3 and S4). Therefore, the catalytic HDO of 4-PrG over various Ru-based γ-Al2O3 catalysts was carried out in H2O at 200 °C and 2 MPa H2 for 2 h, and the results are summarized in Table 1. Almost complete conversion of 4-PrG was achieved at Ru loadings ≥0.25 wt%, while Ru0.10CN/γ-Al2O3 gave merely 36.4% conversion. The selectivity to 4-propylcyclohexanol (4-PrCHOL) exhibited a volcano-shaped trend with increasing Ru loading, reaching a maximum of 79.4% over Ru0.36CN/γ-Al2O3. In contrast, 2-methoxy-4-propylcyclohexanol (2-Me-4-PrCHOL), formed via arene hydrogenation of 4-PrG, exhibited an opposite trend as the Ru loading increased. The total selectivity of 4-PrCHOL and 2-Me-4-PrCHOL exceeded 90% under these conditions. These results suggested that the formation of 4-PrCHOL and 2-Me-4-PrCHOL likely proceeds via parallel reaction pathways. In these reactions, 4-propylcyclohexanone (4-PrCHOE) was also detected in trace amounts, likely serving as an intermediate to 4-PrCHOL. Small quantities of dehydroxylated products, including propylcyclohexane and propylbenzene, were also formed (3.8–7.8%). Notably, Ru0.36/γ-Al2O3, prepared without doped nitrogen, afforded a lower 4-PrCHOL selectivity (68%) compared with Ru0.36CN/γ-Al2O3, despite achieving a high 4-PrG conversion (96.8%). No conversion of 4-PrG occurred over γ-Al2O3 alone, highlighting the essential role of Ru species, even at low loadings. Overall, Ru0.36CN/γ-Al2O3 achieved a good balance between demethoxylation, ring hydrogenation, and suppression of dehydroxylation, affording 4-PrCHOL in 77% yield.| Entry | Catalyst | Conversion (%) | Selectivity (%) | Yield of 4-PrCHOL (%) | |||
|---|---|---|---|---|---|---|---|
| 4-PrCHOL | 4-PrCHOE | 2-Me-4-PrCHOL | Others | ||||
| Reaction conditions: 4-PrG (1 mmol), catalyst (50 mg), H2O (3 mL), H2 (2 MPa), 200 °C, and 2 h. 4-PrG: 4-propylguaiacol, 4-PrCHOL: 4-propylcyclohexanol, 4-PrCHOE: 4-propylcyclohexanone, and 2-Me-4-PrCHOL: 2-methoxy-4-propylcyclohexanol. | |||||||
| 1 | Ru0.10CN/γ-Al2O3 | 36.4 | 44.2 | 4.3 | 46.8 | 4.6 | 15.5 |
| 2 | Ru0.25CN/γ-Al2O3 | 97.7 | 66.3 | 2.2 | 27.7 | 3.8 | 62.0 |
| 3 | Ru0.36CN/γ-Al2O3 | 98.4 | 79.4 | 3.0 | 11.2 | 6.5 | 76.9 |
| 4 | Ru0.67CN/γ-Al2O3 | 99.1 | 61.6 | 2.0 | 28.6 | 7.8 | 58.6 |
| 5 | Ru0.36/γ-Al2O3 | 96.8 | 68.4 | 2.1 | 25.3 | 4.3 | 64.6 |
| 6 | γ-Al2O3 | 0 | 0 | 0 | 0 | 0 | 0 |
Parameter effects
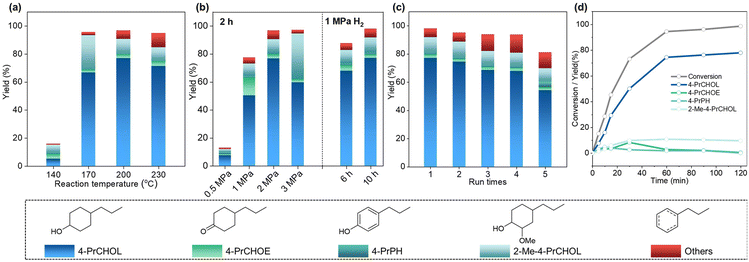
The effects of reaction temperature, time, and H2 pressure on the HDO of 4-PrG over Ru0.36CN/γ-Al2O3 were investigated (Fig. 3a and b). At 2 MPa H2, lowering the temperature to 140 °C resulted in sluggish 4-PrG conversion (18.7%), with trace formation of 4-PrPH. Increasing the temperature to 170 °C enabled complete 4-PrG conversion, affording 4-PrCHOL in 66.8% yield. The maximum yield of 4-PrCHOL was achieved at 200 °C (77%), whereas higher temperatures (230 °C) promoted dehydroxylation due to facilitated cleavage of the Chromatic–OH bond. Given that demethoxylation, ring hydrogenation, and dehydroxylation are all H2-dependent steps, the effect of H2 pressure was examined at 200 °C for 2 h. At 0.5 and 1 MPa H2, the conversion of 4-PrG was limited to 14.3% and 79% in 2 h, with 4-PrCHOL yields of 7.7% and 51%, respectively. Under both conditions, trace amounts of 4-PrPH were observed. Increasing H2 pressure produced a volcano-shaped trend in 4-PrCHOL yield, peaking at 2 MPa (77%), while the yield of 2-Me-4-PrCHOL increased steadily to 32.7% at 3 MPa. These results suggested that high H2 pressure is conducive to ring hydrogenation. At 1 MPa H2 and 200 °C, extending the reaction time to 10 h increased 4-PrG conversion to 99.4% and 4-PrCHOL yield to 77.2%, comparable to the results obtained at 2 MPa H2 in 2 h. These findings suggested that 4-PrCHOL formation is thermodynamically favoured under these catalytic conditions.A time profile of 4-PrG conversion over Ru0.36CN/γ-Al2O3 was measured at 200 °C and 2 MPa H2 (Fig. 3d and Table S7). As the reaction progressed, both the conversion of 4-PrG and the yield of 4-PrCHOL increased, reaching their highest levels at 1 h. Kinetic analysis showed that the consumption of 4-PrG followed first-order behaviour. An initial accumulation followed by a decline in 4-PrCHOE was observed, indicating its role as an intermediate. The TOF values, which depend on the instantaneous concentration of 4-PrG,48 were calculated to be 1014 h−1 at 45% conversion (0.25 h) and 530 h−1 at 94% conversion (1 h).
The recyclability of Ru0.36CN/γ-Al2O3 was evaluated under the conditions of 200 °C and 1 MPa H2 (Fig. 3c and Table S8). The catalyst was readily recovered by simple filtration, washed with water and ethanol, and reused directly. After four cycles, it maintained high activity, giving 98.1% 4-PrG conversion and a 68% 4-PrCHOL yield, comparable to those of the fresh catalyst. In the fifth cycle, however, the performance declined, with conversion and yield dropping to 84.7% and 54%, respectively. ICP-OES analysis showed a reduced Ru content of 0.26 wt% (Table S1), indicating partial leaching. XRD analysis further revealed the disappearance of γ-Al2O3 diffraction peaks and the appearance of boehmite (γ-AlO(OH)) peaks (Fig. S4),43 suggesting hydration of the support at elevated temperatures. Together, Ru loss and support transformation likely accounted for the decreased catalytic activity.
Based on these results, the environmental (E) factor for current transformation was determined to evaluate its sustainability, using the methodology reported in the literature.49,50 As summarized in Table S9, the Ru0.36CN/γ-Al2O3 catalysed-HDO of 4-PrG exhibited an sEF of 1.3 and an EF of 28.7, which falls within the typical range reported for fine chemical processes (E-factor: 5–50).51 These values are comparable to, or even better than, those reported for previously developed systems, underscoring the green and sustainable advantages of this catalytic strategy.
Plausible reaction pathway
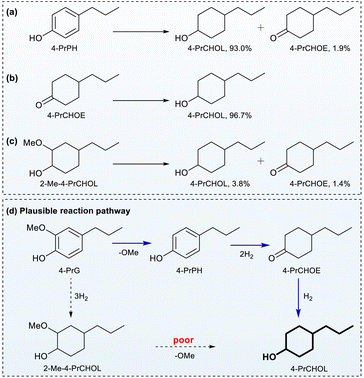
To gain mechanistic insight into the HDO of 4-PrG over Ru0.36CN/γ-Al2O3, the reactivity of several products was examined. When 4-PrPH and 4-PrCHOE, detected in minor amounts during the HDO of 4-PrG, were treated with Ru0.36CN/γ-Al2O3 at 200 °C under 1 MPa H2, both underwent nearly complete conversion, affording 4-PrCHOL in high yields (Fig. 4a and b). A trace amount of 4-PrCHOE was also observed in the transformation of 4-PrPH. These results suggested that both 4-PrPH and 4-PrCHOE act as intermediates enroute to 4-PrCHOL under the present catalytic conditions. In contrast, when 2-Me-4-PrCHOL (isomeric mixture) was used as the substrate, only 8.5% conversion was achieved, yielding 4-PrCHOL (3.8%) and 4-PrCHOE (1.4%) in low amounts, suggesting that the cleavage of C(sp3)–OAr in 2-Me-4-PrCHOL is unfavourable under the current catalytic system. This scenario was different from those of the previous Ru-,52 Pd-,53 or Ni-based54 catalysts, which usually promoted aromatic ring hydrogenation before hydrogenolysis of the Caryl–OCH3 bond.Based on these findings, a plausible reaction pathway was proposed (Fig. 4d). The demethylation of 4-PrG affords 4-PrPH, and this pathway competes with arene hydrogenation, resulting in the formation of 2-Me-4-PrCHOL. The resulting 4-PrPH then undergoes arene hydrogenation to form 4-PrCHOE, followed by ketone hydrogenation to yield 4-PrCHOL.
HDO of various lignin-derived phenols
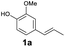
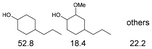
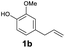
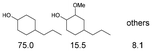
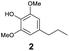
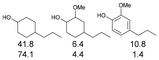
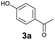
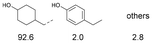
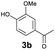
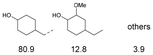
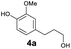
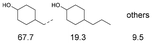
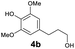
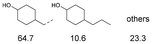
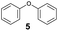
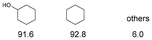
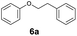
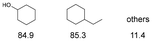
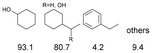
As previously reported, catalytic depolymerisation or pyrolysis of lignin could generate a diverse array of phenolic monomer compounds, typically featuring one or two methoxy groups and various side chains, such as propyl, propanol, propenyl, or allyl groups.8,13 This molecular diversity stems from the intrinsic structural complexity and variability of lignin, as well as the nature of the catalytic systems employed. To assess the general applicability of the Ru0.36CN/γ-Al2O3 catalyst, several representative lignin-derived model compounds were subjected to the current HDO reaction system (Table 2). Substrates 1a and 1b (entries 1 and 2), which contain a single methoxy group and an unsaturated alkyl side chain, were almost completely converted (>99%), resulting in the production of 4-PrCHOL (52.8–75.0%) and 2-Me-4-PrCHOL (15.5–18.4%). The observed product distribution was consistent with the results obtained from the HDO of 4-PrG, indicating a similar reaction pathway involving initial hydrogenation of the side chain and subsequent demethoxylation followed by benzene ring hydrogenation. For 4-propylsyringol 2 (entry 3), having two methoxy groups, HDO using Ru0.36CN/γ-Al2O3 resulted in a 73.8% conversion and a 41.8% yield of 4-PrCHOL. No product from direct ring hydrogenation was detected, demonstrating again that demethoxylation preceded hydrogenation under the current system. Notably, 4-PrG was obtained in 10.8% yield via removal of one methoxy group of 4-propylsyringol 2, revealing that Ru0.36CN/γ-Al2O3 has limited activity in cleaving multiple methoxy groups under the present conditions. Upon increasing the Ru loading to 0.67 wt% (Ru0.67CN/γ-Al2O3), the catalytic performance was significantly enhanced, generating 93.3% conversion and a 74.1% yield of 4-PrCHOL. In the case of ketone-functionalized substrates 3a and 3b (entries 4 and 5), the HDO reaction produced ethyl- and methyl-substituted cyclohexanols with combined yields of 92.6% and 80.9%, respectively, at almost complete conversion (>99%). Similarly, treatment of benzylic alcohol derivatives 4a and 4b (entries 6 and 7, main products from Pd-catalysed depolymerisation of lignin55) with Ru0.67CN/γ-Al2O3 yielded mixtures of 4-PrCHOL and ethyl-/methyl-substituted cyclohexanols with combined yields of 87.0% and 75.3%, respectively, at >99% conversion. The predominant formation of ethyl-substituted cyclohexanol was likely due to the cleavage of the benzylic–CH2OH moiety, as previously reported.56 Compared with 4-PrG, catalytic HDO of the compounds with multifunctional groups (compounds 2–4, entries 2–7) required either higher Ru loading or prolonged reaction time for effective conversion. This scenario might be due to the presence of additional methoxy groups or more complex end-chain functionalities (including ketone and propanol groups), which negatively affected the HDO process, such as the steric effect or stronger electron donating nature of the methoxy group.37,40Given the presence of dimeric compounds in lignin-depolymerised products, catalytic HDO of two simple model compounds with Ru0.36CN/γ-Al2O3 were further conducted under current conditions. In the case of diaryl ether 5 (entry 8), which mimics the 4-O-5 linkage in lignin, effective C–O bond cleavage and benzene ring hydrogenation (>99% conversion) were observed, producing cyclohexanol and cycloalkane as the primary products with 91.6% and 92.8% yields, respectively. For the simple β-O-4-type model compound 6 (entries 9 and 10), selective cleavage of the β-O-4 bond followed by hydrogenation of the aromatic rings led to the formation of cyclohexanol (84.9–93.1%) and ethylcyclohexane (80.7–85.3%). The above results revealed that RuCN/γ-Al2O3 catalysts have good catalytic activity toward both the cleavage of lignin linkages and the HDO of phenolic compounds.
HDO of lignin-derived bio-oil to cyclohexanols
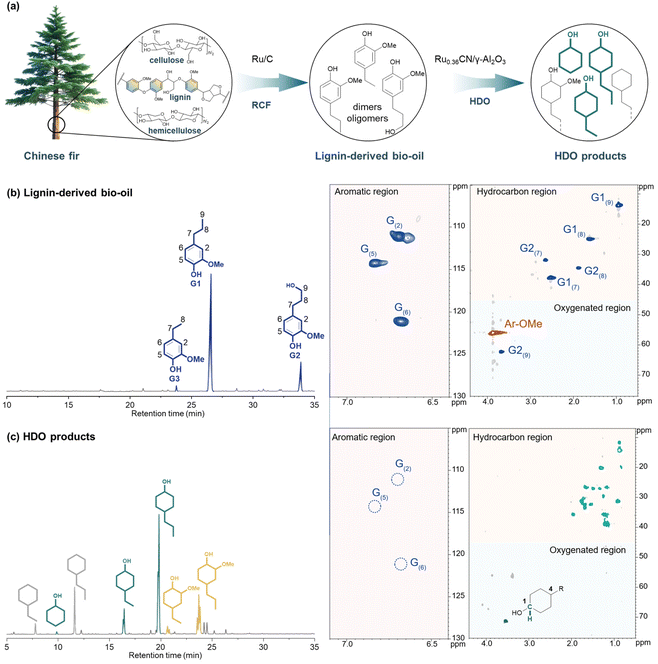
Reductive catalytic fractionation has been proved to be an effective strategy for converting native lignin into aromatic monomers with high yield and selectivity,8,13–15 whereas the subsequent upgrading of the lignin-derived phenolics into cyclohexanols using low-loaded metal catalysts has been rarely reported. Building on our established low-loaded Ru catalytic system, we further performed the direct Ru0.36CN/γ-Al2O3-catalysed HDO of lignin-derived bio-oil, which was obtained from the RCF of Chinese fir (Cunninghamia lanceolata) sawdust over commercial Ru/C (Fig. 5). As depicted in Fig. S5, gel permeation chromatography (GPC) analysis revealed that the bio-oil had an average molecular weight (Mw) of 522 g mol−1, consisting primarily of monomers, dimers, and oligomers. Gas chromatography (GC) and GC–mass spectrometry (GC-MS) analyses identified 4-propylguaiacol (G1) and 4-propanolguaiacol (G2) as the major components, along with trace amounts of 4-ethylguaiacol (G3) (Fig. 5b). A monomer yield of 207.5 mg per gram of bio-oil (ca. 1.2 mmolmonomer gbio-oil−1) was determined by comparison with authentic standards, either commercially purchased or synthesized independently. These RCF results were consistent with a previous result.57 Furthermore, 2D HSQC NMR analysis also confirmed that 4-propylguaiacol and 4-propanolguaiacol were the predominant products in the resulting bio-oil.Catalytic HDO of lignin-derived bio-oil (209 mg) was conducted in H2O with Ru0.36CN/γ-Al2O3 at 200 °C under 2 MPa H2. Upon completion of the HDO reaction, the liquid products were extracted with deuterated chloroform, dried over anhydrous MgSO4, and subsequently analysed by GC and 2D HSQC NMR. GC analysis revealed the formation of ethyl- and propyl-substituted cyclohexanols and cycloalkanes, with a total yield of 23.2 wt% based on the mass of the initial bio-oil. The molar yield of HDO products was calculated to be approximately 1.61 mmolmonomer gbio-oil−1, exceeding that of the phenolic monomers in the untreated bio-oil. This suggested that, in addition to monomers, some dimeric and oligomeric compounds were also converted into relative products. Among the HDO products, cyclohexanols were the predominant compounds, exhibiting a high selectivity of 69.5%, particularly for 4-PrCHOL. These results were consistent with those obtained from the catalytic HDO of 4-propylguaiacol (4-PrG). Further insights were provided by 2D HSQC NMR analysis of the HDO products (Fig. 5c). In both the aromatic and aliphatic regions, the cross-signals corresponding to 4-propylguaiacol and 4-propanolguaiacol, which presented mainly in lignin-derived bio-oil, were no longer detectable, demonstrating the efficient HDO of lignin-derived bio-oil. Meanwhile, a new set of correlation signals in the aliphatic region, attributed to the 13C–1H NMR interactions of ethyl- and propyl-substituted cyclohexanols and cycloalkanes, was also observed (labeled as light green). In particular, the 13C–1H cross signal of the C1 position in cyclohexanols (labeled as blackish green) was observed at δC/δH = 71.2/3.5 ppm, as demonstrated by the NMR spectra of the standard sample (Fig. S6). The prevalence of cyclohexanol derivatives in the 2D HSQC spectra was in line with the results of GC analysis. The above results indicated that Ru0.36CN/γ-Al2O3 exhibited high HDO activity and selectivity toward the conversion of both authentic and complex lignin-derived phenolics into cyclohexanols.
Conclusions
In summary, we developed a low-loaded Ru0.36CN/γ-Al2O3 catalyst with nanometric RuO2 species as active sites, displaying excellent performance in the selective HDO of 4-propylguaiacol into 4-propylcyclohexanol (77% yield) under the conditions of 200 °C and 2 MPa H2 for 2 h. Reaction pathway studies revealed that the HDO reaction should proceed via initial demethylation followed by hydrogenation to cyclohexanols. The catalyst showed broad tolerance toward lignin-derived monomers and dimers with varying methoxy group contents and side-chain types. Furthermore, using lignin-derived bio-oils containing phenolic monomers, dimers and oligomers from the depolymerisation of Chinese fir, the Ru0.36CN/γ-Al2O3 catalyst achieved a total mass yield of 23.2% and a selectivity of 69.5% for alkyl cyclohexanols. This work provides an efficient and sustainable approach to producing cyclohexanols from lignin-derived biophenolics using a low loading of a noble metal catalyst.Author contributions
G. S., S. W., and R. M. conceived the project. R. M. performed the experiments. S. W. and R. M. analysed the data and wrote the manuscript. X. G. and X. L. performed data curation. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the Supplementary Information (SI), which provides detailed catalyst preparation procedures, characterization results (ICP, BET, H2-TPR, etc.), experimental details for the hydrodeoxygenation reactions, relevant catalytic results, E-factor calculations, and calibration curves. See DOI: https://doi.org/10.1039/d5gc04644k.Acknowledgements
This work was funded by the State Key Program of the National Natural Science Foundation of China (52430002), the National Natural Science Foundation of China (22208024) and the Fundamental Research Funds for the Central Universities (JCYJ202509).References
- W. B. Fisher and J. F. VanPeppen, in Kirk–Othmer Encyclopedia of Chemical Technology, 2000, DOI:10.1002/0471238961.0325031206091908.a01.
- S. Schmidt, C. Scherkus, J. Muschiol, U. Menyes, T. Winkler, W. Hummel, H. Gröger, A. Liese, H.-G. Herz and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2015, 54, 2784–2787 CrossRef CAS.
- Y. Zhu, L. Gao, L. Wen, B. Zong, H. Wang and M. Qiao, Green Chem., 2021, 23, 1185–1192 RSC.
- X. Fang, Z. Yin, H. Wang, J. Li, X. Liang, J. Kang and B. He, J. Catal., 2015, 329, 187–194 CrossRef CAS.
- A. Moharir , Cyclohexanol Market Report 2025 (Global Edition), https://www.cognitivemarketresearch.com/cyclohexanol-market-report#:~:text=Cyclohexanol%20Market%20Analysis-,According%20to%20Cognitive%20Market%20Research%2C%20the%20global%20Cyclohexanol%20market%20size,due%20to%20the%20pharmaceuticals%20manufacturing, (accessed June 2025 2025).
- K. C. Hwang and A. Sagadevan, Science, 2014, 346, 1495–1498 CrossRef CAS PubMed.
- K. Zhang, Q. Meng, H. Wu, J. Yan, X. Mei, P. An, L. Zheng, J. Zhang, M. He and B. Han, J. Am. Chem. Soc., 2022, 144, 20834–20846 CrossRef CAS.
- S. Wang, X. Li, R. Ma and G. Song, Acc. Chem. Res., 2025, 58, 529–542 CrossRef CAS PubMed.
- S. Wang, Q. Shen, S. Su, J. Lin and G. Song, Trends Chem., 2022, 4, 948–961 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed.
- W. Boerjan, J. Ralph and M. Baucher, Annu. Rev. Plant Biol., 2003, 54, 519–546 CrossRef CAS.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 8164–8215 CrossRef CAS PubMed.
- C. Li, X. Zhao, A. Wang, G. W. Huber and T. Zhang, Chem. Rev., 2015, 115, 11559–11624 CrossRef CAS.
- W. Schutyser, T. Renders, S. Van den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- G.-Y. Xu, J.-H. Guo, Y.-C. Qu, Y. Zhang, Y. Fu and Q.-X. Guo, Green Chem., 2016, 18, 5510–5517 RSC.
- Y. Nakagawa, M. Ishikawa, M. Tamura and K. Tomishige, Green Chem., 2014, 16, 2197–2203 RSC.
- T. Liu, Z. Tian, W. Zhang, B. Luo, L. Lei, C. Wang, J. Liu, R. Shu and Y. Chen, Fuel, 2023, 339, 126916 CrossRef CAS.
- C. E. J. J. Vriamont, T. Chen, C. Romain, P. Corbett, P. Manageracharath, J. Peet, C. M. Conifer, J. P. Hallett and G. J. P. Britovsek, ACS Catal., 2019, 9, 2345–2354 CrossRef CAS.
- Y. Zhao, J. Zhan, R. Hu, G. Luo, J. Fan, J. H. Clark and S. Zhang, Chem. Eng. J., 2024, 485, 149934 CrossRef CAS.
- G. S. More, D. R. Kanchan, A. Banerjee and R. Srivastava, Chem. Eng. J., 2023, 462, 142110 CrossRef.
- S. Verma, M. N. Nadagouda and R. S. Varma, Green Chem., 2019, 21, 1253–1257 RSC.
- Q. Dai, Z. Xu, S. Wang, X. Zeng, F. He, F. Yue, Z. Zhang, C. Ye, Y. Wang, C. Liu, P. Wang, M. Hou, G. Meng, W. Lan and D. Wang, Angew. Chem., 2025, 137, e202504347 CrossRef.
- X. Wang and R. Rinaldi, Energy Environ. Sci., 2012, 5, 8244–8260 RSC.
- W. Schutyser, S. Van den Bosch, J. Dijkmans, S. Turner, M. Meledina, G. Van Tendeloo, D. P. Debecker and B. F. Sels, ChemSusChem, 2015, 8, 1805–1818 CrossRef CAS PubMed.
- W. Song, Y. He, S. Lai, W. Lai, X. Yi, W. Yang and X. Jiang, Green Chem., 2020, 22, 1662–1670 RSC.
- J. Long, S. Shu, Q. Wu, Z. Yuan, T. Wang, Y. Xu, X. Zhang, Q. Zhang and L. Ma, Energy Convers. Manage., 2015, 105, 570–577 CrossRef CAS.
- Y. Yu, Y. Li, Y. Lou, M. Chen, Y. Liu and H. Yu, Adv. Sci., 2025, 12, 2500687 CrossRef CAS PubMed.
- Y. Zhu, Y. Ma, Y. Sun, W. Ji, L. Wang, F. Lin, Y. Li and H. Xia, ACS Catal., 2024, 14, 17004–17013 CrossRef CAS.
- X. Liu, L. Xu, G. Xu, W. Jia, Y. Ma and Y. Zhang, ACS Catal., 2016, 6, 7611–7620 CrossRef CAS.
- B. Wang, P. Zhou, X. Yan, H. Li, H. Wu and Z. Zhang, J. Energy Chem., 2023, 79, 535–549 CrossRef CAS.
- Z. Tian, X. Chen, T. Liu, J. Wang, C. Wang, R. Shu, J. Liu and Y. Chen, Renewable Energy, 2023, 218, 119304 CrossRef CAS.
- C. Chen, P. Liu, H. Xia, J. Jiang, X. Yang and M. Zhou, ACS Sustainable Chem. Eng., 2021, 9, 13937–13952 CrossRef CAS.
- M. Zhao, J. Hu, S. Wu, L. Yang, X. An, P. Yuan and P. Lu, Fuel, 2022, 308, 121979 CrossRef CAS.
- Q. Li, Q. Zhang, W. Xu, R. Zhao, M. Jiang, Y. Gao, W. Zhong, K. Chen, Y. Chen, X. Li and N. Yang, Adv. Energy Mater., 2023, 13, 2203955 CrossRef CAS.
- O. Lori and L. Elbaz, ChemCatChem, 2020, 12, 3434–3446 CrossRef CAS.
- Q. Gan, W. Zhou, X. Zhang, Y. Lin, S. Huang and G.-P. Lu, ChemSusChem, 2024, 17, e202400644 CrossRef CAS PubMed.
- S. Li, Z. Li, L. Duan, G. Yao, X. Wang, Y. Yang and Y. Wan, ACS Catal., 2025, 15, 8426–8441 CrossRef CAS.
- C. Buttersack, Phys. Chem. Chem. Phys., 2019, 21, 5614–5626 RSC.
- X. Gao, R. Ma, Z. Liu, S. Wang, Y. Wu and G. Song, Appl. Catal., B, 2024, 352, 124059 CrossRef CAS.
- R. Ge, L. Li, J. Su, Y. Lin, Z. Tian and L. Chen, Adv. Energy Mater., 2019, 9, 1901313 CrossRef.
- Z. Wu, Y. Wang, D. Liu, B. Zhou, P. Yang, R. Liu, W. Xiao, T. Ma, J. Wang and L. Wang, Adv. Funct. Mater., 2023, 33, 2307010 CrossRef CAS.
- T. De Saegher, J. E. Nordlander, F. Hallböök, B. Atanasova, P. Vermeir, K. M. Van Geem, J. De Clercq, A. Verberckmoes, C. Hulteberg and J. Lauwaert, Chem. Eng. J., 2025, 503, 158216 CrossRef CAS.
- S.-C. Sun, H. Jiang, Z.-Y. Chen, Q. Chen, M.-Y. Ma, L. Zhen, B. Song and C.-Y. Xu, Angew. Chem., Int. Ed., 2022, 61, e202202519 CrossRef CAS PubMed.
- D. Li, Y. Li, X. Liu, Y. Guo, C.-W. Pao, J.-L. Chen, Y. Hu and Y. Wang, ACS Catal., 2019, 9, 9671–9682 CrossRef CAS.
- Z. Liu, H. Li, X. Gao, X. Guo, S. Wang, Y. Fang and G. Song, Nat. Commun., 2022, 13, 4716 CrossRef CAS.
- S. Van den Bosch, W. Schutyser, R. Vanholme, T. Driessen, S. F. Koelewijn, T. Renders, B. De Meester, W. J. J. Huijgen, W. Dehaen, C. M. Courtin, B. Lagrain, W. Boerjan and B. F. Sels, Energy Environ. Sci., 2015, 8, 1748–1763 RSC.
- S. Kozuch and J. M. L. Martin, ACS Catal., 2012, 2, 2787–2794 CrossRef CAS.
- S. Fadlallah, P. S. Roy, G. Garnier, K. Saito and F. Allais, Green Chem., 2021, 23, 1495–1535 RSC.
- C. Libretti, L. S. Correa and M. A. R. Meier, Green Chem., 2024, 26, 4358–4386 RSC.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- M.-Y. Chen, Y.-B. Huang, H. Pang, X.-X. Liu and Y. Fu, Green Chem., 2015, 17, 1710–1717 RSC.
- C. Zhao, Y. Kou, A. A. Lemonidou, X. Li and J. A. Lercher, Angew. Chem., Int. Ed., 2009, 48, 3987–3990 CrossRef CAS PubMed.
- W. Song, Y. Liu, E. Baráth, C. Zhao and J. A. Lercher, Green Chem., 2015, 17, 1204–1218 RSC.
- S. Van den Bosch, W. Schutyser, S. F. Koelewijn, T. Renders, C. M. Courtin and B. F. Sels, Chem. Commun., 2015, 51, 13158–13161 RSC.
- X. Gao, H. Li, S. Wang, Z. Liu, J.-F. Ma, X.-E. Liu and G. Song, Renewable Energy, 2022, 201, 724–733 CrossRef CAS.
- S. Su, L.-P. Xiao, X. Chen, S. Wang, X.-H. Chen, Y. Guo and S.-R. Zhai, ChemSusChem, 2022, 15, e202200365 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |